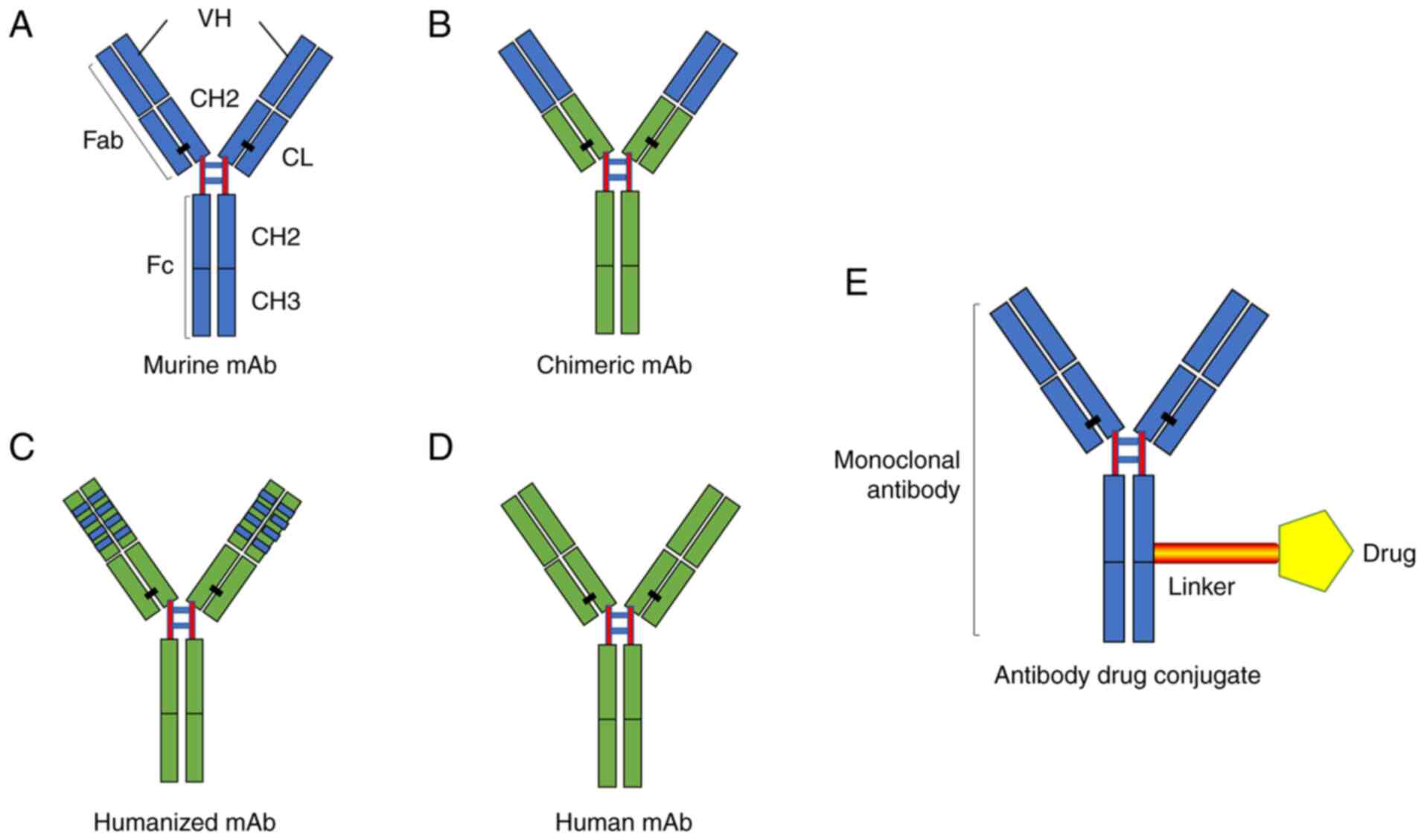
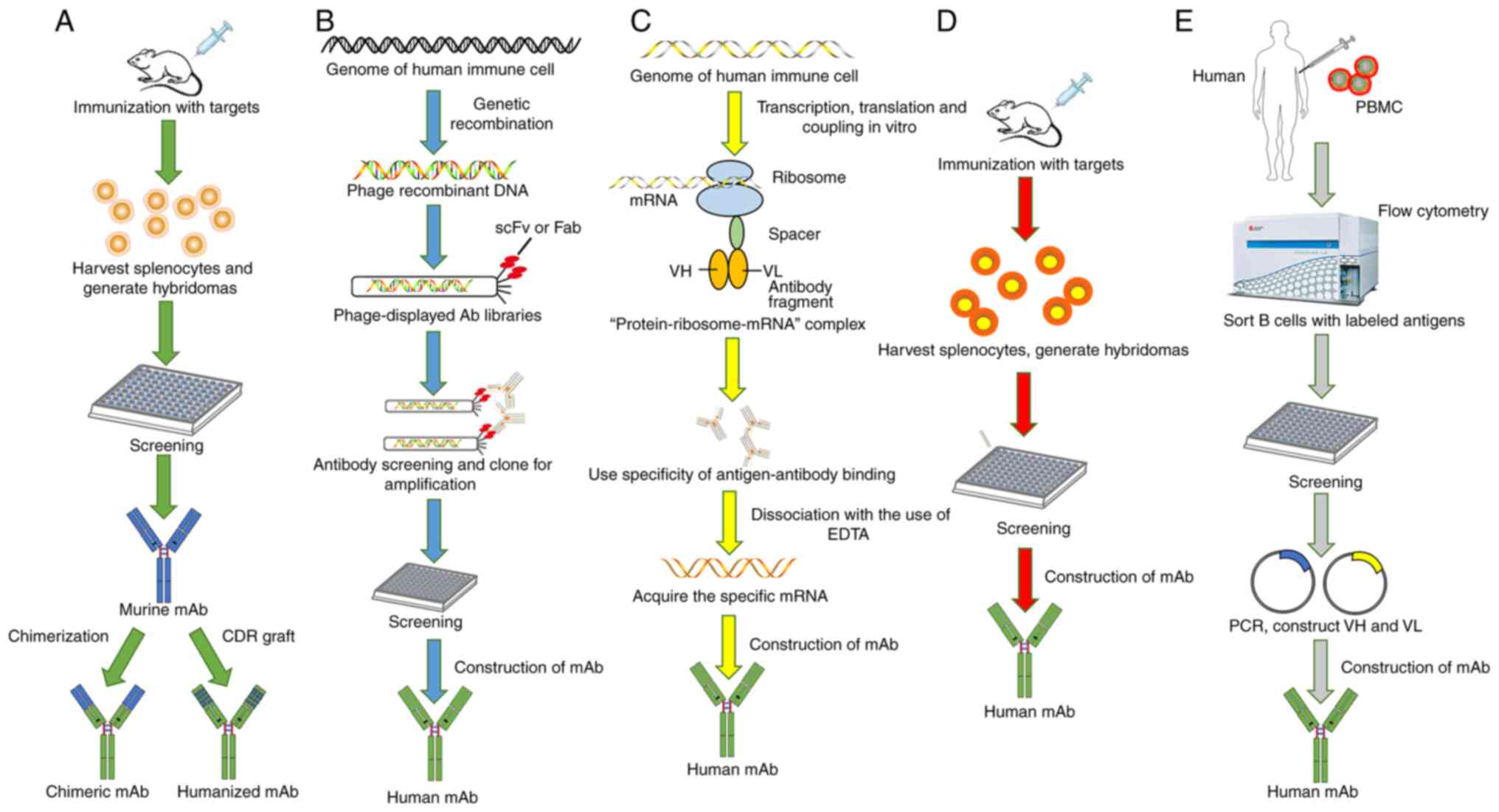
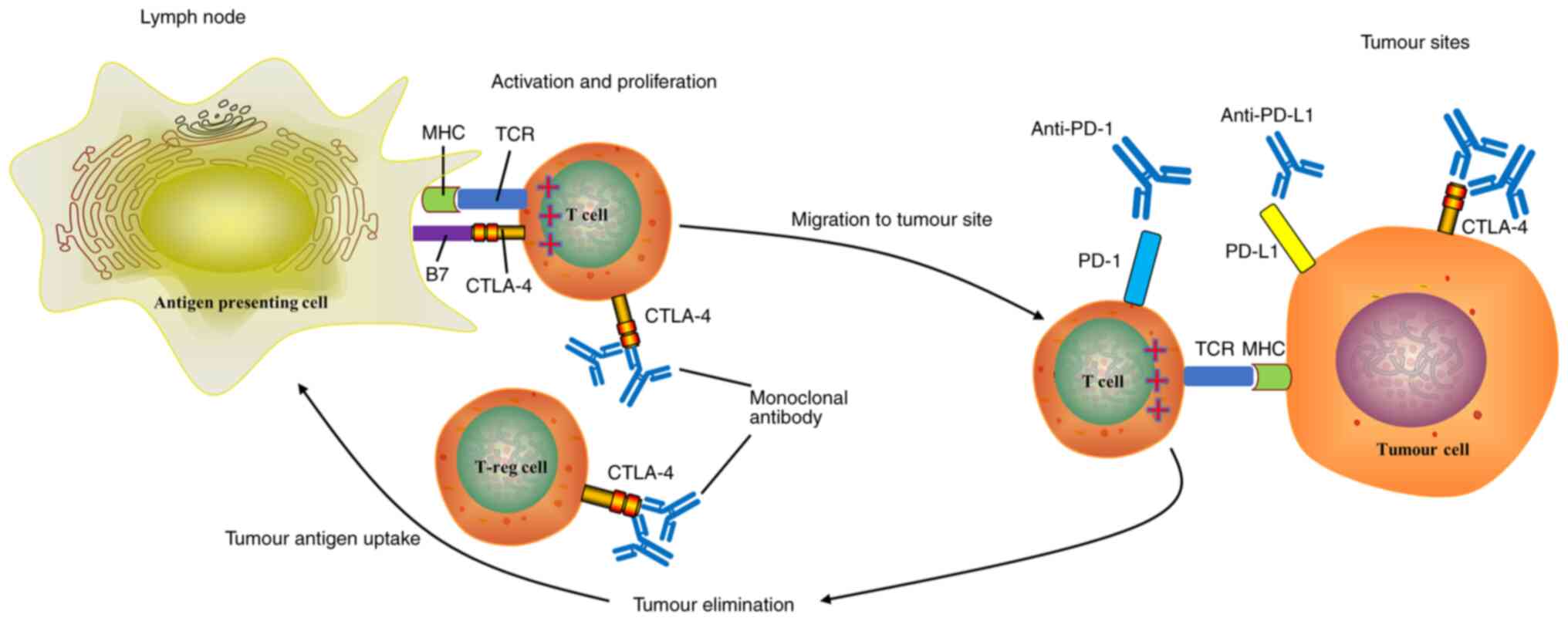
|
1
|
Patel A: Benign vs malignant tumors. JAMA
Oncol. 6:14882020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murray PG and Young LS: An etiological
role for the Epstein-Barr virus in the pathogenesis of classical
Hodgkin lymphoma. Blood. 134:591–596. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keum N and Giovannucci E: Global burden of
colorectal cancer: Emerging trends, risk factors and prevention
strategies. Nat Rev Gastroenterol Hepatol. 16:713–732. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hasanpourghadi M, Pandurangan AK and
Mustafa MR: Modulation of oncogenic transcription factors by
bioactive natural products in breast cancer. Pharmacol Res.
128:376–388. 2018. View Article : Google Scholar
|
|
5
|
Stark A, Donahue TR, Reber HA and Hines
OJ: Pancreatic cyst disease: A review. JAMA. 315:1882–1893. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis WD, Asamura H, Bankier AA, Beasley
MB, Detterbeck F, Flieder DB, Goo JM, MacMahon H, Naidich D,
Nicholson AG, et al: The IASLC lung cancer staging project:
Proposals for coding T categories for subsolid nodules and
assessment of tumor size in part-solid tumors in the forthcoming
eighth edition of the TNM classification of lung cancer. J Thorac
Oncol. 11:1204–1223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clara-Trujillo S, Gallego Ferrer G and
Gómez Ribelles JL: In vitro modeling of non-solid tumors: How far
can tissue engineering go? Int J Mol Sci. 21:57472020. View Article : Google Scholar :
|
|
8
|
Shimada A: Hematological malignancies and
molecular targeting therapy. Eur J Pharmacol. 862:1726412019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dunn-Pirio AM and Vlahovic G:
Immunotherapy approaches in the treatment of malignant brain
tumors. Cancer. 123:734–750. 2017. View Article : Google Scholar
|
|
10
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov.
17:887–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schweizer C, Schubert P, Rutzner S,
Eckstein M, Haderlein M, Lettmaier S, Semrau S, Gostian AO, Frey B,
Gaipl US, et al: Prospective evaluation of the prognostic value of
immune-related adverse events in patients with non-melanoma solid
