Introduction
In 1927, Warburg et al proposed the ‘Warburg
effect’, which states that even in the case of sufficient oxygen,
compared with normal tissues, the glucose metabolism in tumor cells
is significantly enhanced (1,2).
However, the behavior of some tumor cells is different from the
Warburg effect. These tumor cells can oxidize mitochondria to
metabolize the oxidative phosphorylation system (OXPHOS) (3), and glycolysis produces ATP at 1 to
64%. OXPHOS remains the main ATP supplier in cancer cells (4). In addition, numerous studies have
shown that OXPHOS and aerobic glycolysis are not always mutually
exclusive. To some extent, their contribution to ATP production
varies with the tumor environment, such as under normoxia and
hypoxia (5,6). Therefore, studies have proposed a
‘two-compartment’ model, also known as the ‘Reverse Warburg
effect’, to reassess the metabolic pattern of tumor cells (7,8). In
this model, cancer cells secrete hydrogen peroxide into the tumor
microenvironment (TME) to induce oxidative stress in neighboring
stromal cells, so that cancer-associated fibroblasts (CAFs) undergo
aerobic glycolysis and produce high levels of energy-rich
intermediate metabolites (such as pyruvate and ketones). These
energy-rich intermediate metabolites will be used in the
mitochondrial TCA cycle and OXPHOS in cancer cells to produce a
large quantity of ATP (9,10), such that tumor cells have a higher
proliferation ability, which establishes the metabolic interaction
between the tumor and the stromal cells. It enables tumor cells to
better adapt to changes in oxygen levels, switch metabolic states
between glycolysis and oxidative phosphorylation, and survive.
Tumor cells exhibit the Warburg effect under hypoxic conditions and
the ‘Reverse Warburg effect’ under normoxic conditions, which
support tumor growth and metastasis (11).
In addition, in the TME, CAFs are the main tumor
stromal cells, which are prone to metabolic reprogramming, and play
an important role in tumorigenesis, progression, and metastasis
(9,12). Therefore, it is important to study
the differentiation of CAFs and the metabolic reprogramming during
their crosstalk with cancer cells, which will help to develop new
therapeutic strategies and targets (9,13). In
the present review, the metabolic reprogramming mechanism of CAFs
and cancer crosstalk were summarized, and potential therapeutic
strategies involving CAFs reprogramming-associated molecules was
also explored.
Literature searches were performed using the PubMed
database (https://pubmed.ncbi.nlm.nih.gov/). The key words
searched for included: ‘CAFs’, ‘cancer-associated fibroblasts’,
‘metabolic reprogramming’, ‘oxidative stress’, ‘Cav-1’, ‘Warburg
effect’, ‘cancer crosstalk’, ‘mitochondrial oxidative
phosphorylation’, and ‘reverse Warburg effect’. There were 133
studies covering 1956 to 2022, that were included in the present
review.
Origin and differentiation of CAFs
CAFs originate from the activation of resident
fibroblasts or other precursor cells. CAFs can be derived from bone
marrow mesenchymal stem cells, epithelial cells, cancer cells,
endothelial cells, pericytes, smooth muscle cells, adipocytes,
fibroblasts, or certain special cells, such as stellate cells,
pancreas and liver cells, myoepithelial cells in the breast, and
crypt myofibroblasts in the gastrointestinal tract (14). The diversity of sources indicates
the heterogeneity of CAFs. Numerous factors can activate CAFs,
including cancer cell-derived transforming growth factor-β (TGF-β),
epidermal growth factor (EGF), platelet-derived growth factor
(PDGFα, PDGFβ), basic fibroblast growth factor (bFGF, also called
FGF2), interleukin-6 (IL-6) and interleukin-1β (IL-1β) (15–17),
as well as environmental stimuli such as hypoxia, oxidative stress,
and the cell matrix. These stimuli may jointly determine different
CAF phenotypes, further leading to CAF heterogeneity (18,19).
After CAFs are activated, they secrete a large number of growth
factors, such as hepatocyte growth factor (HGF), EGF, connective
tissue growth factor (CTGF), and insulin-like growth factor (IGF),
cytokines, including CXCL12 and IL-6, extracellular vesicles (EVs),
metabolites, extracellular matrix (ECM) components, especially
collagen, fibronectin, and troponin C (TNC), and ECM remodeling
enzymes, such as matrix metalloproteinase (MMP), lysyl oxidase
(LOX), and thyroid microsomes (TGMs). These will directly affect
the behavior of surrounding cells and reshape the ECM (20). Therefore, CAFs contribute to tumor
development, metastasis, tumor metabolism, TME remodeling, and
resistance to treatment (21).
CAF differentiation is mainly triggered by factors
secreted by cancer cells, but it is also induced by mediators
produced by other cell types (such as immune cells). The most
important of these is the ‘classical’ TGF-β signaling pathway,
which activates the transcription factor Smad, an essential factor
for the activation of myofibroblasts. However, the activation of
small GTPase RhoA by the ‘non-classical’ signaling pathway also
plays a regulatory role. TGF-β depends on the cell type and
crosstalk with other signaling pathways, transcription factors, and
epigenetic modifications (22). In
addition, the differentiation of CAFs may also be affected by
released cytokines and growth factors. Another important process of
CAF differentiation is metabolic reprogramming, which is related to
multiple processes, including oxidative stress, induced by cancer
cells. Oxidative stress changes mitochondrial function, leading to
higher glucose uptake and levels of reactive oxygen species (ROS),
ultimately leading to CAF differentiation (23). However, metabolic reprogramming of
tumor-stromal interactions is currently less discussed and rarely
recognized as a potential therapeutic avenue. Therefore, both the
activation and differentiation of CAFs are inseparable from tumor
cells, and are affected by tumor cells and secreted factors, and
mediators related to the microenvironment. In turn, CAFs affect
tumor cells and form a mutual influence circle.
‘Reverse Warburg effect’ of CAFs
In the 1927, Otto Warburg (2) first proposed the ‘Warburg effect’.
Even in the presence of sufficient oxygen, malignant tumor cells
tend to produce adenosine triphosphate (ATP). Glycolysis replaces
OXPHOS (24), also known as
‘aerobic glycolysis’. The Warburg effect is different, and some
tumor cells show a high proportion of OXPHOS (3). In addition, numerous studies have
shown that OXPHOS and aerobic glycolysis are not always mutually
exclusive. To a certain extent, their contribution to ATP
production varies with the tumor environment, such as under
normoxia and hypoxia (5,6). The interaction between cancer cells
and the surrounding CAFs markedly affects the growth, metabolism,
metastasis, and progression of cancer (25). Therefore, a ‘two-compartment’ model,
also known as the ‘Reverse Warburg effect’, was proposed to
reconsider the metabolism in tumors (7,8).
Cancer cells secrete hydrogen peroxide (H2O2)
into the TME to induce oxidative stress in neighboring stromal
cells. The CAFs derived from stromal cells undergo aerobic
glycolysis and produce high levels of energy-rich mitochondrial
‘fuels’ (such as pyruvate, ketone bodies, fatty acids, and lactic
acid). In turn, these energy-rich ‘fuels’ are then ‘fed’ to
mitochondria in cancer cells, where they are oxidatively
metabolized via OXPHOS to produce large quantities of ATP (9,10).
Among them, lactic acid is the most important metabolic ‘fuel’. The
lactic acid produced by hypoxic cancer cells and CAFs is
transported through the lactic acid transporter monocarboxylic acid
transporter (MCT)4, then absorbed through MCT1, and distributed to
the cancer cells (26,27). After entering cancer cells, lactic
acid is metabolized via mitochondrial OXPHOS to produce large
quantities of ATP (28), such that
cancer cells can achieve self-sufficiency (29). Therefore, the ‘Reverse Warburg
effect’ is proposed to link the microenvironment of stromal cells
with cancer cells, and provide energy for cancer cells. When there
is sufficient oxygen in the tumor microenvironment, tumor cells
will experience the ‘Warburg effect’ (30), and pyruvate is converted to lactate
by lactate dehydrogenase (LDH) (27,31).
The excess lactate produced is taken up by CAF, which increases
MCT1 and LDH-1 expression and PDH activity in CAF cells; CAFs
incorporate excess lactate into mitochondria for oxidative
phosphorylation, thereby removing lactate from the tumor
microenvironment (32). When the
tumor microenvironment is hypoxic, CAFs undergo metabolic
reprogramming and glycolysis, and export lactate to tumor cells for
oxidative phosphorylation, providing energy for tumor cells and
promoting their proliferation (11).
In the theory of the ‘autophagic tumor stromal model
of cancer metabolism’, cancer cells secrete ROS in the TME and
induce oxidative stress in CAFs, leading to autophagy and the
production of autophagosomes fused with lysosomes. This in turn
degrades mitochondria and caveolin 1 (Cav-1). However, the absence
of Cav-1 will cause more ROS to be produced in cancer cells, which
initiates the oxidative stress cascade in CAFs through a positive
feedback mechanism (33). In
pancreatic cancer (PDAC), knockdown of Cav-1 promotes ROS
production, which in turn reduces Cav-1 expression. Thereby
promoting PDAC growth and inducing stroma-tumor metabolic coupling
in PDAC (34). Of course, there may
be other mechanisms involved in the dysregulation of Cav-1 in CAFs.
Studies have found that activation of the TGF-β signaling pathway,
the inactivation of tumor suppressor genes (such as p53), and
oncogenes (e.g., h-ras, v-abl, brc-abl, and TGF) (35) also participate. Cav-1 binds to
nitric oxide synthase (NOS) and inhibits its activity in stromal
cells. When Cav-1 is absent, CAFs cannot limit the synthesis of NO.
In addition, the accumulation of NO can lead to mitochondrial
dysfunction and glycolytic metabolism (36,37).
Cav-1 is a negative regulator of ROS produced by the NADPH oxidase
(NOX) enzymes, which is achieved through a variety of mechanisms.
Cav-1 inhibits NOX2 and NOX5 from producing ROS by directly binding
to these enzymes. In addition, Cav-1 can also inhibit NOX2
and NOX4 gene expression and protein synthesis by inhibiting
the nuclear factor-κB (NF-κB) pathway (38). The same pathway regulates Cav-1 and
ROS, and both contribute to the metabolic transformation of CAFs
from mitochondrial OXPHOS to glycolysis (39). In addition, Cav-1 knockdown
induces the expression of pyruvate kinase M (PKM)2, which in
turn triggers aerobic glycolysis and regulates mitochondrial
OXPHOS. The latest study found that Cav-1 directly activates E2F1
in CAFs of lung cancer, which in turn regulates BNIP3, which
ultimately induces mitochondrial defects and regulates the
metabolic reprogramming pathway of tumor cells (40).
