Introduction
Brain-derived neurotrophic factor (BDNF), an
important member of the nerve growth factor family, mainly
activates downstream signaling by binding with two receptors,
namely high-affinity receptor TrkB (Tyrosine Kinase Receptor B) and
low-affinity receptor p75NTR (p75 neurotrophin receptor) (1–3).
BDNF gene contains eleven exons (I–V, Vh, VI–VIII, VIIIh, IX),
which include functional promotors and alternative splice sites.
The sequence that encodes precursor of BDNF (proBDNF) is located
within exon IX (4). proBDNF can be
processed into mature BDNF by protease. ProBDNF and BDNF exert
opposing effects by regulating different signaling: proBDNF often
promotes cell apoptosis and inhibits growth and migration through
p75NTR/sortilin, while BDNF increases cell proliferation, invasion
and migration through TrkB (5–8).
BDNF rs6265 (G196A) single nucleotide polymorphism (SNP) is an A→G
substitution at nucleotide position 196 that directly results in a
valine (Val) changing to methionine (Met) at amino acid position 66
(Val66Met) of the preprotein, and this SNP is significantly
associated with the variability of BDNF activity (9,10).
More than that, epigenetic regulation of BDNF, particularly DNA
methylation and non-coding RNA (ncRNA), has been widely studied and
often regulates the mRNA or/and protein expression of BDNF. BDNF
methylation often happens in CpG island at promoter regions within
BDNF exon I, IV and IX, and is significantly associated with BDNF
expression (11,12). ncRNAs include long non-coding RNA
(lncRNA), microRNA (miRNA), circular RNA (circRNA), rRNA and tRNA.
In recent years, multiple studies have proved that ncRNA, mainly
including miRNA, lncRNA and circRNA, could directly target BDNF and
regulate the expression of BDNF.
Generally, BDNF regulates a variety of cellular
processes by binding and activating TrkB, then results in the
activation of a variety of downstream kinases, i.e., PI3K/Akt
(Phosphoinositide 3-kinase/protein kinase-B), MAPK
(mitogen-activated protein kinase) and PLC-γ (Phospholipase C-γ)
(1–3,13).
BDNF and its downstream signaling pathways are involved in the
development of neurological and neuropsychiatric diseases and can
serve as a biomarker in their adjuvant diagnosis, prognostic
monitoring and therapeutic effects (14–16).
The abnormal expression of BDNF is also found in numerous cancers.
BDNF can regulate tumor cell proliferation, invasion and
metastasis, anoikis and drug resistance, and often acts as cancer
suppressor or cancer promoter in context-dependent signaling
pathways and tumor microenvironment (1–3).
However, little progress has been made in the application of BDNF
in cancer diagnosis and treatment.
miRNA is a non-coding single-stranded RNA molecule
with a length of 21–25 nucleotides transcripts and regulates gene
expression by degradation or translation inhibition of target
mRNAs. miRNAs have been identified to play important roles in
tumorigenesis and showed great potential applications in cancer
diagnosis, prognosis and therapy (17–19).
Recent studies from PubMed, Web of Science, Scopus, Willy, EBSCO
have shown that the expression of the BDNF was regulated by a
cluster of miRNAs (20–51). In the present review, the role and
mechanism of miRNAs directly targeting BDNF in cancers was
summarized and their potential applications in cancer diagnosis and
treatment were discussed.
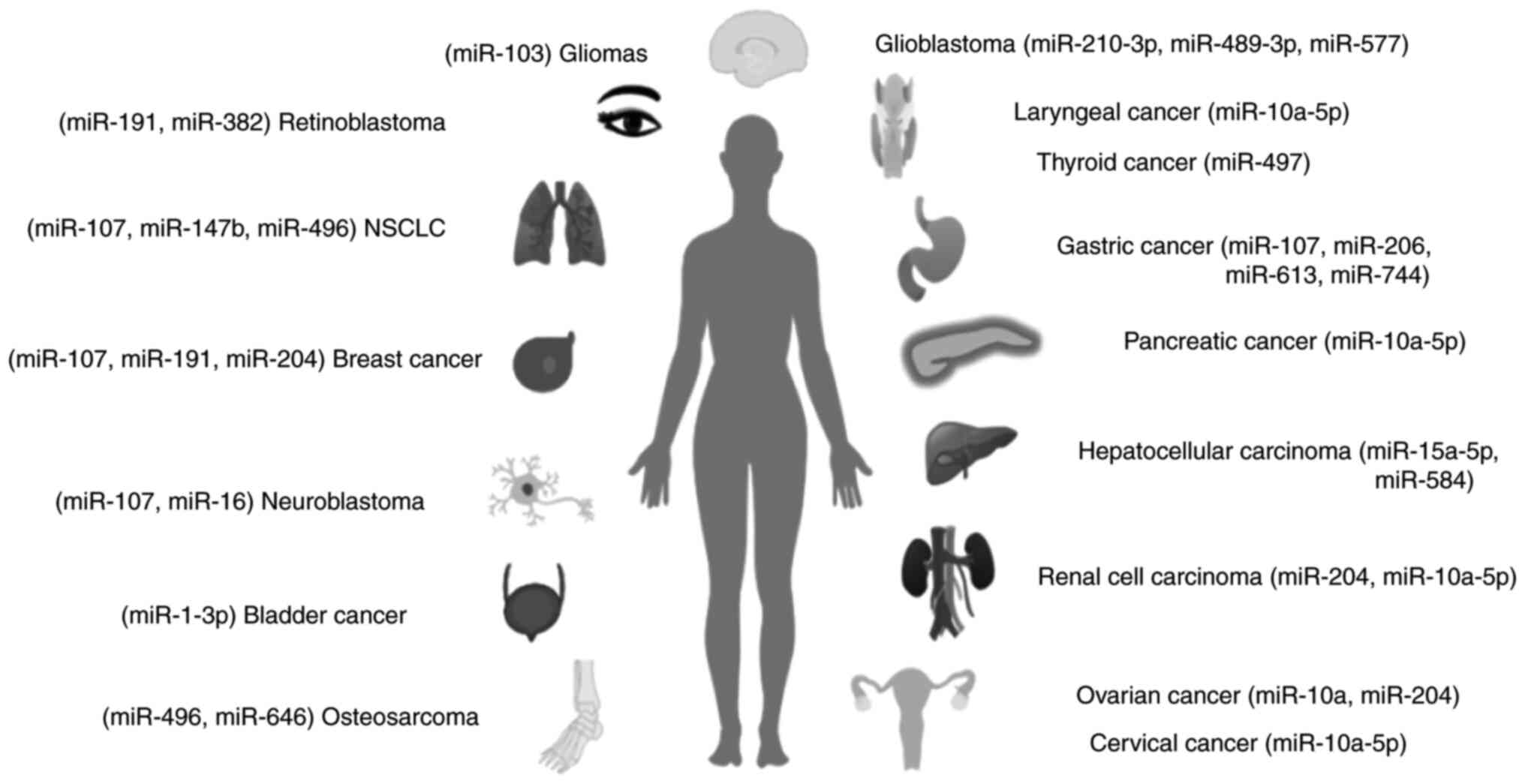
Multiple miRNAs directly targeting BDNF were
identified in human cancers
The expression of miRNAs directly
targeting BDNF in cancers
miRNA regulates the expression of BDNF at the
transcriptional level by pairing with the base of 3′-untranslated
region (3′-UTR) of BDNF mRNA. At present, 20 miRNAs were found to
directly regulate the expression and functions of BDNF in 16
cancers, i.e., miR107, miR191 and miR-204 in breast cancer,
miR-1-3p in bladder cancer, miR-10a-5p in cervical carcinoma,
miR-210-3p, miR-489-3p and miR-577 in glioblastoma, miR-103 in
gliomas, miR-107, miR-206 and miR-613 in gastric cancer, miR-15a-5p
and miR-584 in hepatocellular carcinoma (HCC), miR-204 and
miR-10a-5p in renal cell carcinoma (RCC), miR-10a-5p in laryngeal
cancer, miR-107 and miR-16 in neuroblastoma, miR-107, miR-147b and
miR-496 in non-small cell lung cancer (NSCLC), miR-10a and miR-204
in ovarian cancer, miR-496 and miR-646 in osteosarcoma, miR-10a-5p
in pancreatic cancer (PAAD), miR-191 and miR-382 in retinoblastoma,
and miR-497 in thyroid cancer (20–51)
(Fig. 1 and Table I).
 | Table I.The expression, role and regulatory
mechanism of miRNAs directly targeting BDNF in cancers. |
Table I.
