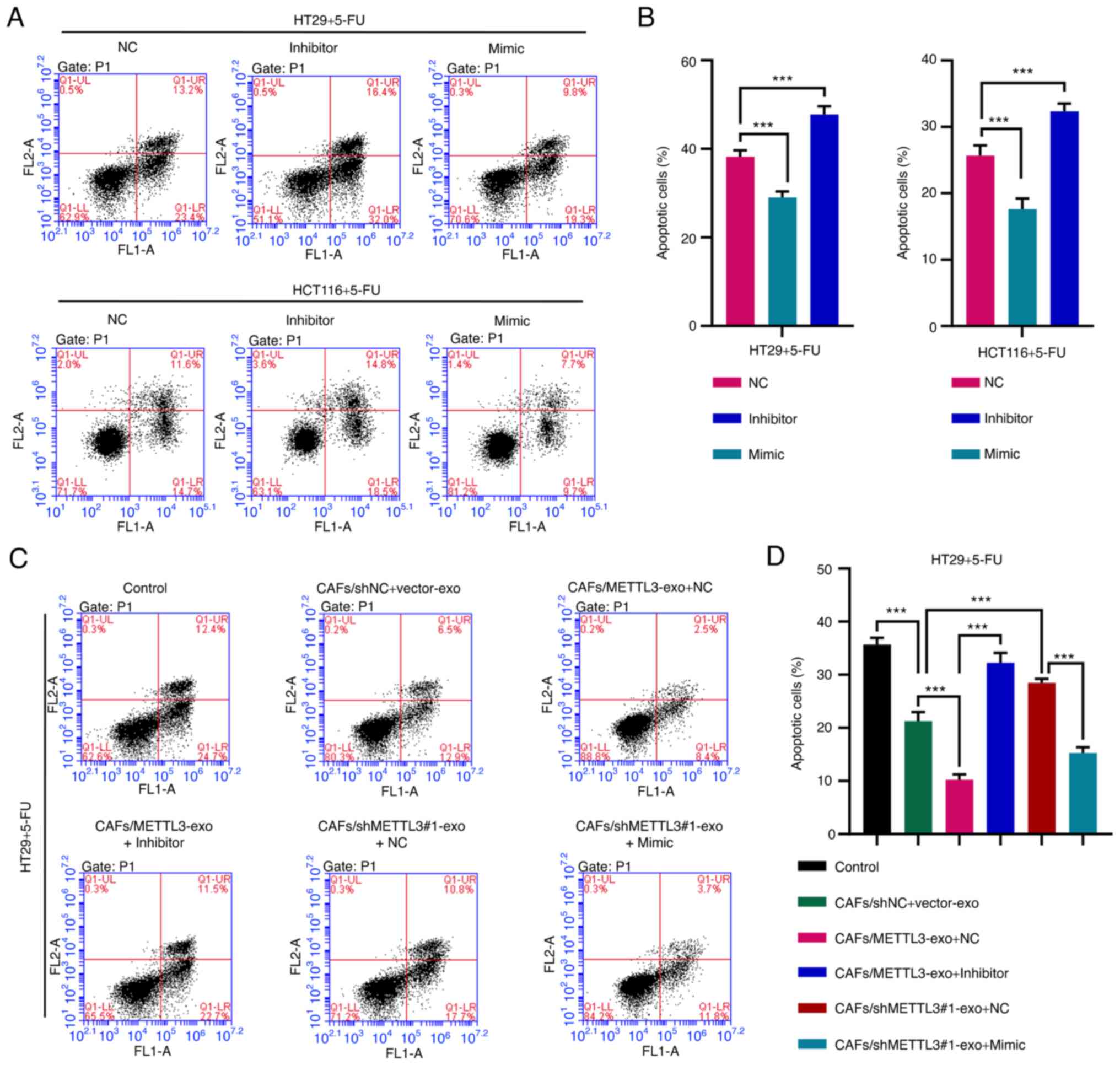
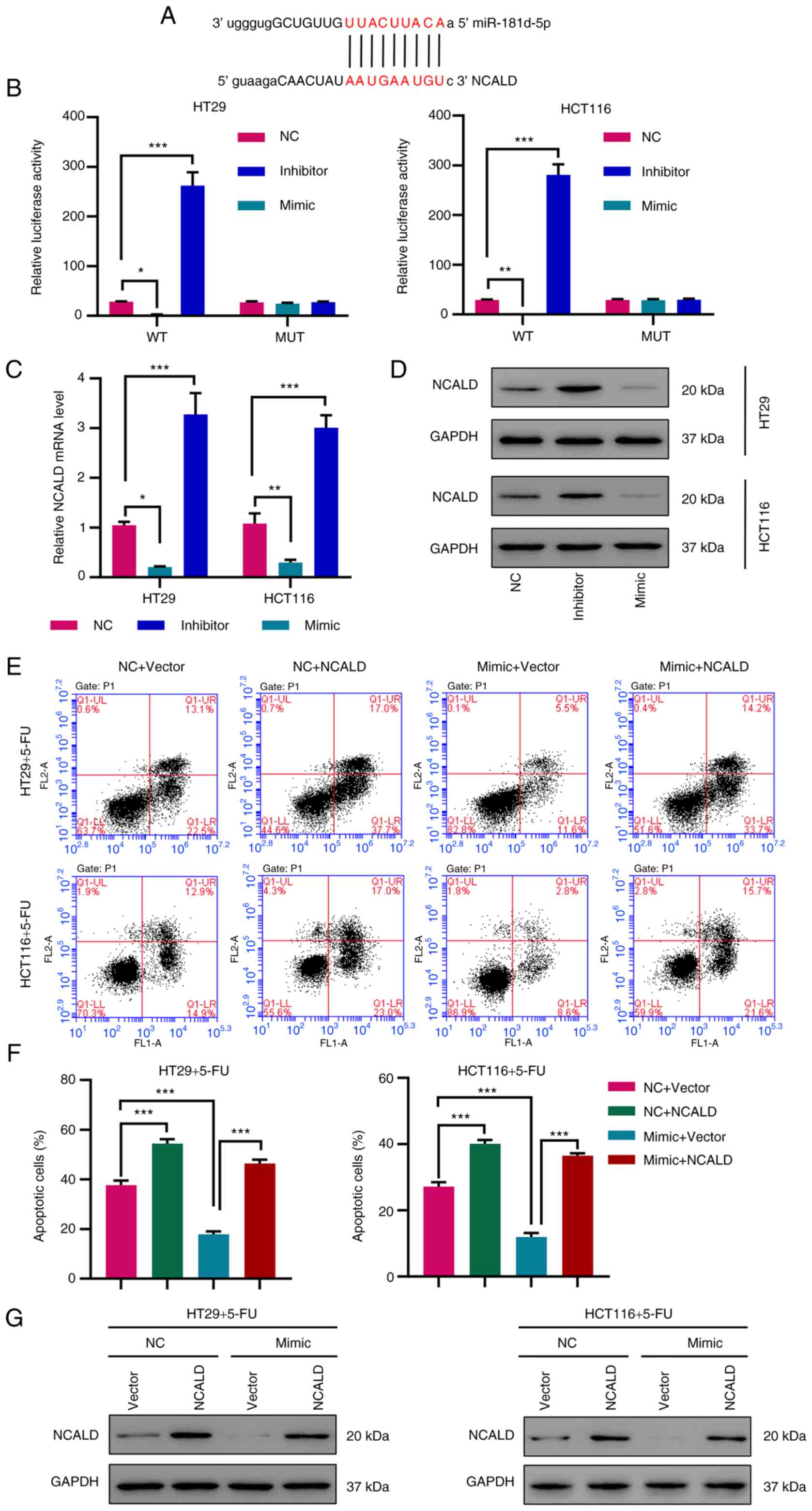
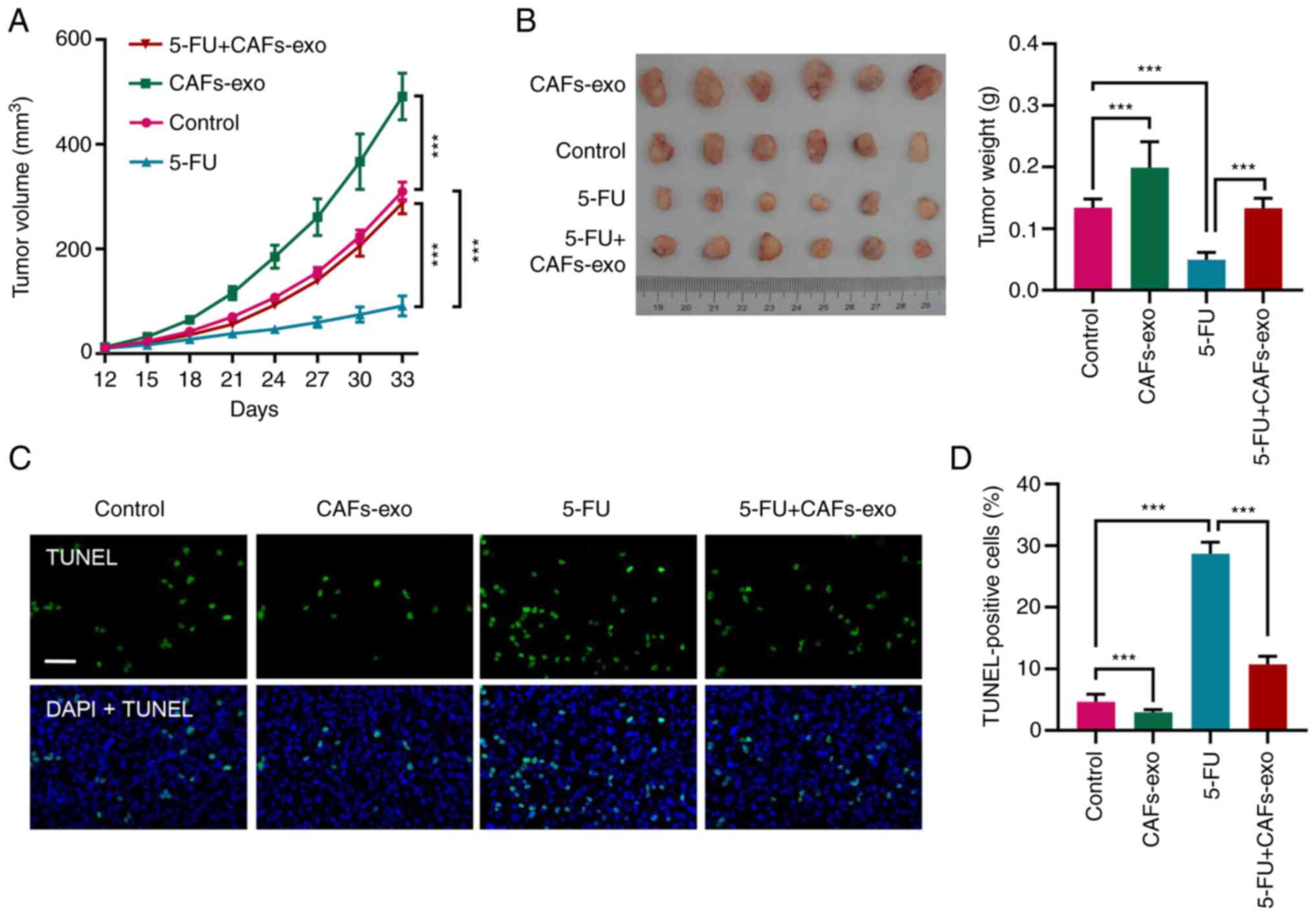
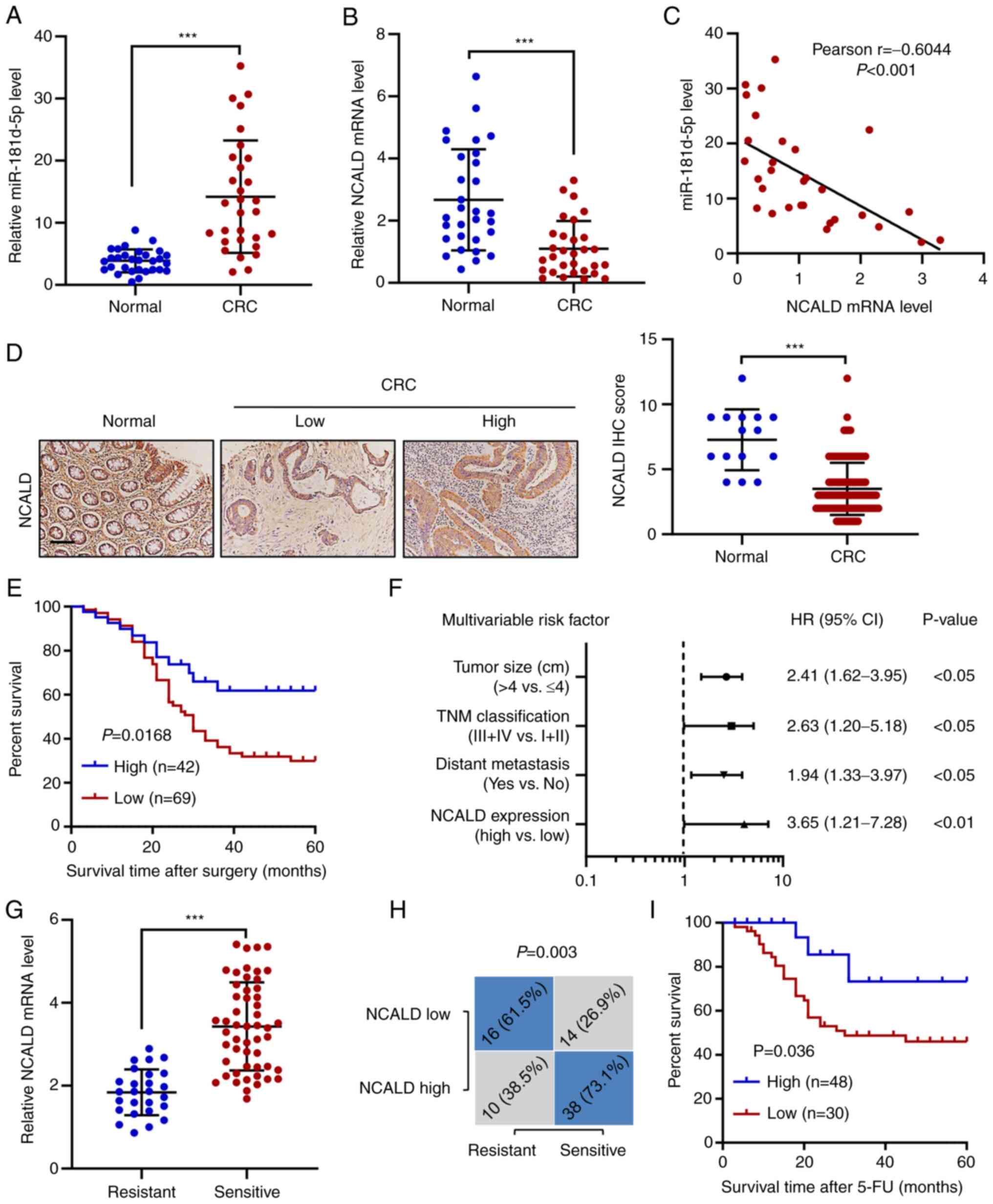
|
1
|
Røed Skårderud M, Polk A, Kjeldgaard
Vistisen K, Larsen FO and Nielsen DL: Efficacy and safety of
regorafenib in the treatment of metastatic colorectal cancer: A
systematic review. Cancer Treat Rev. 62:61–73. 2018. View Article : Google Scholar
|
|
2
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Provenzale D and Gray RN: Colorectal
cancer screening and treatment: Review of outcomes research. J Natl
Cancer Inst Monogr. 45–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jullumstrø E, Lydersen S, Møller B, Dahl O
and Edna TH: Duration of symptoms, stage at diagnosis and relative
survival in colon and rectal cancer. Eur J Cancer. 45:2383–2390.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsikitis VL, Larson DW, Huebner M, Lohse
CM and Thompson PA: Predictors of recurrence free survival for
patients with stage II and III colon cancer. BMC Cancer.
