The tumor microenvironment (TME) is a complex and
special environment consisting of interrelated components and has a
bidirectional impact on tumor growth. Immune cells are the main
component of the TME (1). An
important subset of immune cells in this context are macrophages,
which can change their phenotype and status during tumor
progression. These changes have a dual effect on tumor growth.
Therefore, targeting macrophages as part of an antitumor strategy
is a promising approach.
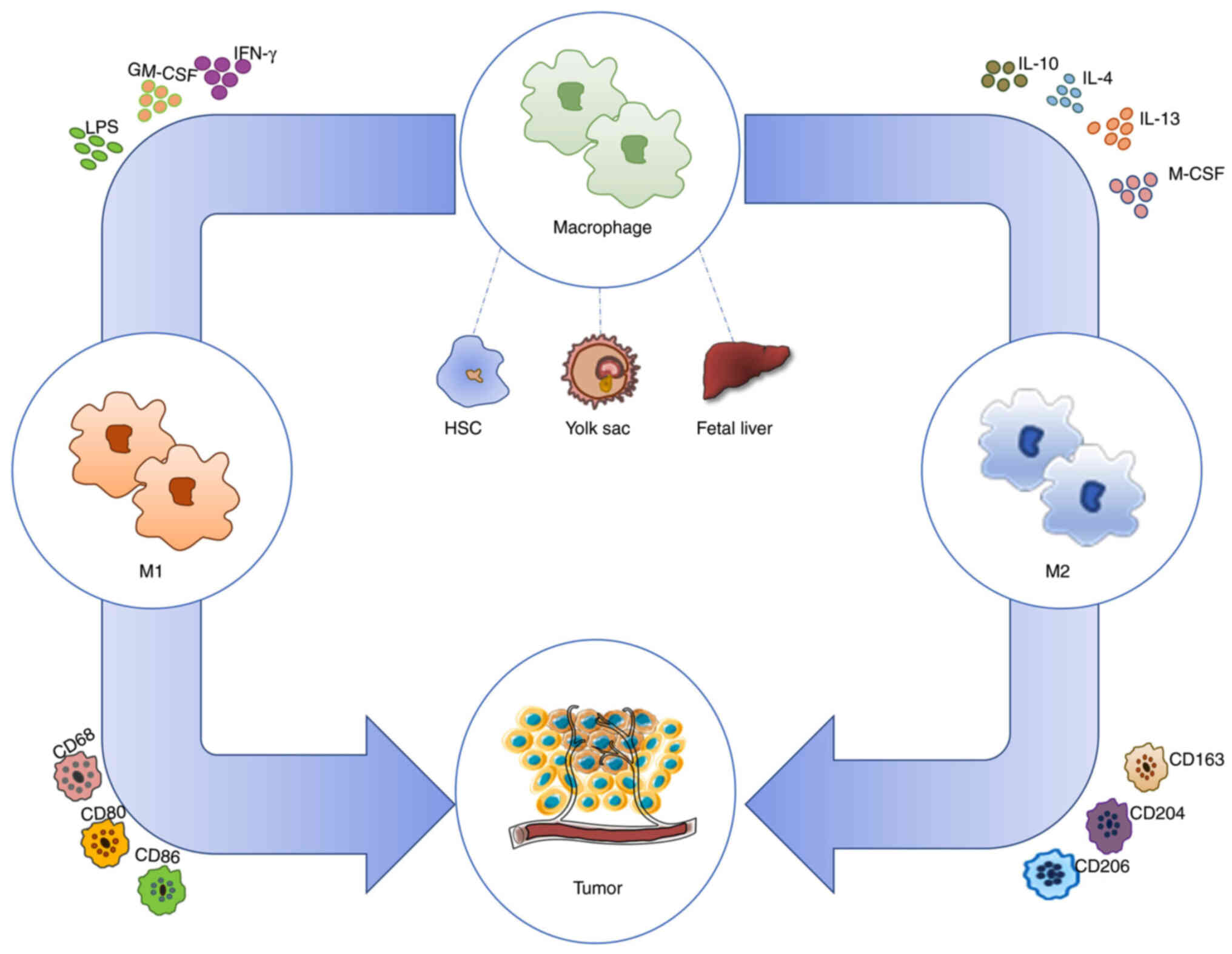
Previously, it was maintained that macrophages
develop from circulating monocytes derived from bone marrow
hematopoietic stem cells. Subsequently, macrophages were found to
originate from the yolk sac or fetal liver in healthy individuals
and to not be involved in bone marrow monocyte circulation during
local self-renewal (2).
Macrophages can be subdivided into three categories. The first one
is monocyte-derived tumor-associated macrophages (TAMs), which are
the main immune cells in the TME. Accounting for approximately half
of tumor-infiltrating immune cells (3), the other two categories are
tissue-resident macrophages and myeloid suppressor cells from the
yolk sac and fetal liver (4).
This review is primarily focused on TAMs.
Studies on macrophage polarization are being
actively conducted, and the prevailing theory in this regard is
that macrophages can be categorized into classically activated M1
macrophages and alternatively activated M2 macrophages on the basis
of interactions of the cytokines secreted by CD4+ T
helper (TH) cell subpopulations (5). Macrophages stimulated by interferon
γ (IFN-γ) and Toll-like receptor (TLR) ligands [e.g.,
lipopolysaccharide (LPS)] or granulocyte-macrophage
colony-stimulating factor (GM-CSF) acquire a distinct
proinflammatory M1 phenotype characterized by the secretion of
proinflammatory cytokines and high levels of major
histocompatibility complex II (MHC II). Production of nitric oxide
and reactive oxygen species (ROS) inhibits tumor growth.
Conversely, TH2 cytokines such as interleukin (IL)-4, IL-13, and
IL-10 polarize macrophages toward the anti-inflammatory M2
phenotype characterized by the production of anti-inflammatory
cytokines that promote tumor progression (6). M2 macrophages further differentiate
into M2a-M2d macrophages according to different stimuli, where M2d
cells are considered TAMs (7)
(Fig. 1).
Macrophages show high plasticity and heterogeneity.
It is generally considered that M1 macrophages play an antitumor
part in the early stages of tumor progression, and gradually
transform into M2 macrophages to stimulate tumor growth. Generally,
TAMs are M2 macrophages (8).
The number and phenotype of macrophages vary at
different stages of tumor progression. The number of macrophages
markedly increases during the early stages of tumor growth
(9). Nonetheless, the association
between macrophages and clinical indicators is still uncertain, and
whether macrophages can be used as prognostic indicators at early
cancer stages remains to be determined. At early stages of lung
cancer, macrophages are significantly recruited to the tumor site
and manifest a mixed phenotype, but no significant association has
been identified with major clinical indicators such as tumor size
and stage (10). With cancer
progression, tumor cells generate relevant signaling molecules to
induce macrophages to recruit themselves to the tumor site and to
polarize toward the M2 phenotype for tumor growth promotion. For
example, colony-stimulating factor 1 (CSF1) produced by tumor cells
can facilitate TAM aggregation and polarization toward the M2
phenotype by inhibiting CD8+ T-cell recruitment to
accelerate the tumor's own growth (11). As a tumor enlarges, macrophages
promote its growth by secreting a series of signaling molecules
including vascular endothelial growth factor (VEGF),
proinflammatory factor IL-6, anti-inflammatory mediator IL-10, ROS
and the corresponding proteases, tumor necrosis factor (TNF), and
transforming growth factor β (TGF-β) (12). Tumor cells stimulate their own
malignant progression by interacting with macrophages, and as the
tumor progresses, macrophages become an indicator of tumor
malignancy and reflect cancer prognosis (8) (Fig.
2).
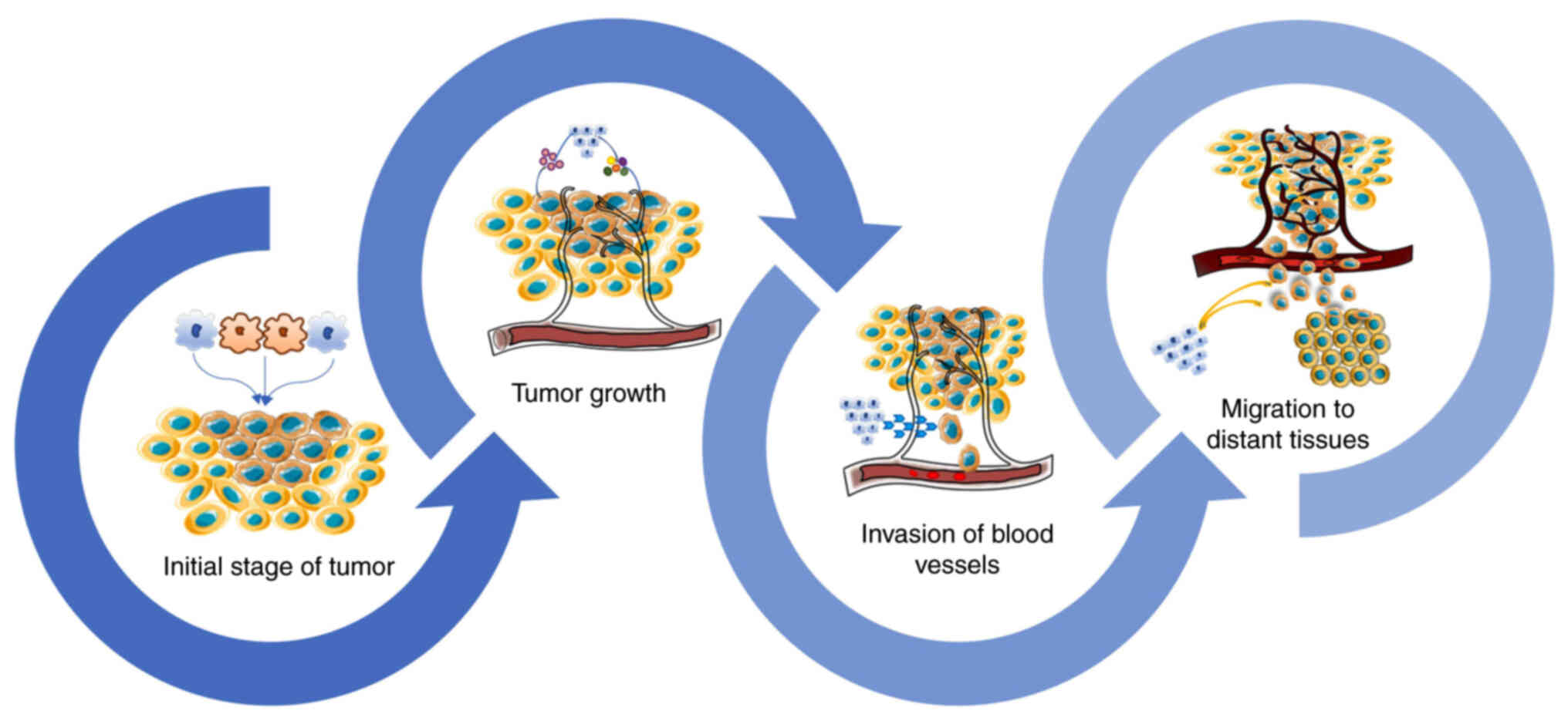
Metastasis is the leading cause of death in patients
with cancer. As a cascade process, metastasis consists of four
steps, i.e., tumor cell detachment from the primary site, invasion
of blood vessels or lymphatic vessels, migration to distant
tissues, and proliferation at the new site (13,14). Epithelial-mesenchymal transition
(EMT) is the first step in epithelial cell carcinoma metastasis
(15). Epithelial cells are
usually involved in human metastases (16) and can acquire the characteristics
of mesenchymal cells at this step (17). Tumor cells can promote EMT by
influencing macrophages. For example, gastric cancer (GC) cells
interact with macrophages by secreting TNF to generate CXCL1 and
CXCL5, which trigger the CXCR2/STAT3 pathway to facilitate EMT and
enable the migration of GC cells (15). GC mesenchymal stem cells (GC-MSCS)
can accelerate metastasis by secreting IL-6 and IL-8 to launch the
JAK2/STAT3 signaling pathway. This event contributes to macrophage
M2 polarization and stimulates EMT (18). Macrophages may participate in
every stage of tumor metastasis. Secreted matrix metalloproteinases
(MMPs) can help tumor cells degrade the extracellular matrix (ECM).
