Patients with recurrent or metastatic (R/M) head and
neck squamous cell carcinomas (HNSCC) have few treatment options
with little or no permanent response to therapy. Novel treatment
regimens are essential as conventional treatment pillars exert
substantial toxicities and are associated with unfavorable
outcomes. This limited response may be partly explained by distinct
intratumoral and intertumoral heterogeneity of HNSCC represented by
a complex mutational landscape. HNSCC carcinogenesis is propagated
by frequent chromosomal instability and multiple genetic drivers
undergoing evolution due to selective pressure during treatment
(1). In a relevant amount of
HNSCC cases, an inflammatory phenotype with tumor-infiltrating
lymphocytes is apparent (2). Yet,
often immunomodulatory molecules are expressed. For instance, a
number of tumor entities, including HNSCC, express programmed cell
death ligand (PD-L)1 and PD-L2, which interact with their
programmed cell death-1 (PD-1) receptor to limit the function of
activated T cells (3-5). At present there is growing knowledge
of the molecular processes that induce the expression of PD-L1 and
PD-L2 or modulate protein stability (6,7).
However, the influence of established therapies such as
radiotherapy (RT), chemotherapy (CT), combined radiochemotherapy
(RCT) and the antibody against the epidermal growth factor receptor
(EGFR) cetuximab on PD-L1 and PD-L2 expression is only
insufficiently known.
That HNSCC frequently express immunomodulatory
molecules and bear inflammatory phenotypes establish the potential
effectiveness of immunomodulatory therapeutic agents (8). The development of so-called
checkpoint inhibitors, which influence the specific T cell activity
as part of the adaptive immune system, was a major breakthrough in
immunotherapy. With the discovery of the first checkpoints
cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and PD-1, a
key regulatory principle of the immune system was revealed
(9-11). PD-1 antibodies are the first
immunotherapeutics that have been able to achieve a stable response
and a reduced mortality rate in R/M HNSCC patients (12-15). The safety and clinical anti-tumor
activity of pembrolizumab and nivolumab, two antibodies against
PD-1, have already been confirmed in several clinical studies in
patients with R/M HNSCC. The two antibodies have been approved by
the FDA (Food and Drug Administration) since 2016 and have already
established themselves as a new standard therapy option for
patients with advanced HNSCC after failure of conventional
approaches, especially after platinum-based CT. Despite these
promising data, the number of patients responding to immune
modulation is low. The overall response rate (ORR) in the CheckMate
141 study is only 13.3% (13) and
18% in the KEYNOTE-012 study (14). If a durable response is attained,
which is rarely the case, this is mostly due to other types of
anticancer therapies (16).
Biomarkers are useful tools and are meant to support
stratification of patients when randomizing the cohort, assist
monitoring under treatment and aid in predicting which subgroups of
patients may derive the greatest benefit from specific treatments
(17). The identification of
these markers, whether they are molecular, histologic, radiologic,
or physiologic, is challenging in a number of tumor types,
including HNSCC. The ideal biomarker is required to encompass the
presentation of molecular therapeutic targets considering the
mutational spectrum but also the tumor microenvironment (TME)
features affecting the clinical course and therapeutic sensitivity
of the disease (18).
As only some patients respond to CPI, it is
necessary to identify and select the subset that might benefit from
CPI beforehand. For monitoring the response to standard oncological
treatments of HNSCC, a variety of biomarkers has been described,
demonstrating predictive value (17). For instance, Sailer et al
(19) found that paired-like
homeodomain transcription factor 2 (PITX2) methylation functions as
an identifier for patients that potentially require additional
measures such as intensified surveillance or adjuvant therapy. DNA
repair protein expression including excision repair cross
complementation group 1 (ERCC1) is also proposed as a predictive
and prognostic marker with decreased overall survival (OS) in HNSCC
(20) and in patients undergoing
definitive platinum-based RCT for HNSCC, irrespective of human
papillomavirus (HPV) status (21). Regarding EGFR blockade,
phosphatase and hensin homolog (PTEN) loss exerts a negative
prognostic impact in patients treated with cetuximab + CT, but not
in the CT only group (22).
Negative effects of CD44 and EGFR and positive effects of p16 on RT
results have been published (23).
PD-1 and PD-L1 expression currently remain the most
significant tissue biomarkers to predict success or failure of
immuno-oncological approaches. Interferon (IFN)y induced
upregulation of PD-1/PD-L1 suppresses the immune response by
downregulating cytokine expression in the TME. PD-1/PD-L1
inhibitors induce tumor regression and this process is affected by
TME-related parameters such as PD-L1 status and tumor-infiltrating
lymphocytes (TILs) (24).
Adaptive immune resistance is one mechanism by which tumors escape
the immune system and is represented by the process of expression
of PD-L1 in response to cytokines. IFN-y induces upregulation of
PD-1/PD-L1, cytokines could be decreased and the immune response in
the TME is consecutively impaired (25,26). Anti-PD-1/PD-L1 treatment causes
tumor regression. Factors that are tumor cell intrinsic and related
to the TME such as PD-L1 status and TILs are supposed to affect
this process (27). Accordingly,
PD-L1 expression and CD8+ T cell density are suggested as
predictive biomarkers for anti-PD-1/PD-L1 efficacy There is an
interrelation between docetaxel, platinum and fluorouracil (TPF)
induction CT in advanced HNSCC and PD-L1 positivity on
tumor-infiltrating immune cells as well as CD8+ lymphocytes
density. These features suggest that combination strategies of
concomitant cytotoxic therapies and anti-PD-1/PD-L1 therapies might
be relevant for HNSCC (28).
In esophageal and colorectal cancer, TIL and Treg
cells are associated with a favorable outcome (33,34). Conversely, the expression of the
forkhead box P3 (FoxP3) protein, the most specific Treg marker, in
HNSCC tissue samples was significantly associated with inferior
survival (35). An enhanced
infiltration of Tregs in both intratumoral and stromal compartments
is associated with improved clinical outcomes (36). By analyzing pre-therapeutic tissue
samples of 280 patients with locally advanced HNSCC treated with
RCT for expression of CD8+ cytotoxic T cells and FoxP3+ Tregs,
Echarti et al (37)
describe a classification into different subsets defined by
intraepithelial and stromal cytotoxic T cells. They found opposing
effects of Tregs between the groups. While in 'immune desert' and
'immune excluded' tumors high Tregs lead to worse survival, the OS
was improved in 'inflamed' tumors with the same Treg constellation.
This finding might in part explain why the prognostic significance
of Tregs is inconsistent in earlier publications (37). Cho and Lim (38) refer to these discrepancies by
differences in tumor type, molecular features, distribution
patterns of Tregs and markers of Treg cells. Recently it was shown
that an increase in the levels of circulating Treg cells not only
in tumor tissue but also in peripheral blood could serve as a
prognostic factor of survival in patients with oral cancer.
Notably, in this study, intratumoral-infiltrating Tregs had no
prognostic significance, while circulating Treg cells in peripheral
blood were associated with a favorable clinical outcome (38). When evaluating the impact of Treg
cells on prognosis, HPV-association of the tumor should be
considered as a relevant factor as the immunologic landscape of
HPV-driven HNSCC is modified (1).
Although PD-1 and PD-L1 expression is not altered by HPV status,
HPV-infected tumors display higher expression of anti-CTLA-4 as
well as Treg infiltration and Tregs/CD8 ratio, indicating that HPV
positivity might enhance the sensitivity to CPI (1). In accordance, most studies report an
increased density of TILs in HPV-driven HNSCC compared with HPV
non-driven tumors. These observations imply a more effective
anti-tumoral immune response and consecutively an improved clinical
outcome for HPV-negative as well as HPV-positive patients with a
more intense tumor infiltration of high CD8+ T cells This
observation suggests a more effective anti-tumoral immune response
and hence an improved clinical outcome for HPV-negative as well as
HPV-positive patients with a more intense tumor infiltration of
high CD8+ T cells (31). However,
Cho and Lim (38) found the Treg
cellular impact on clinical outcome and HPV status not to be
interrelated. In contrast, Lukesova et al (39) state that oropharyngeal squamous
cell carcinoma patients show improved survival when their tumors
are HPV-associated and display elevated Treg levels in peripheral
blood samples.
In addition to Tregs, natural killer (NK) cells
serve an important role in the immune system. NK cells are
characterized as CD56+ CD3-lymphocytes that are part of the innate
immune system and the first line of defense against viruses,
pathogens and cancer (40).
Again, Lukesova et al (39) found a significant difference in
the levels of NK cells between the groups of HPV-positive and HPV
negative patients. Higher levels of NK cells are observed in
HPV-positive patients with improved prognosis. According to Renoux
et al (41) NK cells are
stimulated to higher cytotoxicity and increased cytokine production
by the binding of HPV-specific virus-like particles via the C16
receptor, which could mechanistically explain the finding from
Lukesova et al (39).
Furthermore, smoking as one of the main risk factors for HNSCC may
also contribute to the sensitivity of the tumor towards PD-L1
agonists. Certain genetic smoking signatures of the tumor reflect
smoking. In such tumors, immune infiltration is diminished, often
combined with local immunosuppression and lower levels of
cytotoxicity within the immune microenvironment. This is associated
with an unfavorable prognosis (2,42,43). These subsets of patients may well
respond to CPI. CPI could probably be combined with immune agonists
targeting co-stimulatory molecules expressed on the surface of T
cells, such as 4-1BB, OX40, CD40, GITR) thereby directly
stimulating immune response (1,44,45). Suppressive effects on NK cells,
CD8+ T cells and dendritic cells (DCs) are likely to be involved,
but the precise mechanisms remain to be elucidated (46). In turn, a higher mutational load
is associated with higher response rate towards anti-PD-1 therapy
as shown in a study on lung cancer patients (47). Mandal et al speculate from
their data on HNSCC that smoking signature-high HNSCC may benefit
from immune modulators such as IL-2, toll-like receptor (TLR) and
stimulator of interferon genes (STING) agonists. These compounds
are already applied in other entities to enhance overall host
immunity (1). De la Iglesia et
al (48) analyzed a cohort of
HNSCC for their expression of CD3, CD8, FOXP3, PD-1, PD-L1 and
pan-cytokeratin by multiplex immunofluo-rescence. They report
decreased numbers of cytotoxic T cells in tumors of current smokers
and lower gene expression in the interferon cascades compared with
former-and never-smokers. They conclude that the tumor immune
microenvironment is actively modulated by smoking. This might be
depicted by the presence of higher numbers of immune cells in
certain areas of the tumor, such as the tumor margin (48). The data underscore the need to
investigate novel agents that target modulators of Tregs [e.g.,
CTLA-4, Glucocorticoid-Induced TNFR-Related (GITR), inducible T
cell co-stimulator (ICOS), IDO and vascular endothelial growth
factor A (VEGF-A)] as well as NK cells (e.g., KIR, TIGIT and 4-1BB)
in addition to anti-PD-1 compounds in the treatment of advanced
HNSCC (1).
Tumor mutational burden (TMB) has been investigated
as a potential predictive biomarker to immune checkpoint blockade
across 27 tumor types (49). TMB
is defined as a median number of coding somatic mutations per DNA
mega-base (N mut/MB) (49). HNSCC
is among the neoplasms with the highest TMB. However, why is TMB
associated with the response to PD-1/PD-L1 inhibitors? In theory,
an increased number of missense mutations is related to a higher
number of tumor neo-antigens, which may induce a more substantial
immune reaction and increase the response to CPI treatment. TMB,
PD-L1 and T cell inflamed gene expression profile (GEP) are
independently predictive of response to pembrolizumab in HNSCC
patients, in general regardless of the HPV status. They were also
correlated with progression-free survival (PFS). TMB, the combined
positive score (CPS) and a T cell inflamed GEP were all associated
with best ORs (50). By contrast,
in a cohort of 126 HNSCC patients, responders to immunotherapy
displayed significantly higher levels of TMB than did
non-responders. Notably, virus-positive [HPV-positive)/Epstein-Barr
virus (EBV)-positive] patients had a lower TMB (P<0.01) and
improved OS (P=0.02) (51). High
TMB is often used as a surrogate of immune response to tumors as it
is associated with a larger number of tumor neo-antigens, due to a
higher somatic mutation level. Those neo-antigens boost the
development of the anti-tumor immune response by facilitating the
immune recognition as foreign (52). Additionally, plasma-based TMB
(bTMB) was evaluated as a prognosticator in the phase III EAGLE
study for HNSCC. Patients with high bTMB levels had significantly
improved OS and PFS after immunotherapy compared with patients who
were administered platinum-based CT (53). In current smokers, a suppressive
immune microenvironment is mirrored by a decreased numbers of
cytotoxic T cells in the tumor and suppression of IFN response
pathways. Notably, in this study there was no evidence for an
association between smoking status and TMB and tumor clonality by
the MATH (mutant-allele tumor heterogeneity) score (48) which is not in line with former
observations that carcinogens in tobacco smoke are expected to
cause permanent DNA damage reflected in TMB (54).
A T cell inflamed TME persists in the major subset
of patients with advanced solid tumor diseases. This phenomenon
probably reflects an anti-tumor response resulting in a more
favorable clinical outcome (55).
Tumors with T cell infiltration into the TME are more likely to
respond to immunotherapy including CPI. Hanna et al
(51) investigated a cohort of
126 HNSCC patients treated with anti-PD-1/L1 therapy and found
higher TMB and CD8+ T cell infiltrates to predict a potential
benefit from anti-PD-1/L1 treatment significantly among
virus-negative tumors. B cells and myeloid-derived suppressor cells
are increased in PD-1 blockade responders (56) and the authors now hypothesize an
expanded CD8+ effector memory T cell population among the
responders. The inflammation-induced enzyme Indoleamine
2,3-dioxygenase (IDO) normally controls harmful inflammatory
responses by propagating immunosuppression. IDO is increasingly
expressed in tumor, stroma and immune cells and is hypothesized to
contribute to cancer immune evasion (57). Accordingly, Jia et al
reported that higher expression of IDO was associated with poorer
OS in head and neck cancer patients (P=0.011) and classified it as
a prognostic predictor in head and neck cancer (58). IDO activity has already been
linked to resistance against PD-1 CPI in non-small cell lung cancer
(NSCLC) (59). For HNSCC, Phase
I/II clinical trials have been conducted and showed ORs (34-55%)
and disease control rates (62-70%) for IDO1 inhibitor in
combination with a PD-1 inhibitor, as recently summarized by Lin
et al (60). Combining IDO
inhibitors revealed similar safety profiles with the compound given
as monotherapy. IDO gene expression is referred to as a predictive
biomarker for response to PD-L1 therapy. Although there is a body
of evidence for the application of IDO-based treatment, in all of
the trials IDO inhibitors have been combined with anti-PD1/PD-L1
agents. Furthermore, the IDO immune-based regimen have not been
compared with current standard of care (SOC) regimens for HNSCC
(60).
In summary, it is necessary to unveil the molecular
mechanisms causing a reduction of effector T cell infiltration in
the TME and causing a decreased susceptibility towards CPI.
Consequently, the development of novel compounds that permit to
restore T cell infiltration will promote the efficacy of
immunotherapy. The majority of HNSCC displays immune infiltration,
which is an indicator of an ongoing natural immune response.