tumour treated with PD-1/PD-L1 inhibitors alone and in combination
with radiotherapy. Eur J Cancer. 140:55–62. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ovacik M and Lin K: Tutorial on monoclonal
antibody pharmacokinetics and its considerations in early
development. Clin Transl Sci. 11:540–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cymer F, Beck H, Rohde A and Reusch D:
Therapeutic monoclonal antibody N-glycosylation-structure, function
and therapeutic potential. Biologicals. 52:1–11. 2018. View Article : Google Scholar
|
|
14
|
Alkan SS: Legends of allergy/immunology:
Georges Köhler and the discovery of MONOCLONAL antibodies. Allergy.
74:1412–1414. 2019.PubMed/NCBI
|
|
15
|
Seaman S, Zhu Z, Saha S, Zhang XM, Yang
MY, Hilton MB, Morris K, Szot C, Morris H, Swing DA, et al:
Eradication of tumors through simultaneous ablation of
CD276/B7H3-positive tumor cells and tumor vasculature. Cancer Cell.
31:501–515.e8. 2017. View Article : Google Scholar
|
|
16
|
Fay EK and Graff JN: Immunotherapy in
prostate cancer. Cancers (Basel). 12:17522020. View Article : Google Scholar
|
|
17
|
Arlotta KJ and Owen SC: Antibody and
antibody derivatives as cancer therapeutics. Wiley Interdiscip Rev
Nanomed Nanobiotechnol. 11:e15562019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Starr CG and Tessier PM: Selecting and
engineering monoclonal antibodies with drug-like specificity. Curr
Opin Biotechnol. 60:119–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiu ML and Gilliland GL: Engineering
antibody therapeutics. Curr Opin Struct Biol. 38:163–173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wootla B, Denic A and Rodriguez M:
Polyclonal and monoclonal antibodies in clinic. Methods Mol Biol.
1060:79–110. 2014. View Article : Google Scholar
|
|
21
|
Köhler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller RA, Maloney DG, Warnke R and Levy
R: Treatment of B-cell lymphoma with monoclonal anti-idiotype
antibody. N Engl J Med. 306:517–522. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
An Z: Monoclonal antibodies-a proven and
rapidly expanding therapeutic modality for human diseases. Protein
Cell. 1:319–330. 2010. View Article : Google Scholar
|
|
24
|
Grilo AL and Mantalaris A: The
increasingly human and profitable monoclonal antibody market.
