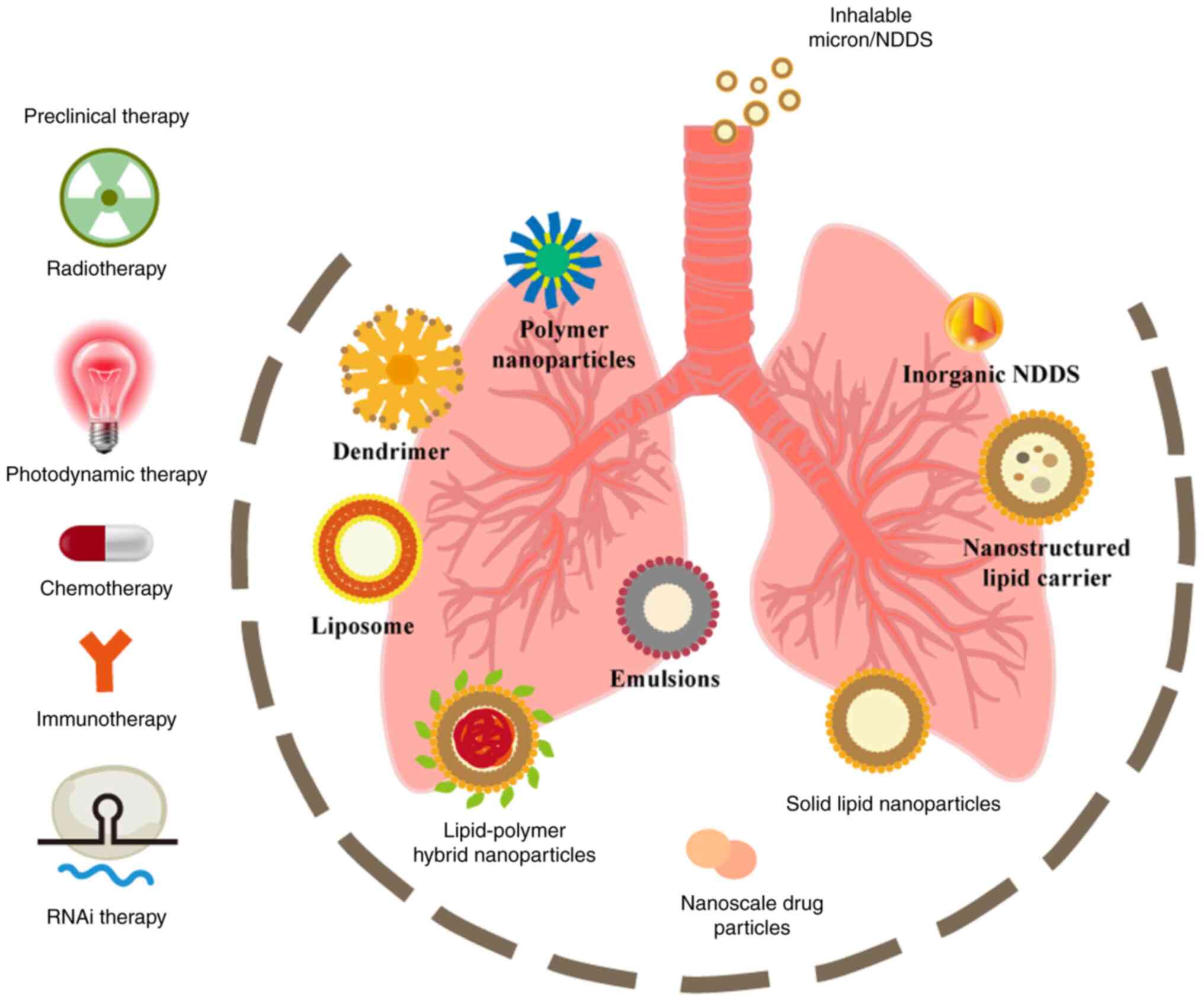
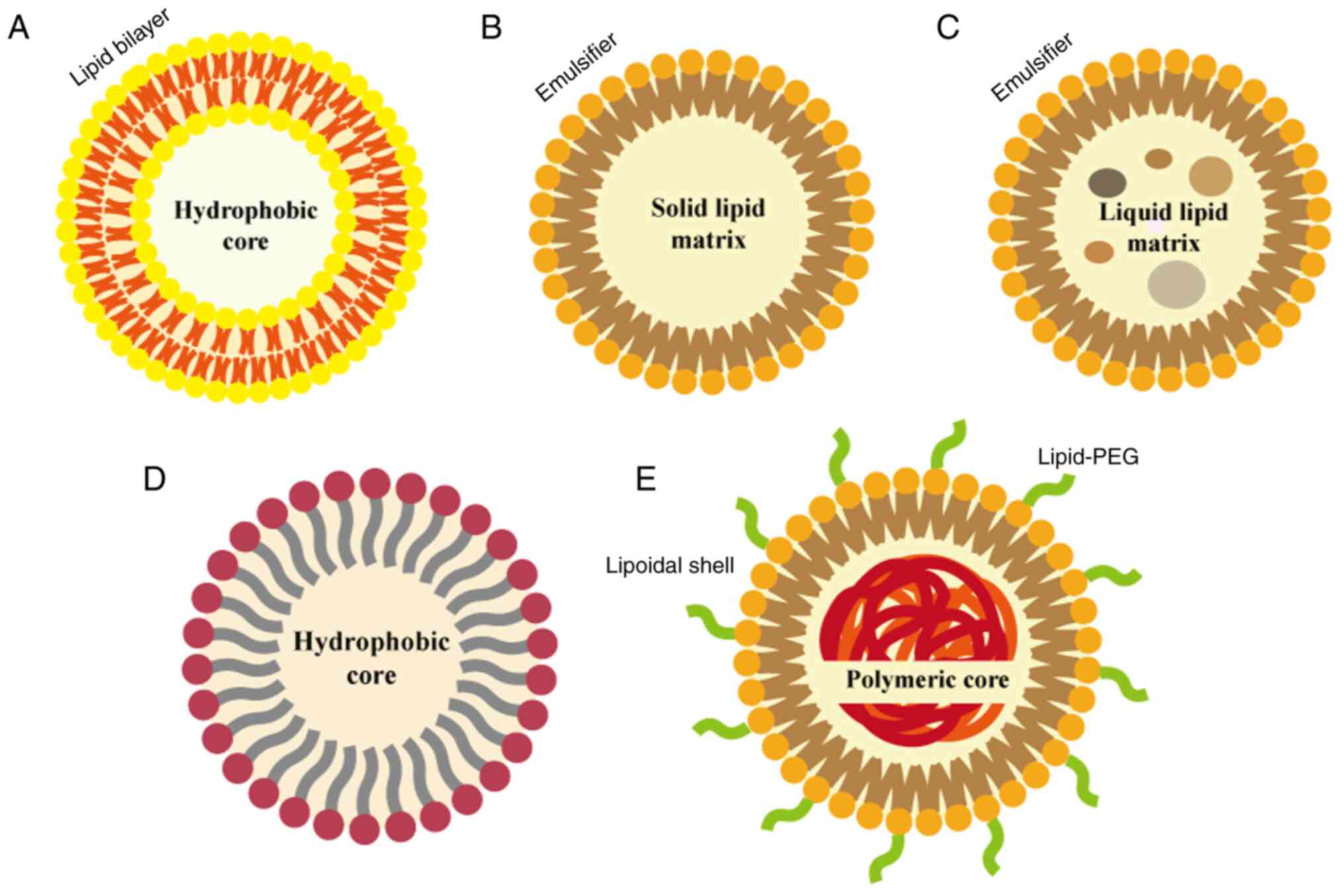
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
|
|
2
|
Reck M, Remon J and Hellmann MD:
First-Line immunotherapy for non-small-cell lung cancer. J Clin
Oncol. 40:586–597. 2022.
