1. Introduction
Lung cancer is the second most common neoplasm in
the world, but the first in males, and the leading cause of
cancer-associated death. In 2020, there were >2.2 million new
cases globally, causing >1.8 million deaths (1). Despite the remarkable advances made
in the diagnosis, immunotherapy and monitoring of the disease, the
average 5-year survival rate is ~23-27%. Lung cancer is
characterized as having a high lethality and comorbidity, and the
majority of patients are diagnosed in advanced stages where
curative surgical options are limited (2). It should also be noted that mortality
data are similar in developed and emerging countries. Even for
patients who underwent curative surgery for the clinical management
of localized tumors, the 5-year survival rate is only 65%,
demonstrating the aggressiveness of these tumors despite those
patients having been diagnosed in early stages. In addition,
>70% of patients diagnosed with lung cancer have locoregional or
metastatic lymphatic spread, decreasing the probability of survival
at 5 years to ~33 and ~7%, respectively (3). In developed countries, the incidence
of lung cancer has decreased in recent years thanks to measures to
prevent tobacco use and control occupational exposure to asbestos,
since the former represents the main risk factor in the general
population. These primary prevention measures have caused a 45%
decrease in lung cancer mortality in males in the 1990-2015 period
and a 19% decrease in females in the 2002-2015 period (4). Other risk factors for lung cancer
have been described and include a multitude of agents such as
radon, arsenic, benzopyrenes, asbestos, infections such as
tuberculosis, and environmental pollution, although their
contribution to global cases is only minor, as tobacco smoking is
responsible for up to 90% of lung cancer cases (5). This is due to the fact that
combustion of tobacco causes the release of polycyclic
hydrocarbons, nitrosamines, nitrates and 60 other carcinogens,
which induce alterations in DNA repair mechanisms, cell cycle
control and dysplasia processes that lead to histological
degeneration, followed by predominating proliferation and invasion
of aberrant malignant cells (6).
Smoking cessation is associated with a clear decrease in the
relative risk of developing lung cancer after 10-15 years (7). It should be noted that ~1.1 billion
individuals smoke in the world and of these, 10-20% of smokers may
develop lung cancer (8). On the
other hand, lung cancer is more frequent in males and the maximum
incidence by age is between 80 and 90 years (9).
The histological varieties of lung cancer have been
traditionally classified by prognostic, pathological and
therapeutic factors. They have been differentiated into small cell
lung cancer (SCLC) characterized by small cells with a very poor
prognosis and mostly associated with paraneoplastic syndromes
(Cushing's syndrome, Syndrome of inappropriate antidiuretic hormone
secretion) and non-SCLC (NSCLC), which are subdivided into squamous
cell carcinoma, large cell carcinoma and lung adenocarcinoma
(10). The subtype most associated
with tobacco exposure is squamous cell cancer, but the most
frequent subtype is lung adenocarcinoma, which accounts for >60%
of non-small cell tumors (11).
Lung adenocarcinoma has unique histological, radiological,
epidemiological, molecular and clinical characteristics compared
with other tumors. For instance, the incidence of lung
adenocarcinoma is similar in males and females, and it is the lung
neoplasm with the highest incidence in individuals who have never
smoked and <45 years of age (12). Histologically, lung adenocarcinoma
is composed of bronchial glands with a tendency to papillary
configuration that degenerate due to deterioration of type II
pneumocytes, generating atypical alveolar hyperplasia and later a
truly invasive neoplasia. At the pathological level, the 2021 World
Health Organization (WHO) classification allows lesions to be
differentiated according to their invasive potential, classifying
them into minimally invasive mucinous or non-mucinous lesions, and
invasive non-mucinous adenocarcinoma, which in turn may be
subclassified in acinar, papillary, micropapillary and solid
tumors. Other less frequent types include invasive mixed mucinous
lesions, colloid adenocarcinoma, fetal or enteric type, each of
them with diagnostic, prognostic and clinical peculiarities
(13).
Regarding the early diagnosis of this disease, it
should be noted that there are screening programs for smokers that
have been evaluated by low-dose computed tomography (CT) of the
chest as approved by the US Preventive Task Force, but in clinical
practice, they are difficult to apply, which means that most
patients are diagnosed in advanced stages of the disease (14). With respect to the clinical
management of pulmonary nodules, evaluation with CT, thoracoscopy,
mediastinoscopy and positron emission tomography-CT have allowed to
improve the diagnosis in the initial stages in these patients,
which still represent a small proportion of them and it is
associated with a complex management, subjecting the patients to a
great level of emotional stress with a follow-up that may last
several months (15). Unlike other
tumors, such as pancreatic adenocarcinoma, ovarian, breast and
testicular neoplasms, where markers such as CA19-9, CA125, CA15.3
or carcinoembryonic antigen (CEA) may be used, there are currently
no serological markers in daily clinical practice that may help
diagnose lung cancer (16). Of
note, most patients initially present with constitutional syndrome,
hemoptysis or cough. It is also common for numerous patients to
present with superior vena cava syndrome, Horner's syndrome,
compression of the brachial nerve or pericardial effusion (17). Although it is true that SCLC is the
lung neoplasm that has been most clearly associated with
paraneoplastic syndromes, patients with lung adenocarcinoma may
present with acanthosis nigricans, dermatomyositis or and Trousseau
syndrome (18).
The therapy of these tumors is different according
to the time-point of diagnosis. In initial stages (I, II and IIIA),
where the tumor is susceptible to surgical treatment, patients may
undergo radiotherapy, neoadjuvant chemotherapy and subsequent
surgery if they are surgical candidates. In more advanced stages,
such as IIIB and IV, where there is mediastinal or subcarinal
involvement, contralateral pulmonary invasion or metastatic spread,
they are treated with chemoradiotherapy. Furthermore, it is
possible to perform a histopathological study in order to
administer specific immunotherapy regimes (19,20).
In this sense, the use of immunotherapy has been a real advance in
recent years as it has improved the prognosis in patients with
disseminated disease, but even so, >80% of patients diagnosed
with lung adenocarcinoma in the advanced stage do not survive for
>5 years (21). Similarly, the
lack of early diagnosis or serological markers is a real challenge
in the early diagnosis of this disease. Hence, the purpose of the
present review was to discuss the main immunohistochemical and
diagnostic markers that not only help in the initial staging but
may also be useful in the follow-up of patients in both advanced
and early stages. Furthermore, the state-of-the-art of potential
serological markers used in these patients was equally revisited,
including promising approaches such as circulating tumor cells
(CTCs), microRNAs (miRNAs/miRs) and exosomes.
2. Molecular and histological markers in
lung cancer
In recent years, the histopathological
classification of lung cancer has been modified by the discovery of
novel histological markers, targeted therapies and numerous
molecular markers, which have completely changed the diagnosis and
prognosis of these patients. Since 2015, the WHO molecular markers
have guided the treatment to be followed based on the expression of
markers with a last update in 2021, taking even more into account
the relationship of molecular markers with the diagnosis of lung
adenocarcinoma (22). Classically,
the treatment of metastatic lung adenocarcinoma has been based on
the use of systemic chemotherapy regimens not only to limit the
extension and limit tumor progression, but also for palliative
purposes to reduce tumor burden and improve the symptoms of
patients in the final stages of the disease. In the last 20 years,
discoveries in different molecular pathways have provided a better
understanding of the underlying pathophysiology of the mechanisms
of carcinogenesis, vascular invasion, proliferation and metastatic
capacity. These mechanisms range from epigenetic and genetic
markers to cytoplasmic receptors and metabolic pathways, which has
led to the development of molecular targets in selected patients
(23). Despite their efficacy,
chemotherapy regimens have multiple common adverse effects that may
even be lethal for numerous patients, so the use of immunotherapy
not only reduces the risk of adverse effects, but its
administration is also associated with better long-term results
(24). Of note, prior to the
application of directed therapies, the expression of different
biomarkers must be analyzed to establish which patients may be
candidates for these therapies. Among the numerous biomarkers
available, the most notable are somatic genetic alterations called
driver mutations. Driver mutations are genetic alterations that
occur in the preneoplastic phase of tumor cells and that confer
mitotic and invasive activity (25). Likewise, there are so-called
passenger mutations that occur in tumor cells but with limited
action in the invasive neoplastic process and that may be observed
in non-tumor cells (26).
