Introduction
Glioblastoma multiforme (GBM) is the most invasive
and malignant tumor of the central nervous system (CNS) with a
median survival rate of <14.6 months (1,2).
Currently, in clinical practice, the common treatment strategy for
patients with GBM is combined neurosurgical resection with
chemotherapy or radiotherapy; however, the recurrence rate of GBM
in patients remains very high. With the development of
molecular-targeted therapies, there have been numerous pre-clinical
studies on novel targeted drugs (3-5);
however, an appropriate drug carrier has not yet been discovered in
order to solve certain key issues, particularly the restrictive
ability of the blood-brain-barrier (BBB) and drug maldistribution
(4,5).
Neural stem cells (NSCs) are CNS stem cells and
reside in two major neurogenic niches of the brain: the
subventricular and subgranular zone. They have self-renewal
properties and multipotential differentiation abilities (they can
differentiate into neurons, astrocytes and oligodendrocytes)
(6). Previous studies have
demonstrated that NSCs can migrate to brain lesions, including
tumors, which is closely associated with the cell paracrine
mechanism (7-10). Exosomes (EXOs) secreted by cells
are the main functional paracrine molecules (11,12).
EXOs have a lipid bilayer structure with a diameter of 30-200 nm.
They contain proteins, lipids, mRNAs and microRNAs (miRNAs/miRs),
and are critically involve in intercellular communications through
the transfer of exosomal cargo from the source cells to targeted
cells. Moreover, EXOs have greater advantages, including the
presence of active molecules from source cells, the ability to
easily cross the BBB, immune privilege, the ability to deliver
drugs and they can be used in targeted therapy (11-13).
In addition, when EXOs are taken up by cells, such as tumor cells,
they regulate the immune response, tumor occurrence and metastasis
by transferring exosomal cargos (14-16).
Of note, EXOs can be modified to carry small molecular drugs,
including miRNAs, for individual-based targeted therapy.
miRNAs, as small non-coding RNAs, modulate gene
expression at the post-transcription level by binding to ≥1 gene in
their specific 3'-untranslated region (UTR). Furthermore, miRNAs
are crucially involved in the occurrence and development of GBM
(17). Previous studies, including
a previous study by the authors, have demonstrated that
miR-124-3p overexpression significantly inhibits GBM cell
proliferation and migration, as well as angiogenesis and enhances
glioma chemosensitivity (18-20).
The small molecule miR-124-3p can be considered as a tumor
suppressor gene in gliomas.
Thus, the present study aimed to use natural
biological carrier EXOs derived from NSCs to load miR-124-3p as a
molecular drug for GBM treatment, as well as to further explore the
potential mechanisms underlying the regulation of GBM progression
by EXOs carrying miR-124-3p.
Materials and methods
Glioma cells and NSC culture
The U87MG (glioblastoma of unknown origin) and
U251MG (glioblastoma) cell lines were preserved in the authors'
laboratory. U87MG cells were matched with the ATCC database by
short tandem repeat (STR) analysis. These cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS,
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
The medium was changed at 2-day intervals and the cells were
passaged on the 4th or 5th day through trypsinization (Trypsin;
Gibco; Thermo Fisher Scientific, Inc.).
NSCs were generously provided by Professor Lukui
Chen (Southern Medical University, Guangzhou, China); moreover,
details regarding the cells have been previously described
(21,22). NSCs were maintained in serum-free
DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
2% B27 (Gibco; Thermo Fisher Scientific, Inc.), 20 ng/ml basic
fibroblast growth factor (PeproTech, Inc.), 20 ng/ml epidermal
growth factor (PeproTech, Inc.) and 1% penicillin-streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). The medium was changed at
3-day intervals and the cells were passaged on the 6th or 7th day
with dissociation using Accutase® dissociation reagent
(MilliporeSigma).
EXO isolation and characterization
EXOs were isolated from NSC culture supernatants
using the Exo-spin™ Exosome Isolation and Purification kit (Cell
Guidance Systems) as per the manufacturer's instructions. Briefly,
conditioned medium (CM) was collected, centrifuged at 2,000 × g,
4°C for 10 min and filtered to remove cell debris using a filter
unit (MilliporeSigma) with a 0.22-µm membrane. As regards
EXO isolation and purification, 1/2 volume of Exo-spin™ Buffer was
added and mixed overnight, followed by centrifugation at 16,000 ×
g, 4°C for 1 h. EXO pellets were resuspended in 100 µl cold
PBS. The purified EXOs were then collected using the Exo-spin™
column through careful centrifugation at 50 × g, 4°C for 1 min.
For transmission electron microscopy (TEM), a drop
of EXOs was placed on a carbon-coated copper grid followed by the
addition of a drop of 2% phosphotungstic acid to the stain for 5
min at room temperature. Subsequently, it was allowed to dry with a
final examination using TEM (H-7650, Hitachi, Inc.). For
nanoparticle tracking analysis (NTA), EXOs were added to the
NanoSight instrument (Zetaview®, Particle Metrix) to
measure the nanoparticle size. The EXOs were then separated and
examined using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (10% SDS-PAGE), followed by immunoreaction with
primary antibodies, anti-Alix (1:1,000, cat. no. 12422-1-AP),
anti-Hsp90 (1:1,000, cat. no. 13171-1-AP), anti-Tsg101 (1:1,000,
cat. no. 28283-1-AP), anti-GM130 (1:1,000, cat. no. 11308-1-AP)
(all from ProteinTech Group, Inc.) at 4°C for overnight. Following
incubation with horseradish peroxidase-conjugated secondary
antibodies (goat anti-rabbit, 1:10,000, cat. no. 111-035-003; goat
anti-mouse, 1:10,000, cat. no. 115-035-003; both from Jackson
ImmunoResearch Laboratories, Inc.) at room temperature for 1 h, the
proteins were reacted with enhanced chemiluminescence reagent (ECL,
Pierce; Thermo Fisher Scientific, Inc.).
PKH67-labeled EXOs and their
endocytosis
Briefly, regarding PKH67 fluorescently-labeled EXOs,
the EXOs were initially mixed with Diluent C (MIDI67,
MilliporeSigma). Subsequently, PKH67-Diluent C dye solution was
rapidly added, mixed and incubated at room temperature for 15 min,
with subsequent termination using 0.5% BSA, which was followed by
centrifugation at 12,000 × g, 4°C for 30 min and re-extraction as
per the manufacturer's instructions. In vitro, PKH67-labeled
EXOs were added to the U87MG and U251MG cells for 12 h.
Subsequently, they were stained using DAPI (DA0001, Leagene, Inc.)
at room temperature for 5 min and observed under a fluorescence
microscope (IX73, Olympus Corporation).
Loading of EXOs with miRNA-124-3p via
electroporation
EXOs were loaded with miR-124-3p through
electroporation using a Gene Pulser Xcell (Bio-Rad Laboratories,
Inc.) electroporation system. First, miR-124-3p mimics (Guangzhou
RiboBio Co., Ltd.) were mixed with EXOs in an electroporation
buffer at a final concentration of 200 nM. The mixtures were then
cultured at 4°C for 10 min and transferred to ice-cold 0.2-cm
cuvettes for electroporation at 400 V and 125 µF capacitance
with a single pulse. Subsequently, the EXOs were maintained on ice
for ≥15 min following electroporation. Negative control (NC) mimics
(Guangzhou RiboBio Co., Ltd.) mixed with EXOs were electroporated
to serve as a control.
