Introduction
Cancer is a serious public health concern with
~20,000,000 new cases diagnosed and 10,000,000 cases of
cancer-related mortality reported worldwide in 2020. According to a
World Health Organization (WHO) report, the incidence of cancer is
increasing, where it has been predicted that there may be ~28.4
million diagnosed cases worldwide by 2040 (1). Several factors have been reported to
contribute to carcinogenesis, although excessive uncontrolled cell
division caused by irregular cell signaling is common across most
if not all cancer types (2).
Hanahan and Weinberg (3)
previously proposed six hallmarks of cancer. Specifically, these
six hallmarks are resistance to inhibitory growth signals,
persistence of proliferative signaling, evasion of cell death,
initiation of angiogenesis, invasion and metastasis (3). Subsequently, Hanahan and Weinberg
(4) added two more details to this
six-hallmark concept in 2011, including dysregulation of cell
metabolism and circumvention of immune destruction to the critical
characteristics of cancer. A decade later, Hanahan (5) then expanded the hallmarks of cancer
to include the importance of non-mutational epigenetic
reprogramming, phenotype plasticity and the host-microbiome as
recent insights in cancer studies emerged. Among these integrative
concepts of cancer, the present review aimed to discuss the
association between the microbiome and cancer development, in
addition to between the microbiome and malignant progression.
The human body contains trillions of symbiotic
microbiomes, including a community of bacteria, viruses, fungi and
protozoa, which is collectively known as the human microbiota
(6,7). Although the skin, oral cavity,
respiratory tract and other body parts all harbor microbiomes, the
majority of the microbiome population is predominantly contained
within the gastrointestinal tract (8). Specifically, the host-associated
microbial community within the gastrointestinal system is called
the gut microbiome (1). They have
been documented to influence various physiological and
pathophysiological processes in the body (9). For example, the metabolites produced
by an imbalanced microbiota can result in the pathogenesis of
certain neurological conditions. Furthermore, physiological energy
homeostasis has been reported to be regulated by microbiota, and
microbiota-generated butyrate provides energy to colonocytes and
prevents autophagy in the colon (9). In addition, microbiota also disrupt
physiological homeostasis, host metabolism and contribute to the
immune dysregulation (9).
Over the past decade, high-throughput sequencing and
multi-omics methods have contributed to the understanding of gut
microbiome diversity, elucidating its functional potential
(10). Explorative research in the
possible relationship between the gut microbiota and various
diseases has revealed that alterations in the microbiome profile of
the human gut are associated with a number of diseases, including
obesity, cancer, diabetes and neurodegenerative diseases (11). However, the causative role mediated
by these microbiomes in various disease conditions remains to be
fully characterized. This altered population of microbiota has been
documented to be great influencers of the host physiology, in
processes including digestion, metabolism, cognition and immunity,
though additional as yet unrevealed functions are highly likely
(12). Several microbiome studies
have previously found that other established factors in cancer,
including obesity, inflammation and genotoxicity, are either
directly or indirectly associated with the interaction between the
human microbiota and the human system and metabolism (13,14).
Association between obesity and cancer
Obesity is becoming a major global issue and already
affects millions of individuals. A person is considered obese when
their body mass index (BMI) is >30 kg/m2. By this
metric, ~650,000,000 adults >18 years old are estimated to be
clinically obese worldwide, as reported by the WHO (15). Obesity has been reported to
associate the incidence of numerous ailments, including type 2
diabetes, hypertension, cardiovascular disease, osteoarthritis,
kidney failure, liver inflammation and cancer. In particular,
chronic inflammation and altered phenotype due to obesity are the
main driving factors in disease progression (16,17).
Previous studies have reported links between cancer
and obesity, specifically in terms of the associated mortality
rate. Obesity is an important contributing factor to the
development of various types of cancer. According to a previous
report, ~20% of cancer cases are associated with obesity (18). In addition, obesity enhances
colorectal cancer-associated mortality by 14 and 20% in men and
women, respectively (19).
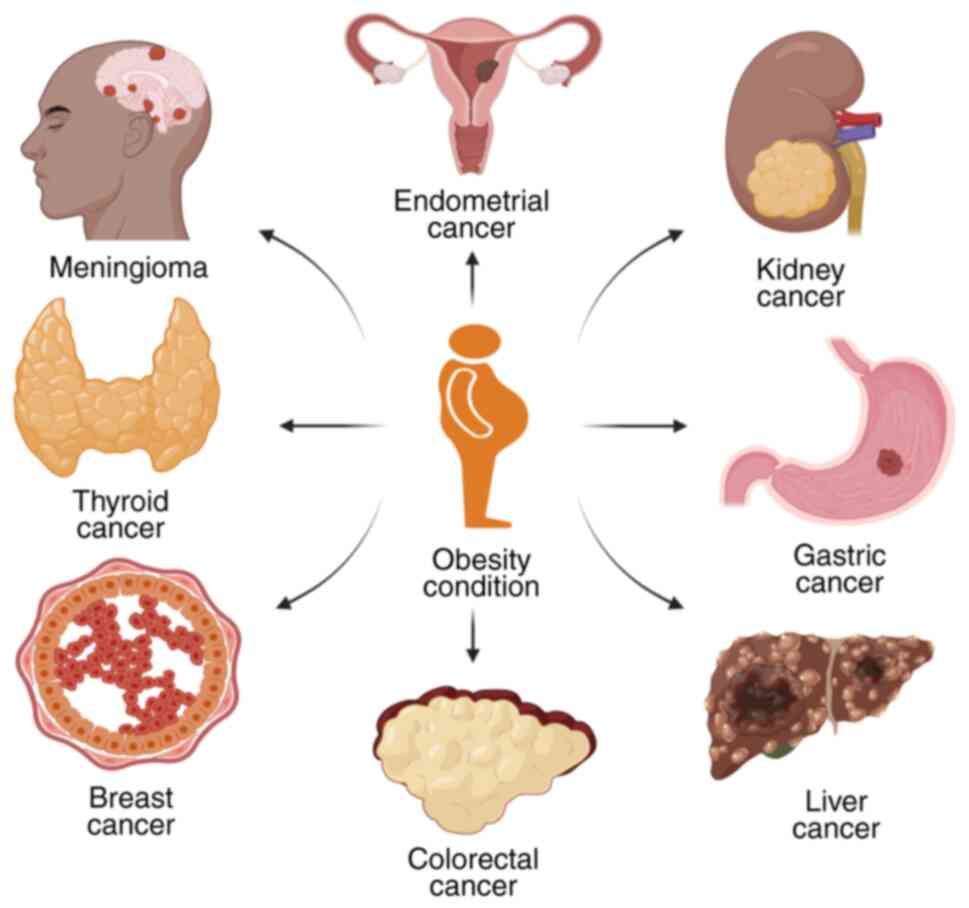
According to the International Agency for Research on Cancer,
obesity is associated with 13 different types of cancer, including
breast, colorectal, endometrial, gall bladder, kidney, gastric
cardia, ovarian, liver, pancreatic and thyroid cancer, and
adenocarcinoma, meningioma and multiple myeloma (20). However, obesity is not the only
isolated factor associated with cancer. Age, family history,
resident environment, body fat, alcohol, smoking, sex hormone
levels, insulin and nutrient levels have all been observed to
contribute to cancer development (21).
The excessive deposition of nutrients into the white
adipose tissue causes inflammation in obese individuals. Adipocytes
in this specialized type of tissue normally functions to store
triglycerides as a long-term source of energy in the form of
cytoplasmic lipid droplets. White adipose tissues can also secrete
various adipokines, including cytokines, hormones and growth
factors, which have a role in obesity-associated disease conditions
(22,23). These cytokines and growth factors
can activate a number of signaling pathways, including NF-κB, JNK
and protein kinase R pathways. Low-grade inflammation is induced
upon the stimulation of these signaling pathways, which causes the
release of common inflammatory cytokines, including IL-1, IL-6 and
TNF-α (24). Hyperplasia and
hypertrophy of adipocytes can also aggravate this low-grade
inflammatory response by increasing the production of free fatty
acids, tissue remodeling and changing the profile of adipokine
production (25). Furthermore,
overproduction of unfolded proteins and subsequent activation of
metabolic pathways by endoplasmic stress in obese individuals can
activate inflammatory responses (26). Although white adipocyte tissues
serve as the primary area for inflammatory pathway activation,
other tissues, such as the liver, brain and pancreas, are also
associated with obesity-induced inflammation (27). Due to the reported involvement of
adipokines in cancer cell growth, the role of leptin and
adiponectin in cancer have been widely studied (28,29).
It has been reported that low levels of adiponectin may have a
permissive role in triggering the neoplastic growth of ERα-positive
breast cancer cells through the MAPK-activating signaling pathway
(30). Obesity-associated
insulin-like growth factor I and insulin resistance are also
involved in the stimulation of several types of cancer, according
to data produced from previous in vitro and in vivo
analyses (31,32).
According to a previous meta-analysis, malignant
melanoma, colon, gallbladder, pancreatic and renal cancer are
significantly more common in obese men (33). In obese women, esophageal
adenocarcinoma, leukemia, endometrial, colon, gallbladder,
pancreatic, postmenopausal breast and renal cancer tended to be
more common (Fig. 1). Obese female
patients with breast cancer have been reported to show more
aggressive characteristics of tumor formation and metastasis
(34). In addition, the severity
of breast cancer in patients is greatly influenced by age, where
women with advancing age are more likely to gain weight and develop
obesity. A particularly high risk of breast cancer has been
reported in menopausal women between 50 and 60 years old, with
weight gain also proposed to be a contributing factor (35). Primary liver cancer is the sixth
most common type of cancer globally (36). Several factors have been documented
to impact the development of liver cancer. Being overweight is one
of the main factors that is associated with the mortality rate of
patients with liver cancer and hepatitis viral infection (37,38).
