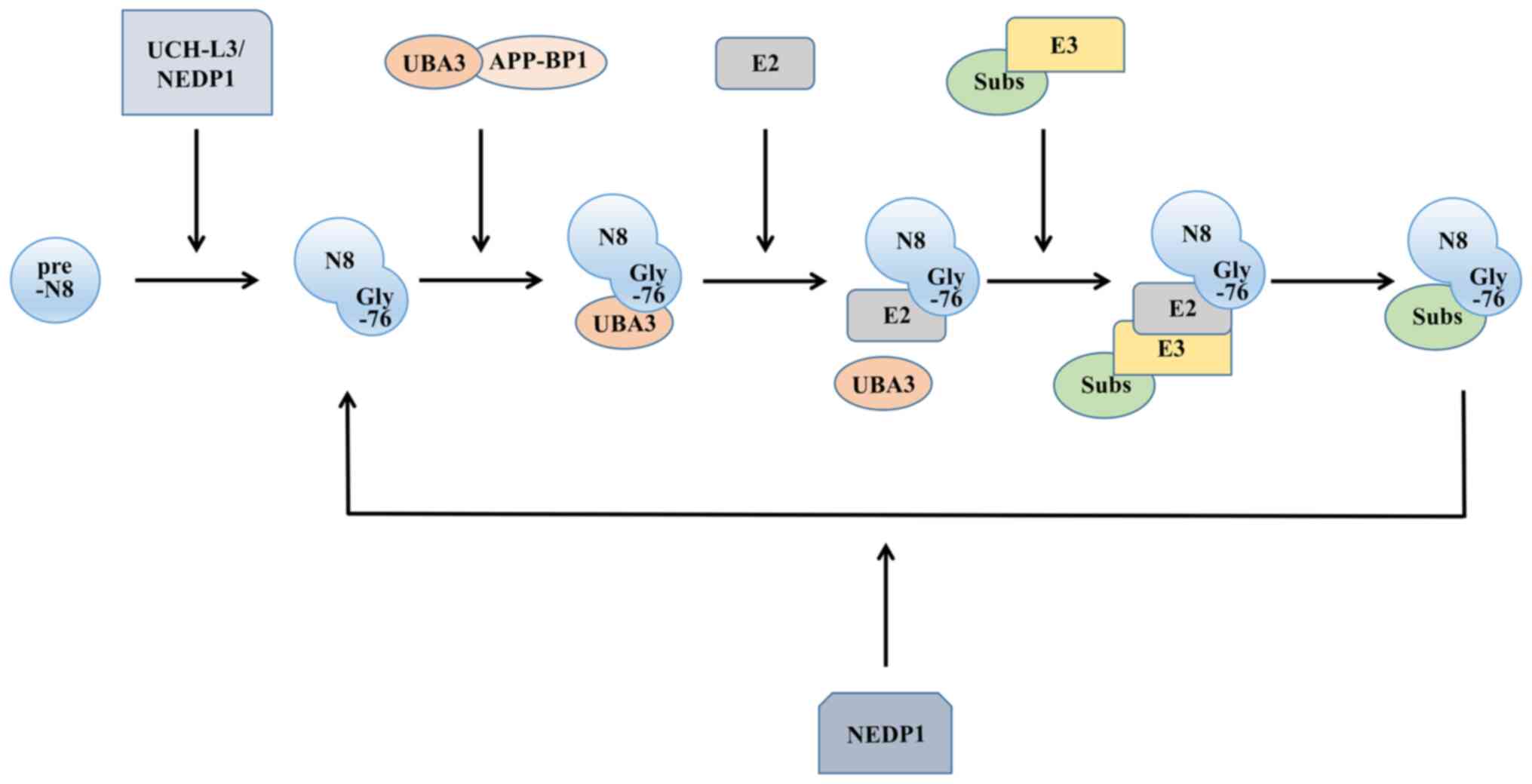
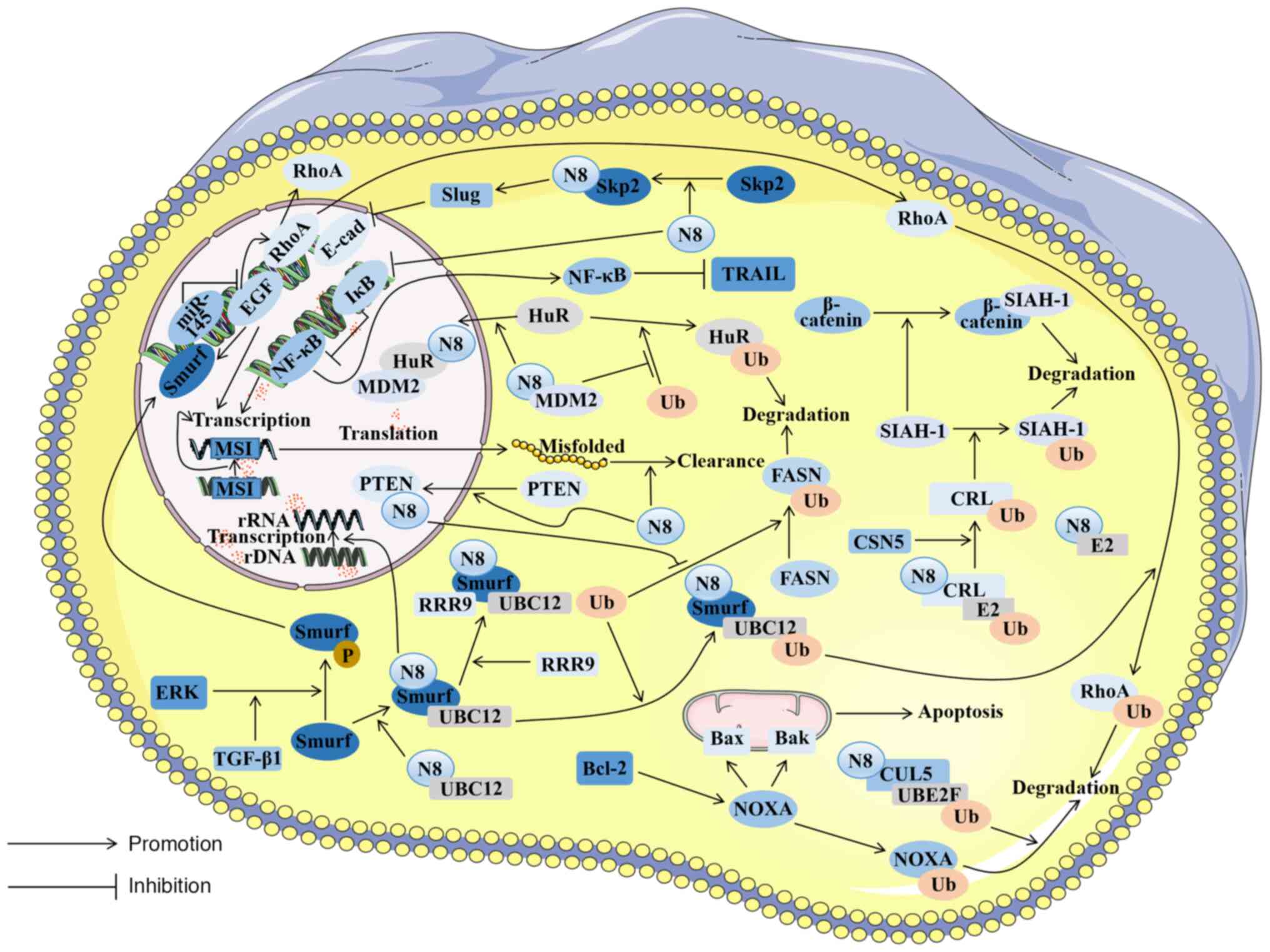
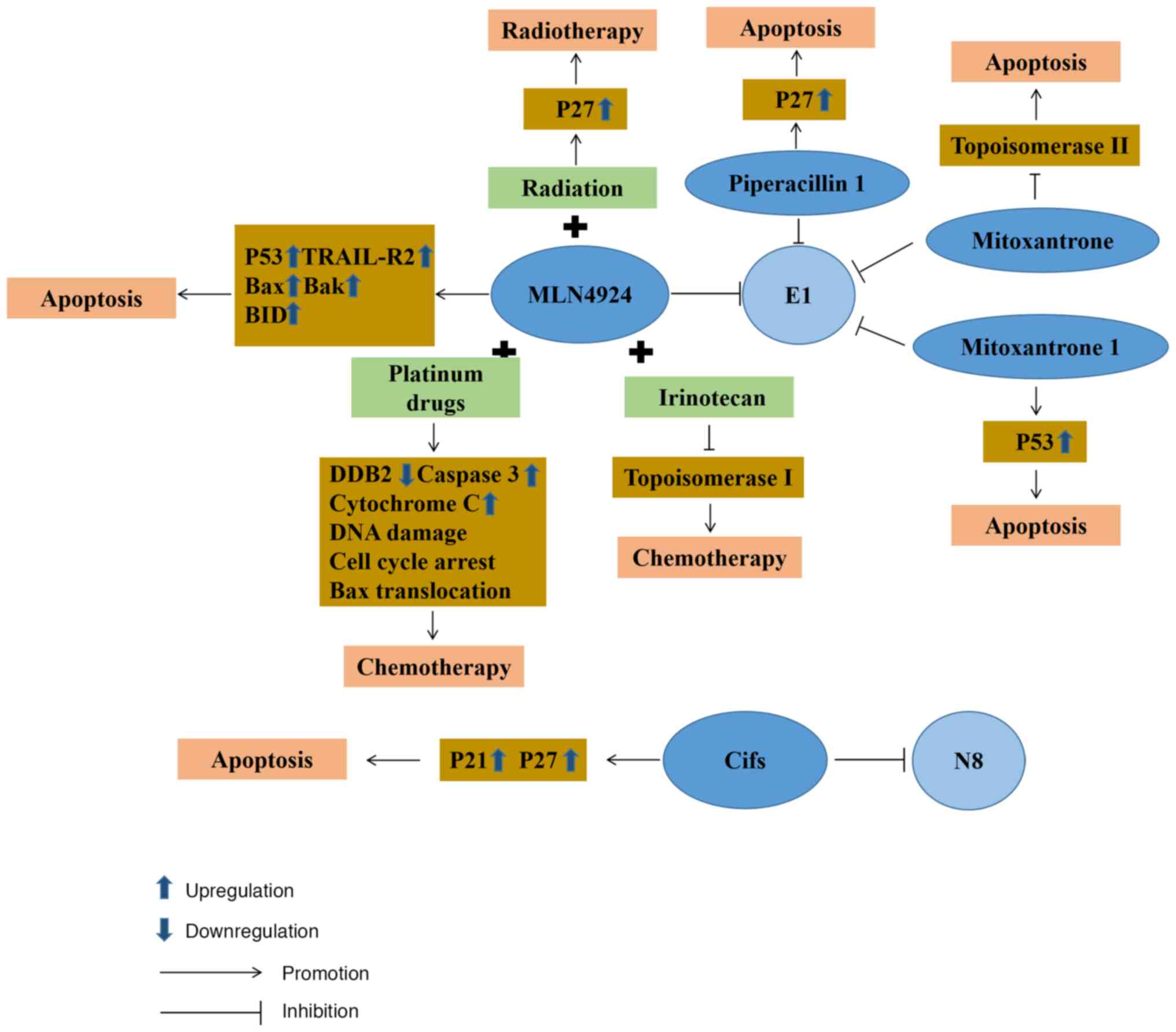
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roque-Castellano C, Fariña-Castro R,
Nogués-Ramia EM, Artiles-Armas M and Marchena-Gómez J: Colorectal
cancer surgery in selected nonagenarians is relatively safe and it
is associated with a good long-term survival: An observational
study. World J Surg Oncol. 18:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salibasic M, Pusina S, Bicakcic E, Pasic
A, Gavric I, Kulovic E, Rovcanin A and Beslija S: Colorectal cancer
surgical treatment, our experience. Med Arch. 73:412–414. 2019.