The metabolic pathways that confer survival to tumor
cells in these patients derived from the expression of driver
mutations have been studied in recent years. The identification of
driver mutations has been and remains to be a central subject of
study. For instance, Kris et al (27) examined 1,007 patients with lung
adenocarcinoma and found driver mutations in 64% of them, and
genetic alterations in KRAS, EGFR and anaplastic lymphoma kinase
(ALK) were frequent. Of the 1,007 patients, 28% were candidates for
receiving targeted therapy, achieving a median survival of 3.5
years compared to 2.4 years in those who did not receive targeted
therapy (27). Currently, the
development of new drugs and novel formulations of existing ones
allow for expanding the number of candidates, which has undoubtedly
made it possible to improve average survival. That is why in recent
years, associations such as the WHO, College of American
Pathologists, Spanish Society of Medical Oncology and Spanish
Society of Pathology, among others, recommend genotyping for EGFR
(Epidermal growth factor receptor) and BRAF (V-Raf murine sarcoma
viral oncogene homolog B) V600E mutations, ALK (anaplastic lymphoma
kinase) and c-ros oncogene 1 (ROS1) rearrangement and, in the case
of non-smokers, light smokers or people <50 years of age,
request the examination of programmed death ligand 1 (PDL1)
expression (28-30). Genotyping may be obtained by
different techniques, such as DNA sequencing, next-generation
sequencing (NGS), DNA allele-specific testing, immunohistochemistry
or in situ fluorescence (31). It should be noted that NGS has
provided a true progression in the analysis of mutations in lung
cancer, since it allows the analysis of a wide variety of genes
whose information may be stored and later studied to analyze the
relevance of undescribed mutations, which makes it possible to have
a mutation database (32). In
addition, the efficacy of studying driver mutations in CTCs in
peripheral blood has been noted, although with a sensitivity
limited to 60-80%, which may restrict the possibility it of being
applicable in targeted treatments in addition to not allowing the
measurement of PDL1 under certain conditions (33). For instance, the mutation of EGFR,
present in ~15% of adenocarcinomas in western countries, determines
the activation of metabolic pathways such as RAS, PI3K or
phospholipase C that cause an increase in cell survival and tumoral
growth (34). The EGFR mutation is
subsidiary to treatment with tyrosine kinase inhibitors, such as
erlotinib, afatinib or osimertinib. Of note, in Asian populations,
the presence of EGFR mutations has been described in >50% of
adenocarcinomas (35). It should
also be highlighted that there are mutations within the EGFR gene
in exons 18-21, being the most frequent those occurred in exons 19
and 21, each with different prognostic implications (36). In this sense, Yoon et al
(37) analyzed 1,020 patients with
lung adenocarcinoma and obtained a positive result for EGFR in 388
patients (~38%). Of the 388 patients, 51% had a mutation in exon 19
with a median survival of 29.9 months, whereas for those who had a
mutation in exon 21, the median survival was 20.6 months. In a
meta-analysis conducted by Zhang et al (38) that included 13 different studies,
exon 19 deletion appeared to be associated with longer
progression-free survival compared to L858 mutation at exon 21
after treatment with first-line EGFR-tyrosine kinase inhibitors
(EGFR-TKIs). Likewise, patients with the T790M mutation of exon 20,
where a threonine is replaced by a methionine in position 790, may
be resistant to first- and second-generation EGFR-TKI, although
this resistance does not occur with third-generation agents, such
as osimertinib, which has been evaluated in clinical trials such as
AURA 3 (39,40).
The rearrangement of ALK has also been described,
which has an incidence of ~4% in patients with lung adenocarcinoma
(41). ALK rearrangement leads to
alterations in the signaling of a type of insulin-related receptor
tyrosine kinase present in neurons of the central nervous system
that is frequently altered in anaplastic long cell lymphoma,
inflammatory myofibroblastic tumor and neuroblastoma. All of this
determines the activation of the echinoderm
microtubule-associated-protein-like 4-ALK complex, which causes
alterations in the correct formation of microtubules and thus
proliferation and migration of tumor cells (42). It should be noted that ALK
rearrangement has special clinical features. For instance, patients
with ALK rearrangement are more likely to develop brain metastases
(43). In addition, these patients
tend to be younger, with a mean age of 52 years, and non-smokers
(44). It should be noted that
cases with ALK rearrangement respond to treatments with crizotinib,
ceritinib or lorlatinib, among others, determining average survival
times of up to 48 months in advanced stages (45).
On the other hand, cases with abnormalities of
mesenchymal epithelial transition factor receptor (MET) tyrosine
kinase in exon 14-skipping mutations and amplifications present in
up to 6% of lung adenocarcinomas-responded to therapies with
capmatinib, tepotinib and crizotinib, which made it possible to
control the progression of adenocarcinoma in these patients
(46). MET is activated by a
single ligand known as hepatocyte growth factor, driving the
activation of pathways such as AKT, ERK/MAPK or STAT3, promoting an
increase in cell survival, proliferation and migration (47).
Likewise, the rearrangement of the protooncogene RET
has been described, which determines an activation of cytosolic
kinases derived from RET, which occurs in 1-2% of patients with
lung adenocarcinoma (48). RET
rearrangement is mainly found in young patients without a history
of smoking and, like EFGFR or ALK mutations, it is associated with
a high probability of metastatic progression in the brain (49). RET rearrangement is amenable to
treatment with selpercatinib and pralsetinib therapy as
demonstrated in the LIBRETTO-001 and ARROW clinical trials with
response rates of 64% maintained for ~18 months (50,51).
Mutations in BRAF and the subsequent activation of
the MAPK pathway are present in ~4% of patients and are usually
associated with non-smoking patients (52). Given the favorable responses shown
in patients with other aggressive neoplasms such as melanoma, the
use of different targeted therapies has been studied, with the
V600E mutation being the most frequent in lung adenocarcinoma.
Patients with BRAF mutations tend to have a better prognosis than
those without mutations, as this conditions a better response to
immunotherapeutic treatment with dabrafenib and trametinib
(53). Another type of molecular
alteration described are alterations in tropomyosin receptor
kinases, which are present in <1% of adenocarcinomas that are
candidates for treatment with larotrectinib and entrectatinib,
reaching response rates of up to 80% and a median survival of 90
months (54).
Rearrangement of ROS1, which is present in up to 2%
of lung adenocarcinomas, is also worth mentioning (55). This alteration, similar to the
others, is much more frequent in patients with adenocarcinoma,
being more common in young patients and non-smokers. This mutation
usually occurs between the ROS1 and CD74 oncogene and is
accompanied by an activation of metabolic pathways such as
JAK/STAT, PI3K/AKT and MAPK/ERK, causing an increase in cell
proliferation, cell survival and histological invasion capacity
(56). One of the therapies that
has demonstrated greater efficacy in these patients is the ROS1/MET
tyrosine kinase inhibitor crizotinib. The outcome of the EUCROSS
clinical trial was a mean survival rate of 55% at 48 months in
patients with NSCLC with ROS rearrangement (57,58).
On the other hand, the efficacy of entrectinib, a ROS1/tropomyosin
tyrosine kinase inhibitor, has also been demonstrated by different
studies. Response rates of 67.1% were obtained, with a response
rate of 72.9% for intracranial metastases and a progression-free
survival of 15.7 months (59). As
with EGFR-TKIs, there are mechanisms of immunoresistance to
crizotinib, with lorlatinib exhibiting a greater efficacy in
patients who had previously received crizotinib (60). Despite the fact that only a small
number of patients are candidates for immunotherapy with ROS1
rearrangement, they obtain very relevant response rates with high
average survival rates. Since the vast majority of patients do not
carry any driver mutations, other histological markers are being
evaluated with different types of targeted therapies, where the
programmed death receptor (PD) and its ligands PDL1 and -2 stand
out, which act as inhibitory factors of the immune response
(61). The expression of PDL1 has
been demonstrated in different tumors and authors have described
its expression level by immunohistochemistry in NSCLC. Aggarwal
et al (62) analyzed 4,784
patients and observed that 28% had a PDL1 expression of ≥50%, 38%
had PDL1 expression between 1 and 49 and 33% had an expression of
<1%. Of these, 80% corresponded to the non-squamous variant
(mainly adenocarcinoma) and 20% to the squamous variant (62). Treatment targeting PDL1 expression
is based on several clinical trials, including KEYNOTE-189,
KEYNOTE-407, KEYNOTE 024 or IMpower 110. According to the results
of the previous clinical trials, in patients with PDL1 expression
<50% or unknown metastatic status, the combination of
pembrolizumab (anti-PD1) plus pemetrexed and carboplatin in
non-squamous NSCL are recommended (63,64).
In those patients with >50% expression of PDL1 who do not have
any rapidly progressive disease, it is recommended as a first-line
treatment monotherapy with pembrolizumab or atezolizumab
(anti-PDL1) (65,66). In cases of rapidly progressive
disease, it is recommended to start pembrolizumab plus chemotherapy
(67). Immunotherapy may be
applied based on different molecular alterations and a large
therapeutic arsenal is available, which allows clinicians to offer
new therapeutic opportunities to patients diagnosed with NSCLC.