Immunofluorescence
The NSCs (including neurospheres and monoplasts) and
their differentiated cells (neurons, astrocytes and
oligodendrocytes) (cell differentiation was induced using
differentiation medium as follows: DMEM/F12 supplemented with 1%
B27, 2% FBS and 1% penicillin-streptomycin, cultured for 7-10 days)
were washed and fixed using 4% paraformaldehyde at room temperature
for 30 min. After washing and fixation, the cells were incubated
with primary rabbit anti-Nestin (1:200, cat. no. ab221660, Abcam),
anti-SOX2 (1:200, cat. no. ab92494, Abcam), anti-βIII τubulin
(Tuj1, 1:200, cat. no. ab18207, Abcam), anti-glial fibrillary
acidic protein (GFAP; 1:200, cat. no. GB11096, Wuhan Servicebio
Technology Co., Ltd.), anti-myelin oligodendrocyte glycoprotein
(MOG; 1:200, cat. no. 12690-1-AP, ProteinTech Group, Inc.) and
mouse anti-Musashi1 (1:200, cat. no. ab129819, Abcam), antibodies
at 4°C overnight. Following incubation with secondary antibodies
[goat anti-rabbit 488 (1:600, cat. no. 4412, Cell Signaling
Technology, Inc.), anti-rabbit 555 (1:600, cat. no. 4413, Cell
Signaling Technology, Inc.) and goat anti-mouse 555 (1:600, cat.
no. 4409, Cell Signaling Technology, Inc.) at room temperature for
1 h, the slides were mounted with DAPI (Beyotime Institute of
Biotechnology), followed by examination under an inverted
fluorescence microscope (IX73, Olympus Corporation) with light
incubation being avoided.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from all samples (glioma
cells) using the Total RNA kit (Omega Bio-Tek, Inc.) as per the
manufacturer's instructions. cDNA synthesis was performed using the
Transcriptor First Strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.) and qPCR was performed using the ABI
Detection System with FastStart Universal SYBR-Green Master (Roche
Diagnostics) following the manufacturer's instructions. The primers
used were as follows: miR-124-3p forward, 5′-ACA CTC CAG CTG
GGT AAG GCA CGC GGT GAA-3′ and reverse, 5′-CTC AAC TGG TGT CGT GGA
GTC GGC AAT TCA GTT GAG TTG GCA TT-3′; flotillin 2 (FLOT2)
forward, 5′-TTG CTG ACT CTA AGC GAG CC-3′ and reverse, 5′-TCC ACG
GCA ATC TGT TTC TTG-3′; U6 forward, 5′-CTC GCT TCG GCA GCA
CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′; GAPDH
forward, 5′-GTC TCC TCT GAC TTC AAC AGC G-3′ and reverse, 5′-ACC
ACC CTG TTG CTG TAG CCA A-3′; ANXA5 forward, AAC CCT CTC GGC
TTT ATG ATG C-3′ and reverse, 5′-CGC TGG TAG TAC CCT GAA GTG-3′;
SUB1 forward, 5′-GGT GAG ACT TCG AGA GCC CT-3′ and reverse,
5′-GCG AAC ACT AAC GTA CCT CAT TT-3′; MYH9 forward, 5′-CCT
CAA GGA GCG TTA CTA CTC A-3′ and reverse, 5′-CTG TAG GCG GTG TCT
GTG AT; RHOG forward, 5′-ACT AAC GCT TTC CCC AAA GAG and
reverse, 5′-GTG TAC GGA GGC GGT CAT AC-3′; and SMAD forward,
5′-CCA GCA GTA AAG CGA TTG TTG G-3′ and reverse, 5′-GGG GTA AGC CTT
TTC TGT GAG-3′. RT-qPCR was performed under the following
conditions: 95°C for 5 sec, followed by 45 cycles at 95°C for 10
sec, 60°C for 20 sec, and 72°C for 20 sec. At least three
biological replicates were used. The results were then analyzed
using the 2−ΔΔCq method (23), ΔCq=Cq (gene)-Cq (U6/GAPDH), and the
2−ΔΔCq indicated the difference in mRNA.
Western blot analysis
To analyze the protein levels, protein (glioma cells
and tissues) was isolated using the Minute™ Total Protein
Extraction kit (SD-001, Invent Biotechnologies), and the
quantification of protein was performed using the Pierce BCA
Protein Assay kit (cat. no. 23223, Thermo Fisher Scientific, Inc.).
Equal amounts of total proteins (20-60 µg) from each cell
group were subjected to SDS-PAGE (10%) and transferred onto
polyvinylidene difluoride membranes (MilliporeSigma). The membranes
were then blocked using 5% milk at room temperature for 1 h and
probed with primary rabbit anti-FLOT2 (1:1,000, cat. no.
66881-1-Ig.), anti-PI3K (1:1,000, cat. no. bs-0128R, BIOSS),
anti-AKT1 (1:1,000, cat. no. bs-0115R, BIOSS), anti-phosphorylated
(p-)AKT1 (1:1,000, cat. no. bs-10996R, BIOSS), anti-GAPDH (1:1,000,
cat. no. 10494-1-AP, ProteinTech Group, Inc.) and anti-β-actin
antibodies (1:1,000, cat. no. bs-0061R, BIOSS) at 4°C for
overnight. Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit, 1:10,000, cat. no. 111-035-003; goat anti-mouse,
1:10,000, cat. no. 115-035-003; both from Jackson ImmunoResearch
Laboratories, Inc.); subsequently, they were reacted with an
enhanced ECL substrate (32106, Pierce; Thermo Fisher Scientific,
Inc.), imaged using a Chemiluminescence imager (ChemiScope 6300,
Clinx Science Instruments Co., Ltd.), and analyzed using ImageJ
software (version 1.50i; National Institutes of Health).
Plasmid construction and dual-luciferase
reporter assay
The target sequences of the FLOT2 wild-type (WT)
3'-UTR containing the predicted miR-124-3p binding sites and its
mutant (MUT) 3'-UTR were synthesized. The fragments were sub-cloned
into the pmirGLO dual-luciferase reporter vector (Promega
Corporation) as per the manufacturer's instructions. For the
luciferase reporter assay, glioma cells were cultured in 24-well
plates and transfected with a complex of 50 nM miR-124-3p or NC
mimics (Ribobio, Inc.) and the WT or MUT luciferase reporter
vectors (OBiO Technology Corp., Ltd.) at 37°C for 48 h.
Normalization was performed using the Renilla luciferase
construct. Following 48 h of incubation at 37°C, the luciferase
activity was measured using a dual-luciferase reporter system
(E1960, Promega Corporation).
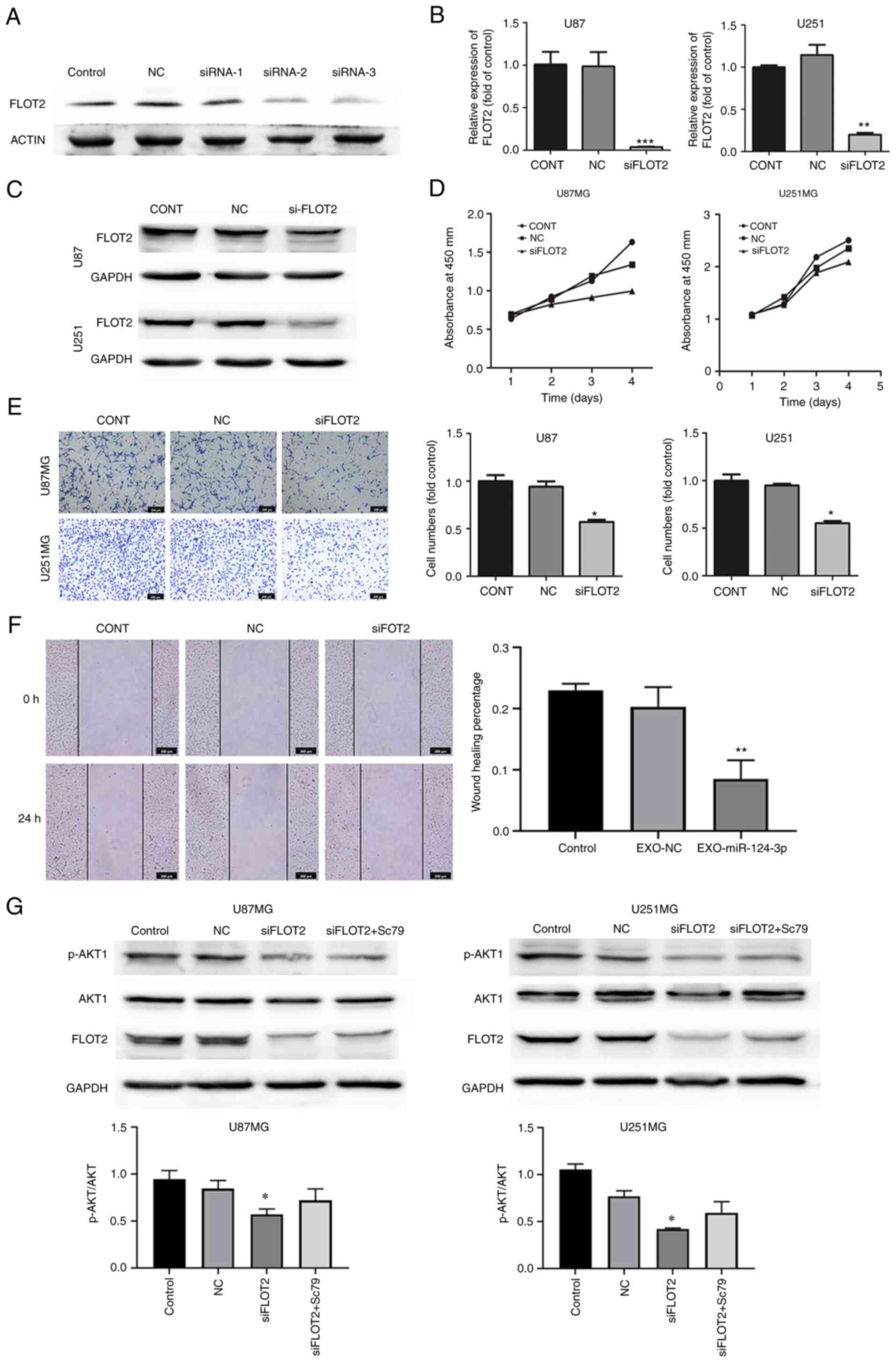
RNA interference and transfection
Duplex siRNA targeting human FLOT2 were purchased
from GengPharma. Co., Ltd. Following a 24-h incubation at 37°C with
an antibiotic-free medium, the glioma cells were transfected with
anti-FLOT2 small interfering RNA (siFLOT2, 20 µM) using
Lipofectamine 2000® Transfection Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 3 days prior to the
start of the subsequent experiments. The FLOT2-siRNA sequences were
as follows: siRNA-1 sense, 5′-GCU GUU GUG GUU CCG ACU A-3′ and
antisense, 3′-CGA CAA CAC CAA GGC UGA U-5′; siRNA-2 sense, 5′-GCA
GUU UCU GGG UAA GAA U-3′ and antisense, 3′-CGU CAA AGA CCC AUU CUU
A-5′; siRNA-3 sense, 5′-CCA AGA UUG CUG ACU CUA A-3′ and antisense,
3′-GGU UCU AAC GAC UGA GAU U-5′. For the experiment using the AKT1
agonist, Sc79 (S7863, Selleck Chemicals LLC.), Sc79 (10
µg/ml) was used to treat the cells for 3 days following
transfection with siRNA FLOT2; total protein was then extracted for
western blot analysis.
