Introduction
Breast cancer (BC) frequently metastasizes to bone
(1), inducing bone pain (BP)
(2). BC-BP disrupts the quality of
life and limits physical and mental activity of patients with BC,
resulting in poor outcomes (3).
Proper and satisfactory management of BP is therefore an important
challenge for the improvement of quality of life and survival of
these patients. The pathophysiology of BC-BP, however, remains
poorly understood.
BC metastasized to bone produces increased amounts
of various growth factors, cytokines and chemokines, such as TGF-β
and insulin-like growth factor, that promote tumor colonization in
bone and osteoclastic bone destruction, leading to the progression
of bone metastasis (4,5). Of note, recent studies have shown
that bone metastatic BC cells also secrete large amounts of lactic
acid and protons via the plasma membrane pH regulators, including
monocarboxylate transporter (MCT)1 and MCT4, carbonic anhydrases
and vacuolar type ATPase (6),
creating an acidic tumor microenvironment (7,8). It
has been reported that under the acidic tumor microenvironment, the
acid-sensing nociceptors, such as transient receptor potential
vanilloid 1 and acid-sensing ion channel 3 of the sensory neurons
are activated by protons, exciting sensory neurons and inducing
bone pain (8-10). On the other hand, the role of
lactate in the activation of sensory neurons and induction of bone
pain is still unclear.
Lactate has long been considered as a waste product
of cellular metabolism in anaerobic conditions. However, data have
shown that lactate plays an important role in brain, skeletal
muscle and cancer as an energy source, a gluconeogenic precursor
and a signaling molecule (11). Of
relevance, lactate is shown to fulfil the neuronal energy needs and
provide signals to regulate neuronal functions, including
excitability, plasticity and memory consolidation in the central
nervous system (12). Therefore,
we hypothesized that lactate also contributes to the induction of
BC-BP by exciting the peripheral sensory nerves.
MCT4 is one of the key transporters involved in the
regulation of intracellular translocation and extracellular
secretion of lactate and protons (9) and shown to predict poor outcome in
patients with triple-negative BC (13). The aim of the present study was to
determine the effects of silencing MCT4 expression on the
excitation of sensory neurons and induction of BC-BP. Furthermore,
the role of G-protein-coupled receptor 81 (GPR81), which is
recently identified as a lactate receptor (14) that regulates the neuronal network
and activity (15), in the
excitation of the sensory neurons was determined. The present
findings demonstrated that lactate released from BC via MCT4
activates neuronal GPR81 to excite sensory neurons, leading to the
induction of BC-BP.
Materials and methods
Tissue microarrays and
immunohistochemical analysis
MCT4 expression in specimens of human BC and normal
breast tissue was determined using tissue microarrays (cat. no.
BC081116d; TissueArray.Com LLC, Rockville, MD,
USA). Normal and breast cancer tissue were from different
individuals. Slides were deparaffinized in xylene twice for 5 min
each time, and rehydrated in 100 (twice), 95, 70 and 50% alcohol
for 3 min each. Antigen retrieval was performed by heating in, 10
mM citric acid solution at 95°C for 15 min. The slides were blocked
with 3% BSA (cat. no. 011-27055; FUJIFILM Wako Pure Chemical
Corporation) for 30 min at room temperature. For
immunohistochemical analysis, specimens were incubated with
anti-MCT4 antibody (1:100; rabbit polyclonal; cat. no. sc-50329;
Santa Cruz Biotechnology, Inc.) overnight at 4°C, followed by
treatment with streptavidin-biotin complex (1:100; cat. no.
K400311-2; EnVision System HRP-labeled polymer; Dako; Agilent
Technologies, Inc.) for 60 min at room temperature and visualized
with the use of a diaminobenzidine (DAB) substrate-chromogen
solution (DakoCytomation Liquid DAB Substrate Chromogen System;
Dako; Agilent Technologies, Inc.). Counterstaining with hematoxylin
solution at room temperature for 10 sec was performed.
Immunohistochemical staining positive area (%) was determined by
calculating the positive area/total area ratio. Images were
captured and quantification was performed using a hybrid cell count
system (light microscope mode of BZ-X800 multiple analyzer BZ-H3A;
Keyence Corporation) and ImageJ (Fiji) software (National
Institutes of Health).
Ethics approval was not required for this research
involving the use of human tissue microarray, due to the fact that
this particular research fits into Section C, Paragraph 1, Part 3,
Chapter 1 of Ethical Guidelines for Medical and Biological Research
Involving Human Subjects issued by the Ministry of Education,
Culture, Sports, Science and Technology, Japan. A waiver of ethics
approval was provided by the Okayama University Ethics Committee
(Okayama, Japan).
Reagents
Puromycin dihydrochloride (cat. no. sc-108071),
control short hairpin (sh)RNA plasmid-A (cat. no. sc-108060), MCT4
shRNA lentivirus particles (mouse, cat. no. sc-40120-V; human, cat.
no. sc-45892-V), anti-MCT4 antibody (rabbit polyclonal; cat. no.
sc-50329) and anti-MCT1 antibody (mouse monoclonal; cat. no.
sc-365501) were purchased from Santa Cruz Biotechnology, Inc.
Anti-phosphorylated (p)-p44/42 MAPK antibody (pERK1/2; rabbit
monoclonal; cat. no. 4370), anti-p44/42 MAPK antibody (ERK1/2;
rabbit monoclonal; cat. no. 4695), anti-p-cAMP response element
binding protein (pCREB) antibody (rabbit monoclonal; cat. no.
9198), anti-CREB antibody (rabbit monoclonal; cat. no. 9197),
HRP-conjugated IgG antibody (goat anti-rabbit; cat. no. 7074),
HRP-conjugated IgG antibody (goat anti-mouse; cat. no. 7076) and
Alexa Fluor 488-conjugated IgG (H+L) F(ab')2 fragment
(goat anti-rabbit; cat. no. 4412) were purchased from Cell
Signaling Technology, Inc. Anti-calcitonin gene-related peptide
(CGRP) antibody (goat polyclonal; cat. no. ab36001) and Alexa Fluor
647-conjugated IgG H&L (donkey anti-goat; cat. no. ab150135)
were purchased from Abcam. Anti-GPR81 antibody (rabbit polyclonal;
cat. no. NLS2095) was purchased from Novus Biologicals, LLC.
Anti-β-actin antibody (rabbit polyclonal; cat. no. ab8227) was
purchased from Abcam and used as loading control.
Cell lines and culture conditions
The human BC cell lines MDA-MB-231 and MCF-7 and
mouse BC cell line 4T1 were obtained from the Japanese Collection
of Research Bioresources Cell Bank. Mouse BC cell line EO771 was
purchased from CH3 BioSystems. Rat dorsal root ganglion (DRG) cell
line F11 (a mouse N18TG2 neuroblastoma and rat DRG sensory neuron
hybrid cell line) (16) has been
maintained in TY's laboratory obtained from American Type Culture
Collection (17). All cells were
cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
10% heat-inactivated FBS (Biosera) and 1% penicillin-streptomycin
in an atmosphere of 5% CO2 at 37°C. All cell lines were
analyzed and authenticated by targeted genomic and RNA
sequencing.
Cell viability assay
BC cells were plated at 1×105 cells/well
(6-well plate), cultured for 48 h and the cell number was counted
using a TC20 automated cell counter (Bio-Rad Laboratories,
Inc.).
Determination of extracellular pH
(pHe)
EO771 cells (1×105; 96-well plate) were
cultured for 48 h in the presence of Adriamycin (cat. no. HY-15142;
MedChemExpress) (30 nmol/l) to prevent cell proliferation, and pHe
was measured using a FiveEasy pH meter (Mettler Toledo) immediately
after cell removal from CO2 incubator, as previously
described (9).
Determination of lactate
concentration
The lactate concentrations of the bone marrow fluids
of tibiae, BC cell (1×105) culture supernatants cultured
in 12-well plates for 48 h and cell lysates of sub-confluent
sh-MCT4 or sh-control EO771 cells seeded in 12-well plates for 24
h, were measured using a lactate assay kit (cat. no. ECLC-100;
EnzyChrom™; BioAssay Systems) according to the manufacturer's
instructions.
Intracellular Ca2+
mobilization
Intracellular calcium changes were determined as
previously described (9). In
brief, 1×103 F11 sensory neuron cells (seeded onto 35 mm
glass bottom dishes) were exposed to Fura-2AM at 3 mM (Invitrogen;
Thermo Fisher Scientific, Inc.) for 15 min at room temperature.
Intracellular calcium changes were monitored in a balanced salt
solution (BSS) composed of NaCl 140 mM, HEPES 10 mM, glucose 10 mM,
KCl 5 mM, CaCl2 2 mM and MgCl2 1 mM. Following Fura-2AM
exposure, the coverslips were rinsed a total of three times in
BSS.