Trends Biotechnol. 37:9–16. 2019. View Article : Google Scholar
|
|
25
|
Garrard LJ and Zhukovsky EA: Antibody
expression in bacteriophage systems: The future of monoclonal
antibodies? Curr Opin Biotechnol. 3:474–480. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parray HA, Shukla S, Samal S, Shrivastava
T, Ahmed S, Sharma C and Kumar R: Hybridoma technology a versatile
method for isolation of monoclonal antibodies, its applicability
across species, limitations, advancement and future perspectives.
Int Immunopharmacol. 85:1066392020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shim H: Antibody phage display. Adv Exp
Med Biol. 1053:21–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Groves MA and Osbourn JK: Applications of
ribosome display to antibody drug discovery. Expert Opin Biol Ther.
5:125–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ,
Li HJ and Wu HC: Development of therapeutic antibodies for the
treatment of diseases. J Biomed Sci. 27:12020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmid AS and Neri D: Advances in antibody
engineering for rheumatic diseases. Nat Rev Rheumatol. 15:197–207.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuramochi T, Igawa T, Tsunoda H and
Hattori K: Humanization and simultaneous optimization of monoclonal
antibody. Methods Mol Biol. 1904:213–230. 2019. View Article : Google Scholar
|
|
32
|
Goydel RS, Weber J, Peng H, Qi J, Soden J,
Freeth J, Park H and Rader C: Affinity maturation, humanization,
and co-crystallization of a rabbit anti-human ROR2 monoclonal
antibody for therapeutic applications. J Biol Chem. 295:5995–6006.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Li C, Xia S, Tian X, Kong Y, Wang Z,
Gu C, Zhang R, Tu C, Xie Y, et al: Identification of human
single-domain antibodies against SARS-CoV-2. Cell Host Microbe.
27:891–898.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frenzel A, Schirrmann T and Hust M: Phage
display-derived human antibodies in clinical development and
therapy. MAbs. 8:1177–1194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pucca MB, Cerni FA, Janke R,
Bermúdez-Méndez E, Ledsgaard L, Barbosa JE and Laustsen AH: History
of envenoming therapy and current perspectives. Front Immunol.
10:15982019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ribatti D: From the discovery of
monoclonal antibodies to their therapeutic application: An
historical reappraisal. Immunol Lett. 161:96–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elgundi Z, Reslan M, Cruz E, Sifniotis V
and Kayser V: The state-of-play and future of antibody
therapeutics. Adv Drug Deliv Rev. 122:2–19. 2017. View Article : Google Scholar
|
|
38
|
Paci A, Desnoyer A, Delahousse J, Blondel
L, Maritaz C, Chaput N, Mir O and Broutin S:
Pharmacokinetic/pharmacodynamic relationship of therapeutic
monoclonal antibodies used in oncology: Part 1, monoclonal
antibodies, antibody-drug conjugates and bispecific T-cell
engagers. Eur J Cancer. 128:107–118. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zaroff S and Tan G: Hybridoma technology:
The preferred method for monoclonal antibody generation for in vivo
applications. Biotechniques. 67:90–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schroff RW, Foon KA, Beatty SM, Oldham RK
and Morgan AC Jr: Human anti-murine immunoglobulin responses in
patients receiving monoclonal antibody therapy. Cancer Res.
45:879–885. 1985.PubMed/NCBI
|
|
41
|
Angus DC, Birmingham MC, Balk RA, Scannon
PJ, Collins D, Kruse JA, Graham DR, Dedhia HV, Homann S and
MacIntyre N: E5 murine monoclonal antiendotoxin antibody in
gram-negative sepsis: A randomized controlled trial. E5 study
investigators JAMA. 283:1723–1730. 2000.
|
|
42
|
Karmali R, Kimby E, Ghielmini M, Flinn IW,
Gordon LI and Zucca E: Rituximab: A benchmark in the development of
chemotherapy-free treatment strategies for follicular lymphomas.
Ann Oncol. 29:332–340. 2018. View Article : Google Scholar
|
|
43
|
Crowe JE Jr: Recent advances in the study
of human antibody responses to influenza virus using optimized
human hybridoma approaches. Vaccine. 27(Suppl 6): G47–G51. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gonzales NR, De Pascalis R, Schlom J and
Kashmiri SV: Minimizing the immunogenicity of antibodies for
clinical application. Tumour Biol. 26:31–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Alfaleh MA, Alsaab HO, Mahmoud AB,
Alkayyal AA, Jones ML, Mahler SM and Hashem AM: Phage display
derived monoclonal antibodies: From bench to bedside. Front
Immunol. 11:19862020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
LoBuglio AF, Wheeler RH, Trang J, Haynes
A, Rogers K, Harvey EB, Sun L, Ghrayeb J and Khazaeli MB:
Mouse/human chimeric monoclonal antibody in man: Kinetics and
immune response. Proc Natl Acad Sci USA. 86:4220–4224. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Targan SR, Hanauer SB, van Deventer SJ,
Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF and
Rutgeerts PJ: A short-term study of chimeric monoclonal antibody
cA2 to tumor necrosis factor alpha for Crohn.s disease. Crohn.s
disease cA2 study group. N Engl J Med. 337:1029–1035. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu AY, Robinson RR, Murray ED Jr,
Ledbetter JA, Hellström I and Hellström KE: Production of a
mouse-human chimeric monoclonal antibody to CD20 with potent
Fc-dependent biologic activity. J Immunol. 139:3521–3526.
1987.PubMed/NCBI
|
|
49
|
McLaughlin P, Grillo-López AJ, Link BK,
Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I,
White CA, Cabanillas F, et al: Rituximab chimeric anti-CD20
monoclonal antibody therapy for relapsed indolent lymphoma: Half of
patients respond to a four-dose treatment program. J Clin Oncol.
16:2825–2833. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Piccolo R, Eitel I, Galasso G,
Dominguez-Rodriguez A, Iversen AZ, Abreu-Gonzalez P, Windecker S,
Thiele H and Piscione F: 1-Year outcomes with intracoronary
abciximab in diabetic patients undergoing primary percutaneous
coronary intervention. J Am Coll Cardiol. 68:727–738. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bachlava E, Loukopoulou S, Karanasios E,
Chrousos G and Michos A: Management of coronary artery aneurysms
using abciximab in children with Kawasaki disease. Int J Cardiol.
220:65–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu SN, Zhang XH, Xu LP, Wang Y, Yan CH,
Chen H, Chen YH, Han W, Wang FR, Wang JZ, et al: Prognostic factors
and long-term follow-up of basiliximab for steroid-refractory acute
graft-versus-host disease: Updated experience from a large-scale
study. Am J Hematol. 95:927–936. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Furuya Y, Jayarajan SN, Taghavi S, Cordova
FC, Patel N, Shiose A, Leotta E, Criner GJ, Guy TS, Wheatley GH, et
al: The impact of alemtuzumab and basiliximab induction on patient
survival and time to bronchiolitis obliterans syndrome in double
lung transplantation recipients. Am J Transplant. 16:2334–2341.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aranda E, García-Alfonso P, Benavides M,
Sánchez Ruiz A, Guillén-Ponce C, Safont MJ, Alcaide J, Gómez A,
López R, Manzano JL, et al: First-line mFOLFOX plus cetuximab
followed by mFOLFOX plus cetuximab or single-agent cetuximab as
maintenance therapy in patients with metastatic colorectal cancer:
Phase II randomised MACRO2 TTD study. Eur J Cancer. 101:263–272.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Strohbehn GW and Vokes EE: Palbociclib: A
new partner for cetuximab? Lancet Oncol. 20:1195–1196. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Strohl WR: Current progress in innovative
engineered antibodies. Protein Cell. 9:86–120. 2018. View Article : Google Scholar :
|
|
57
|
Presta LG: Engineering of therapeutic
antibodies to minimize immunogenicity and optimize function. Adv
Drug Deliv Rev. 58:640–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kumar R, Parray HA, Shrivastava T, Sinha S
and Luthra K: Phage display antibody libraries: A robust approach
for generation of recombinant human monoclonal antibodies. Int J
Biol Macromol. 135:907–918. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Saw PE and Song EW: Phage display
screening of therapeutic peptide for cancer targeting and therapy.
