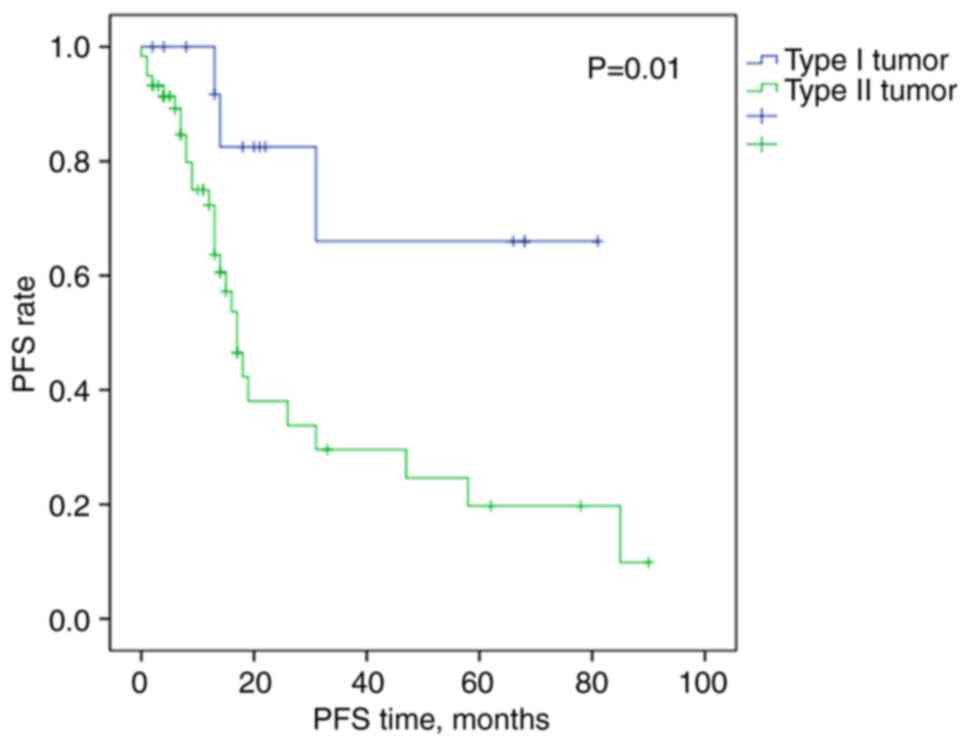
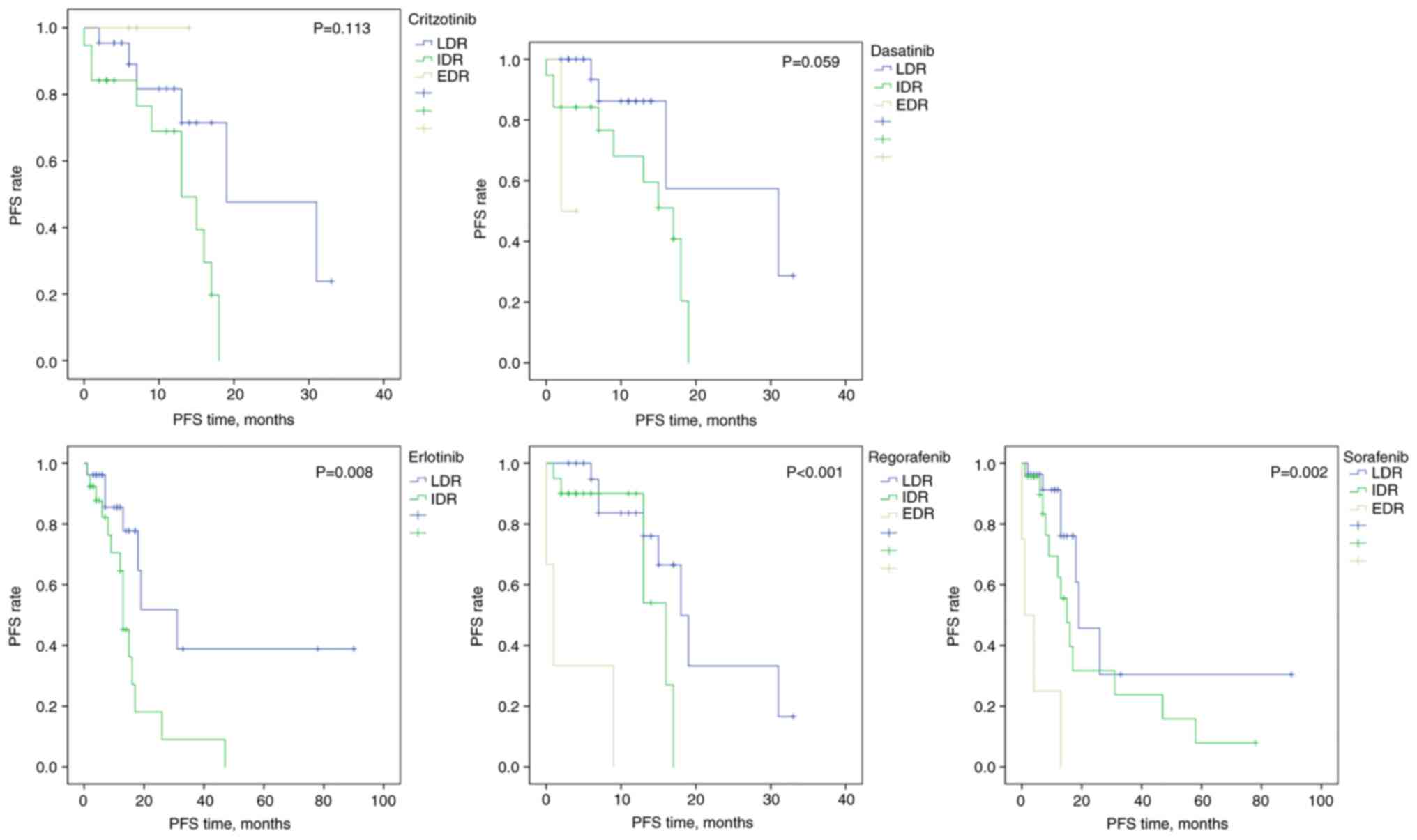
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang S, Dolgalev I, Zhang T, Ran H,
Levine DA and Neel BG: Both fallopian tube and ovarian surface
epithelium are cells-of-origin for high-grade serous ovarian
carcinoma. Nat Commun. 10:53672019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shih IeM and Kurman RJ: Ovarian
Tumorigenesis: A Proposed model based on morphological and
molecular genetic analysis. Am J Pathol. 164:1511–1518. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang SJ, Bristow RE, Chi DS and Cliby WA:
Role of aggressive surgical cytoreduction in advanced ovarian
cancer. J Gynecol Oncol. 26:336–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and Cisplatin compared with paclitaxel and
Cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bookman MA, McGuire WP III, Kilpatrick D,
Keenan E, Hogan WM, Johnson SW, O'Dwyer P, Rowinsky E, Gallion HH
and Ozols RF: Carboplatin and paclitaxel in ovarian carcinoma: A
phase I study of the gynecologic oncology group. J Clin Oncol.
14:1895–1902. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lheureux S, Karakasis K, Kohn EC and Oza
AM: Ovarian cancer treatment: The end of empiricism? Cancer.
121:3203–3211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Despierre E, Yesilyurt BT, Lambrechts S,
Johnson N, Verheijen R, van der Burg M, Casado A, Rustin G, Berns
E, Leunen K, et al: Epithelial ovarian cancer: Rationale for
changing the one-fits-all standard treatment regimen to
subtype-specific treatment. Int J Gynecol Cancer. 24:468–477. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ntanasis-Stathopoulos I, Fotopoulos G,
Tzanninis IG and Kotteas EA: The emerging role of tyrosine kinase
inhibitors in ovarian cancer treatment: A systematic review. Cancer
Invest. 34:313–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farley J, Brady WE, Vathipadiekal V,
Lankes HA, Coleman R, Morgan MA, Mannel R, Yamada SD, Mutch D,
Rodgers WH, et al: Selumetinib in women with recurrent low-grade
serous carcinoma of the ovary or peritoneum: An open-label,
Single-arm, Phase 2 study. Lancet Oncol. 14:134–140. 2013.
