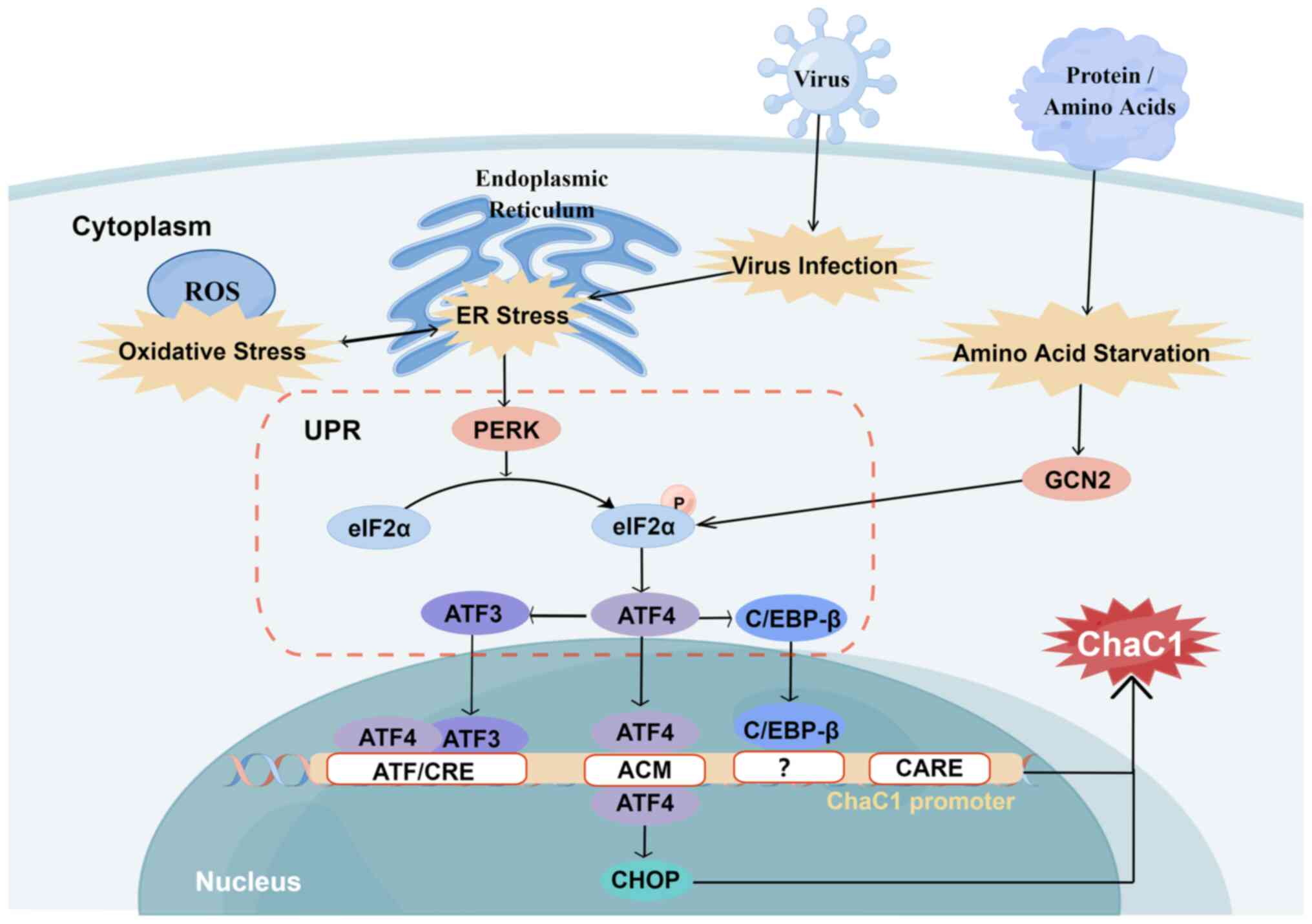
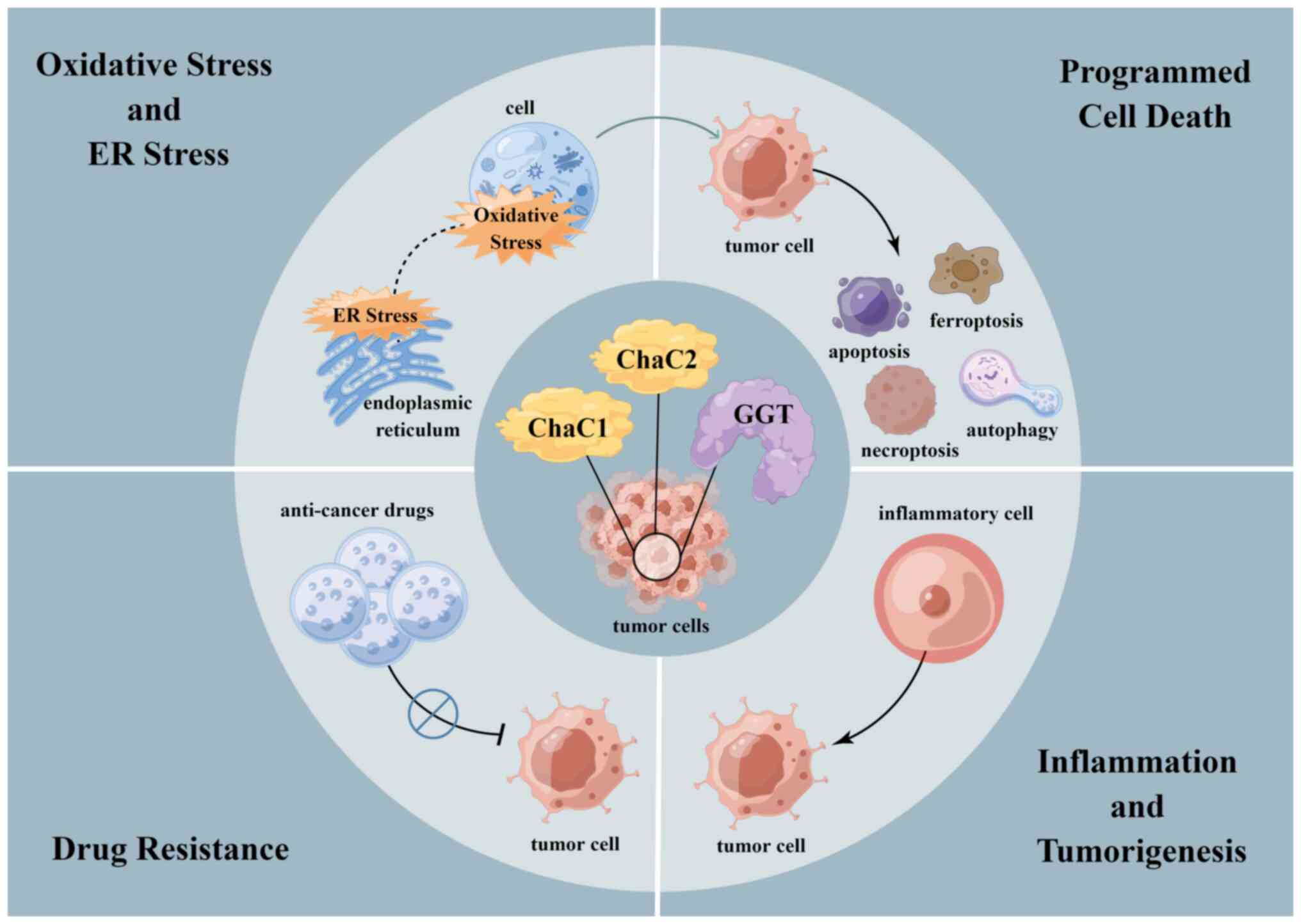
|
1
|
Lushchak VI: Glutathione homeostasis and
functions: potential targets for medical interventions. J Amino
Acids. 2012:7368372012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar :
|
|
3
|
Kennedy L, Sandhu JK, Harper ME and
Cuperlovic-Culf M: Role of glutathione in cancer: From mechanisms
to therapies. Biomolecules. 10:14292020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bansal A and Simon MC: Glutathione
metabolism in cancer progression and treatment resistance. J Cell
Biol. 217:2291–2298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernández-Checa J, Hirano T, Tsukamoto H
and Kaplowitz N: Mitochondrial glutathione depletion in alcoholic
liver disease. Alcohol. 10:469–475. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guarino MP, Afonso RA, Raimundo N, Raposo
JF and Macedo MP: Hepatic glutathione and nitric oxide are critical
for hepatic insulin-sensitizing substance action. Am J Physiol
Gastrointest Liver Physiol. 284:G588–G594. 2003. View Article : Google Scholar
|
|
7
|
Mandal PK, Roy RG and Samkaria A:
Oxidative stress: Glutathione and its potential to protect
methionine-35 of abeta peptide from oxidation. ACS Omega.
