1. Introduction
Epidemiology
Pancreatic ductal adenocarcinoma (PDAC) is the sixth
leading cause of mortality worldwide and the fourth in Europe. In
2018, 458,918 and 132,559 new cases were diagnosed worldwide and in
Europe, with 432,242 and 128,000 deaths, respectively (1). Observed trends in pancreatic cancer
mortality rates in Europe are stable or slightly increasing, and
the number of deaths caused by pancreatic cancer is expected to
increase with the continuous ageing of the European population
(1). At present, survival rates
for pancreatic cancer are not improving in Europe and are unequal
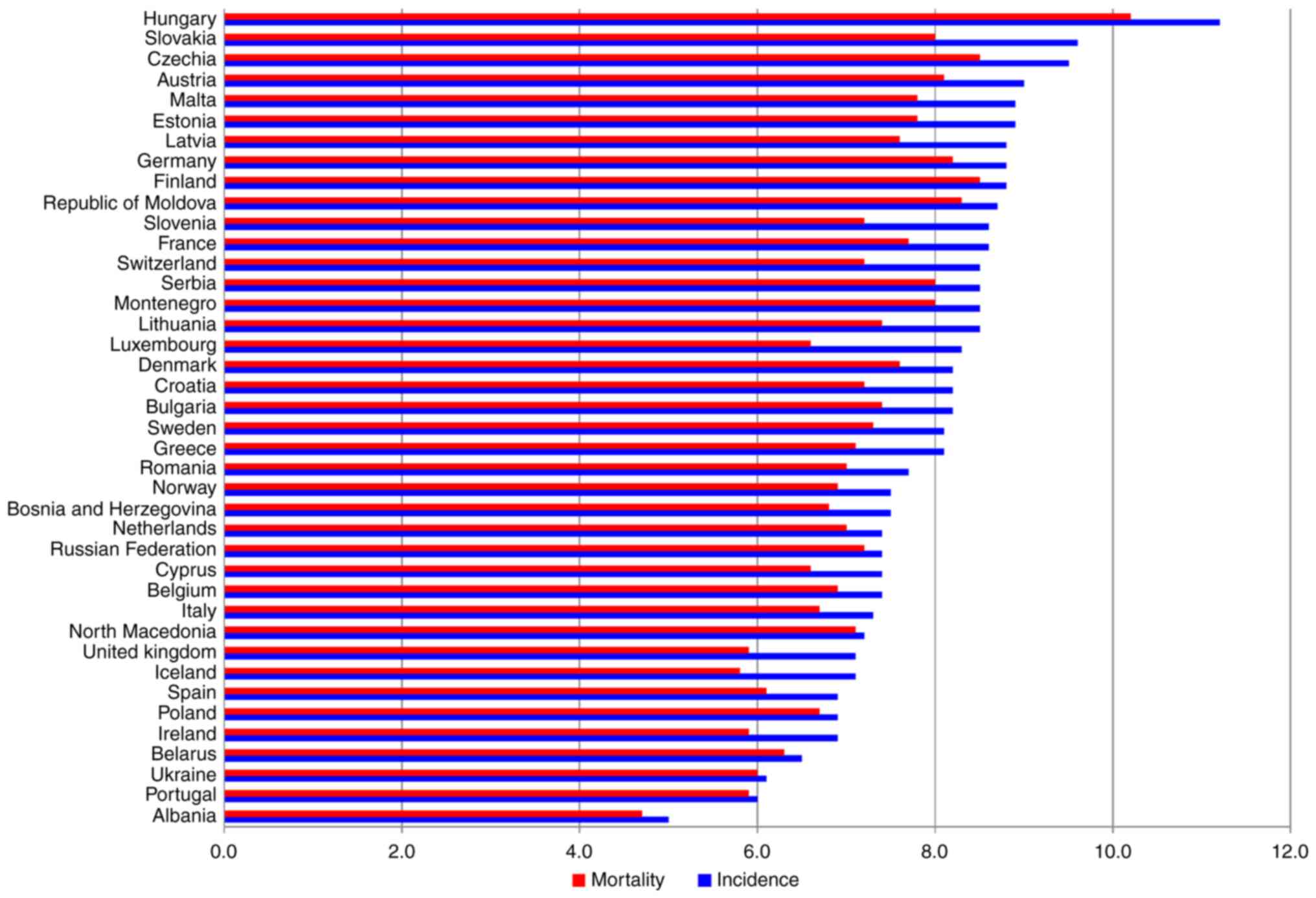
in different European countries. Fig.
1 represents the estimated age-standardized rates of pancreatic
cancer incidence (blue bars) and mortality (red bars) in 2020. Data
from Globocan (2) shows that the
gaps between the countries are substantial. For example, both the
incidence and mortality rates in Albania and Portugal are the
lowest, as opposed to Hungary and Czech Republic with one of the
highest incidence and mortality rates (3). These discrepancies may be attributed
to distinct genetic and cultural backgrounds, as well as exposure
to diverse environmental risk factors and different reporting
methods. Diagnosis and access to new treatment options could also
contribute to variations among different countries. The high
incidence rate in Europe, with 30.7% of the global incidence, can
reflect not only the epidemiology but also the ability for
diagnosis and the diagnostic approach (2,4).
PDAC is predominantly a disease affecting elderly
individuals (5,6). Its incidence peaks at >70 years,
and almost 90% of cases are diagnosed after the age of 55 years
(5,6). As expected, due to PDAC being one of
the most fatal malignant tumor types, the mortality/incidence ratio
is 98% (2,6). In Europe, 1-year survival rates
range from 10 to 23% and the 5-year survival rate is 3% (7).
At present, since there is no established screening
for early detection, and the causes of pancreatic cancer remain
elusive, primary prevention is of the utmost importance for
reducing PDAC burden (6,8).
Risk factors
Epidemiological studies have suggested different
modifiable risk factors for PDAC, including overweight and obesity
(8,9), physical inactivity (10), smoking (11,12), alcohol consumption (13,14) and diabetes mellitus (DM) (15), as well as non-modifiable risk
factors, such as age (16),
chronic pancreatitis (13,14)
and genetic factors/family history of PDAC (17,18). A previous systematic review and
meta-analysis of 23 prospective studies including 9,504 patients
revealed that a BMI >25 kg/m2 and high waist
circumference increase the risk of pancreatic cancer (8).
Smoking is a well-established modifiable risk factor
for PDAC (11). Nevertheless, it
accounts for <30% of the PDAC incidence (15). In total, 82 cohort and case
control studies published between 1950 and 2007 reported a relative
risk (RR) of 1.7 (95% CI, 1.6-1.9) for current smokers and 1.2 (95%
CI, 1.1-1.3) for former smokers, with the risk being maintained 10
years after smoking cessation (12).
The association of DM with PDAC is widely
recognized. A meta-analysis of 35 cohort studies, published in
2011, showed that DM was associated with an increased risk of PDAC
(RR=1.94; 95% CI, 1.66-2.27). These authors concluded that this
risk occurs in both sexes and that DM is considered both as an
early manifestation and an etiologic factor for PDAC (15).
Chronic pancreatitis is a well-known risk factor for
PDAC, although it can also be a consequence of the tumor due to
duct obstruction. A pooled analysis from the International
Pancreatic Cancer Case-Control Consortium demonstrated an
association between pancreatitis and PDAC (13). Mutations in the cationic
trypsinogen gene, serine protease 1 and in the serine protease
inhibitor gene (serine peptidase inhibitor Kazal type 1) cause
autosomal dominant and recessive forms of hereditary pancreatitis,
respectively (19-21). Patients with hereditary
pancreatitis have a 58-fold (95% CI, 23-105) increased risk of
developing PDAC compared with those without (22).
A pooled analysis from the Pancreatic Cancer Cohort
Consortium examined the association between family history of
cancer (pancreatic, prostate, ovarian, breast and colorectal) and
the risk of PDAC (17), and only
a family history of PDAC or prostate cancer were found to be
associated with an increased risk of PDAC.
Inherited conditions, such as familial Peutz-Jeghers
syndrome, confer very high relative risk for gastrointestinal
cancer types, namely PDAC (23).
Germline mutations in BRCA1 and BRCA2 also predispose
to PDAC. For instance, women that carry mutations in these genes
have twice the risk of PDAC compared with the women without
BRCA1 and BRCA2 mutations (24). Another inherited associated
condition of PDAC is Lynch syndrome caused by mutations in DNA
mismatch repair (MMR) genes, which shows a cumulative risk of PDAC
of 1.13% (95% CI, 0.31-2.32%) at <50 years of age and 3.68% (95%
CI, 1.45-5.88%) at <70 years of age (25). Other genetic alterations have been
associated with an increased risk of developing PDAC. For example,
deletion of P16 (p16-Leiden) carriers have a 17%
lifetime (by the age of 75 years) risk of developing pancreatic
cancer, and a mutation in P16/CDKN2A leads to a 38-fold
increased risk of developing PDAC and TP53 germline
mutations (18,26-29).
2. Pathogenesis
Pancreatic cancer types are usually adenocarcinomas
from the exocrine part of the gland, but a minority represents
neuroendocrine tumors arising from the endocrine pancreas. In most
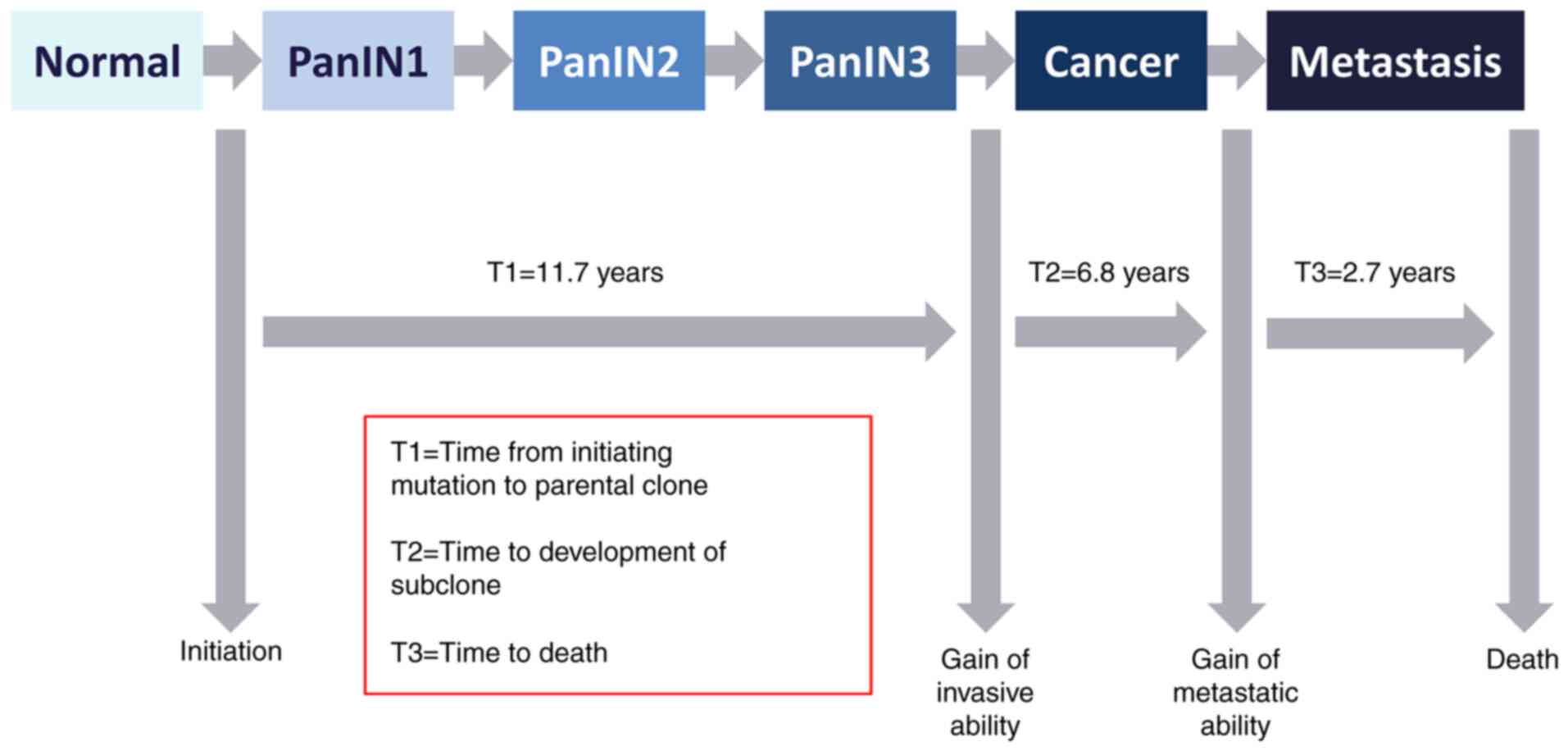
cases PDAC is the result of cumulative genetic alterations from
precursor lesions, pancreatic intraepithelial neoplasia (PanIN),
that are microscopic (<5 mm) and arise from pancreatic ducts.
PanIN1 represents mucinous differentiation of the ductal cells with
minimal atypia, and PanIN3 corresponds to carcinoma in situ.
The average time between the two grades could be 11.7 years
(30,31). Fig.
2 schematically depicts the evolution of PDAC.
