Introduction
Cervical cancer ranks as the fourth most common
cancer type among gynaecological malignancies, according to a
worldwide analysis in 2018 (1). In
total, ~570,000 cases of cervical cancer and 311,000 cervical
cancer-related deaths occurred in 2018, with 106,000 cases and
48,000 deaths in China (1). Despite
recent advances in diagnostic criteria and clinical therapeutic
strategies, a significant number of women suffer from advanced
disease and the therapeutic efficiency remains unsatisfactory
(2,3).
Connexin (Cx) is an important component of gap
junction (GJ), which modulates various cellular processes,
including electrical coupling, proliferation, differentiation and
apoptosis (4). Previous studies
revealed that GJ and Cx are defective in tumour progression, while
GJ recovery impedes cell proliferation and metabolism in tumours
(5,6). However, there have been emerging
studies that have demonstrated that the Cx protein itself affects
the development of carcinoma in a GJ-independent manner (7–9). Our
previous studies showed that Cx32 was aberrantly upregulated and
mislocalized in human cervical cancer tissue. Moreover, abnormal
Cx32 mediates anti-apoptotic and pro-tumour effects via the EGFR
and NF-κB pathways in cervical cancer (10–12).
It has also been reported that the high expression of Cx32 was
correlated with advanced FIGO stage, augmented tumour size and
poorer differentiation in human cervical cancer, especially in
Xinjiang, China (12). However,
besides apoptosis, the role of Cx32 on other types of cell death
(e.g., autophagic cell death or necrosis) remains to be further
investigated in cervical cancer.
Autophagy is a conserved process for bulk
degradation and recycling of cytoplasmic proteins and organelles in
lysosomes (13,14), contributing to the turnover of
membrane proteins, including surface receptors and structural
components (15,16). As membrane protein-based structures,
the stability and degradation of GJ plaques and Cx are regulated by
macroautophagy via the ubiquitin-proteasome system and lysosomes
(17–19). On the other hand, Cx inversely acts
as a negative regulator of autophagic flux (20,21).
However, to the best of our knowledge, the role of Cx32 in
autophagy formation remains unknown, especially in cervical cancer.
In addition, investigating the correlation of Cx32 and autophagy in
isolation cannot reflect the practical significance of Cx32 in
cervical cancer progression. Autophagy and apoptosis are two
distinct mechanisms that may be antagonistic in cancer cells
(22). It has been shown that
inhibition of autophagy enhances N, N-diethylnorspermine-induced
apoptosis in colon cancer cells (23). Conversely, activation of autophagy
by globular adiponectin attenuates ethanol-induced apoptosis in
liver cancer cells (24). However,
whether autophagy is involved in the anti-apoptotic effect of Cx32
in cervical cancer remains unknown, to the best of our knowledge.
Therefore, the present study investigated the relationship between
Cx32 and autophagy in the presence or absence of GJ formation, in
order to provide new findings or novel mechanisms of Cx32 in
cervical cancer.
Materials and methods
Reagents and antibodies
Streptonigrin (SN), chloroquine (CQ), bafilomycin A1
(Baf-A1), rapamycin (Rap), doxycycline (Dox), compound C and
anti-GAPDH antibody (cat. no. G8795) were obtained from
Sigma-Aldrich (Merck KGaA). G418 and hygromycin B were purchased
from Calbiochem (Merck KGaA). Primary antibodies against GAPDH,
β-tubulin (cat. no. 86298), β-actin (cat. no. 3700), LC3 (cat. no.
4108), autophagy-related (Atg)4 (cat. no. 7613), Atg5 (cat. no.
12994), Atg7 (cat. no. 8558), cleaved caspase-3 (cat. no. 9664),
cleaved poly(ADP-ribose) polymerase (PARP, cat. no. 9532),
AMP-activated protein kinase (AMPK, cat. no. 2795)/phosphorylated
(p)-AMPK (cat. no. 4184) and mTOR (cat. no. 2983)/p-mTOR (cat. no.
5536) were purchased from Cell Signaling Technology, Inc. Anti-Cx32
antibody was obtained from Santa Cruz Biotechnology, Inc. Small
interfering RNA (siRNA) targeting Cx32, Atg5 and AMPK were
constructed by Guangzhou RiboBio Co., Ltd. Enhanced green
fluorescent protein (EGFP)-LC3 plasmids were constructed by
Genecopoeia, Inc. The BCA protein assay kit was purchased from
Bio-Rad Laboratories, Inc. An Annexin V-FITC apoptosis detection
kit was purchased from Biotool, LLC. A Chemiluminescent HRP
substrate kit was obtained from MilliporeSigma.
Lipofectamine® 2000, DMEM and EMEM media were purchased
from Invitrogen (Thermo Fisher Scientific, Inc.).
Peroxidase-AffiniPure goat anti-mouse IgG (H+L) (cat. no.
115-035-003) and Peroxidase-AffiniPure goat anti-rabbit IgG (H+L)
(cat. no. 111-035-003) were obtained from Jackson ImmunoResearch
Laboratories, Inc.
Human cervical specimens and clinical
data
The study was approved by the Research Committee of
Ethics of the Affiliated Cancer Hospital of Xinjiang Medical
University (12). The patients who
were diagnosed with cervical cancer and planned to undergo surgery
were included (age, 40–65 years). Human cervical tissue samples
were collected from patients between October 2012 and June 2014 at
The Affiliated Cancer Hospital of Xinjiang Medical University,
Xinjiang, China. The patients who had received any
chemoradiotherapeutic agents, radiotherapy preoperatively or did
not receive surgical treatment were excluded. In total, 50
specimens of cervical cancer were collected from patients who
underwent total hysterectomy, and 30 specimens of benign multiple
uterine fibroids were collected as normal cervical controls. All
the cervical cancer tissue specimens, corresponding peritumoural
tissues (<3 cm distance from the tumour tissue) and remote
normal liver tissues (5 cm away from the tumour tissue) were
collected within 10 min. The normal vs. cancer tissues were not
from the same patients. After hysterectomy, cervical specimens were
stored in liquid nitrogen at −196°C for protein extraction. The
expression levels of Cx32 and LC3 were detected via western
blotting.
Cell lines and low-density
cultures
The human cervical cancer cell line (C-33A) was
purchased from the American Type Culture Collection and was
cultured in EMEM. HeLa-Cx32 cells (a gift from Professor Andrew L.
Harris in Department of Pharmacology, Physiology and Neuroscience,
New Jersey Medical School, Rutgers University) are a stable
transgenic cell line expressing Cx32 under the control of a
bidirectional tetracycline-inducible promoter that was previously
described and characterized (9).
The cells were grown as monolayer cultures in DMEM supplemented
with 100 µg/ml G418 sulphate and 200 µg/ml hygromycin B. Cx32
expression was induced with 1 µg/ml Dox at 37°C for 48 h. All the
cell lines were supplemented with 10% FBS (Beyotime Institute of
Biotechnology) and were grown at 37°C in a 5% CO2
atmosphere.
To physically inhibit GP formation, the low-density
culture method was used, and 1×105 cells were seeded in a 150-mm
dish to ensure that the cells were not in direct contact with each
other, as previously described (10).
Western blotting
Tissue or cells were lysed with RIPA buffer (50 mM
Tris-HCl pH 7.8, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100 and 0.1%
SDS) containing a protease inhibitor cocktail (Sigma-Aldrich; Merck
KGaA). The proteins were quantified by using a Bio-Rad protein BCA
assay kit, and equivalent amounts of protein (20 µg) were resolved
on 12 or 9% SDS-PAGE gels and transferred onto 0.2 µm or 0.45 µm
Immobilon-P transfer membranes (MilliporeSigma). The membranes were
subsequently incubated in blocking buffer (5% non-fat milk) for 1 h
at room temperature and then incubated with appropriate primary
antibodies in blocking buffer at 4°C overnight. The dilutions of
the antibodies were as follows: Anti-LC3 and anti-Cx32 were
1:1,000; anti-cleaved caspase-3, anti-cleaved PARP, anti-Atg4,
anti-Atg5, anti-Atg7, anti-AMPK/p-AMPK and anti-mTOR/p-mTOR were
1:1,500; and anti-β-tubulin, anti-β-actin and anti-GAPDH were
1:10,000. The proteins were probed with the relevant secondary
antibody, detected with an ECL reagent and semi-quantified using
ImageQuant LAS 4000™ (Cytiva) and ImageJ software 1.8.0 (National
Institutes of Health). Anti-β-tubulin, anti-β-actin and anti-GAPDH
were used as loading controls.
Hoechst 33258 staining
Briefly, HeLa cells were fixed with 4%
paraformaldehyde at room temperature for 20 min and permeabilized
with a solution containing 1% BSA (Beyotime Institute of
Biotechnology) and 0.5% Triton X-100 for 15 min at 37°C. Then, the
apoptotic cells were detected by staining with 0.1 µg/ml Hoechst
33258 at room temperature for 15 min in the dark and washed with
PBS. Finally, morphological changes in apoptotic nuclei were
observed under a fluorescence microscope (IX71; Olympus
Corporation) with an ultraviolet filter.
Apoptosis analysis
Apoptosis was induced in cells via an incubation
with 1 µM SN at 37°C for 7 h (10,12).
Apoptosis was assessed via flow cytometry using an Annexin V-FITC
apoptosis detection kit according to the manufacturer's protocol.