7:27052–27061. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charisis S, Ntanasi E, Stamelou M,
Xiromerisiou G, Maraki M, Veskoukis AS, Yannakoulia M, Kosmidis MH,
Anastasiou CA, Giagkou N, et al: Plasma glutathione and prodromal
parkinson's disease probability. Mov Disord. 37:200–205. 2022.
View Article : Google Scholar
|
|
9
|
Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan
X and Wu C: Application of glutathione depletion in cancer therapy:
Enhanced ROS-based therapy, ferroptosis, and chemotherapy.
Biomaterials. 277:1211102021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bachhawat AK and Yadav S: The glutathione
cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB
Life. 70:585–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bachhawat AK and Kaur A: Glutathione
degradation. Antioxid Redox Signal. 27:1200–1216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mungrue I, Pagnon J, Kohannim O,
Gargalovic P and Lusis A: CHAC1/MGC4504 is a novel proapoptotic
component of the unfolded protein response, downstream of the
ATF4-ATF3-CHOP cascade. J Immunol. 182:466–476. 2009. View Article : Google Scholar
|
|
13
|
Kumar A, Tikoo S, Maity S, Sengupta S,
Sengupta S, Kaur A and Bachhawat AK: Mammalian proapoptotic factor
ChaC1 and its homologues function as γ-glutamyl cyclotransferases
acting specifically on glutathione. EMBO Rep. 13:1095–1101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Ma S, Zhang J, Lou L, Liu W, Gao
C, Miao L, Sun F, Chen W, Cao X and Wei J: MicroRNA-142-3p promotes
renal cell carcinoma progression by targeting RhoBTB3 to regulate
HIF-1 signaling and GGT/GSH pathways. Sci Rep. 13:59352023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Li W, Ma Y, Zhao X, He L, Sun P
and Wang H: High-fat diet aggravates colitis-associated
carcinogenesis by evading ferroptosis in the ER stress-mediated
pathway. Free Radic Biol Med. 177:156–166. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alborzinia H, Flórez AF, Kreth S, Brückner
LM, Yildiz U, Gartlgruber M, Odoni DI, Poschet G, Garbowicz K, Shao
C, et al: MYCN mediates cysteine addiction and sensitizes
neuroblastoma to ferroptosis. Nat Cancer. 3:471–485. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stepulak A, Rola R, Polberg K and
Ikonomidou C: Glutamate and its receptors in cancer. J Neural
Transm (Vienna). 121:933–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Locasale JW: Serine, glycine and
one-carbon units: Cancer metabolism in full circle. Nat Rev Cancer.
13:572–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cole SP and Deeley RG: Transport of
glutathione and glutathione conjugates by MRP1. Trends Pharmacol
Sci. 27:438–446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen YTK, Park JS, Jang JY, Kim KR, Vo
TTL, Kim KW and Han BW: Structural and functional analyses of human
ChaC2 in glutathione metabolism. Biomolecules. 10:312019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orlowski M and Meister A: The
gamma-glutamyl cycle: A possible transport system for amino acids.
Proc Natl Acad Sci USA. 67:1248–1255. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanigan MH: Gamma-glutamyl transpeptidase:
Redox regulation and drug resistance. Adv Cancer Res. 122:103–141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verma VV, Gupta R and Goel M: Phylogenetic
and evolutionary analysis of functional divergence among gamma
glutamyl transpeptidase (GGT) subfamilies. Biol Direct. 10:492015.
View Article : Google Scholar
|
|
24
|
Mistry D and Stockley RA: Gamma-glutamyl
transferase: The silent partner? COPD. 7:285–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanigan MH and Frierson HF Jr:
Immunohistochemical detection of gamma-glutamyl transpeptidase in
normal human tissue. J Histochem Cytochem. 44:1101–1108. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Figlewicz DA, Delattre O, Guellaen G,
Krizus A, Thomas G, Zucman J and Rouleau GA: Mapping of human
gamma-glutamyl transpeptidase genes on chromosome 22 and other
human autosomes. Genomics. 17:299–305. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morris C, Courtay C, van Kessel AG, ten
Hoeve J, Heisterkamp N and Groffen J: Localization of a
gamma-glutamyl-transferase-related gene family on chromosome 22.
Hum Genet. 91:31–36. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heisterkamp N, Groffen J, Warburton D and
Sneddon TP: The human gamma-glutamyltransferase gene family. Hum
Genet. 123:321–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wickham S, West MB, Cook PF and Hanigan
MH: Gamma-glutamyl compounds: Substrate specificity of
gamma-glutamyl transpeptidase enzymes. Anal Biochem. 414:208–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mottaghitalab F, Lanjanian H and
Masoudi-Nejad A: Revealing transcriptional and post-transcriptional
regulatory mechanisms of γ-glutamyl transferase and keratin
isoforms as novel cooperative biomarkers in low-grade glioma and
glioblastoma multiforme. Genomics. 113:2623–2633. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Zhang L, Chan FKL, Ji J, Yu J and
Liang JQ: Gamma-glutamyltransferase 7 suppresses gastric cancer by
cooperating with RAB7 to induce mitophagy. Oncogene. 41:3485–3497.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian S, Li J, Guo Y, Dong W and Zheng X:
Expression status and prognostic significance of gamma-glutamyl
transpeptidase family genes in hepatocellular carcinoma. Front
Oncol. 11:7311442021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samgina TA and Lazarenko VA: The role of
polymorphic variants rs11546155 and rs6119534 of the GGT7 gene and
risk factors in the development of acute pancreatitis. Vopr Pitan.
91:43–50. 2022.In Russian.
|
|
34
|
West MB, Wickham S, Parks EE, Sherry DM
and Hanigan MH: Human GGT2 does not autocleave into a functional
enzyme: A cautionary tale for interpretation of microarray data on
redox signaling. Antioxid Redox Signal. 19:1877–1888. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moon DO, Kim BY, Jang JH, Kim MO,
Jayasooriya RG, Kang CH, Choi YH, Moon SK, Kim WJ, Ahn JS and Kim
GY: K-RAS transformation in prostate epithelial cell overcomes
H2O2-induced apoptosis via upregulation of
gamma-glutamyltransferase-2. Toxicol In Vitro. 26:429–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Courtay C, Heisterkamp N, Siest G and
Groffen J: Expression of multiple gamma-glutamyltransferase genes
in man. Biochem J. 297:503–508. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taniguchi N and Ikeda Y: Gamma-glutamyl
transpeptidase: Catalytic mechanism and gene expression. Adv
Enzymol Relat Areas Mol Biol. 72:239–278. 1998.PubMed/NCBI
|
|
38
|
Zhang H and Forman HJ: Redox regulation of
gamma-glutamyl transpeptidase. Am J Respir Cell Mol Biol.
