Introduction
Liver cancer is a frequently occurring malignant
cancer that results in more than 700,000 deaths annually worldwide
(1,2). Although there are an increasing number
of diagnostics methods and therapeutic regimens under development,
the prognosis of patients with liver cancer remains poor, and one
reason for this poor prognosis is the efficient ability of liver
cancer tumors to undergo metastasis leading to the development of
secondary tumors in several different organs, ultimately resulting
in mortality (3,4). Thus, there is a pressing need to
identify novel mechanisms that regulate liver cancer initiation,
development and progression to identify new therapeutic targets for
management of this disease.
The Wnt signal transduction pathway plays a key
biological role in embryonic development, cell fate, and
tumorigenesis (5–7). There is a close association between
the Wnt signaling pathway and the different factors that regulate
early epithelial-mesenchymal transition (EMT) events during tumor
metastasis, including cell adhesion markers (CDH1, N-CDH2 and
CD44), transcription factors (Snail and Slug) and matrix
metalloproteases (MMPs) (8–13). The Wnt signal transduction pathway
is commonly divided into β-catenin-dependent (canonical) and
β-catenin-independent (non-canonical) signaling arms, which
regulate different biochemical events (5,14). The
Wnt signaling pathway has been frequently implicated in liver
cancer metastasis and disease development (15–17).
Tryptophan 2,3-dioxygenase (TDO2) is an
oxidoreductase, and one of the rate-limiting enzymes involved in
the conversion of L-tryptophan to L-kyneurinine, which is the first
step in pathway(s) leading to the provision of key biochemical
compounds including NAD+, picolinic acid and
glutaryl-CoA. The kyneurinine pathway is primarily expressed in
liver and cells of the nervous system, such as neurons (18,19).
TDO2 has a key function in not only regulating the catabolic
breakdown of tryptophan, but also functions to maintain
steady-state homeostasis of cellular tryptophan levels.
Upregulation of TDO2 can increase the breakdown of tryptophan and
result in the accumulation of kynurenine, thereby inactivating or
inhibiting immune cells within the tumor microenvironment,
especially T cells. Such biochemical changes can cause tumor cells
to evade immune surveillance, allowing tumors to grow and spread
rapidly (20,21). Recent studies have found that TDO2
is also highly expressed in tumor cells, such as malignant glioma,
liver cancer, malignant melanoma and bladder cancer (22–25).
Such findings highlight an association between TDO2 levels with
tumor initiation and disease progression; furthermore, TDO2 is
implicated in a functional role in the host immune response to
tumors.
The aim of the present study was to investigate the
functional association between TDO2 and liver cancer, and provide a
mechanistic explanation. The results showed that the upregulation
of TDO2 expression in liver cancer cells was dependent on a
specific microRNA. Increased TDO2 levels impacted signaling via the
Wnt5a pathway, leading to increased expression of secreted cancer
biomarkers, such as CD44 and MMP7, which was associated with
increased liver cancer cell migration and invasion. Furthermore,
there was a strong association between TDO2 expression and liver
cancer development in vivo using a mouse xenograft model of
liver cancer.
Materials and methods
Cell culture
Normal liver cells THLE-3 (cat. no. CRL-11233) and
hepatocellular carcinoma HepG2 cells (cat. no. HB-8065) were
obtained from the American Type Culture Collection. Hepatocellular
carcinoma cells Huh7 (cat. no. SCSP-526) were obtained from the
Cell Bank of the Chinese Academy of Sciences. All cells were grown
in DMEM (Hyclone, Cytiva) supplemented with 10% FBS (Biological
Industries) in a humidified incubator (5% CO2, 37°C).
Liver cancer cells were treated with pan-PKC inhibitor GÖ 6983
(cat. no. HY-13689; MedChemExpress, Inc.) MEK inhibitor U026 (cat.
no. HY-12031A; MedChemExpress, Inc.) or calcium channel blocker
azelnidipine (cat. no. HY-B0023; MedChemExpress, Inc.) at the
indicated concentrations for 0, 24 or 48 h, before being subjected
to further analyses, as detailed below. To identify drugs that can
reduce TDO2 expression, a library of 1,000 US FDA-approved drugs
were screened (Targetmol Co., Ltd.). Briefly, the cells were
inoculated in a 6-well plate and then treated with different drugs
at a concentration of 25 µM. After 24 h, the cells were collected
for protein extraction and validation. All cell lines were free of
mycoplasma and were authenticated by genetic profiling using
polymorphic short tandem repeat loci.
Collection of clinical samples and
analysis
A total of 83 normal (control) liver tissue samples
(age range, 38–68 years; mean age, 52; 40 male and 43 female
patients) and 98 liver cancer tissue samples (age range, 40–60
years; mean age, 51; 51 male and 47 female patients) were collected
from patients who underwent liver biopsy at the Cancer Hospital of
Hubei (Wuhan, China). The normal liver samples and the cancer
samples were derived from different individuals. All samples were
collected from January 2018 to June 2020. All patients provided
written informed consent. The present study was approved by the
Scientific Ethics Committee of the Cancer Hospital of Hubei
(HZ20201018) (Wuhan, China) and the Scientific Ethics Committee of
Wuhan University of Science and Technology (WUST200913) (Wuhan,
China). The clinical samples were fixed with formalin for
immunohistochemical analysis before rapidly freezing in liquid
nitrogen for RNA and protein analysis.
Animal studies
For the mouse xenograft studies, 36 4-week-old male
BALB/c nude mice (weight, 10–15 g) were purchased from SLAC
Laboratory Animal Co. Ltd., and randomly assigned to different
groups (n=3 per group), and maintained under pathogen-free
conditions. A total of 1×106 HepG2 or Huh7 stable cells
were subcutaneously injected into the lower right flank of the nude
mice. Measurement of tumor volume, calculated using the formula:
V=L × W2 × 0.5236 (L=long axis, W=short axis). The tumor
weight of the each mouse was not allowed to exceed 10% of the body
weight, and the average tumor diameter did not exceed 20 mm. In
case of ulceration, infection or necrosis, the experiment was
stopped immediately, and the animals were euthanized. All mice were
euthanized 4 weeks later, the tumors were excised carefully, and
the final tumor weight measured. Mice were euthanized by a
peritoneal injection of 200 mg/kg sodium pentobarbital. When the
mice stopped breathing and beating for 1 min and the pupils were
dilated, the euthanasia was considered complete.
To induce hepatocarcinogenesis, 21 male C57BL/6 mice
(2-weeks of age; body weight, 5 g) were intraperitoneally injected
with 20 µg/g body weight diethylnitrosamine (DEN; Sigma-Aldrich;
Merck KGaA). To increase the expression of TDO2 in liver tissues,
from the second week onwards, 100 µl adenovirus solution containing
1×109 adenoviruses were introduced into the mouse
peritoneum, once a week for 3 weeks. When the growth of the tumor
caused pain to the animal, or the animal lost more than 20% of the
body weight of the normal animal, or ulcers, infection, or necrosis
appeared, the experiment was stopped immediately and the animal was
euthanized. All mice were euthanized after 12 months, and the liver
was dissected for the subsequent experiments. Mice were euthanized
by a peritoneal injection of 200 mg/kg sodium pentobarbital. When
the mice stopped breathing and beating for 1 min and the pupils
were dilated, the euthanasia was considered complete. All mice were
housed at 23–25°C, with 50–60% humidity, 12-h light/dark cycle,
with food and water ad libitum. All experiments on mice were
performed under the guidance of the Animal Ethics Committee of
Wuhan University of Science and Technology (WS267891).
Molecular biology
The human TDO2 overexpression plasmid pTDO2 was
obtained from Addgene. Small interfering (si)RNAs targeting TDO2,
Wnt5a, CD44 and MMP7 were obtained from Shanghai GenePharma Co.,
Ltd. microRNA (miRNA/miR) mimics and the miR-140-5p inhibitor were
chemically synthesized by Guangzhou Ribobio Co., Ltd. The siRNA
sequences were: siTDO2, 5′-CGUUAAUCGCGUAUAAUACGCGUATT-3′; siCD44,
5′-CUCCCAGUAUGACACAUAUTT-3′; siMMP7, 5′-CUGCUGACAUCAUGAUUGGTT-3′;
siWnt5a, 5′-GAAACUGUGCCACUUGUAUTT-3′; siNC
5′-AAUUCUCCGAACGUGUCACGUTT-3′; miR-140-5p mimic,
5′-CAGUGGUUUUACCCUAUGGUAG-3′; miR-140-5p inhibitor,
5′-CUACCAUAGGGUAAAACCACUG-3′ and the corresponding negative
control, 5′-CAGUACUUUUGUGUAGUACAA-3′. These reagents were used
according to standard molecular biology protocols or the
manufacturer's instructions.
