Introduction
Ovarian cancer is the most lethal gynecological
malignancy, responsible for >200,000 deaths globally in 2020
(1). The most common form is
epithelial ovarian cancer (EOC). However, despite major research
efforts, the exact origin and early pathogenesis of EOC are still
not fully understood. EOC can include cells of origin from both the
ovarian surface epithelium and the fallopian tube (2). Moreover, EOC is not a single disease,
but a heterogenic group of tumors that can be classified by their
genetic and histological features. In 2004, Shih and Kurman
(3) suggested a dualistic model,
with type I and type II EOC, and this model has been helpful in
understanding EOC development and tumor biology. Thus, type I
(low-grade serous G1, low-grade endometrioid G1/G2, mucinous or
clear cell) tumors are associated with corresponding benign ovarian
cystic neoplasms, often developing through an intermediate
borderline step and have a better prognosis. Type II (high-grade
serous G2/G3, high-grade endometrioid G3 or carcinosarcoma) tumors
are highly aggressive and genetically unstable tumors that most
often present at advanced stages and are responsible for the
majority of EOC-associated deaths (3,4).
In most cases, modern treatment of ovarian cancer
includes radical cytoreductive surgery, followed by platinum- and
paclitaxel-based systemic chemotherapy (5-7).
However, current treatment regimens are far from optimal (8). The histopathology of ovarian cancer
is heterogeneous, and each subtype harbors specific genetic
mutations that can be used for diagnostics and targeted treatment.
New drugs target some of the stepwise genetic mutations the
neoplastic cells gain to become masters of their growth and
proliferation (9). For example,
tyrosine kinases play a critical role in growth factor signaling
and sustained proliferation, and all histopathological subtypes of
EOC seem to have modifications in growth factor signaling. For
instance, EOC type I tumors typically are chemoresistant tumors
that harbor mutations in BRAF, KRAS and PIK3CA (10).
Tyrosine kinase inhibitors (TKIs) have been trialled
in EOC, and one review has summarized the results of 75 completed
and ongoing clinical trials (11).
While there is still some promise for a few TKIs, the overall
findings point to low efficacy (12-15).
Furthermore, with the exception of poly(ADP-ribose)
polymerase-inhibitors and BRCA1/2 mutations, current systemic
treatments are usually based on group-level clinical trial data and
do not consider histopathology, molecular characterization or
individual drug sensitivity, even though it is well known that
response rates to cancer drugs vary (16). As a result, individuals are at risk
of side effects, while the tumor may be unresponsive to therapy
(17). As clinicopathological
parameters are insufficient for the prediction of response to
chemotherapy, additional methods are needed to individualize
treatment.
The present study used a short-term culture
chemotherapy sensitivity assay to evaluate ex vivo EOC tumor
cell sensitivity to established cytotoxic drugs and TKIs. The aim
was to explore sensitivity patterns in the two EOC subtypes and in
samples with or without previous exposure to cytotoxic drugs.
Furthermore, the study aimed to explore cross-resistance between
drugs and evaluate the association between drug sensitivity and
progression-free survival (PFS) in the patients.
Materials and methods
Patients and tumor samples
In total, 128 patients scheduled for ovarian cancer
surgery between May 2006 and December 2016 at Uppsala University
Hospital (Uppsala, Sweden), Örebro University Hospital (Örebro,
Sweden), Falun hospital (Falun, Sweden) and the private Uppsala
Cancer Clinic (Uppsala, Sweden) were included in the study, with
the majority included during the last 5 years. A successful
chemotherapy sensitivity assay was obtained in 120 patients, and
these were included for further analysis. Of these, 93 patients
were scheduled for potentially curative cytoreductive surgery,
whereas 18 underwent laparotomy, but were too advanced for
cytoreductive surgery and underwent debulking surgery for symptom
relief or only diagnostic laparotomy. As the private clinic closed
during the study, information about surgery could not be obtained
for the remaining 9 patients that were included at this site.
However, at all sites, surgery was performed by gynecological
surgeons and patient tumor burden was assessed according to the
Peritoneal Cancer Index (PCI) (18) at the start of surgery. Residual
disease after surgery was quantified according to the completeness
of cytoreduction (CC) score (19,20),
where a CC score of 0 (no macroscopic tumor left) and 1 (residual
tumor <0.25 cm) were considered as complete cytoreduction.
Preoperative performance status was classified according to the
American Society of Anesthesiologists (ASA) Physical Status
Classification System (21). Tumor
samples were collected during surgery and immediately sent for
ex vivo drug activity assessment.
Tumor sample classifications of type I (low-grade
serous G1, low-grade endometrioid G1/G2, mucinous or clear cell) or
type II (high-grade serous G2/G3, high-grade endometroid G3 or
carcinosarcoma) made by an experienced pathologist at a Swedish
tertiary care hospital were collected from the patient medical
records (3).
Following surgery, patients started chemotherapy
within 4 to 6 weeks, most commonly with paclitaxel 175
mg/m2 and carboplatin (area under the curve, 5). After
completing treatment, patients were followed up with computed
tomography (CT) scans, and then clinical examination, a
transvaginal ultrasound and cancer antigen 125 assessment every 3
months for 2 years, every 6 months for another 3 years, and every
12 months up to 10 years. Findings at the clinical examination
and/or increased tumor marker levels would trigger a CT scan for
the verification of relapse (22).
Characteristics of the patients included are detailed in Table I. Information on histopathological
subtype, clinical characteristics, chemotherapy, surgery, disease
status and survival were obtained from the medical records of
Uppsala University Hospital and the other participating centers.
