Introduction
Osteosarcoma (OS) is the most common primary bone
tumor in children and adolescents; it is characterized by early
metastasis and rapid progression (1). The treatment regimens of OS
currently include neoadjuvant chemotherapy, surgical treatment and
postoperative chemotherapy; however, the 5-year survival rate of
patients remains at ~60% (2). The
prognosis of patients with metastasis is considerably worse, with a
5-year survival rate of 20-30% (3). Therefore, there is an urgent
requirement to identify novel therapeutic regimens or targets to
improve the diagnosis and prognosis of patients with OS.
Circular (circ)RNAs are endogenous non-coding RNAs
that are hundreds or thousands of bases in length and comprise a
covalently closed structure (4).
In recent years, an increasing number of studies have demonstrated
that circRNAs are associated with a variety of human diseases,
including cerebrovascular diseases, inflammatory diseases and
neurological diseases (5-7). circRNAs are also closely associated
with the occurrence of malignant tumors (8-11).
Previous studies have revealed that circRNAs serve important roles
in the progression of OS, directly regulating cell proliferation,
apoptosis and metastasis (12,13). For instance, Ma et al
(8) demonstrated that
overexpression of circRNA ubiquitin associated protein 2
(circ-UBAP2) in OS cells promoted cell proliferation, invasion and
migration by regulating microRNA (miRNA or
miR)-204-3p/high-mobility group AT-hook 2 (HMGA2) signaling. In
addition, Wen et al (11)
reported that circRNA homeodomain-inter- acting protein kinase 3
(circ-HIPK3) promoted OS progression by modulating the
miR-637/circ-HIPK3/histone deacetylase 4 (HDAC4) axis. Chen et
al (12) also confirmed that
circRNA calmodulin-regulated spectrin-associated protein 1
(circ-CAMSAP1) facilitated OS progression and metastasis by
sponging miR-145-5p and subsequently regulating the expression of
Friend leukemia integration 1. circRNA low-density lipoprotein
receptor-related protein 6 (circ-LRP6), a newly discovered circRNA,
has been detected in vascular smooth muscle cells, and has been
determined to facilitate OS, oral squamous cell carcinoma and
esophageal squamous cell cancer tumorigenesis (13-16). However, the role and mechanism of
circ-LRP6 has not been fully elucidated in OS.
As a class of non-coding RNA, miRNAs are short
endogenous RNAs that are 22-25 nucleotides in length (17,18). A large number of miRNAs have been
reported to participate in OS progression, including miR-873,
miR-9, miR-708-5p, miR-183 and miR-17-5p (19-23). miR-141-3p functions as a tumor
suppressor in OS, colorectal cancer and gastric cancer (24-27). Additionally, in recent years, a
number of studies have demonstrated that circRNAs can competitively
bind to miRNAs, thereby eliminating the regulatory effect of miRNAs
on their target genes and participating in tumor progression
(28,29). For instance, in non-small cell
lung carcinoma (NSCLC), circ_100395 inhibited NSCLC malignancy by
sponging miR-141-3p and thereby increasing the expression levels of
large tumor suppressor kinase 2 (30). Another study revealed that
circRNA-100338 functions as a sponge of miR-141-3p, inhibiting the
progression of hepatocellular carcinoma (31). Furthermore, circ-sine
oculis-binding protein homolog, a novel circRNA, promoted cell
migration by regulating the miR-141-3p/myosin phosphatase target
subunit 1/phosphorylated myosin light chain 2 axis in prostate
cancer (32). It has been
reported that circ-LRP6 functions as an endogenous sponge for
miR-145, miR-9-5p and miR-455 (14,33-34). However, it has not been reported
whether circ-LRP6 sponges miR-141-3p, thus regulating the
proliferation, invasion and migration of OS cells.
The HDAC4 gene is located in human chromatin 2q37.3
and is 8,980 base pairs in length, containing a total of 37 exons
(35). Studies have determined
that HDAC4 expression differs in various tissues and organs and
serves an important role in the development and prognosis of tumors
(35,36). In esophageal cancer, HDAC4
upregulation promotes tumor progression and is associated with poor
survival (37). HDAC4 was also
reported to promote OS cell proliferation and invasion (38). High mobility group box-1 (HMGB1)
is highly expressed in a number of tumors, including lung, breast,
head and neck, nasopharyngeal and colorectal cancer, indicating
that it may be associated with the occurrence, invasion and
metastasis of tumors (39-43).
miRNAs exert their biological functions by
regulating the expression of their target genes at the
transcriptional or post-transcriptional level (44). For example, it has been determined
that HDAC4 is regulated by miR-200b-3p, miR-206, miR-29b and
miR-873-3p (45-48). Furthermore, HMGB1 was demonstrated
to be regulated by miR-451, miR-548b, miR-665, miR-142-3p and
miR-129-5p (49-53). However, to the best of our
knowledge, there are no studies that have assessed whether HDAC4
and HMGB1 are regulated by miR-141-3p and circ-LRP6 in OS.
The present study aimed to elucidate the expression,
role and potential molecular mechanism of circ-LRP6 in OS and to
explore the role of the circ-LRP6/miR-141-3p/HDAC4/HMGB1 axis in OS
progression. The results of the current study may provide a
potential target for the early diagnosis and clinical treatment of
OS.
Materials and methods
Human tissue collection
OS and normal paracancerous tissues were obtained
from 50 patients with OS (age range, 7-55 years; mean age,
21.52±10.15 years; 21 male and 29 female patients) who underwent
radical resection at the Zhengzhou Orthopedic Hospital (Zhengzhou,
China) between January 2018 and January 2019. Inclusion criteria
for patient recruitment were as follows: i) Tissue obtained during
surgery and diagnosed as OS by two pathologists; ii) the patient
had not received adjuvant therapy, such as chemotherapy or
radiotherapy, prior to surgery; and iii) the patient is willing to
participate. The exclusion criteria were as follows: i) Patients
with other diseases, including other tumors; ii) patients who
received treatment prior to participation in the present study; and
iii) patients who refused to participate in the study. The patients
with OS were diagnosed by histopathology according to the
tumor-node-metastasis (TNM) classification of the Union for
International Cancer Control (UICC), and lung metastasis was
determined according to CT imaging of lungs. All 50 patients with
OS were studied in a follow-up. The median follow-up was 31 months
(range, 3-60 months). All experiments were approved by the Ethics
Committee of Zhengzhou Orthopedic Hospital (approval no. 201908)
and samples were collected with the written informed consent of
patients.
Cell culture
hFOB1.19 normal human osteoblast cells, along with
OS cell lines (MG63, U-2OS, HOS and SaOS-2), were purchased from
the American Type Culture Collection. hFOB1.19 cells were cultured
in DMEM/F12 (Sangon Biotech Co., Ltd.) containing 10% FBS (HyClone;
Cytiva) at 33.5°C in 5% CO2. All OS cell lines were
cultured in DMEM (Sangon Biotech Co., Ltd.) containing 10% FBS at
37°C in 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissue samples and cell
lines using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), after which the purified RNA was reverse
transcribed into cDNA using the PrimeScript RT Reagent Kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
The SYBR Green PCR Master Mix (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for qPCR using an ABI 7500 Real-Time PCR
Instrument. β-actin was used as an internal control for circ-LRP6,
HDAC4 and HMGB1, whereas U6 was used as an internal control for
miR-141-3p. The relative expression level of each target gene was
calculated using the 2−ΔΔCq method (54). The thermocycling conditions were
as follows: 95°C for 15 sec; followed by 35 cycles at 60°C for 60
sec, 72°C for 40 sec. The expressions of circ-chaperonin-containing
TCP1 subunit 2 (CCT2), circ-tripartite motif-containing 33
(TRIM33), circ-eukaryotic translation initiation factor 4 γ3
(EIF4G3) and circ-LRP6 in OS tissues and paracancerous tissues was
also performed by RT-qPCR assay following the aforementioned
experimental procedures. All the primer sequences used for qPCR are
presented in Table I.
 | Table ISequences of siRNAs, miR-141-3p
inhibitor and mimic used for transfections, and primers used for
reverse transcription- quantitative PCR. |
Table I
Sequences of siRNAs, miR-141-3p
inhibitor and mimic used for transfections, and primers used for
reverse transcription- quantitative PCR.
| Gene | Sequence (5′ to
3′) |
|---|
|
si-circ-LRP6#1 |
AAGGATGTATTTATGTTATAATG |
|
si-circ-LRP6#2 |
AACTAATGTATTTTTAGCTTAAG |
| si-NC |
GCAGGGAGACTCGTCGCAATACC |
| miR-141-3p
inhibitor |
CCAUCUUUACCAGACAGUGUUA |
| Inhibitor NC |
GCUGUCCCGGAGGAUCUUCACG |
| miR-141-3p
mimic |
UAACACUGUCUGGUAAAGAUGG |
| Mimic NC |
CUCGACAAUCAGGUCACAGCGA |
| circ-LRP6
primer | F:
CTTCTGTGCCTCTTGGTTA |
| R:
ACTTGATGATGCTCCTGTAA |
| miR-141-3p
primer | F:
CGGGCTAACACTGTCTGGTAAAG |
| R:
GTGCAGGGTCCGAGGTATTC |
| HDAC4 | F:
GTATGACACGCTGATGCT |
| R:
GCCACGGAGTTGAAGTAG |
| HMGB1 | F:
TCTTCCTCTTCTGCTCTGA |
| R:
ATCTTCCTCCTCTTCCTTCT |
| U6 | F:
CTCGCTTCGGCAGCACA |
| R:
AACGCTTCACGAATTTGCGT |
| β-actin | F:
CCTGTACGCCAACACAGTGC |
| R:
ATACTCCTGCTTGCTGATCC |
| LRP6 | F:
TATTGTCCCCCGATGGGCTG |
| R:
AGTACATGAACCCACTTGAAGGA |
| circ-eukaryotic
translation initiation factor 4 γ3 | F:
CCTACCCCATCCCCTTATTC |
| R:
ACCGTGCTGTAGACTGCTGAG |
|
circ-chaperonin-containing TCP1 subunit
2 | F:
TCTTTGCATAGTCCCGGCAG |
| R:
AGAGAGGCATCTCGTCCACT |
| circ-tripartite
motif-containing 33 | F:
GTATGCCGCCAAGAATGCAG |
| R:
CTTTGCCCAGAAGGTGGGAT |
Construction of the pcDNA3.1-HMGB1 and
HDAC4 overexpress plasmids
HOS and SaOS-2 cells in the logarithmic growth phase
were trypsin digested and collected. HDAC4 and HMGB1 cDNA were
subsequently cloned into pcDNA3.1 (pc; Invitrogen; Thermo Fisher
Scientific, Inc.) to construct overexpression vectors. PCR was used
to copy the HMGB1 (position of PCR amplified fragment on the
chromosome, GRCh38:13:30462604-30461435) and HDAC4 (position of PCR
amplified fragment on the chromosome,
GRCh38:13:239108176-239084216) DNA from the genome of both HOS and
SaOS-2 cells using the following primers: HMGB1 forward, 5′-TCT TCC
TCT TCT GCT CTG A-3′ and reverse, 5′-ATC TTC CTC CTC TTC CTT CT-3′;
and HDAC4 forward, 5′-GTA TGA CAC GCT GAT GCT-3′ and reverse,
5′-GCC ACG GAG TTG AAG TAG-3′. Thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min; followed by 34
cycles of denaturation at 95°C for 30 sec, annealing at 62°C for 30
sec, and extension at 72°C for 60 sec; and a final extension at
72°C for 7 min. PCR products were cleaned up by using the
AxyGen® AxyPrep PCR Clean-Up Kit (Corning, Inc.)
according to the manufacturer's protocol and linked to pGEM-T easy
vector (Promega Corporation). ApaI and NotI enzyme
were used to cut the pGEM-T and pcDNA3.1(-) vectors). T4 DNA ligase
was used to ligate the pcDNA3.1(-) and the excised DNA fragments at
16°C for 2 h using the T4 DNA Ligase kit (Takara Bio, Inc.)
according to the manufacturer's protocol. Plasmids (GeneChem,
Shanghai, China) were mixed in Opti-MEM (Gibco, Burlington, Canada)
and incubated with the transfection reagent
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) for 20 min at room temperature. The resulting
vectors (2 µg/µl) were transformed into
Escherichia coli DH5α cells and cultured on LB plates
containing 100 µg/ml ampicillin.