For the metabolic crosstalk between CAFs and cancer
cells, hypoxia inducible factor 1α (HIF-1α) and nuclear NF-κB are
particularly important. The activation of HIF-1α and NF-κB is
mainly mediated by the decreased expression of prolyl hydroxylase
domain protein (PHD) (41). Hypoxia
has been found to reduce levels of the methyl donor
S-adenosylmethionine (42). PKM2
directly interacts with the HIF-1α subunit, promoting the
transactivation of HIF-1α target gene by enhancing p300 recruitment
and enhancing the binding of HIF-1 to hypoxia response elements. In
addition, prolyl hydroxylase 3 (PHD3) interacts with PKM2, which
can enhance the combination of PKM2 and HIF-1α, enhance the
function of PKM2 coactivator, and hydroxylated PKM2 proline
403/408. Knockdown of PHD3 inhibits PKM2 coactivator function,
reduces glucose intake and lactic acid production, and increases O2
consumption in cancer cells. Moreover, JMJD5 interacts directly
with PKM2. JMJD5-PKM2 interaction is located in the interface
region between PKM2 subunits, blocking PKM2 tetramerization and
blocking pyruvate kinase activity. JMJD5-PKM2 interaction makes
PKM2 shift to the nucleus and promotes HIF-1α mediated
transactivation. JMJD5 and PKM2 are co-recruited to HRE sites of
LDHA and PKM2 loci, which promotes HIF-1α-mediated
activity and reprograms glucose metabolism in cancer cells
(43,44). It was found that oxidative stress
triggers the activation of NF-κB and STAT3 in CAFs to upregulate
CCL2, thereby promoting oral cancer growth (45). IL-6 secreted by CAFs can promote the
growth, migration and invasion of cancer cells in head and neck
squamous cell carcinoma (HNSCC) through the integrin
αvβ3/NF-κB pathway. And CAF-driven
pro-inflammatory signaling is dependent on NF-κB (46). These data suggest that CAF-driven
NF-κB signaling plays a central role in mediating inflammation in
tumor precursors.
HIF-1α mediates Sirtuin1 (SIRT1) signaling to affect
aerobic glycolysis, downregulates oxisome proliferator-activated
receptor-γ coactivator 1α (PGC-1α) or mitochondrial deacetylase
SIRT3, and increases inactive superoxidation and the level of
acetylation of superoxide dismutase 2 (SOD2) (47,48).
In prostate cancer (PCa), lactate released from CAFs unbalances the
NAD+/NADH ratio in PCa cells and increases
NAD+ levels, which in turn enhances SIRT1-mediated
PGC-1α activation and PCa cell mitochondrial mass and activity
(49). In turn, this accumulates
ROS and PKM2, and increases the ability to regulate mitochondria.
PKM2 is oxidized by excessive ROS, is phosphorylated by activated
Src kinase, and then migrates to the nucleus where it recruits
HIF-1 and the related embryonic chondrocyte expression gene 1
(DEC1), thereby inhibiting the expression of miR-205,
resulting in metabolic reprogramming. Mitochondria OXPHOS improves
the survival rate and epithelial-mesenchymal transition (EMT) of
cancer cells (50).
In CAFs, glycolysis-related enzymes, such as
hexokinase 2 (HK2) and 6-phosphofructokinase liver type (PFKL) are
significantly upregulated (51–53).
HK2 is a key glycolytic enzyme that is overexpressed in tumors and
contributes to the ‘Warburg effect’ (54). During the differentiation of CAFs
induced by TGF-β1, the level of the HK2 protein increases. HK2
upregulates p27 protein expression through its downstream
metabolite α-KG. In turn, p27 inhibits cyclin-dependent kinase 2
(CDK2) and activates the G1/S checkpoint. This regulatory mechanism
links glycolysis with cell cycle control; in fact, the HK2 enzyme
regulates both glycolysis and cell cycle checkpoints (55). In addition, there are other
glycolytic enzymes that are also closely related, such as the two
isoforms PKM1 and PKM2, MCT, and LDHA, LDHB (56–58).
The upregulation of PKM1 mediates the production of lactic acid in
CAFs. The upregulation of PKM2 promotes the autophagy program in
CAFs and stimulates ketone body storage (59,60). A
previous in vivo study revealed that overexpression of
PKM1 promotes tumor-related inflammation, and overexpression
of PKM2 can enhance mitochondrial OXPHOS in cancer cells.
High levels of LDHs and MCTs will accelerate the production and
transportation of energy-rich ‘fuel’ to cancer cells, thereby
promoting their survival, progression, and invasion. In addition,
knockdown of encoding isocitrate dehydrogenase [NAD(+)] 3 catalytic
subunit α (IDH3α) increased glycolysis and inhibited
oxidative phosphorylation in fibroblasts (60).
Another previous study revealed that integrin β2
(ITGB2) is highly expressed in the CAFs of oral squamous cell
carcinoma (OSCC). ITGB2 enhances the glycolytic activity of CAFs by
regulating the phosphatidylinositol-4,5-bisphosphate 3-kinase
(PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin
(mTOR) pathway. The lactic acid produced by ITGB2 in CAFs is
absorbed by tumor cells, metabolized to produce NADH, and then
oxidized via mitochondrial OXPHOS to produce ATP. In addition,
integrin β 4 (ITGB4) is overexpressed in TNBC cells, and cancer
cells provide ITGB4 protein to CAFs through exosomes, after which
CAFs induce BNIP3L-dependent mitochondrial autophagy to increase
glycolysis levels (61).
The crosstalk between cancer cells and CAFs is
closely related to cellular metabolic reprogramming, which
contributes to the activation of CAFs, cancer growth, and the
progression and escape of cancer treatment. Studying the metabolic
reprogramming of CAFs will provide a better understanding of the
activation process and the interaction between the stroma and
cancer cells, and provide new therapeutic strategies for the
development of the original tumor activity of CAFs (Fig. 1).
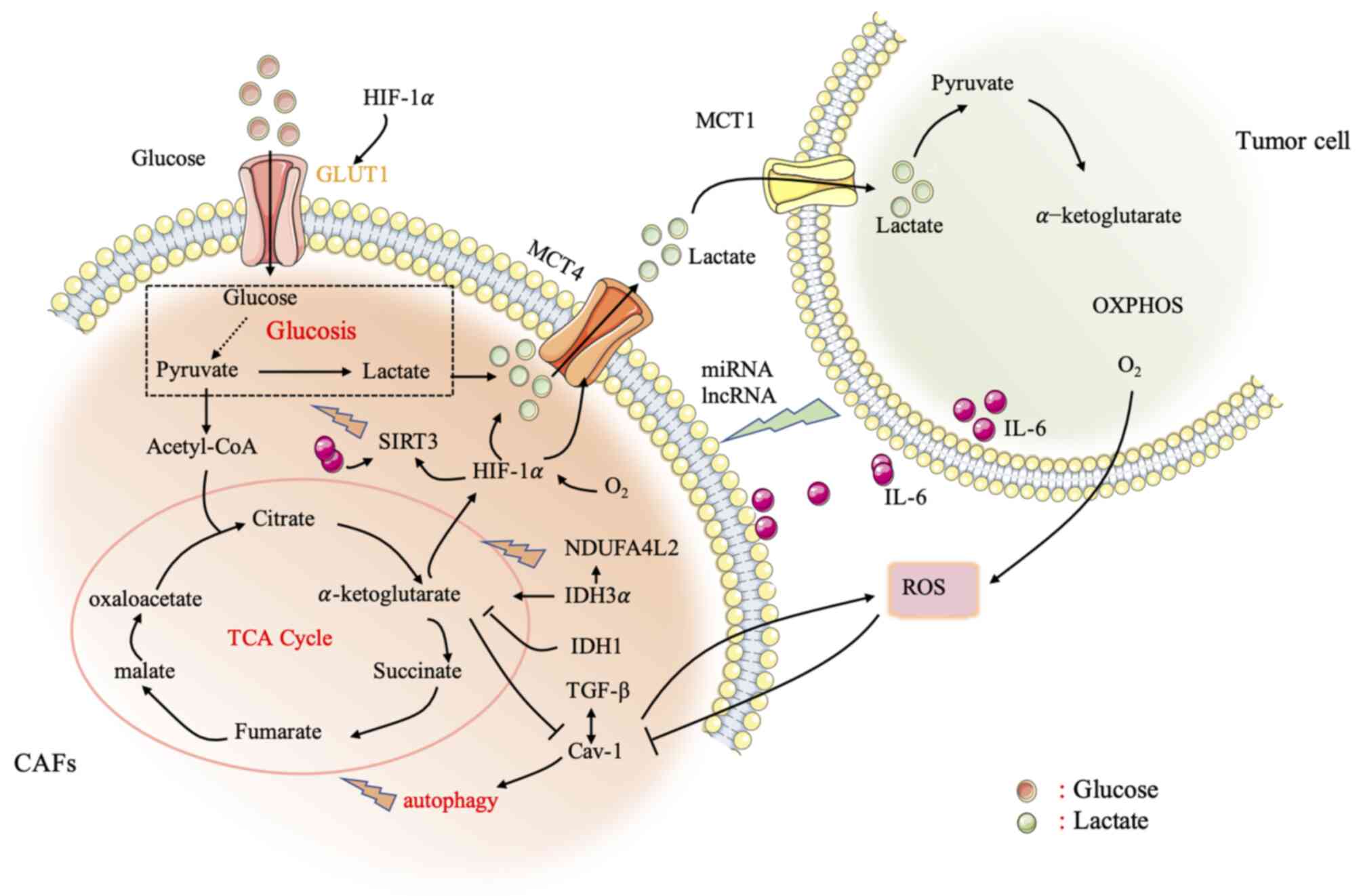 | Figure 1.Metabolic crosstalk between CAFs and
cancer cells. CAFs undergo aerobic glycolysis and produce high
levels of energy-rich mitochondrial ‘fuels’ (such as pyruvate,
ketone bodies, fatty acids, and lactic acid). In turn, these
energy-rich ‘fuels’ are then ‘fed’ to mitochondria in cancer cells
via OXPHOS and produce large quantities of ATP. The lactic acid
produced by CAFs is transported by the lactate transporter MCT4,
and then absorbed by MCT1 and distributed to cancer cells. After
entering cancer cells, lactic acid metabolizes OXPHOS through
mitochondria to produce a large quantity of ATP, allowing the
cancer cells to achieve self-sufficiency. Cancer cells secrete ROS
in the tumor microenvironment, degrade Cav-1, trigger aerobic
glycolysis, and regulate mitochondrial OXPHOS. HIF-1α and NF-κB are
also particularly important in affecting the metabolic crosstalk
between CAFs and cancer cells. In addition, lncRNAs and miRNAs, as
relatively recently discovered biomolecules, play an important role
in the interaction between CAFs and cancer cells, affecting
metabolic reprogramming. Thus, lncRNAs and miRNAs can be developed
as therapeutic targets. The study of the metabolic reprogramming of
CAFs will help to better understand the activation process, and the
interaction between the stroma and cancer cells, and provide new
therapeutic strategies for the development of the original tumor
activity of CAFs. CAFs, cancer-associated fibroblasts; OXPHOS,
oxidative phosphorylation; MCT, monocarboxylic acid transporter;
ROS, reactive oxygen species; CAV-1, caveolin-1; HIF-1α,
hypoxia-inducible factor 1α; NF-κB, nuclear factor-κB; lncRNAs,
long non-coding RNAs; miRNAs, microRNAs. |
Effect of ‘Reverse Warburg effect’ on
metabolic signaling pathways related to CAFs
Studies have shown that the differentiation of CAFs
is necessary for the occurrence and development of cancer, which
can occur in the early stages of cancer (12), or before genetic changes in
epithelial cells, triggering the malignant transformation of
neighboring cells (62). Metabolic
reprogramming of CAFs has been detected in breast cancer, lung
cancer, PCa, gastric cancer, HNSCC, lymphoma and other solid
cancers (63). Factors secreted by
tumor cells, as well as cytokines and growth factors, will affect
the differentiation of CAFs, including TGF-β, EGF, PDGFα, PDGFβ,
bFGF, IL-6 and IL-1β (14,64). Therefore, CAFs are closely related
to the signal transmission pathways of tumor cells, and a deeper
understanding of the signaling mechanisms between them is required.