After exposure to SN, the cells were trypsinized, washed and
collected. Then, the cells were resuspended in Annexin V binding
buffer and incubated with 5 µl FITC-Annexin V and 2 µl PI for 15
min in the dark at 37°C. The apoptotic cells were immediately
analysed using FlowJo 7.6 software (FlowJo LLC). The ratio of early
apoptotic cells was compared with that of the controls for each
experiment.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde at room
temperature for 20 min, permeabilized with 0.5% Triton X-100 for 15
min, washed and blocked in 10% normal goat serum (Cell Signaling
Technology, Inc.) at room temperature for 1 h. After blocking, the
cells were incubated with a primary antibody against Cx32 (1:200)
at 4°C overnight, washed and incubated with FITC-conjugated goat
anti-mouse secondary antibody (1:400) in the dark at 37°C for 1 h.
For identification of the nucleus, the cells were stained with 0.1
µg/ml Hoechst 33342 at room temperature for 10 min in the dark. The
cells were observed under a confocal microscope (Olympus IX83;
Olympus Corporation).
Transmission electron microscopy
Cells were harvested via trypsinization, washed and
fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer at 4°C for
30 min. After washing in phosphate buffer, the samples were
post-fixed in 1% osmium tetroxide at 4°C for 30 min, washed again
and dehydrated in a graded series of ethanol. Then, the cells were
embedded in spur resin at 60°C for 2–4 days for cutting into
ultrathin sections. Transmission electron microscopy (JEOL, Ltd.)
was used to observe all autophagosomal structures after staining
the sections (a thickness of 50 nm) with uranyl acetate and lead
citrate at room temperature for 10 min (25).
EGFP-LC3 plasmid transfection and
siRNA interference
Cells were seeded in 6-well plates at the density of
1×105 cells/well and grown to 80% confluence and then transfected
with 0.8 g EGFP-LC3 plasmid and 5 µl Lipofectamine 2000 per well at
37°C for 36 h. After the corresponding treatment, green
fluorescence LC3-II aggregation was observed under a microscope.
For siRNA interference, the cells were grown to 30–50% confluence,
and then the cells were transfected for 6 h with the corresponding
non-specific siRNA, targeted siRNA (50 nM) or scrambled control
siRNA (cat. no. siN0000001-1-5) with Lipofectamine 2000. The cells
were cultured in complete medium. EGFP-LC3 plasmid transfection and
siRNA interference were detected via immunofluorescence and western
blotting after 48 h. The sequences for the synthetic DNA targeting
EGFP-LC3 were as follows: Forward primer,
5′-ATGCCGTCGGAGAAGACCTTCAAG-3′ and reverse primer,
5′-TTACACTGACAATTTCATCCCGAACGT-3′. The sequences of the synthetic
siRNAs targeting Cx32 (siCx32) were as follows: siCx32-1,
5′-CACCAACAACACATAGAAA-3′; siCx32-2, 5′-GCATCTGCATTATCCTCAA-3′; and
siCx32-3, 5′-GCCTCTCACCTGAATACAA-3′. The sequences of the synthetic
siRNAs targeting Atg5 (siAtg5) were as follows: siAtg5-1,
5′-GGAATATCCTGCAGAAGAA-3′; siAtg5-2, 5′-GGAACATCACAGTACATTT-3′; and
siAtg5-3, 5′-GTGAGATATGGTTTGAATA-3′. The sequences of the synthetic
siRNAs targeting AMPK (siAMPK) were as follows: siAMPK-1,
5′-GAGGAGAGCTATTTGATTA-3′; siAMPK-2, 5′-GCAGAAGTATGTAGAGCAA-3′; and
siAMPK-3, 5′-GATTGATGATGAAGCCTTA-3′.
Statistical analysis
All data are representative of at least three
independent experiments and are presented as the mean ± SEM. Normal
distribution test has been performed prior to statistical analysis.
An independent samples t-test for two groups and one-way ANOVA
followed by Bonferroni post test for >2 groups were used to
evaluate statistical significance, respectively. Pearson's
correlation analysis was used to analyse the correlation between
Cx32 and LC3-II expression, and GraphPad Prism 6.0 software
(GraphPad Software, Inc.) was used to create the histograms and
scatter plots. P<0.05 was considered to indicate a statistically
significant difference.
Results
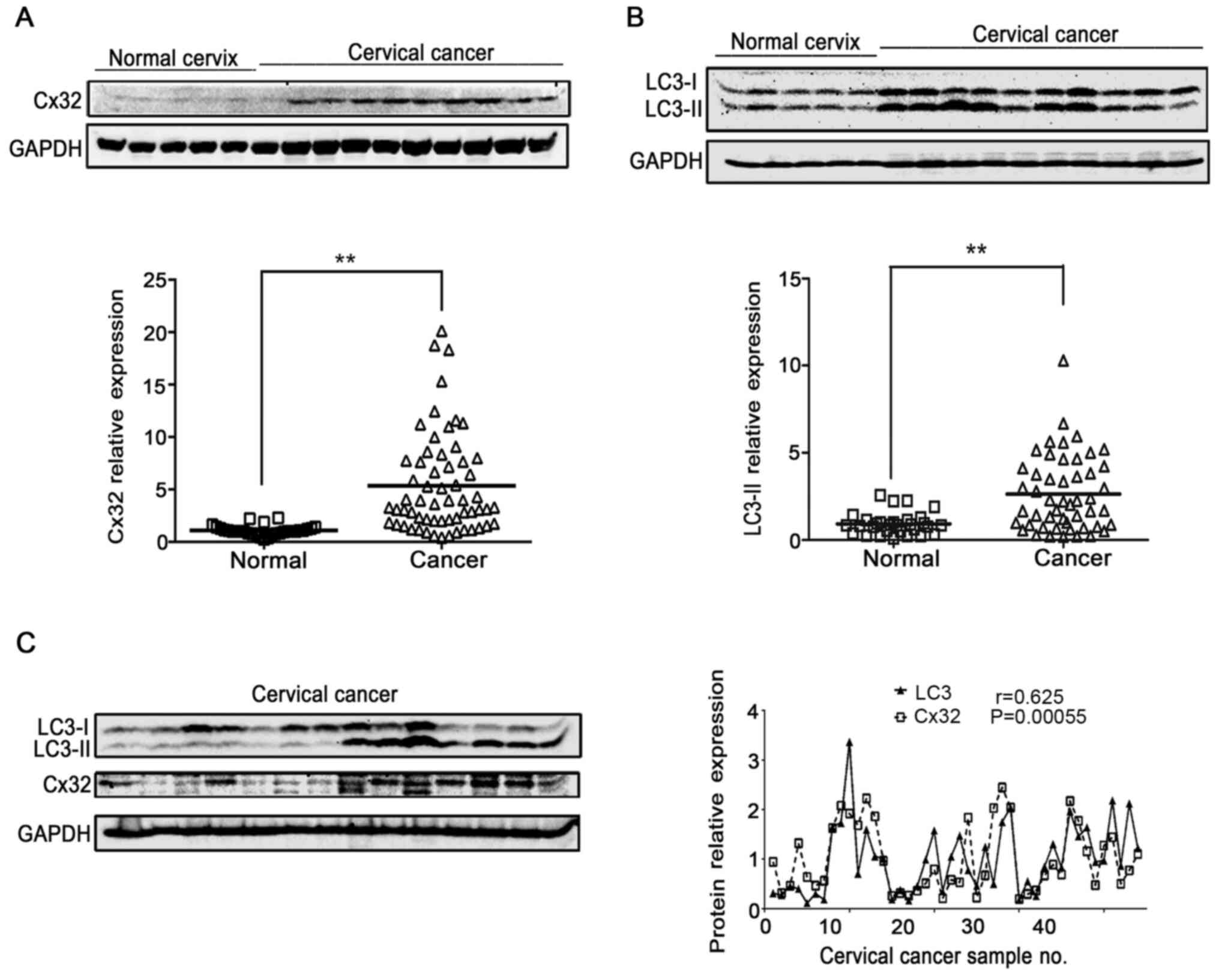
Cx32 positively associates with
autophagy in human cervical cancer
LC3-II is a well-known indicator of autophagosome
formation (26). Thus, the
expression of Cx32 and LC3-II was detected in normal cervix
controls (n=30) and cervical cancer tissue (n=50) to evaluate the
relationship between Cx32 and autophagy. Both Cx32 and LC3 were
significantly increased in cervical cancer tissues compared with
those in normal cervical tissues (Fig.
1A and B). Moreover, LC3-II expression was increased in
cervical tissues with upregulated Cx32 expression. Pearson's
correlation analysis showed that there was a significant
association between Cx32 and LC3-II (n=45, r=0.625, P=0.00055;
Fig. 1C).
Overexpression of Cx32 promotes
autophagy in HeLa cells
Next, the effect of Cx32 on autophagy was examined
in HeLa-Cx32 cells, a stable transgenic cell line expressing Cx32
through a bidirectional tetracycline-inducible promoter, since Cx32
is not naturally expressed in HeLa cells (5). Western blotting and immunofluorescence
results demonstrated that Cx32 expression was induced by Dox (1
µg/ml, 48 h) (Fig. 2A and B). In
control Hela cells, LC3-II expression was unchanged in the presence
of Dox (Fig. 2C). In HeLa-Cx32
cells, Dox treatment also upregulated the expression of LC3-II,
Atg4, Atg5 and Atg7 (Fig. 2D).
