Introduction
There are ~8,300 cases and 1,280 related deaths due
to carcinoma of the anal canal each year in the United Sates
(1). The current standard treatment
for the majority of anal cancers includes the use of 5-fluorouracil
(5-FU) and mitomycin-C (MMC) delivered concurrently with intensity
modulated radiation therapy (IMRT), with the aim of anal sphincter
preservation (2). The use of IMRT
compared to 3-dimensional conformal radiotherapy (3D-CRT) in the
treatment of anal carcinoma has been shown to reduce acute grade 3
or higher skin toxicity (from 49 to 23%), reduce grade 3 or higher
gastrointestinal toxicity (from 36 to 21%), and reduce grade 2 or
higher hematologic toxicity (2,3).
Regardless, the morbidity associated with treatment with
chemoradiation for anal carcinoma remains substantial. Aside from
an impact on the quality of life of patients, severe toxicity
during chemoradiotherapy for anal cancer can lead to a need for
treatment breaks and an extension in treatment duration, which has
been associated with an increased local recurrence and higher
colostomy rates (2).
Radiation dermatitis is one of the most common
severe toxicities observed during chemoradiation for anal cancer.
In Radiation Therapy Oncology Group (RTOG) 0529 (2), 75% of patients with anal carcinoma
treated with chemoradiation experienced grade 2 or higher
dermatologic toxicity, and 23% experienced grade 3 or higher
dermatologic toxicity. The perineal, perianal, genital and inguinal
regions are at a higher risk of skin breakdown and radiation
dermatitis due to the numerous skin folds contributing to a ‘bolus’
effect that increases skin dose and the inherent moisture in the
area. In addition to causing significant pain and discomfort for
patients, severe dermatitis can lead to an environment conducive to
superinfection.
MTS-01 (Tempo1;
4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl) is a nitroxide
oxygen radical scavenger that has been formulated as a topical gel
(tempol 70 mg/ml in water, ethanol and hydroxylpropyl cellulose).
The nitroxides are a class of stable free radical compounds that
exhibit antioxidant activity, protecting mammalian cells against
hydrogen peroxide, superoxide and t-butyl hydroperoxide
cytotoxicity (4–7). Tempol has been shown to protect
against lethal total body radiation exposures, while having no
effect on the tumor radioresponse (8–11). The
MTS-01 formulation of Tempol has been found to protect against
radiation-induced skin toxicity, specifically alopecia, in animal
models and clinical studies (12–14).
The possible mechanisms of Tempol radioprotection include the
oxidation of reduced transition metals, superoxide dismutase-like
activity, and the scavenging of oxy- and carbon-based free radicals
(15).
The primary objective of the present study was to
assess the safety and tolerability of delivering a topical Tempol
application on a daily basis prior to irradiation in the inguinal
area and gluteal cleft of patients receiving combined therapy with
MMC, 5-FU and radiation therapy for carcinoma of the anal canal.
The secondary objectives included the description of the severity
of skin toxicity with this regimen and the need for treatment
breaks.
Patients and methods
Patients with histologically proven invasive primary
squamous carcinoma of the anal canal, stage T1-4, N0-3, M0, with no
previous therapy for anal cancer were eligible for this National
Cancer Institute Institutional Review Board-approved clinical trial
(NCT01324141; registered on March 28, 2011). All research was
performed in accordance with relevant guidelines and regulations.
All studies reported were outlined in an informed consent document
signed by all participants. The study subjects were >18 years of
age with an Eastern Cooperative Oncology Group (ECOG) performance
status ≤2 and adequate bone marrow, renal and hepatic functions.
Patients with human immunodeficiency virus (HIV) and a CD4 T-cell
count >100 cells/µl and an ECOG performance status <2 were
eligible.
Participants were simulated in the supine position
at 1 h following oral contrast administration with a marker placed
at the anal verge. CT images were obtained through the pelvis and
inguinal regions. Contouring or targets and critical structures was
performed using Eclipse software (v4, Varian Medical Systems, Inc.)
and based on the RTOG Consensus guidelines for rectal and anal
cancer planning (16). Radiation
fractionation, the total dose to target structures and normal
tissue constraints (described in Data
S1) were based on RTOG 0529. As per these guidelines, the anal
canal with a 2.5 cm expansion received a dose of 50.4–54 Gy in 28
fractions and the elective nodal regions (mesorectal, inguinal,
external iliac and internal iliac) received 42–45 Gy in 28
fractions. Radiation was delivered as a single daily fraction, 5
days per week. Treatment breaks were allowed for grade 4 skin
reactions, absolute neutrophil counts <500/mm3, grade
3 diarrhea, grade 3 vomiting, or localized or generalized
infection.
MMC was delivered intravenously at a dose of 10
mg/m2 (maximum 20 mg) on days 1 and 29. 5-FU was
delivered at the dose of 1,000 mg/m2/day as a 96-h
continuous venous infusion on days 1 and 29. Radiotherapy commenced
concurrently with chemotherapy (day 1) using IMRT.