Cell Counting Kit-8 (CCK-8) assay for
cell viability assessment
The glioma cells were seeded in 96-well plates for
cell viability analysis using the CCK-8 assay. After 24 h, EXOs or
small molecules were added to the co-culture for 1-4 days;
subsequently, 10 µl CCK-8 (Nanjing KeyGen Biotech Co., Ltd.)
was added into each pore. After 2-4 h of incubation at 37°C, the
absorbance value (OD 450 nm) of each pore was colorimetrically
determined (Multiskan MK3, Thermo Fisher Scientific, Inc.).
Transwell invasion assays
Briefly, as regards the Transwell invasion assay,
the U87MG and U251MG cells were added into 24-well plates with an
8-mm-pore polycarbonate membrane coated with 20 mg of Matrigel (BD
Biosciences). The 2×104 cells were cultured in the upper
chamber in serum-free medium; additionally, medium containing 10%
FBS was added to the lower chamber. Following a 24-h incubation at
room temperature, the invasive cells were fixed using 4%
paraformaldehyde, stained using 0.1% crystal violet (Beyotime
Institute of Biotechnology) at room temperature for 30 min, and
photographed using an Inverted Microscope (IX73, Olympus
Corporation). Three independent experiments were performed.
Wound healing assays
Briefly, for the wound healing assays, the U87MG and
U251MG cells were seeded in complete medium of 6-well plates until
at least 80% confluency was reached. Subsequently, a wound was
created through scraper manual scraping. After removing the
floating cells by washing twice using serum-free medium, the cells
were incubated at room temperature for 24 h. Migrated cells were
observed using an inverted microscope (IX73, Olympus Corporation).
The values obtained were expressed as the cell migration
percentage.
Glioma xenograft model
A total of 12 4-week-old BALB/c male nude mice were
purchased from the Guangdong Animal Center. The present study was
reviewed and approved (permit no. A2020-015) by the Institutional
Animal Care and Use Committee of The Second Affiliated Hospital of
Guangzhou Medical University, and was performed according to the
guidelines of the Committee on Animal Research and Ethics. All mice
were kept in an aseptic environment with a constant humidity
(40-60%) and temperature (24±2°C) with a 12-h light/dark cycle. The
animals were also provided with free access to food and water, and
the padding was replaced twice a week. To establish the glioma
model, U87MG cells (1×107 cells in 100 µl PBS for
each mouse) were subcutaneously injected into the right axilla of
nude mice for 10 days; subsequently, EXOs loaded with miR-124-3p
(concentration 4×108 particles in 20 µl PBS/each
time; untreated cells with 20 µl PBS were used as a control,
twice per week, n=6 mice/group) were used for tumor treatment by
intratumoral injection, and the anesthesia method used was gas
isoflurane 3-4% induction, 1-2% maintenance, flow 0.6-0.8 l/min.
The mice were monitored daily for tumor development and sacrificed
on day 28 after the injection of the glioma cells. No toxic
side-effects were observed in the mice. The mice were euthanized by
carbon dioxide inhalation (30% vol/min), and death was verified by
the loss of consciousness, apnea and the fading of eye color.
Subsequently, the tumors were removed and photographed, and the
tumor volume and weight were recorded. The tumors were fixed in
formalin and embedded in paraffin or snap-frozen in liquid
nitrogen.
Bioinformatics analysis
Predicted miRNA targets or predicted genes were
detected using the miRWalk (http://zmf.umm.uniheidelberg.de/apps/zmf/mirwalk2/),
TargetScan (http://www.targetscan.org), and
miRTar-Base (https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2022/php/index.php)
databases. Furthermore, the miRCancer database (http://mircancer.ecu.edu/index.jsp) and GEPIA
database (http://gepia.cancer-pku.cn/index.html) were used to
evaluate the expression of FLOT2 and its association with the
overall survival (Kaplan-Meier method with the log-rank test) of
patients with GBM derived from the database.
Statistical analysis
Statistical analyses were performed using GraphPad
8.0 software (GraphPad Software Inc.). Data were analyzed using a
two-tailed Student's t-test and are presented as the mean ±
standard deviation (mean ± SD). The significance of the differences
between different groups was examined using one-way ANOVA followed
by the Tukey-Kramer post hoc test. Values of P<0.05 and
P<0.01 were considered to indicate statistically significant and
highly statistically significant differences, respectively).
Results
Characterization of EXOs derived from
NSCs
NSCs have self-renewal properties. When they were
cultured in complete medium, the NSCs gradually formed increasingly
large neurospheres over time (Fig.
1A). As shown in Fig. 1B, the
NSCs expressed the typical NESTIN neurosphere marker, as well as
the stem cell markers, SOX2 and Musashi1. Furthermore, NSCs are
capable of multipotential differentiation; specifically, following
induction in differentiation medium (DMEM/F12 supplemented with 1%
B27, 2% FBS and 1% penicillin-streptomycin), they gradually
differentiated into neurons, astrocytes and oligodendrocytes, which
expressed Tuj1, GFAP and MOG, respectively, as revealed by
immunofluorescence analysis (Fig.
1C).