For total RNA extraction, 1 ml TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was first
added to the collected cells for lysis. Chloroform was then added
to the lysate to stratify it, the supernatant was taken and a new
centrifuge tube was added, and isopropyl alcohol was added to
precipitate the RNA. Next, 1 µg RNA was reverse-transcribed into
cDNA using an MLV-reverse transcriptase kit (Invitrogen; Thermo
Fisher Scientific, Inc.). Reverse transcription-quantitative PCR
was performed using SYBR® Premix Ex Taq (Takara Bio,
Inc.). The primer sequences were as follows: human TDO2
forward, 5′-TCCTCAGGCTATCACTACCTGC-3′ and reverse,
5′-ATCTTCGGTATCCAGTGTCGG-3′; human GAPDH forward,
5′-ATGACATCAAGAAGGTGGTG-3′ and reverse 5′-CATACCAGGAAATGAGCTTG-3′.
The thermocycling conditions were: 95°C for 30 sec; followed by 30
cycles of 95°C for 5 sec, 50°C for 30 sec and 72°C for 30 sec. The
results were normalized to GAPDH using the 2−ΔΔCq method
(26).
Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for transfection of all cells.
according to the manufacturer's instructions. To generate stable
cell lines overexpressing TDO2, HepG2 and Huh7 cells were
transduced with a lentivirus construct containing either the TDO2
cDNA or without any insert in the expression cassette; clones were
selected using 100 µg/ml G418. TDO2-expressing clonal lines were
assessed using RT-qPCR.
Western blotting
For the separation of cytoplasmic and nuclear
proteins, a nucleoprotein and cytoplasmic protein extraction kit
was used (cat. no. KGP150; KeyGEN BioTECH, Inc.). Briefly, the
centrifuged cells were added to the cell membrane lysate and lysed
on ice for 10 min to obtain cytoplasmic proteins after
centrifugation. Nuclear lysate was added to the remaining
precipitate and lysed on ice for 10 min. After centrifugation, the
supernatant obtained contained the nuclear proteins. GAPDH was used
as a control protein in the cytoplasm and LaminB1 was used as a
control protein in the nucleus. For total protein extraction, the
cells were lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology) followed by a BCA protein assay, and 30 µg total
protein was loaded per lane on an SDS-gel and resolved using 10%
SDS-PAGE. Subsequent transfer and blotting were performed using
standard protocols. The primary antibodies used were as follows
(1:1,000 dilution): anti-TDO2 (cat. no. 15880-1-AP; ProteinTech
Group, Inc.), anti-Wnt5a (cat. no. ab179824; Abcam), anti-P-PKC
(cat. no. #2060; Cell Signaling Technology, Inc.), anti-P-ERK (cat.
no. 28733-1-AP; ProteinTech Group, Inc.), anti-CD44 (cat. no.
15675-1-AP; ProteinTech Group, Inc.), anti-MMP7 (cat. no.
10374-2-AP; ProteinTech Group, Inc.) and anti-GAPDH (cat. no.
#5174; Cell Signaling Technology, Inc.). The PVDF membrane was
incubated with the primary antibody at 4°C overnight. After being
washed three times with TBST Buffer, the PVDF membrane was then
incubated with the corresponding secondary antibody (1:10,000
dilution; cat. no. SA00001-2; ProteinTech Group, Inc.) for 1 h at
the shaker. Finally, the blotted proteins were observed using
BeyoECL Plus (cat. no. P0018S; Beyotime, Inc.), and the
quantification of the blots was analyzed using ImageJ version 1.8.0
(National Institutes of Health).
Cell monolayer wound closure
assay
Liver cancer cells (4×105) were incubated
in a 6-well plate; 12 h later the confluent cell monolayer was
wounded across the well diameter using a 200-µl sterile pipette
tip. The wounded cell monolayer was then washed twice with fresh
cell culture medium. Digital images of the wounded cell monolayer
were collected at 0 and 48 h using an inverted microscope. The
wound surface area was assessed using ImageJ software (version
1.8.0), and the wound healing activity of the colon cancer cells
was determined by the quantification of wound healing progression.
Wound healing activity=1-(wound surface area at the indicated
time-point/wound surface area at baseline). Serum-free medium was
used during the experiment.
Cell migration and invasion
assays
Transwell assays were performed using Corning
permeable cell culture chambers. Briefly, 1×105 cells
were mixed into 100 µl of serum-free medium, then the cell
suspension was seeded into the upper chamber, while the lower
chamber contained 600 µl media supplemented with 10% FBS. After 24
h of incubation, the Transwell inserts were removed, and cells were
gently wiped away from the upper chamber using cotton swabs, and
next the Transwell filter (containing cells on the bottom) was
fixed in 100% methanol for 10 min, rinsed in PBS and stained with
0.05% (w/v) crystal violet for 30 min. After washing, the filters
were removed and mounted under coverslips on slides. Using bright
field microscopy and digital imaging, five areas were randomly
selected in each field of view at ×100 magnification, and imaged
and analyzed.
Immunohistochemistry
The tissues were immersed in 4% (w/v)
paraformaldehyde and fixed for 24 h, and then the tissues were
dehydrated, embedded in paraffin and cut into 5-µm sections.
Hematoxylin and eosin staining was performed according to standard
protocols. Paraffin sections were deparaffinized in xylene and then
rehydrated using a concentration gradient alcohol. The processed
tissue sections were subjected to heat-induced antigen recovery by
incubation in a citrate buffer at 100°C for 10 min. The tissue
sections were incubated with the antibody in blocking buffer:
anti-TDO2 antibody (cat. no. 15880-1-AP, ProteinTech Group, Inc.),
anti-Wnt5a antibody (cat. no. ab179824, Abcam), anti-MMP7 (cat. no.
10374-2-AP, ProteinTech Group, Inc.) or anti-CD44 antibody (cat.
no. 15675-1-AP, ProteinTech Group, Inc.), overnight at 4°C. After
extensive washing, the sections were incubated with
species-specific HRP-conjugated secondary antibody in blocking
buffer (Sangon Biotech, Co., Ltd.) at 25°C for 45 min. The sections
were washed extensively, incubated with DAB chromogenic reagent,
counterstained with hematoxylin, rinsed and mounted on microscope
glass slides using neutral resin.
Bioinformatics analysis
miRNAs binding to target genes were analyzed by
DIANA (http://diana.imis.athena–innovation.gr/DianaTools/index.php?r=lncBase/index),
MIRDB (http://mirdb.org/), TargetScan (http://www.targetscan.org/vert_71/) and mirDIP
(http://ophid.utoronto.ca/mirDIP/index_confirm.jsp)
databases.
Statistical analysis
All experimental data are presented as the mean ±
SEM of at least 3 independent experiments. Statistical analysis was
performed using GraphPad Prism 8.0.2 software (GraphPad Software,
Inc.). Data were analyzed using an unpaired Student's t-test when
comparing two groups. Comparisons between multiple groups were
performed using a one-way ANOVA followed by a Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
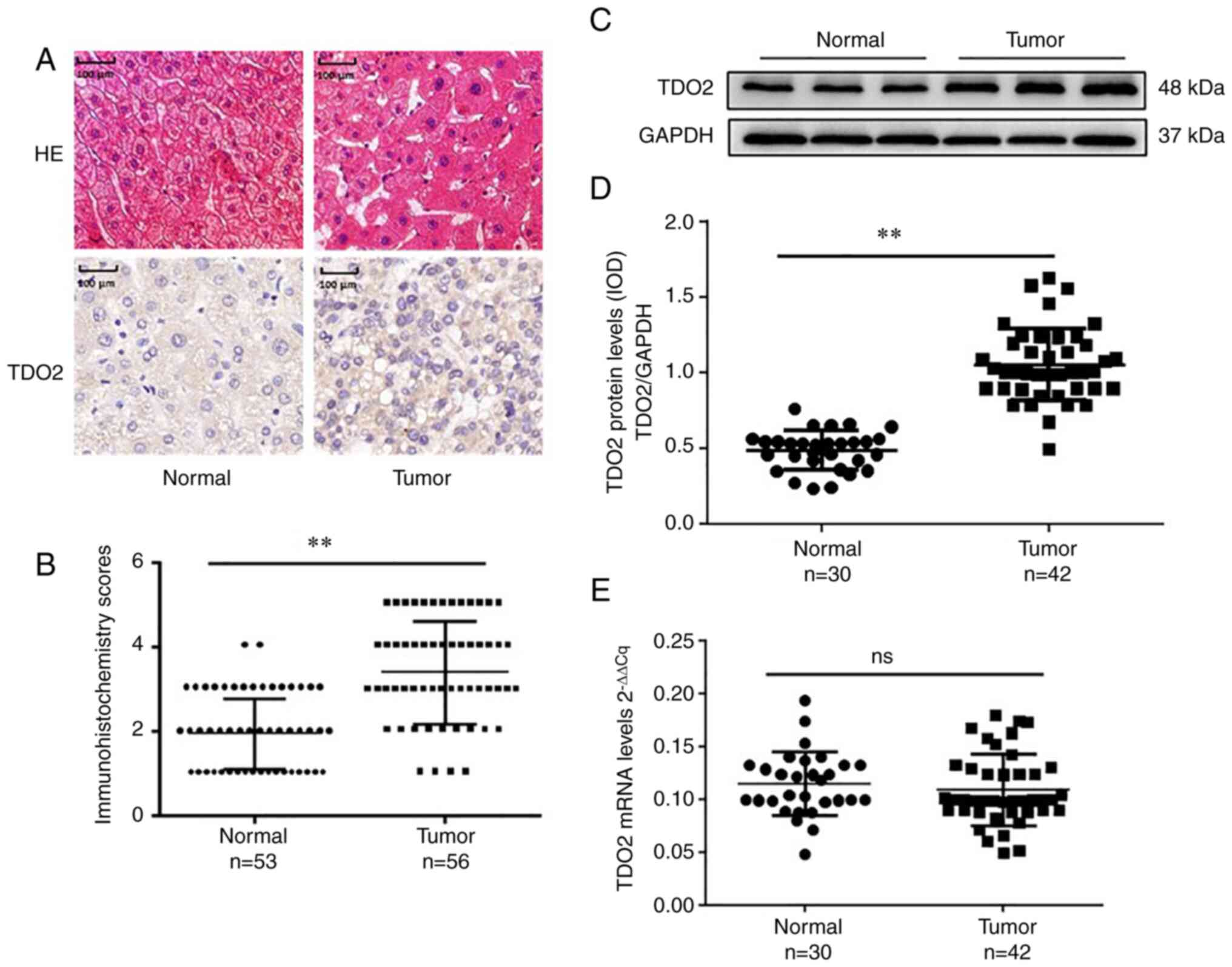
Elevation of TDO2 in liver cancer
disease
To investigate whether TDO2 expression is elevated
in clinical samples from patients with liver cancer, TDO2
expression levels in control and liver cancer clinical specimens
were analyzed using immunohistochemistry. Liver cancer samples
consistently showed upregulation of TDO2 expression (Fig. 1A and B). Western blot analysis
consistently revealed increased TDO2 protein expression in liver
cancer compared with control clinical samples (Fig. 1C and D). RT-qPCR data from control
and liver cancer clinical samples revealed no significant
difference in TDO2 mRNA levels in liver cancer samples
compared with the normal control liver samples (Fig. 1E). These data showed that TDO2
expression is increased in liver cancer disease.