|
|
3
|
Hsu WH, Yang JC, Mok TS and Loong HH:
Overview of current systemic management of EGFR-mutant NSCLC. Ann
Oncol. 29(suppl_1): i3–i9. 2018.
|
|
4
|
Wei J, Luo X, Chen M, Lu J and Li X:
Spatial distribution and antitumor activities after intratumoral
injection of fragmented fibers with loaded hydroxycamptothecin.
Acta Biomater. 23:189–200. 2015.
|
|
5
|
Dhanani J, Fraser JF, Chan HK, Rello J,
Cohen J and Roberts JA: Fundamentals of aerosol therapy in critical
care. Crit Care. 20:2692016.
|
|
6
|
Zhou QT, Tang P, Leung SS, Chan JG and
Chan HK: Emerging inhalation aerosol devices and strategies: where
are we headed? Adv Drug Deliv Rev. 75:3–17. 2014.
|
|
7
|
Matuszak M, Ochowiak M, Włodarczak S,
Krupińska A and Doligalski M: State-of-the-art review of the
application and development of various methods of aerosol therapy.
Int J Pharm. 614:1214322022.
|
|
8
|
Ghosh S, Javia A, Shetty S, Bardoliwala D,
Maiti K, Banerjee S, Khopade A, Misra A, Sawant K and Bhowmick S:
Triple negative breast cancer and non-small cell lung cancer:
Clinical challenges and nano-formulation approaches. J Control
Release. 337:27–58. 2021.
|
|
9
|
Ezhilarasan D, Lakshmi T and Mallineni SK:
Nano-based targeted drug delivery for lung cancer: Therapeutic
avenues and challenges. Nanomedicine (Lond). 17:1855–1869.
2022.
|
|
10
|
Liu Y, Xia Y, Smollar J, Mao W and Wan Y:
The roles of small extracellular vesicles in lung cancer: Molecular
pathology, mechanisms, diagnostics, and therapeutics. Biochim
Biophys Acta Rev Cancer. 1876:1885392021.
|
|
11
|
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y,
Qin L and Wei H: Nanomaterials with enzyme-like characteristics
(nanozymes): Next-generation artificial enzymes (II). Chem Soc Rev.
48:1004–1076. 2019.
|
|
12
|
Abdelaziz HM, Gaber M, Abd-Elwakil MM,
Mabrouk MT, Elgohary MM, Kamel NM, Kabary DM, Freag MS, Samaha MW,
Mortada SM, et al: Inhalable particulate drug delivery systems for
lung cancer therapy: Nanoparticles, microparticles, nanocomposites
and nanoaggregates. J Control Release. 269:374–392. 2018.
|
|
13
|
Lee WH, Loo CY, Traini D and Young PM:
Nano- and micro-based inhaled drug delivery systems for targeting
alveolar macrophages. Expert Opin Drug Deliv. 12:1009–1026.
2015.
|
|
14
|
Zarogoulidis P, Chatzaki E, Porpodis K,
Domvri K, Hohenforst-Schmidt W, Goldberg EP, Karamanos N and
Zarogoulidis K: Inhaled chemotherapy in lung cancer: Future concept
of nanomedicine. Int J Nanomedicine. 7:1551–1572. 2012.
|
|
15
|
Kuzmov A and Minko T: Nanotechnology
approaches for inhalation treatment of lung diseases. J Control
Release. 219:500–518. 2015.
|
|
16
|
Mangal S, Gao W, Li T and Zhou QT:
Pulmonary delivery of nanoparticle chemotherapy for the treatment
of lung cancers: Challenges and opportunities. Acta Pharmacol Sin.
38:782–797. 2017.
|
|
17
|
Gupta C, Jaipuria A and Gupta N: Inhalable
formulations to treat non-small cell lung cancer (NSCLC): Recent
therapies and developments. Pharmaceutics. 15:1392022.
|
|
18
|
Karathanasis E, Ayyagari AL, Bhavane R,
Bellamkonda RV and Annapragada AV: Preparation of in vivo cleavable
agglomerated liposomes suitable for modulated pulmonary drug
delivery. J Control Release. 103:159–175. 2005.
|
|
19
|
Loira-Pastoriza C, Todoroff J and Vanbever
R: Delivery strategies for sustained drug release in the lungs. Adv
Drug Deliv Rev. 75:81–91. 2014.
|
|
20
|
Lee WH, Loo CY, Traini D and Young PM:
Inhalation of nanoparticle-based drug for lung cancer treatment:
Advantages and challenges. Asian J Pharm Sci. 10:481–489. 2015.
|
|
21
|
Choi HS, Ashitate Y, Lee JH, Kim SH,
Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV and
Tsuda A: Rapid translocation of nanoparticles from the lung
airspaces to the body. Nat Biotechnol. 28:1300–1303. 2010.
|
|
22
|
Davis ME, Chen ZG and Shin DM:
Nanoparticle therapeutics: An emerging treatment modality for
cancer. Nat Rev Drug Discov. 7:771–782. 2008.
|
|
23
|
Kumar M, Jha A, Bharti K, Parmar G and
Mishra B: Advances in lipid-based pulmonary nanomedicine for the
management of inflammatory lung disorders. Nanomedicine (Lond).
17:913–934. 2022.
|
|
24
|
Lee WH, Loo CY, Young PM, Traini D, Mason
RS and Rohanizadeh R: Recent advances in curcumin nanoformulation
for cancer therapy. Expert Opin Drug Deliv. 11:1183–1201. 2014.
|
|
25
|
Rosière R, Amighi K and Wauthoz N: Chapter
10-Nanomedicine-Based Inhalation Treatments for Lung Cancer.
