Urokinase-type plasminogen activator receptor (uPAR)
was originally identified on the monocyte/macrophage-like human
cell line U937 as the membrane receptor for the serine protease uPA
in 1985 (1). The n9omenclature of
uPAR reflects its primary function, where uPA binds to uPAR to
activate the conversion of plasminogen into plasmin, followed by
the mobilization of a proteolysis cascade to degrade the
extracellular matrix (ECM), which provides structural support and
an elaborate microenvironment for biological processes to occur
within tissues (2). uPAR is also
named CD87 in the clusters of differentiation classification, which
is expressed by certain immune cell types (3,4).
As uPAR is tethered to the cell surface by a
glycosylphosphatidylinositol (GPI) anchor, it lacks a transcellular
or intracellular framework. Furthermore, uPAR cooperates with
lateral parts, mainly integrin, to assemble complexes and regulate
signaling cascades, which are not restricted to proteolysis
function.
In the first decade after the discovery of uPAR,
researchers have attempted to decipher the molecular structures and
functions of uPAR, uPA, plasmin, inhibitors plasminogen activator
inhibitor type-1 (PAI-1) and PAI-2 (5–7).
In the second decade, preclinical and clinical evidence
demonstrated that uPAR is expressed at high levels in cancer
tissues compared to its undetected expression level in para-cancer
or normal tissues. Overexpression of uPAR promotes carcinogenesis
by influencing proliferation, metastasis, invasion, apoptosis,
dormancy, drug resistance and recurrence of cancer cells,
indicating that uPAR is a major biomarker for cancer invasion and
metastasis (8–10). In the last decade, with the
development of novel therapeutic methods, uPAR and the uPA-uPAR
complex have been established as targets for therapeutic
interventions (11,12). Numerous clinical trials pay
attention to uPAR-specific drugs (13–15).
On the one hand, numerous studies have reported the
construction of uPAR and signaling pathways associated with uPAR in
detail (2,15). However, the non-specific
expression of uPAR in certain normal tissues may discount the
potential of uPAR as a clinically actionable therapeutic target.
Cancer cells display heterogeneity depending on the tumor
microenvironment (TME) and the interactions between stromal and
cancer cells are dependent on the spatial and topographic
distribution of uPAR in stromal and cancer cells, indicating that
the functions of uPAR are not restricted to proteolysis (16–19). Therefore, to exploit whether uPAR
may serve as an efficient therapeutic target, the functions of uPAR
in metabolism, metastasis, invasion and inflammation require to be
discussed.
uPAR is located on the outer leaflet of the cell
plasma membrane as a GPI-anchored cysteine-rich protein receptor.
The primary ligand of uPAR is uPA and the binding of uPA to uPAR
leads to the initiation of the plasminogen activation cascade and
the regulation of pericellular proteolysis in cancer. Furthermore,
uPAR mediates several biological processes, such as inflammation,
cell migration, tissue remodeling, angiogenesis and cell adhesion
in certain normal cells (14,20,21).
uPAR is a member of the lymphocyte antigen-6 (Ly6)
superfamily and this superfamily is characterized by a three-finger
Ly6 domain. Members of the human Ly6 family consist of 35 members,
including uPAR, CD177, CD59 and prostate stem cell antigen. The Ly6
superfamily are involved in similar signaling cascades and
biological functions, e.g. C4.4A, a structural uPAR homolog, which
is also implicated in the invasion and metastasis of numerous
cancer types (28–31).
uPA and its homolog tissue-type PA (tPA) cleave
plasminogen to plasmin, thereby mediating the degradation of fibrin
and other ECM proteins (32). tPA
controls the degradation of intravascular fibrin, while uPA
controls the production of plasmin during cell migration and
invasion (33). Plasmin may
degrade vitronectin, laminin, fibronectin, fibrin and proteoglycans
(33).
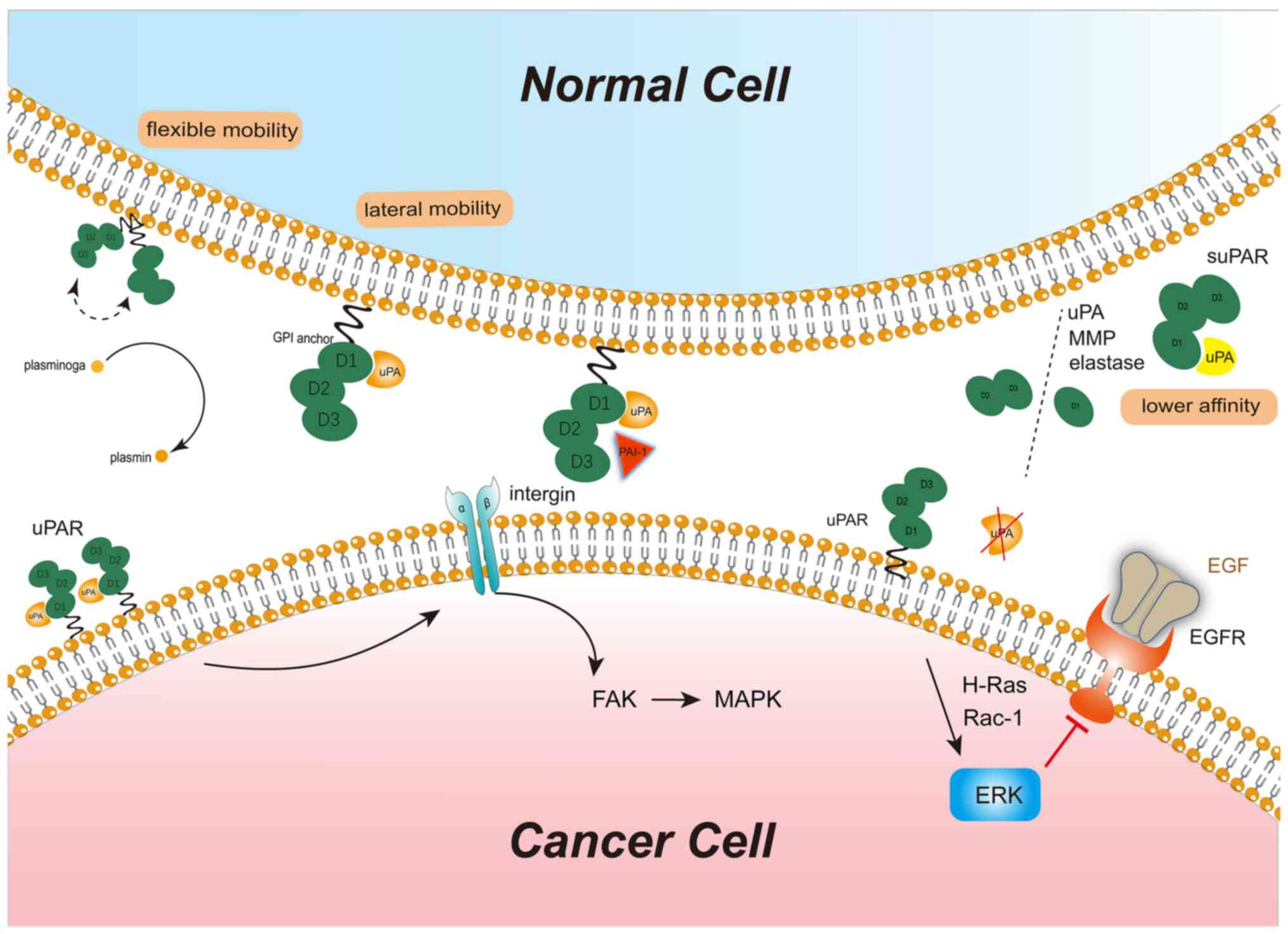
In addition to the fundamental features of
ligand-receptor binding, uPA-uPAR binding has four unique
characteristics that facilitate several disease processes. First,
the binding of uPA to uPAR has a topographical limitation. uPA is
localized at the cell substratum and cell-cell contact sites
through focal adhesions. When uPAR is occupied by uPA, its lateral
mobility is restricted, but the unoccupied uPAR has relatively
flexible mobility (34). This
hallmark of uPAR facilitates active proteolysis within the
cell-cell contact region. Therefore, the uPA-uPAR complex is not
only an activation factor for proteolysis but also a platform for
the actions of proteases. Furthermore, uPA binds to uPAR in a cell
type-specific manner. The expression of uPAR is relatively specific
to the cell type; uPAR is detected at high levels in cancer cells
and cancer-associated stromal cells and moderate levels in
monocytes, as well as certain endometrial cells, under
physiological conditions. However, uPAR is barely detected in most
normal tissues. Even though cells express uPAR, the formation of
the uPA-uPAR complex depends on cellular functions. For instance,
uPAR proteins on rhabdomyosarcoma cells are mostly occupied and
confined to the cell adhesion sites, but uPAR proteins remain
largely unoccupied on fibroblasts (HES cells) (34). Occupied uPAR promotes the
neoplastic transformation of normal cells. In addition, the binding
of uPA to uPAR is not necessary for uPAR to achieve its function.
uPAR functions in downstream signaling without binding to its
ligands. For instance, in estrogen receptor (ER)-positive breast
cancer, but in the absence of estrogen, uPAR without binding to uPA
may activate extracellular signal-regulated kinase (ERK)-epidermal
growth factor receptor (EGFR) pathway depending on H-Ras and Rac-1
to facilitate cell proliferation (35). As another characteristic, although
the uPA-uPAR protein-protein interaction is a tight high-affinity
(dissociation constant KD=1 nM) and stable interaction
(dissociation rate constant koff=10−4
S−1), the affinity depends not only on the binding site
but also on the molecular integrity of uPAR (36). Although the functional binding
site of the intact form of uPAR for uPA is DI, soluble uPAR DI
binds to uPA with low affinity, indicating that the structure and
integrity of the binding site are important for the effective
binding of uPAR to uPA with high affinity (37). Of note, the binding site of uPAR
for uPA is also the site where uPA cleaves uPAR into variant forms
of suPAR. The affinity of uPAR for uPA and the stability of the
uPA-uPAR complex are dependent on the membrane localization of uPA.