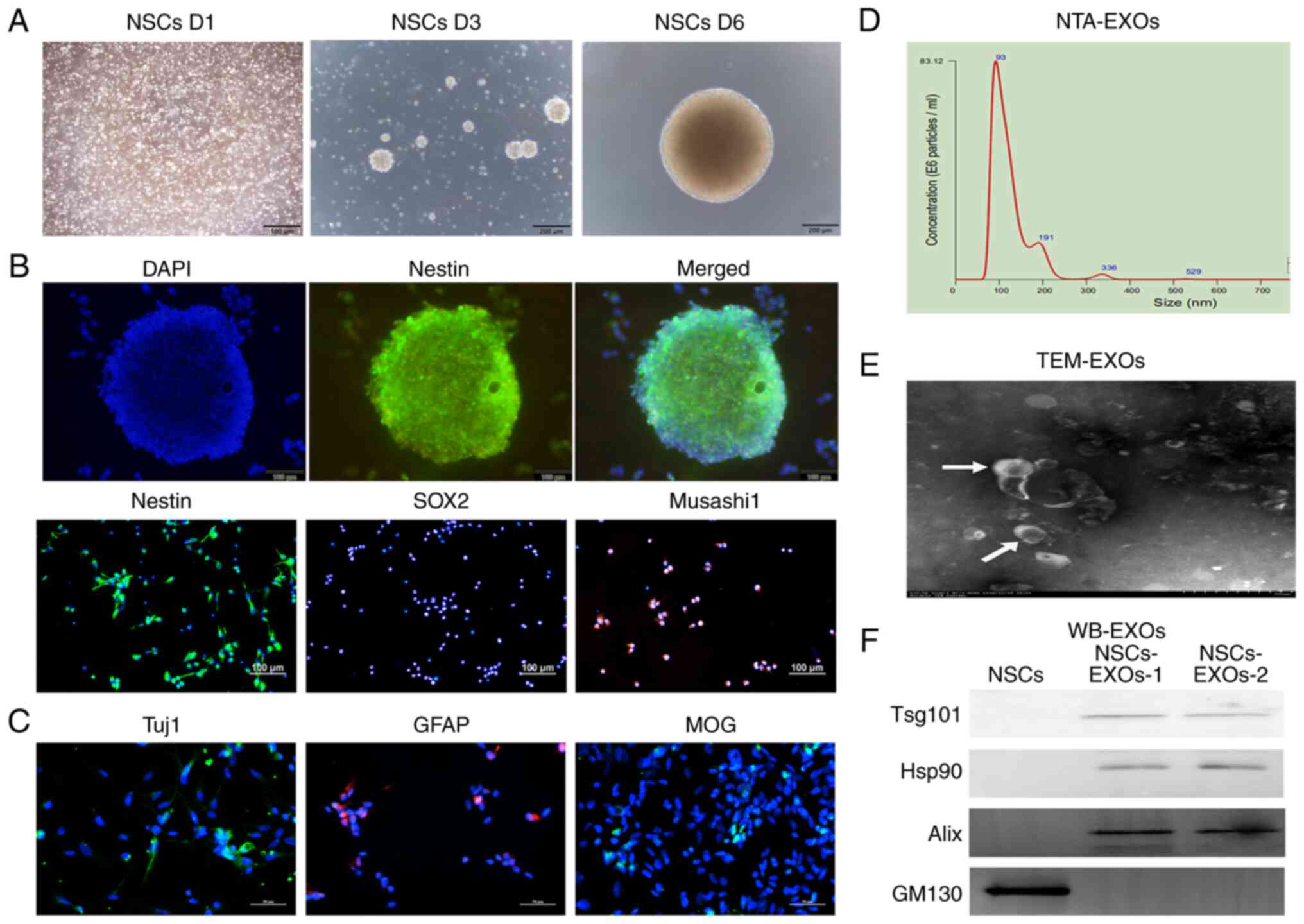 | Figure 1Characterization of exosomes derived
from NSCs. (A) The morphology of NSCs was recorded on day 1, 3 and
6 after cell thawing. Scale bar, 200 µm. (B)
Immunofluorescence detected the stem cell markers, Nestin, SOX2 and
Musashi1. Scale bar, 100 µm. (C) Immunofluorescence detected
neurons (Tuj1), astrocytes (GFAP) and oligodendrocytes (MOG). Scale
bar, 50 µm. (D) NTA analysis revealed the particle
distribution of exosomes derived from NSCs. (E) The exosomes was
detected using TEM. Scale bar, 100 nm. (F) Western blot analysis of
the exosomal markers, Tsg101, Hsp90 and Alix, and the Golgi marker,
GM130. NSCs, neural stem cells; NTA, nanoparticle tracking
analysis; MOG, myelin oligodendrocyte glycoprotein; GFAP, glial
fibrillary acidic protein; TEM, transmission electron microscopy;
EXOs, exosomes. |
To evaluate NSC-derived EXOs, secreted vesicles were
extracted from the NSC medium through standard super-centrifugation
and SEC methods. EXO characterization was performed using NTA, TEM
and western blot analysis methods based on the minimal information
from research on extracellular vesicles 2018 (24). NTA revealed that the vesicle
diameter mainly ranged from 30 to 200 nm (Fig. 1D). TEM revealed that the EXOs had a
concave-cup morphology (Fig. 1E).
Western blot analysis revealed that the EXOs significantly
expressed exosomal classical markers, including Alix, Hsp90 and
Tsg101; however, they did not express the cell protein (Golgi
marker), GM130 (Fig. 1F). Taken
together, these findings represented features of NSC-secreted EXOs;
accordingly, we termed them as NSC-EXOs.
NSC-EXOs loaded with miR-124-3p suppress
glioma cell proliferation, invasion and migration
To assess NSC-EXO uptake by the glioma cells,
NSC-EXOs were labeled using PKH-67 followed by co-incubation with
the U87MG and U251MG cells, and loaded miR-124-3p mimics into
NSC-EXOs through electroporation. Following a 24-h co-incubation,
PKH67-labeled NSC-EXOs and carrier Cy5-miR were significantly
detected in the cytoplasm and nucleus of the glioma cells (Fig. 2A). It was found that NSC-EXOs can
freely enter glioma cells. Subsequently, following the addition of
the EXOs loaded with miR-124-3p to glioma cells, RT-qPCR revealed a
markedly higher miR-124-3p expression in the EXO-miR-124-3p
mimics group than in the control and NC groups for both the U87MG
and U251MG cells (Fig. 2B).
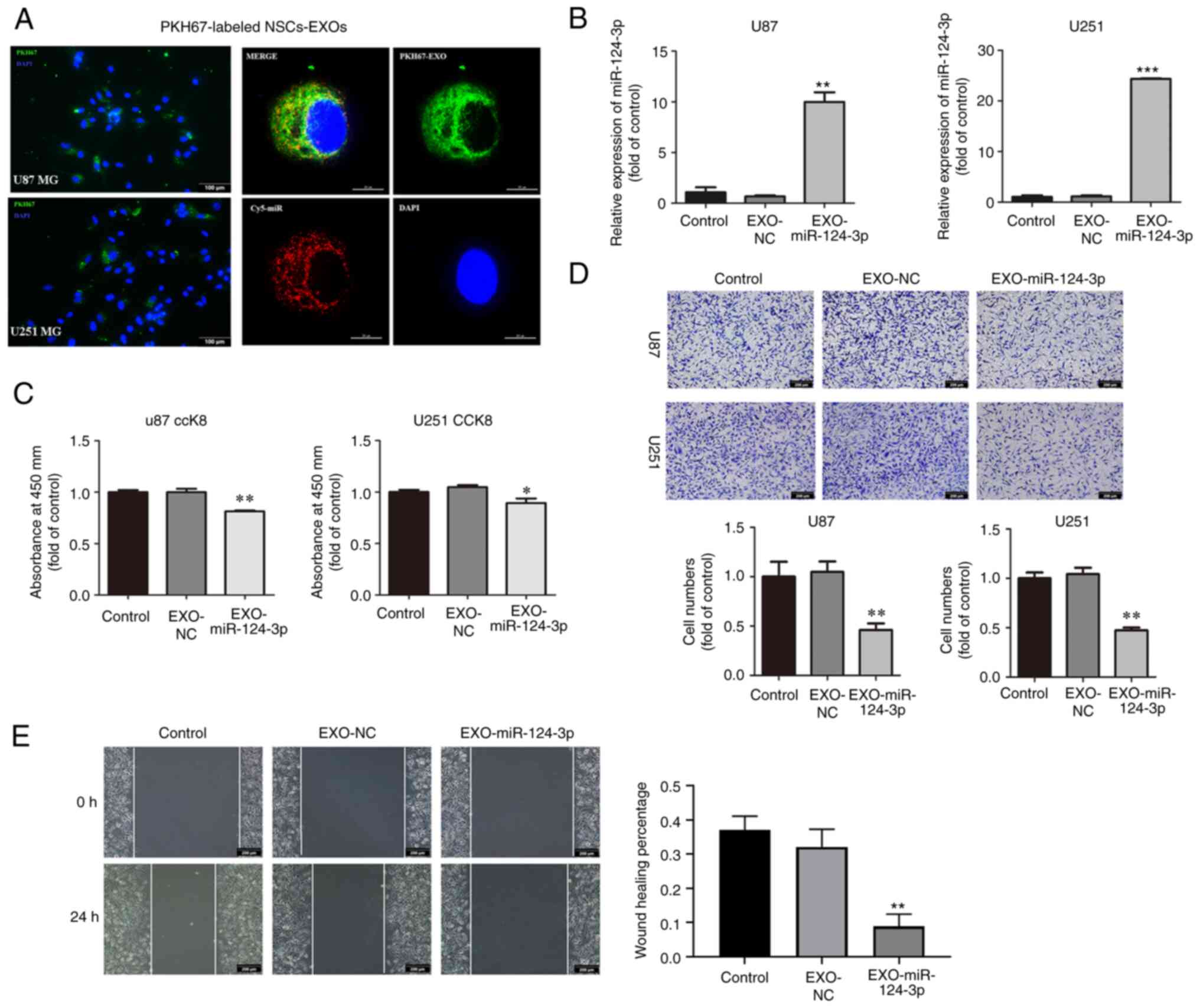 | Figure 2NSC-EXOs loaded with miR-124-3p
suppress glioma cell proliferation, invasion and migration. (A)
EXOs were isolated from neural stem cell supernatant, dyed with
PKH67 (green), and co-cultured with glioma cells for 24 h.
