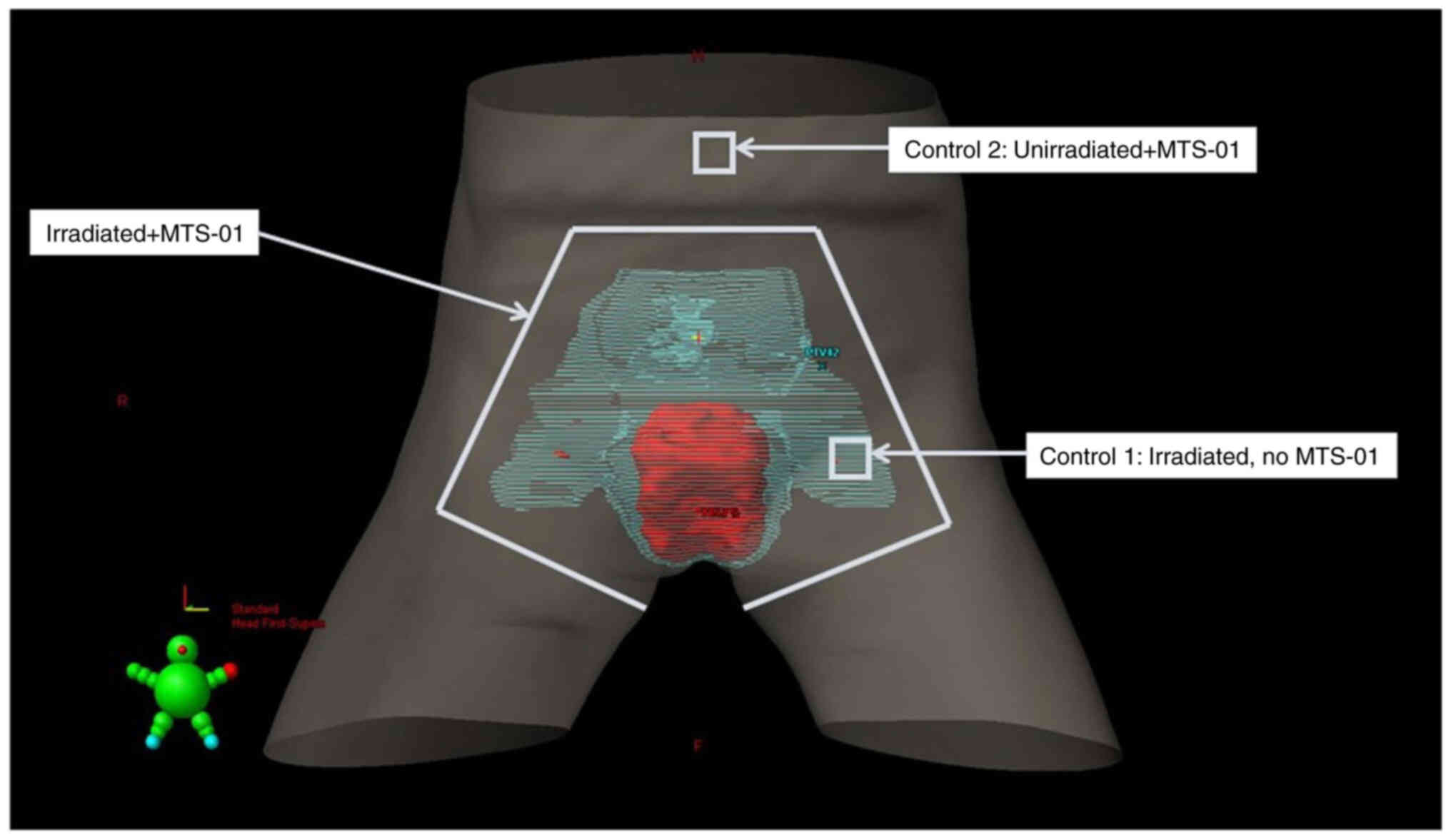
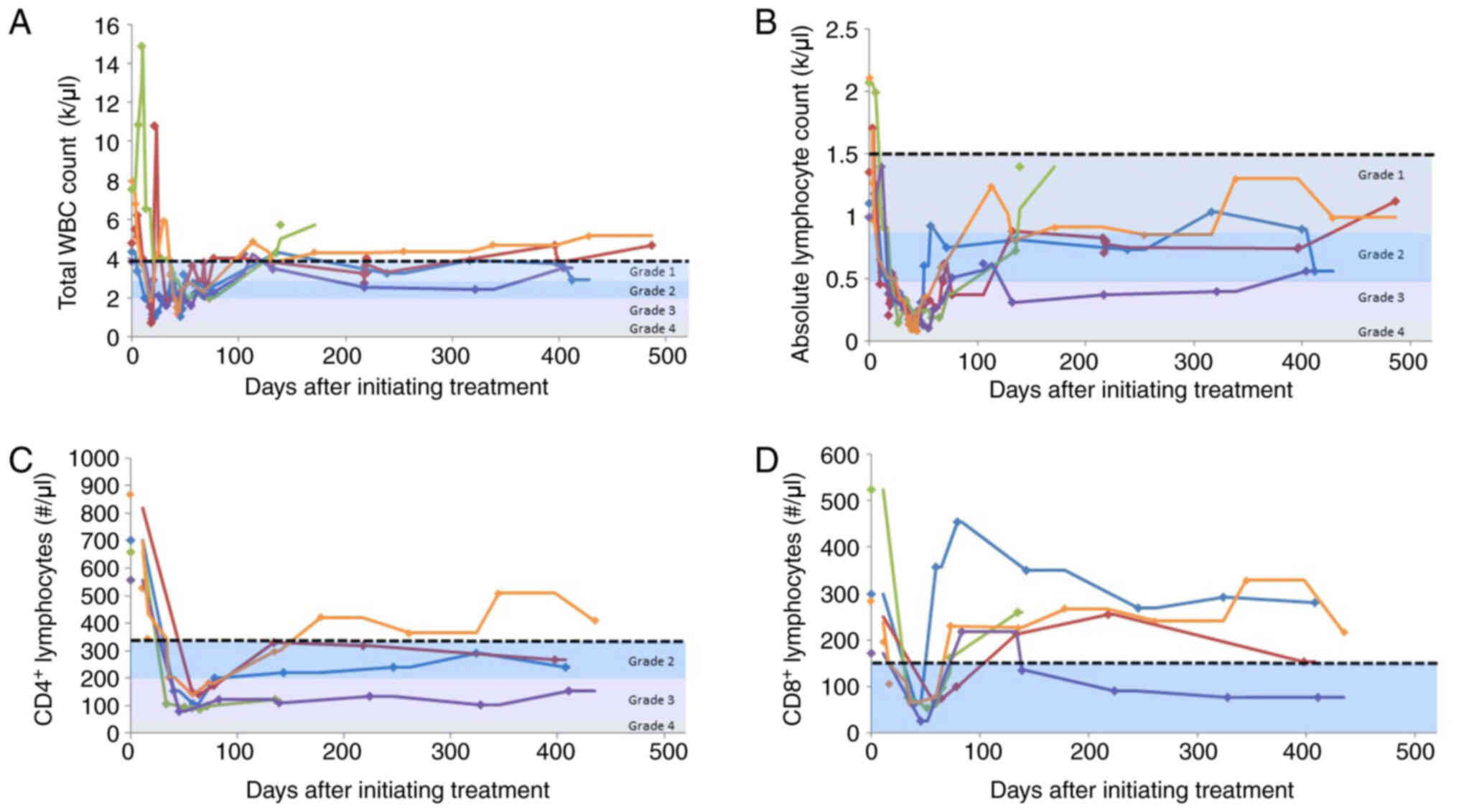
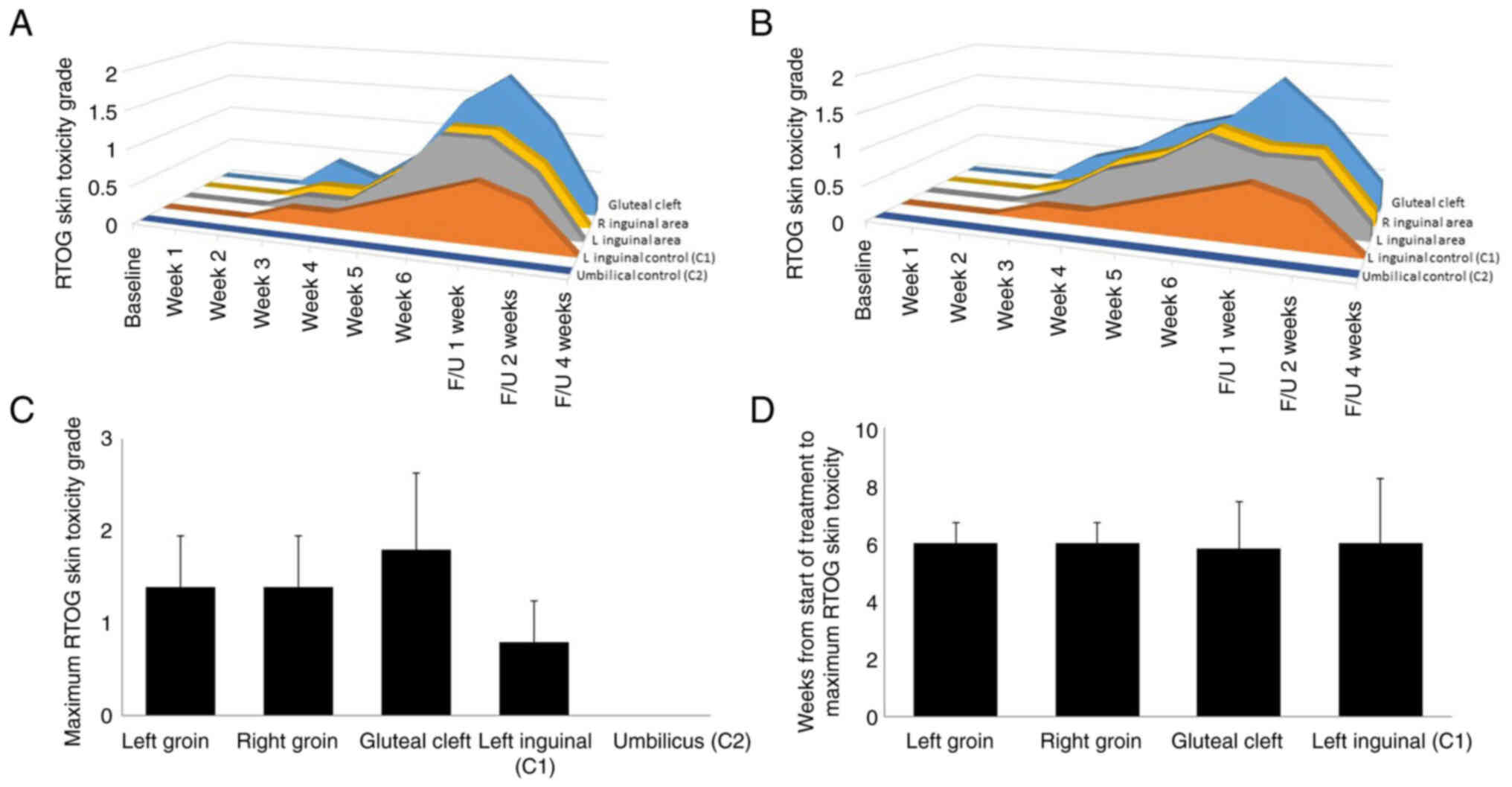