14:3362014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuebler JP, Wieand HS, O'Connell MJ, Smith
RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE,
Atkins JN, et al: Oxaliplatin combined with weekly bolus
fluorouracil and leucovorin as surgical adjuvant chemotherapy for
stage II and III colon cancer: Results from NSABP C-07. J Clin
Oncol. 25:2198–2204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benson AB III, Bekaii-Saab T, Chan E, Chen
YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton
MJ, et al: Metastatic colon cancer, version 3.2013: Featured
updates to the NCCN guidelines. J Natl Compr Canc Netw. 11:141–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu F, Ye ML, Zhang YP, Li WJ, Li MT, Wang
HZ, Qiu X, Xu Y, Yin JW, Hu Q, et al: MicroRNA-375-3p enhances
chemosensitivity to 5-fluorouracil by targeting thymidylate
synthase in colorectal cancer. Cancer Sci. 111:1528–1541. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu T, Han C, Wang S, Fang P, Ma Z, Xu L
and Yin R: Cancer-associated fibroblasts: An emerging target of
anti-cancer immunotherapy. J Hematol Oncol. 12:862019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ziani L, Chouaib S and Thiery J:
Alteration of the antitumor immune response by cancer-associated
fibroblasts. Front Immunol. 9:4142018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lambrechts D, Wauters E, Boeckx B, Aibar
S, Nittner D, Burton O, Bassez A, Decaluwé H, Pircher A, Van den
Eynde K, et al: Phenotype molding of stromal cells in the lung
tumor microenvironment. Nat Med. 24:1277–1289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keller F, Bruch R, Schneider R,
Meier-Hubberten J, Hafner M and Rudolf R: A scaffold-free 3-D
co-culture mimics the major features of the reverse warburg effect
in vitro. Cells. 9:19002020. View Article : Google Scholar :
|
|
14
|
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS,
Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, et al: CAFs secreted
exosomes promote metastasis and chemotherapy resistance by
enhancing cell stemness and epithelial-mesenchymal transition in
colorectal cancer. Mol Cancer. 18:912019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Musa M and Ali A: Cancer-associated
fibroblasts of colorectal cancer and their markers: Updates,
challenges and translational outlook. Future Oncol. 16:2329–2344.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar :
|
|
18
|
Mao L, Li X, Gong S, Yuan H, Jiang Y,
Huang W, Sun X and Dang X: Serum exosomes contain ECRG4 mRNA that
suppresses tumor growth via inhibition of genes involved in
inflammation, cell proliferation, and angiogenesis. Cancer Gene
Ther. 25:248–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaput N and Théry C: Exosomes: Immune
properties and potential clinical implementations. Semin
Immunopathol. 33:419–440. 2011. View Article : Google Scholar
|
|
20
|
Kahlert C and Kalluri R: Exosomes in tumor
microenvironment influence cancer progression and metastasis. J Mol
Med (Berl). 91:431–437. 2013. View Article : Google Scholar
|
|
21
|
Wang H, Wei H, Wang J, Li L, Chen A and Li
Z: MicroRNA-181d-5p-containing exosomes derived from CAFs promote
EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic
Acids. 19:654–667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei H, Wang J, Xu Z, Li W, Wu X, Zhuo C,
Lu Y, Long X, Tang Q and Pu J: Hepatoma cell-derived extracellular
vesicles promote liver cancer metastasis by inducing the
differentiation of bone marrow stem cells through microRNA-181d-5p
and the FAK/Src pathway. Front Cell Dev Biol. 9:6070012021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang H, Weng H and Chen J: The biogenesis