In addition, the use of molecular markers has made
it possible to better classify the subtypes of lung cancer in cases
where the morphology is not clear or when biopsies are not able to
be performed to obtain the necessary material for diagnosis. For
instance, markers such as thyroid transcription factor 1, which is
associated with the EGFR mutation, napsin A and surfactant A, are
much more specific for lung adenocarcinoma, while p63, cytokeratin
5/6, SOX2 and desmoglein-3 are characteristic of squamous cell
carcinoma (68). Likewise, there
are immunohistochemical markers related to a greater extent with
SCLC, such as LMWK, CAM5.2, chromogranin or CD56, among others
(69).
Collectively, the prognostic and therapeutic
implications of driver mutation analysis have led to the creation
of a wide therapeutic armamentarium even in those cases with rare
genetic alterations, which reinforces the importance of molecular
biology in the treatment of lung adenocarcinoma. In conclusion, the
importance of driver mutations as biomarkers and their molecular
analysis have achieved an improvement in the survival of patients
with lung adenocarcinoma in recent years, allowing to guide the use
of therapeutic agents to reduce the complications associated with
chemotherapy treatment and improve the quality of life of
patients.
3. Serological markers
Even though lung adenocarcinoma is one of the main
neoplasms in the current population and despite the numerous driver
preferences that have been described, this type of cancer currently
lacks effective screening programs. The imaging screening test is
based on the use of low-radiation CT of the chest, the
implementation of which, notwithstanding having demonstrated its
usefulness, is limited in daily clinical practice (70). In addition, the presence of
solitary pulmonary nodules in the general population is observed in
up to 13% of chest CT scans, of which ~23% may be due to malignant
pulmonary neoplasms. The evaluation of these solitary pulmonary
neoplasms may involve the use of invasive tests, which are not
exempt from complications (71).
Furthermore, as mentioned above, the majority of patients with lung
adenocarcinoma are diagnosed at an advanced stage of the disease,
so the implementation of serological markers may provide noteworthy
benefits in the early diagnosis of the disease.
In this sense, various authors have attempted to
define the clinical usefulness of potential serum biomarkers. CEA
is one of the most studied markers in lung cancer, being the tumor
serological marker that is mainly used for follow-up and diagnosis
in colorectal cancer. Grunnet and Sorensen (72) evaluated the usefulness of CEA in
lung adenocarcinoma in 217 studies where is observed a correlation
with elevated serum level of CEA and risk of recurrence and death
in this neoplasm. The diagnostic utility of CEA as a serological
marker has also been evaluated together with other potential serum
biomarkers. In this sense, Xu et al (73) defined the sensitivity and
specificity of CEA, neuron-specific enolase (NSE) and matrix
metalloproteinase-9 in 36 patients with lung cancer, obtaining an
area under the curve (AUC) of 0.84, 0.8 and 0.89, respectively. In
the receiver operating characteristics analysis, the AUC is a
measure of the diagnostic performance of a test by comparing
sensitivity and specificity, with a value of 1 being the highest
score attainable. Despite this, Yang et al (74) obtained AUCs that did not exceed
0.65 when markers such as CEA, CA125, CY211 (cytokeratin 19
fragment), NSE or squamous cell carcinoma antigen (SCC) were used
independently in 2,063 individuals, of whom 780 were healthy
controls, 650 patients had pneumonia and 633 patients had lung
cancer to differentiate lung cancer from patients without cancer.
Conversely, these authors also evaluated the combination of
different markers to increase the AUC. For instance, the use of
markers such as CEA+CA125+CY211+SCC reaches an AUC of 0.867 when
comparing patients with lung adenocarcinoma vs. healthy patients.
The limited utility of the markers to be used individually relies
on the fact that numerous benign entities, such as bronchiectasis,
pneumonia or chronic lung diseases, may raise different markers on
their own. For this reason, combinations of serological markers
have only reached AUCs of >0.715 in differentiating pneumonia
from pulmonary neoplasia (74).
The association between serological markers and
histology in lung adenocarcinoma is limited and histological
confirmation would always be necessary to analyze driver mutations
for therapeutic planning of patients. In 155 patients with lung
adenocarcinoma, Gao et al (75) examined how the serological CEA
concentration is related to neoplastic lesions that carry EGFR
mutations, indicating that this was associated with an unfavorable
prognosis and progression during the use of EGFR inhibitors
(75). In this light, other
studies such as that by Molina et al (76) indicated the usefulness of
serological markers in combination, such as CEA, CA15.3, SCC, CY
211, NSE and progastrin-releasing peptide, in the initial
diagnostic of 3,144 individuals with suspected lung cancer, of
which the diagnosis of neoplasia was confirmed in 1,828. They
reported a sensitivity of 88.5% and a specificity of 82%, in
addition to a positive predictive value of 87.3%, therefore
demonstrating the relevance of serological markers in the early
diagnosis of this pathology. It should be noted that in patients
with nodules <3 cm, the negative predictive value was 71.8%. In
other words, 71.8% of the patients who did not have any elevated
serological markers did not have a true malignant neoplasm, which
allows for more conservative management, limiting the use of
aggressive thoracic surgeries and biopsies (76). The prognostic utility of different
serological markers in lung cancer has been evidenced by different
authors. For instance, Chen et al (77) evaluated the preoperative levels of
the serological markers CEA, CYFRA21-1, NSE, CA 19-9, CA 153 and
CA125 in 2,654 patients with NSCLC who were candidates for
resection surgery, demonstrating how high levels of CEA, CYFRA 21-1
or CA125 are associated with unfavorable survival and higher rates
of recurrence. In this light, Bes-Scartezini and Saad Junior
(78) determined that in 112
patients with non-squamous NSCLC, elevation of CA125 or Ca15-3 was
associated with unfavorable survival (77,78).
Therefore, in recent years, the use of serological
markers has not been applied in daily clinical practice, despite
the fact that combination panels have demonstrated their usefulness
not only in the initial diagnosis of these patients, but also in
the follow-up or evaluation and prognosis, acting as potential
prognostic and predictive biomarkers.
4. CTCs
The concept of CTCs is based on the existence of
epithelial cells in the blood circulatory system after a process of
angioinvasion and metastatic dissemination, which are not normally
seen in patients without cancer. Usually, 1 CTC may be found for
every 10 million leukocytes in peripheral blood. There are
non-tumor conditions in which CTCs are present, generally due to
inflammatory diseases such as Crohn's disease or endometriosis, but
to a lesser extent due to tumor processes (79). The relevance of CTCs has already
been described in prostate, breast and colon cancer, where their
presence is accompanied by a worse prognosis and higher rates of
recurrence after chemotherapy or surgery (80). The identification of driver
mutations by different techniques has represented a real advance in
the treatment and prognosis of patients with NSCLC. It is important
to remark that patients may acquire new driver mutations during
targeted therapies, hence developing therapy resistance, which
would require a re-biopsy in most patients. Conceptually, rebiopsy
would be useful not only to study the mechanisms of
immunoresistance (such as the T790M mutation in patients with
EGFR-mutated lung adenocarcinoma), but also to understand the
underlying pathophysiology of the molecular pathways that
ultimately cause immunoresistance (81). The utility of peripheral blood
liquid biopsy would allow the detection of CTCs in order to study
new therapies and develop cell cultures to facilitate a deeper
understanding of the physiological mechanisms of the metastatic
process (82). Simultaneously,
CTCs also allow the monitoring of immunochemotherapy treatment,
since a decrease in these cells would indicate a better response to
the therapies received (83).
Multiple methods have been studied for the detection
of CTCs. The gold standard method approved by the FDA is based on
the detection of the epithelial proteins epithelial cell adhesion
molecule (EpCAM), cytokeratins 8, 18 and or 19 using the Cellsearch
method, which is approved for metastatic breast cancer, prostate
adenocarcinoma and colorectal cancer (84-86).
With respect to lung cancer, numerous authors have used the
Cellsearch method to detect CTCs. In this sense, the detection of
CTCs has been observed in both NSCLC and SCLC. Hou et al
(87) studied these cells in 97
patients with SCLC, detecting CTCs in 85% of them. Furthermore, the
average survival of patients with >50 CTCs per 7.5 ml of blood
was limited to 5.4 months, while in patients with <50 CTCs, a
higher median survival was reported (87). This is in consonance with the
results of Naito et al (88), who reported that 51 patients with
lung cancer with elevated CTCs had unfavorable survival. Regarding
lung adenocarcinoma, the role of CTCs was also demonstrated in the
diagnosis and monitoring of these patients. Indeed, previous works
have been able to detect ALK rearrangements in CTCs, which may
allow starting therapy with tyrosine kinase inhibitors such as
crizotinib in the future, demonstrating its usefulness in the
follow-up of patients diagnosed with lung cancer (89,90).