Among patients in which complete cytoreduction (n=74) was achieved,
data for PFS were collected until February 2017. All tumor sampling
and data collection was performed once written informed consent had
been obtained, and the study was approved by the Regional Ethical
Committee in Uppsala (approval no. Dnr 2007/237).
 | Table IClinical characteristics of the
ovarian cancer samples successfully analyzed ex vivo
(n=120). |
Table I
Clinical characteristics of the
ovarian cancer samples successfully analyzed ex vivo
(n=120).
| Characteristic | Value |
|---|
| Mean age (range),
years | 59 (19-81) |
| Mean BMI (range),
kg/m2 | 25 (16-44) |
| ASA, n (%) | |
| 1 | 16 (13.3) |
| 2 | 62 (51.7) |
| 3 | 21 (17.5) |
| Unknown | 21 (17.5) |
| Histopathology, n
(%) | |
| Type I | 21 (17.5) |
| Low-grade
serous | 13 (10.8) |
| Low-grade
endometroid | 3 (2.5) |
| Mucinous | 2 (1.7) |
| Clear cell | 3 (2.5) |
| Type II | 99 (82.5) |
| High-grade
serous | 93 (77.5) |
| High-grade
endometroid | 3 (2.5) |
|
Carcinosarcoma | 3 (2.5) |
| Prior chemotherapy,
n (%) | 52 (43.3) |
| Peritoneal cancer
index, n (%) | |
| 1-10 | 11 (9.2) |
| 11-20 | 30 (25.0) |
| 21-39 | 44 (36.7) |
| Unknown | 35 (29.2) |
| Operable, n
(%) | |
| Yes | 93 (77.5) |
| No | 18 (15.0) |
| Unknown | 9 (7.5) |
| Complete
cytoreductive surgery, n (%)a | |
| Yes | 74 (79.6) |
| No | 18 (19.4) |
| Not detailed | 1 (1.1) |
Ex vivo assessment of drug
sensitivity
The tumor specimens were kept in a transport culture
medium at room temperature until cell preparation, which mostly
started within 3 h of tumor sampling. Tumor cells were prepared by
collagenase digestion as described previously (23). The cells obtained were mostly
single cells or small cell clusters, in cell suspension, with ≥90%
viability and <30% contaminating non-malignant cells, as judged
by viability staining, using toluidine blue, and morphological
examinations of May-Grünwald-Giemsa-stained cytocentrifuge
preparations, respectively (Fig.
S1). Cytocentrifuge glasses (100 µl, 700 g; 5 min at
room temperature) were stained using May-Grünwald for 5 min
followed by Giemsa stain for 10 min. The glasses were then left to
air dry, in room temperature, before examination using light
microscopy.
Seven standard solid tumor cytotoxic drugs and nine
recently introduced TKIs with different indications were tested
ex vivo. The drugs were commercially available clinical
preparations (cisplatin) or obtained from Selleck Chemicals
(oxaliplatin and crizotinib), MilliporeSigma (irinotecan and
5-fluorouracil) or LC Laboratories (gemcitabine, dasatinib,
docetaxel, doxorubicin, erlotinib, lapatinib, nintedanib,
regorafenib, sorafenib and sunitinib). From 2006 until mid-2013,
the drugs were tested at three 10-fold dilutions from the maximal
concentration of 1,000 µM for 5-fluorouracil (5-FU),
gemcitabine and irinotecan, and 100 µM for oxaliplatin,
cisplatin, docetaxel, doxorubicin, erlotinib, lapatinib, sorafenib
and sunitinib. From mid-2013, five concentrations were tested with
four three-fold dilutions from a lowered maximal concentration,
including some recently introduced TKIs: 180 µM for 5-FU and
irinotecan, 90 µM for oxaliplatin, docetaxel, gemcitabine,
crizotinib, dasatinib, erlotinib, lapatinib, nintedanib,
regorafenib, sorafenib and sunitinib, 45 µM for doxorubicin
and vemurafenib, and finally 30 µM for cisplatin. The drug
concentrations used ex vivo were chosen empirically to
produce concentration-response curves allowing estimation of the
half maximal inhibitory concentrations (IC50), i.e., the
drug concentration producing a cell survival rate of 50% compared
with the unexposed control. From 2006 until mid-2013, 384-well
microplates (Nalge Nunc International) were prepared with 5
µl drug solution at 10 times the final drug concentration
using the pipetting robot BioMek 2000 (Beckman Coulter, Inc.). The
plates were prepared freshly every 3 months, tested for stability,
and then stored at -70°C until further use.
The semiautomated fluorometric microculture
cytotoxicity assay (FMCA) assessed drug sensitivity (24,25).
Briefly, tumor cells from patient samples (5,000 cells/well) in 45
µl RPMI 1640 culture medium [supplemented with 10% fetal
calf serum, glutamine and penicillin-streptomycin (all from
MilliporeSigma)] were seeded in the drug-prepared 384-well plates
using the pipetting robot Precision 2000 (Bio-Tek Instruments,
Inc.). From mid-2013, the drugs were added immediately after cell
seeding using the liquid handling system ECHO® 550
(Labcyte, Inc.). This allowed for fast transfer of volumes ≥2.5 nl
from source plates into destination wells. In ECHO®
experiments, source plates were prepared with appropriate
concentrations of drugs in DMSO (except cisplatin, where the
clinical preparation was used) and stored in the oxygen and
moisture free MiniPod™ system (Roylan Developments Ltd.) until
further use. The method for drug addition did not affect the assay
results. Three columns without drugs served as negative controls,
and one column with medium only served as a blank control.
The culture plates were incubated at 37°C in a
humidified atmosphere containing 95% air and 5% CO2.
After 72 h of incubation, the culture medium was washed away and 50
µl/well of a physiological buffer containing 10 µg/ml
of the vital dye fluorescein diacetate (FDA) was added to the
negative control, experimental and blank control wells. After
incubation for 30-45 min at 37°C, the fluorescence from each well
was read in a FluosStar Optima (BMG Labtech GmbH).
Quality criteria for a successful assay were: ≥70%
tumor cells in the cell preparation before incubation and/or on the
assay day, a fluorescence signal in control cultures of ≥5 times
the mean blank values and a coefficient of variation of cell
survival in control cultures of ≤30%. A total of 8 out of the 128
samples (6%) did not fulfill these quality criteria and were not
included in the results presentation. The results obtained by the
viability indicator FDA were calculated as the survival index (SI),
defined as the fluorescence of the drug-exposed wells as a
percentage of control cultures, with blank values subtracted: SI
(%)=100×[(Fexperimental-Fblank
control)/(Fnegative control-Fblank
control)], where Fi corresponds to the average
fluorescence signal in i=experimental, negative control and blank
control wells, respectively.