Another previous cohort study reported a 17 and 89% liver cancer
risk in overweight and obese individuals, respectively, compared
with the expected average (39).
In addition, this previous study showed that the relative risk of
liver cancer for overweight individuals was 1.17 and for obese
individuals was 1.89 compared with that in individuals of a healthy
weight (39). Liver cancer
associated with obesity or excess body weight was reported to be
more prevalent in men compared with women, with individuals with
excess body weight suffering from liver cirrhosis or hepatitis C
viral infection being more prevalent (40).
The increased incidence and prevalence of obesity
among populations have elevated the risk of gastrointestinal cancer
development. It has been suggested that excess adiposity is a
potential cause of gastrointestinal tract, pancreatic, colorectal,
esophageal, stomach and gallbladder cancer. The relationship
between obesity and gastrointestinal cancer has been proposed to be
associated with changes in insulin and insulin-like growth factor I
signaling, altered sex hormone metabolism and chronic low-grade
inflammation (41). Previous
studies have indicated a strong connection between overall fat
content and elevated gastro-esophageal reflux, Barrett's esophagus
and esophageal adenocarcinoma (42,43).
Although reflux can be adjusted in obese individuals, rates of
esophageal adenocarcinoma have been shown to be more prevalent
whereas waist circumference (a measure of obesity) is associated
with the high occurrence of Barrett's esophagus (44). The importance of obesity
(specifically central obesity) as a predisposing factor for
colorectal cancer, which is the third most common cause of global
cancer mortality, has been frequently reported (45,46).
The increased deposition of abdominal fat and associated obesity is
highly associated with the prevalence of colorectal cancer
(47).
Mechanistic role of bacteria in
carcinogenesis
The human gut is inhabited by a diverse microbiome,
which forms a complex community along the gastrointestinal tract
and contributes to the health and well-being of the host. Although
it is well-known that these microbiomes interact and maintain a
status of symbiosis with the host, alterations in the microbial
flora can adversely affect biological functions in the body
(4). A recent study by Hou et
al (48) on the role of the
human microbiota reported the dynamic role of the gut microbiota in
host health and its relation to pathogenesis of cancer. The gut
microbiome can influence the systemic functioning of the body
through the gut-brain axis, colonization resistance and
immunomodulation (48). In
addition, the strong influence of the human gut microbiota in
developing various ailments, including cancer, respiratory,
neurophysiological, hepatic and kidney diseases, has been
previously reported (Table I).
 | Table I.Mechanistic role of microbes in
carcinogenesis. |
Table I.
Mechanistic role of microbes in
carcinogenesis.
| Microbe | Associated
cancer | Key mechanisms | (Refs.) |
|---|
| Porphyromonas
gingivalis | Oral cancer | The
epithelial-mesenchymal conversion of oral epithelial cells,
inducing MMP-9 and IL-8 | (59) |
| Chlamydia
psittaci | Ocular adnexal
lymphoma | Regulating
oxidative DNA damage and modulating the NF-κB pathway connected
with anti-apoptotic effects | (57) |
| Mycobacterium
tuberculosis | Lung cancer | Modulating T-cell
immune response by elevating expression of the programmed cell
death protein-1/programmed death-ligand 1 pathway | (60) |
| Chlamydia
pneumoniae | Lung cancer | Triggering
monocytes to secrete TNF, IL-8 and superoxide radicals, which
promote cellular and DNA damage | (61) |
| Salmonella
typhi | Gallbladder
cancer | Typhoid
toxin-mediated alteration of cell cycle and DNA damage | (62) |
| Streptococcus
bovis/Streptococcus gallolyticus | Colorectal
cancer | Degrading
anticancer substances, including tannic acid, and triggering
inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-8, to
cause free radical formation, which results in DNA alteration and
leads to cancer condition | (63) |
|
Parabacteroides | Colorectal
cancer | Antagonize the
toll-like receptor 4 and AKT signaling pathways, which lead to
cancer development and progression | (125) |
| Helicobacter
pylori | Gastric cancer | Increase
accumulation of inflammatory cytokines including IFN-γ, IL-1, IL-6,
IL-7, IL-8, IL-10, IL-18 and TNF-α, and stimulate diverse ranges of
immune cells, including lymphocytes, eosinophils, macrophages, mast
cells and neutrophils | (49,50) |
Cancer is caused by a multitude of factors,
including both genome modulation and environmental causes. However,
the association between the human microbiota and cancer development
has not been considered until the 1990s. Microbiome involvement in
carcinogenesis was first detected in gastric cancer in 1994,
resulting in the subsequent recognition of Helicobacter
pylori as a group 1 carcinogen by the WHO (49,50).
The role of the human microbiota in several types of cancer has
since been reported, including oral squamous cell carcinoma
(51,52), lung cancer (53), breast cancer (54) and genitourinary cancer (55). Several mechanisms contributing to
cancer development have been observed to be mediated by the gut
microbiome, including the production of microbiota-derived
metabolites, immune dysregulation and the modification of genetic
and epigenetic factors (56).
Previous studies have demonstrated that the intracellular bacteria
Chlamydia psittaci can cause ocular adnexal lymphoma by
regulating oxidative DNA damage and modulating the NF-κB pathway
connected with anti-apoptotic effects (57,58).
In addition, the non-motile gram-negative bacteria Porphyromonas
gingivalis has been shown to be associated with oral cancer
through the epithelial-mesenchymal transition of oral epithelial
cells and by inducing MMP-9 and IL-8 expression (59). The pathogenic Mycobacterium
tuberculosis can also promote lung cancer development and
metastasis by modulating the T-cell immune response by elevating
the activity of the programmed cell death protein-1/programmed
death-ligand 1 pathway (60). In
addition, the intracellular pathogen Chlamydia pneumoniae
can promote lung cancer through by provoking the monocytes into
secreting TNF-α, IL-8 and superoxide radicals, which promote
cellular and DNA damage (61). The
gram-negative bacteria Salmonella typhi leads to gallbladder
cancer through typhoid toxin-mediated alteration of the cell cycle
and DNA damage (62). Other
non-motile gram-positive bacteria, including Streptococcus
bovis and Streptococcus gallolyticus, have also been
documented to contribute to colorectal cancer by degrading
anticancer substances, including tannic acid (63). Furthermore, bacteria can trigger
the release of inflammatory cytokines, including TNF-α, IL-1β, IL-6
and IL-8, to cause free radical formation, resulting in DNA
alterations and cancer (64).
Over the last two decades, there has been an
increase in studies investigating the impact of the gut microbiota
on cancer. However, the fundamental cause of gut microbiota-induced
tumor initiation remains unknown, meaning that it cannot yet be
exploited for the treatment of cancer. A number of different
hypotheses regarding the impact of the microbiota on cancer
prognosis and therapy have been proposed. Microbial metabolites can
interrupt cell signaling pathways involved in various processes,
such as cell proliferation, division, programmed cell death and
interaction with cell from other organs (65). It is also well-known that
inflammatory conditions can significantly increase the risk of
cancer, representing a pivotal hallmark of the complex process of
carcinogenesis. Previous studies have revealed that enteric
bacteria can significantly impact the immune system, serving a
crucial role in the development of local and systemic inflammation
(66,67). Notably, the gut microbiome can
inhibit infection by adjusting the niche environment and regulating
host immune defense (68). The
interaction between cancer cells and other cell types, such as
immune cells, myeloid cells and cancer-associated fibroblasts,
forms the tumor microenvironment (TME) (69). It is important to note that there
is accumulating evidence suggesting an essential link among gut
microbiome changes, inflammation and cancer development, in a
complex and multifaceted mechanism. High-throughput sequencing has
revealed that aspects of bacterial niches can be detected in TME of
various types of cancer, which may influence the development of
cancer by modulating the immune system (65,68).
The mechanistic role of microbes in carcinogenesis is detailed in
Table I. In a recent review on the
TME in various types of cancer, Chen et al (70) reported that the microbiota tended
to differ between cancer and normal tissues (70). A previous comparative case study of
gut microbial diversity and composition involving healthy
individuals and patients with colon cancer through 16S rDNA
sequencing revealed that the levels of beneficial bacteria were
decreased, whilst the levels of several harmful bacteria were
increased, in the cancer group (71). In another review of the interaction
between the microbiota and the immune system, it was described that
alterations in the gut microbiome can significantly impact the
immune system and related signaling mechanisms through direct cell
interaction or microbiota-derived metabolites (72).
Previous studies on the interaction between the gut
microbiome and immunity has revealed that the gut microbiome can
participate in immune modulation and can promote liver cancer
through bile acid metabolism (73,74).
Furthermore, assessing the role of microbial metabolites in immune
regulation revealed that some of these small to large
macromolecules can modulate cell signaling pathways that evoke a
positive or negative response in cells (72). Polyamine derivatives found in the
microbial metabolites have been reported to activate the Myc
oncogene, promoting cancer progression. The c-Myc class of
transcription factors are crucial for controlling the expression of
genes involved in cell growth, proliferation and differentiation.
In addition, butyrate derivatives have been demonstrated to exhibit
antitumor proprieties, inhibiting inflammation and carcinogenesis
by regulating Wnt and NF-κB signaling pathways
(75). The interplay among gut
microbiota, inflammation and immune responses can also affect the
systemic immune response. These aforementioned observations suggest
that the gut microbiome and its metabolites can significantly
impact various biological processes, such as cell metabolism and
immune regulation, and thus affect health (Fig. 2). Fig.
2 illustrates the intricate interplay between the gut
microbiome, cellular processes and cancer progression.