In addition, MMP2 and MMP9 can promote the production of new tumor
blood vessels and facilitate metastasis in many respects (19). Tumor cells penetrate blood vessels
and enter the bloodstream as circulating tumor cells (CTCs)
(20). In one study, mouse T241
fibrosarcoma cells and Lewis lung carcinoma-derived macrophages
activated by IL-6 and TNF were coimplanted in zebra fish, and this
experiment confirmed that M2 macrophages promote metastasis by
driving endosmosis, suggesting that M2 macrophages are necessary
for the intravascular metastatic stage (21). As CTCs enter distant tissues, the
complex interaction between tumor and target tissues results in the
establishment of a microenvironment that is conducive to the
survival of CTCs, which is a key step for colonization by tumor
cells. TAMs are implicated in the formation of a premetastatic
niche (22).
Angiogenesis is a crucial factor in metastasis.
There is evidence that the degree of tumor angiogenesis is
positively correlated with the number of M2 macrophages in the
tumor. Macrophages are major promoters of angiogenesis in the TME
and act by secreting vascular growth factors and MMPs (23,24). Common proangiogenic factors
include VEGF, epidermal growth factor (EGF), placental-derived
growth factor (PlGF), platelet-derived growth factor, TGF-α and
TGF-β, and angiogenins 1 and 2. Among them, the top proangiogenic
factors are PlGF and members of the VEGF family, i.e., VEGF-A,
VEGF-B, VEGF-C, and VEGF-D (14).
In triple negative breast cancer, macrophages can secrete VEGF to
produce PCAT6, which upregulates VEGFR2 to accelerate angiogenesis
and facilitate metastasis (25).
MMPs can degrade all components of the ECM, among which MMP2, MMP9
and MMP14 are closely associated to tumor angiogenesis (26). Changes in the number and activity
of macrophages can affect protease production, reduce angiogenesis,
and slow tumor growth. By infecting tumor-bearing mice with
Plasmodium, Wang et al revealed that
Plasmodium hemozoin can reduce the number of infiltrating
TAMs and decrease the expression of MMP9 and MMP2, thus suppressing
tumor angiogenesis and slowing down metastasis and tumor growth
(27).
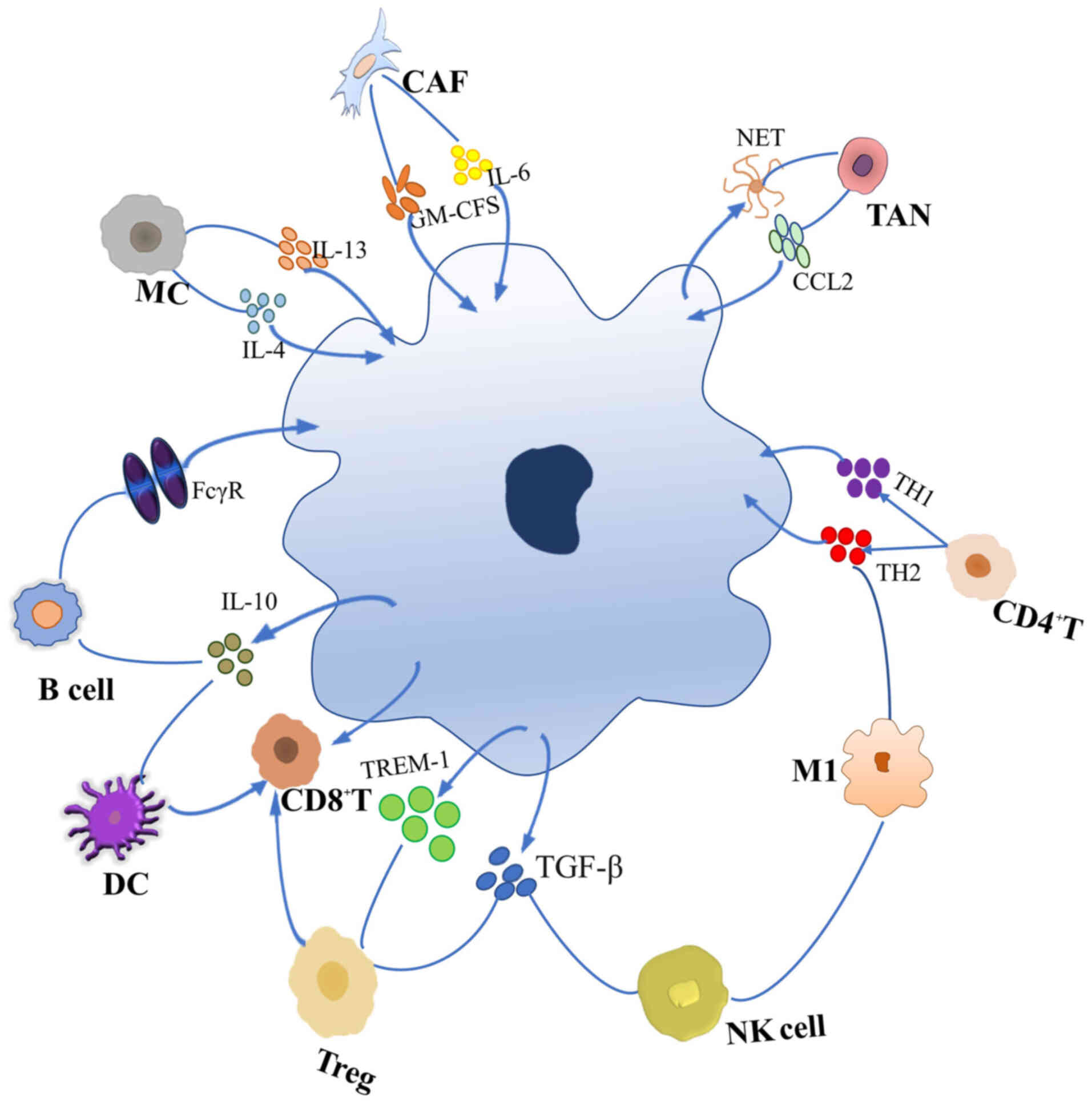
The TME serves as a place where tumor cells and
stromal cells can interact, including fibroblasts, endothelial
cells, and both innate- and adaptive-immunity cells (28). The migration of leukocytes into
the TME gives rise to the tumor immune microenvironment (29). Macrophages constitute half of
these leukocytes and play a major role in the TME (30). Therefore, focusing on the
interactions between macrophages and other cells in the TME offers
unique opportunities for cancer treatment (Fig. 3).
CAFs are the most abundant nontumor cells in the TME
and perform a prominent function in tumor growth and metastasis.
CAFs can simultaneously affect macrophage recruitment and
polarization toward the M2 phenotype (31). CAFs are recruited and attach to
macrophages under the influence of endostatin in hepatocellular
carcinoma (HCC) and secrete GAS6 to promote macrophage M2-type
polarization; injection of human antibody IgG78 into tumor-bearing
mice specifically attenuated the impact of endostatin on the
interaction of CAFs with macrophages, thereby slowing tumor growth
(32). When cultured in
vitro, CAFs from different tumors have been found to produce a
large number of cytokines to drive the differentiation of monocytes
into M2 macrophages; among these cytokines, the presence of the
most representative factors IL-6 and GM-CSF has been confirmed in a
variety of tumors, and a combination of these two factors can
promote M2 polarization of TAMs and affect cancer prognosis
(33). The ECM, a set of
connective substances produced by CAFs, primarily consists of
special fibrin, and is related to all steps of metastasis. As the
most important stromal cells, CAFs determine the hardness and
structure of the ECM. The interaction between CAFs and TAMs can
contribute to ECM remodeling and tumor metastasis (34). There have been few studies on the
effect of TAMs on CAFs. Nonetheless, a positive correlation between
the numbers of TAMs and CAFs has been found in prostate cancer, and
macrophages can induce the transformation of stromal fibroblasts
into CAFs (31). Macrophages
stimulate fibroblast activation via paracrine production of TGF-β1
in nontumor tissues. MMP9 production promotes fibroblast migration
(35,36). The interaction between these two
factors can synergistically accelerate tumor progression. In
neuroblastomas, TAMs are mainly distributed near CAFs, and the two
cell types can promote one another and jointly influence tumor
growth (37). CAFs and TAMs
jointly affect the clinical stage and prognosis of patients with
cancer. In oral squamous cell carcinoma, CAF grade was found not
only to be an independent prognostic factor of tumor progression
but also to accelerate tumor progression by influencing the number
and phenotype of TAMs and by creating an immunosuppressive TME
(38). Tumor cells can also
promote their own growth by influencing both CAFs and TAMs.
Exosomes derived from colorectal cancer (CRC) cells can reduce the
secretion of substances by fibroblasts and convert them into CAFs,
stimulating the polarization of macrophages toward the M2
phenotype, thereby promoting CRC cell growth (39).
The two cell types mutually facilitate tumor growth,
and their interplay results in the emergence of immunosuppressive
activities, suggesting that a better understanding of their action
is necessary for exploring effective antitumor strategies that are
based on the targeting of macrophages.
The immune cells in the TME include innate- and
adaptive-immunity cells. Innate-immunity cells include macrophages,
mast cells (MCs), neutrophils, dendritic cells (DCs), myeloid
inhibitory cells, and natural killer (NK) cells. Adaptive-immunity
cells include T and B cells (40).
After TAMs, T cells are the most important immune
cells in the TME. They can recognize tumor-associated antigens and
play a key role in tumor destruction (41). T cells differentiate into
different subtypes with different immune functions according to the
surface proteins and cytokines produced (42).
Tregs can interact with TAMs in a variety of ways,
and the crosstalk between Tregs and TAMs suggests that the
interaction between macrophages and other components of the TME
should be considered carefully. To maximize the therapeutic effect,
joint targeted measures should be taken instead of targeting only
macrophages.
Thus, it can be concluded from the existing
literature that while TAMs reduce the activity of CD8+ T
cells, CD8+ T cells can kill TAMs. Finding the
equilibrium point of their interaction can reduce tumor immune
escape and improve the efficacy of immunotherapy.
Mature DCs link the innate immune system to the
adaptive immune system through their unique cross-rendering
functions; as the main antigen-presenting cells, they can
internalize extracellular antigens and provide them to
CD4+ T cells, present tumor antigens to CD8+
T cells, and promote the activation of cytotoxic T lymphocytes. The
association between DCs and the activation of T cells forms the
basis of tumor immunotherapy (57,58). DCs have different subtypes,
including plasmacytoid DCs, conventional DCs (cDC1 and cDC2 cells),
and monocyte-derived DCs. Among these, cDC1 cells are the main
antigen-presenting cells and are especially important for the
activation of CD8+ T cells (59,60). Single-cell sequencing has revealed
that TAMs and cDCs form the core network of cellular action in
tumors (61). To date, DC studies
have largely dealt with DC vaccines, but DC vaccines have poor
immunogenicity. On the other hand, various TAM inhibitors combined
with a DC vaccine can improve the efficacy of the latter. In a
mesothelioma mouse model, a TAM inhibitor combined with a DC
vaccine was found to increase the infiltration by CD8+ T
cells, reduce PD-L1 expression, and enhance TAM depletion.
Improving the TME composition increases the efficacy of antitumor
immunity (62). IL-10 also
affects the immunogenicity of DCs, and TAMs are the main source of
IL-10 for breast cancer cells. IL-10 attenuates the activation of
CD8+ T cells by reducing the production of IL-12 by DCs,
thereby affecting the efficacy of chemotherapy. In a colon cancer
model, a combination of a TAM inhibitor, IL-10 antagonist, and DC
vaccine significantly increased CD8+ T-cell infiltration
and optimized tumor shrinkage (63,64).
A combination of a DC vaccine and TAM inhibitor has
been shown to significantly improve the immunogenicity of DC
vaccines. The combination of the two modalities offers another
feasible approach to the targeting of TAMs for improving the
efficacy of antitumor immunity in the future.
NK cells are a part of the innate immune system and
can directly target and kill tumor cells without sensitization. By
secreting cytokines, NK cells can promote mutual crosstalk of
immune cells in the TME, and conversely, the cytokines in the TME
can reduce the killing ability of NK cells (65). NKs can interact with macrophages
in different polarization states. In coculture of TAMs (or PECs),
bone marrow-derived M2 macrophages, and NK cells, TAMs produced a
large amount of TGF-β and reduced the expression of CD27 in NK
cells through cellular contact, thus altering the phenotype of NK
cells; the CD27low NK cells have a higher activation
threshold and poor cytotoxicity (66). Coculture of M1-type or LPS-treated
M0 and M2 macrophages with resting NK cells can increase IFN-γ and
CCR7 production by means of IL-18. CCR7 can promote NK cells to a
lymph node metastasis and upregulate their cytotoxicity, and the
activated NK cells kill the remaining TAMs (67). TAM status can be affected by
enhancement of NK cell activity during treatment, and TAMs, as an
essential component of the TME, correlate with cancer prognosis. In
a melanoma mouse model, anti-MARCO antibodies improved prognosis by
activating NK cells and increasing their activity in lymph node
metastases thereby driving the M1-type polarization of macrophages
(68). Sorafenib is a tyrosinase
inhibitor. In coculture of NK cells with TAMs, sorafenib could
stimulate the production of proinflammatory cytokines, promote NK
cell migration to TAMs, increase NK cell degranulation and IFN-γ
secretion by TAMs, and amplify the killing ability of NK cells
(69).
