Cilia are evolutionally conserved cell structures
protruding from the cell surface, which are found ubiquitously
across species from ancient protozoa to humans. They are usually
divided into two categories: Motile cilia and primary cilia, both
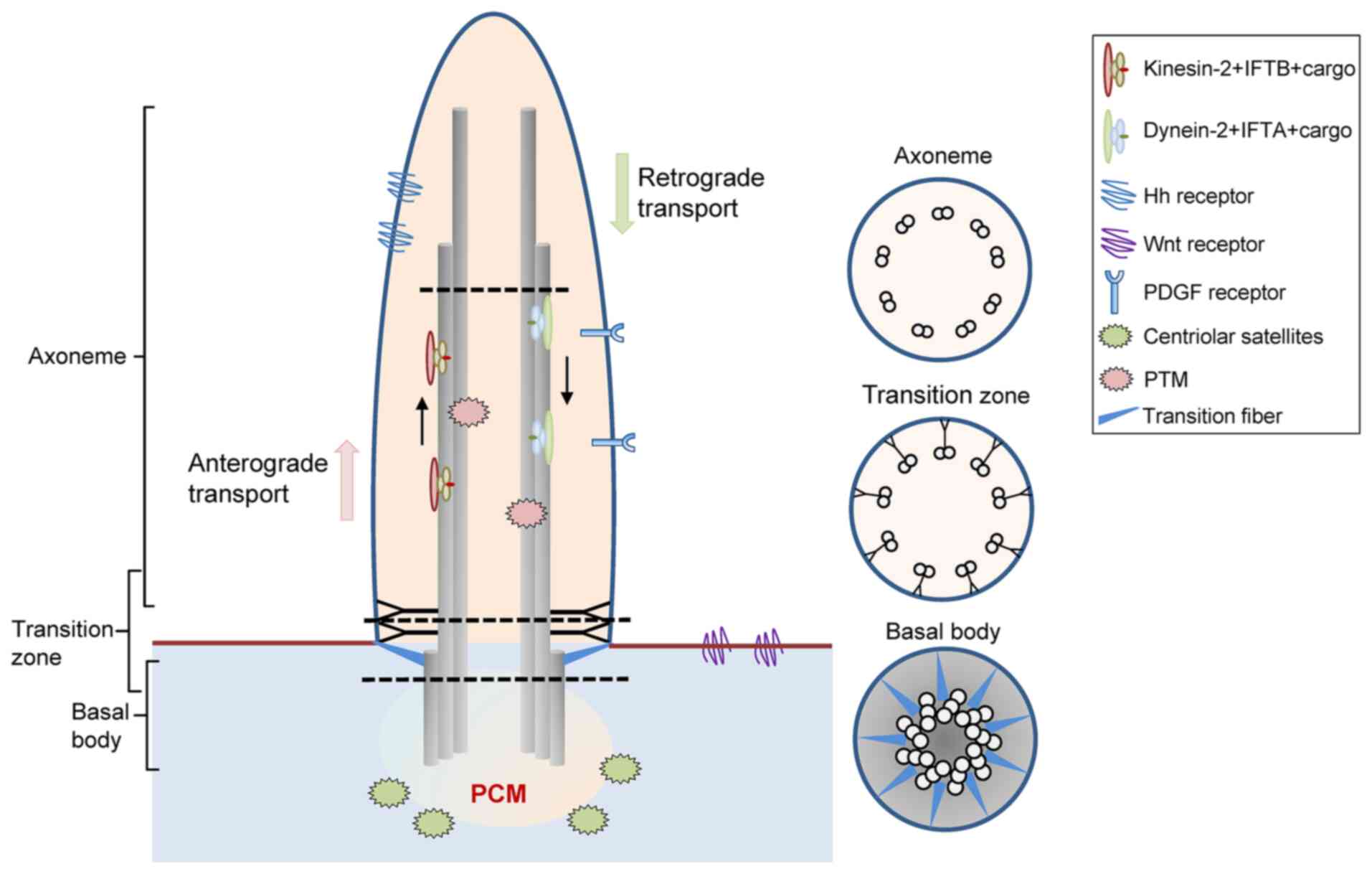
of which consist of the axoneme, matrix and ciliary membrane
(Fig. 1). Unlike motile cilia,
primary cilia usually lack the dynein motor proteins that power
axonemal beating and are therefore immotile (1). For almost 100 years, cilia were
considered as anomalous structures without any function, until they
were found to be associated with the onset of multiple syndromes,
such as Bardet-Biedl (2), Joubert
(3), oral-facial-digital (OFD)
(4), and Ellis-van Creveld
syndrome (5).
There is abundant evidence to indicate that primary
cilia tightly regulate numerous critical signaling pathways, such
as the Hh, Wnt and Notch signaling pathways. Therefore, primary
cilia are regarded as the regulatory ‘hub’ of cell functions
(6,7). It is worth noting that, apart from
basal cell carcinoma (BCC) (8)
and medulloblastoma (9), in which
tumorigenesis is cilia-dependent, cilia formation is compromised in
multiple tumor types, including melanoma (10), pancreatic cancer (11), breast cancer (12), cholangiocarcinoma (CCA) (13), prostate cancer (14), renal cell carcinoma (RCC)
(15) and oral squamous cell
carcinoma (OSCC) (16). Since
hundreds of proteins and numerous critical signaling pathways are
regulated by primary cilia, their proper formation is critical for
the integrity of signal transduction and various cellular processes
(17). Therefore, molecules that
cause primary cilia defects may provide novel approaches with which
to attenuate tumor progression, which is the focus of the present
review.
Uncontrolled cell proliferation and deregulation of
the cell cycle are hallmarks of malignant tumor formation (18). This section mainly provides a
description of the mechanisms through which ciliogenesis is closely
associated with the cell cycle.
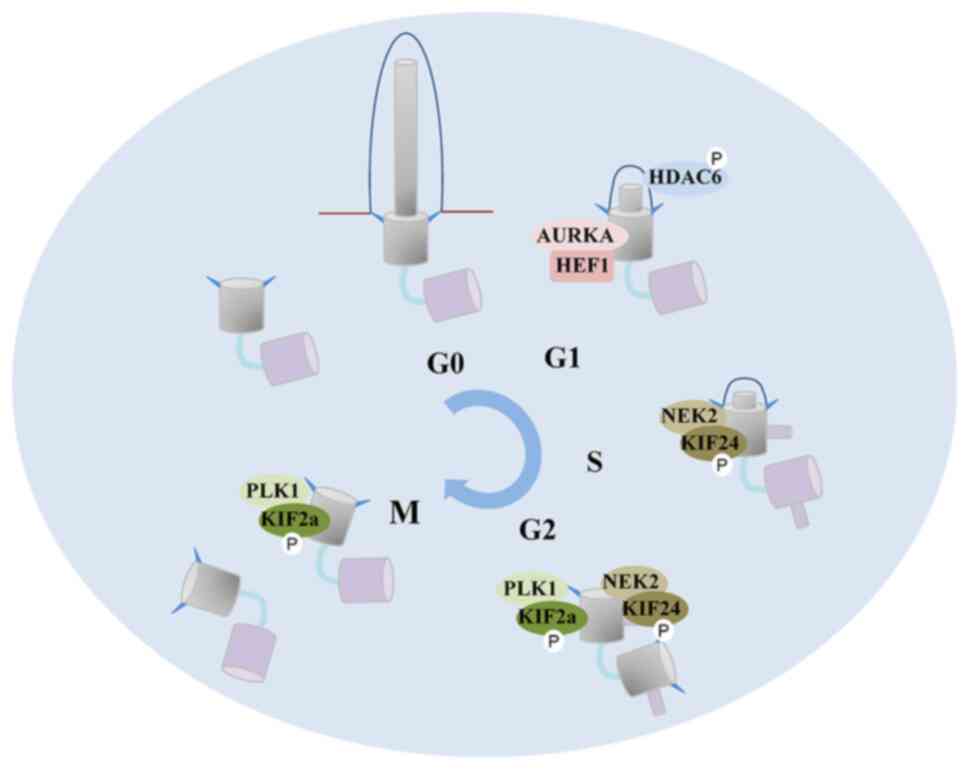
Ciliogenesis is an elaborately regulated process
that begins when cells exit the mitotic cycle (Fig. 2). Ciliogenesis occurs through two
main routes: The classic intracellular pathway, in which the short
axoneme extends from the basal body before the latter reaches the
cytoplasmic membrane (19); and
the alternative pathway, in which the axoneme extends from the
basal body only after the latter is anchored to the cytoplasmic
membrane (20). In fibroblasts,
cilia are assembled through the intracellular pathway. When the
cell exits the cell cycle, cytoplasmic vesicles are anchored to the
distal appendage of the mother centriole and then trigger the
remodeling of the mother centriole into the basal body, which is
the earliest ciliogenesis event that can be detected (21). The basal body-vesicle complex then
migrates to the cell surface and is anchored to the plasma
membrane. Under the transport of intraflagellar transport (IFT)
complexes, proteins accumulate into cilia through the ‘ciliary
gate’, and cilia gradually bulge from the cell surface. Conversely,
in polarized epithelial and multiciliated cells, ciliogenesis
occurs through the alternative pathway. The basal body localizes at
the center of the apical membrane and interacts directly with the
membrane, with cilia assembly occurring entirely in the plasma
membrane [further details on the ciliogenic pathways observed have
been previously described (22)].