Additionally it will be a major challenge to develop new
therapeutic interventions that will amend the mode of action of
immunotherapies towards enhanced efficacy in patients whose tumors
bear the non-T cell-inflamed phenotype. In HNSCC, an inflamed
cancer phenotype is very common, but even those tumors eventually
manage to evade host immunity (61). Therefore Chen et al
(62) propose to stratify HNSCC
into the active and exhausted immune classes. The active immune
class incorporates factors correlating with increased survival
including HPV association, an abundance of TILs, increased
cytolytic activity and pro-inflammatory M1 macrophage signature
while the exhausted immune class is linked with enriched activated
stroma, M2 macrophage signatures, activation of Wnt signaling and
unfavorable outcome. Understanding the immune responses and their
regulation by tumor-intrinsic and extrinsic factors in the TME is
the prerequisite for optimizing the immunotherapeutic response.
Patients assigned to the active immune class may benefit from CPI
as a monotherapy while those with the exhausted immune class may
benefit from combinations including TGF-β inhibitors or anti-CTLA-4
therapy, respectively, combined with anti-PD-1/PD-L1 agents
(62).
HNSCC harbor complex molecular pathology features
and a distinct heterogeneity and vary between localization and
etiology. HPV association in oropharyngeal HNSCC serves a major
role in prognosis and treatment and has recently been classified as
a separate disease entity by the 8th Ed UICC/AJCC TNM staging
system (63). Signaling pathways
are widely affected in HNSCC and are known to be involved in
processes underlying immune evasion in HNSCC. Wondergem et
al (64) explored three
pathways: STAT3, PI3K/AKT/mTOR and Wnt, which are assumed to
represent promising targets to possibly facilitate immunotherapy
response. These pathways are of particular importance, because they
are involved in cellular processes considered to account for
primary or acquired resistance to immunotherapy. The authors
hypothesize that immune responses will be augmented by
pharmacological interference with the signaling components and
their immune-modulatory features to enhance their sensitivity to
CPI. It has been demonstrated that CD8+ T cell infiltration is
pushed through inhibition of the PI3K/AKT/mTOR or Wnt pathways
inducing immunologically 'hot' tumors. The aim is to stop the
propagation of tumor cells and stimulate the immune response at the
same time by blocking responsible signaling cascades. The emphasis
should be on the comparison between HPV-driven and non-driven head
and neck cancer patients as different pathways seem to be relevant
for their respective immune response and response to drugs
(64). Oncogenic pathways that
are activated through gain-of-function alterations in oncogenes or
loss-of-function alterations in tumor suppressor genes are known to
influence the local anti-tumor immune response. Spranger and
Gajewski (65) state that CPI is
more likely to be effective if T cells infiltrate the TME. There
are several pathways involved in the reduction of the effector T
cell infil-tration and new therapeutic strategies should aim on
boosting the efficacy of immunotherapy by restoration of T cell
infiltration by molecularly targeting these biochemical cascades.
PI3K inhibitors, which have already been approved for cancers such
as B cell lymphoma, leukemia and breast cancer, could help to
overcome the dismal response rates to CPI in cancers. The idea is
to boost a T cell-inflamed TME by inhibiting relevant pathways with
specific compounds that should be administered in a combined
regimen along with anti-PD-1 or anti-PD-L1 treatment to promote the
response of cancer to these mAbs. Spranger and Gajewski (65) refer in their review to a synergism
between a PI3Kβ isoform-preferential inhibitor and CPI in an in
vivo model, pointing out that these inhibitors might be capable
of boosting the susceptibility to immunotherapy. Peng et al
(66) show that loss of PTEN in
tumor cells of preclinical melanoma models lead to an inhibition of
T cell mediated tumor cell killing and diminishes T cell
infiltration into the tumor. PTEN loss is known to be associated
with a decrease of T cell infiltration and successful T cell
expansion after tumor resection, concomitantly with lower
susceptibility to PD-1 mAb and unfavorable clinical outcome. As a
mechanism, immunosuppressive cytokine expression is stimulated by
the loss of PTEN, with consecutively low levels of T cell
infiltration in tumors and inhibition of autophagy by which T
cell-mediated tumor cell killing is reduced. After a combined
treatment with a selective PI3Kβ inhibitor and
anti-PD-1/anti-CTLA-4 antibodies, respectively, the susceptibility
to immunotherapy was enhanced in vivo (66). Using a triple negative breast
cancer (TNBC) patient-derived xenograft (PDX) model, PI3K
inhibition by BKM120 is followed by a more inflammatory tumor
leukocyte infiltrate. Accordingly, the combined application of
BKM20 and anti-PD-1 consistently inhibits the tumor growth compared
with monotherapy. In conclusion, the susceptibility to CPI is
boosted by PI3K pathway inhibition (67). This combination regimen might be a
strategy for TNBC but also for other types of cancer with low
response rates to immunotherapy including HNSCC. These studies
warrant the need for new strategies to transform the TME of
non-responsive patients into a milieu supporting T cell-based
inflammation. However, one has to take into account that some of
these pathways identified are critical for the maintenance of
normal tissues. New compounds should therefore selectively target
the relevant immune components while preserving globally relevant
pathways (65).
MAPK cascades activate a number of important
cellular processes by inducing mediators leading to cell growth,
proliferation, differentiation, migration, invasion and survival
(68). The ability of
immunocompetent MAPK mutations in HNSCC are proven to drive a CD8+
T cell-inflamed status in in vivo models (69). Patients with MAPK mutations
consistent with CD8+ T cell-inflamed phenotypes were investigated.
This subset of patients survived 3.3-4 times longer than wildtype
patients under anti-PD-1/PD-L1 immunotherapies. The phenomenon was
seen independently of the TMB. Notably, in this context pathway
mutations were linked to remarkably long patient survival rates.
The prognostic power was found to be independent of the HPV status.
As a mechanism, phosphorylated human epidermal growth factor
receptor 3 (p-ErbB3) regulation by MAPK pathway-mutants is
featured. Low tumoral p-ErbB3 levels and elevated CD8+ T cell
infiltrations are described as indicators of prolonged survival in
HNSCC (70,71). The two events have now been shown
to be governed by MAPK mutations and are likely to be independent
of each other. These findings are likely to contribute to the
ongoing search for predictive biomarkers in HNSCC. MAPK pathway
mutations could help identifying HNSCC patients with CD8+ T
cell-inflamed tumors that might benefit from immunotherapy
(69).
Another approach, which may offer new therapeutic
opportunities, is the activation of the STING pathway through
polymer-induced STING condensation. The cyclic guanosine
monophosphate-adenosine monophosphate synthase-stimulator of
interferon genes (cGAS-STING) is a major regulator of innate immune
sensing of cancer, with potential to enhance tumor rejection
through the induction of a pro-inflammatory response dominated by
Type IIFNs. The first STING agonist is currently in phase I
clinical development. Although in pre-clinical trials assessing a
plethora of natural and synthetic cyclic dinucleotides and
non-nucleotidyl STING agonists these compounds have been promising,
clinical early phase studies on various tumor entities revealed
only modest anti-cancer activity (72). A number of early phase trials are
continuing. For R/M HNSCC, a phase II study is currently examining
the combination of the STING pathway activator ADU-S100 plus
pembrolizumab in patients with no prior systemic treatment.
Endpoints include safety, preliminary anti-tumor activity,
pharmacokinetics and immunomodulation. The results provided
evidence for a robust toleration of ADU-S100 plus pembrolizumab
(73). One limitation of these
efforts is the inherent instability of dinucleotides. The
administration of most of the STING agonists in ongoing clinical
trials, namely directly intra-tumoral (i.t.) is not ideal, as their
application is limited to a narrow spectrum of tumors. An promising
exception is stable STING agonists as identified by Pan et
al (74) and Chin et
al (75) inducing the same
'closed' conformation as the natural STING ligand. The advantage of
these small molecules over previously designed i.t. administered
STING agonists is the possibility of an oral application. The
agonists were shown to activate STING and diverse immune cell types
thereby promoting antitumor immunity by activation of CD8+ T,
natural killer and DCs and exhibiting anti-cancer activity.
Notably, the compound SR-717 stimulates the expression of relevant
target proteins including PD-L1 in a STING-dependent way (75). Efforts should be made in the
progress towards the clinical practice of viable STING agonists as
these compounds are hypothesized to be conveniently applied in a
low-cost regimen.
Unfortunately, it has not yet been possible to
predict adequately the success of CPI for the individual patient.
Clinical studies are searching for biomarkers that would enable
such a prediction. In general, poor prognostic factors such as high
tumor burden, rapidly progressive tumor growth and poor general
condition and performance status seem to be rather unfavorable
regarding the response to immunotherapy (76).
For individual indications, the expression rate of
PD-L1 in tumor tissue has been proven to be a response marker to
PD-1/PD-L1 therapy (i.e. PD-L1 as a predictive biomarker). In most
cases, PD-L1 expression on tumor and/or immune cells is also
associated with OS independently of treatment (i.e. PD-L1 as a
prognostic biomarker) (76).
The KEYNOTE 040 study is an example for this
assertion. Patients with R/M HNSCC after progression on previous
platinum-based therapy were treated with either pembrolizumab or
standard therapy of the investigator's choice (methotrexate,
docetaxel, or cetuximab). Patients had a significantly improved
survival in case their tumor tissue samples showed positivity for
PD-L1. The positivity was defined by a TPS (tumor proportion score)
of ≥50%, which means PD-L1 expression in tumor cells only. Those
patients displayed both an improved response in the pembrolizumab
treatment arm and poorer survival in the CT arm compared with
patients with PD-L1 TPS <50% (77). These differing responses in PD-L1
high and low-expressing tumors led to a restriction of approval for
patients with PD-L1 high-expressing tumors. Szturz and Vermorken
(5) give recommendations for the
translation of the KEYNOTE-048 data in the clinical practice. They
scrutinize the motivation to apply immunotherapy in tumors with
lower PD-L1 expression as given cut-off values should not be
regarded as a dogma and the results can be biased by various
factors. They indicate that high CPS should not be considered
equally with CPI response. Indeed several factors could influence
the decision process in the R/M situation including biological age,
tumor burden, or pace of disease (5). The KEYNOTE-048 study of
pembrolizumab alone or with CT vs. cetuximab with CT in R/M HNSCC
preceded the extended approval of pembrolizumab for first-line
treatment. PD-L1 expression is most predictive when applying the
CPS with a cutoff of ≥20 but already predictive in those with CPS
≥1 (78). Consequently,
pembrolizumab is only approved for patients whose tumors show a
≥50% TPS for PD-L1 expression in the second-line treatment.
Pembrolizumab as a monotherapy can be applied with a CPS ≥1
independently of a platinum-based CT as first-line option. In the
CHECKMATE-141 study, nivolumab appears to be superior to SOC,
regardless of tumor PD-L1 expression or p16 status (13). This is surprising as nivolumab is
assumed to exhibit the same mode of action as pembrolizumab, but
can be in part explained as follows: In principle, nivolumab and
pembrolizumab are interchangeable (79). The incongruity in biomarker
references (CPS, TPS, or none in CheckMate-141) is obviously driven
by the composition of the respective trial (5,80).
For nivolumab, it was observed that the compound is effective in
both PD-L1-positive (PD-L1+) and PD-L1-negative (PD-L1-) patients
(13). Similar to the conclusions
made for pembrolizumab, these findings suggest that improved
markers need to be identified to stratify patients for
immunotherapy. The challenge in making the PD-L1 status a condition
for CPI treatment is that PD-L1 status has been performed with
different tests using different antibodies and different cutoffs
for positivity. However these methods are not conclusive even if
the same antibody is applied. It needs to be mentioned that in
KEYNOTE 055, a phase II study of pembrolizumab as second-line
treatment for R/M HNSCC, PD-L1 negative patients (besides those
with positive PD-L1 status) also benefits from CPI at a
statistically significant level although the response rate is
higher in PD-L1+ patients. These data clearly indicate that
stratification of patients for CPI is complex and challenging and
should not be based on PD-L1 as the only determinant (81). Similar observations are reported
from the phase III CHECKMATE 141 study investigating nivolumab
treatment for R/M HNSCC. Patients with >1% TPS have a more
favorable outcome in terms of improved PFS, while regarding OS, no
significant difference is found between PD-L1+ and PD-L1-patients
(13,82). Intratumoral heterogeneity, one of
the landmark features in HNSCC, could at least in part explain
these ambiguous clinical data on PD-L1 status as a predictor for
immunotherapy. There are observations of Rasmussen et al
(83) that could shed light on
the response to CPI of PD-L1 negative tumors on the one hand and on
treatment failure of CPI in PD-L1 expressing HNSCC on the other
hand. They find PD-L1 varying positivity within the tumor, both
with TPS and CPS, a finding that questions the applicability of the
biomarker. The authors suggest using repeated biopsies or multiple
tumor sampling to improve the predictive power of PD-L1 for
response to CPI (80-83). It is comprehensible that the PD-L1
status alone should not determine patient exclusion from
immunotherapy and alternative biomarkers need to be established in
clinical routine. Despite these controversial results from clinical
trials, PD-1 and PD-L1 expression still remains the most
significant tissue biomarkers for response to immunotherapy. A
total of 175 oral squamous cell carcinomas and 33 corresponding
lymph node metastases were screened for expression levels of PD-L1
and PD-L2. Results were correlated with clinicopathological data
and the study reveals worse OS in case of high expression levels of
PD-L1 and a higher risk of developing neck node metastases,
indicating that CPI may be appropriate even in early tumor stage to
prevent further disease progression (84). Feng et al (85) perform a meta-analysis of the
combination of PD-1/PD-L1 and CTLA-4 inhibitors in patients with
malignant tumors to address the significance of PD-L1. Notably,
they find higher PD-L1 expression to be associated with longer PFS.
Tardy et al (86) present
a case of an R/M HNSCC patient with negative PD-L1 or PD-L2
expression where a microsatel-lite instability (MSI)-high status is
associated with durable complete response to anti-PD-L1 therapy.
The continuing prospective trial PRECISION-01 (NCT03917537,
www.clinicaltrials.gov) aims to
eliminate ambiguities on response markers for CPI and to stratify a
patient subgroup who could clearly benefit from nivolumab. The
study defines mutational signatures by Whole-genome study analyses
on archival tumor tissue samples from platinum-refractory patients
who received at least four cycles of nivolumab.
In summary, the administration of CPI in the
clinical routine is based on PD-L1 cutoffs corresponding with
immunotherapy. However, a valid PD-L1 cutoff that is generally
accepted has yet to be defined. Evrard et al (87) give an overview on clinical and
preclinical studies about cutoffs for PD-L1 positivity in HNSCC. In
most trials a 1% cutoff was applied as this value easily
differentiates between a tumor with positive expression and one
that does not express PD-L1 at all (14,81). In this regard, the importance of
soluble immunological markers should be specifically
considered.
There are two forms of PD-L1. One is membrane-bound
PD-L1 (mPD-L1) that is located mainly on the membrane of tumor
cells, the other is soluble PD-L1 (sPD-L1) in the peripheral blood
from cancer patients but also from chronically sick individuals
(88,89). The soluble form is derived from
the membrane-bound form after proteolytic cleavage (90). sPD-L1 has been suggested as a
potential biomarker to predict response to immunotherapy in
different types of cancer (91).
High mPD-L1 expression is associated with worse survival in cancer
patients (92,93). There is prognostic significance of
circulating tumor cells (CTC) in head and neck cancer as well. A
study by Strati et al (94) demonstrates that detection of CTC
overexpressing PD-L1 may provide important prognostic information
in HNSCC. In case of an overexpression of PD-L1 on CTC post
treatment, patients had significantly shorter PFS and OS, an
observation helping PD-L1 to emerge as an independent prognostic
factor for PFS and OS. Patients whose CTC did not overexpress PD-L1
had a more favorable prognosis in terms of complete response to
immunotherapy (94). These data
imply that biomarkers not only from tissue samples but also from
liquid biopsies are clearly needed to improve customized treatment.