The expression, role and regulatory
mechanism of miRNAs directly targeting BDNF in cancers.
|
| miRNAs targeting
BDNF in cancers |
|---|
|
|
|
|---|
| MicroRNA
family | Seed | MiRNAs | Regulation to
BDNF | Cancer type | Expression | Role in cancer | Significant with
Clinicopathologic characteristics | Regulation
mechanism | (Refs.) |
|---|
| miR-1/206 | GGAAUGU | miR-1-3p | Negative | Bladder cancer | ↓ | Suppressor | - | BDNF-TrkB | (30) |
|
|
| miR-206 | Negative | Gastric cancer | ↓ | Suppressor | Lymphatic
metastasis, local invasion, advanced TNM | BDNF/PI3K/AKT | (22) |
|
|
| miR-613 | Negative | Gastric cancer | ↓ | Suppressor | Lymph node
metastasis, advanced TNM | BDNF | (23) |
| miR-10 | ACCCUGU | miR-10a-5p | Positive | Pancreatic
cancer | ↑ | Promoter | - | BDNF/SEMA4C | (37) |
|
|
|
| Negative | Cervical
cancer | ↓ | Suppressor | - | BDNF | (47) |
|
|
|
| Negative | Laryngeal
Cancer | ↓ | Suppressor | - | BDNF/TrkB | (34) |
|
|
|
| Negative | RCC | ↓ | Suppressor | Tumor size, TNM
stage, lymph node metastasis, OS | BDNF | (43) |
|
miR-15/16/195/424/497 | AGCAGCA | miR-15a-5p | Negative | HCC | ↓ | Suppressor | 5-year survival
rate | BDNF | (31) |
|
|
| miR-16 | Negative | Neuroblastoma | ↓ | Suppressor |
Cisplatin-treatment | BDNF | (32) |
|
|
| miR-497 | Negative | Thyroid cancer | ↓ | Suppressor | - |
LINC00152/miR-497/BDNF | (33) |
| miR-103/107 | GCAGCAU | miR-103 | Negative | Gliomas | ↓ | Suppressor | - | BDNF | (49) |
|
|
| miR-107 | Negative | Gastric cancer | ↓ | Suppressor | TNM stage | BDNF/PI3K/AKT;
circHIPK3/miR-107/BDNF | (20,21) |
|
|
|
| Negative | Breast cancer | ↓ | Suppressor | - | BDNF | (28) |
|
|
|
| Negative | Neuroblastoma | ↓ | Suppressor | - | lncRNA
DLX6-AS1/miR107/BDNF | (29) |
|
|
|
| Negative | NSCLC | ↓ | Suppressor | TNM, regional lymph
node involvement, differentiation, OS, DFS |
circHIPK3/miR-107/BDNF | (25,46) |
| miR-147/147b | UGUGCGG | miR-147b | Negative | NSCLC | ↓ | Suppressor | Lymph nodes
metastasis, tumor stage | BDNF/PI3K/AKT | (26) |
| miR-191 | AACGGAA | miR-191 | Positive | Breast cancer | ↑ | Promoter | HER2, Family
history of cancer |
MED1/ER-α/miR-191/SATB1 | (38–39) |
|
|
|
| Negative | Retinoblastoma | ↓ | Suppressor | - |
XIST/miR-191/BDNF | (40) |
| miR-204/211 | UCCCUUU | miR-204 | Negative | Ovarian cancer | ↓ | Suppressor | - |
BDNF/TrkB/PI3K/AKT/mTOR/Rac1 | (35,45) |
|
|
|
| Negative | RCC | ↓ | Suppressor | - |
BDNF/TrkB/AKT/mTOR/Rac1 | (45) |
|
|
|
| Negative | Breast cancer | ↓ | Suppressor | TNM, metastasis,
OS, DFS |
BDNF/TrkB/AKT/mTOR/Rac1 | (45) |
| miR-210 | UGUGCGU | miR-210-3p | Negative | Glioblastoma | ↓ | Suppressor | Histological grade,
shorter OS | BDNF | (44) |
| miR-382 | AAGUUGU | miR-382 | Negative | Retinoblastoma | ↓ | Suppressor | - | BDNF/PI3K/AKT | (36) |
| miR-489.h | UGACAUC | miR-489-3p | Negative | Glioblastoma | ↓ | Suppressor | clinical grade | BDNF/PI3K/AKT | (48) |
| miR-496 | GAGUAUU | miR-496 | Negative | NSCLC | ↓ | Suppressor | - | BDNF/PI3K/AKT | (27) |
|
|
|
| Negative | Osteosarcoma | ↓ | Suppressor | survival rate | BDNF | (50) |
| miR-577 | AGAUAAA | miR-577 | Negative | Glioblastoma | ↓ | Suppressor | - |
LINC01094/miR-577/BDNF | (41) |
| miR-584 | UAUGGUU | miR-584 | Negative | HCC | ↓ | Suppressor | Tumor size, TNM,
lymph node metastasis | BDNF | (51) |
| miR-646 | AGCAGCU | miR-646 | Negative | Osteosarcoma | ↓ | Suppressor | Sensitivity to
DOX |
circ_0000006/miR-646/BDNF | (42) |
| miR-744 | GCGGGGC | miR-744 | Negative | Gastric cancer | ↓ | Suppressor | Lymph node
metastasis, invasive depth, TNM | BDNF | (24) |
Except for miR-10a-5p in pancreatic cancer and
miR-191 in breast cancer, the vast majority of the miRNAs directly
targeting BDNF serve as suppressors in numerous cancers (Table I). For example, in gastric cancer,
4 miRNAs of miR-107, miR-206, miR-613 and miR-744 are downregulated
and act as tumor inhibitors by suppressing BDNF expression
(20–24); and in NSCLC, the expression of 3
miRNAs of miR-107, miR-147b and miR-496 are also decreased to
inhibit the expression of BDNF (25–27).
In addition, the expression of the same miRNA regulating BDNF has
been discovered in different cancers. For example, miR-107 was
downregulated in gastric cancer, NSCLC, breast cancer and
neuroblastoma (20,21,25,28,29);
and the expression level of miR-204 was reduced in ovarian cancer,
breast cancer and RCC. The varied expression patterns of miRNA
directly targeting BDNF suggested that BDNF regulated by miRNA is a
very complex regulation network.
The miRNA families directly targeting
BDNF in cancers
At present, ~772 miRNA families have been identified
(https://www.targetscan.org/cgi-bin/targetscan/mirna_families.cgi?db=vert50,
Release 5.2: June 2011). Normally, the members of miRNAs in the
same miRNA family share a common seed sequence. The known 21 miRNAs
targeting BDNF belong to 15 different miRNA families including
miR-1/206, miR-10, miR-15/16/195/424/497, miR-103/107,
miR-147/147b, miR-191, miR-204/211, miR-210, miR-382, miR-489.h,
miR-496, miR-577, miR-584, miR-646 and miR-744 (20–51).
The details of miRNA families are shown in Table I.
The members of miRNA family also have similar
physiological functions. For example, human miR-1/206 family,
including miR-1-3p, miR-206 and miR-613, not only inhibit the
cancer cell proliferation, metastasis and invasion, but also play
important roles on myogenesis, oxidative stress in both heart and
lung pathologies (22,23,30,52,53).
miR-15a-5p, miR-16 and miR-497, belonging to miR-15/16/195/424/497
family, have been discovered to target both BDNF and Bcl2, and
inhibit cell proliferation and induce cell apoptosis in HCC,
neuroblastoma and thyroid cancer, respectively (31–33,41,54,55).
Those findings would further provide information and help to
investigate the potential roles and mechanism of members in the
same miRNA family.
The dual roles of miRNAs directly targeting
BDNF in cancers
BDNF act as a cancer suppressor or/and
an oncogene
BDNF is well known to not only act as an oncogene,
but also serve as a cancer suppressor (Fig. 2). BDNF always upregulates and
performs its cancer-promoting function through activating TrkB
and/or PI3K/Akt, Ras-Raf-MEK-ERK, EGFR (1–3,56).
