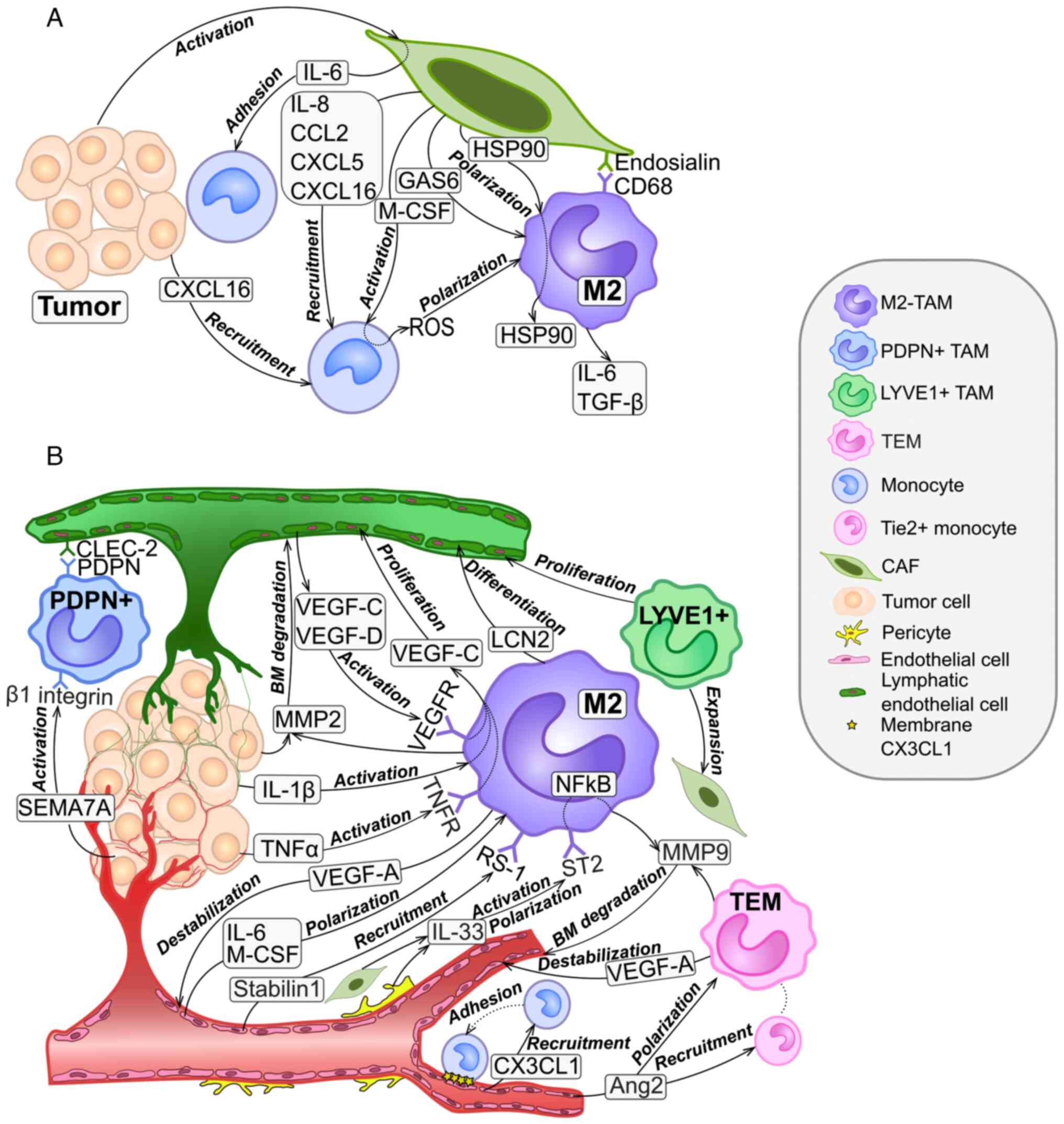
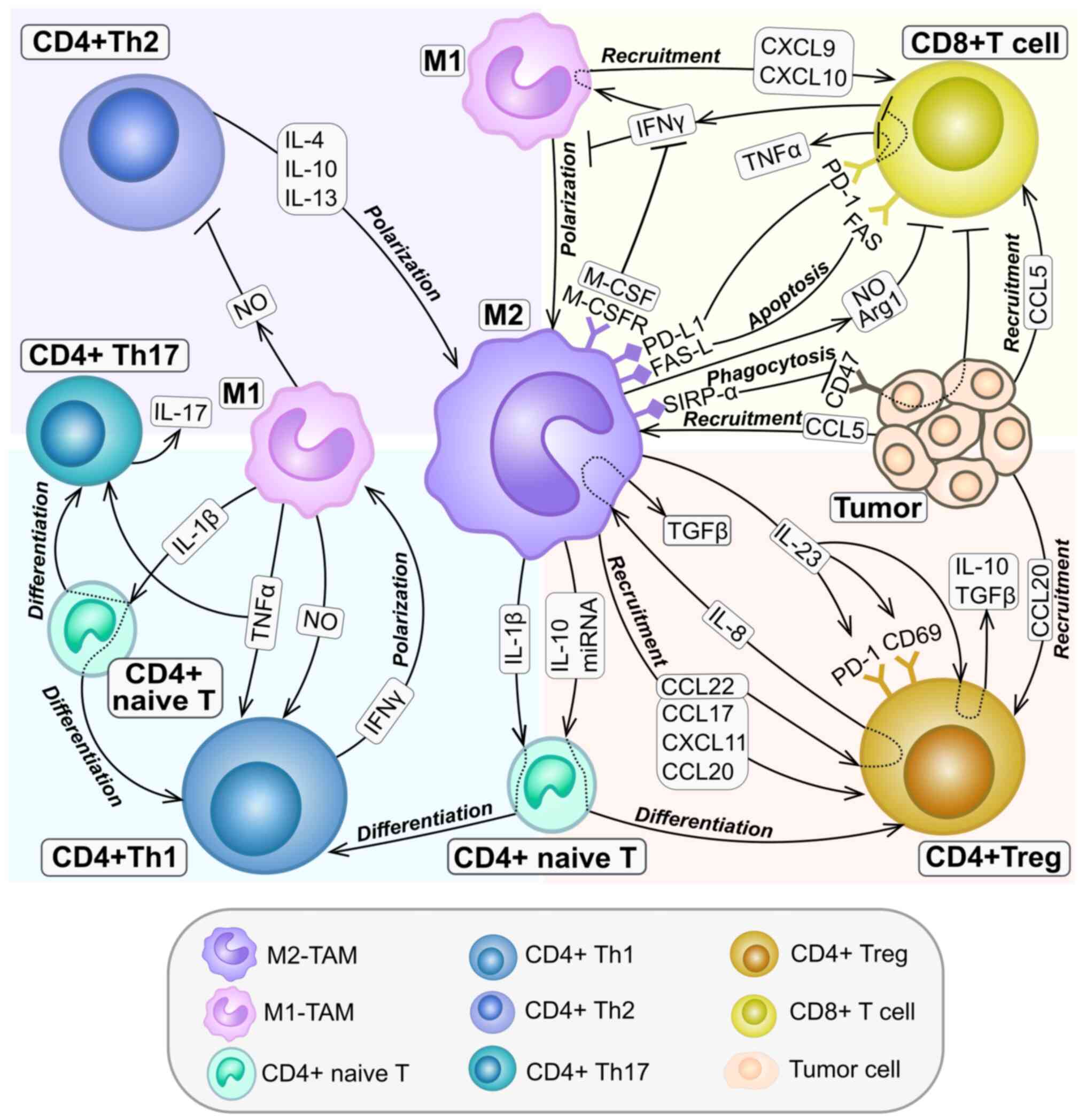
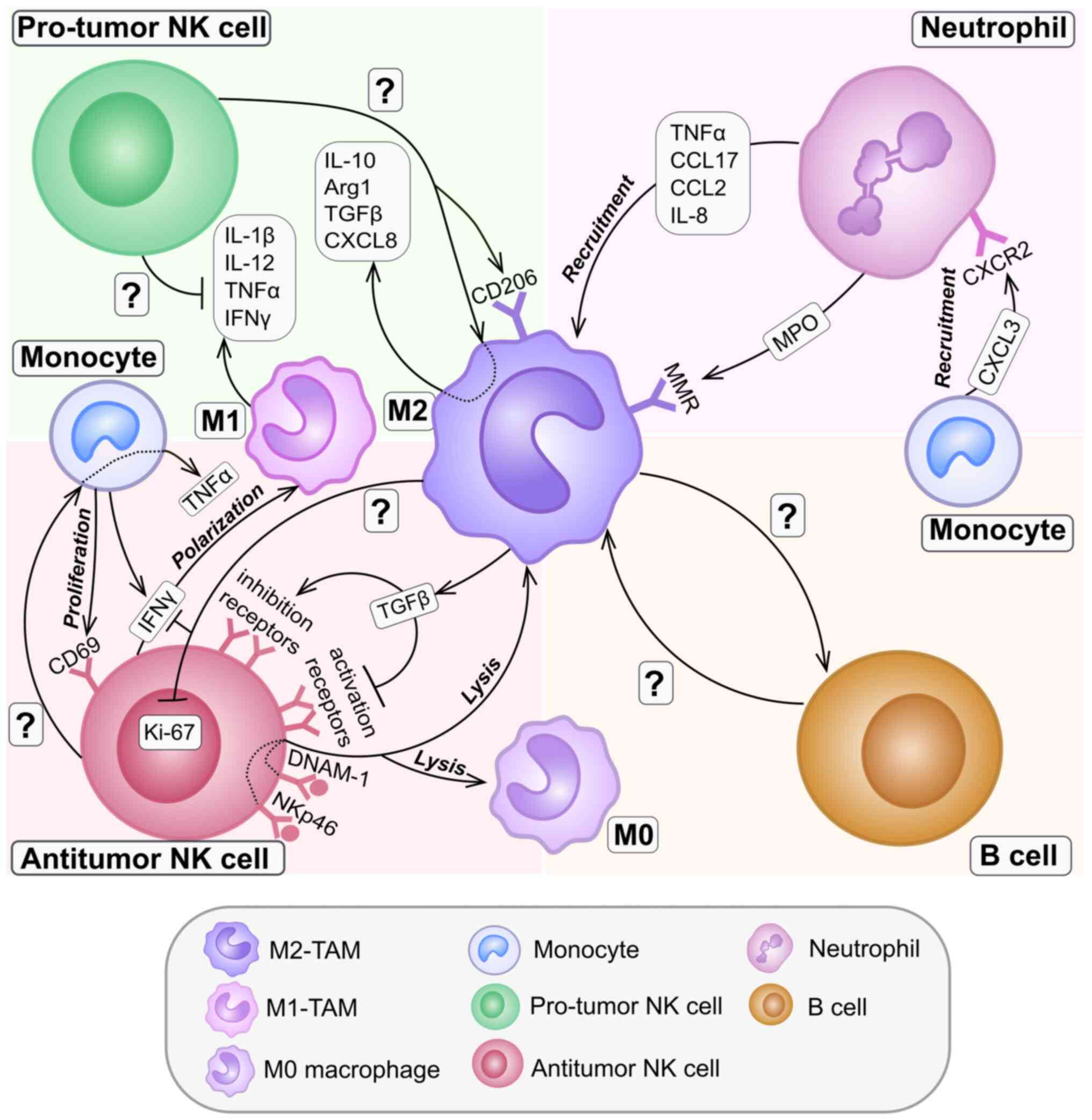
|
1
|
Yuan Y, Jiang YC, Sun CK and Chen QM: Role
of the tumor microenvironment in tumor progression and the clinical
applications (Review). Oncol Rep. 35:2499–2515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Werb Z and Lu P: The role of stroma in
tumor development. Cancer J. 21:250–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baghban R, Roshangar L, Jahanban-Esfahlan
R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T and
Zare P: Tumor microenvironment complexity and therapeutic
implications at a glance. Cell Commun Signal. 18:592020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larionova I, Tuguzbaeva G, Ponomaryova A,
Stakheyeva M, Cherdyntseva N, Pavlov V, Choinzonov E and
Kzhyshkowska J: Tumor-associated macrophages in human breast,
colorectal, lung, ovarian and prostate cancers. Front Oncol.
10:5665112020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Y, Xu J and Lan H: Tumor-associated
macrophages in tumor metastasis: Biological roles and clinical
therapeutic applications. J Hematol Oncol. 12:762019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan Y, Yu Y, Wang X and Zhang T:
Tumor-associated macrophages in tumor immunity. Front Immunol.
11:5830842020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larionova I, Kazakova E, Patysheva M and
Kzhyshkowska J: Transcriptional, epigenetic and metabolic
programming of tumor-associated macrophages. Cancers (Basel).
12:14112020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao J, Liang Y and Wang L: Shaping
polarization of tumor-associated macrophages in cancer
immunotherapy. Front Immunol. 13:8887132022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marusyk A, Almendro V and Polyak K:
Intra-tumour heterogeneity: A looking glass for cancer? Nat Rev
Cancer. 12:323–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gordillo CH, Sa ndova l P, Muñoz-Her
nández P, Pascual-Antón L, López-Cabrera M and Jiménez-Heffernan
JA: Mesothelial-to-mesenchymal transition contributes to the
generation of carcinoma-associated fibroblasts in locally advanced
primary colorectal carcinomas. Cancers (Basel). 12:4992020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiga K, Hara M, Nagasaki T, Sato T,
Takahashi H and Takeyama H: Cancer-associated fibroblasts: Their
characteristics and their roles in tumor growth. Cancers (Basel).
7:2443–2458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adjuto-Saccone M, Soubeyran P, Garcia J,
Audebert S, Camoin L, Rubis M, Roques J, Binétruy B, Iovanna JL and
Tournaire R: TNF-α induces endothelial-mesenchymal transition
promoting stromal development of pancreatic adenocarcinoma. Cell
Death Dis. 12:6492021. View Article : Google Scholar
|
|
15
|
Ciszewski WM, Sobierajska K, Wawro ME,
Klopocka W, Chefczyńska N, Muzyczuk A, Siekacz K, Wujkowska A and
Niewiarowska J: The ILK-MMP9-MRTF axis is crucial for EndMT
differentiation of endothelial cells in a tumor microenvironment.
Biochim Biophys Acta Mol Cell Res. 1864:2283–2296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan CS, Chen LL, Hsu TA, Chen CC, Chua KV,
Li CP and Huang TS: Endothelial-mesenchymal transition harnesses
HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal
adenocarcinoma. J Hematol Oncol. 12:1382019. View Article : Google Scholar
|
|
17
|
Fan CS, Chen WS, Chen LL, Chen CC, Hsu YT,
Chua KV, Wang HD and Huang TS: Osteopontin-integrin engagement
induces HIF-1α-TCF12-mediated endothelial-mesenchymal transition to