Cell grouping and transfections
The miR-141-3p mimic, mimic negative control (NC),
miR-141-3p inhibitor, inhibitor NC, small interfering
(si)RNA-circ-LRP6#1, si-circ-LRP6#2 and si-NC
were designed and synthesized by Shanghai GenePharma Co., Ltd. HOS
and SaOS-2 cells (5×106 cells/ml) were transfected as
follows: i) The si-NC group transfected with 75 nM si-NC; ii) the
si-circ-LRP6#1 group transfected with 75 nM
si-circ-LRP6#1; iii) the si-circ-LRP6#2 group
transfected with 75 nM si-circ-LRP6#2; iv) the
si-circ-LRP6 + miR-141-3p inhibitor group transfected with 75 nM
si-circ-LRP6#1 + 50 nM miR-141-3p inhibitor; v) the
si-circ-LRP6#1 + pc-HDAC4 group transfected with 75 nM
si-circ-LRP6#1 + 2 µg/µl pcDNA3.1-HDAC4;
and vi) the si-circ-LRP6#1 + pc-HMGB1 group transfected
with 75 nM si-circ-LRP6#1 + 1.8 µg/µl
pcDNA3.1-HMGB1. All cells were transfected using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at room tempera- ture for 48 h and then used for
subsequent experimentation. A total of 50 nM miR-141-3p mimic or
mimic NC were transfected into HOS and SaOS-2 cells
(5×106 cells/ml) using Lipofectamine 3000, as
aforementioned, and used in subsequent experiments. The sequences
of the transfected siRNAs, miRNA inhibitor, miRNA mimic and the
respective controls are presented in Table I.
Cell Counting Kit (CCK)-8 assay
Transfected HOS and SaOS-2 cells were collected and
the cell density was adjusted to 5×104 cells/ml, after
which 100 µl cell suspension was inoculated onto 96-well
plates and cultured at 37°C and 5% CO2. After incubation
for 1, 2, 3 and 4 days, 10 µl CCK-8 solution (Beyotime
Institute of Biotechnology) was added and the cells were incubated
at 37°C for a further 4 h. The absorbance value at 450 nm was
determined using a microplate reader.
Transwell assays
At 48 h after transfection, HOS and SaOS-2 cells
were resuspended in serum-free RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.). To assess migration, 150 µl cell
suspension was added into the upper chamber of a 24-well Transwell
insert (pore size, 8 µM) at a density of 3×105
cells/ml. To assess invasion, the upper chamber was pretreated with
50 µl Matrigel and air-dried at room temperature for 4 h,
after which 150 µl cell suspension was added at a density of
3×105 cells/ml. A total of 500 µl RPMI-1640
medium containing 10% FBS was added to the lower chamber and the
cells were placed in an incubator at 37°C and 5% CO2 for
24 h. Subsequently, the cells in the lower chamber were washed
twice with PBS, fixed with 4% paraformaldehyde for 30 min at room
temperature and stained in 0.1% crystal violet for 15 min at room
temperature. Five random fields of view of were observed, images
were captured and cells were counted under a IX51 inverted light
microscope (Olympus Corporation; magnification, ×200).
Western blotting
Total protein was extracted from cells
(5×106 cells/ml)using RIPA lysis buffer (Thermo Fisher
Scientific, Inc.). After denaturing the protein samples at 100°C
for 10 min, their concentration was measured with a BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Protein (50 µg/lane) was then
separated by 10% SDS-PAGE. The protein isolates were transferred
onto PVDF membranes using the wet transfer method, after which the
membranes were blocked with 1X TBS + 0.1% Tween-20 (TBST; Sangon
Biotech Co., Ltd.) containing 10% skimmed milk powder for 2 h at
room temperature. The membranes were washed with TBST for 3 times
(10 minutes each time) and subsequently incubated with anti-HDAC4
(cat. no. ab235583; 1:1,000; Abcam), anti-HMGB1 (cat. no. ab79823;
1:10,000; Abcam) and anti-β-actin (cat. no. ab8227; 1:5,000; Abcam)
primary antibodies at 4°C overnight. HRP-conjugated secondary
antibodies (cat. no. ab6721; 1:5,000; Abcam) were then added to
membranes, which were then incubated for 2 h at room temperature.
The membranes were washed with TBST for 3 times (10 minutes each
time), and the protein bands were visualized using BeyoECL Plus
ECL-like Western reagent (Beyotime Institute of Biotechnology) was
used to visualize the bands. ImageJ version 1.8.0 (National
Institutes of Health) was used for densitometric analysis; β-actin
was used as an internal control.
RNA immunoprecipitation (RIP) assay
The binding of circ-LRP6 to argonaute RISC catalytic
component 2 (AGO2) protein was detected using a Magna RIP
RNA-Binding Protein Immunoprecipitation kit (MilliporeSigma). HOS
and SaOS-2 cells (8×106 cells/ml) were collected and
lysed according to the manufacturer's protocol, after which the
cell extract was incubated for 10 min with antibodies against
Argonaute2 (cat. no. ab32381; 1:2,000; Abcam) or immunoglobulin G
(cat. no. ab109489; 1:5,000; Abcam) at room temperature. The
magnetic bead antibody complex was resuspended in 900 µl RIP
wash buffer. Cell extract (100 µl) was then added and
incubated overnight at 4°C. RNA was extracted from samples
following digestion with protease K, after which the expression
levels of circ-LRP6 and miR-141-3p were detected by RT-qPCR,
aforementioned.
RNase R treatment
RNase R digestion was used to test the stability of
RNA. RNA was isolated from HOS and SaOS-2 cells (7×106
cells/ml) using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), after which 10 µg RNA was digested with
40 U RNase R (Epicentre; Illumina, Inc.). Cells were divided into
two groups: Mock control group and RNase R treatment group. The
enrichment of circ-LRP6 and LRP6 mRNA was then determined by
RT-qPCR, aforementioned.
Bioinformatics analysis
The Circular RNA Interactome (https://circinteractome.irp.nia.nih.gov/index.html)
was used to predict the candidate downstream targets of circ-LRP6.
TargetScan 7.2 (www.targetscan.org/vert_71) was conducted to predict
the target genes of miR-141-3p. OS chip (GSE96964 dataset;
https://www.ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE96964)
was used for analyzing the expression of circRNAs (circ-CCT2,
circ-TRIM33 and circ-EIF4G3) (55).
Dual-luciferase reporter assay
Bioinformatics prediction analysis confirmed that
miR-141-3p could bind with circ-LRP6, HDAC4 and HMGB1. The
wild-type (Wt) 3′-untranslated regions (UTRs) of circ-LRP6, HDAC4
and HMGB1 were incorporated into pmirGLO plasmids (Promega
Corporation). Complementary sequence mutation sites were designed
and introduced into the 3′-UTRs circ-LRP6, HDAC4 and HMGB1, which
were also constructed using the pMIR-reporter plasmid. The Wt or
mutant (Mut) luciferase reporter plasmids were co-transfected with
either miR-141-3p mimic (50 nM) or NC (50 nM) into HOS and SaOS-2
cells (5×104 cells/ml) using Lipofectamine®
3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). At room
temperature 48 h after transfection, luciferase activity was
measured using the pmirGLO Dual-Luciferase Assay (Promega
Corporation). Firefly lucif- erase activities were normalized to
that of Renilla luciferase.
Statistical analysis
The data of the present study were statistically
analyzed using SPSS 22.0 software (IBM Corp.). Data are expressed
as the mean ± SD. Differences between OS and adjacent normal
tissues were evaluated using a paired Student's t-test, whereas the
differences between two groups of OS cells were evaluated using an
unpaired Student's t-test. Differences between multiple groups of
OS cells were evaluated using one-way ANOVA followed by a Tukey's
post-hoc test. Kaplan-Meier analysis followed by log-rank tests
were used to assess survival curves. Receiver operating
characteristic (ROC) curve analysis was performed to assess the
sensitivity and specificity of the measured markers. Fisher's exact
test was used to investigate the relationship between
clinicopathological features and circ-LRP6 expression, according to
median expression levels. Correlations between circ-LRP6 and
miR-141-3p, miR-141-3p and HDAC4, and miR-141-3p and HMGB1 were
determined by performing Pearson's correlation analysis if the data
are parametric and continuous. P<0.05 was considered to indicate
a statistically significant difference.
