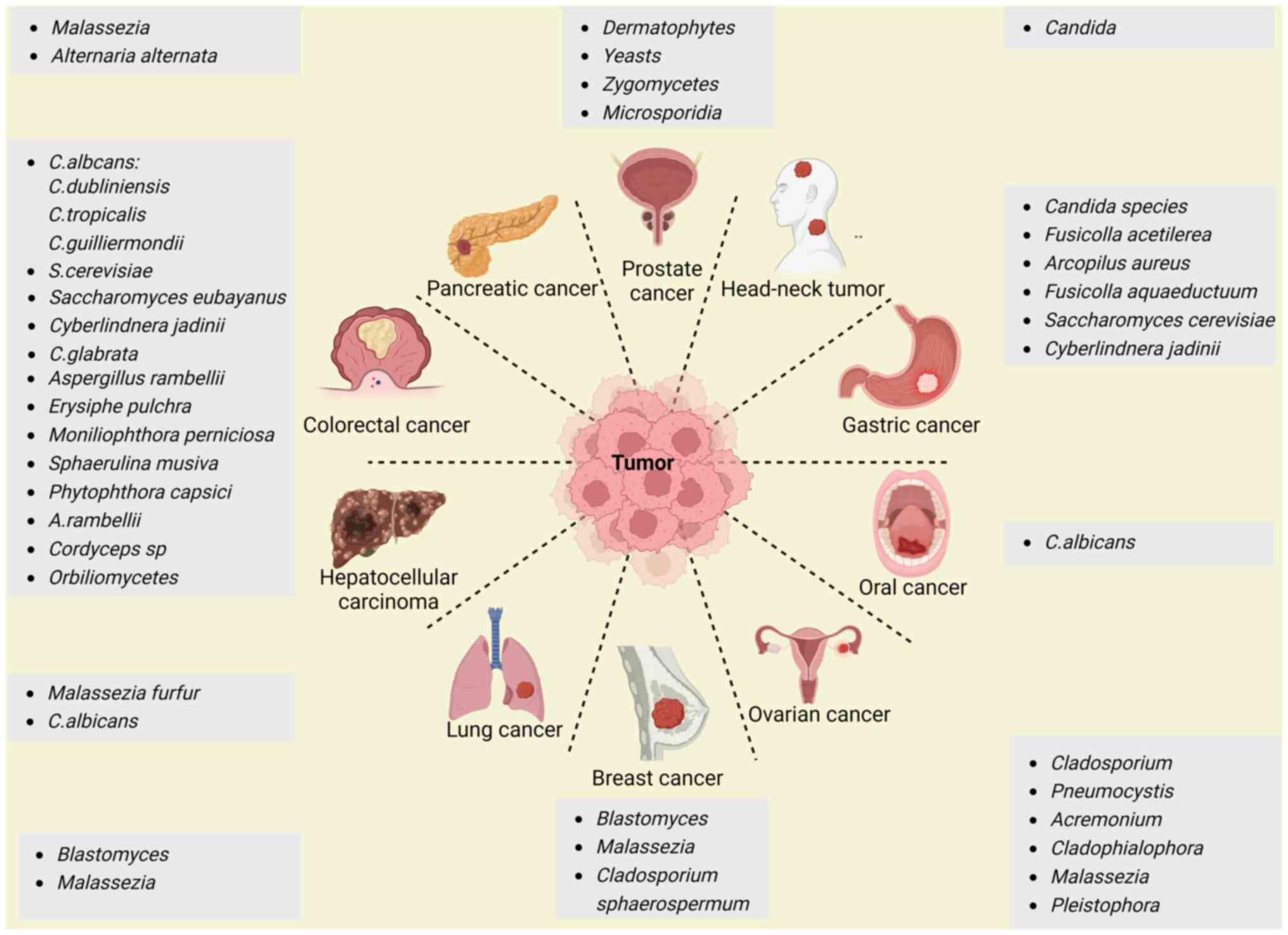
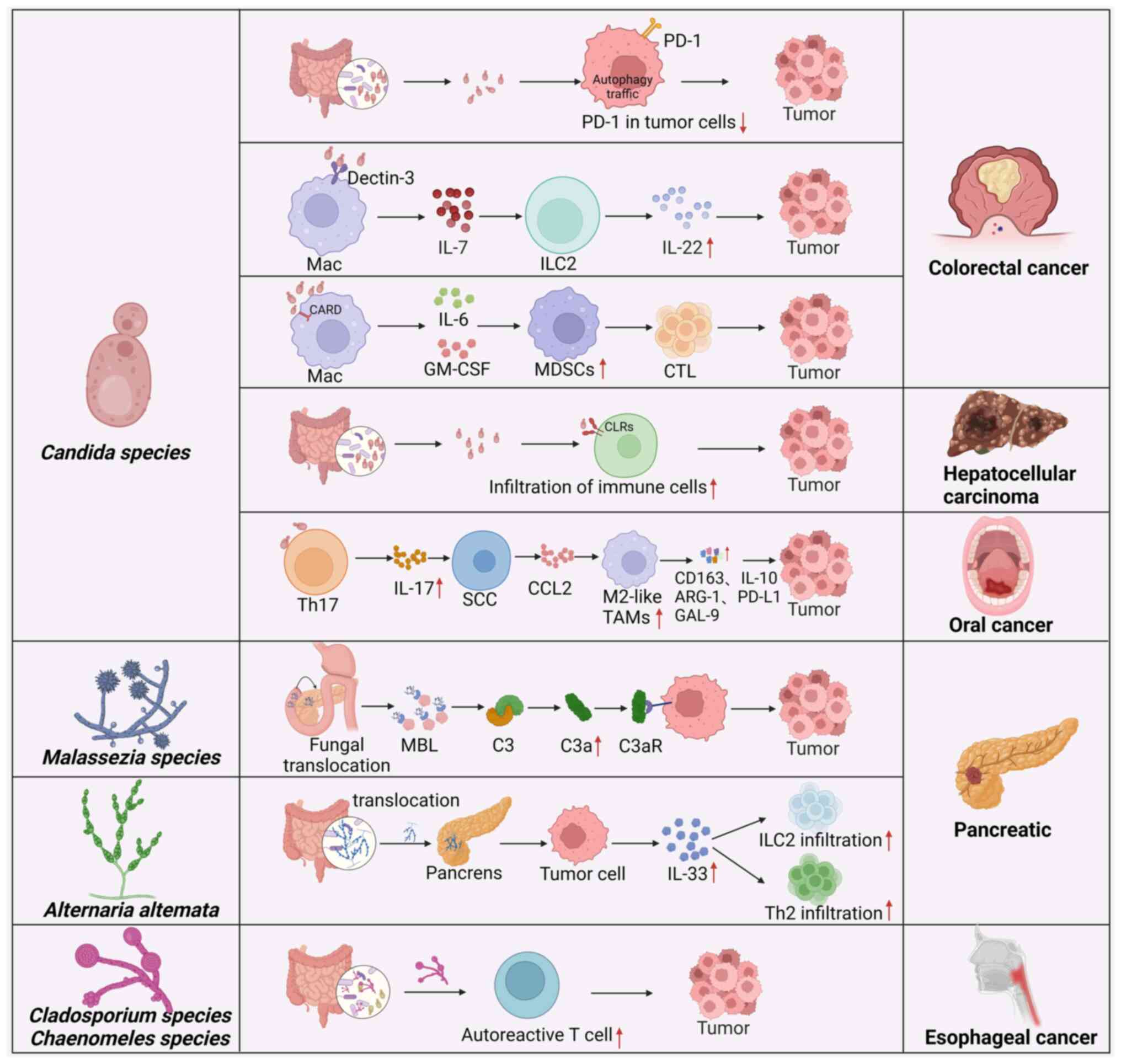
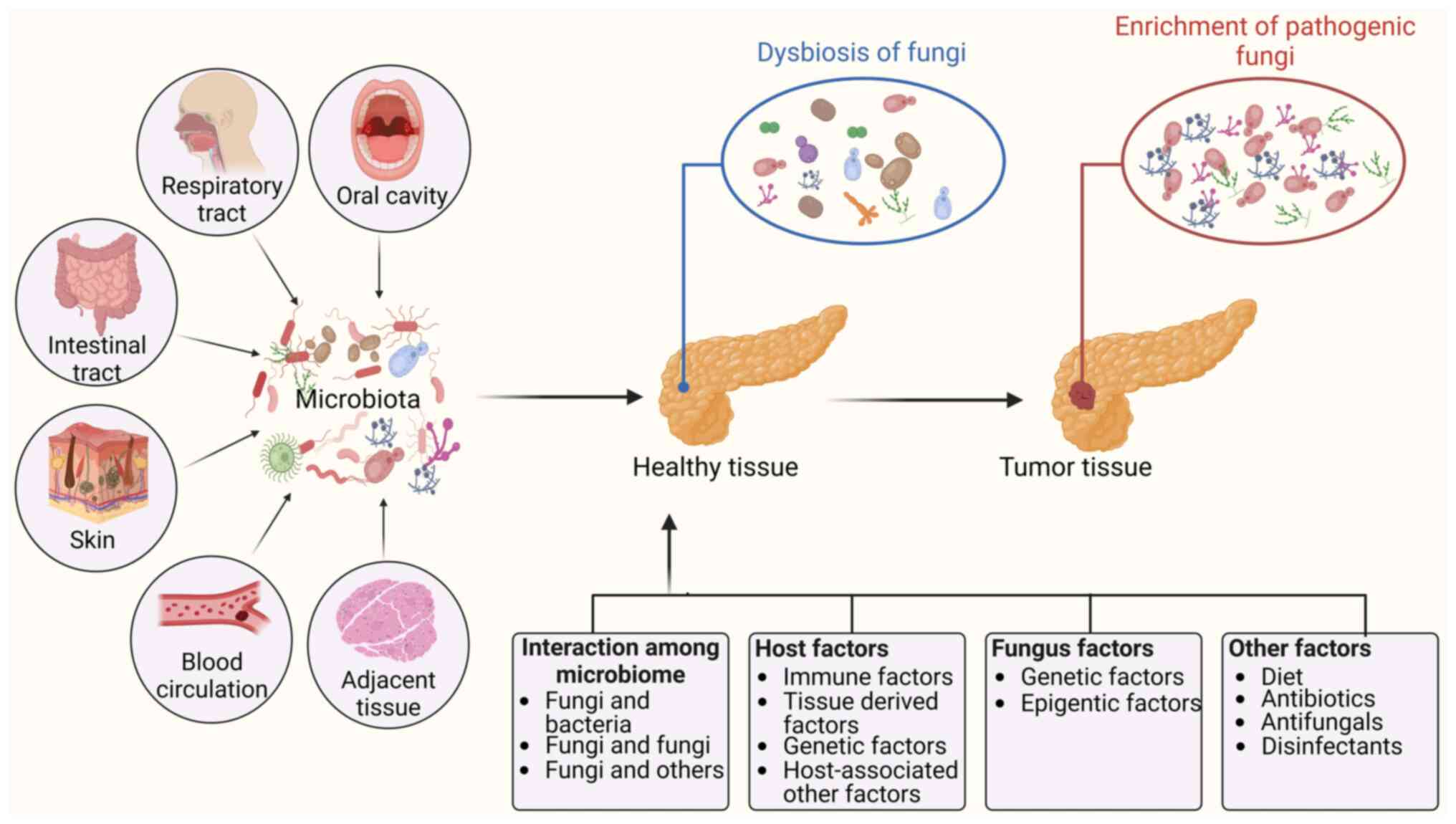
|
1
|
Wheeler ML, Limon JJ and Underhill DM:
Immunity to commensal fungi: Detente and disease. Annu Rev Pathol.
12:359–385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iliev ID, Funari VA, Taylor KD, Nguyen Q,
Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et
al: Interactions between commensal fungi and the C-type lectin
receptor Dectin-1 influence colitis. Science. 336:1314–1317. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okuno K, Tokunaga M, Von Hoff D, Kinugasa
Y and Goel A; PDAC Biomarker Working Group: Intratumoral
malasseziaglobosa levels predict survival and therapeutic response
to adjuvant chemotherapy in patients with pancreatic ductal
adenocarcinoma. Gastroenterology. 165:502–504 e2. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang T, Fan C, Yao A, Xu X, Zheng G, You
Y, Jiang C, Zhao X, Hou Y, Hung MC and Lin X: The Adaptor Protein