The following summarizes some related important signaling
pathways.
ROS-mediated CAFs and the ‘Reverse
Warburg effect’ of cancer cells
Metabolic reprogramming is mainly controlled by
oxidative stress and hypoxia. The high levels of ROS produced by
cancer cells can induce oxidative stress in CAFs, produce
autophagosomes fused with lysosomes, destroy mitochondria, and
degrade Cav-1 (65,66). A reduction or mutation of Cav-1 (a
marker of autophagy, glycolysis, and oxidative stress) is one of
the characteristics of fibroblasts in tumor tissues (67). However, downregulation of Cav-1 in
CAFs will lead to higher ROS levels in cancer cells, which will
induce oxidative stress in CAFs via a positive feedback loop
(68). CAFs and cancer cells
maintain their tumor-promoting potential through self-stimulation
and cross-communication. In addition, downregulation of Cav-1 in
fibroblasts is related to the induction of TGF-β signaling
(69). Activation of TGF-β
downregulates isocitrate dehydrogenase 1 (IDH1), allowing
intracellular accumulation of α-ketoglutarate, leading to
downregulation of Cav-1 (70).
Downregulation of Cav-1 downregulates prolyl hydroxylase
domain-containing protein (PHD) and activity (19), while PHD is involved in the
hydroxylation of HIF-1α and IκB. Activation of HIF and NF-κB leads
to transcription of glycolytic genes (71). In addition, Cav-1 inhibits NOX2 and
NOX5 from producing ROS by directly binding to these enzymes.
Furthermore, Cav-1 can also inhibit NOX2 and NOX4
gene expression and protein synthesis by inhibiting the NF-κB
pathway (38).
Under normal physiological oxygen concentrations,
the α-subunit of HIF is hydroxylated by PHD and then degraded by
the E3-ligase and Hippel-Lindau (VHL) protein (72). Accumulated ROS or a hypoxic
environment inhibits the production of PHD, reduces the
hydroxylation level of HIF-1α, and activates it to stabilize the
HIF protein. However, the detailed mechanism of this process is not
clear (73). HIF-1α induces
hypoxia, promotes the transcription of angiogenic factors (such as
VEGF), and mediates autophagy, mitochondrial autophagy, and aerobic
glycolysis (74,75), thereby affecting metabolic
reprogramming. In addition, SIRT3 (a mitochondrial NAD-dependent
deacetylase) in CAFs also affects the stability of ROS and HIF
(76). A previous study
demonstrated that HIF can regulate and drive the glycolysis of CAFs
through SIRT3. In addition, HIF-1 regulates several genes involved
in glucose metabolism, such as MCT4 and those encoding
glucose transporters (GLUT1 and GLUT3) to increase
the uptake of glucose by cells, and affects the output of lactic
acid (77). HIF also activates
PKM2 transcription, which affects the glucose metabolism of
epithelial cells (43). Hypoxia
also induces oxidative ATM to promote the glycolytic activity of
CAFs by phosphorylating GLUT1 at S490 and increasing the expression
of PKM2. In addition, the lactic acid produced by CAFs under
hypoxic conditions will act as a metabolic conjugate between CAFs
and breast cancer cells, and by activating the TGF-β1/p38
MAPK/MMP2/9 signal axis, it promotes the mitochondrial activity of
cancer cells, thereby promoting breast cancer cell invasion
(78). Therefore, the oxidative
stress induced by ROS production has a significant impact on CAF
metabolism, driving CAF aerobic glycolysis, which in turn has a
profound impact on the TME and affects the metabolic reprogramming
of tumor cells. Put simply, CAFs provide cancer cells with a rich
array of compounds and induce antioxidant defenses in cancer cells,
thereby allowing cancer cells to proliferate.
Increased ROS levels in the tumor environment also
induce the activity of the pro-inflammatory transcription factor
NF-κB in fibroblasts, resulting in a CAF-like phenotype (79). The NF-κB target gene
cyclooxygenase-2 (COX2) is upregulated in solid tumors and
CAFs (80). In addition, activation
of NF-κB inhibits the accumulation of ROS by GPX, which in turn
causes oxidative stress in CAFs (81).
In general, the signaling pathways activated by ROS
activate the metabolic reprogramming of CAFs, which in turn affects
the TME, and thus the metabolism of tumor cells (Fig. 2).
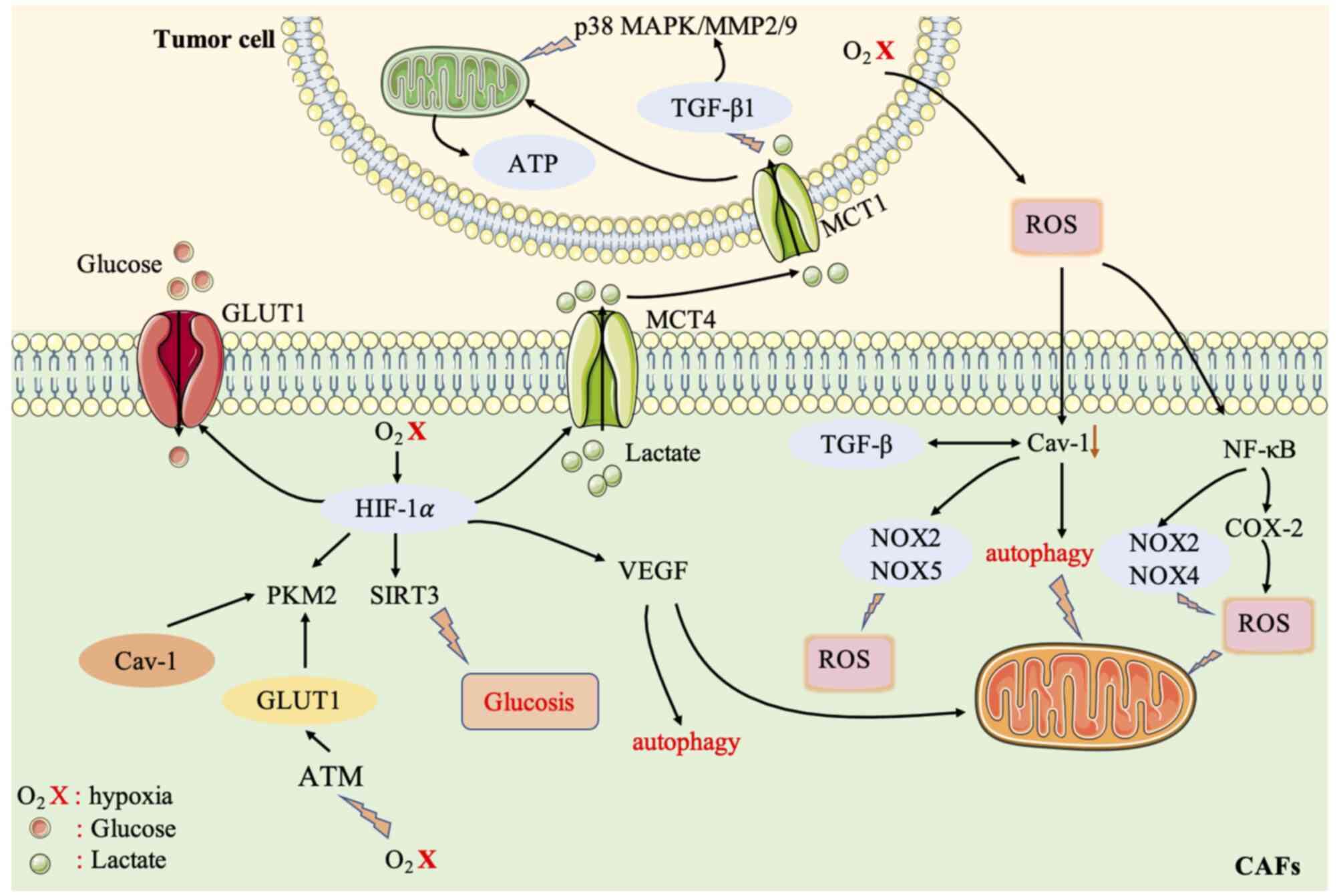 | Figure 2.Crosstalk between CAFs and cancer
cells is mediated by the ROS signaling pathway. The high level of
ROS produced by cancer cells can induce oxidative stress in CAFs,
induce autophagosomes to fuse with lysosomes, and degrade
mitochondria and Cav-1. Cav-1 inhibits NOX2 and NOX5 from producing
ROS by directly binding to the enzymes. Cav-1 can also inhibit
NOX2 and NOX4 gene expression and protein synthesis
by inhibiting the NF-κB pathway, thereby regulating the
re-metabolic programming of CAFs. Accumulated ROS or a hypoxic
environment reduces the hydroxylation level of HIF-1α and affects
the HIF protein. HIF-1α can induce hypoxia, promote the
transcription of angiogenic factors (such as VEGF) and mediate
autophagy, mitochondrial autophagy, and aerobic glycolysis. HIF-1α
affects several important metabolic enzymes of glycolysis. HIF-1α
affects glucose transporters (GLUT1) to increase the uptake
of glucose by cells, affects MCT4, and affects the output of lactic
acid. HIF-1α also activates PKM2 transcription, which alters
the glucose metabolism of epithelial cells. Hypoxia also induces
oxidative ATM to promote the glycolytic activity of CAFs by
phosphorylating GLUT1 at S490 and increasing the expression of
PKM2. Lactate produced by CAFs under hypoxic conditions acts as a
metabolic conjugate between CAFs and breast cancer cells, and
promotes the mitochondrial activity of cancer cells by activating
the TGF-β1/p38 MAPK/MMP2/9 signaling axis. Increased ROS levels in
the tumor environment also induce the activity of the
pro-inflammatory transcription factor NF-κB in fibroblasts, leading
to a CAF-like phenotype and affecting the glycolysis of CAFs. CAFs,
cancer-associated fibroblasts; ROS, reactive oxygen species; CAV-1,
caveolin-1; NOX, NADPH oxidase; NF-κB, nuclear factor-κB; HIF-1α,
hypoxia-inducible factor 1α; VEGF, vascular endothelial growth
factor; MCT, monocarboxylic acid transporter; PKM2, pyruvate kinase
M2; TGF, transforming growth factor; MMP, matrix
metalloproteinase. |
TGF-β signaling pathway mediates the
‘Reverse Warburg effect’ between CAFs and cancer cells
TGF-β plays a key role in the differentiation of
CAFs and participates in their differentiation and metabolic
regulation (70). The activation of
TGF-β in fibroblasts increases oxidative stress,
autophagy/mitochondrial autophagy, aerobic glycolysis, and the
downregulation of Cav-1, thereby affecting surrounding fibroblasts
and supporting cancer cell growth (82). The loss of Cav-1 in stromal cells
drives the activation of TGF-β signaling, thereby increasing the
transcription of TGF-β target genes, such as encoding connective
tissue growth factor (CTGF). Overexpression of CTGF
can activate HIF1, promote autophagy, glycolysis, senescence, and
metabolism of tumor-associated fibroblasts, thereby promoting tumor
growth. On the one hand, TGF-β can regulate the expression of α-SMA
in fibroblasts to affect ROS levels (9), stimulate oxidative stress, thus
affecting CAFs and tumor cells. On the other hand, TGF-β can
upregulate the production of NOX4 and ROS to induce the
differentiation of prostate CAFs (83).