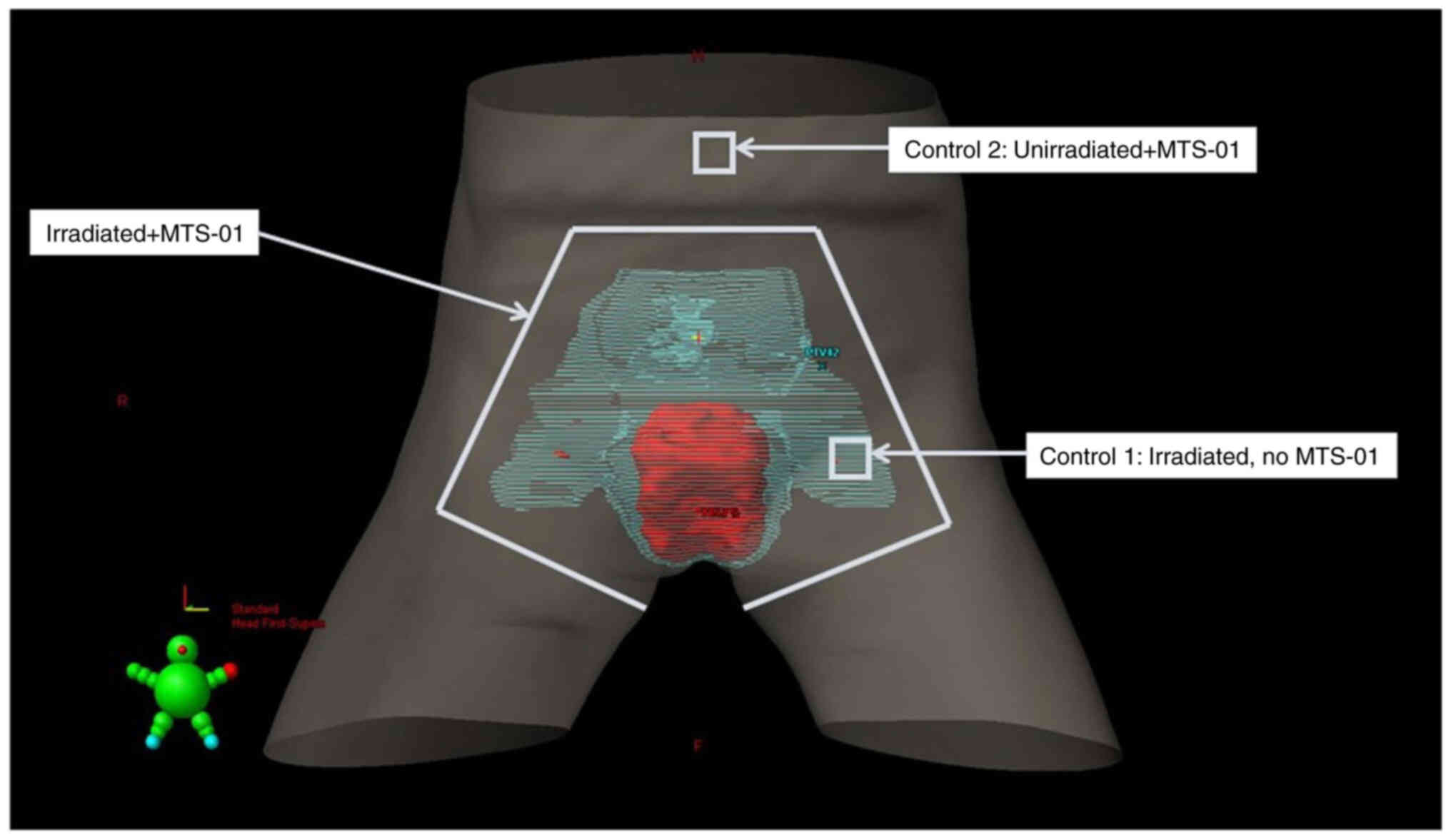
To guide the MTS-01 application, an anterior
projection of the body surface with the prescription isodose
volumes [primary planning target volume (PTV) and nodal PTV] was
generated using Eclipse software v4 (Fig. 1). Treatment planning images were
reviewed to assess skin dose and to guide MTS-01 application. The
corresponding areas were outlined with markers on the skin surface
prior to the first MTS-01 application and maintained throughout
treatment. The gluteal cleft region and a 3 cm radius from the anal
verge border were also marked. Up to 100 ml MTS-01 (70 mg/ml, Mitos
Pharmaceuticals, Inc.) was applied uniformly to the patient's
targeted skin area 15–30 min prior to each fraction of radiation by
trained personnel. Tempol was withheld if the patients were unable
to tolerate the agent, if moist desquamation occurred within the
treatment area, or if other grade 3 or 4 toxicities deemed related
to Tempol manifested. In total, two control sites (2×2 cm each)
were marked (Fig. 1), including a
site receiving radiation only without MTS-01 (left inguinal area,
C1) as well as a site outside of the treatment field receiving
Tempol only (umbilical area, C2). The use of Aquaphor and
Biafine® cream was allowed; however, these agents were
not applied prior to daily treatment.
Adverse events (AE) were assessed throughout
treatment and until 4 weeks of follow-up using the Common
Terminology Criteria for Adverse Events (CTCAE v4.0, http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
The attribution of AEs from standard chemoradiation, MTS-01 or
research other components were assessed. Skin toxicity was
evaluated at five different sites (L inguinal, R inguinal, gluteal
cleft, C1 and C2) weekly using the RTOG Acute Radiation Morbidity
Scoring Schema provided by the study principal investigator, DC.
All five sites were professionally photographed weekly during
treatment and at multiple intervals during follow-up to allow for
toxicity scoring by a second blinded radiation oncologist. The
validated brief pain inventory questionnaire (17) was administered over the same time
points, and additional exploratory laboratory tests and clinical
lymphocyte phenotyping assay were conducted throughout treatment
and follow-up.
Optional rectal mucosal snag biopsies were obtained
during flexible sigmoidoscopy performed at baseline and at 12
months following the completion of treatment, and processed to a
single cell suspension as previously described (18,19)
and as detailed in Data S1. Cells
extracted from the biopsy specimens were stained for 30 min at room
temperature with the following antibodies: anti-CD3 PE-Cy7 (Clone:
SK7, cat. no. BD-341101, Becton, Dickinson and Company), anti-CD4
APC-Cy7 (Clone: SK3, cat. no. BD341105), anti-CD8 PacBlue (Clone:
RPA-T8, cat. no. MHCD0828, Invitrogen; Thermo Fisher Scientific,
Inc.), anti-CD8 PerCP (Clone: SK1, cat. no. MABF1687 EMD
Millipore). All antibodies were used at 50% of the manufacturer's
recommended dilution. The proportion of CD4+ and
CD8+ T-cells in tissue was assessed after gating in
CD3+ cells. Absolute numbers of CD4+ and
CD8+ T-cells per gram of gut tissue were calculated by
dividing the viable cell count by the tissue weight. This number
was then multiplied by percentages obtained from flow cytometric
analysis (LSRII Flow Cytometer, Becton, Dickinson and Company), to
determine the absolute cell count of the T-cell subsets.
Aliquots of plasma were collected prior to treatment
(baseline) and course 1 (day 28) and stored at −80°C until use. The
concentrations of interleukin (IL)-7, transforming growth factor-β1
(TGF-β1), tumor necrosis factor-α (TNF-α) and vascular endothelial
growth factor A (VEGF-A) in plasma were determined using Meso Scale
Discovery multiplex chemiluminescent assays as per the
manufacturer's recommended protocol, and analyzed using a S6000
Instrument (Meso Scale Diagnostics LLC).
Results
A total of 5 patients were enrolled in the study.
All participants completed chemoradiation and MTS-01 treatment. The
patient demographics are summarized in Table I. The median age of the study
participants was 57 years (range, 49–63 years). In total, 1 patient
was African-American and 4 were Caucasian, and 1 patient had HIV.
In addition, 4 patients had stage II and 1 patient had stage III
disease [American Joint Committee on Cancer (AJCC) 7th edition
(20)] (Table I). The study was closed early due to
slow accrual.
 | Table I.Patient demographics. |
Table I.