and precise control of RNA m6A methylation. Trends
Genet. 36:44–52. 2020. View Article : Google Scholar
|
|
24
|
Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G,
Yuan W, Kan Q and Sun Z: The interplay between m6A RNA methylation
and noncoding RNA in cancer. J Hematol Oncol. 12:1212019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi M, Ohsugi M, Sasako T, Awazawa
M, Umehara T, Iwane A, Kobayashi N, Okazaki Y, Kubota N, Suzuki R,
et al: The RNA methyltransferase complex of WTAP, METTL3, and
METTL14 regulates mitotic clonal expansion in adipogenesis. Mol
Cell Biol. 38:e00116–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Liu L, Dong Z, Li J, Yu Y, Chen X,
Ren F, Cui G and Sun R: Expression patterns and prognostic value of
m6A-related genes in colorectal cancer. Am J Transl Res.
11:3972–3991. 2019.
|
|
27
|
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C,
Li S, Tan L, Mai D, Li G, et al: Excessive miR-25-3p maturation via
N6-methyladenosine stimulated by cigarette smoke promotes
pancreatic cancer progression. Nat Commun. 10:18582019. View Article : Google Scholar :
|
|
28
|
Dang TL, Le CT, Le MN, Nguyen TD, Nguyen
TL, Bao S, Li S and Nguyen TA: Select amino acids in DGCR8 are
essential for the UGU-pri-miRNA interaction and processing. Commun
Biol. 3:3442020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH,
Wang F, Wang TT, Xu QG, Zhou WP and Sun SH: METTL14 suppresses the
metastatic potential of hepatocellular carcinoma by modulating
N6-methyladenosine-dependent primary MicroRNA
processing. Hepatology. 65:529–543. 2017. View Article : Google Scholar
|
|
30
|
Alarcón CR, Lee H, Goodarzi H, Halberg N
and Tavazoie SF: N6-methyladenosine marks primary microRNAs for
processing. Nature. 519:482–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yasuda T, Koiwa M, Yonemura A, Akiyama T,
Baba H and Ishimoto T: Protocol to establish cancer-associated
fibroblasts from surgically resected tissues and generate senescent
fibroblasts. STAR Protoc. 2:1005532021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu S, Xia S, Yang D, Wang K, Yeh S, Gao Z
and Chang C: Androgen receptor in human prostate cancer-associated
fibroblasts promotes prostate cancer epithelial cell growth and
invasion. Med Oncol. 30:6742013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kashima H, Wu RC, Wang Y, Sinno AK,
Miyamoto T, Shiozawa T, Wang TL, Fader AN and Shih IeM: Laminin C1
expression by uterine carcinoma cells is associated with tumor
progression. Gynecol Oncol. 139:338–344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu
J, Liu X, Chen CH, Fadare O, Pizzo DP, et al: Cancer-cell-secreted
exosomal miR-105 promotes tumour growth through the MYC-dependent
metabolic reprogramming of stromal cells. Nat Cell Biol.
20:597–609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uchihara T, Miyake K, Yonemura A, Komohara
Y, Itoyama R, Koiwa M, Yasuda T, Arima K, Harada K, Eto K, et al:
Extracellular vesicles from cancer-associated fibroblasts
containing annexin A6 induces FAK-YAP activation by stabilizing β1
integrin, enhancing drug resistance. Cancer Res. 80:3222–3235.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tabet F, Vickers KC, Cuesta Torres LF,
Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L,
Levin MG, Thacker S, et al: HDL-transferred microRNA-223 regulates
ICAM-1 expression in endothelial cells. Nat Commun. 5:32922014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhome R, Del Vecchio F, Lee GH, Bullock
MD, Primrose JN, Sayan AE and Mirnezami AH: Exosomal microRNAs
(exomiRs): Small molecules with a big role in cancer. Cancer Lett.