Potential predictors for treatment response to immunotherapy are
high blood TMB and low baselines levels of sPD-L1 in lung cancer,
as recently reviewed (95).
Patterns with high PD-1+/CD4+ T cell count are related to poor OS,
while PD-1+/CD8+ T cells are associated with a favorable prognosis.
CTC with PD-L1 expression are in most cases connected with a failed
response to immunotherapy and consecutively with worse clinical
outcome. Recently, the translational potential of plasma Semaphorin
4D (sSema4D) as an immune marker in plasma of HNSCC patients was
assessed in matched blood and tumor tissue samples (n=104). The
data suggest that HNSCC with elevated sSema4D could be a distinct
phenotype. An association between sSema4D and the histological
inflammatory subtype was assumed. Younis et al (96) hypothesize that changes in sSema4D
can monitor the underlying dynamics of tumor and stromal
inflammation in real time. Notably, the humanized anti-Sema4D
antibody is currently assessed in advanced solid tumors (97,98).
Altogether, these data indicate that improved tools
are needed to predict patient outcome. The following sections will
introduce and discuss different predictive systems for cancer
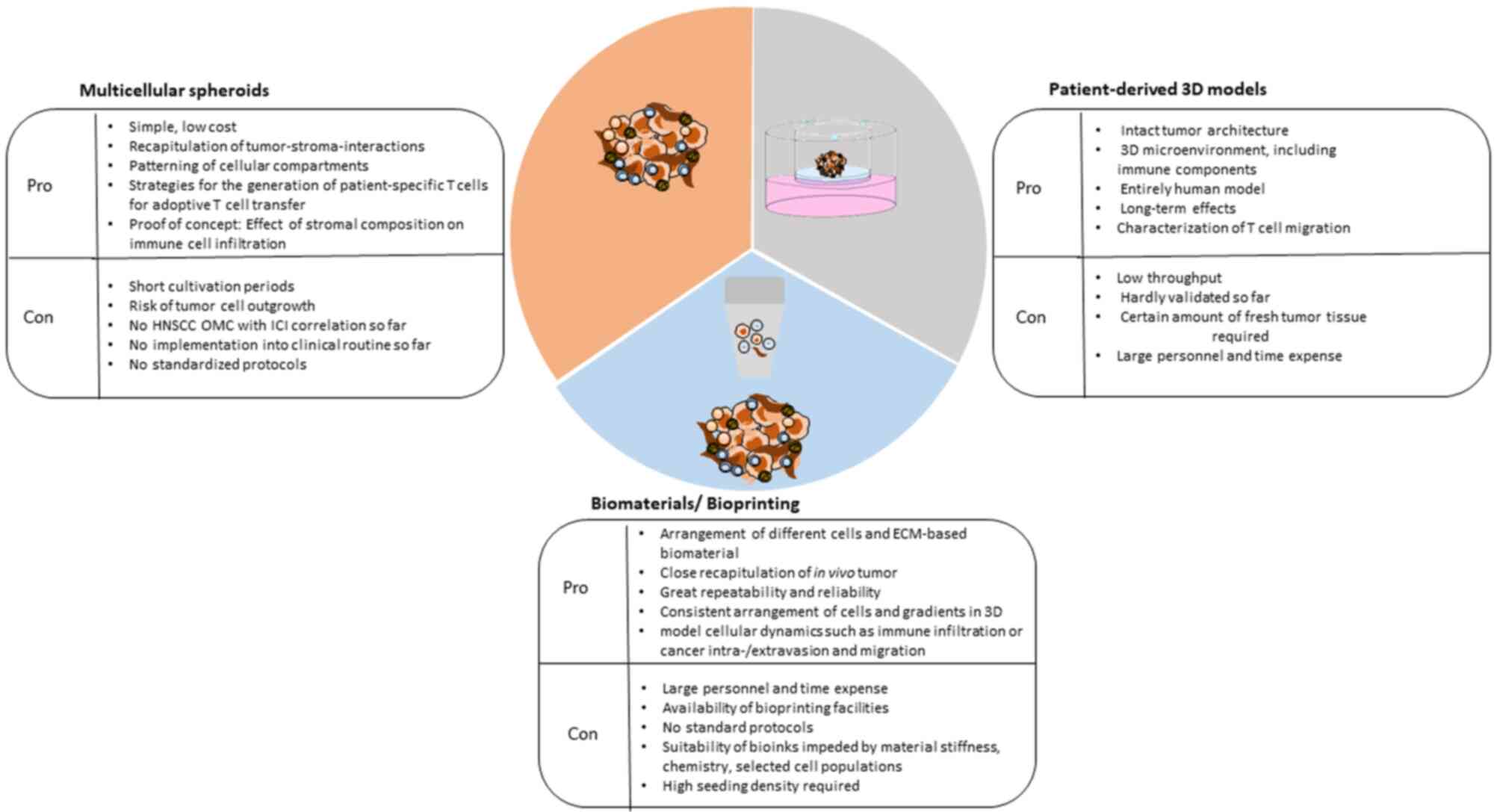
therapy, especially in the light of immunooncology.
In contrast to monolayer models, 3D culture systems
where tumor and stromal cells can interact in a tissue-like
architecture, have been created to assess sensitivity towards
immunotherapies and to bridge the gap between 2D cell cultures and
animal models. One obvious advantage is their increased stability
and longer lifespan, By extended culturing periods, they are
improved suited for the assessment of long-term effects, in
particular of clonal outgrowth which is a major mechanism in
resistance development and essential for the evaluation of
therapeutic resistance (106).
When it comes to testing immunotherapy, it is of particular
importance to choose the right model. For instance, Marrella et
al (107) observed that
immune-checkpoint molecules including B7-H3, PDL2 and PVR more
closely resemble immunophenotypic variants of human tumors in 3D
alginate-based hydrogel neuroblastoma cultures than under standard
2D culture conditions. Appleton et al (108) evaluate immune-related cell
composition and PD-1/PD-L1 expression status in their 3D culture
based on recomposing dissociated cells from vital ovarian cancer
samples. They screen patient-specific alterations in the immune
composition, activation, cytokine secretion and drug response with
the spheroid models. Although the results are based on only two
patients, they appear promising. The authors propose their 3D
system as suitable to address basic biology questions and
immune-oncological drug development (108).
One example for tissue derived tumor spheres, which
are generated after enzymatic digestion of the original tumor
tissue are colospheres from colorectal cancer tissue, established
by Weiswald et al (109,110). Colospheres are implanted as
patient-derived xenografts in mice, then extracted and taken in
culture to build spheroids. To address the interaction between the
TME and tumor immunologic processes, Koeck et al (4) propose a multicellular 3D co-culture
system to address the effect between CAFs and TME-derived cytokines
on the infiltration ability of CD3-CD8 cytotoxic T lympho-cyte
subpopulations. Cancer cell lines are co-cultivated with
fibroblasts and peripheral blood mononuclear cells (PMBCs).
Aggregation and chemokine/cytokine expression are assessed.
Notably, in case of cancer cell monocultures, peripheral blood
mononuclear cells (PBMCs) are able to invade the whole spheroid
while in the presence of fibroblasts co-cultured with tumor cells,
they locate at the margin. By contrast, infiltration is enhanced in
activated CD69 and CD49d cells in the presence of fibroblasts
suggesting that immune cell infiltration is affected by the
composition of the tumor stroma. The incidence of fibroblasts seems
to result in a shift from T lymphocyte infiltration to activated T
lymphocytes (4). The immune
compartment is been described in a pancreatic ductal adenocarcinoma
culture slice model where CD8+ T cells (CD3+ CD8+), Tregs (CD3+,
FoxP3+) and macrophages (CD68+, CD163+, HLA-DR+) are present
throughout the culture period of six days (111). Herter et al (112) recognize that it is a challenge
to mimic the complexity of the TME in vitro, particularly
regarding tumor-host interactions. Therefore, they establish a 3D
heterotypic spheroid model consisting of human colon adenocarcinoma
cells, fibroblasts and immune cells that allow the assessment of
cancer immunotherapy agents. So far, reports on the use of HNSCC
organotypic multicellular spheroids to assess immunomodulatory
aspects are lacking in the literature.
As previously outlined, the major advances in the
development of new drugs targeting cell mechanisms that control
antitumor immunity have aroused an enormous interest in tumor
immunobiology and immunotherapy. There are unprecedented numbers of
preclinical and clinical studies. However, the percentage of
responder patients remains very low (13,113) and due to this small percentage
the majority of patients undergo ineffective therapies with
avoidable side effects. 3D models to study tumor-immune
interactions and responses to PD-1 blockade are clearly needed
especially for cancer entities with rather low response rates such
as HNSCC. New approaches for CPI have been tested in
patient-derived explant models for various cancer entities
including pancreatic ductal carcinomas, endometrial cancer and
prostate cancer (114-116). Recently a patient-derived in
vitro model for colorectal cancer enabled induction and
analysis of tumor-specific T cell response. Organoids and
peripheral blood lymphocytes were co-cultured, the organoids
embedded in Geltrex and the lymphocytes in suspension in the
culture media. Tumor-reactive T cells expand after co-cultivation.
These T cells kill the organoids derived from tumor tissue but not
those from benign tissue. The authors aim at discovering
personalized determinants of response to immunotherapy (117). The response of murine-and
patient-derived organotypic tumor spheroids from various entities
to immune checkpoint blockade was assessed using a 3D microfluidic
device (118). Autologous
lymphoid and myeloid cell populations are preserved in the presence
of cancer spheroids. The authors modulate the response to PD-1
blockade by TBK1/IKKε inhibition, which is predictive for treatment
response. Neal et al (119) demonstrate that the PD-1/PD-L1
axis is conserved in mouse and patient-derived organoids (PDOs)
from NSCLC. These models mimic the immune compartment as CD3+CD8+
and CD3+CD4+T lymphocytes as well as B cells, natural killer cells
and macrophages are visible. They use an air-liquid interface (ALI)
method to propagate PDOs or mouse tumors in syngeneic
immuno-competent hosts. The PDO model is generated preserving tumor
epithelial cells with embedded endogenous immune cells including
native TILs. The growth features of human PDOs correlate with the
tumor grading and the condition of the biopsied tissue. The system
allows modelling intratumoral aspects of CPI as anti-PD-1-dependent
human TIL activation is assessed. TIL expansion and activation are
evoked by PD-1 axis blockade within the TME in human and mouse
PDOs. Correlation studies between patient and organoid treatment
response are still pending (119). Augustine et al (120) describe the establishment of a 3D
Matrigel system for the analysis of interactions between Treg
lymphocytes and NK cells with breast cancer. They use luminal
phenotype MCF-7 cells and basal phenotype MDA-MB-231 cells with
regulatory T-lymphocytes and NK cells and found that breast cancer
phenotype and immune stimulation affect the level of CCL4 secretion
(120). For HNSCC some
remarkable approaches have already been made. Majumder et al
(121) propose a
multi-compartment ex vivo platform maintaining the tumor
architecture and heterogeneity as well as morphologic features and
characteristics of signaling. The cultivation time of samples
deriving from HNSCC and colorectal cancer is three days. During
this period, anticancer drugs are applied to the co-culture of
immune cells and autologous patient plasma and tumor matrix
proteins. The utility of this system for addressing the biology of
CPI treatments has yet to be studied (121). Al-Samadi et al (122) introduce a humanized in
vitro microfluidic chip assay. The assay allows the testing of
immunotherapeutic drugs against HNSCC patient samples. Freshly
isolated cancer cells, patients' serum and immune cells are used on
the chips. Immune cell migration towards cancer cells is assessed
under the effect of a PD-L1 antibody and an IDO 1 pathway
inhibitor. The IDO 1 inhibitor, but not the PD-L1 antibody, induces
the migration of immune cells towards cancer cells. There is a
patient-dependent efficacy of the PD-L1 antibody and the IDO 1
inhibitor. They conclude that the assay is helpful to predict the
efficacy of immunotherapeutic drugs for individual patients
(122). The variability of the
drugs' efficacy apparently reflects the situation in the clinical
practice. Aref et al (123) discuss the challenges in
translating diagnostic assays such as their 3D microfluidic ex
vivo culture of organotypic tumor spheroids to the clinic.
Samples were taken from various solid tumors including HNSCC. They
describe a screening tool for the response of patient tumors to
immune checkpoint blockade therapy evaluating murine-and
patient-derived organotypic tumor spheroids (MDOTS/PDOTS). By the
use of this system, it is feasible to evaluate the requirement for
3D microfluidic culture in MDOTS and the sensitivity towards
immune-checkpoint agents of PDOTS, Using RNA sequencing to
extrapolate changes in the TME tumor-immune interactions were
assessed. Although the results from this approach are promising,
there are certain limitations. The current version of the
spheroids, either murine or human, is only capable of evaluating
tumor-immune interactions of immune cells that have already
infiltrated the tumor. They cannot recapitulate T cell priming
(which occurs primarily in lymph nodes) or recruitment of naïve
immune cells to the TME. The usage of core needle biopsies and fine
needle aspiration material instead of bigger samples such as wedge
biopsies is also described as demanding (123). The authors of the present study
and others (124-126) have demonstrated the feasibility
to culture immune cells ideally in their original TME, which is the
essential condition to study the individual tumor's susceptibility
to immunotherapy. However, so far no data on the usability of head
and neck cancer ex vivo models to assess sensitivity to
immunotherapies in correlation to clinical response has been
reported. Bougherara et al (127) use an ex vivo assay for
lung and ovarian cancer to track resident immunostained CD8 T
cells. T cell migration is influenced by the extracellular matrix
and affecting the control of tumor growth. Such models will make
quite a substantial contribution to the development of novel
immunotherapeutics to boost T cell migration in cancers (Table I).
Animal models are frequently employed (and also
exploited) in preclinical examinations. At least historically, they
serve an essential role in the exploration and characterization of
disease pathogenesis and physiology for various diseases including
cancer. They have also contributed to the identification of targets
and the evaluation of new therapeutic agents.
Currently, the advent of cancer immunotherapy
particularly scrutinizes the validity of animal models by enclosing
further intricacy. When it comes to modelling the immune system,
despite some success, there are severe limitations of animal
models. In order to minimize the subset of patients that are
unlikely to respond to CPI, trustful immunocompetent tumor models
that robustly recapitulate the contexture of immune cells within
the TME are urgently required. In this regard, it is obvious that
the traditional utilization of human tumor cells xenotransplanted
into immunocompromised mice which is considered standard to
evaluate pharmacology, efficacy and safety profiles of cytotoxic
anticancer drugs (147) is not
applicable in questions regarding immunotherapy. Here, only models
with a functionally intact immune system can be employed. Nearly
all animal models lack a functional TME, in particular the immune
system environment, comparable to the human equivalent. The
following aspects contribute to the defective representability of
human immune responses in animal models. Mice are too young with a
very active immune cell production to compare with the immune
status of elderly patients receiving CPI and this limitation needs
to be taken into account when analyzing the data (148). Another factor that is not
generally considered is the influence of the sterile environment
coming along with animal housing that retrains microbial exposure
(146). There is evidence on an
increasing role of the oral and intestinal microbiota discussed as
a candidate marker of response to anti-PD-1/PD-L1 agents in HNSCC
as this ecosystem correlates microenvironment and TME (149,150) and which entails deficiencies of
immune functions and lymphoid tissue architecture (151).