The TGF-β signaling pathway is also related to the
expression level of some metabolic enzymes, such as IDH1. A
study has found that the TGF-β receptor (TGFBR)-IDH1-Cav-1 axis can
trigger TGF-β signaling in fibroblasts (56). IDH1 is an enzyme that converts
isocitrate into α-ketoglutarate (α-KG) in an
NADP+-dependent manner. Downregulation of IDH1 will
increase cellular α-KG levels and inhibit Cav-1 expression. Cav-1
downregulation inhibits TGFBR protein degradation and induces TGF-β
signaling. Therefore, the TGFBR-IDH1-Cav-1 axis affects fibroblast
TGF-β signaling and is an autocrine loop (56).
In addition, some research groups have found that in
CAFs, although IDH3α and IDH1 are downregulated, IDH3α
knockdown increases glycolysis and inhibits oxidative
phosphorylation in fibroblasts, while IDH1 or IDH2
knockdown does not. Moreover, overexpression of IDH3α
prevents fibroblasts from converting into CAFs (84). Downregulation of IDH3α
reduces the level of α-KG by reducing the ratio of α-KG to fumaric
acid and succinic acid, thereby inhibiting PHD2 activity in CAFs,
which increases the stability and the level of HIF-1α. The
accumulation of HIF-1α in turn increases the uptake of glucose and
the production of lactic acid, thus reducing oxygen consumption and
promoting glycolysis, and inhibits OXPHOS by upregulating NDUFA4L2,
a negative regulator of complex I. This demonstrates that
IDH3α is a key metabolic switch in CAFs (84). A recent study revealed that TGF-β
type II receptor (TGF-βRII) nuclear translocation of PKM2 inhibits
glucose metabolism in CAFs, thereby inhibiting oral cancer tumor
growth (85).
A previous study also determined that TGF-β1
downregulates the biosynthesis of acetyl-CoA by regulating the
pyruvate dehydrogenase complex (PDC), thereby regulating CAF
activation. The reduction of acetyl-CoA and acetylated substrates
also leads to the general loss of acetylation of TGF-β1 protein,
which in turn affects CAFs (86).
It can thus be concluded that the TGF-β signaling pathway is a key
metabolic pathway between CAFs and tumor cells, and it is
particularly important to understand the relevant mechanisms of the
TGF-β signaling pathway in CAFs (Fig.
3).
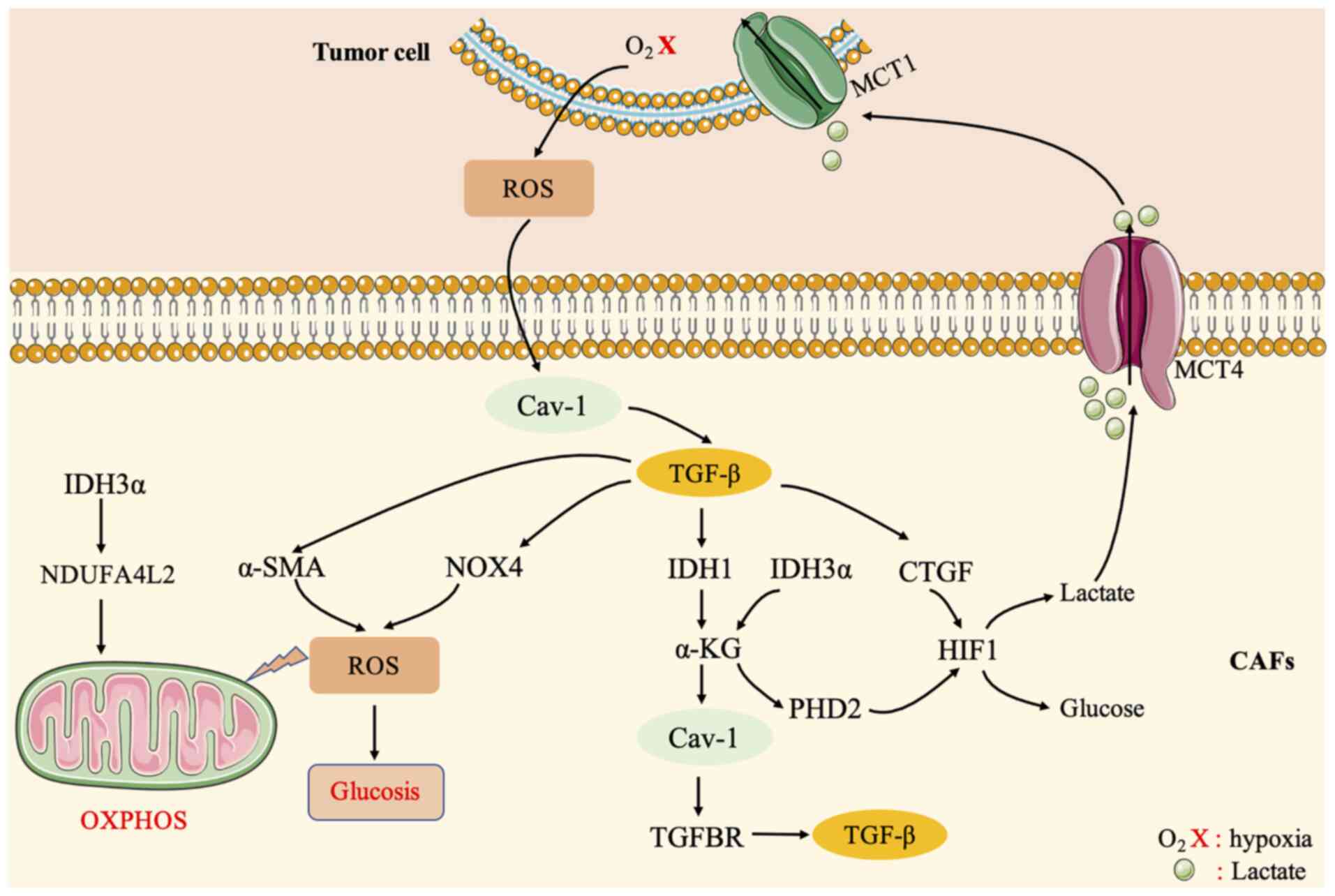 | Figure 3.Crosstalk between CAFs and cancer
cells is mediated by the TGF-β signaling pathway. TGF-β plays a key
role in the differentiation of CAFs and participates in the
differentiation and metabolic regulation of CAFs. The activation of
TGF-β in fibroblasts increases oxidative stress,
autophagy/mitochondrial autophagy, aerobic glycolysis, and
downregulation of Cav-1, thereby affecting surrounding fibroblasts
and supporting cancer cell growth. The loss of Cav-1 drives the
activation of TGF-β signaling and affects CTGF. CTGF can activate
HIF1 and promote autophagy, glycolysis, and senescence of
tumor-related fibroblasts. In turn, metabolism promotes tumor
growth. On the one hand, TGF-β can regulate the expression of α-SMA
in fibroblasts to affect ROS levels, stimulate oxidative stress,
and then affect CAFs and tumor cells. On the other hand, TGF-β can
upregulate the production of NOX4 and ROS to induce the
differentiation of prostate CAFs. The TGFBR-IDH1-Cav-1 axis can
trigger TGF-β signaling in fibroblasts. In addition, knockdown of
IDH3α increases glycolysis and inhibits oxidative
phosphorylation in fibroblasts. IDH3α enhances the negative
regulation of complex I because NDUFA4L2 inhibits OXPHOS. CAFs,
cancer-associated fibroblasts; TGF, transforming growth factor;
CAV-1, caveolin-1; CTGF, connective tissue growth factor; HIF1,
hypoxia-inducible factor 1; ROS, reactive oxygen species; NOX,
NADPH oxidase; TGFBR, TGF-β receptor; IDH1, isocitrate
dehydrogenase 1; IDH3α, isocitrate dehydrogenase [NAD(+)] 3
catalytic subunit α; OXPHOS, oxidative phosphorylation. |
G protein-coupled estrogen receptor
(GPER)-mediated CAFs and the ‘Reverse Warburg effect’ of cancer
cells
GPER is a seven-transmembrane-related estrogen
receptor belonging to the G protein-coupled receptor family, and is
usually upregulated in breast CAFs. GPER, originally known as
GPR30, is an alternative estrogen receptor that is structurally
distinct from ERα and ERβ and primarily mediates rapid non-genomic
responses (87). GPER can regulate
cell signaling pathways, and promote breast cancer proliferation,
chemoresistance, metastasis, and drug resistance. In breast cancer,
80% of fibroblasts are activated as CAFs (88,89).
In vitro experiments have shown that GPER can be expressed
in breast cancer CAFs and affects the proliferation and migration
of breast cancer cells in response to estrogen signals (90). Therefore, the mechanism of GPER in
breast cancer may be related to CAFs in the TME. Compared with
normal breast tissues and cells, GPER expression in breast cancer
tissues and cells decreased, and GPER expression in CAFs increased.
Overexpression of GPER in CAF promotes the expression of
Col-1, and its downregulation inhibits the expression of
Col-1. The upregulation of GPER in CAFs can promote the
proliferation, invasion, and migration of cancer cells, while its
downregulation in CAFs has the opposite effects on cancer cells.
Therefore, GPER can promote the proliferation, migration, and
invasion of triple-negative breast cancer (TNBC) cells through CAFs
(91).
Recent research has investigated the role of GPER
and hypoxia in the differentiation of CAFs. The expression of GPER
in breast CAFs is related to the activation of CAFs induced by
hypoxia and the invasion of breast cancer cells. GPER
knockdown eliminated hypoxia-driven CAF formation, inhibited breast
cancer cell invasion induced by CAF-conditioned medium, and
eliminated hypoxia-activated CTGF, VEGF and IL-6 secretion from
CAFs (23).
In addition, GPER mediates multidrug resistance in
ER positive and negative breast cancer and CAFs in the TME. Thus,
it may be a new agent that promotes drug resistance of tumor cells.
The PI3K/AKT signaling pathway, activated by breast tumor cells,
induces GPER translocation in CAFs in a chromosome region
maintenance 1 (CRM1)-dependent manner, and triggers the aerobic
glycolysis switch in CAFs through the estrogen/GPER/cAMP/PKA/CREB
signal axis. Glycolysis in CAFs supplies additional pyruvate and
lactic acid to tumor cells to enhance mitochondrial activity,
leading to multi-drug resistance (63).
In summary, GPER in breast CAFs can not only promote
the proliferation, migration, and invasion of TNBC cells, but also
eliminate the formation of CAFs driven by hypoxia, and affect the
crosstalk between CAFs and tumor cells. Notably, the aerobic
glycolysis switch in CAFs can be triggered through the
estrogen/GPER/cAMP/PKA/CREB signaling axis, such that tumor cells
develop drug resistance, suggesting the GPER could be used as a
target for drug resistance therapy.