Results
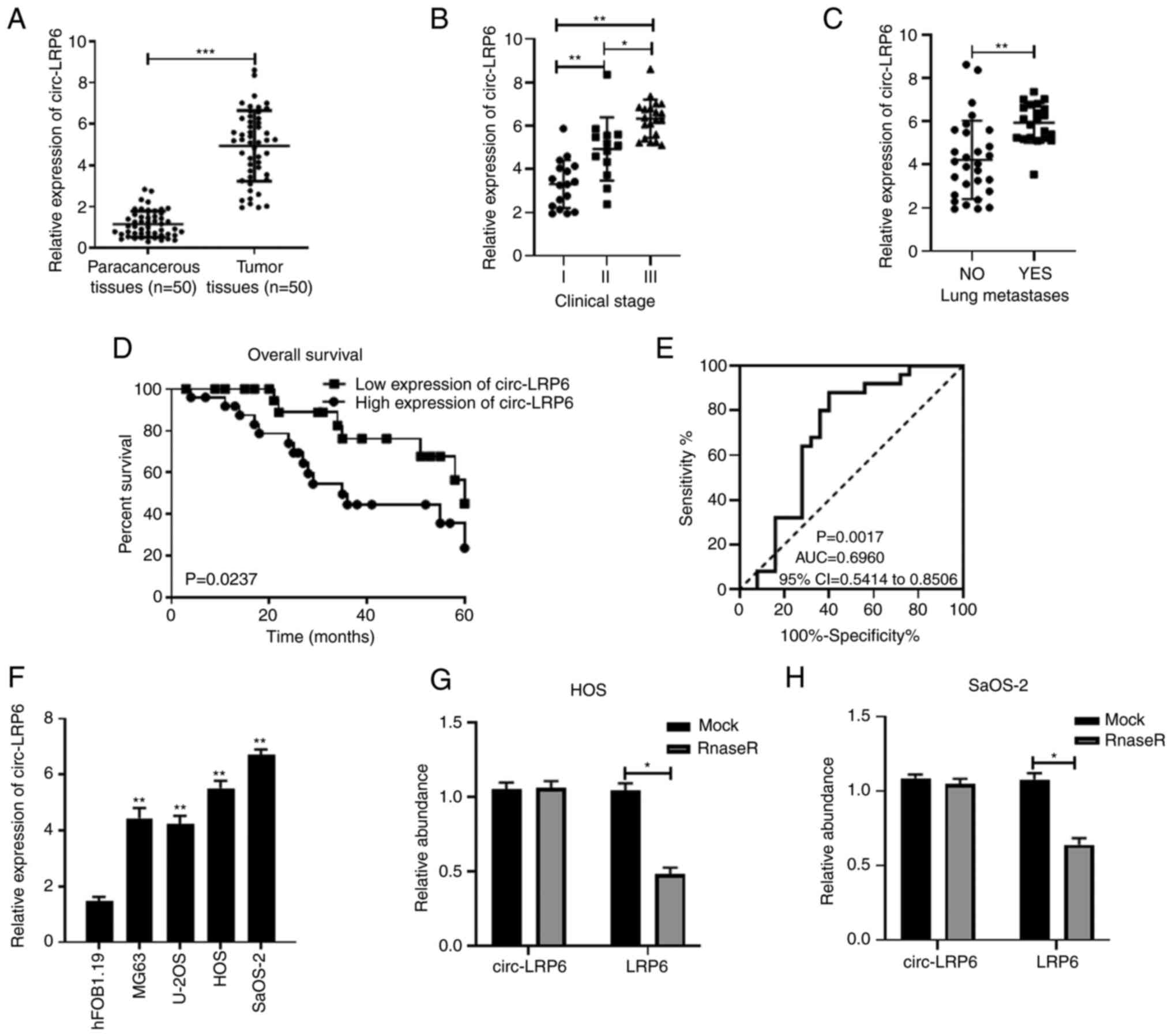
circ-LRP6 expression is upregulated in OS
and is associated with poor prognosis
The OS chip dataset GSE96964 was analyzed and
relevant literature was consulted; from these investigations
several circRNAs (circ-CCT2, circ-TRIM33 and circ-EIF4G3 from
GSE96964) and also circ-LRP6, from previous studies (14,55), were selected for RT-qPCR analysis
in OS tissues (Fig. 1A and
S1). It was found that
circ-CCT2, circ-TRIM33, circ-EIF4G3 and circ-LRP6 was all
overexpressed in OS tissues compared with expression in the
paracancerous tissue (Fig. S1).
As circ-LRP6 appeared to be more highly expressed compared with the
other circRNAs, it was selected for further analysis. To
investigate the relationship between circ-LRP6 expression and the
clinicopathological features of patients, the 50 patients with OS
were divided into two groups according to the median expression of
circ-LRP6: A high expression group (n=25) and a low expression
group (n=25) (Table II). The
association between circ-LRP6 expression levels and the clinical
stage and lung metastases of patients was subsequently assessed.
The results revealed that circ-LRP6 was significantly higher at
clinical stage II and III compared with clinical stage I (Fig. 1B). Furthermore, an increased
expression of circ-LRP6 was determined in patients exhibiting lung
metastases compared with those that did not exhibit OS lung
metastasis (Fig. 1C). circ-LRP6
expression was significantly associated with TNM stage and distant
metastasis (Table II).
Kaplan-Meier survival analysis demonstrated that the total survival
rate of patients with high circ-LRP6 expression levels was
significantly lower compared with that of patients with low
circ-LRP6 expression levels (Fig.
1D). ROC curve analysis was used to evaluate the diagnostic
value of circ-LRP6 expression levels in OS patients. The area under
the curve value of circ-LRP6 was determined to be 0.6960 (95%
confidence interval, 0.5414-0.8506; Fig. 1E).
 | Table IIAssociation of circ-LRP6 expression
with clinicopathological factors in osteosarcoma. |
Table II
Association of circ-LRP6 expression
with clinicopathological factors in osteosarcoma.
| Clinicopathological
feature | Total (n=50) | Expression level of
circ-LRP6
| P-value |
|---|
| Low (n=25) | High (n=25) |
|---|
| Sex | | | | |
| Male | 21 | 10 | 11 | 0.5 |
| Female | 29 | 15 | 14 | |
| Age, years | | | | |
| <20 | 26 | 14 | 12 | 0.389 |
| ≥20 | 24 | 11 | 13 | |
| Tumor size, cm | | | | |
| <8 | 23 | 13 | 10 | 0.285 |
| ≥8 | 27 | 12 | 15 | |
|
Tumor-node-metastasis stage | | | | |
| I-II | 30 | 19 | 11 | 0.021 |
| III | 20 | 6 | 14 | |
| Distant
metastasis | | | | |
| Absent | 29 | 20 | 9 | 0.002 |
| Present | 21 | 5 | 16 | |
The relative expression levels of circ-LRP6 in OS
cell lines were also significantly higher compared with the
hFOB1.19 normal osteoblast cell line (Fig. 1F). As the relative expression
levels of circ-LRP6 were the highest in HOS and SaOS-2 cell lines,
they were selected for subsequent experimentation. Additionally, to
verify the stability of circ-LRP6, RNase treatment was applied, the
results of which revealed that the expression of circ-LRP6 was not
significantly altered when compared with the mock group, whereas
the expression of LRP6 was significantly decreased, indicating the
stability of circ-LRP6 (Fig. 1G and
H).
circ-LRP6 knockdown inhibits the
proliferation, migration and invasion of OS cells
The expression of circ-LRP6 in si-NC,
si-circ-LRP61# and si-circ-LRP62# transfected
OS cells was detected using RT-qPCR. The results revealed that the
expression level of circ-LRP6 in the si-circ-LRP61# and
si-circ-LRP62# groups was significantly lower compared
with the NC group (Fig. 2A),
which indicated that transfection had been a success and that
transfected cells could be used for subsequent experiments. As
si-circ-LRP6#1 had a better transfection efficiency, it
was chosen for subsequent experiments. CCK-8, Transwell and
Matrigel assays were performed to detect OS cell proliferation,
migration and invasion, respectively. The results demonstrated that
HOS and SaOS-2 cell proliferation in the si-circ-LRP6 groups was
significantly inhibited compared with the respective si-NC group
(Fig. 2B and C). In addition,
compared with the si-NC group, the number of migratory and invasive
cells in the si-circ-LRP6 group was significantly decreased
(Fig. 2D and E, respectively). In
conclusion, these data suggested that circ-LRP6 may serve as an
oncogene in the progression of OS.
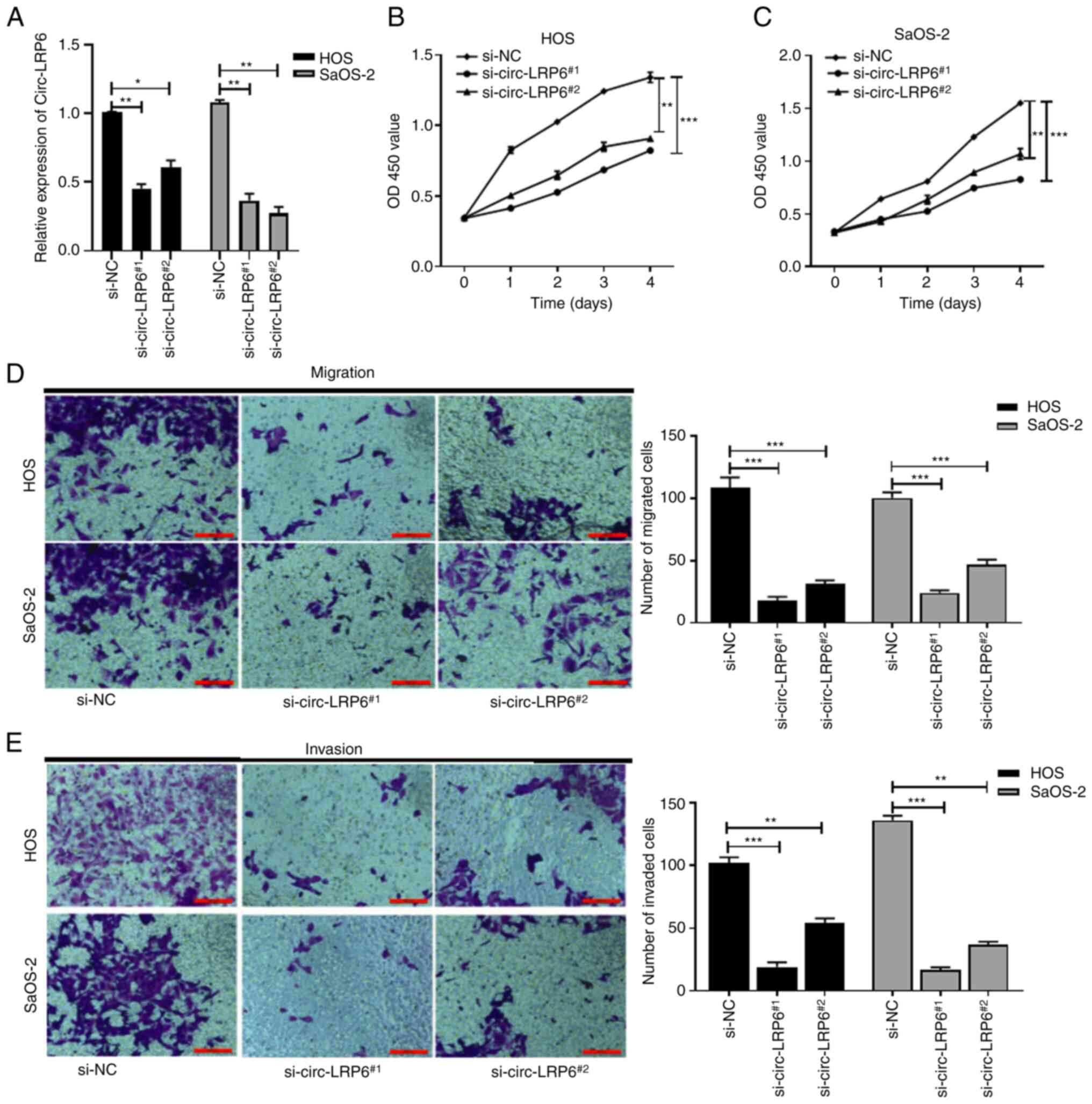 | Figure 2Knockdown of circ-LRP6 inhibits the
proliferation, migration and invasion of OS cells.