In the emerging field of immunooncology, several
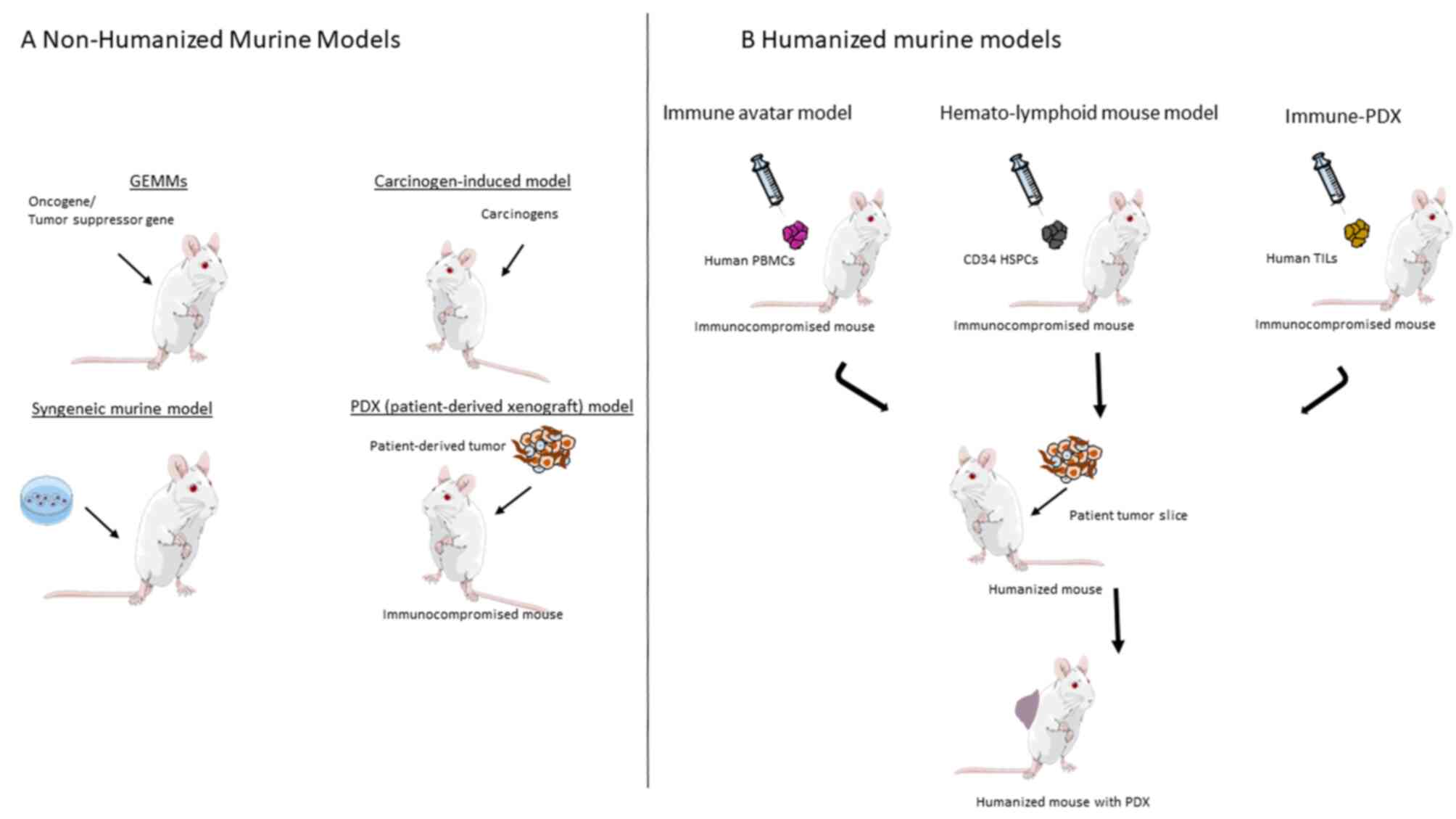
preclinical murine cancer models have been described (Fig. 2) including syngeneic mouse tumor
cell lines, autochthonous tumors that occur spontaneously in
contrast to transplanted tumors, conventional xenograft models or
immunologically humanized mice (148). PDX and cancer cell line-derived
xenografts (CDX) are achieved by transplanting either cancer cell
line or vital human tumor tissue into immunodeficient mice. CDX
head and neck cancer models have been developed with different
HNSCC cell lines (152-154). Brand et al (155) describe an HPV-associated CDX
model by bilateral injection of HPV-positive HNSCC cell lines
(UM-SCC47 (SCC47) and UPCI-SCC90 (SCC90) into athymic nude mice for
evaluating anti-HER3 therapy. However, relevant drawbacks of this
system are the lack of lymphocytes, the perpetuation of murine
stroma and normal/reduced innate immunity (156). PDX and CDX have the potential to
model individual patient tumors and different cancer subtypes and
to study the efficiency of standard and novel agents for specific
patient tumors preclinically. However, the engrafted tumors arise
in immunocompromised animals, which hampers the information on the
role of the immune system in carcinogenesis and response to
treatment. In CDX, it is obvious that immune cells that infiltrate
the grafted tumor are completely murine while the immune cell
portion of the PDX from the first transplantation is lost in the
course of passaging to other host mice.
Syngeneic models consist of tumor cells from the
same genetic background as the immune-competent recipient
mouse-strain. The origin of transplanted cells, the TME and the
host from the same strain is commonly indicated as an advantage. It
is anticipated that mechanisms how cancer therapies perform could
be evaluated in the presence of a functional immune system.
However, cancer cells engrafted in syngeneic models show rapid
growth, a kinetics that provides an inadequate time frame for
assessing the susceptibility to immunotherapy (157). The established tumor constructs
are barely heterogeneous, even though heterogeneity is one major
characteristics in HNSCC that makes every tumor unique (158). On the contrary, in syngeneic
mouse models tumor cells and the TME cells are abnormally
homogeneous. This is due to the absence of progenitor cancer stem
cells or due to the adaptation of tumor cells to their artificial
environment after transplantation. Anti-PD-1/PD-L1 effects have
been investigated in syngeneic mouse models. The combination of
irradiation and immunotherapy is assessed by measuring tumor size,
animal survival and animal body weight in a syngeneic murine
melanoma model. It is observed that treatments with immunotherapy
alone shows only modest effects while combination causes increased
survival and decreased growth pace of the tumor and the model is
considered as suitable for this question (159). Another report evaluated whether
SGT-53, a tumor-targeting nanomedicine carrying the p53 gene, could
enhance anti-PD-1 treatment in a syngeneic glioblastoma model
(160). In this context, tumor
cells interacting with a fully competent immune system are
considered as the major benefit of syngeneic models. However, rapid
tumor growth hampers the development of the chronic inflammatory
environment, although this is a key feature of human cancers
(161). Additionally, syngeneic
tumors do not go through the typical process of oncogenesis from
initiation to a completely developed tumor (162). Syngeneic tumor models are also
used to investigate the antitumor activity of other CPI, including
anti-CTLA-4 (163). Yu et
al (162) propose that four
commonly used syngeneic models are an effective instrument as
different tumor-immune landscapes enable to stratify into
responders and non-responders to CPI. The features on which this
segregation is thought to be based on depend on pre-existing immune
infiltrates. However, injection of xeno-material into mice will
entail inflammation and thereby trigger the innate immune system.
This previously activated immune reaction might inadequately mimic
the TME (161). Finally, the
mutational load in murine tumors is lower compared with human
neoplasms. For instance, in mice the perturbation of only two
signaling pathways involving p53 and RAF/MAPK seems to be
sufficient for tumorigenic conversion of fibroblasts compared with
five altered cascades in humans (164). In 1997, O'Malley et al
(165) proposed an orthotopic
immunocompetent murine model for head and neck cancer, where lung
metastases were present but without the evidence of metastatic
lymph nodes. This was explained by a potential spillage of
transplanted tumor cells into the vasculature of the animals
causing pulmonary lesions which did not undergo the normal process
of metastases (166). Vahle
et al (167) found
neither distant nor lymph node metastases in their orthotopic
murine model of HNSCC in fully immunocompetent mice although the
tumors had a broad invasive front and were not encapsulated.
To optimize syngeneic models, GEMMs were evolved to
create spontaneous tumors in a mouse model. GEMMs are supposed to
depict the TME and the tumor configuration in a more sophisticated
manner. Tumor development in these systems is due to the inclusion
of certain molecular changes in the genome. In these models, mostly
oncogenes are expressed and/or tumor suppressor genes are deleted
by specific genetic engineering techniques (168). GEMMs provide the opportunity to
introduce more than one transgene or knock-in gene for human
check-points. The system is suggested to be suitable for examining
the prospect of immunotherapy as fresh tumors develop against the
backdrop of a functional immune system (168). GEMMS are used for evaluation of
candidate drug targets, therapeutic effects and the identification
of drug resistance in the presence of a competent immune system.
Immunocompromised genetically-engineered models bear specific
immune relevant characteristics as some models preserve NK cell
response. In chemosensitivity assays, specific drugs for the human
checkpoint molecule can be tested in the scenario of an intact
immune system. Autoimmune effects observed in patients treated with
anti-CTLA-4 agents can be modelled in CTLA-4 knock-in mice
(169). Again, low mutational
burden of the tumor model is an issue (170). After all, knock-in mice,
GEMMs and syngeneic models share one severe disadvantage, as they
are all based on a fully murine immune system, This might be in
part overcome by the usability of the clustered regularly
interspaced short palindromic repeats-CRISPR-associated 9
(CRISPR/Cas9) system (171,172). This technology enables the
knockout of different genes in order to illustrate their
contribution to cancer-related processes and response to
therapeutics. Regarding immunotherapy, strategies have been
developed where, through CRISPR/Cas9-based editing, PD-1 and CTLA-4
are removed in order to enhance the effect of T cell-based
immunotherapy (173). The
technology has already made the step into a phase I trial on
metastatic non-small-cell lung cancer where CRISPR/Cas9-mediated
PD-1 knock-out in T cells is currently under clinical
evaluation (174).
Immunocompromised mice [athymic nude mouse (nu/nu)]
and subsequently the severe combined immunodeficient (SCID) mice
have provided the opportunity to screen anticancer drugs in human
tumor xenografts in mice for decades (175). Due to the compromised immune
function of the mice, these analyses have limited potential to
assess tumor-immune interactions and investigations with checkpoint
blockers. The usefulness of athymic mice in oncology research is
due to their lacking mature T cells, which renders them unable to
reject allogenic and xenogeneic engraftments (176). However, mice keep an innate
immunity and immune functions apart from T cells are still
existent.
In athymic mice and rats tumors tend to grow in a
capsule without spreading to distant organs regardless of the
manner they are implanted (orthotopically or heterotopically). It
is noticeable that immune responses from innate components (DCs,
neutrophils and monocytes/macrophages) are not only preserved but
also enhanced compared with euthymic mice (177,178). This gives rise to the question
of how this modified immune response is comparable with the one in
euthymic humans when addressing immunotherapeutic questions. It is
questionable whether these findings have relevance for testing
immunotherapeutic approaches since the animals are
immunocompromised. The complex dynamics of
tumor-immune-surveillance and immune-mediated editing (179) is not adequately reflected
(170). In parallel, attempts
are being made to establish immunocompetent models, where DNA
damage caused by chemicals is thought to convey heterogeneity of
the constructs. Atypical precursor epithelial cells and chronic
inflammation of the connective tissue, similar to the setting in
HNSCC, are supposed to represent human tumor development. By use of
the Epithelial Atypia Index Nauta et al (180) compare the successive stages of
4NQO-induced rat epithelial dysplasia with specimens of human oral
epithelial dysplasia and find close histological similarity. One
other example for chemically-induced head and neck tumor models is
the hamster cheek pouch (181,182). Although the tissue in the human
oral cavity histologically resembles the check pouch, the pouch
lacks lymphatic drainage. Therefore, the model can only be utilized
to a limited extent. The thin pouch skin is considered as
specifically immune-privileged, with sparsely distributed
lymphatics, preventing antigen escape and enabling engraftment and
acquisition of blood supply for xenograft transplants (183). Variations by applying tumor
cells in the tongue led to a broad histological inconsistency as
reactive papilloma started to develop probably after causing an
injury on the back of the tongue in order to enhance carcinogen
exposure (184). When comparing
the model with original oral epithelial lesions, certain
similarities in the expression of cytokines/cytokine receptors and
Cox2 and NF-κB activation were observed (185). However, these artificially
developed models do not show any metastases (186) and are usually less aggressive
than human HNSCC. Eventually, this could be amended by a high
increase in drug concentration in the drinking water of the animals
and extension of treatment and observation times (187). However, there are sparse reports
of metastatic spread in a chemically-induced mouse model to the
best of the authors' knowledge. The model can be considered as a
tool for evaluating cancer development in the early stages,
however, various drawbacks such as additional unintended tumor
development at other sites including paws due to, for example,
grooming (156) restrict the
applicability of chemically-induced tumor models. Another aspect
that should never be ignored is the wellbeing of animals in
chemically-induced models. Especially when establishing colon
cancers, animals suffered from significant weight loss and
diarrhea. Along with the extended observation periods such
procedures are burdening for the animals and not in accordance with
the concept of animal welfare (188).
After all these attempts, it is still unclear
whether animal models are a significant and reliable basis for
therapies of individual patients. To date, comparisons of a
sufficiently big cohort of PDX models with the clinical outcome of
the respective patients are still lacking in HNSCC to the best of
the authors' knowledge. The inability of defining one or more
possible endpoints to determine drug efficacy in mice is considered
as one major issue. Indeed, studies have shown that following
alignment of all relevant factors, there is a high correlation
between the responses of original tumor and avatar, as summarized
by Durinikova et al (189). In case of HNSCC, the PDX model
was used to represent genetic alterations and susceptibility to
anticancer drugs such as cetuximab and the PI3K inhibitor PX-866,
respectively, in a study from 2013 (190). However, PI3K inhibitors have not
been approved for the treatment of HNSCC yet, although they are
repeatedly tested in different clinical trial settings alone or in
combination (NCT00897988; NCT04997902). Additionally, a PDX
approach is not feasible in a number of patients as their tumors
fail to engraft (191). Another
issue is the long cultivation time as it takes up to 6 months for a
PDX model to establish, which considerably impedes the
synchronization of avatar and patient. HNSCC is a distinctly
heterogeneous disease, a feature, which might be inadequately
emulated in serial passaging if the heterogeneous pattern is not
observed in the explanted and passaged tumor slices (192). High costs and high personnel
expenditure also hinder the widespread use of PDX models (193). Moreover, the engraftment of
HPV-driven HNSCC compared with HPV non-driven HNSCC appears to be
complicated as reported in various studies. In particular, the
establishment of HPV-induced HNSCC xenografts still encounters
substantial technical challenges as summarized by Facompre et
al (194). Altogether, the
study of immunomodulatory effects of new anticancer drugs such as
CPI in immunodeficient mice might have led to an inadequate
prediction of clinical outcome in patients (132,170).
Humanized mouse models have recently been presented
as an option for developing and testing immunotherapeutic regimens.
Following whole body gamma irradiation, human PBMCs are engrafted
into immune-deficient mice, followed by transplantation of human
tumor tissue or cell lines. This so-called immune-avatar model is
limited to a rather short lifespan, for instance compared with the
implantation of hematopoietic stem cells (HSCs) and is reduced to
4-8 weeks to investigate immunotherapeutic effects (195). Furthermore, there is a constant
risk of graft vs. host reactions in the presence of T cells
(196). Mosier et al
(195) described the so-called
SCID-hu-peripheral blood leukocytes (PBL) model as a first attempt
of a human-mouse model system in 1988. They set up a xenograft
model where PBMC were i.v. injected into SCID mice in order to
stably reconstitute a functional human immune system inside the
mice. Immune responses were represented by spontaneous secretion of
human immunoglobulin and through detection of human immune cells in
the murine blood. As a side effect, mice rapidly developed
EBV-positive B-cell neoplasms after engraftment with EBV-positive
PBL. Eventually, the human immune cells were eliminated and
supplanted by the murine innate immune system (195). The model was refined by various
approaches, such as transplantation of combined human fetal
thymus/liver and CD34+ cells (197) in order to represent the immune
system at an advanced setting. Eventually, the high complexity of
the models left them unsuitable for high-throughput assays. Hidalgo
et al (198) suggested a
so-called co-clinical trial concept where the avatar model is
established from a patient enrolled in a clinical trial and treated
synchronously to mimic drug sensitivity and clinical response.