Data evaluation and statistical
analysis
IC50 calculations and statistical
analyses thereof were performed using GraphPad Prism version 5.0
for Mac (GraphPad Software, Inc.). Drug IC50 was
calculated using non-linear regression to a standard sigmoidal
dose-response model. Sample sensitivity for regression analysis was
categorized as follows: Low drug resistance (LDR), IC50
below the median; intermediate drug resistance (IDR),
IC50 between the median and the median plus one standard
deviation (SD); or extreme drug resistance (EDR), IC50
above the median plus one SD, based on all samples investigated
ex vivo (24-26). Drug sensitivity correlations for
assessment of cross-resistance were calculated at the drug
concentration where the tumor samples showed the greatest scatter
of SI-values and evaluated using the Pearson correlation test in
GraphPad Prism (Graphpad Software, Inc.).
As the IC50 values for the drugs did not
follow a normal distribution as evaluated by Shapiro-Wilk and
Kolmogorov-Smirnov tests, comparisons between histopathological
subtypes and those who had or had not received preoperative
cytotoxic drug treatment were made by Mann-Whitney U test. The
prognostic importance of ex vivo drug sensitivity on PFS was
evaluated using the Cox proportional hazard model in SPSS version
28.0 (IBM Corp.). Several confounders were tested in these
analyses, but the only one with significant influence was the EOC
tumor type. Due to the prognostic value of the EOC tumor type,
subsequent analysis of the importance of ex vivo drug
sensitivity was performed in patients with type II tumors only
(n=61), with adjustment for ASA class and PCI. The significance
level for all statistical tests was set to P<0.05. Data are
presented as the mean ± SD unless otherwise stated.
Results
A successful ex vivo assay was obtained in
120 out of 128 samples (94%). The remaining 8 samples did not pass
technical quality control (see Materials and methods section
for details). A total of 99 patients had type II tumors, of which
93 had high-grade serous histology (Table I). Among the patients with type I
tumors (n=21), low-grade serous histology was the most common type.
A total of 52 patients (43%) had received chemotherapy prior to
surgery, 50 of these with paclitaxel and carboplatin. According to
the ASA classification, most patients had no or mild functional
limitation (Table I). Curative
cytoreductive surgery was attempted in 93 patients and was achieved
in 74 (80%), of which 13 had type I and 61 type II tumors,
respectively.
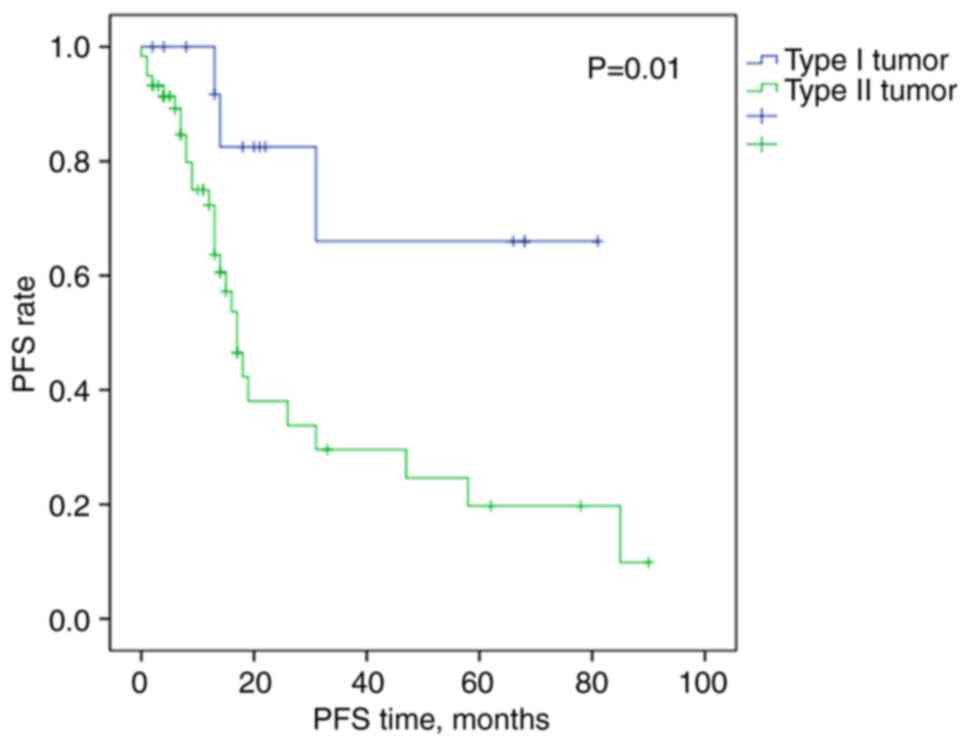
As expected, patients with complete cytoreduction
and type I tumors had longer PFS times than patients with type II
tumors, at 60.9 months (95% CI, 41.7-80.2) vs. 32.0 months (95% CI,
20.9-43.0) (Fig. 1).
Cytotoxic drug sensitivity varied considerably
between patient samples, as indicated by the high SDs in the
IC50 values for the tested drugs (Table II). Tumors previously exposed to
chemotherapy were less sensitive, i.e., had higher IC50,
to all cytotoxic drugs and to three out of the nine kinase
inhibitors, reaching statistical significance for 5-FU, irinotecan,
dasatinib and nintedanib. Notably, for cisplatin, the difference in
sensitivity with respect to treatment status was minimal, and for
erlotinib, sorafenib and sunitinib, samples from previously treated
patients were slightly more, although not statistically
significantly, sensitive compared with treatment naïve samples.
 | Table IIHalf maximal inhibitory concentration
values for standard drugs in ovarian cancer samples (n=120),
according to preoperative cytotoxic drug treatment and
histopathological subtype. |
Table II
Half maximal inhibitory concentration
values for standard drugs in ovarian cancer samples (n=120),
according to preoperative cytotoxic drug treatment and
histopathological subtype.