View Article : Google Scholar
|
|
6
|
Luo W, Wu M and Chen Y: Laparoscopic
versus open surgery for elderly patients with colorectal cancer: A
systematic review and meta-analysis of matched studies. ANZ J Surg.
92:2003–2017. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ustuner MA, Deniz A and Simsek A:
Laparoscopic <em>versus</em> open surgery in colorectal
cancer: Is laparoscopy safe enough? J Coll Physicians Surg Pak.
32:1170–1174. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu G, Zhang S, Zhang Y, Fu X and Liu X:
Robotic surgery in rectal cancer: Potential, challenges, and
opportunities. Curr Treat Options Oncol. 23:961–979. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riesco-Martinez MC, Modrego A,
Espinosa-Olarte P, La Salvia A and Garcia-Carbonero R:
Perioperative chemotherapy for liver metastasis of colorectal
cancer: Lessons learned and future perspectives. Curr Treat Options
Oncol. 23:1320–1337. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Habr-Gama A, Perez RO, São Julião GP,
Proscurshim I and Gama-Rodrigues J: Nonoperative approaches to
rectal cancer: A critical evaluation. Semin Radiat Oncol.
21:234–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu YJ, Chern YJ, Lai IL, Chiang SF, Liao
CK, Tsai WS, Hung HY, Hsieh PS, Yeh CY, Chiang JM, et al:
Usefulness of close surveillance for rectal cancer patients after
neoadjuvant chemoradiotherapy. Open Med (Wars). 17:1438–1448. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McWhirter D, Kitteringham N, Jones RP,
Malik H, Park K and Palmer D: Chemotherapy induced hepatotoxicity
in metastatic colorectal cancer: A review of mechanisms and
outcomes. Crit Rev Oncol Hematol. 88:404–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH: Chemotherapy for colorectal cancer
in the elderly. World J Gastroenterol. 21:5158–5166. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piawah S and Venook AP: Targeted therapy
for colorectal cancer metastases: A review of current methods of
molecularly targeted therapy and the use of tumor biomarkers in the
treatment of metastatic colorectal cancer. Cancer. 125:4139–4147.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar S, Tomooka Y and Noda M:
Identification of a set of genes with developmentally
down-regulated expression in the mouse brain. Biochem Biophys Res
Commun. 185:1155–1161. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar S, Yoshida Y and Noda M: Cloning of
a cDNA which encodes a novel ubiquitin-like protein. Biochem
Biophys Res Commun. 195:393–399. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamitani T, Kito K, Nguyen HP and Yeh ET:
Characterization of NEDD8, a developmentally down-regulated
ubiquitin-like protein. J Biol Chem. 272:28557–28562. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wada H, Kito K, Caskey LS, Yeh ET and
Kamitani T: Cleavage of the C-terminus of NEDD8 by UCH-L3. Biochem
Biophys Res Commun. 251:688–692. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mendoza HM, Shen LN, Botting C, Lewis A,
Chen J, Ink B and Hay RT: NEDP1, a highly conserved cysteine
protease that deNEDDylates Cullins. J Biol Chem. 278:25637–25643.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong L and Yeh ET: Identification of the
activating and conjugating enzymes of the NEDD8 conjugation
pathway. J Biol Chem. 274:12036–12042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang DT, Zhuang M, Ayrault O and Schulman
BA: Identification of conjugation specificity determinants unmasks
vestigial preference for ubiquitin within the NEDD8 E2. Nat Struct
Mol Biol. 15:280–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang DT, Ayrault O, Hunt HW, Taherbhoy
AM, Duda DM, Scott DC, Borg LA, Neale G, Murray PJ, Roussel MF and
Schulman BA: E2-RING expansion of the NEDD8 cascade confers
specificity to cullin modification. Mol Cell. 33:483–495. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baek K, Scott DC and Schulman BA: NEDD8
and ubiquitin ligation by cullin-RING E3 ligases. Curr Opin Struct
Biol. 67:101–109. 2021. View Article : Google Scholar :
|
|
24
|
Lydeard JR, Schulman BA and Harper JW:
Building and remodelling Cullin-RING E3 ubiquitin ligases. EMBO
Rep. 14:1050–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xirodimas DP, Saville MK, Bourdon JC, Hay
RT and Lane DP: Mdm2-mediated NEDD8 conjugation of p53 inhibits its
transcriptional activity. Cell. 118:83–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stickle NH, Chung J, Klco JM, Hill RP,
Kaelin WG Jr and Ohh M: pVHL modification by NEDD8 is required for
fibronectin matrix assembly and suppression of tumor development.
Mol Cell Biol. 24:3251–3261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan J, Yu S and Zheng X: NEDDylation
antagonizes ubiquitination of proliferating cell nuclear antigen
and regulates the recruitment of polymerase η in response to
oxidative DNA damage. Protein Cell. 9:365–379. 2018.
|
|
28
|
Ryu JH, Li SH, Park HS, Park JW, Lee B and
Chun YS: Hypoxia-inducible factor α subunit stabilization by NEDD8
conjugation is reactive oxygen species-dependent. J Biol Chem.
286:6963–6970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brown JS and Jackson SP: Ubiquitylation,
neddylation and the DNA damage response. Open Biol. 5:1500182015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J and Nussinov R: Flexible cullins in
cullin-RING E3 ligases allosterically regulate ubiquitination. J
Biol Chem. 286:40934–40942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sarikas A, Hartmann T and Pan ZQ: The
cullin protein family. Genome Biol. 12:2202011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Osaka F, Kawasaki H, Aida N, Saeki M,
Chiba T, Kawashima S, Tanaka K and Kato S: A new NEDD8-ligating
system for cullin-4A. Genes Dev. 12:2263–2268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petroski MD and Deshaies RJ: Function and
regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol.