Increased TDO2 expression promotes
liver cancer cell migration and invasion
To further explore the biological role of TDO2 in
liver cancer, HepG2 and Huh7 cell lines were transduced with a TDO2
expression plasmid (pTDO2) or a control plasmid (empty vector;
pcDNA3.1). In TDO2-overexpressing HepG2 and HuH7 cells, a
significant increase in liver cancer cell migration and invasion
was observed compared with cells transduced with the control
plasmid (Fig. S1A and B). A
similar picture emerged when RNA interference (RNAi) was used to
assess the role of TDO2 expression in liver cancer cell migration
and invasion. Compared with the control group (siCon), TDO2
knockdown (siTDO2) significantly reduced the wound closure
(Fig. S1C) and migratory and
invasive abilities of the cells (Fig.
S1D). Increased TDO2 expression thus correlates with increased
liver cancer cell migration and invasion.
miR-140-5p targets the TDO2 mRNA
3′-untranslated region (UTR) to inhibit TDO2 expression
Bioinformatics analysis of miRNAs from different
databases (TargetScan, miRDB, mirDIP and DIANA) suggest that
miR-140-5p is a potential regulator of TDO2 expression in normal
vs. liver cancer disease states (Fig.
2A). Using RT-qPCR analysis, miR-140-5p was found to be
significantly downregulated in liver cancer cells (HepG2 and Huh7)
compared with the control liver cells (THLE-3) (Fig. 2B). Furthermore, RT-qPCR analysis of
miR-140-5p in the control and liver cancer specimen showed that
miR-140-5p levels were significantly downregulated in the liver
cancer samples compared with the controls (Fig. 2C). Analysis of TDO2 vs. miR-140-5p
levels showed an inverse correlation in the liver cancer clinical
samples (Fig. 2D); that is reduced
miR-140-5p levels correlated with increased TDO2 levels in liver
cancer disease.
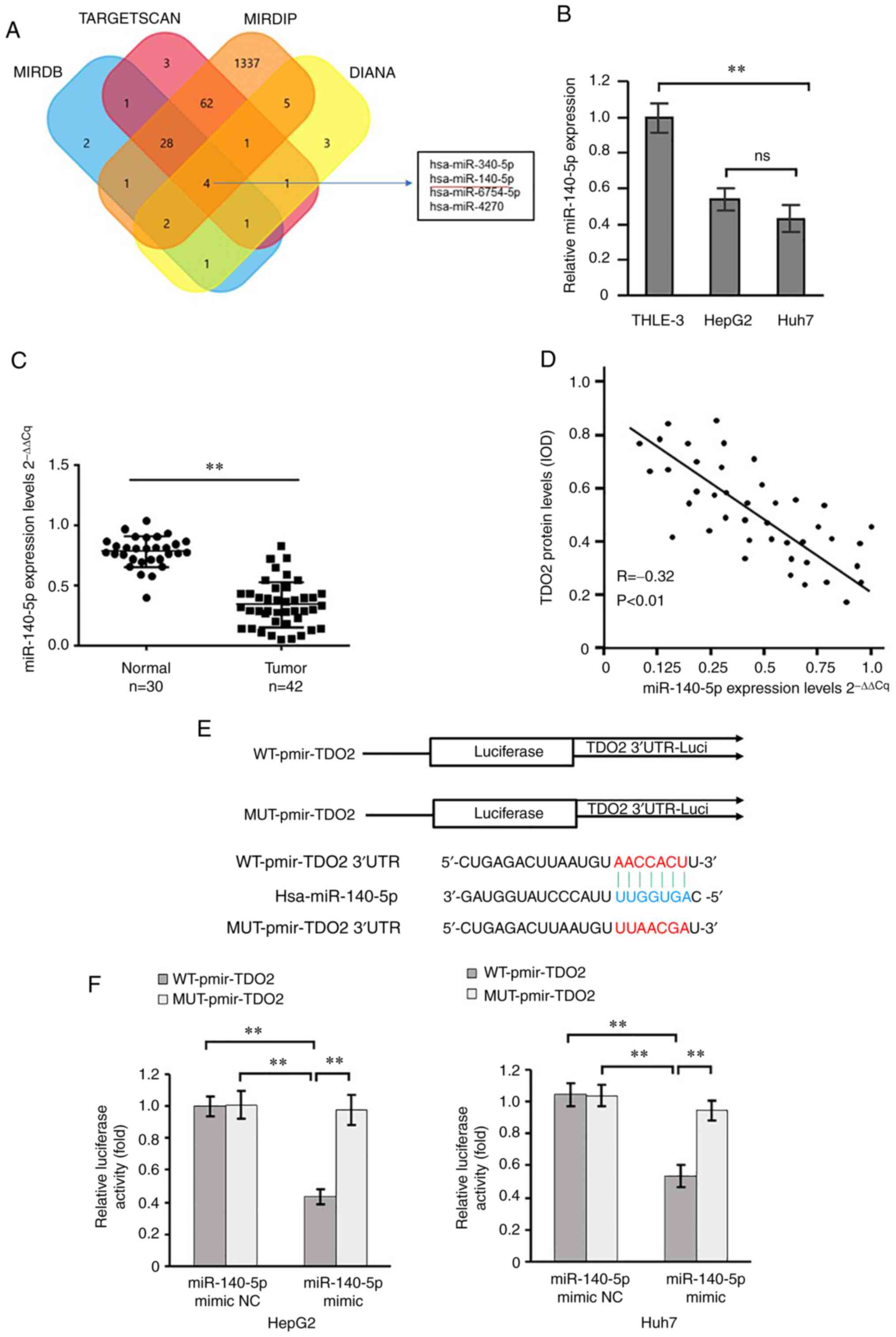 | Figure 2.TDO2 mRNA is a direct target
of miR-140-5p. (A) The four-way Venn diagram indicates the numbers
of miRNAs that overlapped in four publicly available bioinformatics
algorithms with a TDO2 signature. The four-way Venn diagram was
analyzed by DIANA (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=lncBase/index),
MIRDB (http://mirdb.org/), TargetScan
(http://www.targetscan.org/vert_71/)
and mirDIP (http://ophid.utoronto.ca/mirDIP/index_confirm.jsp)
databases. (B) Reverse transcription-quantitative PCR analysis of
miR-140-5p levels in control liver (THLE-3) and liver cancer (HepG2
and HuH7) cells. (C) miR-140-5p levels were quantified in 30 normal
control liver tissues and 42 liver cancer samples from patients.
(D) Correlation between TDO2 protein levels and miR-140-5p levels
in 42 liver cancer patient samples that we collected. (E) Schematic
showing the synthesis of the WT and MUT TDO2 mRNA 3′UTR
sequences to luciferase reporters. The potential miR-140-5p-binding
site in the TDO2 mRNA 3′UTR is highlighted. It was from
TargetScan (http://www.targetscan.org/vert_71/). (F) Luciferase
activity measurements in HepG2 and Huh7 cells transfected with
luciferase reporter plasmids carrying either WT-pmir-TDO2 or
MUT-pmir-TDO2 sequences, and co-expressing either miR-140-5p mimic
or miR-140-5p mimic NC. Data are presented as the mean ± SEM.