Subsequently, they were dyed with DAPI (blue) and examined (EXOs
labeled with PKH67, miR labeled with Cy5; original magnifications,
×100 (left panel) and ×400 (right panel). (B) miR-124-3p expression
was detected using reverse transcription-quantitative PCR following
incubation of the glioma cells for 48 h. (C) The proliferative
ability of U87 and U251MG cells was tested using CCK-8 assay. (D)
Transwell invasion assays in glioma cells were used to determine
cell invasion. (E) Cell migration was detected through a
wound-healing assay in glioma cells (original magnifications, ×100)
Data represent the mean ± SD from three independent experiments.
*P<0.05, **P<0.01 and
***P<0.001, statistically significant differences
between the NC and EXO-miR-124-3p group. NC, miR negative control;
NSC, neural stem cell; EXOs, exosomes. |
Subsequently, the effects of NSC-EXOs loaded with
miR-124-3p on glioma cell proliferation, invasion and migration
were evaluated using CCK-8, Transwell and wound healing assays.
Compared with the control and NC groups, the EXO-miR-124-3p group
exhibited a significantly decreased cell proliferation,
particularly at 48 h following the addition of EXOs to glioma cells
(Fig. 2C). Compared with the
control and NC groups, the EXO-miR-124-3p group exhibited a
significantly lower number of invaded and migrated cells in the
Transwell and wound healing assays, respectively (Fig. 2D and E). These results indicated
that the NSC-EXOs could successfully transfer miR-124-3p into
glioma cells to suppress tumor cell proliferation, invasion and
migration.
NSC-EXO-miR-124-3p inhibits glioma growth
by targeting FLOT2
Three miRNA bioinformatics databases (TargetScan,
miRTarBase and miRWalk) were used to predict the potential target
genes of miR-124-3p; subsequently, six key candidate genes
were selected (Fig. 3A).
Subsequently, the NSC-EXOs loaded with miR-124-3p were incubated
with the glioma cells for 48 h; moreover, RT-qPCR was used to
determine the mRNA expression of the six candidate genes in the
glioma cells. Compared with the control and NC groups, the
EXO-miR-124-3p group exhibited a significantly lower FLOT2
expression (Fig. 3B). However,
relative to the control/NC group, the expression of SUB1 was
inconsistent in the EXO-miR-124-3p group of U87 and U251 cells
(higher in U87 and lower in U251 cells), SMAD expression was
only significantly lower in the U251 cells, but not in the U87
cells; and RHOG expression was higher in the U251 cells, and
lower in the U87 cells (vs. the control). Thus, FLOT2 protein
expression was then examined. Similarly, compared with the control
and NC groups, the EXO-miR-124-3p group exhibited a significantly
lower FLOT2 protein expression (Fig.
3C).
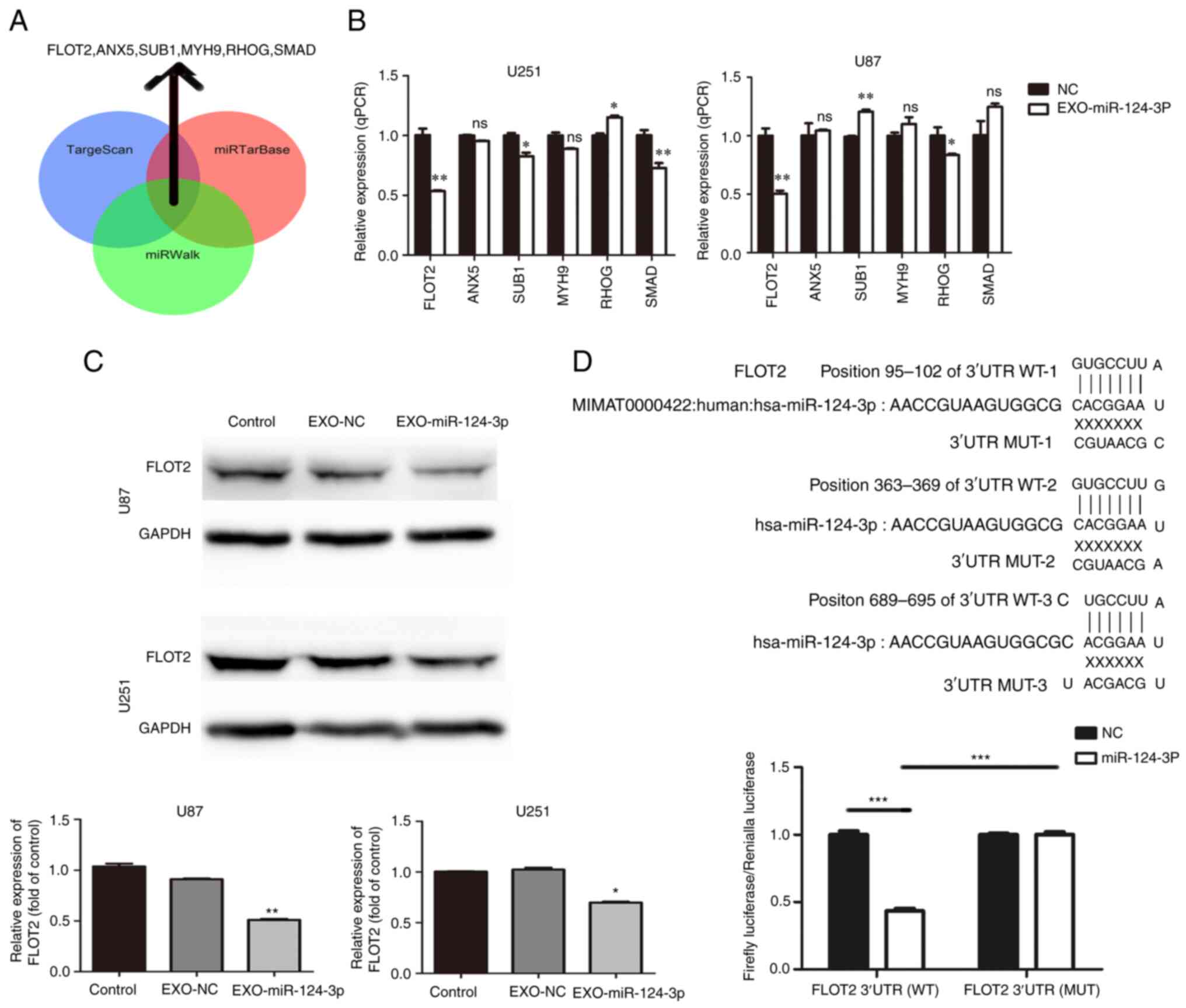 | Figure 3miR-124-3p delivery through NSC-EXOs
is involved in glioma by targeting FLOT2. (A) Venn diagrams
illustrating the six potential miR-124-3p targets identified from
TargetScan, miRTarBase and miRWalk. (B) Reverse
transcription-quantitative PCR analysis of six potential
miR-124-3p target genes in glioma cells. (C) FLOT2
protein levels were detected using western blot analysis in glioma
cells transfected with control and NSC-EXOs loaded with miR-124-3p
mimic or negative control. (D) The potential binding sites of
miR-124-3p within the FLOT2 WT and MUT 3'-UTR.
Luciferase reporter gene assays were used to analyze the miR-124-3p
effect on luciferase activity. *P<0.05,
**P<0.01 and ***P<0.001, statistically
significant differences between the NC and EXO-miR-124-3p group.