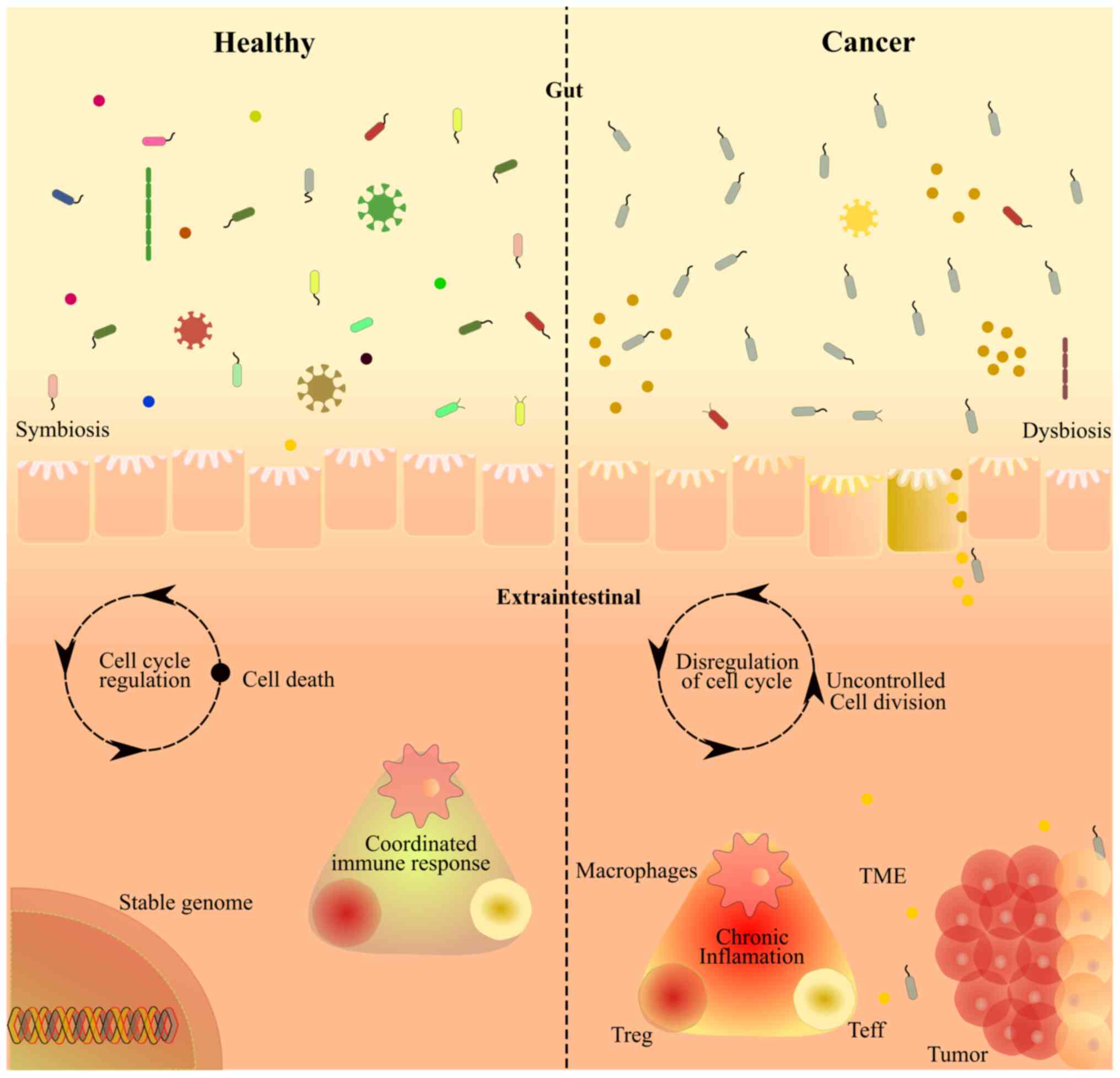 | Figure 2.An illustrative representation of the
intricate relationship between the gut microbiome, cellular
processes and cancer progression. The left side of the illustration
depicts a healthy symbiotic condition, characterized by its
diversity, with various microbial species maintaining a balanced
relationship with the host. By contrast, on the right side, the
illustration focuses on an inflamed condition, showing altered
microbiota composition and its impact on the cellular processes,
resulting in uncontrolled cell division through cell cycle
dysregulation. In this state, the microbial diversity is decreased,
which causes an imbalance in the microbiota. This type of dysbiosis
leads to disruption of the gut barrier, allowing the translocation
of microbial components and inflammatory molecules into the
bloodstream and nearby cells. It is proposed that a dysbiotic
microbiome enhances inflammation, promoting tumor growth and
metastasis. TME, tumor microenvironment; Treg, regulatory T cell;
Teff, effector T cell. |
Gut microbiome, obesity and gastric
cancer
According to the GLOBOCAN database, gastric cancer
is the fourth leading cause of cancer-related mortality worldwide
(1,76). Gastric cancer is particularly
prevalent in developing countries, with a two-fold higher incidence
rate in men compared with that in women (74). Several epidemiological studies have
highlighted the robust association between obesity and gastric
cancer. In a previous meta-analysis, Lin et al identified an
association between increased BMI and gastric cancer. In addition,
obesity (BMI, ≥30 kg/m2) was found to be associated with
an increased risk of gastric cancer compared with being overweight
(BMI, 25.1-30 kg/m2) and normal weight (BMI, 18.5-25
kg/m2) (77).
In 2014, Donohoe et al demonstrated the
inter-relationship between adipose and the TME in gastric cancer
(44). Specifically, inflammation,
hypoxia, energy metabolism and angiogenesis were all found to be
key factors associating obesity with gastric cancer, which are
commonly dysregulated in the TME and adipose tissue Karczewski
et al (41) previously
demonstrated that the primary pathophysiological mechanisms
connecting obesity and gastric cancer include altered levels of
insulin signaling, chronic low-grade inflammation in the adipose
tissue and altered steroid hormone production and metabolism. Lee
et al (78), in a Health
Examinees-Gem study, reported that obesity during early adulthood
(20-39 years) was significantly associated with an increased risk
of gastric cancer.
The interaction between microorganisms and tumors
has garnered widespread attention, with the aim of delineating the
features of the complex microbial communities and their possible
role in carcinogenesis (13).
Numerous studies have discussed the connection between gut microbes
and low-grade inflammation, which can regulate the innate and
adaptive immune responses, at least in part contributing to the
initiation and progression of oncogenic conditions (5,79).
H. pylori-mediated infection can stimulate
immune responses and inflammation to regulate gastric cancer
(80). The immune response of
epithelial and myeloid cells to H. pylori bacteria is
regulated by the gastric microbiota, which in turn determines the
outcome of the disease. The contact between H. pylori and
gastric epithelial and myeloid cells has been shown to induce
signaling through innate pathways, leading to changes in cellular
homeostasis, and the release of cytokines and chemokines that can
trigger the inflammatory responses. H. pylori infection
disturbs the balance between the host gastric microbiota and
mucosa-related factors leading to inflammatory changes, dysbiosis
and subsequently gastric cancer (81). H. pylori is a group 1
carcinogen that can promote gastric carcinogenesis by aggravating
inflammation in the gastric mucosa (81,82).
The key virulence factors of H. pylori, namely
cytotoxin-associated gene (Cag)A and vacuolating toxin A (VacA),
are associated with an increased risk of gastric cancer (13,83).
Infection with the H. pylori CagA strain results in an
increased accumulation of inflammatory cytokines, including
IFN-γ, IL-1, IL-6, IL-7, IL-8, IL-10, IL-18 and
TNF-α, which stimulates a diverse range of immune cells,
including peripheral mononuclear cells, lymphocytes, eosinophils,
macrophages, neutrophils and mast cells (13,84).
In addition, infection with the Cag+ strain leads to the
upregulation of the ERK/MAPK, PI3K/AKT, NF-κB,
Wnt/β-Catenin, Sonic Hedgehog and STAT3 signaling
pathways, whilst downregulating the activity of tumor suppressor
pathways (74,85).
The VacA virulence factor leads to cellular
vacuolation and autophagy in human gastric epithelial cells
(86,87). VacA has been reported to
upregulate MAPK, ERK1/2 and vascular endothelial growth
factor expression, whilst also stimulating the Wnt/β-catenin
signaling pathway, which is required for cell proliferation and
differentiation (88,89). Simultaneously, H. pylori
infection can also induce methylation in genes expressing the cell
adhesion glycoprotein E-cadherin and various tumor suppressors,
such as trefoil factor 2 and fork-head box transcriptional
regulator D3, leading to the increased risk of gastric
adenocarcinoma (90–92).
Advancements in sequencing technology have improved
the understanding into the complexity of the gastric microbiota
(13,93). H. pylori-negative
individuals harbor distinct microbial populations, including
Proteobacteria, Firmicutes and Actinobacteria (94,95).
Sequencing-based and quantitative PCR approaches have confirmed
that patients with gastric cancer tended to harbor a diverse
population of microbiota with a reduced level of Porphyromonas,
Neisseria, TM7 group and Streptococcus sinensis, but an
enriched level of Klebsiella pneumoniae, Lactobacillus
coleohominis and Lachnospiraceae (95,96).
The pathogenic components, including the outer-membrane,
nickel-binding and BAK proteins of non-H. pylori microbiota,
including Helicobacter cinaedi, Lactobacillus coleohominis
and Klebsiella pneumoniae, assist their colonization on the
gastric mucosal layer, leading to tumorigenesis in the stomach
(97).
A high-fat diet large quantities of fatty acids and
low amount of vitamins, fibers and minerals are considered the
primary cause of obesity (98).