exacerbate colorectal cancer. Oncotarget. 9:4998–5015. 2017.
View Article : Google Scholar
|
|
18
|
Chen C, Li WJ, Weng JJ, Chen ZJ, Wen YY,
Deng T, Le HB, Zhang YK and Zhang BJ: Cancer-associated
fibroblasts, matrix metalloproteinase-9 and lymphatic vessel
density are associated with progression from adenocarcinoma in situ
to invasive adenocarcinoma of the lung. Oncol Lett. 20:1302020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie J, Qi X, Wang Y, Yin X, Xu W, Han S,
Cai Y and Han W: Cancer-associated fibroblasts secrete
hypoxia-induced serglycin to promote head and neck squamous cell
carcinoma tumor cell growth in vitro and in vivo by activating the
Wnt/β-catenin pathway. Cell Oncol (Dordr). 44:661–671. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fullár A, Dudás J, Oláh L, Hollósi P, Papp
Z, Sobel G, Karászi K, Paku S, Baghy K and Kovalszky I: Remodeling
of extracellular matrix by normal and tumor-associated fibroblasts
promotes cervical cancer progression. BMC Cancer. 15:2562015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erdogan B and Webb DJ: Cancer-associated
fibroblasts modulate growth factor signaling and extracellular
matrix remodeling to regulate tumor metastasis. Biochem Soc Trans.
45:229–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren J, Smid M, Iaria J, Salvatori DCF, van
Dam H, Zhu HJ, Martens JWM and Ten Dijke P: Cancer-associated
fibroblast-derived Gremlin 1 promotes breast cancer progression.
Breast Cancer Res. 21:1092019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi M, Kobayashi H, Mizutani Y, Hara
A, Iida T, Miyai Y, Asai N and Enomoto A: Roles of the mesenchymal
stromal/stem cell marker meflin/Islr in cancer fibrosis. Front Cell
Dev Biol. 9:7499242021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Attieh Y, Clark AG, Grass C, Richon S,
Pocard M, Mariani P, Elkhatib N, Betz T, Gurchenkov B and Vignjevic
DM: Cancer-associated fibroblasts lead tumor invasion through
integrin-β3-dependent fibronectin assembly. J Cell Biol.
216:3509–3520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Fu B, Li M and Mi S: Secretome of
activated fibroblasts induced by exosomes for the discovery of
biomarkers in non-small cell lung cancer. Small. 17:e20047502021.
View Article : Google Scholar
|
|
26
|
Glentis A, Oertle P, Mariani P, Chikina A,
El Marjou F, Attieh Y, Zaccarini F, Lae M, Loew D, Dingli F, et al:
Cancer-associated fibroblasts induce metalloprotease-independent
cancer cell invasion of the basement membrane. Nat Commun.
8:9242017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stadler M, Pudelko K, Biermeier A,
Walterskirchen N, Gaigneaux A, Weindorfer C, Harrer N, Klett H,
Hengstschläger M, Schüler J, et al: Stromal fibroblasts shape the
myeloid phenotype in normal colon and colorectal cancer and induce
CD163 and CCL2 expression in macrophages. Cancer Lett. 520:184–200.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang
X, Yuan L and Feng Y: Cancer-associated fibroblasts enhance
tumor-associated macrophages enrichment and suppress NK cells
function in colorectal cancer. Cell Death Dis. 10:2732019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, Morine Y, Tokuda K, Yamada S,
Saito Y, Nishi M, Ikemoto T and Shimada M: Cancer-associated
fibroblast-induced M2-polarized macrophages promote hepatocellular
carcinoma progression via the plasminogen activator inhibitor-1
pathway. Int J Oncol. 59:592021. View Article : Google Scholar :
|
|
30
|
Olingy CE, Dinh HQ and Hedrick CC:
Monocyte heterogeneity and functions in cancer. J Leukoc Biol.