| Drug | Total patients,
n | Preoperative
cytotoxic drug treatment
| Histopathological
subtype
|
|---|
| Yes (n=52) | No (n=68) | P-value | Type I (n=21) | Type II (n=99) | P-value |
|---|
| Cytotoxic | | | | | | | |
| 5-FU,
µM | 119 | 309±328 | 171±181 | 0.015a | 267±313 | 224±254 | 0.806 |
| Oxaliplatin,
µM | 118 | 32.9±32.1 | 22.8±24.2 | 0.055 | 35.3±37.0 | 25.4±25.9 | 0.557 |
| Cisplatin,
µM | 106 | 11.9±15.4 | 10.0±14.2 | 0.126 | 16.5±22.5 | 9.81±12.6 | 0.030a |
| Docetaxel,
µM | 105 | 45.9±46.7 | 42.0±38.0 | 0.895 | 65.7±66.5 | 39.2±34.6 | 0.321 |
| Irinotecan,
µM | 119 | 90.8±79.9 | 66.7±62.2 | 0.021a | 85.4±75.1 | 75.5±70.6 | 0.378 |
| Doxorubicin,
µM | 107 | 1.77±3.37 | 1.10±1.53 | 0.085 | 1.66±1.64 | 1.37±2.73 | 0.081 |
| Gemcitabine,
µM | 92 | 396±386 | 240±335 | 0.058 | 253±330 | 314±370 | 0.651 |
| TKI | | | | | | | |
| Crizotinib,
µM | 69 | 16.7±23.6 | 9.44±16.1 | 0.053 | 20.2±27.1 | 11.0±18.0 | 0.064 |
| Dasatinib,
µM | 67 | 11.3±11.2 | 6.64±9.04 | 0.013a | 18.3±13.6 | 6.71±8.35 | 0.002a |
| Erlotinib,
µM | 92 | 61.3±35.6 | 62.0±36.8 | 0.874 | 57.3±37.0 | 62.6±36.0 | 0.612 |
| Lapatinib,
µM | 75 | 15.9±24.2 | 14.2±18.5 | 0.877 | 16.0±19.9 | 14.6±20.9 | 0.686 |
| Nintedanib,
µM | 44 | 23.8±29.5 | 11.5±21.7 | 0.008a | 26.4±36.0 | 14.0±23.3 | 0.171 |
| Regorafenib,
µM | 71 | 15.4±7.91 | 12.4±9.05 | 0.054 | 14.0±7.68 | 13.6±8.88 | 0.607 |
| Sorafenib,
µM | 99 | 13.8±9.89 | 15.4±18.4 | 0.560 | 12.5±7.44 | 15.1±16.3 | 0.879 |
| Sunitinib,
µM | 104 | 5.14±3.72 | 6.42±6.89 | 0.559 | 6.93±6.52 | 5.62±5.49 | 0.735 |
| Vemurafenib,
µM | 62 | 32.3±11.9 | 29.7±12.3 | 0.362 | 37.7±11.9 | 29.5±11.8 | 0.066 |
Compared with type I tumors, type II tumors were
more sensitive to all drugs except gemcitabine, reaching
statistical significance for cisplatin (Table II). The pattern was similar for
the TKIs, with type II tumors being more sensitive to all TKIs
except for erlotinib and sorafenib, with statistical significance
for dasatinib.
To remove the prognostic influence of tumor type,
the analysis of PFS was performed separately for patients with type
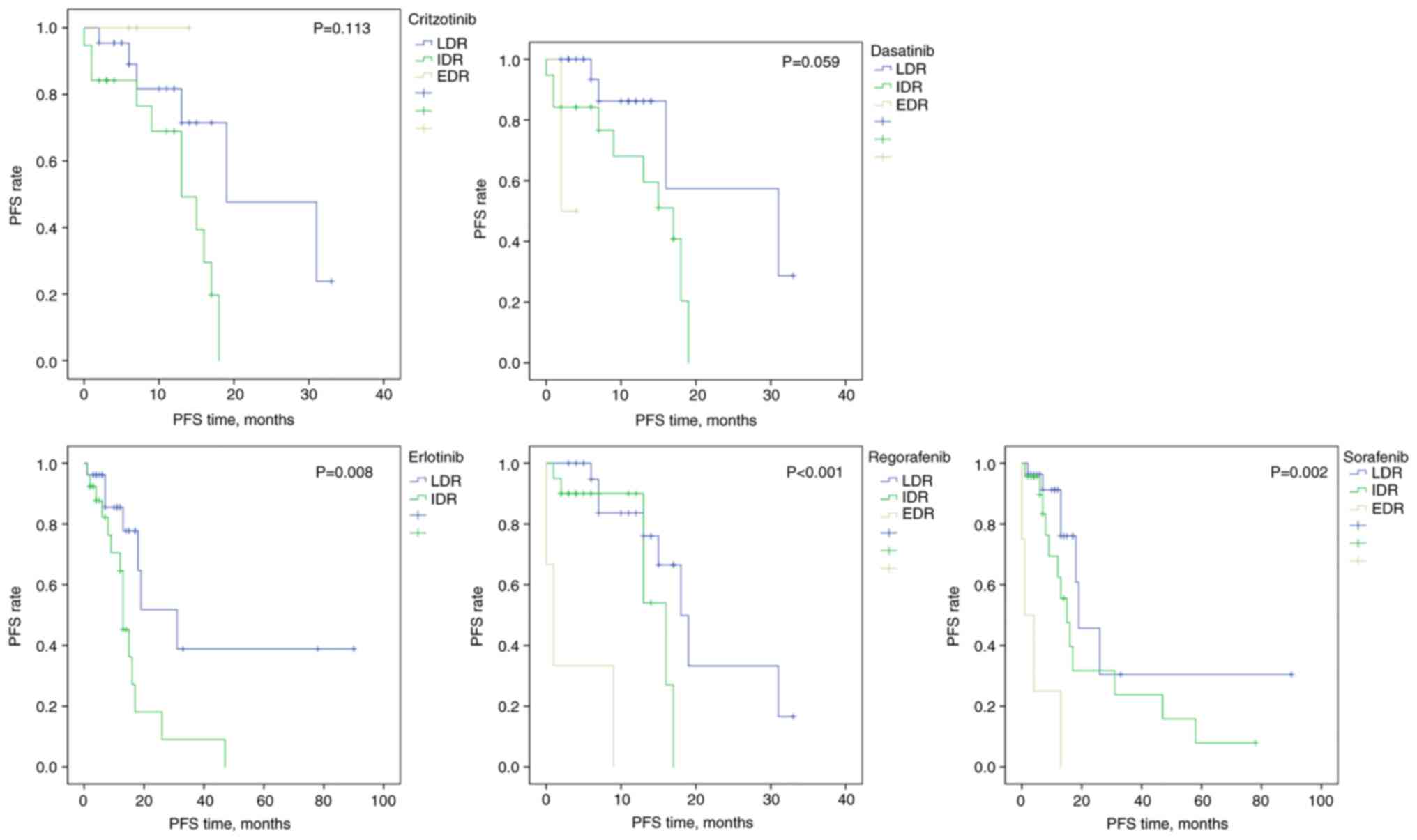
II tumors with complete cytoreduction (n=61). For all drugs either
IDR and/or EDR were associated with a higher risk of progression
compared with those with LDR (adjusted HR >1), reaching
statistical significance for the kinase inhibitors crizotinib,
dasatinib, erlotinib, regorafenib and sorafenib (Table III), although the resistance
classification was not significant overall for crizotinib and
dasatinib (Fig. 2).