**P<0.01. TDO2, tryptophan 2,3-dioxygenase; miR, microRNA; UTR,
untranslated region; NC, negative control; WT, wild-type; MUT,
mutant; RIP, RNA immunoprecipitation. |
Bioinformatics algorithms (TargetScan, miRDB, mirDIP
and DIANA) were used, and they revealed a potential binding site
for miR-140-5p in the 3′UTR of the TDO2 mRNA (Fig. 2E). To test this potential
interaction, two reporter vectors consisting of the
luciferase-coding sequence followed by the 3′UTR of wild-type TDO2
(WT-TDO2 3′-UTR) or mutant 3′-UTR (Mut-TDO2 3′-UTR) were
constructed (Fig. 2E). Transient
transfection of these constructs into HepG2 or Huh7 liver cancer
cells followed by luciferase assay showed that miR-140-5p decreased
luciferase activity when co-expressed alongside WT-TDO2 3′UTR;
however, miR-140-5p had little to no effect on luciferase activity
with Mut-TDO2 3′UTR (Fig. 2F). As
these data suggested that miR-140-5p could directly bind to the
TDO2 3′UTR, miR-140-5p mimics or mimic NC were transfected into
HepG2 and Huh7 cells. The results showed that miR-140-5p mimics
decreased both HepG2 and Huh7 monolayer wound closure, cell
migration and invasion (Fig. S2A and
B). Consistent with this, an inhibitor of miR-140-5p showed the
reverse effect; an increase in HepG2 and Huh7 monolayer wound
closure, cell migration and invasion was observed (Fig. S2C and D). These data suggest that
miR-140-5p inhibits TDO2 expression by directly binding to the
3′UTR of TDO2 mRNA in HepG2 and Huh7 cell lines.
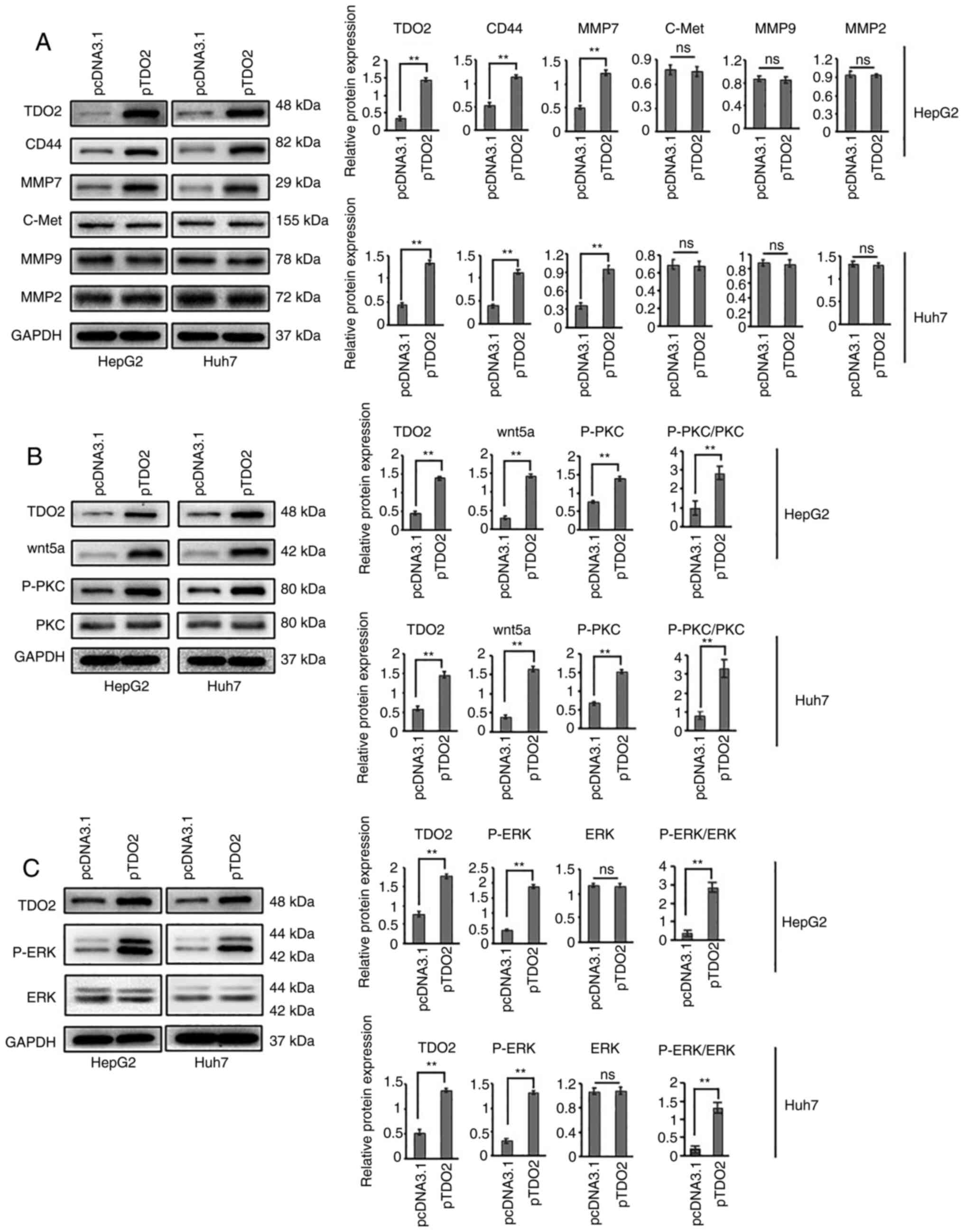
Elevated levels of TDO2 are associated
with increased CD44 and MMP7 expression
To investigate the mechanism underlying the effects
of TDO2-mediated liver cancer cell migration and invasion,
expression of EMT biomarkers associated with liver cancer
development and progression was assessed. Overexpression of TDO2
resulted in a significant increase in N-cadherin and fibronectin
levels; conversely, TDO2 overexpression resulted in a decrease in
the expression of the tumor suppressor, E-cadherin (Fig. S3A). Next, the expression of other
biomarkers related to cancer metastasis were examined. Western
blotting showed that overexpression of TDO2 significantly increased
CD44 and MMP7 levels (Fig. 3A).
However, expression of other cancer biomarkers, such as MMP9, MMP2
and c-Met were not affected (Fig.
3A).
Next, whether CD44 and MMP7 contribute to liver
cancer cell properties, such as wound closure, cell migration and
invasion was assessed (Figs. S4
and S5). CD44 expression was
knocked down using siRNA (siCD44) in both HepG2 and Huh7 cell lines
overexpressing TDO2 (Fig. S4A).
Consistently, the enhancement of monolayer wound closure, cell
migration and invasion caused by TDO2 overexpression was
significantly inhibited following siCD44 transfection (Fig. S4B and C). Next, MMP7 expression was
knocked down using siRNA (siMMP7) in both HepG2 and Huh7 cells
overexpressing TDO2 (Fig. S5A). In
HepG2 or Huh7 cells, downregulation of the MMP7 gene also offsets
the enhancement of wound healing, cell migration and invasion
caused by TDO2 overexpression (Fig.
S5B and C). These data suggest that the elevated expression of
CD44 and MMP7 biomarkers provide important supporting roles in
liver cancer cell migration and invasion.
TDO2 activates the Wnt5a signal
transduction pathway
CD44 and MMP7 are both downstream target genes of
the Wnt/β-catenin signal transduction pathway (11,27,28).
This then raised the question as to whether TDO2 affects signaling
through the Wnt/β-catenin signaling pathway. Western blotting
showed that TDO2 overexpression did not affect components of the
Wnt/β-catenin pathway such as β-catenin and c-Myc (Fig. S3B).
Importantly, upon TDO2 overexpression, other
components of the Wnt5a signal transduction pathway exhibited
changes in both HepG2 and Huh7 cells (Fig. 3B). Overexpression of TDO2 caused a
significant increase in Wnt5a levels (Fig. 3B). Furthermore, the levels of
phospho-protein kinase C (ζ) (P-PKC) as in the figure were
significantly elevated in the TDO2 overexpressing cells (Fig. 3B). In HepG2 and Huh7 cells
overexpressing TDO2, phospho-ERK1/2 (P-ERK) levels were elevated
(Fig. 3C), indicating activation of
the MAPK pathway.
To examine the association between TDO2 and the
Wnt5a signal transduction pathway, the effects of Wnt5a knockdown
(siwnt5a) in HepG2 and Huh7 cells overexpressing TDO2 were examined
(Fig. S6). Interestingly,
knockdown of Wnt5a in TDO2-overexpressing HepG2 or Huh7 cells
completely blocked the increase in P-PKC, CD44 and MMP7 levels
(Fig. S6A). Furthermore, knockdown
of Wnt5a consistently caused a decrease in monolayer wound closure,
cell migration and invasion in both the control and
TDO2-overexpressing HepG2 or Huh7 cells (Fig. S6B and C).
To examine the association between TDO2 and the
Wnt5a signal transduction pathway, the effects of pan-PKC inhibitor
GÖ 6983 treatment on HepG2 and Huh7 cells overexpressing TDO2 were
determined (Fig. S7). Again,
pan-PKC inhibition blocked PKC phosphorylation (P-PKC), and
decreased expression of CD44 and MMP7 (Fig. S7A), consistent with PKC signaling
influencing expression of these key secreted cancer biomarkers.