IL-6 signaling molecule mediates the
‘Reverse Warburg effect’ of CAFs and cancer cells
The pro-inflammatory cytokine IL-6 in the TME is
closely related to CAFs. IL-6 is a multifunctional cytokine
originally considered to be a regulator of immune and inflammatory
responses (92). IL-6 exerts its
effects by binding to the IL-6α chain and the cytokine receptor
signaling subunit gp130, activating the Janus kinase (JAK) family
of tyrosine kinases and the signal transducer and activator of
transcription (STAT)3 family, thereby regulating target gene
transcription (93).
Phosphorylation of STAT proteins (1 and 3) can induce the
trans-differentiation of normal fibroblasts into CAFs. A previous
study showed that when exposed to IL-6 secreted by adjacent gastric
cancer cells, primary gastric fibroblasts that constitutively
express phosphorylated STAT3 will convert to CAFs (94). In addition, the exosomes exuded by
prostate cancer cells under hypoxic conditions contain high levels
of IL-6. IL-6 and several other signaling molecules regulate
microenvironmental remodeling, EMT, cancer cell stemness, and CAF
conversion and differentiation (95). Increased expression of IL-6 has also
been reported in CAFs of breast and ovarian tumors (96), indicating that the association
between IL-6 and CAFs is particularly important. However, at
present, it is not clear how IL-6 regulates the metabolic
reprogramming of CAFs. It is worth noting that IL-6 is likely to
link inflammation with the enhanced glycolysis in CAFs. This
process may be the key to the stimulation of STAT3 by IL-6 and the
regulation of certain glycolytic enzymes, such as
phosphofructokinase, hexokinase, and fructose-2,6-bisphosphatase
(F2,6BP) (97). In addition,
studies have found that the transcription factor Twist1 regulates
the IL-6 signaling cascade and affects the differentiation of CAFs.
When the TME of a solid tumor mass is hypoxic, the overexpression
of Twist1 inhibits the senescence of normal fibroblasts and the
tumor-promoting effect of CAFs on gastric cancer in the TME.
Therefore, Twist1 has been proven to be a highly effective target
molecule for anticancer therapy, and the hypoxia-induced
Twist1/IL-6 axis provides an important target for regulating the
metabolism of CAFs (98,99). Thus, the pro-inflammatory cytokine
IL-6 in the TME links inflammation and CAF metabolic reprogramming,
which could provide a new target for targeting CAF metabolism, and
exhibits favorable development and application prospects.
lncRNAs and microRNAs associated with the
‘Reverse Warburg effect’ of CAFs
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNAs with a length of more than 200 nucleotides that
have no protein coding ability. LncRNAs participate in various
physiological and pathological processes, including development,
the immune response, and tumorigenesis (100,101). LncRNAs can control biological
processes by interacting with other cellular molecules, including
DNA, protein, and RNA. It has been demonstrated that lncRNAs can
combine directly with key glycolytic enzymes (102). The enhanced transcription of
glycolytic enzyme genes activated by lncRNAs regulates glycolysis
in cancer cells (103). In
addition, studies have found that the regulation of glycolysis by
lncRNAs can be linked to CAFs. CAF-secreted CXCL14 acts on ovarian
cancer cells in a paracrine manner. Microarray analysis showed that
numerous lncRNAs are dysregulated in ovarian cancer. Among them,
lncRNA LINC00092 plays an important role in ovarian cancer cells by
interacting with 6-phosphate
fructose-2-kinase/fructose-2,6-bisphosphatase 2 (PFKFB2), which
increases glycolysis levels and ovarian cancer metastasis. In turn,
the glycolytic phenotype of ovarian cancer cells maintains the
CAF-like fibroblasts in the TME, forming a regulatory feedback loop
in the TME (104). Another lncRNA,
urothelial carcinoma associated 1 (UCA1), is induced in
glioma cells through the paracrine activity of CXCL14 on
glioblastoma stromal cells (105).
In addition, the CXCL14-UCA1-miR-182-PFKFB2 axis regulates the
interaction between glioma cells and glioblastoma-related stromal
cells, and promotes glycolysis and glioma invasion (106).
MicroRNAs (miRNAs) are small noncoding RNAs that
regulate numerous biological functions and are key players in
regulating cancer development and metastasis. Studies have found
that several miRNAs are related to CAF conversion; however, their
mechanism of action in CAF cell formation has not yet been fully
determined. In addition, some changes in miRNA expression levels
have been observed during the formation of activated CAFs in normal
fibroblasts (107,108). Among them, the expression levels
of miR-221-5p, miR-31-3p, and miR-221-3p were enhanced, and the
levels of miR-205, miR-200b, miR200c, miR-141, miR-101, and miR-342
were decreased. These miRNAs affect the conversion of CAFs by
regulating the activity of a variety of growth factors, cytokines,
and other cell signaling molecules, including HGF, insulin, MAPK,
tight and adhesion junction proteins, EGF1, androgen receptor, Wnt,
IL-7, and the TGF-β/IL-6 signaling pathway (109).
miRNAs can directly or indirectly regulate the
metabolism of glucose and lipids, amino acid biosynthesis, other
multi-level and multi-path metabolic enzymes, oncogenes, and tumor
suppressor genes, thereby affecting tumor metabolism. Studies have
also found that miRNAs can affect the metabolism of tumor stromal
cells, thereby affecting tumor progression (110–112). miR-186 is downregulated during the
formation of CAFs. This miRNA regulates the level of the
membrane-bound glucose transporter GLUT1 by binding to the
3’-untranslated region (UTR) of GLUT1, which promotes its
degradation. A study has also found that miR-181c can inhibit
glycolysis in CAFs by inhibiting HK2 expression (113). In addition, a study indicated that
after miR-21-inhibitor treatment, the degree of glycolysis in CAFs
decreased. Following indirect co-culture with CAFs, the oxidative
phosphorylation and the expression of SDH, FH, and MCT in BxPc-3
cells increased. After co-culture with miR-21-inhibitor-CAFs, the
oxidative phosphorylation and invasion ability of pancreatic cancer
cells were reduced. Therefore, miR-21 participates in the metabolic
changes of CAFs and affects the development of cancer cells, and
thus may be a therapeutic target for the treatment of pancreatic
cancer (114).
As relatively recently discovered biomolecules,
lncRNAs and miRNAs have attracted increased attention from cancer
researchers. They play an important role in the interaction of CAFs
with cancer cells, affecting metabolic reprogramming, and can
target mRNAs and proteins. As therapeutic targets, they provide a
new clinical treatment paradigm.
CAFs and potential therapeutic targets
The crosstalk between cancer cells and CAFs is
closely related to the reprogramming of cellular metabolism. By
providing the energy needed by tumor cells, CAFs lead to increased
mitochondrial activity in tumor cells, and tumor cells acquire drug
resistance (23). Therefore,
potential therapeutic targets for blocking the metabolic crosstalk
between CAFs and tumor cells are very important.
HK2 is an important anticancer drug target (115). Various HK2 inhibitors have been
identified, including 2-deoxyglucose (2-DG), 3-bromopyruvate
(3-BP), and lonidamine (LND). 2-DG and LND were discontinued
because of adverse reactions in clinical trials (116,117). 3-BP can reduce ATP reserves to
reverse chemoresistance (118).
3-BP has some adverse reactions, and therefore, it was encapsulated
into liposome nanocarriers (T-Lipo-3-BP), which were specifically
delivered to the tumor after systemic administration in a mouse
tumor model, to eliminate severe side effects and inhibit tumor
growth (119).
6-Phosphofructo-2-kinase fructose-2,6-bisphosphatase-3 (PFKFB3) is
a potent regulator of glycolysis (120). PFK15, a derivative of a PFKFB3
inhibitor (3PO), was able to induce the rapid induction of
apoptosis in transformed cells, exerting an antitumor effect
(121). Phase I/II trials of
PFK158 in combination with targeted agents are underway (122).
MCT4 is an exporter of glycolysis products and
lactate and MCT1 imports lactate into tumors (123). The inhibition of MCT1/MCT4 blocks
the metabolic crosstalk between CAFs and tumor cells (124). Syrosingopine, a dual MCT1/MCT4
inhibitor, reduced NAD+ levels by causing lactate
accumulation and inhibiting LDH in a mouse model of liver cancer
(124). Most other current small
molecule MCT inhibitors target MCT1, and one drug, AZD3965, is
currently in clinical trials. However, the drug was revealed to be
ineffective in the presence of MCT4 expression (125). A clinical trial demonstrated that
the antioxidant N-acetylcysteine (NAC) can reduce the expression of
MCT4 in CAFs of patients with cancer and reduces the proliferation
rate of cancer cells in women with stage 0 and stage I breast
cancer. It is effective and safe in breast cancer treatment
(126). In addition, quercetin,
metformin, and chloroquine also significantly reduced the
expression of MCT4 in CAFs (19,127,128). In some patients with aggressive
B-cell lymphoma with lactic acidosis, metformin and sirolimus can
inhibit the activation of the ‘lactate shuttle’ (or high expression
of MCT4), reduce the expression of LDHB and PKM1, and inhibit
CAF-carcinoma cellular metabolism coupling (129).
Antioxidant treatment can also block the metabolic
cross-talk between CAFs and tumor cells, which should inhibit ROS
(122). NAC treatment effectively
inhibits tumor growth by preventing DNA damage and genetic
instability, and reducing HIF-1 levels (130). Metformin acts as a complex I
inhibitor and blocks mitochondria-dependent ROS production
(131). In addition, NAC,
metformin, L-NAME (a nitric oxide NO inhibitor), quercetin, and
chloroquine can restore Cav-1 expression, thereby inhibiting ROS
generation. Clinical trials of metformin alone and in combination
with standard therapy are ongoing in multiple tumor types (19,132).
Furthermore, based on the regulation of Cav-1, MCT4, and HIF-1α
expression in CAFs, the combination of acetylcysteine
(N-acetyl-L-cysteine) and topotecan is being tested in patients
with ovarian cancer in phase II clinical trials (Fig. 4) (133).