 | Table IIIMultivariable Cox regression model
for progression-free survival according to drug sensitivity in
patients with type II ovarian cancer who underwent complete
cytoreductive surgery (n=61)a. |
Table III
Multivariable Cox regression model
for progression-free survival according to drug sensitivity in
patients with type II ovarian cancer who underwent complete
cytoreductive surgery (n=61)a.
| Drug | n | Adjusted HR | 95% CI | P-value |
|---|
| 5-FU | | | | |
| Low drug
resistance | 29 | 1.00 | | |
| Intermediate drug
resistance | 26 | 1.27 | 0.55-2.92 | 0.575 |
| Extreme drug
resistance | 4 | 1.67 | 0.52-5.42 | 0.391 |
| Oxaliplatin | | | | |
| Low drug
resistance | 28 | 1.00 | | |
| Intermediate drug
resistance | 22 | 1.81 | 0.73-4.47 | 0.198 |
| Extreme drug
resistance | 8 | 2.44 | 0.73-8.17 | 0.148 |
| Cisplatin | | | | |
| Low drug
resistance | 28 | 1.00 | | |
| Intermediate drug
resistance | 26 | 0.92 | 0.41-2.07 | 0.841 |
| Extreme drug
resistance | 2 | 3.65 | 0.76-17.21 | 0.102 |
| Docetaxel | | | | |
| Low drug
resistance | 27 | 1.00 | | |
| Intermediate drug
resistance | 24 | 1.18 | 0.48-2.93 | 0.715 |
| Extreme drug
resistance | 5 | 2.69 | 0.64-11.34 | 0.179 |
| Irinotecan | | | | |
| Low drug
resistance | 29 | 1.00 | | |
| Intermediate drug
resistance | 26 | 1.56 | 0.67-3.62 | 0.305 |
| Extreme drug
resistance | 4 | 1.40 | 0.37-5.22 | 0.617 |
| Doxorubicin | | | | |
| Low drug
resistance | 28 | 1.00 | | |
| Intermediate drug
resistance | 27 | 1.63 | 0.69-3.89 | 0.268 |
| Extreme drug
resistance | 2 | 1.57 | 0.17-14.43 | 0.693 |
| Gemcitabin | | | | |
| Low drug
resistance | 25 | 1.00 | | |
| Intermediate drug
resistance | 17 | 1.60 | 0.58-4.42 | 0.367 |
| Extreme drug
resistance | 9 | 1.71 | 0.55-5.32 | 0.358 |
| Crizotinib | | | | |
| Low drug
resistance | 22 | 1.00 | | |
| Intermediate drug
resistance | 19 | 3.49 | 1.08-11.25 | 0.037b |
| Extreme drug
resistance | 3 | - | - | 0.988c |
| Dasatinib | | | | |
| Low drug
resistance | 22 | 1.00 | | |
| Intermediate drug
resistance | 19 | 3.34 | 0.90-12.39 | 0.072 |
| Extreme drug
resistance | 2 | 16.37 | 1.25-213 | 0.033b |
| Erlotinib | | | | |
| Low drug
resistance | 26 | 1.00 | | |
| Intermediate drug
resistance | 25 | 3.83 | 1.42-10.35 | 0.008b |
| Extreme drug
resistance | 0 | | | |
| Lapatinib | | | | |
| Low drug
resistance | 24 | 1.00 | | |
| Intermediate drug
resistance | 20 | 1.39 | 0.52-3.77 | 0.514 |
| Extreme drug
resistance | 3 | 1.09 | 0.23-5.30 | 0.913 |
| Nintedanib | | | | |
| Low drug
resistance | 17 | 1.00 | | |
| Intermediate drug
resistance | 14 | 1.28 | 0.17-9.39 | 0.812 |
| Extreme drug
resistance | 3 | 4.72 | 0.59-37.87 | 0.144 |
| Regorafenib | | | | |
| Low drug
resistance | 22 | 1.00 | | |
| Intermediate drug
resistance | 20 | 3.07 | 0.89-15.38 | 0.071 |
| Extreme drug
resistance | 3 | 28.31 | 4.95-161 | 0.001b |
| Sorafenib | | | | |
| Low drug
resistance | 26 | 1.00 | | |
| Intermediate drug
resistance | 23 | 2.28 | 0.85-6.13 | 0.102 |
| Extreme drug
resistance | 4 | 11.22 | 2.96-42.55 | 0.001b |
| Sunitinib | | | | |
| Low drug
resistance | 28 | 1.00 | | |
| Intermediate drug
resistance | 26 | 1.21 | 0.53-2.78 | 0.646 |
| Extreme drug
resistance | 2 | 1.88 | 0.22-15.79 | 0.563 |
| Vemurafenib | | | | |
| Low drug
resistance | 22 | 1.00 | | |
| Intermediate drug
resistance | 18 | 1.51 | 0.49-4.65 | 0.472 |
| Extreme drug
resistance | 0 | | | |
Cross-resistance between the key ovarian cancer drug
cisplatin and certain selected cytotoxic drugs and TKIs was modest
or statistically significant in most cases, except for between
cisplatin and nintedanib, where there was an absence of
cross-resistance (Table IV).
