|
1
|
Danneskiold-Samsøe NB, Dias de Freitas
Queiroz Barros H, Santos R, Bicas JL, Cazarin CBB, Madsen L,
Kristiansen K, Pastore GM, Brix S and Maróstica Júnior MR:
Interplay between food and gut microbiota in health and disease.
Food Res Int. 115:23–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
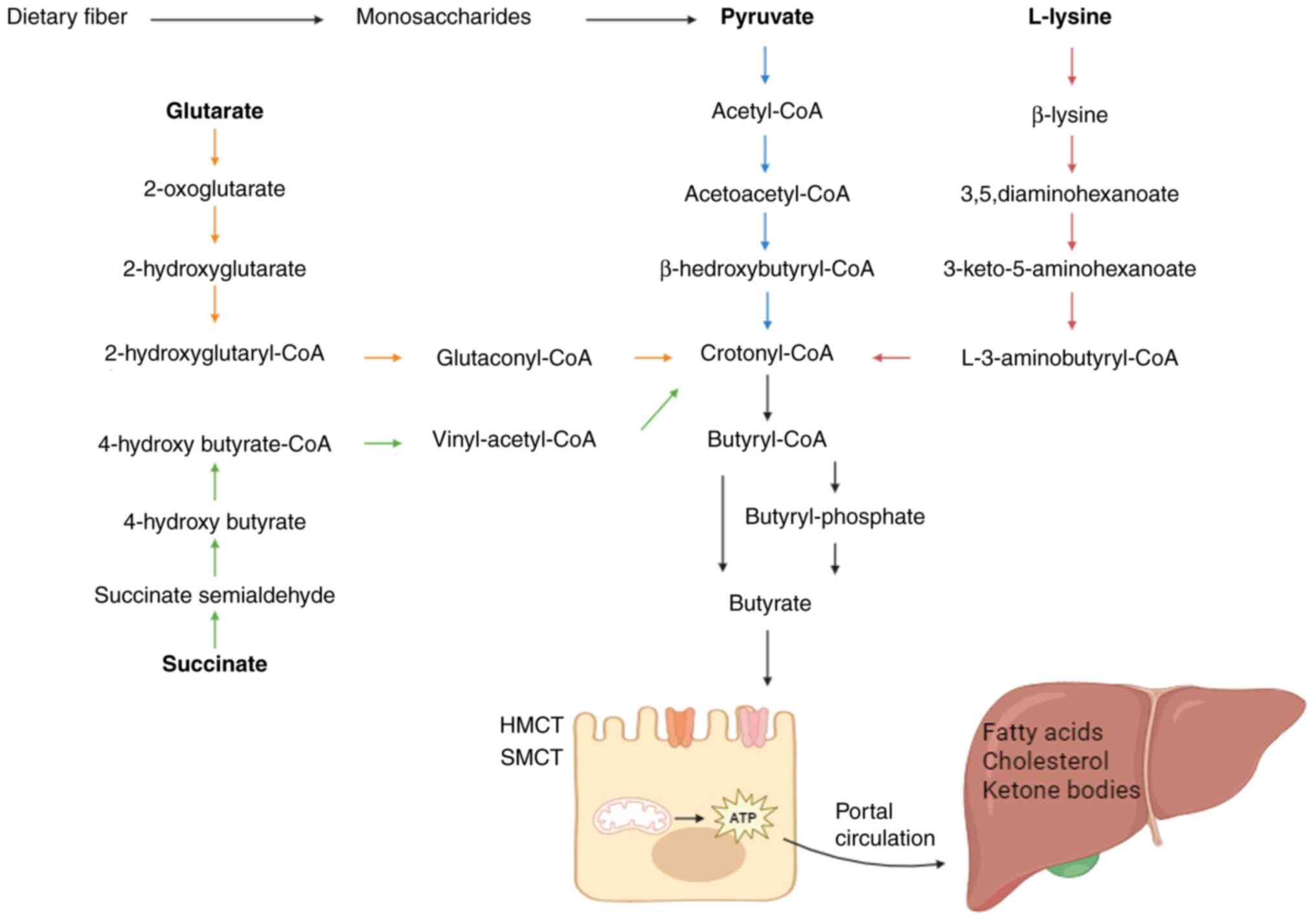
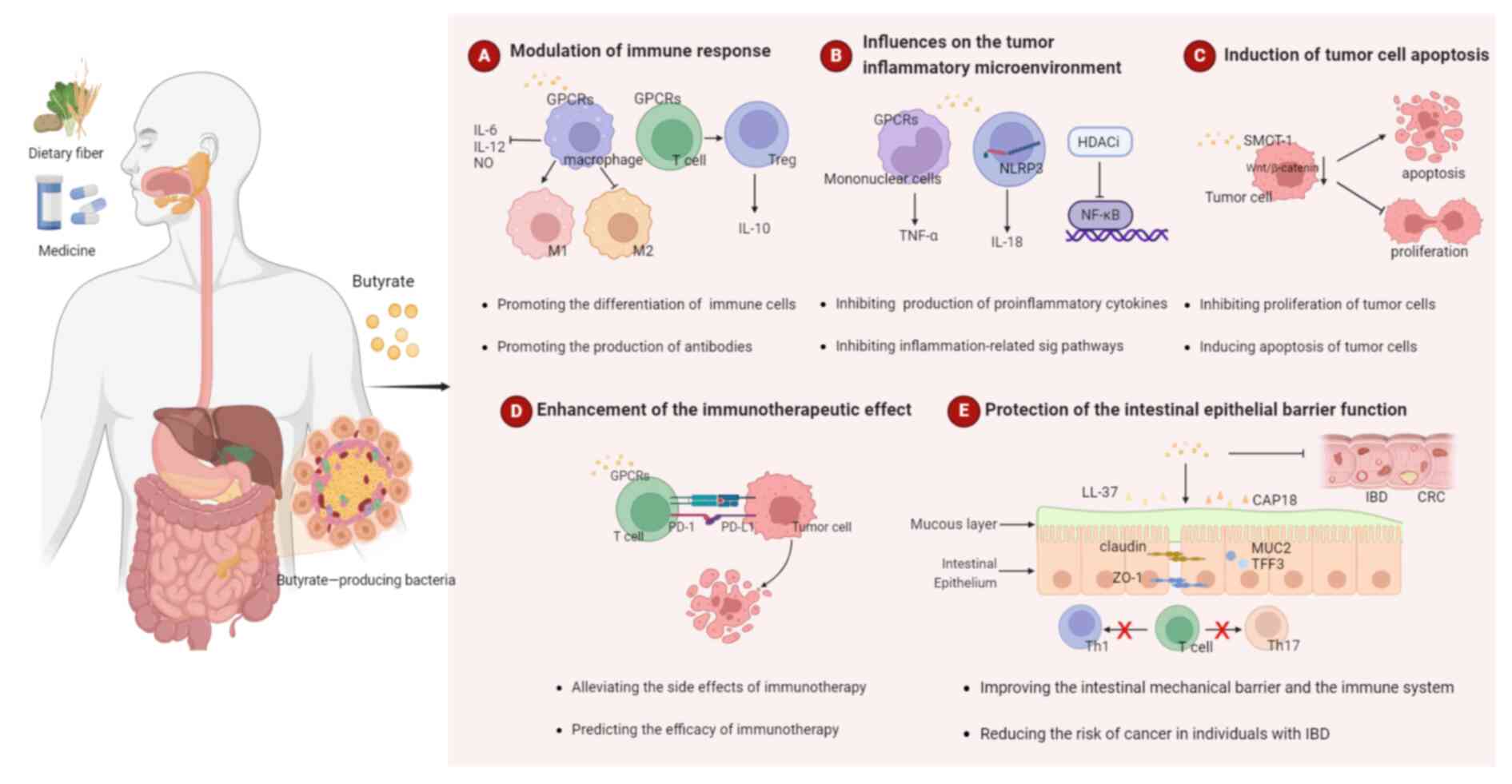
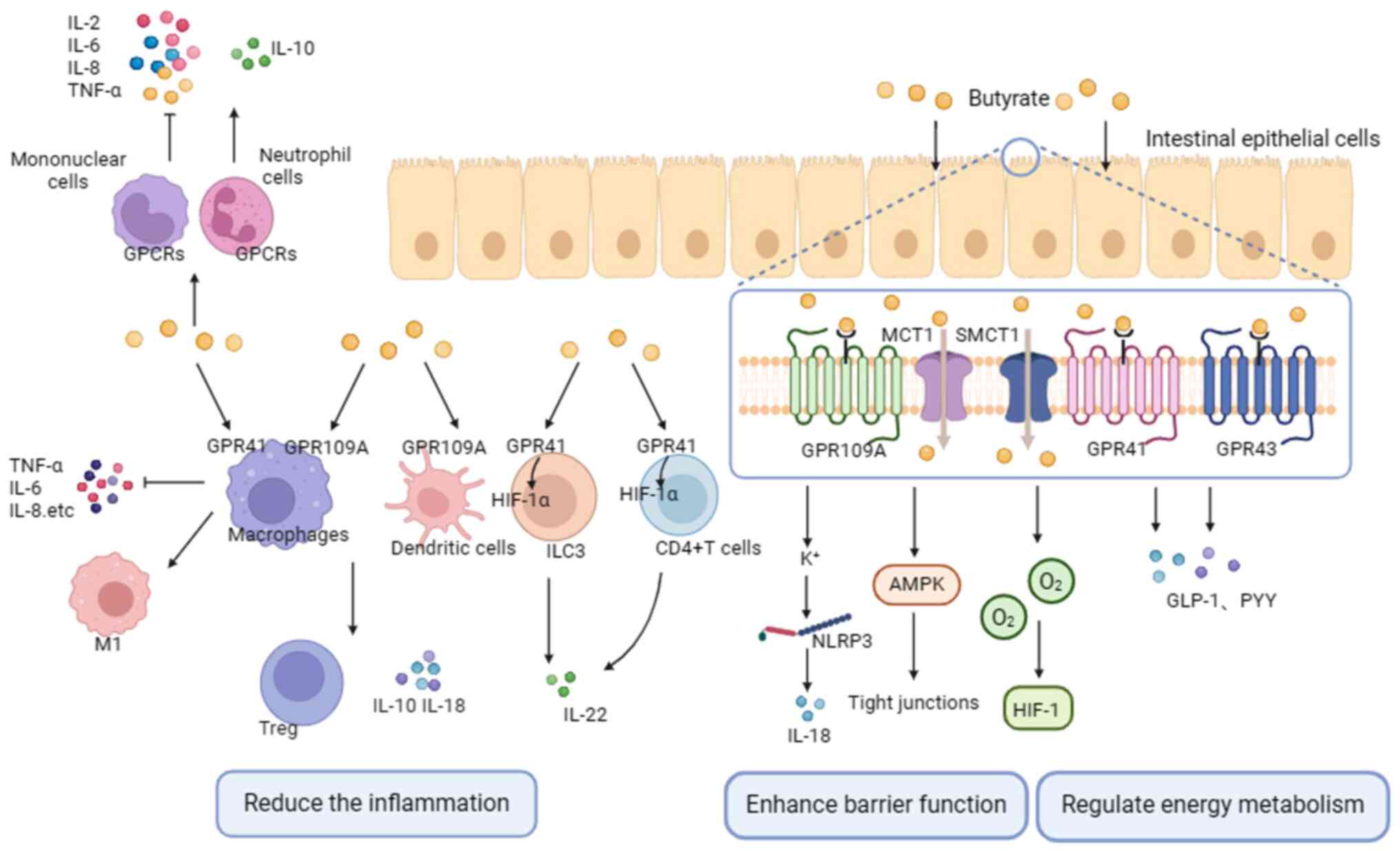
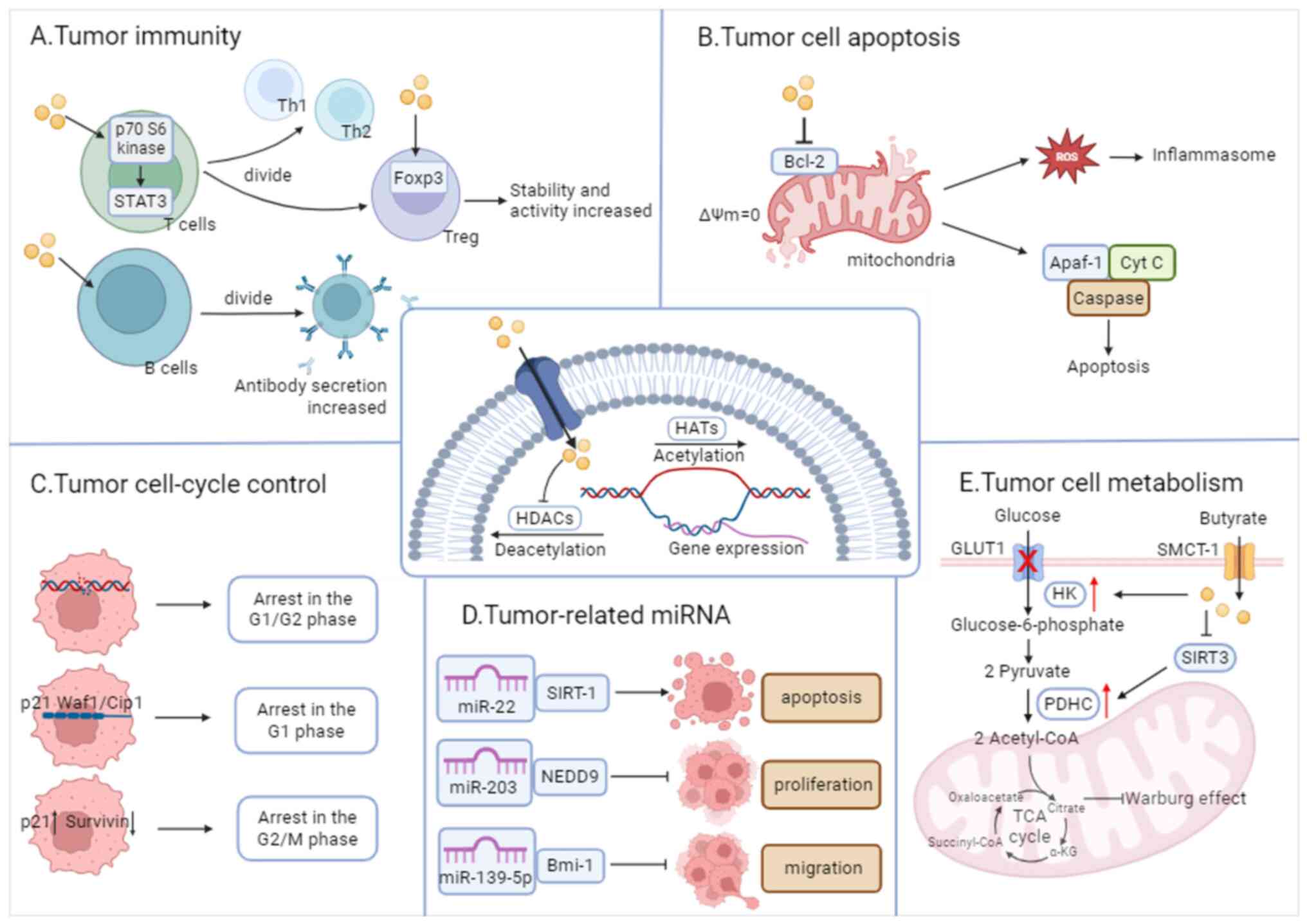
2
|
Kayama H and Takeda K: Functions of innate
immune cells and commensal bacteria in gut homeostasis. J Biochem.
