Introduction
Esophageal cancer ranks 11th in terms of incidence
(511,000 newly diagnosed cases) as well as seventh in mortality
overall (445,000 death cases) in 2022 globally (1). At present, surgical resection,
radio- and chemotherapies are the primary therapeutic options for
ESCC (2). Nevertheless, the
outcome remains unsatisfactory because of limited efficacy and
severe adverse effects (3,4).
Recent clinical trials have demonstrated that immune-checkpoint
inhibitors are promising agents for first-line therapy for advanced
ESCC (5-7). The first-line treatment with
monoclonal antibodies nivolumab or toripalimab combined with
chemotherapy and nivolumab combined with ipilimumab remarkably
improves overall survival in comparison with chemotherapy alone
(5-7). Nevertheless, 54.2% of patients
respond to immunotherapy (8).
Thus, probing the molecular mechanisms underlying ESCC is key for
developing more effective therapeutic strategies.
Production of reactive oxygen species (ROS) within
the endoplasmic reticulum (ER) can promote immunogenic cell death
in bladder cancer cells (9).
Calreticulin (CALR) is a Ca2+-binding ER protein, which
facilitates the folding of proteins that are secreted and inserted
into the plasma membrane (10).
The accumulation of unfolded proteins induces ER stress, and
sustained ER stress can activate apoptosis signals. These functions
are associated with chaperone proteins in the ER, including
calnexin (CANX) and protein disulfide isomerase A3 (PDIA3)
(11,12). ER serves key roles in protein
synthesis and folding, which exhibits high sensitivity to cellular
redox dynamics (13). The
CANX-CALR cycle restores the ER by monitoring the glycosylation
status of ER proteins and promoting the correct folding of newly
synthesized proteins (14).
Dysregulated disulfide bond formation because of ER stress
facilitates ER stress and dysfunction (15). The main function of PDIA3 is to
mediate the formation of correct disulfide bonds within the
molecules of newborn proteins; therefore, the expression of PDIA3
increases after ER stress (14).
IP3R is primarily involved in the regulation of calcium homeostasis
in the ER. IP3R regulates the transfer of calcium from the ER to
mitochondria by forming a 'quasi-synaptic' mechanism with
GRP75-VDAC/ mitochondrial calcium uniporter (16,17). The present study aimed to
determine whether the IP3R1-GRP75-VDAC1 axis is regulated by
CALR.
In healthy cells, CALR functions as a chaperone and
Ca2+ buffer, which assists correct protein folding
within the ER (18). It not only
maintains cellular protein homeostasis but also supports
Ca2+-mediated processes (adhesin and integrin signals),
as well as ensuring normal antigen presentation via major
histocompatibility complex (MHC) class I molecules. For example,
CALR expression on non-small cell lung cancer cellular membranes is
associated with dendritic cell infiltration and triggers the
migration and maturation of dendritic cells (19). Tumor cells that succumb to
immunogenic cell death exhibit CALR on the surface, which
facilitates the uptake of dead cells by phagocytes as well as the
onset of antitumor immunity (20,21). CALR/Melanoma antigen gene A3
(MAGE-A3)-infected dendritic cells stimulate CD8+
cytotoxic T cells, and leading to increased secretion of
interferon-γ, thereby inducing cytotoxic effects on ESCC cells that
express MAGE-A3 (22). Despite
this, the biological implications of CALR expression and the
mechanisms underlying CALR-mediated effects are unclear in ESCC.
Therefore, the present study aimed to assess the biological roles
and molecular mechanisms CALR in ESCC.
Materials and methods
CALR gene expression levels in Esophageal
squamous cell carcinoma and paired normal tissues by the Gene
Expression Profiling Interactive Analysis (GEPIA) database
Differential expression analysis of CALR in tumor
and normal tissues and the correlation analysis between CALR and
CANX/PDIA3 provided by GEPIA were obtained from
gepia.cancer-pku.cn/. Paired Student's t test was used for
statistical analysis, and the cut-off was P<0.01.
Patients and specimens
In total, 79 ESCC along with paired paracancerous
tissue samples (>5 cm distal to tumor lesions) were collected
from The General Hospital of Ningxia Medical University (Yinchuan,
China) from March 2021 to March 2022. There were 44 males and 35
females, with a mean age of 63.72±10.84 years (range, 46-84 years).
The inclusion criteria were patients with esophageal cancer
confirmed by postoperative pathological diagnosis who had not
received radiotherapy or chemotherapy before surgery. Clinical data
were acquired from the clinical data registry.
Immunohistochemical staining
Tissue samples were fixed in 4% paraformaldehyde at
room temperature for 12 h, then embedded in paraffin. Cut the
tissue into 5 µm slices. Tissue slices were deparaffinized
and rehydrated using xylene and graded ethanol, then sealed with
endogenous peroxidase and antigen retrieval was performed at 92°C
for 10 min. Samples were incubated with 3% hydrogen peroxide
solution at 37°C for 15 min and 10% goat serum (cat. no. ZLI-9056,
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) was used for
endogenous peroxidase/phosphatase activity blocking at 37°C for 30
min. Incubation with primary antibodies against CALR (1:200; cat.
no. 27298-1-AP; Proteintech Group, Inc.), CANX (1:100; cat. no.
BF0515, Affinity Biosciences), and PDIA3 (1:200; cat. no.
15967-1-AP; Proteintech Group, Inc.) was conducted overnight at
4°C, followed by undiluted horseradish peroxidase (HRP) labeled
secondary antibody (cat. no. PV-6000; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) incubation at room temperature for 20 min.
Slices were visualized utilizing DAB and counterstained in
hematoxylin at room temperature for 3 min, followed by examination
under an inverted fluorescence microscope (Carl Zeiss GmbH, 400×),
and quantified with Image Pro Plus 6.0 software (Media
Cybernetics). According to the method of Wang et al
(23), immunohistochemical
staining intensity was scored, 0-3 was classified as low expression
of CALR, and 4-12 was classified as high expression of CALR.
Subcutaneous xenograft assay
A total of 106-week-old male BALB/c nude mice (17-21
g) were purchased from Charles River Laboratories, Inc. All animal
experiments complied with ARRIVE guidelines. Animals were housed as
20-26°C, humidity of 40-70%, 12/12-h light/darkness, ad
libitum water and food. The xenograft tumor model was
established by subcutaneous injection of 1×107 KYSE-150
cells (Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd.) into the
upper back. After 21 days, the mice were anesthetized with 50 mg/kg
pentobarbital sodium and sacrificed by cervical dislocation
following anesthesia and the tissues were collected for further
experiments.