6:9–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nam SY, Ko YS, Jung J, Yoon J, Kim YH,
Choi YJ, Park JW, Chang MS, Kim WH and Lee BL: A hypoxia-dependent
upregulation of hypoxia-inducible factor-1 by nuclear factor-κB
promotes gastric tumour growth and angiogenesis. Br J Cancer.
104:166–174. 2011. View Article : Google Scholar
|
|
35
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kittai AS, Danilova OV, Lam V, Liu T,
Bruss N, Best S, Fan G and Danilov AV: NEDD8-activating enzyme
inhibition induces cell cycle arrest and anaphase catastrophe in
malignant T-cells. Oncotarget. 12:2068–2074. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lan H, Tang Z, Jin H and Sun Y:
Neddylation inhibitor MLN4924 suppresses growth and migration of
human gastric cancer cells. Sci Rep. 6:242182016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Bei Q and Luo X: MLN4924 inhibits
cell proliferation by targeting the activated neddylation pathway
in endometrial carcinoma. J Int Med Res.
49:30006052110185922021.PubMed/NCBI
|
|
40
|
Picco G, Petti C, Sassi F, Grillone K,
Migliardi G, Rossi T, Isella C, Di Nicolantonio F, Sarotto I,
Sapino A, et al: Efficacy of NEDD8 pathway inhibition in
preclinical models of poorly differentiated, clinically aggressive
colorectal cancer. J Natl Cancer Inst. 109:djw2092017. View Article : Google Scholar
|
|
41
|
Xie P, Zhang M, He S, Lu K, Chen Y, Xing
G, Lu Y, Liu P, Li Y, Wang S, et al: The covalent modifier Nedd8 is
critical for the activation of Smurf1 ubiquitin ligase in
tumorigenesis. Nat Commun. 5:37332014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang Y, Liang Y, Li L, Zhou L, Cheng W,
Yang X, Yang X, Qi H, Yu J, Jeong LS, et al: Targeting neddylation
inhibits intravascular survival and extravasation of cancer cells
to prevent lung-cancer metastasis. Cell Biol Toxicol. 35:233–245.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mickova A, Kharaishvili G, Kurfurstova D,
Gachechiladze M, Kral M, Vacek O, Pokryvkova B, Mistrik M, Soucek K
and Bouchal J: Skp2 and slug are coexpressed in aggressive prostate
cancer and inhibited by neddylation blockade. Int J Mol Sci.
22:28442021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan KL and Pezzella F: Inhibition of NEDD8
and FAT10 ligase activities through the degrading enzyme NEDD8
ultimate buster 1: A potential anticancer approach. Oncol Lett.
12:4287–4296. 2016. View Article : Google Scholar
|
|
45
|
Watson IR, Blanch A, Lin DC, Ohh M and
Irwin MS: Mdm2-mediated NEDD8 modification of TAp73 regulates its
transactivation function. J Biol Chem. 281:34096–34103. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aoki I, Higuchi M and Gotoh Y: NEDDylation
controls the target specificity of E2F1 and apoptosis induction.
Oncogene. 32:3954–3964. 2013. View Article : Google Scholar
|
|
47
|
Halazonetis TD, Gorgoulis VG and Bartek J:
An oncogene-induced DNA damage model for cancer development.
Science. 319:1352–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garvin AJ: Beyond reversal: ubiquitin and
ubiquitin-like proteases and the orchestration of the DNA double
strand break repair response. Biochem Soc Trans. 47:1881–1893.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Meir M, Galanty Y, Kashani L, Blank M,
Khosravi R, Fernández-Ávila MJ, Cruz-García A, Star A, Shochot L,
Thomas Y, et al: The COP9 signalosome is vital for timely repair of
DNA double-strand breaks. Nucleic Acids Res. 43:4517–4530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gâtel P, Piechaczyk M and Bossis G:
Ubiquitin, SUMO, and Nedd8 as therapeutic targets in cancer. Adv
Exp Med Biol. 1233:29–54. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun Y, Baechler SA, Zhang X, Kumar S,
Factor VM, Arakawa Y, Chau CH, Okamoto K, Parikh A, Walker B, et
al: Targeting neddylation sensitizes colorectal cancer to
topoisomerase I inhibitors by inactivating the DCAF13-CRL4
ubiquitin ligase complex. Nat Commun. 14:37622023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wan J, Zhu J, Li G and Zhang Z:
Radiosensitization of human colorectal cancer cells by MLN4924: An
Inhibitor of NEDD8-Activating Enzyme. Technol Cancer Res Treat.
15:527–534. 2016. View Article : Google Scholar
|
|
54
|
Shao Y, Liu Z, Song X, Sun R, Zhou Y,
Zhang D, Sun H, Huang J, Wu C, Gu W, et al: ALKBH5/YTHDF2-mediated
m6A modification of circAFF2 enhances radiosensitivity of
colorectal cancer by inhibiting Cullin neddylation. Clin Transl
Med. 13:e13182023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou L, Jiang Y, Luo Q, Li L and Jia L:
Neddylation: A novel modulator of the tumor microenvironment. Mol
Cancer. 18:772019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chang FM, Reyna SM, Granados JC, Wei SJ,
Innis-Whitehouse W, Maffi SK, Rodriguez E, Slaga TJ and Short JD:
Inhibition of neddylation represses lipopolysaccharide-induced
proinflammatory cytokine production in macrophage cells. J Biol
Chem. 287:35756–35767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li L, Liu B, Dong T, Lee HW, Yu J, Zheng
Y, Gao H, Zhang Y, Chu Y, Liu G, et al: Neddylation pathway
regulates the proliferation and survival of macrophages. Biochem
Biophys Res Commun. 432:494–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou L, Jiang Y, Liu X, Li L, Yang X, Dong
C, Liu X, Lin Y, Li Y, Yu J, et al: Promotion of tumor-associated
macrophages infiltration by elevated neddylation pathway via
NF-κB-CCL2 signaling in lung cancer. Oncogene. 38:5792–5804. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Joyce JA and Fearon DT: T cell exclusion,
immune privilege, and the tumor microenvironment. Science.