Patient demographics.
| Characteristic | No. of patients
(%) |
|---|
| Sex |
|
|
Male | 2 (40) |
|
Female | 3 (60) |
| Age, years |
|
|
Range | 49-63 |
|
Median | 57 |
| Race |
|
|
African-American | 1 (20) |
|
Caucasian | 4 (80) |
| ECOG status |
|
| 0 | 3 (60) |
| 1 | 2 (40) |
| 2 | 0 (0) |
| 3 | 0 (0) |
| 4 | 0 (0) |
| 5 | 0 (0) |
| HIV status |
|
|
Positive | 1 (20) |
|
Negative | 4 (80) |
| HPV status (anal
swab) |
|
|
Positive | 0 (0) |
|
Negative | 5 (100) |
| T stage (AJCC 7th
edition) |
|
| T1 | 0 (0) |
| T2 | 2 (40) |
| T3 | 3 (60) |
| T4 | 0 (0) |
| N stage (AJCC 7th
edition) |
|
| N0 | 4 (80) |
| N1 | 0 (0) |
| N2 | 0 (0) |
| N3 | 1 (20) |
| Disease stage (AJCC
7th edition) |
|
| I | 0 (0) |
| II | 4 (80) |
|
IIIA | 0 (0) |
|
IIIB | 1 (20) |
| IV | 0 (0) |
AEs attributed to MTS-01 were rare, with the
majority of AEs being attributed to chemotherapy or radiation.
There were no dose-limiting toxicities. In all cases, toxicities
possibly attributed to MTS-01 were also possible toxicities of
chemoradiotherapy or concurrent medications. For example, the only
grade 3 toxicities possibly attributable to MTS-01 were a decrease
in the CD4+ T cell count in a single patient, and a
single brief episode of grade 3 fatigue, which were also
attributable to chemoradiotherapy. Another patient experienced
grade 2 diarrhea, and all remaining toxicities were grade 1,
including fatigue and hypoglycemia (Table II).
 | Table II.Adverse events observed in the
present study trial. |
Table II.
Adverse events observed in the
present study trial.
| Type of adverse
event | MTS-01 Grade 1 | MTS-01 grade 2 | MTS-01 Grade 3 | 5-FU/MMC/IMRT Grade
2 | 5-FU/MMC/IMRT
Grades 3–4 |
|---|
|
Non-hematologic |
|
|
|
|
|
|
Radiation dermatitis |
|
|
| 2 | 3 |
|
Nausea |
|
|
| 3 |
|
|
Vomiting |
|
|
| 2 |
|
|
Abdominal pain |
|
|
| 1 |
|
|
Diarrhea | 1 | 1 |
| 1 | 2 |
|
Gastroesophageal reflux |
|
|
| 1 |
|
|
Mucositis |
|
|
| 1 |
|
| Urinary
tract pain |
|
|
| 2 |
|
| Bladder
spasm |
|
|
| 1 |
|
| Urinary
incontinence |
|
|
| 1 |
|
|
Fatigue | 1 |
| 1 | 1 |
|
|
Hypoglycemia | 1 |
|
|
|
|
|
Transaminitis |
|
|
| 1 |
|
|
Hypoalbuminemia |
|
|
| 1 |
|
|
Hypocalcemia |
|
|
| 1 |
|
|
Myalgia |
|
|
| 1 |
|
|
Headache |
|
|
|
| 1 |
|
Syncope |
|
|
|
| 1 |
|
Infection |
|
|
| 1 | 1 |
|
Insomnia |
|
|
| 1 |
|
|
Pain |
|
|
| 2 | 2 |
| Hematologic |
|
|
|
|
|
|
Leukopenia |
|
|
|
| 5 |
|
Lymphopenia |
|
|
|
| 5 |
|
Neutropenia |
|
|
| 2 | 3 |
| Febrile
neutropenia |
|
|
|
| 1 |
|
Thrombocytopenia |
|
|
| 2 |
|
|
Anemia |
|
|
| 3 |
|
| CD4
count decrease |
|
| 1 |
|
|
There were several grade 3 or higher AEs attributed
to either IMRT, MMC or 5-FU treatment that are summarized in
Table II. Radiation dermatitis was
more severe in the perianal area where MTS-01 was not applied, with
all patients developing grade 2 or higher dermatitis in that area.
Other common non-hematologic AEs of chemoradiotherapy included
diarrhea, perianal and perineal pain, nausea, vomiting and dysuria.
There was one incidence of a skin infection and one urinary tract
infection. All participants had hematologic AEs, including grade 3+
leukopenia, lymphocytopenia and CD4 count decreases, while 3
patients had neutropenia, including an episode of febrile
neutropenia. None of the 5 patients required a treatment break.
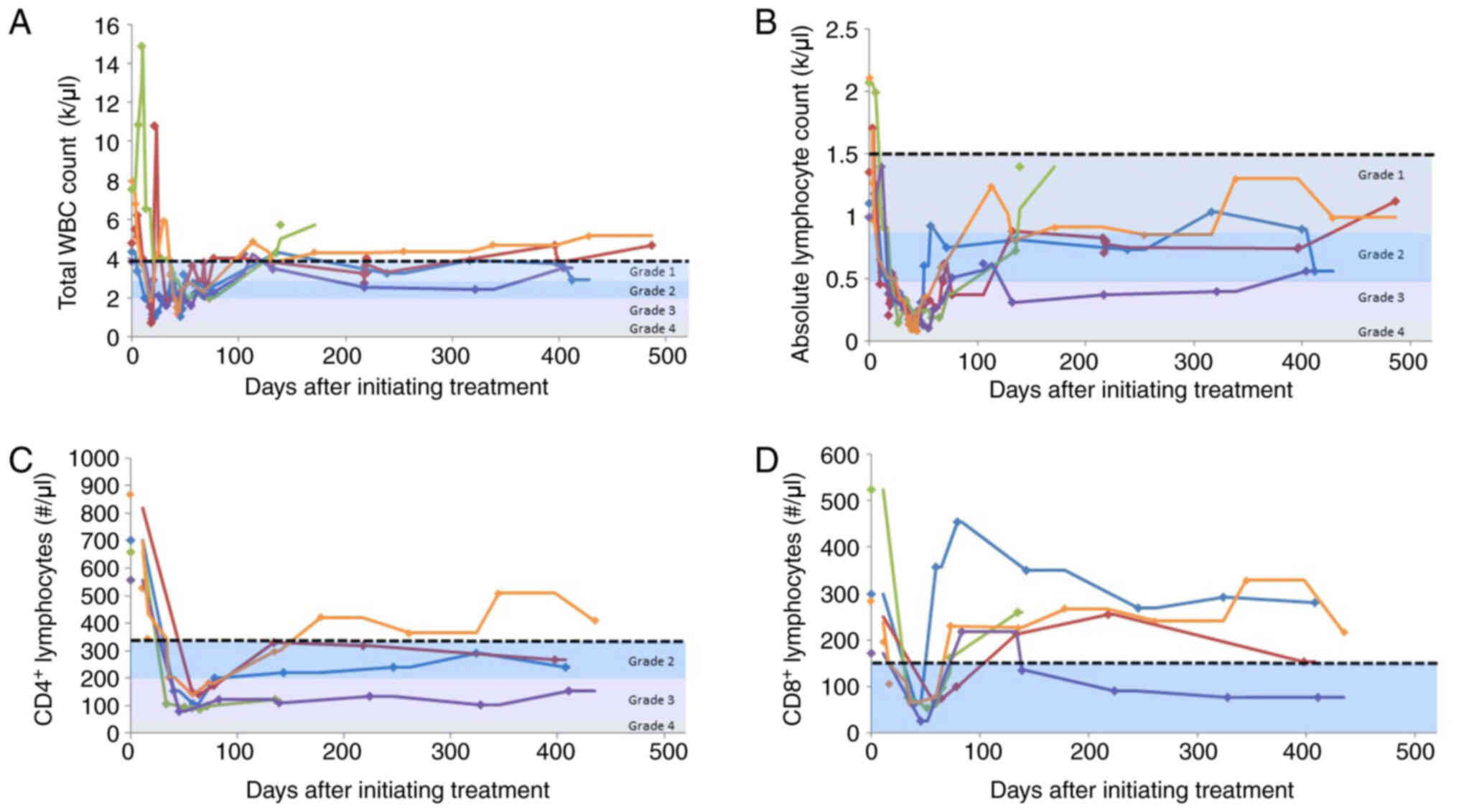
As this trial aimed to assess gut-associated
lymphoid tissue as an exploratory endpoint, the serial lymphocyte
phenotyping of blood was performed as a comparator for tissue
studies throughout treatment and follow-up. As expected with
chemoradiotherapy, leukopenia was pronounced soon following
treatment initiation (Fig. 2A).