Changes in intracellular Ca2+ of F11
cells after treatment with EO771 conditioned medium (CM; 30% v/v)
or 10 µM 3,5-dihydroxybenzoic acid (3,5-DHBA) (R&D
Systems, Inc.) or 20 mM lactic acid (FUJIFILM Wako Pure Chemical
Corporation) at room temperature were observed and recorded with
digital video microfluorometry for 5 min, 90 sec and 150 sec,
respectively, using an intensified CCD camera coupled to a Nikon
Eclipse TE2000U microscope with Nikon Elements® software
(Nikon Instruments Inc.). With the use of a 150 Watt xenon arc
lamp, the coverslips were illuminated, and the 340/380 nm
excitation wavelengths of Fura-2AM were selected by a Lambda DG-5
plus illumination system (Sutter Instrument Company). Prior to the
administration of CM, 3,5-DHBA or lactic acid, sterile BSS was
applied, and any cells showing a response to buffer alone were
excluded from data collection.
Silencing MCT4 expression in BC
cells
MDA-MB-231 and EO771 BC cells were infected with 1
µg control shRNA (cat. no. sc-108080-V; Santa Cruz
Biotechnology, Inc.) or MCT4 shRNA using a lentiviral particle
transduction system (multiplicity of infection, 2.0) (cat. nos.
sc-45892-V and sc-40120-V; Santa Cruz Biotechnology, Inc.) in the
presence of 5 µg/ml polybrene (sc-134220; Santa Cruz
Biotechnology, Inc) for 24 h. Subsequently, infected cells were
cultured in DMEM with 10% FBS for 7 days in the presence of 2
µg/ml puromycin to select cells stably expressing the
shRNAs, following which subsequent experiments were performed.
Santa Cruz Biotechnology shRNAs are provided as pools of 3 target
specific 19-25 nucleotide molecules designed to knock down
expression of specific genes of interest.
Silencing of GPR81 in sensory neuron
cells
F11 cells were infected with 1 µg of control
shRNA or GPR81 shRNA using a lentiviral particle transduction
system (multiplicity of infection, 2.0) (cat. nos. sc-108080-V and
sc-44644-V; Santa Cruz Biotechnology, Inc.) in the presence of 5
µg/ml polybrene for 24 h, and cultured in DMEM with 5% FBS
for 7 days in the presence of 4 µg/ml puromycin to select
cells stably expressing the shRNAs, following which subsequent
experiments were performed.
Western blot analysis
F11 cells were cultured with or without sh-control
or sh-MCT4 EO771 CM (30% v/v) at 37°C for 15min. sh-GPR81 F11 cells
were cultured with or without EO771 CM (30% v/v) at 37°C for 15
min. Proteins from each cell line (MDA-MB-231, EO771, MCF-7, 4T1
and F11) were obtained after lysis with RIPA lysis buffer (cat. no.
9803; Cell Signaling Technology, Inc.) and protein concentrations
were measured using BCA protein assay kit (cat. no. T9300A; Takara
Bio, Inc.). A total of 10 µg of protein from the cell
lysates were mixed with 4X Laemmli sample buffer (Bio-Rad
Laboratories, Inc.) and heated at 95°C for 5 min. The samples were
electrophoresed using 4-12% SDS-PAGE, and the proteins were
transferred onto PVDF membranes. Membranes were blocked with 5% BSA
for 60 min at room temperature. The membranes were incubated with
the following antibodies: Primary antibodies against MCT4
(1:1,000), pERK1/2 (1:1,000), ERK1/2 (1:1,000), CREB (1:1,000),
pCREB (1:1,000) and GPR81 (1:200) were incubated overnight at 4°C.
Secondary HRP-conjugated anti-rabbit (1:2,000) and HRP-conjugated
anti-mouse antibodies (1:2,000) were incubated for 60 min at room
temperature. An ECL reagent (cat. No. RPN2109; Amersham; Cytiva)
was used to detect secondary antibody binding. A ChemiDoc MP system
(Bio-Rad Laboratories, Inc.) and Image Lab software v6.1 (Bio-Rad
Laboratories, Inc.) were used for the analysis of blots. β-actin
(1:2,000) antibody was used as a protein loading control.
Animal model of BC-BP
All animal studies were approved by the
Institutional Animal Care and Use Committee at the Indiana
University School of Medicine (IACUC protocol no. 11170;
Indianapolis, USA) and conducted according to the ARRIVE
guidelines.
A mouse model of bone metastasis of human BC cells
in 5-week-old female BALB/c athymic nude mice (n=20) and mouse BC
cells in 5-week-old female C57BL/6 mice (n=20) was established
(n=5/group; mean body weight, 19.5 g) (Envigo). Mice were
maintained at temperature of 20°C, humidity of 50% with 12/12 h
light/dark cycle and were provided with water and food ad
libitum. Mice were injected with 1×105 MDA-MB-231
human BC or EO771 mouse BC cells (parental, sh-control or sh-MCT4)
using a 29-gauze needle into the bone marrow cavity of the right
tibiae under anesthesia with xylazine (10 mg/kg) + ketamine (100
mg/kg) administered intraperitoneally. The sham mice received only
PBS with a 29-gauze needle into the right tibial bone marrow
cavity. Injection of parental, sh-control, sh-MCT4, MDA-MB-231 and
EO771 cells were performed with a 29-gauze needle into the right
tibial bone marrow cavity. Injection was performed by a single
researcher who was blinded to the experimental conditions.
Radiological analysis of osteolytic
lesions
The bone destruction in tibiae associated with
MDA-MB-231 and EO771 BC tumor progression was assessed by
radiography. The bones harvested at sacrifice were placed against
films (22×27 cm; Fuji industrial film; FUJIFILM Wako Pure Chemical
Corporation) and exposed to soft X-rays at 35 kV for 15 sec with an
X-ray device (CMB-2; Softex Co., Ltd.). The area of the radiolucent
osteolytic lesions was quantified using Lumina Vision/OL v2.5
analysis software (MITANI Corporation) with a light microscope
(IX81; Olympus Corporation) and ImageJ software as previously
described (18).
Pain behavior assay
Mice were individually placed in a cage with a glass
floor, over a movable infrared light source. The light source was
positioned under the center of the hind paw, and the time (sec)
from stimulus onset to the paw's withdrawal was recorded.
Mechanical allodynia was evaluated using von Frey tests and a
Dynamic Plantar Aesthesiometer (Ugo Basile SRL) as previously
described (19). These tests are
widely used for pain assessment in rodents (9). The tests were performed prior to BC
cell injection to determine baseline behaviors and then every 3
days following cell injection. Throughout the experiment,
behavioral tests were performed by a single researcher who was
blinded to the animal experimental conditions.
At day 15 after BC cell injection, 100 µl of
blood was collected from each mouse through cardiac puncture under
anesthesia with 4% isoflurane inhalation, followed by cervical
dislocation. Animal death was verified by cessation of
cardiovascular and respiratory movements. Ipsilateral lumbar DRGs
(L3-L5) and the right tibia were then harvested for subsequent
analyses. The criteria of humane endpoints for euthanasia included
loss of >20% body weight compared with age-matched controls.
Harvesting of DRGs, tibiae, sera and bone
marrow fluids
The collected mouse DRGs were homogenized in RIPA
lysis buffer with 1 mM PMSF and phosphatase inhibitors
(Na3VO4 and NaF). The lysate was centrifuged
at 15,000 × g for 5 min at 4°C, and the supernatant was collected
as total protein. Some of the collected DRGs were fixed for 12 h at
room temperature with 10% neutral-buffered formalin and then
embedded in paraffin. Western blotting and immunofluorescence were
subsequently performed. For the collection of bone marrow fluids
from tibias, the mouse muscles and connective tissues were removed,
both ends of the tibias were cut, and whole bone marrow was flushed
out with 100 µl PBS, followed by centrifugation (1,500 × g;
4°C; 10 min) and collection of the supernatants.
Immunofluorescence analysis
An immunofluorescence analysis was conducted to
determine the expression of pERK1/2 and CGRP, a widely used marker
for sensory neurons (20), in DRGs
from each group of mice using an epifluorescence microscope
(BZ-X810; Keyence Corporation). Formalin-fixed paraffin-embedded
tissues (as aforementioned) were sectioned with 5-µm
thickness. Slides were deparaffinized in xylene twice for 5 min
each time, and rehydrated in 100 (twice), 95, 70 and 50% alcohol
for 3 min each. Antigen retrieval was performed by heating in, 10
mM citric acid solution at 95°C for 5min. The specimens were
incubated with 3% BSA in PBS blocking solution for 1 h at room
temperature and then with primary pERK1/2 antibody (1:200) and
anti-CGRP antibody (1:200) overnight at 4°C, followed by incubation
with Alexa Fluor 488 anti-rabbit IgG (1:1,000) and Alexa Fluor 647
anti-goat IgG (1:1,000) for 60 min at room temperature. Nuclei were
counterstained with Fluoroshield mounting medium with DAPI (cat.
no. ab104139; Abcam).