CARD9 protects against colon cancer by restricting
mycobiota-mediated expansion of myeloid-derived suppressor cells.
Immunity. 49:504–514 e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alam A, Levanduski E, Denz P,
Villavicencio HS, Bhatta M, Alhorebi L, Zhang Y, Gomez EC, Morreale
B, Senchanthisai S, et al: Fungal mycobiome drives IL-33 secretion
and type 2 immunity in pancreatic cancer. Cancer Cell. 40:153–167
e11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malik A, Sharma D, Malireddi RKS, Guy CS,
Chang TC, Olsen SR, Neale G, Vogel P and Kanneganti TD: SYK-CARD9
Signaling axis promotes gut fungi-mediated inflammasome activation
to restrict colitis and colon cancer. Immunity. 49:515–530 e5.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu J, Chen Q, Bing Z, Shen S, Hou Y, Lv M
and Wang T: C. tropicalis promotes CRC by down-regulating tumor
cell-intrinsic PD-1 receptor via autophagy. J Cancer. 14:1794–1808.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Li A, Wang Y and Zhang Y:
Intratumoral microbiota: Roles in cancer initiation, development
and therapeutic efficacy. Signal Transduct Target Ther. 8:352023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Azevedo MM, Pina-Vaz C and Baltazar F:
Microbes and Cancer: Friends or Faux? Int J Mol Sci. 21:31152020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shkoporov AN and Hill C: Bacteriophages of
the Human Gut: The 'Known Unknown' of the Microbiome. Cell Host
Microbe. 25:195–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poore GD, Kopylova E, Zhu Q, Carpenter C,
Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ,
et al: Microbiome analyses of blood and tissues suggest cancer
diagnostic approach. Nature. 579:567–574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narunsky-Haziza L, Sepich-Poore GD,
Livyatan I, Asraf O, Martino C, Nejman D, Gavert N, Stajich JE,
Amit G, González A, et al: Pan-cancer analyses reveal
cancer-type-specific fungal ecologies and bacteriome interactions.