View Article : Google Scholar :
|
|
13
|
Matei D, Sill MW, Lankes HA, DeGeest K,
Bristow RE, Mutch D, Yamada SD, Cohn D, Calvert V, Farley J, et al:
Activity of sorafenib in recurrent ovarian cancer and primary
peritoneal carcinomatosis: A Gynecologic Oncology Group trial. J
Clin Oncol. 29:69–75. 2011. View Article : Google Scholar :
|
|
14
|
Hainsworth JD, Thompson DS, Bismayer JA,
Gian VG, Merritt WM, Whorf RC, Finney LH and Dudley BS:
Paclitaxel/carboplatin with or without sorafenib in the first-line
treatment of patients with stage III/IV epithelial ovarian cancer:
A randomized phase II study of the Sarah Cannon Research Institute.
Cancer Med. 4:673–681. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vergote IB, Jimeno A, Joly F, Katsaros D,
Coens C, Despierre E, Marth C, Hall M, Steer CB, Colombo N, et al:
Randomized phase III study of erlotinib versus observation in
patients with no evidence of disease progression after first-line
platin-based chemotherapy for ovarian carcinoma: A European
Organisation for Research and Treatment of Cancer-Gynaecological
Cancer Group, and Gynecologic Cancer Intergroup study. J Clin
Oncol. 32:320–326. 2014. View Article : Google Scholar
|
|
16
|
Omura GA, Brady MF, Homesley HD, Yordan E,
Major FJ, Buchsbaum HJ and Park RC: Long-term follow-up and
prognostic factor analysis in advanced ovarian carcinoma: The
Gynecologic Oncology Group experience. J Clin Oncol. 9:1138–1150.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blumenthal RD and Goldenberg DM: Methods
and goals for the use of in vitro and in vivo chemosensitivity
testing. Mol Biotechnol. 35:185–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugarbaker PH: Peritonectomy procedures.
Ann Surg. 221:29–42. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugarbaker PH: Management of
peritoneal-surface malignancy: The surgeon's role. Langenbecks Arch
Surg. 384:576–487. 1999. View Article : Google Scholar
|
|
20
|
Moran B, Baratti D, Yan TD, Kusamura S and
Deraco M: Consensus statement on the loco-regional treatment of
appendiceal mucinous neoplasms with peritoneal dissemination
(pseudomyxoma peritonei). J Surg Oncol. 98:277–282. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
ASA Physical Status Classification System.
American Society of Anesthesiologists (ASA); December 13–2020
|
|
22
|
Moore RG, MacLaughlan S and Bast RC Jr:
Current state of biomarker development for clinical application in
epithelial ovarian cancer. Gynecol Oncol. 116:240–245. 2010.
View Article : Google Scholar
|
|
23
|
Csóka K, Tholander B, Gerdin E, de La
Torre M, Larsson R and Nygren P: In vitro determination of
cytotoxic drug response in ovarian carcinoma using the fluorometric
microculture cytotoxicity assay (FMCA). Int J Cancer. 72:1008–1012.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindhagen E, Nygren P and Larsson R: The
fluorometric microculture cytotoxicity assay. Nat Protoc.
3:1364–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blom K, Nygren P, Alvarsson J, Larsson R
and Andersson CR: Ex vivo assessment of drug activity in patient
tumor cells as a basis for tailored cancer therapy. J Lab Autom.