41:509–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Daubeuf S, Duvoix A, Wellman-Rousseau M,
Diederich M and Visvikis A: Phorbol ester regulation of the human
gamma-glutamyltransferase gene promoter. Biochem Biophys Res
Commun. 313:300–307. 2004. View Article : Google Scholar
|
|
40
|
Pawlak A, Lahuna O, Bulle F, Suzuki A,
Ferry N, Siegrist S, Chikhi N, Chobert MN, Guellaen G and Laperche
Y: Gamma-glutamyl transpeptidase: A single copy gene in the rat and
a multigene family in the human genome. J Biol Chem. 263:9913–9916.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pandur S, Pankiv S, Johannessen M, Moens U
and Huseby NE: Gamma-glutamyltransferase is upregulated after
oxidative stress through the Ras signal transduction pathway in rat
colon carcinoma cells. Free Radic Res. 41:1376–1384. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Liu H, Iles KE, Liu RM,
Postlethwait EM, Laperche Y and Forman HJ: 4-Hydroxynonenal induces
rat gamma-glutamyl transpeptidase through mitogen-activated protein
kinase-mediated electrophile response element/nuclear factor
erythroid 2-related factor 2 signaling. Am J Respir Cell Mol Biol.
34:174–181. 2006. View Article : Google Scholar
|
|
44
|
Mussbacher M, Derler M, Basilio J and
Schmid JA: NF-κB in monocytes and macrophages-an inflammatory
master regulator in multitalented immune cells. Front Immunol.
14:11346612023. View Article : Google Scholar
|
|
45
|
Yao C, Ren J, Huang R, Tang C, Cheng Y, Lv
Z, Kong L, Fang S, Tao J, Fu Y, et al: Transcriptome profiling of
microRNAs reveals potential mechanisms of manual therapy
alleviating neuropathic pain through microRNA-547-3p-mediated
Map4k4/NF-κb signaling pathway. J Neuroinflammation. 19:2112022.
View Article : Google Scholar
|
|
46
|
Reuter S, Schnekenburger M, Cristofanon S,
Buck I, Teiten MH, Daubeuf S, Eifes S, Dicato M, Aggarwal BB,
Visvikis A and Diederich M: Tumor necrosis factor alpha induces
gamma-glutamyltransferase expression via nuclear factor-kappaB in
cooperation with Sp1. Biochem Pharmacol. 77:397–411. 2009.
View Article : Google Scholar
|
|
47
|
Kaur A, Gautam R, Srivastava R, Chandel A,
Kumar A, Karthikeyan S and Bachhawat AK: ChaC2, an enzyme for slow
turnover of cytosolic glutathione. J Biol Chem. 292:638–651. 2017.
View Article : Google Scholar :
|
|
48
|
Crawford RR, Prescott ET, Sylvester CF,
Higdon AN, Shan J, Kilberg MS and Mungrue IN: Human CHAC1 protein
degrades glutathione, and mRNA induction is regulated by the
transcription factors ATF4 and ATF3 and a bipartite ATF/CRE
regulatory element. J Biol Chem. 290:15878–15891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang B, Jiang M and Zheng M: Biological
characteristics and functions of ChaC1-catalyzed clutathione
degradation in the cytoplasm. Chin J Biochem Mol Biol. 38:284–289.
2022.
|
|
50
|
Chand S, Mehta V, Sharma RK, Anvikar AR
and Chander H: Cancer informatics analysis indicates high CHAC2
associated with unfavorable prognosis in breast cancer. Front
Oncol. 12:10589312022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsunoda S, Avezov E, Zyryanova A, Konno T,
Mendes-Silva L, Pinho Melo E, Harding HP and Ron D: Intact protein
folding in the glutathione-depleted endoplasmic reticulum
implicates alternative protein thiol reductants. Elife.
3:e034212014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suyal S, Choudhury C and Bachhawat AK: The
ChaC1 active site: Defining the residues and determining the role
of ChaC1-exclusive residues in the structural and functional
stability. Proteins. 91:567–580. 2023. View Article : Google Scholar
|
|
53
|
Oh-Hashi K, Nomura Y, Shimada K, Koga H,
Hirata Y and Kiuchi K: Transcriptional and post-translational
regulation of mouse cation transport regulator homolog 1. Mol Cell
Biochem. 380:97–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS,
Lee HC and Tseng LM: CHAC1 degradation of glutathione enhances
cystine-starvation-induced necroptosis and ferroptosis in human
triple negative breast cancer cells via the GCN2-eIF2α-ATF4
pathway. Oncotarget. 8:114588–114602. 2017. View Article : Google Scholar
|
|
55
|
Grimm C, Hofstetter G, Aust S,
Mutz-Dehbalaie I, Bruch M, Heinze G, Rahhal-Schupp J, Reinthaller
A, Concin N and Polterauer S: Association of
gamma-glutamyltransferase with severity of disease at diagnosis and
prognosis of ovarian cancer. Br J Cancer. 109:610–614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hofbauer SL, Stangl KI, de Martino M,
Lucca I, Haitel A, Shariat SF and Klatte T: Pretherapeutic
gamma-glutamyltransferase is an independent prognostic factor for
patients with renal cell carcinoma. Br J Cancer. 111:1526–1531.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wei JR, Dong J and Li L: Cancer-associated
fibroblasts-derived gamma-glutamyltransferase 5 promotes tumor
growth and drug resistance in lung adenocarcinoma. Aging (Albany
NY). 12:13220–13233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang Y, Zhou H, Zou J and Wang D: GGT5 is
an independent prognostic biomarker in stomach adenocarcinoma. Can
J Gastroenterol Hepatol. 2022:99833512022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xiao Y, Yang H, Lu J, Li D, Xu C and Risch
HA: Serum gamma-glutamyltransferase and the overall survival of
metastatic pancreatic cancer. BMC Cancer. 19:10202019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Q, Shu X, Dong Y, Zhou J, Teng R,
Shen J, Chen Y, Dong M, Zhang W, Huang Y, et al: Tumor and serum
gamma-glutamyl transpeptidase, new prognostic and molecular
interpretation of an old biomarker in gastric cancer. Oncotarget.
8:361712017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Durham JR, Frierson HF and Hanigan MH:
Gamma-glutamyl transpeptidase immunoreactivity in benign and
malignant breast tissue. Breast Cancer Res Treat. 45:55–62. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu J, Wang X, Wang N, Ma L, Xie X, Zhang
H, Kang H and Zhou Z: Identification of novel antioxidant gene
signature to predict the prognosis of patients with gastric cancer.
World J Surg Oncol. 19:2192021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang L, Li C, Zhang Y, Zhang J and Yang
X: Ophiopogonin B induces gastric cancer cell death by blocking the
GPX4/xCT-dependent ferroptosis pathway. Oncol Lett. 23:1042022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gagliardi M, Cotella D, Santoro C, Cora D,
Barlev NA, Piacentini M and Corazzari M: Aldo-keto reductases
protect metastatic melanoma from ER stress-independent ferroptosis.