In healthy individuals, only intact suPAR may be detected in plasma
and/or serum, while in patients with cancer, both intact and
cleaved suPAR may be identified; thus, cleavage is unique for
cancer (37).
Several ligands have been reported to bind to uPAR,
out of which uPA is the most widely known ligand. The interaction
of uPAR and integrin activates proteolytic cell signals and
promotes the localization of proteases to the cell surface
(38). In hepatocellular
carcinoma cells, overexpressed uPAR, through interaction with α5β1
integrin, initiates an intracellular signaling cascade through the
conversion of focal adhesion kinase (FAK) to active the MAPK ERK to
induce cell growth (39).
Blocking this pathway using FAK-related non-kinase resulted in cell
dormancy without any changes in the apoptotic rate or the
expression of proteins associated with the Akt signaling pathway
(39). Furthermore, in ovarian
cancer cells, the inhibition of the co-localization of α5 integrin
and uPAR affects apoptosis but not angiogenesis (microvessel
density) or proliferation (Ki-67) (40). α2β2 integrin (also known as MAC1)
and uPAR co-localize on the surface of monocytes and neutrophils
and induce leukocyte recruitment to the tumor environment in a
uPA-independent manner (41,42). However, a study indicated that
uPAR interacts with α5β6 integrin in ovarian and colorectal cancer
cells to modulate cell migration, revealing that α5β6 integrin
binds to the DII and DIII of uPAR (38).
The endogenous inhibitors of the uPA/uPAR system,
namely PAI-1 and PAI-2, regulate the activity of uPA-uPAR through
direct inhibition or their effect on cell surface expression of
uPAR and internalization of uPA (43). uPAR-uPA-plasmin degrades ECM,
mainly collagen, in homeostasis. Excessive PAI-1 results in the
accumulation of collagen by inhibition of uPAR-uPA-plasmin to form
fibrosis in ECM (44).
Furthermore, PAI-1 has controversial roles in tumors: It inhibits
the activation of uPA-uPAR to suppress invasion and metastasis,
while on the other hand, it promotes angiogenesis and cancer growth
(45). The processes of ligands
binding with uPAR and activation of downstream signals are
illustrated in Fig. 1.
Although vast research focused on uPAR in cancer,
uPAR and its ligands are not obsoletely exclusive to cancer. Its
roles in non-cancer contexts should not be ignored and its
functions in physiological processes should be explored to
comprehensively appraise uPAR. Virtually, without any set standard
for its expression levels, the uPA-uPAR system exists in every body
system, such as the digestive and respiratory tracts, bones, skin,
breast, genital organs and brain, as well as the urinary, central
nervous, endocrine and hematopoietic systems (21). Within those systems, under
physiological conditions, uPAR is expressed in dynamic biological
processes, such as the migration of trophoblast cells and
keratinocytes at the site of epidermal injury, and is expressed on
cells with low numbers, and it mainly localizes at focal contacts
and cell-cell contact sites (46). The activated plasmin-uPA-uPAR axis
causes the retraction of endothelial cells, thereby exposing the
basement membrane for its digestion and degradation (47). The in-depth understanding of the
restricted expression of uPAR in non-cancerous tissues will
facilitate the identification of malignancies.
In the nervous system, uPAR is involved in both
physiological and pathological processes. A mouse model deficient
in uPA and uPAR indicated an aggravated form of autoimmune
encephalomyelitis, proving that the uPA-uPAR complex is
indispensable for central nervous system inflammation (48). Furthermore, the results suggested
that T lymphocytes in those mice had reduced reactivity towards
encephalitogenic antigens (48).
Diaz et al (49) observed
that neurons are able to release uPA and that astrocytes recruit
uPAR to their plasma membrane during the recovery phase from a
hypoxic injury. The binding of neuronal uPA to astrocytic uPAR
induces astrocytic activation by a mechanism that does not require
plasmin generation, but is mediated by the ERK1/2-regulated
phosphorylation of the STAT3, astrocytic thrombospondin-1 and
synaptic low-density lipoprotein receptor-related protein-1,
demonstrating that the binding of uPA to uPAR initiates functions
not limited to proteolysis (49).
In cardiac myocytes, the beneficial effects of
uPA-uPAR binding are independent of the proteolytic function. For
instance, in human adult cardiac myocytes (HACMs), both uPA and
amino-terminal fragment (ATF) of uPA, which is devoid of the
proteolytic domain but is able to bind to uPAR as uPA, may protect
HACMs from oxidative damage by increasing the expression of
8-hydroxyguanine DNA glycosylase for DNA damage repair and
inhibiting p53-induced apoptosis (50). In the hematological system,
patients with paroxysmal nocturnal hemoglobinuria have decreased
expression of GPI-anchored uPAR on granulocytes and platelets but
exhibit increased suPAR in plasma. Somatic mutations in the gene
encoding phosphatidylinositol-glycan biosynthesis class A protein
result in the failure of synthesis of the GPI-moiety for protein
anchoring and suPAR is not shed from the cell surface but secreted
from leukocytes directly. The absence of membrane uPAR contributes
to decreased activation of plasmin based on uPA-uPAR binding,
giving rise to complications such as thromboembolism (51,52). In the immune response against
pathogen infections, uPAR in monocytes and neutrophils is
implicated in leukocyte recruitment, and is a potential maturation
marker for leukocytes (29,41,42,53). For instance, when the lungs of
uPAR-deficient mice were infected by Pseudomonas aeruginosa,
there was no recruitment of neutrophils to the sites of infection.
However, in wild-type mice, uPAR binding to β2 integrin induced
neutrophil recruitment to the sites of infection in a uPA-and
proteolysis-independent manner (54). Furthermore, during lung injury
caused by the decreased expression of surfactant protein C, p53
directly bound to 3′UTR sequences of uPA, uPAR and PAI-1 and
contributed to the apoptosis of alveolar epithelial cells (AECs).
However, the suppressed expression of uPA and uPAR caused by p53
and the increased expression of PAI-1 indicated that the inhibition
of the uPA system may induce apoptosis in AECs (55).
In airway epithelial cells, membrane-bound uPAR
rather than suPAR orchestrates events such as proliferation,
apoptosis and migration under normal conditions; however, the
expression of uPAR alone is upregulated in the sputum of patients
with asthma, chronic obstructive pulmonary disease and cystic
fibrosis (56). In the bronchial
epithelium, human airway trypsin-like protease (HAT) truncates
full-length uPAR (DI-DII-DIII) into DII-DIII, which does not bind
to its ligands-uPA and vitronectin. Therefore, uPAR is regulated by
HAT in a ligand-independent manner, but the process involves the
proteolytic function of uPAR in tissue remodeling (57). In normal lung epithelial cells,
the uPA-uPAR-PAI-1 axis mediates fibrinolytic activity, while in
acute lung injury and pulmonary fibrosis, all of the three
components are active, which is similar to the scenario observed in
cancer (58).
In the normal renal filtration barrier, uPAR is
dispensable and the components of the plasminogen system, uPA, tPA
and PAI-1, are expressed at relatively low levels. However, during
podocyte damage, overexpression of uPA, uPAR and PAI-1 induces
podocyte migration and podocyte apoptosis by plasminogen activation
through the uPA-uPAR-α5β3 integrin axis (59). The levels of uPAR are also
elevated in patients with kidney graft rejection (59). The expression of suPAR directly
correlates with the degree of proteinuria and its interaction with
apolipoprotein L1 serves as a strong predictor of kidney disease
(60). In the digestive system,
the upregulated uPA-uPAR pathway damages the epithelial barrier
integrity of the intestinal mucosa, which is a cause of
inflammatory bowel disease (61).
In colonic crypt luminal surface epithelial cells, the expression
of uPAR is regulated by Kruppel-like 4 transcription factor
(62). The primary cause of liver
fibrosis is cellular senescence and senescent cells exhibit
upregulation of uPAR. Therefore, chimeric antigen receptor (CAR) T
cells targeting uPAR have been proved to be suitable for the
treatment of liver fibrosis (63).
Of note, uPAR is indispensable for the menstruation
cycle and decidualization in the uterus; however, uPAR is also
associated with poor prognosis for endometrial cancer (64,65). The expression of uPAR is always at
moderate levels in non-cancerous tissues with certain beneficial
effects, which are summarized in Table I.