Nanotechnology-Based Targeted Drug Delivery Systems for Lung
Cancer. Kesharwani P: Academic Press; pp. 249–268. 2019
|
|
26
|
Sorino C, Negri S, Spanevello A, Visca D
and Scichilone N: Inhalation therapy devices for the treatment of
obstructive lung diseases: The history of inhalers towards the
ideal inhaler. Eur J Intern Med. 75:15–18. 2020.
|
|
27
|
French DL, Edwards DA and Niven RW: The
influence of formulation on emission, deaggregation and deposition
of dry powders for inhalation. J Aerosol Sci. 27:769–783. 1996.
|
|
28
|
Vanbever R, Mintzes JD, Wang J, Nice J,
Chen D, Batycky R, Langer R and Edwards DA: Formulation and
physical characterization of large porous particles for inhalation.
Pharm Res. 16:1735–1742. 1999.
|
|
29
|
Kuehl PJ, Grimes MJ, Dubose D, Burke M,
Revelli DA, Gigliotti AP, Belinsky SA and Tessema M: Inhalation
delivery of topotecan is superior to intravenous exposure for
suppressing lung cancer in a preclinical model. Drug Deliv.
25:1127–1136. 2018.
|
|
30
|
Chraibi S, Rosière R, De Prez E, Gérard P,
Antoine MH, Langer I, Nortier J, Remmelink M, Amighi K and Wauthoz
N: Preclinical tolerance evaluation of the addition of a
cisplatin-based dry powder for inhalation to the conventional
carboplatin-paclitaxel doublet for treatment of non-small cell lung
cancer. Biomed Pharmacother. 139:1117162021.
|
|
31
|
Adams GP and Weiner LM: Monoclonal
antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005.
|
|
32
|
Coleman N, Yap TA, Heymach JV,
Meric-Bernstam F and Le X: Antibody-drug conjugates in lung cancer:
Dawn of a new era? NPJ Precis Oncol. 7:52023.
|
|
33
|
Shepard KB, Vodak DT, Kuehl PJ, Revelli D,
Zhou Y, Pluntze AM, Adam MS, Oddo JC, Switala L, Cape JL, et al:
Local treatment of non-small cell lung cancer with a spray-dried
bevacizumab formulation. AAPS PharmSciTech. 22:2302021.
|
|
34
|
Maillet A, Congy-Jolivet N, Le Guellec S,
Vecellio L, Hamard S, Courty Y, Courtois A, Gauthier F, Diot P,
Thibault G, et al: Aerodynamical, immunological and pharmacological
properties of the anticancer antibody cetuximab following
nebulization. Pharm Res. 25:1318–1326. 2008.
|
|
35
|
Guilleminault L, Azzopardi N, Arnoult C,
Sobilo J, Hervé V, Montharu J, Guillon A, Andres C, Herault O, Le
Pape A, et al: Fate of inhaled monoclonal antibodies after the
deposition of aerosolized particles in the respiratory system. J
Control Release. 196:344–354. 2014.
|
|
36
|
Brunaugh AD, Ding L, Wu T, Schneider M,
Khalaf R and Smyth HDC: Identification of stability constraints in
the particle engineering of an inhaled monoclonal antibody dried
powder. J Pharm Sci. 111:403–416. 2022.
|
|
37
|
Cortez-Jugo C, Qi A, Rajapaksa A, Friend
JR and Yeo LY: Pulmonary monoclonal antibody delivery via a
portable microfluidic nebulization platform. Biomicrofluidics.
9:0526032015.
|
|
38
|
Wang X, Chen H, Zeng X, Guo W, Jin Y, Wang
S, Tian R, Han Y, Guo L, Han J, et al: Efficient lung
cancer-targeted drug delivery via a nanoparticle/MSC system. Acta
Pharm Sin B. 9:167–176. 2019.
|
|
39
|
Ying N, Liu S, Zhang M, Cheng J, Luo L,
Jiang J, Shi G, Wu S, Ji J, Su H, et al: Nano delivery system for
paclitaxel: Recent advances in cancer theranostics. Colloids Surf B
Biointerfaces. 228:1134192023.
|
|
40
|
Kumar R: Lipid-Based Nanoparticles for
Drug-Delivery Systems-ScienceDirect. Nanocarriers for Drug
Delivery. 249–284. 2019.
|
|
41
|
Gandhi S and Roy I: Lipid-Based inhalable
micro- and nanocarriers of active agents for treating
non-small-cell lung cancer. Pharmaceutics. 15:14572023.
|
|
42
|
Sedighi M, Sieber S, Rahimi F, Shahbazi
MA, Rezayan AH, Huwyler J and Witzigmann D: Rapid optimization of
liposome characteristics using a combined microfluidics and
design-of-experiment approach. Drug Deliv Transl Res. 9:404–413.
2019.
|
|
43
|
Minko T, Khandare JJ, Vetcher AA,
Soldatenkov VA and Pozharov VP: Multifunctional Nanotherapeutics
for Cancer. Fundamental Biomedical Technologies. 2008.
|
|
44
|
Szabová J, Mišík O, Fučík J, Mrázová K,
Mravcová L, Elcner J, Lízal F, Krzyžánek V and Mravec F: Liposomal
form of erlotinib for local inhalation administration and
efficiency of its transport to the lungs. Int J Pharm.
634:1226952023.
|
|
45
|
Sarvepalli S, Parvathaneni V, Chauhan G,
Shukla SK and Gupta V: Inhaled indomethacin-loaded liposomes as
potential therapeutics against non-small cell lung cancer (NSCLC).
Pharm Res. 39:2801–2815. 2022.
|
|
46
|
Fu F, Wang W, Wu L, Wang W, Huang Z, Huang
Y, Wu C and Pan X: Inhalable biomineralized liposomes for cyclic
Ca2+-burst-centered endoplasmic reticulum stress
enhanced lung cancer ferroptosis therapy. ACS Nano. 17:5486–5502.
2023.
|
|
47
|
Zhang M, Li M, Du L, Zeng J, Yao T and Jin
Y: Paclitaxel-in-liposome-in-bacteria for inhalation treatment of
primary lung cancer. Int J Pharm. 578:1191772020.
|
|
48
|
Kulkarni JA, Witzigmann D, Leung J, Tam
YYC and Cullis PR: On the role of helper lipids in lipid
nanoparticle formulations of siRNA. Nanoscale. 11:21733–21739.