These results suggest that Cx32 promotes autophagy in HeLa-Cx32
cells.
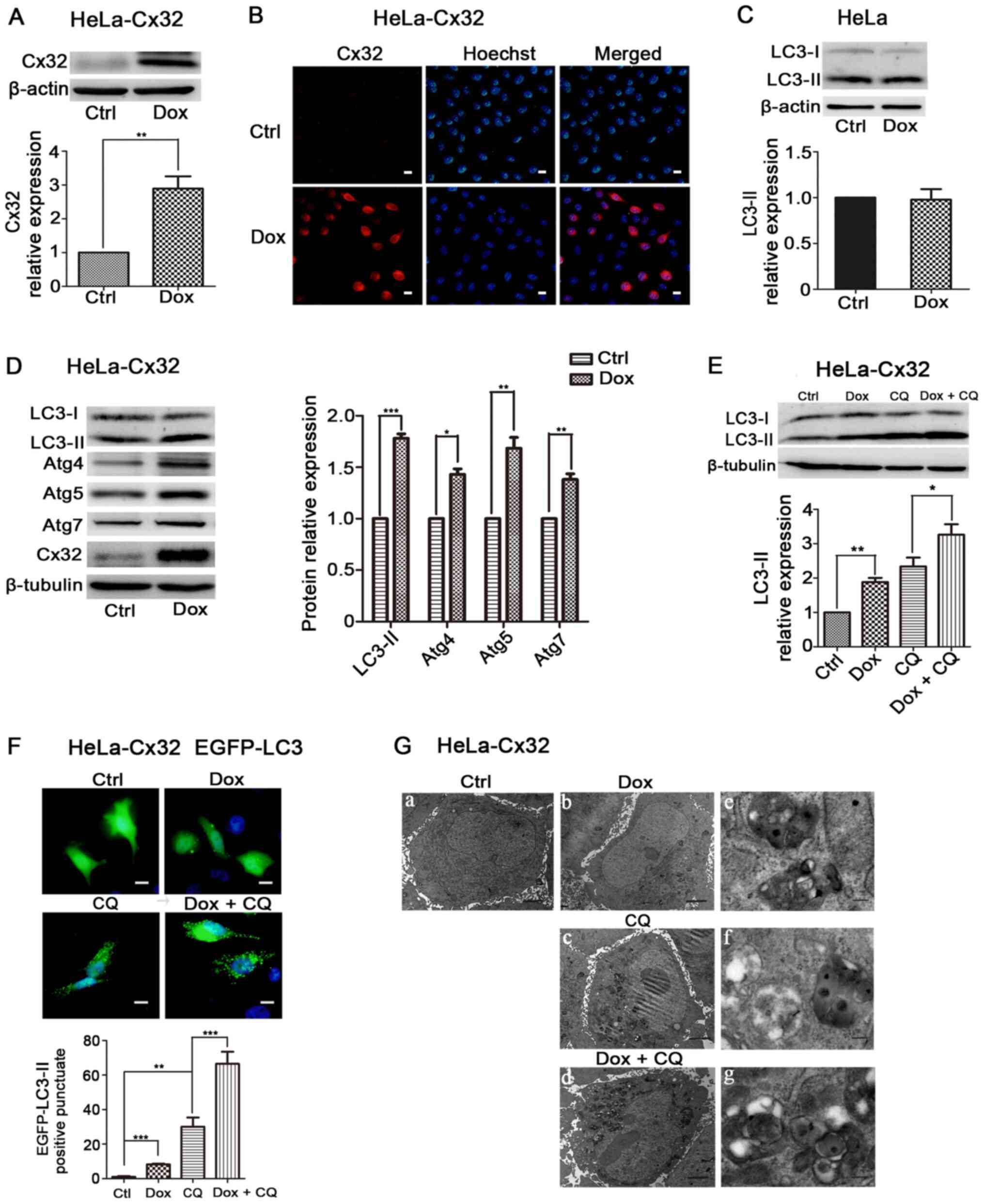 | Figure 2.Cx32 overexpression promotes
autophagy in HeLa-Cx32 cells. HeLa-Cx32 cells were treated with Dox
(1 µg/ml, 48 h), and the expression of Cx32 was detected via (A)
western blotting (n=4) and (B) immunofluorescence (magnification,
×200, n=3). Scale bars, 20 µm. (C) LC3 expression remained
unchanged in HeLa cells with Dox treatment, as determined via
western blotting (n=5). (D) Western blot analysis identified that
the expression levels of autophagy-associated proteins, LC3, Atg4,
Atg5 and Atg7, were increased with Dox treatment (1 µg/ml, 48 h) in
HeLa-Cx32 cells (n=5). (E) LC3-II protein expression in the
presence of CQ (25 µM, 4 h) was determined via western blotting
(n=4). (F) Enhanced green fluorescent protein-LC3 puncta were
examined via fluorescence microscopy and the number of puncta per
cell was quantified. Scale bar, 20 µm (n=3). (G) Autophagic
vesicles containing cell organelles were observed via electron
microscopy analysis. Scale bars, 2 µm in G-a-d and 0.25 µm in
G-e-g. The data are presented as the mean ± SEM. *P<0.05,
**P<0.01, ***P<0.001. Cx32, connexin 32; Atg, autophagy
related; Ctrl, control; Dox, doxycycline; CQ, chloroquine. |
The upregulation of LC3-II may be due to autophagy
induction or blockage of late steps of autophagy, such as
autophagosome fusion with lysosomes and lysosomal degradation. To
determine this mechanism, HeLa-Cx32 cells were treated
simultaneously with Dox and the lysosomal inhibitor CQ (25 µM, 4
h), which blocks autophagic flux by impairing
autophagosome-lysosome fusion (27). The expression of LC3-II in the Dox +
CQ group was significantly increased compared with that of the Dox
group (Fig. 2E), indicating an
effect of Cx32 on autophagic flux. LC3-II's punctate aggregation in
cells indicated autophagosome formation and elevated autophagy
(28). Dox-induced expression of
Cx32 significantly increased LC3 puncta aggregation, and treatment
with CQ further increased LC3 puncta accumulation induced by Cx32
(Fig. 2F). The transmission
electron microscopy observation revealed that Cx32 induction
increased the number and size of autophagic vacuoles, whereas few
vacuoles were observed in control cells. Additionally, lysosomal
inhibition by CQ further increased the number and size of
autophagic vacuoles mediated by Cx32 (Fig. 2G). Taken together, these results
suggest that Cx32 promotes the autophagosome formation and
autophagic flux.
Knockdown of Cx32 negatively modulates
autophagy in C-33A cells
Unlike HeLa cells, C-33A cells endogenously express
Cx32 in cell membrane and cytoplasm. Cx32 expression in C-33A cells
was knocked down via transfection with siCx32-1-3. The results
demonstrated that both siCx32-2 and siCx32-3 efficiently decreased
Cx32 expression (Fig. 3A and B).
Knockdown of Cx32 decreased the protein expression levels of
LC3-II, Atg4, Atg5 and Atg7 (Fig.
3C), indicating that silencing of Cx32 inhibited autophagy in
C-33A cells. Interestingly, the downregulated expression of LC3-II
by Cx32 knockdown was restored by CQ treatment (25 µM, 4 h)
(Fig. 3D).
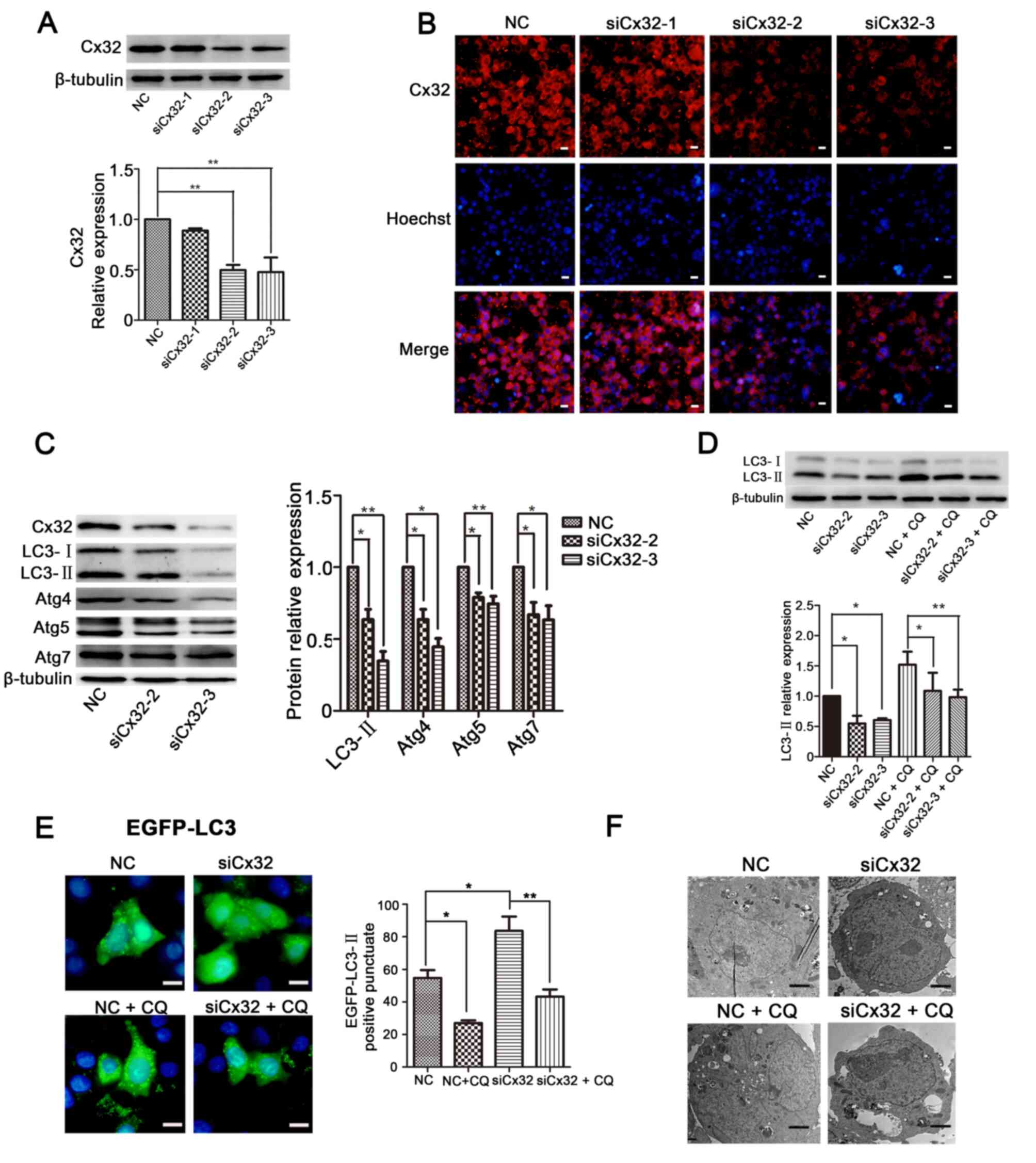 | Figure 3.Knockdown of Cx32 negatively
modulates autophagy in C-33A cells. (A) Western blotting and (B)
immunofluorescence results demonstrated that both siCx32-2 and
siCx32-3 effectively silenced the expression of Cx32
(magnification, ×200, n=3). Scale bars, 20 µm. (C) Protein
expression levels of Cx32, LC3, Atg4, Atg5 and Atg7 were reduced
when Cx32 was knocked down, as detected via western blotting (n=5).