In contrast to cilia assembly, the mechanisms
underlying cilia disassembly in normal or pathological conditions
remain largely unknown (23).
Following the stimulation of mitogens, the scaffolding protein
human enhancer of filamentation 1 (HEF1) activates aurora kinase A
(AURKA) in the basal body, which in turn phosphorylates histone
deacetylase 6 (HDAC6) (24).
Phosphorylated HDAC6 enters the cilia to remove the acetylation
modification of axonemal microtubules, thus inducing cilia
disassembly. However, the mechansims through which HEF1 is
upregulated and recruited to the basal body following mitogen
stimulation remain unclear. In addition to HEF1, Trichoplein
(25) and Pitchfork (26) have also been proven to be
activators of AURKA in the G1 and S phases, respectively.
In addition to HDAC6, kinesin family member (KIF)24
and KIF2a are also implicated in the de-polymerization of axonemal
microtubules. The activity of KIF24 is enhanced by
never-in-mitosis-A-related kinase 2 (NEK2) during the S and G2
phases (27), and KIF2a is
activated by polo-like kinase 1 (PLK1) in the G2 and M phases
(28). Notably, the regulatory
mechanisms of AURKA, NEK2 and PLK1 involved in cilia disassembly
are relatively conservative and in chronological order, ensuring
the irreversibility of cilia resorption following cell cycle
entry.
Since the centriole dually functions as the
microtubule-organizing center during mitosis and as the basal body
for ciliogenesis, a common hypothesis is that cilia anchor the
centriole to the cell membrane, thereby depriving the cell of cycle
entry (23). The abnormal
presence of cilia has been shown to lead to cell cycle arrest
observed in in vivo and in vitro models. Trichoplein
or AURKA knockdown induce cilia to fail to disassemble, thus
inducing G0/G1 arrest. More notably, this phenotype was reversed
when cilia formation was prevented by the simultaneous knockdown of
IFT20 (25). In a previous study,
NudE neurodevelopment protein 1, localizing on the mother
centriole, was found to be highly expressed during mitosis and then
rapidly degraded when the cell became quiescent. Its silencing in
zebrafish and cultured cells led to a significant increase in
ciliary length and S phase arrest (29). In addition, in another study,
Tctex1 deletion, mainly localizing on the transition zone and
regulating ciliary resorption, was shown to resulted in cilia
persistence and G1/S phase arrest (30). These studies suggest that cells
may sense the presence of primary cilia to limit cell cycle entry;
however, further research is required to prove this hypothesis.
Ciliogenesis is a cell cycle-regulated event in
normal cells. However, few studies have closely implicated the
ciliogenesis changes when the cell cycle is under the influence of
malignancies. The most fundamental trait of malignant cells
involves their ability to sustain proliferation (31). Therefore, a possible cause of
cilia loss in malignant cells could be the increased proliferation.
However, Menzl et al (12)
found that the loss of primary cilia was not associated with an
increased proliferative index (Ki67-positive cells) in breast
cancer. Nobutani et al (32) hardly detected primary cilia in
cell cycle-arrested human breast cancer cells. Moreover, in a study
involving 110 patients with kidney cancer, reduced ciliary ratio
was observed to be independent of cell proliferation (15). It is worth noting that
ciliogenesis is not restrained in all malignant tumors. The ciliary
ratio has been shown to be significantly increased in BCC tissues
(33) and cilia have been
identified in some subsets of human medulloblastomas which had
activation in either Hh or Wnt signaling (9). These studies suggest that cilia
defects are characteristic of oncogenic transformation, and this is
not only due to the increased proliferation in malignancies.
The abnormal expression of certain critical ciliary
proteins, which have a number of the hallmarks of oncogenes, can
result in severe cilia defects. Although only a few protein
inhibitors have been used in clinical practice or in clinical
trials (34), targeting these
molecules still holds promise for cancer treatment (35). Nevertheless, a number of studies
have been primarily observational and do not elaborate on the
mechanisms of cancer containment by targeting these ciliary
molecules. This section provides a summary and discussion of the
mechanisms through which these molecules can cause cilia defects in
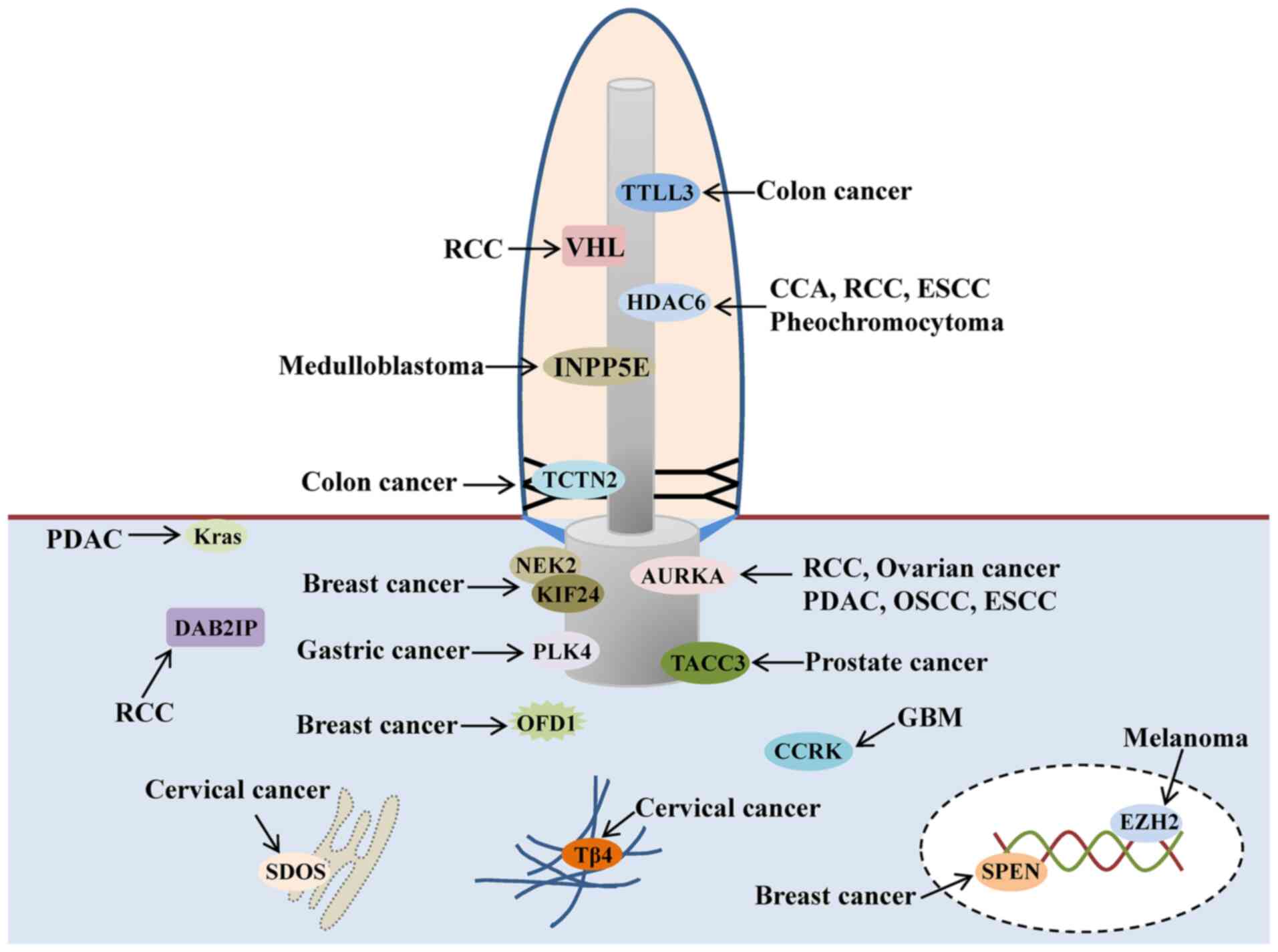
cancer (Fig. 3).
CCRK plays a critical role in promoting G1 phase
entry and is of interest as it is the closest vertebrate homolog to
the long flagella 2, a critical protein controlling the length of
flagella in chlamydomonas (36).