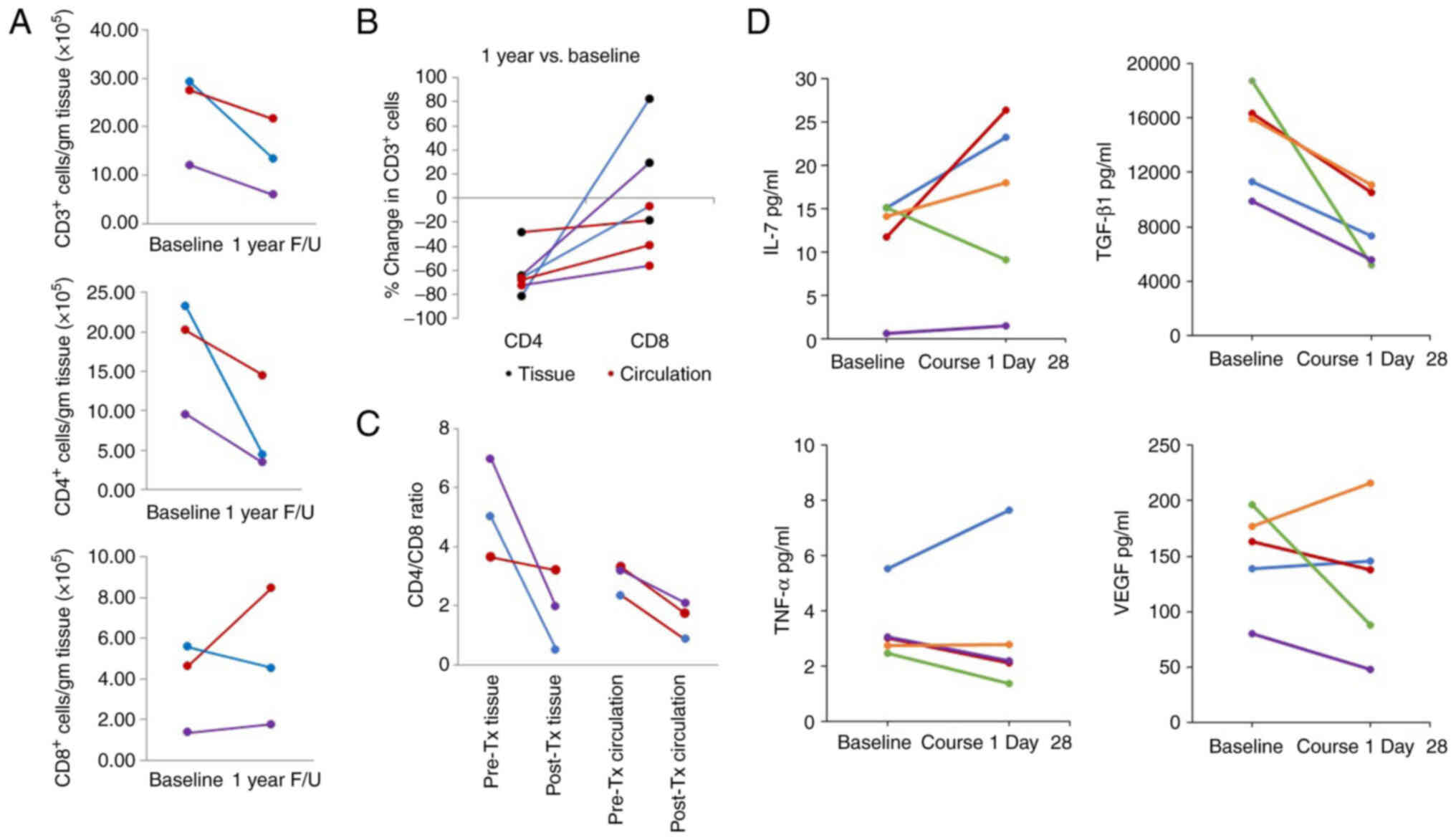
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kachnic LA, Winter K, Myerson RJ, Goodyear
MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran
H and Willett CG: RTOG 0529: A phase 2 evaluation of dose-painted
intensity modulated radiation therapy in combination with
5-fluorouracil and mitomycin-C for the reduction of acute morbidity
in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys.