Pan-PKC inhibition caused a significant decrease in monolayer wound
closure, cell migration and invasion in both control and
TDO2-overexpressing HepG2 or Huh7 cells. The use of GÖ 6983 offset
the enhancement of wound healing, cell migration and invasion
caused by TDO2 overexpression. (Fig.
S7B and C). Another pharmacological inhibitor, U0126, was found
to inhibit MEK protein kinase and signaling through the canonical
MAPK pathway (Fig. S8). Testing
U0126 in this context again revealed a block in the TDO2-induced
increase in CD44 and MMP7 levels (Fig.
S8A). Furthermore, U0126 also caused a decrease in liver cancer
cell migration and invasion in the control and TDO2-overexpressing
cells in the liver cancer cells (Fig.
S8B and C). These data suggest a close functional link between
the TDO2, Wnt5a, PKC and MAPK pathways in controlling HepG2 and
Huh7 cell migration and invasion.
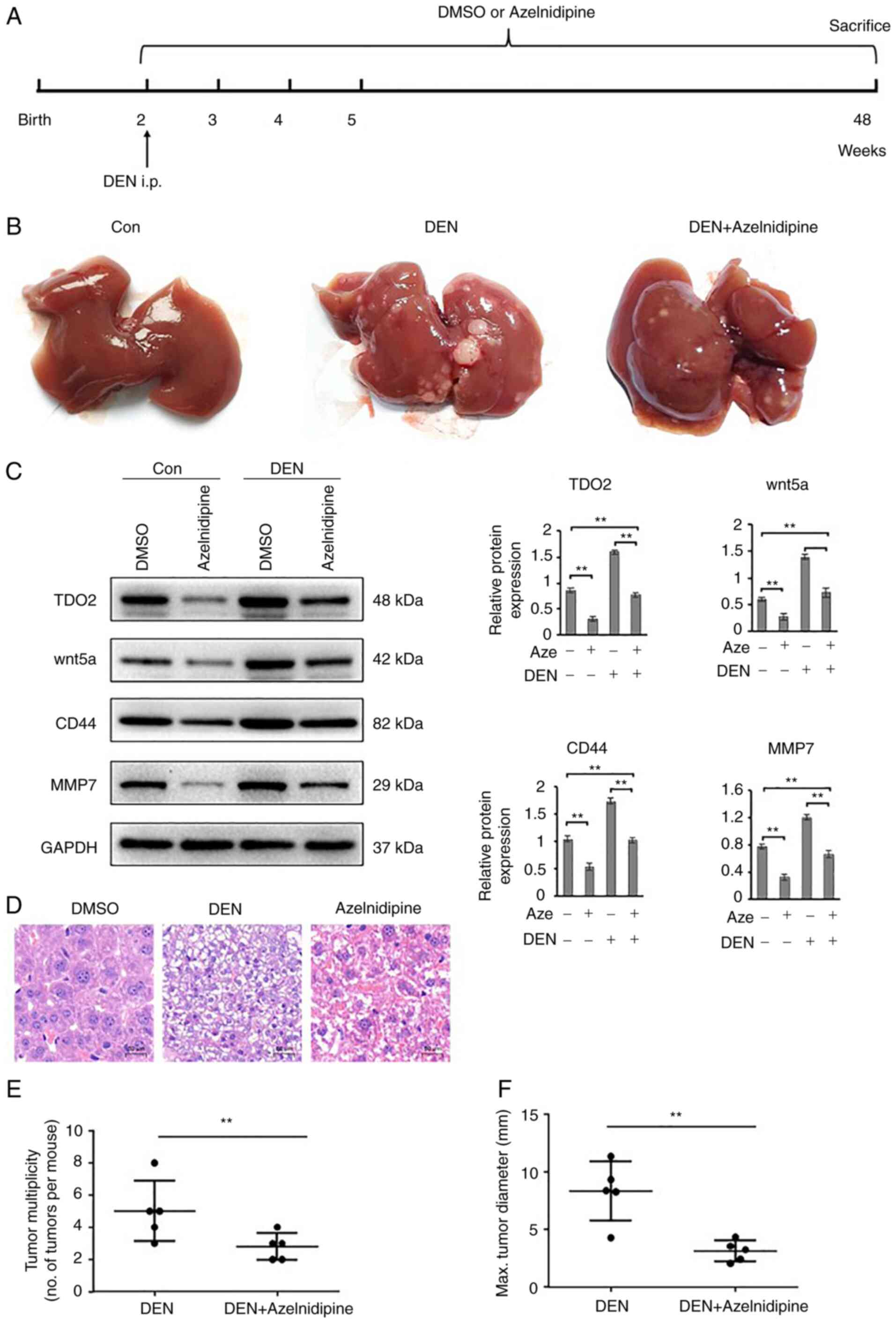
TDO2 overexpression promotes
tumorigenesis in vivo
In order to study the association between TDO2
expression with liver cancer tumor development and progression
in vivo, the effect of TDO2 on the prevalence of DEN-induced
liver cancer in C57BL/6 mice was assessed. C57BL/6 mice were
intraperitoneally injected with 20 µg/g body weight
diethylnitrosamine. To increase the expression of TDO2 in liver
tissues, from the second week onwards, 100 µl adenovirus solution
containing 1×109 adenoviruses were introduced into the
mouse peritoneum, once a week for 3 weeks (Fig. 4A). The results showed that TDO2
overexpression in the liver tissues significantly enhanced tumor
multiplicity (Fig. 4B). Western
blotting showed increased expression of TDO2 was paralleled with
increased Wnt5a, CD44 and MMP7 expression (Fig. 4C). H&E staining showed that TDO2
increased the atypia of liver cancer in mice (Fig. 4D). TDO2 overexpression in the liver
tissues of liver cancer mouse models not only enhanced the severity
of liver cancer, but also markedly enhanced the number of liver
tumors (Fig. 4E and F).
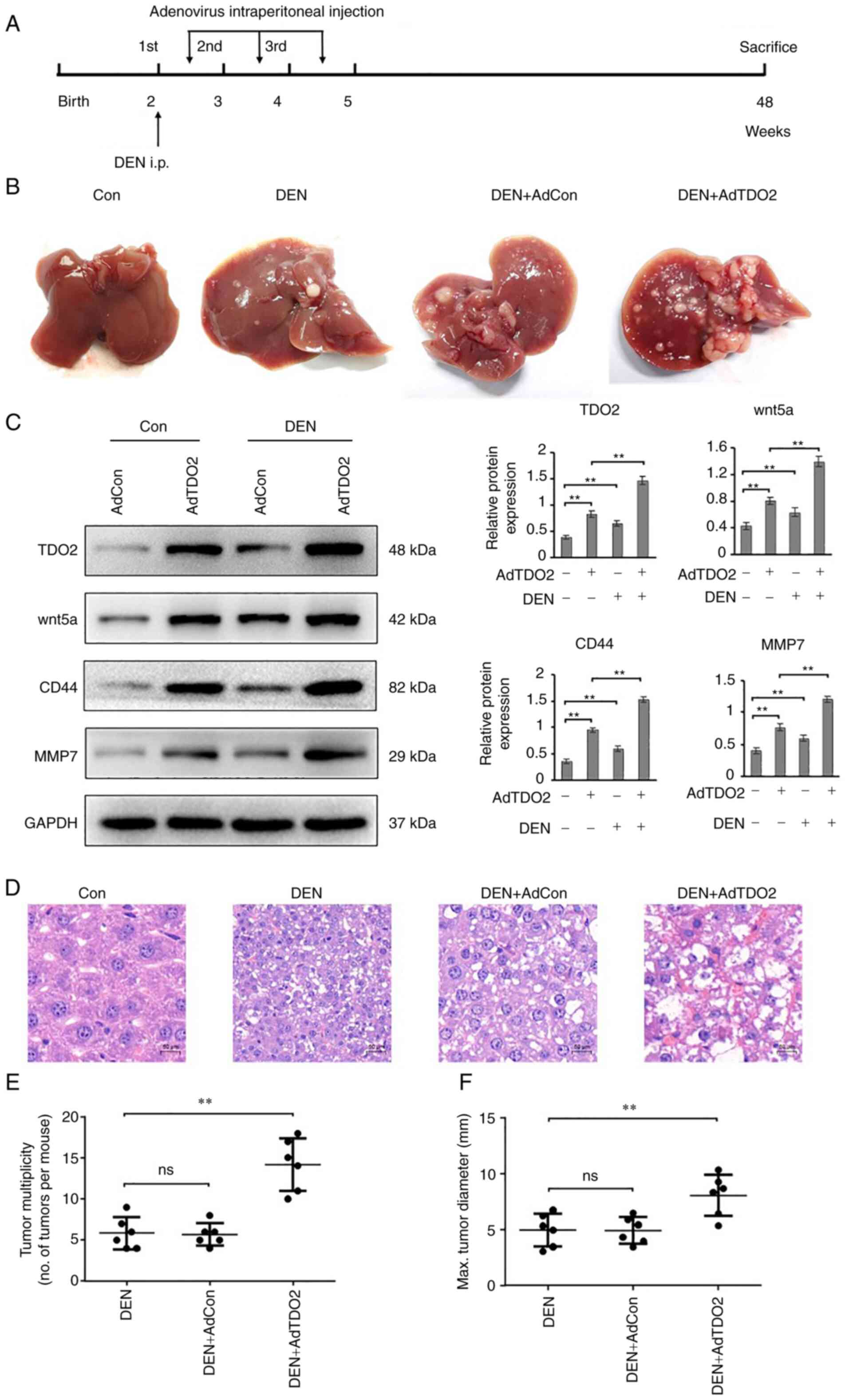 | Figure 4.TDO2 accelerates DEN-induced liver
tumor progression in C57BL/6 mice. (A) Schematic of the
experimental setup: 2-week-old male C57BL/6 mice were injected
intraperitoneally (i.p.) with 20 µg/g DEN. To increase TDO2
expression in liver tissues, adenoviruses containing the TDO2
expression cassette was introduced into the mouse peritoneum, once
a week for 3 weeks. All mice were euthanized at 12 months of age.
