The tumor microenvironment (TME) refers to the
unique environment around the tumor consisting of blood vessels,
immune cells, fibroblasts and an extracellular matrix, which is
more conducive for tumor cells than normal cells (1). Reciprocal crosstalk between cancer
cells and the TME is a complex process contributing to uncontrolled
tumor proliferation, invasion, metastasis and resistance to therapy
(2,3). Multiple signaling pathways are
involved in the crosstalk between cancer cells and the TME, and
annexin A1 (ANXA1) is considered to be an important regulatory
protein involved in this process.
Therefore, the aim of the present review was to
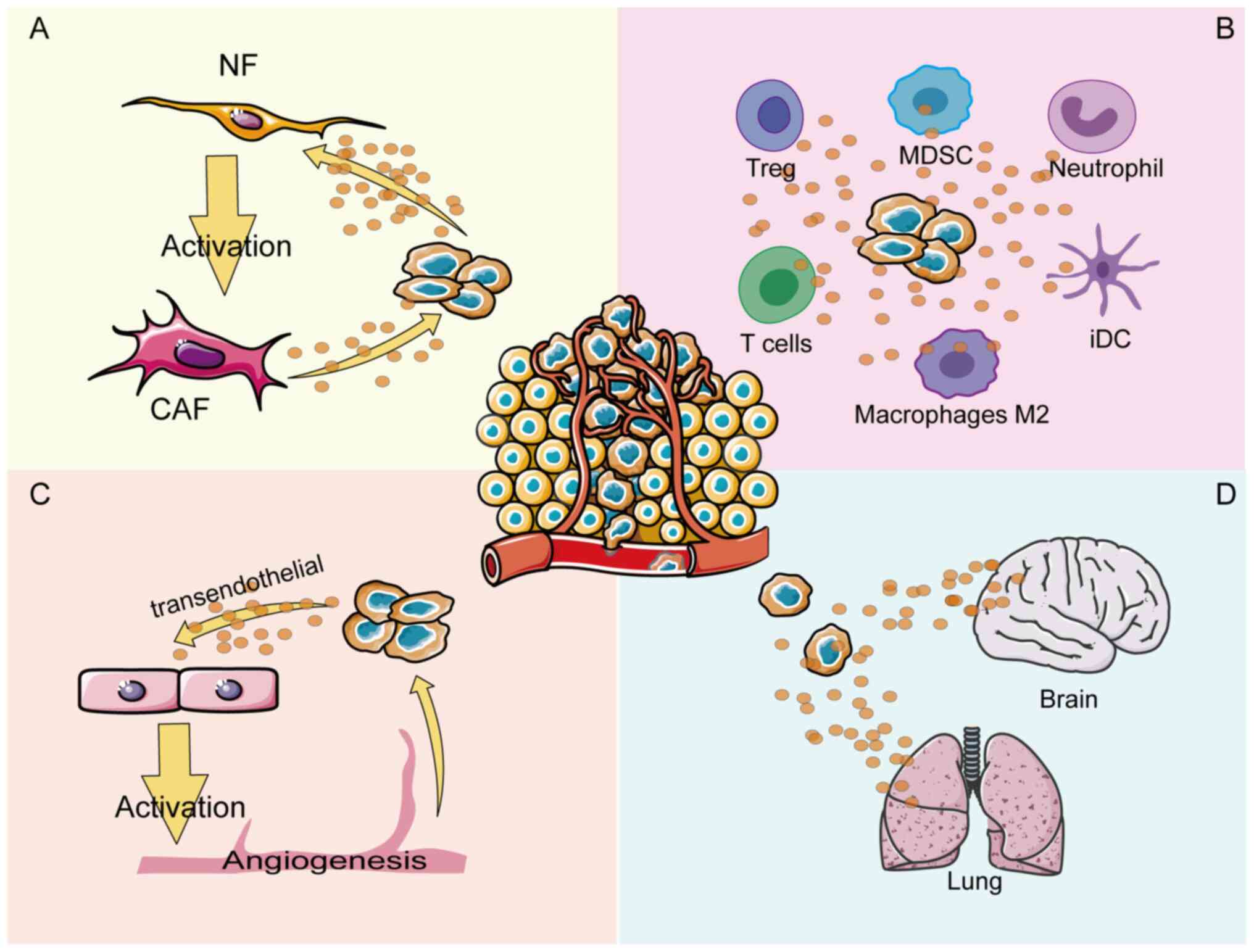
discuss the mechanism of ANXA1 in each compartment of the TME. A
number of efforts have been made to explore how ANXA1 regulates
crosstalk in different compartments of the TME, thereby regulating
tumor cell angiogenesis, tumor immune cells infiltration and tumor
fibroblasts activation (11-13) (Fig.
1). The conflicting pro- or anti-cancer effects of ANXA1 may
originate from the complex TME and elucidating these mechanisms
from a therapeutic perspective may lead to the discovery of
potential anti-cancer strategies that target cancer cells within
the TME.
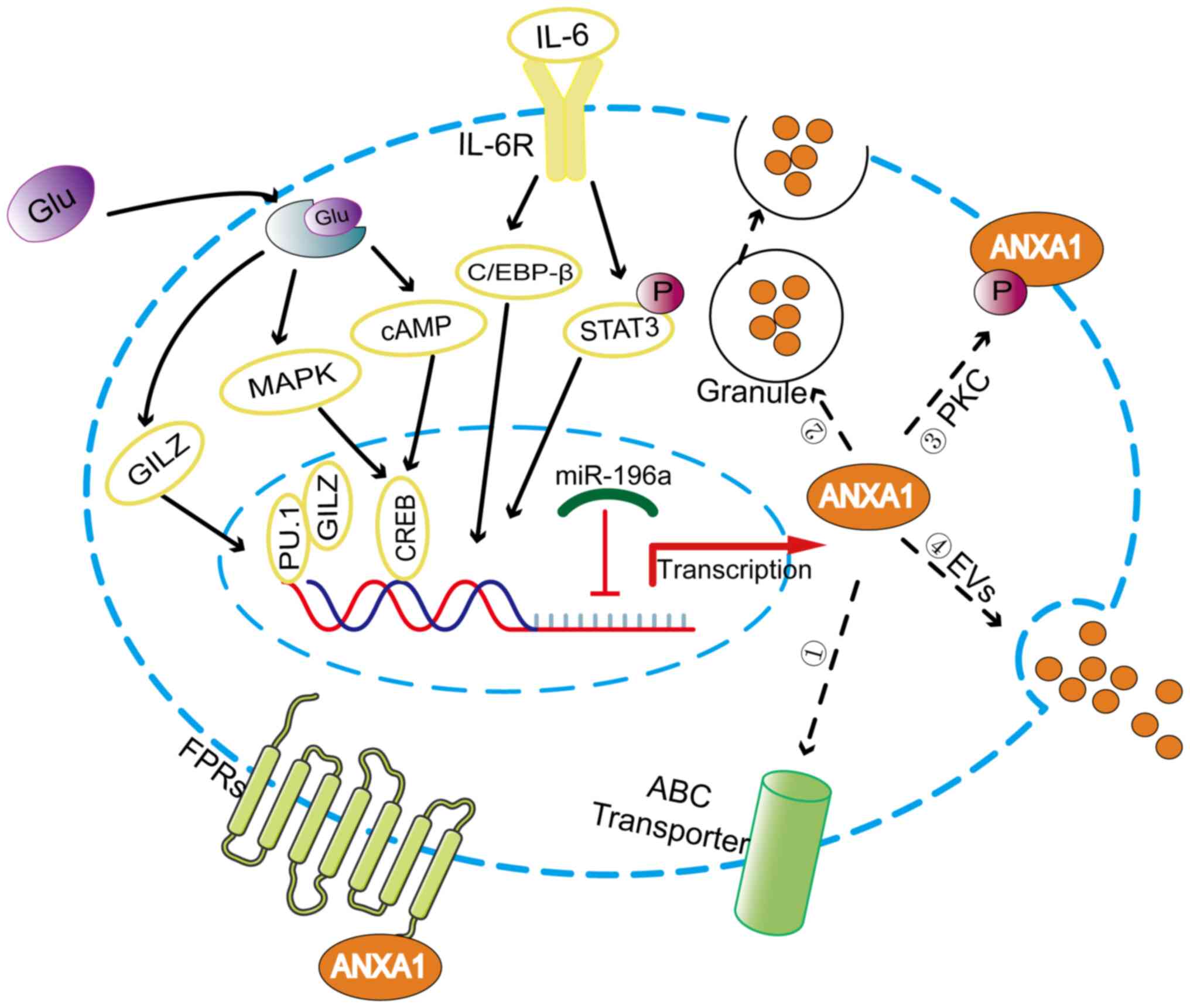
ANXA1, a 37 kDa protein containing 346 amino acids
encoded by a gene located on chromosome 9q21.13 (14), is a glucocorticoid
anti-inflammatory mediator that has been proven to be induced by
glucocorticoids in vitro and in vivo (15,16). Glucocorticoids activate ANXA1
transcription by upregulating glucocorticoid-induced leucine zipper
to bind to PU.1 (17). The ANXA1
promoter region contains cyclic adenosine monophosphate (cAMP)
response element-binding protein (CREB)-binding sites that mediate
the expression of ANXA1 induced by cAMP and P38 MAPK, which can be
upregulated by glucocorticoids (18). Additionally, IL-6 can upregulate
the expression of ANXA1 by activating transcription factors C/EBP-β
and STAT3 (19,20) (Fig.