Several studies have demonstrated that a high-fat diet can promote
gastric cancer through metabolic reprogramming and alteration of
intestinal microbes. Arita and Inagaki-Ohara (99) reported that a high-fat diet can
increase leptin signaling and promote gastric microbial dysbiosis,
leading to intestinal metaplasia. In addition, this previous study
found that a high-fat diet given to mice elevated
Lactobacillus levels and reduced Bifidobacteria
levels. Lactobacillus is a homofermentative probiotic
bacterium that converts lactose to lactic acid. Lactic acid is
associated with gastric carcinogenesis as it regulates
inflammation, metastasis and epithelial-mesenchymal transition
(100).
He et al (101) found that 12 weeks of a high-fat
diet feeding in C57BL/6 mice resulted in dysbiosis of the gastric
microbiota, with a reduced level of community diversity. This
high-fat diet also increased the levels of Proteobacteria and
Firmicutes, whilst downregulating those of Bacteroidetes and
Verrucomicrobiota. Furthermore, enriched levels of
Enterobacteriaceae were detected, which are associated with
increased plasma and fecal endotoxin production. Endotoxin is known
to trigger chronic inflammation and can induce obesity (102). Previously, Xiao et al
(103) reported that
endotoxin-producing opportunistic pathogens, such as
Enterobacteriaceae and Desulfovibrionaceae, can produce
lipopolysaccharide (LPS) and lead to metabolic endotoxemia. LPS can
enhance C-X-C chemokine receptor type 7 expression in gastric
cancer, subsequently regulating the proliferation and migration of
gastric cancer cells through the toll-like receptor 4
(TLR4)/myeloid differentiation factor 2 signaling pathway (104). Furthermore, Desulfovibrionaceae
can reduce sulfate to hydrogen sulfide (H2S) (105). H2S then activates the
fatty acid receptor CD36 in gastric cancer cells to stimulate lipid
metabolic reprogramming and gastric cancer metastasis (7).
Gut microbiome, obesity and colorectal
cancer
Colorectal cancer is the third most common cancer
diagnosed worldwide, with incidence rates 30% higher in men
compared with those in women (2).
Several epidemiological studies have demonstrated the relationship
between obesity and colorectal cancer. Bardou et al
(106) reported that obesity is
associated with 11% colorectal cancer cases in the European
population. Among the list of obesity conditions, visceral fat and
abdominal obesity are associated with a higher risk of colorectal
cancer compared with subcutaneous obesity. It has also been
previously reported that obese individuals exhibit a 20-40% higher
risk of colorectal cancer compared with normal-weight individuals
(107,108).
Obesity leads to colorectal cancer development
through inflammation, metabolic regulation and signaling processes
(109). In a meta-analysis on the
relationship between BMI and colorectal cancer, Ma et al
(110) reported the relevant risk
of colorectal cancer to be 1.334 (95% CI, 1.253-1.420) for obese
individuals compared with individuals of a healthy weight. In a
pooled analysis of eight population-based cohort studies, Matsuo
et al (111) also revealed
a strong association between obesity and colorectal cancer. The
association was stronger in men compared with that in women, with
the association higher in the proximal colon compared with the
rectum. Recently, Socol et al (112) demonstrated that genetic variation
and aberrant signaling in the leptin pathway were associated with
obesity and colorectal cancer. Leptin receptor (LEPR)
expression is critical for the proliferation of colorectal
carcinoma cells (Table II).
Higher expression of LEPR has been shown to lead to
neoangiogenesis and increased metastatic potential of colorectal
carcinoma. By contrast, a lack of LEPR expression reduces
tumor proliferation in colorectal carcinoma (113).
 | Table II.Association between obesity,
microbiome alteration and carcinogenesis. |
Table II.
Association between obesity,
microbiome alteration and carcinogenesis.
| Obesity
condition | Changes in gut
microbiome level | Associated
cancer | Mechanism | (Refs.) |
|---|
| High-fat diet
associated with obesity | Upregulation of
Lactobacillus and downregulation of
Bifidobacteria | Gastric cancer |
Lactobacillus converts lactose to
lactic acid, which acts as a major source of gastric
carcinogenesis | (98) |
|
| Enriched levels of
Enterobacteriaceae and Desulfovibrionaceae | Gastric cancer | Endotoxin-producing
opportunistic pathogens produce lipopolysaccharide, which enhances
C-X-C motif chemokine receptor R7 expression and promotes migration
of gastric cancer cells | (102,103) |
|
| Upregulated levels
of Desulfovibrionaceae | Gastric cancer | The bacteria reduce
sulfate to hydrogen sulfide, which induces fatty acid receptor CD36
in gastric cancer cells | (104, 105) |
| Western
diet-associated obesity | Enhances the level
of collagenase-producing microbes | Colorectal
cancer | Colonization of
collagenase-producing microbes leads to the transmigration of
colorectal cancer cells and results in recurrence | (122) |
|
| Upregulated levels
of Clostridium | Colorectal
cancer | Clostridium
converts the primary bile acids to secondary bile acids. Excessive
accumulation of bile acids leads to the development of colorectal
cancer | (124) |
| Western diet and
high-fat diet-associated obesity | Abundance of
Parabacteroides in intestine | Colorectal
cancer | Antagonizes the
toll-like receptor 4 and AKT signaling pathways, which leads to
colorectal cancer development and progression | (125) |
|
| Enrichment of
pro-inflammatory pathogens and reduction of butyrate-producing gut
microbes | Colorectal
cancer | Results in
dysbiosis and leads to tumor formation through enhanced levels of
the microbial metabolite trimethylamine N-oxide, the
pro-inflammatory cytokine IL-1β and the intestinal permeability
marker Zonulin, and reduced levels of the anti-inflammatory factor
IL-10 | (120) |
| Obesity with
non-alcoholic fatty liver | Increased levels of
liver proteobacteria | Liver cancer | Causes gut
dysbiosis, which promotes hepatocellular carcinoma | (131) |
| Dietary
obesity | Enhanced levels of
gram-positive intestinal microbiota | Liver cancer | Increases the
levels of gut bacterial metabolite deoxycholic acid, which causes
DNA damage and induces liver carcinoma | (132) |
A high-fat diet has been shown to promote colorectal
cancer progression and metastasis. Niku et al (114) previously demonstrated that a
Western diet, containing high fat and low fiber, calcium, vitamin D
and folate, acts as a risk factor for the development of colorectal
adenoma, through the heterozygous loss of adenomatous polyposis
coli and overactivation of the AKT, mTOR and ERK1/2 signaling
pathways in C57BL/6 J Min/+ mice. Park et al (115) demonstrated in another study that
high-fat diet-related obesity can lead to inflammation-associated
colorectal cancer by activating the PI3K/AKT signaling pathway and
triggering the expression of IL-12, monocyte chemoattractant
protein-1 and TNF-α in the TME.
Several studies have highlighted the altered gut
microbiota signatures in colorectal cancer development (6,7). The
lower population of the butyrate-producing Clostridium
cluster IV and XIV bacteria has previously been associated with
colorectal cancer (116).
Besides, the population of Firmicutes, Actinobacteria and
Lachnospiraceae have also been reported to be increased in
pre-malignant colorectal adenoma, whereas Proteobacteria,
Enterobacteriaceae and Sutterella species have been shown to
be increased during colorectal cancer progression (117). Apart from the bacterial
population, temperate phages have also been associated with the
development of colorectal cancer. The bacteriophages interact with
the host bacteria and stimulate the progression of colorectal
cancer by changing the bacterial community structure and regulating
the immune microenvironment (118).
Intestinal microbes can regulate polyamine
synthesis, LPS production, butyrate metabolism and oxidative
phosphorylation to facilitate the occurrence and development of
colorectal cancer (119,120). Schulz et al (121) reported that a high-fat diet can
lead to small intestinal tumor formation in
K-rasG12Dint mice by altering the composition of
intestinal microbes. Gaines et al showed that a Western
pattern diet can enhance the levels of collagenase-producing
microbes, including Proteus mirabilis, Candida parapsilosis
and Enterococcus faecalis, in the intestine of the BALB/c
mice (122). Colonization by
these microbes leads to the transmigration of colorectal cancer
cells and promotes colorectal cancer recurrence. Furthermore, the
intestinal microbes in obesity can enhance bile acid secretion in
the intestine (123). The
gram-positive Clostridium converts primary bile acids to
secondary bile acids, where the excessive accumulation of secondary
bile acids can lead to colorectal cancer (124). In addition, high-fat diet- and
Western diet-mediated obesity can reduce the abundance of
Parabacteroides in the intestine. Parabacteroides antagonize the
TLR4 and AKT signaling pathways (125), which are upregulated during
colorectal cancer development and progression. Therefore,
antagonizing Parabacteroides in the intestine through
high-fat diet-related obesity can promote colorectal cancer.
Sánchez-Alcoholado et al demonstrated that obese patients
with colorectal cancer exhibit a reduced population of
butyrate-producing gut microbes, including Butyricimonas,
Roseburia, Faecalibacterium and Ruminococcus, with an
abundance of opportunistic pathogens, including Fusobacterium,
Desulfovibrio, Clostridium and Enterococcus (120). The enrichment of these
proinflammatory pathogens and reduction in butyrate-producing
bacteria results in dysbiosis and leads to tumor formation, through
elevated levels of the deleterious microbial metabolite
trimethylamine N-oxide, the proinflammatory cytokine
IL-1β and the intestinal permeability marker Zonulin, in
addition to the reduced levels of the anti-inflammatory factor
IL-10.
Gut microbiome and liver cancer
Obesity and high-fat-content food are closely
associated with the progression of liver cancer. Liver cancer
progression is associated with the modified gut microbiome.