Because of the complexity of the TME, NK cells
cannot become fully active and cannot exert a cytotoxic action
there, and macrophages with different polarization states have
different effects on NK cell activity. Incorporating NK cells into
macrophage-reprogramming therapy can strengthen its suppressive
influence on tumors.
Neutrophils make up the largest class of circulating
myeloid leukocytes, can quickly respond to invasive pathogens, and
are the first line of immune defense (70). In the past, neutrophils have not
been considered a significant factor in tumor growth due to their
short cell cycle. Subsequent studies have revealed that the cell
cycle of neutrophils in the TME is significantly prolonged, and
tumor cell-derived cytokines and/or chemokines contribute to the
TME accumulation of neutrophils in vivo (71,72). Similar to M1 and M2 macrophages,
neutrophils can be categorized into N1 and N2 populations based on
their different functions. TGF-β expression can render them prone
to differentiation into tumor-promoting N2 cells, whereas IFN-β can
contribute to the conversion of TANs into the antitumor N1 type
(73,74). Most data indicate that TANs can
promote tumor growth, but invasive TANs are positively correlated
with prognosis in only a small number of tumor types (75). Neutrophils affect tumor growth
mainly by secreting proteases, generating ROS, altering
angiogenesis, and speeding up metastasis. TANs and TAMs have
similarities and play partially overlapping roles in tumor
progression, which means that they can jointly enhance tumor
growth. Both are almost always found in cluster intrahepatic
cholangiocarcinoma tissues, increase downstream target expression,
and stimulate tumor growth and metastasis by generating Oncostatin
M and IL-11 to trigger the STAT3 pathway (76). TANs and TAMs are both important
sources of MMPs in primary tumors, and MMP2 and MMP9 are closely
related to tumor angiogenesis (19). Nevertheless, a quantitative
association between TAMs and TANs in tumors has not been clearly
identified, and a negative correlation has been found in TNBC: A
decrease in the number of TAMs accompanied by an increase in the
number of TANs (71). In an HCC
mouse model, the tumor volume and the metastatic rate in mice
injected with TANs and tumor cells was demonstrated to be
significantly increased as compared with the mice injected with the
tumor cells alone. TANs can secrete CCL2 and CCL17 to recruit
macrophages and Tregs in order to induce tumor invasion and
angiogenesis and increase tumor microvascular density, thereby
accelerating tumor growth. Experiments performed in vitro
indicate that TANs can recruit macrophages in the same way
(77). Neutrophil-derived CSF1
can promote the polarization of macrophages toward an
immunosuppressive (Ly6Clow/M2) phenotype in a nontumor
model resulting in transplant tolerance in mice (72). In addition, activated neutrophils
can release a special form of reticular ultrastructure, which is
mostly composed of DNA and granular proteins that can assemble into
special structures called neutrophil extracellular bactericidal
networks (NETs). These can be formed after neutrophil necrosis or
apoptosis to combat microorganisms. Macrophages of different
phenotypes can dissolve NETs, and the proinflammatory type (M1) has
the strongest effect on the dissolution of NETs (78).
In view of their overlapping sources and functions,
as well as the uncertainty about their mutual influence in the TME,
the correlation between TAMs and TANs in the TME cannot be
exploited at present. Nonetheless, the interaction between the two
cell types occurs in terms of almost every parameter of tumor
growth, and research on the methods for strengthening the
interaction between the two can make their crosstalk useful for
improving the efficacy of cancer treatment.
MCs were first considered to be the primary effector
cells of allergic reactions, and are mainly distributed in tissues
and at the junction point of the host. In a tumor, MCs are mostly
located at the edges of the tumor or near blood vessels; according
to protease expression in MC granules, MCs are subdivided into two
categories, namely, MCTs expressing only trypsinlike proteases and
MCTCs expressing trypsinlike, chymotrypsin, and other proteases
(79,80). MC population is highly
heterogeneous and performs a dual function in tumor growth, but MCs
largely contribute to tumor growth by influencing angiogenesis. In
tumors, the interaction between MCs and macrophages is primarily
manifested in the recruitment of macrophages (81) and induces the polarization of
macrophages toward the M2 phenotype through the production of
cytokines IL-4 and IL-13 (82).
On the one hand, the association between the number and activity of
MCs and TAMs in different tumors is unclear. It is reported that a
large number of MCs in lymphoma can suppress TAM activity and
reduce the promotion of the TAMs involved in tumor growth (83). On the other hand,
immunohistochemical analysis of CRC tissues from patients has shown
that there is a positive correlation between the numbers of MCs and
TAMs (84), while there is no
significant association between the two numbers in oral squamous
cell carcinoma (85). Considering
that both cell types are highly heterogeneous, they have dissimilar
influences on tumor growth in different tumors due to the disparity
in the number and state of infiltrating cells in the TME as well as
in cancer stage and in the location of macrophages and MCs in the
tumor. Nonetheless, because both are closely related to tumor
angiogenesis, there is a distinct association between the number of
both types of cells and new angiogenesis in various tumors. For
example, both MC and TAM counts were independent of angiogenesis in
non-small cell lung carcinoma (86). In HCC, however, both counts were
positively correlated with new angiogenesis, with TAMs highly
correlating with new angiogenesis (87).
Due to the high heterogeneity of the two cell types,
targeted measures can be taken during cancer treatment according to
the correlation between the two in a given tumor, and the
inhibition of tumor neovascularization can be better utilized to
reduce metastasis.
B cells play a crucial role in adaptive immunity
because they can present antigens to T cells. They can also produce
immunoglobulins and have an immunomodulatory influence on antitumor
immunity (88). B cells can exert
different actions on tumors depending on their phenotypes and
interactions with other components of the TME. IL-10 is key for the
ability of B cells to influence the phenotype of macrophages and
can accelerate the polarization of macrophages toward the M2
phenotype without affecting the number of macrophages (89). Depletion of B cells was revealed
to promote the M1-type repolarization of macrophages in a mouse
model of squamous cell carcinoma (88). Macrophages upregulate CD40/CD40L
via the TLR4-MyD88 pathway through cell-to-cell contact with B
cells to support the activation of tumor exosomes and enhance the
antigen-presenting function of B cells (90). Because B cells are the source of
some hematological cancers, the depletion of B cells by antibodies
binding to the B-cell-specific surface molecules, CD19 and CD20,
has become an important treatment method.
By expressing FcγR, macrophages interact with
immunoglobulins produced by B cells thereby affecting the depletion
of B cells by the anti-CD19 and anti-CD20 therapy. Therefore,
altering the number and activity of macrophages during treatment
can affect the therapeutic effects of monoclonal antibodies
(91). Upregulation of type I IFN
gene expression by stimulator of interferon genes (STING) in
lymphoma increases the production of type I IFN and enhances
macrophage phagocytosis in vitro and in vivo. As a
consequence, the FcγR A:I ratio of macrophages is increased and the
depletion of B cells by anti-CD20 therapy is enhanced (92).
B cells, as independent regulators of the macrophage
phenotype, can be used in relatively independent therapeutic
approaches for the treatment of relevant tumors, and the
interaction between B cells and macrophages is expected not only to
improve the efficacy of monoclonal antibodies but also to become a
major method for reprogramming macrophages.
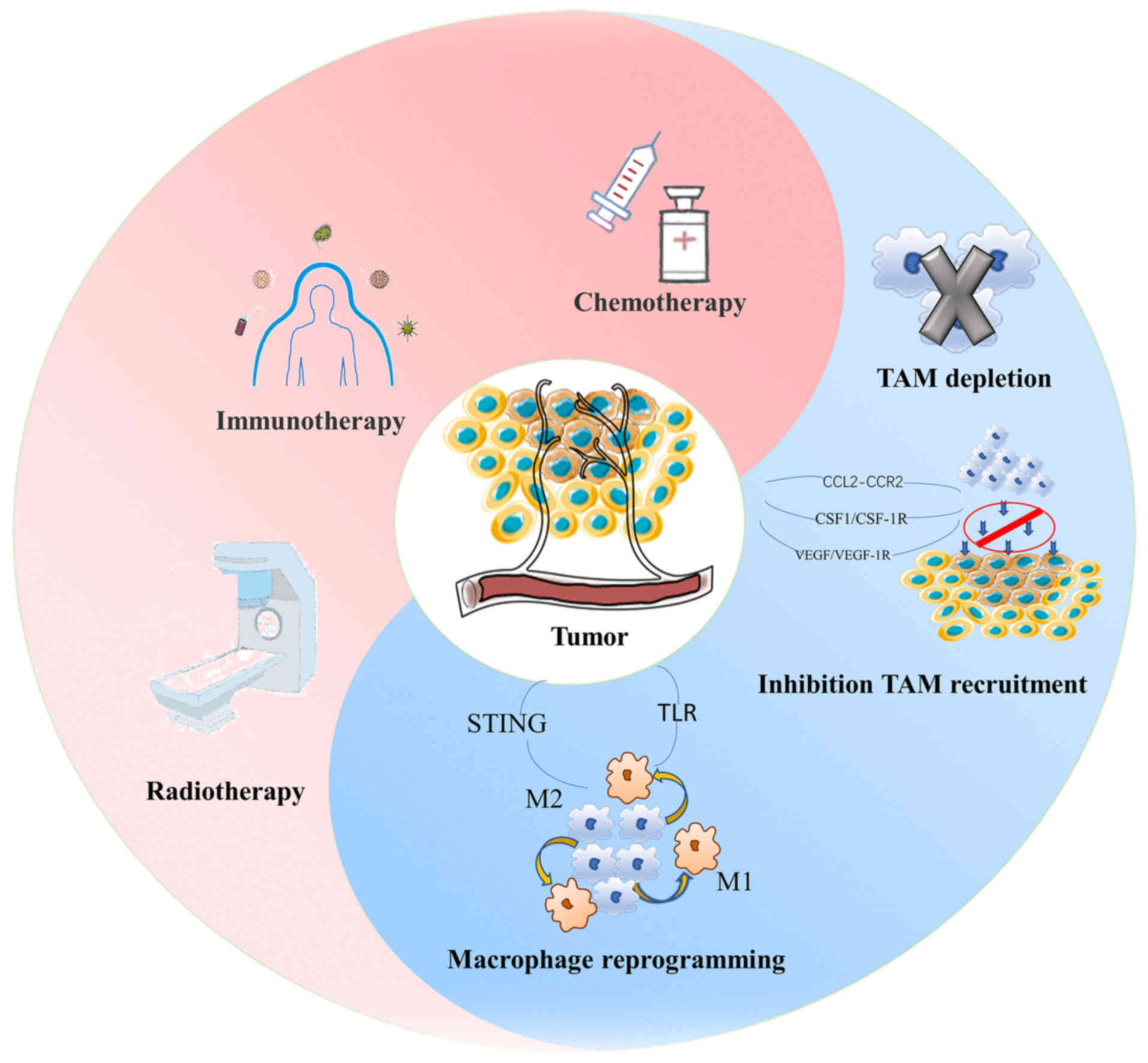
Based on the impact of macrophages on tumor
progression, current treatment strategies targeting macrophages in
tumors are mainly focused on altering macrophage recruitment,
promoting M1-type polarization of macrophages, depletion of
macrophages, delivery of antitumor drugs through macrophages, and
on using these cells in combination with other therapies (93).
In terms of the TME, TCM can slow down tumor growth
by affecting the activity of immune cells; hence, TCM can change
the composition of the TME (94).
The effect of TCM on macrophages largely manifests itself by
altering the polarization state of macrophages and inhibiting the
recruitment of macrophages to tumors (95).