159:141–149. 2016. View Article : Google Scholar :
|
|
3
|
Kim KN, Yao Y and Ju SY: Short chain fatty
acids and fecal microbiota abundance in humans with obesity: A
systematic review and meta-analysis. Nutrients. 11:25122019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyamoto J, Kasubuchi M, Nakajima A, Irie
J, Itoh H and Kimura I: The role of short-chain fatty acid on blood
pressure regulation. Curr Opin Nephrol Hypertens. 25:379–383. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonçalves P, Araújo JR and Di Santo JP: A
Cross-Talk between microbiota-derived short-chain fatty acids and
the host mucosal immune system regulates intestinal homeostasis and
inflammatory bowel disease. Inflamm Bowel Dis. 24:558–572. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohara T and Mori T: Antiproliferative
effects of short-chain fatty acids on human colorectal cancer cells
via gene expression inhibition. Anticancer Res. 39:4659–4666. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gui Q, Li H, Wang A, Zhao X, Tan Z, Chen
L, Xu K and Xiao C: The association between gut butyrate-producing
bacteria and non-small-cell lung cancer. J Clin Lab Anal.
34:e233182020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shuwen H, Miao D, Quan Q, Zhongshan Z,
Chun Z and Xi Y: Protective effect of the
'food-microorganism-SCFAs' axis on colorectal cancer: From basic
research to practical application. J Cancer Res Clin Oncol.
145:2169–2197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chambers ES, Preston T, Frost G and
Morrison DJ: Role of gut microbiota-generated short-chain fatty
acids in metabolic and cardiovascular health. Curr Nutr Rep.
7:198–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vital M, Howe AC and Tiedje JM: Revealing
the bacterial butyrate synthesis pathways by analyzing
(meta)genomic data. mBio. 5:e008892014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu J, Lin S, Zheng B and Cheung PCK:
Short-chain fatty acids in control of energy metabolism. Crit Rev
Food Sci Nutr. 58:1243–1249. 2018. View Article : Google Scholar
|
|
12
|
Ríos-Covián D, Ruas-Madiedo P, Margolles
A, Gueimonde M, de Los Reyes-Gavilán CG and Salazar N: Intestinal
short chain fatty acids and their link with diet and human health.
Front Microbiol. 7:1852016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keshteli AH, Madsen KL and Dieleman LA:
Diet in the pathogenesis and management of ulcerative colitis; A
review of randomized controlled dietary interventions. Nutrients.
11:14982019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mueller NT, Zhang M, Juraschek SP, Miller
ER and Appel LJ: Effects of high-fiber diets enriched with
carbohydrate, protein, or unsaturated fat on circulating short
chain fatty acids: Results from the OmniHeart randomized trial. Am
J Clin Nutr. 111:545–554. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
David LA, Maurice CF, Carmody RN,
Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y,
Fischbach MA, et al: Diet rapidly and reproducibly alters the human
gut microbiome. Nature. 505:559–563. 2014. View Article : Google Scholar :
|
|
16
|
Havenaar R: Intestinal health functions of
colonic microbial metabolites: A review. Benef Microbes. 2:103–114.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Zhang J, Guo Z, Kwok L, Ma C,
Zhang W, Lv Q, Huang W and Zhang H: Effect of oral consumption of
probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA,
SCFAs, and TBAs of adults of different ages. Nutrition.
30:776–783.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi D and Sakata T: Influence of
temperature on short-chain fatty acid production by pig cecal
bacteria in vitro. J Nutr Sci Vitaminol (Tokyo). 52:66–69. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boets E, Deroover L, Houben E, Vermeulen
K, Gomand SV, Delcour JA and Verbeke K: Quantification of in vivo
colonic short chain fatty acid production from inulin. Nutrients.
7:8916–8929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Wang J, He T, Becker S, Zhang G, Li
D and Ma X: Butyrate: A double-edged sword for health? Adv Nutr.
9:21–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Titus E and Ahearn GA: Short-chain fatty
acid transport in the intestine of a herbivorous teleost. J Exp
Biol. 135:77–94. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moschen I, Bröer A, Galić S, Lang F and
Bröer S: Significance of short chain fatty acid transport by
members of the monocarboxylate transporter family (MCT). Neurochem
Res. 37:2562–2568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Donohoe DR, Garge N, Zhang X, Sun W,
O'Connell TM, Bunger MK and Bultman SJ: The microbiome and butyrate
regulate energy metabolism and autophagy in the mammalian colon.
Cell Metab. 13:517–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurata N, Tokashiki N, Fukushima K, Misao
T, Hasuoka N, Kitagawa K, Mashimo M, Regan JW, Murayama T and
Fujino H: Short chain fatty acid butyrate uptake reduces
expressions of prostanoid EP4 receptors and their
mediation of cyclooxygenase-2 induction in HCA-7 human colon cancer
cells. Eur J Pharmacol. 853:308–315. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Beek CM, Canfora EE, Kip AM,
Gorissen SHM, Olde Damink SWM, van Eijk HM, Holst JJ, Blaak EE,
Dejong CHC and Lenaerts K: The prebiotic inulin improves substrate
metabolism and promotes short-chain fatty acid production in
overweight to obese men. Metabolism. 87:25–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McNabney SM and Henagan TM: Short chain
fatty acids in the colon and peripheral tissues: A focus on
butyrate, colon cancer, obesity and insulin resistance. Nutrients.