si-circ-LRP6#1, si-circ-LRP#2 or si-NC was
transfected into HOS and SaOS-2 cells. (A) Expression levels of
circ-LRP6 in the transfected OS cells were analyzed by reverse
transcription-quantitative PCR. The prolifera- tive ability of (B)
HOS and (C) SaOS-2 cells was detected by CCK-8 assay. (D) Migratory
and (E) invasive abilities of HOS and SaOS-2 cells was detected by
Transwell and Matrigel assay, respectively. *P<0.05,
**P<0.01, ***P<0.001. CCK-8, Cell
Counting Kit-8; circ, circular RNA; LRP6, lipoprotein receptor 6;
NC, negative control; OS, osteosarcoma; si, small interfering
RNA. |
circ-LRP6 sponges miR-141-3p in OS
As circRNAs can competitively bind to miRNAs
(12,13), the miRNAs that could bind to
circ-LRP6 were predicted using the Circular RNA Interactome
database. The top five miRNAs (miR-141-3p, miR-1208, miR-326,
miR-330-5p and miR-513a-3p) that match circ-LRP6 along with their
'match degrees' (Fig. SII).
miR-141-3p was selected for further analysis as the matched-degree
between miR-141-3p and circ-LRP6 was relatively high compared with
other matches, and due to the fact that miR-141-3p has been
previously demonstrated to function as a tumor suppressor gene
(24-27). Fig.
3A shows the pairing between miR-141-3p and circ-LRP6.
miR-141-3p levels in the miR-141-3p mimic-transfected group were
higher compared with the mimic NC group, indicating a successful
transfection (Fig. 3B). The
dual-luciferase assay results revealed that, compared with NC
group, luciferase activity in the circ-LRP6-Wt + miR-141-3p group
was significantly decreased, whereas no significant difference was
identified between the luciferase activities of the circ-LRP6-Mut +
miR-141-3p compared with the respective control group (Fig. 3C).
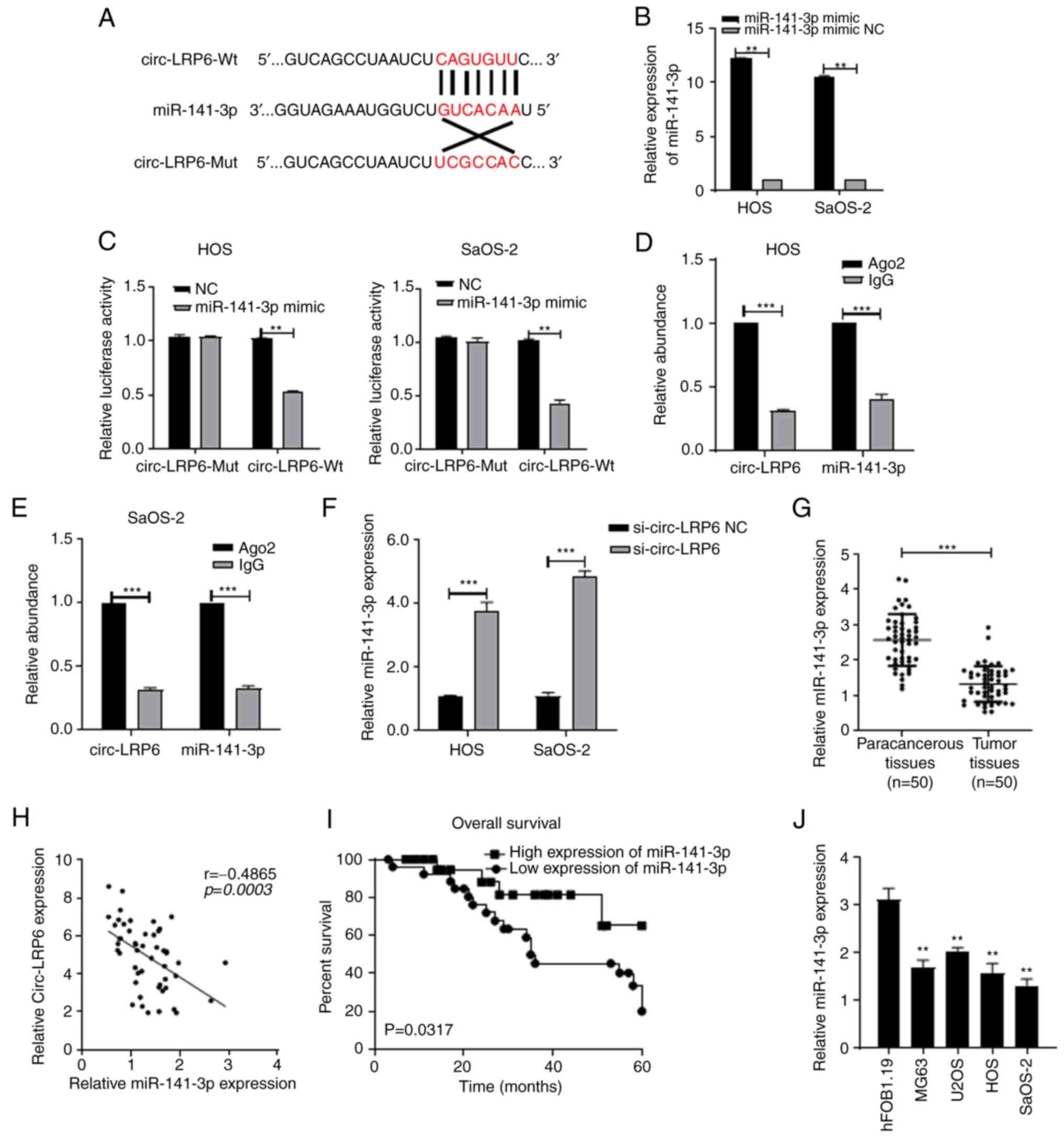 | Figure 3circ-LRP6 sponges miR-141 3p in OS.
(A) Binding sites between circ-LRP6 and miR-141-3p. (B) Expression
levels of miR-141-3p in HOS and SaOS-2 cells transfected with
miR-141-3p inhibitor were analyzed by RT-qPCR. (C) The binding
relationship between circ-LRP6 and miR-141-3p was verified by
dual-luciferase assays in HOS and SaOS-2 cells. circ-LRP6 binding
to miR-141-3p was verified by RIP assays in (D) HOS and (E) SaOS-2
cells. (F) Expression levels of miR-141-3p in HOS and SaOS-2 cells
transfected with si-circ-LRP6 were analyzed by RT-qPCR. (G)
Expression levels of miR-141-3p in 50 pairs of OS tissues and
paracancerous tissues were analyzed by RT-qPCR.
**P<0.05, ***P<0.001. (H) miR-141-3p
expression is negatively correlated with circ-LRP6 expression in OS
tissues. (I) Comparison of overall survival in patients with high
and low expression levels of miR-141-3p. (J) Expression levels of
miR-141-3p in OS cells and hFOB1.19 normal osteoblast cells were
analyzed by RT-qPCR. **P<0.01 vs. hFOB1.19. Ago2,
argonaute RISC catalytic component 2; circ, circular RNA; LRP6,
lipoprotein receptor 6; miR, microRNA; Mut, mutant; NC, negative
control; OS, osteosarcoma; RIP, RNA immunoprecipitation; RT-qPCR,
reverse transcription-quantitative PCR; Wt, wild-type. |
Results of the RIP experiments demonstrated that
circ-LRP6 competitively bound to miR-141-3p in HOS and SaOS-2 cells
(Fig. 3D and E). In addition, the
results of RT-qPCR demonstrated that, compared with the si-NC
group, the relative expression levels of miR-141-3p in the
si-circ-LRP6 group was significantly increased (Fig. 3F), indicating that the expression
of miR-141-3p may be regulated by circ-LRP6. RT-qPCR results
further demonstrated that miR-141-3p expression was downregulated
in OS tissues (Fig. 3G).
miR-141-3p expression was determined to be negatively associated
with circ-LRP6 expression (Fig.
3H). Kaplan-Meier survival curve analysis was subsequently
demonstrated that the survival rate of patients with high
miR-141-3p expression levels was significantly higher compared with
patients with low miR-141-3p expression levels (Fig. 3I). The expression of miR-141-3p
was also determined in OS cells. It was revealed that miR-141-3p
was decreased in these cell lines (Fig. 3J).
HDAC4 and HMGB1 are targets of miR-141-3p
in OS
It has been reported that miRNAs can bind to the
3′-UTR of target genes and subsequently regulate the occurrence and
progression of cancer (47-51). Therefore, the current study aimed
to elucidate the targets of miR-141-3p. The TargetScan
bioinformatics database predicted that miR-141-3p could bind to
HDAC4 and HMGB1 (Fig. 4A and D).
To verify these results, a dual-luciferase assay was performed, the
data of which revealed that, when compared with the respective NC
group, the luciferase activity of the HDAC4- or HMGB1-Wt +
miR-141-3p mimic group was inhibited, whereas no significant
difference was identified in the luciferase activities between
HDAC4- or HMGB1-Mut+ miR-141-3p mimic group and the NC groups in
the OS cell lines (Fig. 4B, C, E and
F). RT-qPCR and western blotting data revealed that the mRNA
and protein expression levels of HDAC4 and HMGB1 in the miR-141-3p
mimic- transfected group was significantly decreased compared with
the NC group (Fig. 4G-J). HDAC4
mRNA expression levels were then determined in OS tissues, and the
results demon- strated that HDAC4 expression was upregulated
compared with expression in the paracancerous tissue (Fig. 4K); this increased HDAC4 expression
was negatively correlated with miR-141-3p expression in OS tissues
(Fig. 4L). Similarly, HDAC4 was
upregulated in OS cells compared with expres- sion in hFOB1.19
cells (Fig. 4M). HMGB1 mRNA
expression levels were also upregulated and negatively correlated
with miR-141-3p expression in OS tissues (Fig. 4N and O); similarly, HMGB1 mRNA
expression was upregulated in OS cells compared with hFOB1.19
(Fig. 4P).