Statistical analysis
Data are presented as the mean ± SD. The data were
analyzed using an unpaired Student's t-test for comparisons between
two groups or one-way ANOVA with Bonferroni's, Dunnett's or Tukey's
post hoc tests for the analysis among multiple groups, using
GraphPad Prism v7.0 software (GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
MCT4 expression in the samples of
patients with BC and BC cell lines
It was first determined whether the lactate
transporter MCT4 is expressed in BC tissues compared with normal
breast tissues using immunohistochemistry. Representative
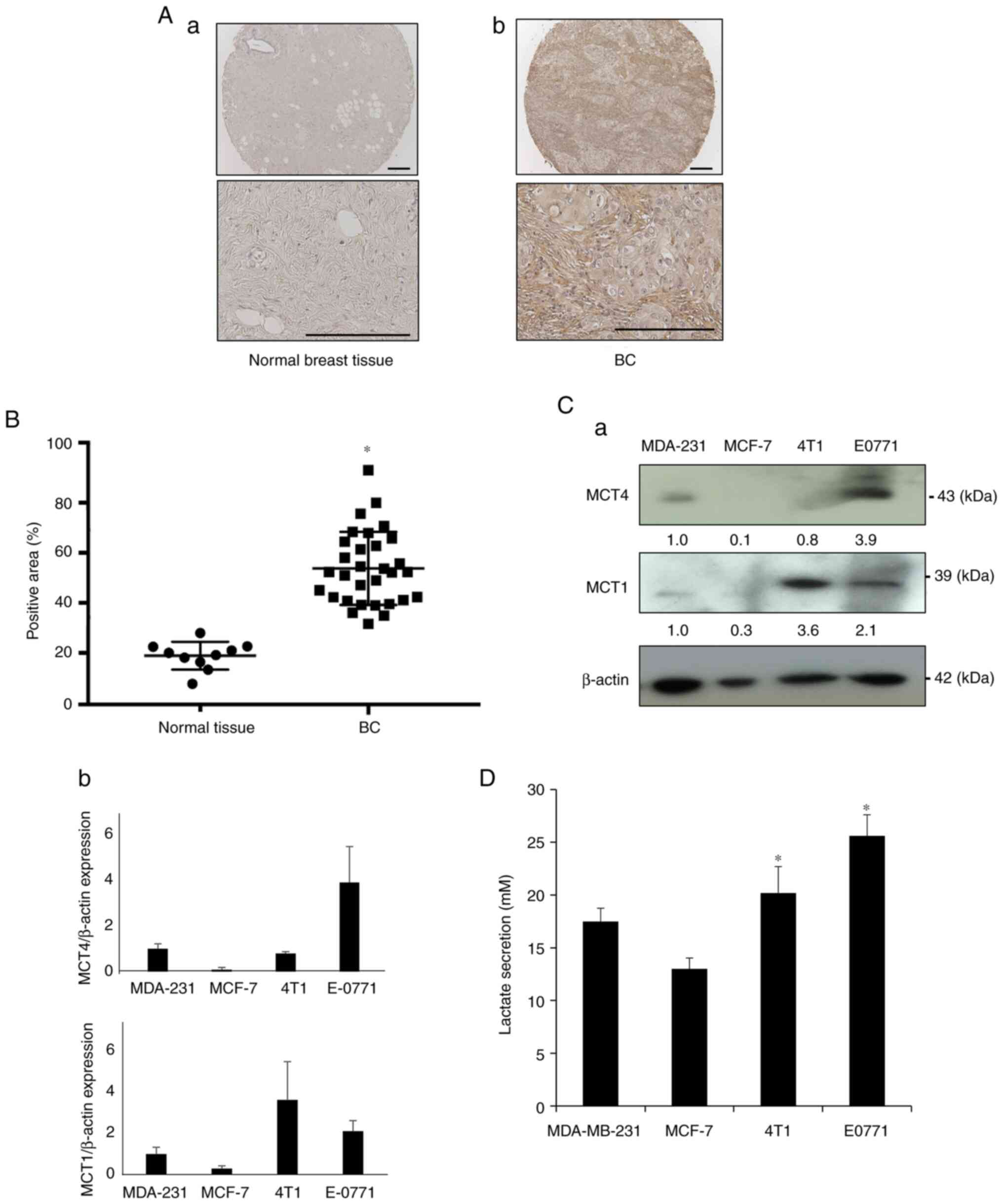
histological images showed that MCT4 expression in BC tissues was
more intense than that in normal breast tissues (Fig. 1A). Tissue microarray analysis
revealed that the MCT4-positive area of BC samples was
significantly increased compared with that in normal breast tissues
(Fig. 1B). These results suggested
that MCT4 expression is associated with BC tumor development.
Based on these data, the expression of MCT4 and MCT1
in human (MDA-MB-231 and MCF-7) and mouse BC cell lines (4T1 and
EO771) was next determined by western blot analysis, to discover
human and mouse BC cell lines that exhibited high MCT4 expression
and thus were suitable for further experiments. MCT1 is also the
lactate transporter that controls the intra- and extracellular
levels of lactate (6). Since
normal breast epithelial cells are currently unavailable and the
data depicted in Fig. 1A
demonstrated that normal breast tissues rarely expressed MCT4,
control cells were not included in the western blot analysis. EO771
BC cells showed strong expression of MCT4 and MCT1 (Fig. 1C), MDA-MB-231 cells showed moderate
expression of MCT4 and MCT1, while MCT4 expression was undetectable
in human MCF-7 and mouse 4T1 BC cells. Of note, these BC cells
released lactate into the culture supernatant in agreement with the
degree of MCT4 and MCT1 expression (Fig. 1D), demonstrating that human and
mouse BC cell lines express functional MCT4.
Role of MCT4 in the excitation of sensory
neurons and induction of BC-BP in mice intratibially injected with
EO771 mouse BC cells
To determine the role of MCT4 in sensory neuron
excitation and following BC-BP induction, MCT4 was silenced in
mouse EO771 BC cells using a lentiviral system (sh-MCT4 EO771 BC
cells). The expression of MCT4 protein was >80% decreased in the
sh-MCT4 EO771 BC cells compared with the parental cells and
sh-control cells (Fig. 2A). The
expression of MCT4 in the sh-control EO771 cells did not differ
from that of the parental EO771 cells. The sh-MCT4 EO771 BC cells
were then injected into the bone marrow cavity of tibiae in the
syngeneic female C57BL/6 mice, and the pain behavior of these mice
was determined compared with the mice intratibially injected with
parental or sh-control cells. No mice that received intratibial
injection of mouse EO771 BC cells died until sacrifice at day 15.
As previously reported using mouse 4T1 BC cells (21), the parental EO771 BC cells
aggressively colonized the bone marrow cavity of tibiae accompanied
with evident osteolysis (Fig. 2B,
left), and sh-MCT4/EO771 BC cells also appeared to grow to an
equivalent size to that of the parental EO771 BC cells in tibiae
(Fig. 2B, right), suggesting that
MCT4 silencing had no effects on EO771 BC growth in the bone. It is
noteworthy that our previous study showed that the area of
osteolysis detected on radiographs correlated well with the tumor
size in bone determined by histomorphometry (18). Tumor growth could not be
macroscopically observed, because the EO771 tumors grew in the bone
marrow cavity. Furthermore, no tumor development was
macroscopically detected at sites other than bone at day 15,
although microscopic metastases could possibly develop in visceral
organs including lung, liver and brain (18,21).
There were no differences in body weight between mice injected with
the EO771 parental, sh-control and sh-MCT4 EO771 cells at day 15
(Fig. S1A).