From a pathology standpoint there are classical key
characteristics of PDAC that contribute to its aggressiveness and
poor prognosis, such as the type of pre-cancer lesions, stage at
diagnosis, including lymph node (N+) metastases,
metastatic spread, cell proliferation and programmed cell death,
genetic signature, cellular differentiation, epithelialmesenchymal
interaction, characteristics of the tumor stroma, immune response,
perineural invasion and cancerization of ducts (30,31). Another component, venous invasion,
may have importance in this context. In fact, 2/3 of patients with
PDAC who underwent surgical resection had identifiable venous
invasion (32,33), which could explain the rapid
dissemination to the liver.
The aggressiveness of PDAC has also been associated
with a complex genomic landscape (including frequent copy number
changes and point mutations, as well as SMAD4 loss), the
release of exosomes by the neoplastic cells, which facilitates
liver metastasis (34), the 'seed
and soil' hypothesis and the presence of circulating tumor cells.
The seed and soil hypothesis states that metastatic tumor cells
will metastasize to a site where the local microenvironment is
favorable and that the mechanical process of metastization is
determined by the pattern of blood flow containing circulating
tumor cells (35).
Although cancer gene amplification mainly occurs in
the earlier disease phases, genomic instability is maintained
throughout the different steps of the metastatic cascade, and the
genetic heterogeneity found in the different sites of metastases
may stem from the primary non-metastatic tissue, since the distinct
clones are shown to be already present in this tissue. Thus, early
genomic events in the tumor evolution can determine the later
molecular subtypes of PDAC (36).
Even though the different molecular subtypes are the
result of intra-tumoral subpopulations, there are recurrent
mutations that occur in KRAS, CDKN2A, TP53 and
SMAD4 pathways (37-40). These mutations appear to occur
after pre-neoplastic changes, which helps to explain the different
invasive phenotypes of PDAC. Although PDAC has been divided in two
subtypes, basal-like and classic, detailed genomic and
transcriptomic analyses have revealed that even within these
subtypes there is heterogeneity (38).
3. Treatment of patients with borderline
resectable and locally advanced tumors
The only curative treatment for PDAC is surgery with
negative margins. However, up to 30% of patients have locally
advanced disease at diagnosis and are not eligible for potentially
curative surgery (6). Patients
with locally advanced PDAC have an intermediate prognosis between
resectable and metastatic disease, with a median overall survival
(OS) of 9-11 months (41). The
classification of locally advanced PDAC is summarized in Table I.
 | Table INational Comprehensive Cancer Network
classification of locally advanced PDAC (40). |
Table I
National Comprehensive Cancer Network
classification of locally advanced PDAC (40).
| Classification
criteria of locally advanced PDAC | Borderline
resectable | Unresectable |
|---|
| SMA | Tumor abutment
≤180° | Solid tumor contact
of >180° |
| CHA | Reconstructible
short segment abutment | Contact with
extension to CA or hepatic artery bifurcation |
| CA | Tumor without
encasement/abutment | Solid tumor contact
of >180° or any contact with aortic involvement |
| SMV-PV | Tumor abutment of
SMV/PV of >180° or abutting ≤180° with irregularity of the vein
± thrombosis with anatomical structures that still allow for safe,
complete resection and vein reconstruction | Unreconstructible
due to tumor involvement or occlusion, or contact with most
proximal draining jejunal branch into SMV (can be due to tumor or
bland thrombus) |
The treatment approach, based on these criteria,
should be decided at multidisciplinary meetings (42). Moreover, due to the difficulty of
strict criteria of unresectability, to clearly differentiate
between locally advanced and borderline resectable tumors it should
be recommended that all cases of non-metastatic tumors are
discussed by a multidisciplinary team after induction therapy to
confirm definitive unresectability (43). Some key concepts used in this
setting do not have a universal definition, and so it is important
to clarify that henceforth the term 'induction chemotherapy (CT)'
will be used referring to the CT administered before radiotherapy
as part of a standard set of treatments (including CT, followed by
radiation and eventually surgery), and neoadjuvant CT will be used
referring to the CT administered before surgery as a first step to
shrink the tumor before the main treatment with curative intent
(44-46).
At present, the therapeutic strategy for locally
advanced PDAC is not optimized. The CT regimens applied in this
setting are based on data from trials with patients with metastatic
disease, with the selection between mFOLFIRINOX or gemcitabine +
nab-paclitaxel (GEM/nab-P) being determined by availability and
risk profile. The only consensus is that patients without surgical
indication should receive CT (46).
Another challenge is neoadjuvant therapy. FOLFIRINOX
or GEM/nab-P have been evaluated in this context (47,48). The efficacy of FOLFIRINOX and
GEM/nab-P was determined in the neoadjuvant setting in a
multicenter study including 274 patients with borderline resectable
(46.4%) and locally advanced (25.5%) pancreatic cancers. The
results demonstrated that both neoadjuvant chemotherapy regimens
improved biochemical, pathological and clinical responses, leading
to OS improvement (48). Another
retrospective study also evaluated the efficacy of FOLFIRINOX or
GEM/nab-P in the neoadjuvant setting in 56 patients with locally
advanced PDAC. Among the 35 patients treated with FOLFIRINOX and
the 21 treated with GEM/nab-P, 14 (40%) and 6 (28%) patients,
respectively, underwent surgery after CT. It was found that
disease-free survival in resected patients was not statistically
different between the two treatments. In the unresected group, the
progression-free survival (PFS) was 49.4 weeks with FOLFIRINOX and
was 30.9 weeks with GEM/nab-P (P=0.0029), whilst the median OS was
72.10 weeks with FOLFIRINOX and 53.30 weeks with GEM/nab-P
(P=0.06). In the resected group, after a follow-up of >12
months, the median OS was not reached in the FOLFIRINOX group and
was ~93.79 weeks in GEM/nab-P group. These differences were
significant when compared with the unresectable patients (P=0.0006
in the FOLFIRINOX group; P=0.0166 in the GEM/nab-P group). These
results indicated that, even in patients who did not undergo
surgery, there was a gain in PFS. However, although neoadjuvant
therapy plus surgery is valuable, there is a need for a long-term
follow-up (47).
In the American Society of Clinical Oncology (ASCO)
2020 meeting, an important trial was presented, which was the
ESPAC-5F trial, a four-arm prospective phase II trial of immediate
surgery compared with neoadjuvant GEM/capecitabine (CAP),
FOLFIRINOX or chemoradiotherapy in patients with borderline
resectable pancreatic cancer. This trial showed that this group of
patients had a survival advantage from neoadjuvant therapy (12
months survival of 40% for immediate surgery vs. 77% in the
neoadjuvant treatment) (49).
These data, referring to patients considered to have resectable
disease at diagnosis lend additional support to the use of a
neoadjuvant approach for advanced disease, although doubts remain
regarding the best therapy regimen. Regardless of these
aforementioned results, further research is necessary to understand
which factors could predict response, perioperative outcomes and
survival in patients submitted to induction CT. A recent study
revealed that, in both borderline and locally advanced PDAC, an
extended duration of CT with associated biochemical and
pathological responses was highly predictive of postoperative
survival (50). Modifying the
initial CT regimen and/or extending treatment duration until the
normalization of cancer antigen 19.9 (CA 19.9) or achieving
complete metabolic response may be beneficial. These factors may
improve survival despite the limited radiological response, which
is mandatory before surgery. Overall, it is crucial to complete the
most appropriate chemotherapy (50).
It can be concluded that both FOLFIRINOX and
GEM/nab-P could be proposed as induction therapy to patients with
locally advanced PDAC and in the neoadjuvant setting for
unresectable or borderline resectable cases. Furthermore, in some
patients, they can be used in sequence with or without
radiotherapy, which corresponds to a total neoadjuvant approach
(47,51).
Although different clinical trials are aiming to
address the impact of chemoradiotherapy in locally advanced PDAC,
its use remains controversial (52-55). LAP07 was an open-label phase III
trial that evaluated the effect of chemoradiotherapy (54 Gy plus
CAP) vs. CT (same regimen as the initial 4 months) after 4 months
of GEM with or without erlotinib in locally advanced PDAC. This
trial reported no differences in OS between groups, including
chemoradiotherapy vs. CT and GEM alone or GEM/erlotinib as
maintenance therapy. However, the chemoradiotherapy group
experienced a decrease in local progression (32 vs. 46%, P=0.03)
(52).
The SCALOP study was a multicenter phase II study
designed to evaluate the safety and efficacy of GEM-based and
CAP-based chemoradiation in 74 patients with locally advanced PDAC.
Patients underwent a 12-week induction period with GEM/CAP, and
patients with stable/responsive disease, tumor ≤6 cm and
performance status (PS) 0-1 were randomized to receive one
additional cycle of CAP or GEM with radiation (50.4 Gy in 28
fractions) (55). The first
results suggested that not only was the CAP-based regimen
preferable to the GEM-based regimen after the induction phase, it
was also more well tolerated. Notwithstanding, the difference in
the 9-month PFS, the primary endpoint, was not statistically
significant (55). Long-term
results of the SCALOP study revealed that the CAP-based
chemoradiation was superior regarding OS and PFS. These long-term
results also showed that patients with CA 19.9 <46 IU/ml were
more likely to benefit from chemoradiation following the induction
phase (54).
The Association des Gastro-Entérologues Oncologues
group recently published the data of a retrospective non-randomized
study including 203 patients with borderline or locally advanced
PDAC. This study evaluated the impact of the addition of
neoadjuvant chemoradiotherapy to a FOLFIRINOX induction regimen,
and showed a significantly higher R0 resection rate (89.2 vs.
76.3%; P=0.017), and increased downstaging and OS (57.8 vs. 35.5
months; P=0.007), suggesting that additional chemoradiotherapy may
be beneficial in the neoadjuvant setting (56).
The ALLIANCE A021501 study randomized patients with
borderline resectable pancreatic cancer to either eight cycles of
preoperative mFOLFIRINOX or seven cycles of preoperative
mFOLFIRINOX, followed by stereotactic body radiation therapy
(SBRT). The results demonstrated that neoadjuvant mFOLFIRINOX was
associated with favorable OS. Moreover, mFOLFIRINOX with
hypofractionated radiation therapy (RT) did not improve OS compared
with the historical data (57).
The use of SBRT in locally advanced PDAC has been
attempted. Compared with conventional fractionated radiation, SBRT
appears to have a limited role in this stage of PDAC. Although the
results of a retrospective study that matched 631 patients
undergoing SBRT with 7819 patients undergoing conventional
fractionated radiotherapy demonstrated that SBRT was associated
with an improvement in OS (58),
further studies are required to evaluate the precise role of this
new therapeutic option.
After neoadjuvant therapy some patients will become
surgical candidates and resection will be possible. Nevertheless,
for patients with an inadequate response to neoadjuvant CT,
radiotherapy could be added to increase the probability of
resection in locally advanced or borderline resectable tumors. This
is a case-by-case decision, and every patient must be discussed
after each therapy modality in a multidisciplinary board.
Currently, the role of radiotherapy in PDAC is not conclusively
established. However, novel RT techniques and the combination with
systemic therapy remain an active area of research.
4. Options in the treatment of metastatic
disease
Multidisciplinary collaboration is standard in the
care of patients with metastatic pancreatic cancer. The treatment
goals are to improve disease-related symptoms and prolong survival.
Since the currently available therapy is palliative and not
curative, additional clinical trials should be performed (59). Early initiation of palliative
care, alongside with disease-modifying treatment, should be
considered, especially for patients with high symptom burden. The
European Cooperative Oncology Group (ECOG) scale relating to
patient PS, comorbidity profile, nutritional status, patient
preferences, symptom burden and psychosocial issues should guide
treatment decision (60).
First line setting
Several trials and meta-analyses demonstrated that
CT, mainly 5-fluorouracil (5-FU), is superior to isolated best
supportive care (BSC) in prolonging OS and improving quality of
life (QOL) in patients with advanced pancreatic cancer (61). Table II summarizes the most important
randomized controlled trials (RCTs) in the first line setting.
 | Table IIMost important RCTs in the first line
setting. |
Table II
Most important RCTs in the first line
setting.
| Trial | Arms | Study
population |
ORRa | mPFS, months | mOS, months | (Refs.) |
|---|
| Burris et
al, 1997 | 5-FU (n=63) vs. GEM
(n=63) | - Patients with
advanced (locally advanced or meta static) symptomatic pancreas
cancer with a Karnofsky PS ≥50 and an estimated life expectancy ≥12
weeks ;
- Prior CT not allowed.
- Prior radiotherapy allowed if non-irradiated measur
able/assessable disease available. | 0.0 vs. 5.4% (not
statistically significant) | 0.92 vs. 2.3
(P=0.0002) | 4.41 vs. 5.65
(P=0.0025) | (62) |
| Moore et al,
2007 | GEM/placebo (n=284)
vs. GEM/erlotinib (n=285) | - Patients with
advanced (locally advanced or metastatic) pancreatic
adenocarcinoma, with measur able/assessable disease, and an ECOG PS
≤2;
- Prior CT not allowed, except for 5-FU or GEM given as a
radiosensitizer.
- Prior radiotherapy for local disease allowed. | 8.0 vs. 8.6% (not
statistically significant) | 3.55 vs. 3.75 (HR,
0.77; 95% CI, 0.64-0.92; P=0.004) | 5.91 vs. 6.24 (HR,
0.82; 95% CI, 0.69-0.99; P=0.038) | (63) |
| ACCORD 11 | GEM (n=169) vs.
FOLFIRINOX (n=167) | - Patients 75 years
old or younger, with measurable metastatic pancreatic
adenocarcinoma with an ECOG PS≤1.
- Prior CT not allowed.