420:228–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Geng X, Li Q, Xu J, Tan Y, Xiao
M, Song J, Liu F, Fang C and Wang H: m6A modification in RNA:
Biogenesis, functions and roles in gliomas. J Exp Clin Cancer Res.
39:1922020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen XY, Zhang J and Zhu JS: The role of
m6A RNA methylation in human cancer. Mol Cancer.
18:1032019. View Article : Google Scholar
|
|
43
|
Deng X, Su R, Weng H, Huang H, Li Z and
Chen J: RNA N6-methyladenosine modification in cancers:
Current status and perspectives. Cell Res. 28:507–517. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X,
Zhang X, Cao Y, Ma D, Zhu X, et al: m6A-dependent
glycolysis enhances colorectal cancer progression. Mol Cancer.
19:722020. View Article : Google Scholar
|
|
45
|
Liu J, Eckert MA, Harada BT, Liu SM, Lu Z,
Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, et al:
m6A mRNA methylation regulates AKT activity to promote
the proliferation and tumorigenicity of endometrial cancer. Nat
Cell Biol. 20:1074–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang X, Feng J, Xue Y, Guan Z, Zhang D,
Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al: Structural basis of
N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature.
534:575–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN,
Chen ZH, Zeng ZL, Wang F, Zheng J, et al: METTL3 facilitates tumor
progression via an m6A-IGF2BP2-dependent mechanism in
colorectal carcinoma. Mol Cancer. 18:1122019. View Article : Google Scholar
|
|
48
|
Wang H, Xu B and Shi J: N6-methyladenosine
METTL3 promotes the breast cancer progression via targeting Bcl-2.
Gene. 722:1440762020. View Article : Google Scholar
|
|
49
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of microRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9:4022018. View Article : Google Scholar
|
|
50
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun S, Hang T, Zhang B, Zhu L, Wu Y, Lv X,
Huang Q and Yao H: miRNA-708 functions as a tumor suppressor in
colorectal cancer by targeting ZEB1 through Akt/mTOR signaling
pathway. Am J Transl Res. 11:5338–5356. 2019.PubMed/NCBI
|
|
53
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar
|
|
54
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of primary microRNAs by the
microprocessor complex. Nature. 432:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guo Y, Tian P, Yang C, Liang Z, Li M, Sims
M, Lu L, Zhang Z, Li H, Pfeffer LM and Yue J: Silencing the
double-stranded RNA binding protein DGCR8 inhibits ovarian cancer
cell proliferation, migration, and invasion. Pharm Res. 32:769–778.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gao LM, Zheng Y, Wang P, Zheng L, Zhang
WL, Di Y, Chen LL, Yin XB, Tian Q, Shi SS and Xu SF:
Tumor-suppressive effects of microRNA-181d-5p on non-small-cell
lung cancer through the CDKN3-mediated Akt signaling pathway in
vivo and in vitro. Am J Physiol Lung Cell Mol Physiol.
316:L918–L933. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang L, Wen C, Yang X, Lou Q, Wang X, Che
J, Chen J, Yang Z, Wu X, Huang M, et al: PEAK1, acting as a tumor
promoter in colorectal cancer, is regulated by the EGFR/KRas
signaling axis and miR-181d. Cell Death Dis. 9:2712018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Feng LY and Li L: Low expression of NCALD
is associated with chemotherapy resistance and poor prognosis in
epithelial ovarian cancer. J Ovarian Res. 13:352020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dong C, Yin F, Zhu D, Cai X, Chen C and
Liu X: NCALD affects drug resistance and prognosis by acting as a
ceRNA of CX3CL1 in ovarian cancer. J Cell Biochem. 121:4470–4483.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|