2). It is well known that IL-6, as an inflammatory factor,
participates in the process of shaping the TME (21).
Post-transcriptional regulatory mechanisms play an
important role in regulating ANXA1 expression. microRNAs
(miRNAs/miRs) are noncoding RNA composed of ~22 nucleotides that
downregulate gene expression by recognizing the 3′UTR sequence of
the target mRNAs (22). miR-196a
can directly target ANXA1 in head and neck cancer, laryngeal cancer
and triple negative breast cancer to promote the proliferation,
migration and radioresistance of cancer cells (23-25).
Post-translational modifications are essential for
the functional regulation of ANXA1, which when activated is
externalized or secreted into the extracellular environment
(26,27), as shown in Fig. 2. Phosphorylation of the Ser27
residue induced by protein kinase C facilitates the movement of
ANXA1 protein to the cell surface (28). In addition, ANXA1 has been found
to be externalized by the ATP-binding cassette (ABC) transporter,
with ABC family member ABC-A1 being specifically involved in this
process (29). However, in
neutrophils where ANXA1 is predominantly localized to gelatin
granules, the gelatin granules are degranulated, resulting in a
high concentration of ANXA1 on the cell surface (30). Researchers have confirmed another
externalization mechanism by which ANXA1 is released from cells as
a component of the extracellular vesicles (EVs) (31,32). EVs can be dispersed either in the
extracellular space near the release point or far away as a
function of cell-to-cell communication.
Externalized ANXA1 can interact with formyl peptide
receptors (FPRs) in an autocrine, juxtacrine, or paracrine manner
(26,33). FPRs belong to the family of G
protein-coupled receptors and consist of three members, FPR1, FPR2
and FPR3 (4). FPRs can interact
with a range of ligands, including the N-terminal peptide of ANXA1,
lipoxin A4 and serum amyloid A (34). Moreover, FPR2 form different
complexes, such as monomers or homo/heterodimer (in combination
with FPR1 or FPR3), that trigger multiple downstream signaling
pathways to exert the pleiotropic function of ANXA1 proteins
(35). The ANXA1/FPRs axis is an
important crosstalk pathway between different compartments of the
TME and plays an important role in remodeling the TME.
Angiogenesis involves the growth of new blood
vessels from the existing vasculature, which provide oxygen and
nutrients for tumor growth. Without vascular support, tumor growth
is difficult to sustain, even during necrosis and apoptosis
(36,37). Angiogenesis is not only necessary
for cancer to invade surrounding tissues, but it also supports the
development of metastatic cancer cells in new location (38). Angiogenesis is a vital cancer
marker that is associated with poor prognosis in patients with
various tumors.
ANXA1 is essential for the formation of tumor blood
vessels. Studies have shown that ANXA1-knockout mice can grow
normally without evident vascular defects, but ANXA1 deficiency
impedes the formation of tumor neovascularization, thus inhibiting
tumor growth and metastasis (39,40). ANXA1 is highly expressed in the
vasculature of various tumor types, such as colon cancer, lung
cancer and melanoma (41), but
the specific mechanisms of ANXA1 function have not been deeply
explored. Researchers have found that activated ANXA1 interacts
with FPR receptors in an autocrine manner in endothelial cells to
promote the externalization of VEGF (42), which is an important angiogenic
factor (43). When externalized,
VEGF-A further promotes the angiogenesis of endothelial cells by
inducing the phosphorylation of ANXA1 mediated by p38/MAPKAP
kinase-2/LIMK1 activation (44).
Therefore, it is possible that VEGF and ANXA1 form a positive
feedback loop that synergistically promotes tumor angiogenesis.
Angiogenesis can provide favorable conditions for
cancer cell proliferation. Meanwhile, tumor cells can further
regulate angiogenesis through ANXA1 by inducing the formation of a
microenvironment that is conducive to tumor cell growth. ANXA1 has
been shown to promote tube formation in endothelial cells by
regulating NF-κB targeted by miR26b* and miR56 in MCF-7 breast
cancer cells with low ANXA1 expression (45). By constructing ANXA1 knockout cell
lines, the researchers demonstrated that pancreatic cancer cells
secreted ANXA1 in the form of EVs, which can regulate the
activation of endothelial cells and promote angiogenesis in a
paracrine manner by interacting with FPR receptors (46). In conclusion, blocking the
signaling of ANXA1 between tumor cells and the tumor vasculature
may lead to the development of a novel therapeutic approach for
impeding cancer development.
ANXA1 is highly expressed in neutrophils, mast cells
and monocytes-macrophages but is lowly expressed in T lymphocytes
and B cell subsets (47), playing
an immunomodulatory role in innate and adaptive immune responses.
During the innate immune response, ANXA1 can inhibit the rolling,
adhesion and migration of neutrophils (48). Meanwhile, ANXA1 can also affect
the clearance of apoptotic neutrophils by macrophages and influence
their differentiation, exerting anti-inflammatory and pro-resolving
effects (49).
There have been relatively few studies on the role
of ANXA1 in the adaptive immune system due to its relatively low
expression level (48), but it is
known that ANXA1 functions differently in adaptive immune cells.
ANXA1 is involved in the proliferation and activation of T cells,
plays a balancing role in T cell differentiation and may be
associated with the initiation of T cell receptor (TCR) signaling
(50). ANXA1 can further
strengthen the TCR signaling pathway by binding to FPRs and
promoting T cell differentiation towards the TH1 phenotype
(47).
However, the function of ANXA1 can be altered by
disturbances in the intracellular and extracellular environment
under different tumor conditions. Therefore, the present study
aimed to further explore the regulatory effects of ANXA1 on various
immune cells within the TME and attempted to explain the functional
pleiotropy of ANXA1 in different TMEs.