NC, miR negative control; NSC, neural stem cell; EXOs, exosomes;
FLOT2, flotillin 2; siFLOT2, siRNA FLOT2; WT, wild-type; MUT,
mutant. |
Consequently, the present study further investigated
whether FLOT2 was the direct miR-124-3p target in glioma cells. A
luciferase reporter plasmid containing the functional 3'-UTR and
mutant 3'-UTR site of FLOT2 (termed pMIR-REPORT
Luciferase-FLOT2 3'-UTR WT and MUT) was constructed (Fig. 3D). Following co-transfection of
FLOT2 WT or MUT plasmid with either miR-124-3p mimics or NC
in glioma cells for 48 h, a significantly decreased luciferase
activity by ~57% was observed in the group co-transfected with
miR-124-3p mimics and luc-FLOT2 3'-UTR WT than in the other groups
(Fig. 3D). These findings
demonstrated that EXOs-miR-124-3p directly targeted the 3'-UTR of
FLOT2 and mediated glioma cell progression mainly by
regulating FLOT2 expression.
NSC-EXOs loaded with miR-124-3p suppress
glioma cell progression via the FLOT2/AKT pathway
siRNA targeting FLOT2 (siFLOT2) was constructed to
knockdown FLOT2 expression in glioma cells and siRNA-2 was screened
as the most efficient siFLOT2 (Fig.
4A), with the findings confirming a marked decrease in both the
mRNA and protein expression of FLOT2 in glioma cells (Fig. 4B and C). Subsequently, the effects
of siFLOT2 on glioma cell proliferation, invasion and migration
were evaluated. CCK-8, Transwell and wound healing assays confirmed
that siFLOT2 significantly suppressed glioma cell proliferation,
invasion and migration, respectively (Fig. 4D-F). Taken together, these findings
suggested that NSC-EXOs loaded with miR-124-3p regulated glioma
cell progression mainly through the direct suppression of
FLOT2 expression.
A previous study by the authors demonstrated that
miR-124-3p promoted glioma progression mainly via the
PI3K/AKT pathway (20). As
shown in Fig. 4G, PI3K/AKT protein
expression was examined and it was found that compared with the
control and NC groups, there was a decreased p-AKT1/AKT1 expression
in the siFLOT2 group; however, PI3K expression was unaltered (data
not shown). Subsequently, the AKT1 agonist, Sc79, was used to
rescue AKT1 expression in the siFLOT2 group. Compared with the
si-FLOT2 group, the combined treatment group exhibited a higher
AKT1 expression (Fig. 4G). These
findings suggested that EXO-miRNA-124-3p/FLOT2 mediated glioma cell
progression via the AKT1 signaling pathway. Thus, NSC-EXOs
loaded with miR-124-3p suppressed glioma cell progression mainly
via the FLOT2/AKT1 pathway.
NSC-EXOs loading with miR-124-3p inhibits
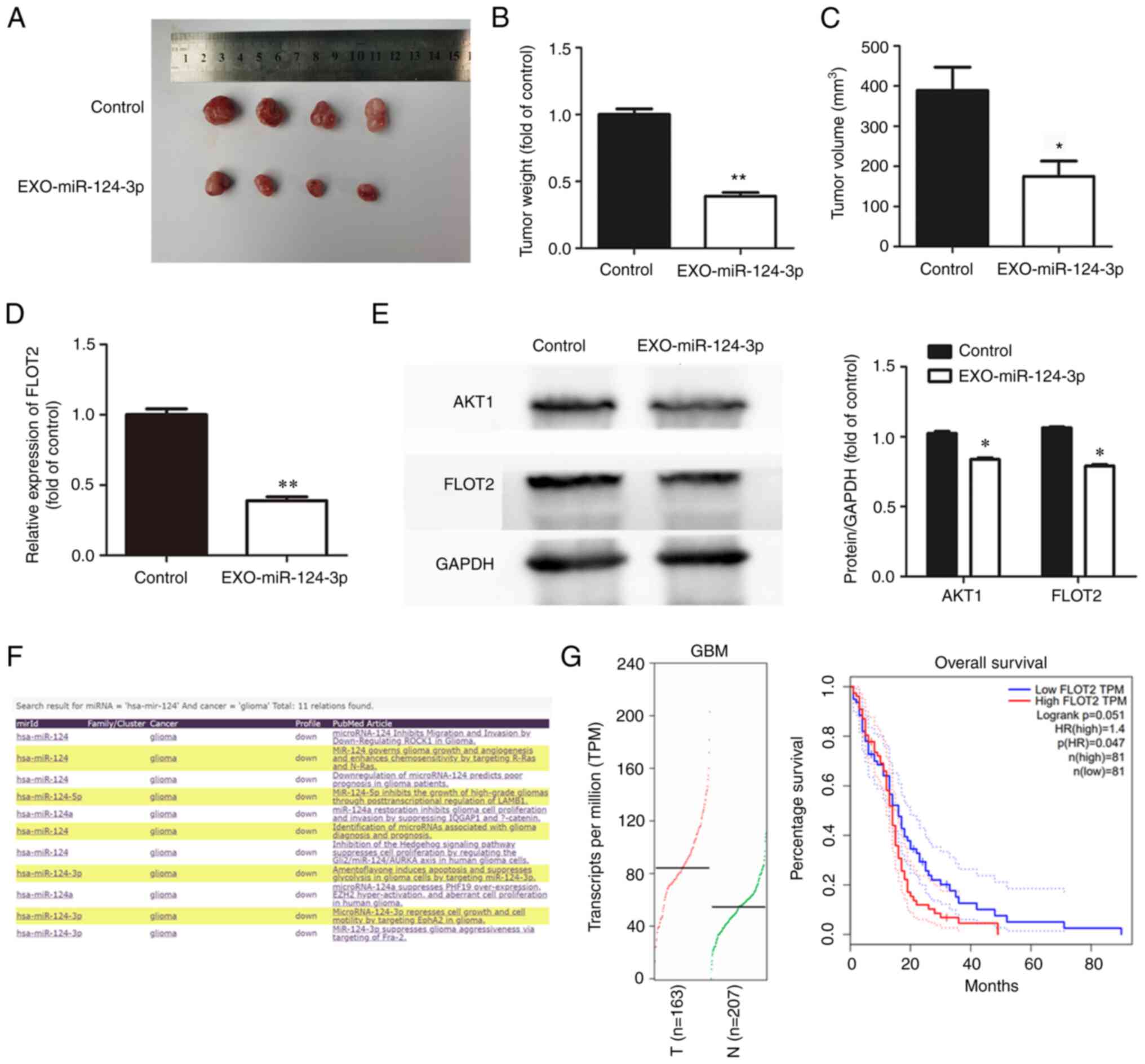
tumor growth in vivo
To explore the effects of NSC-EXOs loaded with
miR-124-3p on glioma cell growth in vivo, glioma cells were
initially injected into the subcutaneous tissue of nude mice after
10 days to allow tumor growth at an appropriate volume.
Subsequently, NSC-EXOs loaded with miR-124-3p were used for
treatment, with PBS as a control. Compared with the control group,
treatment using NSC-EXOs-miR-124-3p markedly suppressed tumor
growth (Fig. 5A). Additionally,
compared with the control group, the tumor-bearing mice in the
treatment group exhibited a significantly smaller tumor volume and
weight in (Fig. 5B and C). This
demonstrated that NSC-EXOs loaded with miR-124-3p also had a marked
inhibitory effect on glioma cells in vivo with no toxic
side-effects observed in the mice. The present study further
examined FLOT2 expression in tumor tissue using RT-qPCR and western
blot analysis. The results revealed the significant inhibition of
FLOT2 and AKT1 expression (Fig. 5D and
E) in the group treated with the NSC-EXOs loaded with
miR-124-3p. In addition, the miRCancer and GEPIA databases were
used to evaluate gene expression, as well as the association
between FLOT2 expression and the overall survival of
patients with GBM (Fig. 5F and G).
The expression of hsa-miR-124 was found to be decreased in
gliomas, which was consistent with the findings from a previous
study (20) by the authors. The
expression of FLOT2 was significantly increased in the
tissues of patients with GBM. Furthermore, the overall survival of
patients with GBM with a high FLOT2 expression was
significantly lower than that of patients with a low expression.
There was a negative association between miR-124-3p and
FLOT2 in gliomas. Thus, NSC-EXOs loading with miR-124-3p
also inhibited glioma growth in vivo, which was consistent
with the results obtained in vitro; moreover,
NSC-EXO-miR-124-3p suppressed glioma cell progression mainly via
the FLOT2/AKT1 pathway.