The uPA system is involved in the proliferation,
adhesion, migration and angiogenesis of tumor cells in common
cancer types (22). Accumulating
evidence has suggested that uPAR has a critical role in
malignancies and the relatively specific functions of the uPA-uPAR
complex include the regulation of cell metastasis, invasion and
cell metabolism (64). The main
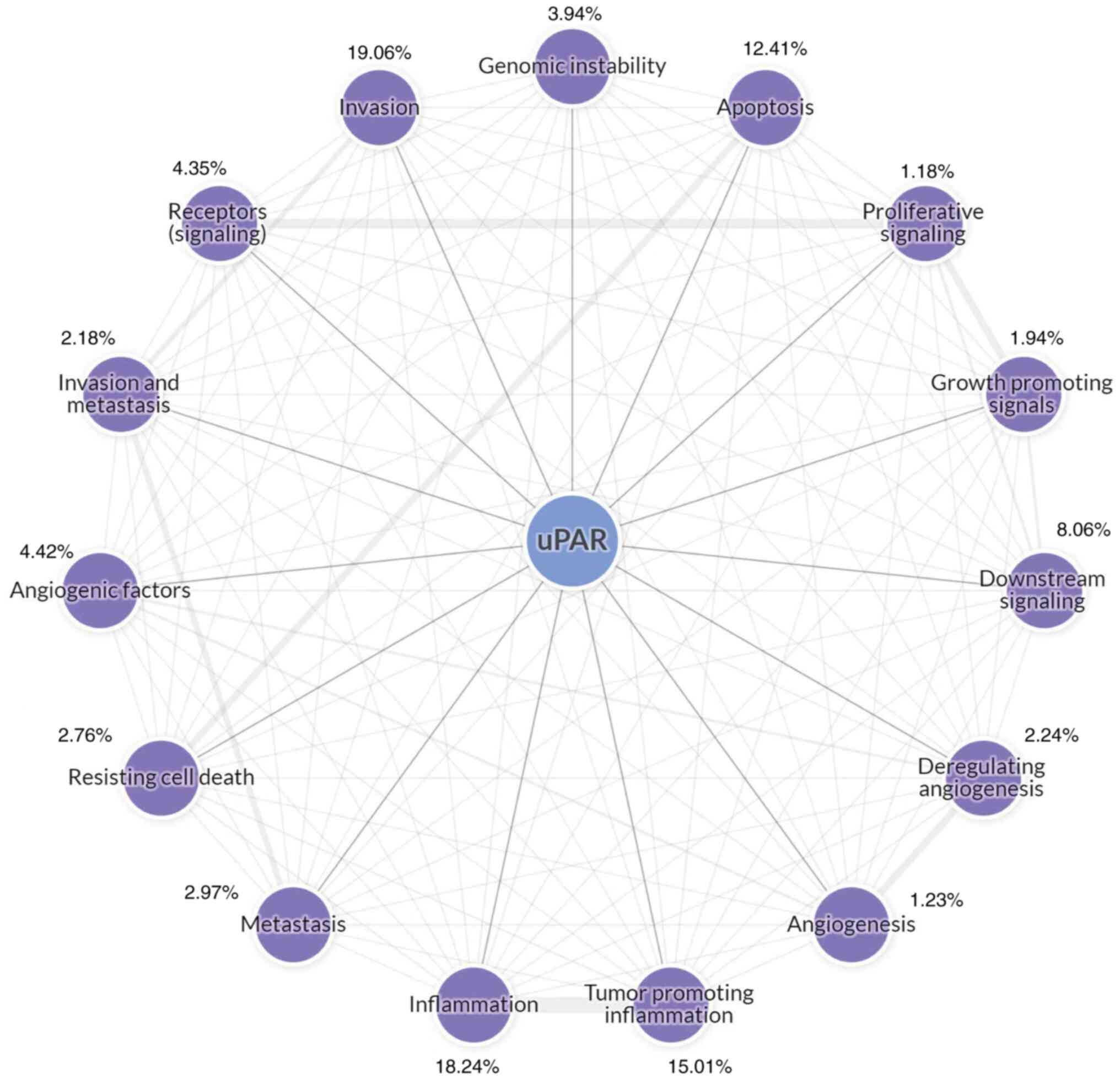
functions of uPAR determined by most studies are presented in
Fig. 2, which were collected by
the Cancer Hallmarks Analytics Tool (http://chat.lionproject.net/). However, its role in
cell energetics and expression in stromal cells have remained
largely unexplored.
In cancer cell metabolism and energetics, as opposed
to normal cells, cancer cells use glycolysis to produce ATP by a
process called aerobic glycolysis or the Warburg effect (65). At present, cellular energetics is
a minority field for uPAR, as indicated with the Cancer Hallmarks
Analytics Tool (Fig. 2), but uPAR
has been demonstrated to be involved in both glycolysis and
reactive oxygen species (ROS). Laurenzana et al (65) reported that uPAR overexpression
promotes glycolysis depending on the uPA-uPAR-a5b1 integrin axis
connected with EGFR involved in the PI3K-mTOR-HIFα pathway in
melanoma cells. In melanoma, the number of mitochondria in cancer
cells increased when uPAR was knocked out by CRISPR/Cas9 (66). High glucose and insulin levels
increase the production of ROS to activate uPA-uPAR and PAI-1
proteolysis function in breast cancer (67). In cell metastasis and cell
invasion, epithelial-mesenchymal transition (EMT) is a non-binary
process where epithelial cells gradually acquire characteristics of
mesenchymal cells, wherein cancer cells express markers with
different types and levels (68).
Highly activated plasmin-uPA-uPAR signaling induces increases in
interleukin-like EMT inducer (ILEI) secretion and changes in the
subcellular location of ILEI, which facilitates EMT to affect
cancer progression in breast cancer (69). The crosstalk of uPAR with formyl
peptide receptor type 1 (FPR1) through both autocrine and paracrine
signaling induces invasion in melanoma cells (70). The 84–95 sequence of uPAR triggers
the activation of FPR1 in a uPA-dependent manner (70). To inhibit invasiveness of
glioblastoma cells, the solute carrier family 8 member 2 protein
was utilized to inactivate the ERK1/2-NF-κB-MMPs/uPA-uPAR pathway
(71). In hepatocellular
carcinoma, PDZ-binding kinase enhanced uPAR expression by
inducing the binding of ETS translocation variant 4 to the promoter
region of uPAR, thus leading to cancer invasion and metastasis
(72). In gastric cancer,
overexpression of uPA and uPAR and knockdown of PAI-1 resulted in
increased propensity for peritoneal metastasis in mice (73). Diallyl disulfide was reported to
downregulate uPAR by inhibiting the ERK/Fra-1 pathway to suppress
metastasis of gastric cancer cells (74). In addition, upregulation of uPAR
may be an early marker from chronic inflammation to cancer in the
stomach. uPAR was detected at high levels in gastric epithelial
cells of mice during persistent infection with Helicobacter
pylori (75). Of note, the
baseline soluble uPAR level in serum prior to chemotherapy may
predict adverse outcomes for patients; those cancers are types
associated with an inflammatory state, such as lung and colon
cancer (76).
Increasing evidence indicates that stromal
non-neoplastic cells have an important role in the TME; the
interaction of stromal cells with tumor cells promotes tumor
progression (22). uPAR has been
reported to be abundantly expressed in stromal cells, including
tumor fibroblasts, macrophages and endothelial cells, as well as
tumor-infiltrating lymphocytes. uPAR expression in endothelial
cells is involved in angiogenesis in cancer tissue. When the
plasmin-uPA-uPAR axis is over-activated, endothelial cells undergo
basement membrane degradation, migrate into the interstitial matrix
and proliferate to form new vessels within the tumor tissue
(77). In malignancies, uPAR may
regulate angiogenic growth factor-induced endothelial cell
migration and survival (78).
uPAR uses the proteolytic activity of uPA on the endothelial cell
surface to promote angiogenesis via the uPAR/uPA-plasmin-TGFβ1
positive feedback loop in a protease-dependent manner (79). Unseld et al (80) observed that uPAR cooperated with
a5 integrin to activate FAK in an NF-κB-dependent manner, leading
to downregulation of phosphatase and tensin homolog to promote
angiogenesis in endothelial cells. However, in endothelial cells,
suPAR may promote angiogenesis by its chemotactic sequence
Ser88-Arg-Ser-Arg-Tyr92 via a direct mechanism involving the
vitronectin receptor and G-protein-coupled formyl peptide receptor
independent of uPAR proteolytic activity (77). Owing to the characteristics of
expression of uPAR in stromal cells, selective targeting of uPAR in
stromal cells may inhibit cancer progression (81,82). Stromal-targeting oncolytic measles
virus, which was designed by exploiting the binding affinity of uPA
for uPAR, induced apoptosis of breast cancer cells (82). This type of oncolytic virus is
able to disintegrate tumor vasculature by targeting uPAR in
epithelial cells. The addition of ATF to stromal uPAR-targeting
oncolytic viruses is associated with increased apoptosis in breast
cancer cells (82).
Besides metabolism, metastasis, invasion and
inflammation, uPAR has special functions for gynecological cancers
and breast cancers in females beyond common cancer types. Those
functions are related to the characteristics of each cancer type,
which may provide a research direction or treatment approach for
female cancers.
Normal-appearing epithelium and normal female breast
tissue were reported to be negative for uPAR (83). Of note, uPAR has special relations
with the specific biomarkers HER2 and ER in breast cancer. First,
HER2 has a strong co-expression with the level of uPAR. In a
paraffin-embedded breast cancer tissue reverse-phase protein
microarray containing 106 patient samples, uPAR was found to be
significantly expressed and strongly correlated with HER2
overexpression; however, uPA expression was not correlated with
HER2 (84). Gene amplification
and overexpression of HER2 and uPAR occur in most cancers and
decreased expression was induced by the other one being silenced by
RNA interference. HER2 upregulates uPAR by activating Src and
protein kinase C; furthermore, uPAR and HER2 may cooperate to
activate ERK to induce cancer cell growth and to evade apoptosis in
breast cancer (85). Second, the
expression of ER is a major hallmark of most breast cancers, as
breast cancer is a sex hormone-dependent tumor. uPA-uPAR performs
functions depending on the ER and estrogen. In ER-positive breast
cancer cells, the binding of uPA with uPAR in the presence of
estrogen induces the activation of ERK to phospho-ERK, a cell
survival factor; however, in ER-negative breast cancer cells, in
the absence of estrogen, uPAR induces the activation of ERK without
uPA through a partially different pathway (35).