9:13482017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borthakur A, Gill RK, Hodges K, Ramaswamy
K, Hecht G and Dudeja PK: Enteropathogenic Escherichia coli
inhibits butyrate uptake in Caco-2 cells by altering the apical
membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol.
290:G30–G35. 2006. View Article : Google Scholar
|
|
28
|
Hoving LR, Heijink M, van Harmelen V, van
Dijk KW and Giera M: GC-MS analysis of short-chain fatty acids in
feces, cecum content, and blood samples. Methods Mol Biol.
1730:247–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stoeva MK, Garcia-So J, Justice N, Myers
J, Tyagi S, Nemchek M, McMurdie PJ, Kolterman O and Eid J:
Butyrate-producing human gut symbiont, Clostridium butyricum, and
its role in health and diesease. Gut Microbes. 13:1–28. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schulthess J, Pandey S, Capitani M,
Rue-Albrecht KC, Arnold I, Franchini F, Chomka A, Ilott NE,
Johnston DGW, Pires E, et al: The short chain fatty acid butyrate
imprints an antimicrobial program in macrophages. Immunity.
50:432–445.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang C, Du W, Ni Y, Lan G and Shi G: The
effect of short-chain fatty acids on M2 macrophages polarization in
vitro and in vivo. Clin Exp Immunol. 207:53–64. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bishehsari F, Engen PA, Preite NZ, Tuncil
YE, Naqib A, Shaikh M, Rossi M, Wilber S, Green SJ, Hamaker BR, et
al: Dietary fiber treatment corrects the composition of gut
microbiota, promotes SCFA production, and suppresses colon
carcinogenesis. Genes (Basel). 9:1022018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian P, Yang W, Guo X, Wang T, Tan S, Sun
R, Xiao R, Wang Y, Jiao D, Xu Y, et al: Early life gut microbiota
sustains liver-resident natural killer cells maturation via the
butyrate-IL-18 axis. Nat Commun. 14:17102023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furusawa Y, Obata Y, Fukuda S, Endo TA,
Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et
al: Commensal microbe-derived butyrate induces the differentiation
of colonic regulatory T cells. Nature. 504:446–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang M, Zhou Q, Dorfman RG, Huang X, Fan
T, Zhang H, Zhang J and Yu C: Butyrate inhibits interleukin-17 and
generates Tregs to ameliorate colorectal colitis in rats. BMC
Gastroenterol. 16:842016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith PM, Howitt MR, Panikov N, Michaud M,
Gallini CA, Bohlooly-Y M, Glickman JN and Garrett WS: The microbial
metabolites, short-chain fatty acids, regulate colonic Treg cell
homeostasis. Science. 341:569–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Malczewski AB, Navarro S, Coward JI and
Ketheesan N: Microbiome-derived metabolome as a potential predictor
of response to cancer immunotherapy. J Immunother Cancer.
8:e0013832020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen L, Zhou X, Wang Y, Wang D, Ke Y and
Zeng X: Propionate and butyrate produced by gut microbiota after
probiotic supplementation attenuate lung metastasis of melanoma
cells in mice. Mol Nutr Food Res. 65:e21000962021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim M, Qie Y, Park J and Kim CH: Gut
microbial metabolites fuel host antibody responses. Cell Host
Microbe. 20:202–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim M and Kim CH: Regulation of humoral
immunity by gut microbial products. Gut Microbes. 8:392–399. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murata M: Inflammation and cancer. Environ
Health Prev Med. 23:502018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim K, Kwon O, Ryu TY, Jung CR, Kim J, Min
JK, Kim DS, Son MY and Cho HS: Propionate of a microbiota
metabolite induces cell apoptosis and cell cycle arrest in lung
cancer. Mol Med Rep. 20:1569–1574. 2019.PubMed/NCBI
|
|
46
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov.
17:887–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Macia L, Tan J, Vieira AT, Leach K,
Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C,
et al: Metabolite-sensing receptors GPR43 and GPR109A facilitate
dietary fibre-induced gut homeostasis through regulation of the
inflammasome. Nat Commun. 6:67342015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bindels LB, Dewulf EM and Delzenne NM:
GPR43/FFA2: Physiopathological relevance and therapeutic prospects.
Trends Pharmacol Sci. 34:226–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tian Y, Xu Q, Sun L, Ye Y and Ji G:
Short-chain fatty acids administration is protective in
colitis-associated colorectal cancer development. J Nutr Biochem.
57:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li M, van Esch BCAM, Henricks PAJ,
Folkerts G and Garssen J: The anti-inflammatory effects of short
chain fatty acids on lipopolysaccharide- or tumor necrosis factor
α-stimulated endothelial cells via activation of GPR41/43 and
inhibition of HDACs. Front Pharmacol. 9:5332018. View Article : Google Scholar
|
|
51
|
Rooks MG and Garrett WS: Gut microbiota,
metabolites and host immunity. Nat Rev Immunol. 16:341–352. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hayashi A, Sato T, Kamada N, Mikami Y,
Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, et
al: A single strain of Clostridium butyricum induces intestinal
IL-10-producing macrophages to suppress acute experimental colitis
in mice. Cell Host Microbe. 13:711–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Russo E, Giudici F, Fiorindi C, Ficari F,
Scaringi S and Amedei A: Immunomodulating activity and therapeutic
effects of short chain fatty acids and tryptophan post-biotics in
inflammatory bowel disease. Front Immunol. 10:27542019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Melo AD, Silveira H, Bortoluzzi C, Lara
LJ, Garbossa CA, Preis G, Costa LB and Rostagno MH: Intestinal
alkaline phosphatase and sodium butyrate may be beneficial in
attenuating LPS-induced intestinal inflammation. Genet Mol Res.
15:2016. View Article : Google Scholar
|
|
55
|
Simeoli R, Mattace Raso G, Pirozzi C, Lama
A, Santoro A, Russo R, Montero-Melendez T, Berni Canani R,
Calignano A, Perretti M and Meli R: An orally administered
butyrate-releasing derivative reduces neutrophil recruitment and
inflammation in dextran sulphate sodium-induced murine colitis. Br
J Pharmacol. 174:1484–1496. 2017. View Article : Google Scholar
|
|
56
|
Li G, Lin J, Zhang C, Gao H, Lu H, Gao X,
Zhu R, Li Z, Li M and Liu Z: Microbiota metabolite butyrate
constrains neutrophil functions and ameliorates mucosal
inflammation in inflammatory bowel disease. Gut Microbes.