Cell Death Dis. 10:9022019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
He S, Zhang M, Ye Y, Zhuang J, Ma X, Song
Y and Xia W: ChaC glutathione specific γ-glutamylcyclotransferase 1
inhibits cell viability and increases the sensitivity of prostate
cancer cells to docetaxel by inducing endoplasmic reticulum stress
and ferroptosis. Exp Ther Med. 22:9972021. View Article : Google Scholar
|
|
66
|
Wang Z, Li M, Liu Y, Qiao Z, Bai T, Yang L
and Liu B: Dihydroartemisinin triggers ferroptosis in primary liver
cancer cells by promoting and unfolded protein response-induced
upregulation of CHAC1 expression. Oncol Rep. 46:2402021. View Article : Google Scholar :
|
|
67
|
Wang N, Zeng GZ, Yin JL and Bian ZX:
Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects
ferroptosis in Burkitt's Lymphoma. Biochem Biophys Res Commun.
519:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Joo NE, Ritchie K, Kamarajan P, Miao D and
Kapila YL: Nisin, an apoptogenic bacteriocin and food preservative,
attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 1:295–305.
2012. View Article : Google Scholar
|
|
69
|
Chen PH, Shen WL, Shih CM, Ho KH, Cheng
CH, Lin CW, Lee CC, Liu AJ and Chen KC: The CHAC1-inhibited Notch3
pathway is involved in temozolomide-induced glioma cytotoxicity.
Neuropharmacology. 116:300–314. 2017. View Article : Google Scholar
|
|
70
|
Li D, Liu S, Xu J, Chen L, Xu C, Chen F,
Xu Z, Zhang Y, Xia S, Shao Y and Wang Y: Ferroptosis-related gene
CHAC1 is a valid indicator for the poor prognosis of kidney renal
clear cell carcinoma. J Cell Mol Med. 25:3610–3621. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tseng HH, Chen YZ, Chou NH, Chen YC, Wu
CC, Liu LF, Yang YF, Yeh CY, Kung ML, Tu YT and Tsai KW: Metformin
inhibits gastric cancer cell proliferation by regulation of a novel
Loc100506691-CHAC1 axis. Mol Ther Oncolytics. 22:180–194. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xiao R, Wang S, Guo J, Liu S, Ding A, Wang
G, Li W, Zhang Y, Bian X, Zhao S and Qiu W: Ferroptosis-related
gene NOX4, CHAC1 and HIF1A are valid biomarkers for stomach
adenocarcinoma. J Cell Mol Med. 26:1183–1193. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Q, Yu M and Zhang T: Construction of
oxidative stress-related genes risk model predicts the prognosis of
uterine corpus endometrial cancer patients. Cancers (Basel).
14:55722022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mehta V, Suman P and Chander H: High
levels of unfolded protein response component CHAC1 associates with
cancer progression signatures in malignant breast cancer tissues.
Clin Transl Oncol. 24:2351–2365. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu Y, Li M, Shi D and Zhu Y: Higher
expression of cation transport regulator-like protein 1 (CHAC1)
predicts of poor outcomes in uveal melanoma (UM) patients. Int
Ophthalmol. 39:2825–2832. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu C, Liu Y, Yu Y, Zhao Y and Yu A:
Comprehensive analysis of ferroptosis-related genes and prognosis
of cutaneous melanoma. BMC Med Genomics. 15:392022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Goebel G, Berger R, Strasak AM, Egle D,
Müller-Holzner E, Schmidt S, Rainer J, Presul E, Parson W, Lang S,
et al: Elevated mRNA expression of CHAC1 splicing variants is
associated with poor outcome for breast and ovarian cancer
patients. Br J Cancer. 106:189–198. 2012. View Article : Google Scholar :
|
|
78
|
Wang CK, Yang SC, Hsu SC, Chang FP, Lin
YT, Chen SF, Cheng CL, Hsiao M, Lu FL and Lu J: CHAC2 is essential
for self-renewal and glutathione maintenance in human embryonic
stem cells. Free Radic Biol Med. 113:439–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu S, Fei W, Shi Q, Li Q, Kuang Y, Wang
C, He C and Hu X: CHAC2, downregulated in gastric and colorectal
cancers, acted as a tumor suppressor inducing apoptosis and
autophagy through unfolded protein response. Cell Death Dis.
8:e30092017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tchantchou F, Graves M, Ashline D, Morin
A, Pimenta A, Ortiz D, Rogers E and Shea TB: Increased
transcription and activity of glutathione synthase in response to
deficiencies in folate, vitamin E, and apolipoprotein E. J Neurosci
Res. 75:508–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Peng W, Wen L, Jiang R, Deng J and Chen M:
CHAC2 promotes lung adenocarcinoma by regulating ROS-mediated MAPK
pathway activation. J Cancer. 14:1309–1320. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tian Y, Lu J and Qiao Y: A
metabolism-associated gene signature for prognosis prediction of
hepatocellular carcinoma. Front Mol Biosci. 9:9883232022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lisek K, Campaner E, Ciani Y, Walerych D
and Del Sal G: Mutant p53 tunes the NRF2-dependent antioxidant
response to support survival of cancer cells. Oncotarget.
9:20508–20523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Joyce-Brady M, Jean JC and Hughey RP:
Gamma-glutamyltransferase and its isoform mediate an endoplasmic
reticulum stress response. J Biol Chem. 276:9468–9477. 2001.
View Article : Google Scholar
|
|
85
|
Hayashima K and Katoh H: Expression of
gamma-glutamyltransferase 1 in glioblastoma cells confers
resistance to cystine deprivation-induced ferroptosis. J Biol Chem.
298:1017032022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ramsay EE and Dilda PJ: Glutathione
S-conjugates as prodrugs to target drug-resistant tumors. Front
Pharmacol. 5:1812014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu Y, Hyde AS, Simpson MA and Barycki JJ:
Emerging regulatory paradigms in glutathione metabolism. Adv Cancer
Res. 122:69–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lieberman MW, Wiseman AL, Shi ZZ, Carter
BZ, Barrios R, Ou CN, Chévez-Barrios P, Wang Y, Habib GM, Goodman
JC, et al: Growth retardation and cysteine deficiency in
gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci
USA. 93:7923–7926. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Barrios R, Shi ZZ, Kala SV, Wiseman AL,
Welty SE, Kala G, Bahler AA, Ou CN and Lieberman MW: Oxygen-induced
pulmonary injury in gamma-glutamyl transpeptidase-deficient mice.
Lung. 179:319–330. 2001. View Article : Google Scholar
|
|
90
|
Rojas E, Valverde M, Kala SV, Kala G and
Lieberman MW: Accumulation of DNA damage in the organs of mice
deficient in gamma-glutamyltranspeptidase. Mutat Res. 447:305–316.
2000. View Article : Google Scholar
|
|
91
|
Darin N, Leckström K, Sikora P, Lindgren
J, Almén G and Asin-Cayuela J: γ-glutamyl transpeptidase deficiency
caused by a large homozygous intragenic deletion in GGT1. Eur J Hum
Genet. 26:808–817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Alessandro C, Maria F, Aldo P and Alfonso
P: Gamma-glutamyltransferase of cancer cells at the crossroads of
tumor progression, drug resistance and drug targeting. Anticancer
Res. 30:1169–1181. 2010.
|
|
93
|
Giommarelli C, Corti A, Supino R, Favini
E, Paolicchi A, Pompella A and Zunino F: Cellular response to
oxidative stress and ascorbic acid in melanoma cells overexpressing
gamma-glutamyltransferase. Eur J Cancer. 44:750–759. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Panis C, Victorino VJ, Herrera AC, Freitas
LF, De Rossi T, Campos FC, Simão AN, Barbosa DS, Pinge-Filho P,
Cecchini R and Cecchini AL: Differential oxidative status and
immune characterization of the early and advanced stages of human
breast cancer. Breast Cancer Res Treat. 133:881–888. 2012.