Discussion
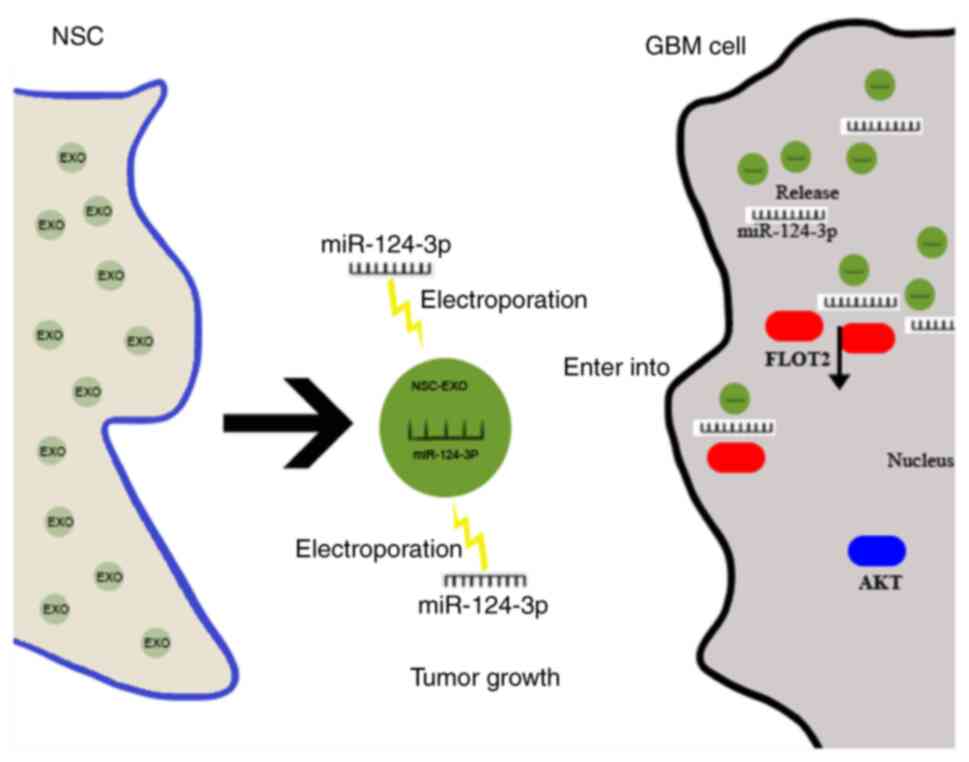
The present study mainly explored the ability of
natural biological drug carrier EXOs to deliver small molecular
miRNA into glioma cells, and found that NSC-EXO-miR-124-3p
significantly inhibited glioma growth. This was achieved by
specifically suppressing downstream FLOT2 expression and
downregulating the AKT1 signal pathway in gliomas (Fig. 6). Currently, EXOs are being
developed as diagnostic biomarkers for some diseases (25-27);
however, the therapeutic advantages of EXOs remain to be completely
exploited. Recent studies have confirmed that EXOs can be developed
as new drug carriers, and can be used to deliver small molecules,
including miRNAs, to target cells, which has provided new insight
into disease treatment, particularly for numerous refractory
diseases, including tumors (28,29).
Furthermore, studies have confirmed that EXOs can successful
transfer miRNAs to regulate the cell biological phenotype For
example, Wu et al (30)
utilized bone marrow mesenchymal stem cell-derived exosomal
miR-193a to reduce drug resistance in non-small cell lung cancer;
Huang et al (31)
demonstrated that EXOs from plasma of patients with medulloblastoma
could transfer miR-130b-3p to inhibit medulloblastoma
tumorigenesis; Munson et al (32) discovered that exosomal miR-16-5P
was a target suppressor miRNA for malignant mesothelioma.
Furthermore, the present study used bio-carrier NSC-EXOs for
loading miR-124-3p and found that they could successfully deliver
miR-124-3p into glioma cells to suppress tumor progression.
NSCs are exclusive brain stem cells involved in
nerve tissue growth, development and repair. Moreover, NSCs are
considered the most promising natural resource in the CNS and are
capable of chemotaxis to lesions, including gliomas (7,8,10,33).
The authors previously found that miR-124-3p expression was
downregulated in glioma cells and tissues (20). Therefore, the present study used
the advantages of NSCs and the capacities of carrier EXOs to load
miR-124-3p into NSC-EXOs, named EXO-miR-124-3p. Subsequently, this
molecular drug miRNA was successfully transferred into glioma cells
to suppress tumor progression. The findings presented herein
confirmed the hypothesis that carrier NSC-EXOs deliver miRNA-124-3p
into glioma cells and are involved in inhibiting tumor cell
proliferation, invasion and migration. Moreover, the effect of
EXOs-miR-124-3p on glioma cell proliferation was about 20%, and the
effects on cell invasion and migration were much more obvious,
which was ~50%; therefore, EXO-miR-124-3p mediated the invasion and
migration of glioma cells possibly via key downstream targets.
The present study then further investigated the
potential mechanisms underlying the treatment effects of NSC-EXOs
loaded with miR-124-3p on GBM progression. For this purpose, six
potential target genes of miR-124-3p from three bioinformatics
databases (TargetScan, miRTarBase and miRWalk) were selected, with
FLOT2 being the key candidate. FLOT2 expression was
significantly decreased by NSC-EXOs loaded with miR-124-3p and
glioma cells. The FLOT2 gene is associated with numerous
cancer types (34-36). FLOT2 is involved in the
formation of caveolae or caveolae-like vesicles, has the functions
of mediating cell adhesion, and mainly regulates the process of
cell invasion and migration. Hazarika et al (34) revealed that the increased
expression of FLOT2 was associated with melanoma
progression. Wang et al (35) reported an association of a poor
survival outcome of patients with breast cancer with a high
FLOT2 expression level. Wang et al (36) also confirmed that FLOT2
promoted hepatocellular carcinoma by modulating the cell cycle and
inducing epithelial-mesenchymal transition. Herein, when assessing
whether FLOT2 was the direct miR-124-3p target in
glioma cells, double luciferase reporter gene assay revealed that
miR-124-3p directly targeted the 3'-UTR of FLOT2. The
proliferation, invasion and migration of U87 and U251 glioma cells
were significantly reduced by the knockdown of FLOT2 expression in
tumor cells. Thus, bio-carrier NSC-EXOs loaded with miR-124-3p
mediated glioma cell progression (particularly cell invasion and
migration) mainly by inhibiting FLOT2.
Additionally, AKT, which is a classical
signaling pathway, is involved in cancer progression (37,38).
The present study found that NSC-EXOs loaded with miR-124-3p mimics
downregulated FLOT2 expression and simultaneously inhibited p-AKT1
expression. This was reversed by the addition of the AKT1 agonist,
Sc79. This suggested that NSC-EXOs loaded with miR-124-3p mimics
suppressed glioma progression mainly by inhibiting FLOT2 and
downregulating the AKT1 pathway. Notably, NSC-EXOs loaded
with miR-124-3p significantly reduced glioma growth in mice; as PBS
group was used as a control in vivo, the use of NSC-EXOs
loaded with miR-124-3p demonstrated the inhibitory effects of these
NSC-EXOs on tumor growth. Taken together, these findings confirmed
that bio-carrier NSC-EXOs could be used to deliver molecular drug
miR-124-3p into glioma cells, with subsequent EXO-miR-124-3p to
target the downregulation of the FLOT2/AKT1 pathway.
This eventually suppressed the proliferation, invasion and
migration abilities of glioma.
However, the present study also has some
limitations. These include the fact that the stimulatory effect of
the BBB was not examined, multiple targets may be involved in in
EXO-miR-124-3p treatment which need to be determined, and no in
situ xenograft model of glioma was used. Furthermore, the
contents in EXOs involved in the regulation of glioma cells need to
be further investigated. The authors aim to further explore other
applications of bio-carrier NSC-EXOs, including the key components
of NSC-EXOs in the future.