106:309–322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hao Q, Vadgama JV and Wang P: CCL2/CCR2
signaling in cancer pathogenesis. Cell Commun Signal. 18:822020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gomez-Roca CA, Italiano A, Le Tourneau C,
Cassier PA, Toulmonde M, D'Angelo SP, Campone M, Weber KL, Loirat
D, Cannarile MA, et al: Phase I study of emactuzumab single agent
or in combination with paclitaxel in patients with
advanced/metastatic solid tumors reveals depletion of
immunosuppressive M2-like macrophages. Ann Oncol. 30:1381–1392.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cannarile MA, Weisser M, Jacob W, Jegg AM,
Ries CH and Rüttinger D: Colony-stimulating factor 1 receptor
(CSF1R) inhibitors in cancer therapy. J Immunother Cancer.
5:532017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cho H, Seo Y, Loke KM, Kim SW, Oh SM, Kim
JH, Soh J, Kim HS, Lee H, Kim J, et al: Cancer-stimulated CAFs
enhance monocyte differentiation and protumoral TAM Activation via
IL6 and GM-CSF secretion. Clin Cancer Res. 24:5407–5421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiang H, Ramil CP, Hai J, Zhang C, Wang H,
Watkins AA, Afshar R, Georgiev P, Sze MA, Song XS, et al:
Cancer-associated fibroblasts promote immunosuppression by inducing
ROS-generating monocytic MDSCs in lung squamous cell carcinoma.
Cancer Immunol Res. 8:436–450. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Higashino N, Koma YI, Hosono M, Takase N,
Okamoto M, Kodaira H, Nishio M, Shigeoka M, Kakeji Y and Yokozaki
H: Fibroblast activation protein-positive fibroblasts promote tumor
progression through secretion of CCL2 and interleukin-6 in
esophageal squamous cell carcinoma. Lab Invest. 99:777–792. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gok Yavuz B, Gunaydin G, Gedik ME,
Kosemehmetoglu K, Karakoc D, Ozgur F and Guc D: Cancer associated
fibroblasts sculpt tumour microenvironment by recruiting monocytes
and inducing immunosuppressive PD-1+ TAMs. Sci Rep.
9:31722019. View Article : Google Scholar
|
|
38
|
Yang F, Wei Y, Han D, Li Y, Shi S, Jiao D,
Wu J, Zhang Q, Shi C, Yang L, et al: Interaction with CD68 and
regulation of GAS6 expression by endosialin in fibroblasts drives
recruitment and polarization of macrophages in hepatocellular
carcinoma. Cancer Res. 80:3892–3905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Allaoui R, Bergenfelz C, Mohlin S,
Hagerling C, Salari K, Werb Z, Anderson RL, Ethier SP, Jirström K,
Påhlman S, et al: Cancer-associated fibroblast-secreted CXCL16
attracts monocytes to promote stroma activation in triple-negative
breast cancers. Nat Commun. 7:130502016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Comito G, Giannoni E, Segura CP,
Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S
and Chiarugi P: Cancer-associated fibroblasts and M2-polarized
macrophages synergize during prostate carcinoma progression.
Oncogene. 33:2423–2431. 2014. View Article : Google Scholar
|
|
41
|
Hegab AE, Ozaki M, Kameyama N, Gao J,
Kagawa S, Yasuda H, Soejima K, Yin Y, Guzy RD, Nakamura Y, et al:
Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its
cancer-associated fibroblasts. J Pathol. 249:193–205. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang A, Qian Y, Ye Z, Chen H, Xie H, Zhou
L, Shen Y and Zheng S: Cancer-associated fibroblasts promote M2
polarization of macrophages in pancreatic ductal adenocarcinoma.
Cancer Med. 6:463–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chua KV, Fan CS, Chen CC, Chen LL, Hsieh
SC and Huang TS: Octyl gallate induces pancreatic ductal
adenocarcinoma cell apoptosis and suppresses
endothelial-mesenchymal transition-promoted M2-macrophages, HSP90α
secretion, and tumor growth. Cells. 9:912019. View Article : Google Scholar
|
|
44
|
Cohen N, Shani O, Raz Y, Sharon Y, Hoffman
D, Abramovitz L and Erez N: Fibroblasts drive an immunosuppressive
and growth-promoting microenvironment in breast cancer via
secretion of Chitinase 3-like 1. Oncogene. 36:4457–4468. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou Q, Wu X, Wang X, Yu Z, Pan T, Li Z,
Chang X, Jin Z, Li J, Zhu Z, et al: The reciprocal interaction
between tumor cells and activated fibroblasts mediated by
TNF-α/IL-33/ST2L signaling promotes gastric cancer metastasis.
Oncogene. 39:1414–1428. 2020. View Article : Google Scholar
|
|
46
|
Andersson P, Yang Y, Hosaka K, Zhang Y,
Fischer C, Braun H, Liu S, Yu G, Liu S, Beyaert R, et al: Molecular
mechanisms of IL-33-mediated stromal interactions in cancer
metastasis. JCI Insight. 3:e1223752018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mazur A, Holthoff E, Vadali S, Kelly T and
Post SR: Cleavage of type I collagen by fibroblast activation
protein-α enhances class A scavenger receptor mediated macrophage
adhesion. PLoS One. 11:e01502872016. View Article : Google Scholar
|
|
48
|
Cat B, Stuhlmann D, Steinbrenner H, Alili
L, Holtkötter O, Sies H and Brenneisen P: Enhancement of tumor
invasion depends on transdifferentiation of skin fibroblasts
mediated by reactive oxygen species. J Cell Sci. 119:2727–2738.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W,
Dang Y, Chu Y, Fan J and He R: FAP promotes immunosuppression by
cancer-associated fibroblasts in the tumor microenvironment via
STAT3-CCL2 signaling. Cancer Res. 76:4124–4135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Potente M and Carmeliet P: The Link
between angiogenesis and endothelial metabolism. Annu Rev Physiol.
79:43–66. 2017. View Article : Google Scholar
|
|
51
|
Papetti M and Herman IM: Mechanisms of
normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol.
282:C947–C970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hida K and Maishi N: Abnormalities of
tumor endothelial cells and cancer progression. Oral Sci Int.
15:1–6. 2018. View Article : Google Scholar
|
|
53
|
Hida K, Maishi N, Takeda R and Hida Y: The
roles of tumor endothelial cells in cancer metastasis. Metastasis
Brisbane (AU): Sergi CM: Exon Publications; 2022, View Article : Google Scholar
|
|
54
|
Wang WY, Lin D, Jarman EH, Polacheck WJ
and Baker BM: Functional angiogenesis requires microenvironmental
cues balancing endothelial cell migration and proliferation. Lab
Chip. 20:1153–1166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang D, Guo P, He T and Powell CA: Role of
endothelial cells in tumor microenvironment. Clin Transl Med.
11:e4502021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wei K, Ma Z, Yang F, Zhao X, Jiang W, Pan
C, Li Z, Pan X, He Z, Xu J, et al: M2 macrophage-derived exosomes
promote lung adenocarcinoma progression by delivering miR-942.
Cancer Lett. 526:205–216. 2022. View Article : Google Scholar
|
|
57
|
Baradaran A, Asadzadeh Z, Hemmat N,
Baghbanzadeh A, Shadbad MA, Khosravi N, Derakhshani A, Alemohammad
H, Afrashteh Nour M, Safarpour H, et al: The cross-talk between
tumor-associated macrophages and tumor endothelium: Recent advances
in macrophage-based cancer immunotherapy. Biomed Pharmacother.
146:1125882022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Laoui D, Van Overmeire E, Di Conza G,
Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe
E, Elkrim Y, et al: Tumor hypoxia does not drive differentiation of
tumor-associated macrophages but rather fine-tunes the M2-like
macrophage population. Cancer Res. 74:24–30. 2014. View Article : Google Scholar
|
|
59
|
Hughes R, Qian BZ, Rowan C, Muthana M,
Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons
M, et al: Perivascular M2 macrophages stimulate tumor relapse after
chemotherapy. Cancer Res. 75:3479–3491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cheng N, Bei Y, Song Y, Zhang W, Xu L,
Zhang W, Yang N, Bai X, Shu Y and Shen P: B7-H3 augments the
pro-angiogenic function of tumor-associated macrophages and acts as
a novel adjuvant target for triple-negative breast cancer therapy.
Biochem Pharmacol. 183:1142982021. View Article : Google Scholar
|
|
61
|
Treps L, Faure S and Clere N: Vasculogenic
mimicry, a complex and devious process favoring
tumorigenesis-Interest in making it a therapeutic target. Pharmacol
Ther. 223:1078052021. View Article : Google Scholar
|
|
62
|
Zhang L, Xu Y, Sun J, Chen W, Zhao L, Ma
C, Wang Q, Sun J, Huang B, Zhang Y, et al: M2-like tumor-associated
macrophages drive vasculogenic mimicry through amplification of
IL-6 expression in glioma cells. Oncotarget. 8:819–832. 2017.