Neutrophils are the first line of defense against
infection, but their role in tumor progression is highly
controversial because of the ability of tumor-associated
neutrophils (TANs) to differentiate into different phenotypes in
distinct microenvironments. TANs can differentiate into N2
immunosuppressive phenotypes following transforming growth factor-β
(TGF-β) treatment to promote tumor progression, while N1 phenotypes
are formed through blocking TGF-β to exert an anti-tumor immune
response (51).
It has been indicated that ANXA1 can stimulate TGF-β
expression to promote the development of neutrophils into the N2
phenotype in melanoma. ANXA1 secreted by neutrophils can promote
melanoma invasion and metastasis via the FPR pathway (52). Moreover, in the ANXA1-knockown
glioblastoma animal model, inhibition of tumor growth and
infiltration of myeloid cells dominated by Ly6G+
granulocytes were observed (53).
The findings suggest that ANXA1 may promote the deterioration of
tumors by inducing neutrophil infiltration and differentiation.
Macrophages are heterogeneous cells that can
differentiate into two subsets of classically activated macrophages
(M1) and selectively activated macrophages (M2) according to
different molecular signals in the TME. M1 macrophages responding
to interferon-γ (INF-γ) can produce a number of pro-inflammatory
cytokines, including IL1β, TNF-α, IL-6, or IL-12, to induce the
subsequent TH1 response, consequently showing pro-inflammatory
activity. By contrast, M2 macrophages can drive the TH2 response by
secreting TGF-β1 and IL-10 (54,55). Therefore, the macrophage
polarization phenotype plays a crucial role in shaping the tumor
immune microenvironment.
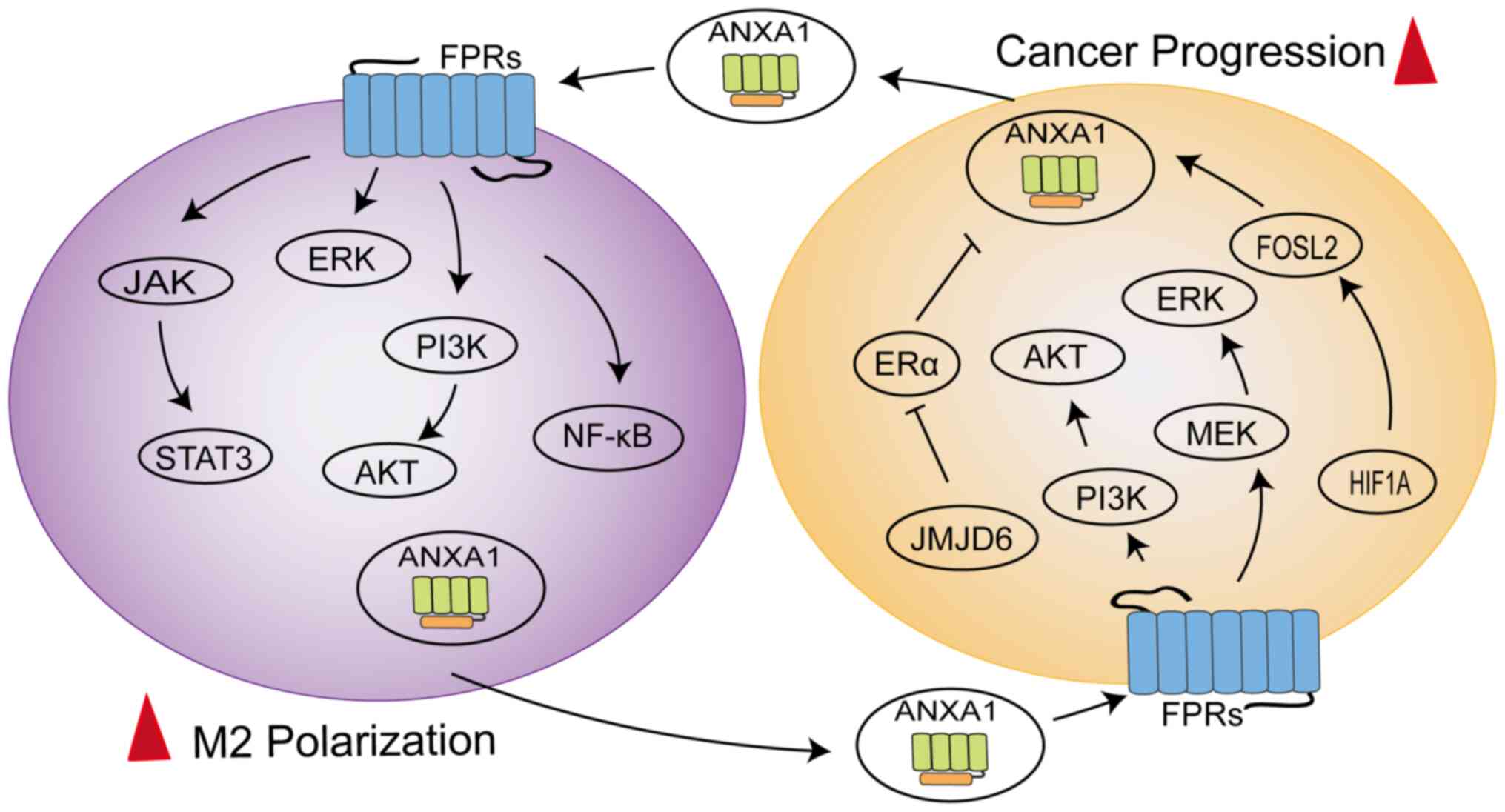
ANXA1 can mediate the polarization of macrophages
towards the M2 phenotype to exert immunosuppressive effects
(Fig. 3). In triple negative
breast cancer 4T1 cells, ANXA1 perform a critical role by inducing
the differentiation of macrophages into the M2 phenotype, which
enhances activation of ERK and NF-κB following ANXA1 communication
with FPR2, subsequently enhancing the invasiveness of 4T1 cells
(56). A similar concept was
described for ERα-positive breast cells, where the presence of
Ac2-26 successfully reversed the M1 phenotypic polarization of
macrophages induced by JMJD6 knockdown and JMJD6 promoted the
expression and secretion of ANXA1 in a lipid drop dependent manner,
thereby promoting tumor growth (57). Moreover, ANXA1 is secreted
extracellularly in the form of exosomes from pancreatic cancer
cells and was observed to enhance the differentiation and
recruitment of macrophages into the M2 phenotype and support the
formation of liver metastases (11).
The latest study found ANXA1 to be overexpressed in
the macrophages of hepatocellular carcinoma (HCC) patients,
exhibiting a loop between macrophages and tumor cells (58). The N-terminal of ANXA1 binding to
FPR2 might promote macrophage M2 polarization and infiltration to
sustain an immunosuppressive TME by downregulating the NF-κB and
NOTCH1 pathways and upregulating the JAK/STAT, AKT and ERK
pathways, while ANXA1 generated by macrophages might promote HCC
cell proliferation and migration by activating the PI3K/AKT and
MEK/ERK pathways to negatively regulate FOXO3 (58). The dynamic regulation of
macrophages by ANXA1 promotes disease progression (59). The HIF1A/FOSL2/ANXA1 axis is
involved in the natural evolution of tumors in glioblastoma (GBM).
As the disease progresses, ANXA1 secretion can increase the
aggregation of M2 macrophages, thereby reducing the proliferation
of CD8+T cells, which exerts an immunosuppressive effect
(59). At the same time,
polarized M2 macrophages produce CCL2 to further accelerate tumor
progression (59). These data
indicate that ANXA1 is also involved in bidirectional crosstalk
between macrophages and tumor cells and is involved in the
activation of multiple signaling pathways.