Leukocyte counts recovered to a normal range at 12 months of
follow-up in only 2 patients. A similar trend was observed in
lymphocyte counts following treatment initiation, with increasing
lymphocyte counts over time (Fig.
2B); however, none of the 4 remaining patients in the study
recovered to a normal lymphocyte range at 1 year after completing
treatment. A more profound decrease in circulating lymphocytes was
observed in the CD4+ lymphocyte subset relative to the
CD8+ lymphocytes (Fig. 2C
and D).
In total, 1 patient (HIV-positive, stage T4 disease)
developed rapid disease progression outside of the radiation field
following treatment and was removed from the study. The remaining 4
patients are alive and relapse-free with no evidence of disease
throughout the duration of follow-up.
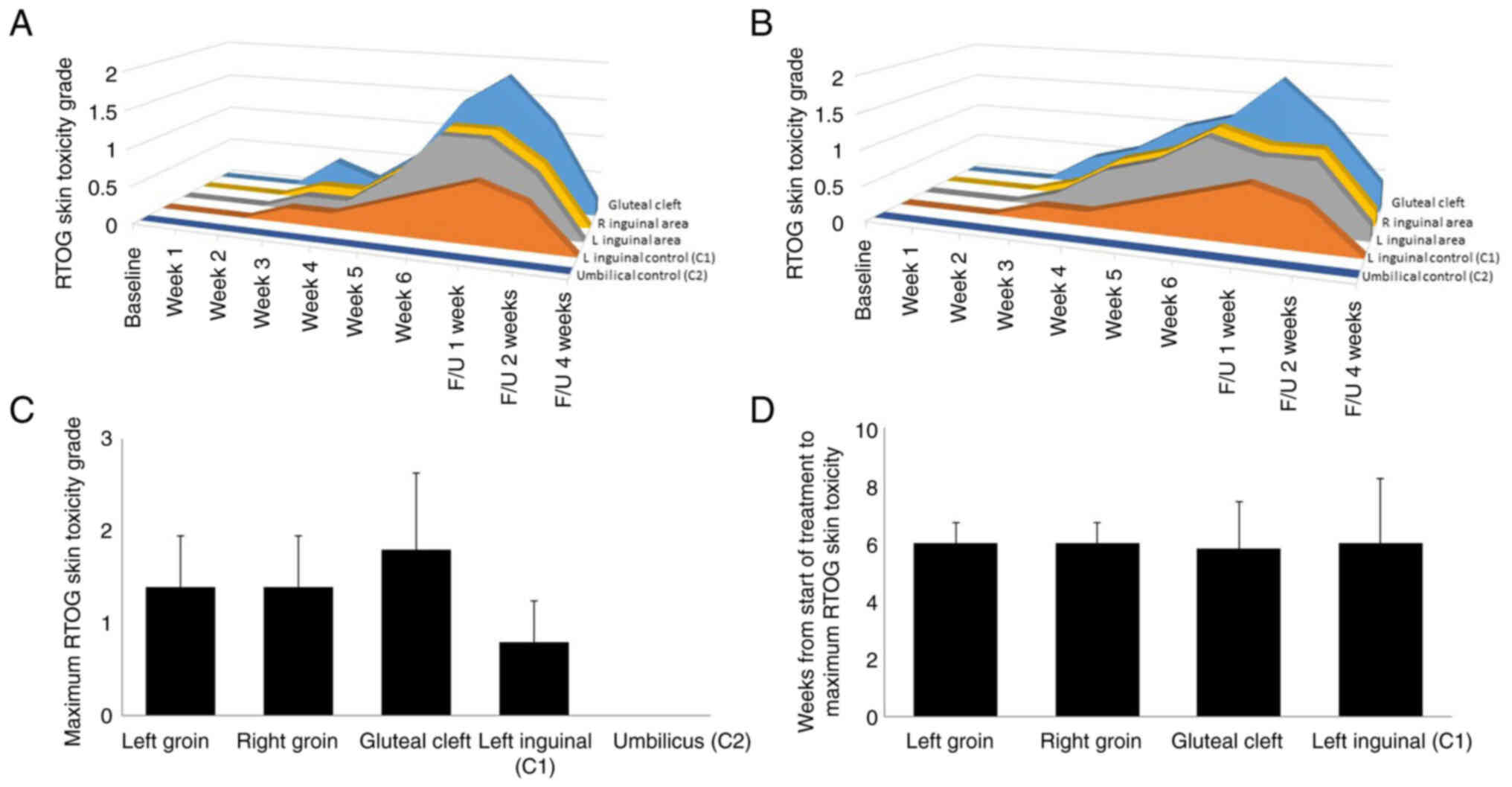
All 5 patients experienced radiation dermatitis in
the radiation treatment field. Radiation dermatitis within the
MTS-01-treated areas and the control areas was assessed at each
time point during examination by the treating physician (Fig. 3A) or by a blinded observer reviewing
professional medical images (Fig.
3B). The mean RTOG acute skin toxicity score at each site per
time point was similar with both techniques of assessment. In the
umbilicus control (C2), there was no noticeable reaction at any
timepoint in any patient with either examiner.