CCRK localizes in the cytoplasm and tunes ciliary length and shape
by coordinating the assembly of the ciliary axoneme and membrane
(37,38). In addition, cell cycle regulation
by CCRK has been found to be dependent on primary cilia. The
mutation of CCRK in mice induces shortened and swollen cilia, which
impairs the Hh pathway and leads to a constellation of
developmental defects, including exencephaly, cleft palate and mild
preaxial polydactyly/limb skeletal defects (39). Immature cilia with ultrastructural
malformation are considered to be a true hallmark of glioblastoma
tumors and to be associated with their unrestrained growth
(40). Yang et al
(41) found that the arrested
ciliogenesis, and therefore tumor growth, were caused by the
elevation of CCRK in glioblastoma. Furthermore, the depletion of
CCRK markedly increased the ratio and length of primary
cilia in glioblastoma cell lines, and notably, cells with restored
cilia exhibited a block or severe delay in cell cycle progression,
thus inhibiting tumor cell growth (41). It would be of interest to explore
the mechanisms of action of CCRK in primary cilia in glioblastoma
in vivo. On the whole, these finding indicate that the
suppression of primary ciliogenesis by the upregulation of CCRK may
be one of the mechanisms used by glioblastoma cells to provide a
growth advantage.
PLK4 is a conserved centrosomal protein (CEP) that
plays a key role in the centriole duplication cycle in a
concentration-dependent manner. The centrioles fail to duplicate
when PLK4 malfunctions, while they overamplify when PLK4 is
overexpressed (42). However,
ciliogenesis is blocked, regardless of its deficiency or
overexpression. Although abnormalities in centriole number and
structure are commonly observed in cancer, cilia defects caused by
PLK4 dysfunction in cancer were not reported until 2014. Shinmura
et al (43) reported that
the abnormally elevated expression of PLK4 was related to the
absence of primary cilia in human gastric cancer. The upregulation
of PLK4 mRNA expression was detected in half of the primary gastric
cancers. Moreover, an in vitro experiment demonstrated that
its overexpression directly caused centrosome amplification,
leading to the suppression of ciliogenesis in gastric cancer cell
lines (43). Another study
overexpressed PLK4 in mice and found that the formation of primary
cilia was prevented, cilia signaling was disrupted, and the
formation of lymphomas and sarcomas in p53 null mice was advanced
(44).
Thus far, the presence of cilia defects in cervical
cancer remains contradictable and elusive. Primary cilia were rare
in HeLa cells in the study by Alieva et al (52); however, in the study by Kowal and
Falk (53), the ciliated
population was significantly higher than previously anticipated. In
addition, due to the extensive presence of primary cilia, the HeLa
cell line was used as a model to explore cilia biology (54). However, these studies only
examined primary cilia in the cultured cells and did not set up a
strict control group of normal cervical epithelial cells.
Therefore, further research is warranted at the tissue level.
The TCTN family (including TCTN1, TCTN2 and TCTN3)
forms complexes with proteins that localize to the transition zone
of cilia, where they regulate the composition of the ciliary
membrane in a tissue-dependent manner (55). Mutations in TCTNs lead to
tissue-specific defects in cilia assembly and trafficking that
underlie several ciliopathies (56). However, the available studies on
the role of TCTNs in cancer are limited. TCTN1 elevation predicts
poor clinical outcomes for patients with glioblastoma (57) and is indispensable for pancreatic
cancer cell proliferation (58).
Its association with primary cilia in these two cancer types has
not yet been investigated, at least to the best of our knowledge. A
previous study identified TCTN2 as an oncogene and a tumor marker
in several cancer types, including colorectal, lung and ovarian
cancer (59). Moreover,
inhibiting the expression of TCTN2 has been found to significantly
reduce colony formation and cell invasiveness, and impair the
assembly of primary cilia in colon cancer cell lines. However, the
existing studies are contradictory. Yasar et al (60) observed that the cilia number was
elevated in colon adenocarcinoma compared with normal tissues,
whereas the study by Rocha et al (61) demonstrated that the cilia number
was decreased in colon cancer.
DAB2IP, a member of the RasGTPase-activating protein
family, has been identified as a tumor suppressor in several cancer
types (62,63). Its loss is highly prevalent in
different subtypes of RCC and is associated with a poor patient
survival (64). A recent study
found that DAB2IP knockdown impaired primary cilia formation by
decreasing the expression of KIF3a (essential for cilia assembly
and length maintenance) in kidney epithelial cells (65). In addition, the loss of KIF3a
further promotes renal tumorigenesis, suggesting that primary cilia
stability is part of the critical homeostatic machinery in renal
epithelia (65).
TACC3, characterized by a highly conserved
C-terminal coil domain, is a key component of
centrosome-microtubule dynamic networks (66). Recently, TACC3 has been identified
as a potential prognostic marker and therapeutic target for various
cancer types, such as breast (67) and lung cancer (68). Cilia ratio and length are
gradually decreased during the progression from normal prostate to
prostatic intraepithelial neoplasia and invasive prostate cancer,
and that increase is accompanied by the activation of Wnt signaling
(14). However, the mechanisms
responsible for cilia defects in prostate cancer remain elusive. A
recently published study demonstrated that TACC3 upregulation
restrained ciliogenesis in prostate cancer cells (69). TACC3 can competitively bind
filamin A and disrupt the michelin-filamin A interaction, which is
necessary for centrosome migration to the apical membrane and cilia
formation (70). Furthermore,
TACC3 knockdown significantly restored the formation of primary
cilia and inhibited tumorigenesis and tumor growth in vitro
and in vivo, suggesting that targeting TACC3 may represent a
novel approach for prostate cancer therapeutics (69).
As mentioned above, the AURKA-HDAC6 signaling axis
plays a critical role in cilia disassembly. AURKA activation is
common in multiple cancer types characterized by centrosomal
amplification and genomic instability (71,72). The oncogenic AURKA appears to be
the key node for the suppression of primary cilia in several types
of cancer, including pancreatic ductal adenocarcinoma (PDAC)
(73), clear cell RCC (ccRCC)
(74), ovarian cancer (75), pheochromocytoma (76) and esophageal squamous cell
carcinoma (ESCC) (77). In
addition, in a previous study, the number of cilia was
significantly decreased in AURKA-activated tissues from patients
with OSCC (16). More
importantly, AURKA inhibition has been shown to result in primary
cilia re-expression and significantly inhibit tumor progression in
PDAC, ccRCC and OSCC (16,73,78).
However, the target of AURKA for cilia absorption
may vary between tumor types. Unlike ccRCC (74), pheochromocytoma (76) and ESCC (77), cilia loss induced by AURKA
activation is independent of HDAC6 in PDAC (73), ovarian cancer (75) and OSCC (16). The specific target of AURKA in
these cancer types is unclear. A previous study demonstrated that,
in addition to HDAC6, AURKA also activated inositol polyphosphate
5-phosphatase E (INPP5E) (79), a
ciliary protein whose absence results in ciliary destabilization
and tumor progression in medulloblastoma (80). AURKA may promote cilia loss
through INPP5E in PDAC, ovarian cancer and OSCC, which requires
further verification.
KIF24, localizing to the distal end of the
centriole, depolymerizes the cilia microtubules and provokes cilia
disassembly shortly following the stimulation of mitogens (27). The depolymerizing activity of
KIF24 is enhanced by NEK2, a kinase only expressed in the S and G2
phases (83). The NEK2-KIF24
action at centriole prevents the aberrant assembly of cilia and
keeps the de-ciliated state necessary for mitosis. This mechanism
of inhibiting primary ciliogenesis is temporally distinct from the
well-established AURKA-HDAC6 pathway by blocking the nucleation of
cilia from the basal body (83).
NEK2 has been identified as an oncogene in various
cancer types, including myeloma and breast cancer (84–86). Cilia loss is considered to be a
characteristic of breast cancer, and the mouse model further
confirmed its importance in the development of breast cancer.
Although the absence of primary cilia was not found to directly
cause breast cancer in mice, it led to the earlier formation and
accelerated the growth of cancer, and was associated with a higher
grade and metastasis (87). Kim
et al (83) found that
NEK2-KIF24 was overexpressed in breast cancer cell lines, and their
ablation resulted in the re-expression of primary cilia, thereby
reducing the proliferation of cancer cells. However, NEK2 has not
been reported to induce the loss of primary cilia in other tumors
apart from breast cancer.