As members of the mononuclear macrophage family,
microglia are involved in the innate immunity of the central
nervous system (60) and can
migrate to the pre-metastatic niche to produce a wide range of
anti-inflammatory factors, forming a microenvironment that supports
brain metastasis (61). It is
noteworthy that when released, ANXA1 induced brain metastasis and
colonization of breast cancer cells by recruiting microglia
mediated by the FPR1/2-STAT3 axis in a model of brain metastasis of
breast cancer (9). Therefore, it
is possible to delay the brain metastasis of breast cancer cells by
blocking the function of ANXA1.
Dendritic cells (DCs) are specialized
antigen-presenting cells (APCs). Mature DCs can perform an
anti-tumor immunity role by processing tumor cell antigens and
presenting co-stimulatory molecules to CD4+ and
CD8+ T cells. DCs can also differentiate into tolerant
DCs (tDCs) and immature DCs (iDCs) during differentiation to hinder
immune responses (62).
The exposed ANXA1 observed in on the apoptotic
cell-surface supports a tolerogenic phenotype in DCs (63), which can be mediated through the
interaction between the core region of ANXA1 and Dectin-1 (64). Similarly, high ANXA1 expression
was found to be positively correlated with the infiltration of tDCs
and iDC in GBM patients (65).
However, during breast cancer chemotherapy, the loss of ANXA1
caused dying cells to fail to interact with FPR1 expressed on DCs,
further activating the anti-cancer immune response (66). Low ANXA1 expression was hardly
infiltrated by DCs and cytotoxic T lymphocytes, suggesting that
ANXA1 deficiency might contribute to the immune escape of breast,
colorectal and lung cancer cells (67). It was hypothesized that the
paradoxical immunomodulatory effects of ANXA1 in DCs might be
caused by the different release modes and functional domains of
ANXA1. When ANXA1 interacts with receptors on the surface of DCs
through the core region, it can enhance the secretion of
anti-inflammatory factors and mediate immune tolerance. However,
the immune function was enhanced by the interaction of FPRs
expressed in DCs within the N-terminal of ANXA1.
Naive T cells receive co-stimulation through the
combination of CD28 expressed on T cells, with B7 expressed on
APCs, which activates their proliferation and differentiation into
effector T cells. Effector T cells can be divided into
CD4+ T helper cells (TH1, TH2 and TH17), regulatory T
cells (Tregs) and cytotoxic T lymphocytes (CTLs) (68). TH1 cells can produce IFN-γ, which
is involved in the elimination of pathogens and the destruction of
cancer cells (69). TH2 cells can
secrete cytokines, such as IL-4, IL-10 and TGF-α, which suppress
the anti-tumor immune microenvironment to promote immune escape
(70). Tregs have
anti-inflammatory properties via the generation of cytokines,
including IL-10, IL-35 and TGF-β, which can mitigate the occurrence
of autoimmune and allergic diseases (71). However, in the context of cancer,
Tregs can hinder the anti-tumor T cell response for fighting cancer
(72).
Other studies have highlighted the role of ANXA1 in
promoting the differentiation of TH1 cells (48,73). However, researchers have observed
that ANXA1 can induce the infiltration of TH2 cells, which is
positively correlated with the poor prognosis of patients with
pancreatic cancer (74). The
authors suspected that the opposite phenomenon may be related to
the different activation status of the TCR in T cells. When T cells
are in an activated state, ANXA1 tends to induce T cell
differentiation into the TH1 phenotype (47). Conversely, ANXA1 can induce T
cells toward the TH2 phenotype in an immunosuppressive state. This
hypothesis can explain why ANXA1 acting directly on T cells could
increase INF-γ secretion, but could inhibit INF-γ secretion when
DCs exhibit a tolerant phenotype (75).
In addition, the regulatory effects of ANXA1 on
Tregs were observed in triple negative breast patients (76). ANXA1 promoted the
immunosuppressive function of Tregs through FPR2, which led to poor
prognosis in breast cancer patients. ANXA1 blocked by FPR2
inhibitor N-tert-butyloxycarbonyl-Met-Leu-Phe (Boc1) can damage the
function of Tregs, resulting in tumor volume reduction in mice
(76); therefore, ANXA1/FPR2 may
be a potential target for breast cancer treatment. A number of
studies have demonstrated that ANXA1 is a central regulator of
various immune cells, capable of synergistically shaping the immune
microenvironment of tumor cells (57,65,67). However, the specific mechanism by
which ANXA1 signaling plays a role in immune infiltration remains
controversial and elucidating the role of ANXA1 signaling in tumor
immune regulation is crucial for cancer treatment.
Cancer-associated fibroblasts (CAFs) are the most
dominant components of the TME, participating in multiple
processes, including tumor cell extracellular matrix (ECM)
remodeling, immune escape, angiogenesis and therapy resistance
(77). CAFs mainly include three
subtypes; myofibroblasts (myCAFs) that directly interact with
cancer cells, inflammatory CAFs (iCAFs) that regulate the
microenvironment by secreting cytokines and antigen-presenting CAFs
with antigen-presenting ability (78). The origin and function of CAFs are
heterogeneous (77), mainly
characterized by the upregulation of proteins such as fibroblast
specific protein 1, vimentin (VIM), α-smooth muscle actin (α-SMA),
fibroblast-activated protein and platelet-derived growth factor
receptor (79,80).
ANXA1 is expressed and secreted in fibroblasts,
where it modulates fibroblast function in the TME (81,82). ANXA1 secretion is significantly
higher in prostate-derived cancer-associated fibroblasts (CAFs)
than in normal prostate fibroblasts (NPFs). Secreted ANXA1 could
increase the activation of pERK1/2 and TGF-β1 and has been shown to
support the prostate cancer stem cell niche by maintaining and
de novo inducing basal stem-like cancer cells through two
independent but complementary pathways, in vitro and in
vivo (13). CAFs-specific
miR-196a directly targets ANXA1, which not only promotes the
inflammatory characteristics of CAFs to activate cancer cells by
accumulating the inflammatory cytokine CCL2, but also by
upregulating the expression of αSMA and FAP to induce the
myofibroblast activity of CAFs, which promotes the invasion of lung
cancer cells through direct interaction (82).
ANXA1 produced by tumor cells also affects the
activity of fibroblasts. In pancreatic cancer cells, Ac2-26 or
containing ANXA1-EVs accelerates fibroblast migration and
upregulates MMP-9, FAP1α and F-actin expression to acquire CAFs
properties (11). A similar
conclusion was found in triple-negative breast cancer, where the
supernatant of ANXA1 wild-type breast cancer cells promotes
fibroblast migration, possibly through an interplay with FPR1
receptors, to create a microenvironment conducive to tumor growth
(20). By contrast, loss of the
ANXA1 protein leads to a cut-off of the ANXA1-FPR2 signaling
pathway, which improves the phosphorylation levels of AKT, ERK and
SMAD3 accompanied by the upregulation of FAP, VIM, MMP1 and α-SMA
expression, ultimately inducing normal fibroblasts to transform
into myCAFs with the evolution of esophageal squamous cell
carcinoma (83). The complexity
of ANXA1-mediated fibroblast functions may be attributed to the
heterogeneity of CAFs origins and the different receptor subtypes
in which ANXA1 acts.