Protein Cell. 10:787–807. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ledsgaard L, Kilstrup M, Karatt-Vellatt A,
McCafferty J and Laustsen AH: Basics of antibody phage display
technology. Toxins (Basel). 10:2362018. View Article : Google Scholar
|
|
61
|
Greenwood J, Willis AE and Perham RN:
Multiple display of foreign peptides on a filamentous
bacteriophage. Peptides from plasmodium falciparum circumsporozoite
protein as antigens. J Mol Biol. 220:821–827. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Smith GP: Filamentous fusion phage: Novel
expression vectors that display cloned antigens on the virion
surface. Science. 228:1315–1317. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Geysen HM, Tainer JA, Rodda SJ, Mason TJ,
Alexander H, Getzoff ED and Lerner RA: Chemistry of antibody
binding to a protein. Science. 235:1184–1190. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Parmley SF and Smith GP:
Antibody-selectable filamentous fd phage vectors: Affinity
purification of target genes. Gene. 73:305–318. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Scott JK and Smith GP: Searching for
peptide ligands with an epitope library. Science. 249:386–390.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
McCafferty J, Griffiths AD, Winter G and
Chiswell DJ: Phage antibodies: Filamentous phage displaying
antibody variable domains. Nature. 348:552–554. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tan Y, Tian T, Liu W, Zhu Z and C JY:
Advance in phage display technology for bioanalysis. Biotechnol J.
11:732–745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Deng X, Wang L, You X, Dai P and Zeng Y:
Advances in the T7 phage display system (Review). Mol Med Rep.
17:714–720. 2018.
|
|
69
|
Burritt JB, Bond CW, Doss KW and Jesaitis
AJ: Filamentous phage display of oligopeptide libraries. Anal
Biochem. 238:1–13. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang J, Doria-Rose NA, Longo NS, Laub L,
Lin CL, Turk E, Kang BH, Migueles SA, Bailer RT, Mascola JR and
Connors M: Isolation of human monoclonal antibodies from peripheral
blood B cells. Nat Protoc. 8:1907–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Z, Li Y, Hou B, Pronobis MI, Wang M,
Wang Y, Cheng G, Weng W, Wang Y, Tang Y, et al: An array of 60,000
antibodies for proteome-scale antibody generation and target
discovery. Sci Adv. 6:eaax22712020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Galán A, Comor L, Horvatić A, Kuleš J,
Guillemin N, Mrljak V and Bhide M: Library-based display
technologies: Where do we stand? Mol Biosyst. 12:2342–2358. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lim CC, Choong YS and Lim TS: Cognizance
of molecular methods for the generation of mutagenic phage display
antibody libraries for affinity maturation. Int J Mol Sci.
20:18612019. View Article : Google Scholar :
|
|
74
|
Goracci M, Pignochino Y and Marchiò S:
Phage display-based nanotechnology applications in cancer
immunotherapy. Molecules. 25:8432020. View Article : Google Scholar :
|
|
75
|
Rahbarnia L, Farajnia S, Babaei H, Majidi
J, Veisi K, Ahmadzadeh V and Akbari B: Evolution of phage display
technology: From discovery to application. J Drug Target.
25:216–224. 2017. View Article : Google Scholar
|
|
76
|
Petrenko VA: Landscape phage: Evolution
from phage display to nanobiotechnology. Viruses. 10:3112018.
View Article : Google Scholar :
|
|
77
|
Brüggemann M and Neuberger MS: Strategies
for expressing human antibody repertoires in transgenic mice.
Immunol Today. 17:391–397. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brüggemann M, Osborn MJ, Ma B, Hayre J,
Avis S, Lundstrom B and Buelow R: Human antibody production in
transgenic animals. Arch Immunol Ther Exp (Warsz). 63:101–108.
2015. View Article : Google Scholar
|
|
79
|
Laffleur B, Pascal V, Sirac C and Cogné M:
Production of human or humanized antibodies in mice. Methods Mol
Biol. 901:149–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen WC and Murawsky CM: Strategies for
generating diverse antibody repertoires using transgenic animals
expressing human antibodies. Front Immunol. 9:4602018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Alt FW, Blackwell TK and Yancopoulos GD:
Immunoglobulin genes in transgenic mice. Trends Genet. 1:231–236.
1985. View Article : Google Scholar
|
|
82
|
Frippiat JP, Williams SC, Tomlinson IM,
Cook GP, Cherif D, Le Paslier D, Collins JE, Dunham I, Winter G and
Lefranc MP: Organization of the human immunoglobulin lambda
light-chain locus on chromosome 22q11.2. Hum Mol Genet. 4:983–991.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fishwild DM, O'Donnell SL, Bengoechea T,
Hudson DV, Harding F, Bernhard SL, Jones D, Kay RM, Higgins KM,
Schramm SR and Lonberg N: High-avidity human IgG kappa monoclonal
antibodies from a novel strain of minilocus transgenic mice. Nat
Biotechnol. 14:845–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mendez MJ, Green LL, Corvalan JR, Jia XC,
Maynard-Currie CE, Yang XD, Gallo ML, Louie DM, Lee DV, Erickson
KL, et al: Functional transplant of megabase human immunoglobulin
loci recapitulates human antibody response in mice. Nat Genet.
15:146–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lonberg N: Human antibodies from
transgenic animals. Nat Biotechnol. 23:1117–1125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Siegel SA, Shealy DJ, Nakada MT, Le J,
Woulfe DS, Probert L, Kollias G, Ghrayeb J, Vilcek J and Daddona
PE: The mouse/human chimeric monoclonal antibody cA2 neutralizes
TNF in vitro and protects transgenic mice from cachexia and TNF
lethality in vivo. Cytokine. 7:15–25. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tsujinaka T, Fujita J, Ebisui C, Yano M,
Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y, Shiozaki H and
Monden M: Interleukin 6 receptor antibody inhibits muscle atrophy
and modulates proteolytic systems in interleukin 6 transgenic mice.
J Clin Invest. 97:244–249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Crombet-Ramos T, Rak J, Pérez R and
Viloria-Petit A: Antiproliferative, antiangiogenic and proapoptotic
activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer.
101:567–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jakobovits A, Amado RG, Yang X, Roskos L
and Schwab G: From XenoMouse technology to panitumumab, the first
fully human antibody product from transgenic mice. Nat Biotechnol.
25:1134–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ros F, Offner S, Klostermann S, Thorey I,
Niersbach H, Breuer S, Zarnt G, Lorenz S, Puels J, Siewe B, et al:
Rabbits transgenic for human IgG genes recapitulating rabbit B-cell
biology to generate human antibodies of high specificity and
affinity. MAbs. 12:18469002020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
He M and Taussig MJ: Eukaryotic ribosome
display with in situ DNA recovery. Nat Methods. 4:281–288. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Thom G and Groves M: Ribosome display.