Previous reports have explored the involvement of the gut
microbiome in chronic liver disease and liver cancer (126,127). Liver inflammation causes changes
in the gut microbiome, causing microbiome dysbiosis and variations
in the intestinal barrier, resulting in a leaky gut (128). In the majority of cases, liver
cirrhosis leads to leakiness and dysbiosis, whilst increasing the
risk of liver cancer. Patients with liver cirrhosis can also
exhibit sudden bacterial peritonitis, contributing to
hepatocellular carcinoma (129).
Fatty liver disease is also associated with liver carcinoma, even
in non-alcoholic patients without cirrhosis (130). Intestinal microbiome dysbiosis in
non-alcoholic patients has also been characterized in the
pathogenesis of fatty liver disease (130).
Inflammatory bowel disease, type 1 diabetes,
autism, cardiovascular diseases, and obesity favor gut microbiome
alterations. Gut metabolites produced during gut dysbiosis induced
by obesity in the enterohepatic circulation have been reported to
promote hepatocellular carcinoma (7,131).
A previous study reported that circulation of deoxycholic acid
triggers the senescence-associated secretory phenotype in hepatic
stellate cells (HSCs), which can result in the secretion of several
tumor-promoting factors in the liver of C57BL/6 mice (131,132). The gut microflora-derived
bacterial products, such as LPS and bacterial DNA, and endogenous
substances, including free fatty acids, trigger the activation of
hepatic TLR4, which leads to liver inflammation, fibrosis and
cancer (132).
In a previous microbiome analysis of patients and
healthy individuals, patients with carcinoma induced by hepatitis B
were reported to possess an abundance of Escherichia,
Shigella and Enterococcus, whereas decreased levels of
Faecalibacterium, Ruminococcus, Ruminoclostridium sp.
Clostridium, Corynebacterium, Bacillus, Desulfovibrio and
Rhodococcus sp. were observed in non-alcoholic patients with
steatohepatitis-associated hepatocellular carcinoma. By contrast,
in patients with cirrhosis-related carcinoma, higher levels of
Epsilonproteobacteria, Actinobacteria, Clostridia,
Fusobacterium and Oribacterium were observed compared
with in healthy volunteers (133).
Gut microbiota interventions in cancer:
Pre-clinical and clinical studies
Data from model systems, such as rodents, coupled
with those from robust clinical trials, have provided evidence on
the role of the gut microbiome in the progression of
obesity-associated cancer, where insights have been gained on
potential therapeutic interventions. Previous pre-clinical and
clinical trials have studied the effect of microbial inflammation
on the development of the pathophysiology of different types of
cancer (14,134). Several pre-biotics have shown
anticancer properties against colorectal cancer models. Prenyl
flavonoids have been reported to improve the gut microbiome in
in vitro models of colorectal cancer and to exert anticancer
activity (134). In addition, a
high-fat diet (HFD) is known to induce gut dysbiosis. The oral
intake of agaro-oligosaccharides (AGO) has been shown to prevent
HFD-mediated gut dysbiosis and to thus inhibit colon carcinogenesis
in C57BL/6N mice. This previous study reported that phospholipids
and bile acids were downregulation in C57BL/6N mice receiving a HFD
alone, whereas this downregulation was recovered with the
administration of AGO supplements (135). A double-blind crossover study
involving healthy human adult volunteers (n=31) reported that
polydextrose (PDX) can modulate the composition and function of the
colonic microbiota. Besides, PDX was found to be associated with
the change in microbial metabolism, including production of
butyrate and reduction in metabolic byproducts of bacterial
putrefaction including branched-chain fatty acids. This previous
study demonstrated that PDX significant reduced the fecal water
genotoxicity of volunteers after consumption, thus indicating the
potential of PDX for reducing the risk factors associated with
colorectal cancer (136).
Fermentation of sugar in the colon tends to favor
butyrate-producing bacteria, Ruminococcus and Clostridium
clusters I, II and IV (136).
Administration of the antibiotic cefoxitin in a murine model has
been shown to reduce enterotoxigenic Bacteroides
fragilis-driven inflammation and colon cancer (13,137). In addition, short-chain fatty
acids favor the restoration of intestinal health and the gut
microbiome to prevent colon cancer. Several mice and human studies
have demonstrated the role of short-chain fatty acid-synthesizing
bacteria in the treatment and prevention of cancer (138,139).
In a mouse model, altered intestinal microbiota can
in turn alter antitumor immune surveillance, which can increase the
risk of liver disease and therefore cancer development.
NEMO∆EMOc/Nlrp6−/− mice exhibited the
hallmarks of intestinal dysbiosis, as well as aggravated
steatohepatitis and increased tumor burden. A significant finding
of this previous study was that the loss of intestinal
Akkermansia muciniphila could increase the abundance of
hepatic monocytic myeloid-derived suppressor cells and T cells
associated with the proliferation and expansion of liver cancer
cells (140). Understanding the
gut-liver axis and microbiome involvement in liver carcinoma
development may facilitate the design of effective therapeutic
methods. Data from rodent models and clinical trials have suggested
using the gut-liver axis as a target for inhibiting liver cancer
but not for the complete treatment. Several drugs, such as
antibiotics, probiotics, TLR4 antagonists and prokinetics, have
been shown to control non-cancerous liver disease and carcinoma
progression in rodent models and human patients (141). In a previous retrospective study,
patients with liver cirrhosis who received rifaximin exhibited a
reduced risk of liver carcinoma development (142). In another study involving
patients with liver cirrhosis, administration of different
antibiotics and probiotics was found to reduce the development of
primary hepatocellular carcinoma and mortality (143). Fecal microbiota transplantation
(FMT) is one of the methods used to modulate the gut microbiota and
reverse dysbiosis. During this process, a new bacterial population
is transferred to the recipient to reverse the dysbiosis that
occurred. Microbial species equilibrium is maintained in the gut by
introducing fecal transplants from healthy individuals (140). FMT has been reported to improve
survival in patients with metastatic gastro-esophageal cancer
studied in randomized, double-blind, placebo-controlled pilot
trials (143,144). By contrast, the transfer of
allogenic FMT from an obese donor to a lean recipient can induce
the obese phenotype and its associated metabolic dysfunctions in
the lean recipient (145).
Another study previously revealed the effectiveness of FMT along
with anti-programmed cell death protein 1 in six out of 15 patients
with melanoma (146). Several
mouse model studies and clinical trials have provided information
on FMT and its ability to reverse the intestinal dysbiosis, relieve
colon and hepatocellular carcinoma symptoms, whilst facilitating
their management (147,148).
Future directions and conclusions
The Gut microbiota aids in the homeostasis of
health and disease. The commensal gut microbiome helps to maintain
homeostasis by producing beneficial metabolites. However, under the
conditions of altered microbiomes induced by various factors, it
can promote carcinogenesis. The present review discussed the close
association between gut microbes and gastric, colorectal and liver
cancer, whilst also discussing potential prevention strategies. It
has been observed that microbiome dysbiosis leads to alterations in
the profile of essential metabolites, which in turn causes the
production of toxins. These metabolites enhance inflammation in the
host and can lead to the formation of tumors. Obesity has been
reported to serve as a factor associated with the initiation and
progression of 13 types of cancer. Therefore, exploring and
employing appropriate microbes or microbial-derived molecules is
recommended to develop a beneficial gut microbiota that can elicit
appropriate immune responses against cancer cells. Probiotics and
other cancer therapies are recommended for re-establishing gut
microbiota and producing an anti-tumorigenesis environment. In the
future, more personalized trials or approaches are sorted to verify
the effects of probiotics on different types of cancer by
modulating the microbiome.
Acknowledgements
Not applicable.
Funding
RK thanks the Science and Engineering Research Board, Govt. of
India (grant no. SERB/2018/001085) for partial financial
assistance. HP thanks the ICMR-SRF fellowship (Grant no.
2021-12759) Govt. of India.
Availability of data and materials
Not applicable.
Authors' contributions
RK designed the study. HP, SP, and VA prepared the
figures. RK, HP, SP, and VA wrote the manuscript. RK, SB, SP, and
MD critically revised the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deo SVS, Sharma J and Kumar S: GLOBOCAN
2020 report on global cancer burden: Challenges and opportunities
for surgical oncologists. Ann Surg Oncol. 29:6497–6500. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ursell LK, Metcalf JL, Parfrey LW and
Knight R: Defining the human microbiome. Nutr Rev. 70:S38–S44.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang R, Tang R, Li B, Ma X, Schnabl B and
Tilg H: Gut microbiome, liver immunology, and liver diseases. Cell
Mol Immunol. 18:4–17. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dekaboruah E, Suryavanshi MV, Chettri D
and Verma AK: Human microbiome: An academic update on human body
site specific surveillance and its possible role. Arch Microbiol.
8:2147–2167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuźniar A, Szawica D, Wąsiewicz E,
Fularska K and Oleszko M: Human gut microbiome-how intestinal
bacteria influence our health. J Educ Health Sport. 1:30–35. 2023.
View Article : Google Scholar
|
|
10
|
Zhu X, Li B, Lou P, Dai T, Chen Y, Zhuge
A, Yuan Y and Li L: The relationship between the gut microbiome and
neurodegenerative diseases. Neurosci Bull. 37:1510–1522. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:1–15.
2013. View Article : Google Scholar
|
|
12
|
Guinane CM and Cotter PD: Role of the gut
microbiota in health and chronic gastrointestinal disease:
Understanding a hidden metabolic organ. Ther Adv Gastroenterol.
4:295–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng C, Bai C, Brown TD, Hood LE and Tian
Q: Human gut microbiota and gastrointestinal cancer. Genomics
Proteomics Bioinformatics. 16:33–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatt AP, Redinbo MR and Bultman SJ: The
role of the microbiome in cancer development and therapy. CA Cancer
J Clin. 67:326–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boutari C and Mantzoros CS: A 2022 update
on the epidemiology of obesity and a call to action: As its twin
COVID-19 pandemic appears to be receding, the obesity and
dysmetabolism pandemic continues to rage on. Metabolism.