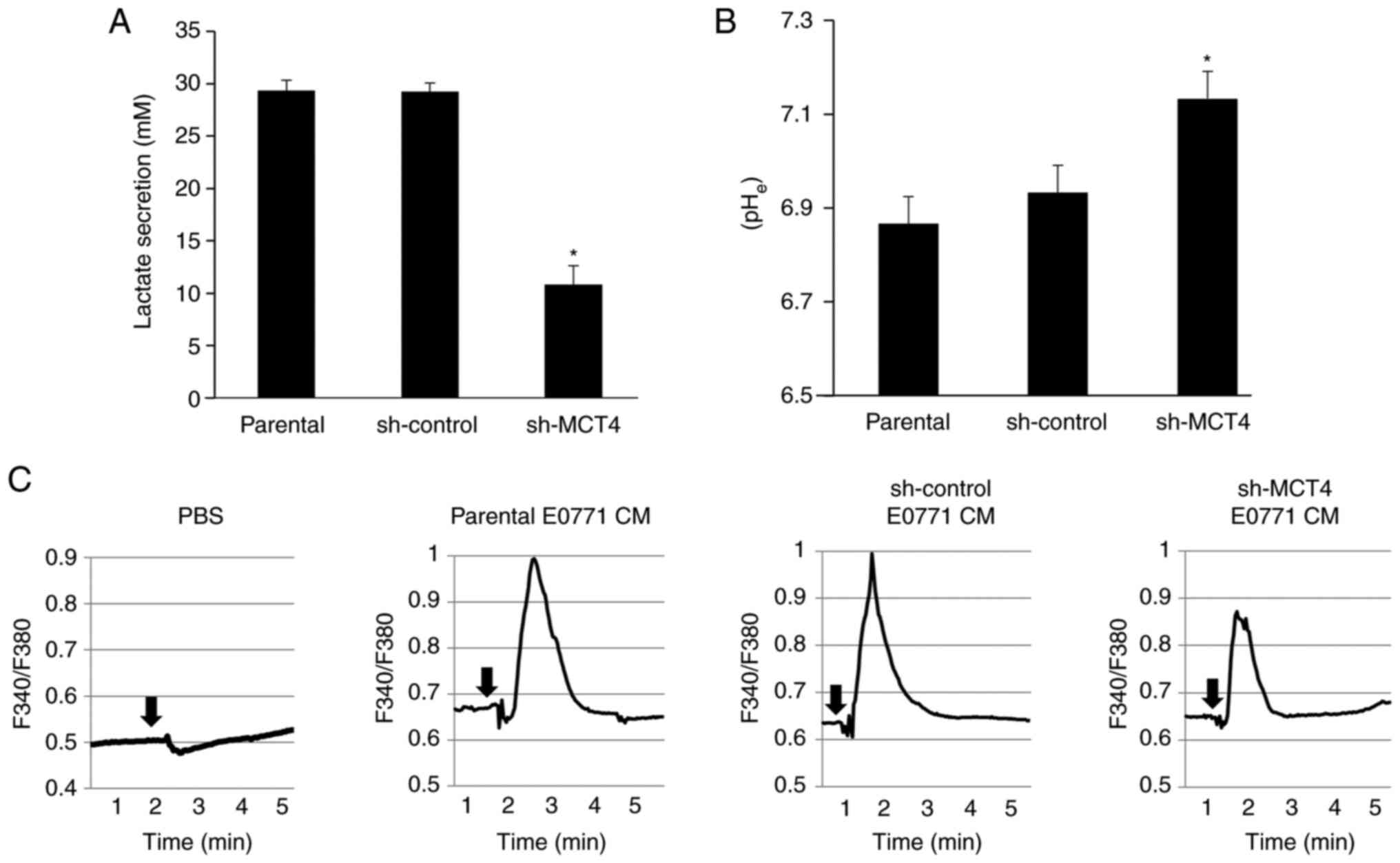
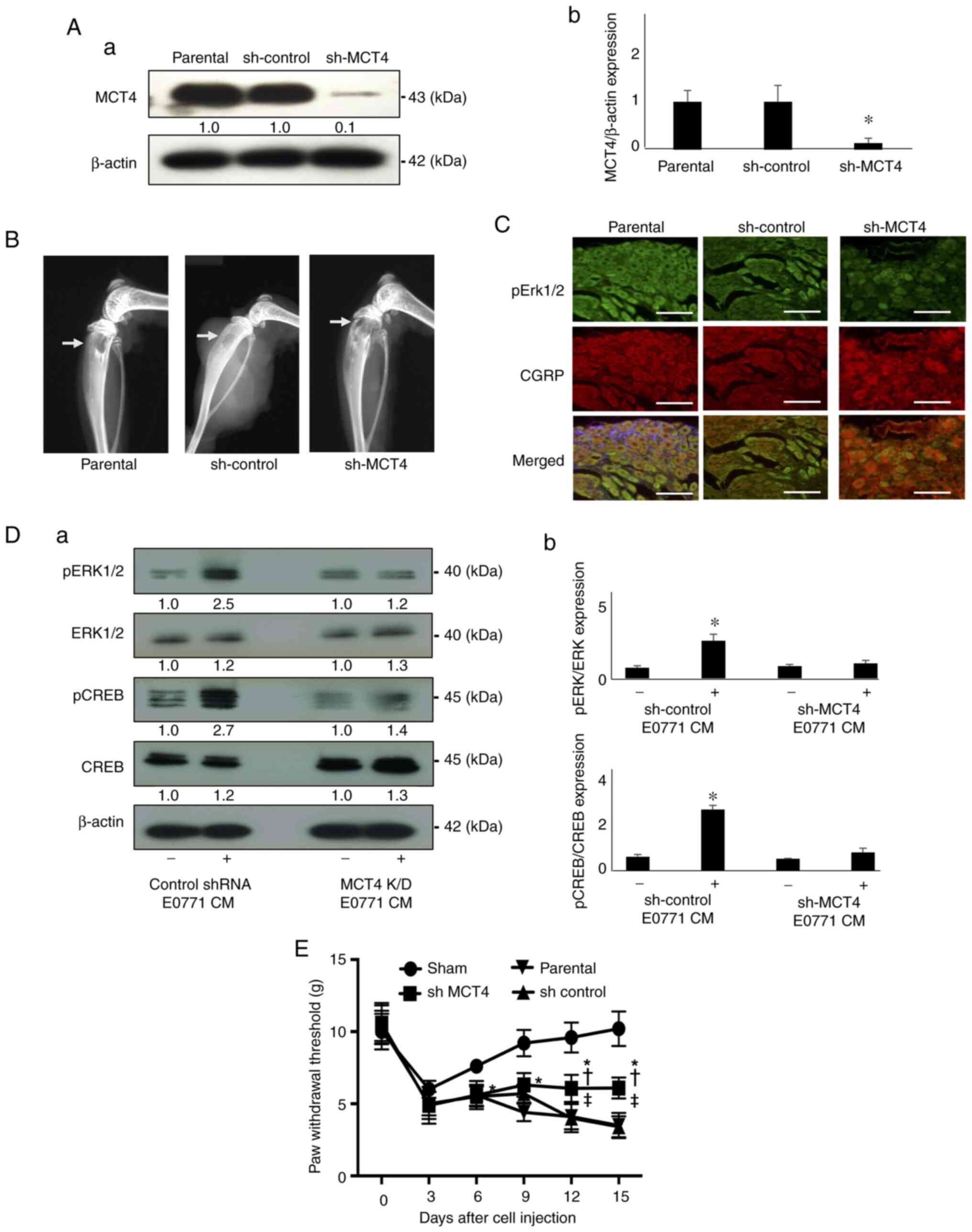 | Figure 2DRG sensory neuron excitation and
mechanical allodynia determined in mice intratibially injected with
EO771 mouse BC cells. (A) Expression of MCT4 in parental,
sh-control and sh-MCT4 EO771 mouse BC cells examined by western
analysis; (a) representative blot images and (b) relative ratio
(parental is indicated as 1.0). *P<0.05 vs. parental.
(B) Radiograph of tumor growth showing bone destruction in the
tibia of mice injected with sh-control and sh-MCT4 mouse EO771 BC
cells at day 15 (arrows). (C) Expression of pERK1/2 (green, upper
panel) and CGRP (red, middle panel) in DRGs harvested from mice
intratibially injected with parental, sh-control and sh-MCT4 EO771
cells at day 15 by immunofluorescence. Scale bar, 50 µm. (D)
Excitation of the F11 DRG sensory neuron cells as determined by
pERK1/2 and pCREB expression. The F11 DRG neuron cells were
cultured in neuron basal medium in the absence or presence of
conditioned medium of the sh-control and sh-MCT4 EO771 cell
cultures (30%, v/v) for 15 min, lysed and the expression of pERK1/2
and pCREB was determined by western blot analysis; (a)
representative blot images (control is indicated as 1.0 normalized
to β-actin) and (b) relative ratio of pERK1/2 and pCREB expression
(pERK1/2/ERK1/2 and pCREB/CREB) *P<0.05 vs. sh-MCT4
CM. (E) Progression of the hind-paw mechanical allodynia in tibias
of mice that received sham, parental, sh-control and sh-MCT4 EO771
cells (n=5 per group). The pain behavior test was performed every 3
days after cell injection. Mechanical allodynia seen in all mice at
day 3 is due to the surgical trauma at day 0. Data are presented as
the mean ± SD. *P<0.05 vs. sham;
†P<0.05 vs. parental; ‡P<0.05 vs.
sh-control. DRG, dorsal root ganglion; MCT4, monocarboxylate
transporter 4; CGRP, calcitonin gene-related peptide; CREB, cAMP
response element binding protein; sh, short hairpin; p,
phosphorylated; CM, conditioned medium. |
The excitation of the DRG neurons was evaluated by
immunohistochemistry using pERK1/2 as a biochemical marker for
neuron excitation (17). DRGs
harvested from mice injected with parental or sh-control EO771 BC
cells at sacrifice (day 15) demonstrated elevated expression of
pERK1/2, while the DRG of mice injected with sh-MCT4 EO771 cells
showed minimum pERK1/2 expression (Fig. 2C). Interestingly, the number of the
CGRP-positive sensory neurons appeared unchanged among the DRGs of
the three groups of mice (Fig.
2C).
Consistent with these in vivo results, the
F11 DRG neuron cells showed increased expression of pERK1/2 and
pCREB (a biochemical marker for neuron excitation) (22) in the presence of CM (30% v/v)
harvested from the cultures of the sh-control EO771 BC cells
(Fig. 2D). By contrast, the
expression of pERK1/2 and pCREB was not upregulated in the F11 DRG
neuron cells treated with the CM of sh-MCT4 EO771 BC cells
(Fig. 2D). These results supported
the hypothesis that MCT4-mediated lactate release from BC leads to
the excitation of the DRG sensory neurons. These in vitro
results were also consistent with the assumption there is
sufficient time and chance for the nociceptive soluble tumor
products that are released as a consequence of the interactions
between GPR81 at the sensory endings and cancer cells to upregulate
the expression of pERK1/2 and pCREB in the DRG.
It was then determined whether MCT4 plays a role in
BC-BP induction using the pain behavior assay. Mice carrying the
parental or sh-control EO771 BC cells began to exhibit decreased
paw withdrawal threshold in the pain behavior assay using von Frey
test at day 6 and 9 (Fig. 2E),
demonstrating that these mice were developing mechanical allodynia.