However, so far there are no reports about a successful
synchronization. Remaining issues are on the one hand the time gap
between engraftment of patient tumors in mice and the patient
treatment schedule and lack of sufficient amounts of tumor tissue
and low tumor take rates on the other hand. Additionally study
designs and protocols need to be aligned and standardized (198).
In the immune-PDX (iPDX) model severely
immunodeficient mice are engrafted with patient-derived tumor
slices after 'humanizing' the immune system of the mice.
Alternatively, CD34+ human hematopoietic stem and progenitor cells
(HSPCs) are used which can be obtained from different sources,
including umbilical cord blood, bone marrow, fetal liver and
peripheral blood, for the establishment of so-called human
hemato-lymphoid mouse models (148,170,199). In an HNSCC engrafted model into
HSC-NOG-hIL-6 Tg mice, human TAMs could be found that expressed
CD163 as a marker of immunoregulatory myeloid cells and produced
immunosuppressive molecules (200). iPDX models are thought to
provide a platform to analyze tumor growth in SCID mice in a
similar situation as they grow in patients. They allow the
investigation of human immune responses by analyzing TILs,
cytokines and anti-bodies (192). Prior to the first passaging, the
human TME is still present so iPDX provides access to the analysis
of tumor-stroma/immune interactions in the first engraftment.
During consecutive passages, however, human stroma is replaced by
murine stroma. In this model, human TILs are available in the TME
and are suitable to be targeted with mAbs administered systemically
to the mice (170). However, the
xenografted tumor constructs take up to at least 1-2 months to
start growing (201).
Restrictions in patient tumor material is another issue as only
very few animals can be transplanted per tumor sample. This is
particularly true for small head and neck cancer specimens. These
aspects limit the broader appli-cation of iPDX in the preclinical
and clinical routine (170).
Occasional approaches for HNSCC iPDX models have been proposed
(202) where a correlation
between immune therapy effects and HLA matching in preclinical
models is described. It remains doubtful to what extent a
reproduced human immune system in murine adequately resembles the
human immune system. The novel technique of engineering so-called
Xact mice has been proposed by Morton et al (203). Thereby HSPCs reconstitute the
bone marrow from NSG mice that has been previously suppressed by
irradiation. Patient tumor tissue is then engrafted into the mice
and human HSPCs now form immune cells that grow into the xenograft
and recreate the natural TME. Consecutively the expression patterns
of epithelial, stromal and immunological genes in Xact mice tumors
match the patient's tumor to a greater extent than tumors from
non-humanized mice. This model is, however, limited by the fact
that there are still murine stromal cells present and innate immune
cells reside. Other issues are linked to the interaction of human
with murine components, lack of HLA matching and heterotopic
engraftment (156). Animal
models discussed in the present review are presented in tabular
form (Table II).
Against the background of questions concerning
animal welfare or animal ethics, and in light of limitations and
inaccuracies of murine models for immunotherapeutic options, there
have been several new approaches published to comply with the 3R
principle.
Bioprinting is emerging as a promising tool for
creating 3D human cancer models that recapitulate critical features
of the TME architecture in an advanced manner. It is a promising
novel technique to more closely represent the TME, compared with
current methods (217). 3D
bioprinting enables the spatially defined placement of cells in a
3D microenvironment in order to create viable 3D constructs.
Microfluidic platforms have already proven beneficial for
representing spatial compartmentalization and migration of immune
cells. Despite bioprinting becoming applied more and more, there
are few approaches in oncology at present (218,219). As comprehensively summarized in
a recent review, by using bioprinting protocols the TME could
already be adequately and dynamically mimicked by microfluidic
channels or by channels printed using sacrificial ink. Perfusing
cells through channels allowed detecting migration properties or
immune cells including PBMC, DCs, macrophages and cytotoxic CD8+ T
cells, which have been shown to exert prognostic relevance in
cancer (217). Drawbacks such as
the secretion of matrix metalloproteinases causing degradation of
the hydrogels limit the applicability of the techniques as certain
features of immune cells, including long-term viability, is
affected and highlights the need for a straight revision of current
protocols. Swaminathan and Clyne (220) recently published a first
description of bioprinting breast spheroids in their 3D
architecture for co-culture with endothelial cells also in their 3D
architecture. Despite limitations, such as migration tendency of
cells out of the spheroids, the technique is anticipated as a
powerful tool for precision medicine through testing of drug
efficacy in a patient-specific model. Browning et al
developed a 3D bioprinted skin model of cutaneous squamous cell
carcinomas together with a microscopy assay allowing the testing of
the efficacy and general toxicity of chemotherapeutics (221). Almela et al (222) established a 3D printed
bone-mimicking scaffold, which was used to investigate the bone
invasion of oral squamous cancer cells to develop a cancerous bone
oral mucosal model. So far, a protocol to create a bioprinted head
and neck tumor model has yet to be published. Unpublished data form
our group already indicate a great potential of this technology to
develop various naturalistic head and neck tumor models (Table I).
In the course of optimizing the response rates of
HNSCC to immunotherapy reliable models that are predictive of
clinical efficacy remain few. At present, no description of a
successful translation of drug sensitivity assays or predictive
models into the clinical routine has been released for HNSCC.
Certainly, none of the markers reviewed in the present review is
adequate to sufficiently give overall predictive data on the
response to CPI so far. It appears essential to establish
predictive 3D tumor models to recapitulate the heterogeneity of
clinical HNSCC and especially to assess the interactions between
tumor and stroma and immune cells. In the context of optimizing
immunotherapeutic response rates in HNSCC, human immune components
need to be included to qualify the respective model to evaluate
novel immunotherapeutic compounds as well as additional new drugs
to be combined with CPI. Various approaches have already been
developed and are discussed in the present review. So far, however,
there are only anecdotal reports of accurate response
prognostication. The aims of novel 3D models studying
immunotherapeutic response are to unveil the underlying mechanisms
of therapeutic resistance in order to develop strategies for
circumvention. It has been hypothesized that combinatorial regimens
of CPI and additional inhibitors might be advantageous. However,
there are still no standardized tumor models available that allow
the prediction of the efficacy of these combinations and the
testing of the individual sensitivity of the tumor to be able to
select from an abundance of therapeutic agents. Having identified a
suitable therapeutic approach, the tumor should ideally be
constantly monitored to discover the outgrowth of resistant clones
as early as possible. The facilitation of a long overdue comparison
between the clinical response of the original tumor and the model
will pave the way for co-clinical trials. Unfortunately, short
cultivation times of 3D HNSCC enabling the analysis of long-term
effects limits their wide-spread use in precision medicine.
Extension of the cultivation time of ex vivo cultures is
therefore one of the most important directions. One major issue is
the performance of clinical validation studies. The ambitious aim
is to account for the guidance of patients' therapy using the
response of ex vivo models in the frame of adaptive clinical
trials or to conduct co-clinical trials that parallel ongoing human
phase I/II clinical trials. Parallelization of pre-clinical and
clinical trials will become of paramount importance in future
decades. It is envisioned that eventually bioprinted or
scaffold-based multicellular 3D cancer models could be applied
'from bench to bedside' to tumor and stroma material cells from
patient tumor biopsies.
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
All authors were involved in the conception of the
study. AA, CS, JK and AL were involved in the literature search.
AA, JK, CS and AL were involved in the writing and preparation of
the original draft of the manuscript. All authors were involved in
the writing and reviewing of the article. NR and KB supervised the
study. All authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The authors' work on 3D HNSCC models is substantially supported
by the 3R network Baden-Wuerttemberg, Ministry of Science
Baden-Wuerttemberg, Germany and by the research funding program
Development of Replacement and Complementary Methods to Reduce
Animal Testing provided by the Ministry of Rural Affairs, Food and
Consumer Protection Baden-Wuerttemberg, Germany
(Staatshaushaltsplan 2020/2021 Kap. 0802, Tit. Gr. 74).
|
1
|
Mandal R, Şenbabaoğlu Y, Desrichard A,
Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA and
Morris LG: The head and neck cancer immune landscape and its
immunotherapeutic implications. JCI Insight. 1:e898292016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Q, Wang C, Yuan X, Feng Z and Han Z:
Prognostic value of tumor-infiltrating lymphocytes for patients
with head and neck squamous cell carcinoma. Transl Oncol. 10:10–16.
2017. View Article : Google Scholar
|
|
3
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koeck S, Kern J, Zwierzina M, Gamerith G,
Lorenz E, Sopper S, Zwierzina H and Amann A: The influence of
stromal cells and tumor-microenvironment-derived cytokines and
chemokines on CD3+CD8+ tumor infiltrating
lymphocyte subpopulations. Oncoimmunology. 6:e13236172017.
View Article : Google Scholar
|
|
5
|
Szturz P and Vermorken JB: Translating
KEYNOTE-048 into practice recommendations for head and neck cancer.
Ann Transl Med. 8:9752020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pai SI, Zandberg DP and Strome SE: The
role of antagonists of the PD-1:PD-L1/PD-L2 axis in head and neck
cancer treatment. Oral oncol. 61:152–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and Function of the PD-L1 Checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keck MK, Zuo Z, Khattri A, Stricker TP,
Brown CD, Imanguli M, Rieke D, Endhardt K, Fang P, Brägelmann J, et
al: Integrative analysis of head and neck cancer identifies two
biologically distinct HPV and three non-HPV subtypes. Clin Cancer
Res. 21:870–881. 2015. View Article : Google Scholar
|
|
9
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Elsas A, Hurwitz AA and Allison JP:
Combination immunotherapy of B16 melanoma using anti-cytotoxic T
lympho-cyte-associated antigen 4 (CTLA-4) and
granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing
vaccines induces rejection of subcutaneous and metastatic tumors
accompanied by autoimmune depigmentation. J Exp Med. 190:355–366.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krummel MF and Allison JP: CD28 and CTLA-4
have opposing effects on the response of T cells to stimulation. J
Exp Med. 182:459–465. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chow LQM, Haddad R, Gupta S, Mahipal A,
Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee SH, et al:
Antitumor activity of pembrolizumab in biomarker-unselected
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma: Results from the phase ib KEYNOTE-012 expansion
cohort. J Clin Oncol. 34:3838–3845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seiwert TY, Burtness B, Mehra R, Weiss J,
Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et
al: Safety and clinical activity of pembrolizumab for treatment of
recurrent or metastatic squamous cell carcinoma of the head and
neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial.
Lancet Oncol. 17:956–965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soulieres D, Cohen E, Le Tourneau C, Dinis
J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, et al:
Abstract CT115: Updated survival results of the KEYNOTE-040 study
of pembrolizumab vs standard-of-care chemotherapy for recurrent or
metastatic head and neck squamous cell carcinoma. Cancer Res.
78:CT1152018.
|
|
16
|
Economopoulou P, Agelaki S, Perisanidis C,
Giotakis E and Psyrri A: The promise of immunotherapy in head and
neck squamous cell carcinoma. Ann Oncol. 27:1675–1685. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsieh JC, Wang HM, Wu MH, Chang KP, Chang
PH, Liao CT and Liau CT: Review of emerging biomarkers in head and
neck squamous cell carcinoma in the era of immunotherapy and
targeted therapy. Head Neck. 41(Suppl 1): S19–S45. 2019. View Article : Google Scholar
|
|
18
|
Lee TW, Lai A, Harms JK, Singleton DC,
Dickson BD, Macann AMJ, Hay MP and Jamieson SMF: Patient-Derived
xenograft and organoid models for precision medicine targeting of
the tumour microenvironment in head and neck cancer. Cancers
(Basel). 12:37432020. View Article : Google Scholar
|
|
19
|
Sailer V, Gevensleben H, Dietrich J, Goltz
D, Kristiansen G, Bootz F and Dietrich D: Clinical performance
validation of PITX2 DNA methylation as prognostic biomarker in
patients with head and neck squamous cell carcinoma. PLoS One.
12:e01794122017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prochnow S, Wilczak W, Bosch V, Clauditz
TS and Muenscher A: ERCC1, XPF and XPA-locoregional differences and
prognostic value of DNA repair protein expression in patients with
head and neck squamous cell carcinoma. Clin Oral Investig.
23:3319–3329. 2019. View Article : Google Scholar
|
|
21
|
Bauman JE, Austin MC, Schmidt R, Kurland
BF, Vaezi A, Hayes DN, Mendez E, Parvathaneni U, Chai X, Sampath S
and Martins RG: ERCC1 is a prognostic biomarker in locally advanced
head and neck cancer: Results from a randomised, phase II trial. Br
J Cancer. 109:2096–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
da Costa AA, D'Almeida Costa F, Ribeiro
AR, Guimarães AP, Chinen LT, Lopes CA and de Lima VC: Low PTEN
expression is associated with worse overall survival in head and
neck squamous cell carcinoma patients treated with chemotherapy and
cetuximab. Int J Clin Oncol. 20:282–289. 2015. View Article : Google Scholar
|
|
23
|
Slavik M, Shatokhina T, Sana J, Ahmad P,
Kazda T, Selingerova I, Hermanova M, Cervena R, Novotny T, Burkon
P, et al: Expression of CD44, EGFR, p16, and their mutual
combinations in patients with head and neck cancer: Impact on
outcomes of intensity-modulated radiation therapy. Head Neck.
41:940–949. 2019. View Article : Google Scholar
|
|
24
|
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X
and Wu K: Biomarkers for predicting efficacy of PD-1/PD-L1
inhibitors. Mol Cancer. 17:129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Shao C, Shi Y and Han W: Lessons
learned from the blockade of immune checkpoints in cancer
immunotherapy. J Hematol Oncol. 11:312018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ribas A: Adaptive immune resistance: How
cancer protects from immune attack. Cancer Discov. 5:915–919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leduc C, Adam J, Louvet E, Sourisseau T,
Dorvault N, Bernard M, Maingot E, Faivre L, Cassin-Kuo MS, Boissier
E, et al: TPF induction chemotherapy increases PD-L1 expression in
tumour cells and immune cells in head and neck squamous cell
carcinoma. ESMO Open. 3:e0002572018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ling DC, Bakkenist CJ, Ferris RL and Clump
DA: Role of immunotherapy in head and neck cancer. Semin Radiat
Oncol. 28:12–16. 2018. View Article : Google Scholar
|
|
30
|
Knocke S, Fleischmann-Mundt B, Saborowski
M, Manns MP, Kühnel F, Wirth TC and Woller N: Tailored tumor
immunogenicity reveals regulation of CD4 and CD8 T cell responses
against cancer. Cell Rep. 17:2234–2246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Ruiter EJ, Ooft ML, Devriese LA and
Willems SM: The prognostic role of tumor infiltrating T-lymphocytes
in squamous cell carcinoma of the head and neck: A systematic
review and meta-analysis. Oncoimmunology. 6:e13561482017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gooden MJ, de Bock GH, Leffers N, Daemen T
and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: A systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noble F, Mellows T, McCormick Matthews LH,
Bateman AC, Harris S, Underwood TJ, Byrne JP, Bailey IS, Sharland
DM, Kelly JJ, et al: Tumour infiltrating lymphocytes correlate with
improved survival in patients with oesophageal adenocarcinoma.