Toxicity in the MTS-01-treated gluteal cleft was
more severe than that in other assessed sites (Fig. 3C), with 1 patient developing grade 3
dermatitis in the gluteal cleft, 2 patients developing grade 2
dermatitis and 2 patients developing grade 1 dermatitis only.
Toxicity in the radiation-treated (no MTS-01) area in the left
inguinal region was less than that observed in the remainder of the
MTS-01-treated left inguinal area. The most severe dermatologic
toxicity in the inguinal regions tended to be the most medial
areas, and the control site (C2) was generally situated more
laterally in the inguinal region, possibly explaining the slightly
reduced toxicity scoring at that site. In general, the severity of
dermatitis increased until peaking at 6 weeks following treatment
initiation, and the time of maximum toxicity was not obviously
different between the sites (Fig.
3D). A total of 3 patients developed grade 2 dermatitis in the
inguinal regions, whereas 2 patients developed grade 1 only.
To describe global pain during treatment, the brief
pain inventory was administered weekly during treatment and in the
subsequent follow-up period. As demonstrated in Table III, pain was most severe at the
completion of treatment, 6 weeks after initiating therapy. At this
time point, pain was also most refractory to relief from medication
and interfered most with daily activities (Table III).
 | Table III.Brief pain inventory scores. |
Table III.
Brief pain inventory scores.
|
| Baseline | Treatment week
3 | Treatment week
6 | 1-Month
follow-up | 3-Month
follow-up |
|---|
|
|
|
|
|
|
|
|---|
| Pain inventory | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range |
|---|
| Worst pain | 4.8 | 0-10 | 3.5 | 0-8 | 7.2 | 4-10 | 3.1 | 0-9 | 2.1 | 0-5 |
| Least pain | 1.4 | 0-4 | 1.5 | 0-4 | 3.6 | 1-5 | 1.2 | 0-3 | 2.8 | 0-5 |
| Average pain | 1.8 | 0-4 | 1.9 | 0-3.5 | 5.0 | 2-7 | 2.0 | 0-6 | 3.0 | 0-6 |
| Pain at time of
survey | 1.6 | 0-6 | 1.3 | 0-5 | 5.4 | 2-9 | 2.2 | 0-6 | 2.2 | 0-5 |
| % Pain relief after
medication | 90.0 | 60-100 | 93.8 | 85-100 | 44.0 | 10-90 | 89.0 | 75-100 | 83.0 | 50-100 |
| Pain
interference | 1.73 | 0.33-3.89 | 3.00 | 0.44-8.00 | 6.70 | 2.89-9.44 | 3.17 | 0-8.33 | 1.87 | 0-4.56 |
Rectal mucosal biopsies were obtained from 3
consenting patients at baseline and at 1 year following the
completion of treatment. The analysis of lymphocyte subsets in
these biopsy tissues revealed a reduction in CD3+ and
CD4+ T-cells in all patients at the 1-year follow-up
(Fig. 4A). By contrast, the numbers
of CD8+ cells largely recovered at the 1-year follow-up
time point. Simultaneously collected blood was analyzed,
demonstrating a consistent decline in CD4+ lymphocytes
in tissue and circulation at 1 year following treatment relative to
baseline levels (Fig. 4B). By
contrast, although CD8+ lymphocytes were reduced in the
circulation relative to baseline, CD8+ lymphocytes were
similar or increased relative to baseline levels. This association
was more clearly demonstrated when comparing the
CD4+/CD8+ ratio (Fig. 4C) and suggests that the
CD8+ lymphocyte subset was more effectively regenerated
in irradiated tissue compared to the circulation and compared to
CD4+ lymphocyte subsets (representative flow cytometry
plots for these data are available from the corresponding author on
reasonable request).
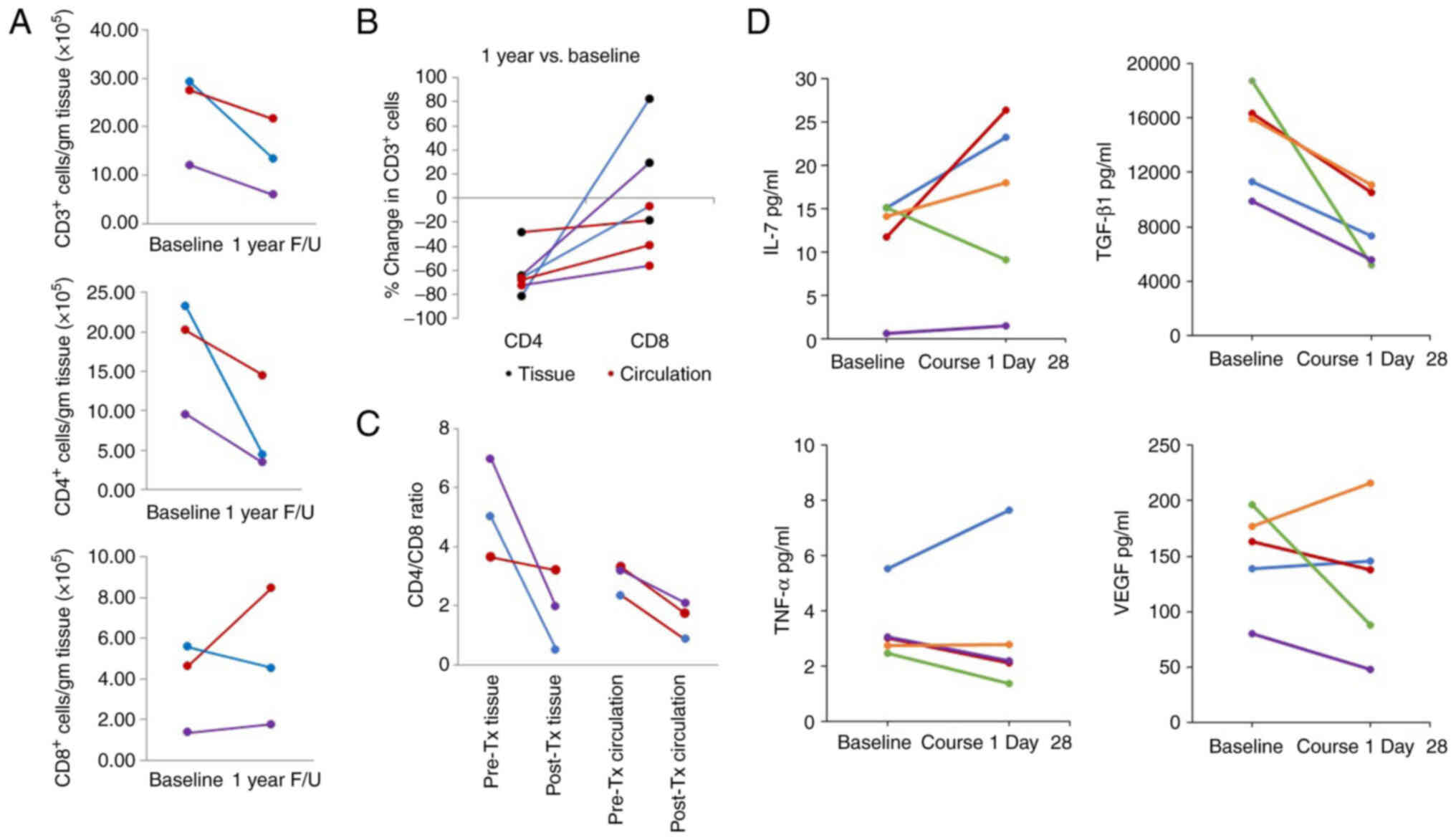 | Figure 4.Circulating and peripheral lymphocyte
subsets. Biopsy tissue from the colon and blood was collected at
baseline and at 1 year of follow-up. Tissue was dissociated to
individual cells, fixed and subjected to flow cytometric analyses
for cell surface markers (representative flow cytometry plots for
these data are available from the corresponding author on
reasonable request). All patients who consented to the tissue
biopsy were HIV-negative. Colors correspond to individual patients,
and those reported in Fig. 1. (A)
The numbers of CD3+, CD4+ and CD8+
cell subsets were determined per gram of rectal mucosal tissue
obtained in the biopsy using flow cytometry. The proportion of
CD4+ and CD8+ T-cells in tissue was assessed
after gating in CD3+ cells. (B) The percentage change in
CD4+ and CD8+ lymphocytes between baseline
and 1 year post-treatment was compared between tissue and the
circulation. (C) The ratio of CD4+ lymphocytes to
CD8+ lymphocytes was calculated in baseline tissue and
blood relative to 1 year of follow-up. (D) Plasma concentrations of
IL-7, TGF-β1, TNF-α and VEGF were measured in plasma obtained at
baseline and at day 28 following the initiation of treatment. The
data of the 1 patient who was HIV-positive are represented by the
green-colored line. F/U, follow-up; HIV, human immunodeficiency
virus; IL-7, interleukin 7; TGF-β1 transforming growth factor-β1;
TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth
factor. |
As aforementioned, leukopenia and lymphopenia were
rapid and often profound with the chemoradiotherapy delivered in
this trial. The evaluation of cytokines known to play a role in