The dual functions of BDNF in cancer are usually significantly
associated with the TrkB isoforms. TrkB is the high-affinity
receptor of BDNF, and BDNF/TrkB signaling pathway plays important
roles in cancer occurrence and progression (2,3,14).
TrkB mainly contains two isoforms, namely the full length of TrkB
(TrkB-FL) and the truncated TrkB (TrkB-T1) (2,57).
In most cancers, BDNF promotes cancer cell proliferation, invasion
and metastasis by binding to TrkB, actually it is TrkB-FL isoform.
TrkB-T1, lacking the intracellular tyrosine kinase domain, is a
natural antagonist of TrkB-FL and also can stimulate cancer cell
growth; However, its tumor-promoting effect is not dependent on
BDNF but is associated with Nfe2l2 response, retinol metabolism,
and hedgehog signaling (57,58).
More than that, the downstream signaling of RhoA (a small G protein
belonging to the Ras superfamily) is also involved in the dual
function of BDNF in cancers. For example, BDNF increases cancer
cell metastasis in HCC by stimulating the activity of RhoA, which
is activated by TrkB-FL; while in glioma, BDNF reduces cancer cell
migration by inhibiting RhoA through the truncated TrkB-T1 receptor
(57,59). The regulatory mechanism is that
BDNF binds with Rho GDI1 (Rho guanine nucleotide dissociation
inhibitor 1) and then causes Rho GDI1 to dissociate from the
COOH-terminal tail of TrkB-T1, which leads to inhibiting the
activity of RhoA (59,60).
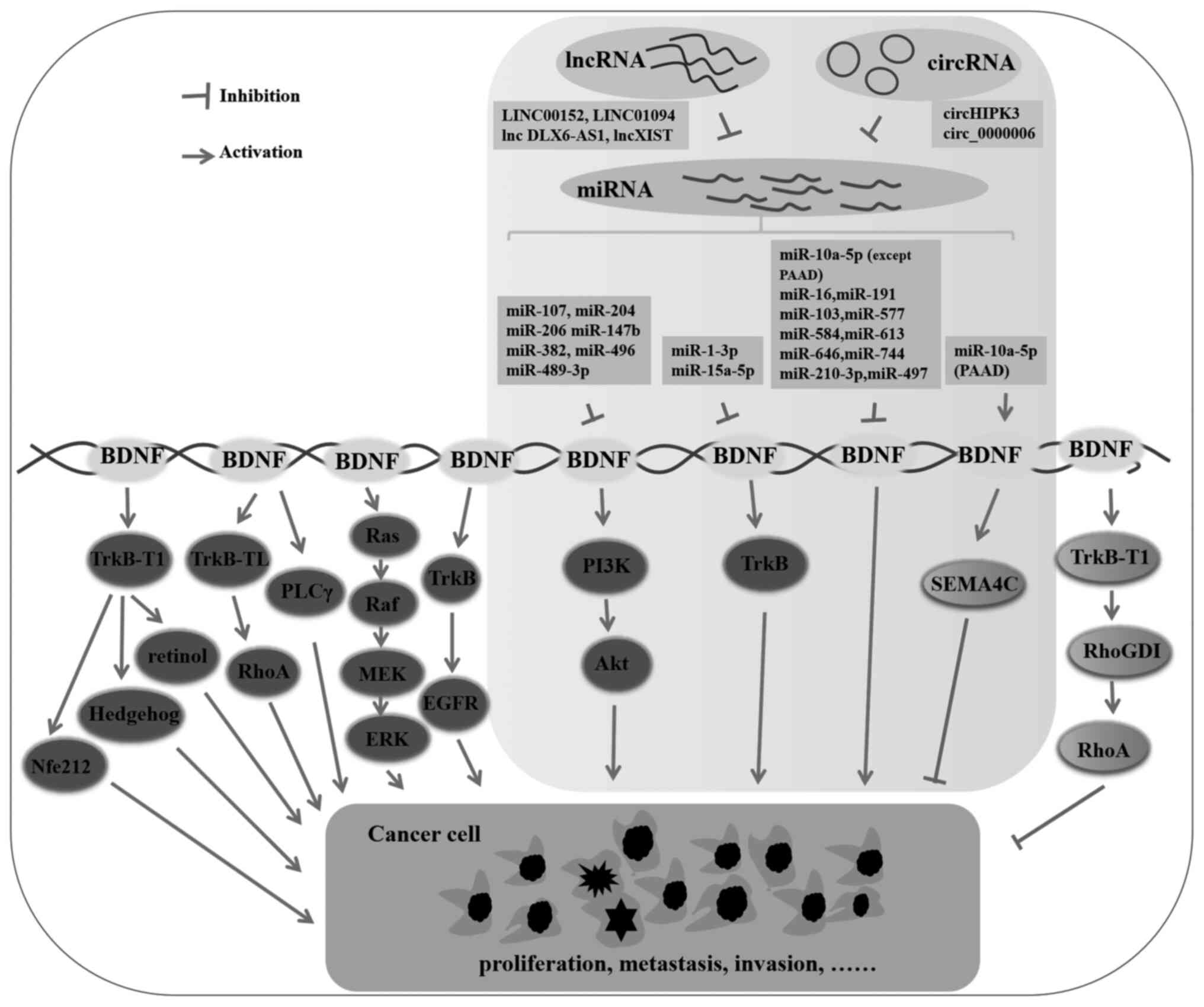 | Figure 2.The role and mechanism of miRNA-BDNF
regulatory network in cancer. BDNF promote cancer development
always by regulating Ras-Raf-MEK-ERK, TrkB, PI3K/Akt, EGFR,
TrkB-TL/RhoA, TrkB-T1/Nfe2l2, TrkB-T1/retinol and TrkB-T1/hedgehog
signaling, while inhibit cancer progression mainly associated with
enriched environment and TrkB-T1/Rho GDI/RhoA signaling. miRNAs can
target BDNF and exert anticancer effect by decreasing BDNF
expression and inhibiting TrkB and PI3K/Akt signaling pathways, and
it can promote cancer development through SEMA4A in PAAD. miRNA,
microRNA; BDNF, brain derived neurotrophic factor; PAAD, pancreatic
adenocarcinoma. |
The cancer microenvironment, including tumor cells,
fibroblasts, endothelial cells, immune cells and extracellular
components, exerts a significant impact on cancer occurrence,
development and therapy (61,62).
Previous studies have revealed that cancer microenvironment affects
the function of BDNF in cancer (2,60,63–65).
For example, in melanoma, colon, glioma and breast cancer, BDNF is
upregulated by enriched environment and reduces the malignance
development, which is associated with obesity and the immune
system. However, these findings are only reported in the cancer
models of mice (60,63–65).
miRNAs directly targeting BDNF also
serve as cancer suppressor and cancer promoter
miRNAs directly targeting BDNF are also found to
have both pro-cancer and anticancer effects in cancer (Fig. 2). The majority of miRNAs act as
suppressor in cancer by downregulating BDNF and/or its downstream
signal pathways, which include TrkB and PI3K/Akt. These miRNAs
contain miR-1-3p, miR-10a-5p (except in pancreatic cancer),
miR-103, miR-107, miR-147, miR-15a-5p, miR-16, miR-191 (in
Retinoblastoma), miR-204, miR-206, miR-210, miR-382, miR-489-3p,
miR-496, miR-497, miR-577, miR-584, miR-613, miR-646 and miR-744
(Table I and Fig. 2). In laryngeal cancer, miR-10a-5p
binds with the 3′-UTR of BDNF and protects cancer cells from
apoptosis and promotes cell proliferation by inducing the
expression of TrkB (34). miR-1-3p
is downregulated in bladder cancer and affects the cell viability,
proliferation, invasion and apoptosis via targeting BDNF to inhibit
TrkB phosphorylation (30).
PI3K/Akt is a well-known signaling pathway and has been considered
as an important target for cancer therapy. In most cancers,
PI3K/Akt signaling pathway can modulate cancer cell proliferation,
apoptosis, invasion and metastasis. Previous studies showed that
miRNAs affected the PI3K/Akt signaling pathway by directly
downregulating the expression of BDNF in cancer. For example, by
downregulating the expression of BDNF and its downstream signaling
of PI3K/Akt, the increased expression of miR-204 leads to ovarian
cancer cells acquiring more sensitivity to anoikis and presenting
decreased invasive and metastatic behavior (35). Another example is miR-147b, it
blocks epithelial-mesenchymal transformation and inhibits the cell
activity and metastasis of NSLC by downregulating of BDNF and
inhibiting the phosphorylation levels of PI3K and Akt (26). Furthermore, the miRNA/BDNF/PI3K/Akt
signaling pathway is also clearly confirmed by the studies on the
effect of miR-107, miR-206 in gastric cancer, miR-496 in NSCLC, and
miR-382 in retinoblastoma (20,22,27,36).