Cancer Immunol Immunother. 65:651–662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu P, Fan W, Zhang Z, Wang J, Wang P, Li Y
and Yu M: The clinicopathological and prognostic implications of
FoxP3+ Regulatory T cells in patients with colorectal
cancer: A meta-analysis. Front Physiol. 8:9502017. View Article : Google Scholar
|
|
35
|
Weller P, Bankfalvi A, Gu X, Dominas N,
Lehnerdt GF, Zeidler R, Lang S, Brandau S and Dumitru CA: The role
of tumour FoxP3 as prognostic marker in different subtypes of head
and neck cancer. Eur J Cancer. 50:1291–1300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seminerio I, Descamps G, Dupont S, de
Marrez L, Laigle JA, Lechien JR, Kindt N, Journe F and Saussez S:
Infiltration of FoxP3+ Regulatory T cells is a strong and
independent prognostic factor in head and neck squamous cell
carcinoma. Cancers (Basel). 11:2272019. View Article : Google Scholar
|
|
37
|
Echarti A, Hecht M, Büttner-Herold M,
Haderlein M, Hartmann A, Fietkau R and Distel L: CD8+ and
regulatory T cells differentiate tumor immune phenotypes and
predict survival in locally advanced head and neck cancer. Cancers
(Basel). 11:13982019. View Article : Google Scholar
|
|
38
|
Cho JH and Lim YC: Prognostic impact of
regulatory T cell in head and neck squamous cell carcinoma: A
systematic review and meta-analysis. Oral Oncol. 112:1050842021.
View Article : Google Scholar
|
|
39
|
Lukesova E, Boucek J, Rotnaglova E,
Salakova M, Koslabova E, Grega M, Eckschlager T, Rihova B,
Prochazka B, Klozar J and Tachezy R: High level of tregs is a
positive prognostic marker in patients with HPV-positive oral and
oropharyngeal squamous cell carcinomas. Biomed Res Int.
2014:3039292014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pedroza-Pacheco I, Madrigal A and
Saudemont A: Interaction between natural killer cells and
regulatory T cells: Perspectives for immunotherapy. Cell Mol
Immunol. 10:222–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Renoux VM, Bisig B, Langers I, Dortu E,
Clémenceau B, Thiry M, Deroanne C, Colige A, Boniver J, Delvenne P
and Jacobs N: Human papillomavirus entry into NK cells requires
CD16 expression and triggers cytotoxic activity and cytokine
secretion. Eur J Immunol. 41:3240–3252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wolf GT, Chepeha DB, Bellile E, Nguyen A,
Thomas D and McHugh J: University of Michigan Head and Neck SPORE
Program: Tumor infiltrating lymphocytes (TIL) and prognosis in oral
cavity squamous carcinoma: A preliminary study. Oral Oncol.
51:90–95. 2015. View Article : Google Scholar
|
|
43
|
Desrichard A, Kuo F, Chowell D, Lee KW,
Riaz N, Wong RJ, Chan TA and Morris LGT: Tobacco smoking-associated
alterations in the immune microenvironment of squamous cell
carcinomas. J Natl Cancer Inst. 110:1386–1392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi Y, Shi Y, Haymaker CL, Naing A,
Ciliberto G and Hajjar J: T-cell agonists in cancer immunotherapy.
J Immunother Cancer. 8:e0009662020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Discov. 14:561–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stämpfli MR and Anderson GP: How cigarette
smoke skews immune responses to promote infection, lung disease and
cancer. Nat Rev Immunol. 9:377–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
de la Iglesia JV, Slebos RJC, Martin-Gomez
L, Wang X, Teer JK, Tan AC, Gerke TA, Aden-Buie G, van Veen T,
Masannat J, et al: Effects of tobacco smoking on the tumor immune
microenvironment in Head and Neck squamous cell carcinoma. Clin
Cancer Res. 26:1474–1485. 2020. View Article : Google Scholar :
|
|
49
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Seiwert TY, Haddad R, Bauml J, Weiss J,
Pfister DG, Gupta S, Mehra R, Gluck I, Kang H, Worden F, et al:
Abstract LB-339: Biomarkers predictive of response to pembrolizumab
in head and neck cancer (HNSCC). Cancer Res. 78:LB-339. 2018.
|
|
51
|
Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC,
Shivdasani P, Frieden A, Chau NG, Schoenfeld JD, Lorch JH, Uppaluri
R, et al: Frameshift events predict anti-PD-1/L1 response in head
and neck cancer. JCI Insight. 3:e988112018. View Article : Google Scholar
|
|
52
|
Samstein RM, Lee C-H, Shoushtari AN,
Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ,
Omuro A, et al: Tumor mutational load predicts survival after
immunotherapy across multiple cancer types. Nat Genet. 51:202–206.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li W, Wildsmith S, Ye J, Si H, Morsli N,
He P, Shetty J, Yovine AJ, Holoweckyj N, Raja R, et al:
Plasma-based tumor mutational burden (bTMB) as predictor for
survival in phase III EAGLE study: Durvalumab (D) ± tremelimumab
(T) versus chemotherapy (CT) in recurrent/metastatic head and neck
squamous cell carcinoma (R/M HNSCC) after platinum failure. J Clin
Oncol. 38:65112020. View Article : Google Scholar
|
|
54
|
Alexandrov LB, Ju YS, Haase K, Van Loo P,
Martincorena I, Nik-Zainal S, Totoki Y, Fujimoto A, Nakagawa H,
Shibata T, et al: Mutational signatures associated with tobacco
smoking in human cancer. Science. 354:618–622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gajewski TF: The next hurdle in cancer
immunotherapy: Overcoming the Non-T-Cell-inflamed tumor
microenvironment. Semin Oncol. 42:663–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ribas A, Shin DS, Zaretsky J, Frederiksen
J, Cornish A, Avramis E, Seja E, Kivork C, Siebert J, Kaplan-Lefko
P, et al: PD-1 blockade expands intratumoral Memory T cells. Cancer
Immunol Res. 4:194–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gavrielatou N, Doumas S, Economopoulou P,
Foukas PG and Psyrri A: Biomarkers for immunotherapy response in
head and neck cancer. Cancer Treat Rev. 84:1019772020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jia YQ, Yang B, Wen LL, Mu WX, Wang Z and
Cheng B: Prognostic value of immune checkpoint molecules in head
and neck cancer: A meta-analysis. Aging. 11:501–522. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Botticelli A, Cerbelli B, Lionetto L,
Zizzari I, Salati M, Pisano A, Federica M, Simmaco M, Nuti M and
Marchetti P: Can IDO activity predict primary resistance to
anti-PD-1 treatment in NSCLC? J Transl Med. 16:2192018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin DJ, Ng JCK, Huang L, Robinson M,
O'Hara J, Wilson JA and Mellor AL: The immunotherapeutic role of
indoleamine 2,3-dioxygenase in head and neck squamous cell
carcinoma: A systematic review. Clin Otolaryngol. 46:919–934. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Messerschmidt C, Obermayer B, Klinghammer
K, Ochsenreither S, Treue D, Stenzinger A, Glimm H, Fröhling S,
Kindler T, Brandts CH, et al: Distinct immune evasion in
APOBEC-enriched, HPV-negative HNSCC. Int J Cancer. 147:2293–2302.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen YP, Wang YQ, Lv JW, Li YQ, Chua MLK,
Le QT, Lee N, Colevas AD, Seiwert T, Hayes DN, et al:
Identification and validation of novel microenvironment-based
immune molecular subgroups of head and neck squamous cell
carcinoma: Implications for immunotherapy. Ann Oncol. 30:68–75.
2019. View Article : Google Scholar
|
|
63
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. John Wiley
& Sons; 2017
|
|
64
|
Wondergem NE, Nijenhuis DNLM, Poell JB,
Leemans CR, Brakenhoff RH and van de Ven R: At the crossroads of
molecular biology and immunology: Molecular pathways for
immunological targeting of head and neck squamous cell carcinoma.
Front Oral Health. 2:6479802021. View Article : Google Scholar
|
|
65
|
Spranger S and Gajewski TF: Impact of
oncogenic pathways on evasion of antitumour immune responses. Nat
Rev Cancer. 18:139–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar :
|
|
67
|
Sai J, Owens P, Novitskiy SV, Hawkins OE,
Vilgelm AE, Yang J, Sobolik T, Lavender N, Johnson AC, McClain C,
et al: PI3K inhibition reduces mammary tumor growth and facilitates
anti-tumor immunity and Anti-PD1 responses. Clin Cancer Res.
23:3371–3384. 2017. View Article : Google Scholar :
|
|
68
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
69
|
Ngan HL, Liu Y, Fong AY, Poon PHY, Yeung
CK, Chan SSM, Lau A, Piao W, Li H, Tse JSW, et al: MAPK pathway
mutations in head and neck cancer affect immune microenvironments
and ErbB3 signaling. Life Sci Alliance. 3:e2019005452020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
de Ruiter EJ, Ooft ML, Devriese LA and
Willems SM: The prognostic role of tumor infiltrating T-lymphocytes
in squamous cell carcinoma of the head and neck: A systematic
review and meta-analysis. Oncoimmunology. 6:e13561482017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Takikita M, Xie R, Chung JY, Cho H, Ylaya
K, Hong SM, Moskaluk CA and Hewitt SM: Membranous expression of
Her3 is associated with a decreased survival in head and neck
squamous cell carcinoma. J Transl Med. 9:1262011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Motedayen Aval L, Pease JE, Sharma R and
Pinato DJ: Challenges and opportunities in the clinical development
of STING agonists for cancer immunotherapy. J Clin Med. 9:33232020.
View Article : Google Scholar :
|
|
73
|
Zandberg D, Ferris R, Laux D, Mehra R,
Nabell L, Kaczmar J, Gibson MK, Kim YJ, Neupane P, Bauman J, et al:
71P A phase II study of ADU-S100 in combination with pembrolizumab
in adult patients with PD-L1+ recurrent or metastatic HNSCC:
Preliminary safety, efficacy and PK/PD results. Ann Oncol.
31:S1446–S1447. 2020. View Article : Google Scholar
|
|
74
|
Pan BS, Perera SA, Piesvaux JA, Presland
JP, Schroeder GK, Cumming JN, Trotter BW, Altman MD, Buevich AV,
Cash B, et al: An orally available non-nucleotide STING agonist
with antitumor activity. Science. 369:eaba60982020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chin EN, Yu C, Vartabedian VF, Jia Y,
Kumar M, Gamo AM, Vernier W, Ali SH, Kissai M, Lazar DC, et al:
Antitumor activity of a systemic STING-activating non-nucleotide
cGAMP mimetic. Science. 369:993–999. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zander H, Müller-Egert S, Zwiewka M, Groß
S, van Zandbergen G and Engelbergs J: Checkpoint inhibitors for
cancer therapy. Bundesgesundheitsblatt Gesundheitsforschung
Gesundheitsschutz. 63:1322–1330. 2020.In German. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar
|
|
78
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Prasad V and Kaestner V: Nivolumab and
pembrolizumab: Monoclonal antibodies against programmed cell
death-1 (PD-1) that are interchangeable. Semin Oncol. 44:132–135.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fessas P, Lee H, Ikemizu S and Janowitz T:
A molecular and preclinical comparison of the PD-1-targeted T-cell
checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol.
44:136–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bauml J, Seiwert TY, Pfister DG, Worden F,
Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, et al:
Pembrolizumab for Platinum-and Cetuximab-refractory head and neck
cancer: Results from a single-arm, phase II study. J Clin Oncol.
35:1542–1549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE,
Even C, et al: Nivolumab vs investigator's choice in recurrent or
metastatic squamous cell carcinoma of the head and neck: 2-year
long-term survival update of CheckMate 141 with analyses by tumor
PD-L1 expression. Oral Oncol. 81:45–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rasmussen JH, Lelkaitis G, Håkansson K,
Vogelius IR, Johannesen HH, Fischer BM, Bentzen SM, Specht L,
Kristensen CA, von Buchwald C, et al: Intratumor heterogeneity of
PD-L1 expression in head and neck squamous cell carcinoma. Br J
Cancer. 120:1003–1006. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Moratin J, Metzger K, Safaltin A, Herpel
E, Hoffmann J, Freier K, Hess J and Horn D: Upregulation of PD-L1
and PD-L2 in neck node metastases of head and neck squamous cell
carcinoma. Head Neck. 41:2484–2491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Feng Y, Jin H, Guo K, Xiang Y, Zhang Y, Du
W, Shen M and Ruan S: Results from a Meta-analysis of Combination
of PD-1/PD-L1 and CTLA-4 inhibitors in malignant cancer patients:
Does PD-L1 matter? Front Pharmacol. 12:572845. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tardy MP, Di Mauro I, Ebran N, Refae S,
Bozec A, Benezery K, Peyrade F, Guigay J, Sudaka-Bahadoran A,
Badoual C, et al: Microsatellite instability associated with
durable complete response to PD-L1 inhibitor in head and neck
squamous cell carcinoma. Oral Oncol. 80:104–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Evrard D, Hourseau M, Couvelard A, Paradis
V, Gauthier H, Raymond E, Halimi C, Barry B and Faivre S: PD-L1
expression in the microenvironment and the response to checkpoint
inhibitors in head and neck squamous cell carcinoma.
Oncoimmunology. 9:18444032020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nielsen C, Ohm-Laursen L, Barington T,
Husby S and Lillevang ST: Alternative splice variants of the human
PD-1 gene. Cell Immunol. 235:109–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Greisen SR, Rasmussen TK,
Stengaard-Pedersen K, Hetland ML, Hørslev-Petersen K, Hvid M and
Deleuran B: Increased soluble programmed death-1 (sPD-1) is
associated with disease activity and radiographic progression in
early rheumatoid arthritis. Scand J Rheumatol. 43:101–108. 2014.
View Article : Google Scholar
|
|
90
|
Zhu X and Lang J: Soluble PD-1 and PD-L1:
Predictive and prognostic significance in cancer. Oncotarget.
8:97671–97682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wei W, Xu B, Wang Y and Wu C, Jiang J and
Wu C: Prognostic significance of circulating soluble programmed
death ligand-1 in patients with solid tumors: A meta-analysis.
Medicine (Baltimore). 97:e96172018. View Article : Google Scholar
|
|
92
|
Wu P, Wu D, Li L, Chai Y and Huang J:
PD-L1 and survival in solid tumors: A meta-analysis. PLoS One.
10:e01314032015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gandini S, Massi D and Mandalà M: PD-L1
expression in cancer patients receiving anti PD-1/PD-L1 antibodies:
A systematic review and meta-analysis. Crit Rev Oncol Hematol.
100:88–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Strati A, Koutsodontis G, Papaxoinis G,
Angelidis I, Zavridou M, Economopoulou P, Kotsantis I, Avgeris M,
Mazel M, Perisanidis C, et al: Prognostic significance of PD-L1
expression on circulating tumor cells in patients with head and
neck squamous cell carcinoma. Ann Oncol. 28:1923–1933. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mildner F, Sopper S, Amann A, Pircher A,
Pall G, Köck S, Naismith E, Wolf D and Gamerith G: Systematic
review: Soluble immunological biomarkers in advanced non-small-cell
lung cancer (NSCLC). Crit Rev Oncol Hematol. 153:1029482020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Younis RH, Ghita I, Elnaggar M,
Chaisuparat R, Theofilou VI, Dyalram D, Ord RA, Davila E, Tallon
LJ, Papadimitriou JC, et al: Soluble Sema4D in plasma of head and
neck squamous cell carcinoma patients is associated with underlying
non-inflamed tumor profile. Front Immunol. 12:5966462021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Leonard JE, Fisher TL, Winter LA,
Cornelius CA, Reilly C, Smith ES and Zauderer M: Nonclinical safety
evaluation of VX15/2503, a humanized IgG4 Anti-SEMA4D antibody. Mol
Cancer Ther. 14:964–972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Patnaik A, Weiss GJ, Leonard JE, Rasco DW,
Sachdev JC, Fisher TL, Winter LA, Reilly C, Parker RB, Mutz D, et
al: Safety, pharmacokinetics, and pharmacodynamics of a humanized
anti-semaphorin 4D antibody, in a First-In-Human study of patients
with advanced solid tumors. Clin Cancer Res. 22:827–836. 2016.