Animal histopathological analysis
Tumor tissue was collected, fixed with 4%
paraformaldehyde at room temperature for 12 h, embedded in paraffin
and cut into 5 µm slices for Masson and reticular fiber
staining. For Masson staining, after dewaxing, sections were
stained with Weiger's iron hematoxylin at room temperature for 5
min, then differentiated using 1% hydrochloric ethanol. Following
rinsing with tap water for 30 min, sections were stained with
ponceau acid fuchsin solution at room temperature for 5 min,
phosphomolybdate solution at room temperature for 3 min and aniline
blue at room temperature for 5 min before addition of 1% glacial
acetic acid at room temperature for 1 min and sealing with neutral
gum. For reticular fiber staining, the dewaxed slices were oxidized
with 0.25% potassium permanganate at room temperature for 3 min,
bleached with 1% oxalic acid, stained with 2.5% iron alum and
silver diaminohydroxide, reduced with 10% formaldehyde, stained
with 0.2% gold chloride, fixed with 5% sodium thiosulfate at room
temperature for 5 min, and sealed with neutral gum. Images were
captured under an inverted light microscope (Olympus, 400X
magnification).
Cell culture
Human ESCC cell lines KYSE150 and KYSE410 (Shanghai
Zhongqiao Xinzhou Biotechnology Co., Ltd.) were cultured in DMEM
(Gibco, Thermo Fisher SCIENTIFIC) and maintained in a cell
incubator (37°C, 5% CO2). Cells were passaged every 2-3
days. 1 µM thapsigargin was used for cell culture to induce
ER stress, and DMSO was used as the negative control of
thapsigargin.
Transfection
Short hairpin (sh)RNA-CALR#1, 2 and 3 were designed
and synthesized by Sangon Biotech (Shanghai) Co., Ltd (cat. no.
PLVE4646-1) to knockdown the expression of CALR. KYSE150 or KYSE410
cells were seeded into a 6-well plate at 1×105
cells/well and incubated at 37°C with antibiotic-free DMEM
[containing 10% FBS (Gibco, Thermo Fisher SCIENTIFIC)] for 24 h.
When the cell confluence reached 70%, Lipofectamine 8,000
transfection kit (cat. no. C0533, Beyotime Institute of
Biotechnology) was used to transfect 100 pmol sh-CALR#1, 2 and 3
for each well into cells at 37°C for 12 h. After replacing medium
with complete DMEM and incubating at 37°C for 48 h, the
transfection efficiency was detected by western blotting. The
sequences of shRNAs were as follows: sh-CALR#1, 5′-CGT CTA CTT CAA
GGA GCA GTT-3′, sh-CALR#2, 5′-GCA GTT CAC GGT GAA ACA TGA-3′;
sh-CALR#3, 5′-GCA AGA ACG TGC TGA TCA ACA-3′ and sh-negative
control (NC), 5′-GTT CTC CGA ACT GTC ACT A-3′. CALR overexpression
(OE) plasmid was purchased from Sangon Biotech (Shanghai) Co., Ltd.
and vector pcDNA 3.1 was used as NC. 2.5 µg plasmid were
added into each well, and transfected with Lipofectamine 8000
transfection kit (cat. no. C0533, Beyotime Institute of
Biotechnology) at 37°C for 12 h. Then replacing with complete DMEM
and incubating at 37°C for 48 h before subsequent
experimentation.
Wound healing assay
Serum starved KYSE150 or KYSE410 cells were seeded
into a 6-well culture plate and grown to 90% confluence. Linear
scratch wound was created using sterile 200 µl pipette tips
in the cell monolayer. Each well was washed with PBS three times to
remove detached cells. Under an inverted light microscope (Carl
Zeiss GmbH, 40×), the images were captured for calculating the
wound area at 0 and 24 h.
Transwell assay
A single cell suspension of KYSE150 or KYSE410 cells
was prepared using serum-free DMEM and 200 µl cell
suspension was added to the upper chamber (1.5×104
cells). A total of 1 ml DMEM containing 10% FBS was added to the
lower chamber. After 24 h incubation at 37°C, the medium in the
upper and lower chambers was discarded and the non-migratory cells
remaining in the upper chamber were wiped off with a cotton swab.
The upper and lower chambers were washed twice with PBS, and the
cells were fixed with 4% paraformaldehyde at room temperature for
20 min and stained with 0.1% crystal violet at room temperature for
30 min. A total of three fields of view were randomly selected
under an inverted light microscope (Carl Zeiss GmbH, 200×) and the
stained cells were counted manually and photographed.
Detection of intracellular
Ca2+ concentration
KYSE150 or KYSE410 cells were inoculated in 6-well
plates (5×105 cells/well) and cultured at 37°C for 48 h.
Thereafter, 1 ml each medium and Flou-4 AM staining working
solution (cat. no. #F14201, Invitrogen; Thermo Fisher Scientific,
Inc.) were separately added to the cells, followed by incubation at
37°C for 20 min. The supernatant was removed by suction, and the
cells were washed twice with Flou-4 AM staining buffer, followed by
addition of 2 ml DMEM. The results were observed under a confocal
laser scanning microscope (Carl Zeiss GmbH; magnification,
×200).
ER-Tracker Red staining
KYSE150 or KYSE410 cells were incubated with
ER-Tracker Red staining solution (cat. no. C1041, Beyotime
Institute of Biotechnology) for 30 min at 37°C. Following removal
of ER-Tracker Red staining working solution, the cells were washed
with PBS three times, and investigated with a confocal laser
scanning microscope (Carl Zeiss GmbH; magnification, ×400).
Mito-Tracker Red staining
Mitochondrial network structure was assessed with
Mito Tracker Red staining (cat. no. C1049B, Beyotime Institute of
Biotechnology). Briefly, KYSE150 or KYSE410 cells were incubated
with 100 nM Mito Tracker Red reagent in DMEM for 30 min at 37°C.
Images were acquired under a laser scanning confocal microscope
(Carl Zeiss GmbH, ×400). The mitochondrial perimeter was quantified
using Image J 2X software (National Institutes of Health).