Other methods for the detection of CTCs, such as positive
immunoselection of EpCAM, negative immunoselection of leukocytes,
filtration, immunomagnetics, electrophoresis or flow cytometry have
also demonstrated their utility but are not currently approved by
the FDA, as they are based on complex techniques that require very
well-trained personnel, which may not be accessible in daily
clinical practice (84). In
reference to liquid biopsy using CTCs, the main limitations of this
technique are mainly based on sample collection and processing
techniques, given that CTCs may become fragile and cannot be
processed properly. In addition, its high price and technical
complexity must be highlighted, which frequently requires a support
laboratory that not all hospitals are able to afford. All of this
may affect the diagnostic performance, decreasing both sensitivity
and specificity, and since these techniques have
diagnostic-therapeutic repercussions, they must be validated in
large clinical trials. Therefore, despite the immense benefits in
the detection of CTCs, these techniques are accompanied by
limitations that may restrict their use in real clinical practice
(91).
Therefore, in recent years, advances in the genetic
analysis of lung cancer have revolutionized the treatment and
management of this disease. The possibility of obtaining the
necessary material through liquid biopsy in peripheral blood and
the possibility of evaluating the immunohistochemical and genetic
expression of CTCs is accompanied not only by an improvement in the
diagnosis of this disease, but also in the monitoring and early
detection of mechanisms of immunoresistance that may cause a fatal
outcome of these patients.
5. MicroRNAs
MiRNAs are small non-coding RNA molecules with a
length of ~20 nucleotides that regulate the post-transcriptional
expression of genes that may be related to cell differentiation,
proliferation and apoptosis processes by promoting or suppressing
the expression of a gene after transcription. A miRNA molecule
regulates the post-transcriptional expression of up to 200
different genes and its study may expand the understanding of the
underlying pathophysiology of the metastatic process (92). In relation to lung cancer, the
implications of miRNAs are numerous-they may promote processes such
as cellular proliferation, metastatic invasion and therapy
resistance through the upregulation or downregulation of either
tumor suppressor genes or oncogenes (93). As previously mentioned, tobacco is
the main cause of lung cancer in the general population and it has
been indicated how the levels of miR-532-5p, miR-25-3p and
miR-133a-3p were significantly higher in patients with lung
carcinoma compared to healthy controls, also observing differences
between expression levels depending on the smoking status (94). On the other hand, Nymark et
al (95) indicated how
numerous miRNAs are dysregulated according to exposure to asbestos
and its relationship with lung cancer. Likewise, there are numerous
miRNAs that have been implicated in EGFR mutations, such as miR-7,
miR-27a-3p, miR-30 and miR-34, which led to the activation of the
RAS/MEK and PI3K/mTOR pathways with consequently uncontrolled cell
proliferation (96,97). Similarly, a role of miR-96 has been
described in the altered levels of ALK and the activation of the
RAS/MEK and PI3K/mTOR metabolic pathways (98). miR-760 causes alterations in the
expression of ROS1, while let-7, miR-193a-3p or miR-148a-3p are
related to KRAS mutations, previously demonstrating their
importance in tumor progression (99-102).
The diagnostic utility of miRNAs has also been
studied by different authors. For instance, the presence of miR-205
was specific for squamous cell carcinoma compared to miR-124a,
which is more characteristic for lung adenocarcinoma (103,104). The relationship between histology
and miRNA expression has made it possible to demonstrate how
miR-93, miR-221 and miR-30e are specific for squamous cell
carcinoma, while miR-29b, miR-29c, let-7e and miR-125a-5p are more
specific for lung adenocarcinoma (105). One of the uses of miRNAs is based
on the possibility of them being used in screening programs that
determine the blood levels of multiple miRNAs, which simplifies the
diagnostic process as well as its ease of performance and improves
its diagnostic performance. Studies such as that by Montani et
al (106) have evaluated the
use of a kit with 34 miRNAs in 1,115 individuals with a high risk
of lung cancer, obtaining a sensitivity of 75.9%, a specificity of
77.8% and an AUC for the diagnostic yield of 85% (106). These results are in agreement
with those obtained by Sozzi et al (107), who analyzed 69 patients with lung
cancer using a kit of 24 miRNAs, obtaining a sensitivity of 87% and
a specificity of 81% (107).
Asakura et al (108)
reported that after analyzing up to 2,588 miRNAs in 208 patients
with lung cancer compared to healthy controls, the highest
diagnostic yield using miRNAs was obtained with miR-1268b and
miR-6075, obtaining a sensitivity and specificity of 99% and an AUC
of 0.993 for lung cancer screening (108).
On the other hand, the expression of different
miRNAs has been studied to analyze its relationship with the
prognosis of patients. Xiao et al (109), in a meta-analysis of 15 studies
that included a total of 1,753 patients with both SCLC and NSCLC,
described that upregulation of miR-125b, miR-21, miR-141, miR-200c,
miR-197, miR-41, miR-370, miR-376α, miR-192 and miR-662 and the
downregulation of miR-26b, miR-381, miR-146α, miR-148α, miR-204,
miR-374α, miR-638 or miR-148b were associated with poor median
survival, evidencing the complex role of miRNAs in lung cancer.
It should be noted that alterations in different
miRNAs have been related to mechanisms of chemoresistance and
sensitivity to immunotherapy. For instance, overexpression of
miR-106b leads to a decrease in the P-glycoprotein responsible for
chemoresistance mechanisms to cisplatin, which causes greater
sensitivity to cisplatin (110).
In turn, Qiu et al (111)
have demonstrated that downregulation of miR-503 alters the
expression of proteins related to chemoresistance processes, such
as the antiapoptotic protein Bcl-2, while another study indicated
that overexpression of miR-196a leads to decreased efficacy of
cisplatin (112). Given that in
recent years, immunotherapy has laid a foundation for the
management of patients with lung cancer, the expression of miRNA in
this context has been evaluated in numerous studies. For instance,
Bisagni et al (113)
examined 32 patients with lung adenocarcinoma receiving second- or
third-line treatment with erlotinib, an EGFR tyrosine kinase
inhibitor, and miR-133b upregulation was associated with better
progression-free survival. However, the main limitation of miRNAs
in lung cancer in terms of their usefulness for screening is their
limited specificity. For instance, miR-21-5-p, miR-155-5p and
miR-210-3p are expressed in different neoplasms, such as breast or
colon cancer, among others, which would require patients to undergo
multiple diagnostic tests with the probability of adverse effects
without a clear diagnostic suspicion. In addition, both
upregulation and downregulation of the same miRNA may be observed
in different neoplasms, which increases the diagnostic uncertainty.
In addition, large clinical trials should be implemented to
specifically validate detection kits that are cost-efficient in
different neoplasms so that they may be systematically applied in
different malignant neoplasms (114).
Examination of miRNAs, which may be performed by
liquid biopsy in peripheral blood, has useful implications in the
diagnosis, follow-up and treatment of patients with pancreatic
adenocarcinoma that may improve diagnosis in early stages and
improve the understanding of the mechanisms of immunochemical
resistance in these patients.
6. Conclusions
Lung cancer is one of the most frequent neoplasms
and the deadliest type of cancer, which is specifically associated
with tobacco consumption. Despite numerous efforts and screening
programs that have been performed, most patients are diagnosed in
the advanced stages of the disease. Lung adenocarcinoma is a
specific subtype of NSCLC with unique histological, radiological,
epidemiologica and clinical characteristics. Recent advances in the
molecular biology of these tumors have permitted the identification
of multiple markers, as summarized in Fig. 1. The study of these markers has
allowed the development of numerous targeted therapies, also aiding
to improve the prognosis, diagnosis and prediction of the response
to different therapeutic regimes. Likewise, numerous serological
markers have been studied, demonstrating promising translational
uses. The main markers studied with their most important
translational/clinical applications are summarized in Table I. Overall, there is still much to
explore in the field of biomarkers in lung adenocarcinoma,
particularly regarding the aim to improve early detection of the
disease and identifying new molecular routes that may be used for
targeted therapies, which is proving to be one of the most
important advances in the field of oncology.