Methods Mol Biol. 901:101–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Plückthun A: Ribosome display: A
perspective. Methods Mol Biol. 805:3–28. 2012. View Article : Google Scholar
|
|
94
|
Rothe A, Hosse RJ and Power BE: Ribosome
display for improved biotherapeutic molecules. Expert Opin Biol
Ther. 6:177–187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ministro J, Manuel AM and Goncalves J:
Therapeutic antibody engineering and selection strategies. Adv
Biochem Eng Biotechnol. 171:55–86. 2020.
|
|
96
|
Mattheakis LC, Bhatt RR and Dower WJ: An
in vitro polysome display system for identifying ligands from very
large peptide libraries. Proc Natl Acad Sci USA. 91:9022–9026.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hammers CM and Stanley JR: Antibody phage
display: Technique and applications. J Invest Dermatol. 134:1–5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Loh B, Kuhn A and Leptihn S: The
fascinating biology behind phage display: Filamentous phage
assembly. Mol Microbiol. 111:1132–1138. 2019. View Article : Google Scholar
|
|
99
|
Zahnd C, Amstutz P and Plückthun A:
Ribosome display: Selecting and evolving proteins in vitro that
specifically bind to a target. Nat Methods. 4:269–279. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hammerling MJ, Fritz BR, Yoesep DJ, Kim
DS, Carlson ED and Jewett MC: In vitro ribosome synthesis and
evolution through ribosome display. Nat Commun. 11:11082020.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
He M and Khan F: Ribosome display:
Next-generation display technologies for production of antibodies
in vitro. Expert Rev Proteomics. 2:421–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lagoutte P, Lugari A, Elie C, Potisopon S,
Donnat S, Mignon C, Mariano N, Troesch A, Werle B and Stadthagen G:
Combination of ribosome display and next generation sequencing as a
powerful method for identification of affibody binders against
β-lactamase CTX-M15. N Biotechnol. 50:60–69. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rouet R, Jackson KJL, Langley DB and
Christ D: Next-generation sequencing of antibody display
repertoires. Front Immunol. 9:1182018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yamaguchi J, Naimuddin M, Biyani M, Sasaki
T, Machida M, Kubo T, Funatsu T, Husimi Y and Nemoto N: cDNA
display: A novel screening method for functional disulfide-rich
peptides by solid-phase synthesis and stabilization of mRNA-protein
fusions. Nucleic Acids Res. 37:e1082009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lipovsek D and Plückthun A: In-vitro
protein evolution by ribosome display and mRNA display. J Immunol
Methods. 290:51–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ueda T, Kanamori T and Ohashi H: Ribosome
display with the PURE technology. Methods Mol Biol. 607:219–225.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Roberts RW and Szostak JW: RNA-peptide
fusions for the in vitro selection of peptides and proteins. Proc
Natl Acad Sci USA. 94:12297–12302. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Muranaka N, Hohsaka T and Sisido M:
Four-base codon mediated mRNA display to construct peptide
libraries that contain multiple nonnatural amino acids. Nucleic
Acids Res. 34:e72006. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Dufner P, Jermutus L and Minter RR:
Harnessing phage and ribosome display for antibody optimisation.
Trends Biotechnol. 24:523–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wardemann H, Yurasov S, Schaefer A, Young
JW, Meffre E and Nussenzweig MC: Predominant autoantibody
production by early human B cell precursors. Science.
301:1374–1377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Traggiai E, Becker S, Subbarao K,
Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R and
Lanzavecchia A: An efficient method to make human monoclonal
antibodies from memory B cells: Potent neutralization of SARS
coronavirus. Nat Med. 10:871–875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Bushey RT, Moody MA, Nicely NL, Haynes BF,
Alam SM, Keir ST, Bentley RC, Roy Choudhury K, Gottlin EB, Campa
MJ, et al: A Therapeutic antibody for cancer, derived from single
human B cells. Cell Rep. 15:1505–1513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tiller T: Single B cell antibody
technologies. N Biotechnol. 28:453–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Rudkin FM, Raziunaite I, Workman H, Essono
S, Belmonte R, MacCallum DM, Johnson EM, Silva LM, Palma AS, Feizi
T, et al: Single human B cell-derived monoclonal anti-Candida
antibodies enhance phagocytosis and protect against disseminated
candidiasis. Nat Commun. 9:52882018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rajan S, Kierny MR, Mercer A, Wu J,
Tovchigrechko A, Wu H, Dall Acqua WF, Xiao X and Chowdhury PS:
Recombinant human B cell repertoires enable screening for rare,
specific, and natively paired antibodies. Commun Biol. 1:52018.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Buisman AM, de Rond CG, Oztürk K, Ten
Hulscher HI and van Binnendijk RS: Long-term presence of memory
B-cells specific for different vaccine components. Vaccine.
28:179–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jilg W, Schmidt M and Deinhardt F: Decline
of anti-HBs after hepatitis B vaccination and timing of
revaccination. Lancet. 335:173–174. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Inoue T, Moran I, Shinnakasu R, Phan TG
and Kurosaki T: Generation of memory B cells and their
reactivation. Immunol Rev. 283:138–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
von Bredow B, Arias JF, Heyer LN, Moldt B,
Le K, Robinson JE, Zolla-Pazner S, Burton DR and Evans DT:
Comparison of antibody-dependent cell-mediated cytotoxicity and
virus neutralization by HIV-1 Env-specific monoclonal antibodies. J
Virol. 90:6127–6139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
von Boehmer L, Liu C, Ackerman S, Gitlin
AD, Wang Q, Gazumyan A and Nussenzweig MC: Sequencing and cloning
of antigen-specific antibodies from mouse memory B cells. Nat
Protoc. 11:1908–1923. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lei L, Tran K, Wang Y, Steinhardt JJ, Xiao
Y, Chiang CI, Wyatt RT and Li Y: Antigen-specific single B cell
sorting and monoclonal antibody cloning in guinea pigs. Front
Microbiol. 10:6722019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L,
Zhu Q, Zhang X, Zheng Y, Geng C, et al: Potent neutralizing
antibodies against SARS-CoV-2 identified by high-throughput
single-cell sequencing of convalescent patients. B cells. Cell.
182:73–84.e16. 2020. View Article : Google Scholar
|
|
123
|
Lanzavecchia A, Corti D and Sallusto F:
Human monoclonal antibodies by immortalization of memory B cells.
Curr Opin Biotechnol. 18:523–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wrammert J, Koutsonanos D, Li GM,
Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig
M, Lipkin WI, et al: Broadly cross-reactive antibodies dominate the
human B cell response against 2009 pandemic H1N1 influenza virus
infection. J Exp Med. 208:181–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hafeez U, Gan HK and Scott AM: Monoclonal
antibodies as immunomodulatory therapy against cancer and
autoimmune diseases. Curr Opin Pharmacol. 41:114–121. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Walcheck B and Wu J: iNK-CD64/16A cells: A
promising approach for ADCC? Expert Opin Biol Ther. 19:1229–1232.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Decaup E, Rossi C, Gravelle P, Laurent C,
Bordenave J, Tosolini M, Tourette A, Perrial E, Dumontet C, Poupot
M, et al: A tridimensional model for NK cell-mediated ADCC of
follicular lymphoma. Front Immunol. 10:19432019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Giles AJ, Hao S, Padget M, Song H, Zhang
W, Lynes J, Sanchez V, Liu Y, Jung J, Cao X, et al: Efficient ADCC
killing of meningioma by avelumab and a high-affinity natural
killer cell line, haNK. JCI Insight. 4:e1306882019. View Article : Google Scholar :
|
|
129
|
Pockley AG, Vaupel P and Multhoff G: NK
cell-based therapeutics for lung cancer. Expert Opin Biol Ther.