Intracellular ROS level measurement
KYSE150 or KYSE410 cells were treated with 10
µM 2,7-dichlorodi-hydrofluorescein diacetate (cat. no.
S0033S; Beyotime Institute of Biotechnology) fluorescent probe for
30 min at 37°C. Intercellular ROS images were captured under a
laser scanning confocal microscope (Carl Zeiss GmbH; magnification,
×200).
Immunofluorescent staining
KYSE150 or KYSE410 cells were fixed with 4%
paraformaldehyde at room temperature for 1 h. Mouse tissues were
fixed in 4% paraformaldehyde at room temperature for 12 h, then
embedded in paraffin and cut the tissue into 5 µm slices.
Mouse tissue sections were dewaxed with xylene and graded ethanol
and permeabilized utilizing Triton X-100. Antigen retrieval was
performed under 92°C for 10 min. Incubated the tissues with 10%
goat serum (cat. no. ZLI-9056, Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) for endogenous peroxidase/phosphatase
activity blocking at 37°C for 30 min. Incubation with primary
antibodies against CALR (1:200; cat. no. 27298-1-AP; Proteintech
Group, Inc.), CANX (1:100; cat. no. BF0515; Affinity Biosciences)
and PDIA3 (1:200; cat. no. 15967-1-AP; Proteintech Group, Inc.) was
performed at 4°C overnight, followed by incubation with Alexa
Fluor® 488 Conjugate (cat. no. ZF-0512) and Alexa
Fluor® 594 Conjugate (both 1:100; cat. no. ZF-0513; both
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) at 37°C for 2 h
in the dark. DAPI was utilized for nuclear staining at room
temperature for 5 min. All sections were scanned utilizing a
confocal laser scanning microscope (Carl Zeiss GmbH; magnification,
×400) and analyzed by Image-Pro Plus 6.0 (MEDIA CYBERNETICS).
Western blotting
Protein was extracted from cells using Whole Cell
Lysis Assay kit (cat. no. KGB5303; Nanjing KeyGen Biotech Co.,
Ltd.) according to the manufacturer's instructions. The protein
concentration was measured using a BCA kit. A total of 20 µg
protein/lane was separated by 10% SDS-PAGE, transferred to PVDF
membranes and blocked with 5% non-fat dry milk at room temperature
for 1 h. The blocked PVDF membranes were incubated overnight at 4°C
with primary antibodies against CALR (1:1,000; cat. no. 27298-1-AP;
Proteintech Group, Inc.), CANX (1:500; cat. no. BF0515; Affinity
Biosciences), PDIA3 (cat. no. 15967-1-AP; Proteintech Group, Inc.),
vimentin (cat. no. bs-8533R), N-cadherin (cat. no. bs-1172R),
glucose regulatory protein 78 (GRP78) (cat. no. bs-1219R; all
BIOSS), α-smooth muscle actin (SMA; cat. no. Bs70000; Biogot
Technology Co., Ltd.), fibroblast activation protein (FAP; all
1:1,000; cat. no. bs-5758R; BIOSS), ferroptosis suppressor protein
1 (FSP-1) (1:4,000; cat. no. 20886-1-AP), Platelet-derived growth
factor receptors (PDGFR) (cat. no. 13449-1-AP; both Proteintech
Group, Inc.), TGF-β (both 1:1,000; cat. no. Ab66043, Abcam). GRP75
(1:20,000; cat. no. 14887-1-AP), Voltage dependent anion channel 1
(VDAC1) (cat. no. 55259-1-AP, both Proteintech Group, Inc.) and
inositol 1,4,5-Trisphosphate Receptor (IP3R1) (both 1:2,000; cat.
no. DF3000, Affinity Biosciences). The horseradish enzyme labeled
secondary antibody (1:5,000; cat. nos. ZB-5301 and ZB-2305; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.) was added at room
temperature for 2 h. The proteins were visualized using the ECL
Chemiluminescence detection kit (Applygen Technologies Inc.). The
protein bands were analyzed with Quantity One software using
β-actin (1:2,000; cat. no. AF7018; Affinity Biosciences) as an
internal reference. Relative protein expression was measured with
ImageJ 2X software (National Institutes of Health).
Co-immunoprecipitation
Immunoprecipitation kit with Protein A + G Magnetic
Beads (cat. no. P2179S, Beyotime Institute of Biotechnology) was
used for co-immunoprecipitation assay. A total of 5×105
KYSE150 and KYSE410 cells were fully lysed with 100 µl Lysis
Buffer (Beyotime, dilute in a ratio of 1:100), centrifuged at
12,000 × g, at 4°C for 5 min and supernatant was collected. A total
of 500 µl VDAC1 (cat. no. 600-401-882) or IP3R1 antibody
(both 1:2,000, cat. no. PA1-901; both Thermo Fisher Scientific,
Inc.) or normal IgG working solution was added to 20 µl
Protein A + G magnetic beads for incubation at room temperature for
2 h. Sample protein and magnetic beads bound with antibodies or
normal IgG were added at a volume ratio of 25:1 and incubated at
4°C overnight. Magnetic beads were separated and the supernatant
was retained for western blotting, as aforementioned.
EdU proliferation assay
The EdU working solution from BeyoClick EdU Cell
Proliferation kit with Alexa Fluor 594 (cat. no. C0078S, Beyotime
Institute of Biotechnology) was added to transfected cells at 37°C
for 2 h. The cells were incubated with 4% paraformaldehyde and PBS
containing 0.3% Triton X-100 at room temperature successively for
15 min. Click Reaction Solution was added at room temperature for
30 min away from light before observation under a fluorescence
microscope (magnification, ×200).
Caspase-3 activity and apoptosis
detection
Following removal of the culture medium, Annexin
V-mCherry Binding Buffer, Annexin V-mCherry and GreenNuc Caspase-3
Substrate in Caspase-3 Activity and Apoptosis Detection Kit for
Live Cell (cat. no. C1077S, Beyotime Institute of Biotechnology)
were added to transfected cells in sequence at room temperature in
the dark for 30 min before observation under a fluorescence
microscope (×200).
Mitochondrial membrane potential
detection
Mitochondrial membrane potential test kit (cat. no.