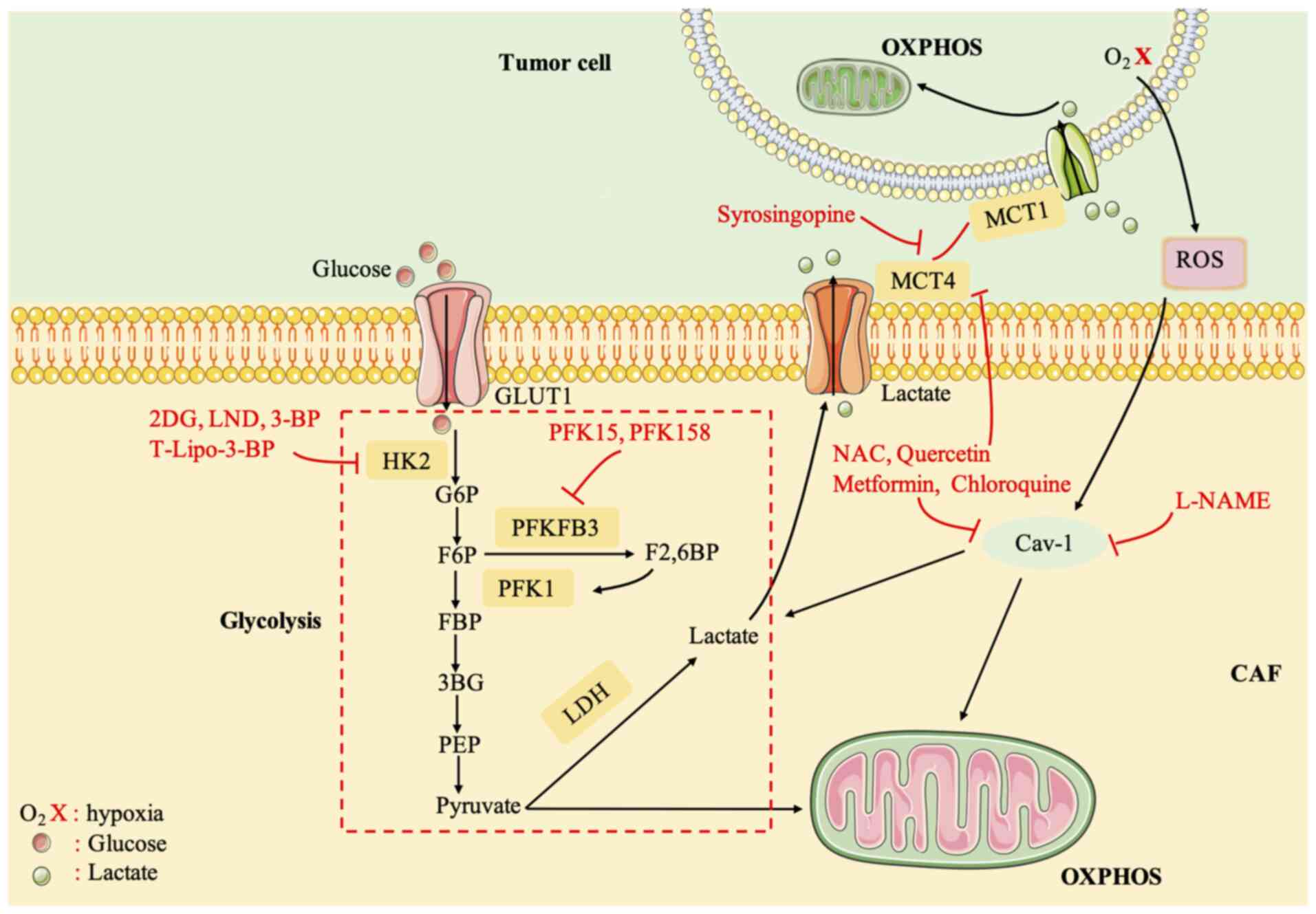 | Figure 4.Compounds targeting CAF metabolism.
HK2, hexokinase 2; G-6-P, glucose-6-phosphate; F6P,
fructose-6-phosphate; FBP, D-fructose-1,6-bisphosphate; PFK1,
6-phosphofructo-1-kinase; 3PG, 3-phosphoglycerate; F2,6BP,
fructose-2,6-bisphosphatase; PEP, phosphoenolpyruvate; PFKFB3,
6-phosphofructo-2-kinase fructose-2,6-bisphosphatase-3; LDH,
lactate dehydrogenase; CAF, cancer-associated fibroblast; OXPHOS,
oxidative phosphorylation. |
Conclusions
The occurrence and development of tumors are related
to the metabolic reprogramming of the TME, in which the metabolic
reprogramming of CAFs is very important. The metabolic
reprogramming of CAFs activates a variety of signaling pathways
that act on tumor cells, and in turn, tumor cells will also affect
CAFs, such as in the differentiation and the activation of related
signaling pathways. The metabolic reprogramming of CAFs is closely
related to the proliferation, metastasis, angiogenesis, drug
resistance, and other aggressive behaviors of cancer cells.
Therefore, the metabolic reprogramming of CAFs and cancer crosstalk
increase the heterogeneity and plasticity of cancer metabolism.
This will closely link the study of tumor cells with the TME, and
help researchers to study the occurrence and development of tumors,
providing a series of new predictive biomarkers and strategies for
anticancer treatment. Therefore, in-depth study of the metabolic
reprogramming of CAFs is of great significance and will provide new
methods for future cancer treatments.
Acknowledgements
Not applicable.
Funding
This work was supported by the Hunan Provincial Natural Science
Foundation (grant no. 2021JJ30915), and the Fundamental Research
Funds for the Central Universities of Central South University.
Availability of data and materials
Not applicable.
Authors' contributions
LL wrote the manuscript and drew the figures. XL,
XJ, WL, and QL collected the related studies and helped to revise
the manuscript. YZ and YL designed and revised the manuscript. Data
authentication is not applicable to the present review. All the
authors read and approved the final version of the review.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang P, Du W and Wu M: Regulation of the
pentose phosphate pathway in cancer. Protein Cell. 5:592–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng J: Energy metabolism of cancer:
Glycolysis versus oxidative phosphorylation (Review). Oncol Lett.
4:1151–1157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alfarouk KO, Shayoub ME, Muddathir AK,
Elhassan GO and Bashir AH: Evolution of tumor metabolism might
reflect carcinogenesis as a reverse evolution process (Dismantling
of Multicellularity). Cancers (Basel). 3:3002–3017. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu XD, Shao SX, Jiang HP, Cao YW, Wang YH,
Yang XC, Wang YL, Wang XS and Niu HT: Warburg effect or reverse
Warburg effect? A review of cancer metabolism. Oncol Res Treat.
38:117–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshida GJ: Metabolic reprogramming: The
emerging concept and associated therapeutic strategies. J Exp Clin
Cancer Res. 34:1112015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saada A: Mitochondria: Mitochondrial
OXPHOS (dys) function ex vivo-the use of primary fibroblasts. Int J
Biochem Cell Biol. 48:60–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arcucci A, Ruocco MR, Granato G, Sacco AM
and Montagnani S: Cancer: An oxidative crosstalk between solid
tumor cells and cancer associated fibroblasts. Biomed Res Int.
2016:45028462016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pertega-Gomes N, Vizcaino JR, Attig J,
Jurmeister S, Lopes C and Baltazar F: A lactate shuttle system
between tumour and stromal cells is associated with poor prognosis
in prostate cancer. BMC Cancer. 14:3522014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee M and Yoon JH: Metabolic interplay
between glycolysis and mitochondrial oxidation: The reverse Warburg
effect and its therapeutic implication. World J Biol Chem.
6:148–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buchsbaum RJ and Oh SY: Breast
cancer-associated fibroblasts: Where we are and where we need to
go. Cancers (Basel). 8:192016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Catalano V, Turdo A, Di Franco S, Dieli F,
Todaro M and Stassi G: Tumor and its microenvironment: A
synergistic interplay. Semin Cancer Biol. 23:522–532. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santi A, Kugeratski FG and Zanivan S:
Cancer associated fibroblasts: The architects of stroma remodeling.
Proteomics. 18:e17001672018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giannoni E, Bianchini F, Masieri L, Serni
S, Torre E, Calorini L and Chiarugi P: Reciprocal activation of
prostate cancer cells and cancer-associated fibroblasts stimulates
epithelial-mesenchymal transition and cancer stemness. Cancer Res.
70:6945–6956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lohr M, Schmidt C, Ringel J, Kluth M,
Müller P, Nizze H and Jesnowski R: Transforming growth factor-beta1
induces desmoplasia in an experimental model of human pancreatic
carcinoma. Cancer Res. 61:550–555. 2001.PubMed/NCBI
|
|
17
|
Shao ZM, Nguyen M and Barsky SH: Human
breast carcinoma desmoplasia is PDGF initiated. Oncogene.
19:4337–4345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G and Sahai E: Mechanotransduction and YAP-dependent
matrix remodelling is required for the generation and maintenance
of cancer-associated fibroblasts. Nat Cell Biol. 15:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinez-Outschoorn UE, Balliet RM,
Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes
D, Daumer KM, Lin Z, Witkiewicz AK, et al: Oxidative stress in
cancer associated fibroblasts drives tumor-stroma co-evolution: A
new paradigm for understanding tumor metabolism, the field effect
and genomic instability in cancer cells. Cell Cycle. 9:3256–3276.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cirri P and Chiarugi P: Cancer associated
fibroblasts: The dark side of the coin. Am J Cancer Res. 1:482–497.
2011.PubMed/NCBI
|
|
21
|
Hirata E, Girotti MR, Viros A, Hooper S,
Spencer-Dene B, Matsuda M, Larkin J, Marais R and Sahai E:
Intravital imaging reveals how BRAF inhibition generates
drug-tolerant microenvironments with high integrin β1/FAK
signaling. Cancer Cell. 27:574–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massague J: TGFβ signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avagliano A, Granato G, Ruocco MR, Romano
V, Belviso I, Carfora A, Montagnani S and Arcucci A: Metabolic
reprogramming of cancer associated fibroblasts: The slavery of
stromal fibroblasts. Biomed Res Int. 2018:60754032018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marin D and Sabater B: The cancer Warburg
effect may be a testable example of the minimum entropy production
rate principle. Phys Biol. 14:0240012017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pereira-Nunes A, Afonso J, Granja S and
Baltazar F: Lactate and lactate transporters as key players in the
maintenance of the warburg effect. Adv Exp Med Biol. 1219:51–74.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Draoui N and Feron O: Lactate shuttles at
a glance: From physiological paradigms to anti-cancer treatments.
Dis Model Mech. 4:727–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee N and Kim D: Cancer metabolism:
Fueling more than just growth. Mol Cells. 39:847–854. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson RB, Solass W, Archid R, Weinreich
FJ, Konigsrainer A and Reymond MA: Resistance to anoikis in
transcoelomic shedding: The role of glycolytic enzymes. Pleura
Peritoneum. 4:201900032019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Konjević G, Jurisić V, Jakovljević B and
Spuzić I: Lactate dehydrogenase (LDH) in peripheral blood
lymphocytes (PBL) of patients with solid tumors. Glas Srp Akad
Nauka Med. 137–147. 2002.(In Serbian). PubMed/NCBI
|
|
32
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Gatter KC and Harris AL: Pyruvate dehydrogenase and pyruvate
dehydrogenase kinase expression in non small cell lung cancer and
tumor-associated stroma. Neoplasia. 7:1–6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen D and Che G: Value of caveolin-1 in
cancer progression and prognosis: Emphasis on cancer-associated
fibroblasts, human cancer cells and mechanism of caveolin-1
expression (Review). Oncol Lett. 8:1409–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao S, Qin T, Qian W, Yue Y, Xiao Y, Li
X, Zhang D, Wang Z, Ma Q and Lei J: Positive feedback in Cav-1-ROS
signalling in PSCs mediates metabolic coupling between PSCs and
tumour cells. J Cell Mol Med. 24:9397–9408. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bist A, Fielding CJ and Fielding PE: p53
regulates caveolin gene transcription, cell cholesterol, and growth
by a novel mechanism. Biochemistry. 39:1966–1972. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Witkiewicz AK, Dasgupta A, Nguyen KH, Liu
C, Kovatich AJ, Schwartz GF, Pestell RG, Sotgia F, Rui H and
Lisanti MP: Stromal caveolin-1 levels predict early DCIS
progression to invasive breast cancer. Cancer Biol Ther.