Among gynecologic malignancies, ovarian cancer is
the second most commonly diagnosed cancer and the second leading
cause of cancer-related death; besides the high mortality, ovarian
cancer, with large amounts of ascites, seriously affects the
quality of life of patients (86,87). Approximately 70% of ovarian
cancers are diagnosed at a terminal stage, and only 30% of patients
with ovarian cancer may expect to survive for five years (88). uPAR has an integral role in
promoting ovarian cancer proliferation, invasion and particularly
metastasis. To reveal peritoneal metastasis, a study using
uPAR−/− and uPAR+/+ mice suggested that
ablation of uPAR prevented tumor growth and peritoneal implants and
also prolonged the survival, decreased ascitic fluid accumulation
and decreased macrophage infiltration in uPAR−/− mice
compared with their uPAR+/+ counterparts (89). The results indicated that uPAR has
an important role in increasing vascular permeability and the
formation of an inflammatory environment around ovarian cancer
(89). Furthermore, uPAR was
observed to be involved in a series of peritoneal metastatic
cascade reactions. The host uPAR promotes ovarian cancer cell
proliferation, mesothelial adhesion and invasion (89). To inhibit metastasis, humanized
monoclonal antibody, ATN-658, which is an antibody against uPAR,
may be used to inhibit metastasis and invasion of ovarian cancer
cells (40).
The uPAR system has emerged as a biomarker for the
diagnosis and poor prognosis of patients with ovarian cancer. The
expression of uPAR was studied in 162 epithelial ovarian cancer
tissues, which indicated that 92% of the ovarian tumors expressed
uPAR (40). Chambers et al
(90) measured the levels of
uPAR, uPA, PAI-1 and PAI-2 in the ascites of patients with
epithelial ovarian cancer (EOC) and indicated that the uPA/uPAR
system affects the biology of ovarian cancer. Upregulation of uPA
and PAI-1 has been found in >75% of primary ovarian carcinomas,
most metastatic EOC samples and EOC cell lines (91). Patients with advanced ovarian
carcinoma with elevated levels of uPA and PAI-1 are more prone to
earlier disease recurrence and poorer survival outcomes compared
with patients with low levels of these proteolytic factors in tumor
tissues (92). The differential
expression of uPA and PAI-1 has been detected between the stages of
primary neoformation and omentum metastasis in patients with
terminal ovarian cancer, indicating that high levels of uPA are
associated with a larger volume of residual tumor after surgery
(93).
Endometrial cancer is the most common gynecologic
malignancy in developing countries, as well as in certain developed
cities in China, such as Beijing and Shanghai (94). Most endometrial cancers are
endometriosis adenocarcinomas, out of which 80% are type 1
endometrial carcinomas, which are estrogen-related and low-grade
(International Federation of Gynecology and Obstetrics grades 1 and
2) with a better prognosis (95,96). However, patients with type 2
endometrial cancers do not respond to estrogen, thus exhibiting a
poor prognosis (95,96). Abnormal uterine bleeding is an
early symptom of endometrial cancer, which is often diagnosed at
stage I with an endometrial biopsy, thereby facilitating the
treatment of this cancer if detected (95,97). Studies on endometrial cancer
pathogenesis mostly focus on the estrogen-stimulated carcinogenesis
pathway, while the role of uPAR in carcinogenesis has not received
much attention. Prifti et al (98) indicated that integrins α4β1, α5β1
and α6β1 mediate adhesion and invasion of endometrial cancer cell
lines. However, they did not detect any significant change in the
expression of uPA, PAI-1 and uPAR after the addition of an
anti-integrin antibody to endometrial cancer cells and they deduced
that low levels of these proteins may be detected by sensitive
methods such as ELISA (98). EMT
is regulated by estrogen and progesterone through the PI3K/AKT,
EGF, IL-6, PDGF, TGF-β, VEGF and Wnt/β-catenin signaling pathways
in endometrial cancer (96). A
study containing 54 samples of endometrial cancer tissues indicated
that uPAR was not significantly related to clinical
characteristics; however, a review on 65 endometrial cancer samples
reported that uPAR is correlated with the disease stage, rate of
recurrence and mortality, indicating that uPAR is a prognostic
factor for endometrial cancer (99,100). Furthermore, the mRNA and protein
levels of uPAR were indicated to be 33 times higher in advanced
endometrial cancer than in normal endometrial tissues (99). In addition, a study on 293
patients with endometrial cancer suggested that PAI-1 and u-PA are
promising prognostic factors and that active proteolysis
facilitates endometrial cancer development (101).
As for normal endometrium, high amounts of plasmin
are detected in the menstrual outflow during the shedding process
to activate MMP in the endometrium. However, high levels of plasmin
are not detected in cancers (102). Endometrial stromal cells express
high levels of uPAR and PAI-1, and the internalization of uPA/PAI-1
complexes is enhanced under progesterone stimulation during the
shedding process of the menstrual cycle, suggesting that
uPA-uPAR/PAI-1 are activated under the effect of estrogen and
progesterone (102). During the
decidualization of uterine stromal cells, progesterone stimulates
the activation of the Wnt pathway and the expression of uPAR,
totally depending on estradiol, which proved that uPAR has a
significant role in the menstrual cycle and successful reproduction
in the uterine endometrium (103). The presence of uPAR in both
normal endometrium and endometrial carcinoma revealed the
difference between the normal physiological and carcinogenic
effects of uPAR.
Cervical cancer is the second most frequently
diagnosed cancer type among females globally, mostly in developing
countries. Persistent infection of keratinocytes with human
papillomavirus (HPV) is associated with most cases, and most of
them progress from cervical intraepithelial neoplasia (104). During the HPV infection process,
the basement membrane and ECM have an important role in primary HPV
infection and cancer initiation in the uterine cervix (104). The uPA/uPAR proteolysis system
facilitates HPV-associated carcinogenesis by means of cell-derived
MMP-9 (105).
In breast cancer and gynecological malignancies,
uPAR is associated with clinical prognosis and characteristics;
thus, exploration of the roles of uPAR in the pathogenetic
mechanism is significant in the future. It has two forms, the
soluble receptor and membrane receptor; they participate in unique
malignant behaviors of various tumors and may be used as clinical
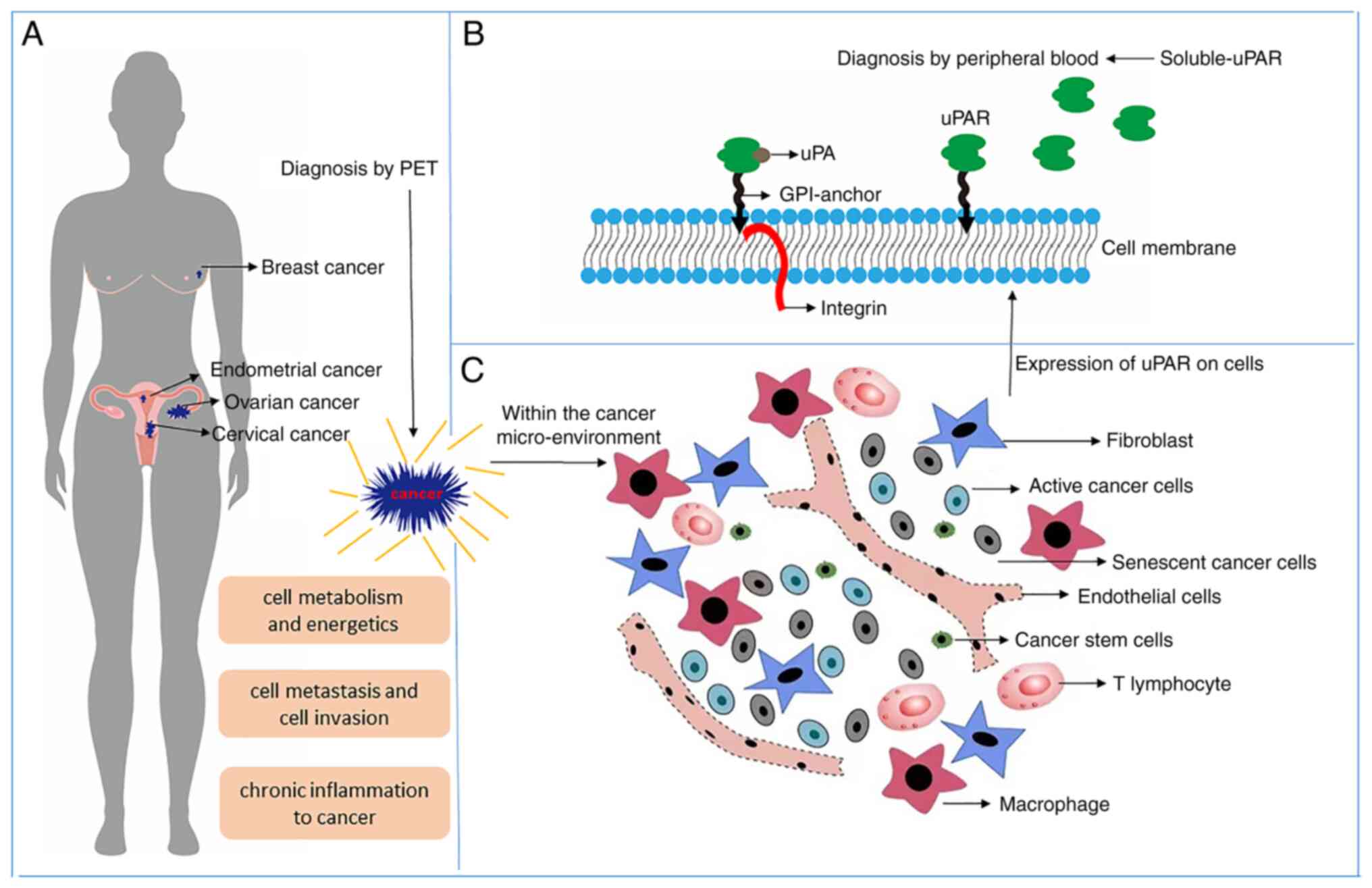
indicators to guide prognosis. A schematic of the roles of uPAR in
breast cancer and gynecologic tumors is provided in Fig. 3. The mechanisms by which uPAR
contributes to pathogeneses in a proteolysis-independent manner
require to be further explored.