View Article : Google Scholar
|
|
95
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar :
|
|
96
|
Shi Y, Jiang B and Zhao J: Induction
mechanisms of autophagy and endoplasmic reticulum stress in
intestinal ischemia-reperfusion injury, inflammatory bowel disease,
and colorectal cancer. Biomed Pharmacother. 170:1159842024.
View Article : Google Scholar
|
|
97
|
Chen H, Xu N, Xu J, Zhang C, Li X, Xu H,
Zhu W, Li J, Liang D and Zhou W: A risk signature based on
endoplasmic reticulum stress-associated genes predicts prognosis
and immunity in pancreatic cancer. Front Mol Biosci.
10:12980772023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Joyce-Brady M, Jean JC and Hughey RP:
Gamma-glutamyltransferase and its isoform mediate an endoplasmic
reticulum stress response. J Biol Chem. 276:9468–9477. 2001.
View Article : Google Scholar
|
|
99
|
Cui Y, Zhou X, Chen L, Tang Z, Mo F, Li
XC, Mao H, Wei X, Wang C and Wang H: Crosstalk between endoplasmic
reticulum stress and oxidative stress in heat exposure-induced
apoptosis is dependent on the ATF4-CHOP-CHAC1 signal pathway in
IPEC-J2 cells. J Agric Food Chem. 69:15495–15511. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tomonobu N, Komalasari NLGY, Sumardika IW,
Jiang F, Chen Y, Yamamoto KI, Kinoshita R, Murata H, Inoue Y and
Sakaguchi M: Xylitol acts as an anticancer monosaccharide to induce
selective cancer death via regulation of the glutathione level.
Chem Biol Interact. 324:1090852020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lv H, Zhen C, Liu J, Yang P, Hu L and
Shang P: Unraveling the potential role of glutathione in multiple
forms of cell death in cancer therapy. Oxid Med Cell Longev.
2019:31501452019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wen RJ, Dong X, Zhuang HW, Pang FX, Ding
SC, Li N, Mai YX, Zhou ST, Wang JY and Zhang JF: Baicalin induces
ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4
regulatory axis. Phytomedicine. 116:1548812023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brancaccio M, Russo M, Masullo M, Palumbo
A, Russo GL and Castellano I: Sulfur-containing histidine compounds
inhibit γ-glutamyl transpeptidase activity in human cancer cells. J
Biol Chem. 294:14603–14614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bui TT, Nitta RT, Kahn SA, Razavi SM,
Agarwal M, Aujla P, Gholamin S, Recht L and Li G: γ-Glutamyl
transferase 7 is a novel regulator of glioblastoma growth. BMC
Cancer. 15:2252015. View Article : Google Scholar
|
|
107
|
Hirschhorn T and Stockwell BR: The
development of the concept of ferroptosis. Free Radic Biol Med.
133:130–143. 2019. View Article : Google Scholar :
|
|
108
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wu J, Wen T, Marzio A, Song D, Chen S,
Yang C, Zhao F, Zhang B, Zhao G, Ferri A, et al: FBXO32-mediated
degradation of PTEN promotes lung adenocarcinoma progression. Cell
Death Dis. 15:2822024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhan CH, Ding DS, Zhang W, Wang HL, Mao ZY
and Liu GJ: The cancer-testis antigen a-kinase anchor protein 3
facilitates breast cancer progression via activation of the
PTEN/PI3K/AKT/mTOR signaling. Bioengineered. 13:8478–8489. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yi J, Zhu J, Wu J, Thompson CB and Jiang
X: Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Accaoui MJ, Enoiu M, Mergny M, Masson C,
Dominici S, Wellman M and Visvikis A:
Gamma-glutamyltranspeptidase-dependent glutathione catabolism
results in activation of NF-kB. Biochem Biophys Res Commun.
276:1062–1067. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Djavaheri-Mergny M, Accaoui MJ, Rouillard
D and Wietzerbin J: Gamma-glutamyl transpeptidase activity mediates
NF-kappaB activation through lipid peroxidation in human leukemia
U937 cells. Mol Cell Biochem. 232:103–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Franzini M, Corti A, Lorenzini E,
Paolicchi A, Pompella A, De Cesare M, Perego P, Gatti L, Leone R,
Apostoli P and Zunino F: Modulation of cell growth and cisplatin
sensitivity by membrane gamma-glutamyltransferase in melanoma
cells. Eur J Cancer. 42:2623–2630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yu X and Long YC: Crosstalk between
cystine and glutathione is critical for the regulation of amino
acid signaling pathways and ferroptosis. Sci Rep. 6:300332016.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tang X, Ding CK, Wu J, Sjol J, Wardell S,
Spasojevic I, George D, McDonnell DP, Hsu DS, Chang JT and Chi JT:
Cystine addiction of triple-negative breast cancer associated with
EMT augmented death signaling. Oncogene. 36:4235–4242. 2017.
View Article : Google Scholar
|
|
117
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Galluzzi L, Kepp O, Chan FKM and Kroemer
G: Necroptosis: Mechanisms and relevance to disease. Annu Rev
Pathol. 12:103–130. 2017. View Article : Google Scholar
|
|
119
|
Zhang G, Wang J, Zhao Z, Xin T, Fan X,
Shen Q, Raheem A, Lee CR, Jiang H and Ding J: Regulated necrosis, a
proinflammatory cell death, potentially counteracts pathogenic
infections. Cell Death Dis. 13:6372022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang Z and Klionsky DJ: An overview of the
molecular mechanism of autophagy. Curr Top Microbiol Immunol.
335:1–32. 2009.PubMed/NCBI
|
|
121
|
Xu G, Wang J, Zhang Y, Chen Z and Deng R:
GGT1 suppresses the development of ferroptosis and autophagy in
mouse retinal ganglion cell through targeting GCLC. Eye Brain.
15:139–151. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sohrab SS, Raj R, Nagar A, Hawthorne S,
Paiva-Santos AC, Kamal MA, El-Daly MM, Azhar EI and Sharma A:
Chronic inflammation's transformation to cancer: A nanotherapeutic
paradigm. Molecules. 28:44132023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ying HQ, Liao YC, Luo YR, Xiong G, Huang
Y, Nie RW, Xiong CF and Cheng XX: Cancer-elicited inflammation
attenuates response and outcome in tyrosine kinase inhibitor naive
patients with advanced NSCLC. Pharmacol Res. 170:1057342021.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Carr B and Guerra V: Serum inflammation
parameters and survival in hepatocellular carcinoma patients:
Importance of albumin and gamma-glutamyltranspeptidase. Oncology.
101:313–320. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ozcelik F: Prognostic value of
gamma-glutamyl transpeptidase in liver cirrhosis and hepatocellular
cancer regardless of other parameters. Clin Res Hepatol
Gastroenterol. 45:1017082021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Singh J, Chander J, Singh S, Singh G and
Atal CK: Gamma-glutamyl transpeptidase: A novel biochemical marker
in inflammation. Biochem Pharmacol. 35:3753–3760. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ricci V, Giannouli M, Romano M and
Zarrilli R: Helicobacter pylori gamma-glutamyl transpeptidase and
its pathogenic role. World J Gastroenterol. 20:630–638. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Oertli M, Noben M, Engler DB, Semper RP,
Reuter S, Maxeiner J, Gerhard M, Taube C and Müller A: Helicobacter
pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote
gastric persistence and immune tolerance. Proc Natl Acad Sci USA.