Cell. 185:3789–3806 e17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dohlman AB, Klug J, Mesko M, Gao IH,
Lipkin SM, Shen X and Iliev ID: A pan-cancer mycobiome analysis
reveals fungal involvement in gastrointestinal and lung tumors.
Cell. 185:3807–3822 e12. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu NN, Jiao N, Tan JC, Wang Z, Wu D, Wang
AJ, Chen J, Tao L, Zhou C, Fang W, et al: Multi-kingdom microbiota
analyses identify bacterial-fungal interactions and biomarkers of
colorectal cancer across cohorts. Nat Microbiol. 7:238–250. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang M, Yu F and Li P: Intratumor
microbiota in cancer pathogenesis and immunity: From mechanisms of
action to therapeutic opportunities. Front Immunol. 14:12690542023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nejman D, Livyatan I, Fuks G, Gavert N,
Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E,
et al: The human tumor microbiome is composed of tumor
type-specific intracellular bacteria. Science. 368:973–980. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galeano Nino JL, Wu H, LaCourse KD,
Kempchinsky AG, Baryiames A, Barber B, Futran N and Houlton J:
Effect of the intratumoral microbiota on spatial and cellular
heterogeneity in cancer. Nature. 611:810–817. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu A, Yao B, Dong T and Cai S: Emerging
roles of intratumor microbiota in cancer metastasis. Trends Cell
Biol. 33:583–593. 2023. View Article : Google Scholar
|
|
19
|
Zong Z, Zhou F and Zhang L: The fungal
mycobiome: a new hallmark of cancer revealed by pan-cancer
analyses. Signal Transduct Target Ther. 8:502023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luan C, Xie L, Yang X, Miao H, Lv N, Zhang
R, Xiao X, Hu Y, Liu Y, Wu N, et al: Dysbiosis of fungal microbiota
in the intestinal mucosa of patients with colorectal adenomas. Sci
Rep. 5:79802015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Y, Lau HC, Liu Y, Kang X, Wang Y, Ting
NL, Kwong TN, Han J, Liu W, Liu C, et al: Altered mycobiota
signatures and enriched pathogenic aspergillus rambellii are
associated with colorectal cancer based on multicohort fecal
metagenomic analyses. Gastroenterology. 163:908–921. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong
SH, Ng SC, Chan FKL, Sung JJY and Yu J: Enteric fungal microbiota
dysbiosis and ecological alterations in colorectal cancer. Gut.
68:654–662. 2019. View Article : Google Scholar
|
|
23
|
Aykut B, Pushalkar S, Chen R, Li Q,
Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, et al:
The fungal mycobiome promotes pancreatic oncogenesis via activation
of MBL. Nature. 574:264–267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banerjee S, Tian T, Wei Z, Shih N, Feldman
MD, Peck KN, DeMichele AM, Alwine JC and Robertson ES: Distinct
microbial signatures associated with different breast cancer types.
Front Microbiol. 9:9512018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banerjee S, Alwine JC, Wei Z, Tian T, Shih
N, Sperling C, Guzzo T, Feldman MD and Robertson ES: Microbiome
signatures in prostate cancer. Carcinogenesis. 40:749–764. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Banerjee S, Tian T, Wei Z, Shih N, Feldman
MD, Alwine JC, Coukos G and Robertson ES: The ovarian cancer
oncobiome. Oncotarget. 8:36225–36245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu F, Willette-Brown J, Song NY, Lomada
D, Song Y, Xue L, Gray Z, Zhao Z, Davis SR, Sun Z, et al:
Autoreactive T cells and chronic fungal infection drive esophageal
carcinogenesis. Cell Host Microbe. 21:478–493 e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao R, Kong C, Li H, Huang L, Qu X, Qin N
and Qin H: Dysbiosis signature of mycobiota in colon polyp and
colorectal cancer. Eur J Clin Microbiol Infect Dis. 36:2457–2468.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alnuaimi AD, Wiesenfeld D, O'Brien-Simpson
NM, Reynolds EC, Peng B and McCullough MJ: The development and
validation of a rapid genetic method for species identification and
genotyping of medically important fungal pathogens using
high-resolution melting curve analysis. Mol Oral Microbiol.
29:117–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li JQ, Li JL, Xie YH, Wang Y, Shen XN,
Qian Y, Han JX, Chen YX and Fang JY: Saccharomyces cerevisiae may
serve as a probiotic in colorectal cancer by promoting cancer cell
apoptosis. J Dig Dis. 21:571–582. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Zheng Y, Chen Y, Yin Y, Chen Y,
Chen Q, Hou Y, Shen S, Lv M and Wang T: Gut fungi enhances
immunosuppressive function of myeloid-derived suppressor cells by
activating PKM2-dependent glycolysis to promote colorectal
tumorigenesis. Exp Hematol Oncol. 11:882022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:41022020. View Article : Google Scholar
|
|
34
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542. 2020.