To date, several experimental and preclinical
studies have used uPAR as a target to inhibit cancer. First,
regarding immunotherapy, the use of antibodies against uPAR is the
main therapeutic strategy against tumors. A total of 12 unique
human fragment of antigen bindings that target uPAR have been
identified (111). Blocking the
binding of uPA to uPAR and integrin to uPAR proved effective in
inhibiting cell invasion (111).
Monoclonal antibodies against uPAR, such as ANT-658 and ANT-615,
have also been developed (112).
ANT-658, which was demonstrated to have antitumor effects, was used
to inhibit the growth of ovarian (40), liver (113) and prostate cancers (114). Of note, CAR T cells targeting
uPAR proved beneficial for killing cancer cells in ovarian and lung
cancer (63,115). Programmed death 1 (PD-1)
antibody has been proven effective in the clinic and in a
pre-clinical study, targeting uPAR potentiated anti-PD-1 efficacy
in gastric cancer using uPAR antibody or uPAR CAR-T cells (116). Furthermore, bio-toxicity
medicine and synthetic peptides that interfere with the binding of
uPA to uPAR have been developed. A study exploited the specific
binding of the EGF domain of uPA to uPAR and the antitumor effects
of melittin to design and express a fusion protein in Pichia
pastoris that contained uPA amino acids 1–43 and melittin (117). The fusion protein product, named
rhupa1-43-melittin, resulted in cell cycle arrest, inhibited growth
and induced apoptosis in ovarian cancer cells (117). Apart from this, the
amino-terminal fragment (ATF) of uPA, which includes the
uPAR-binding region but completely lacks the catalytic domain of
uPA, has been used to design fusion proteins for the delivery of
toxins, such as diphtheria toxin (118–120), the antiangiogenic domain of
vasostatin (121), Kunitz-type
protease inhibitor (122),
ribosome-inactivating protein saporin (123) and scorpion toxin
analgesic-antitumor peptide (124). Most of these toxin-delivery
systems are efficient in cancer cell lines, but none of them are
clinically applicable. A uPA-derived small-sized synthetic peptide
(Å6) exerted a therapeutic effect by interfering with uPA/uPAR
interaction in patients with advanced ovarian cancer (125). In non-cancer disease, a
C-terminal domain of endostatin, known as E4, was able to bind to
uPAR to exert antifibrotic effects in idiopathic pulmonary fibrosis
and systemic sclerosis (126).
In atomic medicine, uPAR has also been applied in positron emission
tomography (PET) in non-invasive molecular imaging technology
(127,128). The latest progress on uPAR in
atomic medicine is a prospective phase II trial, which suggested
that using 68Ga-NOTA-AE105 PET for imaging uPAR is relevant for
risk stratification for patients with neuroendocrine neoplasms
(129). Researchers are aware
that single anti-uPAR therapy is not effective for cancer and
combination therapy is widely studied, e.g., designed bi-specific
anti-uPAR and EGFR toxins may kill solid tumor cells (130). Furthermore, naturopathy
targeting uPAR has also been developed; photodynamic therapy and
photothermal therapy essentially utilizes the binding of ATF to
uPAR for capturing tumor cells (131). Overall, uPAR, a promising target
for cancer therapy, has broad-anti-tumor applications. Inevitably,
uPAR is expressed in numerous non-cancer tissues, as mentioned
above in this review, and lessons learned from therapy failure and
adverse reactions are that ‘on-target off-tumor’ effects should be
avoided (132).
However, to date, no uPAR-targeted therapy has been
approved in the clinic. The pre-clinical and clinical studies
mentioned above implied that limitations of uPAR still represent a
bottleneck to therapy. uPAR is not an exclusively-specific
biomarker for cancer disease, which may cause on target-off tumor
effects and different administration routes may have different
degrees of this side effect (125,127). A higher affinity of
uPAR-targeting is not directly linked to better efficacy. It is a
challenge to control affinity at a moderate level to avoid
unnecessary destruction of non-cancer cells (109,110,119,121,122). However, due to certain
limitations, the untility of uPAR as an ideal biomarker for cancer
is restricted. First, it is expressed in cancer, but not all
cancers; furthermore, it is expressed not only in cancer cells but
also stroma cells (81,128). Thus, novel modification
strategies for uPAR targeting are indeed in need, such as those
bispecific with other biomarkers such as EGFR (128). The limitations of uPAR in
targeted therapy are summarized and corresponding possible
solutions are suggested in Table
II.
Virtually, elucidating the physiological roles of a
molecule may help understand its function in cancer.
Microscopically, within the TME, communications between cancers and
stromal cells are orchestrated signals. Comparison of a molecule in
both stromal cells and parenchymal cells may be an objective
method. Furthermore, it is challenging to pinpoint a single
molecule as a suppressor or promoter of a cancer. In physiological
processes, uPAR is expressed at a moderate level, while in cancer,
uPAR is usually overexpressed, which is associated with a malignant
biological behavior. Therefore, it is important to study the
function of uPAR under both physiological and pathological
conditions to identify its particular role in tumorigenesis in the
future.
Not applicable.
This study was supported by grants from the Natural Science
Foundation of Fujian Province (grant no. 2021J05077), the Science
and Technology Project of Fujian Provincial Health Commission
(grant no. 2020GGB014) and the Fujian Provincial Health Commission
(grant no. 2020J02059).
Not applicable.
LW and XL performed the literature search. LW and PS
conceived the study. LW, XL and PS were involved in writing the
manuscript. LW, XL and PS read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Vassalli JD, Baccino D and Belin D: A
cellular binding site for the Mr 55,000 form of the human
plasminogen activator, urokinase. J Cell Biol. 100:86–92. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith HW and Marshall CJ: Regulation of
cell signalling by uPAR. Nat Rev Mol Cell Biol. 11:23–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gyetko MR, Libre EA, Fuller JA, Chen GH
and Toews G: Urokinase is required for T lymphocyte proliferation
and activation in vitro. J Lab Clin Med. 133:274–288. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alfano M, Sidenius N, Panzeri B, Blasi F
and Poli G: Urokinase-urokinase receptor interaction mediates an
inhibitory signal for HIV-1 replication. Proc Natl Acad Sci USA.
99:8862–8867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ploug MRE, Behrendt N, Jensen AL, Blasi F
and Danø K: Cellular receptor for urokinase plasminogen activator.
Carboxyl-terminal processing and membrane anchoring by
glycosyl-phosphatidylinositol. J Biol Chem. 266:1926–1933. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ploug M, Behrendt N, Løber D and Danø K:
Protein structure and membrane anchorage of the cellular receptor
for urokinase-type plasminogen activator. Semin Thromb Hemost.
17:183–193. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ellis V, Wun TC, Behrendt N, Rønne E and
Danø K: Inhibition of receptor-bound urokinase by
plasminogen-activator inhibitors. J Biol Chem. 265:9904–9908. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ploug M, Gårdsvoll H, Jørgensen TJ,
Lønborg Hansen L and Danø K: Structural analysis of the interaction
between urokinase-type plasminogen activator and its receptor: A
potential target for anti-invasive cancer therapy. Biochem Soc
Trans. 30:177–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andreasen PA, Egelund R and Petersen HH:
The plasminogen activation system in tumor growth, invasion, and
metastasis. Cell Mol Life Sci. 57:25–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Danø K, Romer J, Nielsen BS, Bjørn S, Pyke
C, Rygaard J and Lund LR: Cancer invasion and tissue
remodeling-cooperation of protease systems and cell types. APMIS.
107:120–127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berkenblit A, Matulonis UA, Kroener JF,
Dezube BJ, Lam GN, Cuasay LC, Brünner N, Jones TR, Silverman MH and
Gold MA: A6, a urokinase plasminogen activator (uPA)-derived
peptide in patients with advanced gynecologic cancer: A phase I
trial. Gynecol Oncol. 99:50–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gold MA, Brady WE, Lankes HA, Rose PG,
Kelley JL, De Geest K, Crispens MA, Resnick KE and Howell SB: A
phase II study of a urokinase-derived peptide (A6) in the treatment
of persistent or recurrent epithelial ovarian, fallopian tube, or
primary peritoneal carcinoma: A gynecologic oncology group study.
Gynecol Oncol. 125:635–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ploug M: Structure-driven design of
radionuclide tracers for non-invasive imaging of uPAR and targeted
radiotherapy. The tale of a synthetic peptide antagonist.
Theranostics. 3:467–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noh H, Hong S and Huang S: Role of
urokinase receptor in tumor progression and development.
Theranostics. 3:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Halloran TV, Ahn R, Hankins P, Swindell
E and Mazar AP: The many spaces of uPAR: Delivery of theranostic
agents and nanobins to multiple tumor compartments through a single
target. Theranostics. 3:496–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuang X, Zhang H and Hu G: Cancer and
microenvironment plasticity: Double-edged swords in metastasis.
Trends Pharmacol Sci. 40:419–429. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishii G, Ochiai A and Neri S: Phenotypic
and functional heterogeneity of cancer-associated fibroblast within
the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Najafi M, Goradel NH, Farhood B, Salehi E,
Solhjoo S, Toolee H, Kharazinejad E and Mortezaee K: Tumor
microenvironment: Interactions and therapy. J Cell Physiol.