lymphopoiesis were analyzed at baseline vs. the end of the first
course of chemotherapy (course 1, day 28). In 4 of the 5
participants, the IL-7 levels increased at course 1 (day 28)
relative to baseline levels, whereas the TGF-β1 concentrations in
the circulation decreased universally. No clear patterns were
observed in the plasma concentrations of TNF-α and VEGF (Fig. 4D).
Discussion
The primary objective of the present study was to
assess the safety and efficacy of delivering topical MTS-01 daily
prior to irradiation in the bilateral inguinal area and gluteal
cleft of patients receiving combined therapy with MMC, 5-FU and
radiation therapy for carcinoma of the anal canal. In the present
phase I study on 5 patients, minimal toxicity was noted with the
application of MTS-01. A strong signal of efficacy was not
demonstrated, although there are significant limitations to this
observation in this small-scale study. The present study reported a
long-term decrease in leukocyte counts, specifically
CD4+ lymphocytes, associated with standard
chemoradiation for anal carcinoma, with evidence of CD8+
lymphocyte persistence or recovery in tissue relative to
circulation and relative to CD4+ lymphocytes.
Chemoradiotherapy combined with MTS-01 led to a universal decrease
in TGF-β1 levels.
The present study employed several methods to
increase the likelihood of determining the efficacy of
radioprotection. Dermatitis was scored in real-time by a single
trained physician using control sites in each patient. A blinded
observer assessed response using deidentified professionally
captured medical photographs of the sites scored for toxicity.
These images were collected in identical locations, with identical
lighting conditions and identical photography equipment.
However, there were also several limitations to the
ability to demonstrate the efficacy of MTS-01 as a radioprotector
of skin in the present study. An inherent challenge in the
evaluation of topical radioprotectors is the difficulty of
predicting locations of severe dermatitis in an individual patient.
The inguinal control site (no MTS-01 applied) was situated in the
center of the inguinal region, below the inguinal fold, and was
specifically selected to reduce the chance of MTS-01 contamination
of the site during hip flexion when patients rose to walk to
radiation treatment following the MTS-01 application. Although the
skin surrounding this control site, where MTS-01 was not applied,
often had less dermatitis than the more medial portions of the
inguinal region, toxicity was scored based on the most severe
toxicity within the inguinal region, which may have resulted in
toxicity grading spuriously appearing to reflect less toxicity at
the inguinal control site (MTS-01 not applied, radiated) relative
to the remaining inguinal areas. Thus, even if zones of toxicity
can be accurately predicted, control sites must be carefully
selected to ensure accurate comparisons of efficacy, the
simultaneous goals of both minimizing contamination and ensuring
toxicity grading accounts for regional variation in dermatitis. The
inclusion of only 5 patients prior to study closure also prevented
firm conclusions regarding the efficacy of MTS-01. Regardless, the
lessons learned from the techniques utilized in the present study
may be useful in designing future studies assessing topical
radioprotectors or mitigators.
Despite an inability to demonstrate a reduction in
dermatitis with MTS-01, there was minimal toxicity to its
application, even in the setting of evolving dermatitis. Consistent
with the findings of other clinical trials in cancer patients
receiving topical formulations of Tempol, the most commonly
reported AEs were gastrointestinal, constitutional, dermatological
and metabolic (14). These
toxicities of MTS-01 are also commonly associated with
chemoradiation for carcinoma of the anal canal, such as diarrhea,
mild hypoglycemia and fatigue. With the caveat of a limited sample
size, the minimal MTS-01-associated systemic toxicities, and the
consistent lack of AEs at the umbilical control site, suggest that
the topical application of MTS-01 has limited toxicity in this
clinical setting. Prior clinical and preclinical studies have
suggested that systemic exposure is negligible following the
topical application of this formulation (12–14).
Out of concern that desquamation at the site of application may
increase the likelihood of systemic absorption, MTS-01 was withheld
in any region with moist desquamation; thus, the safety in the
setting of severe skin toxicity remains uncertain.
The lack of systemic absorption of MTS-01 in
previous research (14) is
encouraging, not only as it limits potential toxicity, but also as
it is unlikely to adversely impact tumor control. Although the
assessment of Tempol on the tumor response to radiation was not an
end point of the present study, 4 out of the 5 patients were alive,
with no evidence of disease at the extended follow-up. Future
studies on MTS-01 are required however, to address this important
issue in a larger group of patients.