348:74–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jin HS, Liao L, Park Y and Liu YC:
Neddylation pathway regulates T-cell function by targeting an
adaptor protein Shc and a protein kinase Erk signaling. Proc Natl
Acad Sci USA. 110:624–629. 2013. View Article : Google Scholar :
|
|
62
|
Jiang Y, Li L, Li Y, Liu G, Hoffman RM and
Jia L: Neddylation regulates macrophages and implications for
cancer therapy. Front Cell Dev Biol. 9:6811862021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Best S, Lam V, Liu T, Bruss N, Kittai A,
Danilova OV, Murray S, Berger A, Pennock ND, Lind EF and Danilov
AV: Immunomodulatory effects of pevonedistat, a NEDD8-activating
enzyme inhibitor, in chronic lymphocytic leukemia-derived T cells.
Leukemia. 35:156–168. 2021. View Article : Google Scholar :
|
|
64
|
Maishi N and Hida K: Tumor endothelial
cells accelerate tumor metastasis. Cancer Sci. 108:1921–1926. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tang N, Wang L, Esko J, Giordano FJ, Huang
Y, Gerber HP, Ferrara N and Johnson RS: Loss of HIF-1alpha in
endothelial cells disrupts a hypoxia-driven VEGF autocrine loop
necessary for tumorigenesis. Cancer Cell. 6:485–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shi CS, Kuo KL, Lin WC, Chen MS, Liu SH,
Liao SM, Hsu CH, Chang YW, Chang HC and Huang KH: Neddylation
inhibitor, MLN4924 suppresses angiogenesis in huvecs and solid
cancers: in vitro and in vivo study. Am J Cancer Res. 10:953–964.
2020.PubMed/NCBI
|
|
67
|
Yao WT, Wu JF, Yu GY, Wang R, Wang K, Li
LH, Chen P, Jiang YN, Cheng H, Lee HW, et al: Suppression of tumor
angiogenesis by targeting the protein neddylation pathway. Cell
Death Dis. 5:e10592014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tauriello DVF, Palomo-Ponce S, Stork D,
Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M,
Ibiza S, Cañellas A, Hernando-Momblona X, et al: TGFβ drives immune
evasion in genetically reconstituted colon cancer metastasis.
Nature. 554:538–543. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kerscher O, Felberbaum R and Hochstrasser
M: Modification of proteins by ubiquitin and ubiquitin-like
proteins. Annu Rev Cell Dev Biol. 22:159–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
He S, Cao Y, Xie P, Dong G and Zhang L:
The Nedd8 Non-covalent Binding Region in the Smurf HECT Domain is
Critical to its Ubiquitn Ligase Function. Sci Rep. 7:413642017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zheng J, Shi Z, Yang P, Zhao Y, Tang W, Ye
S, Xuan Z, Chen C, Shao C, Wu Q and Sun H: ERK-Smurf1-RhoA
signaling is critical for TGFβ-drived EMT and tumor metastasis.
Life Sci Alliance. 5:e2021013302022. View Article : Google Scholar
|
|
72
|
Xie L, Law BK, Chytil AM, Brown KA, Aakre
ME and Moses HL: Activation of the Erk pathway is required for
TGF-beta1-induced EMT in vitro. Neoplasia. 6:603–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cardoso AP, Pinto ML, Pinto AT, Oliveira
MI, Pinto MT, Gonçalves R, Relvas JB, Figueiredo C, Seruca R,
Mantovani A, et al: Macrophages stimulate gastric and colorectal
cancer invasion through EGFR Y(1086), c-Src, Erk1/2 and Akt
phosphorylation and smallGTPase activity. Oncogene. 33:2123–2133.
2014. View Article : Google Scholar
|
|
75
|
Price JT, Wilson HM and Haites NE:
Epidermal growth factor (EGF) increases the in vitro invasion,
motility and adhesion interactions of the primary renal carcinoma
cell line, A704. Eur J Cancer. 32A:1977–1982. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li S, Wu X, Xu Y, Wu S, Li Z, Chen R,
Huang N, Zhu Z and Xu X: miR-145 suppresses colorectal cancer cell
migration and invasion by targeting an ETS-related gene. Oncol Rep.
36:1917–1926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kwon A, Lee HL, Woo KM, Ryoo HM and Baek
JH: SMURF1 plays a role in EGF-induced breast cancer cell migration
and invasion. Mol Cells. 36:548–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Du MG, Liu F, Chang Y, Tong S, Liu W, Chen
YJ and Xie P: Neddylation modification of the U3 snoRNA-binding
protein RRP9 by Smurf1 promotes tumorigenesis. J Biol Chem.
297:1013072021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Barrios-Rodiles M, Brown KR, Ozdamar B,
Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW,
et al: High-throughput mapping of a dynamic signaling network in
mammalian cells. Science. 307:1621–1625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Clerget G, Bourguignon-Igel V,
Marmier-Gourrier N, Rolland N, Wacheul L, Manival X, Charron C,
Kufel J, Méreau A, Senty-Ségault V, et al: Synergistic defects in
pre-rRNA processing from mutations in the U3-specific protein Rrp9
and U3 snoRNA. Nucleic Acids Res. 48:3848–3868. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pecoraro A, Pagano M, Russo G and Russo A:
Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins. Int
J Mol Sci. 22:54962021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pelletier J, Thomas G and Volarević S:
Ribosome biogenesis in cancer: New players and therapeutic avenues.
Nat Rev Cancer. 18:51–63. 2018. View Article : Google Scholar
|
|
84
|
Guo J, Xu G, Mao C and Wei R: Low
Expression of Smurf1 Enhances the Chemosensitivity of Human
Colorectal Cancer to Gemcitabine and Cisplatin in Patient-Derived
Xenograft Models. Transl Oncol. 13:1008042020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Salmena L, Carracedo A and Pandolfi PP:
Tenets of PTEN tumor suppression. Cell. 133:403–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xie P, Peng Z, Chen Y, Li H, Du M, Tan Y,
Zhang X, Lu Z, Cui CP, Liu CH, et al: Neddylation of PTEN regulates
its nuclear import and promotes tumor development. Cell Res.