86:27–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajani JA, Winter KA, Gunderson LL,
Pedersen J, Benson AB III, Thomas CR Jr, Mayer RJ, Haddock MG, Rich
TA and Willett C: Fluorouracil, mitomycin, and radiotherapy vs
fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal
canal: A randomized controlled trial. JAMA. 299:1914–1921. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitchell JB, Samuni A, Krishna MC, DeGraff
WG, Ahn MS, Samuni U and Russo A: Biologically active
metal-independent superoxide dismutase mimics. Biochemistry.
29:2802–2807. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samuni A, Krishna CM, Mitchell JB, Collins
CR and Russo A: Superoxide reaction with nitroxides. Free Radic Res
Commun. 9:241–249. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samuni A, Godinger D, Aronovitch J, Russo
A and Mitchell JB: Nitroxides block DNA scission and protect cells
from oxidative damage. Biochemistry. 30:555–561. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samuni A, Mitchell JB, DeGraff W, Krishna
CM, Samuni U and Russo A: Nitroxide SOD-mimics: Modes of action.
Free Radic Res Commun 12–13 Pt. 1:187–194. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hahn SM, Tochner Z, Krishna CM, Glass J,
Wilson L, Samuni A, Sprague M, Venzon D, Glatstein E, Mitchell JB,
et al: Tempol, a stable free radical, is a novel murine radiation
protector. Cancer Res. 52:1750–1753. 1992.PubMed/NCBI
|
|
9
|
Hahn SM, Sullivan FJ, DeLuca AM, Krishna
CM, Wersto N, Venzon D, Russo A and Mitchell JB: Evaluation of
tempol radioprotection in a murine tumor model. Free Radic Biol
Med. 22:1211–1216. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cotrim AP, Hyodo F, Matsumoto K, Sowers
AL, Cook JA, Baum BJ, Krishna MC and Mitchell JB: Differential
radiation protection of salivary glands versus tumor by Tempol with
accompanying tissue assessment of Tempol by magnetic resonance
imaging. Clin Cancer Res. 13:4928–4933. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cotrim AP, Yoshikawa M, Sunshine AN, Zheng
C, Sowers AL, Thetford AD, Cook JA, Mitchell JB and Baum BJ:
Pharmacological protection from radiation +/-cisplatin-induced oral
mucositis. Int J Radiat Oncol Biol Phys. 83:1284–1290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goffman T, Cuscela D, Glass J, Hahn S,
Krishna CM, Lupton G and Mitchell JB: Topical application of
nitroxide protects radiation-induced alopecia in guinea pigs. Int J
Radiat Oncol Biol Phys. 22:803–806. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cuscela D, Coffin D, Lupton GP, Cook JA,
Krishna MC, Bonner RF and Mitchell JB: Protection from
radiation-induced alopecia with topical application of nitroxides:
Fractionated studies. Cancer J Sci Am. 2:273–278. 1996.PubMed/NCBI
|
|
14
|
Metz JM, Smith D, Mick R, Lustig R,
Mitchell J, Cherakuri M, Glatstein E and Hahn SM: A phase I study
of topical Tempol for the prevention of alopecia induced by whole
brain radiotherapy. Clin Cancer Res. 10:6411–6417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitchell JB, DeGraff W, Kaufman D, Krishna
MC, Samuni A, Finkelstein E, Ahn MS, Hahn SM, Gamson J and Russo A:
Inhibition of oxygen-dependent radiation-induced damage by the
nitroxide superoxide dismutase mimic, tempol. Arch Biochem Biophys.
289:62–70. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Myerson RJ, Garofalo MC, El Naqa I, Abrams
RA, Apte A, Bosch WR, Das P, Gunderson LL, Hong TS, Kim JJ, et al:
Elective clinical target volumes for conformal therapy in anorectal
cancer: A radiation therapy oncology group consensus panel
contouring atlas. Int J Radiat Oncol Biol Phys. 74:824–830. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cleeland CS and Ryan KM: Pain assessment:
Global use of the brief pain inventory. Ann Acad Med Singap.