M8650, Beijing Solarbio Science & Technology Co., Ltd.) was
used for mitochondrial membrane potential detection. A total of
5×105 cells were cultured in a 6-well plate at 37°C for
24 h, then 1 ml JC-1 staining solution was added to each well at
37°C for 20 min. Cells were washed with JC-1 staining buffer twice,
2 ml cell culture DMEM was added to each well for observation under
a fluorescence microscope (×400).
NAD+/NADH assay
NAD+/NADH Assay kit with WST-8 (cat. no.
S0175; Beyotime Institute of Biotechnology) was used to assay
NAD+ and NADH levels. Transfected 5×105 cells
were cultured in 6-well plates at 37°C for 48 h.
NAD+/NADH extract was added to lyse the cells and
supernatant was obtained after centrifugation at room temperature
and 1,000 × g for 5 min for the determination of the total amount
of NAD+ and NADH. The supernatant was heated in a water
bath at 60°C for 30 min to decompose NAD+ for the
determination of NADH content. A total of 20 µl of sample or
gradient diluted standard to each well of the 96-well plate, and
then add 90 µl of alcohol dehydrogenase working solution to
each well. Following incubation at 37°C for 10 min in the dark, 10
µl color developing solution was added and incubated at 37°C
for 30 min in the dark. Absorbance at 450 nm was measured to
calculate the NAD+ and NADH content.
ActinRed cytoskeleton staining
A total of 5×105 KYSE150 or KYSE410 cells
were fixed with 4% paraformaldehyde at room temperature for 10 min,
then washed with PBS containing 0.1% Triton X-100 for 5 min. Cells
were incubated with PBS containing 1% BSA at room temperature for
20 min and stained with 1:20 ActinRed (cat. no. KGMP0012, Nanjing
KeyGen Biotech Co., Ltd.) at room temperature for 20 min. The
staining was observed under a fluorescence microscope (×400) after
sealing with DAPI at room for 5 min.
Statistical analysis
All statistical analyses were performed with SPSS
23.0 software (IBM Corp.). Unpaired or paired Student's t-test was
utilized to compare two groups. χ2 test was performed to
compute the association between CALR expression and
clinicopathological factors. One-way ANOVA followed by LSD post hoc
test was used to compare >2 groups. All data are presented as
the mean ± SD of three independent experimental repeats. P<0.05
was considered to indicate a statistically significant
difference.
Results
CALR is upregulated in ESCC and
positively associated with CANX and PDIA3
Using Gene Expression Profiling Interactive Analysis
2 (gepia2.cancer-pku.cn/#index) (24), CALR expression levels were
examined in The Cancer Genome Atlas (TCGA)-esophageal carcinoma
(ESCA) cohort. Higher CALR expression was observed in ESCA (n=182)
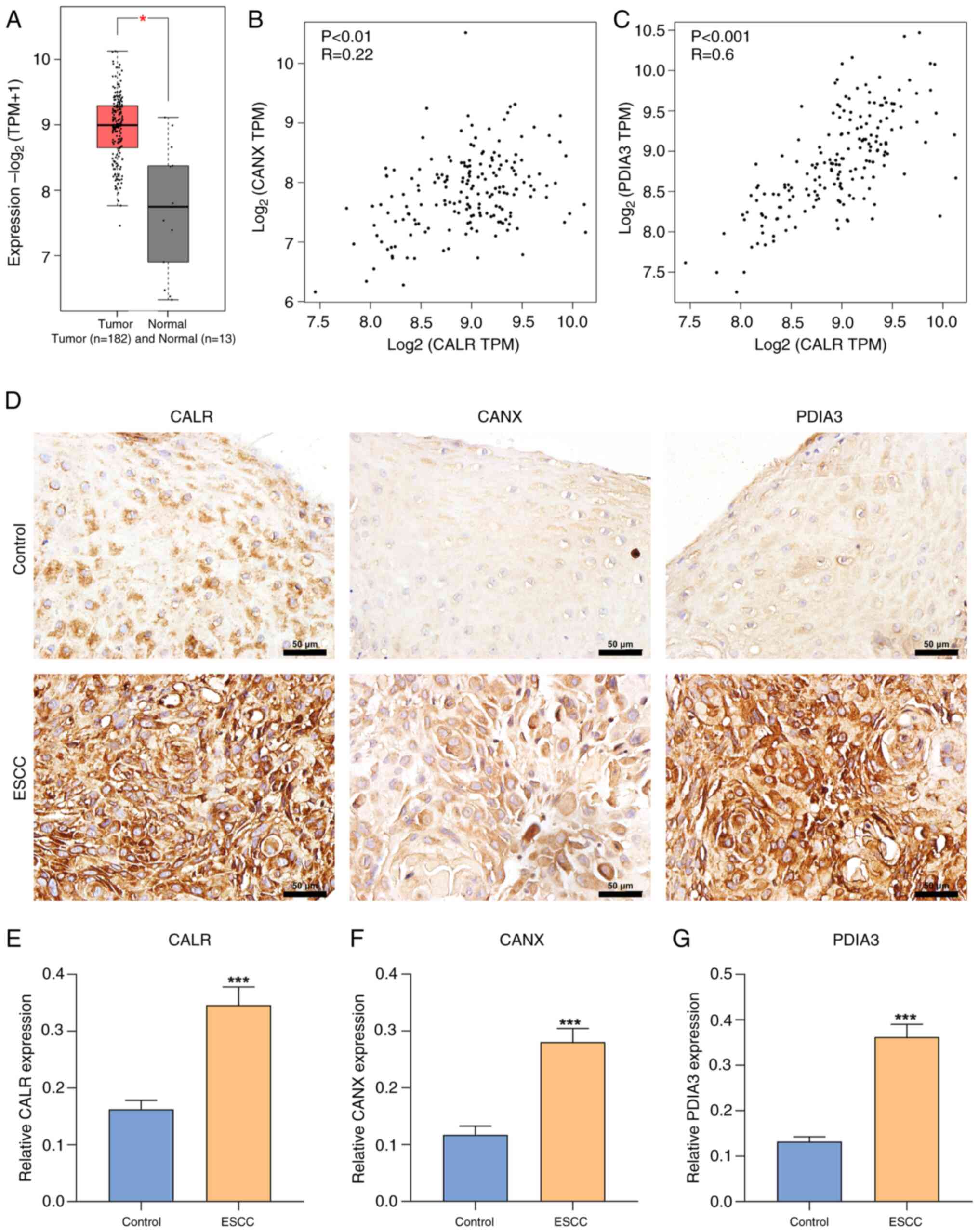
in comparison with normal specimens (n=13; Fig. 1A). CALR, CANX and PDIA3 are
critical for the proper assembly of MHC class I heavy chains with
β2 microglobulin in the lumen of ER 10). In TCGA-ESCA cohort, CALR
expression was positively associated with CANX as well as PDIA3
expression (Fig. 1B and C),
indicating interactions in ESCC. In total, 79 ESCC and matched
normal tissue samples were included for immunohistochemical
straining, which demonstrated significant upregulation of CALR,
CANX and PDIA3 in ESCC compared with normal tissue (Fig. 1D-G).