The glutamylation of tubulin in mammals can be
catalyzed by nine glutamate ligases, in which only two enzymes,
TTLL3 and TTLL8, are capable of initiating glycylation on
microtubules (88). Glycylation
has been found to function as a critical regulator of ciliary
disassembly in motile cilia and flagella, while previous studies
that used zebrafish or mouse models proved that the integrity of
primary cilia was also dependent on this post-translational
modification on tubulin (89,90). TTLL3 is the only expressed
glycylase in colon tissue, and its knockout in mice has been shown
to not only cause a marked reduction in the number of cilia, but to
also lead to a markedly increased cell proliferation rate in the
colon epithelium. In addition, the lower expression level of TTLL3
has been shown to be significantly associated with the development
of colorectal carcinoma, suggesting that cilia defects caused by
TTLL3 may serve as a prognostic marker for this type cancer
(61).
TSC is a genetic syndrome with widespread dysplastic
and multisystemic tumors, caused by the mutations in the tumor
suppressor genes, TSC1 and TSC2 (91). TSC1 and TSC2 form a heterodimer
that inhibits mTOR signaling by inactivating mTORC1, a type of
complex sensitive to rapamycin (92). TSC1, localizing to the basal body
(93), is frequently
heterozygously lost in ccRCC (94). Activating mTOR signaling by
silencing TSC1 lengthened primary cilia in zebrafish (95) and mouse (96) models, and inhibiting mTOR
signaling by rapamycin, could return the ciliary length to normal
(96). Rosengren et al
(97) also found that mTORC1
inhibition by rapamycin resulted in shortened cilia. These studies
appear to indicate that mTORC1 plays a positive role in ciliary
maintenance. However, another study reported the negative effects
of mTORC1 on primary cilia, in which ciliary length was increased
following rapamycin treatment in a dose-dependent manner in renal
epithelial and vascular endothelial cells (98).
The aforementioned study indicated a positive role
of autophagy in the regulation of primary cilia formation, while
another report recognized basal autophagy as a suppressor in
ciliogenesis in primary Hürthle cell tumors. Cilia loss was caused
by a high basal autophagic flux, and the inhibition of
autophagosome formation notably restored the primary ciliogenesis
in tumor cells (109). Pampliega
et al (110) also found
that autophagy inhibited ciliogenesis and cilia-associated
signaling during normal nutritional conditions. On the other hand,
Maharjan et al (111)
reported that alterations in autophagy during serum-restimulation,
irrespective of whether autophagy was activated or inhibited,
prevented the disassembly of primary cilia in RPE1 cells. These
studies suggest that the regulation of autophagy on primary cilia
is likely dependent on the cellular context. Furthermore, the
association between primary cilia and autophagy is bidirectional,
since the abrogation of ciliogenesis partially inhibits autophagy
(110). The regulatory
mechanisms between autophagy and primary cilia are further
complicated by other cellular events that are usually observed in
cancer, such as the activation of AURKA, HDAC6 or other critical
factors controlling cilia biology, and thus warrant further
investigation (112).
VHL has been widely accepted as a component of an E3
ubiquitin ligase that targets hypoxia-inducible factor α for
ubiquitination and degradation in an oxygen-dependent manner
(113). Its mutation results in
VHL disease, the most well-known familial kidney cancer syndrome
(114). ccRCC is the most common
subtype of renal cancer, and is mainly sporadic. Of note, the
VHL gene is inactivated in up to 87% of sporadic ccRCC cases
(115). In addition, the
re-expression of VHL protein is sufficient to suppress the
formation of renal cancer in vivo, suggesting that VHL
inactivation is a direct cause of renal tumorigenesis (116).
Primary cilia are almost absent in the renal cysts
and ccRCC of patients with VHL (15). In addition, the histological
manifestations of early-stage RCC, including increased disorganized
cilia, occurred in the kidney of the VHL knockout zebrafish
(117). However, in a previous
study, ccRCC was not induced by a specific deletion of VHL
in mouse renal epithelial cells, suggesting that additional
mutations are required in mammals (118). The combined conditional
inactivation of VHL and other tumor suppressor factors, Pten or
tumor protein p53, gave rise to cilia ablation, renal cysts and
neoplastic growth resembling human ccRCC (119,120). Of note, another study detected
the mutations of ciliary genes in ~50% of 448 human ccRCC samples,
suggesting that the dysfunction of primary cilia plays an important
role in at least part of ccRCC (121).
The formation of primary cilia has been shown to be
restored by re-expressing the wild-type VHL in VHL-defective ccRCC
cell lines (122). However, the
mechanisms responsible for cilia defects caused by VHL mutation
remain elusive. Schermer et al (116) found that VHL localized on
primary cilia and regulated the cilia maintenance by directing the
growth of microtubules toward the cell periphery, which is a
prerequisite for ciliogenesis. However, a different study revealed
that VHL-knockdown led to cilia disassembly by upregulating
the expression of NEK8, a cell cycle regulator (123). In addition, VHL knockdown
increased AURKA expression by activating β-catenin, thus leading to
cilia disassembly. Furthermore, the β-catenin responsive
transcription inhibitor rescued cilia defects by inhibiting AURKA,
opening new avenues for treatment with β-catenin inhibitors to
rescue ciliogenesis in ccRCC (74).
Spen, characterized by N-terminal RNA-binding
motifs, is a large nuclear protein and a component of the HDAC
corepressor complex (124). Spen
regulates the expression of key transcriptional effectors in
multiple signaling pathways (125) and has been established as a
tumor suppressor gene by negatively regulating the transcription of
estrogen receptor α targets in breast cancer (126). Recently, Spen was found to be
co-expressed with the ciliogenic transcription factor, regulatory
factor X family member 3. The knockdown of Spen considerably
inhibited the formation of primary cilia, and its re-expression
rescued ciliogenesis in breast cancer cells (127). Furthermore, the regulation of
cell migration by Spen only occurred in those cells harboring
primary cilia, indicating that Spen may coordinate cellular
movement in a cilia-dependent manner in breast cancer (127).
EZH2, a histone methyltransferase, is part of
polycomb repressive complex 2 (PRC2), which silences target genes
epigenetically by catalyzing histone H3 tri-methylation (128). EZH2 is activated in a variety of
cancer types and drives cancer progression by suppressing the
expression of various tumor suppressor genes (129). Cilia loss is considered to be a
promoter and a potential biomarker in melanoma development
(10). A recent study (130) reported an inverse correlation
between primary cilia and EZH2 expression levels during melanoma
development. Activated EZH2 was a driver of melanoma oncogenesis by
silencing genes related to ciliary integrity and thus
deconstructing primary cilia. Cilia deconstruction further led to
the activation of the Wnt/β-catenin pathway, a well-known oncogenic
signaling pathway in melanoma. Strikingly, EZH2 activity blockage
significantly induced primary ciliogenesis and cilia-dependent
tumor growth-arrest, suggesting that rescuing ciliogenesis by
targeting EZH2 may serve as a new strategy for melanoma treatment
(130).
SDOS was initially reported to co-localize with
syndecan 4 cytoplasmic domain in focal contacts and promotes the
assembly of focal adhesions (131). A recent study identified SDOS as
a novel RNA-binding protein that interacted with TNF
receptor-associated protein 1 at the endoplasmic reticulum to
regulate mRNA translation (132). A small subset of mRNAs
responsible for the primary ciliogenesis was regulated
post-transcriptionally by SDOS, including transmembrane protein 67,
coiled-coil and C2 domain containing 2A and Kif7, known as the
ciliopathy-associated genes. Furthermore, the regulatory effect of
SDOS on primary cilia was further proven in HeLa cells. The number
and length of primary cilia were significantly increased in
SDOS-silenced HeLa cells, whereas they were decreased in
SDOS-overexpressing cells.
KRAS, having intrinsic GTPase activity, is a small
GTP-binding protein and functions as a molecular switch for various
cellular processes (133). Its
mutation, a common driver in various cancers, locks the protein
into the GTP-bound state and results in constitutive signaling,
which gives a growth advantage to mutated cells, thereby leading to
the development of cancer (134,135). In PDAC, KRAS is the most
commonly mutated gene, which is present in >90% of tumor cells
(136). In addition, the
activation of the oncogenic KRAS allele directly induced the
formation of PDAC in mice and blocked ciliogenesis in cancer cells
(11). Of note, cilia defects
were rescued by inhibiting KRAS effector pathways. The present
study raises the possibility that aberrant KRAS signaling may
promote carcinogenesis by inducing cilia loss in PDAC.
Primary cilia were previously considered as
vestigial organelles, while recent advances have recognized the
complex biological functions of these unique structures in diseases
and cancer. Abnormal signaling pathways lead to uncontrolled
proliferation (137), drug
resistance (138) and immune
escape in cancer (139). As the
‘hub’ for multiple signaling receptors and downstream effector
molecules, primary cilia are considered to be the critical
regulatory center for inducing pathway defects (140). Primary cilia are likely to
impact cancer development in multiple ways, including affecting the
cell cycle process (83),
mediating signal transduction (141) and regulating the response to
therapy targeted to ciliary proteins or related pathways (142). Therefore, several studies have
highlighted the possible application of cilia dysfunction in the
early diagnosis and prognosis pf cancer.