2019.
|
|
49
|
Ganesan P and Narayanasamy D: Lipid
nanoparticles: Different preparation techniques, characterization,
hurdles, and strategies for the production of solid lipid
nanoparticles and nanostructured lipid carriers for oral drug
delivery. Sustain Chem Pharm. 6:37–56. 2017.
|
|
50
|
Filipczak N, Yalamarty SSK, Li X, Khan MM,
Parveen F and Torchilin V: Lipid-Based drug delivery systems in
regenerative medicine. Materials (Basel). 14:53712021.
|
|
51
|
Mehnert W and Mäder K: Solid lipid
nanoparticles: Production, characterization and applications. Adv
Drug Deliv Rev. 47:165–196. 2001.
|
|
52
|
Zimmermann CM, Baldassi D, Chan K, Adams
NBP, Neumann A, Porras-Gonzalez DL, Wei X, Kneidinger N, Stoleriu
MG, Burgstaller G, et al: Spray drying siRNA-lipid nanoparticles
for dry powder pulmonary delivery. J Control Release. 351:137–150.
2022.
|
|
53
|
Robins MM, Watson AD and Wilde PJ:
Emulsions-creaming and rheology. Current Opinion in Colloid and
Interface Science. 2002.
|
|
54
|
Ngan CL and Asmawi AA: Lipid-based
pulmonary delivery system: A review and future considerations of
formulation strategies and limitations. Drug Deliv Transl Res.
8:1527–1544. 2018.
|
|
55
|
Lovelyn C and Attama AA: Current state of
nanoemulsions in drug delivery. J Biomater Nanobiotechnol.
2:626–639. 2011.
|
|
56
|
Pandey P, Gulati N, Makhija M, Purohit D
and Dureja H: Nanoemulsion: A novel drug delivery approach for
enhancement of bioavailability. Recent Pat Nanotechnol. 14:276–293.
2020.
|
|
57
|
Jyoti K, Kaur K, Pandey RS, Jain UK,
Chandra R and Madan J: Inhalable nanostructured lipid particles of
9-bromo-noscapine, a tubulin-binding cytotoxic agent: in vitro and
in vivo studies. J Colloid Interface Sci. 445:219–230. 2015.
|
|
58
|
Arbain NH, Salim N, Masoumi HRF, Wong TW,
Basri M and Abdul Rahman MB: In vitro evaluation of the inhalable
quercetin loaded nanoemulsion for pulmonary delivery. Drug Deliv
Transl Res. 9:497–507. 2019.
|
|
59
|
Asmawi AA, Salim N, Abdulmalek E and Abdul
Rahman MB: Modeling the effect of composition on formation of
aerosolized nanoemulsion system encapsulating docetaxel and
curcumin using D-Optimal mixture experimental design. Int J Mol
Sci. 21:43572020.
|
|
60
|
Asmawi AA, Salim N, Abdulmalek E and Abdul
Rahman MB: Size-Controlled preparation of docetaxel- and
curcumin-loaded nanoemulsions for potential pulmonar y deliver y.
Pharmaceutics. 15:6522023.
|
|
61
|
Xu M, Zhang L, Guo Y, Bai L, Luo Y, Wang
B, Kuang M, Liu X, Sun M, Wang C and Xie J: Nanoemulsion Co-Loaded
with XIAP siRNA and gambogic acid for inhalation therapy of lung
cancer. Int J Mol Sci. 23:142942022.
|
|
62
|
Chauhan G, Wang X, Yousry C and Gupta V:
Scalable production and in vitro efficacy of inhaled erlotinib
nanoemulsion for enhanced efficacy in non-small cell lung cancer
(NSCLC). Pharmaceutics. 15:9962023.
|
|
63
|
Mukherjee A, Waters AK, Kalyan P, Achrol
AS, Kesari S and Yenugonda VM: Lipid-polymer hybrid nanoparticles
as a next-generation drug delivery platform: State of the art,
emerging technologies, and perspectives. Int J Nanomedicine.
14:1937–1952. 2019.
|
|
64
|
Zhang L, Chan JM, Gu FX, Wang AZ,
Radovic-Moreno AF, Alexis F, Langer R and Farokhzad OC:
Self-assembled lipid-polymer hybrid nanoparticles: A robust drug
delivery platform. ACS Nano. 2:1696–1702. 2008.
|
|
65
|
Bardoliwala D, Patel V, Misra A and Sawant
K: Systematic development and characterization of inhalable dry
powder containing Polymeric Lipid Hybrid Nanocarriers co-loaded
with ABCB1 shRNA and docetaxel using QbD approach.
|
|
66
|
Zielińska A, Carreiró F, Oliveira AM,
Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P,
Silva AM, et al: Polymeric Nanoparticles: Production,
characterization, toxicology and ecotoxicology. Molecules.
25:37312020.
|
|
67
|
Mahar R, Chakraborty A, Nainwal N,
Bahuguna R, Sajwan M and Jakhmola V: Application of PLGA as a
biodegradable and biocompatible polymer for pulmonary delivery of
drugs. AAPS PharmSciTech. 24:392023.
|
|
68
|
Elbatanony RS, Parvathaneni V, Kulkarni
NS, Shukla SK, Chauhan G, Kunda NK and Gupta V: Afatinib-loaded
inhalable PLGA nanoparticles for localized therapy of non-small
cell lung cancer (NSCLC)-development and in-vitro efficacy. Drug
Deliv Transl Res. 11:927–943. 2021.
|
|
69
|
Gaikwad D, Shewale R, Patil V, Mali D,
Gaikwad U and Jadhav N: Enhancement in in vitro anti-angiogenesis
activity and cytotoxicity in lung cancer cell by pectin-PVP based
curcumin particulates. Int J Biol Macromol. 104(Pt A): 656–664.
2017.
|
|
70
|
Rasul RM, Tamilarasi Muniandy M, Zakaria
Z, Shah K, Chee CF, Dabbagh A, Rahman NA and Wong TW: A review on
chitosan and its development as pulmonary particulate
anti-infective and anti-cancer drug carriers. Carbohydr Polym.
250:1168002020.
|
|
71
|
Jin Q, Zhu W, Zhu J, Zhu J, Shen J, Liu Z,
Yang Y and Chen Q: Nanoparticle-Mediated Delivery of Inhaled
Immunotherapeutics for Treating Lung Metastasis. Adv Mater.
33:e20075572021.
|
|
72
|
Zhang M and Zhao X: Alginate hydrogel
dressings for advanced wound management. Int J Biol Macromol.
162:1414–1428. 2020.
|
|
73
|
Karim A, Rehman A, Feng J, Noreen A,
Assadpour E, Kharazmi MS, Lianfu Z and Jafari SM: Alginate-based
nanocarriers for the delivery and controlled-release of bioactive
compounds. Adv Colloid Interface Sci. 307:1027442022.
|
|
74
|
Alsmadi MM, Obaidat RM, Alnaief M, Albiss
BA and Hailat N: Development, In Vitro Characterization, and In
Vivo Toxicity Evaluation of Chitosan-Alginate Nanoporous Carriers
Loaded with Cisplatin for Lung Cancer Treatment. AAPS PharmSciTech.