 | Table IVCorrelations of survival index (%)
and linear regression slope between the pairs of drugs
indicateda. |
Table IV
Correlations of survival index (%)
and linear regression slope between the pairs of drugs
indicateda.
| Drug pair | r | Slope | P-value |
|---|
| Cisplatin/5-FU | 0.499 | 0.398±0.120 | 0.0023b |
|
Cisplatin/oxaliplatin | 0.307 | 0.243±0.088 | 0.0075b |
|
Cisplatin/docetaxel | 0.270 | 0.349±0.137 | 0.0130b |
|
Cisplatin/irinotecan | 0.224 | 0.249±0.189 | 0.1962 |
|
Cisplatin/doxorubicin | 0.301 | 0.276±0.152 | 0.0792 |
|
Cisplatin/lapatinib | 0.344 | 0.0335±0.116 | 0.0051b |
|
Cisplatin/nintedanib | 0.026 | 0.040±0.216 | 0.8539 |
|
Cisplatin/sorafenib | 0.336 | 0.309±0.101 | 0.0030b |
|
Cisplatin/sunitinib | 0.385 | 0.354±0.099 | 0.0006b |
|
Sorafenib/regorafenib | 0.357 | 0.325±0.100 | 0.0018b |
Discussion
Type I epithelial ovarian tumors are reported to
have a better prognosis than the highly aggressive and genetically
more unstable type II tumors (4).
This assumption was also confirmed in the present study. Type II
tumors are characterized by initial sensitivity to cytotoxic agents
that often affect DNA repair pathways. By contrast, type I tumors
show more indolent behavior and are less sensitive to conventional
treatment than type II tumors (27,28).
The ex vivo results reported in the present study are in
line with this clinical experience. The type I tumors were
generally less sensitive to cytotoxic agents than the type II
tumors, typically illustrated by the difference for cisplatin.
Hence, the difference in PFS in favor of the type I histology
combined with the reduced cytotoxic drug sensitivity in type I
tumors suggests that the overall improved prognosis is due to their
more indolent tumor biology. To improve the prognosis in type I
tumors further, the present study points to erlotinib and sorafenib
as drugs with some promise to be relatively active in this
subgroup. However, the limited clinical experience with erlotinib
and sorafenib in EOC points to very low activity, but available
studies have not reported on histopathological subgroups (12-15).
Furthermore, the present study observed a stepwise
increase in risk for disease progression or death with decreasing
drug sensitivity. This finding, that the clinical pattern of drug
activity is reflected in an ex vivo total cell kill assay
like the FMCA, lends support for a clinical role for such assays in
clinical treatment decision-making for cancer drug therapy in EOC,
much in line with the findings by von Heideman et al
(29). This pattern of drug
sensitivity also applied to most TKIs and was also observed when
comparing samples from previously untreated and treated patients,
indicating that cancer drug resistance is somewhat of a general
phenomenon and not isolated to one or a few individual drugs. This
means that once drug resistance has been observed in the clinic,
the probability that another drug, irrespective of mechanistic
class, will work decreases. However, given the considerable
variability and modest cross-resistance between drugs, response in
later line therapy is not excluded.
Samples from patients previously exposed to
cytotoxic drugs generally tended to be more resistant to most drugs
than samples from unexposed patients. This observation is in line
with clinical experience and findings, supporting the notion that
exposure to cytotoxic treatments contributes to development of more
or less general resistance mechanisms (30). Induced chemoresistance seems to be
less or absent for cisplatin, supporting the fact that platinum is
often active even in treating relapses (31). On the other hand, resistance to the
TKIs after exposure varied, but was seemingly less pronounced than
for standard cytotoxic drugs. Sorafenib and sunitinib seemingly
lack development of resistance after prior cytotoxic drug exposure,
and they may be notable drugs for further investigation in the
treatment of resistant disease (32); however, as aforementioned, the
results from limited clinical experience with these drugs is not
very promising (12-15).
A limitation of the present study was that the
genetic constitution of the tumors was not available for use as a
covariate. Such data would enable an integrated precision medicine
study in which novel genetic markers for effect could be
identified.
Drug sensitivity varied considerably between patient
samples, indicating that ex vivo drug sensitivity testing
may be helpful prior to the treatment of patients with EOC.
Previous studies with FMCA and similar assays have proven useful in
providing prognostic information (29,33,34).
On the other hand, assay-based drug selection for EOC treatment has
shown variable results. A randomized controlled trial with 180
patients suggested a trend towards improved responses and more
prolonged PFS time from assay-guided therapy. Still, no significant
impact on overall survival could be demonstrated (35). In another comparative yet
non-randomized trial in patients with EOC relapse, a cell-based
assay was useful and revealed longer PFS and overall survival times
in patients with platinum-sensitive disease (36).
In the present study, the cross-resistance in
vitro between the platinum drugs cisplatin and oxaliplatin was
modest and significant, in line with previous findings in
preclinical and clinical settings (37-40)
Oxaliplatin differs somewhat from cisplatin concerning mechanism of
action and resistance (30).
Oxaliplatin is effective in EOC, but cisplatin is the platinum drug
established in first-line treatment of EOC (41,42).
Previously published FMCA EOC results suggested no cross-resistance
between docetaxel and cisplatin, supporting different pathways of
action and clinical benefits with combinations of platinum and
docetaxel in EOC treatment (29,43-45).
Cross-resistance between cisplatin and docetaxel was modest to low
in the present study and supported the suitability of clinical use
of this combination.