Primary cilia have been recognized as an important
therapeutic target, although the mechanisms of cilia defects are
variable in the context of each cancer type (Fig. 3). As already aforementioned,
several key factors regulating primary cilia, such as AURKA, HDAC6
and NEK2, have been proven to function as critical oncogenes in the
development of various cancer types (13,71,122). Targeting these oncogenic factors
and rescuing ciliogenesis can restrain tumor growth, particularly
when simultaneously targeting multiple proteins that cause cilia
defects. Therefore, targeting these molecules to develop novel
therapeutic approaches has been an emerging field in the research
of pancreatic, lung, kidney and breast cancer (143).
Although a number of researchers have reported that
tumor growth was arrested by re-expressing primary cilia in
vivo and in vitro, further extensive research is
warranted before targeting cilia can be used in cancer treatment.
Firstly, the mechanisms through which primary cilia regulate
carcinogenesis differs between cancer types and within cancer
subtypes. Secondly, targeting cilia can both restrain tumor
progression and induce kinase inhibitor resistance, which appears
to prevent achieving good therapeutic effects (142). Primary cilia are complex
organelles whose structure, arrangement and function are highly
regulated. However, further studies are required to decipher the
complex signals they transmit. Nevertheless, existing studies have
suggested that the status of primary cilia should play a role in
the decision making for precise and personalized treatment.
Not applicable.
The present study was supported by the Key Research and
Development Program of Zhejiang Province (grant no. 2021C03074) and
the Fundamental Research Funds for the Central Universities (grant
no. K20220177).
Not applicable.
FY performed the literature search and wrote the
manuscript. ZW, CX and FC collected the relevant references and
edited the manuscript. QC supervised and revised the manuscript.
All authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Mitchison HM and Valente EM: Motile and
non-motile cilia in human pathology: From function to phenotypes. J
Pathol. 241:294–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ansley SJ, Badano JL, Blacque OE, Hill J,
Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM,
et al: Basal body dysfunction is a likely cause of pleiotropic
Bardet-Biedl syndrome. Nature. 425:628–633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sattar S and Gleeson JG: The ciliopathies
in neuronal development: A clinical approach to investigation of
Joubert syndrome and Joubert syndrome-related disorders. Dev Med
Child Neurol. 53:793–798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franco B and Thauvin-Robinet C: Update on
oral-facial-digital syndromes (OFDS). Cilia. 5:122016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruiz-Perez VL, Blair HJ, Rodriguez-Andres
ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H and Goodship JA:
Evc is a positive mediator of Ihh-regulated bone growth that
localises at the base of chondrocyte cilia. Development.
134:2903–2912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singla V and Reiter JF: The primary cilium
as the cell's antenna: Signaling at a sensory organelle. Science.
313:629–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pala R, Alomari N and Nauli SM: Primary
cilium-dependent signaling mechanisms. Int J Mol Sci. 18:22722017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong SY, Seol AD, So PL, Ermilov AN,
Bichakjian CK, Epstein EH Jr, Dlugosz AA and Reiter JF: Primary
cilia can both mediate and suppress Hedgehog pathway-dependent
tumorigenesis. Nat Med. 15:1055–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han YG, Kim HJ, Dlugosz AA, Ellison DW,
Gilbertson RJ and Alvarez-Buylla A: Dual and opposing roles of
primary cilia in medulloblastoma development. Nat Med.
15:1062–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim J, Dabiri S and Seeley ES: Primary
cilium depletion typifies cutaneous melanoma in situ and malignant
melanoma. PLoS One. 6:e274102011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seeley ES, Carriere C, Goetze T,
Longnecker DS and Korc M: Pancreatic cancer and precursor
pancreatic intraepithelial neoplasia lesions are devoid of primary
cilia. Cancer Res. 69:422–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Menzl I, Lebeau L, Pandey R, Hassounah NB,
Li FW, Nagle R, Weihs K and McDermott KM: Loss of primary cilia
occurs early in breast cancer development. Cilia. 3:72014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mansini AP, Lorenzo Pisarello MJ, Thelen
KM, Cruz-Reyes M, Peixoto E, Jin S, Howard BN, Trussoni CE, Gajdos
GB, LaRusso NF, et al: MicroRNA (miR)-433 and miR-22 dysregulations
induce histone-deacetylase-6 overexpression and ciliary loss in
cholangiocarcinoma. Hepatology. 68:561–573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassounah NB, Nagle R, Saboda K, Roe DJ,
Dalkin BL and McDermott KM: Primary cilia are lost in preinvasive
and invasive prostate cancer. PLoS One. 8:e685212013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basten SG, Willekers S, Vermaat JS, Slaats
GG, Voest EE, van Diest PJ and Giles RH: Reduced cilia frequencies
in human renal cell carcinomas versus neighboring parenchymal
tissue. Cilia. 2:22013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin F, Chen Q, Shi Y, Xu H, Huang J, Qing
M, Zhong L, Li J, Xie L and Zeng X: Activation of EGFR-Aurora A
induces loss of primary cilia in oral squamous cell carcinoma. Oral
Dis. 28:621–630. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin F, Chen Q, Shi Y, Xu H, Huang J, Qing
M, Zhong L, Li J, Xie L and Zeng X: Activation of EGFR-Aurora A
induces loss of primary cilia in oral squamous cell carcinoma. Oral
Dis. 28:621–630. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sorokin S: Centrioles and the formation of
rudimentary cilia by fibroblasts and smooth muscle cells. J Cell
Biol. 15:363–377. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sorokin SP: Reconstructions of centriole
formation and ciliogenesis in mammalian lungs. J Cell Sci.
3:207–230. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reiter JF, Blacque OE and Leroux MR: The
base of the cilium: Roles for transition fibres and the transition
zone in ciliary formation, maintenance and compartmentalization.
EMBO Rep. 13:608–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bernabe-Rubio M and Alonso MA: Routes and
machinery of primary cilium biogenesis. Cell Mol Life Sci.
74:4077–4095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanchez I and Dynlacht BD: Cilium assembly
and disassembly. Nat Cell Biol. 18:711–717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pugacheva EN, Jablonski SA, Hartman TR,
Henske EP and Golemis EA: HEF1-dependent Aurora A activation
induces disassembly of the primary cilium. Cell. 129:1351–1363.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoko A, Matsuyama M, Goto H,
Ohmuro-Matsuyama Y, Hayashi Y, Enomoto M, Ibi M, Urano T, Yonemura
S, Kiyono T, et al: Trichoplein and Aurora A block aberrant primary
cilia assembly in proliferating cells. J Cell Biol. 197:391–405.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kinzel D, Boldt K, Davis EE, Burtscher I,
Trümbach D, Diplas B, Attié-Bitach T, Wurst W, Katsanis N, Ueffing
M and Lickert H: Pitchfork regulates primary cilia disassembly and
left-right asymmetry. Dev Cell. 19:66–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi T, Tsang WY, Li J, Lane W and
Dynlacht BD: Centriolar kinesin Kif24 interacts with CP110 to
remodel microtubules and regulate ciliogenesis. Cell. 145:914–925.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyamoto T, Hosoba K, Ochiai H, Royba E,
Izumi H, Sakuma T, Yamamoto T, Dynlacht BD and Matsuura S: The
Microtubule-depolymerizing activity of a mitotic kinesin protein
KIF2A drives primary cilia disassembly coupled with cell
proliferation. Cell Rep. 10:664–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim S, Zaghloul NA, Bubenshchikova E, Oh
EC, Rankin S, Katsanis N, Obara T and Tsiokas L: Nde1-mediated
inhibition of ciliogenesis affects cell cycle Re-entry. Nat Cell
Biol. 13:351–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li A, Saito M, Chuang JZ, Tseng YY,
Dedesma C, Tomizawa K, Kaitsuka T and Sung CH: Ciliary transition
zone activation of phosphorylated Tctex-1 controls ciliary
resorption, S-phase entry and fate of neural progenitors. Nat Cell
Biol. 13:402–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nobutani K, Shimono Y, Yoshida M, Mizutani
K, Minami A, Kono S, Mukohara T, Yamasaki T, Itoh T, Takao S, et
al: Absence of primary cilia in cell cycle-arrested human breast
cancer cells. Genes Cells. 19:141–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang N, Leung EL, Liu C, Li L, Eguether T,
Jun Yao XJ, Jones EC, Norris DA, Liu A, Clark RA, et al: INTU is
essential for oncogenic Hh signaling through regulating primary
cilia formation in basal cell carcinoma. Oncogene. 36:4997–5005.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frett B, Brown RV, Ma M, Hu W, Han H and
Li HY: Therapeutic melting pot of never in mitosis gene a related
kinase 2 (Nek2): A perspective on Nek2 as an oncology target and
recent advancements in Nek2 small molecule inhibition. J Med Chem.