21:1912020.
|
|
75
|
Jiang X, Du Z, Zhang X, Zaman F, Song Z,
Guan Y, Yu T and Huang Y: Gelatin-based anticancer drug delivery
nanosystems: A mini review. Front Bioeng Biotechnol.
11:11587492023.
|
|
76
|
Gou S, Wang G, Zou Y, Geng W, He T, Qin Z,
Che L, Feng Q and Cai K: Non-Pore Dependent and MMP-9 Responsive
Gelatin/Silk Fibroin Composite Microparticles as Universal Delivery
Platform for Inhaled Treatment of Lung Cancer. Adv Mater.
35:e23037182023.
|
|
77
|
Chowdhury S, Toth I and Stephenson RJ:
Dendrimers in vaccine delivery: Recent progress and advances.
Biomaterials. 280:1213032022.
|
|
78
|
Sapra R, Verma RP, Maurya GP, Dhawan S,
Babu J and Haridas V: Designer Peptide and Protein Dendrimers: A
Cross-Sectional Analysis. Chem Rev. 119:11391–11441. 2019.
|
|
79
|
Kalomiraki M, Thermos K and Chaniotakis
NA: Dendrimers as tunable vectors of drug delivery systems and
biomedical and ocular applications. Int J Nanomedicine. 11:1–12.
2015.
|
|
80
|
Conti DS, Brewer D, Grashik J, Avasarala S
and da Rocha SR: Poly(amidoamine) dendrimer nanocarriers and their
aerosol formulations for siRNA delivery to the lung epithelium. Mol
Pharm. 11:1808–1822. 2014.
|
|
81
|
B FCA, D FZ, E DSD, et al: Bioreducible
and traceable Ru(III) prodrug-loaded mesoporous silica
nanoparticles for sequentially targeted nonsmall cell lung cancer
chemotherapy-ScienceDirect. Applied Materials Today.
|
|
82
|
Ma Z, Wang H, Shi Z, Yan F, Li Q, Chen J,
Cui ZK, Zhang Y, Jin X, Jia YG and Wang L: Inhalable GSH-Triggered
Nanoparticles to Treat Commensal Bacterial Infection in In Situ
Lung Tumors. ACS Nano. 17:5740–5756. 2023.
|
|
83
|
Salama JK and Vokes EE: New radiotherapy
and chemoradiotherapy approaches for non-small-cell lung cancer. J
Clin Oncol. 31:1029–1038. 2013.
|
|
84
|
De Ruysscher D, Niedermann G, Burnet NG,
Siva S, Lee AWM and Hegi-Johnson F: Radiotherapy toxicity. Nat Rev
Dis Primers. 5:132019.
|
|
85
|
Patel V, Papineni RV, Gupta S, Stoyanova R
and Ahmed MM: A realistic utilization of nanotechnology in
molecular imaging and targeted radiotherapy of solid tumors. Radiat
Res. 177:483–495. 2012.
|
|
86
|
Wang H, Mu X, He H and Zhang XD: Cancer
Radiosensitizers. Trends Pharmacol Sci. 39:24–48. 2018.
|
|
87
|
Hao Y, Altundal Y, Moreau M, Sajo E, Kumar
R and Ngwa W: Potential for enhancing external beam radiotherapy
for lung cancer using high-Z nanoparticles administered via
inhalation. Phys Med Biol. 60:7035–7043. 2015.
|
|
88
|
Hou YJ, Yang XX, Liu RQ, Zhao D, Guo CX,
Zhu AC, Wen MN, Liu Z, Qu GF and Meng HX: Pathological Mechanism of
Photodynamic Therapy and Photothermal Therapy Based on
Nanoparticles. Int J Nanomedicine. 15:6827–6838. 2020.
|
|
89
|
Xue S, Jiao J, Miao S, Wang L, Liu Y,
Zhang Q, Wang Q, Xi Y and Zhang Y: Lipid-coated bismuth nanoflower
as the thermos-radio sensiti for therapy of lung metastatic breast
cancer: Preparation, optimisation, and characterisation. IET
Nanobiotechnol. 16:305–315. 2022.
|
|
90
|
Wang Q, Liu J, Chen D, Miao S, Wen J, Liu
C, Xue S, Liu Y, Zhang Q and Shen Y: 'Cluster Bomb' Based Bismuth
Nano-in-Micro Spheres Formed Dry Powder Inhalation for Thermo-Radio
Sensitization Effects of Lung Metastatic Breast Cancer. Adv Healthc
Mater. 12:e22026222023.
|
|
91
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011.
|
|
92
|
Kwiatkowski S, Knap B, Przystupski D,
Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O,
Kotowski K and Kulbacka J: Photodynamic therapy-mechanisms,
photosensitizers and combinations. Biomed Pharmacother.
106:1098–1107. 2018.
|
|
93
|
Baumgartner R, Huber RM, Schulz H, Stepp
H, Rick K, Gamarra F, Leberig A and Roth C: Inhalation of
5-aminolevulinic acid: A new technique for fluorescence detection
of early stage lung cancer. J Photochem Photobiol B. 36:169–174.
1996.
|
|
94
|
Baghdan E, Duse L, Schüer JJ, Pinnapireddy
SR, Pourasghar M, Schäfer J, Schneider M and Bakowsky U:
Development of inhalable curcumin loaded Nano-in-Microparticles for
bronchoscopic photodynamic therapy. Eur J Pharm Sci. 132:63–71.
2019.
|
|
95
|
Lehmann J, Agel MR, Engelhardt KH,
Pinnapireddy SR, Agel S, Duse L, Preis E, Wojcik M and Bakowsky U:
Improvement of pulmonary photodynamic therapy: Nebulisation of
curcumin-loaded tetraether liposomes. Pharmaceutics.
13:12432021.
|
|
96
|
Zhang T, Bao J, Zhang M, Ge Y, Wei J, Li
Y, Wang W, Li M and Jin Y: Chemo-photodynamic therapy by pulmonary
delivery of gefitinib nanoparticles and 5-aminolevulinic acid for
treatment of primary lung cancer of rats. Photodiagnosis Photodyn
Ther. 31:1018072020.
|
|
97
|
Chaft JE, Rimner A, Weder W, Azzoli CG,
Kris MG and Cascone T: Evolution of systemic therapy for stages
I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin
Oncol. 18:547–557. 2021.
|
|
98
|
Bradbury P, Sivajohanathan D, Chan A,
Kulkarni S, Ung Y and Ellis PM: Postoperative Adjuvant Systemic
Therapy in Completely Resected Non-Small-Cell Lung Cancer: A
Systematic Review. Clin Lung Cancer. 18:259–273.e258. 2017.
|
|
99
|
De Ruysscher D, Faivre-Finn C, Nackaerts
K, Jordan K, Arends J, Douillard JY, Ricardi U and Peters S:
Recommendation for supportive care in patients receiving concurrent
chemotherapy and radiotherapy for lung cancer. Ann Oncol. 31:41–49.