(D) LC3-II expression was decreased by siCx32-2 and siCx32-3 but
was restored by incubation with CQ (25 µM, 4 h) (n=4). (E) EGFP-LC3
puncta were examined via fluorescence microscopy. Scale bars, 20
µm. (F) Autophagosomes were detected using transmission electron
microscopy in C-33A cells. Scale bars, 2 µM. The data are presented
as the mean ± SEM. *P<0.05, **P<0.01. Cx32, connexin 32; Atg,
autophagy related; Ctrl, control; Dox, doxycycline; CQ,
chloroquine; si, small interfering RNA; NC, negative control. |
Next, C-33A cells were transfected with siCx32-3 for
24 h, followed by transfection with the EGFP-LC3 plasmid for 36 h
and then treatment with or without CQ. The results demonstrated
that silencing of Cx32 decreased the aggregation of LC3 puncta and
this effect was reversed by CQ (Fig.
3E). A similar result was observed in the transmission electron
microscopy experiment (Fig. 3F).
These results indicate that knockdown of Cx32 blocks autophagy in
C-33A cells.
Cx32 promotes autophagy in cervical
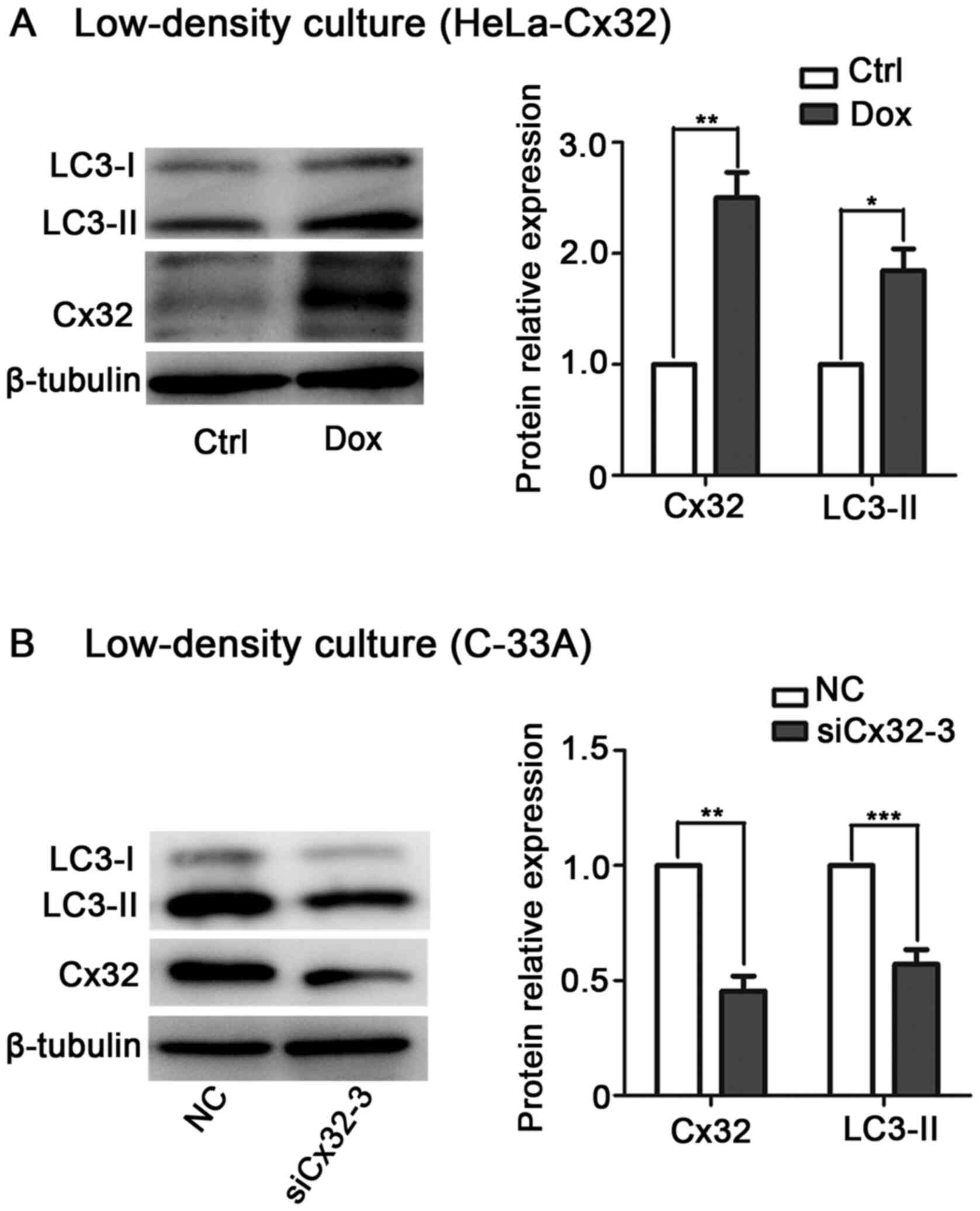
cancer cells in a GJ-independent manner
It is known that Cx exerts its biological function
via GJ-dependent and GJ-independent mechanisms (29–31).
To determine whether Cx32-induced autophagy depends on GJ,
low-density culture was used to physically inhibit gap junctional
intercellular communication (GJIC). In low-density culture,
Dox-induced Cx32 upregulated LC3-II expression in HeLa-Cx32 cells
(Fig. 4A). By contrast, knockdown
of Cx32 decreased LC3-II expression in C-33A cells (Fig. 4B). These data suggest that the
effect of Cx32 on autophagy is mediated by the Cx32 protein rather
than by GJs.
Cx32 promotes autophagy via the AMPK
pathway in cervical cancer cells
Recent studies have shown that AMPK/mTOR signalling
plays an important role in regulating autophagy (32–34).
Then, it was examined whether AMPK/mTOR signalling was involved in
Cx32-induced autophagy. Induction of Cx32 increased the expression
levels of LC3 and p-AMPK/AMPK in HeLa-Cx32 cells (Fig. 5A). By contrast, knockdown of Cx32
decreased the expression levels of LC3 and p-AMPK/AMPK in C-33A
cells (Fig. 5B). To verify the
involvement of AMPK in Cx32-induced autophagy, siAMPK and inhibitor
(compound C) were used to inhibit AMPK expression (35). The upregulation of LC3-II and p-AMPK
expression by Cx32 was reversed by siAMPK or compound C (10 µM, 6
h) in HeLa-Cx32 cells (Fig. 5C and
D). Taken together, these data demonstrate that Cx32 mediates
autophagy via the AMPK signalling pathway.