Currently, only one miRNA, miR-10a-5p, has been
reported to exert cancer-promoting role by targeting BDNF. Compared
with healthy adjacent tissues, pancreatic cancer tissues have
significantly higher expression levels of miR-10a-5p, which
stimulates BDNF/SEMA4C signaling to encourage cell proliferation
and invasion (37). miR-191 was
also found to target BDNF and exert cancer-promoting activity in
breast cancer; however, its cancer-promoting effect is not related
to BDNF, but to the target SATB1 (38,39).
The sponge effect of lncRNA and
circRNA on the miRNAs directly targeting BDNF
miRNAs that target BDNF in cancers are regulated by
lncRNA and circRNA, such as circHIPK3, circ_0000006, LINC00152,
LINC01094, lnc DLX6-AS1 and lncXIST (21,25,29,33,40–42)
(Fig. 2 and Table I).
lncRNA, with a length of more than 200 nt, accounts
for 80~90% of ncRNAs and has the characteristics of the large
number, low expression, poor conservation among species and cell
expression specificity. lncRNAs always function as molecular decoys
or sponges of miRNAs and display vital roles in regulating BDNF
transcription (29,33,40,41).
One example is LncDLX6-AS1, which is highly expressed in
neuroblastoma tissues and cell lines, serves as a molecular sponge
for miR-107 and promotes neuroblastoma cell proliferation, cell
migration and invasion by positively regulating the expression of
BDNF (29). Similar regulation
mechanisms of LINC00152-miR497-BDNF, lnc XIST-miR191-BDNF and
LINC01094-miR577-BDNF are also identified in thyroid cancer,
retinoblastoma and glioblastoma, respectively (33,40,41).
CircRNA is another kind of ncRNAs, which is without
5; cap and 3′ Poly (A) tail and is characterized by its covalently
closed loop structures. CircRNA can target complementary miRNA
response elements and is always used as competitive endogenous RNA
(ceRNA) to have the sponge effect on miRNA. circRNA is also
identified to participate in the initiation and progression of
various cancers (1–3,14,21,25,42).
At present, only two circRNAs, circHIPK3 and circ_0000006, are
declared to target BDNF in cancer. circHIPK3 has been found to
overexpress in NSCLC and gastric cancer cells, and the
cancer-promoting effect of circHIPK3 is mainly associated with the
signaling pathway of CircHIPK3/miR-107/BDNF (21,25).
circ_0000006 is a newly discovered circular RNA in osteosarcoma,
and the circ_0000006/miR-646/BDNF pathway is identified to be
responsible for the DOX-mediated anticancer effects on osteosarcoma
(42).
miRNAs directly targeting BDNF may be used
as potential biomarker for cancer diagnosis and treatment
miRNA is involved in almost all of the important
signaling pathways of tumorigenesis. The expression level of miRNA
in serum and tissues is different in cancer patients than that in
normal individuals, and thus miRNA can be used as a marker for
tumor diagnosis. For example, a plasma miRNA panel (miR-21,
miR-26a, miR-27a, miR-122, miR-192, miR-223 and miR-801) is applied
to the clinical diagnosis of HCC and has improved sensitivity and
specificity than AFP (alpha fetoprotein) (66). miR-92a is a oncomir and has become
a useful biomarker for early detection of colorectal cancer in
stool with 89% sensitivity and 70% specificity (67,68).
The great majority of studies also showed that the abnormal
regulatory of miRNAs targeting BDNF were significant associated
with pathogenesis and progression of cancer. These miRNAs not only
regulate the cancer cell activities, but also are positively
correlated with the clinicopathologic characteristics of cancer
patients. For example, miR-206, miR-613 and miR-744 can inhibit the
gastric cancer cell proliferation, metastasis and invasion, and
their downregulation is particularly related to lymphatic
metastasis and advanced TNM staging of gastric cancer (22–24).
In addition, low expression of miR-10a-5p and miR-210-3p has great
relevance to poorer overall survival (OS) of kidney cancer and
glioblastoma patients, respectively (43,44).
miR-107 and miR-204 are related to shorter OS, [progression-free
survival (PFS) or disease-free survival (DFS)] in patients of NSCLC
and breast cancer, respectively (45,46).
In addition, cisplatin is one of the most common anticancer drugs
and has been found to inhibit neuroblastoma cell proliferation by
downregulating BDNF, which depends on miR-16 (32); and miR-646/BDNF is responsible for
the DOX-mediated effects of Circ_0000006 on osteosarcoma
development (42). Those results
indicated that miRNAs targeting BDNF have the prospective to be
used for cancer diagnosis and therapy.
Dysregulation of ncRNAs, primarily involving siRNA,
miRNA, circRNA and lncRNA, is frequent event in a wide range of
diseases. An increasing number of ncRNAs have been investigated and
have made a breakthrough in drug development for their potential
role as a biomarker in diseases (66–68).
To date, 3 siRNA drugs, Patisiran, Givosiran and Lumasiran have
been approved by FDA; there are three miRNA drugs lademirsen,
MRG-201, RG-125 in phase II trials and two miRNA drugs MRG-106,
MRG-110 in phase I trials; And only one miRNA drug, MRG-106, is in
phase I trials for lymphomas and leukemias (69–71).
Although no BDNF-related miRNA drug has yet been
identified and used in clinical trials, researchers found that
certain drugs and natural compounds exhibited their anticancer
effects by targeting the miRNAs regulating BDNF (72–87)
(Table II). For example,
Bortezomib, an anticancer drug with a significant effect in
treatment of multiple myeloma, was found to induce cell death in
leukemia by upregulating the signal of miR-744/3154/3162/CEPBD
(CCAAT/enhancer binding protein delta) (72). In addition, the known
non-anticancer drugs were also identified to possess anticancer
action via regulating the expression of miRNAs, such as propofol
and metformin. Propofol, a drug commonly used in anesthesia,
suppressed cell proliferation, invasion and migration in colorectal
cancer, breast cancer, glioma and lung adenocarcinoma by targeting
miR-1-3p, miR-204, miR-206 and miR-210, respectively (73–76).
Metformin, the optimal first-line drug for the treatment type 2
diabetes mellitus, was discovered to induce cell pyroptosis through
activating the signal of miR-497/PELP1 in esophageal squamous cell
carcinoma (77). Beyond that, the
natural compounds also showed significant anticancer roles by
modulating the expression of miRNAs. Quercetin, a natural flavonoid
extracted from numerous plants, was found to inhibit cell
proliferation, migration and invasion by targeting miR-1-3p in
esophagus cancer and miR-16 in oral cancer and HCC (78–80).
Curcumin, another natural phenolic compound mainly from Curcuma
longa, exhibited its high anticancer activity via increasing
the expression of miR-206 in NSCLC and miR-210 in prostate cancer
(81,82). At present, however, there is no
study about the anticancer effect of drugs and natural compounds
associated with miRNA/BDNF signaling. Nevertheless, it was found
that the aforementioned drugs and natural compounds could decrease
the expression level of BDNF. For example, when treated with
metformin for 6 h, the nascent and steady-state BDNF transcripts
are slightly decreased in Daoy cells, a human medulloblastoma cell
line (88). In addition, curcumin
was demonstrated to reduce mammary cancer via increasing the
expression of PPAR-gamma and decreasing the expression of BDNF in
SD rats (89). Thus, the
aforementioned studies strongly suggested that the drugs and
natural compounds exerted their anticancer effect maybe through
miRNAs/BDNF signaling. Those studies further indicated that miRNAs
targeting BDNF may become new targets for cancer therapy.
 | Table II.Drugs and natural compounds exhibit
anticancer effects by targeting miRNAs. |
Table II.