110:3047–3052. 2013. View Article : Google Scholar
|
|
129
|
Li N, Ouyang Y, Chen S, Peng C, He C, Hong
J, Yang X, Zhu Y and Lu NH: Integrative analysis of differential
lncRNA/mRNA expression profiling in Helicobacter pylori
infection-associated gastric carcinogenesis. Front Microbiol.
11:8802020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wada Y, Takemura K, Tummala P, Uchida K,
Kitagaki K, Furukawa A, Ishige Y, Ito T, Hara Y, Suzuki T, et al:
Helicobacter pylori induces somatic mutations in TP53 via
overexpression of CHAC1 in infected gastric epithelial cells. FEBS
Open Bio. 8:671–679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ogawa T, Wada Y, Takemura K, Board PG,
Uchida K, Kitagaki K, Tamura T, Suzuki T, Tokairin Y, Nakajima Y
and Eishi Y: CHAC1 overexpression in human gastric parietal cells
with Helicobacter pylori infection in the secretory canaliculi.
Helicobacter. 24:e125982019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Perra L, Balloy V, Foussignière T,
Moissenet D, Petat H, Mungrue IN, Touqui L, Corvol H, Chignard M
and Guillot L: CHAC1 is differentially expressed in normal and
cystic fibrosis bronchial epithelial cells and regulates the
inflammatory response induced by Pseudomonas aeruginosa. Front
Immunol. 9:28232018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rousset-Jablonski C, Dalon F, Reynaud Q,
Lemonnier L, Dehillotte C, Jacoud F, Berard M, Viprey M, Van Ganse
E, Durieu I and Belhassen M: Cancer incidence and prevalence in
cystic fibrosis patients with and without a lung transplant in
France. Front Public Health. 10:10436912022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Daubeuf S, Leroy P, Paolicchi A, Pompella
A, Wellman M, Galteau MM and Visvikis A: Enhanced resistance of
HeLa cells to cisplatin by overexpression of
gamma-glutamyltransferase. Biochem Pharmacol. 64:207–216. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang C, Xu C, Gao X and Yao Q:
Platinum-based drugs for cancer therapy and anti-tumor strategies.
Theranostics. 12:2115–2132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wang L, Liu Z, He S, He S and Wang Y:
Fighting against drug-resistant tumors by the inhibition of
γ-glutamyl transferase with supramolecular platinum prodrug
nano-assemblies. J Mater Chem B. 9:4587–4595. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hanigan MH, Frierson HF Jr, Abeler VM,
Kaern J and Taylor PT Jr: Human germ cell tumours: Expression of
gamma-glutamyl transpeptidase and sensitivity to cisplatin. Br J
Cancer. 81:75–79. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chen J, Zaal EA, Berkers CR, Ruijtenbeek
R, Garssen J and Redegeld FA: Omega-3 fatty acids DHA and EPA
reduce bortezomib resistance in multiple myeloma cells by promoting
glutathione degradation. Cells. 10:22872021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yu X, He Z, Wang Z, Ke S, Wang H, Wang Q
and Li S: Brusatol hinders the progression of bladder cancer by
Chac1/Nrf2/SLC7A11 pathway. Exp Cell Res. 438:1140532024.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang X, He MJ, Chen XJ, Bai YT and Zhou G:
Glaucocalyxin A impairs tumor growth via amplification of the
ATF4/CHOP/CHAC1 cascade in human oral squamous cell carcinoma. J
Ethnopharmacol. 290:1151002022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhou H, Shen Q, Fu J, Jiang F, Wang L and
Wang Y: Analysis of lncRNA UCA1-related downstream pathways and
molecules of cisplatin resistance in lung adenocarcinoma. J Clin
Lab Anal. 34:e233122020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ha Y, Chon YE, Kim MN, Lee JH and Hwang
SG: Gamma-glutamyl transpeptidase dynamics as a biomarker for
advanced fibrosis in non-alcoholic fatty liver disease. J
Gastroenterol Hepatol. 37:1624–1632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhang S, Xu L and Xu M: Gamma-glutamyl
transpeptidase to albumin ratio holds a prognostic significance
after hepatectomy in patients with hepatocellular carcinoma and
liver cirrhosis. Asian J Surg. 46:1327–1328. 2023. View Article : Google Scholar
|
|
144
|
Yamada Y, Ishizaki M, Kido T, Honda R,
Tsuritani I, Ikai E and Yamaya H: Alcohol, high blood pressure, and
serum gamma-glutamyl transpeptidase level. Hypertension.
18:819–826. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Takemura K, Board PG and Koga F: A
systematic review of serum γ-glutamyltransferase as a prognostic
biomarker in patients with genitourinary cancer. Antioxidants
(Basel). 10:5492021. View Article : Google Scholar
|
|
146
|
Chen RQ, Zhang ZL, Jia YM, Chen RX and
Peng L: Preoperative CA19-9 and GGT ratio as a prognostic indicator
in ampullary carcinoma. BMC Gastroenterol. 23:722023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Xie H, Gao J, Sun X, Song Y, Zhang Q,
Zhang P and Ding C: A water-soluble fluorescent probe for the
determination of γ-glutamyltransferase activity and its application
in tumor imaging. Talanta. 253:1239432023. View Article : Google Scholar
|
|
148
|
Gao D, Miao Y, Ye S, Lu C, Lv G, Li K, Yu
C, Lin J and Qiu L: A fluorine-18 labeled radiotracer for PET
imaging of γ-glutamyltranspeptidase in living subjects. RSC Adv.
11:18738–18747. 2021. View Article : Google Scholar
|
|
149
|
Ou-Yang J, Li Y, Jiang WL, He SY, Liu HW
and Li CY: Fluorescence-guided cancer diagnosis and surgery by a
zero cross-talk ratiometric near-infrared γ-glutamyltranspeptidase
fluorescent probe. Anal Chem. 91:1056–1063. 2019. View Article : Google Scholar
|
|
150
|
Jin Y, Wang Z, He D, Zhu Y, Gong L, Xiao
M, Chen X and Cao K: Analysis of ferroptosis-mediated modification
patterns and tumor immune microenvironment characterization in
uveal melanoma. Front Cell Dev Biol. 9:6851202021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mehta V, Meena J, Kasana H, Munshi A and
Chander H: Prognostic significance of CHAC1 expression in breast
cancer. Mol Biol Rep. 49:8517–8526. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Jahn B, Arvandi M, Rochau U, Fiegl H,
Goebel G, Marth C and Siebert U: Development of a novel prognostic
score for breast cancer patients using mRNA expression of CHAC1. J
Comp Eff Res. 6:563–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lin D, Hu B, Zhu S and Wu Y: Exploring a
ferroptosis and oxidative stress-based prognostic model for clear
cell renal cell carcinoma. Front Oncol. 13:11314732023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen W, Lei C, Wang Y, Guo D, Zhang S,
Wang X, Zhang Z, Wang Y and Ma W: Prognostic prediction model for
glioblastoma: A ferroptosis-related gene prediction model and
independent external validation. J Clin Med. 12:13412023.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ahluwalia GS, Grem JL, Hao Z and Cooney
DA: Metabolism and action of amino acid analog anti-cancer agents.
Pharmacol Ther. 46:243–271. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Viña JR, Palacin M, Puertes IR, Hernandez
R and Viña J: Role of the gamma-glutamyl cycle in the regulation of
amino acid translocation. Am J Physiol. 257:E916–E922.
1989.PubMed/NCBI
|
|
157
|
Xie Z, Kawasaki T, Zhou H, Okuzaki D,
Okada N and Tachibana M: Targeting GGT1 eliminates the
tumor-promoting effect and enhanced immunosuppressive function of
myeloid-derived suppressor cells caused by G-CSF. Front Pharmacol.