- Previous radiotherapy for measurable lesions not allowed. | 9.4 vs. 31.6%
(P<0.001). | 3.3 vs 6.4 (HR,
0.47; 95% CI, 0.37-0.59; P<0.001) | 6.8 vs. 11.1 (HR,
0.57; 95% CI, 0.45-0.73; P<0.001). | (65) |
| MPACT | GEM (n=430) vs.
GEM/nab-P (n=431) | - Patients with
measurable pancreatic adenocarcinoma with metastatic disease
diagnosed ≤6 weeks before randomization, and a Karnofsky PS
≥70.
- Prior CT not allowed, except for 5-FU or GEM given as a
radiosensitizer in the adjuvant setting ≥6 months before
randomization. | 7 vs. 23% (HR,
3.19; 95% CI, 2.18-4.66, P<0.001) | 3.7 vs. 5.5 (HR,
0.69; 95% CI, 0.58-0.82; P<0.001) | 6.6 vs. 8.7 (HR,
0.72; 95% CI, 0.62-0.83; P<0.0001). | (66) |
In 1997, Burris et al (62) demonstrated GEM to be more
effective than 5-FU in palliation of disease-related symptoms, as
well as in improving QOL and OS, thereby establishing a new
standard of care. In the following years, several GEM-based
combination regimens were tested in randomized trials, such as the
PA.3 trial (63). When GEM-based
combinations were compared with GEM monotherapy in a 2013
meta-analysis, only a small OS improvement was observed in the
combination regimens [hazard ratio (HR), 0.93; P=0.001], with an
increased relative toxicity (64). However, in 2011, the superiority
of 6 months of FOLFIRINOX over GEM monotherapy was already
established by the phase III ACCORD 11 trial (65). FOLFIRINOX increased overall
response rate (ORR), PFS and OS, and despite the greater toxicity,
at 6 months significantly fewer patients in the FOLFIRINOX group
had a definitive QOL degradation, proving the classic FOLFIRINOX
regimen as an option for the treatment of patients with good PS
(65).
In 2013, the MPACT trial showed the combination of
GEM/nab-P superior to GEM monotherapy with regards to the median
OS, median PFS and ORR (66).
However, the rates of neuropathy and myelosuppression were also
increased.
At present, to the best of our knowledge, there are
no prospective, randomized trials, directly comparing GEM/nab-P to
FOLFIRINOX. The available evidence comes from retrospective studies
and systematic reviews, suggesting there is no major difference in
clinical outcomes apart from the toxicity profiles (67).
The GEST trial, a phase III study conducted in Japan
and Taiwan, showed the non-inferiority of S-1 compared to GEM in
terms of OS (8.8 vs. 9.7 months; HR, 0.96; 97,5% CI, 0.78-1,18;
P<0.001 for non-inferiority), with good tolerability. In the
same study, the combination of GEM and S-1 (GEM/S-1) was not
superior to GEM monotherapy (68). More recently, the combination of
nab-P with S-1 (nab-P/S-1) was compared to nab-P/GEM in a
single-center, Chinese phase II trial, showing comparable efficacy
in ORR, PFS and OS, with an improved safety profile (69). Furthermore, to the best of our
knowledge, in the Asian population, there are currently no
prospective trials comparing GEM/nab-P or nab-P/S-1 to FOLFIRINOX.
Therefore, in non-mutation carriers with metastatic pancreatic
cancer who are unable or unwilling to participate in a clinical
trial, the choice of first line regimen in Europe and United States
is usually made between GEM monotherapy, FOLFIRINOX and GEM/nab-P
(59,70,71). Most guidelines suggest FOLFIRINOX
or GEM/nab-P for PS 0-1 patients with a favorable comorbidity
profile, and a serum total bilirubin level <1.5 times the upper
limit of normal (ULN). The choice between the two regimens is
largely based on physician and patient preferences and toxicity
profiles, with gastrointestinal, hematological toxicities and
fatigue being more common with FOLFIRINOX and alopecia, and
neuropathy more evident with GEM/nab-P. For patients with serum
bilirubin >1.5 times ULN (without a treatable obstruction),
FOLFOX-6 may be considered (72).
In the Asian population, S-1 combinations can also be an
option.
After choosing the regimen, the second question is
the duration at which the patient is deriving a clinical benefit.
This is especially important considering most protocols can cause
cumulative sensory peripheral neuropathy and risk of
hypersensitivity reactions. To the best of our knowledge, there are
currently no randomized phase III trials comparing the maintenance
of the original regimen until disease progression or unacceptable
toxicity vs. original regimen for 4 or 6 months followed by 'CT
holidays' vs. original regimen for 4 or 6 months and then
deescalating to a maintenance regimen ('stop-and-go' strategy).
The phase II PRODIGE 35-PANOPTIMOX study compared 6
months of FOLFIRINOX vs. 4 months of FOLFIRINOX followed by 5-FU
maintenance treatment and FOLFIRINOX reintroduction at progression
vs. alternating GEM and FOLFIRI-3 every 2 months. In a preliminary
report presented at the 2018 annual ASCO meeting, 6-month PFS rates
were 47, 44 and 34% for the FOLFIRINOX, maintenance and alternating
arms, respectively. The median OS was 10.1, 11.2 and 7.3 months,
respectively. Unexpectedly, the rates of severe neurotoxicity were
higher in the maintenance therapy arm (19.8 vs. 10.2% of patients),
although it occurred later, possibly due to a higher cumulative
oxaliplatin dose in this group (73).
Another phase II trial enrolled 32 patients to four
cycles of FOLFOX-6, followed by three cycles of GEM and, in the
event of clinical benefit, followed by a maintenance treatment
based on the investigator's discretion. The median time to
progression and OS were 4 and 10 months, respectively, with no
evident efficacy or safety warning signs when compared with the
literature (72).
While phase III trials addressing this topic are
urgently required, and the available phase II trial evidence offers
novel options for maintenance, clinical decisions should be
individualized.
Elderly and frail patients
Despite contributing to the majority of cases of
pancreatic cancer, elderly patients (≥65 years old) (74) are repeatedly under-represented in
phase III RCTs (75,76). The scarcity of applicable results,
the specificities of this subgroup, including pharmacokinetic and
pharmacodynamic changes, and the irregular access to comprehensive
geriatric assessment, make metastatic pancreatic cancer treatment
even more challenging (76,77). Retrospective studies have shown
systemic therapy to be associated with longer OS in elderly
patients, with a median OS ranging from 8 to 12 months in this
subgroup, without compromising QOL (78-80). The available evidence suggests
that old age should not preclude patients with good PS, adequate
comorbidity profiles and social support from benefiting from
protocols such as FOLFIRINOX, mFOLFIRINOX, GEM/nab-P or GEM/CAP
(78,81-84).
For frail patients, with PS ≥2 or a comorbidity
profile that precludes intensive therapy, GEM monotherapy is
suggested. Where available, S-1 monotherapy is often considered an
alternative for frail patients, who prefer the convenience of an
oral regimen (71,72,85,86).
In selected patients with PS ≥2, especially when the
disability is due to heavy tumor burden, GEM/nab-P, GEM/CAP or
GEM/S-1 (where available) can be considered due to its higher ORR
(68,87,88). Patients with a PS ≥3 or poorly
controlled comorbid conditions are not included in clinical trials,
and BSC and QOL should always be emphasized.
BRCA or PALB2 mutation carriers
In total, 4-7% of patients with pancreatic cancer
have a germline BRCA mutation and up to 1.3% have a germline
PALB2 mutation. BRCA1, BRCA2 and PALB2 genes
are critical to double-strand DNA repair (88,89). Hence, pathogenic mutations in
these genes increase the risk of developing pancreatic cancer
(among others), and also render these cancer types more sensitive
to DNA damaging agents, such as platinum agents, and to drugs
targeting the DNA damage response pathway, including poly adenosine
diphosphate ribose polymerase (PARP) inhibitors (90,91). Therefore, the ASCO expert panel
recommends discussing germline genetic testing for patients with
pancreatic cancer, even when the family history is unremarkable
(92). Recently, the National
Comprehensive Cancer Network (NCCN) guidelines suggest germline
testing for any patient with confirmed pancreatic cancer, using
comprehensive genes panels for hereditary cancer syndromes
(93). Nowadays, the use of this
will also depend on the institution, patient characteristics and
access to PARP inhibitors.
In the phase III POLO trial, 154 patients with
germline BRCA-mutated metastatic PDAC who had not progressed
during ≥16 weeks of first-line platinum-based therapy were randomly
assigned to maintenance olaparib or placebo (94). The ORR was 23% in the olaparib
group (OR, 2.3; 95% CI, 0.89-6.76), with a median duration of
response of 24.9 months (CI could not be calculated). Among the
long-term survivors in the olaparib group (n=34), 14.7% (n=5)
achieved a complete response, as determined by investigator
assessment. No safety red flags or clinically meaningful
deterioration in health-related QOL emerged (95). The median PFS was significantly
longer in the olaparib group (7.4 vs. 3.8 months; HR, 0.53;
P=0.004) (96). At 3 years from
randomization, 21.5% of patients in the olaparib arm remained free
of subsequent cancer therapy vs. 3.6% in the placebo arm (HR, 0.44;
nominal P<0.0001) (96). The
median time from randomization to second disease progression or
death showed a benefiting trend in favor of olaparib but was not α
protected (median 16.9 and 9.3 months; HR, 0.66, 0.43-1.03;
P=0.0613). After a median follow up of 23.9 and 31.3 months, OS was
similar between groups (median 19.0 and 19.2 months; HR, 0.83; 95%
CI, 0.56-1.22; P=0.3487). However, the study was not statistically
powered to evidence survival differences (96).
On the other hand, a phase II trial of GEM and
cisplatin with or without veliparib in germline
BRCA/PALB2-mutated cases with stage III or IV pancreatic
cancer, was not statistically significant with regards the primary
end-point, as the ORR was 74.1 (with cisplatin/GEM and veliparib)
vs. 65.2% (cisplatin/GEM) (P=0.55) (97). These results reinforced the use of
a combination containing a platinum-based therapy in these
patients. Nevertheless, the unprecedented high ORR in the GEM plus
cisplatin arm reinforced this regimen as a highly effective CT
option in this setting.
Despite the absence of trials randomizing patients
with germline BRCA or PALB2 mutation between initial
platinum- vs. non-platinum-containing CT, the high ORR and
prolonged survivals support the recommendation of a platinum-based
CT regimen in the first line treatment of metastatic pancreatic
cancer arising in the setting of a known germline BRCA1 or
PALB2 mutation (98).
Moreover, maintenance with olaparib, based on the POLO trial, only
applies to patients with clinical benefit on initial platinum-based
CT. Depending on the PS, comorbidities, serum bilirubin and
preferences, FOLFIRINOX, mFOLFIRINOX, FOLFOX or GEM plus cisplatin
can be considered. In mutation carriers deriving clinical benefit
from a first line platinum-based CT, whether to maintain the
initial regimen, to deescalate to a maintenance CT regimen without
platinum, to stop all CT after 6 months or to switch to olaparib
remain unanswered questions. It is a matter of debate whether, in
the absence of an OS benefit and in the presence of some design
flaws (absence of an active control arm), the POLO trial can change
the standard of care (99).
Certainly, it is a new option in a field where targeted and
maintenance therapy are urgently required.
In most cases, metastatic pancreatic cancer is
diagnosed in a patient with unknown mutational status. Given that
molecular analysis usually takes ≥4 weeks to complete, the low
probability of mutation and the aggressiveness of pancreatic
cancer, it is not currently recommended to wait for the genetic
results to begin CT. Instead, treatment with a platinum-based
regimen can be started, although it is not wrong to begin with
GEM/nab-P. If the patient starts with GEM/nab-P and then is found
to have a germline BRCA or PALB2 mutation, a
platinum-containing regimen and PARP inhibitors may be considered
in subsequent lines (59,71,86).
FOLFIRINOX and GEM/nab-P are the two most consensual
regiments in the first-line therapy for patients with ECOG PS 0-1,
and the choice must be based on the toxicity profile of each
regimen and the physician and patient preferences. The center
experience in managing toxicities is also crucial in this context.
In frail and elderly patients, the recommended regimen is GEM
monotherapy, although S-1 can be preferred, if available, due to
patient convenience. According to the POLO trial, in the context of
BRCA-mutated metastatic PDAC, olaparib is a valid option as
a maintenance therapy for patients whose PDAC did not progress
during ≥16 weeks of first-line treatment with a platinum-based
protocol (86).
Second line setting
Upon progression, reevaluating the patient's PS,
comorbidities, residual toxicities and expectations is mandatory.
Second line therapy in advanced PDAC is not consensual and
different studies have been conducted with the aim of providing
clinicians with evidence regarding the most effective and secure
second line CT option.
A comprehensive analysis of 44 clinical trials
comparing therapeutic approaches in this setting concluded that
data regarding second line CT in PDAC are limited, and that
additional studies are required (100). Despite this, second line CT is
being increasingly considered in patients with PS <2. For PS ≤1
cases, the choice depends on the previous line of CT. For patients
with PS ≥2, in some cases, second line can be considered, although
controversial, and in several cases, it will be a monotherapy. Once
again, a PS ≥3 favors BSC (46,70,86,101).
After progression on GEM
Different studies have evaluated the best second
line therapeutic options for patients with advanced PDAC upon
progression on a GEM-based first line (100). A meta-analysis published in 2017
included five studies and 895 patients, and concluded that
combinations including oxaliplatin or irinotecan formulations
resulted in an improvement in PFS compared with monotherapy.
However, only the combination with a fluoropyrimidine conferred an
advantage regarding OS, although the evidence was scarce (102).