Clinicopathological value of CALR in
ESCC
A total of 79 ESCC cases were classified as high
(n=61) and low (n=18) expression of CALR and clinicopathological
factors were compared. High CALR expression was significantly
associated with lymph node metastasis, lymphovascular invasion, TNM
stage as well as vascular invasion of ESCC cases (Table I). This indicated the
clinicopathological value of CALR during ESCC progression.
 | Table IDifferences in clinicopathological
factors in patients with esophageal squamous cell carcinoma with
high and low calreticulin expression. |
Table I
Differences in clinicopathological
factors in patients with esophageal squamous cell carcinoma with
high and low calreticulin expression.
| Clinical
factor | Total (n=79) | Low (n=18) | High (n=61) | χ2 | P-value |
|---|
| Age, years | | | | | |
| <60 | 37 | 8 | 29 | 0.054 | 0.817 |
| ≥60 | 42 | 10 | 32 | | |
| Sex | | | | | |
| Male | 44 | 11 | 33 | 0.277 | 0.599 |
| Female | 35 | 7 | 28 | | |
| Maximum tumor
diameter, cm | | | | | |
| >4 | 47 | 14 | 33 | 3.234 | 0.072 |
| ≤4 | 32 | 4 | 28 | | |
| Tumor location | | | | | |
| Upper thoracic
segment | 20 | 4 | 16 | 0.123 | 0.940 |
| Middle thoracic
segment | 30 | 7 | 23 | | |
| Lower thoracic
segment | 29 | 7 | 22 | | |
| Invasion depth | | | | | |
| Mucous layer | 19 | 5 | 14 | 0.332 | 0.847 |
| Muscle layer | 34 | 8 | 26 | | |
| Mantle layer | 26 | 5 | 21 | | |
| Lymph node
metastasis | | | | | |
| Yes | 36 | 4 | 32 | 5.123 | 0.024 |
| No | 43 | 14 | 29 | | |
| TNM stage | | | | | |
| I | 13 | 5 | 8 | 8.924 | 0.023 |
| II | 15 | 6 | 9 | | |
| III | 22 | 5 | 17 | | |
| IV | 29 | 2 | 27 | | |
| Lymphovascular
invasion | | | | | |
| Yes | 39 | 5 | 34 | 4.347 | 0.037 |
| No | 40 | 13 | 27 | | |
| Vascular
invasion | | | | | |
| Yes | 39 | 4 | 35 | 6.872 | 0.009 |
| No | 40 | 14 | 26 | | |
CALR is responsible for ESCC
migration
sh-CALR#1 sequence exhibited the optimal knockdown
effects and was selected for subsequent assays (Fig. 2A-C). Similarly, the transfection
efficiency of CALR overexpression plasmid was also verified
(Fig. 2D-F). After 24 h, the
wound distance was significantly narrower in OE-CALR transfected
KYSE150 or KYSE410 cells than controls (Fig. 2G-I). Meanwhile, compared with
controls, sh-CALR transfected KYSE150 or KYSE410 cells exhibited
significantly wider wound distance. OE-CALR significantly increased
migration of KYSE150 or KYSE410 cells in comparison with controls
(Fig. 2J-L), whereas migration of
sh-CALR transfected KYSE150 or KYSE410 cell cells was significantly
decreased, indicating CALR was responsible for migratory ability of
ESCC cells.
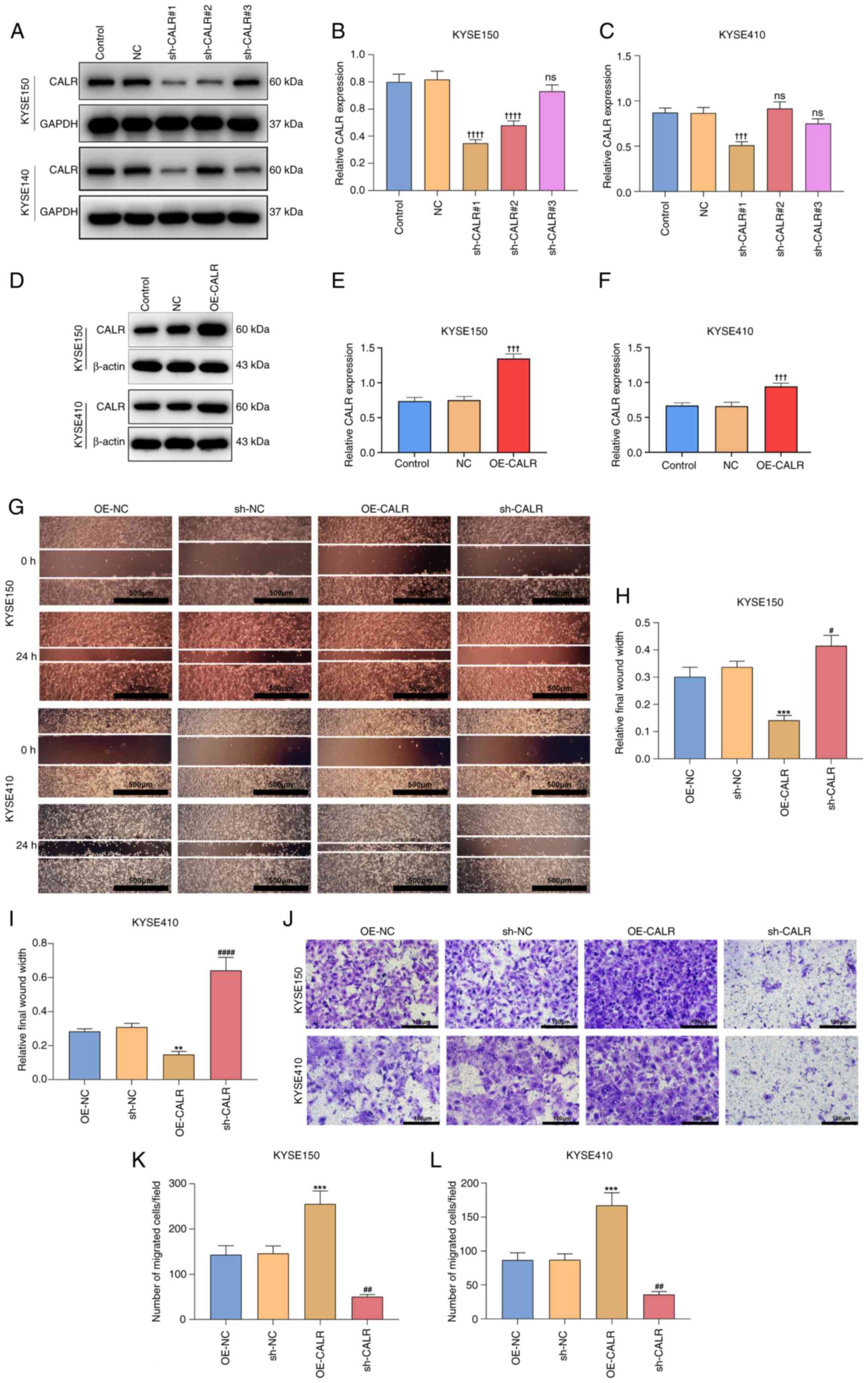 | Figure 2CALR is responsible for esophageal
squamous cell carcinoma migration. (A) Western blots of CALR
expression in KYSE150 or KYSE410 cells with sh-CALR transfection.