View Article : Google Scholar :
|
|
63
|
Delprat V and Michiels C: A bi-directional
dialog between vascular cells and monocytes/macrophages regulates
tumor progression. Cancer Metastasis Rev. 40:477–500. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cortes-Santiago N, Hossain MB,
Gabrusiewicz K, Fan X, Gumin J, Marini FC, Alonso MM, Lang F, Yung
WK, Fueyo J, et al: Soluble Tie2 overrides the heightened invasion
induced by anti-angiogenesis therapies in gliomas. Oncotarget.
7:16146–16157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sopo M, Sallinen H, Hämäläinen K, Kivelä
A, Ylä-Herttuala S, Kosma VM, Keski-Nisula L and Anttila M: High
expression of Tie-2 predicts poor prognosis in primary high grade
serous ovarian cancer. PLoS One. 15:e02414842020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dong Z, Chen J, Yang X, Zheng W, Wang L,
Fang M, Wu M, Yao M and Yao D: Ang-2 promotes lung cancer
metastasis by increasing epithelial-mesenchymal transition.
Oncotarget. 9:12705–12717. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hidalgo M, Martinez-Garcia M, Le Tourneau
C, Massard C, Garralda E, Boni V, Taus A, Albanell J, Sablin MP,
Alt M, et al: First-in-human phase I study of single-agent
vanucizumab, a first-in-class bispecific
anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with
advanced solid tumors. Clin Cancer Res. 24:1536–1545. 2018.
View Article : Google Scholar
|
|
68
|
Karikoski M, Irjala H, Maksimow M,
Miiluniemi M, Granfors K, Hernesniemi S, Elima K, Moldenhauer G,
Schledzewski K, Kzhyshkowska J, et al: Clever-1/stabilin-1
regulates lymphocyte migration within lymphatics and leukocyte
entrance to sites of inflammation. Eur J Immunol. 39:3477–3487.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Karikoski M, Marttila-Ichihara F, Elima K,
Rantakari P, Hollmén M, Kelkka T, Gerke H, Huovinen V, Irjala H,
Holmdahl R, et al: Clever-1/stabilin-1 controls cancer growth and
metastasis. Clin Cancer Res. 20:6452–6464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Riabov V, Yin S, Song B, Avdic A,
Schledzewski K, Ovsiy I, Gratchev A, Llopis Verdiell M, Sticht C,
Schmuttermaier C, et al: Stabilin-1 is expressed in human breast
cancer and supports tumor growth in mammary adenocarcinoma mouse
model. Oncotarget. 7:31097–31110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yin M, Zhou HJ, Zhang J, Lin C, Li H, Li
X, Li Y, Zhang H, Breckenridge DG, Ji W and Min W: ASK1-dependent
endothelial cell activation is critical in ovarian cancer growth
and metastasis. JCI Insight. 2:e918282017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Arwert EN, Harney AS, Entenberg D, Wang Y,
Sahai E, Pollard JW and Condeelis JS: A unidirectional transition
from migratory to perivascular macrophage is required for tumor
cell intravasation. Cell Rep. 23:1239–1248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Leung E, Xue A, Wang Y, Rougerie P, Sharma
VP, Eddy R, Cox D and Condeelis J: Blood vessel
endothelium-directed tumor cell streaming in breast tumors requires
the HGF/C-Met signaling pathway. Oncogene. 36:2680–2692. 2017.
View Article : Google Scholar :
|
|
74
|
Opzoomer JW, Anstee JE, Dean I, Hill EJ,
Bouybayoune I, Caron J, Muliaditan T, Gordon P, Sosnowska D, Nuamah
R, et al: Macrophages orchestrate the expansion of a proangiogenic
perivascular niche during cancer progression. Sci Adv.
7:eabg95182021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lewis CE, Harney AS and Pollard JW: The
multifaceted role of perivascular macrophages in tumors. Cancer
Cell. 30:3652016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sparano JA, Gray R, Oktay MH, Entenberg D,
Rohan T, Xue X, Donovan M, Peterson M, Shuber A, Hamilton DA, et
al: A metastasis biomarker (MetaSite Breast™ Score) is associated
with distant recurrence in hormone receptor-positive, HER2-negative
early-stage breast cancer. NPJ Breast Cancer. 3:422017. View Article : Google Scholar
|
|
77
|
Anstee JE, Opzoomer JW, Dean I, Muller HP,
Bahri M, Liakath-Ali K, Liu Z, Choy D, Caron J, Sosnowska D, et al:
Perivascular macrophages collaborate to facilitate chemotherapy
resistance in cancer. bioRxiv. 2022.2002.2003.478952. 2022.
|
|
78
|
Hongu T, Pein M, Insua-Rodríguez J,
Gutjahr E, Mattavelli G, Meier J, Decker K, Descot A, Bozza M,
Harbottle R, et al: Perivascular tenascin C triggers sequential
activation of macrophages and endothelial cells to generate a
pro-metastatic vascular niche in the lungs. Nat Cancer. 3:486–504.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
He H, Mack JJ, Güç E, Warren CM, Squadrito
ML, Kilarski WW, Baer C, Freshman RD, McDonald AI, Ziyad S, et al:
Perivascular macrophages limit permeability. Arterioscler Thromb
Vasc Biol. 36:2203–2212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang Q, He Z, Huang M, Liu T, Wang Y, Xu
H, Duan H, Ma P, Zhang L, Zamvil SS, et al: Vascular niche IL-6
induces alternative macrophage activation in glioblastoma through
HIF-2α. Nat Commun. 9:5592018. View Article : Google Scholar
|
|
81
|
Chen L, Li J, Wang F, Dai C, Wu F, Liu X,
Li T, Glauben R, Zhang Y, Nie G, et al: Tie2 expression on
macrophages is required for blood vessel reconstruction and tumor
relapse after chemotherapy. Cancer Res. 76:6828–6838. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harney AS, Arwert EN, Entenberg D, Wang Y,
Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG and Condeelis JS:
Real-time imaging reveals local, transient vascular permeability,
and tumor cell intravasation stimulated by TIE2hi
macrophage-derived VEGFA. Cancer Discov. 5:932–943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bieniasz-Krzywiec P, Martín-Pérez R,
Ehling M, García-Caballero M, Pinioti S, Pretto S, Kroes R, Aldeni
C, Di Matteo M, Prenen H, et al: Podoplanin-expressing macrophages
promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell
Metab. 30:917–936.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Elder AM, Tamburini BAJ, Crump LS, Black
SA, Wessells VM, Schedin PJ, Borges VF and Lyons TR: Semaphorin 7A
promotes macrophage-mediated lymphatic remodeling during postpartum
mammary gland involution and in breast cancer. Cancer Res.
78:6473–6485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Evans R, Flores-Borja F, Nassiri S,
Miranda E, Lawler K, Grigoriadis A, Monypenny J, Gillet C, Owen J,
Gordon P, et al: Integrin-mediated macrophage adhesion promotes
lymphovascular dissemination in breast cancer. Cell Rep.
27:1967–1978.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bron S, Henry L, Faes-Van't Hull E,
Turrini R, Vanhecke D, Guex N, Ifticene-Treboux A, Marina Iancu E,
Semilietof A, Rufer N, et al: TIE-2-expressing monocytes are
lymphangiogenic and associate specifically with lymphatics of human
breast cancer. Oncoimmunology. 5:e10738822015. View Article : Google Scholar
|
|
87
|
Moussai D, Mitsui H, Pettersen JS, Pierson
KC, Shah KR, Suárez-Fariñas M, Cardinale IR, Bluth MJ, Krueger JG
and Carucci JA: The human cutaneous squamous cell carcinoma
microenvironment is characterized by increased lymphatic density
and enhanced expression of macrophage-derived VEGF-C. J Invest
Dermatol. 131:229–236. 2011. View Article : Google Scholar
|
|
88
|
Zhang BC, Gao J, Wang J, Rao ZG, Wang BC
and Gao JF: Tumor-associated macrophages infiltration is associated
with peritumoral lymphangiogenesis and poor prognosis in lung
adenocarcinoma. Med Oncol. 28:1447–1452. 2011. View Article : Google Scholar
|
|
89
|
Zhang B, Yao G, Zhang Y and Gao J, Yang B,
Rao Z and Gao J: M2-polarized tumor-associated macrophages are
associated with poor prognoses resulting from accelerated
lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo).
66:1879–1886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yamagata Y, Tomioka H, Sakamoto K, Sato K,
Harada H, Ikeda T and Kayamori K: CD163-positive macrophages within
the tumor stroma are associated with lymphangiogenesis and lymph
node metastasis in oral squamous cell carcinoma. J Oral Maxillofac
Surg. 75:2144–2153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, et al:
M2-polarized tumor-associated macrophage infiltration of regional
lymph nodes is associated with nodal lymphangiogenesis and occult
nodal involvement in pN0 pancreatic cancer. Pancreas. 42:155–159.
2013. View Article : Google Scholar
|
|
92
|
Wu H, Xu JB, He YL, Peng JJ, Zhang XH,
Chen CQ, Li W and Cai SR: Tumor-associated macrophages promote
angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol.