View Article : Google Scholar
|
|
35
|
Zhong M, Xiong Y, Zhao J, Gao Z, Ma J, Wu
Z, Song Y and Hong X: Candida albicans disorder is associated with
gastric carcinogenesis. Theranostics. 11:4945–4956. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vallianou N, Kounatidis D, Christodoulatos
GS, Panagopoulos F, Karampela I and Dalamaga M: Mycobiome and
Cancer: What is the evidence? Cancers (Basel). 13:31492021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Chen C, Chai D, Li C, Qiu Z,
Kuang T, Liu L, Deng W and Wang W: Characterization of the
intestinal fungal microbiome in patients with hepatocellular
carcinoma. J Transl Med. 21:1262023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Del Castillo E, Meier R, Chung M, Koestler
DC, Chen T, Paster BJ, Charpentier KP, Kelsey KT, Izard J and
Michaud DS: The microbiomes of pancreatic and duodenum tissue
overlap and are highly subject specific but differ between
pancreatic cancer and noncancer subjects. Cancer Epidemiol
Biomarkers Prev. 28:370–383. 2019. View Article : Google Scholar
|
|
39
|
Vitiello GA, Cohen DJ and Miller G:
Harnessing the microbiome for pancreatic cancer immunotherapy.
Trends Cancer. 5:670–676. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Y, Yi J, Xiang J, Jia W, Chen A, Chen
L, Zheng L, Zhou W, Wu M, Yu Z and Tang J: Exploration of lung
mycobiome in the patients with non-small-cell lung cancer. BMC
Microbiol. 23:812023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perera M, Al-Hebshi NN, Perera I, Ipe D,
Ulett GC, Speicher DJ, Chen T and Johnson NW: A dysbiotic mycobiome
dominated by Candida albicans is identified within oral
squamous-cell carcinomas. J Oral Microbiol. 9:13853692017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Navarro-Arias MJ, Hernández-Chávez MJ,
García-Carnero LC, Amezcua-Hernández DG, Lozoya-Pérez NE,
Estrada-Mata E, Martínez-Duncker I, Franco B and Mora-Montes HM:
Differential recognition of Candida tropicalis, Candida
guilliermondii, Candida krusei, and Candida auris by human innate
immune cells. Infect Drug Resist. 12:783–794. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang F, Syrjänen S, Wang L and Syrjänen
K: Infectious agents in the etiology of esophageal cancer.
Gastroenterology. 103:1336–1348. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang CS: Research on esophageal cancer in
China: A review. Cancer Res. 40(8 Pt 1): 2633–2644. 1980.PubMed/NCBI
|
|
45
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T and Akagi M: Characterizing inflammatory markers in highly
aggressive soft tissue sarcomas. Medicine (Baltimore).
101:e306882022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu X, Jiang B, Hao H and Liu Z: CARD9
Signaling, inflammation, and diseases. Front Immunol.
13:8808792022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bergmann H, Roth S, Pechloff K, Kiss EA,
Kuhn S, Heikenwälder M, Diefenbach A, Greten FR and Ruland J:
Card9-dependent IL-1β regulates IL-22 production from group 3
innate lymphoid cells and promotes colitis-associated cancer. Eur J
Immunol. 47:1342–1353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Glocker EO, Hennigs A, Nabavi M, Schäffer
AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami
F, et al: A homozygous CARD9 mutation in a family with
susceptibility to fungal infections. N Engl J Med. 361:1727–1735.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Leone RD and Powell JD: Metabolism of
immune cells in cancer. Nat Rev Cancer. 20:516–531. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Deng Y, Yang J, Luo F, Qian J, Liu R,
Zhang D, Yu H and Chu Y: mTOR-mediated glycolysis contributes to
the enhanced suppressive function of murine tumor-infiltrating
monocytic myeloid-derived suppressor cells. Cancer Immunol
Immunother. 67:1355–1364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reinfeld BI, Madden MZ, Wolf MM, Chytil A,
Bader JE, Patterson AR, Sugiura A, Cohen AS, Ali A, Do BT, et al:
Cell-programmed nutrient partitioning in the tumour
microenvironment. Nature. 593:282–288. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhu Y, Shi T, Lu X, Xu Z, Qu J, Zhang Z,
Shi G, Shen S, Hou Y, Chen Y and Wang T: Fungal-induced glycolysis
in macrophages promotes colon cancer by enhancing innate lymphoid
cell secretion of IL-22. EMBO J. 40:e1053202021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
De Monte L, Reni M, Tassi E, Clavenna D,
Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C and Protti MP:
Intratumor T helper type 2 cell infiltrate correlates with
cancer-associated fibroblast thymic stromal lymphopoietin
production and reduced survival in pancreatic cancer. J Exp Med.
208:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang X, Wu S, Wu W, Zhang W, Li L, Liu Q
and Yan Z: Candida albicans promotes oral cancer via
IL-17A/IL-17RA-Macrophage axis. mBio. 14:e00447232023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xia J, Ding H, Liu S, An R, Shi X, Chen M
and Ren H: C-Type lectin receptors-triggered antifungal immunity
may synergize with and optimize the effects of immunotherapy in
hepatocellular carcinoma. J Inflamm Res. 16:19–33. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Erendor F, Sahin EO, Sanlioglu AD, Balci
MK, Griffith TS and Sanlioglu S: Lentiviral gene therapy vectors
encoding VIP suppressed diabetes-related inflammation and augmented
pancreatic beta-cell proliferation. Gene Ther. 28:130–141. 2021.
View Article : Google Scholar
|
|
57
|
Gainza-Cirauqui ML, Nieminen MT, Novak
Frazer L, Aguirre-Urizar JM, Moragues MD and Rautemaa R: Production
of carcinogenic acetaldehyde by Candida albicans from patients with
potentially malignant oral mucosal disorders. J Oral Pathol Med.
42:243–249. 2013. View Article : Google Scholar
|
|
58
|
Smith MT, Guyton KZ, Gibbons CF, Fritz JM,
Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavlock RJ, Lambert
PF, et al: Key characteristics of carcinogens as a basis for
organizing data on mechanisms of carcinogenesis. Environ Health
Perspect. 124:713–721. 2016. View Article : Google Scholar :
|
|
59
|
Rushing BR and Selim MI: Aflatoxin B1: A
review on metabolism, toxicity, occurrence in food, occupational
exposure, and detoxification methods. Food Chem Toxicol.
124:81–100. 2019. View Article : Google Scholar
|
|
60
|
Johnson CH, Dejea CM, Edler D, Hoang LT,
Santidrian AF, Felding BH, Ivanisevic J, Cho K, Wick EC,
Hechenbleikner EM, et al: Metabolism links bacterial biofilms and
colon carcinogenesis. Cell Metab. 21:891–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hold GL and Allen-Vercoe E: Gut microbial
biofilm composition and organisation holds the key to CRC. Nat Rev
Gastroenterol Hepatol. 16:329–330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tomkovich S, Dejea CM, Winglee K, Drewes
JL, Chung L, Housseau F, Pope JL, Gauthier J, Sun X, Mühlbauer M,
et al: Human colon mucosal biofilms from healthy or colon cancer
hosts are carcinogenic. J Clin Invest. 129:1699–1712. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Garcia-Ceron D, Bleackley MR and Anderson
MA: Fungal extracellular vesicles in pathophysiology. Subcell
Biochem. 97:151–177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Freitas MS, Bonato VLD, Pessoni AM,
Rodrigues ML, Casadevall A and Almeida F: Fungal extracellular
vesicles as potential targets for immune interventions. mSphere.
4:e00747–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rodrigues ML and Casadevall A: A two-way
road: Novel roles for fungal extracellular vesicles. Mol Microbiol.
110:11–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Freitas MS, Bitencourt TA, Rezende CP,
Martins NS, Dourado TMH, Tirapelli CR and Almeida F: Aspergillus
fumigatus extracellular vesicles display increased galleria
mellonella survival but partial pro-inflammatory response by
macrophages. J Fungi (Basel). 9:5412023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vargas G, Rocha JD, Oliveira DL,
Albuquerque PC, Frases S, Santos SS, Nosanchuk JD, Gomes AM,
Medeiros LC, Miranda K, et al: Compositional and immunobiological
analyses of extracellular vesicles released by Candida albicans.
Cell Microbiol. 17:389–407. 2015. View Article : Google Scholar
|
|
68
|
Bielska E, Sisquella MA, Aldeieg M, Birch
C, O'Donoghue EJ and May RC: Pathogen-derived extracellular
vesicles mediate virulence in the fatal human pathogen Cryptococcus
gattii. Nat Commun. 9:15562018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hamad I, Ranque S, Azhar EI, Yasir M,
Jiman-Fatani AA, Tissot-Dupont H, Raoult D and Bittar F:
Culturomics and amplicon-based metagenomic approaches for the study
of fungal population in human gut microbiota. Sci Rep. 7:167882017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Leong C, Schmid B, Toi MJ, Wang J,
Irudayaswamy AS, Goh JPZ, Bosshard PP, Glatz M and Dawson TL Jr:
Geographical and ethnic differences influence culturable commensal
yeast diversity on healthy skin. Front Microbiol. 10:18912019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen Y, Chen Z, Guo R, Chen N, Lu H, Huang
S, Wang J and Li L: Correlation between gastrointestinal fungi and
varying degrees of chronic hepatitis B virus infection. Diagn
Microbiol Infect Dis. 70:492–498. 2011. View Article : Google Scholar
|
|
72
|
Proctor DM, Drummond RA, Lionakis MS and
Segre JA: One population, multiple lifestyles: Commensalism and
pathogenesis in the human mycobiome. Cell Host Microbe. 31:539–553.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tsui C, Kong EF and Jabra-Rizk MA:
Pathogenesis of Candida albicans biofilm. Pathog Dis.