More attention has been paid to ANXA1 expression in
tumor cells because of the differential expression of ANXA1 in
normal and tumor samples, but the role of externalized ANXA1 in
extracellular fluid cannot be ignored. The expression of ANXA1 in
the serum of lung cancer and melanoma patients is significantly
higher than that of normal controls (52,84) and high serum ANXA1 levels are
closely correlated with the pathological grade and clinical stage
of lung cancer patients (84).
Therefore, ANXA1 may develop into a novel blood marker for tumor
detection. However, serum ANXA1 levels are tumor-specific, so their
expression varies among patients with different types of cancer.
ANXA1 expression levels in peripheral blood samples from patients
with oral squamous cell carcinoma and esophageal squamous cell
carcinoma (ESCC) are reduced relative to those of healthy
individuals (85,86). However, ANXA1 concentration in
serum increases following chemoradiotherapy in patients with ESCC
and is related to poor prognosis (86). Therefore, it may be beneficial to
develop serum ANXA1 as a predictive marker of treatment outcomes in
patients with chemoradiotherapy. Furthermore, upregulated secretion
of ANXA1 into the extracellular environment could promote brain
metastasis of small cell lung cancer (SCLC) (87). ANXA1 expression levels in the
serum of patients with brain metastases were significantly higher
than those of SCLC patients without brain metastases, suggesting
that ANXA1 might be a diagnostic marker for patients with brain
metastases (87). Compared with
traditional invasive cancer detection methods, serum ANXA1 content
assessment offers the advantages of convenient acquisition, less
trauma and convenient operation, which can open up a new
perspective for the differential diagnosis and prognostic
evaluation of cancer.
ANXA1 is specifically induced on the luminal surface
of tumor vascular endothelial cells, serving as a specific tumor
vascular marker (88). In
response to this characteristic, researchers screened a peptide
that binds specifically to the N-terminal of ANXA1, IF7 (IFLLWQR),
which can be designed as a tumor-specific drug delivery vehicle
(41). IF7 combined with
different anticancer drugs can not only slow the growth of colon
cancer, lung cancer, bladder cancer, melanoma and other tumors in
mouse models (41,89,90), but can even overcome the
blood-brain barrier to further damage brain tumors (91). The anti-tumor drug delivery system
designed for ANXA1 has the characteristics of strong specificity,
high cytotoxicity and low side effects, thus offering a new
direction for the design of anti-tumor drugs.
In addition, conjugating imaging agents, such as
Alexa Fluor 488, to the targeted binding peptide of ANXA1 can be
constructed for non-invasive tumor imaging detection (41), which can be developed into a new
imaging probe for clinical application in the diagnosis of the
tumor vascular system because of strong tumor targeting, high
plasma clearance rate and low background.
Considering the function of ANXA1 in immune
regulation, it is highly promising to apply it to immunotherapy for
tumors. ANXA1 as damage-associated molecular patterns should not be
ignored in immunogenic cell death (ICD) (92). Several chemotherapeutic drugs can
trigger the activation of CTLs by inducing ICD, thereby leading to
the continuous elimination of tumor cells (93). Loss of ANXA1 in cancer cells or
lack of host FPRs function may lead to weakened chemotherapeutic
capacity and shortened patient overall survival (66,94). Therefore, the functions of ANXA1
and FPRs can be evaluated to predict the therapeutic effects of
chemotherapy drugs, leading to the development of personalized
treatment plans for patients.
In addition, ANXA1 has been confirmed to mediate
increased expression of programmed cell death ligand 1 (PD-L1) by
upregulating the phosphorylation of AKT and STAT3 in cancer cells
(95,96). As an immune checkpoint, PD-L1 can
interact with programmed cell death 1 (PD-1) to inactivate CTLs and
trigger the immune escape of tumor cells (97). ANXA1-derived peptide A11 could
compete with the deubiquitinating enzyme USP7 for binding to PD-L1,
thereby reducing the stability of PD-L1 in breast, lung and
melanoma cells (98). The
completely opposite effect of ANXA1 in immunotherapy might be
primarily responsible for different intracellular localizations,
thereby activating different signaling pathways.
The ANXA1/FPRs axis is an important pathway for
communication between various compartments in TME. Therefore,
blocking the effects of ANXA1 and FPRs can provide a new avenue for
targeted cancer therapy. Boc1, a non-selective FPRs antagonist
(99), plays an anti-tumor role
in breast cancer animal models (76). However, it is difficult to use in
clinical research because of its poor specificity. At present,
studies on FPRs inhibitors are mainly limited to inflammatory cells
(100,101). Therefore, more research is
needed to expand the development of FPRs inhibitors for clinical
applications in cancer treatment.
ANXA1 is critical for the regulation of the TME;
however, its reported roles in cancer are contradictory because of
its different origins and temporal and spatial dynamics. Therefore,
the collective study of ANXA1 should be based on a comprehensive
understanding of its diverse biological functions specific to each
compartment of the TME and according to specific cancer types, as
this will eventually lead to the development of more accurate
clinical treatment strategies.
Not applicable.
KG drafted and reviewed the manuscript; SL and XL
reviewed and edited the manuscript; LZ reviewed and edited the
manuscript and was responsible for supervision and funding
acquisition. Data authentication is not applicable. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81673510 and 82073936) and the
Outstanding Scientific Fund of Shengjing Hospital (grant no.
M0779).
|
1
|
Baghban R, Roshangar L, Jahanban-Esfahlan
R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T and
Zare P: Tumor microenvironment complexity and therapeutic
implications at a glance. Cell Commun Signal. 18:592020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelly L, McGrath S, Rodgers L, McCall K,
Tulunay Virlan A, Dempsey F, Crichton S and Goodyear CS:
Annexin-A1: The culprit or the solution? Immunology. 166:2–16.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Foo SL, Yap G, Cui J and Lim LHK:
Annexin-A1-A Blessing or a curse in cancer? Trends Mol Med.
25:315–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng C, Liu X, Zhang C, Li L, Wen S, Gao X
and Liu L: ANXA1GSK3beta interaction and its involvement in NSCLC
metastasis. Acta Biochim Biophys Sin (Shanghai). 53:912–924. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu JF, Huang W, Yi HM, Xiao T, Li JY,
Feng J, Yi H, Lu SS, Li XH, Lu RH, et al: Annexin A1-suppressed
autophagy promotes nasopharyngeal carcinoma cell invasion and
metastasis by PI3K/AKT signaling activation. Cell Death Dis.
9:11542018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Novizio N, Belvedere R, Morretta E,
Tomasini R, Monti MC, Morello S and Petrella A: Role of
intracellular and extracellular annexin A1 in MIA PaCa-2 spheroids
formation and drug sensitivity. Cancers (Basel). 14:47642022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Foo SL, Sachaphibulkij K, Lee CLY, Yap
GLR, Cui J, Arumugam T and Lim LHK: Breast cancer metastasis to
brain results in recruitment and activation of microglia through
annexin-A1/formyl peptide receptor signaling. Breast Cancer Res.
24:252022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khau T, Langenbach SY, Schuliga M, Harris
T, Johnstone CN, Anderson RL and Stewart AG: Annexin-1 signals
mitogen-stimulated breast tumor cell proliferation by activation of
the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 25:483–496.