21:178–187. 2016. View Article : Google Scholar
|
|
26
|
Larsson R, Fridborg H, Kristensen J,
Sundström C and Nygren P: In vitro testing of chemotherapeutic drug
combinations in acute myelocytic leukaemia using the fluorometric
microculture cytotoxicity assay (FMCA). Br J Cancer. 67:969–974.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmeler KM, Sun CC, Bodurka DC, Deavers
MT, Malpica A, Coleman RL, Ramirez PT and Gershenson DM:
Neoadjuvant chemotherapy for low-grade serous carcinoma of the
ovary or peritoneum. Gynecol Oncol. 108:510–514. 2008. View Article : Google Scholar
|
|
28
|
Gershenson DM, Sun CC, Bodurka D, Coleman
RL, Lu KH, Sood AK, Deavers M, Malpica AL and Kavanagh JJ:
Recurrent low-grade serous ovarian carcinoma is relatively
chemoresistant. Gynecol Oncol. 114:48–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
von Heideman A, Tholander B, Grundmark B,
Cajander S, Gerdin E, Holm L, Axelsson A, Rosenberg P, Mahteme H,
Daniel E, et al: Chemotherapeutic drug sensitivity of primary
cultures of epithelial ovarian cancer cells from patients in
relation to tumour characteristics and therapeutic outcome. Acta
Oncol. 53:242–250. 2014. View Article : Google Scholar
|
|
30
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parmar MKB, Ledermann JA, Colombo N, du
Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W,
Torri V, et al: Paclitaxel plus platinum-based chemotherapy versus
conventional platinum-based chemotherapy in women with relapsed
ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet.
361:2099–2106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bjersand K, Mahteme H, Sundström Poromaa
I, Andréasson H, Graf W, Larsson R and Nygren P: Drug sensitivity
testing in cytoreductive surgery and intraperitoneal chemotherapy
of pseudomyxoma peritonei. Ann Surg Oncol. 22(Suppl 3): S810–S816.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herzog TJ, Krivak TC, Fader AN and Coleman
RL: Chemosensitivity testing with ChemoFx and overall survival in
primary ovarian cancer. Am J Obstet Gynecol. 203:68.e1–e6. 2010.
View Article : Google Scholar
|
|
35
|
Cree IA, Kurbacher CM, Lamont A, Hindley
AC and Love S: A prospective randomized controlled trial of tumour
chemosensitivity assay directed chemotherapy versus physician's
choice in patients with recurrent platinum-resistant ovarian
cancer. Anticancer Drugs. 18:1093–1101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loizzi V, Chan JK, Osann K, Cappuccini F,
DiSaia PJ and Berman ML: Survival outcomes in patients with
recurrent ovarian cancer who were treated with chemoresistance
assay-guided chemotherapy. Am J Obstet Gynecol. 189:1301–1307.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rixe O, Ortuzar W, Alvarez M, Parker R,
Reed E, Paull K and Fojo T: Oxaliplatin, tetraplatin, cisplatin,
and carboplatin: Spectrum of activity in drug-resistant cell lines
and in the cell lines of the National Cancer Institute 's
Anticancer Drug Screen panel. Biochem Pharmacol. 52:1855–1865.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fojo T, Farrell N, Ortuzar W, Tanimura H,
Weinstein J and Myers TG: Identification of non-cross-resistant
platinum compounds with novel cytotoxicity profiles using the NCI
anticancer drug screen and clustered image map visualizations. Crit
Rev Oncol Hematol. 53:25–34. 2005. View Article : Google Scholar
|
|
39
|
Bogliolo S, Cassani C, Gardella B,
Musacchi V, Babilonti L, Venturini PL, Ferrero S and Spinillo A:
Oxaliplatin for the treatment of ovarian cancer. Expert Opin
Investig Drugs. 24:1275–1286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vermorken JB: The integration of
paclitaxel and new platinum compounds in the treatment of advanced
ovarian cancer. Int J Gynecol Cancer. 11(Suppl 1): S21–S30. 2001.
View Article : Google Scholar
|
|
41
|
Herzog TJ, Monk BJ, Rose PG, Braly P,
Hines JF, Bell MC, Wenham RM, Secord AA, Roman LD, Einstein MH, et
al: A phase II trial of oxaliplatin, docetaxel, and bevacizumab as
first-line therapy of advanced cancer of the ovary, peritoneum, and
fallopian tube. Gynecol Oncol. 132:517–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: A phase 3 trial of bevacizumab in
ovarian cancer. N Engl J Med. 365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R; Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vasey PA, Jayson GC, Gordon A, Gabra H,
Coleman R, Atkinson R, Parkin D, Paul J, Hay A and Kaye SB;
Scottish Gynaecological Cancer Trials Group: Phase III randomized
trial of docetaxel-carboplatin versus paclitaxel-carboplatin as
first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst.
96:1682–1691. 2004. View Article : Google Scholar : PubMed/NCBI
|