After progression under GEM-based CT in first line,
oxaliplatin, folinic acid and leucovorin (FA) and 5-FU (OFF) vs. FA
and 5-FU alone (FF) were tested in a German phase III study
including 168 patients. The median OS in the OFF group was
significantly increased compared with the FF group (5.9 vs. 3.3
months; P=0.010) (103,104). The rates of adverse effects
(AEs) were similar between groups. In clinical practice this has
become a widely used second line (104).
PANCREOX was a phase III trial that included
patients with advanced PDAC previously treated with GEM-based CT
who had a PS <2. Patients were randomized to receive bi-weekly
mFOLFOX6 vs. infusional 5-FU and leucovorin (5-FU/LV) until
progression. Unexpectedly, oxaliplatin association conferred no
benefit, thereby suggesting 5-FU/LV as a reasonable and well
tolerated second line (105).
For patients with progressive disease on GEM, other
oxaliplatin-based regimens are also active, namely combinations
with capecitabine (101,106), S-1 (107,108), GEM (109), irinotecan or docetaxel (110).
Nanoliposomal irinotecan plus 5-FU/LV was compared
with 5-FU/LV or nanoliposomal irinotecan alone in the NAPOLI-1
trial, which was a phase III study that included 417 patients with
metastatic PDAC previously treated with GEM-based therapy. The
median OS was, respectively, 6.2 vs. 4.2 (HR, 0.75; 95% CI,
0.57-0.99) vs. 4.9 months, favoring the group of nanoliposomal
irinotecan plus 5-FU/LV against the monotherapy arms. PFS, ORR and
disease control rate (DCR) also favored the nanoliposomal
irinotecan plus 5-FU/LV arm. The safety profile was considered
manageable, making this scheme an option in patients treated with
GEM-based CT in first line, according to the European Society for
Medical Oncology (ESMO) and ASCO guidelines (70,86).
After progression on
fluoropyrimidine
After a first line fluoropyrimidine-based CT (mostly
FOLFIRINOX), a second line GEM-based regimen is usually advised.
Moreover, the enrolment on clinical trials if possible is a
favorable choice. GEM/nab-P has been studied in a clinical trial
after FOLFIRINOX failure, which included 75 patients with advanced
pancreatic cancer (111).
Patients were treated with GEM/nab-P until disease progression,
patient refusal or unacceptable toxicity, for a median of four
cycles. A DCR of 58%, an ORR of 17.5%, a median OS of 8.8 months
(95% CI, 6.2-9.7) and a median PFS of 5.1 months (95% CI, 3.2-6.2)
were observed. Although grade 3-4 AEs were reported in 40% of the
patients, the authors considered the toxicity profile manageable,
concluding that GEM/nab-P appeared to be effective as second line
CT in patients with metastatic PDAC after
FOLFIRINOX/fluoropyrimidine-based first line (111).
The ESMO and ASCO guidelines both include GEM-based
CT (GEM/nab-P or GEM/CAP) as second line options in patients with
PS <2. For patients with PS ≥2, monotherapy with GEM and/or BSC
should be considered. For the majority of patients with PS ≥3, BSC
is the best option (59,70).
MMR-deficient/high microsatellite
instability (MSI)
MMR defects can lead to frequent somatic mutations
and high MSI and, consequently, to tumors that may be susceptible
to immune checkpoint blockade (112,113). The efficacy of pembrolizumab, an
anti-programmed cell death protein-1 (PD-1) reagent, was tested in
MSI/MMR-deficient non-colorectal cancer cases in the KEYNOTE-158
trial, a phase II study that included 233 patients with different
types of cancers, with PDAC being one of the most frequent types,
and in tumors that showed failure with prior therapy and had
received pembrolizumab. The ORR was 18.2% (95% CI, 20.3-66.5) and
median PFS was 9.2 months. Taken together, these results
demonstrated a clinical benefit of pembrolizumab in patients with
unresectable metastatic PDAC with high microsatellite
instability/deficiency in DNA MMR (114).
Recently, the COMBAT trial, a single-arm phase IIa
study that enrolled 37 patients, assessed the safety, efficacy and
immunobiological effects of C-X-C motif chemokine receptor 4 and
PD-1 blockade, with BL-8040 and pembrolizumab, respectively, in
patients with metastatic PDAC refractory to one or more previous
lines of CT (115). The DCR was
34.5% in the evaluable population (31% with stable disease and 3.4%
with partial response). The median OS was 3.3 months in the
intention to treat population and 7.5 months in patients receiving
the drugs as second line therapy (115). These data suggested that BL-8040
and pembrolizumab may increase the benefit of CT, but further
randomized trials are required.
A rare subtype of PDAC (0.34-0.5%) has
neutrophic-TRK (NTRK) gene fusions and may benefit from
larotrectinib or entrectinib therapy (116-119). This issue will be addressed
elsewhere in this article.
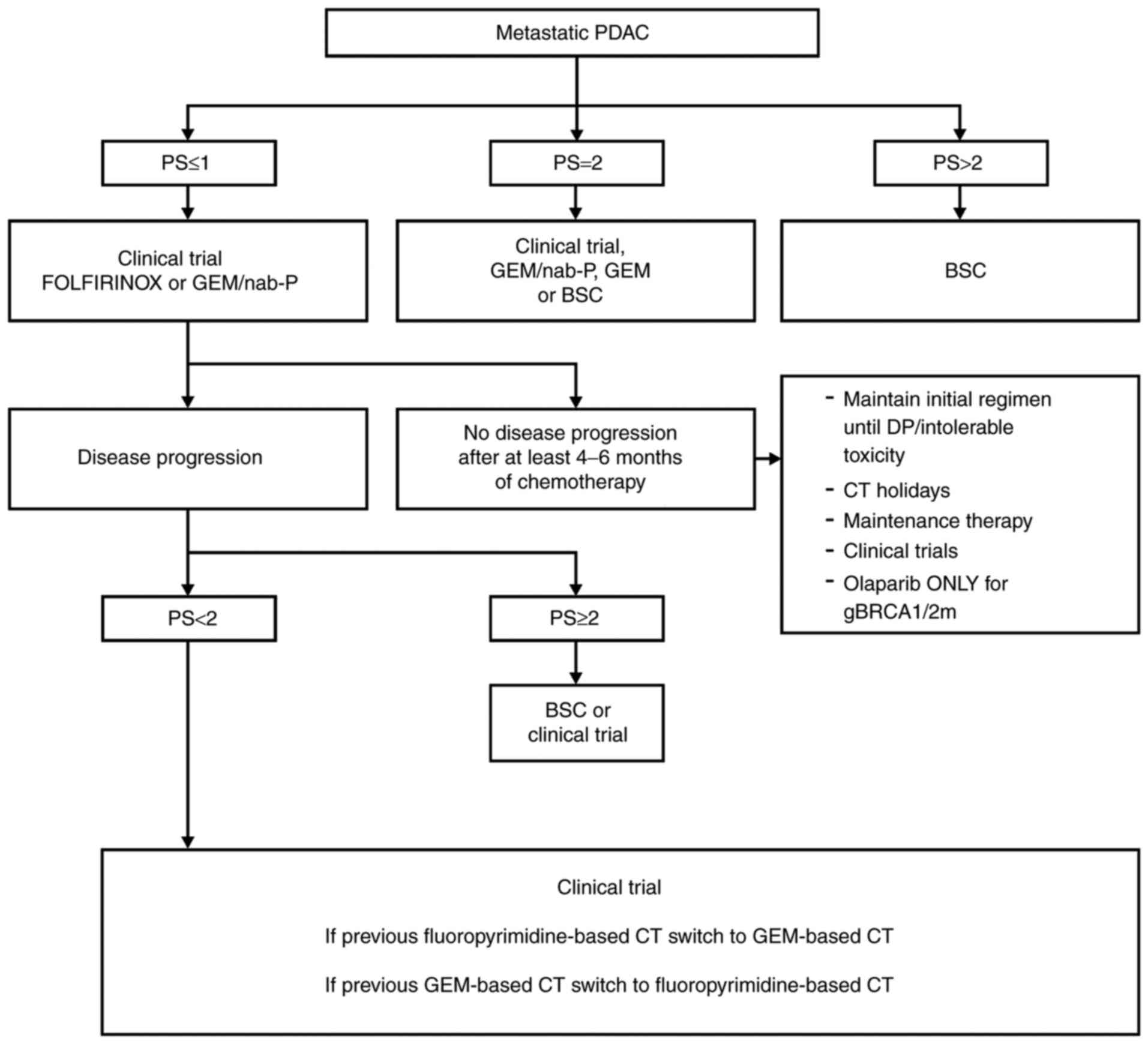
Collectively the second line options depend on
various factors, including the first line therapy and patient
clinical characteristics, such as PS, co-morbidities, residual
toxicities, and patient expectations. For second line
therapy-eligible patients, after progression on gemcitabine, both
the combination of oxaliplatin to 5-FU/LV and nanoliposomal
irinotecan plus 5-FU/LV are options. On the other hand, GEM-based
CT after a fluoropyrimidine-based CT (usually FOLFIRINOX) is
recommended. In patients with MMR-deficient/MSI-high tumors,
immunotherapy can be an option as there are some data regarding
pembrolizumab, irrespectively of first-line treatment. Integrating
this information, Fig. 3
represents a proposal for PDAC treatment in the metastatic setting
(120).
5. New therapeutic targets and
biomarkers
The lack of therapeutic options that significantly
improve the prognosis of patients not eligible for surgical
resection, and the absence of biomarkers able to detect early
lesions, are reflected in the poor prognosis of this disease. Thus,
new therapeutic strategies are required to control and eradicate
PDAC. Since its cells are genetically unstable, and the number of
somatic mutations appears to be higher in the genomes of cancer
types with MMR deficiency, the efforts to apply immunotherapy to
PDAC treatment are a major focus of current research.
Recently, the genome of 385 patients with PDAC was
studied, and the main mutational signatures were defects in DNA
repair, with MMR deficiency identified in 1% of the tumors
harboring different mechanism of mutL homolog 1 and mutS homolog 2
somatic inactivation (121).
Despite these recent data, there remain numerous important research
areas to explore and obstacles to overcome in PDAC, such as who to
treat and how therapy should be delivered (122).
Beyond immune checkpoint inhibitors there are three
other main classes of immunotherapy being evaluated, including:
Vaccines, adaptive T cell therapy and monoclonal antibodies.
Currently, neither vaccines or monoclonal antibodies have shown
significant benefit. Regarding adaptive T cell therapy, there are
very few identified potential antigens and its cost and
time-consuming process hamper its progress in PDAC, which has been
very slow (122).
Various research projects, using different
laboratory models, have been performed in the past years to
overcome PDAC. Global genomic analysis could provide new insights
on its pathogenesis, as most of the mutations found are point
mutations that occur in 67-100% of the tumors and affect 12
cellular core signaling pathways, which could explain the major
features of pancreatic tumorigenesis (122,123).
It is known that epidermal growth factor receptor
(EGFR) is expressed during pancreatic injury and in the
pre-neoplastic lesions. The KRAS oncogenes are dependent on
EGFR signaling, and loss of EGFR increases tumor latency and
survival. In addition, tumor explants lacking p53 and EGFR are
sensitive to the combined inhibition of PI3K and STAT3 (124-127). Based on these findings,
successful strategies for the treatment of advanced human PDAC may
require the inhibition of four signaling cascades driven by KRAS,
EGFR, PI3K and STAT3 (125). A
study with engineered exosomes carrying small interfering RNA
(siRNA) or short hairpin RNA specific to oncogenic
KRASG12D (iExosomes) demonstrated that these
structures were able to suppress pancreatic cancer growth in
multiple mouse models and significantly increase OS (128). Based on these results, a phase I
clinical trial is underway to evaluate the best dose and the side
effects of mesenchymal stromal cells-derived exosomes with
KRASG12D siRNA (iExosomes) in treating PDAC cases
with KRASG12D mutation that has spread (129).
In a previous study in which xenografts of
resectable PDAC were implanted in nude mice, the rate of
engraftment was 61%, and the authors concluded that SMAD4
inactivation in tumors contributed to the engraftment rate
(130). Moreover, engraftment
was a factor of poor prognosis, and these tumors had a metastatic
gene expression signature. This model could be useful to perform
drug screening, biomarkers research and help further understand the
biology of PDAC (130). The use
of mouse models has already identified Hedgehog signaling as a key
biological feature in the metastatic spread (131), which was associated with an
inefficient drug delivery that could contribute to
chemo-resistance, namely to GEM. The co-administration of a
Hedgehog signaling inhibitor and GEM increases the intra-tumoral
concentration of GEM (132). The
ineffective delivery of CT drugs was also restored in murine models
through the ablation of the abundant matrix glycosaminoglycan that
creates a drug-free sanctuary in PDAC (133). This intrinsic chemo-resistance
characteristic is associated with a growth-permissive tumor
environment. Reprograming the tumor stroma could enable an
increased response to CT, which may be accomplished by adding
vitamin D to the standard therapy (134). Although adding calcipotriol to
cell lines and murine models reduces inflammatory markers and
fibrosis, which can favor drug activity (134), data from the largest combination
of European cohort studies did not show a difference in PDAC risk
associated with the pre-diagnostic concentrations of vitamin D
(135). This may be justified by
the simultaneous reduction in T cell effector functions, which
could compromise the patients' tumor immune surveillance. Moreover,
calcipotriol dual effects can play and important role in PDAC
(136).