2011. View Article : Google Scholar
|
|
11
|
Novizio N, Belvedere R, Pessolano E,
Morello S, Tosco A, Campiglia P, Filippelli A and Petrella A: ANXA1
contained in EVs regulates macrophage polarization in tumor
microenvironment and promotes pancreatic cancer progression and
metastasis. Int J Mol Sci. 22:110182021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vecchi L, Alves Pereira Zóia M, Goss
Santos T, de Oliveira Beserra A, Colaço Ramos CM, França Matias
Colombo B, Paiva Maia YC, Piana de Andrade V, Teixeira Soares Mota
S, Gonçalves de Araújo T, et al: Inhibition of the AnxA1/FPR1
autocrine axis reduces MDA-MB-231 breast cancer cell growth and
aggressiveness in vitro and in vivo. Biochim Biophys Acta Mol Cell
Res. 1865:1368–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geary LA, Nash KA, Adisetiyo H, Liang M,
Liao CP, Jeong JH, Zandi E and Roy-Burman P: CAF-Secreted Annexin
A1 induces prostate cancer cells to gain stem cell-like features.
Mol Cancer Res. 12:607–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu Z, Zhang S, Wang B, Huang W, Zheng L
and Cheng A: Annexin A1: A double-edged sword as novel cancer
biomarker. Clin Chim Acta. 504:36–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizuno H, Uemura K, Moriyama A, Wada Y,
Asai K, Kimura S and Kato T: Glucocorticoid induced the expression
of mRNA and the secretion of lipocortin 1 in rat astrocytoma cells.
Brain Res. 746:256–264. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gimenes AD, Andrade TR, Mello CB, Ramos L,
Gil CD and Oliani SM: Beneficial effect of annexin A1 in a model of
experimental allergic conjunctivitis. Exp Eye Res. 134:24–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ricci E, Ronchetti S, Pericolini E,
Gabrielli E, Cari L, Gentili M, Roselletti E, Migliorati G,
Vecchiarelli A and Riccardi C: Role of the glucocorticoid-induced
leucine zipper gene in dexamethasone-induced inhibition of mouse
neutrophil migration via control of annexin A1 expression. FASEB J.
31:3054–3065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castro-Caldas M, Mendes AF, Duarte CB and
Lopes MC: Dexamethasone-induced and estradiol-induced CREB
activation and annexin 1 expression in CCRF-CEM lymphoblastic
cells: Evidence for the involvement of cAMP and p38 MAPK. Mediators
Inflamm. 12:329–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Solito E, de Coupade C, Parente L, Flower
RJ and Russo-Marie F: IL-6 stimulates annexin 1 expression and
translocation and suggests a new biological role as class II acute
phase protein. Cytokine. 10:514–521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vecchi L, Mota STS, Zoia MAP, Martins IC,
de Souza JB, Santos TG, Beserra AO, de Andrade VP, Goulart LR and
Araujo TG: Interleukin-6 signaling in triple negative breast cancer
cells elicits the annexin A1/Formyl peptide receptor 1 axis and
affects the tumor microenvironment. Cells. 11:17052022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soler MF, Abaurrea A, Azcoaga P, Araujo AM
and Caffarel MM: New perspectives in cancer immunotherapy:
Targeting IL-6 cytokine family. J Immunother Cancer.
11:e0075302023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan Y, Anbalagan D, Lee LH, Samy RP,
Shanmugam MK, Kumar AP, Sethi G, Lobie PE and Lim LH: ANXA1
inhibits miRNA-196a in a negative feedback loop through NF-kB and
c-Myc to reduce breast cancer proliferation. Oncotarget.
7:27007–27020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suh YE, Raulf N, Gäken J, Lawler K, Urbano
TG, Bullenkamp J, Gobeil S, Huot J, Odell E and Tavassoli M:
MicroRNA-196a promotes an oncogenic effect in head and neck cancer
cells by suppressing annexin A1 and enhancing radioresistance. Int
J Cancer. 137:1021–1034. 2015. View Article : Google Scholar
|
|
25
|
Hu C, Peng J, Lv L, Wang X, Zhou Y, Huo J
and Liu D: miR-196a regulates the proliferation, invasion and
migration of esophageal squamous carcinoma cells by targeting
ANXA1. Oncol Lett. 17:5201–5209. 2019.PubMed/NCBI
|
|
26
|
Boudhraa Z, Bouchon B, Viallard C, D'Incan
M and Degoul F: Annexin A1 localization and its relevance to
cancer. Clin Sci (Lond). 130:205–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoque M, Rentero C, Cairns R, Tebar F,
Enrich C and Grewal T: Annexins-Scaffolds modulating PKC
localization and signaling. Cell Signal. 26:1213–1225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Solito E CH, Festa M, Mulla A, Tierney T,
Flower RJ and Buckingham JC: Post-translational modification plays
an essential role in the translocation of annexin A1 from the
cytoplasm to the cell surface. FASEB J. 20:1498–1500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Li L, Wang J, Zhang R, Zhang T, Wu
Y, Wang S and Xing D: The ABCA1-efferocytosis axis: A new strategy
to protect against atherosclerosis. Clin Chim Acta. 518:1–8. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Perretti M, Christian H, Wheller SK,
Aiello I, Mugridge KG, Morris JF, Flower RJ and Goulding NJ:
Annexin I is stored within gelatinase granules of human neutrophil
and mobilized on the cell surface upon adhesion but not
phagocytosis. Cell Biol Int. 24:163–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leoni G, Neumann PA, Kamaly N, Quiros M,
Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, et
al: Annexin A1-containing extracellular vesicles and polymeric
nanoparticles promote epithelial wound repair. J Clin Invest.
125:1215–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Q, Liu W, Wang Z, Wang C and Ai Z:
Exosomal ANXA1 derived from thyroid cancer cells is associated with
malignant transformation of human thyroid follicular epithelial
cells by promoting cell proliferation. Int J Oncol. 59:1042021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pessolano E, Belvedere R, Bizzarro V,
Franco P, Marco ID, Porta A, Tosco A, Parente L, Perretti M and
Petrella A: Annexin A1 may induce pancreatic cancer progression as
a key player of extracellular vesicles effects as evidenced in the
in vitro MIA PaCa-2 model system. Int J Mol Sci. 19:38782018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y,
Hao H, Tang K, Yi P, Liu M, et al: Pleiotropic regulation of
macrophage polarization and tumorigenesis by formyl peptide
receptor-2. Oncogene. 30:3887–3899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cooray SN, Gobbetti T, Montero-Melendez T,
McArthur S, Thompson D, Clark AJ, Flower RJ and Perretti M:
Ligand-specific conformational change of the G-protein-coupled
receptor ALX/FPR2 determines proresolving functional responses.
Proc Natl Acad Sci USA. 110:18232–18237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin KT, Yao JY, Fang XL, Di H and Ma YY:
Roles of lncRNAs in cancer: Focusing on angiogenesis. Life Sci.
252:1176472020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Ostoot FH, Salah S, Khamees HA and
Khanum SA: Tumor angiogenesis: Current challenges and therapeutic
opportunities. Cancer Treat Res Commun. 28:1004222021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yi M and Schnitzer JE: Impaired tumor
growth, metastasis, angiogenesis and wound healing in annexin
A1-null mice. Proc Natl Acad Sci USA. 106:17886–17891. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Delorme S, Privat M, Sonnier N, Rouanet J,
Witkowski T, Kossai M, Mishellany F, Radosevic-Robin N, Juban G,
Molnar I, et al: New insight into the role of ANXA1 in melanoma
progression: involvement of stromal expression in dissemination. Am
J Cancer Res. 11:1600–1615. 2021.PubMed/NCBI
|
|
41
|
Hatakeyama S, Sugihara K, Shibata TK,
Nakayama J, Akama TO, Tamura N, Wong SM, Bobkov AA, Takano Y,
Ohyama C, et al: Targeted drug delivery to tumor vasculature by a
carbohydrate mimetic peptide. Proc Natl Acad Sci USA.