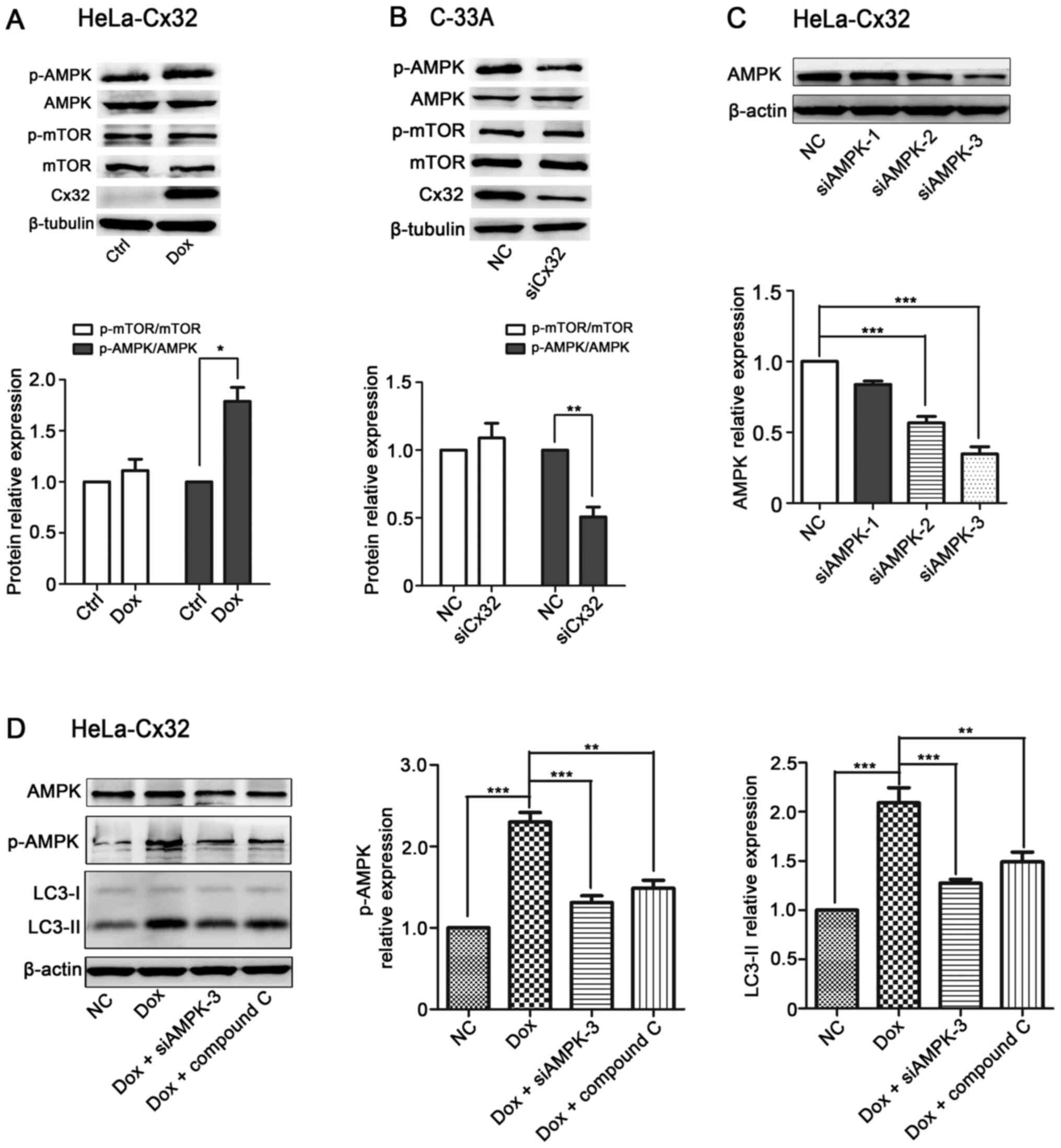 | Figure 5.Cx32 promotes autophagy via the AMPK
pathway in cervical cancer cells. (A) Cx32 overexpression in
HeLa-32 cells increased the protein expression levels of
p-AMPK/AMPK (n=4). (B) Cx32 knockdown in C-33A cells decreased the
protein expression levels of p-AMPK/AMPK (n=4). (C) Western
blotting showed that siAMPK-3 had the greatest efficacy in reducing
the expression of AMPK in HeLa-Cx32 cells (n=4). (D) The
upregulation of LC3-II and p-AMPK/AMPK expression by Cx32 in
HeLa-Cx32 cells was reversed by siAMPK-3 and compound C (10 µM, 6
h) (n=4). The data are presented as the mean ± SEM. *P<0.05,
**P<0.01, ***P<0.001. Cx32, connexin 32; p-, phosphorylated;
AMPK, AMP-activated protein kinase; Dox, doxycycline; si, small
interfering RNA; NC, negative control; Ctrl, control. |
Cx32 inhibits apoptosis by promoting
autophagy
Autophagy is considered to be a crucial modulator of
apoptosis (36). Next, the
relationship between autophagy and apoptosis in cervical cancer
cells was examined, and it was found that pretreatment with an
autophagy activator Rap (2 µM, 4 h) significantly inhibited SN (1
µM, 6 h)-induced apoptosis in HeLa-Cx32 cells (Fig. 6A). By contrast, treatment with
autophagy inhibitors CQ (25 µM, 4 h) or Baf-A1 (100 nM, 4 h)
enhanced apoptosis (Fig. 6A).
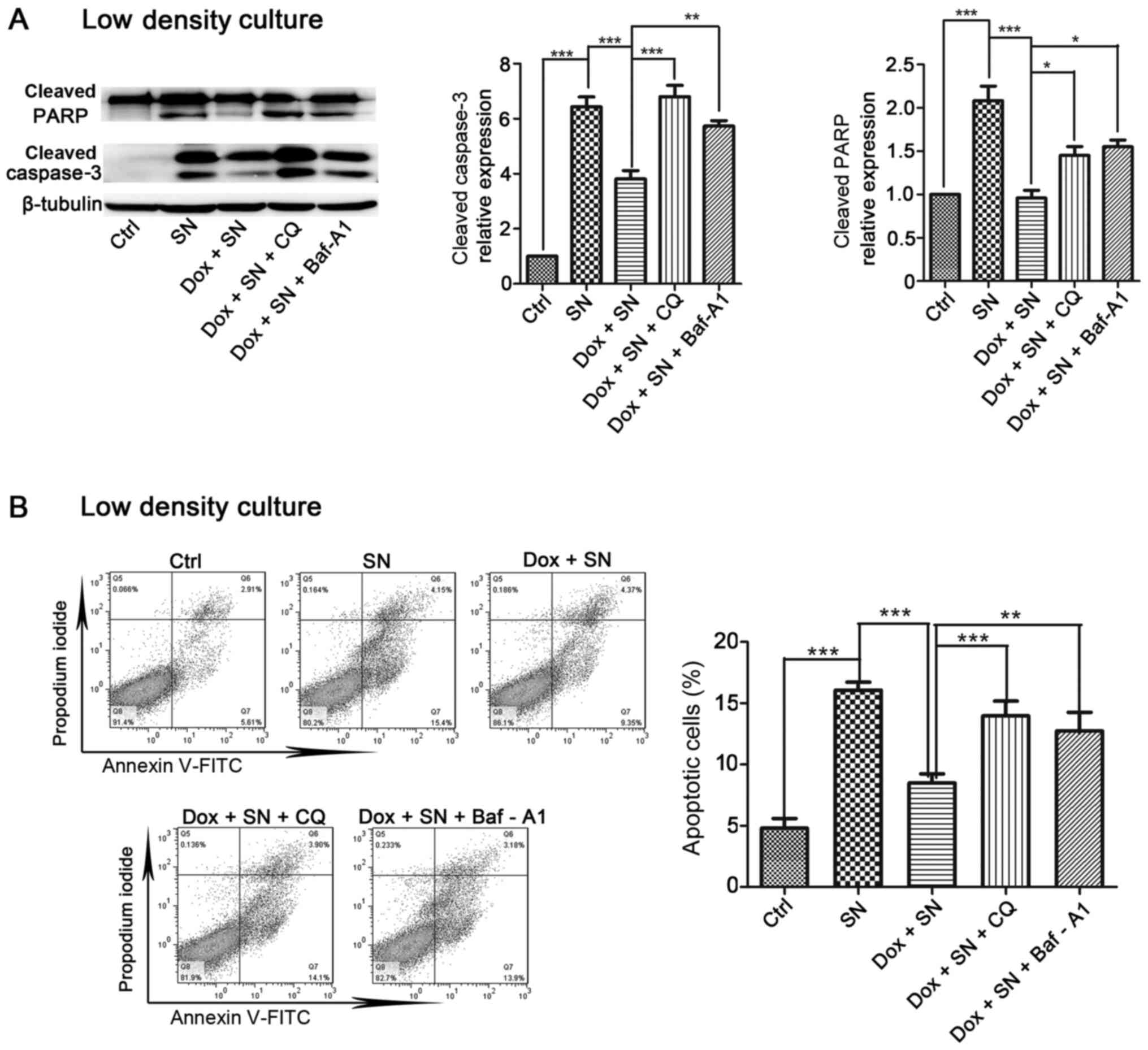
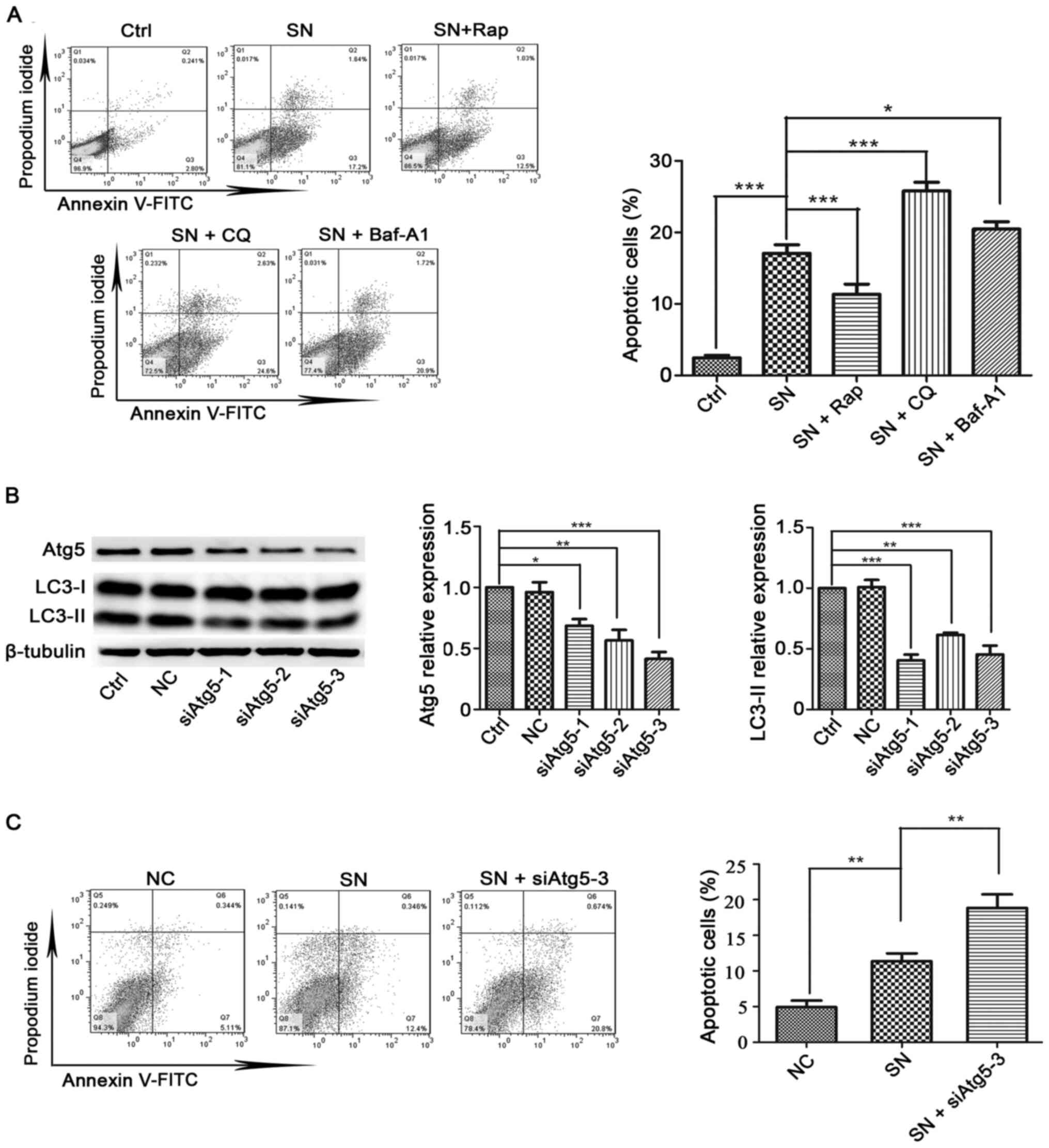 | Figure 6.Effects of autophagy promoters or
autophagy inhibitors on SN-induced apoptosis in HeLa-Cx32 cells.
(A) Autophagy activator (Rap) and autophagy inhibitors (CQ or
Baf-A1) significantly influenced SN-induced cell apoptosis (n=3).
(B) Western blot analysis of the effectiveness of three siAtg5
vectors in reducing the expression of Atg5 and autophagy levels
(n=3). (C) HeLa cells were transfected with siAtg5 for 48 h before
exposure to 1 µM SN for 6 h. Cell apoptosis was analysed using an
Annexin V/PI assay (n=3). The data are presented as the mean ± SEM.
*P<0.05, **P<0.01, ***P<0.001. Atg, autophagy related; si,