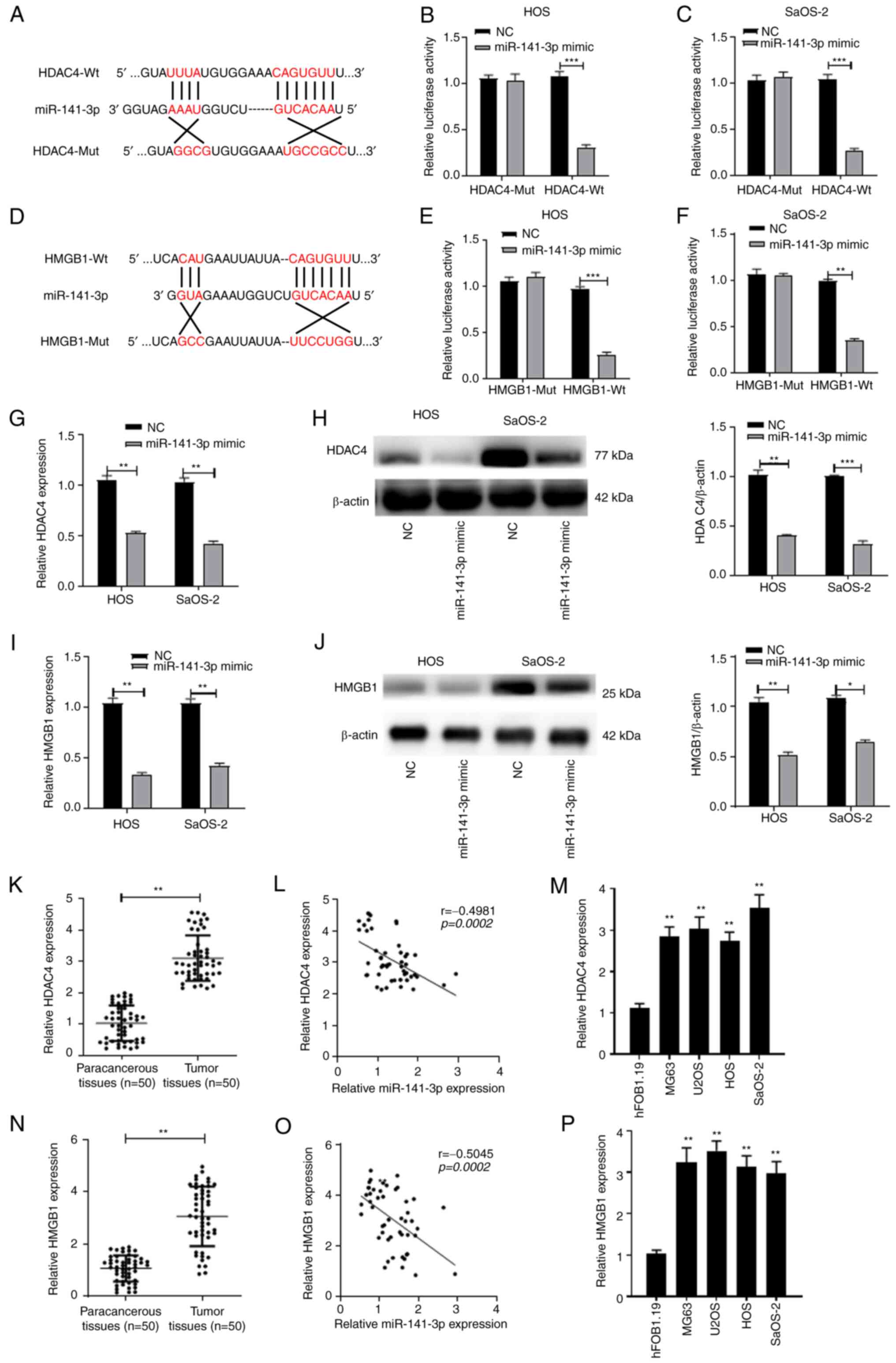 | Figure 4HDAC4 and HMGB1 are the targets of
miR-141-3p in OS. (A) Binding sites between HDAC4 and miR-141-3p.
Binding relationship between HDAC4 and miR-141-3p were verified by
dual-luciferase assays (B) HOS and (C) SaOS-2 cells. (D) Binding
sites between HMGB1 and miR-141-3p. Binding relationship between
HMGB1 and miR-141-3p were verified by dual-luciferase assays in (E)
HOS and (F) SaOS-2 cells. (G) mRNA and (H) protein expression
levels of HDAC4 in HOS and SaOS-2 cells transfected with miR-141-3p
mimic were analyzed by RT-qPCR and western blotting, respectively.
(I) mRNA and (J) protein expression levels of HMGB1 in HOS and
SaOS-2 cells transfected with miR-141-3p mimic were analyzed by
RT-qPCR and western blotting, respectively. (K) Expression levels
of HDAC4 mRNA in 50 pairs of OS tissues and paracancerous tissues
were analyzed by RT-qPCR. *P<0.05,
**P<0.01, ***P<0.001. (L) HDAC4
expression was negatively correlated with miR-141-3p expression in
OS tissues. (M) Expression levels of HDAC4 mRNA in OS cells and in
hFOB1.19 normal osteoblast cells were analyzed by RT-qPCR.
**P<0.01 vs. hFOB1.19. (N) Expression levels of HMGB1
mRNA in 50 pairs of OS tissues and paracancerous tissues were
analyzed by RT-qPCR. **P<0.01. (O) HMGB1 expression
was negatively correlated with miR-141-3p expression in OS tissues.
(P) Expression levels of HMGB1 mRNA in OS cell lines and in
hFOB1.19 normal osteoblast cells were analyzed by RT-qPCR.
**P<0.01 vs. hFOB1.19. HDAC4, histone deacetylase 4;
HMBG1, high mobility group protein 1; miR, microRNA; Mut, mutant;
NC, negative control; OS, osteosarcoma; RT-qPCR, reverse
transcription-quantitative PCR; Wt, wild-type. |
circ-LRP6 promotes OS cell proliferation,
migration and invasion by regulating the miR-141-3p/HDAC4 axis
The aforementioned results indicated that circ-LRP6
may promote OS cell malignancy, that circ-LRP6 sponged miR-141-3p
and that HDAC4 was the target of miR-141-3p in OS. Therefore, to
determine whether circ-LRP6 promoted OS progression by regulating
the miR-141-3p/HDAC4 axis. OS cells transfected with either
pcDNA3.1 empty vector or pc-HDAC4 overexpression vector; the
results demonstrated that HDAC4 expression levels in the pc-HDAC4
group were significantly higher compared with the pcDNA3.1 group,
indicating that transfection was successful (Fig. 5A). Additionally, miR-141-3p levels
in the miR-141-3p inhibitor group were lower compared with the
inhibitor NC group, indicating successful transfection (Fig. 5B). si-circ-LRP6, miR-141-3p
inhibitor and pc-HDAC4 were co-transfected into HOS and SaOS-2
cells in various combinations, after which CCK-8 and Transwell
assays were performed. The data revealed that inhibition of cell
proliferation, migration and invasion induced by circ-LRP6
knockdown were partially reversed by miR-141-3p inhibition or HDAC4
overexpression (Fig. 5C-F). These
data suggested that circ-LRP6 may facilitate OS progression through
the miR-141-3p/HDAC4 axis.
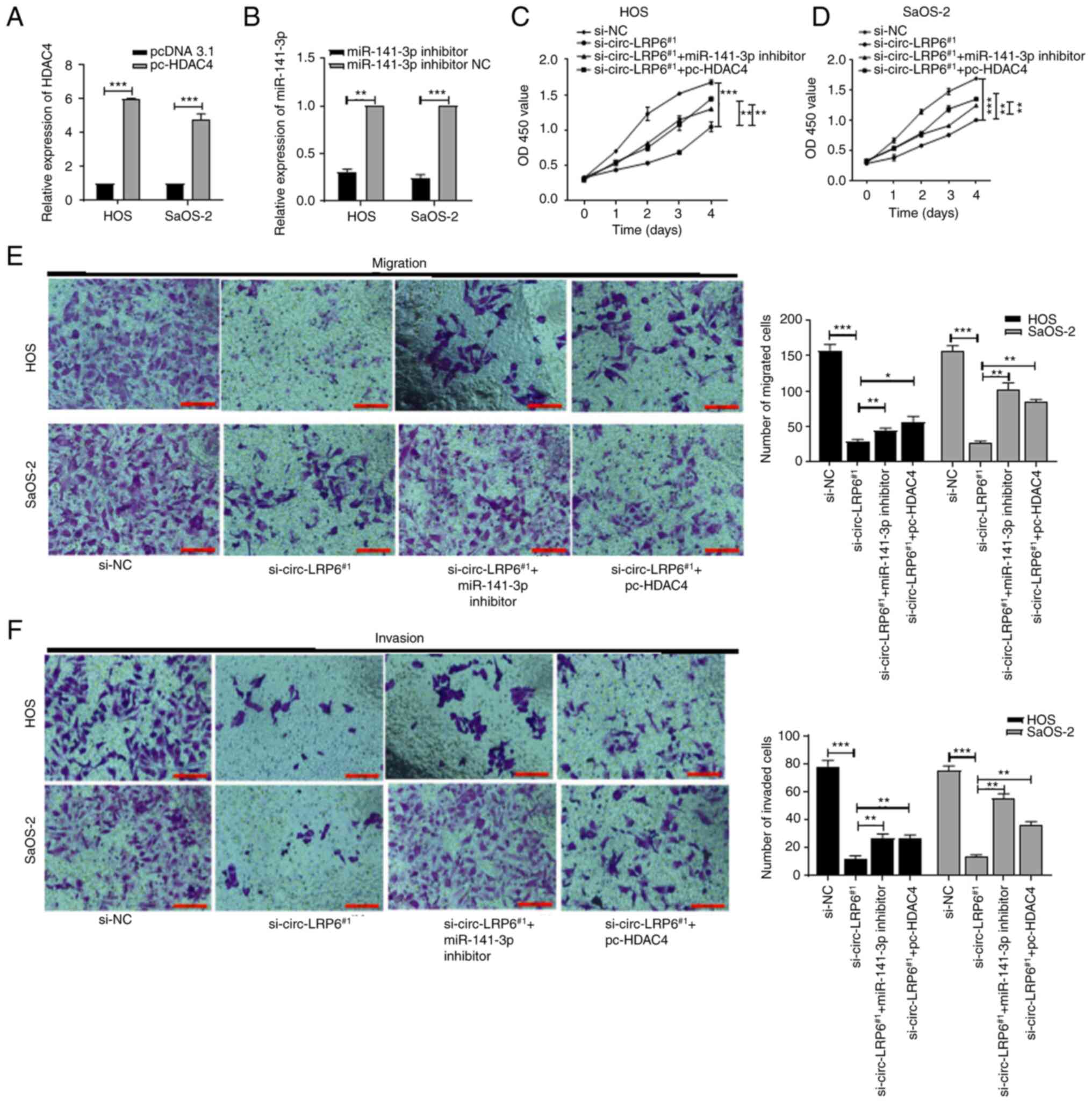 | Figure 5circ-LRP6 promotes OS cell
proliferation, migration and invasion by regulating the
miR-141-3p/HDAC4 axis. HOS and SaOS2 OS cells were co-transfected
with various combination of si-circ-LRP6, miR-141-3p inhibitor and
pc-HDAC4. (A) Expression levels of HDAC4 mRNA in HOS and SaOS-2
cells transfected with pc-HDAC4 were analyzed by RT-qPCR. (B)
Expression levels of miR-141-3p in HOS and SaOS-2 cells transfected
with miR-141-3p mimic were analyzed by RT-qPCR. The proliferative
ability of (C) HOS and (D) SaOS-2 cells was detected by CCK-8
assay. (E) The migratory ability of HOS and SaOS-2 cells was
detected by Transwell assay. (F) The invasive ability of HOS and
SaOS-2 cells was detected by Matrigel assay. *P<0.05,
**P<0.01, ***P<0.001. CCK-8, Cell
Counting Kit-8; circ, circular RNA; HDAC4, histone deacetylase 4;
LRP6, lipoprotein receptor 6; miR, microRNA; NC, negative control;
OS, osteosarcoma; RT-qPCR, reverse transcription-quantitative PCR;
si, small interfering RNA. |
circ-LRP6 promotes OS cell proliferation,
migration and invasion by regulating the miR-141-3p/HMGB1 axis
As aforementioned, circ-LRP6 may promote OS cell
malignancy and sponge miR-141-3p, and the results also revealed
that HMGB1 was a target of miR-141-3p in OS. Therefore, whether
circ-LRP6 promoted OS progression by regulating the
miR-141-3p/HMGB1 axis was investigated. The expression of HMGB1 in
OS cells transfected with pcDNA3.1 empty vector or pc-HMGB1
overexpression vector was detected using RT-qPCR, and the results
demonstrated that HMGB1 expression levels in the pc-HMGB1 group
were significantly higher compared with the pcDNA3.1 group,
indicating that transfection had been successful (Fig. 6A). Subsequently, si-circ-LRP6,
miR-141-3p inhibitor and pc-HMGB1 were variously co-transfected
into HOS and SaOS-2 cells. Results from CCK-8, Transwell and
Matrigel assays demonstrated that the significant inhibition of
proliferation, migration and invasion of OS cells caused by
circ-LRP6 knockdown were partially reversed by miR-141-3p
inhibition or of HDAC4 over- expression (Fig. 6B-E). These results indicated that
circ-LRP6 may facilitate OS progression through the
miR-141-3p/HMGB1 axis.