106:462–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ding H, Cai J, Mao M, Fang Y, Huang Z, Jia
J, Li T, Xu L, Wang J, Zhou J, et al: Tumor-associated macrophages
induce lymphangiogenesis in cervical cancer via interaction with
tumor cells. APMIS. 122:1059–1069. 2014.PubMed/NCBI
|
|
94
|
Utrera-Barillas D, Castro-Manrreza M,
Castellanos E, Gutiérrez-Rodríguez M, Arciniega-Ruíz de Esparza O,
García-Cebada J, Velazquez JR, Flores-Reséndiz D,
Hernández-Hernández D and Benítez-Bribiesca L: The role of
macrophages and mast cells in lymphangiogenesis and angiogenesis in
cervical carcinogenesis. Exp Mol Pathol. 89:190–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang W, Tian J and Hao Q: HMGB1 combining
with tumor-associated macrophages enhanced lymphangiogenesis in
human epithelial ovarian cancer. Tumour Biol. 35:2175–2186. 2014.
View Article : Google Scholar
|
|
96
|
Sweat RS, Sloas DC and Murfee WL: VEGF-C
induces lymphangiogenesis and angiogenesis in the rat mesentery
culture model. Microcirculation. 21:532–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H,
Lim S, Nakamura M, Andersson P, Wang J, Sun Y, et al: TNFR1
mediates TNF-α-induced tumour lymphangiogenesis and metastasis by
modulating VEGF-C-VEGFR3 signalling. Nat Commun. 5:49442014.
View Article : Google Scholar
|
|
98
|
Peppicelli S, Bianchini F and Calorini L:
Inflammatory cytokines induce vascular endothelial growth factor-C
expression in melanoma-associated macrophages and stimulate
melanoma lymph node metastasis. Oncol Lett. 8:1133–1138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Schoppmann SF, Birner P, Stöckl J, Kalt R,
Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K and Kerjaschki
D: Tumor-associated macrophages express lymphatic endothelial
growth factors and are related to peritumoral lymphangiogenesis. Am
J Pathol. 161:947–956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mazzone M and Bergers G: Regulation of
blood and lymphatic vessels by immune cells in tumors and
metastasis. Annu Rev Physiol. 81:535–560. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Weichand B, Popp R, Dziumbla S, Mora J,
Strack E, Elwakeel E, Frank AC, Scholich K, Pierre S, Syed SN, et
al: S1PR1 on tumor-associated macrophages promotes
lymphangiogenesis and metastasis via NLRP3/IL-1β. J Exp Med.
214:2695–2713. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Watari K, Shibata T, Kawahara A, Sata K,
Nabeshima H, Shinoda A, Abe H, Azuma K, Murakami Y, Izumi H, et al:
Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph
node metastasis through M2-type macrophages. PLoS One.
9:e995682014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jung M, Ören B, Mora J, Mertens C,
Dziumbla S, Popp R, Weigert A, Grossmann N, Fleming I and Brüne B:
Lipocalin 2 from macrophages stimulated by tumor cell-derived
sphingosine 1-phosphate promotes lymphangiogenesis and tumor
metastasis. Sci Signal. 9:ra642016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Storr SJ, Safuan S, Ahmad N, El-Refaee M,
Jackson AM and Martin SG: Macrophage-derived interleukin-1beta
promotes human breast cancer cell migration and lymphatic adhesion
in vitro. Cancer Immunol Immunother. 66:1287–1294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen C, He W, Huang J, Wang B, Li H, Cai
Q, Su F, Bi J, Liu H, Zhang B, et al: LNMAT1 promotes lymphatic
metastasis of bladder cancer via CCL2 dependent macrophage
recruitment. Nat Commun. 9:38262018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tauchi Y, Tanaka H, Kumamoto K, Tokumoto
M, Sakimura C, Sakurai K, Kimura K, Toyokawa T, Amano R, Kubo N, et
al: Tumor-associated macrophages induce capillary morphogenesis of
lymphatic endothelial cells derived from human gastric cancer.
Cancer Sci. 107:1101–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kabasawa T, Ohe R, Aung NY, Urano Y,
Kitaoka T, Tamazawa N, Utsunomiya A and Yamakawa M: Potential role
of M2 TAMs around lymphatic vessels during lymphatic invasion in
papillary thyroid carcinoma. Sci Rep. 11:11502021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen XJ, Wei WF, Wang ZC, Wang N, Guo CH,
Zhou CF, Liang LJ, Wu S, Liang L and Wang W: A novel lymphatic
pattern promotes metastasis of cervical cancer in a hypoxic
tumour-associated macrophage-dependent manner. Angiogenesis.
24:549–565. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ding M, Fu X, Tan H, Wang R, Chen Z and
Ding S: The effect of vascular endothelial growth factor C
expression in tumor-associated macrophages on lymphangiogenesis and
lymphatic metastasis in breast cancer. Mol Med Rep. 6:1023–1029.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ran S and Wilber A: Novel role of immature
myeloid cells in formation of new lymphatic vessels associated with
inflammation and tumors. J Leukoc Biol. 102:253–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ran S and Volk-Draper L: Lymphatic
endothelial cell progenitors in the tumor microenvironment. Adv Exp
Med Biol. 1234:87–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ran S and Montgomery KE:
Macrophage-mediated lymphangiogenesis: The emerging role of
macrophages as lymphatic endothelial progenitors. Cancers (Basel).
4:618–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Volk-Draper L, Patel R, Bhattarai N, Yang
J, Wilber A, DeNardo D and Ran S: Myeloid-derived lymphatic
endothelial cell progenitors significantly contribute to lymphatic
metastasis in clinical breast cancer. Am J Pathol. 189:2269–2292.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gordon EJ, Rao S, Pollard JW, Nutt SL,
Lang RA and Harvey NL: Macrophages define dermal lymphatic vessel
calibre during development by regulating lymphatic endothelial cell
proliferation. Development. 137:3899–3910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dannenmann SR, Thielicke J, Stöckli M,
Matter C, von Boehmer L, Cecconi V, Hermanns T, Hefermehl L,
Schraml P, Moch H, et al: Tumor-associated macrophages subvert
T-cell function and correlate with reduced survival in clear cell
renal cell carcinoma. Oncoimmunology. 2:e235622013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
He Z and Zhang S: Tumor-associated
macrophages and their functional transformation in the hypoxic
tumor microenvironment. Front Immunol. 12:7413052021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhang L, Zhang K, Zhang J, Zhu J, Xi Q,
Wang H, Zhang Z, Cheng Y, Yang G, Liu H, et al: Loss of fragile
site-associated tumor suppressor promotes antitumor immunity via
macrophage polarization. Nat Commun. 12:43002021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang W, Marinis JM, Beal AM, Savadkar S,
Wu Y, Khan M, Taunk PS, Wu N, Su W, Wu J, et al: RIP1 kinase drives
macrophage-mediated adaptive immune tolerance in pancreatic cancer.
Cancer Cell. 34:757–774.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ho TTB, Nasti A, Seki A, Komura T, Inui H,
Kozaka T, Kitamura Y, Shiba K, Yamashita T, Yamashita T, et al:
Combination of gemcitabine and anti-PD-1 antibody enhances the
anticancer effect of M1 macrophages and the Th1 response in a
murine model of pancreatic cancer liver metastasis. J Immunother
Cancer. 8:e0013672020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Klug F, Prakash H, Huber PE, Seibel T,
Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et
al: Low-dose irradiation programs macrophage differentiation to an
iNOS+/M1 phenotype that orchestrates effective T cell
immunotherapy. Cancer Cell. 24:589–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Shiao SL, Ruffell B, DeNardo DG, Faddegon
BA, Park CC and Coussens LM: TH2-polarized CD4(+) T cells and
macrophages limit efficacy of radiotherapy. Cancer Immunol Res.
3:518–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
DeNardo DG, Barreto JB, Andreu P, Vasquez
L, Tawfik D, Kolhatkar N and Coussens LM: CD4(+) T cells regulate
pulmonary metastasis of mammary carcinomas by enhancing protumor
properties of macrophages. Cancer Cell. 16:91–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yasuda K, Takeuchi Y and Hirota K: The
pathogenicity of Th17 cells in autoimmune diseases. Semin
Immunopathol. 41:283–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chang SH: T helper 17 (Th17) cells and
interleukin-17 (IL-17) in cancer. Arch Pharm Res. 42:549–559. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kryczek I, Banerjee M, Cheng P, Vatan L,
Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et
al: Phenotype, distribution, generation, and functional and
clinical relevance of Th17 cells in the human tumor environments.
Blood. 114:1141–1149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhou J, Li X, Wu X, Zhang T, Zhu Q and
Wang X, Wang H, Wang K, Lin Y and Wang X: Exosomes released from
tumor-associated macrophages transfer miRNAs that induce a
Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer
Immunol Res. 6:1578–1592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yang JY, Jie Z, Mathews A, Zhou X, Li Y,
Gu M, Xie X, Ko CJ, Cheng X, Qi Y, et al: Intestinal epithelial
tbk1 prevents differentiation of T-helper 17 cells and
tumorigenesis in mice. Gastroenterology. 159:1793–1806. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Maruyama T, Kono K, Mizukami Y, Kawaguchi
Y, Mimura K, Watanabe M, Izawa S and Fujii H: Distribution of Th17
cells and FoxP3(+) regulatory T cells in tumor-infiltrating
lymphocytes, tumor-draining lymph nodes and peripheral blood
lymphocytes in patients with gastric cancer. Cancer Sci.