View Article : Google Scholar
|
|
99
|
Boschert V, Teusch J, Aljasem A, Schmucker
P, Klenk N, Straub A, Bittrich M, Seher A, Linz C, Müller-Richter
UDA and Hartmann S: HGF-Induced PD-L1 expression in head and neck
cancer: Preclinical and clinical findings. Int J Mol Sci.
21:87702020. View Article : Google Scholar :
|
|
100
|
Freeman GJ, Sharpe AH and Kuchroo VK:
Protect the killer: CTLs need defenses against the tumor. Nat Med.
8:787–789. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Merhi M, Raza A, Inchakalody V, Zar AR,
Uddin S and Dermime S: Immunotherapeutic strategies in patients
with advanced head and neck squamous cell carcinoma. Ann Transl
Med. 7(Suppl 1): S222019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rodrigues J, Heinrich MA, Teixeira LM and
Prakash J: 3D in vitro model (R)evolution: Unveiling tumor-stroma
interactions. Trends Cancer. 7:249–264. 2021. View Article : Google Scholar
|
|
104
|
Halfter K, Ditsch N, Kolberg HC, Fischer
H, Hauzenberger T, von Koch FE, Bauerfeind I, von Minckwitz G,
Funke I, Crispin A, et al: Prospective cohort study using the
breast cancer spheroid model as a predictor for response to
neoadjuvant therapy-the SpheroNEO study. BMC Cancer. 15:5192015.
View Article : Google Scholar
|
|
105
|
Bauml JM, Aggarwal C and Cohen RB:
Immunotherapy for head and neck cancer: Where are we now and where
are we going? Ann Transl Med. 7(Suppl 3): S752019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hoarau-Véchot J, Rafii A, Touboul C and
Pasquier J: Halfway between 2D and Animal Models: Are 3D Cultures
the ideal tool to study cancer-microenvironment interactions? Int J
Mol Sci. 19:1812018. View Article : Google Scholar :
|
|
107
|
Marrella A, Dondero A, Aiello M, Casu B,
Olive D, Regis S, Bottino C, Pende D, Meazza R, Caluori G, et al:
Cell-laden hydrogel as a clinical-relevant 3D model for analyzing
neuro-blastoma growth, immunophenotype, and susceptibility to
therapies. Front Immunol. 10:18762019. View Article : Google Scholar
|
|
108
|
Appleton KM, Elrod AK, Lassahn KA, Shuford
S, Holmes LM and DesRochers TM: PD-1/PD-L1 checkpoint inhibitors in
combination with olaparib display antitumor activity in ovarian
cancer patient-derived three-dimensional spheroid cultures. Cancer
Immunol Immunother. 70:843–856. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Weiswald LB, Richon S, Massonnet G,
Guinebretière JM, Vacher S, Laurendeau I, Cottu P, Marangoni E,
Nemati F, Validire P, et al: A short-term colorectal cancer sphere
culture as a relevant tool for human cancer biology investigation.
Br J Cancer. 108:1720–1731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Weiswald LB, Richon S, Validire P, Briffod
M, Lai-Kuen R, Cordelières FP, Bertrand F, Dargere D, Massonnet G,
Marangoni E, et al: Newly characterised ex vivo colospheres as a
three-dimensional colon cancer cell model of tumour aggressiveness.
Br J Cancer. 101:473–482. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jiang X, Seo YD, Chang JH, Coveler A,
Nigjeh EN, Pan S, Jalikis F, Yeung RS, Crispe IN and Pillarisetty
VG: Long-lived pancreatic ductal adenocarcinoma slice cultures
enable precise study of the immune microenvironment.
Oncoimmunology. 6:e13332102017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Herter S, Morra L, Schlenker R, Sulcova J,
Fahrni L, Waldhauer I, Lehmann S, Reisländer T, Agarkova I, Kelm
JM, et al: A novel three-dimensional heterotypic spheroid model for
the assessment of the activity of cancer immunotherapy agents.
Cancer Immunol Immunother. 66:129–140. 2017. View Article : Google Scholar :
|
|
113
|
Larkins E, Blumenthal GM, Yuan W, He K,
Sridhara R, Subramaniam S, Zhao H, Liu C, Yu J, Goldberg KB, et al:
FDA approval summary: Pembrolizumab for the treatment of recurrent
or metastatic head and neck squamous cell carcinoma with disease
progression on or after platinum-containing chemotherapy.
Oncologist. 22:873–878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Collins A, Miles GJ, Wood J, MacFarlane M,
Pritchard C and Moss E: Patient-derived explants, xenografts and
organoids: 3-dimensional patient-relevant pre-clinical models in
endometrial cancer. Gynecol Oncol. 156:251–259. 2020. View Article : Google Scholar
|
|
115
|
Seo YD, Jiang X, Sullivan KM, Jalikis FG,
Smythe KS, Abbasi A, Vignali M, Park JO, Daniel SK, Pollack SM, et
al: Mobilization of CD8+ T cells via CXCR4 blockade
facilitates PD-1 checkpoint therapy in human pancreatic cancer.
Clin Cancer Res. 25:3934–3945. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Muthuswamy R, Corman JM, Dahl K, Chatta GS
and Kalinski P: Functional reprogramming of human prostate cancer
to promote local attraction of effector CD8+ T cells.
Prostate. 76:1095–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Dijkstra KK, Cattaneo CM, Weeber F,
Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL,
Kaing S, Kelderman S, et al: Generation of tumor-reactive T cells
by co-culture of peripheral blood lymphocytes and tumor organoids.
Cell. 174:1586–1598.e2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jenkins RW, Aref AR, Lizotte PH, Ivanova
E, Stinson S, Zhou CW, Bowden M, Deng J, Liu H, Miao D, et al: Ex
vivo profiling of PD-1 blockade using organotypic tumor spheroids.
Cancer Discov. 8:196–215. 2018. View Article : Google Scholar :
|
|
119
|
Neal JT, Li X, Zhu J, Giangarra V,
Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et
al: Organoid modeling of the tumor immune microenvironment. Cell.
175:1972–1988.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Augustine TN, Dix-Peek T, Duarte R and
Candy GP: Establishment of a heterotypic 3D culture system to
evaluate the interaction of TREG lymphocytes and NK cells with
breast cancer. J Immunol Methods. 426:1–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Majumder B, Baraneedharan U, Thiyagarajan
S, Radhakrishnan P, Narasimhan H, Dhandapani M, Brijwani N, Pinto
DD, Prasath A, Shanthappa BU, et al: Predicting clinical response
to anticancer drugs using an ex vivo platform that captures tumour
heterogeneity. Nat Commun. 6:61692015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Al-Samadi A, Poor B, Tuomainen K, Liu V,
Hyytiäinen A, Suleymanova I, Mesimaki K, Wilkman T, Mäkitie A,
Saavalainen P and Salo T: In vitro humanized 3D microfluidic chip
for testing personalized immunotherapeutics for head and neck
cancer patients. Exp Cell Res. 383:1115082019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Aref AR, Campisi M, Ivanova E, Portell A,
Larios D, Piel BP, Mathur N, Zhou C, Coakley RV, Bartels A, et al:
3D microfluidic ex vivo culture of organotypic tumor spheroids to
model immune checkpoint blockade. Lab Chip. 18:3129–3143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Engelmann L, Thierauf J, Koerich Laureano
N, Stark HJ, Prigge ES, Horn D, Freier K, Grabe N, Rong C,
Federspil P, et al: Organotypic Co-cultures as a Novel 3D model for
head and neck squamous cell carcinoma. Cancers (Basel).
12:23302020. View Article : Google Scholar
|
|
125
|
Kross KW, Heimdal JH, Olsnes C, Olofson J
and Aarstad HJ: Tumour-associated macrophages secrete IL-6 and
MCP-1 in head and neck squamous cell carcinoma tissue. Acta
Otolaryngol. 127:532–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Klöss S, Chambron N, Gardlowski T, Weil S,
Koch J, Esser R, Pogge von Strandmann E, Morgan MA, Arseniev L,
Seitz O and Köhl U: Cetuximab reconstitutes pro-inflammatory
cytokine secretions and tumor-infiltrating capabilities of
sMICA-inhibited NK cells in HNSCC tumor spheroids. Front Immunol.
6:5432015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bougherara H, Mansuet-Lupo A, Alifano M,
Ngô C, Damotte D, Le Frère-Belda MA, Donnadieu E and Peranzoni E:
Real-time imaging of resident T cells in human lung and ovarian
carcinomas reveals how different tumor microenvironments control T
lymphocyte migration. Front Immunol. 6:5002015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Laudanski K, Stentz M, DiMeglio M, Furey
W, Steinberg T and Patel A: Potential Pitfalls of the Humanized
Mice in Modeling Sepsis. Int J Inflam. 2018:65634542018.PubMed/NCBI
|
|
129
|
Osuchowski MF, Remick DG, Lederer JA, Lang
CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R,
et al: Abandon the mouse research ship? Not just yet! Shock.
41:463–475. 2014. View Article : Google Scholar
|
|
130
|
Mestas J and Hughes CW: Of mice and not
men: Differences between mouse and human immunology. J Immunol.
172:2731–2738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Seok J, Warren HS, Cuenca AG, Mindrinos
MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy
L, et al: Genomic responses in mouse models poorly mimic human
inflammatory diseases. Proc Natl Acad Sci USA. 110:3507–3512. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Mak IW, Evaniew N and Ghert M: Lost in
translation: Animal models and clinical trials in cancer treatment.
Am J Transl Res. 6:114–118. 2014.PubMed/NCBI
|
|
133
|
Perel P, Roberts I, Sena E, Wheble P,
Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P and Khan
KS: Comparison of treatment effects between animal experiments and
clinical trials: Systematic review. BMJ. 334:1972007. View Article : Google Scholar :
|
|
134
|
Hackam DG and Redelmeier DA: Translation
of research evidence from animals to humans. JAMA. 296:1731–1732.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Nauseef WM: The proper study of mankind. J
Clin Invest. 107:401–403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
McGonigle P and Ruggeri B: Animal models
of human disease: Challenges in enabling translation. Biochem
Pharmacol. 87:162–171. 2014. View Article : Google Scholar
|
|
137
|
Green SB: Can animal data translate to
innovations necessary for a new era of patient-centred and
individualised healthcare? Bias in preclinical animal research. BMC
Med Ethics. 16:53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hameed I and Gaudino M: Commentary: Do not
kill (especially for nothing). J Thoracic Cardiovascular Surg.
158:1557–1558. 2019. View Article : Google Scholar
|
|
139
|
Leenaars CHC, Kouwenaar C, Stafleu FR,
Bleich A, Ritskes-Hoitinga M, De Vries RBM and Meijboom FLB: Animal
to human translation: A systematic scoping review of reported
concordance rates. J Transl Med. 17:2232019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ruggeri BA, Camp F and Miknyoczki S:
Animal models of disease: Pre-clinical animal models of cancer and
their applications and utility in drug discovery. Biochem
Pharmacol. 87:150–161. 2014. View Article : Google Scholar
|
|
141
|
Van Norman GA: Limitations of animal
studies for predicting toxicity in clinical trials: Is it time to
rethink our current approach? JACC Basic Transl Sci. 4:845–854.
2019. View Article : Google Scholar
|
|
142
|
Jüni P, Nartey L, Reichenbach S, Sterchi
R, Dieppe PA and Egger M: Risk of cardiovascular events and
rofecoxib: Cumulative meta-analysis. Lancet. 364:2021–2029. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Knobloch J, Jungck D and Koch A: The
molecular mechanisms of thalidomide teratogenicity and implications
for modern medicine. Curr Mol Med. 17:108–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
O'Collins VE, Macleod MR, Donnan GA, Horky
LL, van der Worp BH and Howells DW: 1,026 experimental treatments
in acute stroke. Ann Neurol. 59:467–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Akhtar A: The flaws and human harms of
animal experimentation. Camb Q Healthc Ethics. 24:407–419. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pound P, Ebrahim S, Sandercock P, Bracken
MB and Roberts I: Where is the evidence that animal research
benefits humans? BMJ. 328:514–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
DeVita VT and Chu E: A history of cancer
chemotherapy. Cancer Res. 68:8643–8653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Saito R, Kobayashi T, Kashima S, Matsumoto
K and Ogawa O: Faithful preclinical mouse models for better
translation to bedside in the field of immuno-oncology. Int J Clin
Oncol. 25:831–841. 2020. View Article : Google Scholar
|
|
149
|
Zheng D, Liwinski T and Elinav E:
Interaction between microbiota and immunity in health and disease.
Cell Res. 30:492–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Affolter A, Lammert A, Kern J, Scherl C
and Rotter N: Precision medicine gains momentum: Novel 3D models
and stem cell-based approaches in head and neck cancer. Front Cell
Dev Biol. 9:6665152021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Bauer H, Horowitz RE, Levenson SM and
Popper H: The response of the lymphatic tissue to the microbial
flora. Studies on germfree mice. Am J Pathol. 42:471–483.
1963.PubMed/NCBI
|
|
152
|
Li J, Huang J, Jeong JH, Park SJ, Wei R,
Peng J, Luo Z, Chen YT, Feng Y and Luo JL: Selective TBK1/IKKi dual
inhibitors with anticancer potency. Int J Cancer. 134:1972–1980.
2014. View Article : Google Scholar
|
|
153
|
Brand TM, Hartmann S, Bhola NE, Li H, Zeng
Y, O'Keefe RA, Ranall MV, Bandyopadhyay S, Soucheray M, Krogan NJ,
et al: Cross-talk signaling between HER3 and HPV16 E6 and E7
mediates resistance to PI3K inhibitors in head and neck cancer.
Cancer Res. 78:2383–2395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Bais MV, Kukuruzinska M and Trackman PC:
Orthotopic non-metastatic and metastatic oral cancer mouse models.
Oral Oncol. 51:476–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Brand TM, Hartmann S, Bhola NE, Peyser ND,
Li H, Zeng Y, Isaacson Wechsler E, Ranall MV, Bandyopadhyay S,
Duvvuri U, et al: Human papillomavirus regulates HER3 expression in
head and neck cancer: Implications for targeted HER3 therapy in
HPV(+) patients. Clin Cancer Res. 23:3072–3083. 2017. View Article : Google Scholar :
|
|
156
|
Rossa C Jr and D'Silva NJ: Immune-relevant
aspects of murine models of head and neck cancer. Oncogene.
38:3973–3988. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Olson B, Li Y, Lin Y, Liu ET and Patnaik
A: Mouse Models for Cancer Immunotherapy Research. Cancer Discov.
8:1358–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Li E, Lin L, Chen CW and Ou DL: Mouse
models for immunotherapy in hepatocellular carcinoma. Cancers
(Basel). 11:18002019. View Article : Google Scholar
|
|
159
|
Jiao R, Allen KJH, Malo ME, Rickles D and
Dadachova E: Evaluating the combination of radioimmunotherapy and
immunotherapy in a melanoma mouse model. Int J Mol Sci. 21:7732020.