23:129–138. 1994.PubMed/NCBI
|
|
18
|
Sereti I, Estes JD, Thompson WL, Morcock
DR, Fischl MA, Croughs T, Beq S, Lafaye de Micheaux S, Yao MD, Ober
A, et al: Decreases in colonic and systemic inflammation in chronic
HIV infection after IL-7 administration. PLoS Pathog.
10:e10038902014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ciccone EJ, Greenwald JH, Lee PI,
Biancotto A, Read SW, Yao MA, Hodge JN, Thompson WL, Kovacs SB,
Chairez CL, et al: CD4+ T cells, including Th17 and
cycling subsets, are intact in the gut mucosa of HIV-1-infected
long-term nonprogressors. J Virol. 85:5880–5888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
21
|
Alfa-Wali M, Allen-Mersh T, Antoniou A,
Tait D, Newsom-Davis T, Gazzard B, Nelson M and Bower M:
Chemoradiotherapy for anal cancer in HIV patients causes prolonged
CD4 cell count suppression. Ann Oncol. 23:141–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bryant AK, Mudgway R, Huynh-Le MP, Simpson
DR, Mell LK, Gupta S, Sharabi AB and Murphy JD: Effect of CD4 count
on treatment toxicity and tumor recurrence in human
immunodeficiency virus-positive patients with anal cancer. Int J
Radiat Oncol Biol Phys. 100:478–485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cattin S, Fellay B, Calderoni A,
Christinat A, Negretti L, Biggiogero M, Badellino A, Schneider AL,
Tsoutsou P, Pellanda AF and Rüegg C: Circulating Immune cell
populations related to primary breast cancer, surgical removal, and
radiotherapy revealed by flow cytometry analysis. Breast Cancer
Res. 23:642021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xi J, Hassan B, Katumba RGN, Khaddour K,
Govindan A, Luo J, Huang J and Campian JL: The predictive value of
absolute lymphocyte counts on tumor progression and
pseudoprogression in patients with glioblastoma. BMC Cancer.
21:2852021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Jin Y, Hu X and Chen M: Effect of
Chemoradiotherapy on the proportion of circulating lymphocyte
subsets in patients with limited-stage small cell lung cancer.
Cancer Immunol Immunother. 70:2867–2876. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Citrin DE and Mitchell JB: Mechanisms of
normal tissue injury from irradiation. Semin Radiat Oncol.
27:316–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schaue D and McBride WH: T lymphocytes and
normal tissue responses to radiation. Front Oncol. 2:1192012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Linard C, Strup-Perrot C, Lacave-Lapalun
JV and Benderitter M: Flagellin preconditioning enhances the
efficacy of mesenchymal stem cells in an irradiation-induced
proctitis model. J Leukoc Biol. 100:569–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ellsworth SG: Field size effects on the
risk and severity of treatment-induced lymphopenia in patients
undergoing radiation therapy for solid tumors. Adv Radiat Oncol.
3:512–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strauss J, Gatti-Mays ME, Cho BC, Hill A,
Salas S, McClay E, Redman JM, Sater HA, Donahue RN, Jochems C, et
al: Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β
and PD-L1, in patients with human papillomavirus-associated
malignancies. J Immunother Cancer. 8:e0013952020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dickson J, Davidson SE, Hunter RD and West
CM: Pretreatment plasma TGF beta 1 levels are prognostic for
survival but not morbidity following radiation therapy of carcinoma
of the cervix. Int J Radiat Oncol Biol Phys. 48:991–995. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anscher MS, Kong FM and Jirtle RL: The
relevance of transforming growth factor beta 1 in pulmonary injury
after radiation therapy. Lung Cancer. 19:109–120. 1998. View Article : Google Scholar : PubMed/NCBI
|