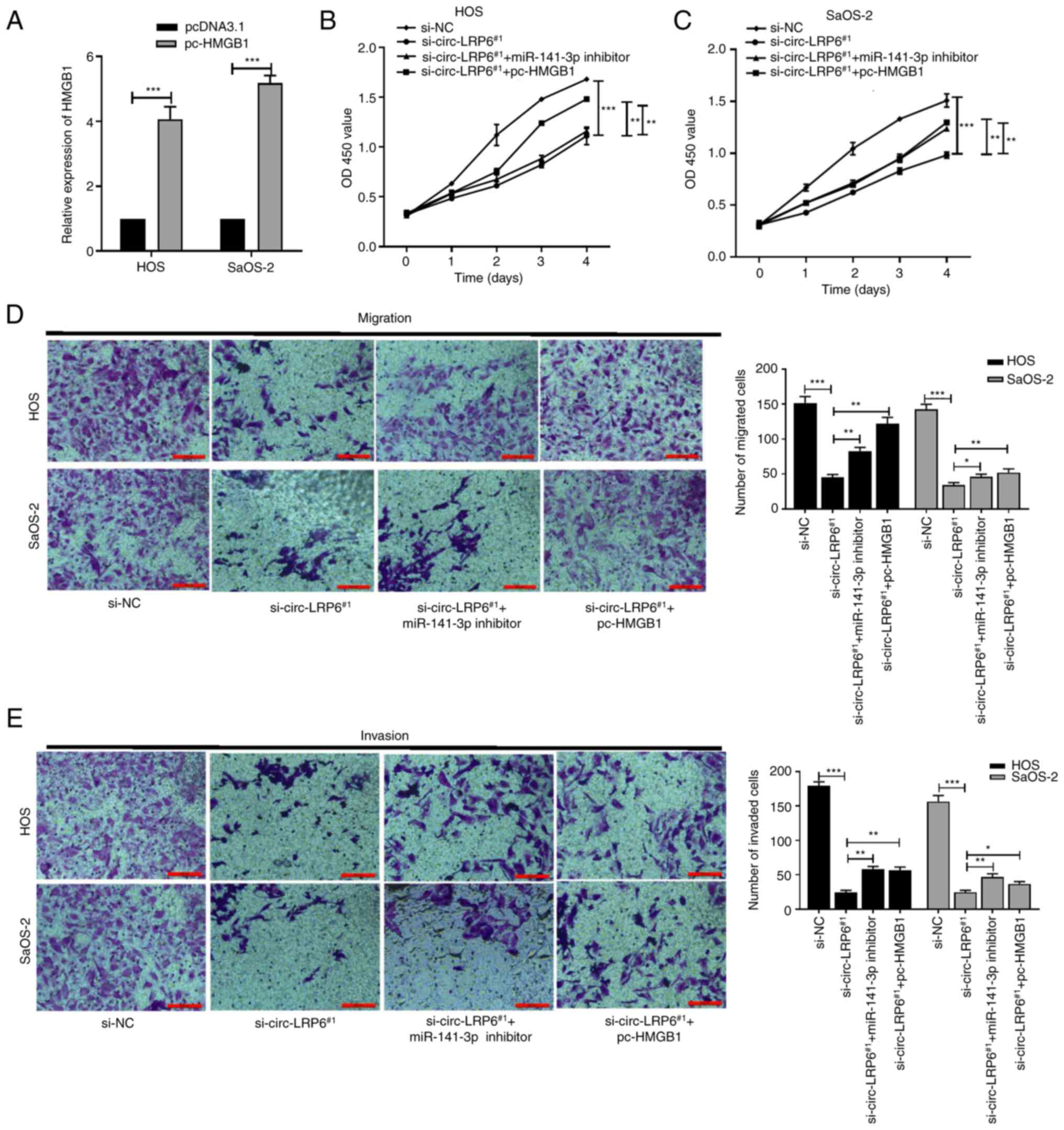 | Figure 6circ-LRP6 promotes OS cell
proliferation, migration and invasion by regulating the
miR-141-3p/HMGB1 axis. si-circ-LRP6, miR-141-3p inhibitor and
pc-HMGB1 were variously co-transfected into OS cells. (A)
Expression levels of HMGB1 mRNA in HOS and SaOS-2 cells transfected
with pc-HMGB1 were analyzed by reverse transcription-quantitative
PCR. The proliferative ability of (B) HOS and (C) SaOS-2 cells was
detected by CCK-8 assay. (D) The migratory ability of HOS and
SaOS-2 cells was detected by Transwell assay. (E) The invasive
ability of HOS and SaOS-2 cells was detected by Matrigel assay.
*P<0.05, **P<0.01,
***P<0.001. CCK-8, Cell Counting Kit-8; circ,
circular RNA; HMBG1, high mobility group protein 1; LRP6,
lipoprotein receptor 6; miR, microRNA; NC, negative control; OS,
osteosarcoma; si, small interfering RNA. |
Discussion
In recent years, non-coding RNAs, including
circRNAs, long non-coding RNAs and miRNAs, have been widely
reported as molecular markers for the genesis and development of
various malignant tumors, including OS (8,23,56). For example, circ_0003074,
circ-cytosolic 5′ nucleotidase II and circ_0081001 can be used as
biomarkers for the early diagnosis of OS (57-59). Additionally, various circRNAs
mediate tumor progression by regulating cancer cell proliferation,
invasion and migration. For example, Ma et al (8) revealed that circ-UBAP2 was highly
expressed in OS tissues, and that high levels of circ-UBAP2 were
positively associated with poor patient survival. Furthermore, Wang
et al (60) demonstrated
that circ-03955 overexpression could significantly promote the
invasion, migration and epithelial to mesenchymal transformation of
OS cells. Wan et al (61)
also stated that circRNA-plasmacytoma variant translocation 1
facilitated OS cell invasion and metastasis. The results of the
present study determined that circ-LRP6 was highly expressed in OS
tissues and cell lines, and that low expression of circ-LRP6
significantly inhibited the proliferation, invasion and migration
of OS cells. The GSE96964 OS microarray data identified several
circRNAs (circ-CCT2, circ-TRIM33, circ-EIF4G3) and also circ-LRP6
were all highly-expressed in OS tissues (14,55). However, only circ-LRP6 was studied
at present, and the roles of circ-CCT2, circ-TRIM33 and circ-EIF4G3
from GSE96964 shall be investigated in future studies.
circRNAs act as competing endogenous RNAs to sponge
miRNAs, and to subsequently regulate the expression of target genes
(28,56,61,62). As Ma et al (8) reported, circ-UBAP2 knockdown
inhibited the progression of OS cells by upregulating the
expression of miR-204-3p and, thus, downregulating the expression
of its target gene, HMGA2. In addition, Wen et al (11) reported that circ_HIPK3 promoted OS
cell proliferation, migration and invasion by regulating miR-637
and HDAC4 signaling. As a tumor suppressor-related miRNA,
miR-141-3p has been reported to serve as a tumor suppressor in
several types of cancer, including OS (24-27). With use of a Circular RNA
Interactome database, the present study predicted that circ-LRP6
may have a binding site with miR-141-3p, which was confirmed by
dual luciferase assay. Following circ-LRP6 knockout, OS cell
proliferation, migration and invasion were inhibited, whereas
combined miR-141-3p inhibition reversed this effect, which
suggested that circ-LRP6 may facilitate the malignant behavior of
OS cells by competitively binding to miR-141-3p.
Previous studies have demonstrated that miRNAs can
bind to the 3′-UTRs of target genes to achieve post-transcriptional
regulation, thereby modulating the occurrence and progression of
related diseases (20-25,45,63). In the present study, TargetScan
bioinformatics analysis and luciferase assays confirmed that
miR-141-3p bound to the 3′-UTR of HDAC4 and HMGB1. HDAC4 belongs to
the HDAC family and serves a role mainly through histone
acetyltransferases (37). HDACs
regulate the expression of a variety of genomic proteins to
regulate cell apoptosis and participate in the occurrence and
development of tumors (38-41). Previous studies have revealed that
HDAC4 is abnormally expressed in a variety of tumors, including
glioma, breast cancer, ovarian cancer and OS (35,38,64,65). HMGB1 is a type of chromatin
nucleoprotein that is associated with tumor invasion and metastasis
(66-68). It is highly expressed in a number
of malignant tumors, including cervical cancer, breast cancer and
endometrial carcinoma (40,52,67). It is also associated with
pathological stage, degree of invasion and degree of tumor
metastasis (68). The present
study showed that HDAC4 and HMGB1 were highly expressed in OS
tissues and cells, which is consistent with previous studies
(11,65). Furthermore, the protein expression
levels of HDAC4 and HMGB1 were significantly decreased after
miR-141-3p overexpression, suggesting that miR-141-3p may bind to
HDAC4 and HMGB1 to downregulate their expression.