2020.
|
|
100
|
Non-Small Cell Lung Cancer Collaborative
Group: Chemotherapy and supportive care versus supportive care
alone for advanced non-small cell lung cancer. Cochrane Database
Syst Rev. Cd0073092010.
|
|
101
|
Zarogoulidis P, Eleftheriadou E,
Sapardanis I, Zarogoulidou V, Lithoxopoulou H, Kontakiotis T,
Karamanos N, Zachariadis G, Mabroudi M, Zisimopoulos A and
Zarogoulidis K: Feasibility and effectiveness of inhaled
carboplatin in NSCLC patients. Invest New Drugs. 30:1628–1640.
2012.
|
|
102
|
Lemarie E, Vecellio L, Hureaux J, Prunier
C, Valat C, Grimbert D, Boidron-Celle M, Giraudeau B, le Pape A,
Pichon E, et al: Aerosolized gemcitabine in patients with carcinoma
of the lung: Feasibility and safety study. J Aerosol Med Pulm Drug
Deliv. 24:261–270. 2011.
|
|
103
|
Tatsumura T, Koyama S, Tsujimoto M,
Kitagawa M and Kagamimori S: Further study of nebulisation
chemotherapy, a new chemotherapeutic method in the treatment of
lung carcinomas: Fundamental and clinical. Br J Cancer.
68:1146–1149. 1993.
|
|
104
|
Otterson GA, Villalona-Calero MA, Sharma
S, Kris MG, Imondi A, Gerber M, White DA, Ratain MJ, Schiller JH,
Sandler A, et al: Phase I study of inhaled Doxorubicin for patients
with metastatic tumors to the lungs. Clin Cancer Res. 13:1246–1252.
2007.
|
|
105
|
Issa JP, Kantarjian HM and Kirkpatrick P:
Azacitidine. Nat Rev Drug Discov. 4:275–276. 2005.
|
|
106
|
Cheng H, Zou Y, Shah CD, Fan N, Bhagat TD,
Gucalp R, Kim M, Verma A, Piperdi B, Spivack SD, et al:
First-in-human study of inhaled Azacitidine in patients with
advanced non-small cell lung cancer. Lung Cancer. 154:99–104.
2021.
|
|
107
|
Patra J K, Das G, Fraceto LF, Campos EVR,
Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R,
Swamy MK, Sharma S, et al: Nano based drug delivery systems: Recent
developments and future prospects. J Nanobiotechnology.
16:712018.
|
|
108
|
Alavi M and Hamidi M: Passive and active
targeting in cancer therapy by liposomes and lipid nanoparticles.
Drug Metab Pers Ther. 34:2019.
|
|
109
|
Danhier F, Ansorena E, Silva JM, Coco R,
Le Breton A and Préat V: PLGA-based nanoparticles: An overview of
biomedical applications. J Control Release. 161:505–522. 2012.
|
|
110
|
Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT,
Shin BS, Lee KC and Youn YS: Doxorubicin-loaded highly porous large
PLGA microparticles as a sustained-release inhalation system for
the treatment of metastatic lung cancer. Biomaterials.
33:5574–5583. 2012.
|
|
111
|
Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT,
Shin BS, Lee KC and Youn YS: Doxorubicin-loaded porous PLGA
microparticles with surface attached TRAIL for the inhalation
treatment of metastatic lung cancer. Biomaterials. 34:6444–6453.
2013.
|
|
112
|
Large DE, Abdelmessih RG, Fink EA and
Auguste DT: Liposome composition in drug delivery design,
synthesis, characterization, and clinical application. Adv Drug
Deliv Rev. 176:1138512021.
|
|
113
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007.
|
|
114
|
Zhang T, Chen Y, Ge Y, Hu Y, Li M and Jin
Y: Inhalation treatment of primary lung cancer using liposomal
curcumin dry powder inhalers. Acta Pharm Sin B. 8:440–448.
2018.
|
|
115
|
Knight V, Koshkina NV, Golunski E, Roberts
LE and Gilbert BE: Cyclosporin A aerosol improves the anticancer
effect of paclitaxel aerosol in mice. Trans Am Clin Climatol Assoc.
115:395–404; discussion 404. 2004.
|
|
116
|
Shukla SK, Kulkarni NS, Farrales P,
Kanabar DD, Parvathaneni V, Kunda NK, Muth A and Gupta V: Sorafenib
loaded inhalable polymeric nanocarriers against non-small cell lung
cancer. Pharm Res. 37:672020.
|
|
117
|
Bakhtiary Z, Barar J, Aghanejad A, Saei
AA, Nemati E, Ezzati Nazhad Dolatabadi J and Omidi Y:
Microparticles containing erlotinib-loaded solid lipid
nanoparticles for treatment of non-small cell lung cancer. Drug Dev
Ind Pharm. 43:1244–1253. 2017.
|
|
118
|
Sawant SS, Patil SM, Shukla SK, Kulkarni
NS, Gupta V and Kunda NK: Pulmonary delivery of osimertinib
liposomes for non-small cell lung cancer treatment: Formulation
development and in vitro evaluation. Drug Deliv Transl Res.
12:2474–2487. 2022.
|
|
119
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020.
|
|
120
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
|
|
121
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: opportunities and
challenges in cancer. J Hematol Oncol. 12:342019.
|
|
122
|
Wang W, Wang W, Jin S, Fu F, Huang Z,
Huang Y, Wu C and Pan X: Open pocket and tighten holes: Inhalable
lung cancer-targeted nanocomposite for enhanced
ferroptosis-apoptosis synergetic therapy. Chem Eng J.
458:1414872023.
|
|
123
|
Wang W, Fu F, Huang Z, Wang W, Chen M, Yue
X, Fu J, Feng X, Huang Y, Wu C and Pan X: Inhalable biomimetic
protein corona-mediated nanoreactor for self-amplified lung
adenocarcinoma ferroptosis therapy. ACS Nano. 16:8370–8387.