To further understand the biology of metastatic
PDAC, large scale multi-omics approaches have been used. A more
mesenchymal transcriptomic subtype has been associated with
metastases and consequently with poor prognosis (127,137). Nevertheless, it appears not to
be sufficient since cancer cells require a pre-metastatic niche to
survive in a distant organ (137). One of the players in the
construction of the immunosuppressive microenvironment in locally
advanced PDAC is the chemokine axis C-C motif chemokine ligand
2/C-C motif chemokine receptor (CCR) (138). In pre-clinical models, it has
been reported that the inhibition of this axis leads to the
restoration of antitumor immunity (138). As aforementioned, based on these
data, a phase Ib clinical trial was performed to test the safety
and tolerability of an CCR2 inhibitor in combination with
FOLFIRINOX. The results showed that the combination is safe and
tolerable (127).
Another important family of receptors that are
associated with cancer include the neutrophic-TRK (NTRK)
genes (139). Fusions involving
these genes appear to be oncogenic drivers (139). These fusions are observed in
0.31% of adult tumors, although other alterations such as
mutations, amplifications and mRNA overexpression were found to
occur in 14.2% of the 13,467 analyzed samples (primary cancer and
matched sampled) from The Cancer Genome Atlas (116). Entrectinib, a selective tyrosine
kinase inhibitor of the C-ros oncogene 1 (ROS1), TRK and anaplastic
lymphoma kinase (ALK), has shown clinical activity in patients with
different locally advanced or metastatic solid tumors (140). In total, 3 cases of PDAC with
gene fusions (2 with translocated promotor region-NTRK and 1
with scarecrow-like protein 4-ROS1) were treated with
entrectinib and showed clinical improvement and CA 19.9
normalization (139). These data
revealed the importance of earlier molecular testing contributing
to a personalized therapy in the context of PDAC and allowing for
improved survival and ORR. It has also been shown that gene fusion
of ALK leads to constitutive activation of oncogenic
pathways (140). Furthermore,
inhibition of the ALK signaling pathway by using crizotinib in
pancreatic cancer cell lines resulted in the inhibition of cell
proliferation/angiogenesis and in apoptosis induction (141). Thus, this drug could potentially
be a new therapeutic agent for PDAC.
The early detection of PDAC without the use of
invasive methods is challenging (142). Currently, the only used serum
marker for PDAC is CA 19.9 (143,144), and its elevation correlates with
advanced PDAC and poor prognosis (145,146). However, high CA 19.9 levels can
also be caused by numerous other conditions, including various
benign diseases (147) or other
cancer types (145). Therefore,
CA 19.9 is not recommended as a screening marker for PDAC (146). Ongoing studies are focusing on
directly exploring if CA 19.9 in combination with other markers
could yield improved sensitivity and specificity.
Recently the potential for using liquid biopsy has
been investigated, more specifically through the analysis of
circulating tumor cells, circulating tumor DNA and exosomes. These
last structures are extracellular vesicles released by all cells,
including cancer cells, which carry genetic and molecular material
providing a view of the content of the cells of origin (148). Exosomes are therefore a
promising biological material from patients with cancer that
require further validation and technological improvement allowing
for their use in early detection and disease monitoring (149,150). Besides these reported studies,
there are other currently ongoing phase III trials in PDAC using
new therapeutic targets and approaches, which are summarized in
Table III.
 | Table IIIOngoing phase III trials in
pancreatic ductal adenocarcinoma. |
Table III
Ongoing phase III trials in
pancreatic ductal adenocarcinoma.
| Study | Main outcome |
|---|
| NAPOLI 3
(NCT04083235) | Condition:
Metastatic Adenocarcinoma of the Pancreas |
| Setting:
Frontline |
| n=750 (Estimated
study completion December 31, 2023) |
| Arms: Irinotecan
liposome injection/Oxaliplatin/5-FU/LV vs. nab-P/GEM |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: Until
progression or unacceptable toxicity |
| Primary outcome
measures: OS |
| PANOVA-3
(NCT03377491) | Condition: Locally
advanced Pancreas Adenocarcinoma |
| Setting:
Frontline |
| n=556 (Estimated
study completion September 2023) |
| Arms:
NovoTTF-100L(P) vs. GEM/nab-P |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: 4
years |
| Primary outcome
measures: OS |
| (NCT03126435) | Condition:
Locally/advanced and/or Metastatic Pancreas Adenocarcinoma who |
| failed on first
line FOLFIRINOX |
| Setting: 2nd
line |
| n=218 (Estimated
study completion June 2022) |
| Arms: EndoTAG-1/GEM
vs. GEM |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: Until
progressive disease, unacceptable toxicity or withdrawal of consent
occurs |
| Primary outcome
measures: OS |
| (NCT01954992) | Condition:
Metastatic Pancreatic Adenocarcinoma previously treated with
GEM |
| Setting: 2nd
line |
| n=480 (Estimated
study completion June 2021) |
| Arms: Glufosfamide
vs. 5-FU |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: 3-6
months |
| Primary outcome
measures: OS |
| Trybeca-1
(NCT03665441) | Condition: Pancreas
Adenocarcinoma who have failed only one prior line of systemic
anti-cancer therapy for advanced pancreatic cancer |
| Setting: 2nd
line |
| n=500 (Estimated
study completion April 2021) |
| Arms:
Eryaspase/nab-P/GEM vs. Irinotecan/LV/5-FU vs. FOLFIRI/LV/5-FU
vs. |
| GEM/nab-P OR
Irinotecan/5-FU/LV |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: 1 year
after last patient randomized |
| Primary outcome
measures: OS |
| (NCT03504423) | Condition:
Metastatic Pancreas Adenocarcinoma |
| Setting: 2nd
line |
| n=500 (Estimated
study completion March 2022) |
| Arms:
CPI-613/mFOLFIRINOX vs. FOLFIRINOX |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: At least
6 months |
| Primary outcome
measures: ORR |
| HEAT
(NCT01077427) | Condition: Resected
Pancreatic Adenocarcinoma |
| Setting:
Frontline |
| n=336 (Estimated
study completion March 2021) |
| Arms: GEM/CAP vs.
GEM/Cisplatin with regional hyperthermia |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: From
date of randomization until the date of first documented
progression or date of death from any cause, whichever came first,
assessed up to 60 months |
| Primary outcome
measures:DFS |
| (NCT04229004) | Condition:
Metastatic Pancreatic Cancer |
| Setting: 1st or 2nd
line |
| n=825 (Estimated
study completion February 20, 2024) |
| Arms: GEM/nab-P vs.
SM-88/methoxsalen/phenytoin/sirolimus vs. mFOLFIRINOX |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: Up to 2
years |
| Primary outcome
measures: OS |
| DIRECT
(NCT03899636) | Condition: Stage
III Pancreatic Cancer |
| Setting: 2nd
line |
| n=528 (Estimated
study completion December 2023) |
| Arms:
mFOLFIRINOX/IRE using NanoKnife System vs. mFOLFIRINOX |
| Allocation:
Randomized |
| Masking: None (Open
Label) |
| Follow-up: At least
24 months |
| Primary outcome
measures:OS |
| RELIANT
(NCT04329949) | Condition:
Metastatic Pancreatic Ductal Adenocarcinoma |
| Setting: 3rd or
subsequent lines |
| n=80 (Estimated
study completion January 2022) |
| Arms:
Relacorilant/nab-P |
| Allocation:
N/A |
| Masking: None (Open
Label) |
| Follow-up:
Enrolment through 24 months |
| Primary outcome
measures: ORR per BICR |
6. Conclusion
Nowadays, PDAC is a key issue in the oncology field
since its incidence is growing as much as its mortality. Among the
different available therapeutic options for the treatment of
advanced PDAC, results are modest, probably due to the complexity
of the disease, and the prognostic remains poor. Further economic
investment in basic research and integration of data provided by
distinct platforms (genomics, transcriptomics, proteomics,
epigenomics and metabolomics) offers a plethora of opportunities to
identify causal relationships occurring between molecular
alterations and phenotypes. The perspective of multi-omics as a
valuable tool to subtype tumors and provide prognosis is exciting
but is far from becoming a reality in medical routine.
Notwithstanding, the use of these high throughput technologies in
the pursuit of novel biomarkers and identification of therapeutic
opportunities is highly significant. This can provide a framework
in which multi-omics data integration can be translated in valuable
biomarkers with clinical utility. Furthermore, understanding the
balance and influence of the stroma in tumor evolution could
represent a significant step forward in therapeutic efficiency. The
development of guidelines for early detection in special risk
groups (genetic risk groups, individuals with relatives diagnosed
with pancreatic cancer, smokers, alcohol consumers, type 2
diabetics, patients with chronic pancreatitis and obese
individuals), as well as the implementation of national public
health plans, thereby creating awareness both in the medical
community and in the public, should also be priorities for
investment. The success of these strategies has been already
witnessed with other tumors and are imperative in pancreatic
cancer.
Availability of data and materials
Not applicable.
Authors' contributions
Study conception and design was developed by HM.
The manuscript draft was written by all authors. A critical
revision of the manuscript was performed by AGB, CFP, MM, NC, OS,
SAM and HM. All authors contributed for the acquisition, analysis,
and interpretation of data for the work, read and approved the
final version of the manuscript and agreed to be accountable for
all aspects of the work, ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. Data sharing is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Author AGB declares to have received fees from
Amgen, Inc., AstraZeneca plc, Bristol Myers Squibb, Incyte Corp,
Eli Lilly and Company, Merck Serono, MSD, Novartis International
AG, Laboratories Pierre Fabre, Roche Diagnostics and Laboratories
Servier, either by participation in advisory boards, as invited
speaker or in the form of research projects grants. Author CFP
declares to have received fees from AstraZeneca plc, Roche
Diagnostics, Pfizer, Inc., Daiichi-Sankyo Co., Ltd., Leo Farma A/S,
Novartis International AG and Grünenthal. Author MM declares to
have received fees from AstraZeneca plc, Roche Diagnostics, Merck
KGaA, MSD, Laboratories Servier, Bayer and Angelini Pharma from
participation in advisory boards. Author NC declares to have
received fees from Laboratories Servier from participation in
advisory boards. Authors MJB and OS declare to have received fees
from AstraZeneca plc. Author HM declares to have received fees from
AstraZeneca plc, Roche Diagnostics, Pfizer, Inc., Laboratories
Pierre Fabre, Incyte Corp., BMS, Novartis International AG, Amgen,
Inc., Laboratories Servier, Merck Serono, and Grünenthal either by
participation in advisory boards or as invited speaker.
Author SAM declares to hold patents in the area of
exosomes biology and that these are licensed to Codiak Biosciences,
Inc. Patents: i) Title: mirna biogenesis in exosomes for diagnosis
and therapy; Patent no. 20200255831; Filed: March 23, 2020;
Publication date: August 13, 2020; Inventors: Raghu Kalluri, Sónia
Melo; United States of America, US. ii) Title: miRNA biogenesis in
exosomes for diagnosis and therapy; Patent no. 20160024503; Filed:
March 14, 2014; Publication date: January 28, 2016; Inventors:
Raghu Kalluri, Sónia Melo; United States of America, US. iii)
Title: use of exosomes for the treatment of disease; Patent no.
20180177727; Filed: June 10, 2016; Publication date: June 28, 2018;
Inventors: Raghu Kalluri, Sónia Melo; United States of America, US.
iv) Title: use of exosomes for the treatment of disease; Patent no.
20190117570; Filed: November 28, 2018; Publication date: April 25,
2019; Inventors: Raghu Kalluri, Sónia Melo; United States of
America, US. v) Title: Use of exosomes for the treatment of
disease; Patent no. 10959952; Filed: November 28, 2018; Date of
Patent: March 30, 2021; Inventors: Raghu Kalluri, Sónia Melo;
United States of America, US. vi) Title: Analysis of genomic DNA,
RNA, and proteins in exosomes for diagnosis and theranosis; Patent
no. 20200200755; Filed: March 4, 2020; Publication date: June 25,
2020; Inventors: Raghu Kalluri, Sónia Melo; United States of
America, US. vii) Analysis of genomic DNA, RNA, and proteins in
exosomes for diagnosis and theranosis; Patent no. 20170059572;
Filed: December 4, 2014; Publication date: March 2, 2017;
Inventors: Raghu Kalluri, Sónia Melo; United States of America, US.
viii) Analysis of genomic DNA, RNA, and proteins in exosomes for
diagnosis and theranosis; Patent no. 20180045728; Filed: November
1, 2017; Publication date: February 15, 2018; Inventors: Raghu
Kalluri, Sónia Melo; United States of America, US. ix) Analysis of
genomic DNA, RNA, and proteins in exosomes for diagnosis and
theranosis; Patent no. 10598665; Filed: November 1, 2017; Date of
Patent: March 24, 2020; Inventors: Raghu Kalluri, Sónia Melo;
United States of America, US. x) Analysis of genomic DNA, RNA, and
proteins in exosomes for diagnosis and theranosis; Patent no.
9921223; Filed: December 4, 2014; Date of Patent: March 20, 2018;
Inventors: Raghu Kalluri, Sónia Melo; United States of America,
US.
Acknowledgments
Not applicable.
Funding
Editorial support, in the form of medical writing and editing
assistance, in the development of this manuscript was provided by
x2-Science Solutions, and was unconditionally funded by AstraZeneca
plc. AstraZeneca plc had no role in the conduct of the research, in
study design, in the collection, analysis and interpretation of
data, in the writing of the report or in the decision to submit the
article for publication.