Drugs and natural compounds exhibit
anticancer effects by targeting miRNAs.
| Natural compounds
and Drug |
| Cancer type | Target miRNA | Effects on
miRNA | The roles of drug
on cancer | Regulatory
mechanism | (Refs.) |
|---|
| Drug | Bortezomib | Leukemia | miR-744 | Upregulate | Induce cell
death | miR-744, 3154 and
3162/CEBPD | (72) |
|
| Propofol | Colorectal
cancer | miR-1-3p | Upregulate | Inhibit cell
proliferation, accelerate apoptosis |
miR-1-3p/IGF1/AKT/mTOR | (73) |
|
|
| Breast cancer | miR-204 | Upregulate | Inhibit cell
invasion, migration and EMT | mir-204/MMP9 | (74) |
|
|
| Glioma | miR-206 | Upregulate | Inhibited cell
migration, invasion |
miR-206/ROCK1/PI3K/AKT | (75) |
|
|
| Lung
adenocarcinoma | miR-210 | Downregulate | Inhibit cell
proliferation and metastasis | HIF-1α/miR-210 | (76) |
|
| Metformin | ESCC | miR-497 | Upregulate | Induces
pyroptosis | miR-497/PELP1 | (77) |
|
| Vitamin D3 | Liver cancer | miR-15a-5p | Upregulate | Suppress cell
proliferation, induce apoptosis |
miR-15a-5p/E2F3 | (78) |
| Natural
compounds | Quercetin | Esophagus
Cancer | miR-1-3p | Upregulate | Inhibit cell growth
and metastasis |
miR-1-3p/TAGLN2 | (79) |
|
|
| Oral cancer | miR-16 | Upregulate | Inhibit cell
viability, migration and invasion | miR-16/HOXA10 | (80) |
|
|
| HCC | miR-16 | Upregulate | Inhibit cellular
proliferation and migration |
TP53/miR-15a/miR-16 | (81) |
|
| Curcumin | NSCLC | miR-206 | Upregulate | Inhibit cell
invasion and migration |
miR-206/PI3K/AKT/mTOR | (82) |
|
|
| Prostate
cancer | miR-210 | Upregulate | Inhibit cell
proliferation, promote apoptosis | miR-210/TLR4
signaling pathway | (83) |
|
| S-equol | Breast cancer | miR-10a-5p | Upregulate | Inhibit cell
proliferation, promote apoptosis |
miR-10a-5p/PI3K/AKT | (84) |
|
| Skullcapflavone
I | Colorectal
cancer | miR-107 | Downregulate | Suppress cell
proliferation and viability |
miR-107/TPM1/MEK/ERK+NF-κB | (85) |
|
| Astragalus IV | TNBC | miR-206/613 | Upregulate | Multi-Drug
Resistance and glycolysis |
circ_0001982-miR-206/613 | (86) |
|
|
Andrographolide | Prostate
cancer | miR-206 | Upregulate | Inhibit cell
proliferation and induce apoptosis | miR-206/STC2 | (87) |
Discussion and conclusion
It is generally known that BDNF and its downstream
signaling pathways are overexpressed in most cancers and play vital
roles in cancer occurrence and development. However, little
progress has been made in the application of BDNF in cancer. This
may be associated with the complex epigenetics and genetic
mechanism of BDNF in the same type of cancer. For example, genetic
variations, particularly proBDNF and BDNF Val66Met polymorphism,
play crucial biological roles in breast cancer occurrence and
development. proBDNF released by breast cancer cells is identified
as a mediator to induce anti-angiogenic effect in brain endothelial
cells, and BDNF Val66Met gene polymorphism has a significant
relation to the risk of breast cancer (90,91).
In addition, Alhusban et al (90) declared that the ratio of
proBDNF/BDNF, which is released by breast cancer cell MDA-MB-231,
has significant correlation with anti-angiogenic effect. Epigenetic
changes of BDNF also perform major role in breast cancer: The
methylation of BDNF promoter serves as biological marker for
suicidality in patients with breast cancer (92); BDNF-AS, the first identified
natural non-coding antisense for BDNF, is overexpressed and
significantly correlated with poor outcomes in hormone
receptor-positive and triple-negative breast cancer patients
(93); miR-107, miR-191 and
miR-204 affect the breast cancer proliferation, metastasis and
invasion by regulating the mRNA and/or protein expression of BDNF
(28,39,45).
Both epigenetic and genetic variations also exist in gastric
cancer, HCC and RCC (5,21–23,31,43,45,94–96).
Currently, accumulating evidence has indicated that
miRNAs provided a new vision to understand the occurrence and
development of cancer. In the present review, the miRNAs targeting
BDNF and their functions, regulatory network and clinical
significance in cancers were discussed. circRNAs and lncRNAs, often
serve as cancer promoter by sponging miRNA, positively regulate the
expression of BDNF, and the miRNAs targeting BDNF usually act as
cancer suppressors by downregulating the BDNF/TrkB and PI3K/Akt
signaling pathways. Previous studies found that decreased miR-16
and miR-107 promoted cell growth, metastasis and invasion by
upregulating the BDNF expression, and increased BDNF effected cell
viability and metastasis by activating the TrkB/PI3K/Akt signaling
pathway in neuroblastoma (29,32,97,98).
Moreover, in cervical cancer, downregulated miR-10a-5p was revealed
to stimulate cell viability, division and cell cycle arrest via
increasing the expression of BDNF, and overexpression of BDNF
promotes cell proliferation, migration and invasion in this type of
cancer through the TrkB/PI3K/AKT signaling pathway (47,99).
Similar results were also identified in gastric cancer, RCC, breast
cancer and glioblastoma (20,22,27,35,36,45,48,100). Thus, these published results help
us confirm that miRNA directly targeting BDNF can downregulate the
TrkB/PI3K/Akt signaling pathway; in other words, the regulatory
network of miRNA/BDNF//PI3K/Akt is of supreme importance in cancer
occurrence and development.
BDNF/TrkB and its downstream signaling PI3K/Akt are
overexpressed in numerous cancers and have close correlations with
poor prognosis and short survival time of cancer patients, and have
become the key targets for drug development and cancer therapy. For
example, TRK inhibitor Larotrectinib, has recently shown broad
clinical activity in multiple tumor types with positive TRK fusion
gene (NTRK1, NTRK2 or NTRK3, which encode the neurotrophin
receptors TrkA, TrkB and TrkC, respectively) (101,102); multiple PI3K/Akt inhibitors,
including idelalisib, copanlisib, duvelisib, linperlisib,
ipatasertib, afuresertib, uprosertib, also have been widely used in
cancer therapy (103–105). Although there are no studies
about drugs associated with miRNAs targeting BDNF, the conclusion
by the authors indicated that drugs and natural compounds exerting
their anticancer effect may be obviously correlated with the
miRNAs/BDNF regulatory network.
In summary, BDNF plays vital roles in cancer
occurrence and development, but blocking BDNF remains a difficult
task due to its complex genetic and epigenetic variation. It has
been widely demonstrated that miRNAs have great potential
applications in cancer diagnosis, prognosis and therapy. In the
present review, the role and mechanism of miRNAs targeting BDNF in
cancer was concluded and it was indicated that miRNAs targeting
BDNF can be used as a potential biomarker for cancer diagnosis and
treatment. However, further investigation must be conducted.
Acknowledgements
The authors would like to thank Dr Yang Hai
(Zhongyuan University of Technology, Zhengzhou, Henan) for
carefully checking and improving the English writing.
Funding
The present study was supported by the Foundation of Henan
Educational Committee (grant no. 22A310024), the Natural Science
Foundation for Young Teachers' Basic Research of Zhengzhou
University (grant no. JC202035025) and the National College
Students Innovation and Entrepreneurship Training Program, grant
number 202110459202.
Availability of data and materials
Not applicable.
Authors' contributions
ZX, XX and WC wrote this manuscript and created
figures. LH, CK, JH and YG consulted and analyzed literature and
created tables. LH, MY and SM designed, edited and revised the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Colucci-D'amato L, Speranza L and
Volpicelli F: Neurotrophic factor BDNF, physiological functions and
therapeutic potential in depression, neurodegeneration and brain
cancer. Int J Mol Sci. 21:77772020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radin DP and Patel P: BDNF: An oncogene or
tumor suppressor? Anticancer Res. 37:3983–3990. 2017.PubMed/NCBI
|
|
3
|
Meng L, Liu B, Ji R, Jiang X, Yan X and
Xin Y: Targeting the BDNF/TrkB pathway for the treatment of tumors.