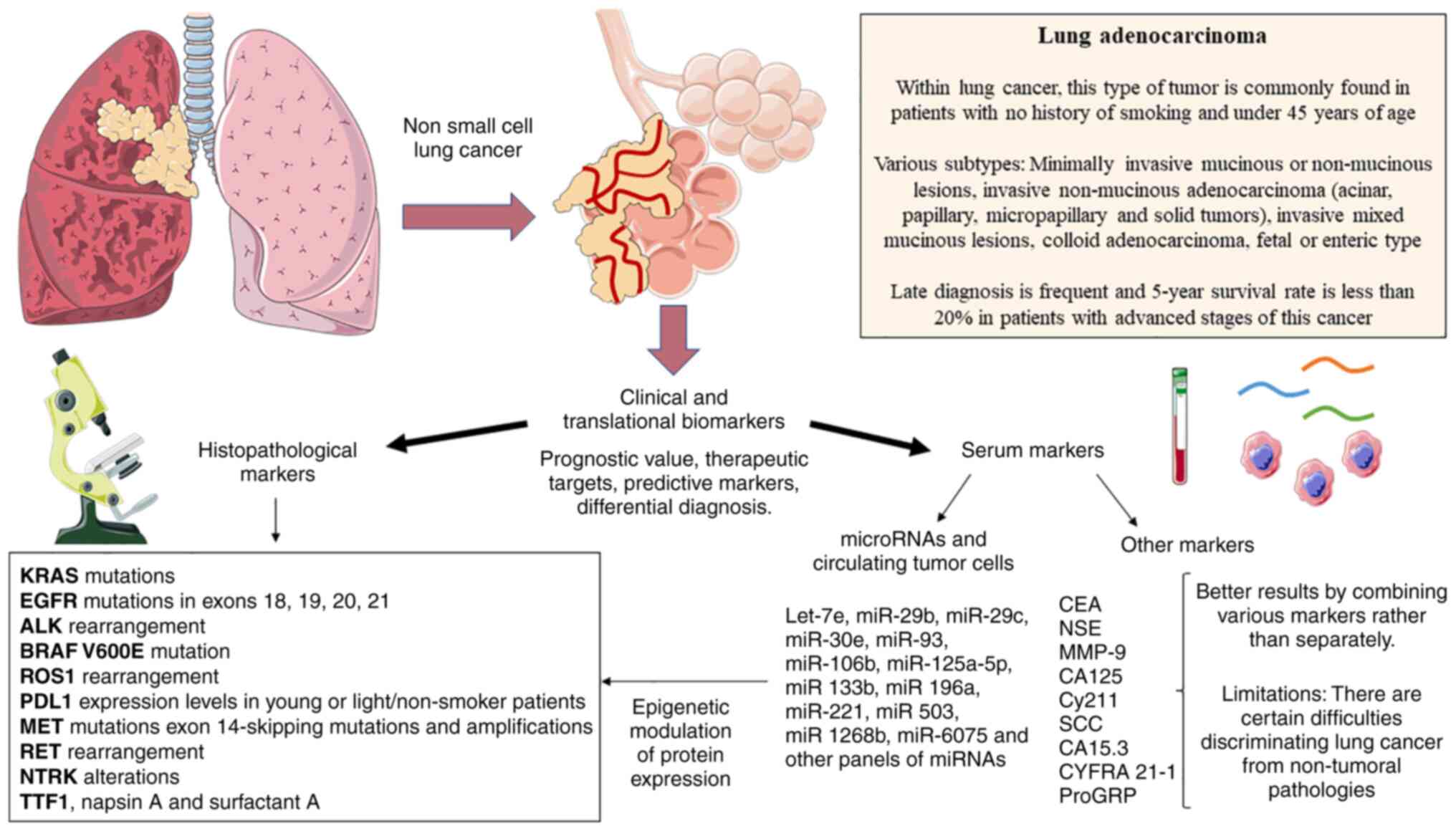 | Figure 1Main clinical and translational
biomarkers studied in lung adenocarcinoma. Both histopathological
and serological markers are available. All of these markers are
involved or appear as a consequence of the tumoral biology, aiding
in the development of novel therapeutic strategies, prognostics,
response to therapy prediction or in differential diagnosis.
miRNA/miR, microRNA; EGFR, epidermal growth factor receptor; ALK,
anaplastic lymphoma kinase; MET, mesenchymal epithelial transition
factor receptor; RET, rearranged during transfection; BRAF, V-Raf
murine sarcoma viral oncogene homolog B; NTRK, neurotrophic
tyrosine receptor kinase; CEA, carcinoembryonic antigen; CA125,
cancer antigen 125; CY211, cytokeratin 19 fragment; SCC, squamous
cell carcinoma antigen; NSE, neuron-specific enolase; ProGRP,
pro-gastrin-releasing peptide; TTF1, thyroid transcription factor
1; ROS1, c-ros oncogene 1; PDL1, programmed death ligand 1. |
 | Table IMain translational applications
derived from the potential biomarkers collected in the present
study. |
Table I
Main translational applications
derived from the potential biomarkers collected in the present
study.
| Biomarker | Type |
Diagnostic/prognostic value |
Predictive/therapeutic utility | (Refs.) |
|---|
| EGFR | Driver
mutation | 15% of
adenocarcinomas (up to 50% in Asian population) | EGFR
inhibitors | (34,35) |
| T790M EGFR
mutation | Driver
mutation | Resistance to
first-and second-generation EGFR inhibitors | Response to third
generation tyrosine kinase inhibitors | (39,40) |
| ALK | Driver
mutation | ~4% of
adenocarcinomas, non-smoking young patients | ALK inhibitor | (41-45) |
| MET | Driver
mutation | ~6% of
adenocarcinomas | MET inhibitor | (46,47) |
| RET | Driver
mutation | 1-2% of
adenocarcinomas, non-smoking young patients | RET inhibitor | (50,51) |
| BRAF | Driver
mutation | 4% of
adenocarcinomas | anti-BRAF
inhibitor | (52,53) |
| NTRK | Driver
mutation | <1% of
adenocarcinomas | Tropomyosin kinase
inhibitor | (54) |
| CEA+CA125+C
Y211+SCC | Serological marker
combination | AUC 86.7% | Screening | (61) |
| CEA, CA15.3, SCC,
CY21, NSE and ProGRP | Serological marker
combination | Sensitivity, 88.5%;
specificity, 82%; positive predictive value, 87.3% | Early
diagnosis | (63) |
| Circulating tumor
cells | Liquid biopsy
diagnostic kit | Detectable in up to
85% of small-cell lung cancers | - | (70-72) |
| miR-93, miR-221 and
miR-30e | miRNA | Specific for
squamous cell lung cancer | - | (90) |
| miR-29b, miR- 29c,
let-7e and miR-125a-5p | miRNA | Specific for lung
adenocarcinoma | - | (90) |
| Panel of 34
miRNAs | miRNA kit evaluated
in 1115 individuals | Sensitivity, 75.9%;
specificity, 77.8%; AUC, 85% | - | (84) |
| Panel of 24
miRNAs | Evaluated in 939
individuals | Sensitivity, 87%;
specificity, 81% | - | (91) |
| miR-1268b and
miR-6075 | miRNA | Sensitivity, 99%;
specificity, 99%; AUC, 0.993 | - | (92) |
| miR-106b | miRNA | - | Increased
sensitivity to cisplatin | (94) |
| miR-196a | miRNA | - | Increased
resistance to cisplatin | (96) |
| miR-503 | miRNA | - | Less sensitive to
chemotherapy | (95) |
| miR-133b | miRNA | - | Better survival in
patients with mutated EGFR | (97) |
Availability of data and materials
Not applicable.
Authors' contributions
MAO, FN, LP, MAM were involved in the
conceptualization of the study. MAO and MAM were involved in
funding acquisition. MAO was involved in project administration.
MAO, FN, LP, OFM, CGM, MAS, MA, JM and MAM were involved in the
investigative aspects of the study. MAO, FN, LP, OFM, CGM, MAS, MA,
JM and MAM were involved in data validation. All authors have read
and agreed to the published version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The study was supported by the Comunidad de Madrid (grant no.
B2017/BMD-3804 MITIC-CM) and the patronage program HALEKULANI, S.
L.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo YH, Luo L, Wampfler JA, Wang Y, Liu D,
Chen YM, Adjei AA, Midthun DE and Yang P: 5-year overall survival
in patients with lung cancer eligible or ineligible for screening
according to US Preventive Services Task Force criteria: A
prospective, observational cohort study. Lancet Oncol.
20:1098–1108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilfinger TV, Albano D, Perwaiz M,
Keresztes R and Nemesure B: Survival outcomes among lung cancer
patients treated using a multidisciplinary team approach. Clin Lung
Cancer. 19:346–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Groot PM, Wu CC, Carter BW and Munden
RF: The epidemiology of lung cancer. Transl Lung Cancer Res.
7:220–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mustafa M, Azizi ARJ, IIIzam EL, Nazirah
A, Sharifa AM and Abbas SA: Lung Cancer: Risk Factors, Management,
And Prognosis. IOSR J Dent Med Sci. 15:94–101. 2016. View Article : Google Scholar
|
|
6
|
Brambilla E and Gazdar A: Pathogenesis of
lung cancer signalling pathways: Roadmap for therapies. Eur Respir
J. 33:1485–1497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reitsma M, Kendrick P, Anderson J, Arian
N, Feldman R, Gakidou E and Gupta V: Reexamining rates of decline
in lung cancer risk after smoking cessation. A meta-analysis. Ann
Am Thorac Soc. 17:1126–1132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weber MF, Sarich PEA, Vaneckova P, Wade S,
Egger S, Ngo P, Joshy G, Goldsbury DE, Yap S, Feletto E, et al:
Cancer incidence and cancer death in relation to tobacco smoking in
a population-based Australian cohort study. Int J Cancer.