57:5835–5844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang PH, Zhang L, Zhang YJ, Zhang J and Xu
WF: HDAC6: Physiological function and its selective inhibitors for
cancer treatment. Drug Discov Ther. 7:233–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tam LW, Wilson NF and Lefebvre PA: A
CDK-related kinase regulates the length and assembly of flagella in
Chlamydomonas. J Cell Biol. 176:819–829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maurya AK, Rogers T and Sengupta P: A CCRK
and a MAK kinase modulate cilia branching and length via regulation
of axonemal microtubule dynamics in caenorhabditis elegans. Curr
Biol. 29:1286–1300.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ko HW, Norman RX, Tran J, Fuller KP,
Fukuda M and Eggenschwiler JT: Broad-minded links cell
cycle-related kinase to cilia assembly and hedgehog signal
transduction. Dev Cell. 18:237–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Snouffer A, Brown D, Lee H, Walsh J, Lupu
F, Norman R, Lechtreck K, Ko HW and Eggenschwiler J: Cell
Cycle-related kinase (CCRK) regulates ciliogenesis and Hedgehog
signaling in mice. PLoS Genet. 13:e10069122017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moser JJ, Fritzler MJ and Rattner JB:
Ultrastructural characterization of primary cilia in pathologically
characterized human glioblastoma multiforme (GBM) tumors. BMC Clin
Pathol. 14:402014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Roine N and Makela TP: CCRK
depletion inhibits glioblastoma cell proliferation in a
cilium-dependent manner. EMBO Rep. 14:741–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hori A, Barnouin K, Snijders AP and Toda
T: A non-canonical function of Plk4 in centriolar satellite
integrity and ciliogenesis through PCM1 phosphorylation. EMBO Rep.
17:326–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shinmura K, Kurabe N, Goto M, Yamada H,
Natsume H, Konno H and Sugimura H: PLK4 overexpression and its
effect on centrosome regulation and chromosome stability in human
gastric cancer. Mol Biol Rep. 41:6635–6644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Coelho PA, Bury L, Shahbazi MN,
Liakath-Ali K, Tate PH, Wormald S, Hindley CJ, Huch M, Archer J,
Skarnes WC, et al: Over-expression of Plk4 induces centrosome
amplification, loss of primary cilia and associated tissue
hyperplasia in the mouse. Open Biol. 5:1502092015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goldstein AL, Hannappel E, Sosne G and
Kleinman HK: Thymosin β4: A multi-functional regenerative peptide.
Basic properties and clinical applications. Expert Opin Biol Ther.
12:37–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Safer D, Elzinga M and Nachmias VT:
Thymosin beta 4 and Fx, an actin-sequestering peptide, are
indistinguishable. J Biol Chem. 266:4029–4032. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cha HJ, Jeong MJ and Kleinman HK: Role of
thymosin beta4 in tumor metastasis and angiogenesis. J Natl Cancer
Inst. 95:1674–1680. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang ZY, Zhang W, Yang JJ, Song DK, Wei JX
and Gao S: Association of thymosin beta4 expression with
clinicopathological parameters and clinical outcomes of bladder
cancer patients. Neoplasma. 63:991–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chi LH, Chang WM, Chang YC, Chan YC, Tai
CC, Leung KW, Chen CL, Wu AT, Lai TC, Li YJ and Hsiao M: Global
Proteomics-based identification and validation of thymosin beta-4
X-Linked as a prognostic marker for head and neck squamous cell
carcinoma. Sci Rep. 7:90312017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee JW, Kim HS and Moon EY: Thymosin
beta-4 is a novel regulator for primary cilium formation by
nephronophthisis 3 in HeLa human cervical cancer cells. Sci Rep.
9:68492019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee JW, Thuy PX, Han HK and Moon EY:
Di-(2-ethylhexyl) phthalate-induced tumor growth is regulated by
primary cilium formation via the axis of H2O2
production-thymosin beta-4 gene expression. Int J Med Sci.
18:1247–1258. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alieva IB, Gorgidze LA, Komarova YA,
Chernobelskaya OA and Vorobjev IA: Experimental model for studying
the primary cilia in tissue culture cells. Membr Cell Biol.
12:895–905. 1999.PubMed/NCBI
|
|
53
|
Kowal TJ and Falk MM: Primary cilia found
on HeLa and other cancer cells. Cell Biol Int. 39:1341–1347. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vorobyeva AG and Saunders AJ: Amyloid-beta
interrupts canonical Sonic hedgehog signaling by distorting primary
cilia structure. Cilia. 7:52018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Garcia-Gonzalo FR, Corbit KC,
Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD,
Robinson JF, Bennett CL, Josifova DJ, et al: A transition zone
complex regulates mammalian ciliogenesis and ciliary membrane
composition. Nat Genet. 43:776–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sang L, Miller JJ, Corbit KC, Giles RH,
Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al:
Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy
disease genes and pathways. Cell. 145:513–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Meng D, Chen Y, Zhao Y, Wang J, Yun D,
Yang S, Chen J, Chen H and Lu D: Expression and prognostic
significance of TCTN1 in human glioblastoma. J Transl Med.
12:2882014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao S, Chen X, Wan M, Jiang X, Li C, Cui
Y and Kang P: Tectonic 1 is a key regulator of cell proliferation
in pancreatic cancer. Cancer Biother Radiopharm. 31:7–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cano-Rodriguez D, Campagnoli S, Grandi A,
Parri M, Camilli E, Song C, Jin B, Lacombe A, Pierleoni A, Bombaci
M, et al: TCTN2: A novel tumor marker with oncogenic properties.
Oncotarget. 8:95256–95269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yasar B, Linton K, Slater C and Byers R:
Primary cilia are increased in number and demonstrate structural
abnormalities in human cancer. J Clin Pathol. 70:571–574. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rocha C, Papon L, Cacheux W, Marques Sousa
P, Lascano V, Tort O, Giordano T, Vacher S, Lemmers B, Mariani P,
et al: Tubulin glycylases are required for primary cilia, control
of cell proliferation and tumor development in colon. EMBO J.
33:2247–2260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Z, Tseng CP, Pong RC, Chen H,
McConnell JD, Navone N and Hsieh JT: The mechanism of
growth-inhibitory effect of DOC-2/DAB2 in prostate cancer.
Characterization of a novel GTPase-activating protein associated
with N-terminal domain of DOC-2/DAB2. J Biol Chem. 277:12622–12631.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shen YJ, Kong ZL, Wan FN, Wang HK, Bian
XJ, Gan HL, Wang CF and Ye DW: Downregulation of DAB2IP results in
cell proliferation and invasion and contributes to unfavorable
outcomes in bladder cancer. Cancer Sci. 105:704–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang ZR, Wei JH, Zhou JC, Haddad A, Zhao
LY, Kapur P, Wu KJ, Wang B, Yu YH, Liao B, et al: Validation of
DAB2IP methylation and its relative significance in predicting
outcome in renal cell carcinoma. Oncotarget. 7:31508–31519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin CJ, Dang A, Hernandez E and Hsieh JT:
DAB2IP modulates primary cilia formation associated with renal
tumorigenesis. Neoplasia. 23:169–180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Schneider L, Essmann F, Kletke A, Rio P,
Hanenberg H, Wetzel W, Schulze-Osthoff K, Nürnberg B and Piekorz
RP: The transforming acidic coiled coil 3 protein is essential for
spindle-dependent chromosome alignment and mitotic survival. J Biol
Chem. 282:29273–29283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Campo L and Breuer EK: Inhibition of TACC3
by a small molecule inhibitor in breast cancer. Biochem Biophys Res
Commun. 498:1085–1092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jiang F, Kuang B, Que Y, Lin Z, Yuan L,
Xiao W, Peng R and Zhang X and Zhang X: The clinical significance
of transforming acidic coiled-coil protein 3 expression in
non-small cell lung cancer. Oncol Rep. 35:436–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Qie Y, Wang L, Du E, Chen S, Lu C, Ding N,
Yang K and Xu Y: TACC3 promotes prostate cancer cell proliferation
and restrains primary cilium formation. Exp Cell Res.