13:19682572021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Couto MR, Gonçalves P, Magro F and Martel
F: Microbiota-derived butyrate regulates intestinal inflammation:
Focus on inflammatory bowel disease. Pharmacol Res. 159:1049472020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee C, Kim BG, Kim JH, Chun J, Im JP and
Kim JS: Sodium butyrate inhibits the NF-kappa B signaling pathway
and histone deacetylation, and attenuates experimental colitis in
an IL-10 independent manner. Int Immunopharmacol. 51:47–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen G, Ran X, Li B, Li Y, He D, Huang B,
Fu S, Liu J and Wang W: Sodium butyrate inhibits inflammation and
maintains epithelium barrier integrity in a TNBS-induced
inflammatory bowel disease mice model. EBioMedicine. 30:317–325.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jahns F, Wilhelm A, Jablonowski N, Mothes
H, Radeva M, Wölfert A, Greulich KO and Glei M: Butyrate suppresses
mRNA increase of osteopontin and cyclooxygenase-2 in human colon
tumor tissue. Carcinogenesis. 32:913–920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ma H, Yu Y, Wang M, Li Z, Xu H, Tian C,
Zhang J, Ye X and Li X: Correlation between microbes and colorectal
cancer: Tumor apoptosis is induced by sitosterols through promoting
gut microbiota to produce short-chain fatty acids. Apoptosis.
24:168–183. 2019. View Article : Google Scholar
|
|
62
|
Shao X, Sun S, Zhou Y, Wang H, Yu Y, Hu T,
Yao Y and Zhou C: Bacteroides fragilis restricts colitis-associated
cancer via negative regulation of the NLRP3 axis. Cancer Lett.
523:170–181. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vinolo MA, Rodrigues HG, Hatanaka E,
Hebeda CB, Farsky SH and Curi R: Short-chain fatty acids stimulate
the migration of neutrophils to inflammatory sites. Clin Sci
(Lond). 117:331–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Donohoe DR, Collins LB, Wali A, Bigler R,
Sun W and Bultman SJ: The Warburg effect dictates the mechanism of
butyrate-mediated histone acetylation and cell proliferation. Mol
Cell. 48:612–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nandi D, Parida S and Sharma D: The gut
microbiota in breast cancer development and treatment: The good the
bad and the useful! Gut Microbes. 15:22214522023. View Article : Google Scholar
|
|
66
|
Xi Y, Jing Z, Wei W, Chun Z, Quan Q, Qing
Z, Jiamin X and Shuwen H: Inhibitory effect of sodium butyrate on
colorectal cancer cells and construction of the related molecular
network. BMC Cancer. 21:1272021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zeng H, Taussig DP, Cheng WH, Johnson LK
and Hakkak R: Butyrate inhibits cancerous HCT116 colon cell
proliferation but to a lesser extent in noncancerous NCM460 colon
cells. Nutrients. 9:252017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen D, Jin D, Huang S, Wu J, Xu M, Liu T,
Dong W, Liu X, Wang S, Zhong W, et al: Clostridium butyricum, a
butyrate-producing probiotic, inhibits intestinal tumor development
through modulating Wnt signaling and gut microbiota. Cancer Lett.
469:456–467. 2020. View Article : Google Scholar
|
|
69
|
Darvin P, Toor SM, Sasidharan Nair V and
Elkord E: Immune checkpoint inhibitors: Recent progress and
potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Santoni M, Piva F, Conti A, Santoni A,
Cimadamore A, Scarpelli M, Battelli N and Montironi R: Re: Gut
microbiome influences efficacy of PD-1-based immunotherapy against
epithelial tumors. Eur Urol. 74:521–522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tanoue T, Morita S, Plichta DR, Skelly AN,
Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et
al: A defined commensal consortium elicits CD8 T cells and
anti-cancer immunity. Nature. 565:600–605. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Spencer CN, McQuade JL, Gopalakrishnan V,
McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG,
Peterson CB, et al: Dietary fiber and probiotics influence the gut
microbiome and melanoma immunotherapy response. Science.
374:1632–1640. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar
|
|
76
|
Nomura M, Nagatomo R, Doi K, Shimizu J,
Baba K, Saito T, Matsumoto S, Inoue K and Muto M: Association of
short-chain fatty acids in the gut microbiome with clinical
response to treatment with nivolumab or pembrolizumab in patients
with solid cancer tumors. JAMA Netw Open. 3:e2028952020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Botticelli A, Vernocchi P, Marini F,
Quagliariello A, Cerbelli B, Reddel S, Del Chierico F, Di Pietro F,
Giusti R, Tomassini A, et al: Gut metabolomics profiling of
non-small cell lung cancer (NSCLC) patients under immunotherapy
treatment. J Transl Med. 18:492020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chuanbing Z, Zhengle Z, Ruili D, Kongfan Z
and Jing T: Genes Modulating butyrate metabolism for assessing
clinical prognosis and responses to systematic therapies in
hepatocellular carcinoma. Biomolecules. 13:522022. View Article : Google Scholar
|
|
79
|
Coutzac C, Jouniaux JM, Paci A, Schmidt J,
Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix
L, et al: Systemic short chain fatty acids limit antitumor effect
of CTLA-4 blockade in hosts with cancer. Nat Commun. 11:21682020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
van der Beek CM, Dejong CHC, Troost FJ,
Masclee AAM and Lenaerts K: Role of short-chain fatty acids in
colonic inflammation, carcinogenesis, and mucosal protection and
healing. Nutr Rev. 75:286–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hudcovic T, Kolinska J, Klepetar J,
Stepankova R, Rezanka T, Srutkova D, Schwarzer M, Erban V, Du Z,
Wells JM, et al: Protective effect of Clostridium tyrobutyricum in
acute dextran sodium sulphate-induced colitis: Differential
regulation of tumour necrosis factor-α and interleukin-18 in BALB/c
and severe combined immunodeficiency mice. Clin Exp Immunol.
167:356–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Levy M, Blacher E and Elinav E:
Microbiome, metabolites and host immunity. Curr Opin Microbiol.
35:8–15. 2017. View Article : Google Scholar
|
|
83
|
Burger-van Paassen N, Vincent A, Puiman
PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van
Seuningen I and Renes IB: The regulation of intestinal mucin MUC2
expression by short-chain fatty acids: implications for epithelial
protection. Biochem J. 420:211–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Koh A, De Vadder F, Kovatcheva-Datchary P
and Bäckhed F: From dietary fiber to host physiology: Short-Chain
fatty acids as key bacterial metabolites. Cell. 165:1332–1345.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang Y, Yu K, Chen H, Su Y and Zhu W:
Caecal infusion of the short-chain fatty acid propionate affects
the microbiota and expression of inflammatory cytokines in the
colon in a fistula pig model. Microb Biotechnol. 11:859–868. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Iraporda C, Errea A, Romanin DE, Cayet D,
Pereyra E, Pignataro O, Sirard JC, Garrote GL, Abraham AG and Rumbo
M: Lactate and short chain fatty acids produced by microbial
fermentation downregulate proinflammatory responses in intestinal
epithelial cells and myeloid cells. Immunobiology. 220:1161–1169.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nastasi C, Fredholm S, Willerslev-Olsen A,
Hansen M, Bonefeld CM, Geisler C, Andersen MH, Ødum N and Woetmann
A: Butyrate and propionate inhibit antigen-specific CD8(+) T cell
activation by suppressing IL-12 production by antigen-presenting
cells. Sci Rep. 7:145162017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dalile B, Van Oudenhove L, Vervliet B and
Verbeke K: The role of short-chain fatty acids in
microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol.