149:1076–1088. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barta JA, Powell CA and Wisnivesky JP:
Global epidemiology of lung cancer. Ann Glob Health. 85:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Houston KA, Mitchell KA, King J, White A
and Ryan BM: Histologic lung cancer incidence rates and trends vary
by race/ethnicity and residential county. J Thorac Oncol.
13:497–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Provencio M, Carcereny E, Rodríguez-Abreu
D, López-Castro R, Guirado M, Camps C, Bosch-Barrera J,
García-Campelo R, Ortega-Granados AL, González-Larriba JL, et al:
Lung cancer in Spain: Information from the thoracic tumors registry
(TTR study). Transl Lung Cancer Res. 8:461–475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Navani N and Spiro SG: The Presentation
and Diagnosis of Lung Cancer and Mesothelioma. Lung Cancer. John
Wiley & Sons, Ltd; Hoboken, NJ: pp. 15–47. 2013, View Article : Google Scholar
|
|
13
|
Nicholson AG, Tsao MS, Beasley MB, Borczuk
AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S,
et al: The 2021 WHO classification of lung tumors: Impact of
advances since 2015. J Thorac Oncol. 17:362–387. 2022. View Article : Google Scholar
|
|
14
|
Ruano-Ravina A, Provencio-Pulla M and
Casan Clarà P: Cribado de cáncer de pulmón con tomografía
computarizada de baja dosis. No es cuestión de logística. Arch
Bronconeumol. 53:593–594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nooreldeen R and Bach H: Current and
future development in lung cancer diagnosis. Int J Mol Sci.
22:86612021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zusman I and Ben-Hur H: Serological
markers for detection of cancer (Review). Int J Mol Med. 7:547–56.
2001.PubMed/NCBI
|
|
17
|
Kim HC, Jung CY, Cho DG, Jeon JH, Lee JE,
Ahn JS, Kim SJ, Kim Y, Kim YC, Kim JE, et al: Clinical
characteristics and prognostic factors of lung cancer in Korea: A
pilot study of data from the Korean nationwide lung cancer
registry. Tuberc Respir Dis (Seoul). 82:118–125. 2019. View Article : Google Scholar
|
|
18
|
Kanaji N, Watanabe N, Kita N, Bandoh S,
Tadokoro A, Ishii T, Dobashi H and Matsunaga T: Paraneoplastic
syndromes associated with lung cancer. World J Clin Oncol.
5:197–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steven A, Fisher SA and Robinson BW:
Immunotherapy for lung cancer. Respirology. 21:821–833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simmons CP, Koinis F, Fallon MT, Fearon
KC, Bowden J, Solheim TS, Gronberg BH, McMillan DC, Gioulbasanis I
and Laird BJ: Prognosis in advanced lung cancer-A prospective study
examining key clinicopathological factors. Lung Cancer. 88:304–309.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarro G, Paolini M and Rossi A: Molecular
Biology of Lung Cancer and Future Perspectives for Screening. Mass
Spectrometry-Future Perceptions and Applications. Kamble G:
IntechOpen; London: 2019, http://dx.doi.org/10.5772/intechopen.85334.
View Article : Google Scholar
|
|
24
|
Lee SH: Chemotherapy for Lung Cancer in
the Era of Personalized Medicine. Tuberc Respir Dis (Seoul).
82:179–189. 2019. View Article : Google Scholar
|
|
25
|
Brown AL, Li M, Goncearenco A and
Panchenko AR: Finding driver mutations in cancer: Elucidating the
role of background mutational processes. PLoS Comput Biol.
15:e10069812019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wodarz D, Newell AC and Komarova NL:
Passenger mutations can accelerate tumour suppressor gene
inactivation in cancer evolution. J R Soc Interface.
15:201709672018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kris MG, Johnson BE, Berry LD, Kwiatkowski
DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson
SL, Su PF, et al: Using multiplexed assays of oncogenic drivers in
lung cancers to select targeted drugs. JAMA. 311:1998–2006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
VanderLaan PA, Rangachari D and Costa DB:
The rapidly evolving landscape of biomarker testing in non-small
cell lung cancer. Cancer Cytopathol. 129:179–181. 2021. View Article : Google Scholar :
|
|
29
|
Rodríguez-Lescure A, de la Peña FA, Aranda
E, Calvo A, Felip E, Garrido P and Vera R: Study of the Spanish
Society of Medical Oncology (SEOM) on the access to oncology drugs
and predictive biomarkers in Spain. Clin Transl Oncol.
22:2253–2263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salas C, Martín-López J, Martínez-Pozo A,
Hernández-Iglesias T, Carcedo D, Ruiz de Alda L, García JF and
Rojo F: Real-world biomarker testing rate and positivity rate in
NSCLC in Spain: Prospective central lung cancer biomarker testing
registry (LungPath) from the Spanish Society of Pathology (SEAP). J
Clin Pathol. 75:193–200. 2022. View Article : Google Scholar
|
|
31
|
Normanno N, Barberis M, De Marinis F and
Gridelli C; On The Behalf Of The Aiot Expert Panel: Molecular and
genomic profiling of lung cancer in the Era of precision medicine:
A position paper from the Italian association of thoracic oncology
(AIOT). Cancers (Basel). 12:16272020. View Article : Google Scholar
|
|
32
|
Cainap C, Balacescu O, Cainap SS and Pop
LA: Next generation sequencing technology in lung cancer diagnosis.
Biology (Basel). 10:8642021.
|
|
33
|
Oxnard GR, Thress KS, Alden RS, Lawrance
R, Paweletz CP, Cantarini M, Yang JC, Barrett JC and Jänne PA:
Association between plasma genotyping and outcomes of treatment
with osimertinib (AZD9291) in advanced non-small-cell lung cancer.
J Clin Oncol. 34:3375–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
da Cunha Santos G, Shepherd FA and Tsao
MS: EGFR mutations and lung cancer. Annu Rev Pathol. 6:49–69. 2011.
View Article : Google Scholar
|
|
35
|
Shi Y, Li J, Zhang S, Wang M, Yang S, Li
N, Wu G, Liu W, Liao G, Cai K, et al: Molecular Epidemiology of
EGFR Mutations in Asian patients with advanced non-small-cell lung
cancer of adenocarcinoma histology-Mainland China subset analysis
of the PIONEER study. PLoS One. 10:e01435152015. View Article : Google Scholar
|
|
36
|
Lohinai Z, Hoda MA, Fabian K, Ostoros G,
Raso E, Barbai T, Timar J, Kovalszky I, Cserepes M, Rozsas A, et
al: Distinct epidemiology and clinical consequence of classic
versus rare EGFR mutations in lung adenocarcinoma. J Thorac Oncol.
10:738–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoon HY, Ryu JS, Sim YS, Kim D, Lee SY,
Choi J, Park S, Ryu YJ, Lee JH and Chang JH: Clinical significance
of EGFR mutation types in lung adenocarcinoma: A multi-centre
Korean study. PLoS One. 15:e02289252020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Sheng J, Kang S, Fang W, Yan Y,
Hu Z, Hong S, Wu X, Qin T, Liang W and Zhang L: Patients with Exon
19 deletion were associated with longer progression-free survival
compared to those with L858R Mutation after First-Line EGFR-TKIs
for advanced non-small cell lung cancer: A Meta-Analysis. PLoS One.
9:e1071612014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu YL, Tsuboi M, He J, John T, Grohe C,
Majem M, Goldman JW, Laktionov K, Kim SW, Kato T, et al:
Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N
Engl J Med. 383:1711–1723. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung
Cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar
|
|
41
|
Chia PL, Mitchell P, Dobrovic A and John
T: Prevalence and natural history of ALK positive non-small-cell
lung cancer and the clinical impact of targeted therapy with ALK
inhibitors. Clin Epidemiol. 6:423–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Selinger CI, Rogers TM, Russell PA,
O'Toole S, Yip P, Wright GM, Wainer Z, Horvath LG, Boyer M,
McCaughan B, et al: Testing for ALK rearrangement in lung
adenocarcinoma: A multicenter comparison of immunohistochemistry
and fluorescent in situ hybridization. Mod Pathol. 26:1545–1553.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Griesinger F, Roeper J, Pöttgen C,
Willborn KC and Eberhardt WEE: Brain metastases in ALK-positive
NSCLC-time to adjust current treatment algorithms. Oncotarget.