31:291–311. 2021. View Article : Google Scholar :
|
|
88
|
Finicle BT, Jayashankar V and Edinger AL:
Nutrient scavenging in cancer. Nat Rev Cancer. 18:619–633. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Murphy N, Song M, Papadimitriou N,
Carreras-Torres R, Langenberg C, Martin RM, Tsilidis KK, Barroso I,
Chen J, Frayling TM, et al: Associations Between Glycemic Traits
and Colorectal Cancer: A Mendelian Randomization Analysis. J Natl
Cancer Inst. 114:740–752. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang W, Lu Y and Li X, Zhang J, Lin W,
Zhang W, Zheng L and Li X: IPO5 promotes the proliferation and
tumourigenicity of colorectal cancer cells by mediating RASAL2
nuclear transportation. J Exp Clin Cancer Res. 38:2962019.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xia Q, Zhang H, Zhang P, Li Y, Xu M, Li X,
Li X and Dong L: Oncogenic Smurf1 promotes PTEN wild-type
glioblastoma growth by mediating PTEN ubiquitylation. Oncogene.
39:5902–5915. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Du MG, Peng ZQ, Gai WB, Liu F, Liu W, Chen
YJ, Li HC, Zhang X, Liu CH, Zhang LQ, et al: The Absence of PTEN in
Breast Cancer Is a Driver of MLN4924 Resistance. Front Cell Dev
Biol. 9:6674352021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Phelps RA, Chidester S, Dehghanizadeh S,
Phelps J, Sandoval IT, Rai K, Broadbent T, Sarkar S, Burt RW and
Jones DA: A two-step model for colon adenoma initiation and
progression caused by APC loss. Cell. 137:623–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schütz AK, Hennes T, Jumpertz S, Fuchs S
and Bernhagen J: Role of CSN5/JAB1 in Wnt/β-catenin activation in
colorectal cancer cells. FEBS Lett. 586:1645–1651. 2012. View Article : Google Scholar
|
|
96
|
Cope GA and Deshaies RJ: COP9 signalosome:
A multifunctional regulator of SCF and other cullin-based ubiquitin
ligases. Cell. 114:663–671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jumpertz S, Hennes T, Asare Y, Vervoorts
J, Bernhagen J and Schütz AK: The β-catenin E3 ubiquitin ligase
SIAH-1 is regulated by CSN5/JAB1 in CRC cells. Cell Signal.
26:2051–2059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sninsky JA, Shore BM, Lupu GV and Crockett
SD: Risk Factors for Colorectal Polyps and Cancer. Gastrointest
Endosc Clin N Am. 32:195–213. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Aiello NM, Maddipati R, Norgard RJ, Balli
D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, et al:
EMT Subtype Influences Epithelial Plasticity and Mode of Cell
Migration. Dev Cell. 45:681–695.e84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Asmamaw MD, Liu Y, Zheng YC, Shi XJ and
Liu HM: Skp2 in the ubiquitin-proteasome system: A comprehensive
review. Med Res Rev. 40:1920–1949. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yu X, Zhou L, Liu W, Liu L, Gao F, Li W
and Liu H: Skp2 stabilizes Mcl-1 and confers radioresistance in
colorectal cancer. Cell Death Dis. 13:2492022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen P, Li X, Zhang R, Liu S, Xiang Y,
Zhang M, Chen X, Pan T, Yan L, Feng J, et al: Combinative treatment
of β-elemene and cetuximab is sensitive to KRAS mutant colorectal
cancer cells by inducing ferroptosis and inhibiting
epithelial-mesenchymal transformation. Theranostics. 10:5107–5119.
2020. View Article : Google Scholar :
|
|
104
|
Wang L, Li S, Luo H, Lu Q and Yu S: PCSK9
promotes the progression and metastasis of colon cancer cells
through regulation of EMT and PI3K/AKT signaling in tumor cells and
phenotypic polarization of macrophages. J Exp Clin Cancer Res.
41:3032022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kunkel TA and Erie DA: DNA mismatch
repair. Annu Rev Biochem. 74:681–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guastadisegni C, Colafranceschi M, Ottini
L and Dogliotti E: Microsatellite instability as a marker of
prognosis and response to therapy: A meta-analysis of colorectal
cancer survival data. Eur J Cancer. 46:2788–2798. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
de la Chapelle A and Hampel H: Clinical
relevance of microsatellite instability in colorectal cancer. J
Clin Oncol. 28:3380–3387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
McGrail DJ, Garnett J, Yin J, Dai H, Shih
DJH, Lam TNA, Li Y, Sun C, Li Y, Schmandt R, et al: Proteome
Instability Is a Therapeutic Vulnerability in Mismatch
Repair-Deficient Cancer. Cancer Cell. 37:371–386.e12. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kim H, Rafiuddin-Shah M, Tu HC, Jeffers
JR, Zambetti GP, Hsieh JJ and Cheng EH: Hierarchical regulation of
mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell
Biol. 8:1348–1358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: The sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ploner C, Kofler R and Villunger A: Noxa:
At the tip of the balance between life and death. Oncogene.
27(Suppl 1): S84–S92. 2008. View Article : Google Scholar
|
|
113
|
Xu S, Ma Y, Tong Q, Yang J, Liu J, Wang Y,
Li G, Zeng J, Fang S, Li F, et al: Cullin-5 neddylation-mediated
NOXA degradation is enhanced by PRDX1 oligomers in colorectal
cancer. Cell Death Dis. 12:2652021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Brennan CM and Steitz JA: HuR and mRNA
stability. Cell Mol Life Sci. 58:266–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang W, Caldwell MC, Lin S, Furneaux H and
Gorospe M: HuR regulates cyclin A and cyclin B1 mRNA stability
during cell proliferation. EMBO J. 19:2340–2350. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Abdelmohsen K and Gorospe M:
Posttranscriptional regulation of cancer traits by HuR. Wiley
Interdiscip Rev RNA. 1:214–229. 2010. View
Article : Google Scholar
|
|
117
|
McLarnon A: Cancer: Mdm2-regulated
stabilization of HuR by neddylation in HCC and colon cancer-a
possible target for therapy. Nat Rev Gastroenterol Hepatol.