101:1947–1954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Knochelmann HM, Dwyer CJ, Bailey SR, Amaya
SM, Elston DM, Mazza-McCrann JM and Paulos CM: When worlds collide:
Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol.
15:458–469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ohue Y and Nishikawa H: Regulatory T
(Treg) cells in cancer: Can Treg cells be a new therapeutic target?
Cancer Sci. 110:2080–2089. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Whiteside TL: What are regulatory T cells
(Treg) regulating in cancer and why? Semin Cancer Biol. 22:327–334.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Tanaka A and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Cell Res. 27:109–118. 2017.
View Article : Google Scholar :
|
|
134
|
Waniczek D, Lorenc Z, Śnietura M, Wesecki
M, Kopec A and Muc-Wierzgoń M: Tumor-associated macrophages and
regulatory T cells infiltration and the clinical outcome in
colorectal cancer. Arch Immunol Ther Exp (Warsz). 65:445–454. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
La Fleur L, Botling J, He F, Pelicano C,
Zhou C, He C, Palano G, Mezheyeuski A, Micke P, Ravetch JV, et al:
Targeting MARCO and IL37R on immunosuppressive macrophages in lung
cancer blocks regulatory T cells and supports cytotoxic lymphocyte
function. Cancer Res. 81:956–967. 2021. View Article : Google Scholar
|
|
136
|
Zhu Q, Wu X, Wu Y and Wang X: Interaction
between Treg cells and tumor-associated macrophages in the tumor
microenvironment of epithelial ovarian cancer. Oncol Rep.
36:3472–3478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wu Q, Zhou W, Yin S, Zhou Y, Chen T, Qian
J, Su R, Hong L, Lu H, Zhang F, et al: Blocking triggering receptor
expressed on myeloid cells-1-positive tumor-associated macrophages
induced by hypoxia reverses immunosuppression and anti-programmed
cell death ligand 1 resistance in liver cancer. Hepatology.
70:198–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Fujimura T, Kambayashi Y, Fujisawa Y,
Hidaka T and Aiba S: Tumor-associated macrophages: Therapeutic
targets for skin cancer. Front Oncol. 8:32018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Furudate S, Fujimura T, Kambayashi Y,
Kakizaki A, Hidaka T and Aiba S: Immunomodulatory effect of
imiquimod through CCL22 produced by tumor-associated macrophages in
B16F10 melanomas. Anticancer Res. 37:3461–3471. 2017.PubMed/NCBI
|
|
140
|
Wang D, Yang L, Yue D, Cao L, Li L, Wang
D, Ping Y, Shen Z, Zheng Y, Wang L and Zhang Y: Macrophage-derived
CCL22 promotes an immunosuppressive tumor microenvironment via IL-8
in malignant pleural effusion. Cancer Lett. 452:244–253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Carbó JM, León TE, Font-Díaz J, De la Rosa
JV, Castrillo A, Picard FR, Staudenraus D, Huber M, Cedó L,
Escolà-Gil JC, et al: Pharmacologic activation of LXR alters the
expression profile of tumor-associated macrophages and the
abundance of regulatory T cells in the tumor microenvironment.
Cancer Res. 81:968–985. 2021. View Article : Google Scholar
|
|
142
|
Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng
Q and Wang H, Chen J and Wang H: Tumor-associated macrophages
recruit CCR6+ regulatory T cells and promote the development of
colorectal cancer via enhancing CCL20 production in mice. PLoS One.
6:e194952011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Yang S, Wang B, Guan C, Wu B, Cai C, Wang
M, Zhang B, Liu T and Yang P: Foxp3+IL-17+ T cells promote
development of cancer-initiating cells in colorectal cancer. J
Leukoc Biol. 89:85–91. 2011. View Article : Google Scholar
|
|
144
|
Liu C, Chikina M, Deshpande R, Menk AV,
Wang T, Tabib T, Brunazzi EA, Vignali KM, Sun M, Stolz DB, et al:
Treg cells promote the SREBP1-dependent metabolic fitness of
tumor-promoting macrophages via repression of CD8+ T
cell-derived interferon-γ. Immunity. 51:381–397.e6. 2019.
View Article : Google Scholar
|
|
145
|
Fu Q, Xu L, Wang Y, Jiang Q, Liu Z, Zhang
J, Zhou Q, Zeng H, Tong S, Wang T, et al: Tumor-associated
macrophage-derived Interleukin-23 interlinks kidney cancer
glutamine addiction with immune evasion. Eur Urol. 75:752–763.
2019. View Article : Google Scholar
|
|
146
|
Sun W, Wei FQ, Li WJ, Wei JW, Zhong H, Wen
YH, Lei WB, Chen L, Li H, Lin HQ, et al: A positive-feedback loop
between tumour infiltrating activated Treg cells and type 2-skewed
macrophages is essential for progression of laryngeal squamous cell
carcinoma. Br J Cancer. 117:1631–1643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Campesato LF, Budhu S, Tchaicha J, Weng
CH, Gigoux M, Cohen IJ, Redmond D, Mangarin L, Pourpe S, Liu C, et
al: Blockade of the AHR restricts a Treg-macrophage suppressive
axis induced by L-Kynurenine. Nat Commun. 11:40112020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Raskov H, Orhan A, Christensen JP and
Gögenur I: Cytotoxic CD8+ T cells in cancer and cancer
immunotherapy. Br J Cancer. 124:359–367. 2021. View Article : Google Scholar
|
|
149
|
Reiser J and Banerjee A: Effector, memory,
and dysfunctional CD8(+) T cell fates in the antitumor immune
response. J Immunol Res. 2016:89412602016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Peranzoni E, Lemoine J, Vimeux L, Feuillet
V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J,
Ouakrim H, et al: Macrophages impede CD8 T cells from reaching
tumor cells and limit the efficacy of anti-PD-1 treatment. Proc
Natl Acad Sci USA. 115:E4041–E4050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Garrido-Martin EM, Mellows TWP, Clarke J,
Ganesan AP, Wood O, Cazaly A, Seumois G, Chee SJ, Alzetani A, King
EV, et al: M1hot tumor-associated macrophages boost
tissue-resident memory T cells infiltration and survival in human
lung cancer. J Immunother Cancer. 8:e0007782020. View Article : Google Scholar
|
|
152
|
Dai X, Lu L, Deng S, Meng J, Wan C, Huang
J, Sun Y, Hu Y, Wu B, Wu G, et al: USP7 targeting modulates
anti-tumor immune response by reprogramming tumor-associated
macrophages in lung cancer. Theranostics. 10:9332–9347. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Casanova-Acebes M, Dalla E, Leader AM,
LeBerichel J, Nikolic J, Morales BM, Brown M, Chang C, Troncoso L,
Chen ST, et al: Tissue-resident macrophages provide a
pro-tumorigenic niche to early NSCLC cells. Nature. 595:578–584.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y,
Wang F, Duan X, Li J, Zhang W and Wang H: A natural CCR2 antagonist
relieves tumor-associated macrophage-mediated immunosuppression to
produce a therapeutic effect for liver cancer. EBioMedicine.
22:58–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Dolina JS, Van Braeckel-Budimir N, Thomas
GD and Salek-Ardakani S: CD8+ T cell exhaustion in
cancer. Front Immunol. 12:7152342021. View Article : Google Scholar
|
|
156
|
Dangaj D, Bruand M, Grimm AJ, Ronet C,
Barras D, Duttagupta PA, Lanitis E, Duraiswamy J, Tanyi JL,
Benencia F, et al: Cooperation between constitutive and inducible
chemokines enables T cell engraftment and immune attack in solid
tumors. Cancer Cell. 35:885–900.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
House IG, Savas P, Lai J, Chen AXY, Oliver
AJ, Teo ZL, Todd KL, Henderson MA, Giuffrida L, Petley EV, et al:
Macrophage-derived CXCL9 and CXCL10 are required for antitumor
immune responses following immune checkpoint blockade. Clin Cancer
Res. 26:487–504. 2020. View Article : Google Scholar
|
|
158
|
Petty AJ, Li A, Wang X, Dai R, Heyman B,
Hsu D, Huang X and Yang Y: Hedgehog signaling promotes
tumor-associated macrophage polarization to suppress intratumoral
CD8+ T cell recruitment. J Clin Invest. 129:5151–5162. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pascual-García M, Bonfill-Teixidor E,
Planas-Rigol E, Rubio-Perez C, Iurlaro R, Arias A, Cuartas I,
Sala-Hojman A, Escudero L, Martínez-Ricarte F, et al: LIF regulates
CXCL9 in tumor-associated macrophages and prevents CD8+
T cell tumor-infiltration impairing anti-PD1 therapy. Nat Commun.
10:24162019. View Article : Google Scholar
|
|
160
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al: Liver
metastasis restrains immunotherapy efficacy via macrophage-mediated
T cell elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Kusmartsev S and Gabrilovich DI: STAT1
signaling regulates tumor-associated macrophage-mediated T cell
deletion. J Immunol. 174:4880–4891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Stanley ER and Chitu V: CSF-1 receptor
signaling in myeloid cells. Cold Spring Harb Perspect Biol.