16:461–478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park JH, Kotani T, Konno T, Setiawan J,
Kitamura Y, Imada S, Usui Y, Hatano N, Shinohara M, Saito Y, et al:
Promotion of intestinal epithelial cell turnover by commensal
bacteria: Role of short-chain fatty acids. PLoS One.
11:e01563342016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Meisel M, Mayassi T, Fehlner-Peach H,
Koval JC, O'Brien SL, Hinterleitner R, Lesko K, Kim S, Bouziat R,
Chen L, et al: Interleukin-15 promotes intestinal dysbiosis with
butyrate deficiency associated with increased susceptibility to
colitis. ISME J. 11:15–30. 2017. View Article : Google Scholar :
|
|
91
|
Ganapathy V, Thangaraju M, Prasad PD,
Martin PM and Singh N: Transporters and receptors for short-chain
fatty acids as the molecular link between colonic bacteria and the
host. Curr Opin Pharmacol. 13:869–874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Halnes I, Baines KJ, Berthon BS,
MacDonald-Wicks LK, Gibson PG and Wood LG: Soluble fibre meal
challenge reduces airway inflammation and expression of GPR43 and
GPR41 in asthma. Nutrients. 9:572017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kelly CJ, Zheng L, Campbell EL, Saeedi B,
Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson
A, et al: Crosstalk between microbiota-derived short-chain fatty
acids and intestinal epithelial HIF augments tissue barrier
function. Cell Host Microbe. 17:662–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kim M, Friesen L, Park J, Kim HM and Kim
CH: Microbial metabolites, short-chain fatty acids, restrain tissue
bacterial load, chronic inflammation, and associated cancer in the
colon of mice. Eur J Immunol. 48:1235–1247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ahmed K, Tunaru S and Offermanns S:
GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid
receptors. Trends Pharmacol Sci. 30:557–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kim CH: Immune regulation by microbiome
metabolites. Immunology. 154:220–229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kim MH, Kang SG, Park JH, Yanagisawa M and
Kim CH: Short-chain fatty acids activate GPR41 and GPR43 on
intestinal epithelial cells to promote inflammatory responses in
mice. Gastroenterology. 145:396-406.e1–10. 2013. View Article : Google Scholar
|
|
98
|
Ohira H, Fujioka Y, Katagiri C, Mamoto R,
Aoyama-Ishikawa M, Amako K, Izumi Y, Nishiumi S, Yoshida M, Usami M
and Ikeda M: Butyrate attenuates inflammation and lipolysis
generated by the interaction of adipocytes and macrophages. J
Atheroscler Thromb. 20:425–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Vinolo MA, Ferguson GJ, Kulkarni S,
Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT and
Curi R: SCFAs induce mouse neutrophil chemotaxis through the GPR43
receptor. PLoS One. 6:e212052011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sun M, Wu W, Chen L, Yang W, Huang X, Ma
C, Chen F, Xiao Y, Zhao Y, Ma C, et al: Microbiota-derived
short-chain fatty acids promote Th1 cell IL-10 production to
maintain intestinal homeostasis. Nat Commun. 9:35552018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Singh N, Gurav A, Sivaprakasam S, Brady E,
Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et
al: Activation of Gpr109a, receptor for niacin and the commensal
metabolite butyrate, suppresses colonic inflammation and
carcinogenesis. Immunity. 40:128–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fachi JL, Felipe JS, Pral LP, da Silva BK,
Corrêa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Câmara NOS, de
Sales E Souza ÉL, et al: Butyrate protects mice from clostridium
difficile-induced colitis through an HIF-1-Dependent mechanism.
Cell Rep. 27:750–761.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang M, Li RW, Yang H, Tan Z and Liu F:
Recent advances in developing butyrogenic functional foods to
promote gut health. Crit Rev Food Sci Nutr. 1–22. 2022.Epub ahead
of print.
|
|
104
|
Maslowski KM, Vieira AT, Ng A, Kranich J,
Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al:
Regulation of inflammatory responses by gut microbiota and
chemoattractant receptor GPR43. Nature. 461:1282–1286. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Samuel BS, Shaito A, Motoike T, Rey FE,
Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J,
Yanagisawa M and Gordon JI: Effects of the gut microbiota on host
adiposity are modulated by the short-chain fatty-acid binding G
protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA.
105:16767–16772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tolhurst G, Heffron H, Lam YS, Parker HE,
Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F and
Gribble FM: Short-chain fatty acids stimulate glucagon-like
peptide-1 secretion via the G-protein-coupled receptor FFAR2.
Diabetes. 61:364–371. 2012. View Article : Google Scholar :
|
|
107
|
Huang W, Zhou L, Guo H and Xu Y and Xu Y:
The role of short-chain fatty acids in kidney injury induced by
gut-derived inflammatory response. Metabolism. 68:20–30. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Licciardi PV, Ververis K and Karagiannis
TC: Histone deacetylase inhibition and dietary short-chain Fatty
acids. ISRN Allergy. 2011:8696472011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kasubuchi M, Hasegawa S, Hiramatsu T,
Ichimura A and Kimura I: Dietary gut microbial metabolites,
short-chain fatty acids, and host metabolic regulation. Nutrients.
7:2839–2849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Park J, Kim M, Kang SG, Jannasch AH,
Cooper B, Patterson J and Kim CH: Short-chain fatty acids induce
both effector and regulatory T cells by suppression of histone
deacetylases and regulation of the mTOR-S6K pathway. Mucosal
Immunol. 8:80–93. 2015. View Article : Google Scholar
|
|
111
|
Luu M, Riester Z, Baldrich A, Reichardt N,
Yuille S, Busetti A, Klein M, Wempe A, Leister H, Raifer H, et al:
Microbial short-chain fatty acids modulate CD8(+) T cell responses
and improve adoptive immunotherapy for cancer. Nat Commun.
12:40772021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Thorburn AN, McKenzie CI, Shen S, Stanley
D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, et al:
Evidence that asthma is a developmental origin disease influenced
by maternal diet and bacterial metabolites. Nat Commun. 6:73202015.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Stempelj M, Kedinger M, Augenlicht L and
Klampfer L: Essential role of the JAK/STAT1 signaling pathway in
the expression of inducible nitric-oxide synthase in intestinal
epithelial cells and its regulation by butyrate. J Biol Chem.