Mechanical allodynia advanced as a function of time in parallel
with EO771 BC growth in the bone. By contrast, mice intratibially
injected with sh-MCT4 EO771 BC cells showed reduced mechanical
allodynia compared with mice carrying parental or sh-control EO771
BC cells (Fig. 2E). These results
suggested that MCT4 plays an important role in BC-BP induction by
mediating lactate release. Lactate acidosis may also contribute to
the induction of BC-BP represented by mechanical allodynia by
activating acid-sensing nociceptors, such as the transient receptor
potential vanilloid 1 and acid-sensing ion channels (23).
Role of MCT4 expression in EO771 mouse BC
cells
The effects of MCT4 knockdown on lactate metabolism
in EO771 mouse BC cells were subsequently investigated in
vitro. Lactate secretion into the culture supernatants was
significantly decreased (Fig. 3A)
and pHe was significantly elevated (Fig. 3B) in sh-MCT4 EO771 cells compared
with those in the parental and sh-control EO771 cells. These
results suggested that MCT4 regulates lactate secretion and pHe in
EO771 mouse BC cells.
In addition, the CM of parental and sh-control EO771
BC cell cultures also increased Ca2+ influx, a widely
used indicator of sensory neuron excitation (24), in the F11 DRG neuron cells, while
sh-MCT4 EO771 CM increased Ca2+ influx to a lesser
extent than the CM of parental and sh-control EO771 BC cells
(Fig. 3C). Consistent with the
present results, the F11 cells are reported to display functional
DRG neuron properties, including unique neuronal morphology of
extended neurites and Ca2+ mobilization in response to
stimuli (25) without the presence
of glia cells. Furthermore, it was recently found that the F11
cells possess neuron-like properties, responding to the soluble
products released from BC cells (data not shown). These data will
be investigated in future studies. Taken together, these results
suggested that lactate released from EO771 mouse BC cells via MCT4
excites sensory neuron cells.
Role of MCT4 expression in lactate
metabolism and BP induction in MDA-MB-231 human BC cells
The role of MCT4 expression in lactate metabolism
and BP induction in human MDA-MB-231 BC cells was next
investigated. To this end, MCT4 was knocked down in MDA-MB-231
cells by introducing shRNA plasmids using a lentiviral system,
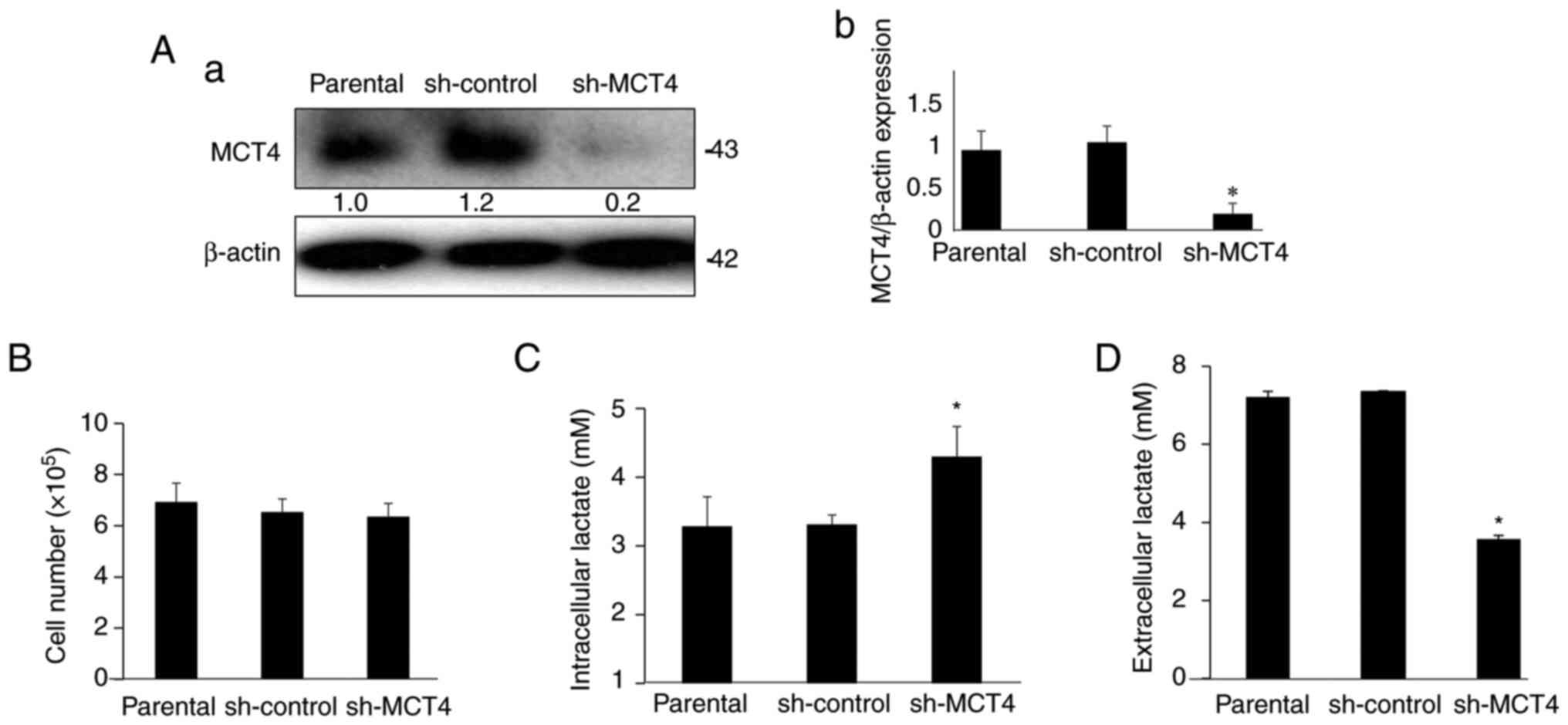
thereby generating sh-MCT4 MDA-MB-231 cells. The expression of MCT4
protein was >80% decreased in the sh-MCT4 MDA-MB-231 cells
compared with the parental cells and sh-control cells (Fig. 4A). The expression of MCT4 in the
sh-control MDA-MB-231 cells did not differ from that of the
parental MDA-MB-231 cells.
Cell proliferation was not changed among parental,
sh-control and sh-MCT4 MDA-MB-231 cells (Fig. 4B). Importantly, the intracellular
lactate level was increased (Fig.
4C) and lactate secretion was reduced by ~40% (Fig. 4D) in sh-MCT4 MDA-MB-231 cells
compared with those in the parental and sh-control MDA-MB-231
cells. These results suggested that MCT4 regulates intracellular
and extracellular levels of lactate in human MDA-MB-231 BC
cells.
Role of MCT4 expression in lactate levels
in bone marrow and BC-BP induction
Subsequently, the role of MCT4 in BC-BP after
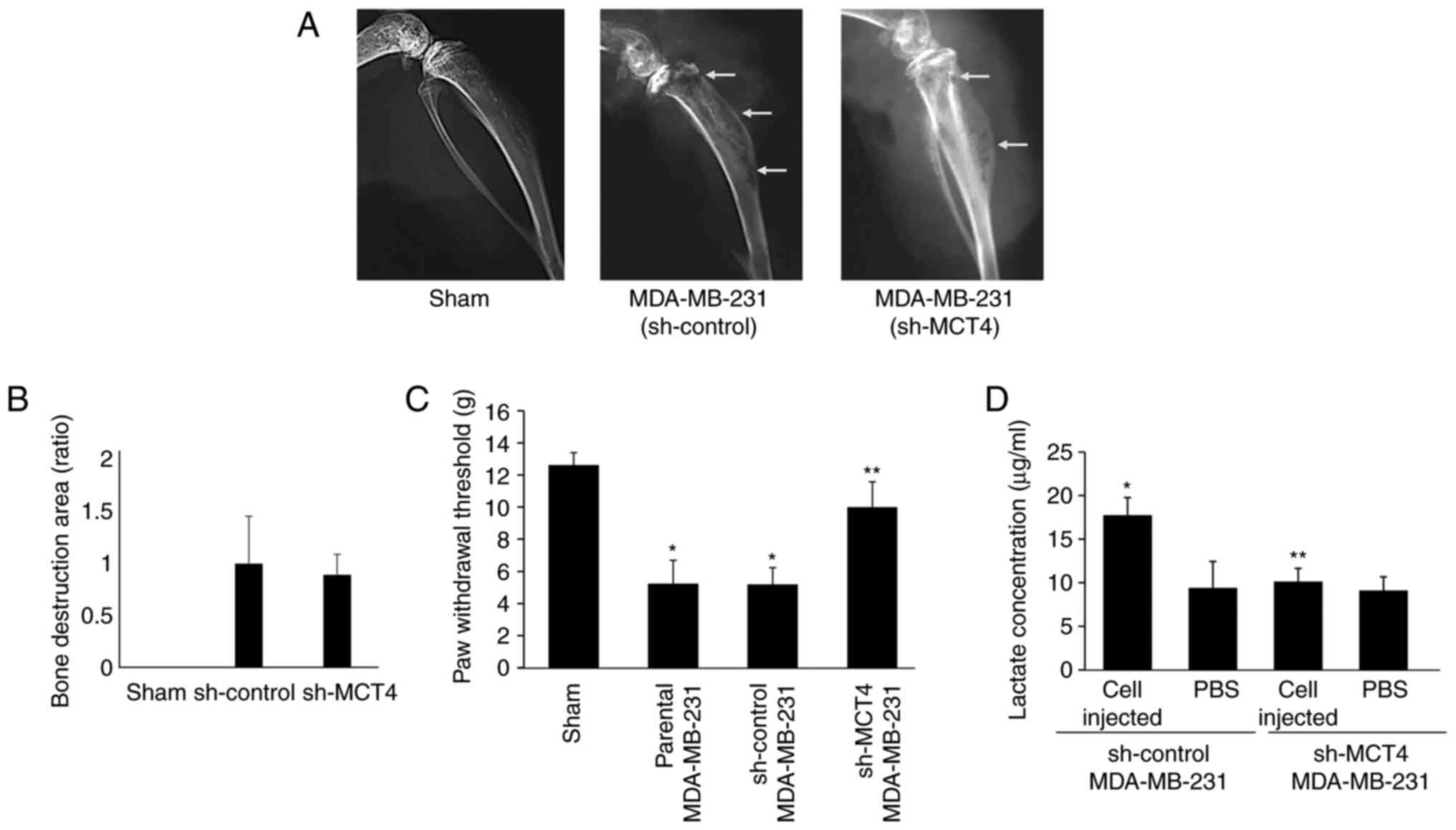
intratibial injection of MDA-MB-231 cells in Balb/c athymic nude
mice was determined. Mice injected with MDA-MB-231 sh-control and
sh-MCT4 cells showed bone destruction associated with tumor growth
on the radiographs at day 7 (Fig.