M2 macrophages in tumor tissues are associated with
a poor prognosis. Inhibition of the polarization of macrophages
toward the M2 phenotype or activation of M1 macrophages is a major
method for targeting macrophages during cancer treatment (96). Monotherapies and complex
formulations of TCM affect tumor growth by regulating the M1/M2
ratio in the TME. For example, Tripterygium wilfordii can
slow tumor growth by affecting apoptosis, angiogenesis, and drug
resistance, among other effects. Triptolide, the active ingredient
of T. wilfordii, can inhibit macrophage M2 polarization
through dose-dependent cytotoxicity in vivo and in
vitro. It also reduces the expression of IL-10 and TGF-β1 in
vivo and in this way decreases the production of TH2 cytokines.
Thus, the M2 polarization of TAMs is blocked thereby weakening the
recruitment of TAMs to the tumor matrix and diminishing tumor
growth (97). In addition,
rhubarb contains emodin, a natural anthraquinone derivative. Emodin
reduces lung infiltration by M2 macrophages by inhibiting STAT6 and
C/EBPβ pathways and attenuates lung metastasis of breast cancer in
mice (98). Ginsenosides can
regulate the communication between macrophages and lung cancer
cells when the two cell types are cocultured, they can reduce the
protein expression of VEGF, MMP2, and MMP9 and they can repolarize
M2 macrophages into the M1 phenotype to slow tumor growth and
metastasis (99). The clinical
dose of sorafenib can effectively inhibit tumor growth but has
strong adverse effects, whereas a subclinical dose exerts milder
adverse effects but has poor antitumor properties. Combining an
injection of a compound kushen injection with a low dose of
sorafenib in the treatment of HCC results in an increase of the
M1/M2 ratio. The TME is remodeled upon the triggering of the TNF
receptor TNFR1 and the downstream launch of NF-κB and MAPK P38
pathways, and these events cause the production of soluble
molecules that indirectly increase the number and activity of
CD8+ T cells. These phenomena lead to an improvement of
the antitumor action of low-dose sorafenib and a reduction in its
adverse effects (100).
Yupingfeng powder, composed of Astragalus membranaceus,
Atractylodes atractylodes, and Fangfeng, increases STAT1
phosphorylation in a dose-dependent manner, contributes to the M1
polarization of TAMs and to the remodeling of the TME, enhances the
antigen-presenting function of M1 macrophages, and promotes the
degranulation of CD4+ T cells. As a consequence, the
growth of tumor cells is slowed down, and the survival of
tumor-bearing mice is prolonged (101).
TAM recruitment to tumors can enhance the stemness
of cancer stem cells and accelerate metastasis (102). Monocyte precursors are the main
source of TAMs, and inhibition of monocyte recruitment into tumor
tissue and of the subsequent influence of macrophage infiltration
is one approach to TAM-targeting therapy (103). By influencing the levels of
chemokines, growth factors, and CSFs produced by cancer cells and
stromal cells in the TME, the recruitment of monocyte macrophages
by tumors can be suppressed (104). Studies (105-107) on the effect of TCM on macrophage
recruitment have mostly addressed the influence of macrophage
recruitment through the CCL2-CCR2 axis. CCL2 is also called
monocyte chemotactic protein-1, and its expression is associated
with metastasis and macrophage recruitment. The corresponding
receptor CCR2 is located on the surface of TAMs, and TCM can
effectively inhibit metastasis by acting on the CCL2-CCR2 axis to
reduce macrophage recruitment (31). Dahuang Zhechong pill was revealed
to significantly reduce the expression of CCL2 in the liver of CRC
tumor-bearing mice, decrease macrophage recruitment, alleviate
liver fibrosis, destroy the premetastatic niche, and diminish
metastasis (105,106). Dihydroisotanshinone I, an active
ingredient of Salvia miltiorrhiza, can reduce the expression
of CCL2 in THP-1 cells or RAW 264.7 cells cocultured with lung
cancer cells, it can inhibit the recruitment of macrophages by
tumor cells, and it can hinder the migration of lung cancer cells
(107).
The number of TAMs in the TME, their polarization
state, and its progression are closely related to cancer prognosis,
and research aimed at macrophage-based tumor targeting has recently
begun. The impact of TCM on macrophages for reshaping the TME is an
effective antitumor strategy targeting macrophages. However, there
are few studies on the specific mechanisms by which TCM acts on
macrophages to cause the observed antitumor effects. Therefore,
because changing the status of macrophages through TCM to reshape
the TME can be regarded as an important means of targeting
macrophages, further research in this area would be warranted.
With respect to the influence of macrophages on
tumors, the approach of conventional medicine has consisted of
targeting macrophages to treat tumors from two perspectives: Either
inhibiting or supporting the presence of macrophages in tumors.
Based on the tumor-promoting properties of M2
macrophages, selective depletion of TAMs and retention of other
macrophage subtypes in the TME are expected to alter the
immunosuppressive properties of the TME and its resistance to
treatment. Liposomes can engulf mouse M2 macrophages, and the
number of lymphatic vessels and blood vessels in mice treated with
chlorophosphonate-containing liposomes was reduced along with the
number of macrophages (108).
Chlorophosphonate liposomes can also engulf macrophages and
suppress angiogenesis in tumor-bearing mice but affect not only M2
macrophages but also tissue macrophages and other immune cells
(109,110). Considering that
chlorophosphonate cannot selectively and completely target
macrophages, the development of a strategy for selective depletion
of macrophages is a relevant research direction in the field of
macrophage-targeted therapies. In mice with PD-1-resistant
melanoma, selective killing of CD163+ macrophages by
doxorubicin-bound antibody-conjugated lipid nanoparticles abrogated
tumor growth without affecting the total number of macrophages
(111). Bivalent T-cell engagers
specifically recognize M2 macrophage markers and redirect
endogenous T cells to M2 macrophages. In malignant ascites, FRβ
bivalent T-cell engager therapy significantly increases the number
of T cells that specifically deplete M2 ascites macrophages and
reshape the TME (112). Although
the combination with nanomaterials can better kill TAMs
selectively, there is uncertainty about the long-term consequences
of the TME alterations after macrophage depletion in tumors; for
example, studies show that when macrophages are depleted,
monoclonal antibodies cannot deplete B cells (113); further research is needed to
determine whether the strategy involving macrophage depletion can
be used in the clinic.
There are three major pathways that inhibit
macrophage recruitment, and one of them is the CCL2 signaling
cascade. Inhibition of CCL2 and its receptors can effectively
suppress macrophage recruitment and reduce metastasis. As the
receptor for CCL2, CCR2 is also associated with macrophage
recruitment and polarization. By combining a CCR2-targeted
single-chain variable fragment (SCFv) antibody with polyvalent
nanoparticles, investigators have demonstrated that high-priced
58C-SCFV can suppress macrophage recruitment to the maximum extent
possible and can support M2 macrophage repolarization into the M1
phenotype (114). Nevertheless,
suppression of the CCL2 pathway may accelerate metastasis. In
breast cancer, inhibition of CCL2 can isolate monocytes in bone
marrow and effectively reduce metastasis, but cessation of CCL2
inhibition can release monocytes into bone marrow. These monocytes
migrate to a tumor site, and with increased IL-6 levels resulting
in a VEGF-A release and elevated angiogenesis, these cells lead to
rapid metastasis (115).
Strategies involving targeted CCL2- or CCR2-based suppression of
macrophage recruitment should take into account the rapid
recurrence of metastasis after discontinuation of anti-CCL2
therapy; combining this approach with nanomultivalent targeting to
improve the effect of macrophage recruitment through the CCL2-CCR2
axis should also be considered. Tumor cells secrete macrophage CSF
(M-CSF) to recruit macrophages to the tumor; therefore, the CSF1R
axis is another effective route for altering macrophage
recruitment. In sarcomas, pexidartinib (PLX3397), a CSF1-CSF1R
signaling inhibitor, reduces macrophage recruitment, remodels the
TME, and slows tumor growth and metastasis (116). In a mouse model of melanoma,
this CSF1R antagonist (PLX3397) was found to reduce the number of
TAMs, and a combination with CD8+ T-mediated
immunotherapy significantly delayed the action of tumor
growth-enhancing intratumor T cells (117). Similarly, CSF1R antagonists have
greater efficacy when applied in combination with other therapies.
The CSF1-CSF1R axis has significant effects on macrophage
recruitment and tumor growth suppression, and the combination of
CSF1R inhibitors with other therapies can delay tumor growth to a
greater extent. VEGF is also associated with macrophage recruitment
and polarization. VEGFR-1 is a tyrosine kinase receptor that
induces the recruitment of TAMs and promotes tumor growth after
upregulation of VEGF-A, VEGF-B, or PlGF (118). VEGF-A has been shown to affect
monocyte macrophage recruitment via VEGF-R1 in melanoma models
(119). VEGF-1 inhibitors can
not only reduce macrophage infiltration in tumors but also enhance
tumor suppression when used in combination with an immune
checkpoint inhibitor (ICI) (71).
Combining the method influencing macrophage recruitment and
remodeling of the TME through VEGF with other therapies may become
a major breakthrough for future combination therapies based on the
targeting of macrophages.
Repolarizing M2 macrophages into M1 macrophages is a
more promising approach than the depletion of TAMs. Toll-like
receptors (TLRs), as sensors in innate immunity, represent an
essential pathway for macrophage reprogramming and influence
inflammatory pathways through corresponding connexins. TLR7, as an
important component of this pathway, can diminish IL-10 production
by delivering LET-7-equivalent TLR7 agonists and by reprogramming
macrophages to reshape the TME (120). Compared with TLR agonists, STING
agonists can not only regulate FcγR expression in vitro but
also reverse the inhibitory FcγR spectrum in vivo, thereby
improving the triggering of FcγR on TAMs and effectively
stimulating macrophage reprogramming (92). Alteration of metabolic patterns of
macrophages is an essential method of their reprogramming. M1 and
M2 macrophages have different metabolic phenotypes, and M2
macrophages obtain energy mainly through fatty acid oxidation and
oxidative phosphorylation. Inhibition of M2 macrophage-related
metabolic pathways can implement macrophage reprogramming. For
instance, via upregulation of RIPK3 in tumor tissues in HCC, ROS
production can be increased and PPAR lysis can be promoted, thereby
effectively suppressing fatty acid metabolism and reducing the
polarization of macrophages toward the M2 phenotype and slowing
tumor progression (121).
Dimethyl malonate treatment of tumor-bearing mice can block
succinic acid production in the oxidative phosphorylation pathway,
reduce macrophage conversion to the M2 phenotype, and delay tumor
growth (122). In addition,
proliferation and polarization of macrophages can be changed by
treatments affecting macrophage RNA. In mice with Dicer1 deletions,
upregulation of M1 macrophage-related cytokines by
cytotoxic-T-lymphocyte-derived IFN-γ and increasing the proportion
of M1 macrophages have been shown to improve tumor suppression
(123). A combination of
macrophage-targeting drugs with various vectors can better
influence the reprogramming of macrophages. For instance, combining
a STAT3 inhibitor called corosolic acid with long-circulating
liposomes to form corosolic acid-long-circulating liposomes acting
on human macrophages can reduce STAT3 expression in
CD163+ macrophages to a greater extent, diminish IL-10
production, and promote the switching of macrophages to the M1
phenotype (124). A combination
with nanoparticles to reprogram macrophages can improve the degree
of reprogramming, and a combination of a photosensitizer and
nanoparticles can alter the polarization state of macrophages by
increasing ROS production and increasing T-cell infiltration to
enhance tumor inhibition (125).
Iron oxide nanoparticles can induce macrophage repolarization and
decelerate tumor growth; in particular, negatively charged iron
oxide nanoparticles can maximize the conversion of M2 macrophages
to the M1 type (126). A CpG
oligodeoxynucleotide (CpG ODN), which is a TLR9 agonist, can
contribute to the repolarization of the macrophages, however it is
prone to inflammatory processes in vivo due to its failure
to penetrate cell membranes. In one study CpG ODN was encapsulated
by the strong acidic nanocellular carrier ferritin and an M2
macrophage-targeting peptide. Intravenous administration of CpG ODN
slowed down tumor growth in tumor-bearing mice and switched
macrophages from the M2 to M1 type. Of note, it emerged that this
method can reverse the phenotype of human macrophages, and there is
hope that it can be translated to clinical practice (127). The combination of
macrophage-specific targeting ligands and various carrier materials
pushes the reprogramming to new heights. The combination of
nanomaterials can implement the reprogramming of macrophages to a
greater extent and is expected to gain popularity in clinical
practice; this is a key research direction for the reprogramming of
macrophages in the future.