8:1071–1079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pavlides S, Tsirigos A, Migneco G,
Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro
MC, Wang C, Pestell RG, et al: The autophagic tumor stroma model of
cancer: Role of oxidative stress and ketone production in fueling
tumor cell metabolism. Cell Cycle. 9:3485–3505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen F, Barman S, Yu Y, Haigh S, Wang Y,
Black SM, Rafikov R, Dou H, Bagi Z, Han W, et al: Caveolin-1 is a
negative regulator of NADPH oxidase-derived reactive oxygen
species. Free Radic Biol Med. 73:201–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sotgia F, Martinez-Outschoorn UE, Pavlides
S, Howell A, Pestell RG and Lisanti MP: Understanding the Warburg
effect and the prognostic value of stromal caveolin-1 as a marker
of a lethal tumor microenvironment. Breast Cancer Res. 13:2132011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen C, Chen X, Xiao K and Che G: New
relationship of E2F1 and BNIP3 with caveolin-1 in lung
cancer-associated fibroblasts. Thorac Cancer. 11:1369–1371. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Q, Liu L, Zhao Y, Zhang J, Wang D,
Chen J, He Y, Wu J, Zhang Z, Liu Z, et al: Hypoxia induces genomic
DNA demethylation through the activation of HIF-1α and
transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer
Ther. 10:1113–1123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang HJ, Hsieh YJ, Cheng WC, Lin CP, Lin
YS, Yang SF, Chen CC, Izumiya Y, Yu JS, Kung HJ and Wang WC: JMJD5
regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated
glucose metabolism. Proc Natl Acad Sci USA. 111:279–284. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li X, Xu Q, Wu Y, Li J, Tang D, Han L and
Fan Q: A CCL2/ROS autoregulation loop is critical for
cancer-associated fibroblasts-enhanced tumor growth of oral
squamous cell carcinoma. Carcinogenesis. 35:1362–1370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qin X, Yan M, Wang X, Xu Q, Wang X, Zhu X,
Shi J, Li Z, Zhang J, Chen W, et al: Cancer-associated
Fibroblast-derived IL-6 promotes head and neck cancer progression
via the osteopontin-NF-kappa B signaling pathway. Theranostics.
8:921–940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chiarugi P and Cirri P: Metabolic
exchanges within tumor microenvironment. Cancer Lett. 380:272–280.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fiaschi T, Marini A, Giannoni E, Taddei
ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P and
Chiarugi P: Reciprocal metabolic reprogramming through lactate
shuttle coordinately influences tumor-stroma interplay. Cancer Res.
72:5130–5140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ippolito L, Morandi A, Taddei ML, Parri M,
Comito G, Iscaro A, Raspollini MR, Magherini F, Rapizzi E,
Masquelier J, et al: Cancer-associated fibroblasts promote prostate
cancer malignancy via metabolic rewiring and mitochondrial
transfer. Oncogene. 38:5339–5355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Martinez-Outschoorn U, Sotgia F and
Lisanti MP: Tumor microenvironment and metabolic synergy in breast
cancers: Critical importance of mitochondrial fuels and function.
Semin Oncol. 41:195–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pavlides S, Whitaker-Menezes D,
Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro
MC, Wang C, Fortina P, Addya S, et al: The reverse Warburg effect:
Aerobic glycolysis in cancer associated fibroblasts and the tumor
stroma. Cell Cycle. 8:3984–4001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roy A and Bera S: CAF cellular glycolysis:
Linking cancer cells with the microenvironment. Tumour Biol.
37:8503–8514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hasebe T, Mukai K, Tsuda H and Ochiai A:
New prognostic histological parameter of invasive ductal carcinoma
of the breast: Clinicopathological significance of fibrotic focus.
Pathol Int. 50:263–272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mao Y, Keller ET, Garfield DH, Shen K and
Wang J: Stromal cells in tumor microenvironment and breast cancer.
Cancer Metastasis Rev. 32:303–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu JW, Sun P, Zhang DX, Xiong WJ and Mi J:
Hexokinase 2 regulates G1/S checkpoint through CDK2 in
cancer-associated fibroblasts. Cell Signal. 26:2210–2216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pavlides S, Tsirigos A, Vera I, Flomenberg
N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn
UE, Howell A, et al: Transcriptional evidence for the ‘Reverse
Warburg Effect’ in human breast cancer tumor stroma and metastasis:
Similarities with oxidative stress, inflammation, Alzheimer's
disease, and ‘Neuron-Glia Metabolic Coupling’. Aging (Albany NY).
2:185–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chiavarina B, Whitaker-Menezes D,
Martinez-Outschoorn UE, Witkiewicz AK, Birbe R, Howell A, Pestell
RG, Smith J, Daniel R, Sotgia F and Lisanti MP: Pyruvate kinase
expression (PKM1 and PKM2) in cancer-associated fibroblasts drives
stromal nutrient production and tumor growth. Cancer Biol Ther.
12:1101–1113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Giannoni E, Taddei ML, Morandi A, Comito
G, Calvani M, Bianchini F, Richichi B, Raugei G, Wong N, Tang D and
Chiarugi P: Targeting stromal-induced pyruvate kinase M2 nuclear
translocation impairs oxphos and prostate cancer metastatic spread.
Oncotarget. 6:24061–24074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li Z, Yang P and Li Z: The multifaceted
regulation and functions of PKM2 in tumor progression. Biochim
Biophys Acta. 1846:285–296. 2014.PubMed/NCBI
|
|
60
|
Hamabe A, Konno M, Tanuma N, Shima H,
Tsunekuni K, Kawamoto K, Nishida N, Koseki J, Mimori K, Gotoh N, et
al: Role of pyruvate kinase M2 in transcriptional regulation
leading to epithelial-mesenchymal transition. Proc Natl Acad Sci
USA. 111:15526–15531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sung JS, Kang CW, Kang S, Jang Y, Chae YC,
Kim BG and Cho NH: ITGB4-mediated metabolic reprogramming of
cancer-associated fibroblasts. Oncogene. 39:664–676. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qiao A, Gu F, Guo X, Zhang X and Fu L:
Breast cancer-associated fibroblasts: Their roles in tumor
initiation, progression and clinical applications. Front Med.
10:33–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yu T, Yang G, Hou Y, Tang X, Wu C, Wu XA,
Guo L, Zhu Q, Luo H, Du YE, et al: Cytoplasmic GPER translocation
in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic
axis to confer tumor cells with multidrug resistance. Oncogene.
36:2131–2145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ziani L, Chouaib S and Thiery J:
Alteration of the antitumor immune response by cancer-associated
fibroblasts. Front Immunol. 9:4142018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fu Y, Liu S, Yin S, Niu W, Xiong W, Tan M,
Li G and Zhou M: The reverse Warburg effect is likely to be an
Achilles' heel of cancer that can be exploited for cancer therapy.
Oncotarget. 8:57813–57825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Martinez-Outschoorn UE, Lisanti MP and
Sotgia F: Catabolic cancer-associated fibroblasts transfer energy
and biomass to anabolic cancer cells, fueling tumor growth. Semin
Cancer Biol. 25:47–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dabiri S, Talebi A, Shahryari J, Meymandi
MS and Safizadeh H: Distribution of myofibroblast cells and
microvessels around invasive ductal carcinoma of the breast and
comparing with the adjacent range of their normal-to-DCIS zones.
Arch Iran Med. 16:93–99. 2013.PubMed/NCBI
|
|
68
|
Capparelli C, Whitaker-Menezes D, Guido C,
Balliet R, Pestell TG, Howell A, Sneddon S, Pestell RG,
Martinez-Outschoorn U, Lisanti MP and Sotgia F: CTGF drives
autophagy, glycolysis and senescence in cancer-associated
fibroblasts via HIF1 activation, metabolically promoting tumor
growth. Cell Cycle. 11:2272–2284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Khan HY and Orimo A: Transforming growth
factor-β: Guardian of catabolic metabolism in carcinoma-associated
fibroblasts. Cell Cycle. 11:4302–4303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hou X, Zhang J, Wang Y, Xiong W and Mi J:
TGFBR-IDH1-Cav1 axis promotes TGF-β signalling in cancer-associated
fibroblast. Oncotarget. 8:83962–83974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Trimmer C, Sotgia F, Whitaker-Menezes D,
Balliet RM, Eaton G, Martinez-Outschoorn UE, Pavlides S, Howell A,
Iozzo RV, Pestell RG, et al: Caveolin-1 and mitochondrial SOD2
(MnSOD) function as tumor suppressors in the stromal
microenvironment: A new genetically tractable model for human
cancer associated fibroblasts. Cancer Biol Ther. 11:383–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Panday A, Inda ME, Bagam P, Sahoo MK,
Osorio D and Batra S: Transcription factor NF-κB: An update on
intervention strategies. Arch Immunol Ther Exp (Warsz). 64:463–483.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zwaans BM and Lombard DB: Interplay
between sirtuins, MYC and hypoxia-inducible factor in
cancer-associated metabolic reprogramming. Dis Model Mech.
7:1023–1032. 2014.PubMed/NCBI
|
|
74
|
De Francesco EM, Lappano R, Santolla MF,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou F, Du J and Wang J: Albendazole
inhibits HIF-1α-dependent glycolysis and VEGF expression in
non-small cell lung cancer cells. Mol Cell Biochem. 428:171–178.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fiaschi T and Chiarugi P: Oxidative
stress, tumor microenvironment, and metabolic reprogramming: A
diabolic liaison. Int J Cell Biol. 2012:7628252012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ullah MS, Davies AJ and Halestrap AP: The
plasma membrane lactate transporter MCT4, but not MCT1, is
up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J
Biol Chem. 281:9030–9037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sun K, Tang S, Hou Y, Xi L, Chen Y, Yin J,
Peng M, Zhao M, Cui X and Liu M: Oxidized ATM-mediated glycolysis
enhancement in breast cancer-associated fibroblasts contributes to
tumor invasion through lactate as metabolic coupling. EBioMedicine.
41:370–383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhu Y, Shi C, Zeng L, Liu G, Jiang W,
Zhang X, Chen S, Guo J, Jian X, Ouyang J, et al: High COX-2
expression in cancer-associated fibiroblasts contributes to poor
survival and promotes migration and invasiveness in nasopharyngeal
carcinoma. Mol Carcinog. 59:265–280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chan JS, Tan MJ, Sng MK, Teo Z, Phua T,
Choo CC, Li L, Zhu P and Tan NS: Cancer-associated fibroblasts
enact field cancerization by promoting extratumoral oxidative
stress. Cell Death Dis. 8:e25622017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Guido C, Whitaker-Menezes D, Capparelli C,
Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, Andò S,
Martinez-Outschoorn U, et al: Metabolic reprogramming of
cancer-associated fibroblasts by TGF-β drives tumor growth:
Connecting TGF-β signaling with ‘Warburg-like’ cancer metabolism
and L-lactate production. Cell Cycle. 11:3019–3035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sampson N, Koziel R, Zenzmaier C,
Bubendorf L, Plas E, Jansen-Dürr P and Berger P: ROS signaling by
NOX4 drives fibroblast-to-myofibroblast differentiation in the
diseased prostatic stroma. Mol Endocrinol. 25:503–515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang D, Wang Y, Shi Z, Liu J, Sun P, Hou
X, Zhang J, Zhao S, Zhou BP and Mi J: Metabolic reprogramming of
cancer-associated fibroblasts by IDH3α downregulation. Cell Rep.
10:1335–1348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wu F, Wang S, Zeng Q, Liu J, Yang J, Mu J,
Xu H, Wu L, Gao Q, He X, et al: TGF-βRII regulates glucose
metabolism in oral cancer-associated fibroblasts via promoting PKM2
nuclear translocation. Cell Death Discov. 8:32022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Smith ER and Hewitson TD: TGF-β1 is a
regulator of the pyruvate dehydrogenase complex in fibroblasts. Sci
Rep. 10:179142020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Pupo M, Maggiolini M and Musti AM: GPER
mediates non-genomic effects of estrogen. Methods Mol Biol.