Mice that received normal drinking water and were not instilled
with adenovirus were used as the control. (B) Representative images
of the gross mouse livers. (C) TDO2, Wnt5a, CD44 and MMP7
expression in liver tissues were determined by western blotting.
(D) Representative microscopic features of liver cancer in
H&E-stained liver sections from mice. (E) Tumor numbers in the
mouse liver. (F) Average maximal diameters of the tumors. Scale
bar, 50 mm. Data are presented as the mean ± SEM of at least 3 mice
per group. **P<0.01; ns, not significant; TDO2, tryptophan
2,3-dioxygenase; DEN, diethylnitrosamine; H&E, hematoxylin and
eosin; MMP, matrix metalloprotease. |
A mouse xenograft model was also used to study the
association between TDO2 expression with liver cancer development
and progression. In these experiments, an immunocompromised nude
mouse was used and subcutaneously injected into HepG2 or Huh7 cells
stably overexpressing TDO2. Consistently, there was an increase in
tumor volume and weight, and TDO2 serum levels in liver cancer
cells overexpressing TDO2 compared with the control (Fig. S9A-C). Immunohistochemistry analysis
of tumor sections showed increased expression of TDO2 was
paralleled with increased Wnt5a, CD44 and MMP7 expression (Fig. S9D). These data suggest that TDO2
overexpression promotes liver cancer development and progression
in vivo.
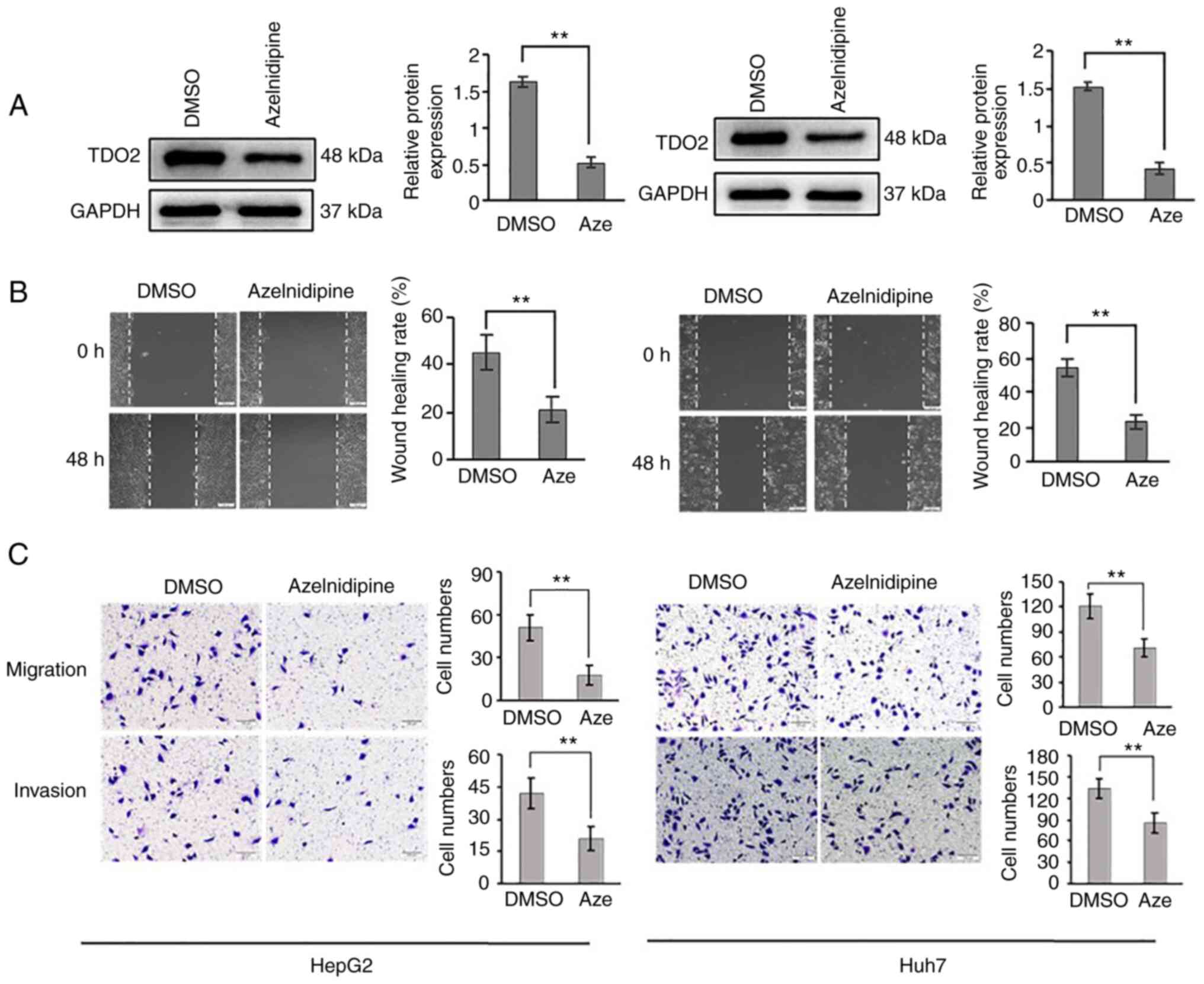
Reduction of TDO2 expression inhibits
the tumorigenesis in liver
To determine a pharmacological route for blocking
TDO2 promotion of liver cancer disease, a small molecule drug
library was screened. A calcium channel blocker, azelnidipine
(Aze), was found to be able to decrease TDO2 levels in the HepG2
and Huh7 cell lines (Fig. 5A).
Treatment with azelnidipine caused a decrease in cell migration and
invasion (Fig. 5B and C).
Overexpression of TDO2 in these liver cancer cells partially
overcame the pharmacological effects of azelnidipine (Fig. S10). Notably, TDO2 overexpression
re-established the elevation in CD44 and MMP7 levels (Fig. S10A); furthermore, although
azelnidipine caused a decrease in cell migration and invasion,
there was a consistent increase in these parameters in
TDO2-overexpressing cells compared with the controls (Fig. S10B and C).
Administration of azelnidipine to the liver cancer
mouse models reduced TDO2 expression in the liver tissues (Fig. 6A-C). Pharmacological reduction of
TDO2 expression in the liver cancer mouse model alleviated the
severity of liver cancer and liver tumor numbers (Fig. 6D-F). Similar to liver cancer mouse
models, azelnidipine reduced tumor volume, weight and serum TDO2
level in the mouse xenograft tumor model (Fig. S11A-D and Fig. S11F-I). Immunohistochemistry
analysis of tumor sections showed that azelnidipine reduced the
expression of TDO2, Wnt5a, CD44 and MMP7 (Fig. S11E and Fig. S11J).
Discussion
In the present study, it was shown that the
expression of tryptophan 2,3-dioxygenase (TDO2) was upregulated in
clinical samples of liver cancer compared with a control patient
group. By combining bioinformatics analysis and experimental
studies, miR-140-5p was determined to mediate an inhibitory effect
on TDO2 expression, largely mediated by the binding of miR-140-5p
to the 3′UTR of the TDO2 mRNA. Analysis of miR-140-5p levels
in the control and liver cancer samples indicated that lower
miR-140-5p levels were correlated with reduced overall survival in
patients with liver cancer. There was a clear association between
elevated TDO2 levels and activation of the Wnt5a signal
transduction pathway: There was increased activation of the PKC
(isoform) and the canonical MAPK pathway, resulting in increased
expression of matrix metalloprotease 7 (MMP7) and the cell adhesion
receptor CD44 cancer biomarkers. These findings showing that TDO2
promotes liver cancer disease progression is supported by other
studies, which have also highlighted a role for TDO2 in promoting
tumor development and progression in multiple disease states
including skin (18), breast
(29) and non-small cell lung
cancer (30).
Aberrant miRNA expression frequently leads to
abnormal expression of a range of target genes. miR-140-5p is known
to suppress tumor growth and metastasis by targeting fibroblast
growth factor 9 and transforming growth factor (TGF)β receptor 1
(31). miR-140-5p functions as a
tumor suppressor in breast, ovarian and non-small cell lung cancer
(32–34). The results of the present study
propose a novel role for miR-140-5p in modulating TDO2 expression,
and this is a major driver in liver cancer development and
progression (Figs. 7 and S12).