Oncol Lett. 17:2031–2039. 2019.PubMed/NCBI
|
|
4
|
Pruunsild P, Kazantseva A, Aid T, Palm K
and Timmusk T: Dissecting the human BDNF locus: Bidirectional
transcription, complex splicing, and multiple promoters. Genomics.
90:397–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De la Cruz-Morcillo MA, Berger J,
Sánchez-Prieto R, Saada S, Naves T, Guillaudeau A, Perraud A,
Sindou P, Lacroix A, Descazeaud A, et al: p75 neurotrophin receptor
and pro-BDNF promote cell survival and migration in clear cell
renal cell carcinoma. Oncotarget. 7:34480–34497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Ma W, Wang T, Yang J, Wu Z, Liu K,
Dai Y, Zang C, Liu W, Liu J, et al: BDNF-TrkB and
proBDNF-p75NTR/sortilin signaling pathways are involved in
mitochondria-mediated neuronal apoptosis in dorsal root ganglia
after sciatic nerve transection. CNS Neurol Disord Drug Targets.
19:66–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong J, Zhou L, Yang M, Lim Y, Zhu YH, Fu
DL, Li ZW, Zhong JH, Xiao ZC and Zhou XF: ProBDNF and its receptors
are upregulated in glioma and inhibit the growth of glioma cells in
vitro. Neuro Oncol. 15:990–1007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiong J, Zhou LI, Lim Y, Yang M, Zhu YH,
Li ZW, Fu DL and Zhou XF: Mature brain-derived neurotrophic factor
and its receptor TrkB are upregulated in human glioma tissues.
Oncol Lett. 10:223–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yap NY, Tan NYT, Tan CJ, Loh KW, Ng RCH,
Ho HK and Chan A: Associations of plasma brain-derived neurotrophic
factor (BDNF) and Val66Met polymorphism (rs6265) with long-term
cancer-related cognitive impairment in survivors of breast cancer.
Breast Cancer Res Treat. 183:683–696. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hall D, Dhilla A, Charalambous A, Gogos JA
and Karayiorgou M: Sequence variants of the brain-derived
neurotrophic factor (BDNF) gene are strongly associated with
obsessive-compulsive disorder. Am J Hum Genet. 73:370–376. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Addario C, Bellia F, Benatti B, Grancini
B, Vismara M, Pucci M, De Carlo V, Viganò C, Galimberti D, Fenoglio
C, et al: Exploring the role of BDNF DNA methylation and
hydroxymethylation in patients with obsessive compulsive disorder.
J Psychiatr Res. 114:17–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleimann A, Kotsiari A, Sperling W,
Gröschl M, Heberlein A, Kahl KG, Hillemacher T, Bleich S, Kornhuber
J and Frieling H: BDNF serum levels and promoter methylation of
BDNF exon I, IV and VI in depressed patients receiving
electroconvulsive therapy. J Neural Transm (Vienna). 122:925–928.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou W, Hu X and Jiang L: Advances in
regulating tumorigenicity and metastasis of cancer through TrkB
signaling. Curr Cancer Drug Targets. 20:779–788. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CS, Kavalali ET and Monteggia LM:
BDNF signaling in context: From synaptic regulation to psychiatric
disorders. Cell. 185:62–76. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arora S, Kanekiyo T and Singh J:
Functionalized nanoparticles for brain targeted BDNF gene therapy
to rescue Alzheimer's disease pathology in transgenic mouse model.
Int J Biol Macromol. 208:901–911. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukherjee S, Kuroiwa M, Oakden W, Paul BT,
Noman A, Chen J, Lin V, Dimitrijevic A, Stanisz G and Le TN: Local
magnetic delivery of adeno-associated virus AAV2(quad Y-F)-mediated
BDNF gene therapy restores hearing after noise injury. Mol Ther.
30:519–533. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi
S, Xie H, Peng X, Yin W, Tao Y and Wang X: miRNA-based biomarkers,
therapies, and resistance in cancer. Int J Biol Sci. 16:2628–2647.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi RU, Prieto-Vila M, Kohama I and
Ochiya T: Development of miRNA-based therapeutic approaches for
cancer patients. Cancer Sci. 110:1140–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng F, Yang Z, Huang F, Yin L, Yan G and
Gong G: microRNA-107 inhibits gastric cancer cell proliferation and
metastasis by targeting PI3K/AKT pathway. Microb Pathog.
121:110–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei J, Xu H, Wei W, Wang Z, Zhang Q, De W
and Shu Y: circHIPK3 promotes cell proliferation and migration of
gastric cancer by sponging miR-107 and regulating BDNF expression.
Onco Targets Ther. 13:1613–1624. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren J, Huang HJ, Gong Y, Yue S, Tang LM
and Cheng SY: MicroRNA-206 suppresses gastric cancer cell growth
and metastasis. Cell Biosci. 4:262014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding D, Hou R, Gao Y and Feng Y: miR-613
inhibits gastric cancer progression through repressing brain
derived neurotrophic factor. Exp Ther Med. 15:1735–1741.
2018.PubMed/NCBI
|
|
24
|
Xu AJ, Fu LN, Wu HX, Yao XL and Meng R:
MicroRNA-744 inhibits tumor cell proliferation and invasion of
gastric cancer via targeting brain derived neurotrophic factor. Mol
Med Rep. 16:5055–5061. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong W, Zhang Y, Ding J, Yang Q, Xie H and
Gao X: circHIPK3 acts as competing endogenous RNA and promotes
non-small-cell lung cancer progression through the miR-107/BDNF
signaling pathway. Biomed Res Int. 2020:60759022020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li F, Wang X and Yang L: MicroRNA-147
targets BDNF to inhibit cell proliferation, migration and invasion
in non-small cell lung cancer. Oncol Lett. 20:1931–1937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma R, Zhu P, Liu S, Gao B and Wang W:
miR-496 suppress tumorigenesis via targeting BDNF-mediated PI3K/Akt
signaling pathway in non-small cell lung cancer. Biochem Biophys
Res Commun. 518:273–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao B, Hao S, Tian W, Jiang Y, Zhang S,
Guo L, Zhao J, Zhang G, Yan J and Luo D: MicroRNA-107 is
downregulated and having tumor suppressive effect in breast cancer
by negatively regulating brain-derived neurotrophic factor. J Gene
Med. 19:e29322017. View Article : Google Scholar
|
|
29
|
Zhang HY, Xing MQ, Guo J, Zhao JC, Chen X,
Jiang Z, Zhang H and Dong Q: Long noncoding RNA DLX6-AS1 promotes
neuroblastoma progression by regulating miR-107/BDNF pathway.
Cancer Cell Int. 19:3132019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao L, Yan P, Guo FF, Liu HJ and Zhao ZF:
MiR-1-3p inhibits cell proliferation and invasion by regulating
BDNF-TrkB signaling pathway in bladder cancer. Neoplasma. 65:89–96.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Long J, Jiang C, Liu B, Fang S and Kuang
M: MicroRNA-15a-5p suppresses cancer proliferation and division in
human hepatocellular carcinoma by targeting BDNF. Tumour Biol.
37:5821–5828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun YX, Yang J, Wang PY, Li YJ, Xie SY and
Sun RP: Cisplatin regulates SH-SY5Y cell growth through
downregulation of BDNF via miR-16. Oncol Rep. 30:2343–2349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Z, Guo X, Zang M, Wang P, Xue S and
Chen G: Long non-coding RNA LINC00152 promotes cell growth and
invasion of papillary thyroid carcinoma by regulating the
miR-497/BDNF axis. J Cell Physiol. 234:1336–1345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu X, Zou W, Liu D, Qin G and Jiang L: The
down-regulation of TrkB alleviates the malignant biological
behavior and cancer stem-like property of laryngeal cancer. Cancer
Manag Res. 12:6865–6875. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan H, Wu W, Ge H, Li P and Wang Z:
Up-regulation of miR-204 enhances anoikis sensitivity in epithelial
ovarian cancer cell line via brain-derived neurotrophic factor
pathway in vitro. Int J Gynecol Cancer. 25:944–952. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song D, Diao J, Yang Y and Chen Y:
MicroRNA-382 inhibits cell proliferation and invasion of
retinoblastoma by targeting BDNFmediated PI3K/AKT signalling
pathway. Mol Med Rep. 16:6428–6436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fei X, Jin HY, Gao Y, Kong LM and Tan XD:
Hsa-miR-10a-5p promotes pancreatic cancer growth by BDNF/SEMA4C
pathway. J Biol Regul Homeost Agents. 34:927–934. 2020.PubMed/NCBI
|
|
38
|
Nagpal N, Ahmad HM, Molparia B and
Kulshreshtha R: MicroRNA-191, an estrogen-responsive microRNA,
functions as an oncogenic regulator in human breast cancer.