282:9797–9804. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Pan X, Fang X, Wang F, Li H, Niu W, Liang
W, Wu C, Li J, Tu X, Pan LL and Sun J: Butyrate ameliorates
caerulein-induced acute pancreatitis and associated intestinal
injury by tissue-specific mechanisms. Br J Pharmacol.
176:4446–4461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ogrodnik M, Salmonowicz H, Jurk D and
Passos JF: Expansion and cell-cycle arrest: Common denominators of
cellular senescence. Trends Biochem Sci. 44:996–1008. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fung KY, Cosgrove L, Lockett T, Head R and
Topping DL: A review of the potential mechanisms for the lowering
of colorectal oncogenesis by butyrate. Br J Nutr. 108:820–831.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Rephaeli A, Blank-Porat D, Tarasenko N,
Entin-Meer M, Levovich I, Cutts SM, Phillips DR, Malik Z and
Nudelman A: In vivo and in vitro antitumor activity of
butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase
inhibitor, in human prostate cancer. Int J Cancer. 116:226–235.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Pellizzaro C, Coradini D, Daniotti A,
Abolafio G and Daidone MG: Modulation of cell cycle-related protein
expression by sodium butyrate in human non-small cell lung cancer
cell lines. Int J Cancer. 91:654–657. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Edmond V, Brambilla C, Brambilla E,
Gazzeri S and Eymin B: SRSF2 is required for sodium
butyrate-mediated p21(WAF1) induction and premature senescence in
human lung carcinoma cell lines. Cell Cycle. 10:1968–1977. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li F, Wu Y, Yan Y, Wu S, Zhu J, Zhang G,
Zhang P, Yuan L, Zeng Y and Liu Z: Transcriptomic landscape of
sodium butyrate-induced growth inhibition of human colorectal
cancer organoids. Mol Omics. 18:754–764. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang Y, Hu PC, Ma YB, Fan R, Gao FF, Zhang
JW and Wei L: Sodium butyrate-induced apoptosis and ultrastructural
changes in MCF-7 breast cancer cells. Ultrastruct Pathol.
40:200–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Qin X, Xu Y, Peng S, Qian S, Zhang X, Shen
S, Yang J and Ye J: Sodium butyrate opens mitochondrial
permeability transition pore (MPTP) to induce a proton leak in
induction of cell apoptosis. Biochem Biophys Res Commun.
527:611–617. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang K, Ji X, Song Z, Wu F, Qu Y, Jin X,
Xue X, Wang F and Huang Y: Butyrate inhibits gastric cancer cells
by inducing mitochondriamediated apoptosis. Comb Chem High
Throughput Screen. 26:630–638. 2023. View Article : Google Scholar
|
|
124
|
Zhang K, Ji X, Song Z, Song W, Huang Q, Yu
T, Shi D, Wang F, Xue X and Guo J: Butyrate inhibits the
mitochondrial complex Ι to mediate mitochondria-dependent apoptosis
of cervical cancer cells. BMC Complement Med Ther. 23:2122023.
View Article : Google Scholar
|
|
125
|
Pajak B, Gajkowska B and Orzechowski A:
Sodium butyrate sensitizes human colon adenocarcinoma COLO 205
cells to both intrinsic and TNF-alpha-dependent extrinsic
apoptosis. Apoptosis. 14:203–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Salimi V, Shahsavari Z, Safizadeh B,
Hosseini A, Khademian N and Tavakoli-Yaraki M: Sodium butyrate
promotes apoptosis in breast cancer cells through reactive oxygen
species (ROS) formation and mitochondrial impairment. Lipids Health
Dis. 16:2082017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li X, Fang F, Gao Y, Tang G, Xu W, Wang Y,
Kong R, Tuyihong A and Wang Z: ROS Induced by KillerRed targeting
mitochondria (mtKR) enhances apoptosis caused by radiation via Cyt
c/Caspase-3 Pathway. Oxid Med Cell Longev.
2019:45286162019.PubMed/NCBI
|
|
128
|
Ferreira AC, Robaina MC, Rezende LM,
Severino P and Klumb CE: Histone deacetylase inhibitor prevents
cell growth in Burkitt's lymphoma by regulating PI3K/Akt pathways
and leads to upregulation of miR-143, miR-145, and miR-101. Ann
Hematol. 93:983–993. 2014.PubMed/NCBI
|
|
129
|
Han R, Sun Q, Wu J, Zheng P and Zhao G:
Sodium Butyrate Upregulates miR-203 expression to exert
anti-proliferation effect on colorectal cancer cells. Cell Physiol
Biochem. 39:1919–1929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Humphreys KJ, Cobiac L, Le Leu RK, Van der
Hoek MB and Michael MZ: Histone deacetylase inhibition in
colorectal cancer cells reveals competing roles for members of the
oncogenic miR-17-92 cluster. Mol Carcinog. 52:459–474. 2013.
View Article : Google Scholar
|
|
131
|
Cho JH, Dimri M and Dimri GP: MicroRNA-31
is a transcriptional target of histone deacetylase inhibitors and a
regulator of cellular senescence. J Biol Chem. 290:10555–10567.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Pant K, Yadav AK, Gupta P, Islam R, Saraya
A and Venugopal SK: Butyrate induces ROS-mediated apoptosis by
modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox
Biol. 12:340–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xu Z, Tao J, Chen P, Chen L, Sharma S,
Wang G and Dong Q: Sodium butyrate inhibits colorectal cancer cell
migration by downregulating Bmi-1 Through Enhanced miR-200c
expression. Mol Nutr Food Res. 62:e17008442018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wang F, Wu H, Fan M, Yu R, Zhang Y, Liu J,
Zhou X, Cai Y, Huang S, Hu Z and Jin X: Sodium butyrate inhibits
migration and induces AMPK-mTOR pathway-dependent autophagy and
ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human
bladder cancer cells. FASEB J. 34:4266–4282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Amoêdo ND, Rodrigues MF, Pezzuto P, Galina
A, da Costa RM, de Almeida FC, El-Bacha T and Rumjanek FD: Energy
metabolism in H460 lung cancer cells: Effects of histone
deacetylase inhibitors. PLoS One. 6:e222642011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Xu S, Liu CX, Xu W, Huang L, Zhao JY and
Zhao SM: Butyrate induces apoptosis by activating PDC and
inhibiting complex I through SIRT3 inactivation. Signal Transduct
Target Ther. 2:160352017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kaczmarek JL, Musaad SM and Holscher HD:
Time of day and eating behaviors are associated with the
composition and function of the human gastrointestinal microbiota.
Am J Clin Nutr. 106:1220–1231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ritchie LE, Sturino JM, Carroll RJ, Rooney
LW, Azcarate-Peril MA and Turner ND: Polyphenol-rich sorghum brans
alter colon microbiota and impact species diversity and species
richness after multiple bouts of dextran sodium sulfate-induced
colitis. FEMS Microbiol Ecol. 91:fiv0082015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ariyoshi T, Hagihara M, Eguchi S, Fukuda
A, Iwasaki K, Oka K, Takahashi M, Yamagishi Y and Mikamo H:
Clostridium butyricum MIYAIRI 588-Induced Protectin D1 Has an
anti-inflammatory effect on antibiotic-induced intestinal disorder.