5A). There was no significant difference in the extent of bone
destruction between the MDA-MB-231 sh-control and sh-MCT4-injected
mice (Fig. 5A and B).
To examine the effects of MCT4 expression on BC-BP
induction in mice, a mechanical allodynia test was performed at day
10. Mice intratibially injected with parental and sh-control
MDA-MB-231 BC cells exhibited mechanical allodynia (Fig. 5C). By contrast, mechanical
allodynia was significantly decreased in mice injected with sh-MCT4
MDA-MB-231 cells compared with mice injected with parental and
sh-control MDA-MB-231 BC cells (Fig.
5C), suggesting that MCT4 expression is critical to BC-BP
induction.
The lactate concentration in the bone marrow fluid
of tibiae injected with MDA-MB-231 human BC cells was also
determined. Lactate concentration was elevated in the fluids
harvested from the bone marrow of tibiae injected with sh-control
MDA-MB-231 BC cells compared with that of sham-operated tibiae
(Fig. 5D). By contrast, lactate
concentration in the fluids harvested from the bone marrow of
tibiae injected with sh-MCT4 MDA-MB-231 cells was not significantly
increased compared with those injected with sh-control MDA-MB-231
cells (Fig. 5D). These results
collectively suggested that inhibition of MCT4 expression decreased
lactate release from BC cells into the bone marrow cavity, which in
turn reduced BC-BP. There were no differences in body weight
between mice injected with the MDA-MB-231 parental, sh-control and
sh-MCT4 MDA-MB-231 cells at day 15 (Fig. S1B).
Role of the lactate receptor GPR81 in
sensory neuron excitation
Finally, the mechanism via which BC-secreted lactate
excites the sensory neurons to induce BC-BP was determined. GPR81
has been initially found as a lactate receptor that mediates
lipolysis in the adipose tissue (14). It has been also shown that GPR81
regulates neuronal network activity in cooperation with other GPRs
(26). However, little is known
about the expression and function of GPR81 in sensory neurons. The
present study found that F11 DRG neuron cells express GPR81 by
western blot analysis (Fig. 6A).
To determine the role of GPR81 in sensory neuron excitation,
GPR81-knockdown F11 DRG neuron cells were established via shRNA
transduction (sh-GPR81 F11 cells). GPR81 protein expression was
reduced by 70% in sh-GPR81 F11 DRG neuron cells compared with
parental and sh-control F11 cells (Fig. 6A). Of note, the CM harvested from
EO771 BC cultures, which contains high levels of lactate, increased
the expression of pERK1/2 in the F11 cells (Fig. 6B), which was almost abolished in
the sh-GPR81 F11 cells. These results suggested that GPR81
contributes to sensory neuron excitation caused by BC-derived
lactate.
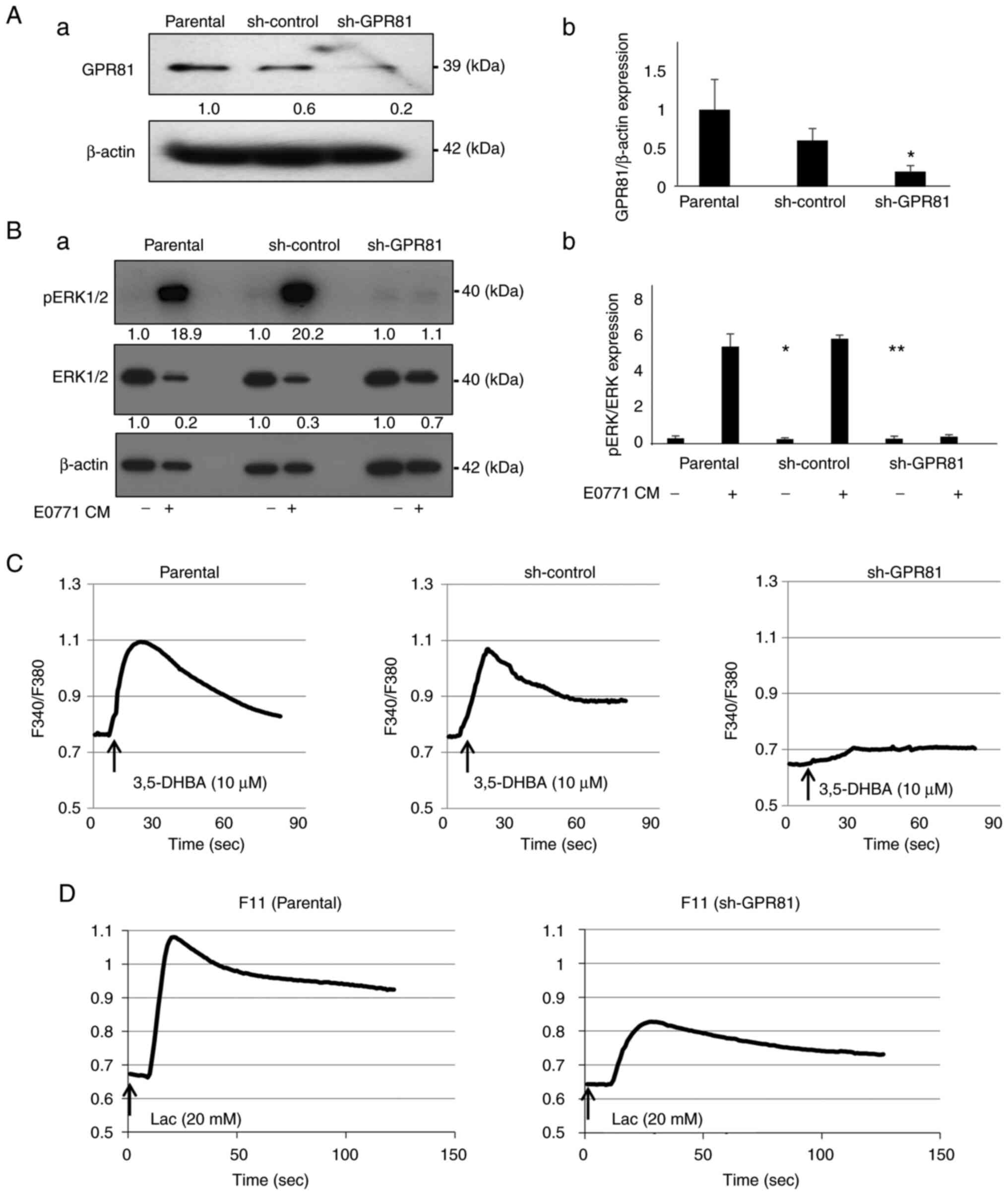 | Figure 6Role of the lactate receptor GPR81 in
the excitation of the F11 DRG sensory neuron cells. (A) Expression
of GPR81 in the parental, sh-control and sh-GPR81 F11 DRG cells
analyzed by western blotting; (a) representative blot images and
(b) relative ratio (parental is indicated as 1.0) (n=3). (B)
pERK1/2 expression in the F11 DRG sensory neuron cells. Cells were
treated with or without EO771 CM (30%, v/v) for 15 min, lysed and
the expression of pERK1/2 was analyzed by western blotting; (a)
representative blot images (control is indicated as 1.0 normalized
to β-actin) and (b) relative ratio of pERK1/2 expression
(pERK1/2/ERK1/2). *P<0.01 vs. parental CM (-);
**P<0.01 vs. sh-control CM (-) (n=3). (C)
Intracellular Ca2+ mobilization in the F11 DRG sensory
neuron cells treated with or without the GPR81 agonist 3,5-DHBA
(n=10). (D) Intracellular Ca2+ mobilization in the F11
DRG sensory neuron cells treated with or without lactic acid
(n=10). GPR81, G-protein-coupled receptor 81; 3,5-DHBA,
3,5-dihydroxybenzoic acid; sh, short hairpin, CM, conditioned
medium; DRG, dorsal root ganglion. |
Consistent with these results, treatment with the
GPR81 agonist 3,5-DHBA (27)
increased Ca2+ influx in parental and sh-control F11
cells (Fig. 6C), which was not
observed in sh-GPR81 F11 cells (Fig.