133:1552172022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S adults. N Engl J Med.
17:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin-Rodriguez E, Guillen-Grima F, Martí
A and Brugos-Larumbe A: Comorbidity associated with obesity in a
large population: The APNA study. Obes Res Clin Pract. 5:435–447.
2003.PubMed/NCBI
|
|
18
|
Wolin KY, Carson K and Colditz GA: Obesity
and cancer. Oncologist. 6:556–565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amersi F, Agustin M and Ko CY: Colorectal
cancer: Epidemiology, risk factors, and health services. Clin Colon
Rectal Surg. 3:133–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lauby-Secretan B, Scoccianti C, Loomis D,
Grosse Y, Bianchini F and Straif K: Body fatness and
cancer-viewpoint of the IARC working group. N Engl J Med.
8:794–798. 2016. View Article : Google Scholar
|
|
21
|
Deslypere JP: Obesity and cancer.
Metabolism. 44:24–27. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fasshauer M and Blüher M: Adipokines in
health and disease. Trends Pharmacol Sci. 7:461–470. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gregor MF and Hotamisligil GS:
Inflammatory mechanisms in obesity. Annu Rev Immunol. 29:415–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Longo M, Zatterale F, Naderi J, Parrillo
L, Formisano P, Raciti GA, Beguinot F and Miele C: Adipose tissue
dysfunction as determinant of obesity-associated metabolic
complications. Int J Mol Sci. 20:23582019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amen OM, Sarker SD, Ghildyal R and Arya A:
Endoplasmic reticulum stress activates unfolded protein response
signaling and mediates inflammation, obesity, and cardiac
dysfunction: Therapeutic and molecular approach. Front Pharmacol.
10:9772019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cnop M, Foufelle F and Velloso LA:
Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med.
18:59–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolb R, Sutterwala FS and Zhang W: Obesity
and cancer: Inflammation bridges the two. Curr Opin Pharmacol.
29:77–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fenton JI, Hord NG, Lavigne JA, Perkins SN
and Hursting SD: Leptin, insulin-like growth factor-1, and
insulin-like growth factor-2 are mitogens in ApcMin/+ but not
Apc+/+ colonic epithelial cell lines. Cancer Epidemiol Biomarkers
Prev. 14:1646–1652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
VanSaun MN: Molecular pathways:
Adiponectin and leptin signaling in cancer. Clin Cancer Res.
19:1926–1932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hursting SD, Nunez ND, Varticovski L and
Vinson C: The obesity-cancer link: Lessons learned from a fatless
mouse. Cancer Res. 67:2391–2393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohen DH and LeRoith D: Obesity, type 2
diabetes, and cancer: The insulin and IGF connection. Endocr Relat
Cancer. 19:F27–F45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amin MN, Hussain MS, Sarwar MS, Moghal MM,
Das A, Hossain MZ, Chowdhury JA, Millat MS and Islam MS: How the
association between obesity and inflammation may lead to insulin
resistance and cancer. Diabetes Metab Syndr. 13:1213–1224. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dobbins M, Decorby K and Choi BCK: The
association between obesity and cancer risk: A meta-analysis of
observational studies from 1985 to 2011. ISRN Prev Med. 2013:1–16.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiralerspong S and Goodwin PJ: Obesity and
breast cancer prognosis: Evidence, challenges, and opportunities. J
Clin Oncol. 34:4203–4216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Osman MA and Hennessy BT: Obesity
correlation with metastases development and response to first-line
metastatic chemotherapy in breast cancer. Clin Med Insights Oncol.
9:105–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rumgay H, Arnold M, Ferlay J, Lesi O,
Cabasag CJ, Vignat J, Laversanne M, McGlynn KA and Soerjomataram I:
Global burden of primary liver cancer in 2020 and predictions to
2040. J Hepatol. 77:1598–1606. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El-Serag HB and Mason AC: Risk factors for
the rising rates of primary liver cancer in the United States. Arch
Intern Med. 160:3227–3230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ohishi W, Fujiwara S, Cologne JB, Suzuki
G, Akahoshi M, Nishi N, Tsuge M and Chayama K: Impact of radiation
and hepatitis virus infection on risk of hepatocellular carcinoma.
Hepatology. 53:1237–1245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Larsson S and Wolk A: Overweight, obesity
and risk of liver cancer: A meta-analysis of cohort studies. Br J
Cancer. 97:1005–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Wang X, Wang J, Yan Z and Luo J:
Excess body weight and the risk of primary liver cancer: An updated
meta-analysis of prospective studies. Eur J Cancer. 48:2137–2145.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karczewski J, Begier-Krasińska B,
Staszewski R, Popławska E, Gulczynska-Elhadi K and Dobrowolska A:
Obesity and the risk of gastrointestinal cancers. Dig Dis Sci.
64:2740–2749. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
El-Serag HB, Ergun GA, Pandolfino J,
Fitzgerald S, Tran T and Kramer JR: Obesity increases oesophageal
acid exposure. Gut. 56:749–755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Long E and Beales IL: The role of obesity
in oesophageal cancer development. Ther Adv Gastroenterol.
7:247–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Donohoe CL, O'Farrell NJ, Doyle SL and
Reynolds JV: The role of obesity in gastrointestinal cancer:
Evidence and opinion. Ther Adv Gastroenterol. 7:38–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pati S, Irfan W, Jameel A, Ahmed S and
Shahid RK: Obesity and cancer: A current overview of epidemiology,
pathogenesis, outcomes, and management. Cancers (Basel).
15:4852023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rawla P, Sunkara T and Barsouk A:
Epidemiology of colorectal cancer: Incidence, mortality, survival,
and risk factors. Prz Gastroenterology. 14:89–103. 2019.
|
|
47
|
Soltani G, Poursheikhani A, Yassi M,
Hayatbakhsh A, Kerachian M and Kerachian MA: Obesity, diabetes and
the risk of colorectal adenoma and cancer. BMC Endocr Disord.
19:1132019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D,
Xiao C, Zhu D, Koya JB, Wei L, Li J and Chen ZS: Microbiota in
health and diseases. Signal Transduct Target Ther. 7:1352022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Barra WF, Sarquis DP, Khayat AS, Khayat
BCM, Demachki S, Anaissi AKM, Ishak G, Santos NPC, Dos Santos SEB,
Burbano RR, et al: Gastric cancer microbiome. Pathobiology.
88:156–169. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chattopadhyay I, Verma M and Panda M: Role
of oral microbiome signatures in diagnosis and prognosis of oral
cancer. Technol Cancer Res Treat. 18:15330338198673542019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang Y, Dai D, Jin W, Huang Y, Zhang Y,
Chen Y, Wang W, Lin W, Chen X, Zhang J, et al: Microbiota and
metabolites alterations in proximal and distal gastric cancer
patients. J Transl Med. 20:4392022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Irfan M, Delgado RZR and Frias-Lopez J:
The oral microbiome and cancer. Front. Immunol.
11:5910882020.PubMed/NCBI
|
|
53
|
Zhao Y, Liu Y, Li S, Peng Z, Liu X, Chen J
and Zheng X: Role of lung and gut microbiota on lung cancer
pathogenesis. J Cancer Res Clin Oncol. 147:2177–2186. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ruo SW, Alkayyali T, Win M, Tara A, Joseph
C, Kannan A, Srivastava K, Ochuba O, Sandhu JK, Went TR, et al:
Role of gut microbiota dysbiosis in breast cancer and novel
approaches in prevention, diagnosis, and treatment. Cureus.
26:e174722021.PubMed/NCBI
|
|
55
|
Nicolaro M, Portal DE, Shinder B, Patel HV
and Singer EA: The human microbiome and genitourinary malignancies.
Ann Transl Med. 8:12452020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Allen J and Sears CL: Impact of the gut
microbiome on the genome and epigenome of colon epithelial cells:
Contributions to colorectal cancer development. Genome Med.
25:112019. View Article : Google Scholar
|
|
57
|
Collina F, Chiara AD, Renzo AD, Rosa GD,
Botti G and Franco R: Chlamydia psittaci in ocular adnexa MALT
lymphoma: A possible role in lymphomagenesis and a different
geographical distribution. Infect Agent Cancer. 7:82012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang T, Wu H, Chen X and Chen L, Liu L, Li
Z, Bai Q, Chen Y and Chen L: The hypothetical inclusion membrane
protein CPSIT_0846 regulates mitochondrial-mediated host cell
apoptosis via the ERK/JNK signaling pathway. Front Cell Infect
Microbiol. 11:6074222021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Olsen I and Yilmaz Ö: Possible role of
Porphyromonas gingivalis in orodigestive cancers. J Oral Microbiol.
11:15634102019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao S, Li J, Lu J, Zhong R and Zhong H:
Mycobacterium tuberculosis antigens repress Th1 immune response
suppression and promotes lung cancer metastasis through PD-1/PDl-1
signaling pathway. Cell Death Dis. 10:442019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Littman AJ, Jackson LA and Vaughan TL:
Chlamydia pneumoniae and lung cancer: Epidemiologic evidence.
Cancer Epidemiol Biomarkers Prev. 14:773–778. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Di Domenico EG, Cavallo I, Pontone M, Toma
L and Ensoli F: Biofilm producing Salmonella Typhi: Chronic
colonization and development of gallbladder cancer. Int J Mol Sci.