2022.
|
|
124
|
Tan AC and Tan DSW: Targeted therapies for
lung cancer patients with oncogenic driver molecular alterations. J
Clin Oncol. 40:611–625. 2022.
|
|
125
|
Min HY and Lee HY: Mechanisms of
resistance to chemotherapy in non-small cell lung cancer. Arch
Pharm Res. 44:146–164. 2021.
|
|
126
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22:402023.
|
|
127
|
Peters S, Reck M, Smit EF, Mok T and
Hellmann MD: How to make the best use of immunotherapy as
first-line treatment of advanced/metastatic non-small-cell lung
cancer. Ann Oncol. 30:884–896. 2019.
|
|
128
|
Sapalidis K, Zarogoulidis P, Pavlidis E,
Laskou S, Katsaounis A, Koulouris C, Giannakidis D, Mantalovas S,
Huang H, Bai C, et al: Aerosol Immunotherapy with or without
Cisplatin for metastatic lung cancer non-small cell lung cancer
disease: In vivo study. A more efficient combination. J Cancer.
9:1973–1977. 2018.
|
|
129
|
Liu Y, Crowe WN, Wang L, Petty WJ, Habib
AA and Zhao D: Aerosolized immunotherapeutic nanoparticle
inhalation potentiates PD-L1 blockade for locally advanced lung
cancer. Nano Res. 16:5300–5310. 2023.
|
|
130
|
Kell SA, Kachura MA, Renn A, Traquina P,
Coffman RL and Campbell JD: Preclinical development of the TLR9
agonist DV281 as an inhaled aerosolized immunotherapeutic for lung
cancer: Pharmacological profile in mice, non-human primates, and
human primary cells. Int Immunopharmacol. 66:296–308. 2019.
|
|
131
|
Fan Q, Li Z, Yin J, Xie M, Cui M, Fan C,
Wang L and Chao J: Inhalable pH-responsive DNA tetrahedron
nanoplatform for boosting anti-tumor immune responses against
metastatic lung cancer. Biomaterials. 301:1222832023.
|
|
132
|
Dougan M, Luoma AM, Dougan SK and
Wucherpfennig KW: Understanding and treating the inflammatory
adverse events of cancer immunotherapy. Cell. 184:1575–1588.
2021.
|
|
133
|
Kara G, Calin GA and Ozpolat B: RNAi-based
therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv
Rev. 182:1141132022.
|
|
134
|
Lamb YN: Inclisiran: First Approval.
Drugs. 81:389–395. 2021.
|
|
135
|
Conde J, Ambrosone A, Hernandez Y, Tian F,
McCully M, Berry CC, Baptista PV, Tortiglione C and de la Fuente
JM: 15 years on siRNA delivery: Beyond the State-of-the-Art on
inorganic nanoparticles for RNAi therapeutics. Nano Today.
10:421–450. 2015.
|
|
136
|
Merkel OM, Rubinstein I and Kissel T:
siRNA delivery to the lung: what's new? Adv Drug Deliv Rev.
75:112–128. 2014.
|
|
137
|
Feldmann DP and Merkel OM: The advantages
of pulmonary delivery of therapeutic siRNA. Ther Deliv. 6:407–409.
2015.
|
|
138
|
Taratula O, Garbuzenko OB, Chen AM and
Minko T: Innovative strategy for treatment of lung cancer: Targeted
nanotechnology-based inhalation co-delivery of anticancer drugs and
siRNA. J Drug Target. 19:900–914. 2011.
|
|
139
|
Zhao G, Ho W, Chu J, Xiong X, Hu B,
Boakye-Yiadom KO, Xu X and Zhang XQ: Inhalable siRNA nanoparticles
for enhanced tumor-targeting treatment of KRAS-Mutant
non-small-cell lung cancer. ACS Appl Mater Interfaces.
15:31273–31284. 2023.
|
|
140
|
Luo CQ, Jang Y, Xing L, Cui PF, Qiao JB,
Lee AY, Kim HJ, Cho MH and Jiang HL: Aerosol delivery of
folate-decorated hyperbranched polyspermine complexes to suppress
lung tumorigenesis via Akt signaling pathway. Int J Pharm.
513:591–601. 2016.
|
|
141
|
Gankhuyag N, Yu KN, Davaadamdin O, Lee S,
Cho WY, Park C, Jiang HL, Singh B, Chae CH, Cho MH and Cho CS:
Suppression of tobacco carcinogen-induced lung tumorigenesis by
aerosol-delivered glycerol propoxylate triacrylate-spermine
copolymer/short hairpin Rab25 RNA Complexes in Female A/J Mice. J
Aerosol Med Pulm Drug Deliv. 30:81–90. 2017.
|
|
142
|
Fukushige K, Tagami T, Naito M, Goto E,
Hirai S, Hatayama N, Yokota H, Yasui T, Baba Y and Ozeki T:
Developing spray-freeze-dried particles containing a hyaluronic
acid-coated liposome-protamine-DNA complex for pulmonary
inhalation. Int J Pharm. 583:1193382020.
|
|
143
|
Garbuzenko OB, Kuzmov A, Taratula O, Pine
SR and Minko T: Strategy to enhance lung cancer treatment by five
essential elements: inhalation delivery, nanotechnology,
tumor-receptor targeting, chemo- and gene therapy. Theranostics.
9:8362–8376. 2019.
|
|
144
|
Babu A, Templeton AK, Munshi A and Ramesh
R: Nanoparticle-based drug delivery for therapy of lung cancer:
Progress and challenges. J Nanomater. 2013.https://doi.org/10.1155/2013/863951.
|
|
145
|
Tharkar P, Madani AU, Lasham A, Shelling
AN and Al-Kassas R: Nanoparticulate carriers: An emerging tool for
breast cancer therapy. J Drug Target. 23:97–108. 2015.
|
|
146
|
Peer D, Karp JM, Hong S, Farokhzad OC,
Margalit R and Langer R: Nanocarriers as an emerging platform for
cancer therapy. Nat Nanotechnol. 2:751–760. 2007.
|
|
147
|
Wittgen BP, Kunst PW, van der Born K, van
Wijk AW, Perkins W, Pilkiewicz FG, Perez-Soler R, Nicholson S,
Peters GJ and Postmus PE: Phase I study of aerosolized SLIT
cisplatin in the treatment of patients with carcinoma of the lung.
Clin Cancer Res. 13:2414–2421. 2007.
|
|
148
|
Otterson GA, Villalona-Calero MA, Hicks W,
Pan X, Ellerton JA, Gettinger SN and Murren JR: Phase I/II study of
inhaled doxorubicin combined with platinum-based therapy for
advanced non-small cell lung cancer. Clin Cancer Res. 16:2466–2473.