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
AE
|
adverse effect
|
|
ALK
|
anaplastic lymphoma kinase
|
|
ASCO
|
American Society of Clinical
Oncology
|
|
BSC
|
best supportive care
|
|
CAP
|
capecitabine
|
|
CA 19.9
|
cancer antigen 19.9
|
|
CT
|
chemotherapy
|
|
DM
|
diabetes mellitus
|
|
ECOG
|
European Cooperative Oncology
Group
|
|
EGFRs
|
epidermal growth factor receptors
|
|
ESMO
|
European Society for Medical
Oncology
|
|
FA
|
folinic acid and leucovirin
|
|
HR
|
hazard ratio
|
|
MMR
|
mismatch-repair
|
|
MSI
|
microsatellite instability
|
|
NCCN
|
National Comprehensive Cancer
Network
|
|
NTRK
|
neutrophic-tropomyosin receptor
kinase
|
|
ORR
|
overall response rate
|
|
OS
|
overall survival
|
|
PanIN
|
pancreatic intraepithelial
neoplasia
|
|
PARP
|
poly adenosine diphosphate-ribose
polymerase
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
PFS
|
progression-free survival
|
|
PS
|
performance status
|
|
QOL
|
quality of life
|
|
RCTs
|
randomized controlled trial
|
|
RR
|
relative risk
|
|
RT
|
radiation therapy
|
|
SBRT
|
stereotactic body radiation
therapy
|
|
TRK
|
tropomyosin receptor kinases
|
|
ULN
|
upper limit of normal
|
References
|
1
|
Karim-Kos HE, de Vries E, Soerjomataram I,
Lemmens V, Siesling S and Coebergh JW: Recent trends of cancer in
Europe: A combined approach of incidence, survival and mortality
for 17 cancer sites since the 1990s. Eur J Cancer. 44:1345–1389.
2008. View Article : Google Scholar
|
|
2
|
International Agency for Research on
Cancer WHO Pancreas: Source: Globocan 2020. Available from:
http://gcoiarcfr/today/data/factsheets/cancers/13-Pancreas-fact-sheetpdf.
2020
|
|
3
|
American Cancer Society: Cancer Facts and
Figures. 2019.
|
|
4
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe Estimates
for 40 countries in 2012. Eur J Cancer. 49:1374–1403. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hidalgo M, Cascinu S, Kleeff J, Labianca
R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL and Heinemann V:
Addressing the challenges of pancreatic cancer: Future directions
for improving outcomes. Pancreatology. 15:8–18. 2015. View Article : Google Scholar
|
|
6
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carrato A, Falcone A, Ducreux M, Valle JW,
Parnaby A, Djazouli K, Alnwick-Allu K, Hutchings A, Palaska C and
Parthenaki I: A systematic review of the burden of pancreatic
cancer in europe: Real-world impact on survival, quality of life
and costs. J Gastrointest Cancer. 46:201–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aune D, Greenwood DC, Chan DS, Vieira R,
Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ and Norat T:
Body mass index, abdominal fatness and pancreatic cancer risk: A
systematic review and non-linear dose-response meta-analysis of
prospective studies. Ann Oncol. 23:843–852. 2012. View Article : Google Scholar
|
|
9
|
Nöthlings U, Wilkens LR, Murphy SP, Hankin
JH, Henderson BE and Kolonel LN: Body mass index and physical
activity as risk factors for pancreatic cancer: The multiethnic
cohort study. Cancer Causes Control. 18:165–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pratapwar M, Stenzel AE, Joseph JM,
Fountzilas C, Etter JL, Mongiovi JM, Cannioto R and Moysich KB:
Physical inactivity and pancreatic cancer mortality. J Gastrointest
Cancer. 51:1088–1093. 2020. View Article : Google Scholar
|
|
11
|
Bosetti C, Lucenteforte E, Silverman DT,
Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH, et
al: Cigarette smoking and pancreatic cancer: An analysis from the
international pancreatic cancer case-control consortium (Panc4).
Ann Oncol. 23:1880–1888. 2012. View Article : Google Scholar :
|
|
12
|
Iodice S, Gandini S, Maisonneuve P and
Lowenfels AB: Tobacco and the risk of pancreatic cancer: A review
and meta-analysis. Langenbecks Arch Surg. 393:535–545. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duell EJ, Lucenteforte E, Olson SH, Bracci
PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA, et
al: Pancreatitis and pancreatic cancer risk: A pooled analysis in
the international pancreatic cancer case-control consortium
(PanC4). Ann Oncol. 23:2964–2970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rebours V, Boutron-Ruault MC, Schnee M,
Férec C, Maire F, Hammel P, Ruszniewski P and Lévy P: Risk of
pancreatic adenocarcinoma in patients with hereditary pancreatitis:
A national exhaustive series. Am J Gastroenterol. 103:111–119.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ben Q, Xu M, Ning X, Liu J, Hong S, Huang
W, Zhang H and Li Z: Diabetes mellitus and risk of pancreatic
cancer: A meta-analysis of cohort studies. Eur J Cancer.
47:1928–1937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McWilliams RR, Maisonneuve P, Bamlet WR,
Petersen GM, Li D, Risch HA, Yu H, Fontham ET, Luckett B, Bosetti
C, et al: Risk factors for early-onset and very-early-onset
pancreatic adenocarcinoma: A pancreatic cancer case-control
consortium (PanC4) analysis. Pancreas. 45:311–316. 2016. View Article : Google Scholar :
|
|
17
|
Pancreatic Cancer Europe Pancreatic Cancer
Inequality Report. https://www.pancreaticcancereuropeeu/campaign/the-pancreatic-cancer-europe-inequality-report/.
2018
|
|
18
|
Klein AP, Lindström S, Mendelsohn JB,
Steplowski E, Arslan AA, Bueno-de-Mesquita HB, Fuchs CS, Gallinger
S, Gross M, Helzlsouer K, et al: An absolute risk model to identify
individuals at elevated risk for pancreatic cancer in the general
population. PLoS One. 8:e723112013. View Article : Google Scholar :
|
|
19
|
Whitcomb DC: Genetics of alcoholic and
nonalcoholic pancreatitis. Curr Opin Gastroenterol. 28:501–506.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whitcomb DC, Gorry MC, Preston RA, Furey
W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST,
Toskes PP, et al: Hereditary pancreatitis is caused by a mutation
in the cationic trypsinogen gene. Nat Genet. 14:141–145. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Witt H, Luck W, Hennies HC, Classen M,
Kage A, Lass U, Landt O and Becker M: Mutations in the gene
encoding the serine protease inhibitor, Kazal type 1 are associated
with chronic pancreatitis. Nat Genet. 25:213–216. 2000. View Article : Google Scholar
|
|
22
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giardiello FM, Brensinger JD, Tersmette
AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M and Offerhaus
JA: Very high risk of cancer in familial Peutz-Jeghers syndrome.
Gastroenterology. 119:1447–1453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iqbal J, Ragone A, Lubinski J, Lynch HT,
Moller P, Ghadirian P, Foulkes WD, Armel S, Eisen A, Neuhausen SL,
et al: The incidence of pancreatic cancer in BRCA1 and BRCA2
mutation carriers. Br J Cancer. 107:2005–2009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kastrinos F, Mukherjee B, Tayob N, Wang F,
Sparr J, Raymond VM, Bandipalliam P, Stoffel EM, Gruber SB and
Syngal S: Risk of pancreatic cancer in families with Lynch
syndrome. JAMA. 302:1790–1795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moskaluk CA, Hruban H, Lietman A, Smyrk T,
Fusaro L, Fusaro R, Lynch J, Yeo CJ, Jackson CE, Lynch HT and Kern
SE: Novel germline p16(INK4) allele (Asp145Cys) in a family with
multiple pancreatic carcinomas. Mutations in brief no 148 Online.
Hum Mutat. 12:701998. View Article : Google Scholar
|
|
27
|
Vasen HF, Gruis NA, Frants RR, van Der
Velden PA, Hille ET and Bergman W: Risk of developing pancreatic
cancer in families with familial atypical multiple mole melanoma
associated with a specific 19 deletion of p16 (p16-Leiden). Int J
Cancer. 87:809–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldstein AM, Struewing JP, Fraser MC,
Smith MW and Tucker MA: Prospective risk of cancer in CDKN2A
germline mutation carriers. J Med Genet. 41:421–424. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rutter JL, Bromley CM, Goldstein AM, Elder
DE, Holly EA, Guerry D IV, Hartge P, Struewing JP, Hogg D, Halpern
A, et al: Heterogeneity of risk for melanoma and pancreatic and
digestive malignancies: A melanoma case-control study. Cancer.
101:2809–2816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iacobuzio-Donahue CA: Genetic evolution of
pancreatic cancer: Lessons learnt from the pancreatic cancer genome
sequencing project. Gut. 61:1085–1094. 2012. View Article : Google Scholar
|
|
32
|
Noë M, Rezaee N, Asrani K, Skaro M, Groot
VP, Wu PH, Olson MT, Hong SM, Kim SJ, Weiss MJ, et al:
Immunolabeling of cleared human pancreata provides insights into
three-dimensional pancreatic anatomy and pathology. Am J Pathol.
188:1530–1535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamada M, Sugiura T, Okamura Y, Ito T,
Yamamoto Y, Ashida R, Sasaki K, Nagino M and Uesaka K: Microscopic
venous invasion in pancreatic cancer. Ann Surg Oncol. 25:1043–1051.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fidler IJ and Poste G: The 'seed and soil'
hypothesis revisited. Lancet Oncol. 9:8082008. View Article : Google Scholar
|
|
36
|
Wang S, Zheng Y, Yang F, Zhu L, Zhu XQ,
Wang ZF, Wu XL, Zhou CH, Yan JY, Hu BY, et al: The molecular
biology of pancreatic adenocarcinoma: Translational challenges and
clinical perspectives. Signal Transduct Target Ther. 6:2492021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Campbell PJ, Yachida S, Mudie LJ, Stephens
PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren
S, Lin ML, et al: The patterns and dynamics of genomic instability
in metastatic pancreatic cancer. Nature. 467:1109–1113. 2010.
View Article : Google Scholar
|
|
38
|
Chan-Seng-Yue M, Kim JC, Wilson GW, Ng K,
Figueroa EF, O'Kane GM, Connor AA, Denroche RE, Grant RC, McLeod J,
et al: Transcription phenotypes of pancreatic cancer are driven by
genomic events during tumor evolution. Nat Genet. 52:231–240. 2020.
View Article : Google Scholar
|
|
39
|
Hruban RH, Gaida MM, Thompson E, Hong SM,
Noë M, Brosens LA, Jongepier M, Offerhaus GJA and Wood LD: Why is
pancreatic cancer so deadly? The Pathologist's view. J Pathol.
248:131–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cancer Genome Atlas Research Network:
Electronic address: simpleandrew_aguirre@dfci.harvard.edu
and Cancer Genome Atlas Research Network: Integrated genomic
characterization of pancreatic ductal adenocarcinoma. Cancer Cell.
32:185–203.e13. 2017. View Article : Google Scholar
|
|
41
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)
Pancreatic Adenocarcinoma. Version 1.2020. November 26–2019.
|
|
43
|
Lambert A, Schwarz L, Borbath I, Henry A,
Van Laethem JL, Malka D, Ducreux M and Conroy T: An update on
treatment options for pancreatic adenocarcinoma. Ther Adv Med
Oncol. 11:17588359198755682019. View Article : Google Scholar :
|
|
44
|
Devisetty K and Wong SJ: Neoadjuvant
versus induction chemotherapy: More than semantics. J Clin Oncol.
31:2971–2972. 2013. View Article : Google Scholar
|
|
45
|
NIH-National Cancer Institute NCI
Dictionary of Cancer Terms. Available from: http://www.cancergov/dictionary. Accessed September
27, 2021.
|
|
46
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)
Pancreatic Adenocarcinoma Version 2.0 2021-February 25 2021.
www.nccn.org. 2021
|
|
47
|
Napolitano F, Formisano L, Giardino A,
Girelli R, Servetto A, Santaniello A, Foschini F, Marciano R,
Mozzillo E, Carratù AC, et al: Neoadjuvant treatment in locally
advanced pancreatic cancer (LAPC) patients with FOLFIRINOX or
gemcitabine NabPaclitaxel: A single-center experience and a
literature review. Cancers (Basel). 11:9812019. View Article : Google Scholar
|
|
48
|
Macedo FI, Ryon E, Maithel SK, Lee RM,
Kooby DA, Fields RC, Hawkins WG, Williams G, Maduekwe U, Kim HJ, et
al: Survival outcomes associated with clinical and pathological
response following neoadjuvant FOLFIRINOX or
gemcitabine/Nab-paclitaxel chemotherapy in resected pancreatic
cancer. Ann Surg. 270:400–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ghaneh P, Palmer DH, Cicconi S, Halloran
C, Psarelli E, Rawcliffe C, Sripadam R, Mukherjee S, Wadsley J,
Al-Mukhtar A, et al: ESPAC-5F: Four-arm, prospective, multi-center,
international randomized phase II trial of immediate surgery
compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or
FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline
resectable pancreatic cancer. J Clin Oncol. 38:4505. 2020.
View Article : Google Scholar
|
|
50
|
Truty MJ, Kendrick ML, Nagorney DM, Smoot
RL, Cleary SP, Graham RP, Goenka AH, Hallemeier CL, Haddock MG,
Harmsen WS, et al: Factors predicting response, perioperative
outcomes, and survival following total neoadjuvant therapy for
Borderline/locally advanced pancreatic cancer. Ann Surg.