18:18872017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Oehmcke-Hecht S, Mandl V, Naatz LT,
Dühring L, Köhler J, Kreikemeyer B and Maletzki C: Streptococcus
gallolyticus abrogates anti-carcinogenic properties of tannic acid
on low-passage colorectal carcinomas. Sci Rep. 10:47142020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Abdulamir AS, Hafidh RR and Bakar FA: The
association of Streptococcus bovis/gallolyticus with colorectal
tumors: The nature and the underlying mechanisms of its etiological
role. J Exp Clin Cancer Res. 30:112011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou X, Kandalai S, Hossain F and Zheng Q:
Tumor microbiome metabolism: A game changer in cancer development
and therapy. Front Oncol. 12:9334072022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Johansson P, Eckstein A and Küppers R:
Biology of ocular adnexal marginal zone lymphomas. Cancers (Basel).
14:12642022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yoo JY, Groer M, Dutra SVO, Sarkar A and
McSkimming DI: Gut microbiota and immune system interactions.
Microorganisms. 8:15872020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ge Y, Wang X, Guo Y, Yan J, Abuduwaili A,
Aximujiang K, Yan J and Wu M: Gut microbiota influence tumor
development and Alter interactions with the human immune system. J
Exp Clin Cancer Res. 40:422021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kovács T, Mikó E, Ujlaki G, Sári Z and Bai
P: The microbiome as a component of the tumor microenvironment.
Tumor Microenviron. 2020:137–153. 2020. View Article : Google Scholar
|
|
70
|
Chen Y, Wu FH, Wu PQ, Xing HY and Ma T:
The role of the tumor microbiome in tumor development and its
treatment. Front Immunol. 13:9358462023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
He T, Cheng X and Xing C: The gut
microbial diversity of colon cancer patients and the clinical
significance. Bioengineered. 12:7046–7060. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng D, Liwinski T and Elinav E:
Interaction between microbiota and immunity in health and disease.
Cell Res. 30:492–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chénard T, Prévost K, Dubé J and Massé E:
Immune system modulations by products of the gut microbiota.
Vaccines (Basel). 8:4612020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention.
Gastroenterol Rev Gastroenterol. 14:26–38. 2018. View Article : Google Scholar
|
|
75
|
Hanus M, Parada-Venegas D, Landskron G,
Wielandt AM, Hurtado C, Alvarez K, Hermoso MA, López-Köstner F and
la Fuente MD: Immune system, microbiota, and microbial metabolites:
The unresolved triad in colorectal cancer microenvironment. Front
Immunol. 12:6128262021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ilic M and Ilic I: Epidemiology of stomach
cancer. World J Gastroenterol. 28:1187–1203. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lin XJ, Wang CP, Liu XD, Yan KK, Li S, Bao
HH, Zhao LY and Liu X: Body mass index and risk of gastric cancer:
A meta-analysis. Jpn J Clin Oncol. 44:783–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee HW, Huang D, Shin WK, de la Torre K,
Yang JJ, Song M, Shin A, Lee JK and Kang D: Obesity at early
adulthood increases risk of gastric cancer from the health
Examinees-Gem (HEXA-G) study. PLoS One. 17:e02608262022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cani PD and Jordan BF: Gut
microbiota-mediated inflammation in obesity: A link with
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 15:671–682.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Doorakkers E, Lagergren J, Engstrand L and
Brusselaers N: Eradication of helicobacter pylori and gastric
cancer: A systematic review and meta-analysis of cohort studies. J
Natl Cancer Inst. 108:djw1322016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Toh JWT and Wilson RB: Pathways of gastric
carcinogenesis, helicobacter pylori virulence and interactions with
antioxidant systems, vitamin C and phytochemicals. Int J Mol Sci.
21:64512020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Colotta F, Allavena A, Sica C, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Khatoon J, Rai RP and Prasad KN: Role of
helicobacter pylori in gastric cancer: Updates. World J
Gastrointest Oncol. 8:147–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Moyat M and Velin D: Immune responses to
Helicobacter pylori infection. World J Gastroenterol. 20:5583–5593.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Udhayakumar G, Jayanthi V, Devaraj N and
Devaraj H: Interaction of MUC1 with β-catenin modulates the Wnt
target Gene cyclinD1 in H. pylori-induced gastric cancer. Mol
Carcinog. 46:807–817. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hotchin NA, Cover TL and Akhtar N: Cell
vacuolation induced by the VacA cytotoxin ofhelicobacter pylori is
regulated by the Rac1 GTPase. J Biol Chem. 275:14009–14012. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yahiro K, Akazawa Y, Nakano M, Suzuki H,
Hisatune J, Isomoto H, Sap J, Noda M, Moss J and Hirayama T:
Helicobacter pylori VacA induces apoptosis by accumulation of
connexin 43 in autophagic vesicles via a Rac1/ERK-dependent
pathway. Cell Death Discov. 1:150352015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Caputo R, Tuccillo C, Manzo BA, Zarrilli
R, Tortora G, Blanco CD, Ricci V, Ciardiello F and Romano M:
Helicobacter pylori VacA toxin up-regulates vascular endothelial
growth factor expression in MKN 28 gastric cells through an
epidermal growth factor receptor-, cyclooxygenase-2-dependent
mechanism1. Clin Cancer Res. 9:2015–2021. 2003.PubMed/NCBI
|
|
89
|
Song X, Xin N, Wang W and Zhao C:
Wnt/β-catenin, an oncogenic pathway targeted by H. pylori in
gastric carcinogenesis. Oncotarget. 6:35579–35588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Muhammad JS, Eladl MA and Khoder G:
Helicobacter pylori-induced DNA methylation as an epigenetic
modulator of gastric cancer: Recent outcomes and future direction.
Pathogens. 8:232019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Peterson AJ, Menheniott TR, O'Connor L,
Walduck AK, Fox JG, Kawakami K, Minamoto T, Ong EK, Wang TC, Judd
LM and Giraud AS: Helicobacter pylori infection promotes
methylation and silencing of trefoil factor 2, leading to gastric
tumor development in mice and humans. Gastroenterology.
139:2005–2017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sato F and Meltzer SJ: CpG island
hypermethylation in progression of esophageal and gastric cancer.
Cancer. 106:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Smet A, Kupcinskas J, Link A, Hold GL and
Bornschein J: The role of microbiota in gastrointestinal cancer and
cancer treatment: Chance or curse? Cell Mol Gastroenterol Hepatol.
13:857–874. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Engstrand L and Lindberg M: Helicobacter
pylori and the gastric microbiota. Best Pract Res Clin
Gastroenterol. 27:39–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Aviles-Jimenez F, Vazquez-Jimenez F,
Medrano-Guzman R, Mantilla A and Torres J: Stomach microbiota
composition varies between patients with non-atrophic gastritis and
patients with intestinal type of gastric cancer. Sci Rep.
4:42022014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Dias-Jácome E, Libânio D, Borges-Canha M,
Galaghar A and Pimentel-Nunes P: Gastric microbiota and
carcinogenesis: The role of non-Helicobacter pylori bacteria-A
systematic review. Rev Esp Enferm Dig. 108:530–540. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
De Witte C, Schulz C, Smet A,
Malfertheiner P and Haesebrouck F: Other Helicobacters and gastric
microbiota. Helicobacter. 21:62–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tong Y, Gao H, Qi Q, Liu X, Li J, Gao J,
Li P, Wang Y, Du L and Wang C: High fat diet, gut microbiome and
gastrointestinal cancer. Theranostics. 11:5889–5910. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Arita S and Inagaki-Ohara K:
High-fat-diet-induced modulations of leptin signaling and gastric
microbiota drive precancerous lesions in the stomach. Nutrition.
67–68. 1105562019.
|
|
100
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
He C, Cheng D, Peng C, Li Y, Zhu Y and Lu
N: High-fat diet induces dysbiosis of gastric microbiota prior to
gut microbiota in association with metabolic disorders in mice.
Front Microbiol. 9:6392018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kim KA, Gu W, Lee IA, Joh EH and Kim DH:
High fat diet-induced gut microbiota exacerbates inflammation and
obesity in mice via the TLR4 signaling pathway. PLoS One.
7:e477132012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xiao S, Fei N, Pang X, Shen J, Wang L,
Zhang B, Zhang M, Zhang X, Zhang C, Li M, et al: A gut
microbiota-targeted dietary intervention for amelioration of
chronic inflammation underlying metabolic syndrome. FEMS Microbiol
Ecol. 87:357–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li N, Xu H, Ou Y, Feng Z, Zhang Q, Zhu Q
and Cai Z: LPS-induced CXCR7 expression promotes gastric Cancer
proliferation and migration via the TLR4/MD-2 pathway. Diagn
Pathol. 14:32019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang XHF, Giuliano M, Trivedi MV, Schiff
R and Osborne CK: Metastasis dormancy in estrogen receptor-positive
breast cancer. Clin Cancer Res. 19:6389–6397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bardou M, Barkun AN and Martel M: Obesity
and colorectal cancer. Gut. 62:933–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pischon T and Nimptsch K: Obesity and risk
of cancer: An introductory overview in obesity and cancer. Recent
Results Cancer Res. 208:1–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chaplin A, Rodriguez RM, Segura-Sampedro
JJ, Ochogavía-Seguí A, Romaguera D and Barceló-Coblijn G: Insights
behind the relationship between colorectal cancer and obesity: Is
visceral adipose tissue the missing link? Int J Mol Sci.