small interfering RNA; NC, negative control; Ctrl, control; SN,
streptonigrin; Rap, rapamycin; Baf-A1, bafilomycin A1; CQ,
chloroquine. |
The Atg5-Atg12 conjugation system is essential for
autophagy, and inhibition of Atg5 specifically inhibits autophagy
(37). Atg5 is key regulator
controlling autophagy. Knockdown of Atg5 reduced LC3-II expression
(Fig. 6B). Moreover, inhibition of
autophagy by siAtg5-3 increased the level of apoptosis (Fig. 6C). Collectively, these data indicate
that autophagy suppresses apoptosis in HeLa-Cx32 cells.
Next, the current study determined the effect of
autophagy on Cx32-mediated apoptosis. Induction of Cx32
significantly inhibited SN-induced apoptosis in HeLa-Cx32 cells and
this inhibition was blocked by pretreatment with autophagy
inhibitor CQ (25 µM, 4 h) or Baf-A1 (100 nM, 4 h) (Fig. 7A). In agreement with this result,
overexpression of Cx32 reduced the levels of apoptosis-related
protein, cleaved caspase-3 and cleaved PARP, which were increased
by SN. Moreover, pretreatment with CQ or Baf-A1 reversed the
suppressive effect of Cx32 (Fig.
7B). Taken together, these results suggest that Cx32 inhibits
apoptosis by activating autophagy in cervical cancer cells. The
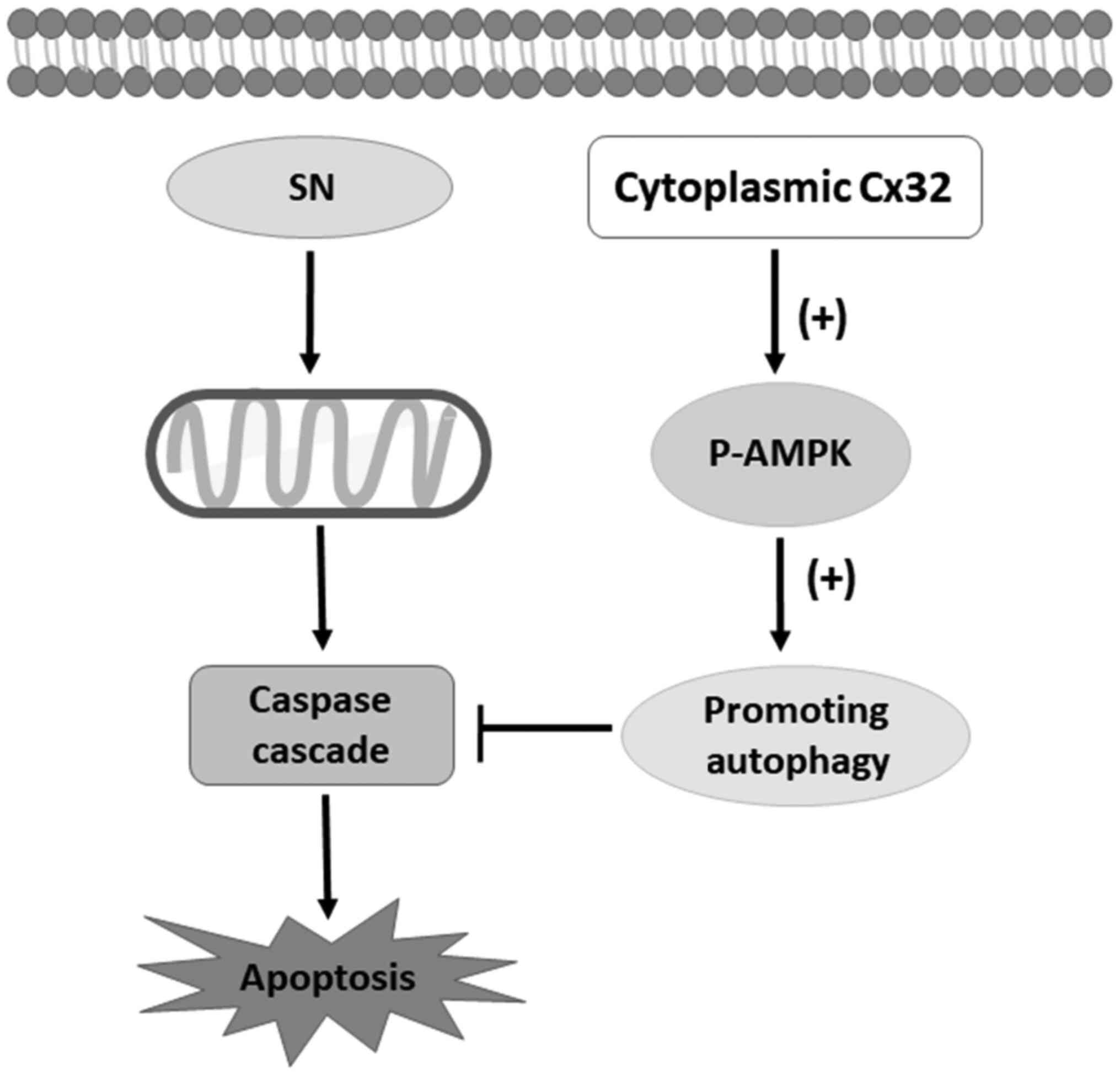
schematic model of this study is shown in Fig. 8.
Discussion
Both autophagy and apoptosis are complex processes.
Our previous study elucidated that the upregulation degree of Cx32
expression correlated with advanced FIGO stages and that Cx32
inhibited apoptosis in cervical cancer via EGFR and NF-κB pathways
(10–12). However, whether the autophagy is
involved in the function of Cx32 on cervical cancer cells remained
unknown. In the present study, a positive correlation between Cx32
and LC3 was observed in cervical cancer specimens. Importantly,
Cx32 promotes autophagy and produces a resistance to SN-induced
apoptosis via activation of AMPK signalling. This work reveals a
previously unknown mechanism between Cx32 and autophagy together
with apoptosis in the process of cervical cancer progression.