74:ftw0182016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li XV, Leonardi I, Putzel GG, Semon A,
Fiers WD, Kusakabe T, Lin WY, Gao IH, Doron I, Gutierrez-Guerrero
A, et al: Immune regulation by fungal strain diversity in
inflammatory bowel disease. Nature. 603:672–678. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Seelbinder B, Lohinai Z, Vazquez-Uribe R,
Brunke S, Chen X, Mirhakkak M, Lopez-Escalera S, Dome B,
Megyesfalvi Z, Berta J, et al: Candida expansion in the gut of lung
cancer patients associates with an ecological signature that
supports growth under dysbiotic conditions. Nat Commun.
14:26732023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zeise KD, Woods RJ and Huffnagle GB:
Interplay between Candida albicans and lactic acid bacteria in the
gastrointestinal tract: Impact on colonization resistance,
microbial carriage, opportunistic infection, and host immunity.
Clin Microbiol Rev. 34:e00323202021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
MacAlpine J, Daniel-Ivad M, Liu Z, Yano J,
Revie NM, Todd RT, Stogios PJ, Sanchez H, O'Meara TR, Tompkins TA,
et al: A small molecule produced by Lactobacillus species blocks
Candida albicans filamentation by inhibiting a DYRK1-family kinase.
Nat Commun. 12:61512021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fan D, Coughlin LA, Neubauer MM, Kim J,
Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hoope LV and Koh AY:
Activation of HIF-1α and LL-37 by commensal bacteria inhibits
Candida albicans colonization. Nat Med. 21:808–814. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Boutin RC, Petersen C, Woodward SE,
Serapio-Palacios A, Bozorgmehr T, Loo R, Chalanuchpong A, Cirstea
M, Lo B, Huus KE, et al: Bacterial-fungal interactions in the
neonatal gut influence asthma outcomes later in life. Elife.
10:e677402021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nash AK, Auchtung TA, Wong MC, Smith DP,
Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, et
al: The gut mycobiome of the Human Microbiome Project healthy
cohort. Microbiome. 5:1532017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gonzalez-Orozco BD, Kosmerl E,
Jiménez-Flores R and Alvarez VB: Enhanced probiotic potential of
Lactobacillus kefiranofaciens OSU-BDGOA1 through co-culture with
Kluyveromyces marxianus bdgo-ym6. Front Microbiol. 14:12366342023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zeng X, Jia H, Shi Y, Chen K, Wang Z, Gao
Z, Yuan Y and Yue T: Lactobacillus kefiranofaciens JKSP109 and
Saccharomyces cerevisiae JKSP39 isolated from Tibetan kefir grain
co-alleviated AOM/DSS induced inflammation and colorectal
carcinogenesis. Food Funct. 13:6947–6961. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rao C, Coyte KZ, Bainter W, Geha RS,
Martin CR and Rakoff-Nahoum S: Multi-kingdom ecological drivers of
microbiota assembly in preterm infants. Nature. 591:633–638. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hoft MA, Hoving JC and Brown GD: Signaling
C-Type lectin receptors in antifungal immunity. Curr Top Microbiol
Immunol. 429:63–101. 2020.PubMed/NCBI
|
|
86
|
Hatinguais R, Willment JA and Brown GD:
PAMPs of the fungal cell wall and mammalian PRRs. Curr Top
Microbiol Immunol. 425:187–223. 2020.PubMed/NCBI
|
|
87
|
Witchley JN, Penumetcha P, Abon NV,
Woolford CA, Mitchell AP and Noble SM: Candida albicans
Morphogenesis Programs Control the Balance between Gut Commensalism
and Invasive Infection. Cell Host Microbe. 25:432–443 e6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pande K, Chen C and Noble SM: Passage
through the mammalian gut triggers a phenotypic switch that
promotes Candida albicans commensalism. Nat Genet. 45:1088–1091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen C, Pande K, French SD, Tuch BB and
Noble SM: An iron homeostasis regulatory circuit with reciprocal
roles in Candida albicans commensalism and pathogenesis. Cell Host
Microbe. 10:118–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Day AM, McNiff MM, da Silva Dantas A, Gow
NAR and Quinn J: Hog1 regulates stress tolerance and virulence in
the emerging fungal pathogen Candida auris. mSphere. 3:e00506–18.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Deorukhkar SC, Saini S and Mathew S:
Non-albicans Candida Infection: An emerging threat. Interdiscip
Perspect Infect Dis. 2014:6159582014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Moyes DL, Wilson D, Richardson JP,
Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J,
Runglall M, et al: Candidalysin is a fungal peptide toxin critical
for mucosal infection. Nature. 532:64–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gao J, Chow EWL, Wang H, Xu X, Cai C, Song
Y, Wang J and Wang Y: LncRNA DINOR is a virulence factor and global
regulator of stress responses in Candida auris. Nat Microbiol.