273:341–349. 2021. View Article : Google Scholar
|
|
51
|
Kaufmann B, Hartmann D, D'Haese JG,
Stupakov P, Radenkovic D, Gloor B and Friess H: Neoadjuvant
treatment for borderline resectable pancreatic ductal
adenocarcinoma. Dig Surg. 36:455–461. 2019. View Article : Google Scholar
|
|
52
|
Hammel P, Huguet F, van Laethem JL,
Goldstein D, Glimelius B, Artru P, Borbath I, Bouché O, Shannon J,
André T, et al: Effect of chemoradiotherapy vs chemotherapy on
survival in patients with locally advanced pancreatic cancer
controlled after 4 months of gemcitabine with or without erlotinib:
The LAP07 randomized clinical trial. JAMA. 315:1844–1853. 2016.
View Article : Google Scholar
|
|
53
|
Hammel P, Lacy J, Portales F, Sobrero AF,
Pazo Cid RA, Mozo JLM, Terrebonne E, Dowden SD, Li JS, Ong TJ, et
al: Phase II LAPACT trial of nab-paclitaxel (nab-P) plus
gemcitabine (G) for patients with locally advanced pancreatic
cancer (LAPC). J Clin Oncol. 36:2042018. View Article : Google Scholar
|
|
54
|
Hurt CN, Falk S, Crosby T, McDonald A, Ray
R, Joseph G, Staffurth J, Abrams RA, Griffiths G, Maughan T and
Mukherjee S: Long-term results and recurrence patterns from SCALOP:
A phase II randomised trial of gemcitabine- or capecitabine-based
chemoradiation for locally advanced pancreatic cancer. Br J Cancer.
116:1264–1270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mukherjee S, Hurt CN, Bridgewater J, Falk
S, Cummins S, Wasan H, Crosby T, Jephcott C, Roy R, Radhakrishna G,
et al: Gemcitabine-based or capecitabine-based chemoradiotherapy
for locally advanced pancreatic cancer (SCALOP): A multicentre,
randomised, phase 2 trial. Lancet Oncol. 14:317–326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pietrasz D, Turrini O, Vendrely V, Simon
JM, Hentic O, Coriat R, Portales F, Le Roy B, Taieb J, Regenet N,
et al: How does chemoradiotherapy following induction FOLFIRINOX
improve the results in resected borderline or locally advanced
pancreatic adenocarcinoma? An AGEO-FRENCH multicentric cohort. Ann
Surg Oncol. 26:109–117. 2019. View Article : Google Scholar
|
|
57
|
Katz MH, Ou FS, Herman JM, Ahmad SA,
Wolpin B, Marsh R, Behr S, Shi Q, Chuong M, Schwartz LH, et al:
Alliance for clinical trials in oncology (ALLIANCE) trial A021501:
Preoperative extended chemotherapy vs. chemotherapy plus
hypofractionated radiation therapy for borderline resectable
adenocarcinoma of the head of the pancreas. BMC Cancer. 17:5052017.
View Article : Google Scholar :
|
|
58
|
Zhong J, Patel K, Switchenko J, Cassidy
RJ, Hall WA, Gillespie T, Patel PR, Kooby D and Landry J: Outcomes
for patients with locally advanced pancreatic adenocarcinoma
treated with stereotactic body radiation therapy versus
conventionally fractionated radiation. Cancer. 123:3486–3493. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sohal DP, Kennedy EB, Khorana A, Copur MS,
Crane CH, Garrido-Laguna I, Krishnamurthi S, Moravek C, O'Reilly
EM, Philip PA, et al: Metastatic pancreatic cancer: ASCO clinical
practice guideline update. J Clin Oncol. 36:2545–2556. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sultana A, Tudur Smith C, Cunningham D,
Starling N, Neoptolemos JP and Ghaneh P: Meta-analyses of
chemotherapy for locally advanced and metastatic pancreatic cancer:
Results of secondary end points analyses. Br J Cancer. 99:6–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ciliberto D, Botta C, Correale P, Rossi M,
Caraglia M, Tassone P and Tagliaferri P: Role of gemcitabine-based
combination therapy in the management of advanced pancreatic
cancer: A meta-analysis of randomised trials. Eur J Cancer.
49:593–603. 2013. View Article : Google Scholar
|
|
65
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar
|
|
66
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar
|
|
67
|
Pusceddu S, Ghidini M, Torchio M, Corti F,
Tomasello G, Niger M, Prinzi N, Nichetti F, Coinu A, Di Bartolomeo
M, et al: Comparative effectiveness of gemcitabine plus
nab-Paclitaxel and FOLFIRINOX in the First-Line setting of
metastatic pancreatic cancer: A systematic review and
Meta-Analysis. cancers (Basel). 11:4842019. View Article : Google Scholar
|
|
68
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zong Y, Yuan J, Peng Z, Lu M, Wang X, Shen
L and Zhou J: Nab-paclitaxel plus S-1 versus nab-paclitaxel plus
gemcitabine as first-line chemotherapy in patients with advanced
pancreatic ductal adenocarcinoma: A randomized study. J Cancer Res
Clin Oncol. 147:1529–1536. 2021. View Article : Google Scholar
|
|
70
|
Ducreux M, Cuhna AS, Caramella C,
Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van
Laethem JL, Conroy T, et al: Cancer of the pancreas: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 26(Suppl 5): v56–v68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sohal DP, Mangu PB, Khorana AA, Shah MA,
Philip PA, O'Reilly EM, Uronis HE, Ramanathan RK, Crane CH,
Engebretson A, et al: Metastatic pancreatic cancer: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 34:2784–2796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ghosn M, Saroufim A, Kattan J, Chahine G,
Nasr F and Farhat F: Sequential FOLFOX-6 and gemcitabine for
locally advanced and/or metastatic pancreatic cancer. Med Oncol.
29:2831–2837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dahan L, Phelip JM, Malicot KL, Williet N,
Desrame J, Volet J, Petorin C, Malka D, Rebischung C, Aparicio T,
et al: FOLFIRINOX until progression, FOLFIRINOX with maintenance
treatment, or sequential treatment with gemcitabine and FOLFIRI.3
for first-line treatment of metastatic pancreatic cancer: A
randomized phase II trial (PRODIGE 35-PANOPTIMOX). 2018 ASCO Annual
Meeting I Gastrointestinal (noncolorectal). J Clin Oncol.
36:40002018. View Article : Google Scholar
|
|
74
|
International Society of Geriatric
Oncology Population Demographics-Cancer Epidemiology. 2021.
|
|
75
|
GBD 2017 Pancreatic Cancer Collaborators:
The global, regional, and national burden of pancreatic cancer and
its attributable risk factors in 195 countries and territories,
1990-2017: A systematic analysis for the Global Burden of Disease
Study 2017. Lancet Gastroenterol Hepatol. 4:934–947. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lamont EB, Schilsky RL, He Y, Muss H,
Cohen HJ, Hurria A, Meilleur A, Kindler HL, Venook A, Lilenbaum R,
et al: Generalizability of trial results to elderly Medicare
patients with advanced solid tumors (Alliance 70802). J Natl Cancer
Inst. 107:3362015. View Article : Google Scholar
|
|
77
|
Higuera O, Ghanem I, Nasimi R, Prieto I,
Koren L and Feliu J: Management of pancreatic cancer in the
elderly. World J Gastroenterol. 22:764–775. 2016. View Article : Google Scholar :
|
|
78
|
Petrillo A, Pappalardo A, Calabrese F,
Tirino G, Pompella L, Ventriglia J, Laterza MM, Caterino M, Sforza
V, Iranzo V, et al: First line nab-paclitaxel plus gemcitabine in
elderly metastatic pancreatic patients: A good choice beyond age. J
Gastrointest Oncol. 10:910–917. 2019. View Article : Google Scholar :
|
|
79
|
Martin JL, Sidhu S, Benhayoun N, Dedonno M
and Brenner WS: Dosing modifications to increase tolerability of
gemcitabine and nab-paclitaxel in treatment of pancreatic cancer in
the elderly. J Clin Oncol. 37:4412019. View Article : Google Scholar
|
|
80
|
Li D, Capanu M, Yu KH, Lowery MA, Kelsen
DP and O'Reilly EM: Treatment, outcomes, and clinical trial
participation in elderly patients with metastatic pancreas
adenocarcinoma. Clin Colorectal Cancer. 14:269–276.e1. 2015.
View Article : Google Scholar
|
|
81
|
Guion-Dusserre JF, Bertaut A, Ghiringhelli
F, Vincent J, Quipourt V, Marilier S, Tharin Z and Bengrine-Lefevre
L: Folfirinox in elderly patients with pancreatic or colorectal
cancer-tolerance and efficacy. World J Gastroenterol. 22:9378–9386.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yamamoto T, Sunakawa Y, Kubota Y, Tagawa
T, Kaga Y, Uto TIY, Kitahara Y, Toshima H, Kobayashi K, Kitahara A,
et al: Tolerability and clinical outcomes in elderly patients with
advanced pancreatic cancer treated with FOLFIRINOX. J Clin Oncol.
34:4042016. View Article : Google Scholar
|
|
83
|
Alessandretti MB, Moreira RB, Brandao EP,
Gomes JR and Amarante PM: Safety and efficacy of modified
dose-attenuated FOLFIRINOX chemotherapy in patients over 65 years
with advanced pancreatic adenocarcinoma. J Clin Oncol. 33:4682015.
View Article : Google Scholar
|
|
84
|
Michalaki V, Poydorou A, Vezakis A,
Frangulidis G, Theodosopoulos T and Papadimitriou C: Gemcitabine
plus capecitabine in elderly patients with advanced pancreatic
cancer. Ann Oncol. 28:iii762017. View Article : Google Scholar
|
|
85
|
Marsanic P, Mellano A, Sottile A and De
Simone M: Irreversible electroporation as treatment of locally
advanced and as margin accentuation in borderline resectable
pancreatic adenocarcinoma. Med Biol Eng Comput. 55:1123–1127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sohal DP, Kennedy EB, Cinar P, Conroy T,
Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T,
Krishnamurthi S, et al: Metastatic pancreatic cancer: ASCO
guideline update. J Clin Oncol. Aug 5–2020.Epub ahead of print.
View Article : Google Scholar
|
|
87
|
Macarulla T, Pazo-Cid R, Guillén-Ponce C,
López R, Vera R, Reboredo M, Muñoz Martin A, Rivera F, Díaz
Beveridge R, La Casta A, et al: Phase I/II Trial to evaluate the
efficacy and safety of nanoparticle albumin-bound paclitaxel in
combination with gemcitabine in patients with pancreatic cancer and
an ECOG performance status of 2. J Clin Oncol. 37:230–238. 2019.
View Article : Google Scholar
|
|
88
|
Cunningham D, Chau I, Stocken DD, Valle
JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J,
et al: Phase III randomized comparison of gemcitabine versus
gemcitabine plus capecitabine in patients with advanced pancreatic
cancer. J Clin Oncol. 27:5513–5518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Smith AL, Wong C, Cuggia A, Borgida A,
Holter S, Hall A, Connor AA, Bascuñana C, Asselah J, Bouganim N, et
al: Reflex Testing for Germline BRCA1, BRCA2, PALB2, and ATM
mutations in pancreatic cancer: Mutation prevalence and clinical
outcomes from two canadian research registries. JCO Precision
Oncology. Jan 19–2018. View Article : Google Scholar
|
|
90
|
Gudmundsdottir K and Ashworth A: The roles
of BRCA1 and BRCA2 and associated proteins in the maintenance of
genomic stability. Oncogene. 25:5864–5874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rebelatto TF, Falavigna M, Pozzari M,
Pozzari M, Spada F, Cella CA, Laffi A, Pellicori S and Fazio N:
Should platinum-based chemotherapy be preferred for germline BReast
CAncer genes (BRCA) 1 and 2-mutated pancreatic ductal
adenocarcinoma (PDAC) patients? A systematic review and
meta-analysis. Cancer Treat Rev. 80:1018952019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Stoffel EM, McKernin SE, Brand R, Canto M,
Goggins M, Moravek C, Nagarajan A, Petersen GM, Simeone DM,
Yurgelun M and Khorana AA: Evaluating susceptibility to pancreatic
cancer: ASCO provisional clinical opinion. J Clin Oncol.
37:153–164. 2019. View Article : Google Scholar
|
|
93
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)
Pancreatic Adenocarcinoma. Version 1.2021. October. 23–2020.
|
|
94
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance olaparib for germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hammel P, Kindler HL, Reni M, Van Cutsem
E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Health-related quality of life in patients with a germline BRCA
mutation and metastatic pancreatic cancer receiving maintenance
olaparib. Ann Oncol. 30:1959–1968. 2019. View Article : Google Scholar
|
|
96
|
Golan T, Hammel P, Reni M, et al: Final
overall survival results from the phase 3 POLO trial: Maintenance
olaparib for germline BRCA-mutated metastatic pancreatic cancer.
In: 2021 Gastrointestinal Cancer Symposium. Oral Presentation GI21
presented at ASCO-GI, 2021;
|
|
97
|
O'Reilly EM, Lee JW, Zalupski M, Capanu M,
Park J, Golan T, Tahover E, Lowery MA, Chou JF, Sahai V, et al:
Randomized, multicenter, phase ii trial of gemcitabine and
cisplatin with or without veliparib in patients with pancreas
adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol.