Carcinogenesis. 34:1889–1899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagpal N, Sharma S, Maji S, Durante G,
Ferracin M, Thakur JK and Kulshreshtha R: Essential role of MED1 in
the transcriptional regulation of ER-dependent oncogenic miRNAs in
breast cancer. Sci Rep. 8:118052018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Y, Fu Z, Gao X, Wang R and Li Q: Long
non-coding RNA XIST promotes retinoblastoma cell proliferation,
migration, and invasion by modulating microRNA-191-5p/brain derived
neurotrophic factor. Bioengineered. 12:1587–1598. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong X, Fu X, Yu M and Li Z: Long
intergenic non-protein coding RNA 1094 promotes initiation and
progression of glioblastoma by promoting microRNA-577-regulated
stabilization of brain-derived neurotrophic factor. Cancer Manag
Res. 12:5619–5631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Amuti A, Liu D, Maimaiti A, Yu Y, Yasen Y,
Ma H, Li R, Deng S, Pang F and Tian Y: Doxorubicin inhibits
osteosarcoma progression by regulating circ_0000006/miR-646/BDNF
axis. J Orthop Surg Res. 16:6452021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Y, Qi L, Zhang K and Wang F:
MicroRNA-10a suppresses cell metastasis by targeting BDNF and
predicted patients survival in renal cell carcinoma. J BUON.
26:250–258. 2021.PubMed/NCBI
|
|
44
|
Liu S, Jiang T, Zhong Y and Yu Y: miR-210
inhibits cell migration and invasion by targeting the brain-derived
neurotrophic factor in glioblastoma. J Cell Biochem.
120:11375–11382. 2019. View Article : Google Scholar
|
|
45
|
Imam JS, Plyler JR, Bansal H, Prajapati S,
Bansal S, Rebeles J, Chen HI, Chang YF, Panneerdoss S, Zoghi B, et
al: Genomic loss of tumor suppressor miRNA-204 promotes cancer cell
migration and invasion by activating AKT/mTOR/Rac1 signaling and
actin reorganization. PLoS One. 7:e523972012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhong KZ, Chen WW, Hu XY, Jiang AL and
Zhao J: Clinicopathological and prognostic significance of
microRNA-107 in human non small cell lung cancer. Int J Clin Exp
Pathol. 7:4545–4551. 2014.PubMed/NCBI
|
|
47
|
Zhai L, Li Y, Lan X and Ai L:
MicroRNA-10a-5p suppresses cancer proliferation and division in
human cervical cancer by targeting BDNF. Exp Ther Med.
14:6147–6151. 2017.PubMed/NCBI
|
|
48
|
Zheng B and Chen T: MiR-489-3p inhibits
cell proliferation, migration, and invasion, and induces apoptosis,
by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma.
Open Life Sci. 15:274–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang L, Liu Y and Song J: MicroRNA-103
suppresses glioma cell proliferation and invasion by targeting the
brain-derived neurotrophic factor. Mol Med Rep. 17:4083–4089.
2018.PubMed/NCBI
|
|
50
|
Ye J, Xie W, Zuo Y, Jing G and Tong J:
MicroRNA-496 suppresses tumor cell proliferation by targeting BDNF
in osteosarcoma. Exp Ther Med. 19:1425–1431. 2020.PubMed/NCBI
|
|
51
|
Song Y, Wang G, Zhuang J, Ni J, Zhang S,
Ye Y and Xia W: MicroRNA-584 prohibits hepatocellular carcinoma
cell proliferation and invasion by directly targeting BDNF. Mol Med
Rep. 20:1994–2001. 2019.PubMed/NCBI
|
|
52
|
Climent M, Viggiani G, Chen YW, Coulis G
and Castaldi A: MicroRNA and ROS crosstalk in cardiac and pulmonary
diseases. Int J Mol Sci. 21:43702020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bjorkman KK, Buvoli M, Pugach EK, Polmear
MM and Leinwand LA: miR-1/206 downregulates splicing factor Srsf9
to promote C2C12 differentiation. Skelet Muscle. 9:312019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et
al: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang
B, Shu Y and Liu P: miR-497 modulates multidrug resistance of human
cancer cell lines by targeting BCL2. Med Oncol. 29:384–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tajbakhsh A, Mokhtari-Zaer A, Rezaee M,
Afzaljavan F, Rivandi M, Hassanian SM, Ferns GA, Pasdar A and Avan
A: Therapeutic potentials of BDNF/TrkB in breast cancer; current
status and perspectives. J Cell Biochem. 118:2502–2515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo D, Sun W, Zhu L, Zhang H, Hou X, Liang
J, Jiang X and Liu C: Knockdown of BDNF suppressed invasion of
HepG2 and HCCLM3 cells, a mechanism associated with inactivation of
RhoA or Rac1 and actin skeleton disorganization. APMIS.
120:469–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou Y, Sinha S, Schwartz JL and Adami GR:
A subtype of oral, laryngeal, esophageal, and lung, squamous cell
carcinoma with high levels of TrkB-T1 neurotrophin receptor mRNA.
BMC Cancer. 19:6072019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Garofalo S, D'Alessandro G, Chece G, Brau
F, Maggi L, Rosa A, Porzia A, Mainiero F, Esposito V, Lauro C, et
al: Enriched environment reduces glioma growth through immune and
non-immune mechanisms in mice. Nat Commun. 6:66232015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li Z, Chang Z, Chiao LJ, Kang Y, Xia Q,
Zhu C, Fleming JB, Evans DB and Chiao PJ: TrkBT1 induces liver
metastasis of pancreatic cancer cells by sequestering Rho GDP
dissociation inhibitor and promoting RhoA activation. Cancer Res.
69:7851–7859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gao J, Zhang X, Jiang L, Li Y and Zheng Q:
Tumor endothelial cell-derived extracellular vesicles contribute to
tumor microenvironment remodeling. Cell Commun Signal. 20:972022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhou C, Liu Q, Xiang Y, Gou X and Li W:
Role of the tumor immune microenvironment in tumor immunotherapy.
Oncol Lett. 23:532022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cao L, Liu X, Lin EJ, Wang C, Choi EY,
Riban V, Lin B and During MJ: Environmental and genetic activation
of a brain-adipocyte BDNF/leptin axis causes cancer remission and
inhibition. Cell. 142:52–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu X, McMurphy T, Xiao R, Slater A, Huang
W and Cao L: Hypothalamic gene transfer of BDNF inhibits breast
cancer progression and metastasis in middle age obese mice. Mol
Ther. 22:1275–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiao R, Bergin SM, Huang W, Slater AM, Liu
X, Judd RT, Lin ED, Widstrom KJ, Scoville SD, Yu J, et al:
Environmental and genetic activation of hypothalamic BDNF modulates
T-cell immunity to exert an anticancer phenotype. Cancer Immunol
Res. 4:488–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z,
Wang JF, Zhang Z, Lu S, Huang X, et al: Plasma microRNA panel to
diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin
Oncol. 29:4781–4788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ng EKO, Chong WWS, Jin H, Lam EKY, Shin
VY, Yu J, Poon TCW, Ng SSM and Sung JJY: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Toden S, Zumwalt TJ and Goel A: Non-coding
RNAs and potential therapeutic targeting in cancer. Biochim Biophys
Acta Rev Cancer. 1875:1884912021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Saw PE, Xu X, Chen J and Song EW:
Non-coding RNAs: The new central dogma of cancer biology. Sci China
Life Sci. 64:22–50. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang S, Cheng Z, Wang Y and Han T: The
risks of miRNA therapeutics: In a drug target perspective. Drug Des
Devel Ther. 15:721–733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chu YY, Ko CY, Wang SM, Lin PI, Wang HY,
Lin WC, Wu DY, Wang LH and Wang JM: Bortezomib-induced miRNAs
direct epigenetic silencing of locus genes and trigger apoptosis in
leukemia. Cell Death Dis. 8:e31672017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ye LL, Cheng ZG, Cheng XE and Huang YL:
Propofol regulates miR-1-3p/IGF1 axis to inhibit the proliferation
and accelerates apoptosis of colorectal cancer cells. Toxicol Res
(Camb). 10:696–705. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cui P, Hu J, Wang X, Xia Y, Ruan X and Cai
M: Effects of propofol on invasion, migration and
epithelial-mesenchymal transition of breast cancer MDA-MB-231 cells
by up-regulating miR-204. Chin J Immunol. 36:2100–2104. 2020.(In
Chinese).