9:42011.
|
|
118
|
Embade N, Fernández-Ramos D, Varela-Rey M,
Beraza N, Sini M, Gutiérrez de Juan V, Woodhoo A, Martínez-López N,
Rodríguez-Iruretagoyena B, Bustamante FJ, et al: Murine double
minute 2 regulates Hu antigen R stability in human liver and colon
cancer through NEDDylation. Hepatology. 55:1237–1248. 2012.
View Article : Google Scholar
|
|
119
|
Greenlee JD, Lopez-Cavestany M,
Ortiz-Otero N, Liu K, Subramanian T, Cagir B and King MR:
Oxaliplatin resistance in colorectal cancer enhances TRAIL
sensitivity via death receptor 4 upregulation and lipid raft
localization. Elife. 10:e677502021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lee SJ, Lee DE, Choi SY and Kwon OS:
OSMI-1 Enhances TRAIL-Induced Apoptosis through ER Stress and NF-κB
Signaling in Colon Cancer Cells. Int J Mol Sci. 22:110732021.
View Article : Google Scholar
|
|
121
|
Paiva C, Godbersen JC, Rowland T, Danilova
OV, Danes C, Berger A and Danilov AV: Pevonedistat, a
Nedd8-activating enzyme inhibitor, sensitizes neoplastic B-cells to
death receptor-mediated apoptosis. Oncotarget. 8:21128–21139. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sakamoto K, Maeda S, Hikiba Y, Nakagawa H,
Hayakawa Y, Shibata W, Yanai A, Ogura K and Omata M: Constitutive
NF-kappaB activation in colorectal carcinoma plays a key role in
angiogenesis, promoting tumor growth. Clin Cancer Res.
15:2248–2258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Linares J, Sallent-Aragay A,
Badia-Ramentol J, Recort-Bascuas A, Méndez A, Manero-Rupérez N, Re
DL, Rivas EI, Guiu M, Zwick M, et al: Long-term platinum-based drug
accumulation in cancer-associated fibroblasts promotes colorectal
cancer progression and resistance to therapy. Nat Commun.
14:7462023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Soucy TA, Smith PG, Milhollen MA, Berger
AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP,
Critchley S, et al: An inhibitor of NEDD8-activating enzyme as a
new approach to treat cancer. Nature. 458:732–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Brownell JE, Sintchak MD, Gavin JM, Liao
H, Bruzzese FJ, Bump NJ, Soucy TA, Milhollen MA, Yang X, Burkhardt
AL, et al: Substrate-assisted inhibition of ubiquitin-like
protein-activating enzymes: The NEDD8 E1 inhibitor MLN4924 forms a
NEDD8-AMP mimetic in situ. Mol Cell. 37:102–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wu KJ, Zhong HJ, Li G, Liu C, Wang HD, Ma
DL and Leung CH: Structure-based identification of a
NEDD8-activating enzyme inhibitor via drug repurposing. Eur J Med
Chem. 143:1021–1027. 2018. View Article : Google Scholar
|
|
127
|
Ferris J, Espona-Fiedler M, Hamilton C,
Holohan C, Crawford N, McIntyre AJ, Roberts JZ, Wappett M, McDade
SS, Longley DB and Coyle V: Pevonedistat (MLN4924): Mechanism of
cell death induction and therapeutic potential in colorectal
cancer. Cell Death Discov. 6:612020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Sekeres MA, Watts J, Radinoff A, Sangerman
MA, Cerrano M, Lopez PF, Zeidner JF, Campelo MD, Graux C, Liesveld
J, et al: Randomized phase 2 trial of pevonedistat plus azacitidine
versus azacitidine for higher-risk MDS/CMML or low-blast AML.
Leukemia. 35:2119–2124. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhou X, Sedarati F, Faller DV, Zhao D,
Faessel HM, Chowdhury S, Bolleddula J, Li Y, Venkatakrishnan K and
Papai Z: Phase I study assessing the mass balance,
pharmacokinetics, and excretion of [14C]-pevonedistat, a
NEDD8-activating enzyme inhibitor in patients with advanced solid
tumors. Invest New Drugs. 39:488–498. 2021. View Article : Google Scholar
|
|
131
|
Zheng W, Luo Z, Zhang J, Min P, Li W, Xu
D, Zhang Z, Xiong P, Liang H and Liu J: Neural precursor cell
expressed, developmentally downregulated 8-activating enzyme
inhibitor MLN4924 sensitizes colorectal cancer cells to oxaliplatin
by inducing DNA damage, G2 cell cycle arrest and apoptosis. Mol Med
Rep. 15:2795–2801. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Toth JI, Yang L, Dahl R and Petroski MD: A
gatekeeper residue for NEDD8-activating enzyme inhibition by
MLN4924. Cell Rep. 1:309–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhang S, You X, Xu T, Chen Q, Li H, Dou L
and Sun Y, Xiong X, Meredith MA and Sun Y: PD-L1 induction via the
MEK-JNK-AP1 axis by a neddylation inhibitor promotes
cancer-associated immunosuppression. Cell Death Dis. 13:8442022.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhou S, Zhao X, Yang Z, Yang R, Chen C,
Zhao K, Wang W, Ma Y, Zhang Q and Wang X: Neddylation inhibition
upregulates PD-L1 expression and enhances the efficacy of immune
checkpoint blockade in glioblastoma. Int J Cancer. 145:763–774.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6:82018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Issa NT, Stathias V, Schürer S and
Dakshanamurthy S: Machine and deep learning approaches for cancer
drug repurposing. Semin Cancer Biol. 68:132–142. 2021. View Article : Google Scholar
|
|
137
|
Gin A, Dilay L, Karlowsky JA, Walkty A,
Rubinstein E and Zhanel GG: Piperacillin-tazobactam: A
beta-lactam/beta-lactamase inhibitor combination. Expert Rev Anti
Infect Ther. 5:365–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Maarbjerg SF, Thorsted A, Friberg LE,
Nielsen EI, Wang M, Schrøder H and Albertsen BK: Continuous
infusion of piperacillin-tazobactam significantly improves target
attainment in children with cancer and fever. Cancer Rep (Hoboken).