Existing antitumor strategies targeting macrophages
are mainly based on the functional characteristics of macrophages.
These strategies have good efficacy either alone or in combination
with other therapies.
Routine therapies of tumors mainly include
chemotherapy, radiotherapy and immunotherapy. Macrophages undergo
known changes during conventional tumor treatments, and synergistic
effects may be achieved by targeting macrophages to perform
relevant adjustments (Fig.
4).
As the most common cancer treatment, chemotherapy
not only exerts cytotoxic actions on tumor cells but also causes a
woundlike reaction in the injured tissue, drives a release of
cytokines altering immune-cell infiltration of the TME, and
contributes to the aggregation of macrophages in the tumor. In
addition, chemotherapy also promotes the M2 polarization of
macrophages, which is associated with chemotherapy resistance
(128). Chemotherapy-treated
pancreatic ductal adenocarcinoma cells can recruit macrophages and
support their differentiation into the M2 type, and M2 macrophages
decrease gemcitabine cytotoxicity by secreting deoxycytidine
(129); the depletion of TAMs by
means of chlorophosphonate in pancreatic ductal adenocarcinoma
significantly increases the tumor sensitivity to gemcitabine and
increases apoptosis of tumor cells (130). The depletion of macrophages can
improve tumor sensitivity to chemotherapy drugs, but direct
depletion of macrophages has uncertain long-term effects. It is
safer and more effective to combine chemotherapy with inhibiting
macrophage recruitment or changing the polarization state of
macrophages. In PDAC mice, gemcitabine combined with blocking TAM
effector cytokines TGF-β1 and GM-CSF could reduce M2-polarized TAM
and generate more CT8+ T cells to remodel the TME,
thereby improving the inhibition of gemcitabine on tumor growth
(131). CSF1R protein is highly
expressed in tumor tissues, which is closely related to
chemotherapy resistance of various cancers (132). Although anti-CSF1R alone can
inhibit macrophage recruitment and reduce the number of macrophages
in tumor tissues, it cannot directly affect tumor growth and
metastasis. Furthermore, a combination of an anti-CSF1R therapeutic
agent and a platinum chemotherapy drug can produce synergistic
effects prolonging the survival of mice with breast cancer, and on
this basis, combined with neutrophil inhibitors, it can further
improve the efficacy (133). The
efficacy of chemotherapy is correlated with the polarization of
macrophages, and paclitaxel can exert LPS-like actions in mouse
models of breast cancer and melanoma by inducing M1-type
repolarization of M2 macrophages in a TLR4-dependent manner,
thereby reducing the immune tolerance toward cancer cells (134). A peptide with hairpin structure
combined with simvastatin was revealed to drive M1-type
repolarization of M2 macrophages by acting on the liver X
receptors/ATP-binding box transporter A1, reduce TGF-β secretion,
reverse EMT, and diminish paclitaxel resistance in lung cancer
cells (Table I) (135).
Due to the association between macrophages and
chemotherapy efficacy, combination treatments involving a
macrophage inhibitor can effectively alleviate chemotherapy
resistance by blocking macrophage recruitment to tumors or by
altering macrophage polarization. In addition, if the interaction
between macrophages and other cells is considered, combined
strategies targeting other cells can further improve the efficacy
of chemotherapy.
Radiotherapy can directly act on DNA and generate
free radicals to induce apoptosis and kill tumor cells while having
indirect effects on macrophages. As for the association between
radiotherapy and the number of macrophages, it has been reported
that radiotherapy can promote the accumulation of macrophages by
affecting macrophage chemokines (136). It has also been suggested that
radiotherapy can alter the gene expression of resident leukocytes,
leading to a significant increase in macrophage numbers during the
tumor regeneration period (14 days) after radiotherapy (137). The general theory in this field
is that low-dose radiotherapy can encourage the polarization of
macrophages toward the proinflammatory (M1) phenotype, whereas
high-dose radiotherapy can promote the anti-inflammatory (M2)
phenotype of macrophages. Nevertheless, no clear conclusion has
been reached regarding the association between the radiotherapy
dose and macrophage status. Research indicates that brachytherapy
at 10 Gy can most effectively reduce tumor growth in mice with
melanoma, significantly increases the number of macrophages in
tissues, and reduces the number of M2 macrophages (138). Other studies suggest that 10 Gy
ionizing radiation can cause DNA damage in macrophages without
affecting their activity and can reduce the anti-inflammatory
phenotype but does not stimulate the conversion to the
proinflammatory phenotype. In vitro manipulations of
ionizing-radiation-exposed macrophages can produce a
proinflammatory or anti-inflammatory phenotype (139,140). In advanced PDA, high-dose
radiotherapy promoted macrophage accumulation and M2-type
polarization through M-CSF, but different from conventional M2
macrophages, the M2 macrophages accumulated after radiotherapy
expressed high levels of TNF-α. Notably, the association between
radiotherapy and macrophage polarization is also time-dependent.
Macrophage recruitment and M2 polarization can be observed in the
early stage of radiotherapy, but such changes disappear after 8
weeks of radiotherapy (141). A
combination of targeted macrophage inhibitors with radiotherapy can
better suppress tumors; in mice receiving 10 Gy radiotherapy, the
serum CSF1 protein content increased, and a combination with
anti-CSF1 therapy reduced the number of TAMs and repolarization to
the M1 type, prolonging the inhibition of tumor growth by
radiotherapy (142). In breast
cancer mice, radiotherapy combined with anti-CSF1 not only
repolarized M2 macrophages to the M1-type, but also reduced the
number of CD4+ T cells and reshaped the
immunosuppressive microenvironment (137). Radiotherapy can recruit TAM
through M-CSF. The combination of α-M-CSF mAb with radiotherapy can
not only inhibit the recruitment of TAM and promote the
repolarization of TAM to the M1 type, but also reduce the
generation of tumor-promoting T cells and improve the inhibition of
tumor growth (141). CD47 binds
to the macrophage ligand SIRPα to inhibit macrophage phagocytosis.
In breast cancer, HER2 activates NF-κB through the PI3K/Akt pathway
to promote CD7 expression. The expression of CD47 is increased in
breast cancer cells after radiotherapy, and the combination of
radiotherapy and anti-CD47 anti-HER2 double receptor inhibition can
maximize the phagocytosis of macrophages and improve the
sensitivity of radiotherapy (143). Radiotherapy can cause local skin
damage. Blocking macrophage recruitment after radiotherapy can
alleviate local skin irritation and reduce local inflammation
(26). Pulmonary fibrosis is a
delayed side effect of thoracic radiotherapy. Pulmonary
interstitial macrophages have an M2 phenotype after radiotherapy,
which can induce fibroblast activation and produce the ECM. The
combination of anti-CSF1R can specifically reduce interstitial
macrophages and slow down the formation of pulmonary fibrosis
(Table II) (144).
TAMs attenuate DNA damage caused by radiation to
tumor cells. The combination of radiotherapy and targeted
macrophage strategy is an effective combination therapy based on
the role of macrophages in radiotherapy, which can greatly improve
the inhibition of tumor growth by radiotherapy and reduce the side
effects caused by radiotherapy. Although there are still
conflicting theories about the association between radiotherapy and
macrophages, the main problem will be solved if investigators
determine the optimal radiotherapy dose that repolarizes
macrophages to the M1 phenotype or suppresses their tumor
recruitment. Grasping the changes of macrophages at different
time-points after radiotherapy and adopting the strategy of
targeting macrophages combined with radiotherapy at the most
appropriate time, can not only improve the growth inhibition of
tumors but also alleviate the side effects brought on by
radiotherapy.
Although immunotherapy has unprecedented
therapeutic effects, it is also accompanied by immune nonresponse
and immune tolerance in most patients (145). Currently, the main
immunotherapies include immune checkpoint inhibitors and adoptive
cell therapy (146). An
important reason limiting the efficacy of immunotherapy is the lack
of antitumor T cells in the tumor cell region. TAMs can slow down
the movement of CD8+ T cells in the tumor matrix and
inhibit the contact between CD8+ T cells and the tumor.
Based on the interaction between macrophages and T cells, the
combination of anti-CSF1R-specific depletion macrophages and
anti-PD-1 therapy can generate T-cell chemokines, promote the
infiltration of CD8+ T cells in tumor islets, and
increase the stable contact between CD8+ T cells and
tumor cells, thereby reducing tumor load (147).
Based on the role of macrophages in conventional
tumor therapy, conventional tumor therapy is combined with
antitumor strategies targeting macrophages. This not only improves
tumor inhibition, but also exerts tumor inhibition through the
adaptive immune system by reshaping the TME. Concurrently, it can
reduce the toxic and side effects of conventional treatment, and
has a considerable possibility of clinical application. More
importantly, compared with the development of new antitumor drugs,
the combination of targeted macrophages and conventional therapy
has a more profound clinical basis, with better safety and
timeliness.
TAMs are a highly heterogeneous population of cells
and have different functional phenotypes during tumor progression.
Other cells that infiltrate the TME also undergo alterations.
Therefore, the dynamic interaction between TAMs and other immune
cells may be a major target for future treatments involving
tumor-specific macrophages. The number and state of macrophages can
be changed through the interaction of the TME itself to achieve the
inhibition of tumor growth. Modern medicine has developed a series
of targeting strategies for macrophages by changing the number and
phenotype of macrophages, starting from the changes of macrophages
in the process of tumor growth and their effects on tumor growth.
Combining these strategies with existing anticancer therapies has
significantly improved the inhibition of tumors. In-depth
investigation into the interaction between macrophages and other
components of the TME and into the different roles of macrophages
in conventional therapies is a key step in the treatment of tumors
by means of macrophages. Due to the impact of TCM on macrophages,
designing a unique multitarget approach aimed at macrophages for
cancer treatment is possible. These insights however, remain only
in theory thus far. There are still very few clinical validations
of targeted macrophage therapies, and most of the relevant research
involves only animal experiments. Therefore, increasing the number
of clinical studies on macrophages is essential for future research
in this field.
Not applicable.
YX wrote the manuscript, searched the literature
and prepared the figures and tables. XW was involved in the design
of the study, and revised the manuscript. CS provided article
ideas, modified the tables and revised the manuscript. LL, JWa and
JWu performed literature research and collected relevant articles.
Data authentication is not applicable. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by the National Natural Science
Foundation of China (grant nos. 82174222 and 81973677).
|
1
|
Maimela NR, Liu S and Zhang Y: Fates of
CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J.
17:1–13. 2019. View Article : Google Scholar
|
|
2
|
Locati M, Curtale G and Mantovani A:
Diversity, mechanisms, and significance of macrophage plasticity.
Annu Rev Pathol. 15:123–147. 2020. View Article : Google Scholar
|
|
3
|
Goswami KK, Ghosh T, Ghosh S, Sarkar M,
Bose A and Baral R: Tumor promoting role of anti-tumor macrophages
in tumor microenvironment. Cell Immunol. 316:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XM, Chen DG, Li SC, Zhu B and Li ZJ:
Embryonic origin and subclonal evolution of tumor-associated
macrophages imply preventive care for cancer. Cells. 10:9032021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Yung MMH, Ngan HYS, Chan KKL and
Chan DW: The impact of the tumor microenvironment on macrophage
polarization in cancer metastatic progression. Int J Mol Sci.
22:65602021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yahaya MAF, Lila MAM, Ismail S, Zainol M
and Afizan N: Tumour-associated macrophages (TAMs) in colon cancer
and how to reeducate them. J Immunol Res. 2019:23682492019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castegna A, Gissi R, Menga A, Montopoli M,
Favia M, Viola A and Canton M: Pharmacological targets of
metabolism in disease: Opportunities from macrophages. Pharmacol
Ther. 210:1075212020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar
|
|
9
|
Liu Q, Li Y, Niu Z, Zong Y, Wang M, Yao L,
Lu Z, Liao Q and Zhao Y: Atorvastatin (Lipitor) attenuates the
effects of aspirin on pancreatic cancerogenesis and the
chemotherapeutic efficacy of gemcitabine on pancreatic cancer by
promoting M2 polarized tumor associated macrophages. J Exp Clin
Cancer Res. 35:332016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singhal S, Stadanlick J, Annunziata MJ,
Rao AS, Bhojnagarwala PS, O'Brien S, Moon EK, Cantu E,
Danet-Desnoyers G, Ra HJ, et al: Human tumor-associated
monocytes/macrophages and their regulation of T cell responses in
early-stage lung cancer. Sci Transl Med. 11:eaat15002019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gyori D, Lim EL, Grant FM, Spensberger D,
Roychoudhuri R, Shuttleworth SJ, Okkenhaug K, Stephens LR and
Hawkins PT: Compensation between CSF1R+ macrophages and Foxp3+ Treg
cells drives resistance to tumor immunotherapy. JCI Insight.