9:35181–35194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who Harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Britschgi C, Addeo A, Rechsteiner M,
Delaloye R, Früh M, Metro G, Banini M, Gautschi O, Rothschild SI,
Wild PJ, et al: Real-World treatment patterns and survival outcome
in advanced anaplastic lymphoma kinase (ALK) rearranged
non-small-cell lung cancer patients. Front Oncol. 10:12992020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liang H and Wang M: MET oncogene in
non-small cell lung cancer: Mechanism of MET dysregulation and
agents targeting the HGF/c-Met axis. Onco Targets Ther.
13:2491–2510. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Salgia R: MET in lung cancer: Biomarker
selection based on scientific rationale. Mol Cancer Ther.
16:555–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Drusbosky LM, Rodriguez E, Dawar R and
Ikpeazu CV: Therapeutic strategies in RET gene rearranged non-small
cell lung cancer. J Hematol Oncol. 14:502021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bronte G, Ulivi P, Verlicchi A, Cravero P,
Delmonte A and Crinò L: Targeting RET-rearranged non-small-cell
lung cancer: Future prospects. Lung Cancer (Auckl). 10:27–36.
2019.
|
|
50
|
Minchom A, Tan AC, Massarelli E, Subbiah
V, Boni V, Robinson B, Wirth LJ, Hess LM, Jen MH, Kherani J, et al:
Patient-Reported outcomes with selpercatinib among patients with
RET fusion-positive non-small cell lung cancer in the Phase I/II
LIBRETTO-001 Trial. Oncologist. 27:22–29. 2022. View Article : Google Scholar
|
|
51
|
Gainor JF, Curigliano G, Kim DW, Lee DH,
Besse B, Baik CS, Doebele RC, Cassier PA, Lopes G, Tan DSW, et al:
Pralsetinib for RET fusion-positive non-small-cell lung cancer
(ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol.
22:959–969. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bustamante JGB and Otterson GA: Agents to
treat BRAF-mutant lung cancer. Drugs in Context. Drugs Context.
8:2125662019. View Article : Google Scholar
|
|
53
|
Roviello G, D'Angelo A, Sirico M,
Pittacolo M, Conter FU and Sobhani N: Advances in anti-BRAF
therapies for lung cancer. Invest New Drugs. 39:879–890. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Briggs A, Paracha N, Rosettie K, et al:
Estimating Long-Term Survival Outcomes for Tumor-Agnostic
Therapies: Larotrectinib Case Study. Oncol. 100:124–129. 2022.
View Article : Google Scholar
|
|
55
|
Rikova K, Guo A, Zeng Q, Possemato A, Yu
J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al: Global Survey
of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung
Cancer. Cell. 131:1190–1203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Joshi A, Pande N, Noronha V, Patil V,
Kumar R, Chougule A, Trivedi V, Janu A, Mahajan A and Prabhash K:
ROS1 mutation non-small cell lung cancer-access to optimal
treatment and outcomes. Ecancermedicalscience. 13:9002019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shaw AT, Riely GJ, Bang YJ, Kim DW,
Camidge DR, Solomon BJ, Varella-Garcia M, Iafrate AJ, Shapiro GI,
Usari T, et al: Crizotinib in ROS1-rearranged advanced
non-small-cell lung cancer (NSCLC): Updated results, including
overall survival, from PROFILE 1001. Ann Oncol. 30:1121–1126. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Michels S, Massutí B, Schildhaus HU,
Franklin J, Sebastian M, Felip E, Grohé C, Rodriguez-Abreu D,
Abdulla DSY, Bischoff H, et al: Safety and Efficacy of Crizotinib
in Patients With Advanced or Metastatic ROS1-Rearranged Lung Cancer
(EUCROSS): A European Phase II Clinical Trial. J Thorac Oncol.
14:1266–1276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dziadziuszko R, Krebs MG, De Braud F,
Siena S, Drilon A, Doebele RC, Patel MR, Cho BC, Liu SV, Ahn MJ, et
al: Updated Integrated Analysis of the Efficacy and Safety of
Entrectinib in Locally Advanced or Metastatic ROS1 Fusion-Positive
Non-Small-Cell Lung Cancer. J Clin Oncol. 39:1253–1263. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shaw AT, Solomon BJ, Chiari R, Riely GJ,
Besse B, Soo RA, Kao S, Lin CC, Bauer TM, Clancy JS, et al:
Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A
multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol.
20:1691–1701. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu H, Boyle TA, Zhou C, Rimm DL and Hirsch
FR: PD-L1 expression in lung cancer. J Thorac Oncol. 11:964–975.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Aggarwal C, Abreu DR, Felip E, et al:
Prevalence of PD-L1 expression in patients with non-small cell lung
cancer screened for enrollment in KEYNOTE-001, -010, and -024.
Annals Oncol. 27:vi3632016. View Article : Google Scholar
|
|
63
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Herbst RS, Giaccone G, de Marinis F,
Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z,
Geater S, et al: Atezolizumab for First-Line Treatment of
PD-L1-Selected Patients with NSCLC. N Engl J Med. 383:1328–1339.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou Y, Lin Z, Zhang X, Chen C, Zhao H,
Hong S and Zhang L: First-line treatment for patients with advanced
non-small cell lung carcinoma and high PD-L1 expression:
Pembrolizumab or pembrolizumab plus chemotherapy. J Immunother
Cancer. 7:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shanzhi W, Yiping H, Ling H, Jianming Z
and Qiang L: The Relationship between TTF-1 Expression and EGFR
mutations in lung adenocarcinomas. PLoS One. 9:e954792014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yatabe Y, Dacic S, Borczuk AC, Warth A,
Russell PA, Lantuejoul S, Beasley MB, Thunnissen E, Pelosi G,
Rekhtman N, et al: Best practices recommendations for diagnostic
immunohistochemistry in lung cancer. J Thorac Oncol. 14:377–407.
2019. View Article : Google Scholar :
|
|
70
|
Potter AL, Bajaj SS and Yang CJ: The 2021
USPSTF lung cancer screening guidelines: A new frontier. Lancet
Respir Med. 9:689–691. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wyker A and Henderson WW: Solitary
Pulmonary Nodule. StatPearls [Internet]. StatPearls Publishing;
Treasure Island, FL: 2022
|
|
72
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012. View Article : Google Scholar
|
|
73
|
Xu L, Lina W and Xuejun Y: The diagnostic
value of serum CEA, NSE and MMP-9 for on-small cell lung cancer.
Open Med (Wars). 11:59–62. 2016. View Article : Google Scholar
|
|
74
|
Yang Q, Zhang P, Wu R, Lu K and Zhou H:
Identifying the Best Marker Combination in CEA, CA125, CY211, NSE,
and SCC for lung cancer screening by combining ROC curve and
logistic regression analyses: Is it feasible? Dis Markers.
2018:1–12. 2018. View Article : Google Scholar
|
|
75
|
Gao Y, Song P, Li H, Jia H and Zhang B:
Elevated serum CEA levels are associated with the explosive
progression of lung adenocarcinoma harboring EGFR mutations. BMC
Cancer. 17:4842017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Molina R, Marrades RM, Augé JM, Escudero
JM, Viñolas N, Reguart N, Ramirez J, Filella X, Molins L and Agustí
A: Assessment of a combined panel of six serum tumor markers for
lung cancer. Am J Respir Crit Care Med. 193:427–437. 2016.
View Article : Google Scholar
|
|
77
|
Chen H, Fu F, Zhao Y, Wu H, Hu H, Sun Y
and Zhang Y, Xiang J and Zhang Y: The Prognostic Value of
Preoperative Serum Tumor Markers in Non-Small Cell Lung Cancer
Varies With Radiological Features and Histological Types. Front
Oncol. 11:6451592021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bes-Scartezini F and Saad Junior R:
Prognostic assessment of tumor markers in lung carcinomas. Rev
Assoc Med Bras (1992). 68:313–317. 2022. View Article : Google Scholar
|
|
79
|
Lin D, Shen L, Luo M, Zhang K, Li J, Yang
Q, Zhu F, Zhou D, Zheng S, Chen Y and Zhou J: Circulating tumor
cells: Biology and clinical significance. Signal Transduct Target
Ther. 6:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yang C, Xia BR, Jin WL and Lou G:
Circulating tumor cells in precision oncology: Clinical
applications in liquid biopsy and 3D organoid model. Cancer Cell
Int. 19:3412019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Scharpenseel H, Hanssen A, Loges S, Mohme
M, Bernreuther C, Peine S, Lamszus K, Goy Y, Petersen C, Westphal
M, et al: EGFR and HER3 expression in circulating tumor cells and
tumor tissue from non-small cell lung cancer patients. Sci Rep.