108:19587–19592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hebeda CB, Sandri S, Benis CM, Paula-Silva
M, Loiola RA, Reutelingsperger C, Perretti M and Farsky SHP:
Annexin A1/Formyl peptide receptor pathway controls uterine
receptivity to the blastocyst. Cells. 9:11812020. View Article : Google Scholar
|
|
43
|
Dianat-Moghadam H, Nedaeinia R, Keshavarz
M, Azizi M, Kazemi M and Salehi R: Immunotherapies targeting tumor
vasculature: Challenges and opportunities. Front Immunol.
14:12263602023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Côté MC, Lavoie JR, Houle F, Poirier A,
Rousseau S and Huot J: Regulation of vascular endothelial growth
factor-induced endothelial cell migration by LIM kinase 1-mediated
phosphorylation of annexin 1. J Biol Chem. 285:8013–8021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Anbalagan D, Yap G, Yuan Y, Pandey VK, Lau
WH, Arora S, Bist P, Wong JS, Sethi G, Nissom PM, et al: Annexin-A1
regulates microRNA-26b* and microRNA-562 to directly target
NF-kappaB and angiogenesis in breast cancer cells. PLoS One.
9:e1145072014. View Article : Google Scholar
|
|
46
|
Novizio N, Belvedere R, Pessolano E, Tosco
A, Porta A, Perretti M, Campiglia P, Filippelli A and Petrella A:
Annexin A1 released in extracellular vesicles by pancreatic cancer
cells activates components of the tumor microenvironment, through
interaction with the Formyl-Peptide receptors. Cells. 9:27192020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
D'Acquisto F, Perretti M and Flower RJ:
Annexin-A1: A pivotal regulator of the innate and adaptive immune
systems. Br J Pharmacol. 155:152–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Perretti M and D'Acquisto F: Annexin A1
and glucocorticoids as effectors of the resolution of inflammation.
Nat Rev Immunol. 9:62–70. 2009. View Article : Google Scholar
|
|
49
|
Solito E, Kamal A, Russo-Marie F,
Buckingham JC, Marullo S and Perretti M: A novel calcium-dependent
proapoptotic effect of annexin 1 on human neutrophils. FASEB J.
17:1–27. 2003. View Article : Google Scholar
|
|
50
|
Gavins FNE and Hickey MJ: Annexin A1 and
the regulation of innate and adaptive immunity. Front Immunol.
3:3542012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shaul ME and Fridlender ZG: Neutrophils as
active regulators of the immune system in the tumor
microenvironment. J Leukoc Biol. 102:343–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sandri S, Hebeda CB, Broering MF, de Paula
Silva M, Moredo LF, de Barros E Silva MJ, Sapata Molina A, Lopes
Pinto CA, Duprat Neto JP, Reutelingsperger CP, et al: Role of
Annexin A1 secreted by neutrophils in melanoma metastasis. Cells.
12:4252023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang Y, Liu Y, Yao X, Ping Y, Jiang T, Liu
Q, Xu S, Huang J, Mou H, Gong W, et al: Annexin 1 released by
necrotic human glioblastoma cells stimulates tumor cell growth
through the formyl peptide receptor 1. Am J Pathol. 179:1504–1512.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moraes LA, Ampomah PB and Lim LHK: Annexin
A1 in inflammation and breast cancer: A new axis in the tumor
microenvironment. Cell Adh Migr. 12:417–423. 2018.PubMed/NCBI
|
|
55
|
Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR
and Yang SM: Macrophages in tumor microenvironments and the
progression of tumors. Clin Dev Immunol. 2012:9480982012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Moraes LA, Kar S, Foo SL, Gu T, Toh YQ,
Ampomah PB, Sachaphibulkij K, Yap G, Zharkova O, Lukman HM, et al:
Annexin-A1 enhances breast cancer growth and migration by promoting
alternative macrophage polarization in the tumour microenvironment.
Sci Rep. 7:179252017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cioni B, Ratti S, Piva A, Tripodi I,
Milani M, Menichetti F, Langella T, Botti L, De Cecco L, Chiodoni
C, et al: JMJD6 shapes a pro-tumor microenvironment via
ANXA1-dependent macrophage polarization in breast cancer. Mol
Cancer Res. 21:614–627. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Song Z, Wang X, Liu X, Luo Y, Qiu J, Yin
A, Liu Y, Yi H, Xiao Z and Li A: Targeting of Annexin A1 in
Tumor-associated Macrophages as a therapeutic strategy for
hepatocellular carcinoma. Biochem Pharmacol. 213:1156122023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu L, Wu W, Zhang J, Zhao Z, Li L, Zhu M,
Wu M, Wu F, Zhou F, Du Y, et al: Natural coevolution of tumor and
immunoenvironment in glioblastoma. Cancer Discov. 12:2820–2837.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Han CJ, Zheng JY, Sun L, Yang HC, Cao ZQ,
Zhang XH, Zheng LT and Zhen XC: The oncometabolite
2-hydroxyglutarate inhibits microglial activation via the
AMPK/mTOR/NF-κB pathway. Acta pharmacol Sin. 40:1292–1302. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li W and Graeber MB: The molecular profile
of microglia under the influence of glioma. Neuro oncol.
14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Domogalla MP, Rostan PV, Raker VK and
Steinbrink K: Tolerance through education: How tolerogenic
dendritic cells shape immunity. Front Immunol. 8:17642017.
View Article : Google Scholar
|
|
63
|
Linke B, Abeler-Dörner L, Jahndel V, Kurz
A, Mahr A, Pfrang S, Linke L, Krammer PH and Weyd H: The
tolerogenic function of annexins on apoptotic cells is mediated by
the annexin core domain. J Immunol. 194:5233–5242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bode K, Bujupi F, Link C, Hein T,
Zimmermann S, Peiris D, Jaquet V, Lepenies B, Weyd H and Krammer
PH: Dectin-1 Binding to annexins on apoptotic cells induces
peripheral immune tolerance via NADPH Oxidase-2. Cell Rep.
29:4435–4446.e9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen R, Chen C, Han N, Guo W, Deng H, Wang
Y, Ding Y and Zhang M: Annexin-1 is an oncogene in glioblastoma and
causes tumour immune escape through the indirect upregulation of
interleukin-8. J Cell Mol Med. 26:4343–4356. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vacchelli E, Ma Y, Baracco EE, Sistigu A,
Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et
al: Chemotherapy-induced antitumor immunity requires formyl peptide
receptor 1. Science. 350:972–978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Baracco EE, Stoll G, Van Endert P,
Zitvogel L, Vacchelli E and Kroemer G: Contribution of annexin A1
to anticancer immunosurveillance. Oncoimmunology. 8:e16477602019.
View Article : Google Scholar
|
|
68
|
Waldman AD, Fritz JM and Lenardo MJ: A
guide to cancer immunotherapy: From T cell basic science to
clinical practice. Nat Rev Immunol. 20:651–668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shang Q, Yu X, Sun Q, Li H, Sun C and Liu
L: Polysaccharides regulate Th1/Th2 balance: A new strategy for
tumor immunotherapy. Biomed Pharmacother. 170:1159762024.
View Article : Google Scholar
|
|
70
|
Bretscher P: On Analyzing How the Th1/Th2
Phenotype of an immune response is determined: Classical
observations must not be ignored. Front Immunol. 10:12342019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cvetanovich GL and Hafler DA: Human
regulatory T cells in autoimmune diseases. Curr Opin Immunol.
22:753–760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ohue Y and Nishikawa H: Regulatory T
(Treg) cells in cancer: Can Treg cells be a new therapeutic target?