6:a0218572014. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Mehta AK, Cheney EM, Hartl CA, Pantelidou
C, Oliwa M, Castrillon JA, Lin JR, Hurst KE, de Oliveira Taveira M,
Johnson NT, et al: Targeting immunosuppressive macrophages
overcomes PARP inhibitor resistance in BRCA1-associated
triple-negative breast cancer. Nat Cancer. 2:66–82. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Xiang W, Shi R, Kang X, Zhang X, Chen P,
Zhang L, Hou A, Wang R, Zhao Y, Zhao K, et al: Monoacylglycerol
lipase regulates cannabinoid receptor 2-dependent macrophage
activation and cancer progression. Nat Commun. 9:25742018.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Chen EP, Markosyan N, Connolly E, Lawson
JA, Li X, Grant GR, Grosser T, FitzGerald GA and Smyth EM: Myeloid
cell COX-2 deletion reduces mammary tumor growth through enhanced
cytotoxic T-lymphocyte function. Carcinogenesis. 35:1788–1797.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Li L, Han L, Sun F, Zhou J, Ohaegbulam KC,
Tang X, Zang X, Steinbrecher KA, Qu Z and Xiao G: NF-κB RelA
renders tumor-associated macrophages resistant to and capable of
directly suppressing CD8+ T cells for tumor promotion.
Oncoimmunology. 7:e14352502018. View Article : Google Scholar
|
|
168
|
Kaneda MM, Messer KS, Ralainirina N, Li H,
Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P,
et al: PI3Kγ is a molecular switch that controls immune
suppression. Nature. 539:437–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
McIntire RH and Hunt JS: Antigen
presenting cells and HLA-G-a review. Placenta. 26(Suppl A):
S104–S109. 2005. View Article : Google Scholar
|
|
170
|
Nixon BG, Kuo F, Liu M, Capistrano K, Do
M, Franklin RA, Wu X, Kansler ER, Srivastava RM, Purohit TA, et al:
IRF8 governs tumor-associated macrophage control of T cell
exhaustion. bioRxiv. 2020.2003.2012.989731. 2020.
|
|
171
|
Tseng D, Volkmer JP, Willingham SB,
Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA,
Weiskopf K, Miyanishi M and Weissman IL: Anti-CD47
antibody-mediated phagocytosis of cancer by macrophages primes an
effective antitumor T-cell response. Proc Natl Acad Sci USA.
110:11103–11108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ruffell B, Chang-Strachan D, Chan V,
Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS and
Coussens LM: Macrophage IL-10 blocks CD8+ T cell-dependent
responses to chemotherapy by suppressing IL-12 expression in
intratumoral dendritic cells. Cancer Cell. 26:623–637. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Fang W, Zhou T, Shi H, Yao M, Zhang D,
Qian H, Zeng Q, Wang Y, Jin F, Chai C and Chen T: Progranulin
induces immune escape in breast cancer via up-regulating PD-L1
expression on tumor-associated macrophages (TAMs) and promoting
CD8+ T cell exclusion. J Exp Clin Cancer Res. 40:42021.
View Article : Google Scholar
|
|
174
|
Lin H, Wei S, Hurt EM, Green MD, Zhao L,
Vatan L, Szeliga W, Herbst R, Harms PW, Fecher LA, et al: Host
expression of PD-L1 determines efficacy of PD-L1 pathway
blockade-mediated tumor regression. J Clin Invest. 128:805–815.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Chow MT, Ozga AJ, Servis RL, Frederick DT,
Lo JA, Fisher DE, Freeman GJ, Boland GM and Luster AD: Intratumoral
activity of the CXCR3 chemokine system is required for the efficacy
of anti-PD-1 therapy. Immunity. 50:1498–1512.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Lee AH, Sun L, Mochizuki AY, Reynoso JG,
Orpilla J, Chow F, Kienzler JC, Everson RG, Nathanson DA, Bensinger
SJ, et al: Neoadjuvant PD-1 blockade induces T cell and cDC1
activation but fails to overcome the immunosuppressive tumor
associated macrophages in recurrent glioblastoma. Nat Commun.
12:69382021. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Tsou P, Katayama H, Ostrin EJ and Hanash
SM: The emerging role of B cells in tumor immunity. Cancer Res.
76:5597–5601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Nelson BH: CD20+ B cells: The other
tumor-infiltrating lymphocytes. J Immunol. 185:4977–4982. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Gunderson AJ, Kaneda MM, Tsujikawa T,
Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt
M, Olson P, et al: Bruton tyrosine kinase-dependent immune cell
cross-talk drives pancreas cancer. Cancer Discov. 6:270–285. 2016.
View Article : Google Scholar
|
|
180
|
Fremd C, Schuetz F, Sohn C, Beckhove P and
Domschke C: B cell-regulated immune responses in tumor models and
cancer patients. Oncoimmunology. 2:e254432013. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wang SS, Liu W, Ly D, Xu H, Qu L and Zhang
L: Tumor-infiltrating B cells: Their role and application in
anti-tumor immunity in lung cancer. Cell Mol Immunol. 16:6–18.
2019. View Article : Google Scholar :
|
|
182
|
Audzevich T, Bashford-Rogers R, Mabbott
NA, Frampton D, Freeman TC, Potocnik A, Kellam P and Gilroy DW:
Pre/pro-B cells generate macrophage populations during homeostasis
and inflammation. Proc Natl Acad Sci USA. 114:E3954–E3963. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Fridman WH, Petitprez F, Meylan M, Chen
TW, Sun CM, Roumenina LT and Sautès-Fridman C: B cells and cancer:
To B or not to B? J Exp Med. 218:e202008512021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Gonzalez H, Hagerling C and Werb Z: Roles
of the immune system in cancer: From tumor initiation to metastatic
progression. Genes Dev. 32:1267–1284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Michel T, Hentges F and Zimmer J:
Consequences of the cross-talk between monocytes/macrophages and
natural killer cells. Front Immunol. 3:4032013. View Article : Google Scholar
|
|
186
|
Sivori S, Pende D, Quatrini L, Pietra G,
Della Chiesa M, Vacca P, Tumino N, Moretta F, Mingari MC, Locatelli
F and Moretta L: NK cells and ILCs in tumor immunotherapy. Mol
Aspects Med. 80:1008702021. View Article : Google Scholar
|
|
187
|
Krneta T, Gillgrass A, Poznanski S, Chew
M, Lee AJ, Kolb M and Ashkar AA: M2-polarized and tumor-associated
macrophages alter NK cell phenotype and function in a
contact-dependent manner. J Leukoc Biol. 101:285–295. 2017.
View Article : Google Scholar
|
|
188
|
Platonova S, Cherfils-Vicini J, Damotte D,
Crozet L, Vieillard V, Validire P, André P, Dieu-Nosjean MC,
Alifano M, Régnard JF, et al: Profound coordinated alterations of
intratumoral NK cell phenotype and function in lung carcinoma.
Cancer Res. 71:5412–5422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Kloss M, Decker P, Baltz KM, Baessler T,
Jung G, Rammensee HG, Steinle A, Krusch M and Salih HR: Interaction
of monocytes with NK cells upon Toll-like receptor-induced
expression of the NKG2D ligand MICA. J Immunol. 181:6711–6719.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Peng LS, Zhang JY, Teng YS, Zhao YL, Wang
TT, Mao FY, Lv YP, Cheng P, Li WH, Chen N, et al: Tumor-associated
monocytes/macrophages impair NK-cell function via TGFβ1 in human
gastric cancer. Cancer Immunol Res. 5:248–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Castriconi R, Cantoni C, Della Chiesa M,
Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L
and Moretta A: Transforming growth factor beta 1 inhibits
expression of NKp30 and NKG2D receptors: Consequences for the
NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA.
100:4120–4125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Gallazzi M, Baci D, Mortara L, Bosi A,
Buono G, Naselli A, Guarneri A, Dehò F, Capogrosso P, Albini A, et
al: Prostate cancer peripheral blood NK cells show enhanced CD9,
CD49a, CXCR4, CXCL8, MMP-9 production and secrete
monocyte-recruiting and polarizing factors. Front Immunol.
11:5861262021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Malekghasemi S, Majidi J, Baghbanzadeh A,
Abdolalizadeh J, Baradaran B and Aghebati-Maleki L:
Tumor-associated macrophages: Protumoral macrophages in
inflammatory tumor microenvironment. Adv Pharm Bull. 10:556–565.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Xu B, Chen L, Li J, Zheng X, Shi L, Wu C
and Jiang J: Prognostic value of tumor infiltrating NK cells and
macrophages in stage II+III esophageal cancer patients. Oncotarget.
7:74904–74916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Eisinger S, Sarhan D, Boura VF,
Ibarlucea-Benitez I, Tyystjärvi S, Oliynyk G, Arsenian-Henriksson
M, Lane D, Wikström SL, Kiessling R, et al: Targeting a scavenger
receptor on tumor-associated macrophages activates tumor cell
killing by natural killer cells. Proc Natl Acad Sci USA.