38:1378–1388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wattenberg MM, Asch D, Yu S, O'Dwyer PJ,
Domchek SM, Nathanson KL, Rosen MA, Beatty GL, Siegelman ES and
Reiss KA: Platinum response characteristics of patients with
pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or
PALB2 mutation. Br J Cancer. 122:333–339. 2020. View Article : Google Scholar :
|
|
99
|
Nishikawa G, Booth C and Prasad V:
Olaparib for BRCA mutant pancreas cancer: Should the POLO trial
change clinical practice? Cancer. 126:4087–4088. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rahma OE, Duffy A, Liewehr DJ, Steinberg
SM and Greten TF: Second-line treatment in advanced pancreatic
cancer: A comprehensive analysis of published clinical trials. Ann
Oncol. 24:1972–1979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lee K, Bang K, Yoo C, Hwang I, Jeong JH,
Chang HM, Oh D, Song TJ, Park DH, Lee SS, et al: Clinical outcomes
of second-line chemotherapy after progression on Nab-paclitaxel
plus gemcitabine in patients with metastatic pancreatic
adenocarcinoma. Cancer Res Treat. 52:254–262. 2020. View Article : Google Scholar :
|
|
102
|
Sonbol MB, Firwana B, Wang Z,
Almader-Douglas D, Borad MJ, Makhoul I, Ramanathan RK, Ahn DH and
Bekaii-Saab T: Second-line treatment in patients with pancreatic
ductal adenocarcinoma: A meta-analysis. Cancer. 123:4680–4686.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cherri S, Noventa S and Zaniboni A:
Pancreatic adenocarcinoma: Beyond first line, where are we? World J
Gastroenterol. 27:1847–1863. 2021. View Article : Google Scholar :
|
|
104
|
Oettle H, Riess H, Stieler JM, Heil G,
Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, et
al: Second-line oxaliplatin, folinic acid, and fluorouracil versus
folinic acid and fluorouracil alone for gemcitabine-refractory
pancreatic cancer: Outcomes from the CONKO-003 trial. J Clin Oncol.
32:2423–2429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gill S, Ko YJ, Cripps C, Beaudoin A,
Dhesy-Thind S, Zulfiqar M, Zalewski P, Do T, Cano P, Lam WYH, et
al: PANCREOX: A randomized phase III study of
Fluorouracil/Leucovorin with or without oxaliplatin for second-line
advanced pancreatic cancer in patients who have received
gemcitabine-based chemotherapy. J Clin Oncol. 34:3914–3920. 2016.
View Article : Google Scholar
|
|
106
|
Xiong HQ, Varadhachary GR, Blais JC, Hess
KR, Abbruzzese JL and Wolff RA: Phase 2 trial of oxaliplatin plus
capecitabine (XELOX) as second-line therapy for patients with
advanced pancreatic cancer. Cancer. 113:2046–2052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ohkawa S, Okusaka T, Isayama H, Fukutomi
A, Yamaguchi K, Ikeda M, Funakoshi A, Nagase M, Hamamoto Y,
Nakamori S, et al: Randomised phase II trial of S-1 plus
oxaliplatin vs S-1 in patients with gemcitabine-refractory
pancreatic cancer. Br J Cancer. 112:1428–1434. 2015. View Article : Google Scholar :
|
|
108
|
Fortune BE, Li X, Kosuri KV, Weatherby LM,
Thomas JP and Bekaii-Saab TS: Fixed-dose-rate gemcitabine in
combination with oxaliplatin in patients with metastatic pancreatic
cancer refractory to standard-dose-rate gemcitabine: A
single-institute study. Oncology. 76:333–337. 2009. View Article : Google Scholar
|
|
109
|
Demols A, Peeters M, Polus M, Marechal R,
Gay F, Monsaert E, Hendlisz A and Van Laethem JL: Gemcitabine and
oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic
adenocarcinoma: A phase II study. Br J Cancer. 94:481–485. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ramfidis VS, Syrigos KN and Saif MW: New
therapeutic strategies in the second line setting of advanced or
metastatic pancreatic adenocarcinoma. JOP. 14:344–346.
2013.PubMed/NCBI
|
|
111
|
Portal A, Pernot S, Tougeron D, Arbaud C,
Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J,
Coriat R, et al: Nab-paclitaxel plus gemcitabine for metastatic
pancreatic adenocarcinoma after Folfirinox failure: An AGEO
prospective multicentre cohort. Br J Cancer. 113:989–995. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite Instability/Mismatch
Repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar
|
|
115
|
Bockorny B, Semenisty V, Macarulla T,
Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ,
Pedersen KS, et al: BL-8040, a CXCR4 antagonist, in combination
with pembrolizumab and chemotherapy for pancreatic cancer: The
COMBAT trial. Nat Med. 26:878–885. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Okamura R, Boichard A, Kato S, Sicklick
JK, Bazhenova L and Kurzrock R: Analysis of NTRK alterations in
pan-cancer adult and pediatric malignancies: Implications for
NTRK-Targeted therapeutics. JCO Precis Oncol 2018. PO18.00183.
2018. View Article : Google Scholar
|
|
117
|
Solomon JP, Linkov I, Rosado A, Mullaney
K, Rosen EY, Frosina D, Jungbluth AA, Zehir A, Benayed R, Drilon A,
et al: NTRK fusion detection across multiple assays and 33,997
cases: Diagnostic implications and pitfalls. Mod Pathol. 33:38–46.
2020. View Article : Google Scholar :
|
|
118
|
O'Reilly EM and Hechtman JF: Tumour
response to TRK inhibition in a patient with pancreatic
adenocarcinoma harbouring an NTRK gene fusion. Ann Oncol. 30(Suppl
8): viii36–viii40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Doebele RC, Drilon A, Paz-Ares L, Siena S,
Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, et al:
Entrectinib in patients with advanced or metastatic NTRK
fusion-positive solid tumours: Integrated analysis of three phase
1-2 trials. Lancet Oncol. 21:271–282. 2020. View Article : Google Scholar
|
|
120
|
Wang-Gillam A, Hubner RA, Siveke JT, Von
Hoff DD, Belanger B, de Jong FA, Mirakhur B and Chen LT: NAPOLI-1
phase 3 study of liposomal irinotecan in metastatic pancreatic
cancer: Final overall survival analysis and characteristics of
long-term survivors. Eur J Cancer. 108:78–87. 2019. View Article : Google Scholar
|
|
121
|
Humphris JL, Patch AM, Nones K, Bailey PJ,
Johns AL, McKay S, Chang DK, Miller DK, Pajic M, Kassahn KS, et al:
Hypermutation in pancreatic cancer. Gastroenterology. 152:68–74.e2.
2017. View Article : Google Scholar
|
|
122
|
Leem G and Song SY: Immunotherapy in
pancreatic cancer; the road less traveled. Immunol Disord
Immunother. 1:1062016. View Article : Google Scholar
|
|
123
|
Hayashi A, Hong J and Iacobuzio-Donahue
CA: The pancreatic cancer genome revisited. Nat Rev Gastroenterol
Hepatol. 18:469–481. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Nedaeinia R, Avan A, Manian M, Salehi R
and Ghayour-Mobarhan M: EGFR as a potential target for the
treatment of pancreatic cancer: Dilemma and controversies. Curr
Drug Targets. 15:1293–1301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Navas C, Hernández-Porras I, Schuhmacher
AJ, Sibilia M, Guerra C and Barbacid M: EGF receptor signaling is
essential for k-ras oncogene-driven pancreatic ductal
adenocarcinoma. Cancer Cell. 22:318–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Matsuoka T and Yashiro M: Molecular
targets for the treatment of pancreatic cancer: Clinical and
experimental studies. World J Gastroenterol. 22:776–789. 2016.
View Article : Google Scholar :
|
|
127
|
Nywening TM, Wang-Gillam A, Sanford DE,
Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA,
Yano M, et al: Targeting tumour-associated macrophages with CCR2
inhibition in combination with FOLFIRINOX in patients with
borderline resectable and locally advanced pancreatic cancer: A
single-centre, open-label, dose-finding, non-randomised, phase 1b
trial. Lancet Oncol. 17:651–662. 2016. View Article : Google Scholar
|
|
128
|
Kamerkar S, LeBleu VS, Sugimoto H, Yang S,
Ruivo CF, Melo SA, Lee JJ and Kalluri R: Exosomes facilitate
therapeutic targeting of oncogenic KRAS in pancreatic cancer.
Nature. 546:498–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
ClinicalTrials.gov: National Library of
Medicine (US): 2000 Feb 29. Identifier NCT03608631, iExosomes in
Treating Participants with Metastatic Pancreas Cancer with KrasG12D
Mutation. August 1, 2018 [cited 2020 June 29]. Bethesda, MD, 2020.
Available from: https://clinicaltrials.gov/ct2/show/NCT03608631.
|
|
130
|
Garrido-Laguna I, Uson M, Rajeshkumar NV,
Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor
G, Sharma R, et al: Tumor engraftment in nude mice and enrichment
in stroma-related gene pathways predict poor survival and
resistance to gemcitabine in patients with pancreatic cancer. Clin
Cancer Res. 17:5793–5800. 2011. View Article : Google Scholar :
|
|
131
|
Yachida S and Iacobuzio-Donahue CA: The
pathology and genetics of metastatic pancreatic cancer. Arch Pathol
Lab Med. 133:413–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Provenzano PP, Cuevas C, Chang AE, Goel
VK, Von Hoff DD and Hingorani SR: Enzymatic targeting of the stroma
ablates physical barriers to treatment of pancreatic ductal
adenocarcinoma. Cancer Cell. 21:418–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sherman MH, Yu RT, Engle DD, Ding N,
Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S,
et al: Vitamin D receptor-mediated stromal reprogramming suppresses
pancreatitis and enhances pancreatic cancer therapy. Cell.
159:80–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
van Duijnhoven FJB, Jenab M, Hveem K,
Siersema PD, Fedirko V, Duell EJ, Kampman E, Halfweeg A, van Kranen
HJ, van den Ouweland JM, et al: Circulating concentrations of
vitamin D in relation to pancreatic cancer risk in European
populations. Int J Cancer. 142:1189–1201. 2018. View Article : Google Scholar :
|
|
136
|
Gorchs L, Ahmed S, Mayer C, Knauf A,
Fernández Moro C, Svensson M, Heuchel R, Rangelova E, Bergman P and
Kaipe H: The vitamin D analogue calcipotriol promotes an
anti-tumorigenic phenotype of human pancreatic CAFs but reduces T
cell mediated immunity. Sci Rep. 10:174442020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Le Large TY, Bijlsma MF, Kazemier G, van
Laarhoven HW, Giovannetti E and Jimenez CR: Key biological
processes driving metastatic spread of pancreatic cancer as
identified by multi-omics studies. Semin Cancer Biol. 44:153–169.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sano M, Ijichi H, Takahashi R, Miyabayashi
K, Fujiwara H, Yamada T, Kato H, Nakatsuka T, Tanaka Y, Tateishi K,
et al: Blocking CXCLs-CXCR2 axis in tumor-stromal interactions
contributes to survival in a mouse model of pancreatic ductal
adenocarcinoma through reduced cell invasion/migration and a shift
of immune-inflammatory microenvironment. Oncogenesis. 8:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Pishvaian MJ, Rolfo CD, Liu SV, Multani
PS, Maneval EC and Garrido-Laguna I: Clinical benefit of
entrectinib for patients with metastatic pancreatic cancer who
harbor NTRK and ROS1 fusions. J Clin Oncol. 36:5212018. View Article : Google Scholar
|
|
140
|
Drilon A, Siena S, Ou SI, Patel M, Ahn MJ,
Lee J, Bauer TM, Farago AF, Wheler JJ, Liu SV, et al: Safety and
antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK
inhibitor entrectinib: Combined results from two phase I trials
(ALKA-372-001 and STARTRK-1). Cancer Discov. 7:400–409. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yan HH, Jung KH, Son MK, Fang Z, Kim SJ,
Ryu YL, Kim J, Kim MH and Hong SS: Crizotinib exhibits antitumor
activity by targeting ALK signaling not c-MET in pancreatic cancer.
Oncotarget. 5:9150–9168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Herreros-Villanueva M and Bujanda L:
Non-invasive biomarkers in pancreatic cancer diagnosis: What we
need versus what we have. Ann Transl Med. 4:1342016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Humphris JL, Chang DK, Johns AL, Scarlett
CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA,
et al: The prognostic and predictive value of serum CA19.9 in
pancreatic cancer. Ann Oncol. 23:1713–1722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhang Y, Yang J, Li H, Wu Y, Zhang H and
Chen W: Tumor markers CA19-9 CA242 and CEA in the diagnosis of
pancreatic cancer: A meta-analysis. Int J Clin Exp Med.
8:11683–11691. 2015.
|
|
145
|
Kaur S, Baine MJ, Jain M, Sasson AR and
Batra SK: Early diagnosis of pancreatic cancer: Challenges and new
developments. Biomark Med. 6:597–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR and Hayes DF: ASCO 2006
update of recommendations for the use of tumor markers in
gastrointestinal cancer. J Clin Oncol. 24:5313–5327. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Goonetilleke KS and Siriwardena AK:
Systematic review of carbohydrate antigen (CA 19-9) as a
biochemical marker in the diagnosis of pancreatic cancer. Eur J
Surg Oncol. 33:266–270. 2007. View Article : Google Scholar
|
|
148
|
Ruivo CF, Adem B, Silva M and Melo SA: The
biology of cancer exosomes: Insights and new perspectives. Cancer
Res. 77:6480–6488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hu W, Liu C, Bi ZY, Zhou Q, Zhang H, Li
LL, Zhang J, Zhu W, Song YY and Zhang F: Comprehensive landscape of
extracellular vesicle-derived RNAs in cancer initiation,
progression, metastasis and cancer immunology. Mol Cancer.
19:1022020. View Article : Google Scholar : PubMed/NCBI
|