Cancer Sci. 110:2080–2089. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
D'Acquisto F, Paschalidis N, Sampaio AL,
Merghani A, Flower RJ and Perretti M: Impaired T cell activation
and increased Th2 lineage commitment in Annexin-1-deficient T
cells. Eur J Immunol. 37:3131–3142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Oshi M, Tokumaru Y, Mukhopadhyay S, Yan L,
Matsuyama R, Endo I and Takabe K: Annexin A1 expression is
associated with epithelial-mesenchymal transition (EMT), cell
proliferation, prognosis and drug response in pancreatic cancer.
Cells. 10:6532021. View Article : Google Scholar
|
|
75
|
Weyd H, Abeler-Dorner L, Linke B, Mahr A,
Jahndel V, Pfrang S, Schnolzer M, Falk CS and Krammer PH: Annexin
A1 on the surface of early apoptotic cells suppresses CD8+ T cell
immunity. PLoS One. 8:e624492013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bai F, Zhang P, Fu Y, Chen H, Zhang M,
Huang Q, Li D, Li B and Wu K: Targeting ANXA1 abrogates
Tregmediated immune suppression in triplenegative breast cancer. J
Immunother Cancer. 8:e0001692020. View Article : Google Scholar
|
|
77
|
Ishii G, Ochiai A and Neri S: Phenotypic
and functional heterogeneity of cancer-associated fibroblast within
the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016.
View Article : Google Scholar
|
|
78
|
Vaish U, Jain T, Are AC and Dudeja V:
Cancer-Associated fibroblasts in pancreatic ductal adenocarcinoma:
An update on heterogeneity and therapeutic targeting. Int J Mol
Sci. 22:134082021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar
|
|
80
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Neymeyer H, Labes R, Reverte V, Saez F,
Stroh T, Dathe C, Hohberger S, Zeisberg M, Muller GA, Salazar J, et
al: Activation of annexin A1 signalling in renal fibroblasts exerts
antifibrotic effects. Acta Physiol (Oxf). 215:144–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lee S, Hong JH, Kim JS, Yoon JS, Chun SH,
Hong SA, Kim EJ, Kang K, Lee Kang J, Ko YH, et al:
Cancer-associated fibroblasts activated by miR-196a promote the
migration and invasion of lung cancer cells. Cancer Lett.
508:92–103. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen Y, Zhu S, Liu T, Zhang S, Lu J, Fan
W, Lin L, Xiang T, Yang J, Zhao X, et al: Epithelial cells activate
fibroblasts to promote esophageal cancer development. Cancer Cell.
41:903–918. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rong B, Zhao C, Liu H, Ming Z, Cai X, Gao
W and Yang S: Elevated serum annexin A1 as potential diagnostic
marker for lung cancer: A retrospective case-control study. Am J
Transl Res. 6:558–569. 2014.PubMed/NCBI
|
|
85
|
Faria PC, Sena AA, Nascimento R, Carvalho
WJ, Loyola AM, Silva SJ, Durighetto AF, Oliveira AD, Oliani SM and
Goulart LR: Expression of annexin A1 mRNA in peripheral blood from
oral squamous cell carcinoma patients. Oral oncol. 46:25–30. 2010.
View Article : Google Scholar
|
|
86
|
Han GH, Lu KJ, Huang JX, Zhang LX, Dai SB
and Dai CL: Association of serum annexin A1 with treatment response
and prognosis in patients with esophageal squamous cell carcinoma.
J Cancer Res Ther. 14(Suppl): S667–S674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu Y, Liu YS, Wu PF, Li Q, Dai WM, Yuan
S, Xu ZH, Liu TT, Miao ZW, Fang WG, et al: Brain microvascular
endothelium induced-annexin A1 secretion contributes to small cell
lung cancer brain metastasis. Int J Biochem Cell Biol. 66:11–19.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Oh P, Li Y, Yu J, Durr E, Krasinska KM,
Carver LA, Testa JE and Schnitzer JE: Subtractive proteomic mapping
of the endothelial surface in lung and solid tumours for
tissue-specific therapy. Nature. 429:629–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yoneyama T, Hatakeyama S, Sutoh Yoneyama
M, Yoshiya T, Uemura T, Ishizu T, Suzuki M, Hachinohe S, Ishiyama
S, Nonaka M, et al: Tumor vasculature-targeted 10B
delivery by an Annexin A1-binding peptide boosts effects of boron
neutron capture therapy. BMC cancer. 21:722021. View Article : Google Scholar
|
|
90
|
Hatakeyama S, Shibata TK, Tobisawa Y,
Ohyama C, Sugihara K and Fukuda MN: Tumor targeting by a
carbohydrate ligand-mimicking peptide. Methods Mol Biol.
1022:369–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Nonaka M, Suzuki-Anekoji M, Nakayama J,
Mabashi-Asazuma H, Jarvis DL, Yeh JC, Yamasaki K, Akama TO, Huang
CT, Campos AR, et al: Overcoming the blood-brain barrier by Annexin
A1-binding peptide to target brain tumours. Br J Cancer.
123:1633–1643. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Baracco EE, Petrazzuolo A and Kroemer G:
Assessment of annexin A1 release during immunogenic cell death.
Methods Enzymol. 629:71–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Arai H, Xiao Y, Loupakis F, Kawanishi N,
Wang J, Battaglin F, Soni S, Zhang W, Mancao C, Salhia B, et al:
Immunogenic cell death pathway polymorphisms for predicting
oxaliplatin efficacy in metastatic colorectal cancer. J Immunother
Cancer. 8:e0017142020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fucikova J, Kepp O, Kasikova L, Petroni G,
Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G and Galluzzi L:
Detection of immunogenic cell death and its relevance for cancer
therapy. Cell Death Dis. 11:10132020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xiong W, Zhang B, Yu H, Zhu L, Yi L and
Jin X: RRM2 Regulates sensitivity to sunitinib and PD-1 blockade in
renal cancer by Stabilizing ANXA1 and Activating the AKT pathway.
Adv Sci (Weinh). 8:21008812021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xiao D, Zeng T, Zhu W, Yu ZZ, Huang W, Yi
H, Lu SS, Feng J, Feng XP, Wu D, et al: ANXA1 promotes tumor immune
evasion by binding PARP1 and upregulating Stat3-induced expression
of PD-L1 in multiple cancers. Cancer Immunol Res. 11:1367–1383.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yu ZZ, Liu YY, Zhu W, Xiao D, Huang W, Lu
SS, Yi H, Zeng T, Feng XP, Yuan L, et al: ANXA1-derived peptide for
targeting PD-L1 degradation inhibits tumor immune evasion in
multiple cancers. J Immunother Cancer. 11:e0063452023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Stenfeldt AL, Karlsson J, Wennerås C,
Bylund J, Fu H and Dahlgren C: Cyclosporin H, Boc-MLF and Boc-FLFLF
are antagonists that preferentially inhibit activity triggered
through the formyl peptide receptor. Inflammation. 30:224–229.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yang SC, Chang SH, Hsieh PW, Huang YT, Ho
CM, Tsai YF and Hwang TL: Dipeptide HCH6-1 inhibits neutrophil
activation and protects against acute lung injury by blocking FPR1.
Free Radic Biol Med. 106:254–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li Z, Li Y, Han J, Zhu Z, Li M, Liu Q,
Wang Y and Shi FD: Formyl peptide receptor 1 signaling potentiates
inflammatory brain injury. Sci Transl Med. 13:eabe98902021.
View Article : Google Scholar : PubMed/NCBI
|