390:1119522020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Adams M, Simms RJ, Abdelhamed Z, Dawe HR,
Szymanska K, Logan CV, Wheway G, Pitt E, Gull K, Knowles MA, et al:
A meckelin-filamin A interaction mediates ciliogenesis. Hum Mol
Genet. 21:1272–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Goepfert TM, Adigun YE, Zhong L, Gay J,
Medina D and Brinkley WR: Centrosome amplification and
overexpression of aurora A are early events in rat mammary
carcinogenesis. Cancer Res. 62:4115–4122. 2002.PubMed/NCBI
|
|
72
|
Gritsko TM, Coppola D, Paciga JE, Yang L,
Sun M, Shelley SA, Fiorica JV, Nicosia SV and Cheng JQ: Activation
and overexpression of centrosome kinase BTAK/Aurora-A in human
ovarian cancer. Clin Cancer Res. 9:1420–1426. 2003.PubMed/NCBI
|
|
73
|
Kobayashi T, Nakazono K, Tokuda M, Mashima
Y, Dynlacht BD and Itoh H: HDAC2 promotes loss of primary cilia in
pancreatic ductal adenocarcinoma. EMBO Rep. 18:334–343. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dere R, Perkins AL, Bawa-Khalfe T, Jonasch
D and Walker CL: β-catenin links von Hippel-Lindau to aurora kinase
A and loss of primary cilia in renal cell carcinoma. J Am Soc
Nephrol. 26:553–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Egeberg DL, Lethan M, Manguso R, Schneider
L, Awan A, Jørgensen TS, Byskov AG, Pedersen LB and Christensen ST:
Primary cilia and aberrant cell signaling in epithelial ovarian
cancer. Cilia. 1:152012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
O'Toole SM, Watson DS, Novoselova TV,
Romano LEL, King PJ, Bradshaw TY, Thompson CL, Knight MM, Sharp TV,
Barnes MR, et al: Oncometabolite induced primary cilia loss in
pheochromocytoma. Endocr Relat Cancer. 26:165–180. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen Q, Li J, Yang X, Ma J, Gong F and Liu
Y: Prdx1 promotes the loss of primary cilia in esophageal squamous
cell carcinoma. BMC Cancer. 20:3722020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xu J, Li H, Wang B, Xu Y, Yang J, Zhang X,
Harten SK, Shukla D, Maxwell PH, Pei D and Esteban MA: VHL
inactivation induces HEF1 and Aurora kinase A. J Am Soc Nephrol.
21:2041–2046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Plotnikova OV, Seo S, Cottle DL, Conduit
S, Hakim S, Dyson JM, Mitchell CA and Smyth IM: INPP5E interacts
with AURKA, linking phosphoinositide signaling to primary cilium
stability. J Cell Sci. 128:364–372. 2015.PubMed/NCBI
|
|
80
|
Conduit SE, Ramaswamy V, Remke M, Watkins
DN, Wainwright BJ, Taylor MD, Mitchell CA and Dyson JM: A
compartmentalized phosphoinositide signaling axis at cilia is
regulated by INPP5E to maintain cilia and promote Sonic Hedgehog
medulloblastoma. Oncogene. 36:5969–5984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gradilone SA, Radtke BN, Bogert PS, Huang
BQ, Gajdos GB and LaRusso NF: HDAC6 inhibition restores ciliary
expression and decreases tumor growth. Cancer Res. 73:2259–2270.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Peixoto E, Jin S, Thelen K, Biswas A,
Richard S, Morleo M, Mansini A, Holtorf S, Carbone F, Pastore N, et
al: HDAC6-dependent ciliophagy is involved in ciliary loss and
cholangiocarcinoma growth in human cells and murine models. Am J
Physiol Gastrointest Liver Physiol. 318:G1022–G1033. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kim S, Lee K, Choi JH, Ringstad N and
Dynlacht BD: Nek2 activation of Kif24 ensures cilium disassembly
during the cell cycle. Nat Commun. 6:80872015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cappello P, Blaser H, Gorrini C, Lin DC,
Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, et
al: Role of Nek2 on centrosome duplication and aneuploidy in breast
cancer cells. Oncogene. 33:2375–2384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou W, Yang Y, Xia J, Wang H, Salama ME,
Xiong W, Xu H, Shetty S, Chen T, Zeng Z, et al: NEK2 induces drug
resistance mainly through activation of efflux drug pumps and is
associated with poor prognosis in myeloma and other cancers. Cancer
Cell. 23:48–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hayward DG, Clarke RB, Faragher AJ, Pillai
MR, Hagan IM and Fry AM: The centrosomal kinase Nek2 displays
elevated levels of protein expression in human breast cancer.
Cancer Res. 64:7370–7376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hassounah NB, Nunez M, Fordyce C, Roe D,
Nagle R, Bunch T and McDermott KM: Inhibition of ciliogenesis
promotes hedgehog signaling, tumorigenesis, and metastasis in
breast cancer. Mol Cancer Res. 15:1421–1430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rogowski K, Juge F, van Dijk J, Wloga D,
Strub JM, Levilliers N, Thomas D, Bré MH, Van Dorsselaer A, Gaertig
J and Janke C: Evolutionary divergence of enzymatic mechanisms for
posttranslational polyglycylation. Cell. 137:1076–1087. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bosch Grau M, Masson C, Gadadhar S, Rocha
C, Tort O, Marques Sousa P, Vacher S, Bieche I and Janke C:
Alterations in the balance of tubulin glycylation and glutamylation
in photoreceptors leads to retinal degeneration. J Cell Sci.
130:938–949. 2017.PubMed/NCBI
|
|
90
|
Pathak N, Austin CA and Drummond IA:
Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain
zebrafish cilia structure and motility. J Biol Chem.
286:11685–11695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Curatolo P, Bombardieri R and Jozwiak S:
Tuberous sclerosis. Lancet. 372:657–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jozwiak J: Hamartin and tuberin: Working
together for tumour suppression. Int J Cancer. 118:1–5. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hartman TR, Liu D, Zilfou JT, Robb V,
Morrison T, Watnick T and Henske EP: The tuberous sclerosis
proteins regulate formation of the primary cilium via a
rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol
Genet. 18:151–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wilson C, Bonnet C, Guy C, Idziaszczyk S,
Colley J, Humphreys V, Maynard J, Sampson JR and Cheadle JP: Tsc1
haploinsufficiency without mammalian target of rapamycin activation
is sufficient for renal cyst formation in Tsc1+/- mice. Cancer Res.
66:7934–7938. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
DiBella LM, Park A and Sun Z: Zebrafish
Tsc1 reveals functional interactions between the cilium and the TOR
pathway. Hum Mol Genet. 18:595–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Armour EA, Carson RP and Ess KC:
Cystogenesis and elongated primary cilia in Tsc1-deficient distal
convoluted tubules. Am J Physiol Renal Physiol. 303:F584–F592.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rosengren T, Larsen LJ, Pedersen LB,
Christensen ST and Moller LB: TSC1 and TSC2 regulate cilia length
and canonical Hedgehog signaling via different mechanisms. Cell Mol
Life Sci. 75:2663–2680. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sherpa RT, Atkinson KF, Ferreira VP and
Nauli SM: Rapamycin increases length and mechanosensory function of
primary cilia in renal epithelial and vascular endothelial cells.
Int Educ Res J. 2:91–97. 2016.PubMed/NCBI
|
|
99
|
Takahashi K, Nagai T, Chiba S, Nakayama K
and Mizuno K: Glucose deprivation induces primary cilium formation
through mTORC1 inactivation. J Cell Sci.
131:jcs2087692018.PubMed/NCBI
|
|
100
|
Huber TB, Walz G and Kuehn EW: mTOR and
rapamycin in the kidney: Signaling and therapeutic implications
beyond immunosuppression. Kidney Int. 79:502–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shillingford JM, Murcia NS, Larson CH, Low
SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA,
Kramer-Zucker A, et al: The mTOR pathway is regulated by
polycystin-1, and its inhibition reverses renal cystogenesis in
polycystic kidney disease. Proc Natl Acad Sci USA. 103:5466–5471.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang T and George DJ: Immunotherapy and
targeted-therapy combinations mark a new era of kidney cancer
treatment. Nat Med. 27:586–588. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fan Y, Sun T, Shao Z, Zhang Q, Ouyang Q,
Tong Z, Wang S, Luo Y, Teng Y, Wang X, et al: Effectiveness of
adding everolimus to the First-line treatment of advanced breast
cancer in premenopausal women who experienced disease progression
while receiving selective estrogen receptor modulators: A phase 2
randomized clinical trial. JAMA Oncol. 7:e2134282021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ferrante MI, Giorgio G, Feather SA,
Bulfone A, Wright V, Ghiani M, Selicorni A, Gammaro L, Scolari F,
Woolf AS, et al: Identification of the gene for oral-facial-digital
type I syndrome. Am J Hum Genet. 68:569–576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang J, Chen X, Wang F, Zhang J, Li P, Li
Z, Xu J, Gao F, Jin C, Tian H, et al: OFD1, as a ciliary protein,
exhibits neuroprotective function in photoreceptor degeneration
models. PLoS One. 11:e01558602016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Singla V, Romaguera-Ros M, Garcia-Verdugo
JM and Reiter JF: Ofd1, a human disease gene, regulates the length
and distal structure of centrioles. Dev Cell. 18:410–424. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lopes CA, Prosser SL, Romio L, Hirst RA,
O'Callaghan C, Woolf AS and Fry AM: Centriolar satellites are
assembly points for proteins implicated in human ciliopathies,
including oral-facial-digital syndrome 1. J Cell Sci. 124:600–612.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tang Z, Lin MG, Stowe TR, Chen S, Zhu M,
Stearns T, Franco B and Zhong Q: Autophagy promotes primary
ciliogenesis by removing OFD1 from centriolar satellites. Nature.