Although hematologic toxicity is frequently
described as a consequence of chemoradiation for carcinoma of the
anal canal, a strength of the present study was a more
comprehensive evaluation of lymphocytopenia in a small patient
subset. All participants underwent the serial assessment of blood
counts and lymphocyte phenotyping. In addition, the four HIV
seronegative participants without progression who were follow-up
for 1 year following the completion of therapy were noted to have
prolonged lymphocytopenia. Lymphocytopenia was largely due to
prolonged decreases in the numbers of CD4+ T-cells,
while circulating CD8+ lymphocytes recovered in the
majority of patients to a normal range within weeks following
treatment. These finding were true even in the setting of increases
in the levels of the homeostatic cytokine, IL-7, suggesting that
physiological responses to lymphocytopenia may be inadequate to
promote CD4 reconstitution over a period of 1 year after standard
chemoradiation for anal cancer.
Previous research has demonstrated the suppression
of CD4+ lymphocytes in HIV-positive individuals
following chemoradiotherapy for anal cancer (21). Indeed, a lower post-treatment CD4
count has been associated with an increased risk of local
recurrence following chemoradiation for anal cancer in HIV-positive
patients (22). However, there are
limited data available on the prevalence or impact of CD4
suppression following treatment in patients without HIV. The
majority of trials demonstrating lymphopenia and a decrease in the
CD4 count following chemoradiation in HIV-negative patients have no
follow-up of patients beyond 4–12 weeks (23–25);
thus, the prolonged suppression observed herein is notable. If
validated in larger patient cohorts, this observation may have
important implications for the long-term monitoring and care of
patients who receive chemoradiotherapy for anal cancer. Further
studies are required to determine the reproducibility and relevance
of this additional therapeutic toxicity.
A notable component of the present study is the
assessment of CD4+ and CD8+ lymphocytes in
rectal mucosal biopsies at 1 year following irradiation compared to
baseline levels. Although both circulating CD4 and CD8 cells
remained suppressed at 1 year, only the numbers of CD4+
lymphocytes were reduced in rectal tissue at 1 year. Preclinical
studies and limited human tissue studies suggest that although
T-cells do not account for the majority of accumulated cells in
irradiated tissue (26), they are
capable of orchestrating immune responses through effector
mechanisms that drive chronic inflammatory diseases with
pathologies similar to those observed after irradiation (27). An altered balance in T-cell subsets
has been implicated as a possible contributor to radiation injury
(27), such as in animal models of
radiation proctitis (28). The
capacity to observe these changes in the rectal mucosa acquired
from asymptomatic individuals suggests that these changes occur as
a consequence of therapy even in asymptomatic individuals and are
not merely a marker of proctitis.
The design of the present study does not allow for
the ruling out of the possibility that these profound and sustained
immunosuppressive effects were related to MTS-01 administration.
However, other clinical studies evaluating MTS-01 have not
identified prolonged immune effects, and the available literature
that has described the lymphocyte count and CD4 count suppression
when combining chemotherapy and radiotherapy for the treatment of
other cancers further supports that the causative agents are
chemotherapy and radiation (29).
Another observation was that treatment with
chemoradiation combined with MTS-01 led to a decrease in plasma
levels of TGF-β1. TGF-β1 has been implicated in the pathogenesis of
human papillomavirus-associated malignancies (30), with elevated levels being associated
with a poor prognosis of patients with cervical cancer treated with
chemoradiation (31), as well as
radiation lung toxicity in non-small cell lung cancer (32). The impact of MTS-01 on these
findings is unknown and the further evaluation of plasma TGF-β1 as
a biomarker is thus warranted in anal cancer and studies evaluating
MTS-01.
In conclusion, as demonstrated herein, MTS-01 is
tolerable when used to manage dermatotoxicity in patients with
localized anal cancer undergoing chemoradiation. The lack of an
efficacy signal that was noted to be due to the inadequate sample
size and control site selection, were significant factors in the
decision to close the study to accrual early. A more suitable
control site that would not be subject to easy cross-contamination
with MTS-01, but would be expected to develop severe dermatitis,
could not be identified. Regardless, there are important lessons to
be learnt for future studies evaluating a topical radiation
protector and attempting to integrate a control site. Despite the
sample size, there are several interesting hypotheses generating
findings related to treatment induced CD4 lymphocytopenia and
TGF-β1 that provide subjects for future studies.
Supplementary Material
Supporting Data
Acknowledgements
The authors are grateful to Ms. Luz Giordano and Ms.
Debbie McNally, both from the National Cancer Institute, for their
contributions to the conduct of this trial. Both were involved in
the application of the investigational agent and the conduct of
research-related assessments. The trial registration number for the
study is NCT01324141 (registered on March 28, 2011).
Funding
The present study was supported in part by the intramural
research program of the National Cancer Institute, Center for
Cancer Research (grant no. ZIA BC 010850).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC and TU were involved in the conception and design
of the study, in the acquisition of data, data analysis,
interpretation of the data and in manuscript preparation. LV was
involved in data analysis, interpretation of the data and in
manuscript preparation. KC was involved the acquisition of data,
data analysis and in manuscript preparation. TCZ, DS and MY were
involved in the acquisition of data and in manuscript preparation.
JBM was involved in the conception of the study and in manuscript
preparation. WT was involved in data analysis and in manuscript
preparation. IS was involved in the acquisition of data, data
analysis, interpretation of the data and in manuscript preparation.
DC and KC confirm the authenticity of the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All analyses reported in the present study relating
to human subjects were reviewed and approved by the National Cancer
Institute Institutional Review Board. All studies reported were
outlined in an informed consent document signed by all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kachnic LA, Winter K, Myerson RJ, Goodyear
MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran
H and Willett CG: RTOG 0529: A phase 2 evaluation of dose-painted
intensity modulated radiation therapy in combination with
5-fluorouracil and mitomycin-C for the reduction of acute morbidity
in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys.
86:27–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajani JA, Winter KA, Gunderson LL,
Pedersen J, Benson AB III, Thomas CR Jr, Mayer RJ, Haddock MG, Rich
TA and Willett C: Fluorouracil, mitomycin, and radiotherapy vs
fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal
canal: A randomized controlled trial. JAMA. 299:1914–1921. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitchell JB, Samuni A, Krishna MC, DeGraff
WG, Ahn MS, Samuni U and Russo A: Biologically active
metal-independent superoxide dismutase mimics. Biochemistry.
29:2802–2807. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samuni A, Krishna CM, Mitchell JB, Collins
CR and Russo A: Superoxide reaction with nitroxides. Free Radic Res
Commun. 9:241–249. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samuni A, Godinger D, Aronovitch J, Russo
A and Mitchell JB: Nitroxides block DNA scission and protect cells
from oxidative damage. Biochemistry. 30:555–561. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samuni A, Mitchell JB, DeGraff W, Krishna
CM, Samuni U and Russo A: Nitroxide SOD-mimics: Modes of action.