5:e15852022. View Article : Google Scholar
|
|
139
|
Rosanova MT, Cuellar-Pompa L and Lede R:
Efficacy and safety of empirical treatment with
piperacillin/tazobactan as monotherapy in episodes of neutropenia
and fever in children with cancer: Systematic review and
meta-analysis. Rev Chilena Infectol. 38:488–494. 2021.In Spanish.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zhong HJ, Liu LJ, Chan DS, Wang HM, Chan
PW, Ma DL and Leung CH: Structure-based repurposing of FDA-approved
drugs as inhibitors of NEDD8-activating enzyme. Biochimie.
102:211–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Evison BJ, Sleebs BE, Watson KG, Phillips
DR and Cutts SM: Mitoxantrone, more than just another topoisomerase
II poison. Med Res Rev. 36:248–299. 2016. View Article : Google Scholar
|
|
142
|
Faulds D, Balfour JA, Chrisp P and Langtry
HD: Mitoxantrone. A review of its pharmacodynamic and
pharmacokinetic properties, and therapeutic potential in the
chemotherapy of cancer. Drugs. 41:400–449. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Taieb F, Nougayrède JP and Oswald E: Cycle
inhibiting factors (cifs): Cyclomodulins that usurp the
ubiquitin-dependent degradation pathway of host cells. Toxins
(Basel). 3:356–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Liu L, Ni J, Zhang J and He X:
Construction and characterization of regulated cycle inhibiting
factors induced upon Tet-On system in human colon cancer cell
lines. Anticancer Drugs. 29:854–860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu L, Zhang J, Gu M, Li G, Ni J and Fan
M: Antitumor effect of cycle inhibiting factor expression in colon
cancer via salmonella VNP20009. Anticancer Agents Med Chem.
20:1722–1727. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wheate NJ, Walker S, Craig GE and Oun R:
The status of platinum anticancer drugs in the clinic and in
clinical trials. Dalton Trans. 39:8113–8127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Tchounwou PB, Dasari S, Noubissi FK, Ray P
and Kumar S: Advances in our understanding of the molecular
mechanisms of action of cisplatin in cancer therapy. J Exp
Pharmacol. 13:303–328. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li W, Sun Y, Chen J, Jiang Z and Yang J:
PEGylated cisplatin nanoparticles for treating colorectal cancer in
a pH-Responsive manner. J Immunol Res. 2022:80239152022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Jones TM, Espitia CM, Ooi A, Bauman JE,
Carew JS and Nawrocki ST: Targeted CUL4A inhibition synergizes with
cisplatin to yield long-term survival in models of head and neck
squamous cell carcinoma through a DDB2-mediated mechanism. Cell
Death Dis. 13:3502022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Misra S, Zhang X, Wani NA, Sizemore S and
Ray A: Both BRCA1-wild type and -mutant triple-negative breast
cancers show sensitivity to the NAE inhibitor MLN4924 which is
enhanced upon MLN4924 and cisplatin combination treatment.
Oncotarget. 11:784–800. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zeng Y, Iv YS, Pan QH, Zhou YG and Li H:
An overactive neddylation pathway serves as a therapeutic target
and MLN4924 enhances the anticancer activity of cisplatin in
pancreatic cancer. Oncol Lett. 18:2724–2732. 2019.PubMed/NCBI
|
|
152
|
Lin WC, Kuo KL, Shi CS, Wu JT, Hsieh JT,
Chang HC, Liao SM, Chou CT, Chiang CK, Chiu WS, et al: MLN4924, a
Novel NEDD8-activating enzyme inhibitor, exhibits antitumor
activity and enhances cisplatin-induced cytotoxicity in human
cervical carcinoma: In vitro and in vivo study. Am J Cancer Res.
5:3350–3362. 2015.
|
|
153
|
Ho GY, Woodward N and Coward JI: Cisplatin
versus carboplatin: Comparative review of therapeutic management in
solid malignancies. Crit Rev Oncol Hematol. 102:37–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Arango D, Wilson AJ, Shi Q, Corner GA,
Arañes MJ, Nicholas C, Lesser M, Mariadason JM and Augenlicht LH:
Molecular mechanisms of action and prediction of response to
oxaliplatin in colorectal cancer cells. Br J Cancer. 91:1931–1946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Shoji H, Takahari D, Hara H, Nagashima K,
Adachi J and Boku N: A phase I study of pevonedistat plus
capecitabine plus oxaliplatin in patients with advanced gastric
cancer refractory to platinum (NCCH-1811). Future Sci OA.
7:FSO7212021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Liu T, Zhang X, Du L, Wang Y, Liu X, Tian
H, Wang L, Li P, Zhao Y, Duan W, et al: Exosome-transmitted
miR-128-3p increase chemosensitivity of oxaliplatin-resistant
colorectal cancer. Mol Cancer. 18:432019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Buyana B, Naki T, Alven S and Aderibigbe
BA: Nanoparticles loaded with platinum drugs for colorectal cancer
therapy. Int J Mol Sci. 23:112612022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Hicks LD, Hyatt JL, Stoddard S, Tsurkan L,
Edwards CC, Wadkins RM and Potter PM: Improved, selective, human
intestinal carboxylesterase inhibitors designed to modulate
7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin
(Irinotecan; CPT-11) toxicity. J Med Chem. 52:3742–3752. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Meisenberg C, Ashour ME, El-Shafie L, Liao
C, Hodgson A, Pilborough A, Khurram SA, Downs JA, Ward SE and
El-Khamisy SF: Epigenetic changes in histone acetylation underpin
resistance to the topoisomerase I inhibitor irinotecan. Nucleic
Acids Res. 45:1159–1176. 2017.PubMed/NCBI
|