|
|
75
|
Wang D, Yang T, Liu J, Liu Y, Xing N, He
J, Yang J and Ai Y: Propofol inhibits the migration and invasion of
glioma cells by blocking the PI3K/AKT pathway through miR-206/ROCK1
axis. Onco Targets Ther. 13:361–370. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shi A, Luo J and Cao H: Propofol affects
invasion and metastasis of lung adenocarcinoma cells by regulating
hypoxia inducible factor-1α/microRNA-210 signaling pathway. Chin J
Clin Pharmacol. 35:2314–2317. 2019.(In Chinese).
|
|
77
|
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong
X, Ning Z, Wang J, Xu X, Jiang Y, et al: Metformin induces human
esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1
axis. Cancer Lett. 450:22–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang Y, Chen X, Li J and Xia C: Quercetin
antagonizes esophagus cancer by modulating miR-1-3p/TAGLN2
pathway-dependent growth and metastasis. Nutr Cancer. 74:1872–1881.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhao J, Fang Z, Zha Z, Sun Q, Wang H, Sun
M and Qiao B: Quercetin inhibits cell viability, migration and
invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur J
Pharmacol. 847:11–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ahmed Youness R, Amr Assal R, Mohamed
Ezzat S, Zakaria Gad M and Abdel Motaal A: A methoxylated quercetin
glycoside harnesses HCC tumor progression in a TP53/miR-15/miR-16
dependent manner. Nat Prod Res. 34:1475–1480. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang N, Feng T, Liu X and Liu Q: Curcumin
inhibits migration and invasion of non-small cell lung cancer cells
through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR
signaling pathway. Acta Pharm. 70:399–409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ran H, Chen H, Pu J, Li M, Zhang Z and He
Y: Effect of curcumin on apoptosis of PC3 cell line via
down-regulating the expressions of MiR210 and TLR4/NF-κB signaling
pathway. Pharmacol Clin Chin Mater. 37:64–68. 2021.(In
Chinese).
|
|
83
|
Li Y, Lin Q, Chang S, Zhang R and Wang J:
Vitamin D3 mediates miR-15a-5p inhibition of liver cancer cell
proliferation via targeting E2F3. Oncol Lett. 20:292–298. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang J, Ren L, Yu M, Liu X, Ma W, Huang
L, Li X and Ye X: S-equol inhibits proliferation and promotes
apoptosis of human breast cancer MCF-7 cells via regulating
miR-10a-5p and PI3K/AKT pathway. Arch Biochem Biophys.
672:1080642019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang W, Li W and Han X: Skullcapflavone I
inhibits proliferation of human colorectal cancer cells via
down-regulation of miR-107 expression. Neoplasma. 66:203–210. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li H, Xia Z, Liu L, Pan G, Ding J, Liu J,
Kang J, Li J, Jiang D and Liu W: Astragalus IV undermines
multi-drug resistance and glycolysis of MDA-MB-231/ADR Cell line by
depressing hsa_circ_0001982-miR-206/miR-613 axis. Cancer Manag Res.
13:5821–5833. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dan H, Lei H and JinMing H:
Andrographolide inhibits proliferation and promotes apoptosis of
prostate cancer cells by regulating miR-206/STC2. Chin J Gerontol.
39:4802–4807. 2019.
|
|
88
|
Buist M, Fuss D and Rastegar M:
Transcriptional regulation of MECP2E1-E2 isoforms and BDNF by
Metformin and Simvastatin through analyzing nascent RNA synthesis
in a human brain cell Line. Biomolecules. 11:12532021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kumar P, Barua CC, Sulakhiya K and Sharma
RK: Curcumin ameliorates cisplatin-induced nephrotoxicity and
potentiates its anticancer activity in SD rats: Potential role of
curcumin in breast cancer chemotherapy. Front Pharmacol. 8:1322017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Alhusban L, Ayoub N and Alhusban A:
ProBDNF is a novel mediator of the interaction between MDA-MB-231
breast cancer cells and brain microvascular endothelial cells. Curr
Mol Med. 21:914–921. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Iqbal MUN, Yaqoob T, Ali SA and Khan TA: A
functional polymorphism (rs6265, G>A) of brain-derived
neurotrophic factor gene and breast cancer: An association study.
Breast Cancer (Auckl). 13:11782234198449772019.PubMed/NCBI
|
|
92
|
Kim JM, Kang HJ, Kim SY, Kim SW, Shin IS,
Kim HR, Park MH, Shin MG, Yoon JH and Yoon JS: BDNF promoter
methylation associated with suicidal ideation in patients with
breast cancer. Int J Psychiatry Med. 49:75–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin X, Dinglin X, Cao S, Zheng S, Wu C,
Chen W, Li Q, Hu Q, Zheng F, Wu Z, et al: Enhancer-driven lncRNA
BDNF-AS induces endocrine resistance and malignant progression of
breast cancer through the RNH1/TRIM21/mTOR cascade. Cell Rep.
31:1077532020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang G, Xiang Z, Wu H, He Q, Dou R, Lin
Z, Yang C, Huang S, Song J, Di Z, et al: The lncRNA
BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer
peritoneal metastasis by regulating VDAC3 ubiquitination. Int J
Biol Sci. 18:1415–1433. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Koh MJ, Jeung HC, Namkoong K, Chung HC and
Kang JI: Influence of the BDNF Val66Met polymorphism on coping
response to stress in patients with advanced gastric cancer. J
Psychosom Res. 77:76–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Guo JC, Yang YJ, Zheng JF, Guo M, Wang XD,
Gao YS, Fu LQ, Jiang XL, Fu LM and Huang T: Functional rs6265
polymorphism in the brain-derived neurotrophic factor gene confers
protection against neurocognitive dysfunction in posttraumatic
stress disorder among Chinese patients with hepatocellular
carcinoma. J Cell Biochem. 120:10434–10443. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bai L, Zhang S, Zhou X, Li Y and Bai J:
Brain-derived neurotrophic factor induces thioredoxin-1 expression
through TrkB/Akt/CREB pathway in SH-SY5Y cells. Biochimie.
160:55–60. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hua Z, Gu X, Dong Y, Tan F, Liu Z, Thiele
CJ and Li Z: PI3K and MAPK pathways mediate the BDNF/TrkB-increased
metastasis in neuroblastoma. Tumour Biol. 37:16227–16236.
2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yuan Y, Ye HQ and Ren QC: Upregulation of
the BDNF/TrKB pathway promotes epithelial-mesenchymal transition,
as well as the migration and invasion of cervical cancer. Int J
Oncol. 52:461–472. 2018.PubMed/NCBI
|
|
100
|
Okugawa Y, Tanaka K, Inoue Y, Kawamura M,
Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, et al:
Brain-derived neurotrophic factor/tropomyosin-related kinase B
pathway in gastric cancer. Br J Cancer. 108:121–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kojadinovic A, Laderian B and Mundi PS:
Targeting TRK: A fast-tracked application of precision oncology and
future directions. Crit Rev Oncol Hematol. 165:1034512021.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bhangoo MS and Sigal D: TRK inhibitors:
Clinical development of larotrectinib. Curr Oncol Rep. 21:142019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Peng Y, Wang Y, Zhou C, Mei W and Zeng C:
PI3K/Akt/mTOR pathway and its role in cancer therapeutics: Are we
making headway? Front Oncol. 12:8191282022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kaboli PJ, Imani S, Jomhori M and Ling KH:
Chemoresistance in breast cancer: PI3K/Akt pathway inhibitors vs
the current chemotherapy. Am J Cancer Res. 11:5155–5183.
2021.PubMed/NCBI
|
|
105
|
Duan Y, Haybaeck J and Yang Z: Therapeutic
potential of PI3K/AKT/mTOR pathway in gastrointestinal stromal
tumors: Rationale and progress. Cancers (Basel). 12:29722020.
View Article : Google Scholar : PubMed/NCBI
|