234:5700–5721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Alessio S and Blasi F: The urokinase
receptor as an entertainer of signal transduction. Front Biosci
(Landmark Ed). 14:4575–4587. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dass K, Ahmad A, Azmi AS, Sarkar SH and
Sarkar FH: Evolving role of uPA/uPAR system in human cancers.
Cancer Treat Rev. 34:122–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ulisse S, Baldini E, Sorrenti S and
D'Armiento M: The urokinase plasminogen activator system: A target
for anti-cancer therapy. Curr Cancer Drug Targets. 9:32–71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blasi F and Sidenius N: The urokinase
receptor: Focused cell surface proteolysis, cell adhesion and
signaling. FEBS Lett. 584:1923–1930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paulick MG and Bertozzi CR: The
glycosylphosphatidylinositol anchor: A complex membrane-anchoring
structure for proteins. Biochemistry. 47:6991–7000. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eden G, Archinti M, Furlan F, Murphy R and
Degryse B: The urokinase receptor interactome. Curr Pharm Des.
17:1874–1889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eugen-Olsen J and Giamarellos-Bourboulis
EJ: suPAR: The unspecific marker for disease presence, severity and
prognosis. Int J Antimicrob Agents. 46 (Suppl 1):S33–S34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desmedt S, Desmedt V, Delanghe JR,
Speeckaert R and Speeckaert MM: The intriguing role of soluble
urokinase receptor in inflammatory diseases. Crit Rev Clin Lab Sci.
54:117–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loughner CL, Bruford EA, McAndrews MS,
Delp EE, Swamynathan S and Swamynathan SK: Organization, evolution
and functions of the human and mouse Ly6/uPAR family genes. Hum
Genomics. 10:102016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong HK and Park JH: Characterization and
function of human Ly-6/uPAR molecules. BMB Rep. 45:595–603. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kriegbaum MC, Jacobsen B and Hald Aand
Ploug M: Expression of C4.4A, a structural uPAR homolog, reflects
squamous epithelial differentiation in the adult mouse and during
embryogenesis. J Histochem Cytochem. 59:188–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hansen LV, Gårdsvoll H, Nielsen BS, Lund
LR, Danø K, Jensen ON and Ploug M: Structural analysis and tissue
localization of human C4.4A: A protein homologue of the urokinase
receptor. Biochem J. 380:845–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davidson B, Trope CG and Reich R: The role
of the tumor stroma in ovarian cancer. Front Oncol. 4:1042014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baig MH, Adil M, Khan R, Dhadi S, Ahmad K,
Rabbani G, Bashir T, Imran MA, Husain FM, Lee EJ, et al: Enzyme
targeting strategies for prevention and treatment of cancer:
Implications for cancer therapy. Semin Cancer Biol. 56:1–11. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Myöhänen HT, Stephens RW, Hedman K,
Tapiovaara H, Rønne E, Høyer-Hansen G, Danø K and Vaheri A:
Distribution and lateral mobility of the urokinase-receptor complex
at the cell surface. J Histochem Cytochem. 41:1291–1301. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eastman BM, Jo M, Webb DL, Takimoto S and
Gonias SL: A transformation in the mechanism by which the urokinase
receptor signals provides a selection advantage for estrogen
receptor-expressing breast cancer cells in the absence of estrogen.
Cell Signal. 24:1847–1855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu D, Xu D, Liu M, Knabe WE, Yuan C, Zhou
D, Huang M and Meroueh SO: Small molecules engage hot spots through
cooperative binding to inhibit a tight protein-protein interaction.
Biochemistry. 56:1768–1784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Høyer-Hansen G and Lund IK: Urokinase
receptor variants in tissue and body fluids. Adv Clin Chem.
44:65–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahn SB, Mohamedali A, Anand S, Cheruku HR,
Birch D, Sowmya G, Cantor D, Ranganathan S, Inglis DW, Frank R, et
al: Characterization of the interaction between heterodimeric αvβ6
integrin and urokinase plasminogen activator receptor (uPAR) using
functional proteomics. J Proteome Res. 13:5956–5964. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aguirre Ghiso JA: Inhibition of FAK
signaling activated by urokinase receptor induces dormancy in human
carcinoma cells in vivo. Oncogene. 21:2513–2524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kenny HA, Leonhardt P, Ladanyi A, Yamada
SD, Montag A, Im HK, Jagadeeswaran S, Shaw DE, Mazar AP and Lengyel
E: Targeting the urokinase plasminogen activator receptor inhibits
ovarian cancer metastasis. Clin Cancer Res. 17:459–471. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gyetko MR, Sud S, Sonstein J, Polak T, Sud
A and Curtis JL: Cutting edge: Antigen-driven lymphocyte
recruitment to the lung is diminished in the absence of
urokinase-type plasminogen activator (uPA) receptor, but is
independent of uPA. J Immunol. 167:5539–5542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li S, Wei X, He J, Tian X, Yuan S and Sun
L: Plasminogen activator inhibitor-1 in cancer research. Biomed
Pharmacother. 105:83–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghosh AK and Vaughan DE: PAI-1 in tissue
fibrosis. J Cell Physiol. 227:493–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Olson D, Pöllänen J, Høyer-Hansen G, Rønne
E, Sakaguchi K, Wun TC, Appella E, Danø K and Blasi F:
Internalization of the urokinase-plasminogen activator inhibitor
type-1 complex is mediated by the urokinase receptor. J Biol Chem.
267:9129–9133. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Solberg H, Ploug M, Høyer-Hansen G,
Nielsen BS and Lund LR: The murine receptor for urokinase-type
plasminogen activator is primarily expressed in tissues actively
undergoing remodeling. J Histochem Cytochem. 49:237–246. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Conforti G, Dominguez-Jimenez C, Rønne E,
Høyer-Hansen G and Dejana E: Cell-surface plasminogen activation
causes a retraction of in vitro cultured human umbilical vein
endothelial cell monolayer. Blood. 83:994–1005. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gur-Wahnon D, Mizrachi T, Maaravi-Pinto
FY, Lourbopoulos A, Grigoriadis N, Higazi AA and Brenner T: The
plasminogen activator system: Involvement in central nervous system
inflammation and a potential site for therapeutic intervention. J
Neuroinflammation. 10:1242013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Diaz A, Merino P, Manrique LG, Ospina JP,
Cheng L, Wu F, Jeanneret V and Yepes M: A cross talk between
neuronal urokinase-type plasminogen activator (uPA) and astrocytic
uPA receptor (uPAR) promotes astrocytic activation and synaptic
recovery in the ischemic brain. J Neurosci. 37:10310–10322. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hohensinner PJ, Takacs N, Kaun C, Thaler
B, Krychtiuk KA, Pfaffenberger S, Aliabadi A, Zuckermann A, Huber K
and Wojta J: Urokinase plasminogen activator protects cardiac
myocytes from oxidative damage and apoptosis via hOGG1 induction.
Apoptosis. 22:1048–1055. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ploug M, Plesner T, Rønne E, Ellis V,
Høyer-Hansen G, Hansen NE and Danø K: The receptor for
urokinase-type plasminogen activator is deficient on peripheral
blood leukocytes in patients with paroxysmal nocturnal
hemoglobinuria. Blood. 79:1447–1455. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sloand EM, Pfannes L, Scheinberg P, More
K, Wu CO, Horne M and Young NS: Increased soluble urokinase
plasminogen activator receptor (suPAR) is associated with
thrombosis and inhibition of plasmin generation in paroxysmal
nocturnal hemoglobinuria (PNH) patients. Exp Hematol. 36:1616–1624.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu W, Hsu AY, Wang Y, Lin T, Sun H,
Pachter JS, Groisman A, Imperioli M, Yungher FW, Hu L, et al:
Mitofusin-2 regulates leukocyte adhesion and β2 integrin
activation. J Leukoc Biol. 111:771–791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gyetko MR, Sud S, Kendall T, Fuller JA,
Newstead MW and Standiford TJ: Urokinase receptor-deficient mice
have impaired neutrophil recruitment in response to pulmonary
Pseudomonas aeruginosa infection. J Immunol. 165:1513–1519. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Puthusseri B, Marudamuthu A, Tiwari N, Fu
J, Idell S and Shetty S: Regulation of p53-mediated changes in the
uPA-fibrinolytic system and in lung injury by loss of surfactant
protein C expression in alveolar epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 312:L783–L796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Stewart CE and Sayers I: Urokinase
receptor orchestrates the plasminogen system in airway epithelial
cell function. Lung. 191:215–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Beaufort N, Leduc D, Eguchi H, Mengele K,
Hellmann D, Masegi T, Kamimura T, Yasuoka S, Fend F, Chignard M and
Pidard D: The human airway trypsin-like protease modulates the
urokinase receptor (uPAR, CD87) structure and functions. Am J
Physiol Lung Cell Mol Physiol. 292:L1263–L1272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shetty S, Padijnayayveetil J, Tucker T,
Stankowska D and Idell S: The fibrinolytic system and the
regulation of lung epithelial cell proteolysis, signaling, and
cellular viability. Am J Physiol Lung Cell Mol Physiol.
295:L967–L975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Svenningsen P, Hinrichs GR, Zachar R,
Ydegaard R and Jensen BL: Physiology and pathophysiology of the
plasminogen system in the kidney. Pflugers Arch. 469:1415–1423.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hayek SS, Koh KH, Grams ME, Wei C, Ko YA,
Li J, Samelko B, Lee H, Dande RR, Lee HW, et al: A tripartite
complex of suPAR, APOL1 risk variants and
αvβ3 integrin on podocytes mediates chronic
kidney disease. Nat Med. 23:945–953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cheng Y, Hall TR, Xu X, Yung I, Souza D,
Zheng J, Schiele F, Hoffmann M, Mbow ML, Garnett JP and Li J:
Targeting uPA-uPAR interaction to improve intestinal epithelial
barrier integrity in inflammatory bowel disease. EBioMedicine.