A number of studies have demonstrated that circRNAs
act as competitive RNAs to adsorb various miRNAs, thus affecting
the expression of target mRNAs (10-15). As aforementioned, circ-LRP6
promoted OS cell malignancy by downregulating miR-141-3p.
Furthermore, as miR-141-3p could bind to HDAC4 and HMGB1, it was
hypothesized that circ-LRP6 promoted OS cell malignancy by
regulating the miR-141-3p/HDAC4/HMGB1 axis. A series of functional
assays revealed that circ-LRP6 silencing inhibited cell
proliferation, migration and invasion, while miR-141-3p inhibition
or HDAC4 and HMGB1 overexpression could reverse these effects,
suggesting that the circ-LRP6/miR-141-3p/HDAC4/HMGB1 axis
participated in OS progression. However, there are certain
limitations to the present study. For example, in vivo
experiments were not performed to elucidate the effects of
circ-LRP6 on OS tumorigenesis and lung metastasis.
In conclusion, the present results revealed that
circ-LRP6 was upregulated in OS tissues and cells, and that
circ-LRP6 could downregulate the expression of miR-141-3p and
upregulate HDAC4 and HMGB1 expression, thereby promoting the
proliferation, migration and invasion of OS cells. To the best of
our knowledge, the current study was the first to demonstrate that
the circ-LRP6/miR-141-3p/HDAC4/HMGB1 axis participated in OS
progression, which provides a new area of research for the
exploration of OS pathogenesis. However, as there have been no
animal experiments to confirm the regulatory mechanism of circ-LRP6
in vivo, further experimentation is required. Additionally,
future studies should assess whether circ-LRP6 is suitable for the
clinical treatment of patients with OS.
Supplementary Data
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YY designed the study, performed the experiments and
drafted the manuscript. GD and ZL analyzed the data. YZ and ZS
analyzed the data for the work and revised the manuscript. GW
contributed to the conception or design of the work, analyzed the
data for the work and managed the project administration. YY and GW
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no. 201908)
by the Ethics Committee of Zhengzhou Orthopedic Hospital
(Zhengzhou, China). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Medical Science and
Technology Project of Henan Province in 2020 (LHGJ20200759).
References
|
1
|
Sangle NA and Layfield LJ: Telangiectatic
osteosarcoma. Arch Pathol Lab Med. 136:572–576. 2012. View Article : Google Scholar
|
|
2
|
Strobel O, Neoptolemos J, Jäger D and
Büchler MW: Optimizing the outcomes of pancreatic cancer surgery.
Nat Rev Clin Oncol. 16:11–26. 2019. View Article : Google Scholar
|
|
3
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar
|
|
4
|
Li Z, Ruan Y, Zhang H, Shen Y, Li T and
Xiao B: Tumor-suppressive circular RNAs: Mechanisms underlying
their suppression of tumor occurrence and use as therapeutic
targets. Cancer Sci. 110:3630–3638. 2019. View Article : Google Scholar
|
|
5
|
Zhang F, Zhang R, Zhang X, Wu Y, Li X,
Zhang S, Hou W, Ding Y, Tian J, Sun L and Kong X: Comprehensive
analysis of circRNA expression pattern and circRNA-miRNA-mRNA
network in the pathogenesis of atherosclerosis in rabbits. Aging
(Albany NY). 10:2266–2283. 2018. View Article : Google Scholar
|
|
6
|
Xu Y, Xu X, Ocansey DKW, Cao H, Qiu W, Tu
Q and Mao F: CircRNAs as promising biomarkers of inflammatory bowel
disease and its associated-colorectal cancer. Am J Transl Res.
13:1580–1593. 2021.
|
|
7
|
Wu F, Han B, Wu S, Yang L, Leng S, Li M,
Liao J, Wang G, Ye Q, Zhang Y, et al: Circular RNA TLK1 aggravates
neuronal injury and neurological deficits after ischemic stroke via
miR-335-3p/TIPARP. J Neurosci. 39:7369–7393. 2019. View Article : Google Scholar
|
|
8
|
Ma W, Xue N, Zhang J, Wang D, Yao X, Lin L
and Xu Q: circUBAP2 regulates osteosarcoma progression via the
miR-204-3p/HMGA2 axis. Int J Oncol. 58:298–311. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Zhang J, Zheng T, Mou X and Xin W:
Circ_WWC3 overexpression decelerates the progression of
osteosarcoma by regulating miR-421/PDE7B axis. Open Life Sci.
16:229–241. 2021. View Article : Google Scholar :
|
|
10
|
Huo S and Dou D: Circ_0056285 regulates
proliferation, apop- tosis and glycolysis of osteosarcoma cells via
miR-1244/TRIM44 axis. Cancer Manag Res. 13:1257–1270. 20210.
View Article : Google Scholar
|
|
11
|
Wen Y, Li B, He M, Teng S, Sun Y and Wang
G: circHIPK3 promotes proliferation and migration and invasion via
regulation of miR-637/HDAC4 signaling in osteosarcoma cells. Oncol
Rep. 45:169–179. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Z, Xu W, Zhang D, Chu J, Shen S, Ma
Y, Wang Q, Liu G, Yao T, Huang Y, et al: circCAMSAP1 promotes
osteosarcoma progression and metastasis by sponging miR-145-5p and
regulating FLI1 expression. Mol Ther Nucleic Acids. 23:1120–1135.
2020. View Article : Google Scholar
|
|
13
|
Hall IF, Climent M, Quintavalle M, Farina
FM, Schorn T, Zani S, Carullo P, Kunderfranco P, Civilini E,
Condorelli G and Elia L: Circ_Lrp6, a circular RNA enriched in
vascular smooth muscle cells, acts as a sponge regulating miRNA-145
function. Circ Res. 124:498–510. 2019. View Article : Google Scholar
|
|
14
|
Zheng S, Qian Z, Jiang F, Ge D, Tang J,
Chen H, Yang J, Yao Y, Yan J, Zhao L, et al: CircRNA LRP6 promotes
the development of osteosarcoma via negatively regulating KLF2 and
APC levels. Am J Transl Res. 11:4126–4138. 2019.
|
|
15
|
Zhang Q, Jiang C, Ren W, Li S, Zheng J,
Gao Y, Zhi K and Gao L: Circ-LRP6 mediates epithelial-mesenchymal
transition and autophagy in oral squamous cell carcinomas. J Oral
Pathol Med. 50:660–667. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Zhu W, Tao G and Wang W: Circular
RNA circ-LRP6 facilitates Myc-driven tumorigenesis in esophageal
squamous cell cancer. Bioengineered. 11:932–938. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu Y, Wang L, Li Z, Zheng Y, Shi Z and
Wang G: Long noncoding RNA CRNDE functions as a diagnostic and
prognostic biomarker in osteosarcoma, as well as promotes its
progression via inhibition of miR-335-3p. J Biochem Mol Toxicol.
35:e227342021. View Article : Google Scholar
|
|
18
|
Gulino R, Forte S, Parenti R, Memeo L and
Gulisano M: MicroRNA and pediatric tumors: Future perspectives.
Acta Histochem. 117:339–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Wang Y, Yang H, Zhao L, Song R, Tan
H and Wang L: MicroRNA-873 targets HOXA9 to inhibit the aggressive
pheno- type of osteosarcoma by deactivating the Wnt/β-catenin
pathway. Int J Oncol. 54:1809–1820. 2019.PubMed/NCBI
|
|
20
|
Gao S, Wang J, Tian S and Luo J: miR-9
depletion suppresses the proliferation of osteosarcoma cells by
targeting p16. Int J Oncol. 54:1921–1932. 2019.PubMed/NCBI
|
|
21
|
Feng T, Zhu Z, Jin Y, Wang H, Mao X, Liu
D, Li Y, Lu L and Zuo G: The microRNA-708-5p/ZEB1/EMT axis mediates
the metastatic potential of osteosarcoma. Oncol Rep. 43:491–502.
2020.PubMed/NCBI
|
|
22
|
Sun X, Xu Y, Zhang S, Li X and Wang Y,
Zhang Y, Zhao X, Li Y and Wang Y: MicroRNA-183 suppresses the
vitality, invasion and migration of human osteosarcoma cells by
targeting metastasis-associated protein 1. Exp Ther Med.
15:5058–5064. 2018.PubMed/NCBI
|
|
23
|
Zhao X, Xu Y, Sun X, Ma Y, Zhang Y and
Wang Y, Guan H, Jia Z, Li Y and Wang Y: miR-17-5p promotes
proliferation and epithelial-mesenchymal transition in human
osteosarcoma cells by targeting SRC kinase signaling inhibitor 1. J
Cell Biochem. 120:5495–5504. 2019. View Article : Google Scholar
|
|
24
|
Wang N, Li P, Liu W, Wang N, Lu Z, Feng J,
Zeng X, Yang J, Wang Y and Zhao W: miR-141-3p suppresses
proliferation and promotes apoptosis by targeting GLI2 in
osteosarcoma cells. Oncol Rep. 39:747–754. 2018.
|
|
25
|
Wang L: MiR-141-3p overexpression
suppresses the malignancy of osteosarcoma by targeting FUS to
degrade LDHB. Biosci Rep. Jun 26–2020.Epub ahead of print.
|
|
26
|
Liang Z, Li X, Liu S, Li C, Wang X and
Xing J: MiR-141-3p inhibits cell proliferation, migration and
invasion by targeting TRAF5 in colorectal cancer. Biochem Biophys
Res Commun. 514:699–705. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Zhong JH, Gong FS and Xiao J:
MiR-141-3p suppresses gastric cancer induced transition of normal
fibroblast and BMSC to cancer-associated fibroblasts via targeting
STAT4. Exp Mol Pathol. 107:85–94. 2019. View Article : Google Scholar
|
|
28
|
Jiang J, Sun Y, Xu G, Wang H and Wang L:
The role of miRNA, lncRNA and circRNA in the development of
intervertebral disk degeneration (review). Exp Ther Med.