502:254–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lee J, Yi S, Kang YE, Chang JY, Kim JT,
Sul HJ, Kim JO, Kim JM, Kim J, Porcelli AM, et al: Defective
ciliogenesis in thyroid hurthle cell tumors is associated with
increased autophagy. Oncotarget. 7:79117–79130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Pampliega O, Orhon I, Patel B, Sridhar S,
Díaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P and Cuervo
AM: Functional interaction between autophagy and ciliogenesis.
Nature. 502:194–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Maharjan Y, Lee JN, Kwak S, Lim H, Dutta
RK, Liu ZQ, So HS and Park R: Autophagy alteration prevents primary
cilium disassembly in RPE1 cells. Biochem Biophys Res Commun.
500:242–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ko JY, Lee EJ and Park JH: Interplay
between primary cilia and autophagy and its controversial roles in
cancer. Biomol Ther (Seoul). 27:337–341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: The central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Maher ER and Kaelin WG Jr: von
Hippel-Lindau disease. Medicine (Baltimore). 76:381–391. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Arjumand W and Sultana S: Role of VHL gene
mutation in human renal cell carcinoma. Tumour Biol. 33:9–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Schermer B, Ghenoiu C, Bartram M, Müller
RU, Kotsis F, Höhne M, Kühn W, Rapka M, Nitschke R, Zentgraf H, et
al: The von Hippel-Lindau tumor suppressor protein controls
ciliogenesis by orienting microtubule growth. J Cell Biol.
175:547–554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Noonan HR, Metelo AM, Kamei CN, Peterson
RT, Drummond IA and Iliopoulos O: Loss of vhl in the zebrafish
pronephros recapitulates early stages of human clear cell renal
cell carcinoma. Dis Model Mech. 9:873–884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Frew IJ and Moch H: A clearer view of the
molecular complexity of clear cell renal cell carcinoma. Annu Rev
Pathol. 10:263–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Albers J, Rajski M, Schonenberger D,
Harlander S, Schraml P, von Teichman A, Georgiev S, Wild PJ, Moch
H, Krek W and Frew IJ: Combined mutation of Vhl and Trp53 causes
renal cysts and tumours in mice. EMBO Mol Med. 5:949–964. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Frew IJ, Thoma CR, Georgiev S, Minola A,
Hitz M, Montani M, Moch H and Krek W: pVHL and PTEN tumour
suppressor proteins cooperatively suppress kidney cyst formation.
EMBO J. 27:1747–1757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Harlander S, Schonenberger D, Toussaint
NC, Prummer M, Catalano A, Brandt L, Moch H, Wild PJ and Frew IJ:
Combined mutation in Vhl, Trp53 and Rb1 causes clear cell renal
cell carcinoma in mice. Nat Med. 23:869–877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Esteban MA, Harten SK, Tran MG and Maxwell
PH: Formation of primary cilia in the renal epithelium is regulated
by the von Hippel-Lindau tumor suppressor protein. J Am Soc
Nephrol. 17:1801–1806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ding XF, Zhou J, Hu QY, Liu SC and Chen G:
The tumor suppressor pVHL down-regulates never-in-mitosis A-related
kinase 8 via hypoxia-inducible factors to maintain cilia in human
renal cancer cells. J Biol Chem. 290:1389–1394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Oswald F, Kostezka U, Astrahantseff K,
Bourteele S, Dillinger K, Zechner U, Ludwig L, Wilda M, Hameister
H, Knöchel W, et al: SHARP is a novel component of the
Notch/RBP-Jkappa signalling pathway. EMBO J. 21:5417–5426. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ariyoshi M and Schwabe JW: A conserved
structural motif reveals the essential transcriptional repression
function of Spen proteins and their role in developmental
signaling. Genes Dev. 17:1909–1920. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Legare S, Cavallone L, Mamo A, Chabot C,
Sirois I, Magliocco A, Klimowicz A, Tonin PN, Buchanan M, Keilty D,
et al: The estrogen receptor cofactor SPEN functions as a tumor
suppressor and candidate biomarker of drug responsiveness in
hormone-dependent breast cancers. Cancer Res. 75:4351–4363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Legare S, Chabot C and Basik M: SPEN, a
new player in primary cilia formation and cell migration in breast
cancer. Breast Cancer Res. 19:1042017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Margueron R and Reinberg D: The Polycomb
complex PRC2 and its mark in life. Nature. 469:343–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang X, Brea LT and Yu J: Immune
modulatory functions of EZH2 in the tumor microenvironment:
Implications in cancer immunotherapy. Am J Clin Exp Urol. 7:85–91.
2019.PubMed/NCBI
|
|
130
|
Zingg D, Debbache J, Peña-Hernández R,
Antunes AT, Schaefer SM, Cheng PF, Zimmerli D, Haeusel J, Calçada
RR, Tuncer E, et al: EZH2-mediated primary cilium deconstruction
drives metastatic melanoma formation. Cancer Cell. 34:69–84.e14.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Denhez F, Wilcox-Adelman SA, Baciu PC,
Saoncella S, Lee S, French B, Neveu W and Goetinck PF: Syndesmos, a
syndecan-4 cytoplasmic domain interactor, binds to the focal
adhesion adaptor proteins paxillin and Hic-5. J Biol Chem.
277:12270–12274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Avolio R, Jarvelin AI, Mohammed S,
Agliarulo I, Condelli V, Zoppoli P, Calice G, Sarnataro D, Bechara
E, Tartaglia GG, et al: Protein syndesmos is a novel RNA-binding
protein that regulates primary cilia formation. Nucleic Acids Res.
46:12067–12086. 2018.PubMed/NCBI
|
|
133
|
Haigis KM: KRAS alleles: The devil is in
the detail. Trends Cancer. 3:686–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kempf E, Rousseau B, Besse B and Paz-Ares
L: KRAS oncogene in lung cancer: Focus on molecularly driven
clinical trials. Eur Respir Rev. 25:71–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Pupo E, Avanzato D, Middonti E, Bussolino
F and Lanzetti L: KRAS-driven metabolic rewiring reveals novel
actionable targets in cancer. Front Oncol. 9:8482019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Eser S, Schnieke A, Schneider G and Saur
D: Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer.
111:817–822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Raleigh DR, Choksi PK, Krup AL, Mayer W,
Santos N and Reiter JF: Hedgehog signaling drives medulloblastoma
growth via CDK6. J Clin Invest. 128:120–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Farooqi AA, de la Roche M, Djamgoz MBA and
Siddik ZH: Overview of the oncogenic signaling pathways in
colorectal cancer: Mechanistic insights. Semin Cancer Biol.
58:65–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Palicelli A, Croci S, Bisagni A, Zanetti
E, De Biase D, Melli B, Sanguedolce F, Ragazzi M, Zanelli M, Chaux
A, et al: What do we have to know about PD-L1 expression in
prostate cancer? a systematic literature review. Part 3: PD-L1,
intracellular signaling pathways and tumor microenvironment. Int J
Mol Sci. 22:123302021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Eguether T, Cordelieres FP and Pazour GJ:
Intraflagellar transport is deeply integrated in hedgehog
signaling. Mol Biol Cell. 29:1178–1189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Deng YZ, Cai Z, Shi S, Jiang H, Shang YR,
Ma N, Wang JJ, Guan DX, Chen TW, Rong YF, et al: Cilia loss
sensitizes cells to transformation by activating the mevalonate
pathway. J Exp Med. 215:177–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Jenks AD, Vyse S, Wong JP, Kostaras E,
Keller D, Burgoyne T, Shoemark A, Tsalikis A, de la Roche M,
Michaelis M, et al: Primary cilia mediate diverse kinase inhibitor
resistance mechanisms in cancer. Cell Rep. 23:3042–3055. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Khan NA, Willemarck N, Talebi A, Marchand
A, Binda MM, Dehairs J, Rueda-Rincon N, Daniels VW, Bagadi M,
Thimiri Govinda Raj DB, et al: Identification of drugs that restore
primary cilium expression in cancer cells. Oncotarget. 7:9975–9992.
2016. View Article : Google Scholar : PubMed/NCBI
|