Cx is an indispensable transmembrane protein with a
short half-life and is suggested to be an autophagy substrate
(20). Endocytosed GJ is degraded
by autophagy (38), while Cx43
degradation and GJIC impairment induced by simulated ischaemia is
prevented by chemical or genetic inhibitors of autophagy (39). However, an increasing number of
studies have reported that Cxs themselves can suppress autophagy by
recruiting pre-autophagosomal Atg proteins and PI3K components
(21). Independent of its ability
to form functional GJs, Cx43 could co-localize with Atg16 and
repress autophagic flux. Moreover, knockdown of Cx26 or Cx32 in MEF
cells resulted in increased autophagy, coinciding with their
ability to bind and recruit Atg16 (21). Zhong et al (40) reported that downregulation of Cx43
and Cx32 in keratocystic odontogenic tumours correlated negatively
with the expression levels of LC3 and p62. Since our previous study
has demonstrated that the expression of Cx32, rather than Cx26 or
Cx43 was correlated with advanced FIGO stage, augmented tumour size
and poorer differentiation in cervical cancer (12). Therefore, the current study aimed to
determine whether autophagy played a role in the function of Cx32
on cervical cancer cells. It was found that Cx32 was involved in
the activation of autophagy.
Cx is an important component of GJ that maintains
the normal activities of cells and it functions beyond GJs or
hemichannels (29). Emerging
studies have reported that in some tumour types, Cx facilitates
specific stages of tumour progression via both junctional and
non-junctional signalling pathways (41,42).
Upregulated and internalized Cx32 exerts anti-apoptotic effects in
hepatocellular carcinoma (9) and
mediates cisplatin resistance in ovarian cancer cells (43,44).
Cytoplasmic expression of Cx26 in colorectal cancer was responsible
for lung metastasis (45). However,
the carboxy-tail of Cx43 localizes to the nucleus and inhibits cell
proliferation (46). Therefore, the
role of non-junctional Cx remains controversial. In the present
study, the low-density cell culture data indicated that the
induction of autophagy was mediated by the Cx32 protein rather than
by its role in intercellular communication. This study further
suggested the role of the Cx32 protein itself in the progression of
cervical cancer. In future studies, uncovering the turnover and
trafficking of Cx would be indispensable in understanding the
association between Cx and cancer progression and could provide
potential strategies for tumour treatment.
The present study also identified the molecular
mechanism via which Cx32 promotes autophagy in cervical cancer. The
AMPK/mTOR pathway is one of the classic autophagy regulatory
pathways and plays an important role in regulating cell metabolism,
proliferation and apoptosis (47,48).
Therefore, the current study examined whether Cx32 promoted
autophagy via this pathway. The results demonstrated that Cx32
stimulated AMPK phosphorylation but had no effect on mTOR and
p-mTOR proteins expression. Accumulating evidence has shown that
AMPK is an upstream molecule of mTOR and directly promotes
autophagy by phosphorylating autophagy-related proteins in the mTOR
complex 1, unc-51 like autophagy activating kinase 1 (ULK1) and
Phosphatidylinositol 3-kinase catalytic subunit 3/class III
phosphoinositide 3-kinase vacuolar protein sorting 34 complexes or
indirectly by regulating the expression of autophagy-related genes
downstream of transcription factors, such as FOXO3, TFEB and
bromodomain containing 4 (32). In
addition, autophagy was reported to be induced by mTOR-independent
activation of the AMPK/ULK1 pathway (49). Therefore, further investigation is
still required to explore the detailed mechanism of AMPK signalling
involved in Cx32-mediated autophagy.
The role of autophagy in tumour growth remains
controversial (50). On the one
hand, autophagy inhibits tumour formation by reducing oxidative
stress and DNA damage in normal tissues. On the other hand,
autophagy provides cells with energy and vital compounds upon
various stress stimuli in developed cancers, thereby promoting
tumour cell survival (51).
Autophagy and apoptosis cross-regulate each other through an
elaborate network (52). Autophagy
reduces apoptosis by regulating the activity of caspase family
proteins (53). By contrast,
autophagy promotes apoptosis via autophagic degradation of certain
types of inhibitors of apoptosis proteins (54,55).
In the present study, the induction of autophagy reversed
SN-induced apoptosis in HeLa cells, suggesting that autophagy
antagonizes apoptosis in cervical cancer. To the best of our
knowledge, this was the first evidence showing that autophagy and
apoptosis are antagonists in cervical cancer cells.
The current results further indicated that
Cx32-induced autophagy antagonizes SN-induced apoptosis. These
findings revealed a new mechanism via which Cx32 promotes autophagy
and inhibits apoptosis, which accounts for the pro-tumour effect
and drug resistance resulting from Cx32 in cervical cancer. This
study may demonstrate a novel mechanism and provide a theoretical
basis for Cx32 as a potential therapeutic target in cervical
cancer.
There are several limitations to the present study.
First, this study only performed the overexpression and knockdown
of Cx32 in HeLa and C-33A cells, respectively. Whether the results
can be applied to other cell lines remains unclear. Second, though
the role of Cx32 on autophagy and apoptosis was clarified in vitro,
the conclusion will be more persuasive using xenograft animal
model-based experiments. Moreover, the clinic samples were from The
Affiliated Cancer Hospital of Xinjiang Medical University, which
may not be able to reflect the pathophysiological features of
cervical cancer from other regions. Further investigations using a
xenograft animal model and cervical cancer from other regionals are
warranted in the future.
In conclusion, it was identified that Cx32 promotes
autophagy via the AMPK signalling pathway to protect cervical
cancer cells from chemotherapy-induced apoptosis, suggesting that
Cx32 may be a potential biomarker for chemotherapy resistance and
may serve as a key link for autophagy and apoptosis in cervical
cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by the Guangdong Basic and Applied Basic
Research Foundation (grant no. 2019A1515012215), the grant from
Department of Science and Technology of Guangdong Province (grant
no. 20160908), the National Natural Science Foundation of China
(grant no. 81473234), the Joint Fund of the National Natural
Science Foundation of China (grant no. U1303221) and the Guangdong
Basic and Applied Basic Research Fund Project (grant no.
2020A1515110058)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LT and QW designed research. LXF, LT and YCL
performed the study. SYC and ZYZ participated in western blot
analysis of cervical tissues tissue. FY and RYS analyzed the data
and prepared the figures and tables. LXF and YCL wrote the paper.
LT and QW are responsible for confirming the authenticity of the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval (approval no. K-201354) of clinical
tissues was obtained from the Research Committee of Ethics in The
Affiliated Cancer Hospital of Xinjiang Medical University. Written
informed consent form for the experimental studies was obtained
from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boussios S, Seraj E, Zarkavelis G,
Petrakis D, Kollas A, Kafantari A, Assi A, Tatsi K, Pavlidis N and
Pentheroudakis G: Management of patients with recurrent/advanced
cervical cancer beyond first line platinum regimens: Where do we
stand? A literature review. Crit Rev Oncol Hematol. 108:164–174.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marth C, Landoni F, Mahner S, McCormack M,
Gonzalez-Martin A and Colombo N; ESMO Guidelines Committee, :
Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28 (Suppl 4):iv72–iv83. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinyuk M, Mulkearns-Hubert EE, Reizes O
and Lathia J: Cancer Connectors: Connexins, gap junctions, and
communication. Front Oncol. 8:6462018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Q, You T, Yuan D, Han X, Hong X, He
B, Wang L, Tong X, Tao L and Harris AL: Cisplatin and oxaliplatin
inhibit gap junctional communication by direct action and by
reduction of connexin expression, thereby counteracting cytotoxic
efficacy. J Pharmacol Exp Ther. 333:903–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Tao L, Fan L, Peng Y, Yang K,
Zhao Y, Song Q and Wang Q: Different gap junction-propagated
effects on cisplatin transfer result in opposite responses to
cisplatin in normal cells versus tumor cells. Sci Rep. 5:125632015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Omori Y, Nishikawa Y, Yoshioka T,
Yamamoto Y and Enomoto K: Cytoplasmic accumulation of connexin32
protein enhances motility and metastatic ability of human hepatoma
cells in vitro and in vivo. Int J Cancer. 121:536–546. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aasen T, Leithe E, Graham SV, Kameritsch
P, Mayán MD, Mesnil M, Pogoda K and Tabernero A: Connexins in
cancer: Bridging the gap to the clinic. Oncogene. 38:4429–4451.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiang Y, Wang Q, Guo Y, Ge H, Fu Y, Wang X
and Tao L: Cx32 exerts anti-apoptotic and pro-tumor effects via the
epidermal growth factor receptor pathway in hepatocellular
carcinoma. J Exp Clin Cancer Res. 38:1452019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai Y, Fan L, Zhao Y, Ge H, Feng X, Wang
Q, Zhang X, Peng Y, Wang X and Tao L: Cx32 suppresses extrinsic
apoptosis in human cervical cancer cells via the NF-κB signalling
pathway. Int J Oncol. 51:1159–1168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai Y, Tao L, Zhao Y, Zhang X, Sun X, Wang
Q and Xu C: Cx32 inhibits TNFα-induced extrinsic apoptosis with and
without EGFR suppression. Oncol Rep. 38:2885–2892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Lai Y, Ge H, Guo Y, Feng X, Song
J, Wang Q, Fan L, Peng Y, Cao M, et al: Non-junctional Cx32
mediates anti-apoptotic and pro-tumor effects via epidermal growth
factor receptor in human cervical cancer cells. Cell Death Dis.