117:32005–32016. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Bellora F, Castriconi R, Dondero A,
Pessino A, Nencioni A, Liggieri G, Moretta L, Mantovani A, Moretta
A and Bottino C: TLR activation of tumor-associated macrophages
from ovarian cancer patients triggers cytolytic activity of NK
cells. Eur J Immunol. 44:1814–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Bellora F, Castriconi R, Dondero A,
Reggiardo G, Moretta L, Mantovani A, Moretta A and Bottino C: The
interaction of human natural killer cells with either unpolarized
or polarized macrophages results in different functional outcomes.
Proc Natl Acad Sci USA. 107:21659–21664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Dalbeth N, Gundle R, Davies RJO, Lee YCG,
McMichael AJ and Callan MFC: CD56bright NK cells are enriched at
inflammatory sites and can engage with monocytes in a reciprocal
program of activation. J Immunol. 173:6418–6426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
O'Sullivan T, Saddawi-Konefka R, Vermi W,
Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF,
Teng MW, et al: Cancer immunoediting by the innate immune system in
the absence of adaptive immunity. J Exp Med. 209:1869–1882. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Ponzetta A, Carriero R, Carnevale S,
Barbagallo M, Molgora M, Perucchini C, Magrini E, Gianni F,
Kunderfranco P, Polentarutti N, et al: Neutrophils driving
unconventional T cells mediate resistance against murine sarcomas
and selected human tumors. Cell. 178:346–360.e24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Wu L and Zhang XH: Tumor-associated
neutrophils and macrophages-heterogenous but not chaotic. Front
Immunol. 11:5539672020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z,
Chen EB, Fan J, Cao Y, Dai Z and Zhou J: Tumor-associated
neutrophils recruit macrophages and T-regulatory cells to promote
progression of hepatocellular carcinoma and resistance to
sorafenib. Gastroenterology. 150:1646–1658.e17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Zilionis R, Engblom C, Pfirschke C, Savova
V, Zemmour D, Saatcioglu HD, Krishnan I, Maroni G, Meyerovitz CV,
Kerwin CM, et al: Single-cell transcriptomics of human and mouse
lung cancers reveals conserved myeloid populations across
individuals and species. Immunity. 50:1317–1334.e10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H,
Luo C, Zhou J, Fan J and Zhou S: Tumor-associated neutrophils and
macrophages interaction contributes to intrahepatic
cholangiocarcinoma progression by activating STAT3. J Immunother
Cancer. 9:e0019462021. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Gunaydin G: CAFs interacting with TAMs in
tumor microenvironment to enhance tumorigenesis and immune evasion.
Front Oncol. 11:6683492021. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Sweeny L, Liu Z, Lancaster W, Hart J,
Hartman YE and Rosenthal EL: Inhibition of fibroblasts reduced head
and neck cancer growth by targeting fibroblast growth factor
receptor. Laryngoscope. 122:1539–1544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Vu HN, Miller WJ, O'Connor SA, He M,
Schafer PH, Payvandi F, Muller GW, Stirling DI and Libutti SK:
CC-5079: A small molecule with MKP1, antiangiogenic, and antitumor
activity. J Surg Res. 164:116–125. 2010. View Article : Google Scholar
|
|
209
|
Donthireddy L, Vonteddu P, Murthy T, Kwak
T, Eraslan RN, Podojil JR, Elhofy A, Boyne MT II, Puisis JJ, Veglia
F, et al: ONP-302 nanoparticles inhibit tumor growth by altering
tumor-associated macrophages and cancer-associated fibroblasts. J
Cancer. 13:1933–1944. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kashyap AS, Schmittnaegel M, Rigamonti N,
Pais-Ferreira D, Mueller P, Buchi M, Ooi CH, Kreuzaler M,
Hirschmann P, Guichard A, et al: Optimized antiangiogenic
reprogramming of the tumor microenvironment potentiates CD40
immunotherapy. Proc Natl Acad Sci USA. 117:541–551. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Zhang L, Qi Y, Min H, Ni C, Wang F, Wang
B, Qin H, Zhang Y, Liu G, Qin Y, et al: Cooperatively responsive
peptide nanotherapeutic that regulates angiopoietin receptor Tie2
activity in tumor microenvironment to prevent breast tumor relapse
after chemotherapy. ACS Nano. 13:5091–5102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Alam A, Blanc I, Gueguen-Dorbes G, Duclos
O, Bonnin J, Barron P, Laplace MC, Morin G, Gaujarengues F, Dol F,
et al: SAR131675, a potent and selective VEGFR-3-TK inhibitor with
antilymphangiogenic, antitumoral, and antimetastatic activities.
Mol Cancer Ther. 11:1637–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Gyori D, Lim EL, Grant FM, Spensberger D,
Roychoudhuri R, Shuttleworth SJ, Okkenhaug K, Stephens LR and
Hawkins PT: Compensation between CSF1R+ macrophages and Foxp3+ Treg
cells drives resistance to tumor immunotherapy. JCI Insight.
3:e1206312018. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Oweida AJ, Darragh L, Phan A, Binder D,
Bhatia S, Mueller A, Court BV, Milner D, Raben D, Woessner R, et
al: STAT3 modulation of regulatory T cells in response to radiation
therapy in head and neck cancer. J Natl Cancer Inst. 111:1339–1349.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM,
West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC and
DeNardo DG: CSF1/CSF1R blockade reprograms tumor-infiltrating
macrophages and improves response to T-cell checkpoint
immunotherapy in pancreatic cancer models. Cancer Res.
74:5057–5069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Sluijter M, van der Sluis TC, van der
Velden PA, Versluis M, West BL, van der Burg SH and van Hall T:
Inhibition of CSF-1R supports T-cell mediated melanoma therapy.
PLoS One. 9:e1042302014. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Doleschel D, Hoff S, Koletnik S, Rix A,
Zopf D, Kiessling F and Lederle W: Regorafenib enhances anti-PD1
immunotherapy efficacy in murine colorectal cancers and their
combination prevents tumor regrowth. J Exp Clin Cancer Res.
40:2882021. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Zhang JQ, Zeng S, Vitiello GA, Seifert AM,
Medina BD, Beckman MJ, Loo JK, Santamaria-Barria J, Maltbaek JH,
Param NJ, et al: Macrophages and CD8+ T cells mediate
the antitumor efficacy of combined CD40 ligation and imatinib
therapy in gastrointestinal stromal tumors. Cancer Immunol Res.
6:434–447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Zhang M, Wen B, Anton OM, Yao Z, Dubois S,
Ju W, Sato N, DiLillo DJ, Bamford RN, Ravetch JV and Waldmann TA:
IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by
NK cells and macrophages. Proc Natl Acad Sci USA.
115:E10915–E10924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Jarosz-Biej M, Kamińska N, Matuszczak S,
Cichoń T, Pamuła-Piłat J, Czapla J, Smolarczyk R, Skwarzyńska D,
Kulik K and Szala S: M1-like macrophages change tumor blood vessels
and microenvironment in murine melanoma. PLoS One. 13:e01910122018.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Gaggero S, Witt K, Carlsten M and Mitra S:
Cytokines orchestrating the natural killer-myeloid cell crosstalk
in the tumor microenvironment: implications for natural killer
cell-based cancer immunotherapy. Front Immunol. 11:6212252021.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Zhou Z, Zhang C, Zhang J and Tian Z:
Macrophages help NK cells to attack tumor cells by stimulatory
NKG2D ligand but protect themselves from NK killing by inhibitory
ligand Qa-1. PLoS One. 7:e369282012. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Wang C, Cui A, Bukenya M, Aung A, Pradhan
D, Whittaker CA, Agarwal Y, Thomas A, Liang S, Amlashi P, et al:
Reprogramming NK cells and macrophages via combined antibody and
cytokine therapy primes tumors for elimination by checkpoint
blockade. Cell Rep. 37:1100212021. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Nywening TM, Belt BA, Cullinan DR, Panni
RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE,
et al: Targeting both tumour-associated CXCR2+
neutrophils and CCR2+ macrophages disrupts myeloid
recruitment and improves chemotherapeutic responses in pancreatic
ductal adenocarcinoma. Gut. 67:1112–1123. 2018. View Article : Google Scholar
|
|
225
|
Li X, Yao W, Yuan Y, Chen P, Li B, Li J,
Chu R, Song H, Xie D, Jiang X and Wang H: Targeting of
tumour-infiltrating macrophages via CCL2/CCR2 signalling as a
therapeutic strategy against hepatocellular carcinoma. Gut.
66:157–167. 2017. View Article : Google Scholar
|
|
226
|
Veillette A and Tang Z: Signaling
regulatory protein (SIRP) α-CD47 blockade joins the ranks of immune
checkpoint inhibition. J Clin Oncol. 37:1012–1014. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Wesolowski R, Sharma N, Reebel L, Rodal
MB, Peck A, West BL, Marimuthu A, Severson P, Karlin DA, Dowlati A,
et al: Phase Ib study of the combination of pexidartinib (PLX3397),
a CSF-1R inhibitor, and paclitaxel in patients with advanced solid
tumors. Ther Adv Med Oncol. 11:17588359198542382019. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q,
Deng S and Zhou H: Signaling pathways in cancer-associated
fibroblasts and targeted therapy for cancer. Signal Transduct
Target Ther. 6:2182021. View Article : Google Scholar : PubMed/NCBI
|