75:1037582022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang H, Yang L, Jamaluddin MS and Boyd DD:
The Kruppel-like KLF4 transcription factor, a novel regulator of
urokinase receptor expression, drives synthesis of this binding
site in colonic crypt luminal surface epithelial cells. J Biol
Chem. 279:22674–22683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Amor C, Feucht J, Leibold J, Ho YJ, Zhu C,
Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, et
al: Senolytic CAR T cells reverse senescence-associated
pathologies. Nature. 583:127–132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lv T, Zhao Y, Jiang X, Yuan H, Wang H, Cui
X, Xu J, Zhao J and Wang J: uPAR: An essential factor for tumor
development. J Cancer. 12:7026–7040. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Laurenzana A, Chillà A, Luciani C,
Peppicelli S, Biagioni A, Bianchini F, Tenedini E, Torre E, Mocali
A, Calorini L, et al: uPA/uPAR system activation drives a
glycolytic phenotype in melanoma cells. Int J Cancer.
141:1190–1200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Biagioni A, Laurenzana A, Chillà A, Del
Rosso M, Andreucci E, Poteti M, Bani D, Guasti D, Fibbi G and
Margheri F: uPAR knockout results in a deep glycolytic and OXPHOS
reprogramming in melanoma and colon carcinoma cell lines. Cells.
9:3082020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Flores-López LA, Martínez-Hernández MG,
Viedma-Rodríguez R, Díaz-Flores M and Baiza-Gutman LA: High glucose
and insulin enhance uPA expression, ROS formation and invasiveness
in breast cancer-derived cells. Cell Oncol (Dordr). 39:365–378.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Csiszar A, Kutay B, Wirth S, Schmidt U,
Macho-Maschler S, Schreiber M, Alacakaptan M, Vogel GF, Aumayr K,
Huber LA and Beug H: Interleukin-like epithelial-to-mesenchymal
transition inducer activity is controlled by proteolytic processing
and plasminogen-urokinase plasminogen activator receptor
system-regulated secretion during breast cancer progression. Breast
Cancer Res. 16:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ragone C, Minopoli M, Ingangi V, Botti G,
Fratangelo F, Pessi A, Stoppelli MP, Ascierto PA, Ciliberto G,
Motti ML and Carriero MV: Targeting the cross-talk between
urokinase receptor and Formyl peptide receptor type 1 to prevent
invasion and trans-endothelial migration of melanoma cells. J Exp
Clin Cancer Res. 36:1802017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Qu M, Yu J, Liu H, Ren Y, Ma C, Bu X and
Lan Q: The candidate tumor suppressor gene SLC8A2 inhibits
invasion, angiogenesis and growth of glioblastoma. Mol Cells.
40:761–772. 2017.PubMed/NCBI
|
|
72
|
Yang QX, Zhong S, He L, Jia XJ, Tang H,
Cheng ST, Ren JH, Yu HB, Zhou L, Zhou HZ, et al: PBK overexpression
promotes metastasis of hepatocellular carcinoma via activating
ETV4-uPAR signaling pathway. Cancer Lett. 452:90–102. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ding Y, Zhang H, Lu A, Zhou Z, Zhong M,
Shen D, Wang X and Zhu Z: Effect of urokinase-type plasminogen
activator system in gastric cancer with peritoneal metastasis.
Oncol Lett. 11:4208–4216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Su B, Su J, He H, Wu Y, Xia H, Zeng X, Dai
W, Ai X, Ling H, Jiang H and Su Q: Identification of potential
targets for diallyl disulfide in human gastric cancer MGC-803 cells
using proteomics approaches. Oncol Rep. 33:2484–2894. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Alpízar-Alpízar W, Skindersoe ME,
Rasmussen L, Kriegbaum MC, Christensen IJ, Lund IK, Illemann M,
Laerum OD, Krogfelt KA, Andersen LP and Ploug M: Helicobacter
pylori colonization drives urokinase receptor (uPAR) expression in
murine gastric epithelium during early pathogenesis.
Microorganisms. 8:10192020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Beleva E, Stoencheva S, Deneva T, Nenova I
and Grudeva-Popova Z: Assessment of clinical utility and predictive
potential of pre-chemotherapy soluble urokinase plasminogen
activator receptor-observational single center study. Bosn J Basic
Med Sci. Sep 8–2022.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bifulco K, Longanesi-Cattani I, Gala M, DI
Carluccio G, Masucci MT, Pavone V, Lista L, Arra C, Stoppelli MP
and Carriero MV: The soluble form of urokinase receptor promotes
angiogenesis through its Ser88-Arg-Ser-Arg-Tyr92 chemotactic
sequence. J Thromb Haemost. 8:2789–2799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Poettler M, Unseld M, Mihaly-Bison J,
Uhrin P, Koban F, Binder BR, Zielinski CC and Prager GW: The
urokinase receptor (CD87) represents a central mediator of growth
factor-induced endothelial cell migration. Thromb Haemost.
108:357–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Boas SEM, Carvalho J, van den Broek M,
Weijers EM, Goumans MJ, Koolwijk P and Merks RMH: A local
uPAR-plasmin-TGFβ1 positive feedback loop in a qualitative
computational model of angiogenic sprouting explains the in vitro
effect of fibrinogen variants. PLoS Comput Biol. 14:e10062392018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Unseld M, Chilla A, Pausz C, Mawas R,
Breuss J, Zielinski C, Schabbauer G and Prager GW: PTEN expression
in endothelial cells is down-regulated by uPAR to promote
angiogenesis. Thromb Haemost. 114:379–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jing Y, Tong C, Zhang J, Nakamura T,
Iankov I, Russell SJ and Merchan JR: Tumor and vascular targeting
of a novel oncolytic measles virus retargeted against the urokinase
receptor. Cancer Res. 69:1459–1468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jing Y, Chavez V, Ban Y, Acquavella N,
El-Ashry D, Pronin A, Chen X and Merchan JR: Molecular effects of
stromal-selective targeting by uPAR-retargeted oncolytic virus in
breast cancer. Mol Cancer Res. 15:1410–1420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pyke C, Graem N, Ralfkiaer E, Rønne E,
Høyer-Hansen G, Brünner N and Danø K: Receptor for urokinase is
present in tumor-associated macrophages in ductal breast carcinoma.
Cancer Res. 53:1911–1915. 1993.PubMed/NCBI
|
|
84
|
Berg D, Wolff C, Malinowsky K, Tran K,
Walch A, Bronger H, Schuster T, Höfler H and Becker KF: Profiling
signalling pathways in formalin-fixed and paraffin-embedded breast
cancer tissues reveals cross-talk between EGFR, HER2, HER3 and
uPAR. J Cell Physiol. 227:204–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li C, Cao S, Liu Z, Ye X, Chen L and Meng
S: RNAi-mediated downregulation of uPAR synergizes with targeting
of HER2 through the ERK pathway in breast cancer cells. Int J
Cancer. 127:1507–1516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Desai A, Xu J, Aysola K, Qin Y, Okoli C,
Hariprasad R, Chinemerem U, Gates C, Reddy A, Danner O, et al:
Epithelial ovarian cancer: An overview. World J Transl Med. 3:1–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kim S, Kim B and Song YS: Ascites
modulates cancer cell behavior, contributing to tumor heterogeneity
in ovarian cancer. Cancer Sci. 107:1173–1178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Al-Hassan NN, Behzadian A, Caldwell R,
Ivanova VS, Syed V, Motamed K and Said NA: Differential roles of
uPAR in peritoneal ovarian carcinomatosis. Neoplasia. 14:259–270.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chambers SK, Gertz RE Jr, Ivins CM and
Kacinski BM: The significance of urokinase-type plasminogen
activator, its inhibitors, and its receptor in ascites of patients
with epithelial ovarian cancer. Cancer. 75:1627–1633. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
van Dam PA, Coelho A and Rolfo C: Is there
a role for urokinase-type plasminogen activator inhibitors as
maintenance therapy in patients with ovarian cancer? Eur J Surg
Oncol. 43:252–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kuhn W, Schmalfeldt B, Reuning U, Pache L,
Berger U, Ulm K, Harbeck N, Späthe K, Dettmar P, Höfler H, et al:
Prognostic significance of urokinase (uPA) and its inhibitor PAI-1
for survival in advanced ovarian carcinoma stage FIGO IIIc. Br J
Cancer. 79:1746–1751. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dorn J, Harbeck N, Kates R, Gkazepis A,
Scorilas A, Soosaipillai A, Diamandis E, Kiechle M, Schmalfeldt B
and Schmitt M: Impact of expression differences of
kallikrein-related peptidases and of uPA and PAI-1 between primary
tumor and omentum metastasis in advanced ovarian cancer. Ann Oncol.
22:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Du J, Li Y, Lv S, Wang Q, Sun C, Dong X,
He M, Ulain Q, Yuan Y, Tuo X, et al: Endometrial sampling devices
for early diagnosis of endometrial lesions. J Cancer Res Clin
Oncol. 142:2515–2522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chiu HC, Li CJ, Yiang GT, Tsai AP and Wu
MY: Epithelial to mesenchymal transition and cell biology of
molecular regulation in endometrial carcinogenesis. J Clin Med.
8:4392019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sorosky JI: Endometrial cancer. Obstet
Gynecol. 120:383–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Prifti S, Zourab Y, Koumouridis A,
Bohlmann M, Strowitzki T and Rabe T: Role of integrins in invasion
of endometrial cancer cell lines. Gynecol Oncol. 84:12–20. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Memarzadeh S, Kozak KR, Chang L, Natarajan
S, Shintaku P, Reddy ST and Farias-Eisner R: Urokinase plasminogen
activator receptor: Prognostic biomarker for endometrial cancer.