(B) Quantification of proteins in KYSE150 cell in each group. (C)
Quantification of proteins in KYSE410 cells. (D) Transfection
efficiency of CALR overexpression plasmid was verified by western
blotting. (E) Quantification of transfection efficiency of KYSE150
cell. (F) Quantification of transfection efficiency of KYSE410
cell. (G) Wound distance at 0 and 24 h for KYSE150 or KYSE410 cells
with OE-CALR or sh-CALR transfection. (H) Quantification of
relative final wound width in KYSE150 cell. (I) Quantification of
relative final wound width in KYSE410 cell. Scale bar, 500
µm. (J) Transwell assay of KYSE150 or KYSE410 cells with
OE-CALR or sh-CALR transfections. (K) Number of migrated
cells/field in KYSE150 cell. (L) Number of migrated cells/field in
KYSE410 cell. Scale bar, 100 µm. ††††P<0.0001,
†††P<0.001 vs. NC, **P<0.01,
***P<0.001 vs. OE-NC, #P<0.05,
##P<0.01, ####P<0.0001 vs. sh-NC. CALR,
Calreticulin; sh, short hairpin; OE, overexpression; NC, negative
control; ns, not significant. |
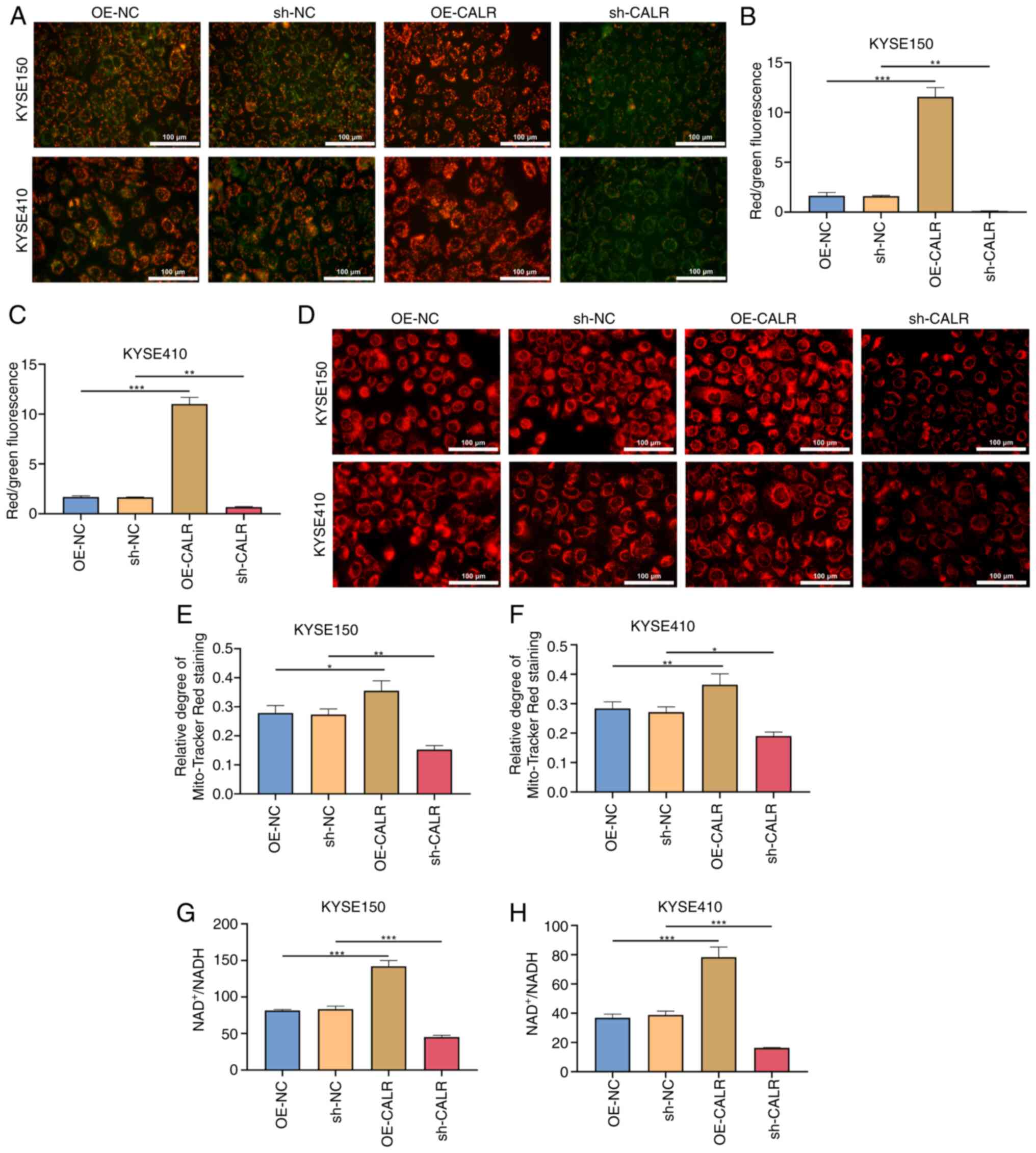
CALR induces ER stress
Channel functions were evaluated by detecting
intracellular Ca2+ concentration utilizing Flou-4
Ca2+ indicator dye. OE-CALR transfected KYSE150 or
KYSE410 cells exhibited enhanced intracellular Ca2+
concentration in comparison with controls (Fig. 3A-C). Additionally, intracellular
Ca2+ concentration was significantly decreased in
KYSE150 or KYSE410 cell lines transfected with sh-CALR.