21:5552021. View Article : Google Scholar :
|
|
29
|
Chen F, He L, Qiu L, Zhou Y, Li Z, Chen G,
Xin F, Dong X, Xu H, Wang G, et al: Circular RNA CircEPB41L2
functions as tumor suppressor in hepatocellular carcinoma through
sponging miR-590-5p. Cancer Manag Res. 13:2969–2981. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang C, Cao J, Lv W and Mou H:
CircRNA_100395 carried by exosomes from adipose-derived mesenchymal
stem cells inhibits the malignant transformation of non-small cell
lung carcinoma through the miR-141-3p-LATS2 axis. Front Cell Dev
Biol. 9:6631472021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang XY, Huang ZL, Zhang PB, Huang XY,
Huang J, Wang HC, Xu B, Zhou J and Tang ZY: CircRNA-100338 is
associated with mTOR signaling pathway and poor prognosis in
hepatocellular carcinoma. Front Oncol. 9:3922019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chao F, Song Z, Wang S, Ma Z, Zhuo Z, Meng
T, Xu G and Chen G: Novel circular RNA circSOBP governs amoeboid
migration through the regulation of the miR-141-3p/MYPT1/p-MLC2
axis in prostate cancer. Clin Transl Med. 11:e3602021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma J, Wu Y and He Y: Silencing circRNA
LRP6 down-regulates PRMT1 to improve the streptozocin-induced
pancreatic β-cell injury and insulin secretion by sponging
miR-9-5p. J Bioenerg Biomembr. 53:333–342. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xue J, Chen C, Luo F, Pan X, Xu H, Yang P,
Sun Q, Liu X, Lu L, Yang Q, et al: CircLRP6 regulation of ZEB1 via
miR-455 is involved in the epithelial-mesenchymal transition during
arsenite-induced malignant transformation of human keratinocytes.
Toxicol Sci. 162:450–461. 2018. View Article : Google Scholar
|
|
35
|
Cai JY, Xu TT, Wang Y, Chang JJ, Li J,
Chen XY, Chen X, Yin YF and Ni XJ: Histone deacetylase HDAC4
promotes the proliferation and invasion of glioma cells. Int J
Oncol. 53:2758–2768. 2018.PubMed/NCBI
|
|
36
|
Xiao Q, Huang L, Zhang Z, Chen X, Luo J,
Zhang Z, Chen S, Shu Y, Han Z and Cao K: Overexpression of miR-140
inhibits proliferation of osteosarcoma cells via suppression of
histone deacetylase 4. Oncol Res. 25:267–275. 2017. View Article : Google Scholar
|
|
37
|
Zeng LS, Yang XZ, Wen YF, Mail SJ, Wang
MH, Zhang MY, Zheng XF and Wang HY: Overexpressed HDAC4 is
associated with poor survival and promotes tumor progression in
esophageal carcinoma. Aging (Albany NY). 8:1236–1249. 2016.
View Article : Google Scholar
|
|
38
|
Cao K, Wang H, Fang Y, Wang Y, Wei L, Chen
X, Jiang Z, Wei X and Hu Y: Histone deacetylase 4 promotes
osteosarcoma cell proliferation and invasion by regulating
expression of proliferating cell nuclear antigen. Front Oncol.
9:8702019. View Article : Google Scholar
|
|
39
|
Wang H, Feng L, Zheng Y, Li W, Liu L, Xie
S, Zhou Y, Chen C and Cheng D: LINC00680 promotes the progression
of non-small cell lung cancer and functions as a sponge of
miR-410-3p to enhance HMGB1 expression. Onco Targets Ther.
13:8183–8196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H, Wang J, Li J, Zhou X, Yin L, Wang
Y, Gu Y, Niu X, Yang Y, Ji H and Zhang Q: HMGB1 is a key factor for
tamoxifen resistance and has the potential to predict the efficacy
of CDK4/6 inhibitors in breast cancer. Cancer Sci. 112:1603–1613.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakamura T, Okui T, Hasegawa K, Ryumon S,
Ibaragi S, Ono K, Kunisada Y, Obata K, Masui M, Shimo T and Sasaki
A: High mobility group box 1 induces bone pain associated with bone
invasion in a mouse model of advanced head and neck cancer. Oncol
Rep. 44:2547–2558. 2020. View Article : Google Scholar :
|
|
42
|
Zhu X, Sun L and Wang Y: High mobility
group box 1 (HMGB1) is upregulated by the Epstein-Barr virus
infection and promotes the proliferation of human nasopharyngeal
carcinoma cells. Acta Otolaryngol. 136:87–94. 2016. View Article : Google Scholar
|
|
43
|
Yuan C and Yang L: Long non-coding RNA
PITPNA-AS1 accelerates the progression of colorectal cancer through
miR-129-5p/HMGB1 axis. Cancer Manag Res. 12:12497–12507. 2020.
View Article : Google Scholar :
|
|
44
|
Guan H, Liu J, Lv P, Zhou L, Zhang J and
Cao W: MicroRNA-590 inhibits migration, invasion and
epithelial-to-mesenchymal transition of esophageal squamous cell
carcinoma by targeting low-density lipoprotein receptor-related
protein 6. Oncol Rep. 44:1385–1392. 2020.
|
|
45
|
Zhang F, Cheng N, Du J, Zhang H and Zhang
C: MicroRNA-200b-3p promotes endothelial cell apoptosis by
targeting HDAC4 in atherosclerosis. BMC Cardiovasc Disord.
21:1722021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu Z, Wang D, Wang X, Zou J, Sun J and Bi
Z: MiR-206 regulates the progression of osteoporosis via targeting
HDAC4. Eur J Med Res. 26:82021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gondaliya P, P Dasare A, Jash K, Tekade
RK, Srivastava A and Kalia K: miR-29b attenuates histone
deacetylase-4 mediated podocyte dysfunction and renal fibrosis in
diabetic nephropathy. J Diabetes Metab Disord. 19:13–27. 2019.
View Article : Google Scholar
|
|
48
|
Malavika D, Shreya S, Raj Priya V, Rohini
M, He Z, Partridge NC and Selvamurugan N: miR-873-3p targets HDAC4
to stimulate matrix metalloproteinase-13 expression upon
parathyroid hormone exposure in rat osteoblasts. J Cell Physiol.
235:7996–8009. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Chu X and Wei Q: MiR-451 Promotes
cell apoptosis and inhibits autophagy in pediatric acute myeloid
leukemia by targeting HMGB1. J Environ Pathol Toxicol Oncol.
40:45–53. 2021. View Article : Google Scholar
|
|
50
|
Feng XE: miR-548b suppresses melanoma cell
growth, migration, and invasion by negatively regulating its target
gene HMGB1. Cancer Biother Radiopharm. 36:189–201. 2021. View Article : Google Scholar
|
|
51
|
Shen H, Xu L, You C, Tang H, Wu H, Zhang Y
and Xie M: miR-665 is downregulated in glioma and inhibits tumor
cell proliferation, migration and invasion by targeting high
mobility group box 1. Oncol Lett. 21:1562021. View Article : Google Scholar
|
|
52
|
Dong H and Song J: miR-142-3p reduces the
viability of human cervical cancer cells by negatively regulating
the cytoplasmic localization of HMGB1. Exp Ther Med. 21:2122021.
View Article : Google Scholar
|
|
53
|
Qiu M, Liu D and Fu Q: MiR-129-5p shuttled
by human synovial mesenchymal stem cell-derived exosomes relieves
IL-1β induced osteoarthritis via targeting HMGB1. Life Sci.
269:1189872021. View Article : Google Scholar
|
|
54
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
55
|
Liu W, Zhang J, Zou C, Xie X, Wang Y, Wang
B, Zhao Z, Tu J, Wang X, Li H, et al: Microarray expression profile
and functional analysis of circular RNAs in osteosarcoma. Cell
Physiol Biochem. 43:969–985. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guan H, Mei Y, Mi Y, Li C, Sun X, Zhao X,
Liu J, Cao W, Li Y and Wang Y: Downregulation of lncRNA ANRIL
suppresses growth and metastasis in human osteosarcoma cells. Onco
Targets Ther. 11:4893–4899. 2018. View Article : Google Scholar
|
|
57
|
Lei S and Xiang L: Up-regulation of
circRNA hsa_circ_0003074 expression is a reliable diagnostic and
prognostic biomarker in patients with osteosarcoma. Cancer Manag
Res. 12:9315–9325. 2020. View Article : Google Scholar
|
|
58
|
Nie WB, Zhao LM, Guo R, Wang MX and Ye FG:
Circular RNA circ-NT5C2 acts as a potential novel biomarker for
prognosis of osteosarcoma. Eur Rev Med Pharmacol Sci. 22:6239–6244.
2018.PubMed/NCBI
|
|
59
|
Kun-Peng Z, Chun-Lin Z, Jian-Ping H and
Lei Z: A novel circulating hsa_circ_0081001 act as a potential
biomarker for diagnosis and prognosis of osteosarcoma. Int J Biol
Sci. 14:1513–1520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Z, Deng M, Chen L, Wang W, Liu G, Liu
D, Han Z and Zhou Y: Circular RNA Circ-03955 promotes
epithelial-mesenchymal transition in osteosarcoma by regulating
miR-3662/metadherin pathway. Front Oncol. 10:5454602020. View Article : Google Scholar :
|
|
61
|
Wan J, Liu Y, Long F, Tian J and Zhang C:
circPVT1 promotes osteosarcoma glycolysis and metastasis by
sponging miR-423-5p to activate Wnt5a/Ror2 signaling. Cancer Sci.
112:1707–1722. 2021. View Article : Google Scholar
|
|
62
|
Yi Y, Liu Y, Wu W, Wu K and Zhang W:
Reconstruction and analysis of circRNA-miRNA-mRNA network in the
pathology of cervical cancer. Oncol Rep. 41:2209–2225.
2019.PubMed/NCBI
|
|
63
|
Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu
S, Wu M, Pan Z and Zhou W: microRNA-22, downregulated in
hepatocellular carcinoma and correlated with prognosis, suppresses
cell proliferation and tumourigenicity. Br J Cancer. 103:1215–1220.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hsieh TH, Hsu CY, Tsai CF, Long CY, Chai
CY, Hou MF, Lee JN, Wu DC, Wang SC and Tsai EM: miR-125a-5p is a
prognostic biomarker that targets HDAC4 to suppress breast
tumorigenesis. Oncotarget. 6:494–509. 2015. View Article : Google Scholar
|
|
65
|
Shen YF, Wei AM, Kou Q, Zhu QY and Zhang
L: Histone deacetylase 4 increases progressive epithelial ovarian
cancer cells via repression of p21 on fibrillar collagen matrices.
Oncol Rep. 35:948–954. 2016. View Article : Google Scholar
|
|
66
|
Zhang Y, Lv F, Qiao L and Zhao Q: MiR-505
inhibits prostate cancer cell invasion, metastasis and
epithelial-to-mesenchymal transition through targeting HMGB-1. J
BUON. 25:2036–2044. 2020.PubMed/NCBI
|
|
67
|
Luan X, Ma C, Wang P and Lou F: HMGB1 is
negatively correlated with the development of endometrial carcinoma
and prevents cancer cell invasion and metastasis by inhibiting the
process of epithelial-to-mesenchymal transition. Onco Targets Ther.
10:1389–1402. 2017. View Article : Google Scholar :
|
|
68
|
Meng Q, Zhao J, Liu H, Zhou G, Zhang W, Xu
X and Zheng M: HMGB1 promotes cellular proliferation and invasion,
suppresses cellular apoptosis in osteosarcoma. Tumour Biol.
35:12265–12274. 2014. View Article : Google Scholar : PubMed/NCBI
|