23:131282022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou
Y and Qin H: Obesity and risk of colorectal cancer: A systematic
review of prospective studies. PLoS One. 8:e539162013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Matsuo K, Mizoue T, Tanaka K, Tsuji I,
Sugawara Y, Sasazuki S, Nagata C, Tamakoshi A, Wakai K, Inoue M, et
al: Association between body mass index and the colorectal cancer
risk in Japan: Pooled analysis of population-based cohort studies
in Japan. Ann Oncol. 23:479–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Socol CT, Chira A, Martinez-Sanchez MA,
Nuñez-Sanchez MA, Maerescu CM, Mierlita D, Rusu AV, Ruiz-Alcaraz
AJ, Trif M and Ramos-Molina B: Leptin signaling in obesity and
colorectal cancer. Int J Mol Sci. 23:47132022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Milosevic VS, Vukmirovic FC, Krstic MC,
Zindovic MM, Stojanovic DL and Jancic SA: Involvement of leptin
receptors expression in proliferation and neoangiogenesis in
colorectal carcinoma. J BUON. 20:100–108. 2015.PubMed/NCBI
|
|
114
|
Niku M, Pajari AM, Sarantaus L, Päivärinta
E, Storvik M, Heiman-Lindh A, Suokas S, Nyström M and Mutanen M:
Western diet enhances intestinal tumorigenesis in Min/+ mice,
associating with mucosal metabolic and inflammatory stress and loss
of Apc heterozygosity. J Nutr Biochem. 39:126–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Park S, Koh E, Koo JS, Kim SI, Park BW and
Kim KS: Lack of both androgen receptor and forkhead box A1 (FOXA1)
expression is a poor prognostic factor in estrogen
receptor-positive breast cancers. Oncotarget. 8:82940–82955. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gheorghe AS, Negru ȘM, Preda M, Mihăilă
RI, Komporaly IA, Dumitrescu EA, Lungulescu CV, Kajanto LA,
Georgescu B, Radu EA and Stănculeanu DL: Biochemical and
metabolical pathways associated with microbiota-derived butyrate in
colorectal cancer and omega-3 fatty acids implications: A narrative
review. Nutrients. 14:11522022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mori G, Rampelli S, Orena BS, Rengucci C,
De Maio G, Barbieri G, Passardi A, Gardini AC, Frassineti GL,
Gaiarsa S, et al: Shifts of faecal microbiota during sporadic
colorectal carcinogenesis. Sci Rep. 8:103292018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hannigan GD, Duhaime MB, Ruffin MT IV,
Koumpouras CC and Schloss PD: Diagnostic potential and interactive
dynamics of the colorectal cancer virome. mBio. 9:e02248–e02218.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Fang Y, Yan C, Zhao Q, Xu J, Liu Z, Gao J,
Zhu H, Dai Z, Wang D and Tang D: The roles of microbial products in
the development of colorectal cancer: A review. Bioengineered.
12:720–735. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sánchez-Alcoholado L, Ramos-Molina B,
Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J
and Queipo-Ortuño MI: The role of the gut microbiome in colorectal
cancer development and therapy response. Cancers (Basel).
12:14062020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Schulz MD, Atay C, Heringer J, Romrig FK,
Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C,
et al: High-fat-diet-mediated dysbiosis promotes intestinal
carcinogenesis independently of obesity. Nature. 514:508–512. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Gaines S, van Praagh JB, Williamson AJ,
Jacobson RA, Hyoju S, Zaborin A, Mao J, Koo HY, Alpert L,
Bissonnette M, et al: Western diet promotes intestinal colonization
by collagenolytic microbes and promotes tumor formation after
colorectal surgery. Gastroenterology. 158:958–970.e2. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zeng H, Umar S, Rust B, Lazarova D and
Bordonaro M: Secondary bile acids and short chain fatty acids in
the colon: A focus on colonic microbiome, cell proliferation,
inflammation, and cancer. Int J Mol Sci. 20:12142019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Schramm C: Bile acids, the microbiome,
immunity, and liver tumors. N Engl J Med. 379:888–890. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Koh GY, Kane A, Lee K, Xu Q, Wu X, Roper
J, Mason JB and Crott JW: Parabacteroides distasonis attenuates
toll-like receptor 4 signaling and Akt activation and blocks colon
tumor formation in high-fat diet-fed azoxymethane-treated mice. Int
J Cancer. 143:1797–1805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Gupta H, Youn GS, Shin MJ and Suk KT: Role
of gut microbiota in hepatocarcinogenesis. Microorganisms.
7:1212019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Trivedi Y, Bolgarina Z, Desai HN,
Senaratne M, Swami SS, Aye SL and Mohammed L: The role of gut
microbiome in hepatocellular carcinoma: A systematic review.
Cureus. 15:e438622023.PubMed/NCBI
|
|
128
|
Plaza-Díaz J, Solís-Urra P,
Rodríguez-Rodríguez F, Olivares-Arancibia J, Navarro-Oliveros M,
Abadía-Molina F and Álvarez-Mercado AI: The gut barrier, intestinal
microbiota, and liver disease: Molecular mechanisms and strategies
to manage. Int J Mol Sci. 21:83512020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Schwabe RF and Greten TF: Gut microbiome
in HCC-Mechanisms, diagnosis and therapy. J Hepatol. 72:230–238.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chu H, Williams B and Schnabl B: Gut
microbiota, fatty liver disease, and hepatocellular carcinoma.
Liver Res. 2:43–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yoshimoto S, Loo TM, Atarashi K, Kanda H,
Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et
al: Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature. 499:97–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Roh YS and Seki E: Toll-like receptors in
alcoholic liver disease, non-alcoholic steatohepatitis and
carcinogenesis. J Gastroenterol Hepatol. 28:38–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bartolini I, Risaliti M, Tucci R, Muiesan
P, Ringressi MN, Taddei A and Amedei A: Gut microbiota and immune
system in liver cancer: Promising therapeutic implication from
development to treatment. World J Gastrointest Oncol. 13:1616–1631.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Allsopp P, Possemiers S, Campbell D, Gill
C and Rowland I: A comparison of the anti-cancer properties of
isoxanthohumol and 8-prenylnaringenin using in vitro models of
colon cancer. Biofactors. 39:441–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Higashimura Y, Naito Y, Takagi T, Uchiyama
K, Mizushima K, Ushiroda C, Ohnogi H, Kudo Y, Yasui M, Inui S, et
al: Protective effect of agaro-oligosaccharides on gut dysbiosis
and colon tumorigenesis in high-fat diet-fed mice. Am J Physiol
Gastrointest Liver Physiol. 310:G367–G375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Costabile A, Fava F, Röytiö H, Forssten
SD, Olli K, Klievink J, Rowland IR, Ouwehand AC, Rastall RA, Gibson
GR and Walton GE: Impact of polydextrose on the faecal microbiota:
A double-blind, crossover, placebo-controlled feeding study in
healthy human subjects. Br J Nutr. 108:471–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Shields CE, Van Meerbeke SW, Housseau F,
Wang H, Huso DL, Casero RA Jr, O'Hagan HM and Sears CL: Reduction
of murine colon tumorigenesis driven by enterotoxigenic bacteroides
fragilis using cefoxitin treatment. J Infect Dis. 214:122–129.
2016. View Article : Google Scholar
|
|
138
|
Hou H, Chen D, Zhang K, Zhang W, Liu T,
Wang S, Dai X, Wang B, Zhong W and Cao H: Gut microbiota-derived
short-chain fatty acids and colorectal cancer: Ready for clinical
translation? Cancer Lett. 526:225–235. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Son MY and Cho HS: Anticancer effects of
gut microbiota-derived short-chain fatty acids in cancers. J
Microbiol Biotechnol. 33:849–856. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Schneider KM, Mohs A, Gui W, Galvez EJC,
Candels LS, Hoenicke L, Muthukumarasamy U, Holland CH, Elfers C,
Kilic K, et al: Imbalanced gut microbiota fuels hepatocellular
carcinoma development by shaping the hepatic inflammatory
microenvironment. Nat Commun. 13:39642022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yu LX and Schwabe RF: The gut microbiome
and liver cancer: Mechanisms and clinical translation. Nat Rev
Gastroenterol Hepatol. 14:527–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Patel AH, Li Y, Minacapelli CD, Catalano K
and Rustgi V: Reduction in gastrointestinal cancers in cirrhotic
patients receiving rifaximin vs lactulose only therapy for hepatic
encephalopathy. Cureus. 15:e352592023.PubMed/NCBI
|
|
143
|
Ting NLN, Lau HCH and Yu J: Cancer
pharmacomicrobiomics: Targeting microbiota to optimise cancer
therapy outcomes. Gut. 71:1412–1425. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
de Clercq NC, van den Ende T, Prodan A,
Hemke R, Davids M, Pedersen HK, Nielsen HB, Groen AK, de Vos WM,
van Laarhoven HWM and Nieuwdorp M: Fecal microbiota transplantation
from overweight or obese donors in cachectic patients with advanced
gastroesophageal cancer: A randomized, double-blind,
placebo-controlled, phase II study. Clin Cancer Res. 27:3784–3792.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Napolitano M and Covasa M: Microbiota
transplant in the treatment of obesity and diabetes: Current and
future perspectives. Front Microbiol. 11:5903702020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Davar D, Dzutsev AK, McCulloch JA,
Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding
Q, Pagliano O, et al: Fecal microbiota transplant overcomes
resistance to anti-PD-1 therapy in melanoma patients. Science.
371:595–602. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Xu H, Cao C, Ren Y, Weng S, Liu L, Guo C,
Wang L, Han X, Ren J and Liu Z: Antitumor effects of fecal
microbiota transplantation: Implications for microbiome modulation
in cancer treatment. Front Immunol. 13:9494902022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zhao LY, Mei JX, Yu G, Lei L, Zhang WH,
Liu K, Chen XL, Kołat D, Yang K and Hu JK: Role of the gut
microbiota in anticancer therapy: from molecular mechanisms to
clinical applications. Signal Transduction Target Ther. 8:2012023.
View Article : Google Scholar : PubMed/NCBI
|