6C). Furthermore, lactic acid treatment also increased
Ca2+ influx in parental F11 cells (Fig. 6D), which was reduced in sh-GPR81
F11 cells. These results provided additional evidence supporting
that GPR81 plays important roles in the excitation of sensory
neurons by lactate.
Discussion
Cancer cells produce large amounts of lactate
resulting from the Warburg effect (28) and actively secrete it via the
lactate transporter MCT4 (13) to
avoid cell death due to intracellular lactate acidosis. Secreted
lactate is then taken up by neighboring cells, such as stromal,
endothelial and immune cells, in the tumor microenvironment via the
lactate transporter MCT1 as an energy source (29). Interestingly, stromal cells in turn
release lactate via MCT4, which is then incorporated by cancer
cells via MCT1 as an energy source for ATP generation (known as
reverse Warburg effect) (30).
However, the effects of lactate released via MCT4 from cancer on
the sensory neurons innervating the tumor microenvironment are
poorly understood. The present study showed that human BC tissues
express increased MCT4 compared with normal breast tissues,
suggesting that MCT4 is associated with BC development.
Furthermore, it was found that mouse and human BC cell lines also
substantially express MCT4 to increase lactate secretion, thereby
lowering pHe, and in turn decrease intracellular lactate levels.
Consistent with these in vitro results, lactate levels in
the bone marrow fluids of tibiae in mice injected with MDA-MB-231
human BC cells were elevated. Importantly, these effects were all
inhibited when MCT4 expression was knocked down in BC cells. In
addition, it was also demonstrated that intratibial injection of
EO771 mouse BC cells and MDA-MB-231 human BC cells in mice caused
sensory nerve excitation and BC-BP induction, which was attenuated
after intratibial injection of EO771 BC cells with MCT4 is
knockdown. Taken together, these results demonstrated that the
supply of lactate by BC to the sensory nerves via MCT4 in the tumor
microenvironment causes the excitation of sensory nerves, which
consequently induces BC-BP. The present results suggested that MCT4
of BC is an important player in the excitation of the sensory
neurons and induction of BC-BP. Blocking MCT4 actions may thus be
an effective novel approach for the treatment of BC-BP.
It should be noted that active secretion of excess
intracellular lactate facilitates the development of an acidic
tumor microenvironment, which has been shown to promote cancer
progression by increasing tumor angiogenesis, proteolytic activity,
metastatic potential and resistance to immune system and anticancer
therapies (31). Furthermore,
increased MCT4 expression is associated with poor outcome in
patients with triple-negative BC (32). It has also been reported that MCT4
expression is increased in head and neck cancer (28). Hence, the present results provide
additional pieces of evidence that MCT4-mediated active lactate
excretion is associated with BC progression, suggesting that MCT4
may also be a potential therapeutic target for BC treatment
(33).
The present study demonstrated that sensory neurons
express GPR81 that mediates the effects of BC-derived lactate to
excite sensory neurons. The current results showed that lactate, as
a signaling molecule, bound to and activated its cognitive receptor
GPR81 to excite sensory neurons leading to the upregulation of
pERK1/2 expression. However, the downstream signaling pathways that
are propagated following lactate binding to GPR81 were not
determined in the present study. It has been reported that GPR81
regulates neuronal network activity in cooperation with other GPRs
(26). Identification of GPR81
signaling pathways involving ERK1/2 and CREB in association with
sensory neuron excitation and BC-BP induction is important to
design pharmacological interventions for BC-BP.
The results of the present study that GPR81 plays a
role in the promotion of the excitation of sensory neurons are not
consistent with previous results reporting that GPR81 activation
inhibits neuron excitation (15,34).
The reasons for this discrepancy are unknown. The present results
could be due to an involvement of certain indirect routes. Along
this line, it has been reported that GPR81 modulates the expression
of MCT1 and MCT4, which are responsible for the regulation of
cellular lactate metabolism by controlling lactate uptake and
release (35,36). It is therefore speculated that
MCT1/MCT4 affects GPR81 role in neurons. Determination of the
interactions between GPR81 and MCT1/MCT4 in the regulation of
neuronal activity is important to define the effects of GPR81 on
neurons.
The present study showed that lactate released from
BC via the lactate transporter MCT4 changes sensory neuron
excitation and BC-BP induction, presumably following the binding to
the lactate receptor GPR81 of the DRG neurons, indicating that
cancer regulates neuronal activity in a paracrine manner. Of
interest, recent studies reported that neuronal activity in turn
modulates cancer progression and metastasis (37-39).
Various types of cancer have been reported to exhibit increased
expression of GPR81, which is associated with their progression
(35,40). In agreement with these reports, BC
tissue of patients showed elevated GPR81 expression compared with
normal human breast tissue by immunohistochemistry, and knockdown
of GPR81 decreased BC growth in subcutis and bone in mice (36). Taken together, these results
suggested that GPR81 plays a role not only in neuron excitation by
lactate released from BC via MCT4 in a paracrine manner but also
promotion of cancer progression by BC-derived lactate in an
autocrine manner. It is intriguing to determine the effects of the
activation or suppression of GPR81 of DRG neurons on cancer
aggressiveness.
There are several limitations in the present study.
Firstly, the present study did not provide compelling evidence that
GPR81 plays a pivotal role in the pathophysiology of BC-BP. Using
von Frey test, which has long and widely been used in pain behavior
assays, it was shown that mice intratibially injected with EO771 BC
cells displayed mechanical allodynia, suggesting that these mice
developed BC-BP. However, the present study did not demonstrate
data of spinal sensitization maintained as a consequence of lactate
activation of GPR81 on the C-nociceptors, which is a prerequisite
for mechanical allodynia. Secondly, the present study did not
present some critical data, including the expression of GPR81 on
the peripheral ending and DRG C-nociceptors, the effects of GPR81
on pain behaviors of GPR81−/− mice intratibially
injected with BC cells compared with wild-type mice and the effects
of GPR81 antagonists on pain behaviors of these mice. These studies
are currently ongoing. Thirdly, it has been previously demonstrated
that the area of bone destruction detected on radiographs
corresponds well with the tumor size in bone determined by
histomorphometry (21); however,
the present study lacks histomorphometric data of EO771 BC tumor
size in bone. Fourthly, the present results may raise the question
whether the tumor growth in bone is correlated with the degree of
lactate production and/or MCT4 expression. This point was not
specifically determined in the present study, although it appeared
that no such correlation existed. It has been demonstrated that
tumor growth is modulated not only by tumor activity but also
surrounding microenvironments (41). The effects of the tumor
microenvironments may mask the effects of lactate/MCT4 on tumor
growth. Finally, since the cell line data had no adequate normal
control, these data should be cautiously interpreted.
In conclusion, the current findings suggested that
lactate transported via MCT4 from BC excites sensory neurons
through binding and activating GPR81 of the sensory neurons.
Unraveling the GPR81 signaling pathways in sensory neurons should
increase the understanding of the mechanism of lactate regulation
of BC-BP and facilitate the design of mechanism-based therapies for
the control of BC-BP, for which effective treatments are currently
limited.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TO and TY conceived and designed the experiments.
TO, MH, KH, TN and KO performed the experiments. TO and TY analyzed
and interpreted the data. TO, MH, KH, TN, KO, SI, TK and AS
performed data acquisition. TO and TY wrote the paper. TO, TY, MH,
KH, TN, KO, AS, TK and SI performed manuscript revision/review. TO,
MH and TY confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies were approved by the
Institutional Animal Care and Use Committee at the Indiana
University School of Medicine (IACUC protocol no. 11170;
Indianapolis, USA) and conducted according to the ARRIVE
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present work was supported by a Grant-in-Aid for Research
Activity and Grant-in-Aid for Young Scientists from JSPS KAKENHI to
TO from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (grant nos. 16H0699219 and 18K1722500), a
Grant-in-Aid for Scientific Research from JSPS KAKENHI to TY (grant
no. 17H04377) and the IU Health Strategic Research Initiative in
Oncology to TY (grant no. 46-875-54) and start-up fund of the
Indiana University School of Medicine to TY (grant no. 2382696
YONED).
References
|
1
|
Clézardin P, Coleman R, Puppo M, Ottewell
P, Bonnelye E, Paycha F, Confavreux CB and Holen I: Bone
metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev.
101:797–855. 2021. View Article : Google Scholar
|
|
2
|
Falk S and Dickenson AH: Pain and
nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol.