6:842–851. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Boutin RCT, Sbihi H, McLaughlin RJ, Hahn
AS, Konwar KM, Loo RS, Dai D, Petersen C, Brinkman FSL, Winsor GL,
et al: Composition and associations of the infant gut fungal
microbiota with environmental factors and childhood allergic
outcomes. mBio. 12:e03396202021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yamaguchi N, Sonoyama K, Kikuchi H, Nagura
T, Aritsuka T and Kawabata J: Gastric colonization of Candida
albicans differs in mice fed commercial and purified diets. J Nutr.
135:109–115. 2005. View Article : Google Scholar
|
|
96
|
Robbins J, Passmore GM, Abogadie FC,
Reilly JM and Brown DA: Effects of KCNQ2 gene truncation on M-type
Kv7 potassium currents. PLoS One. 8:e718092013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Goncalves B, Ferreira C, Alves CT,
Henriques M, Azeredo J and Silva S: Vulvovaginal candidiasis:
Epidemiology, microbiology and risk factors. Crit Rev Microbiol.
42:905–927. 2016. View Article : Google Scholar
|
|
98
|
Seelbinder B, Chen J, Brunke S,
Vazquez-Uribe R, Santhaman R, Meyer AC, de Oliveira Lino FS, Chan
KF, Loos D, Imamovic L, et al: Antibiotics create a shift from
mutualism to competition in human gut communities with a
longer-lasting impact on fungi than bacteria. Microbiome.
8:1332020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhai B, Ola M, Rolling T, Tosini NL,
Joshowitz S, Littmann ER, Amoretti LA, Fontana E, Wright RJ,
Miranda E, et al: High-resolution mycobiota analysis reveals
dynamic intestinal translocation preceding invasive candidiasis.
Nat Med. 26:59–64. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chandra D, Selvanesan BC, Yuan Z, Libutti
SK, Koba W, Beck A, Zhu K, Casadevall A, Dadachova E and Gravekamp
C: 32-Phosphorus selectively delivered by listeria to pancreatic
cancer demonstrates a strong therapeutic effect. Oncotarget.
8:20729–20740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sepich-Poore GD, Zitvogel L, Straussman R,
Hasty J, Wargo JA and Knight R: The microbiome and human cancer.
Science. 371:eabc45522021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dhankhar R, Gupta V, Kumar S, Kapoor RK
and Gulati P: Microbial enzymes for deprivation of amino acid
metabolism in malignant cells: Biological strategy for cancer
treatment. Appl Microbiol Biotechnol. 104:2857–2869. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Halley A, Leonetti A, Gregori A, Tiseo M,
Deng DM, Giovannetti E and Peters GJ: The role of the microbiome in
cancer and therapy efficacy: Focus on lung cancer. Anticancer Res.
40:4807–4818. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Brandi G, Turroni S, McAllister F and
Frega G: The human microbiomes in pancreatic cancer: Towards
evidence-based manipulation strategies? Int J Mol Sci. 22:99142021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Fazzino L, Anisman J, Chacón JM and
Harcombe WR: Phage cocktail strategies for the suppression of a
pathogen in a cross-feeding coculture. Microb Biotechnol.
13:1997–2007. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wong CC and Yu J: Gut microbiota in
colorectal cancer development and therapy. Nat Rev Clin Oncol.
20:429–452. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Elkrief A, Derosa L, Kroemer G, Zitvogel L
and Routy B: The negative impact of antibiotics on outcomes in
cancer patients treated with immunotherapy: A new independent
prognostic factor? Ann Oncol. 30:1572–1579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mayne ST, Playdon MC and Rock CL: Diet
nutrition, and cancer: Past present and future. Nat Rev Clin Oncol.
13:504–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
David LA, Maurice CF, Carmody RN,
Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y,
Fischbach MA, et al: Diet rapidly and reproducibly alters the human
gut microbiome. Nature. 505:559–563. 2014. View Article : Google Scholar :
|
|
110
|
Roy S and Dhaneshwar S: Role of
prebiotics, probiotics, and synbiotics in management of
inflammatory bowel disease: Current perspectives. World J
Gastroenterol. 29:2078–2100. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Clark MJ, Robien K and Slavin JL: Effect
of prebiotics on biomarkers of colorectal cancer in humans: A
systematic review. Nutr Rev. 70:436–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Canale FP, Basso C, Antonini G, Perotti M,
Li N, Sokolovska A, Neumann J, James MJ, Geiger S, Jin W, et al:
Metabolic modulation of tumours with engineered bacteria for
immunotherapy. Nature. 598:662–666. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Geiger R, Rieckmann JC, Wolf T, Basso C,
Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et
al: L-Arginine Modulates T cell metabolism and enhances survival
and anti-tumor activity. Cell. 167:829–842 e13. 2016. View Article : Google Scholar : PubMed/NCBI
|