13:8737922022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Stevens AM, Xiang M, Heppler LN, Tošić I,
Jiang K, Munoz JO, Gaikwad AS, Horton TM, Long X, Narayanan P, et
al: Atovaquone is active against AML by upregulating the integrated
stress pathway and suppressing oxidative phosphorylation. Blood
Adv. 3:4215–4227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
U Ferreira MJ: Natural product-derived
compounds for targeting multidrug resistance in cancer and
microorganisms. Int J Mol Sci. 24:143212023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ashrafizadeh M, Aref AR, Sethi G, Ertas YN
and Wang L: Natural product/diet-based regulation of macrophage
polarization: Implications in treatment of inflammatory-related
diseases and cancer. J Nutr Biochem. 1096472024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lyons SD, Sant ME and Christopherson RI:
Cytotoxic mechanisms of glutamine antagonists in mouse L1210
leukemia. J Biol Chem. 265:11377–11381. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Guan Z, Chen J, Li X and Dong N:
Tanshinone IIA induces ferroptosis in gastric cancer cells through
p53-mediated SLC7A11 down-regulation. Biosci Rep.
40:BSR202018072020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Townsend DM, Deng M, Zhang L, Lapus MG and
Hanigan MH: Metabolism of cisplatin to a nephrotoxin in proximal
tubule cells. J Am Soc Nephrol. 14:1–10. 2003. View Article : Google Scholar
|
|
164
|
Azouz AA, Abdel-Nassir Abdel-Razek E and
Abo-Youssef AM: Amlodipine alleviates cisplatin-induced
nephrotoxicity in rats through gamma-glutamyl transpeptidase (GGT)
enzyme inhibition, associated with regulation of Nrf2/HO-1,
MAPK/NF-κB, and Bax/Bcl-2 signaling. Saudi Pharm J. 28:1317–1325.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Xue Y, Lu F, Chang Z, Li J, Gao Y, Zhou J,
Luo Y, Lai Y, Cao S, Li X, et al: Intermittent dietary methionine
deprivation facilitates tumoral ferroptosis and synergizes with
checkpoint blockade. Nat Commun. 14:47582023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Sumi D, Taguchi H, Takeuchi K and
Fujishiro H: CHAC1 exacerbates arsenite cytotoxicity by lowering
intracellular glutathione levels. J Toxicol Sci. 48:487–494. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Chen S, Fan J, Xie P, Ahn J, Fernandez M,
Billingham LK, Miska J, Wu JD, Wainwright DA, Fang D, et al: CD8+ T
cells sustain antitumor response by mediating crosstalk between
adenosine A2A receptor and glutathione/GPX4. J Clin Invest.
134:e1700712024. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Xiong Y, Liu T and Chen J: Anisomycin has
the potential to induce human ovarian cancer stem cell ferroptosis
by influencing glutathione metabolism and autophagy signal
transduction pathways. J Cancer. 14:1202–1215. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liu CX, Gao Y, Xu XF, Jin X, Zhang Y, Xu
Q, Ding HX, Li BJ, Du FK, Li LC, et al: Bile acids inhibit
ferroptosis sensitivity through activating farnesoid X receptor in
gastric cancer cells. World J Gastroenterol. 30:485–498. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Mitrić A and Castellano I: Targeting
gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in
glutathione metabolism and in the control of redox homeostasis.
Free Radic Biol Med. 208:672–683. 2023. View Article : Google Scholar
|
|
171
|
Pompella A, Corti A, Paolicchi A,
Giommarelli C and Zunino F: Gamma-glutamyltransferase, redox
regulation and cancer drug resistance. Curr Opin Pharmacol.
7:360–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Bai C, Zhang M, Zhang Y, He Y, Dou H, Wang
Z, Wang Z, Li Z and Zhang L: Gamma-glutamyltransferase activity
(GGT) is a long-sought biomarker of redox status in blood
circulation: A retrospective clinical study of 44 types of human
diseases. Oxid Med Cell Longev. 2022:84940762022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Hamano M, Tomonaga S, Osaki Y, Oda H, Kato
H and Furuya S: Transcriptional activation of Chac1 and other
Atf4-target genes induced by extracellular l-serine depletion is
negated with glycine consumption in Hepa1-6 hepatocarcinoma cells.
Nutrients. 12:30182020. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Ge X, Cai Q, Zhang S, Wu X, Ying P, Ke J
and Yang Z: Treatment with paraquat affects the expression of
ferroptosis-related genes. Hum Exp Toxicol.
42:96032712311675852023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Dosumu OA, Rotimi SO, Adeleye OO, Akamo
AJ, Osinuga KT, Taiwo OA, Omotosho OO and Sani LO: Vitamin K
protects against 7,12-dimethylbenz(A)anthracene induced
hepatotoxicity in Wistar rats. Environ Toxicol. 36:362–373. 2021.
View Article : Google Scholar
|
|
176
|
Schreiber CL and Smith BD: Molecular
imaging of aminopeptidase N in cancer and angiogenesis. Contrast
Media Mol Imaging. 2018:53151722018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Amin SA, Adhikari N and Jha T: Design of
aminopeptidase N inhibitors as anti-cancer agents. J Med Chem.
61:6468–6490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Guzman-Rojas L, Rangel R, Salameh A,
Edwards JK, Dondossola E, Kim YG, Saghatelian A, Giordano RJ,
Kolonin MG, Staquicini FI, et al: Cooperative effects of
aminopeptidase N (CD13) expressed by nonmalignant and cancer cells
within the tumor microenvironment. Proc Natl Acad Sci USA.
109:1637–1642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Wang X, Shi L, Deng Y, Qu M, Mao S, Xu L,
Xu W and Fang C: Inhibition of leucine aminopeptidase 3 suppresses
invasion of ovarian cancer cells through down-regulation of fascin
and MMP-2/9. Eur J Pharmacol. 768:116–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Xu Y, Zhang N, Chen C, Xu X, Luo A, Yan Y,
Lu Y, Liu J, Ou X, Tan Y, et al: Sevoflurane induces ferroptosis of
glioma cells through activating the ATF4-CHAC1 pathway. Front
Oncol. 12:8596212022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wen YF, Yang XZ, Zeng LS, Peng HH, Huang
WJ, Cai LM, Zhou TC and Lin XD: Prognostic impact of pretherapeutic
gamma-glutamyltransferase on patients with nasopharyngeal
carcinoma. PLoS One. 12:e01723452017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Mujawar SJ, Suchitra G, Kosandal KA,
Choudhari S, Inamdar NA and Ahmed KB: Evaluation of salivary
gamma-glutamyl transpeptidase as a biomarker in oral squamous cell
carcinoma and precancerous lesions. J Oral Maxillofac Pathol.