32:1647–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiao RQ, Zhang HR, Ma RX, Li RF and Hu YC:
Prognostic factors for bone survival and functional outcomes in
patients with breast cancer spine metastases. Technol Cancer Res
Treat. 21:153303382211226422022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu W, Zhang L, Dong Y, Tian Z, Chen Y and
Dong S: Tumour dormancy in inflammatory microenvironment: A
promising therapeutic strategy for cancer-related bone metastasis.
Cell Mol Life Sci. 77:5149–5169. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Infante M, Fabi A, Cognetti F, Gorini S,
Caprio M and Fabbri A: RANKL/RANK/OPG system beyond bone
remodeling: Involvement in breast cancer and clinical perspectives.
J Exp Clin Cancer Res. 38:122019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ngo DC, Ververis K, Tortorella SM and
Karagiannis TC: Introduction to the molecular basis of cancer
metabolism and the Warburg effect. Mol Biol Rep. 42:819–823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tiedemann K, Hussein O and Komarova SV:
Role of altered metabolic microenvironment in osteolytic
metastasis. Front Cell Dev Biol. 8:4352020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avnet S, Di Pompo G, Lemma S and Baldini
N: Cause and effect of microenvironmental acidosis on bone
metastases. Cancer Metastasis Rev. 38:133–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hiasa M, Okui T, Allette YM, Ripsch MS,
Sun-Wada GH, Wakabayashi H, Roodman GD, White FA and Yoneda T: Bone
pain induced by multiple myeloma is reduced by targeting V-ATPase
and ASIC3. Cancer Res. 77:1283–1295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoneda T, Hiasa M, Nagata Y, Okui T and
White FA: Acidic microenvironment and bone pain in cancer-colonized
bone. Bonekey Rep. 4:6902015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brooks GA: The science and translation of
lactate shuttle theory. Cell Metab. 27:757–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magistretti PJ and Allaman I: Lactate in
the brain: From metabolic end-product to signalling molecule. Nat
Rev Neurosci. 19:235–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Payen VL, Mina E, Van Hée VF, Porporato PE
and Sonveaux P: Monocarboxylate transporters in cancer. Mol Metab.
33:48–66. 2020. View Article : Google Scholar :
|
|
14
|
Cai TQ, Ren N, Jin L, Cheng K, Kash S,
Chen R, Wright SD, Taggart AK and Waters MG: Role of GPR81 in
lactate-mediated reduction of adipose lipolysis. Biochem Biophys
Res Commun. 377:987–991. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Castro Abrantes H, Briquet M,
Schmuziger C, Restivo L, Puyal J, Rosenberg N, Rocher AB,
Offermanns S and Chatton JY: The Lactate Receptor HCAR1 Modulates
neuronal network activity through the activation of Gα
and Gβγ Subunits. J Neurosci. 39:4422–4433. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Francel PC, Harris K, Smith M, Fishman MC,
Dawson G and Miller RJ: Neurochemical characteristics of a novel
dorsal root ganglion X neuroblastoma hybrid cell line, F-11. J
Neurochem. 48:1624–1631. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakanishi M, Hata K, Nagayama T, Sakurai
T, Nishisho T, Wakabayashi H, Hiraga T, Ebisu S and Yoneda T: Acid
activation of Trpv1 leads to an up-regulation of calcitonin
gene-related peptide expression in dorsal root ganglion neurons via
the CaMK-CREB cascade: A potential mechanism of inflammatory pain.
Mol Biol Cell. 21:2568–2577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okui T, Shimo T, Fukazawa T, Kurio N,
Hassan NM, Honami T, Takaoka M, Naomoto Y and Sasaki A: Antitumor
effect of temsirolimus against oral squamous cell carcinoma
associated with bone destruction. Mol Cancer Ther. 9:2960–2969.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okui T, Hiasa M, Ryumon S, Ono K, Kunisada
Y, Ibaragi S, Sasaki A, Roodman GD, White FA and Yoneda T: The
HMGB1/RAGE axis induces bone pain associated with colonization of
4T1 mouse breast cancer in bone. J Bone Oncol. 26:1003302021.
View Article : Google Scholar
|
|
20
|
Russell FA, King R, Smillie SJ, Kodji X
and Brain SD: Calcitonin gene-related peptide: Physiology and
pathophysiology. Physiol Rev. 94:1099–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiraga T, Myoui A, Hashimoto N, Sasaki A,
Hata K, Morita Y, Yoshikawa H, Rosen CJ, Mundy GR and Yoneda T:
Bone-derived IGF mediates crosstalk between bone and breast cancer
cells in bony metastases. Cancer Res. 72:4238–4249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawasaki Y, Kohno T, Zhuang ZY, Brenner
GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ and Ji RR:
Ionotropic and metabotropic receptors, protein kinase A, protein
kinase C, and Src contribute to C-fiber-induced ERK activation and
cAMP response element-binding protein phosphorylation in dorsal
horn neurons, leading to central sensitization. J Neurosci.
24:8310–8321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leffler A, Mönter B and Koltzenburg M: The
role of the capsaicin receptor TRPV1 and acid-sensing ion channels
(ASICS) in proton sensitivity of subpopulations of primary
nociceptive neurons in rats and mice. Neuroscience. 139:699–709.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ouyang K, Zheng H, Qin X, Zhang C, Yang D,
Wang X, Wu C, Zhou Z and Cheng H: Ca2+ sparks and secretion in
dorsal root ganglion neurons. Proc Natl Acad Sci USA.
102:12259–12264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pastori V, D'Aloia A, Blasa S and Lecchi
M: Serum-deprived differentiated neuroblastoma F-11 cells express
functional dorsal root ganglion neuron properties. PeerJ.
7:e79512019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lauritzen KH, Morland C, Puchades M,
Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H,
Storm-Mathisen J, Gjedde A and Bergersen LH: Lactate receptor sites
link neurotransmission, neurovascular coupling, and brain energy
metabolism. Cereb Cortex. 24:2784–2795. 2014. View Article : Google Scholar
|
|
27
|
Liu C, Kuei C, Zhu J, Yu J, Zhang L, Shih
A, Mirzadegan T, Shelton J, Sutton S, Connelly MA, et al:
3,5-Dihydroxybenzoic acid, a specific agonist for hydroxycarboxylic
acid 1, inhibits lipolysis in adipocytes. J Pharmacol Exp Ther.
341:794–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hasegawa K, Okui T, Shimo T, Ibaragi S,
Kawai H, Ryumon S, Kishimoto K, Okusha Y, Monsur Hassan NM and
Sasaki A: Lactate transporter monocarboxylate transporter 4 induces
bone pain in head and neck squamous cell carcinoma. Int J Mol Sci.
19:33172018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang L, Li W, Li X, Jin X, Liao Q, Li Y
and Zhou Y: 'Reverse Warburg effect' of cancer-associated
fibroblasts (Review). Int J Oncol. 60:672022. View Article : Google Scholar
|
|
31
|
Corbet C and Feron O: Tumour acidosis:
From the passenger to the driver's seat. Nat Rev Cancer.
17:577–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doyen J, Trastour C, Ettore F, Peyrottes
I, Toussant N, Gal J, Ilc K, Roux D, Parks SK, Ferrero JM and
Pouysségur J: Expression of the hypoxia-inducible monocarboxylate
transporter MCT4 is increased in triple negative breast cancer and
correlates independently with clinical outcome. Biochem Biophys Res
Commun. 451:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong CS, Graham NA, Gu W, Espindola
Camacho C, Mah V, Maresh EL, Alavi M, Bagryanova L, Krotee PAL,
Gardner BK, et al: MCT1 modulates cancer cell pyruvate export and
growth of tumors that Co-express MCT1 and MCT4. Cell Rep.
14:1590–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Órdenes P, Villar PS, Tarifeño-Saldivia E,
Salgado M, Elizondo-Vega R, Araneda RC and García-Robles MA:
Lactate activates hypothalamic POMC neurons by intercellular
signaling. Sci Rep. 11:216442021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roland CL, Arumugam T, Deng D, Liu SH,
Philip B, Gomez S, Burns WR, Ramachandran V, Wang H,
Cruz-Monserrate Z and Logsdon CD: Cell surface lactate receptor
GPR81 is crucial for cancer cell survival. Cancer Res.
74:5301–5310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishihara S, Hata K, Hirose K, Okui T,
Toyosawa S, Uzawa N, Nishimura R and Yoneda T: The lactate sensor
GPR81 regulates glycolysis and tumor growth of breast cancer. Sci
Rep. 12:62612022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Venkatesh HS: The neural regulation of
cancer. Science. 366:9652019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zahalka AH and Frenette PS: Nerves in
cancer. Nat Rev Cancer. 20:143–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Silverman DA, Martinez VK, Dougherty PM,
Myers JN, Calin GA and Amit M: Cancer-associated neurogenesis and
nerve-cancer cross-talk. Cancer Res. 81:1431–1440. 2021. View Article : Google Scholar
|
|
40
|
Brown TP, Bhattacharjee P, Ramachandran S,
Sivaprakasam S, Ristic B, Sikder MOF and Ganapathy V: The lactate
receptor GPR81 promotes breast cancer growth via a paracrine
mechanism involving antigen-presenting cells in the tumor
microenvironment. Oncogene. 39:3292–3304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|