In conclusion, GBM is the most malignant and
invasive tumor in the CNS, with a very poor prognosis. The present
study exploited the ability of new biological carrier NSC-EXOs to
deliver miR-124-3p mimics into gliomas. It was found that
EXO-miR-124-3p could specifically target the 3'-UTR of FLOT2
to inhibit downstream FLOT2 expression. Furthermore, these EXOs
downregulated the AKT1 signaling pathway and suppressed
glioma cell growth. The findings of the present study provide
evidence of stem cell-free molecular targeted therapy based on
bio-carrier EXOs, and these findings may aid in the development of
novel therapeutic strategies for neurological diseases in the
future.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ and YeW conceptualized the study. YoW, DC and CW
were involved in the study methodology. CQ, YoW, YJ and GZ
performed the experiments. CQ, YoW and YJ were involved in the
writing of the original draft. GZ and YeW were involved in the
writing, reviewing and editing of the manuscript, and supervised
the study. GZ and YeW confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved (permit
no. A2020-015) by the Institutional Animal Care and Use Committee
of The Second Affiliated Hospital of Guangzhou Medical University,
and was performed according to the guidelines of the Committee on
Animal Research and Ethics.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Guangdong Province (grant no. 2022A1515011328) and
the China Postdoctoral Science Foundation (grant nos. 2019TQ0071
and 2020M672592).
References
|
1
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bedard PL, Hyman DM, Davids MS and Siu LL:
Small molecules, big impact: 20 years of targeted therapy in
oncology. Lancet. 395:1078–1088. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reifenberger G, Wirsching HG,
Knobbe-Thomsen CB and Weller M: Advances in the molecular genetics
of gliomas-implications for classification and therapy. Nat Rev
Clin Oncol. 14:434–452. 2017. View Article : Google Scholar
|
|
5
|
Ammirati M, Chotai S, Newton H, Lamki T,
Wei L and Grecula J: Hypofractionated intensity modulated
radiotherapy with temozolomide in newly diagnosed glioblastoma
multiforme. J Clin Neurosci. 21:633–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bond AM, Ming GL and Song H: Adult
mammalian neural stem cells and neurogenesis: Five decades later.
Cell Stem Cell. 17:385–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagó JR, Alfonso-Pecchio A, Okolie O,
Dumitru R, Rinkenbaugh A, Baldwin AS, Miller CR, Magness ST and
Hingtgen SD: Therapeutically engineered induced neural stem cells
are tumour-homing and inhibit progression of glioblastoma. Nat
Commun. 7:105932016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mutukula N and Elkabetz Y: 'Neural killer'
cells: Autologous cytotoxic neural stem cells for fighting glioma.
Cell Stem Cell. 20:426–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng Y, Morshed R, Cheng SH, Tobias A,
Auffinger B, Wainwright DA, Zhang L, Yunis C, Han Y, Chen CT, et
al: Nanoparticle-programmed self-destructive neural stem cells for
glioblastoma targeting and therapy. Small. 9:4123–4129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Portnow J, Synold TW, Badie B, Tirughana
R, Lacey SF, D'Apuzzo M, Metz MZ, Najbauer J, Bedell V, Vo T, et
al: Neural stem cell-based anticancer gene therapy: A
first-in-human study in recurrent high-grade glioma patients. Clin
Cancer Res. 23:2951–2960. 2017. View Article : Google Scholar
|
|
11
|
Marban E: The secret life of exosomes:
What bees can teach us about next-generation therapeutics. J Am
Coll Cardiol. 71:193–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Niel G, D'Angelo G and Raposo G:
Shedding light on the cell biology of extracellular vesicles. Nat
Rev Mol Cell Biol. 19:213–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mathieu M, Martin-Jaular L, Lavieu G and
Thery C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Nat
Cell Biol. 21:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J,
Zhou K, Liu X, Ren X, Wang F, et al: Cancer-derived exosomal
miR-25-3p promotes pre-metastatic niche formation by inducing
vascular permeability and angiogenesis. Nat Commun. 9:53952018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yue X, Lan F and Xia T: Hypoxic glioma
cell-secreted exosomal miR-301a activates Wnt/β-catenin signaling
and promotes radiation resistance by targeting TCEAL7. Mol Ther.
27:1939–1949. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng H, Zhao H, Xiao X, Huang Q, Zeng W,
Tian B, Ma T, Lu D, Jin Y and Li Y: Long non-coding RNA MALAT1
upregulates ZEB2 expression to promote malignant progression of
glioma by attenuating miR-124. Mol Neurobiol. 58:1006–1016. 2021.
View Article : Google Scholar
|
|
19
|
Shi Z, Chen Q, Li C, Wang L, Qian X, Jiang
C, Liu X, Wang X, Li H, Kang C, et al: MiR-124 governs glioma
growth and angiogenesis and enhances chemosensitivity by targeting
R-Ras and N-Ras. Neuro Oncol. 16:1341–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang G, Chen L, Khan AA, Li B, Gu B, Lin
F, Su X and Yan J: miRNA-124-3p/neuropilin-1(NRP-1) axis plays an
important role in mediating glioblastoma growth and angiogenesis.
Int J Cancer. 143:635–644. 2018. View Article : Google Scholar
|
|
21
|
Zhang G, Chen L, Guo X, Wang H, Chen W, Wu
G, Gu B, Miao W, Kong J, Jin X, et al: Comparative analysis of
microRNA expression profiles of exosomes derived from normal and
hypoxic preconditioning human neural stem cells by next generation
sequencing. J Biomed Nanotechnol. 14:1075–1089. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang G, Zhu Z, Wang H, Yu Y, Chen W,
Waqas A, Wang Y and Chen L: Exosomes derived from human neural stem
cells stimulated by interferon gamma improve therapeutic ability in
ischemic stroke model. J Adv Res. 24:435–445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extra-cellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar
|
|
25
|
Saadatpour L, Fadaee E, Fadaei S, Nassiri
Mansour R, Mohammadi M, Mousavi SM, Goodarzi M, Verdi J and Mirzaei
H: Glioblastoma: Exosome and microRNA as novel diagnosis
biomarkers. Cancer Gene Ther. 23:415–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Liu J, Sun G, Meng H, Wang J, Guan
Y, Yin Y, Zhao Z, Dong X, Yin S, et al: Glioblastoma extracellular
vesicles induce the tumour-promoting transformation of neural stem
cells. Cancer Lett. 466:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katakowski M and Chopp M: Exosomes as
tools to suppress primary brain tumor. Cell Mol Neurobiol.
36:343–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo S, Chen J, Chen F, Zeng Q, Liu WL and
Zhang G: Exosomes derived from Fusobacterium nucleatum-infected
colorectal cancer cells facilitate tumour metastasis by selectively
carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut. Nov 10–2020.Epub
ahead of print.
|
|
30
|
Wu H, Mu X, Liu L, Wu H, Hu X, Chen L, Liu
J, Mu Y, Yuan F, Liu W and Zhao Y: Bone marrow mesenchymal stem
cells-derived exosomal microRNA-193a reduces cisplatin resistance
of non-small cell lung cancer cells via targeting LRRC1. Cell Death
Dis. 11:8012020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang S, Xue P, Han X, Zhang C, Yang L,
Liu L, Wang X, Li H, Fu J and Zhou Y: Exosomal miR-130b-3p targets
SIK1 to inhibit medulloblastoma tumorigenesis. Cell Death Dis.
11:4082020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munson PB, Hall EM, Farina NH, Pass HI and
Shukla A: Exosomal miR-16-5p as a target for malignant
mesothelioma. Sci Rep. 9:116882019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin EY, Cooper DD, Abbott KL, Lennon J,
Nagaraja S, Mackay A, Jones C, Vogel H, Jackson PK and Monje M:
Neural precursor-derived pleiotrophin mediates subventricular zone
invasion by glioma. Cell. 170:845–859.e19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hazarika P, McCarty MF, Prieto VG, George
S, Babu D, Koul D, Bar-Eli M and Duvic M: Up-regulation of
Flotillin-2 is associated with melanoma progression and modulates
expression of the thrombin receptor protease activated receptor 1.
Cancer Res. 64:7361–7369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Yang Q, Guo L, Li XH, Zhao XH,
Song LB and Lin HX: Flotillin-2 is associated with breast cancer
progression and poor survival outcomes. J Transl Med. 11:1902013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang CH, Zhu XD, Ma DN, Sun HC, Gao DM,
Zhang N, Qin CD, Zhang YY, Ye BG, Cai H, et al: Flot2 promotes
tumor growth and metastasis through modulating cell cycle and
inducing epithelial-mesenchymal transition of hepatocellular
carcinoma. Am J Cancer Res. 7:1068–1083. 2017.
|
|
37
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoxhaj G and Manning BD: The PI3K-AKT
network at the interface of oncogenic signalling and cancer
metabolism. Nat Rev Cancer. 20:74–88. 2020. View Article : Google Scholar
|