8:e27732017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ravanan P, Srikumar IF and Talwar P:
Autophagy: The spotlight for cellular stress responses. Life Sci.
188:53–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kardideh B, Samimi Z, Norooznezhad F,
Kiani S and Mansouri K: Autophagy, cancer and angiogenesis: Where
is the link? Cell Biosci. 9:652019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin EJ, Kiral FR and Hiesinger PR: The
where, what, and when of membrane protein degradation in neurons.
Dev Neurobiol. 78:283–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Zhu X, Xiong L, Zhang Y and Ren J:
Toll-like receptor 4 knockout alleviates paraquat-induced
cardiomyocyte contractile dysfunction through an
autophagy-dependent mechanism. Toxicol Lett. 257:11–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu X, Ruan Z, Yang X, Chu K, Wu H, Li Y
and Huang Y: Connexin 31.1 degradation requires the
Clathrin-mediated autophagy in NSCLC cell H1299. J Cell Mol Med.
19:257–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen Z, Chen Q, Jin T, Wang M, Ying H, Lu
J, Wang M, Zhang W, Qiu F, Jin C, et al: Theaflavin 3,3′-digallate
reverses the downregulation of connexin 43 and autophagy induced by
high glucose via AMPK activation in cardiomyocytes. J Cell Physiol.
234:17999–18016. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Xu S, Su Y, Chen B, Yuan H, Xu A
and Wu L: Autophagy-Src Regulates Connexin43-Mediated Gap Junction
Intercellular Communication in Irradiated HepG2 Cells. Radiat Res.
190:494–503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iyyathurai J, Decuypere JP, Leybaert L,
D'hondt C and Bultynck G: Connexins: Substrates and regulators of
autophagy. BMC Cell Biol. 17 (Suppl 1):202016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bejarano E, Yuste A, Patel B, Stout RF Jr,
Spray DC and Cuervo AM: Connexins modulate autophagosome
biogenesis. Nat Cell Biol. 16:401–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gurkan AC, Arisan ED, Yerlikaya PO, Ilhan
H and Unsal NP: Inhibition of autophagy enhances DENSpm-induced
apoptosis in human colon cancer cells in a p53 independent manner.
Cell Oncol (Dordr). 41:297–317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nepal S and Park PH: Activation of
autophagy by globular adiponectin attenuates ethanol-induced
apoptosis in HepG2 cells: Involvement of AMPK/FoxO3A axis. Biochim
Biophys Acta. 1833:2111–2125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinet W, Timmermans JP and De Meyer GR:
Methods to assess autophagy in situ - transmission electron
microscopy versus immunohistochemistry. Methods Enzymol.
543:89–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr
M, Hijlkema KJ, Coppes RP, Engedal N, Mari M and Reggiori F:
Chloroquine inhibits autophagic flux by decreasing
autophagosome-lysosome fusion. Autophagy. 14:1435–1455. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sheng J, Sun LB, Zhao SF, Qi WW, Lv J,
Zhang ZG, Ding AP and Qiu WS: Acidic stress induces protective
autophagy in SGC7901 cells. J Int Med Res. 46:3285–3295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goodenough DA and Paul DL: Beyond the gap:
Functions of unpaired connexon channels. Nat Rev Mol Cell Biol.
4:285–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plotkin LI and Bellido T: Beyond gap
junctions: Connexin43 and bone cell signaling. Bone. 52:157–166.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martins-Marques T, Ribeiro-Rodrigues T,
Batista-Almeida D, Aasen T, Kwak BR and Girao H: Biological
Functions of Connexin43 Beyond Intercellular Communication. Trends
Cell Biol. 29:835–847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y and Chen Y: AMPK and Autophagy. Adv
Exp Med Biol. 1206:85–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li SX, Li C, Pang XR, Zhang J, Yu GC, Yeo
AJ, Lavin MF, Shao H, Jia Q and Peng C: Metformin Attenuates
Silica-Induced Pulmonary Fibrosis by Activating Autophagy via the
AMPK-mTOR Signaling Pathway. Front Pharmacol. 12:7195892021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu J, Ao H, Liu M, Cao K and Ma J: UBE2T
promotes autophagy via the p53/AMPK/mTOR signaling pathway in lung
adenocarcinoma. J Transl Med. 19:3742021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hardie DG: AMPK and autophagy get
connected. EMBO J. 30:634–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun X, Momen A, Wu J, Noyan H, Li R, von
Harsdorf R and Husain M: p27 protein protects metabolically
stressed cardiomyocytes from apoptosis by promoting autophagy. J
Biol Chem. 289:16924–16935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fong JT, Kells RM, Gumpert AM, Marzillier
JY, Davidson MW and Falk MM: Internalized gap junctions are
degraded by autophagy. Autophagy. 8:794–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martins-Marques T, Catarino S, Zuzarte M,
Marques C, Matafome P, Pereira P and Girão H: Ischaemia-induced
autophagy leads to degradation of gap junction protein connexin43
in cardiomyocytes. Biochem J. 467:231–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhong WQ, Chen G, Zhang W, Xiong XP, Ren
JG, Zhao Y, Liu B and Zhao YF: Down-regulation of connexin43 and
connexin32 in keratocystic odontogenic tumours: Potential
association with clinical features. Histopathology. 66:798–807.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Polusani SR, Kalmykov EA, Chandrasekhar A,
Zucker SN and Nicholson BJ: Cell coupling mediated by connexin 26
selectively contributes to reduced adhesivity and increased
migration. J Cell Sci. 129:4399–4410. 2016.PubMed/NCBI
|
|
42
|
Chen Q, Boire A, Jin X, Valiente M, Er EE,
Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K, et al:
Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP
transfer. Nature. 533:493–498. 2016.Erratum in: Nature 544: 124,
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu W, Fan L, Bao Z, Zhang Y, Peng Y, Shao
M, Xiang Y, Zhang X, Wang Q and Tao L: The cytoplasmic
translocation of Cx32 mediates cisplatin resistance in ovarian
cancer cells. Biochem Biophys Res Commun. 487:292–299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Tao L, Fan LX, Huang K, Luo HM,
Ge H, Wang X and Wang Q: Cx32 mediates cisplatin resistance in
human ovarian cancer cells by affecting drug efflux transporter
expression and activating the EGFR-Akt pathway. Mol Med Rep.
19:2287–2296. 2019.PubMed/NCBI
|
|
45
|
Ezumi K, Yamamoto H, Murata K, Higashiyama
M, Damdinsuren B, Nakamura Y, Kyo N, Okami J, Ngan CY, Takemasa I,
et al: Aberrant expression of connexin 26 is associated with lung
metastasis of colorectal cancer. Clin Cancer Res. 14:677–684. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dang X, Doble BW and Kardami E: The
carboxy-tail of connexin-43 localizes to the nucleus and inhibits
cell growth. Mol Cell Biochem. 242:35–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tamargo-Gómez I and Mariño G: AMPK:
Regulation of Metabolic Dynamics in the Context of Autophagy. Int J
Mol Sci. 19:E38122018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Visnjic D, Dembitz V and Lalic H: The Role
of AMPK/mTOR Modulators in the Therapy of Acute Myeloid Leukemia.
Curr Med Chem. 26:2208–2229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li RL, Wu SS, Wu Y, Wang XX, Chen HY, Xin
JJ, Li H, Lan J, Xue KY, Li X, et al: Irisin alleviates pressure
overload-induced cardiac hypertrophy by inducing protective
autophagy via mTOR-independent activation of the AMPK-ULK1 pathway.
J Mol Cell Cardiol. 121:242–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cristofani R, Montagnani Marelli M,
Cicardi ME, Fontana F, Marzagalli M, Limonta P, Poletti A and
Moretti RM: Dual role of autophagy on docetaxel-sensitivity in
prostate cancer cells. Cell Death Dis. 9:8892018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cotzomi-Ortega I, Aguilar-Alonso P,
Reyes-Leyva J and Maycotte P: Autophagy and Its Role in Protein
Secretion: Implications for Cancer Therapy. Mediators Inflamm.
2018:42315912018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chung Y, Lee J, Jung S, Lee Y, Cho JW and
Oh YJ: Dysregulated autophagy contributes to caspase-dependent
neuronal apoptosis. Cell Death Dis. 9:11892018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tsapras P and Nezis IP: Caspase
involvement in autophagy. Cell Death Differ. 24:1369–1379. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He W, Wang Q, Srinivasan B, Xu J, Padilla
MT, Li Z, Wang X, Liu Y, Gou X, Shen HM, et al: A JNK-mediated
autophagy pathway that triggers c-IAP degradation and necroptosis
for anticancer chemotherapy. Oncogene. 33:3004–3013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu J, Xu X, Shi S, Wang Q, Saxton B, He W,
Gou X, Jang JH, Nyunoya T, Wang X, et al: Autophagy-mediated
degradation of IAPs and c-FLIP(L) potentiates apoptosis induced by
combination of TRAIL and Chal-24. J Cell Biochem. 117:1136–1144.
2016. View Article : Google Scholar : PubMed/NCBI
|