In conclusion, ex vivo assessment of drug
activity based on total cell kill reveals that EOC type I and II
are differently sensitive to standard cytotoxic drugs and recently
introduced TKIs, and that none of these seem very promising for the
treatment of drug-resistant type I disease. Ex vivo reported
tumor cell drug sensitivity in EOC is in line with clinical
experience and outcome, pointing towards a role for such assays to
optimize drug therapy in EOC.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KBj, ISP, RL and PN were responsible for study
conception and design. Drug sensitivity assays were performed by
KBl. KBj, KBl, ISP, KS, AML, FB, ÅN, CA, RL and PN were responsible
for data acquisition, analysis and interpretation. KBj and PN
drafted the manuscript followed by its review and revision by all
authors. All authors approved the final manuscript and take
responsibility for its content. KBj, KBl and PN confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All tumor sampling and data collection was performed
once written informed consent had been obtained, and the study was
approved by the Regional Ethical Committee in Uppsala (approval no.
Dnr 2007/237).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Annika Jonasson
and Mrs. Anna-Karin Lannergård from Uppsala University Hospital
(Uppsala, Sweden) for providing skillful technical assistance.
Funding
This study was supported by grants from the Swedish Cancer
Society (no. 17 0661), the Uppsala-Örebro Regional Research Fund
(no. RFR-228691) and the Lions Research Cancer Fund (no. 2014).
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
ASA
|
American Society of Anesthesiology
|
|
CC
|
completeness of cytoreduction
|
|
CT
|
computed tomography
|
|
EDR
|
extreme drug resistance
|
|
EOC
|
epithelial ovarian cancer
|
|
FMCA
|
fluorometric microculture cytotoxicity
assay
|
|
LDR
|
low drug resistance
|
|
PCI
|
Peritoneal Cancer Index
|
|
PFS
|
progression-free survival
|
|
SD
|
standard deviation
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang S, Dolgalev I, Zhang T, Ran H,
Levine DA and Neel BG: Both fallopian tube and ovarian surface
epithelium are cells-of-origin for high-grade serous ovarian
carcinoma. Nat Commun. 10:53672019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shih IeM and Kurman RJ: Ovarian
Tumorigenesis: A Proposed model based on morphological and
molecular genetic analysis. Am J Pathol. 164:1511–1518. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang SJ, Bristow RE, Chi DS and Cliby WA:
Role of aggressive surgical cytoreduction in advanced ovarian
cancer. J Gynecol Oncol. 26:336–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and Cisplatin compared with paclitaxel and
Cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bookman MA, McGuire WP III, Kilpatrick D,
Keenan E, Hogan WM, Johnson SW, O'Dwyer P, Rowinsky E, Gallion HH
and Ozols RF: Carboplatin and paclitaxel in ovarian carcinoma: A
phase I study of the gynecologic oncology group. J Clin Oncol.
14:1895–1902. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lheureux S, Karakasis K, Kohn EC and Oza
AM: Ovarian cancer treatment: The end of empiricism? Cancer.
121:3203–3211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Despierre E, Yesilyurt BT, Lambrechts S,
Johnson N, Verheijen R, van der Burg M, Casado A, Rustin G, Berns
E, Leunen K, et al: Epithelial ovarian cancer: Rationale for
changing the one-fits-all standard treatment regimen to
subtype-specific treatment. Int J Gynecol Cancer. 24:468–477. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ntanasis-Stathopoulos I, Fotopoulos G,
Tzanninis IG and Kotteas EA: The emerging role of tyrosine kinase
inhibitors in ovarian cancer treatment: A systematic review. Cancer
Invest. 34:313–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farley J, Brady WE, Vathipadiekal V,
Lankes HA, Coleman R, Morgan MA, Mannel R, Yamada SD, Mutch D,
Rodgers WH, et al: Selumetinib in women with recurrent low-grade
serous carcinoma of the ovary or peritoneum: An open-label,
Single-arm, Phase 2 study. Lancet Oncol. 14:134–140. 2013.
View Article : Google Scholar :
|
|
13
|
Matei D, Sill MW, Lankes HA, DeGeest K,
Bristow RE, Mutch D, Yamada SD, Cohn D, Calvert V, Farley J, et al:
Activity of sorafenib in recurrent ovarian cancer and primary
peritoneal carcinomatosis: A Gynecologic Oncology Group trial. J
Clin Oncol. 29:69–75. 2011. View Article : Google Scholar :
|
|
14
|
Hainsworth JD, Thompson DS, Bismayer JA,
Gian VG, Merritt WM, Whorf RC, Finney LH and Dudley BS:
Paclitaxel/carboplatin with or without sorafenib in the first-line
treatment of patients with stage III/IV epithelial ovarian cancer:
A randomized phase II study of the Sarah Cannon Research Institute.
Cancer Med. 4:673–681. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vergote IB, Jimeno A, Joly F, Katsaros D,
Coens C, Despierre E, Marth C, Hall M, Steer CB, Colombo N, et al:
Randomized phase III study of erlotinib versus observation in
patients with no evidence of disease progression after first-line
platin-based chemotherapy for ovarian carcinoma: A European
Organisation for Research and Treatment of Cancer-Gynaecological
Cancer Group, and Gynecologic Cancer Intergroup study. J Clin
Oncol. 32:320–326. 2014. View Article : Google Scholar
|
|
16
|
Omura GA, Brady MF, Homesley HD, Yordan E,
Major FJ, Buchsbaum HJ and Park RC: Long-term follow-up and
prognostic factor analysis in advanced ovarian carcinoma: The
Gynecologic Oncology Group experience. J Clin Oncol. 9:1138–1150.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blumenthal RD and Goldenberg DM: Methods
and goals for the use of in vitro and in vivo chemosensitivity
testing. Mol Biotechnol. 35:185–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugarbaker PH: Peritonectomy procedures.