1366:471–488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Madeo A and Maggiolini M: Nuclear
alternate estrogen receptor GPR30 mediates 17beta-estradiol-induced
gene expression and migration in breast cancer-associated
fibroblasts. Cancer Res. 70:6036–6046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Vivacqua A, Romeo E, De Marco P, De
Francesco EM, Abonante S and Maggiolini M: GPER mediates the Egr-1
expression induced by 17β-estradiol and 4-hydroxitamoxifen in
breast and endometrial cancer cells. Breast Cancer Res Treat.
133:1025–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
De Francesco EM, Sims AH, Maggiolini M,
Sotgia F, Lisanti MP and Clarke RB: GPER mediates the angiocrine
actions induced by IGF1 through the HIF-1alpha/VEGF pathway in the
breast tumor microenvironment. Breast Cancer Res. 19:1292017.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang K and Yao Y: Mechanism of GPER
promoting proliferation, migration and invasion of triple-negative
breast cancer cells through CAF. Am J Transl Res. 11:5858–5868.
2019.PubMed/NCBI
|
|
92
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bromberg J and Wang TC: Inflammation and
cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kinoshita H, Hirata Y, Nakagawa H,
Sakamoto K, Hayakawa Y, Takahashi R, Nakata W, Sakitani K, Serizawa
T, Hikiba Y, et al: Interleukin-6 mediates epithelial-stromal
interactions and promotes gastric tumorigenesis. PLoS One.
8:e609142013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ramteke A, Ting H, Agarwal C, Mateen S,
Somasagara R, Hussain A, Graner M, Frederick B, Agarwal R and Deep
G: Exosomes secreted under hypoxia enhance invasiveness and
stemness of prostate cancer cells by targeting adherens junction
molecules. Mol Carcinog. 54:554–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Erez N, Glanz S, Raz Y, Avivi C and
Barshack I: Cancer associated fibroblasts express pro-inflammatory
factors in human breast and ovarian tumors. Biochem Biophys Res
Commun. 437:397–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ando M, Uehara I, Kogure K, Asano Y,
Nakajima W, Abe Y, Kawauchi K and Tanaka N: Interleukin 6 enhances
glycolysis through expression of the glycolytic enzymes hexokinase
2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3. J
Nippon Med Sch. 77:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee KW, Yeo SY, Sung CO and Kim SH: Twist1
is a key regulator of cancer-associated fibroblasts. Cancer Res.
75:73–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Garen A: From a retrovirus infection of
mice to a long noncoding RNA that induces proto-oncogene
transcription and oncogenesis via an epigenetic transcription
switch. Signal Transduct Target Ther. 1:160072016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ma MZ, Zhang Y, Weng MZ, Wang SH, Hu Y,
Hou ZY, Qin YY, Gong W, Zhang YJ, Kong X, et al: Long Noncoding RNA
GCASPC, a Target of miR-17-3p, negatively regulates pyruvate
carboxylase-dependent cell proliferation in gallbladder cancer.
Cancer Res. 76:5361–5371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhao L, Ji G, Le X, Wang C, Xu L, Feng M,
Zhang Y, Yang H, Xuan Y, Yang Y, et al: Long Noncoding RNA
LINC00092 acts in cancer-associated fibroblasts to drive glycolysis
and progression of ovarian cancer. Cancer Res. 77:1369–1382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
He Z, You C and Zhao D: Long non-coding
RNA UCA1/miR-182/PFKFB2 axis modulates glioblastoma-associated
stromal cells-mediated glycolysis and invasion of glioma cells.
Biochem Biophys Res Commun. 500:569–576. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ahn YH and Kim JS: Long Non-Coding RNAs as
regulators of interactions between cancer-associated fibroblasts
and cancer cells in the tumor microenvironment. Int J Mol Sci.
21:74842020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mitra AK, Zillhardt M, Hua Y, Tiwari P,
Murmann AE, Peter ME and Lengyel E: MicroRNAs reprogram normal
fibroblasts into cancer-associated fibroblasts in ovarian cancer.
Cancer Discov. 2:1100–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li P, Shan JX, Chen XH, Zhang D, Su LP,
Huang XY, Yu BQ, Zhi QM, Li CL, Wang YQ, et al: Epigenetic
silencing of microRNA-149 in cancer-associated fibroblasts mediates
prostaglandin E2/interleukin-6 signaling in the tumor
microenvironment. Cell Res. 25:588–603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zeng Z, Hu P, Tang X, Zhang H, Du Y, Wen S
and Liu M: Dectection and analysis of miRNA expression in breast
cancer-associated fibroblasts. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
30:1071–1075. 2014.(In Chinese). PubMed/NCBI
|
|
110
|
Wang Z, Tan Y, Yu W, Zheng S, Zhang S, Sun
L and Ding K: Small role with big impact: miRNAs as communicators
in the cross-talk between cancer-associated fibroblasts and cancer
cells. Int J Biol Sci. 13:339–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Vallabhaneni KC, Hassler MY, Abraham A,
Whitt J, Mo YY, Atfi A and Pochampally R: Mesenchymal Stem/Stromal
cells under stress increase osteosarcoma migration and apoptosis
resistance via extracellular vesicle mediated communication. PLoS
One. 11:e01660272016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tang H, Lee M, Sharpe O, Salamone L,
Noonan EJ, Hoang CD, Levine S, Robinson WH and Shrager JB:
Oxidative stress-responsive microRNA-320 regulates glycolysis in
diverse biological systems. FASEB J. 26:4710–4721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sun P, Hu JW, Xiong WJ and Mi J: miR-186
regulates glycolysis through Glut1 during the formation of
cancer-associated fibroblasts. Asian Pac J Cancer Prev.
15:4245–4250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen S, Chen X, Shan T, Ma J, Lin W, Li W
and Kang Y: MiR-21-mediated Metabolic alteration of
cancer-associated fibroblasts and its effect on pancreatic cancer
cell behavior. Int J Biol Sci. 14:100–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Grasso C, Jansen G and Giovannetti E: Drug
resistance in pancreatic cancer: Impact of altered energy
metabolism. Crit Rev Oncol Hematol. 114:139–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kurtoglu M, Maher JC and Lampidis TJ:
Differential toxic mechanisms of 2-deoxy-D-glucose versus
2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells.
Antioxid Redox Signal. 9:1383–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nancolas B, Guo L, Zhou R, Nath K, Nelson
DS, Leeper DB, Blair IA, Glickson JD and Halestrap AP: The
anti-tumour agent lonidamine is a potent inhibitor of the
mitochondrial pyruvate carrier and plasma membrane monocarboxylate
transporters. Biochem J. 473:929–936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gatenby RA and Gillies RJ: Glycolysis in
cancer: A potential target for therapy. Int J Biochem Cell Biol.
39:1358–1366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang Y, Wei J, Xu J, Leong WS, Liu G, Ji
T, Cheng Z, Wang J, Lang J, Zhao Y, et al: Suppression of tumor
energy supply by liposomal nanoparticle-mediated inhibition of
aerobic glycolysis. ACS Appl Mater Interfaces. 10:2347–2353. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lu L, Chen Y and Zhu Y: The molecular
basis of targeting PFKFB3 as a therapeutic strategy against cancer.
Oncotarget. 8:62793–62802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Clem BF, O'Neal J, Tapolsky G, Clem AL,
Imbert-Fernandez Y, Kerr DA II, Klarer AC, Redman R, Miller DM,
Trent JO, et al: Targeting 6-phosphofructo-2-kinase (PFKFB3) as a
therapeutic strategy against cancer. Mol Cancer Ther. 12:1461–1470.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lisanti MP, Martinez-Outschoorn UE and
Sotgia F: Oncogenes induce the cancer-associated fibroblast
phenotype: Metabolic symbiosis and ‘fibroblast addiction’ are new
therapeutic targets for drug discovery. Cell Cycle. 12:2723–2732.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lamb R, Ozsvari B, Bonuccelli G, Smith DL,
Pestell RG, Martinez-Outschoorn UE, Clarke RB, Sotgia F and Lisanti
MP: Dissecting tumor metabolic heterogeneity: Telomerase and large
cell size metabolically define a sub-population of stem-like,
mitochondrial-rich, cancer cells. Oncotarget. 6:21892–21905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Benjamin D, Robay D, Hindupur SK, Pohlmann
J, Colombi M, El-Shemerly MY, Maira SM, Moroni C, Lane HA and Hall
MN: Dual Inhibition of the lactate transporters MCT1 and MCT4 is
synthetic lethal with metformin due to NAD+ depletion in cancer
cells. Cell Rep. 25:3047–3058.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Polanski R, Hodgkinson CL, Fusi A, Nonaka
D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE,
et al: Activity of the monocarboxylate transporter 1 inhibitor
AZD3965 in small cell lung cancer. Clin Cancer Res. 20:926–937.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Monti D, Sotgia F, Whitaker-Menezes D,
Tuluc M, Birbe R, Berger A, Lazar M, Cotzia P, Draganova-Tacheva R,
Lin Z, et al: Pilot study demonstrating metabolic and
anti-proliferative effects of in vivo anti-oxidant supplementation
with N-Acetylcysteine in Breast Cancer. Semin Oncol. 44:226–232.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Crawford S: Anti-inflammatory/antioxidant
use in long-term maintenance cancer therapy: A new therapeutic
approach to disease progression and recurrence. Ther Adv Med Oncol.
6:52–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Martinez-Outschoorn UE, Pavlides S,
Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco
G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, et al: Tumor
cells induce the cancer associated fibroblast phenotype via
caveolin-1 degradation: Implications for breast cancer and DCIS
therapy with autophagy inhibitors. Cell Cycle. 9:2423–2433. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Martinez-Outschoorn UE, Whitaker-Menezes
D, Valsecchi M, Martinez-Cantarin MP, Dulau-Florea A, Gong J,
Howell A, Flomenberg N, Pestell RG, Wagner J, et al: Reverse
Warburg effect in a patient with aggressive B-cell lymphoma: Is
lactic acidosis a paraneoplastic syndrome? Semin Oncol. 40:403–418.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Gao P, Zhang H, Dinavahi R, Li F, Xiang Y,
Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, et al:
HIF-dependent antitumorigenic effect of antioxidants in vivo.
Cancer Cell. 12:230–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Morales AI, Detaille D, Prieto M, Puente
A, Briones E, Arévalo M, Leverve X, López-Novoa JM and El-Mir MY:
Metformin prevents experimental gentamicin-induced nephropathy by a
mitochondria-dependent pathway. Kidney Int. 77:861–869. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sonnenblick A, Agbor-Tarh D, Bradbury I,
Di Cosimo S, Azim HA Jr, Fumagalli D, Sarp S, Wolff AC, Andersson
M, Kroep J, et al: Impact of diabetes, insulin, and metformin use
on the outcome of patients with human epidermal growth factor
receptor 2-positive primary breast cancer: Analysis from the ALTTO
PHASE III randomized Trial. J Clin Oncol. 35:1421–1429. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: A diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar : PubMed/NCBI
|


