2010.
|
|
149
|
Verschraegen CF, Gilbert BE, Loyer E,
Huaringa A, Walsh G, Newman RA and Knight V: Clinical evaluation of
the delivery and safety of aerosolized liposomal
9-nitro-20(s)-camptothecin in patients with advanced pulmonary
malignancies. Clin Cancer Res. 10:2319–2326. 2004.
|
|
150
|
Rosière R, Berghmans T, De Vuyst P, Amighi
K and Wauthoz N: the position of inhaled chemotherapy in the care
of patients with lung tumors: Clinical feasibility and indications
according to recent pharmaceutical progresses. Cancers (Basel).
11:3292019.
|
|
151
|
Zarogouldis P, Karamanos NK, Porpodis K,
Domvri K, Huang H, Hohenforst-Schimdt W, Goldberg EP and
Zarogoulidis K: Vectors for inhaled gene therapy in lung cancer.
Application for nano oncology and safety of bio nanotechnology. Int
J Mol Sci. 13:10828–10862. 2012.
|
|
152
|
Durcan N, Murphy C and Cryan SA: Inhalable
siRNA: Potential as a therapeutic agent in the lungs. Mol Pharm.
5:559–566. 2008.
|
|
153
|
Garon EB, Spira AI, Johnson M, Bazhenova
L, Leach J, Cummings AL, Candia A, Coffman RL, Janatpour MJ,
Janssen R, et al: A Phase Ib Open-Label, Multicenter Study of
Inhaled DV281, a TLR9 agonist, in combination with nivolumab in
patients with advanced or metastatic non-small cell lung cancer.
Clin Cancer Res. 27:4566–4573. 2021.
|
|
154
|
Shevchenko IT and Resnik GE: Inhalation of
chemical substances and oxygen in radiotherapy of bronchial cancer.
Neoplasma. 15:419–426. 1968.
|
|
155
|
Rizvi NA, Riely GJ, Azzoli CG, Miller VA,
Ng KK, Fiore J, Chia G, Brower M, Heelan R, Hawkins MJ and Kris MG:
Phase I/II trial of weekly intravenous 130-nm albumin-bound
paclitaxel as initial chemotherapy in patients with stage IV
non-small-cell lung cancer. J Clin Oncol. 26:639–643. 2008.
|
|
156
|
Forest V and Pourchez J: Nano-delivery to
the lung - by inhalation or other routes and why nano when micro is
largely sufficient? Adv Drug Deliv Rev. 183:1141732022.
|
|
157
|
Luo MX, Hua S and Shang QY: Application of
nanotechnology in drug delivery systems for respiratory diseases
(Review). Mol Med Rep. 23:3252021.
|
|
158
|
Ali ME, McConville JT and Lamprecht A:
Pulmonary delivery of anti-inflammatory agents. Expert Opin Drug
Deliv. 12:929–945. 2015.
|
|
159
|
Mansour HM, Rhee YS and Wu X: Nanomedicine
in pulmonary delivery. Int J Nanomedicine. 4:299–319. 2009.
|
|
160
|
Patton JS, Fishburn CS and Weers JG: The
lungs as a portal of entry for systemic drug delivery. Proc Am
Thorac Soc. 1:338–344. 2004.
|
|
161
|
Yeagle P: Nanoparticles for drug delivery
in lungs. Science. 356:37–38. 2017.
|
|
162
|
Zhong W, Zhang X, Zeng Y, Lin D and Wu J:
Recent applications and strategies in nanotechnology for lung
diseases. Nano Res. 14:2067–2089. 2021.
|
|
163
|
Rau JL: The inhalation of drugs:
Advantages and problems. Respir Care. 50:367–382. 2005.
|
|
164
|
Darwiche K, Zarogoulidis P, Karamanos NK,
Domvri K, Chatzaki E, Constantinidis TC, Kakolyris S and
Zarogoulidis K: Efficacy versus safety concerns for aerosol
chemotherapy in non-small-cell lung cancer: A future dilemma for
micro-oncology. Future Oncol. 9:505–525. 2013.
|
|
165
|
Rosière R, Hureaux J, Levet V, Amighi K
and Wauthoz N: Inhaled chemotherapy-Part 1: General concept and
current technological challenges. Rev Mal Respir. 35:357–377.
2018.In French.
|
|
166
|
Hadrup N, Zhernovkov V, Jacobsen NR, Voss
C, Strunz M, Ansari M, Schiller HB, Halappanavar S, Poulsen SS,
Kholodenko B, et al: Acute phase response as a biological
mechanism-of-action of (Nano)particle-Induced cardiovascular
disease. Small. 16:e19074762020.
|
|
167
|
Ruge CA, Kirch J and Lehr CM: Pulmonary
drug delivery: From generating aerosols to overcoming biological
barriers-therapeutic possibilities and technological challenges.
Lancet Respir Med. 1:402–413. 2013.
|
|
168
|
Skibba M, Drelich A, Poellmann M, Hong S
and Brasier AR: Nanoapproaches to modifying epigenetics of
epithelial mesenchymal transition for treatment of pulmonary
fibrosis. Front Pharmacol. 11:6076892020.
|
|
169
|
Osman NM, Sexton DW and Saleem IY:
Toxicological assessment of nanoparticle interactions with the
pulmonary system. Nanotoxicology. 14:21–58. 2020.
|
|
170
|
Wauthoz N, Rosière R and Amighi K: Inhaled
cytotoxic chemotherapy: Clinical challenges, recent developments,
and future prospects. Expert Opin Drug Deliv. 18:333–354. 2021.
|
|
171
|
Osman N, Kaneko K, Carini V and Saleem I:
Carriers for the targeted delivery of aerosolized macromolecules
for pulmonary pathologies. Expert Opin Drug Deliv. 15:821–834.
2018.
|
|
172
|
Pilcer G and Amighi K: Formulation
strategy and use of excipients in pulmonary drug delivery. Int J
Pharm. 392:1–19. 2010.
|
|
173
|
Wauthoz N, Deleuze P, Hecq J, Roland I,
Saussez S, Adanja I, Debeir O, Decaestecker C, Mathieu V, Kiss R
and Amighi K: In vivo assessment of temozolomide local delivery for
lung cancer inhalation therapy. Eur J Pharm Sci. 39:402–411.
2010.
|
|
174
|
Fulzele SV, Chatterjee A, Shaik MS,
Jackson T and Singh M: Inhalation delivery and anti-tumor activity
of celecoxib in human orthotopic non-small cell lung cancer
xenograft model. Pharm Res. 23:2094–2106. 2006.
|
|
175
|
Shaik MS, Haynes A, McSween J, Ikediobi O,
Kanikkannan N and Singh M: Inhalation delivery of anticancer agents
via HFA-based metered dose inhaler using methotrexate as a model
drug. J Aerosol Med. 15:261–270. 2002.
|