Proc Natl Acad Sci USA. 99:10647–10652. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tecimer C, Doering DL, Goldsmith LJ, Meyer
JS, Abdulhay G and Wittliff JL: Clinical relevance of
urokinase-type plasminogen activator, its receptor, and its
inhibitor type 1 in endometrial cancer. Gynecol Oncol. 80:48–55.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fredstorp-Lidebring M, Bendahl PO, Brünner
N, Casslén B, Högberg T, Långström-Einarsson E, Willén R and Fernö
M: Urokinase plasminogen activator and its inhibitor, PAI-1, in
association with progression-free survival in early stage
endometrial cancer. Eur J Cancer. 37:2339–2348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Makieva S, Giacomini E, Ottolina J,
Sanchez AM, Papaleo E and Viganò P: Inside the endometrial cell
signaling subway: Mind the Gap(s). Int J Mol Sci. 19:24772018.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rider V, Isuzugawa K, Twarog M, Jones S,
Cameron B, Imakawa K and Fang J: Progesterone initiates
Wnt-beta-catenin signaling but estradiol is required for nuclear
activation and synchronous proliferation of rat uterine stromal
cells. J Endocrinol. 191:537–548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sahebali S, Van den Eynden G, Murta EF,
Michelin MA, Cusumano P, Petignat P and Bogers JJ: Stromal issues
in cervical cancer: A review of the role and function of basement
membrane, stroma, immune response and angiogenesis in cervical
cancer development. Eur J Cancer Prev. 19:204–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Smola S: Immunopathogenesis of
HPV-associated cancers and prospects for immunotherapy. Viruses.
9:254–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jing J, Zheng S, Han C, Du L, Guo Y and
Wang P: Evaluating the value of uPAR of serum and tissue on
patients with cervical cancer. J Clin Lab Anal. 26:16–21. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sasaki T, Nishi H, Nagata C, Nagai T,
Nagao T, Terauchi F and Isaka K: A retrospective study of
urokinase-type plasminogen activator receptor (uPAR) as a
prognostic factor in cancer of the uterine cervix. Int J Clin
Oncol. 19:1059–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nishi H, Sasaki T, Nagamitsu Y, Terauchi
F, Nagai T, Nagao T and Isaka K: Hypoxia inducible factor-1
mediates upregulation of urokinase-type plasminogen activator
receptor gene transcription during hypoxia in cervical cancer
cells. Oncol Rep. 35:992–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chaudary N and Hill RP: Increased
expression of metastasis-related genes in hypoxic cells sorted from
cervical and lymph nodal xenograft tumors. Lab Invest. 89:587–596.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sato M, Kawana K, Adachi K, Fujimoto A,
Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Takahashi J,
et al: Decreased expression of the plasminogen activator inhibitor
type 1 is involved in degradation of extracellular matrix
surrounding cervical cancer stem cells. Int J Oncol. 48:829–835.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Duriseti S, Goetz DH, Hostetter DR, LeBeau
AM, Wei Y and Craik CS: Antagonistic anti-urokinase plasminogen
activator receptor (uPAR) antibodies significantly inhibit
uPAR-mediated cellular signaling and migration. J Biol Chem.
285:26878–26888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Xu X, Cai Y, Wei Y, Donate F, Juarez J,
Parry G, Chen L, Meehan EJ, Ahn RW, Ugolkov A, et al:
Identification of a new epitope in uPAR as a target for the cancer
therapeutic monoclonal antibody ATN-658, a structural homolog of
the uPAR binding integrin CD11b (αM). PLoS One. 9:e853492014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Van Buren G II, Gray MJ, Dallas NA, Xia L,
Lim SJ, Fan F, Mazar AP and Ellis LM: Targeting the urokinase
plasminogen activator receptor with a monoclonal antibody impairs
the growth of human colorectal cancer in the liver. Cancer.
115:3360–3368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Rabbani SA, Ateeq B, Arakelian A,
Valentino ML, Shaw DE, Dauffenbach LM, Kerfoot CA and Mazar AP: An
anti-urokinase plasminogen activator receptor antibody (ATN-658)
blocks prostate cancer invasion, migration, growth, and
experimental skeletal metastasis in vitro and in vivo. Neoplasia.
12:778–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang L, Yang R, Zhao L, Zhang X, Xu T and
Cui M: Basing on uPAR-binding fragment to design chimeric antigen
receptors triggers antitumor efficacy against uPAR expressing
ovarian cancer cells. Biomed Pharmacother. 117:1091732019.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Qin L, Wang L, Zhang J, Zhou H, Yang Z,
Wang Y, Cai W, Wen F, Jiang X, Zhang T, et al: Therapeutic
strategies targeting uPAR potentiate anti-PD-1 efficacy in
diffuse-type gastric cancer. Sci Adv. 8:eabn37742022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Su M, Chang W, Cui M, Lin Y, Wu S and Xu
T: Expression and anticancer activity analysis of recombinant human
uPA1-43-melittin. IntJ Oncol. 46:619–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hall WA and Vallera DA: Efficacy of
antiangiogenic targeted toxins against glioblastoma multiforme.
Neurosurg Focus. 20:E232006. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Todhunter DA, Hall WA, Rustamzadeh E, Shu
Y, Doumbia SO and Vallera DA: A bispecific immunotoxin (DTAT13)
targeting human IL-13 receptor (IL-13R) and urokinase-type
plasminogen activator receptor (uPAR) in a mouse xenograft model.
Protein Eng Des Sel. 17:157–164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Vallera DA, Li C, Jin N,
Panoskaltsis-Mortari A and Hall WA: Targeting urokinase-type
plasminogen activator receptor on human glioblastoma tumors with
diphtheria toxin fusion protein DTAT. J Natl Cancer Inst.
94:597–606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sun Q, Xu Q, Dong X, Cao L, Huang X, Hu Q
and Hua ZC: A hybrid protein comprising ATF domain of pro-UK and
VAS, an angiogenesis inhibitor, is a potent candidate for targeted
cancer therapy. Int J Cancer. 123:942–950. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Takei Y, Mizukami H, Saga Y, Kobayashi H
and Suzuki M, Matsushita T, Ozawa K and Suzuki M: Overexpression of
a hybrid gene consisting of the amino-terminal fragment of
urokinase and carboxyl-terminal domain of bikunin suppresses
invasion and migration of human ovarian cancer cells in vitro. Int
J Cancer. 113:54–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Errico Provenzano A, Posteri R, Giansanti
F, Angelucci F, Flavell SU, Flavell DJ, Fabbrini MS, Porro D,
Ippoliti R, Ceriotti A, et al: Optimization of construct design and
fermentation strategy for the production of bioactive ATF-SAP, a
saporin based anti-tumoral uPAR-targeted chimera. Microb Cell Fact.
15:1942016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Liu X, Liu X, Sunchen S, Liu M, Shen C, Wu
J, Zhao W, Yu B and Liu J: A novel tumor-activated ALA fusion
protein for specific inhibition on the growth and invasion of
breast cancer cells MDA-MB-231. Drug Deliv. 24:1811–1817. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Schmitt M, Harbeck N, Brünner N, Jänicke
F, Meisner C, Mühlenweg B, Jansen H, Dorn J, Nitz U, Kantelhardt EJ
and Thomssen C: Cancer therapy trials employing level-of-evidence-1
disease forecast cancer biomarkers uPA and its inhibitor PAI-1.
Expert Rev Mol Diagn. 11:617–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sharma S, Watanabe T, Nishimoto T,
Takihara T, Mlakar L, Nguyen XX, Sanderson M, Su Y, Chambers RA and
Feghali-Bostwick C: E4 engages uPAR and enolase-1 and activates
urokinase to exert antifibrotic effects. JCI Insight.
6:e1449352021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Gao N, Bozeman EN, Qian W, Wang L, Chen H,
Lipowska M, Staley CA, Wang YA, Mao H and Yang L: Tumor penetrating
theranostic nanoparticles for enhancement of targeted and
image-guided drug delivery into peritoneal tumors following
intraperitoneal delivery. Theranostics. 7:1689–16704. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kriegbaum MC, Persson M, Haldager L,
Alpízar-Alpízar W, Jacobsen B, Gårdsvoll H, Kjær A and Ploug M:
Rational targeting of the urokinase receptor (uPAR): Development of
antagonists and non-invasive imaging probes. Curr Drug Targets.
12:1711–1728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Carlsen EA, Loft M, Loft A, Berthelsen AK,
Langer SW, Knigge U and Kjaer A: Prospective phase II trial of
prognostication by 68Ga-NOTA-AE105 uPAR PET in patients
with neuroendocrine neoplasms: Implications for uPAR targeted
therapy. J Nucl Med. 63:1371–1377. 2022.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Oh F, Modiano JF, Bachanova V and Vallera
DA: Bispecific targeting of EGFR and urokinase receptor (uPAR)
using ligand-targeted toxins in solid tumors. Biomolecules.
10:9562020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhai BT, Tian H, Sun J, Zou JB, Zhang XF,
Cheng JX, Shi YJ, Fan Y and Guo DY: Urokinase-type plasminogen
activator receptor (uPAR) as a therapeutic target in cancer. J
Transl Med. 20:1352022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Metrangolo V, Ploug M and Engelholm LH:
The urokinase receptor (uPAR) as a ‘trojan horse’ in targeted
cancer therapy: Challenges and opportunities. Cancers (Basel).
13:53762021. View Article : Google Scholar : PubMed/NCBI
|