20:23–33. 2020. View Article : Google Scholar
|
|
130
|
Adams GP and Weiner LM: Monoclonal
antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Seguin-Devaux C, Plesseria JM, Verschueren
C, Masquelier C, Iserentant G, Fullana M, Józsi M, Cohen JHM and
Dervillez X: FHR4-based immunoconjugates direct
complement-dependent cytotoxicity and phagocytosis towards
HER2-positive cancer cells. Mol Oncol. 13:2531–2553. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wyant T, Fedyk E and Abhyankar B: An
overview of the mechanism of action of the monoclonal antibody
vedolizumab. J Crohns Colitis. 10:1437–1444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Czyz M: Fibroblast growth factor receptor
signaling in skin cancers. Cells. 8:5402019. View Article : Google Scholar :
|
|
134
|
Jimenez-Pascual A and Siebzehnrubl FA:
Fibroblast growth factor receptor functions in glioblastoma. Cells.
8:7152019. View Article : Google Scholar :
|
|
135
|
Lee YT, Tan YJ and Oon CE: Molecular
targeted therapy: Treating cancer with specificity. Eur J
Pharmacol. 834:188–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Weiner GJ: Building better monoclonal
antibody-based therapeutics. Nat Rev Cancer. 15:361–370. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Howie LJ, Scher NS, Amiri-Kordestani L,
Zhang L, King-Kallimanis BL, Choudhry Y, Schroeder J, Goldberg KB,
Kluetz PG, Ibrahim A, et al: FDA approval summary: Pertuzumab for
adjuvant treatment of HER2-positive early breast cancer. Clin
Cancer Res. 25:2949–2955. 2019. View Article : Google Scholar
|
|
138
|
Touat M, Idbaih A, Sanson M and Ligon KL:
Glioblastoma targeted therapy: Updated approaches from recent
biological insights. Ann Oncol. 28:1457–1472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Xu MJ, Johnson DE and Grandis JR:
EGFR-targeted therapies in the post-genomic era. Cancer Metastasis
Rev. 36:463–473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Frezzetti D, Gallo M, Maiello MR,
D'Alessio A, Esposito C, Chicchinelli N, Normanno N and De Luca A:
VEGF as a potential target in lung cancer. Expert Opin Ther
Targets. 21:959–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yalcin F, Dzaye O and Xia S: Tenascin-C
function in glioma: Immunomodulation and beyond. Adv Exp Med Biol.
1272:149–172. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Lieverse RIY, Van Limbergen EJ, Oberije
CJG, Troost EGC, Hadrup SR, Dingemans AC, Hendriks LEL, Eckert F,
Hiley C, Dooms C, et al: Stereotactic ablative body radiotherapy
(SABR) combined with immunotherapy (L19-IL2) versus standard of
care in stage IV NSCLC patients, ImmunoSABR: A multicentre,
randomised controlled open-label phase II trial. BMC Cancer.
20:5572020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wester HJ and Schottelius M: PSMA-targeted
radiopharmaceuticals for imaging and therapy. Semin Nucl Med.
49:302–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang JC, Chen WD, Alvarez JB, Jia K, Shi
L, Wang Q, Zou N, He K and Zhu H: Cancer immune checkpoint blockade
therapy and its associated autoimmune cardiotoxicity. Acta
Pharmacol Sin. 39:1693–1698. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chamoto K, Al-Habsi M and Honjo T: Role of
PD-1 in immunity and diseases. Curr Top Microbiol Immunol.
410:75–97. 2017.PubMed/NCBI
|
|
148
|
Darvin P, Toor SM, Sasidharan Nair V and
Elkord E: Immune checkpoint inhibitors: Recent progress and
potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Gotwals P, Cameron S, Cipolletta D,
Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S,
Sabatos-Peyton C, Petruzzelli L, et al: Prospects for combining
targeted and conventional cancer therapy with immunotherapy. Nat
Rev Cancer. 17:286–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Abril-Rodriguez G and Ribas A: SnapShot:
Immune checkpoint inhibitors. Cancer Cell. 31:848–848.e1. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Rowshanravan B, Halliday N and Sansom DM:
CTLA-4: A moving target in immunotherapy. Blood. 131:58–67. 2018.
View Article : Google Scholar
|
|
153
|
Lo B and Abdel-Motal UM: Lessons from
CTLA-4 deficiency and checkpoint inhibition. Curr Opin Immunol.
49:14–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Duperret EK, Trautz A, Stoltz R, Patel A,
Wise MC, Perales-Puchalt A, Smith T, Broderick KE, Masteller E, Kim
JJ, et al: Synthetic DNA-encoded monoclonal antibody delivery of
anti-CTLA-4 antibodies induces tumor shrinkage in vivo. Cancer Res.
78:6363–6370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Specenier P: Ipilimumab in melanoma.
Expert Rev Anticancer Ther. 16:811–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Carter BW, Bhosale PR and Yang WT:
Immunotherapy and the role of imaging. Cancer. 124:2906–2922. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kuehn HS, Ouyang W, Lo B, Deenick EK,
Niemela JE, Avery DT, Schickel JN, Tran DQ, Stoddard J, Zhang Y, et
al: Immune dysregulation in human subjects with heterozygous
germline mutations in CTLA4. Science. 345:1623–1627. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Schubert D, Bode C, Kenefeck R, Hou TZ,
Wing JB, Kennedy A, Bulashevska A, Petersen BS, Schäffer AA,
Grüning BA, et al: Autosomal dominant immune dysregulation syndrome
in humans with CTLA4 mutations. Nat Med. 20:1410–1416. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Chesney J, Puzanov I, Collichio F, Singh
P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et
al: Randomized, open-label phase II study evaluating the efficacy
and safety of talimogene laherparepvec in combination with
ipilimumab versus ipilimumab alone in patients with advanced,
unresectable melanoma. J Clin Oncol. 36:1658–1667. 2018. View Article : Google Scholar :
|
|
160
|
Soularue E, Lepage P, Colombel JF, Coutzac
C, Faleck D, Marthey L, Collins M, Chaput N, Robert C and Carbonnel
F: Enterocolitis due to immune checkpoint inhibitors: A systematic
review. Gut. 67:2056–2067. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Spain L, Diem S and Larkin J: Management
of toxicities of immune checkpoint inhibitors. Cancer Treat Rev.
44:51–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Rotte A: Combination of CTLA-4 and PD-1
blockers for treatment of cancer. J Exp Clin Cancer Res.
38:2552019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Five-year survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 381:1535–1546. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Sidaway P: Immunotherapy: Local
chemotherapy synergizes with CTLA-4 inhibition. Nat Rev Clin Oncol.
15:2022018.PubMed/NCBI
|
|
165
|
Weber JS, Kähler KC and Hauschild A:
Management of immune-related adverse events and kinetics of
response with ipilimumab. J Clin Oncol. 30:2691–2697. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
167
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Xu-Monette ZY, Zhou J and Young KH: PD-1
expression and clinical PD-1 blockade in B-cell lymphomas. Blood.