Co-immunoprecipitation showed that CALR overexpression increased
the binding between GRP75 and VDAC1, as well as between GRP75 and
IP3R1 (Fig. 4M-N). ER
fluorescence intensity was enhanced by CALR overexpression and
decreased by knockdown (Fig.
3D-F). Moreover, the expression of CALR, CANX and PDIA3 in ESCC
cells significantly increased after treatment with ER stress
agonist thapsigargin (Fig. 4A-H).
GRP78 expression was increased in KYSE150 or KYSE410 cells with
OE-CALR transfection compared with controls (Fig. 4I-L). These indicated that CALR
induced ER stress of ESCC cells.
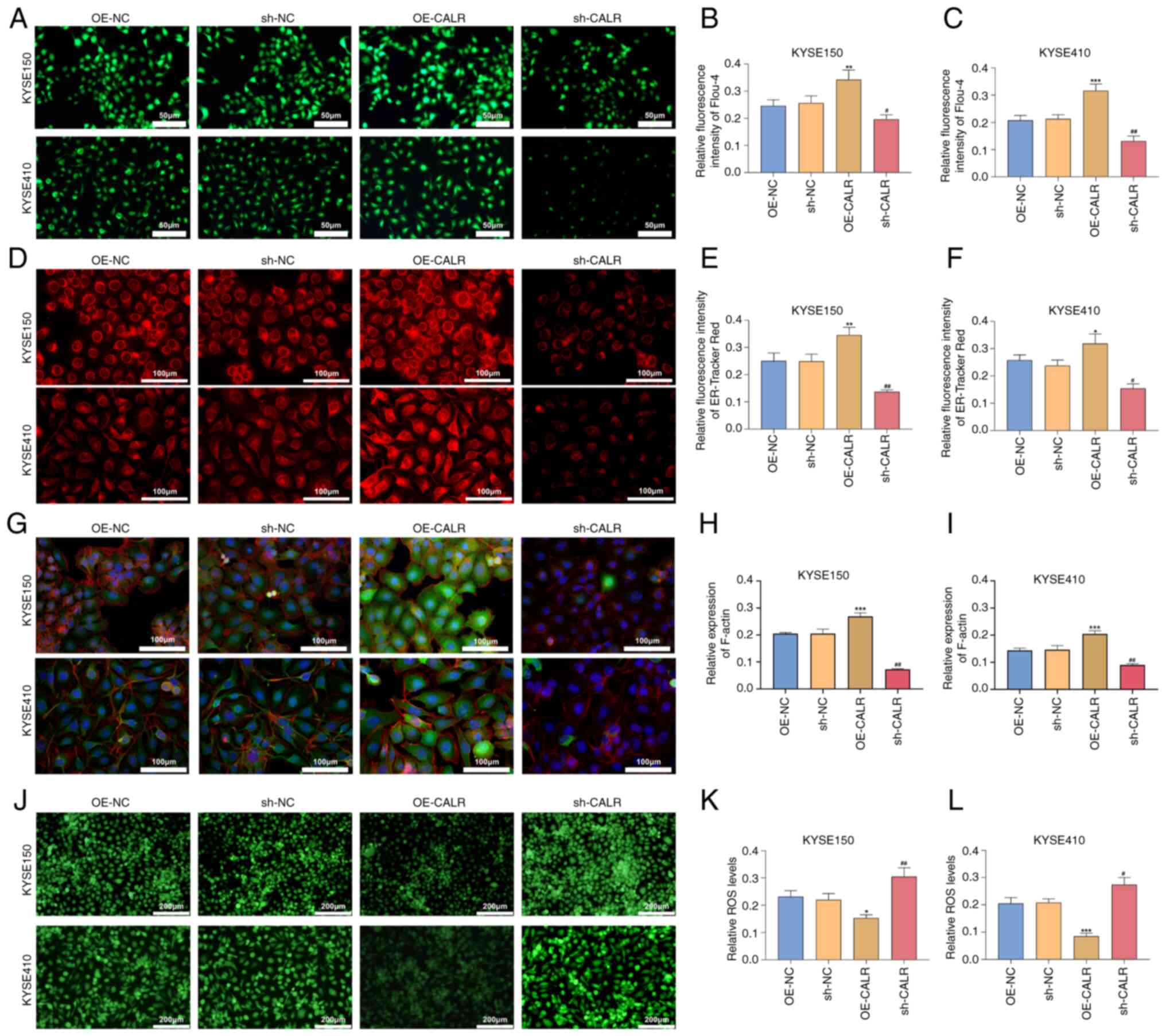 | Figure 3CALR effect on intracellular
Ca2+ concentration, endoplasmic reticulum stress,
cytoskeletal remodeling and ROS accumulation of esophageal squamous
cell carcinoma cells. (A) Flou-4 AM fluorescent staining for
intracellular Ca2+ concentration of KYSE150 or KYSE410
cells with OE-CALR or sh-CALR transfections. Scale bar, 50
µm. (B) Quantification of relative fluorescence intensity of
flou-4 in (C) KYSE150. (C) Quantification of relative fluorescence
intensity of flou-4 in KYSE410. (D) ER-Tracker Red staining of
KYSE150 or KYSE410 cell line with OE-CALR or sh-CALR transfection.