Ann Surg. 221:29–42. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugarbaker PH: Management of
peritoneal-surface malignancy: The surgeon's role. Langenbecks Arch
Surg. 384:576–487. 1999. View Article : Google Scholar
|
|
20
|
Moran B, Baratti D, Yan TD, Kusamura S and
Deraco M: Consensus statement on the loco-regional treatment of
appendiceal mucinous neoplasms with peritoneal dissemination
(pseudomyxoma peritonei). J Surg Oncol. 98:277–282. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
ASA Physical Status Classification System.
American Society of Anesthesiologists (ASA); December 13–2020
|
|
22
|
Moore RG, MacLaughlan S and Bast RC Jr:
Current state of biomarker development for clinical application in
epithelial ovarian cancer. Gynecol Oncol. 116:240–245. 2010.
View Article : Google Scholar
|
|
23
|
Csóka K, Tholander B, Gerdin E, de La
Torre M, Larsson R and Nygren P: In vitro determination of
cytotoxic drug response in ovarian carcinoma using the fluorometric
microculture cytotoxicity assay (FMCA). Int J Cancer. 72:1008–1012.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindhagen E, Nygren P and Larsson R: The
fluorometric microculture cytotoxicity assay. Nat Protoc.
3:1364–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blom K, Nygren P, Alvarsson J, Larsson R
and Andersson CR: Ex vivo assessment of drug activity in patient
tumor cells as a basis for tailored cancer therapy. J Lab Autom.
21:178–187. 2016. View Article : Google Scholar
|
|
26
|
Larsson R, Fridborg H, Kristensen J,
Sundström C and Nygren P: In vitro testing of chemotherapeutic drug
combinations in acute myelocytic leukaemia using the fluorometric
microculture cytotoxicity assay (FMCA). Br J Cancer. 67:969–974.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmeler KM, Sun CC, Bodurka DC, Deavers
MT, Malpica A, Coleman RL, Ramirez PT and Gershenson DM:
Neoadjuvant chemotherapy for low-grade serous carcinoma of the
ovary or peritoneum. Gynecol Oncol. 108:510–514. 2008. View Article : Google Scholar
|
|
28
|
Gershenson DM, Sun CC, Bodurka D, Coleman
RL, Lu KH, Sood AK, Deavers M, Malpica AL and Kavanagh JJ:
Recurrent low-grade serous ovarian carcinoma is relatively
chemoresistant. Gynecol Oncol. 114:48–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
von Heideman A, Tholander B, Grundmark B,
Cajander S, Gerdin E, Holm L, Axelsson A, Rosenberg P, Mahteme H,
Daniel E, et al: Chemotherapeutic drug sensitivity of primary
cultures of epithelial ovarian cancer cells from patients in
relation to tumour characteristics and therapeutic outcome. Acta
Oncol. 53:242–250. 2014. View Article : Google Scholar
|
|
30
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parmar MKB, Ledermann JA, Colombo N, du
Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W,
Torri V, et al: Paclitaxel plus platinum-based chemotherapy versus
conventional platinum-based chemotherapy in women with relapsed
ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet.
361:2099–2106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bjersand K, Mahteme H, Sundström Poromaa
I, Andréasson H, Graf W, Larsson R and Nygren P: Drug sensitivity
testing in cytoreductive surgery and intraperitoneal chemotherapy
of pseudomyxoma peritonei. Ann Surg Oncol. 22(Suppl 3): S810–S816.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herzog TJ, Krivak TC, Fader AN and Coleman
RL: Chemosensitivity testing with ChemoFx and overall survival in
primary ovarian cancer. Am J Obstet Gynecol. 203:68.e1–e6. 2010.
View Article : Google Scholar
|
|
35
|
Cree IA, Kurbacher CM, Lamont A, Hindley
AC and Love S: A prospective randomized controlled trial of tumour
chemosensitivity assay directed chemotherapy versus physician's
choice in patients with recurrent platinum-resistant ovarian
cancer. Anticancer Drugs. 18:1093–1101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loizzi V, Chan JK, Osann K, Cappuccini F,
DiSaia PJ and Berman ML: Survival outcomes in patients with
recurrent ovarian cancer who were treated with chemoresistance
assay-guided chemotherapy. Am J Obstet Gynecol. 189:1301–1307.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rixe O, Ortuzar W, Alvarez M, Parker R,
Reed E, Paull K and Fojo T: Oxaliplatin, tetraplatin, cisplatin,
and carboplatin: Spectrum of activity in drug-resistant cell lines
and in the cell lines of the National Cancer Institute 's
Anticancer Drug Screen panel. Biochem Pharmacol. 52:1855–1865.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fojo T, Farrell N, Ortuzar W, Tanimura H,
Weinstein J and Myers TG: Identification of non-cross-resistant
platinum compounds with novel cytotoxicity profiles using the NCI
anticancer drug screen and clustered image map visualizations. Crit
Rev Oncol Hematol. 53:25–34. 2005. View Article : Google Scholar
|
|
39
|
Bogliolo S, Cassani C, Gardella B,
Musacchi V, Babilonti L, Venturini PL, Ferrero S and Spinillo A:
Oxaliplatin for the treatment of ovarian cancer. Expert Opin
Investig Drugs. 24:1275–1286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vermorken JB: The integration of
paclitaxel and new platinum compounds in the treatment of advanced
ovarian cancer. Int J Gynecol Cancer. 11(Suppl 1): S21–S30. 2001.
View Article : Google Scholar
|
|
41
|
Herzog TJ, Monk BJ, Rose PG, Braly P,
Hines JF, Bell MC, Wenham RM, Secord AA, Roman LD, Einstein MH, et
al: A phase II trial of oxaliplatin, docetaxel, and bevacizumab as
first-line therapy of advanced cancer of the ovary, peritoneum, and
fallopian tube. Gynecol Oncol. 132:517–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: A phase 3 trial of bevacizumab in
ovarian cancer. N Engl J Med. 365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R; Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vasey PA, Jayson GC, Gordon A, Gabra H,
Coleman R, Atkinson R, Parkin D, Paul J, Hay A and Kaye SB;
Scottish Gynaecological Cancer Trials Group: Phase III randomized
trial of docetaxel-carboplatin versus paclitaxel-carboplatin as
first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst.
96:1682–1691. 2004. View Article : Google Scholar : PubMed/NCBI
|