131:68–83. 2018. View Article : Google Scholar :
|
|
169
|
Du S, McCall N, Park K, Guan Q, Fontina P,
Ertel A, Zhan T, Dicker AP and Lu B: Blockade of tumor-expressed
PD-1 promotes lung cancer growth. Oncoimmunology. 7:e14087472018.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X
and Wu K: Biomarkers for predicting efficacy of PD-1/PD-L1
inhibitors. Mol Cancer. 17:1292018. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Naidoo J, Page DB, Li BT, Connell LC,
Schindler K, Lacouture ME, Postow MA and Wolchok JD: Toxicities of
the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann
Oncol. 26:2375–2391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Patel SA and Minn AJ: Combination cancer
therapy with immune checkpoint blockade: Mechanisms and strategies.
Immunity. 48:417–433. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Hayashi H and Nakagawa K: Combination
therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol.
25:818–830. 2020. View Article : Google Scholar
|
|
175
|
Aggen DH, Drake CG and Rini BI: Targeting
PD-1 or PD-L1 in metastatic kidney cancer: Combination therapy in
the first-line setting. Clin Cancer Res. 26:2087–2095. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Mathew M, Enzler T, Shu CA and Rizvi NA:
Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther.
186:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Kong Y, Zhao X, Zou L, Xing P, Ma Y, Tian
Y and Zhang L: PD-1 inhibitor combined with radiotherapy and GM-CSF
as salvage therapy in patients with chemotherapy-refractory
metastatic solid tumors. J Clin Oncol. 38(Suppl 15): e151732020.
View Article : Google Scholar
|
|
178
|
Kordbacheh T, Honeychurch J, Blackhall F,
Faivre-Finn C and Illidge T: Radiotherapy and anti-PD-1/PD-L1
combinations in lung cancer: Building better translational research
platforms. Ann Oncol. 29:301–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Walshaw RC, Honeychurch J, Illidge TM and
Choudhury A: The anti-PD-1 era-an opportunity to enhance
radiotherapy for patients with bladder cancer. Nat Rev Urol.
15:251–259. 2018. View Article : Google Scholar
|
|
180
|
Sheng X, Yan X, Chi Z, Si L, Cui C, Tang
B, Li S, Mao L, Lian B, Wang X, et al: Axitinib in combination with
toripalimab, a humanized immunoglobulin G4 monoclonal
antibody against programmed cell death-1, in patients with
metastatic mucosal melanoma: An open-label phase IB trial. J Clin
Oncol. 37:2987–2999. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar
|
|
182
|
Atkins MB, Plimack ER, Puzanov I, Fishman
MN, McDermott DF, Cho DC, Vaishampayan U, George S, Olencki TE,
Tarazi JC, et al: Axitinib in combination with pembrolizumab in
patients with advanced renal cell cancer: A non-randomised,
open-label, dose-finding, and dose-expansion phase 1b trial. Lancet
Oncol. 19:405–415. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Kohlhapp FJ and Kaufman HL: Molecular
pathways: Mechanism of action for talimogene laherparepvec, a new
oncolytic virus immunotherapy. Clin Cancer Res. 22:1048–1054. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Kowalsky SJ, Liu Z, Feist M, Berkey SE, Ma
C, Ravindranathan R, Dai E, Roy EJ, Guo ZS and Bartlett DL:
Superagonist IL-15-armed oncolytic virus elicits potent antitumor
immunity and therapy that are enhanced with PD-1 blockade. Mol
Ther. 26:2476–2486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Sahin U and Türeci Ö: Personalized
vaccines for cancer immunotherapy. Science. 359:1355–1360. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Sui H, Ma N, Wang Y, Li H, Liu X, Su Y and
Yang J: Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer:
Toward personalized medicine and combination strategies. J Immunol
Res. 2018:69849482018. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Rafiq S, Yeku OO, Jackson HJ, Purdon TJ,
van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, et al:
Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances
anti-tumor efficacy in vivo. Nat Biotechnol. 36:847–856. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Shi X, Zhang D, Li F, Zhang Z, Wang S,
Xuan Y, Ping Y and Zhang Y: Targeting glycosylation of PD-1 to
enhance CAR-T cell cytotoxicity. J Hematol Oncol. 12:1272019.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Xu J, Sun HH, Fletcher CD, Hornick JL,
Morgan EA, Freeman GJ, Hodi FS, Pinkus GS and Rodig SJ: Expression
of programmed cell death 1 ligands (PD-L1 and PD-L2) in histiocytic
and dendritic cell disorders. Am J Surg Pathol. 40:443–453. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1
blockade therapy in advanced non-small-cell lung cancer: Current
status and future directions. Oncologist. 24(Suppl 1): S31–S41.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Chaudhri A, Xiao Y, Klee AN, Wang X, Zhu B
and Freeman GJ: PD-L1 binds to B7-1 only in Cis on the same cell
surface. Cancer Immunol Res. 6:921–929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Inman BA, Longo TA, Ramalingam S and
Harrison MR: Atezolizumab: A PD-L1-blocking antibody for bladder
cancer. Clin Cancer Res. 23:1886–1890. 2017. View Article : Google Scholar
|
|
193
|
Garassino MC, Cho BC, Kim JH, Mazières J,
Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J,
Chouaid C, et al: Durvalumab as third-line or later treatment for
advanced non-small-cell lung cancer (ATLANTIC): An open-label,
single-arm, phase 2 study. Lancet Oncol. 19:521–536. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Lui Y and Davis SJ: LAG-3: A very singular
immune checkpoint. Nat Immunol. 19:1278–1279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Maruhashi T, Sugiura D, Okazaki IM and
Okazaki T: LAG-3: From molecular functions to clinical
applications. J Immunother Cancer. 8:e0010142020. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Das M, Zhu C and Kuchroo VK: Tim-3 and its
role in regulating anti-tumor immunity. Immunol Rev. 276:97–111.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Thomas A, Teicher BA and Hassan R:
Antibody-drug conjugates for cancer therapy. Lancet Oncol.
17:e254–e262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Sharabi AB, Lim M, DeWeese TL and Drake
CG: Radiation and checkpoint blockade immunotherapy:
Radiosensitisation and potential mechanisms of synergy. Lancet
Oncol. 16:e498–e509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Yaghoubi S, Karimi MH, Lotfinia M, Gharibi
T, Mahi-Birjand M, Kavi E, Hosseini F, Sineh Sepehr K, Khatami M,
Bagheri N, et al: Potential drugs used in the antibody-drug
conjugate (ADC) architecture for cancer therapy. J Cell Physiol.
235:31–64. 2020. View Article : Google Scholar
|
|
201
|
Qin SY, Cheng YJ, Lei Q, Zhang AQ and
Zhang XZ: Combinational strategy for high-performance cancer
chemotherapy. Biomaterials. 171:178–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Yu WD, Sun G, Li J, Xu J and Wang X:
Mechanisms and therapeutic potentials of cancer immunotherapy in
combination with radiotherapy and/or chemotherapy. Cancer Lett.
452:66–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Pérez-Herrero E and Fernández-Medarde A:
Advanced targeted therapies in cancer: Drug nanocarriers, the
future of chemotherapy. Eur J Pharm Biopharm. 93:52–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Nadal R and Bellmunt J: Management of
metastatic bladder cancer. Cancer Treat Rev. 76:10–21. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Akbari B, Farajnia S, Ahdi Khosroshahi S,
Safari F, Yousefi M, Dariushnejad H and Rahbarnia L: Immunotoxins
in cancer therapy: Review and update. Int Rev Immunol. 36:207–219.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Alewine C, Hassan R and Pastan I: Advances
in anticancer immunotoxin therapy. Oncologist. 20:176–185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Polito L, Djemil A and Bortolotti M: Plant
toxin-based immunotoxins for cancer therapy: A short overview.