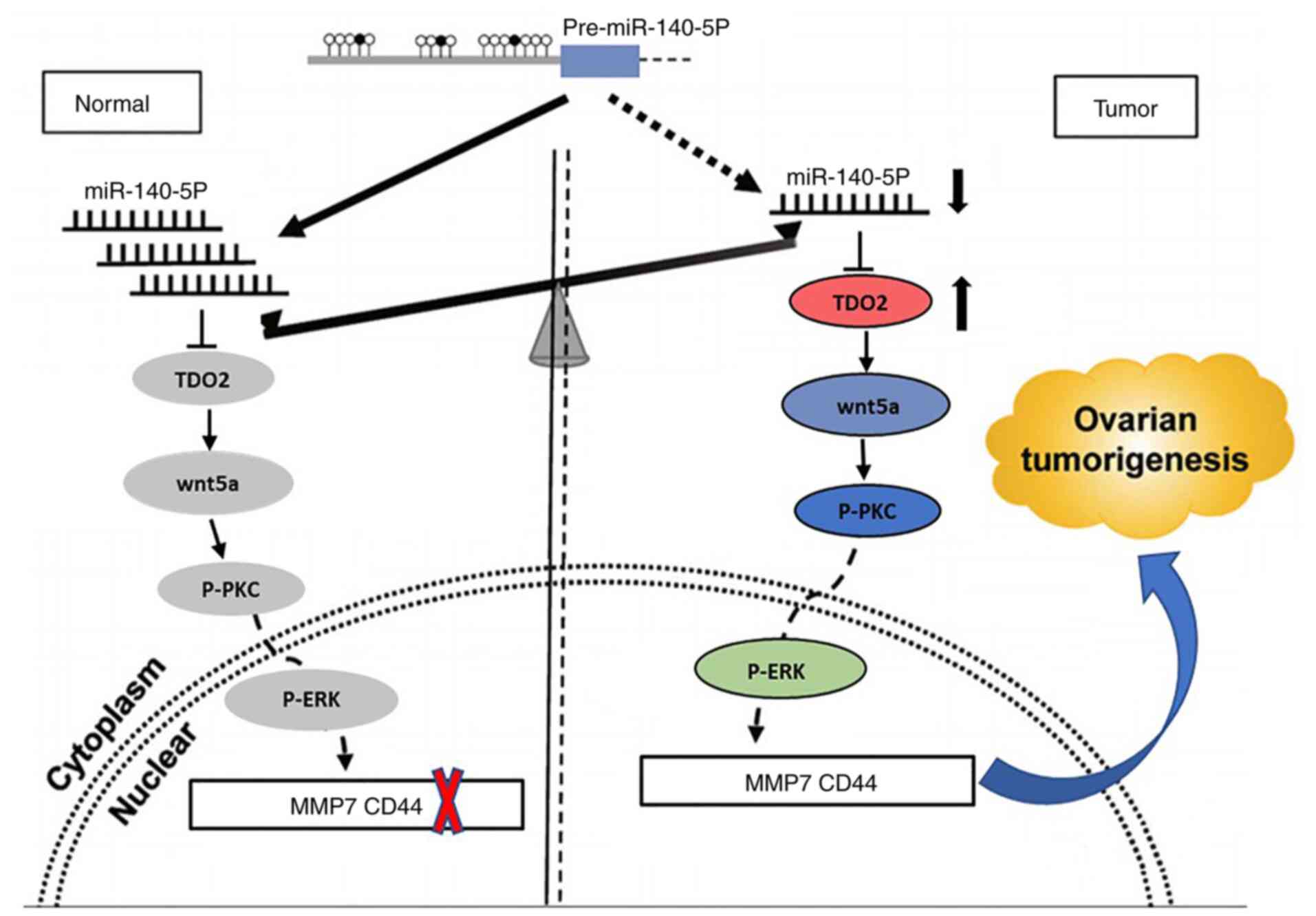 | Figure 7.Schematic diagram showing the
proposed mechanism by which TDO2 regulates liver cancer
tumorigenesis. Under normal non-transformed conditions, miR-140-5p
binds to the 3′UTR in the TDO2 mRNA and downregulates TDO2
protein levels. In liver cancer, there are reduced levels of
miR-140-5p, leading to elevated TDO2 expression levels, and thus
activation of the Wnt5a signaling pathway, and increased expression
of MMP7 and CD44. TDO2, tryptophan 2,3-dioxygenase; MMP, matrix
metalloprotease; P-PKC, phospho-protein kinase C; UTR, untranslated
region. |
How TDO2 enzymatic activity influences cancer
development and progression was also assessed. TDO2 encodes a
heme-containing enzyme and oxidoreductase that plays a critical
role in tryptophan metabolism by catalyzing the first and
rate-limiting step of the kynurenine pathway. Previous reports have
found that TDO2 plays an important role in the occurrence and
development of breast and brain cancer; this enzyme also promotes
the activation of AHR and increases the Kyn/TPR ratio in a range of
cancers (29,34–37).
In the present study, the increased levels of TDO2 activated the
Wnt5a signal transduction pathway causing an increase in MMP7 and
CD44 expression in liver cancer cells and xenograft tumors.
Administration of an miR-140-5p mimic or RNAi-mediated TDO2
knockdown caused a corresponding decrease in CD44 and MMP7 levels.
Interestingly, in the liver cancer samples, decreased levels of
miR140-5p were correlated with increased TDO2 levels, and reduced
overall liver cancer patient survival. These findings provide a
strong mechanistic link between the increased TDO2 levels and
increased signaling through the Wnt5a pathway in liver cancer;
increased expression of CD44 and MMP7 could thus contribute to
liver cancer development and progression. Furthermore, increased
TDO2 expression also modulates epithelial-mesenchymal transition
(EMT) biomarkers such as E-cadherin, N-cadherin and fibronectin
suggesting further effects on the EMT phenotype, which is
implicated in tumor metastasis.
Azelnidipine is a dihydropyridine calcium channel
blocker, and is widely used as a first-line hypertension treatment
due to the reliability of its antihypertensive effect. Azelnidipine
has diuretic, cardioprotective, renal protective and
anti-arteriosclerotic effects (38,39).
In the present study, it was found that azelnidipine could be used
to inhibit liver cancer cell migration and metastasis by inhibiting
TDO2 expression. Although the molecular mechanism by which
azelnidipine inhibits TDO2 expression is still unclear, it is
unlikely to directly involve cytosolic calcium ion levels. This was
based on the result that nifedipine, another dihydropyridine and
long-acting calcium antagonist, did not affect TDO2 expression
(Fig. S13).
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank Dr Hao Hu and Dr Jizao Yang
(The First Affiliated Hospital of Nanjing Medical University,
China) for their assistance with the pathology techniques.
Funding
This study was financially supported by the National Natural
Science Foundation of China (grant nos. 31501149, 31770815 and
31570764) and Hubei Natural Science Foundation (grant no.
2017CFB537) and the Educational Commission of Hubei (grant no.
B2020001), Hubei Province Health and Family Planning Scientific
Research Project (grant nos. WJ2021Q051 and WJ2019M255), the
Frontier Project of Applied Basic Research in Wuhan (grant no.
2020020601012250) and The Royal Society International Exchanges
UK-China Award (grant no. IECNSFC181262).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XHL conceived and devised the study. XHL, QBZ, ZTD,
HW, SP, KC, YW, HL, ZXL, TCZ and YX designed the experiments and
performed the analysis. HL, YX, ZTD, HMZ, YH, CS, KC, YW and JW
performed the experiments and analyzed the data. HW, ZXL and TCZ
confirm the authenticity of all the raw data. SP and XHL wrote the
manuscript. All authors read and approved the final manuscript for
publication.
Ethics approval and consent to
participate
The study was approved by the Scientific Ethics
Committee of the Cancer Hospital of Hubei (HZ20201018) and the
Scientific Ethics Committee of Wuhan University of Science and
Technology (WUST200913) (Wuhan, China) and was conducted in
accordance with the Declaration of Helsinki. Written informed
consents were obtained from all participants. The animal study was
reviewed and approved by the Animal Ethics Committee of Wuhan
University of Science and Technology (WS267891) (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IOD
|
integrated optical density
|
|
UTR
|
untranslated region
|
|
H&E
|
hematoxylin and eosin
|
|
TDO2
|
tryptophan 2,3-dioxygenase
|
References
|
1
|
Li LY, Yang JF, Rong F, Luo ZP, Hu S, Fang
H, Wu Y, Yao R, Kong WH, Feng XW, et al: ZEB1 serves an oncogenic
role in the tumourigenesis of HCC by promoting cell proliferation,
migration, and inhibiting apoptosis via Wnt/β-catenin signaling
pathway. Acta Pharmacol Sin. 42:1676–1689. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ichikawa T, Sano K and Morisaka H:
Diagnosis of pathologically early hcc with eob-mri: Experiences and
current consensus. Liver Cancer. 3:97–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gentile D, Donadon M, Lleo A, Aghemo A,
Roncalli M, di Tommaso L and Torzilli G: Surgical treatment of
hepatocholangiocarcinoma: A systematic review. Liver Cancer.