9:74062019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kloten V, Lampignano R, Krahn T and
Schlange T: Circulating Tumor Cell PD-L1 expression as biomarker
for therapeutic efficacy of immune checkpoint inhibition in NSCLC.
Cells. 8:8092019. View Article : Google Scholar :
|
|
83
|
Maly V, Maly O, Kolostova K and Bobek V:
Circulating tumor cells in diagnosis and treatment of lung cancer.
In Vivo. 33:1027–1037. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang H, Lin X, Huang Y, Wang M, Cen C,
Tang S, Dique MR, Cai L, Luis MA, Smollar J, et al: Detection
methods and clinical applications of circulating tumor cells in
breast cancer. Front Oncol. 11:6522532021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Galletti G, Portella L, Tagawa ST, Kirby
BJ, Giannakakou P and Nanus DM: Circulating tumor cells in prostate
cancer diagnosis and monitoring: An appraisal of clinical
potential. Mol Diagn Ther. 18:389–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hardingham JE, Grover P, Winter M, Hewett
PJ, Price TJ and Thierry B: Detection and clinical significance of
circulating tumor cells in colorectal cancer-20 years of progress.
Mol Med. 21(Suppl 1): S25–S31. 2015. View Article : Google Scholar :
|
|
87
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Naito T, Tanaka F, Ono A, Yoneda K,
Takahashi T, Murakami H, Nakamura Y, Tsuya A, Kenmotsu H, Shukuya
T, et al: Prognostic Impact of Circulating Tumor Cells in Patients
with Small Cell Lung Cancer. J Thorac Oncol. 7:512–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Juan O, Vidal J, Gisbert R, Muñoz J, Maciá
S and Gómez-Codina J: Prognostic significance of circulating tumor
cells in advanced non-small cell lung cancer patients treated with
docetaxel and gemcitabine. Clin Transl Oncol. 16:637–643. 2014.
View Article : Google Scholar
|
|
90
|
Pailler E, Adam J, Barthélémy A, Oulhen M,
Auger N, Valent A, Borget I, Planchard D, Taylor M, André F, et al:
Detection of circulating tumor cells harboring a unique ALK
Rearrangement in ALK-Positive non-small-cell lung cancer. J Clin
Oncol. 31:2273–2281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ilie M, Hofman V, Long E, Bordone O, Selva
E, Washetine K, Marquette CH and Hofman P: Current challenges for
detection of circulating tumor cells and cell-free circulating
nucleic acids, and their characterization in non-small cell lung
carcinoma patients. What is the best blood substrate for
personalized medicine? Ann Transl Med. 2:1072014.PubMed/NCBI
|
|
92
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin PY, Yu SL and Yang PC: MicroRNA in
lung cancer. Br J Cancer. 103:1144–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ramírez-Salazar EG, Gayosso-Gómez LV,
Baez-Saldaña R, Ramírez-Salazar EG, Gayosso-Gómez LV, Baez-Saldaña
R, Falfán-Valencia R, Pérez-Padilla R, Higuera-Iglesias AL,
Vázquez-Manríquez ME and Ortiz-Quintero B: Cigarette smoking
alters the expression of circulating microRNAs and its potential
diagnostic value in female lung cancer patients. Biology (Basel).
10:7932021.
|
|
95
|
Nymark P, Guled M, Borze I, Faisal A,
Lahti L, Salmenkivi K, Kettunen E, Anttila S and Knuutila S:
Integrative analysis of microRNA, mRNA and aCGH data reveals
asbestos- and histology-related changes in lung cancer. Genes
Chromosomes Cancer. 50:585–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wu X, Bhayani MK, Dodge CT, Nicoloso MS,
Chen Y, Yan X, Adachi M, Thomas L, Galer CE, Jiffar T, et al:
Coordinated Targeting of the EGFR Signaling Axis by MicroRNA-27a*.
Oncotarget. 4:1388–1398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Han F, He J, Li F, Yang J, Wei J, Cho WC
and Liu X: Emerging roles of MicroRNAs in EGFR-Targeted therapies
for lung cancer. Biomed Res Int. 2015:6727592015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vishwamitra D, Li Y, Wilson D, Manshouri
R, Curry CV, Shi B, Tang XM, Sheehan AM, Wistuba II, Shi P and Amin
HM: MicroRNA 96 is a post-transcriptional suppressor of anaplastic
lymphoma kinase expression. Am J Pathol. 180:1772–1780. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yan C, Zhang W, Shi X, Zheng J, Jin X and
Huo J: MiR-760 suppresses non-small cell lung cancer proliferation
and metastasis by targeting ROS1. Environ Sci Pollut Res Int.
25:18385–18391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fan Q, Hu X, Zhang H, Wang S, Zhang H, You
C, Zhang CY, Liang H, Chen X and Ba Y: MiR-193a-3p is an important
tumour suppressor in lung cancer and directly targets KRAS. Cell
Physiol Biochem. 44:1311–1324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pop-Bica C, Pintea S, Magdo L, Cojocneanu
R, Gulei D, Ferracin M and Berindan-Neagoe I: The Clinical Utility
of miR-21 and let-7 in Non-small Cell Lung Cancer (NSCLC). A
systematic review and meta-analysis. Front Oncol. 10:5168502020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Xie Q, Yu Z, Lu Y, Fan J, Ni Y and Ma L:
microRNA-148a-3p inhibited the proliferation and
epithelial-mesenchymal transition progression of non-small-cell
lung cancer via modulating Ras/MAPK/Erk signaling. J Cell Physiol.
234:12786–12799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bishop JA, Benjamin H, Cholakh H, Chajut
A, Clark DP and Westra WH: Accurate classification of non-small
cell lung carcinoma using a novel MicroRNA-based approach. Clin
Cancer Res. 16:610–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lebanony D, Benjamin H, Gilad S, Ezagouri
M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H, et
al: Diagnostic Assay Based on hsa-miR-205 expression distinguishes
squamous from nonsquamous non-small-cell lung carcinoma. J Clin
Oncol. 27:2030–2037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang YK, Zhu WY, He JY, Chen DD, Huang
YY, Le HB and Liu XG: MiRNAs expression profiling to distinguish
lung squamous-cell carcinoma from adenocarcinoma subtypes. J Cancer
Res Clin Oncol. 138:1641–1650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Montani F, Marzi MJ, Dezi F, Dama E,
Carletti RM, Bonizzi G, Bertolotti R, Bellomi M, Rampinelli C,
Maisonneuve P, et al: MiR-Test: A blood test for lung cancer early
detection. J Natl Cancer Inst. 107:djv0632015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sozzi G, Boeri M, Rossi M, Verri C,
Suatoni P, Bravi F, Roz L, Conte D, Grassi M, Sverzellati N, et al:
Clinical utility of a plasma-based miRNA signature classifier
within computed tomography lung cancer screening: A correlative
MILD Trial Study. J Clin Oncol. 32:768–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Asakura K, Kadota T, Matsuzaki J, Yoshida
Y, Yamamoto Y, Nakagawa K, Takizawa S, Aoki Y, Nakamura E, Miura J,
et al: A miRNA-based diagnostic model predicts resectable lung
cancer in humans with high accuracy. Commun Biol. 3:1342020.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Xiao W, Zhong Y, Wu L, Yang D, Ye S and
Zhang M: Prognostic value of microRNAs in lung cancer: A systematic
review and meta-analysis. Mol Clin Oncol. 10:67–77. 2019.PubMed/NCBI
|
|
110
|
Yu S, Qin X, Chen T, Zhou L, Xu X and Feng
J: MicroRNA-106b-5p regulates cisplatin chemosensitivity by
targeting polycystic kidney disease-2 in non-small-cell lung
cancer. AntiCancer Drugs. 28:852–860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Qiu T, Zhou L, Wang T, Xu J, Wang J, Chen
W, Zhou X, Huang Z, Zhu W, Shu Y and Liu P: MiR-503 regulates the
resistance of non-small cell lung cancer cells to cisplatin by
targeting Bcl-2. Int J Mol Med. 32:593–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li Q, Yang Z, Chen M and Liu Y:
Downregulation of microRNA-196a enhances the sensitivity of
non-small cell lung cancer cells to cisplatin treatment. Int J Mol
Med. 37:1067–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bisagni A, Pagano M, Maramotti S, Zanelli
F, Bonacini M, Tagliavini E, Braglia L, Paci M, Mozzarelli A and
Croci S: Higher expression of miR-133b is associated with better
efficacy of erlotinib as the second or third line in non-small cell
lung cancer patients. PLoS One. 13:e01963502018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Condrat CE, Thompson DC, Barbu MG, Bugnar
OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM and Voinea SC: MiRNAs
as biomarkers in disease: Latest findings regarding their role in
diagnosis and prognosis. Cells. 9:2762020. View Article : Google Scholar :
|