3:e1206312018. View Article : Google Scholar :
|
|
12
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov.
17:887–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Wang W, Wu H, Zhou Y, Qin X, Wang
Y, Wu J, Sun XY, Yang Y, Xu H, et al: The essential role of PRAK in
tumor metastasis and its therapeutic potential. Nat Commun.
12:17362021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY and
Mou XZ: The roles of tumor-associated macrophages in tumor
angiogenesis and metastasis. Cell Immunol. 353:1041192020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Z, Xia G, Xiang Z, Liu M, Wei Z, Yan
J, Chen W, Zhu J, Awasthi N, Sun X, et al: A C-X-C chemokine
receptor type 2-dominated cross-talk between tumor cells and
macrophages drives gastric cancer metastasis. Clin Cancer Res.
25:3317–3328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar
|
|
17
|
Paolillo M and Schinelli S: Extracellular
matrix alterations in metastatic processes. Int J Mol Sci.
20:49472019. View Article : Google Scholar :
|
|
18
|
Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H,
Zheng P and Zhao S: Gastric cancer-derived mesenchymal stromal
cells trigger M2 macrophage polarization that promotes metastasis
and EMT in gastric cancer. Cell Death Dis. 10:9182019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swierczak A and Pollard JW: Myeloid cells
in metastasis. Cold Spring Harb Perspect Med. 10:a0380262020.
View Article : Google Scholar
|
|
20
|
Zavyalova MV, Denisov EV, Tashireva LA,
Savelieva OE, Kaigorodova EV, Krakhmal NV and Perelmuter VM:
Intravasation as a key step in cancer metastasis. Biochemistry
(Mosc). 84:762–772. 2019. View Article : Google Scholar
|
|
21
|
Wang J, Cao Z, Zhang XM, Nakamura M, Sun
M, Hartman J, Harris RA, Sun Y and Cao Y: Novel mechanism of
macrophage-mediated metastasis revealed in a zebrafish model of
tumor development. Cancer Res. 75:306–315. 2015. View Article : Google Scholar
|
|
22
|
Chen XW, Yu TJ, Zhang J, Li Y, Chen HL,
Yang GF, Yu W, Liu YZ, Liu XX, Duan CF, et al: CYP4A in
tumor-associated macrophages promotes pre-metastatic niche
formation and metastasis. Oncogene. 36:5045–5057. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ludwig N, Yerneni SS, Azambuja JH,
Gillespie DG, Menshikova EV, Jackson EK and Whiteside TL:
Tumor-derived exosomes promote angiogenesis via adenosine
A2B receptor signaling. Angiogenesis. 23:599–610. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Min AKT, Mimura K, Nakajima S, Okayama H,
Saito K, Sakamoto W, Fujita S, Endo H, Saito M, Saze Z, et al:
Therapeutic potential of anti-VEGF receptor 2 therapy targeting for
M2-tumor-associated macrophages in colorectal cancer. Cancer
Immunol Immunother. 70:289–298. 2021. View Article : Google Scholar
|
|
25
|
Dong F, Ruan S, Wang J, Xia Y, Le K, Xiao
X, Hu T and Wang Q: M2 macrophage-induced lncRNA PCAT6 facilitates
tumorigenesis and angiogenesis of triple-negative breast cancer
through modulation of VEGFR2. Cell Death Dis. 11:7282020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang B, Li Q, Wang J, Zhao S, Nashun B,
Qin L and Chen X: Plasmodium infection inhibits tumor angiogenesis
through effects on tumor-associated macrophages in a murine
implanted hepatoma model. Cell Commun Signal. 18:1572020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson NM and Simon MC: The tumor
microenvironment. Curr Biol. 30:R921–R925. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu T, Dai LJ, Wu SY, Xiao Y, Ma D, Jiang
YZ and Shao ZM: Spatial architecture of the immune microenvironment
orchestrates tumor immunity and therapeutic response. J Hematol
Oncol. 14:982021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirkiles-Smith NC, Harding MJ, Shepherd
BR, Fader SA, Yi T, Wang Y, McNiff JM, Snyder EL, Lorber MI,
Tellides G and Pober JS: Development of a humanized mouse model to
study the role of macrophages in allograft injury. Transplantation.
87:189–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Comito G, Giannoni E, Segura CP,
Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S
and Chiarugi P: Cancer-associated fibroblasts and M2-polarized
macrophages synergize during prostate carcinoma progression.
Oncogene. 33:2423–2431. 2014. View Article : Google Scholar
|
|
32
|
Yang F, Wei Y, Han D, Li Y, Shi S, Jiao D,
Wu J, Zhang Q, Shi C, Yang L, et al: Interaction with CD68 and
Regulation of GAS6 expression by endosialin in fibroblasts drives
recruitment and polarization of macrophages in hepatocellular
carcinoma. Cancer Res. 80:3892–3905. 2020.PubMed/NCBI
|
|
33
|
Cho H, Seo Y, Loke KM, Kim SW, Oh SM, Kim
JH, Soh J, Kim HS, Lee H, Kim J, et al: Cancer-Stimulated CAFs
enhance monocyte differentiation and protumoral TAM Activation via
IL6 and GM-CSF secretion. Clin Cancer Res. 24:5407–5421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Najafi M, Farhood B and Mortezaee K:
Extracellular matrix (ECM) stiffness and degradation as cancer
drivers. J Cell Biochem. 120:2782–2790. 2019. View Article : Google Scholar
|
|
35
|
Ueshima E, Fujimori M, Kodama H, Felsen D,
Chen J, Durack JC, Solomon SB, Coleman JA and Srimathveeravalli G:
Macrophage-secreted TGF-β1 contributes to fibroblast
activation and ureteral stricture after ablation injury. Am J
Physiol Renal Physiol. 317:F52–F64. 2019. View Article : Google Scholar
|
|
36
|
Li G, Jin F, Du J, He Q, Yang B and Luo P:
Macrophage-secreted TSLP and MMP9 promote bleomycin-induced
pulmonary fibrosis. Toxicol Appl Pharmacol. 366:10–16. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashimoto O, Yoshida M, Koma Y, Yanai T,
Hasegawa D, Kosaka Y, Nishimura N and Yokozaki H: Collaboration of
cancer-associated fibroblasts and tumour-associated macrophages for
neuroblastoma development. J Pathol. 240:211–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takahashi H, Sakakura K, Kudo T, Toyoda M,
Kaira K, Oyama T and Chikamatsu K: Cancer-associated fibroblasts
promote an immunosuppressive microenvironment through the induction
and accumulation of protumoral macrophages. Oncotarget.
8:8633–8647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang M, Su Z and Amoah Barnie P: Crosstalk
among colon cancer-derived exosomes, fibroblast-derived exosomes,
and macrophage phenotypes in colon cancer metastasis. Int
Immunopharmacol. 81:1062982020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Wang X, Ma X, Liu C, Wu J and Sun C:
Natural polysaccharides and their derivates: A promising natural
adjuvant for tumor immunotherapy. Front Pharmacol. 12:6218132021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kishton RJ, Sukumar M and Restifo NP:
Metabolic Regulation of T cell longevity and function in tumor
immunotherapy. Cell Metab. 26:94–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walsh AJ, Mueller KP, Tweed K, Jones I,
Walsh CM, Piscopo NJ, Niemi NM, Pagliarini DJ, Saha K and Skala MC:
Classification of T-cell activation via autofluorescence lifetime
imaging. Nat Biomed Eng. 5:77–88. 2021. View Article : Google Scholar :
|
|
43
|
Erlandsson A, Carlsson J, Lundholm M, Fält
A, Andersson SO, Andrén O and Davidsson S: M2 macrophages and
regulatory T cells in lethal prostate cancer. Prostate. 79:363–369.
2019. View Article : Google Scholar :
|
|
44
|
Liu C, Chikina M, Deshpande R, Menk AV,
Wang T, Tabib T, Brunazzi EA, Vignali KM, Sun M, Stolz DB, et al:
Treg cells promote the SREBP1-dependent metabolic fitness of
tumor-promoting macrophages via repression of CD8+ T
cell-derived interferon-γ. Immunity. 51:381–397.e6. 2019.
View Article : Google Scholar
|
|
45
|
Wu Q, Zhou W, Yin S, Zhou Y, Chen T, Qian
J, Su R, Hong L, Lu H, Zhang F, et al: Blocking triggering receptor
expressed on myeloid cells-1-positive tumor-associated macrophages
induced by hypoxia reverses immunosuppression and anti-programmed
cell death ligand 1 resistance in liver cancer. Hepatology.
70:198–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
La Fleur L, Botling J, He F, Pelicano C,
Zhou C, He C, Palano G, Mezheyeuski A, Micke P, Ravetch JV, et al:
Targeting MARCO and IL37R on immunosuppressive macrophages in lung
cancer blocks regulatory T cells and supports cytotoxic lymphocyte
function. Cancer Res. 81:956–967. 2021. View Article : Google Scholar
|
|
47
|
Zhou J, Li X, Wu X, Zhang T, Zhu Q and
Wang X, Wang H, Wang K, Lin Y and Wang X: Exosomes released from
tumor-associated macrophages transfer miRNAs That Induce a
Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer
Immunol Res. 6:1578–1592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang D, Yang L, Yue D, Cao L, Li L, Wang
D, Ping Y, Shen Z, Zheng Y, Wang L and Zhang Y: Macrophage-derived
CCL22 promotes an immunosuppressive tumor microenvironment via IL-8
in malignant pleural effusion. Cancer Lett. 452:244–253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li L, Han L, Sun F, Zhou J, Ohaegbulam KC,
Tang X, Zang X, Steinbrecher KA, Qu Z and Xiao G: NF-κB RelA
renders tumor-associated macrophages resistant to and capable of
directly suppressing CD8+ T cells for tumor promotion.
Oncoimmunology. 7:e14352502018. View Article : Google Scholar
|
|
50
|
Fujimori D, Kinoshita J, Yamaguchi T,
Nakamura Y, Gunjigake K, Ohama T, Sato K, Yamamoto M, Tsukamoto T,
Nomura S, et al: Established fibrous peritoneal metastasis in an
immunocompetent mouse model similar to clinical immune
microenvironment of gastric cancer. BMC Cancer. 20:10142020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu B, Wang Z, Zeng H, Qi Y, Chen Y, Wang
T, Wang J, Chang Y, Bai Q, Xia Y, et al: Blockade of
DC-SIGN+ Tumor-Associated macrophages reactivates
antitumor immunity and improves immunotherapy in muscle-invasive
bladder cancer. Cancer Res. 80:1707–1719. 2020.PubMed/NCBI
|
|
52
|
Śledzińska A, Vila de Mucha M, Bergerhoff
K, Hotblack A, Demane DF, Ghorani E, Akarca AU, Marzolini MAV,
Solomon I, Vargas FA, et al: Regulatory T cells restrain
interleukin-2- and Blimp-1-dependent acquisition of cytotoxic
function by CD4+ T cells. Immunity. 52:151–166.e6. 2020.
View Article : Google Scholar
|
|
53
|
Eisel D, Das K, Dickes E, König R, Osen W
and Eichmüller SB: Cognate interaction with CD4+ T cells
instructs tumor-associated macrophages to acquire M1-Like
phenotype. Front Immunol. 10:2192019. View Article : Google Scholar
|
|
54
|
Bogen B, Fauskanger M, Haabeth OA and
Tveita A: CD4+ T cells indirectly kill tumor cells via
induction of cytotoxic macrophages in mouse models. Cancer Immunol
Immunother. 68:1865–1873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nakayama T, Hirahara K, Onodera A, Endo Y,
Hosokawa H, Shinoda K, Tumes DJ and Okamoto Y: Th2 cells in health
and disease. Annu Rev Immunol. 35:53–84. 2017. View Article : Google Scholar
|
|
56
|
Shirabe K, Mano Y, Muto J, Matono R,
Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T,
Taketomi A, et al: Role of tumor-associated macrophages in the
progression of hepatocellular carcinoma. Surg Today. 42:1–7. 2012.
View Article : Google Scholar
|
|
57
|
Fu C and Jiang A: Dendritic cells and CD8
T cell immunity in tumor microenvironment. Front Immunol.