Biomedicines. 4:122016. View Article : Google Scholar
|
|
208
|
Madhumathi J, Devilakshmi S, Sridevi S and
Verma RS: Immunotoxin therapy for hematologic malignancies: Where
are we heading? Drug Discov Today. 21:325–332. 2016. View Article : Google Scholar
|
|
209
|
Kumar M, Thangavel C, Becker RC and
Sadayappan S: Monoclonal antibody-based immunotherapy and its role
in the development of cardiac toxicity. Cancers (Basel). 13:862020.
View Article : Google Scholar
|
|
210
|
Tse BW, Collins A, Oehler MK, Zippelius A
and Heinzelmann-Schwarz VA: Antibody-based immunotherapy for
ovarian cancer: Where are we at? Ann Oncol. 25:322–331. 2014.
View Article : Google Scholar
|
|
211
|
Chau CH, Steeg PS and Figg WD:
Antibody-drug conjugates for cancer. Lancet. 394:793–804. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Khongorzul P, Ling CJ, Khan FU, Ihsan AU
and Zhang J: Antibody-drug conjugates: A comprehensive review. Mol
Cancer Res. 18:3–19. 2020. View Article : Google Scholar
|
|
213
|
Tsuchikama K and An Z: Antibody-drug
conjugates: Recent advances in conjugation and linker chemistries.
Protein Cell. 9:33–46. 2018. View Article : Google Scholar :
|
|
214
|
Erickson HK, Park PU, Widdison WC, Kovtun
YV, Garrett LM, Hoffman K, Lutz RJ, Goldmacher VS and Blättler WA:
Antibody-maytansinoid conjugates are activated in targeted cancer
cells by lysosomal degradation and linker-dependent intracellular
processing. Cancer Res. 66:4426–4433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Huang Y, Lee C, Borgström P and Gjerset
RA: Macrophage-mediated bystander effect triggered by tumor cell
apoptosis. Mol Ther. 15:524–533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Staudacher AH and Brown MP: Antibody drug
conjugates and bystander killing: Is antigen-dependent
internalisation required? Br J Cancer. 117:1736–1742. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Beck A, Goetsch L, Dumontet C and Corvaïa
N: Strategies and challenges for the next generation of
antibody-drug conjugates. Nat Rev Drug Discov. 16:315–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Birrer MJ, Moore KN, Betella I and Bates
RC: Antibody-drug conjugate-based therapeutics: State of the
science. J Natl Cancer Inst. 111:538–549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Mahalingaiah PK, Ciurlionis R, Durbin KR,
Yeager RL, Philip BK, Bawa B, Mantena SR, Enright BP, Liguori MJ
and Van Vleet TR: Potential mechanisms of target-independent uptake
and toxicity of antibody-drug conjugates. Pharmacol Ther.
200:110–125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Lambert JM and Berkenblit A: Antibody-drug
conjugates for cancer treatment. Annu Rev Med. 69:191–207. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Duerr C and Friess W: Antibody-drug
conjugates-stability and formulation. Eur J Pharm Biopharm.
139:168–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Theocharopoulos C, Lialios PP, Gogas H and
Ziogas DC: An overview of antibody-drug conjugates in oncological
practice. Ther Adv Med Oncol. Oct 4–2020.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Abdollahpour-Alitappeh M, Lotfinia M,
Gharibi T, Mardaneh J, Farhadihosseinabadi B, Larki P, Faghfourian
B, Sepehr KS, Abbaszadeh-Goudarzi K, Abbaszadeh-Goudarzi G, et al:
Antibody-drug conjugates (ADCs) for cancer therapy: Strategies,
challenges, and successes. J Cell Physiol. 234:5628–5642. 2019.
View Article : Google Scholar
|
|
224
|
Liu L: Pharmacokinetics of monoclonal
antibodies and Fc-fusion proteins. Protein Cell. 9:15–32. 2018.
View Article : Google Scholar :
|
|
225
|
Yu B and Liu D: Antibody-drug conjugates
in clinical trials for lymphoid malignancies and multiple myeloma.
J Hematol Oncol. 12:942019. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Gébleux R and Casi G: Antibody-drug
conjugates: Current status and future perspectives. Pharmacol Ther.
167:48–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Tiller KE and Tessier PM: Advances in
antibody design. Annu Rev Biomed Eng. 17:191–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Ponziani S, Di Vittorio G, Pitari G,
Cimini AM, Ardini M, Gentile R, Iacobelli S, Sala G, Capone E,
Flavell DJ, et al: Antibody-drug conjugates: The new frontier of
chemotherapy. Int J Mol Sci. 21:55102020. View Article : Google Scholar :
|
|
229
|
Kobayashi H and Choyke PL: Near-infrared
photoimmunotherapy of cancer. Acc Chem Res. 52:2332–2339. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Ozog DM, Rkein AM, Fabi SG, Gold MH,
Goldman MP, Lowe NJ, Martin GM and Munavalli GS: Photodynamic
therapy: A clinical consensus guide. Dermatol Surg. 42:804–827.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Larue L, Myrzakhmetov B, Ben-Mihoub A,
Moussaron A, Thomas N, Arnoux P, Baros F, Vanderesse R, Acherar S
and Frochot C: Fighting hypoxia to improve PDT. Pharmaceuticals
(Basel). 12:1632019. View Article : Google Scholar
|
|
232
|
Nagaya T, Nakamura Y, Okuyama S, Ogata F,
Maruoka Y, Choyke PL, Allen C and Kobayashi H: Syngeneic mouse
models of oral cancer are effectively targeted by anti-CD44-Based
NIR-PIT. Mol Cancer Res. 15:1667–1677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Mew D, Lum V, Wat CK, Towers GH, Sun CH,
Walter RJ, Wright W, Berns MW and Levy JG: Ability of specific
monoclonal antibodies and conventional antisera conjugated to
hematoporphyrin to label and kill selected cell lines subsequent to
light activation. Cancer Res. 45:4380–4386. 1985.PubMed/NCBI
|
|
234
|
Mew D, Wat CK, Towers GH and Levy JG:
Photoimmunotherapy: Treatment of animal tumors with tumor-specific
monoclonal antibody-hematoporphyrin conjugates. J Immunol.
130:1473–1477. 1983.PubMed/NCBI
|
|
235
|
Wang M, Rao J, Wang M, Li X, Liu K, Naylor
MF, Nordquist RE, Chen WR and Zhou F: Cancer photo-immunotherapy:
From bench to bedside. Theranostics. 11:2218–2231. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Mitsunaga M, Ogawa M, Kosaka N, Rosenblum
LT, Choyke PL and Kobayashi H: Cancer cell-selective in vivo near
infrared photoimmunotherapy targeting specific membrane molecules.
Nat Med. 17:1685–1691. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Isobe Y, Sato K, Nishinaga Y, Takahashi K,
Taki S, Yasui H, Shimizu M, Endo R, Koike C, Kuramoto N, et al:
Near infrared photoimmunotherapy targeting DLL3 for small cell lung
cancer. EBioMedicine. 52:1026322020. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Nishimura T, Mitsunaga M, Ito K, Kobayashi
H and Saruta M: Cancer neovasculature-targeted near-infrared
photoimmunotherapy (NIR-PIT) for gastric cancer: Different
mechanisms of phototoxicity compared to cell membrane-targeted
NIR-PIT. Gastric Cancer. 23:82–94. 2020. View Article : Google Scholar
|
|
239
|
Deshaies RJ: Multispecific drugs herald a
new era of biopharmaceutical innovation. Nature. 580:329–338. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Kaplon H, Muralidharan M, Schneider Z and
Reichert JM: Antibodies to watch in 2020. MAbs. 12:17035312020.
View Article : Google Scholar :
|