24:5842020. View Article : Google Scholar
|
|
183
|
Mizushima T, Ohnishi S, Shimizu Y,
Hatanaka Y, Hatanaka KC, Hosono H, Kubota Y, Natsuizaka M, Kamiya
M, Ono S, et al: Fluorescent imaging of superficial head and neck
squamous cell carcinoma using a γ-glutamyltranspeptidase-activated
targeting agent: A pilot study. BMC Cancer. 16:4112016. View Article : Google Scholar
|
|
184
|
Lee YJ, Han KD, Kim DH and Lee CH:
Determining the association between repeatedly elevated serum
gamma-glutamyltransferase levels and risk of respiratory cancer: A
nationwide population-based cohort study. Cancer Med. 10:1366–1376.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Gu J, Xie R, Zhao Y, Zhao Z, Xu D, Ding M,
Lin T, Xu W, Nie Z, Miao E, et al: A machine learning-based
approach to predicting the malignant and metastasis of thyroid
cancer. Front Oncol. 12:9382922022. View Article : Google Scholar
|
|
186
|
Foddis R, Franzini M, Bonotti A, Marino R,
Silvestri R, Fallahi P, Chiappino D, Emdin M, Paolicchi A and
Cristaudo A: Big and free fractions of gamma-glutamyltransferase:
New diagnostic biomarkers for malignant mesothelioma? Diagnostics
(Basel). 12:3112022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Huang H, Wang XP, Li XH, Chen H, Zheng X,
Lin JH, Kang T, Zhang L and Chen PS: Prognostic value of
pretreatment serum alanine aminotransferase/aspartate
aminotransferase (ALT/AST) ratio and gamma glutamyltransferase
(GGT) in patients with esophageal squamous cell carcinoma. BMC
Cancer. 17:5442017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Choi YJ, Lee DH, Han KD, Yoon H, Shin CM,
Park YS and Kim N: Elevated serum gamma-glutamyltransferase is
associated with an increased risk of oesophageal carcinoma in a
cohort of 8,388,256 Korean subjects. PLoS One. 12:e01770532017.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Chen Y, Liu H, Zhang J, Wu Y, Zhou W,
Cheng Z, Lou J, Zheng S, Bi X, Wang J, et al: Prognostic value and
predication model of microvascular invasion in patients with
intrahepatic cholangiocarcinoma: A multicenter study from China.
BMC Cancer. 21:12992021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Catalano M, Roviello G, Aprile G, Ramello
M, Conca R, Petrioli R, Perrone G, Ianza A and Mini E: Prognostic
value of alkaline phosphatase and gamma-glutamyl transferase in
patients with metastatic pancreatic cancer. Future Oncol.
19:937–946. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Liao W, Yang Y, Yang H, Qu Y, Song H and
Li Q: Circulating gamma-glutamyl transpeptidase and risk of
pancreatic cancer: A prospective cohort study in the UK Biobank.
Cancer Med. 12:7877–7887. 2023. View Article : Google Scholar
|
|
192
|
Hong SW, Lee HJ, Han K, Moon JM, Park S,
Soh H, Kang EA, Chun J, Im JP and Kim JS: Risk of gastrointestinal
cancer in patients with an elevated level of
gamma-glutamyltransferase: A nationwide population-based study.
PLoS One. 16:e02450522021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Yang S, He X, Liu Y, Ding X, Jiang H, Tan
Y and Lu H: Prognostic significance of serum uric acid and
gamma-glutamyltransferase in patients with advanced gastric cancer.
Dis Markers. 2019:14154212019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Hong TC, Yang HC, Chen CL, Kao JH, Liu CJ,
Chen MJ, Wang HY, Kuo YC, Yu LY and Hu KC: Relationship between
serum gamma-glutamyl transferase level and colorectal adenoma. PLoS
One. 15:e02404452020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Yang LH, Xu LZ, Huang ZJ, Pan HH, Wu M, Wu
QY, Lu T, Zhang YP, Zhu YB, Wu JB, et al: Comprehensive analysis of
immune ferroptosis gene in renal clear cell carcinoma: Prognosis
and influence of tumor microenvironment. Am J Transl Res.
14:5982–6010. 2022.PubMed/NCBI
|
|
196
|
Horie K, Kawakami K, Fujita Y, Matsuda Y,
Arai T, Suzui N, Miyazaki T, Koie T, Mizutani K and Ito M: Serum
exosomal gamma-glutamyltransferase activity increased in patients
with renal cell carcinoma with advanced clinicopathological
features. Oncology. 98:734–742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Pankevičiūtė-Bukauskienė M, Mikalayeva V,
Žvikas V, Skeberdis VA and Bordel S: Multi-omics analysis revealed
increased de novo synthesis of serine and lower activity of the
methionine cycle in breast cancer cell lines. Molecules.
28:45352023. View Article : Google Scholar
|
|
198
|
Seol A, Wang W, Kim SI, Han Y, Park IS,
Yoo J, Jo H, Han KD and Song YS: Enhanced susceptibility to breast
cancer in Korean women with elevated serum
gamma-glutamyltransferase levels: A nationwide population-based
cohort study. Front Oncol. 11:6686242021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Shi B, Zhang Z, Jin Q, Wang Z, Tang J, Xu
G, Zhu T, Gong X, Tang X and Zhao C: Selective tracking of
ovarian-cancer-specific γ-glutamyltranspeptidase using a
ratiometric two-photon fluorescent probe. J Mater Chem B.
6:7439–7443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Schwameis R, Grimm C, Brodowicz T, Petru
E, Hefler-Frischmuth K, Staudigl C, Reinthaller A, Heinze G,
Polterauer S and Polterauer M: Gamma-glutamyltransferase as novel
biomarker in patients with uterine leiomyosarcoma. Sci Rep.
6:337572016. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Polterauer S, Hofstetter G, Grimm C,
Rahhal J, Mailath-Pokorny M, Kohl M, Concin N, Tempfer C, Marth C
and Reinthaller A: Relevance of gamma-glutamyltransferase-a marker
for apoptotic balance-in predicting tumor stage and prognosis in
cervical cancer. Gynecol Oncol. 122:590–594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kawakami K, Fujita Y, Matsuda Y, Arai T,
Horie K, Kameyama K, Kato T, Masunaga K, Kasuya Y, Tanaka M, et al:
Gamma-glutamyltransferase activity in exosomes as a potential
marker for prostate cancer. BMC Cancer. 17:3162017. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Su S, Liu L, Sun C, Nie Y, Guo H, Hu Y,
Guo S and Pang S: Preoperative serum gamma-glutamyltransferase as a
prognostic biomarker in patients undergoing radical cystectomy for
bladder cancer. Front Oncol. 11:6489042021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Takemura K, Fukushima H, Ito M, Kataoka M,
Nakanishi Y, Sakamoto K, Suzuki H, Tobisu KI and Koga F: Prognostic
significance of serum γ-glutamyltransferase in patients with
advanced urothelial carcinoma. Urol Oncol. 37:108–115. 2019.
View Article : Google Scholar
|
|
205
|
Song Y, Tian S, Zhang P, Zhang N, Shen Y
and Deng J: Construction and validation of a novel
ferroptosis-related prognostic model for acute myeloid leukemia.
Front Genet. 12:7086992022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Watanabe B, Tabuchi Y, Wada K and Hiratake
J: Synthesis and evaluation of the inhibitory activity of the four
stereoisomers of the potent and selective human γ-glutamyl
transpeptidase inhibitor GGsTop. Bioorg Med Chem Lett.
27:4920–4924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Han L, Hiratake J, Kamiyama A and Sakata
K: Design, synthesis, and evaluation of gamma-phosphono diester
analogues of glutamate as highly potent inhibitors and active site
probes of gamma-glutamyl transpeptidase. Biochemistry.
46:1432–1447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
King JB, West MB, Cook PF and Hanigan MH:
A novel, species-specific class of uncompetitive inhibitors of
gamma-glutamyl transpeptidase. J Biol Chem. 284:9059–9065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Zhai X, Dai T, Chi Z, Zhao Z, Wu G, Yang S
and Dong D: Naringin alleviates acetaminophen-induced acute liver
injury by activating Nrf2 via CHAC2 upregulation. Environ Toxicol.
37:1332–1342. 2022. View Article : Google Scholar : PubMed/NCBI
|