Free Radic Res Commun 12–13 Pt. 1:187–194. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hahn SM, Tochner Z, Krishna CM, Glass J,
Wilson L, Samuni A, Sprague M, Venzon D, Glatstein E, Mitchell JB,
et al: Tempol, a stable free radical, is a novel murine radiation
protector. Cancer Res. 52:1750–1753. 1992.PubMed/NCBI
|
|
9
|
Hahn SM, Sullivan FJ, DeLuca AM, Krishna
CM, Wersto N, Venzon D, Russo A and Mitchell JB: Evaluation of
tempol radioprotection in a murine tumor model. Free Radic Biol
Med. 22:1211–1216. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cotrim AP, Hyodo F, Matsumoto K, Sowers
AL, Cook JA, Baum BJ, Krishna MC and Mitchell JB: Differential
radiation protection of salivary glands versus tumor by Tempol with
accompanying tissue assessment of Tempol by magnetic resonance
imaging. Clin Cancer Res. 13:4928–4933. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cotrim AP, Yoshikawa M, Sunshine AN, Zheng
C, Sowers AL, Thetford AD, Cook JA, Mitchell JB and Baum BJ:
Pharmacological protection from radiation +/-cisplatin-induced oral
mucositis. Int J Radiat Oncol Biol Phys. 83:1284–1290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goffman T, Cuscela D, Glass J, Hahn S,
Krishna CM, Lupton G and Mitchell JB: Topical application of
nitroxide protects radiation-induced alopecia in guinea pigs. Int J
Radiat Oncol Biol Phys. 22:803–806. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cuscela D, Coffin D, Lupton GP, Cook JA,
Krishna MC, Bonner RF and Mitchell JB: Protection from
radiation-induced alopecia with topical application of nitroxides:
Fractionated studies. Cancer J Sci Am. 2:273–278. 1996.PubMed/NCBI
|
|
14
|
Metz JM, Smith D, Mick R, Lustig R,
Mitchell J, Cherakuri M, Glatstein E and Hahn SM: A phase I study
of topical Tempol for the prevention of alopecia induced by whole
brain radiotherapy. Clin Cancer Res. 10:6411–6417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitchell JB, DeGraff W, Kaufman D, Krishna
MC, Samuni A, Finkelstein E, Ahn MS, Hahn SM, Gamson J and Russo A:
Inhibition of oxygen-dependent radiation-induced damage by the
nitroxide superoxide dismutase mimic, tempol. Arch Biochem Biophys.
289:62–70. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Myerson RJ, Garofalo MC, El Naqa I, Abrams
RA, Apte A, Bosch WR, Das P, Gunderson LL, Hong TS, Kim JJ, et al:
Elective clinical target volumes for conformal therapy in anorectal
cancer: A radiation therapy oncology group consensus panel
contouring atlas. Int J Radiat Oncol Biol Phys. 74:824–830. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cleeland CS and Ryan KM: Pain assessment:
Global use of the brief pain inventory. Ann Acad Med Singap.
23:129–138. 1994.PubMed/NCBI
|
|
18
|
Sereti I, Estes JD, Thompson WL, Morcock
DR, Fischl MA, Croughs T, Beq S, Lafaye de Micheaux S, Yao MD, Ober
A, et al: Decreases in colonic and systemic inflammation in chronic
HIV infection after IL-7 administration. PLoS Pathog.
10:e10038902014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ciccone EJ, Greenwald JH, Lee PI,
Biancotto A, Read SW, Yao MA, Hodge JN, Thompson WL, Kovacs SB,
Chairez CL, et al: CD4+ T cells, including Th17 and
cycling subsets, are intact in the gut mucosa of HIV-1-infected
long-term nonprogressors. J Virol. 85:5880–5888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
21
|
Alfa-Wali M, Allen-Mersh T, Antoniou A,
Tait D, Newsom-Davis T, Gazzard B, Nelson M and Bower M:
Chemoradiotherapy for anal cancer in HIV patients causes prolonged
CD4 cell count suppression. Ann Oncol. 23:141–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bryant AK, Mudgway R, Huynh-Le MP, Simpson
DR, Mell LK, Gupta S, Sharabi AB and Murphy JD: Effect of CD4 count
on treatment toxicity and tumor recurrence in human
immunodeficiency virus-positive patients with anal cancer. Int J
Radiat Oncol Biol Phys. 100:478–485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cattin S, Fellay B, Calderoni A,
Christinat A, Negretti L, Biggiogero M, Badellino A, Schneider AL,
Tsoutsou P, Pellanda AF and Rüegg C: Circulating Immune cell
populations related to primary breast cancer, surgical removal, and
radiotherapy revealed by flow cytometry analysis. Breast Cancer
Res. 23:642021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xi J, Hassan B, Katumba RGN, Khaddour K,
Govindan A, Luo J, Huang J and Campian JL: The predictive value of
absolute lymphocyte counts on tumor progression and
pseudoprogression in patients with glioblastoma. BMC Cancer.
21:2852021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Jin Y, Hu X and Chen M: Effect of
Chemoradiotherapy on the proportion of circulating lymphocyte
subsets in patients with limited-stage small cell lung cancer.
Cancer Immunol Immunother. 70:2867–2876. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Citrin DE and Mitchell JB: Mechanisms of
normal tissue injury from irradiation. Semin Radiat Oncol.
27:316–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schaue D and McBride WH: T lymphocytes and
normal tissue responses to radiation. Front Oncol. 2:1192012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Linard C, Strup-Perrot C, Lacave-Lapalun
JV and Benderitter M: Flagellin preconditioning enhances the
efficacy of mesenchymal stem cells in an irradiation-induced
proctitis model. J Leukoc Biol. 100:569–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ellsworth SG: Field size effects on the
risk and severity of treatment-induced lymphopenia in patients
undergoing radiation therapy for solid tumors. Adv Radiat Oncol.
3:512–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strauss J, Gatti-Mays ME, Cho BC, Hill A,
Salas S, McClay E, Redman JM, Sater HA, Donahue RN, Jochems C, et
al: Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β
and PD-L1, in patients with human papillomavirus-associated
malignancies. J Immunother Cancer. 8:e0013952020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dickson J, Davidson SE, Hunter RD and West
CM: Pretreatment plasma TGF beta 1 levels are prognostic for
survival but not morbidity following radiation therapy of carcinoma
of the cervix. Int J Radiat Oncol Biol Phys. 48:991–995. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anscher MS, Kong FM and Jirtle RL: The
relevance of transforming growth factor beta 1 in pulmonary injury
after radiation therapy. Lung Cancer. 19:109–120. 1998. View Article : Google Scholar : PubMed/NCBI
|