9:15–27. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie KL, Zhang YG, Liu J, Zeng Y and Wu H:
MicroRNAs associated with HBV infection and HBV-related HCC.
Theranostics. 4:1176–1192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hall CL, Kang S, MacDougald OA and Keller
ET: Role of Wnts in prostate cancer bone metastases. J Cell
Biochem. 97:661–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu HH, Cao G, Wu XQ, Vaziri ND and Zhao
YY: Wnt signaling pathway in aging-related tissue fibrosis and
therapies. Ageing Res Rev. 60:1010632020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caspi M, Wittenstein A, Kazelnik M,
Shor-Nareznoy Y and Rosin-Arbesfeld R: Therapeutic targeting of the
oncogenic Wnt signaling pathway for treating colorectal cancer and
other colonic disorders. Adv Drug Deliv Rev. 169:118–136. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang
H, Gusev Y, Lee MH, Kallakury B, Shivapurkar N, Cahn K, et al:
βII-Spectrin (SPTBN1) suppresses progression of hepatocellular
carcinoma and Wnt signaling by regulation of Wnt inhibitor
kallistatin. Hepatology. 61:598–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Xu J, Wong DS, Li J, Zhao P and Bian
L: Self-assembled N-cadherin mimetic peptide hydrogels promote the
chondrogenesis of mesenchymal stem cells through inhibition of
canonical Wnt/β-catenin signaling. Biomaterials. 145:33–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang S, Liu Y, Li MY, Ng CS, Yang SL, Wang
S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor growth
and metastasis by activating Wnt/β-catenin signaling pathway and
EMT in non-small cell lung cancer. Mol Cancer. 16:1242017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei CY, Zhu MX, Yang YW, Zhang PF, Yang X,
Peng R, Gao C, Lu JC, Wang L, Deng XY, et al: Downregulation of
RNF128 activates Wnt/β-catenin signaling to induce cellular EMT and
stemness via CD44 and CTTN ubiquitination in melanoma. J Hematol
Oncol. 12:212019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Li M, Li Q, Wang CJ and Xie SQ:
DKK1 promotes hepatocellular carcinoma cell migration and invasion
through β-catenin/MMP7 signaling pathway. Mol Cancer. 12:1572013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Villar J, Cabrera NE, Casula M, Valladares
F, Flores C, López-Aguilar J, Blanch L, Zhang H, Kacmarek RM and
Slutsky AS: WNT/β-catenin signaling is modulated by mechanical
ventilation in an experimental model of acute lung injury.
Intensive Care Med. 37:1201–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Russell JO and Monga SP: Wnt/β-catenin
signaling in liver development, homeostasis, and pathobiology.
Annual Rev Pathol. 13:351–378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang R, Lin HM, Broering R, Shi XD, Yu
XH, Xu LB, Wu WR and Liu C: Dickkopf-1 contributes to
hepatocellular carcinoma tumorigenesis by activating the
Wnt/β-catenin signaling pathway. Signal Transduct Target Ther.
4:542019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Rajasekaran M, Xia H, Zhang X,
Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL,
et al: The microtubule-associated protein PRC1 promotes early
recurrence of hepatocellular carcinoma in association with the
Wnt/β-catenin signalling pathway. Gut. 65:1522–1534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuo Q, He J, Zhang S, Wang H, Jin G, Jin
H, Cheng Z, Tao X, Yu C, Li B, et al: PPARγ Coactivator-1α
suppresses metastasis of hepatocellular carcinoma by inhibiting
warburg effect by PPARγ-dependent WNT/β-catenin/Pyruvate
dehydrogenase kinase isozyme 1 axis. Hepatology. 73:644–660. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wardhani LO, Matsushita M, Iwasaki T,
Kuwamoto S, Nonaka D, Nagata K, Kato M, Kitamura Y and Hayashi K:
Expression of the IDO1/TDO2-AhR pathway in tumor cells or the tumor
microenvironment is associated with merkel cell polyomavirus status
and prognosis in merkel cell carcinoma. Hum Pathol. 84:52–61. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheong JE and Sun L: Targeting the
IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy-challenges and
opportunities. Trends Pharmacol Sci. 39:307–325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung DJ, Rossi M, Romano E, Ghith J, Yuan
J, Munn DH and Young JW: Indoleamine 2,3-dioxygenase-expressing
mature human monocyte-derived dendritic cells expand potent
autologous regulatory T cells. Blood. 114:555–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fallarino F, Grohmann U, You S, McGrath
BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi
C, et al: The combined effects of tryptophan starvation and
tryptophan catabolites down-regulate T cell receptor zeta-chain and
induce a regulatory phenotype in naive T cells. J Immunol.
176:6752–6761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Zhang W, Wu C, Gao H, Yu J, Wang X,
Li B, Jun Z, Zhang W, Zhou P, et al: HOXC10 promotes proliferation
and invasion and induces immunosuppressive gene expression in
glioma. FEBS J. 285:2278–2291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li S, Weng J, Song F, Li L, Xiao C, Yang W
and Xu J: Circular RNA circZNF566 promotes hepatocellular carcinoma
progression by sponging miR-4738-3p and regulating TDO2 expression.
Cell Death Dis. 11:4522020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muller AJ, Manfredi MG, Zakharia Y and
Prendergast GC: Inhibiting IDO pathways to treat cancer: Lessons
from the ECHO-301 trial and beyond. Semin Immunopathol. 41:41–48.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Mahendran R, Tham SM, Thamboo TP,
Chionh BJ, Lim YX, Tsang WC, Wu QH, Chia JY, Tay MHW, et al:
Tryptophan-kynurenine ratio as a biomarker of bladder cancer. BJU
Int. 127:445–453. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmitt M, Metzger M, Gradl D, Davidson G
and Orian-Rousseau V: CD44 functions in Wnt signaling by regulating
LRP6 localization and activation. Cell Death Differ. 22:677–689.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lv YF, Dai H, Yan GN, Meng G, Zhang X and
Guo QN: Downregulation of tumor suppressing STF cDNA 3 promotes
epithelial-mesenchymal transition and tumor metastasis of
osteosarcoma by the Wnt/GSK-3β/β-catenin/Snail signaling pathway.
Cancer Lett. 373:164–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Amato NC, Rogers TJ, Gordon MA, Greene
LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC
and Richer JK: A TDO2-AhR signaling axis facilitates anoikis
resistance and metastasis in triple-negative breast cancer. Cancer
Res. 75:4651–4664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Creelan BC, Antonia S, Bepler G, Garrett
TJ, Simon GR and Soliman HH: Indoleamine 2,3-dioxygenase activity
and clinical outcome following induction chemotherapy and
concurrent chemoradiation in Stage III non-small cell lung cancer.
Oncoimmunology. 2:e234282013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang H, Fang F, Chang R and Yang L:
MicroRNA-140-5p suppresses tumor growth and metastasis by targeting
transforming growth factor β receptor 1 and fibroblast growth
factor 9 in hepatocellular carcinoma. Hepatology. 58:205–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu D, Zhang J, Lu Y, Bo S, Li L, Wang L,
Zhang Q and Mao J: miR-140-5p inhibits the proliferation and
enhances the efficacy of doxorubicin to breast cancer stem cells by
targeting Wnt1. Cancer gene therapy. 26:74–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu J, Cai H, Wu Y, Fang S and Wang D:
Elevation of FGD5-AS1 contributes to cell progression by improving
cisplatin resistance against non-small cell lung cancer cells
through regulating miR-140-5p/WEE1 axis. Gene. 755:1448862020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lan H, Chen W, He G and Yang S: miR-140-5p
inhibits ovarian cancer growth partially by repression of PDGFRA.
Biomed Pharmacother. 75:117–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guastella AR, Michelhaugh SK, Klinger NV,
Fadel HA, Kiousis S, Ali-Fehmi R, Kupsky WJ, Juhász C and Mittal S:
Investigation of the aryl hydrocarbon receptor and the intrinsic
tumoral component of the kynurenine pathway of tryptophan
metabolism in primary brain tumors. J Neuro Oncol. 139:239–249.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohapatra SR, Sadik A, Tykocinski LO,
Dietze J, Poschet G, Heiland I and Opitz CA: Hypoxia inducible
factor 1α inhibits the expression of immunosuppressive
tryptophan-2,3-dioxygenase in glioblastoma. Front Immunol.
10:27622019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greene LI, Bruno TC, Christenson JL,
D'Alessandro A, Culp-Hill R, Torkko K, Borges VF, Slansky JE and
Richer JK: A role for tryptophan-2,3-dioxygenase in CD8 T-cell
suppression and evidence of tryptophan catabolism in breast cancer
patient plasma. Mol Cancer Res. 17:131–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Azuma A, Kudoh S, Nakashima M and Nagatake
T: A double-blind study of zaltoprofen for the treatment of upper
respiratory tract infection. Pharmacology. 85:41–47. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ohyama T, Sato K, Kishimoto K, Yamazaki Y,
Horiguchi N, Ichikawa T, Kakizaki S, Takagi H, Izumi T and Mori M:
Azelnidipine is a calcium blocker that attenuates liver fibrosis
and may increase antioxidant defence. Br J Pharmacol.
165:1173–1187. 2012. View Article : Google Scholar : PubMed/NCBI
|