9:30592018. View Article : Google Scholar
|
|
58
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Verneau J, Sautés-Fridman C and Sun CM:
Dendritic cells in the tumor microenvironment: Prognostic and
theranostic impact. Semin Immunol. 48:1014102020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chaib M, Chauhan SC and Makowski L: Friend
or foe? Recent strategies to target myeloid cells in cancer. Front
Cell Dev Biol. 8:3512020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang L, Li Z, Skrzypczynska KM, Fang Q,
Zhang W, O'Brien SA, He Y, Wang L, Zhang Q, Kim A, et al:
Single-cell analyses inform mechanisms of myeloid-targeted
therapies in colon cancer. Cell. 181442–459. (29)2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dammeijer F, Lievense LA, Kaijen-Lambers
ME, van Nimwegen M, Bezemer K, Hegmans JP, van Hall T, Hendriks RW
and Aerts JG: Depletion of tumor-associated macrophages with a
CSF-1R kinase inhibitor enhances antitumor immunity and survival
induced by DC immunotherapy. Cancer Immunol Res. 5:535–546. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ruffell B, Chang-Strachan D, Chan V,
Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS and
Coussens LM: Macrophage IL-10 blocks CD8+ T cell-dependent
responses to chemotherapy by suppressing IL-12 expression in
intratumoral dendritic cells. Cancer Cell. 26:623–637. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Llopiz D, Ruiz M, Silva L, Repáraz D,
Aparicio B, Egea J, Lasarte JJ, Redin E, Calvo A, Angel M, et al:
Inhibition of adjuvant-induced TAM receptors potentiates cancer
vaccine immunogenicity and therapeutic efficacy. Cancer Lett.
499:279–289. 2021. View Article : Google Scholar :
|
|
65
|
Meza Guzman LG, Keating N and Nicholson
SE: Natural killer cells: Tumor surveillance and signaling. Cancers
(Basel). 12:9522020. View Article : Google Scholar
|
|
66
|
Krneta T, Gillgrass A, Poznanski S, Chew
M, Lee AJ, Kolb M and Ashkar AA: M2-polarized and tumor-associated
macrophages alter NK cell phenotype and function in a
contact-dependent manner. J Leukoc Biol. 101:285–295. 2017.
View Article : Google Scholar
|
|
67
|
Bellora F, Castriconi R, Dondero A,
Reggiardo G, Moretta L, Mantovani A, Moretta A and Bottino C: The
interaction of human natural killer cells with either unpolarized
or polarized macrophages results in different functional outcomes.
Proc Natl Acad Sci USA. 107:21659–21664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Eisinger S, Sarhan D, Boura VF,
Ibarlucea-Benitez I, Tyystjärvi S, Oliynyk G, Arsenian-Henriksson
M, Lane D, Wikström SL, Kiessling R, et al: Targeting a scavenger
receptor on tumor-associated macrophages activates tumor cell
killing by natural killer cells. Proc Natl Acad Sci USA.
117:32005–32016. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sprinzl MF, Reisinger F, Puschnik A,
Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A,
Galle PR, Schuchmann M, et al: Sorafenib perpetuates cellular
anticancer effector functions by modulating the crosstalk between
macrophages and natural killer cells. Hepatology. 57:2358–2368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kim IS, Gao Y, Welte T, Wang H, Liu J,
Janghorban M, Sheng K, Niu Y, Goldstein A, Zhao N, et al:
Immuno-subtyping of breast cancer reveals distinct myeloid cell
profiles and immunotherapy resistance mechanisms. Nat Cell Biol.
21:1113–1126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Braza MS, Conde P, Garcia M, Cortegano I,
Brahmachary M, Pothula V, Fay F, Boros P, Werner SA, Ginhoux F, et
al: Neutrophil derived CSF1 induces macrophage polarization and
promotes transplantation tolerance. Am J Transplant. 18:1247–1255.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: 'N1' versus 'N2'
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Andzinski L, Kasnitz N, Stahnke S, Wu CF,
Gereke M, von Köckritz-Blickwede M, Schilling B, Brandau S, Weiss S
and Jablonska J: Type I IFNs induce anti-tumor polarization of
tumor associated neutrophils in mice and human. Int J Cancer.
138:1982–1993. 2016. View Article : Google Scholar
|
|
75
|
Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin
K, Huang Q, Shi X, Ni Z, Ding N, et al: Tumor-infiltrating immune
cells act as a marker for prognosis in colorectal cancer. Front
Immunol. 10:23682019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H,
Luo C, Zhou J, Fan J and Zhou S: Tumor-associated neutrophils and
macrophages interaction contributes to intrahepatic
cholangiocarcinoma progression by activating STAT3. J Immunother
Cancer. 9:e0019462021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z,
Chen EB, Fan J, Cao Y, Dai Z and Zhou J: Tumor-associated
neutrophils recruit macrophages and T-regulatory cells to promote
progression of hepatocellular carcinoma and resistance to
sorafenib. Gastroenterology. 150:1646–1658.e17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Haider P, Kral-Pointner JB, Mayer J,
Richter M, Kaun C, Brostjan C, Eilenberg W, Fischer MB, Speidl WS,
Hengstenberg C, et al: Neutrophil extracellular trap degradation by
differently polarized macrophage subsets. Arterioscler Thromb Vasc
Biol. 40:2265–2278. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Marichal T, Tsai M and Galli SJ: Mast
cells: Potential positive and negative roles in tumor biology.
Cancer Immunol Res. 1:269–279. 2013. View Article : Google Scholar
|
|
80
|
Khazaie K, Blatner NR, Khan MW, Gounari F,
Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, et
al: The significant role of mast cells in cancer. Cancer Metastasis
Rev. 30:45–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Khan MW, Keshavarzian A, Gounaris E,
Melson JE, Cheon EC, Blatner NR, Chen ZE, Tsai FN, Lee G, Ryu H, et
al: PI3K/AKT signaling is essential for communication between
tissue-infiltrating mast cells, macrophages, and epithelial cells
in colitis-induced cancer. Clin Cancer Res. 19:2342–2354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Galli SJ, Borregaard N and Wynn TA:
Phenotypic and functional plasticity of cells of innate immunity:
Macrophages, mast cells and neutrophils. Nat Immunol. 12:1035–1044.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Taskinen M, Karjalainen-Lindsberg ML and
Leppä S: Prognostic influence of tumor-infiltrating mast cells in
patients with follicular lymphoma treated with rituximab and CHOP.
Blood. 111:4664–4667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y and
Zhao LJ: Prognostic significance of cell infiltrations of
immunosurveillance in colorectal cancer. World J Gastroenterol.
11:1210–1214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Attramadal CG, Kumar S, Gao J, Boysen ME,
Halstensen TS and Bryne M: Low mast cell density predicts poor
prognosis in oral squamous cell carcinoma and reduces survival in
head and neck squamous cell carcinoma. Anticancer Res.
36:5499–5506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tataroğlu C, Kargi A, Ozkal S, Eşrefoğlu N
and Akkoçlu A: Association of macrophages, mast cells and
eosinophil leukocytes with angiogenesis and tumor stage in
non-small cell lung carcinomas (NSCLC). Lung Cancer. 43:47–54.
2004. View Article : Google Scholar
|
|
87
|
Peng SH, Deng H, Yang JF, Xie PP, Li C, Li
H and Feng DY: Significance and relationship between infiltrating
inflammatory cell and tumor angiogenesis in hepatocellular
carcinoma tissues. World J Gastroenterol. 11:6521–6524. 2005.
View Article : Google Scholar
|
|
88
|
Affara NI, Ruffell B, Medler TR, Gunderson
AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong
Q, et al: B cells regulate macrophage phenotype and response to
chemotherapy in squamous carcinomas. Cancer Cell. 25:809–821. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wong SC, Puaux AL, Chittezhath M, Shalova
I, Kajiji TS, Wang X, Abastado JP, Lam KP and Biswas SK: Macrophage
polarization to a unique phenotype driven by B cells. Eur J
Immunol. 40:2296–2307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhou M, Li W, Wen Z, Sheng Y, Ren H, Dong
H, Cao M, Hu HM and Wang LX: Macrophages enhance tumor-derived
autophagosomes (DRibbles)-induced B cells activation by TLR4/MyD88
and CD40/CD40L. Exp Cell Res. 331:320–330. 2015. View Article : Google Scholar
|
|
91
|
Lykken JM and Tedder TF: The tumor
microenvironment regulates CD19 and CD20 immunotherapy for
lymphoma. Cancer J. 21:351–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dahal LN, Dou L, Hussain K, Liu R, Earley
A, Cox KL, Murinello S, Tracy I, Forconi F, Steele AJ, et al: STING
activation reverses lymphoma-mediated resistance to antibody
immunotherapy. Cancer Res. 77:3619–3631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sawa-Wejksza K and Kandefer-Szerszeń M:
Tumor-associated macrophages as target for antitumor therapy. Arch
Immunol Ther Exp (Warsz). 66:97–111. 2018. View Article : Google Scholar
|
|
94
|
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H,
Qiu F and Dai Z: Antitumor effects of immunity-enhancing
traditional Chinese medicine. Biomed Pharmacother. 121:1095702020.
View Article : Google Scholar
|
|
95
|
He J, Yin P and Xu K: Effect and molecular
mechanisms of traditional Chinese medicine on tumor targeting
tumor-associated macrophages. Drug Des Devel Ther. 14:907–919.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Guerriero JL: Macrophages: The road less
traveled, changing anticancer therapy. Trends Mol Med. 24:472–489.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li H, Li L, Mei H, Pan G, Wang X, Huang X,
Wang T, Jiang Z, Zhang L and Sun L: Antitumor properties of
triptolide: Phenotype regulation of macrophage differentiation.
Cancer Biol Ther. 21:178–188. 2020. View Article : Google Scholar :
|
|
98
|
Jia X, Yu F, Wang J, Iwanowycz S, Saaoud
F, Wang Y, Hu J, Wang Q and Fan D: Emodin suppresses pulmonary
metastasis of breast cancer accompanied with decreased macrophage
recruitment and M2 polarization in the lungs. Breast Cancer Res
Treat. 148:291–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li H, Huang N, Zhu W, Wu J, Yang X, Teng
W, Tian J, Fang Z, Luo Y, Chen M and Li Y: Modulation the crosstalk
between tumor-associated macrophages and non-small cell lung cancer
to inhibit tumor migration and invasion by ginsenoside Rh2. BMC
Cancer. 18:5792018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yang Y, Sun M, Yao W, Wang F, Li X, Wang
W, Li J, Gao Z, Qiu L, You R, et al: Compound kushen injection
relieves tumor-associated macrophage-mediated immunosuppression
through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib.
J Immunother Cancer. 8:e0003172020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang L, Wu W, Zhu X, Ng W, Gong C, Yao C,
Ni Z, Yan X, Fang C and Zhu S: The Ancient Chinese decoction
Yu-Ping-Feng Suppresses Orthotopic lewis lung cancer tumor growth
through increasing M1 macrophage polarization and CD4(+) T cell
cytotoxicity. Front Pharmacol. 10:13332019. View Article : Google Scholar
|
|
102
|
Wang S, Ma L, Wang Z, He H, Chen H, Duan
Z, Li Y, Si Q, Chuang TH, Chen C and Luo Y: Lactate dehydrogenase-A
(LDH-A) preserves cancer stemness and recruitment of
tumor-associated macrophages to promote breast cancer progression.