View Article : Google Scholar :
|
|
160
|
Kim SS, Harford JB, Moghe M, Slaughter T,
Doherty C and Chang EH: A tumor-targeting nanomedicine carrying the
p53 gene crosses the blood-brain barrier and enhances anti-PD-1
immunotherapy in mouse models of glioblastoma. Int J Cancer.
145:2535–2546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chulpanova DS, Kitaeva KV, Rutland CS,
Rizvanov AA and Solovyeva VV: Mouse tumor models for advanced
cancer immunotherapy. Int J Mol Sci. 21:41182020. View Article : Google Scholar :
|
|
162
|
Yu JW, Bhattacharya S, Yanamandra N,
Kilian D, Shi H, Yadavilli S, Katlinskaya Y, Kaczynski H, Conner M,
Benson W, et al: Tumor-immune profiling of murine syngeneic tumor
models as a framework to guide mechanistic studies and predict
therapy response in distinct tumor microenvironments. PLoS One.
13:e02062232018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang Z, Wu VH, Allevato MM, Gilardi M, He
Y, Luis Callejas-Valera J, Vitale-Cross L, Martin D,
Amornphimoltham P, Mcdermott J, et al: Syngeneic animal models of
tobacco-associated oral cancer reveal the activity of in situ
anti-CTLA-4. Nat Commun. 10:55462019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Rangarajan A and Weinberg RA: Opinion:
Comparative biology of mouse versus human cells: Modelling human
cancer in mice. Nat Rev Cancer. 3:952–959. 2003. View Article : Google Scholar
|
|
165
|
O'Malley BW Jr, Cope KA, Johnson CS and
Schwartz MR: A new immunocompetent murine model for oral cancer.
Arch Otolaryngol Head Neck Surg. 123:20–24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Kim S: Animal models of cancer in the head
and neck region. Clin Exp Otorhinolaryngol. 2:55–60. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Vahle AK, Kerem A, Oztürk E, Bankfalvi A,
Lang S and Brandau S: Optimization of an orthotopic murine model of
head and neck squamous cell carcinoma in fully immunocompetent
mice-role of toll-like-receptor 4 expressed on host cells. Cancer
Lett. 317:199–206. 2012. View Article : Google Scholar
|
|
168
|
Kersten K, de Visser KE, van Miltenburg MH
and Jonkers J: Genetically engineered mouse models in oncology
research and cancer medicine. EMBO Mol Med. 9:137–153. 2017.
View Article : Google Scholar :
|
|
169
|
Lute KD, May KF Jr, Lu P, Zhang H, Kocak
E, Mosinger B, Wolford C, Phillips G, Caligiuri MA, Zheng P and Liu
Y: Human CTLA4 knock-in mice unravel the quantitative link between
tumor immunity and autoimmunity induced by anti-CTLA-4 antibodies.
Blood. 106:3127–3133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Sanmamed MF, Chester C, Melero I and Kohrt
H: Defining the optimal murine models to investigate immune
checkpoint blockers and their combination with other
immunotherapies. Ann Oncol. 27:1190–1198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Sánchez-Rivera FJ and Jacks T:
Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev
Cancer. 15:387–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Azangou-Khyavy M, Ghasemi M, Khanali J,
Boroomand-Saboor M, Jamalkhah M, Soleimani M and Kiani J:
CRISPR/Cas: From tumor gene editing to T Cell-based immunotherapy
of cancer. Front Immunol. 11:20622020. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Ren J, Liu X, Fang C, Jiang S, June CH and
Zhao Y: Multiplex genome editing to generate universal CAR T cells
resistant to PD1 inhibition. Clin Cancer Res. 23:2255–2266. 2017.
View Article : Google Scholar :
|
|
174
|
Cyranoski D: CRISPR gene-editing tested in
a person for the first time. Nature. 539:4792016. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kelland LR: Of mice and men: Values and
liabilities of the athymic nude mouse model in anticancer drug
development. Eur J Cancer. 40:827–836. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Pelleitier M and Montplaisir S: The nude
mouse: A model of deficient T-cell function. Methods Achiev Exp
Pathol. 7:149–166. 1975.PubMed/NCBI
|
|
177
|
Dixon TC, Meselson M, Guillemin J and
Hanna PC: Anthrax. N Engl J Med. 341:815–826. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Watts CJ, Hahn BL and Sohnle PG:
Resistance of athymic nude mice to experimental cutaneous Bacillus
anthracis infection. J Infect Dis. 199:673–679. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Kang Y: Analysis of cancer stem cell
metastasis in xenograft animal models. Methods Mol Biol. 568:7–19.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Nauta JM, Roodenburg JL, Nikkels PG,
Witjes MJ and Vermey A: Comparison of epithelial dysplasia-the 4NQO
rat palate model and human oral mucosa. Int J Oral Maxillofac Surg.
24:53–58. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Aromando RF, Pérez MA, Heber EM, Trivillin
VA, Tomasi VH, Schwint AE and Itoiz ME: Potential role of mast
cells in hamster cheek pouch carcinogenesis. Oral Oncol.
44:1080–1087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Ghiabi M, Gallagher GT and Wong DT:
Eosinophils, tissue eosinophilia, and eosinophil-derived
transforming growth factor alpha in hamster oral carcinogenesis.
Cancer Res. 52:389–393. 1992.PubMed/NCBI
|
|
183
|
Barker CF and Billingham RE: The lymphatic
status of hamster cheek pouch tissue in relation to its properties
as a graft and as a graft site. J Exp Med. 133:620–639. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Li Q, Dong H, Yang G, Song Y, Mou Y and Ni
Y: Mouse tumor-bearing models as preclinical study platforms for
oral squamous cell carcinoma. Front Oncol. 10:2122020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Liu YC, Ho HC, Lee MR, Lai KC, Yeh CM, Lin
YM, Ho TY, Hsiang CY and Chung JG: Early induction of
cytokines/cytokine receptors and Cox2, and activation of NF-κB in
4-nitroquino-line 1-oxide-induced murine oral cancer model. Toxicol
Appl Pharmacol. 262:107–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Eveson JW and MacDonald DG: Hamster tongue
carcinogenesis. I. Characteristics of the experimental model. J
Oral Pathol. 10:322–331. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Chen YF, Chang KW, Yang IT, Tu HF and Lin
SC: Establishment of syngeneic murine model for oral cancer
therapy. Oral Oncol. 95:194–201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Bürtin F, Mullins CS and Linnebacher M:
Mouse models of colorectal cancer: Past, present and future
perspectives. World J Gastroenterol. 26:1394–1426. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Durinikova E, Buzo K and Arena S:
Preclinical models as patients' avatars for precision medicine in
colorectal cancer: Past and future challenges. J Exp Clin Cancer
Res. 40:1852021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Keysar SB, Astling DP, Anderson RT, Vogler
BW, Bowles DW, Morton JJ, Paylor JJ, Glogowska MJ, Le PN,
Eagles-Soukup JR, et al: A patient tumor transplant model of
squamous cell cancer identifies PI3K inhibitors as candidate
therapeutics in defined molecular bins. Mol Oncol. 7:776–790. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Garrido-Laguna I, Uson M, Rajeshkumar NV,
Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor
G, Sharma R, et al: Tumor engraftment in nude mice and enrichment
in stroma-related gene pathways predict poor survival and
resistance to gemcitabine in patients with pancreatic cancer. Clin
Cancer Res. 17:5793–5800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Choi Y, Lee S, Kim K, Kim SH, Chung YJ and
Lee C: Studying cancer immunotherapy using patient-derived
xenografts (PDXs) in humanized mice. Exp Mol Med. 50:1–9. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Choi SY, Lin D, Gout PW, Collins CC, Xu Y
and Wang Y: Lessons from patient-derived xenografts for better in
vitro modeling of human cancer. Adv Drug Deliv Rev. 79-80:222–237.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Facompre ND, Sahu V, Montone KT, Harmeyer
KM, Nakagawa H, Rustgi AK, Weinstein GS, Gimotty PA and Basu D:
Barriers to generating PDX models of HPV-related head and neck
cancer. Laryngoscope. 127:2777–2783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Mosier DE, Gulizia RJ, Baird SM and Wilson
DB: Transfer of a functional human immune system to mice with
severe combined immunodeficiency. Nature. 335:256–259. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Ali N, Flutter B, Sanchez Rodriguez R,
Sharif-Paghaleh E, Barber LD, Lombardi G and Nestle FO: Xenogeneic
graft-versus-host-disease in NOD-scid IL-2Rγ null mice display a
T-effector memory phenotype. PLoS One. 7:e442192012. View Article : Google Scholar
|
|
197
|
Lan P, Tonomura N, Shimizu A, Wang S and
Yang YG: Reconstitution of a functional human immune system in
immunodeficient mice through combined human fetal thymus/liver and
CD34+ cell transplantation. Blood. 108:487–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient-derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Matsumura T, Kametani Y, Ando K, Hirano Y,
Katano I, Ito R, Shiina M, Tsukamoto H, Saito Y, Tokuda Y, et al:
Functional CD5+ B cells develop predominantly in the spleen of
NOD/SCID/gammac(null) (NOG) mice transplanted either with human
umbilical cord blood, bone marrow, or mobilized peripheral blood
CD34+ cells. Exp Hematol. 31:789–797. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Hanazawa A, Ito R, Katano I, Kawai K, Goto
M, Suemizu H, Kawakami Y, Ito M and Takahashi T: Generation of
Human immunosuppressive myeloid cell populations in human
Interleukin-6 transgenic NOG mice. Front Immunol. 9:1522018.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Pan B, Wei X and Xu X: Patient-derived
xenograft models in hepatopancreatobiliary cancer. Cancer Cell Int.
22:41. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Stecklum M, Klinghammer K, Wulf-Goldenberg
A, Brzezicha B, Jöhrens K and Hoffmann J: P0314 Preclinical case
study: Patient-derived head and neck cancer xenograft on mice
humanized with autologous immune cells, a model for personalized
immuno-oncology research. J Immuno Ther Cancer. 8(Suppl 2):
A27–A28. 2020.
|
|
203
|
Morton JJ, Bird G, Keysar SB, Astling DP,
Lyons TR, Anderson RT, Glogowska MJ, Estes P, Eagles JR, Le PN, et
al: XactMice: Humanizing mouse bone marrow enables microenvironment
reconstitution in a patient-derived xenograft model of head and
neck cancer. Oncogene. 35:290–300. 2016. View Article : Google Scholar
|
|
204
|
DeBord LC, Pathak RR, Villaneuva M, Liu
HC, Harrington DA, Yu W, Lewis MT and Sikora AG: The chick
chorioallantoic membrane (CAM) as a versatile patient-derived
xenograft (PDX) platform for precision medicine and preclinical
research. Am J Cancer Res. 8:1642–1660. 2018.PubMed/NCBI
|
|
205
|
Mapanao AK, Che PP, Sarogni P, Sminia P,
Giovannetti E and Voliani V: Tumor grafted-chick chorioallantoic
membrane as an alternative model for biological cancer research and
conventional/nanomaterial-based theranostics evaluation. Expert
Opin Drug Metab Toxicol. 17:947–968. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Garcia P, Wang Y, Viallet J and Macek
Jilkova Z: The chicken embryo model: A novel and relevant model for
immune-based studies. Front Immunol. 12:7910812021. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Rousset X, Dosda E and Viallet J: Use of
an egg grafted with tumor cells in order to study the anti-cancer
effectiveness of immune therapies in the absence of immune effector
cells other than those in the grafted egg. Google Patents.
2020.
|
|
208
|
Moticka EJ: Development of immunological
competence in chickens. Am Z. 15:135–146. 1975. View Article : Google Scholar
|
|
209
|
Schmitd LB, Liu M, Scanlon CS, Banerjee R
and D'Silva NJ: The chick chorioallantoic membrane in vivo model to
assess perineural invasion in head and neck cancer. J Vis Exp. Jun
21–2019.Epub ahead of print. View
Article : Google Scholar : PubMed/NCBI
|
|
210
|
de Medeiros MC, Liu M, Banerjee R, Bellile
E, D'Silva NJ and Rossa C Jr: Galanin mediates tumor-induced
immunosuppression in head and neck squamous cell carcinoma. Cell
Oncol (Dordr). 45:241–256. 2022. View Article : Google Scholar
|
|
211
|
Gu L and Mooney DJ: Biomaterials and
emerging anticancer therapeutics: Engineering the microenvironment.
Nat Rev Cancer. 16:56–66. 2016. View Article : Google Scholar :
|
|
212
|
Kamatar A, Gunay G and Acar H: Natural and
synthetic biomaterials for engineering multicellular tumor
spheroids. Polymers (Basel). 12:25062020. View Article : Google Scholar
|
|
213
|
Park Y, Huh KM and Kang SW: Applications
of biomaterials in 3D cell culture and contributions of 3D cell
culture to drug development and basic biomedical research. Int J
Mol Sci. 22:24912021. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Li J, Luo Y, Li B, Xia Y, Wang H and Fu C:
Implantable and injectable biomaterial scaffolds for cancer
immunotherapy. Front Bioeng Biotechnol. 8:6129502020. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Phuengkham H, Song C, Um SH and Lim YT:
Implantable synthetic immune niche for spatiotemporal modulation of
tumor-derived immunosuppression and systemic antitumor immunity:
Postoperative immunotherapy. Adv Mater. 30:e17067192018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Sanmamed MF and Chen L: A Paradigm shift
in cancer immunotherapy: From enhancement to normalization. Cell.
175:313–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Datta P, Dey M, Ataie Z, Unutmaz D and
Ozbolat IT: 3D bioprinting for reconstituting the cancer
microenvironment. NPJ Precis Oncol. 4:182020. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Asghar W, El Assal R, Shafiee H, Pitteri
S, Paulmurugan R and Demirci U: Engineering cancer
microenvironments for in vitro 3-D tumor models. Mater Today
(Kidlington). 18:539–553. 2015. View Article : Google Scholar
|
|
219
|
Oztan YC, Nawafleh N, Zhou Y, Liyanage PY,
Hettiarachchi SD, Seven ES, Leblanc RM, Ouhtit A and Celik E:
Recent advances on utilization of bioprinting for tumor modeling.
Bioprinting. Jan 29–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Swaminathan S and Clyne AM: Direct
bioprinting of 3D multicellular breast spheroids onto endothelial
networks. J Vis Exp. Nov 2–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Browning JR, Derr P, Derr K, Doudican N,
Michael S, Lish SR, Taylor NA, Krueger JG, Ferrer M, Carucci JA and
Gareau DS: A 3D biofabricated cutaneous squamous cell carcinoma
tissue model with multi-channel confocal microscopy imaging
biomarkers to quantify antitumor effects of chemotherapeutics in
tissue. Oncotarget. 11:2587–2596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Almela T, Al-Sahaf S, Brook IM, Khoshroo
K, Rasoulianboroujeni M, Fahimipour F, Tahriri M, Dashtimoghadam E,
Bolt R, Tayebi L and Moharamzadeh K: 3D printed tissue engineered
model for bone invasion of oral cancer. Tissue Cell. 52:71–77.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Matthews JB, Mason GI, Scully CM and Prime
SS: In situ characterisation of the oral mucosal inflammatory cell
response of rats induced by 4-nitroquinoline-N-oxide.
Carcinogenesis. 7:783–788. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Thomas DW, Matthews JB, Patel V, Game SM
and Prime SS: Inflammatory cell infiltrate associated with primary
and transplanted tumours in an inbred model of oral carcinogenesis.
J Oral Pathol Med. 24:23–31. 1995. View Article : Google Scholar : PubMed/NCBI
|