Front Microbiol. 11:5877252020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Cassir N, Benamar S and La Scola B:
Clostridium butyricum: From beneficial to a new emerging pathogen.
Clin Microbiol Infect. 22:37–45. 2016. View Article : Google Scholar
|
|
141
|
Tomita Y, Ikeda T, Sakata S, Saruwatari K,
Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S and
Sakagami T: Association of probiotic clostridium butyricum therapy
with survival and response to immune checkpoint blockade in
patients with lung cancer. Cancer Immunol Res. 8:1236–1242. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Derosa L, Hellmann MD, Spaziano M,
Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC,
Chaft JE, et al: Negative association of antibiotics on clinical
activity of immune checkpoint inhibitors in patients with advanced
renal cell and non-small-cell lung cancer. Ann Oncol. 29:1437–1444.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Dizman N, Meza L, Bergerot P, Alcantara M,
Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, et al:
Nivolumab plus ipilimumab with or without live bacterial
supplementation in metastatic renal cell carcinoma: A randomized
phase 1 trial. Nat Med. 28:704–712. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Shinnoh M, Horinaka M, Yasuda T, Yoshikawa
S, Morita M, Yamada T, Miki T and Sakai T: Clostridium butyricum
MIYAIRI 588 shows antitumor effects by enhancing the release of
TRAIL from neutrophils through MMP-8. Int J Oncol. 42:903–911.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu M, Xie W, Wan X and Deng T:
Clostridium butyricum modulates gut microbiota and reduces colitis
associated colon cancer in mice. Int Immunopharmacol.
88:1068622020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Traisaeng S, Herr DR, Kao HJ, Chuang TH
and Huang CM: A derivative of butyric acid, the fermentation
metabolite of staphylococcus epidermidis, inhibits the growth of a
staphylococcus aureus strain isolated from atopic dermatitis
patients. Toxins. 11:3112019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zha Z, Lv Y, Tang H, Li T, Miao Y, Cheng
J, Wang G, Tan Y, Zhu Y, Xing X, et al: An orally administered
butyrate-releasing xylan derivative reduces inflammation in dextran
sulphate sodium-induced murine colitis. Int J Biol Macromol.
156:1217–1233. 2020. View Article : Google Scholar
|
|
148
|
El-Sawy HS, Al-Abd AM, Ahmed TA, El-Say KM
and Torchilin VP: Stimuli-Responsive nano-architecture
drug-delivery systems to solid tumor micromilieu: Past, present,
and future perspectives. ACS Nano. 12:10636–10664. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
He Y, Fu L, Li Y, Wang W, Gong M, Zhang J,
Dong X, Huang J, Wang Q, Mackay CR, et al: Gut microbial
metabolites facilitate anticancer therapy efficacy by modulating
cytotoxic CD8(+) T cell immunity. Cell Metab. 33:988–1000.e7. 2021.
View Article : Google Scholar
|
|
150
|
Lin XB, Farhangfar A, Valcheva R, Sawyer
MB, Dieleman L, Schieber A, Gänzle MG and Baracos V: The role of
intestinal microbiota in development of irinotecan toxicity and in
toxicity reduction through dietary fibres in rats. PLoS One.
9:e836442014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Ferreira TM, Leonel AJ, Melo MA, Santos
RR, Cara DC, Cardoso VN, Correia MI and Alvarez-Leite JI: Oral
supplementation of butyrate reduces mucositis and intestinal
permeability associated with 5-Fluorouracil administration. Lipids.
47:669–678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Murga-Garrido SM, Hong Q, Cross TL,
Hutchison ER, Han J, Thomas SP, Vivas EI, Denu J, Ceschin DG, Tang
ZZ and Rey FE: Gut microbiome variation modulates the effects of
dietary fiber on host metabolism. Microbiome. 9:1172021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li L, Chang L, Zhang X, Ning Z, Mayne J,
Ye Y, Stintzi A, Liu J and Figeys D: Berberine and its structural
analogs have differing effects on functional profiles of individual
gut microbiomes. Gut Microbes. 11:1348–1361. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lukovac S, Belzer C, Pellis L, Keijser BJ,
de Vos WM, Montijn RC and Roeselers G: Differential modulation by
Akkermansia muciniphila and Faecalibacterium prausnitzii of host
peripheral lipid metabolism and histone acetylation in mouse gut
organoids. mBio. 5:e014382014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Park M, Kwon J, Shin HJ, Moon SM, Kim SB,
Shin US, Han YH and Kim Y: Butyrate enhances the efficacy of
radiotherapy via FOXO3A in colorectal cancer patient-derived
organoids. Int J Oncol. 57:1307–1318. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Bouges E, Segers C, Leys N, Lebeer S,
Zhang J and Mastroleo F: Human intestinal organoids and
microphysiological systems for modeling radiotoxicity and assessing
radioprotective agents. Cancers (Basel). 15:58592023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Cai J, Cheung J, Cheung SWM, Chin KTC,
Leung RWK, Lam RST, Sharma R, Yiu JHC and Woo CW: Butyrate acts as
a positive allosteric modulator of the 5-HT transporter to decrease
availability of 5-HT in the ileum. Br J Pharmacol. Dec 21–2023.Epub
ahead of print. PubMed/NCBI
|
|
158
|
Mun SJ, Lee J, Chung KS, Son MY and Son
MJ: Effect of microbial short-chain fatty acids on CYP3A4-Mediated
metabolic activation of human pluripotent stem cell-derived liver
organoids. Cells. 10:1262021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wong SH, Zhao L, Zhang X, Nakatsu G, Han
J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, et al: Gavage of fecal
samples from patients with colorectal cancer promotes intestinal
carcinogenesis in germ-free and conventional mice.
Gastroenterology. 153:1621–1633.e6. 2017. View Article : Google Scholar
|
|
160
|
Rosshart SP, Vassallo BG, Angeletti D,
Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA,
Badger JH, Ajami NJ, et al: Wild mouse gut microbiota promotes host
fitness and improves disease resistance. Cell. 171:1015–1028.e13.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chang CW, Lee HC, Li LH, Chiang Chiau JS,
Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY, et al: Fecal
microbiota transplantation prevents intestinal injury, upregulation
of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced
toxicity in colorectal cancer. Int J Mol Sci. 21:3862020.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Pandey SP, Bender MJ, McPherson AC, Phelps
CM, Sanchez LM, Rana M, Hedden L, Sangani KA, Chen L, Shapira JH,
et al: Tet2 deficiency drives liver microbiome dysbiosis triggering
Tc1 cell autoimmune hepatitis. Cell Host Microbe. 30:1003–1019.e10.
2022. View Article : Google Scholar : PubMed/NCBI
|