Front Oncol. 11:6544522021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Laviron M and Boissonnas A: Ontogeny of
tumor-associated macrophages. Front Immunol. 10:17992019.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Chen C, Yao X, Xu Y, Zhang Q, Wang H, Zhao
L, Wen G, Liu Y, Jing L and Sun X: Dahuang Zhechong Pill suppresses
colorectal cancer liver metastasis via ameliorating exosomal CCL2
primed pre-metastatic niche. J Ethnopharmacol. 238:1118782019.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wu CY, Cherng JY, Yang YH, Lin CL, Kuan
FC, Lin YY, Lin YS, Shu LH, Cheng YC, Liu HT, et al: Danshen
improves survival of patients with advanced lung cancer and
targeting the relationship between macrophages and lung cancer
cells. Oncotarget. 8:90925–90947. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wu X, Schulte BC, Zhou Y, Haribhai D,
Mackinnon AC, Plaza JA, Williams CB and Hwang ST: Depletion of
M2-like tumor-associated macrophages delays cutaneous T-cell
lymphoma development in vivo. J Invest Dermatol. 134:2814–2822.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zeisberger SM, Odermatt B, Marty C,
Zehnder-Fjällman AH, Ballmer-Hofer K and Schwendener RA:
Clodronate-liposome-mediated depletion of tumour-associated
macrophages: A new and highly effective antiangiogenic therapy
approach. Br J Cancer. 95:272–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Baert T, Vankerckhoven A, Riva M, Van
Hoylandt A, Thirion G, Holger G, Mathivet T, Vergote I and
Coosemans A: Myeloid derived suppressor cells: Key drivers of
immunosuppression in ovarian cancer. Front Immunol. 10:12732019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Etzerodt A, Tsalkitzi K, Maniecki M,
Damsky W, Delfini M, Baudoin E, Moulin M, Bosenberg M, Graversen
JH, Auphan-Anezin N, et al: Specific targeting of CD163+ TAMs
mobilizes inflammatory monocytes and promotes T cell-mediated tumor
regression. J Exp Med. 216:2394–2411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Scott EM, Jacobus EJ, Lyons B, Frost S,
Freedman JD, Dyer A, Khalique H, Taverner WK, Carr A, Champion BR,
et al: Bi- and tri-valent T cell engagers deplete tumour-associated
macrophages in cancer patient samples. J Immunother Cancer.
7:3202019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Galletti G, Caligaris-Cappio F and
Bertilaccio MT: B cells and macrophages pursue a common path toward
the development and progression of chronic lymphocytic leukemia.
Leukemia. 30:2293–2301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Deci MB, Ferguson SW, Scatigno SL and
Nguyen J: Modulating macrophage polarization through CCR2
inhibition and multivalent engagement. Mol Pharm. 15:2721–2731.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bonapace L, Coissieux MM, Wyckoff J, Mertz
KD, Varga Z, Junt T and Bentires-Alj M: Cessation of CCL2
inhibition accelerates breast cancer metastasis by promoting
angiogenesis. Nature. 515:130–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fujiwara T, Yakoub MA, Chandler A, Christ
AB, Yang G, Ouerfelli O, Rajasekhar VK, Yoshida A, Kondo H, Hata T,
et al: CSF1/CSF1R signaling inhibitor pexidartinib (PLX3397)
reprograms tumor-associated macrophages and stimulates T-cell
infiltration in the sarcoma microenvironment. Mol Cancer Ther.
20:1388–1399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sluijter M, van der Sluis TC, van der
Velden PA, Versluis M, West BL, van der Burg SH and van Hall T:
Inhibition of CSF-1R supports T-cell mediated melanoma therapy.
PLoS One. 9:e1042302014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Atzori MG, Ceci C, Ruffini F, Trapani M,
Barbaccia ML, Tentori L, D'Atri S, Lacal PM and Graziani G: Role of
VEGFR-1 in melanoma acquired resistance to the BRAF inhibitor
vemurafenib. J Cell Mol Med. 24:465–475. 2020. View Article : Google Scholar
|
|
119
|
Linde N, Lederle W, Depner S, van Rooijen
N, Gutschalk CM and Mueller MM: Vascular endothelial growth
factor-induced skin carcinogenesis depends on recruitment and
alternative activation of macrophages. J Pathol. 227:17–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Huang Z, Gan J, Long Z, Guo G, Shi X, Wang
C, Zang Y, Ding Z, Chen J, Zhang J and Dong L: Targeted delivery of
let-7b to reprogramme tumor-associated macrophages and tumor
infiltrating dendritic cells for tumor rejection. Biomaterials.
90:72–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wu L, Zhang X, Zheng L, Zhao H, Yan G,
Zhang Q, Zhou Y, Lei J, Zhang J, Wang J, et al: RIPK3 orchestrates
fatty acid metabolism in tumor-associated macrophages and
hepatocarcinogenesis. Cancer Immunol Res. 8:710–721. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yu Q, Wang Y, Dong L, He Y, Liu R, Yang Q,
Cao Y, Wang Y, Jia A, Bi Y and Liu G: Regulations of Glycolytic
activities on macrophages functions in tumor and infectious
inflammation. Front Cell Infect Microbiol. 10:2872020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Baer C, Squadrito ML, Laoui D, Thompson D,
Hansen SK, Kiialainen A, Hoves S, Ries CH, Ooi CH and De Palma M:
Suppression of microRNA activity amplifies IFN-γ-induced macrophage
activation and promotes anti-tumour immunity. Nat Cell Biol.
18:790–802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Andersen MN, Etzerodt A, Graversen JH,
Holthof LC, Moestrup SK, Hokland M and Møller HJ: STAT3 inhibition
specifically in human monocytes and macrophages by CD163-targeted
corosolic acid-containing liposomes. Cancer Immunol Immunother.
68:489–502. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shi C, Liu T, Guo Z, Zhuang R, Zhang X and
Chen X: Reprogramming Tumor-associated macrophages by
nanoparticle-based reactive oxygen species photogeneration. Nano
Lett. 18:7330–7342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang W, Cao S, Liang S, Tan CH, Luo B, Xu
X and Saw PE: Differently charged super-paramagnetic iron oxide
nanoparticles preferentially induced M1-like phenotype of
macrophages. Front Bioeng Biotechnol. 8:5372020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Shan H, Dou W, Zhang Y and Qi M: Targeted
ferritin nanoparticle encapsulating CpG oligodeoxynucleotides
induces tumor-associated macrophage M2 phenotype polarization into
M1 phenotype and inhibits tumor growth. Nanoscale. 12:22268–22280.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bhattacharya U, Gutter-Kapon L, Kan T,
Boyango I, Barash U, Yang SM, Liu J, Gross-Cohen M, Sanderson RD,
Shaked Y, et al: Heparanase and chemotherapy synergize to drive
macrophage activation and enhance tumor growth. Cancer Res.
80:57–68. 2020. View Article : Google Scholar :
|
|
129
|
Halbrook CJ, Pontious C, Kovalenko I,
Lapienyte L, Dreyer S, Lee HJ, Thurston G, Zhang Y, Lazarus J,
Sajjakulnukit P, et al: Macrophage-released pyrimidines inhibit
gemcitabine therapy in pancreatic cancer. Cell Metab.
29:1390–1399.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Buchholz SM, Goetze RG, Singh SK,
Ammer-Herrmenau C, Richards FM, Jodrell DI, Buchholz M, Michl P,
Ellenrieder V, Hessmann E and Neesse A: Depletion of macrophages
improves therapeutic response to gemcitabine in murine pancreas
cancer. Cancers (Basel). 12:19782020. View Article : Google Scholar
|
|
131
|
Liu Q, Wu H, Li Y, Zhang R, Kleeff J,
Zhang X, Cui M, Liu J, Li T, Gao J, et al: Combined blockade of
TGf-β1 and GM-CSF improves chemotherapeutic effects for pancreatic
cancer by modulating tumor microenvironment. Cancer Immunol
Immunother. 69:1477–1492. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Baghdadi M, Wada H, Nakanishi S, Abe H,
Han N, Putra WE, Endo D, Watari H, Sakuragi N, Hida Y, et al:
Chemotherapy-induced IL34 enhances immunosuppression by
tumor-associated macrophages and mediates survival of
chemoresistant lung cancer cells. Cancer Res. 76:6030–6042. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Salvagno C, Ciampricotti M, Tuit S, Hau
CS, van Weverwijk A, Coffelt SB, Kersten K, Vrijland K, Kos K, Ulas
T, et al: Therapeutic targeting of macrophages enhances
chemotherapy efficacy by unleashing type I interferon response. Nat
Cell Biol. 21:511–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wanderley CW, Colón DF, Luiz JPM, Oliveira
FF, Viacava PR, Leite CA, Pereira JA, Silva CM, Silva CR, Silva RL,
et al: Paclitaxel reduces tumor growth by reprogramming
tumor-associated macrophages to an M1 profile in a TLR4-dependent
manner. Cancer Res. 78:5891–5900. 2018.PubMed/NCBI
|
|
135
|
Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J
and Huang Y: Targeting lipid metabolism to overcome EMT-associated
drug resistance via integrin β3/FAK pathway and tumor-associated
macrophage repolarization using legumain-activatable delivery.
Theranostics. 9:265–278. 2019. View Article : Google Scholar :
|
|
136
|
Inoue T, Fujishima S, Ikeda E, Yoshie O,
Tsukamoto N, Aiso S, Aikawa N, Kubo A, Matsushima K and Yamaguchi
K: CCL22 and CCL17 in rat radiation pneumonitis and in human
idiopathic pulmonary fibrosis. Eur Respir J. 24:49–56. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Shiao SL, Ruffell B, DeNardo DG, Faddegon
BA, Park CC and Coussens LM: TH2-polarized CD4(+) T cells and
macrophages limit efficacy of radiotherapy. Cancer Immunol Res.
3:518–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Jarosz-Biej M, Smolarczyk R, Cichoń T,
Drzyzga A, Czapla J, Urbaś Z, Pilny E, Matuszczak S and Wojcieszek
P: Brachytherapy in a Single dose of 10Gy as an 'in situ'
Vaccination. Int J Mol Sci. 21:45852020. View Article : Google Scholar
|
|
139
|
Teresa Pinto A, Laranjeiro Pinto M,
Patrícia Cardoso A, Monteiro C, Teixeira Pinto M, Filipe Maia A,
Castro P, Figueira R, Monteiro A, Marques M, et al: Ionizing
radiation modulates human macrophages towards a pro-inflammatory
phenotype preserving their pro-invasive and pro-angiogenic
capacities. Sci Rep. 6:187652016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Rödel F, Frey B, Manda K, Hildebrandt G,
Hehlgans S, Keilholz L, Seegenschmiedt MH, Gaipl US and Rödel C:
Immunomodulatory properties and molecular effects in inflammatory
diseases of low-dose x-irradiation. Front Oncol. 2:1202012.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Seifert L, Werba G, Tiwari S, Giao Ly NN,
Nguy S, Alothman S, Alqunaibit D, Avanzi A, Daley D, Barilla R, et
al: Radiation therapy induces macrophages to suppress T-cell
responses against pancreatic tumors in mice. Gastroenterology.
150:1659–1672.e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Jones KI, Tiersma J, Yuzhalin AE,
Gordon-Weeks AN, Buzzelli J, Im JH and Muschel RJ: Radiation
combined with macrophage depletion promotes adaptive immunity and
potentiates checkpoint blockade. EMBO Mol Med. 10:e93422018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Candas-Green D, Xie B, Huang J, Fan M,
Wang A, Menaa C, Zhang Y, Zhang L, Jing D, Azghadi S, et al: Dual
blockade of CD47 and HER2 eliminates radioresistant breast cancer
cells. Nat Commun. 11:45912020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Meziani L, Mondini M, Petit B, Boissonnas
A, Thomas de Montpreville V, Mercier O, Vozenin MC and Deutsch E:
CSF1R inhibition prevents radiation pulmonary fibrosis by depletion
of interstitial macrophages. Eur Respir J. 51:17021202018.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Riley RS, June CH, Langer R and Mitchell
MJ: Delivery technologies for cancer immunotherapy. Nat Rev Drug
Discov. 18:175–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kruger S, Ilmer M, Kobold S, Cadilha BL,
Endres S, Ormanns S, Schuebbe G, Renz BW, D'Haese JG, Schloesser H,
et al: Advances in cancer immunotherapy 2019-latest trends. J Exp
Clin Cancer Res. 38:2682019. View Article : Google Scholar
|
|
147
|
Peranzoni E, Lemoine J, Vimeux L, Feuillet
V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J,
Ouakrim H, et al: Macrophages impede CD8 T cells from reaching
tumor cells and limit the efficacy of anti-PD-1 treatment. Proc
Natl Acad Sci USA. 115:E4041–E4050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gordon SR, Maute RL, Dulken BW, Hutter G,
George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al:
PD-1 expression by tumour-associated macrophages inhibits
phagocytosis and tumour immunity. Nature. 545:495–499. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Klichinsky M, Ruella M, Shestova O, Lu XM,
Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty
NE, et al: Human chimeric antigen receptor macrophages for cancer
immunotherapy. Nat Biotechnol. 38:947–953. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 blockade by Hu5F9-G4 and rituximab in Non-Hodgkin's
lymphoma. N Engl J Med. 379:1711–1721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai
B, Sun Y, Kang F, Yang Z, He L, et al: Activating
macrophage-mediated cancer immunotherapy by genetically edited
nanoparticles. Adv Mater. 32:e20048532020. View Article : Google Scholar : PubMed/NCBI
|