Scale bar, 100 µm. (E) Quantification of relative
fluorescence intensity of ER-Tracker Red in KYSE150. (F)
Quantification of relative fluorescence intensity of ER-Tracker Red
in KYSE410. (G) Cytoskeletal microfilament structure staining,
Scale bar, 100 µm. (H) Quantification of relative expression
of F-actin in KYSE150. (I) Quantification of relative expression of
F-actin in KYSE410. (J) DCFH-DA fluorescent staining for
intracellular ROS levels in KYSE150 or KYSE410 cells with OE-CALR
or sh-CALR transfection. Scale bar, 200 µm. (K) Relative ROS
levels in KYSE150. (L) Relative ROS level in KYSE410.
*P<0.05, **P<0.01,
***P<0.001 vs. OE-NC, #P<0.05,
##P<0.01 vs. sh-NC. CALR, Calreticulin; ROS, reactive
oxygen species; OE, overexpression; sh, short hairpin; NC, negative
control. |
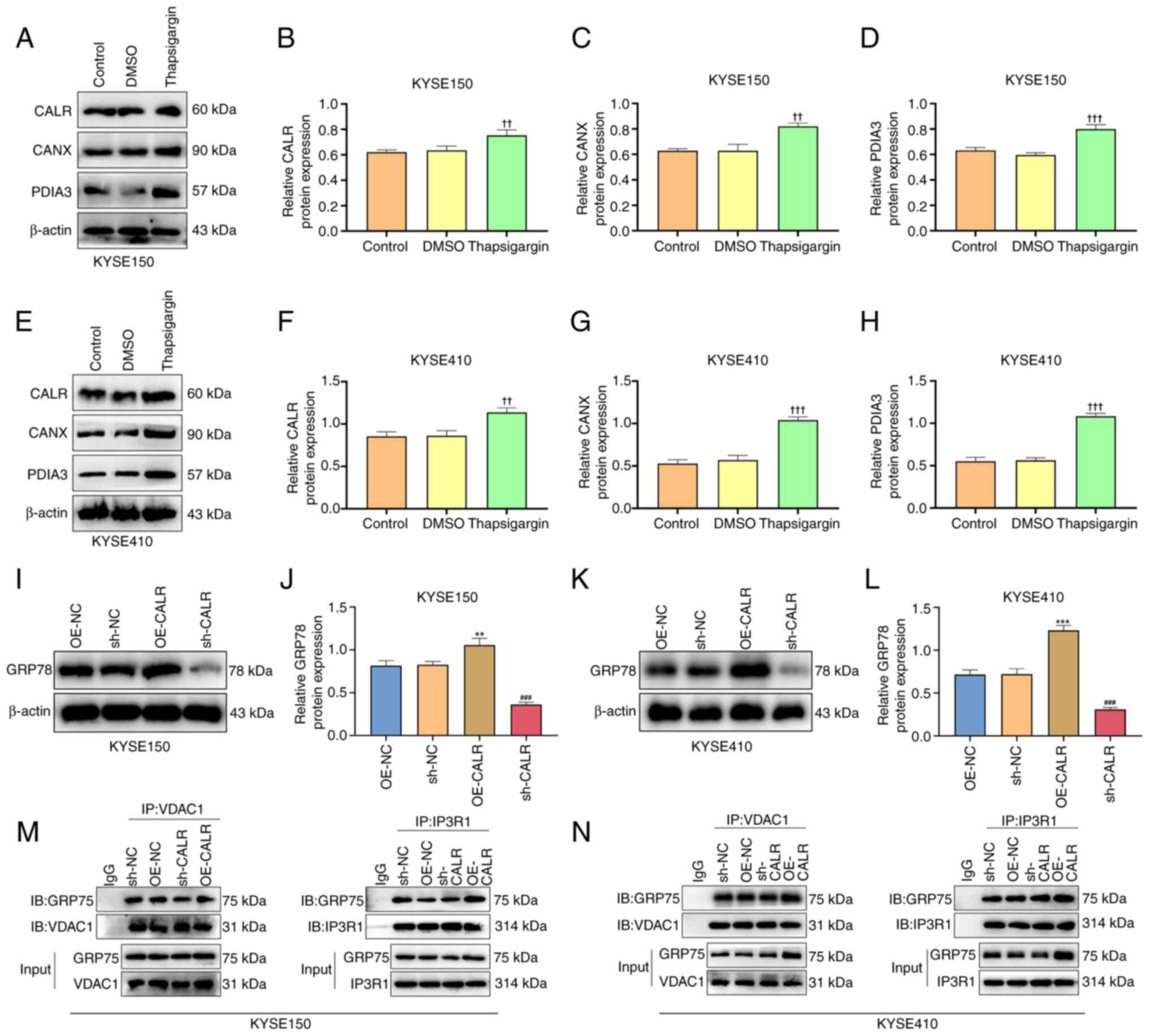 | Figure 4CALR regulates IP3R calcium ion
release channels and ER stress. (A) Protein expression of (B) CALR,
(C) CANX and (D) PDIA3 in KYSE150 after treatment with ER stress
agonist thapsigargin. (E) Protein expression of (F) CALR, (G) CANX
and (H) PDIA3 in KYSE410 cells following treatment with ER stress
agonist thapsigargin. (I) In intracellular GRP78 expression after
overexpression or knockdown of CALR in KYSE150. (J) Protein
expression of GRP78 in KYSE150. (K) In intracellular GRP78
expression after overexpression or knockdown of CALR in KYSE410.
(L) Protein expression of GRP78 in KYSE410. (M) Co-IP revealed the
interaction of GRP75 and VDAC1 and IP3R1 in KYSE150. (N) Co-IP
revealed the interaction of GRP75 and VDAC1 and IP3R1 in KYSE410.
††P<0.01, †††P<0.001 vs. DMSO,
**P<0.01, ***P<0.001 vs. OE-NC,
###P<0.001 vs. sh-NC. CALR, Calreticulin; IP3R,
inositol 1,4,5-Trisphosphate Receptor; ER, endoplasmic reticulum;
CANX, calnexin; PDIA3, protein disulfide isomerase A3; ESCC,
esophageal squamous cell carcinoma; GRP, glucose regulatory
protein; VDAC, voltage-dependent anion channel; IB, immunoblotting;
IP, immunoprecipitation; OE, overexpression; NC, negative control;
sh, short hairpin. |
CALR enhances remodeling of the cellular
cytoskeleton
Following transfection with OE-CALR, the
fluorescence intensity of KYSE150 and KYSE410 cells stained with
ActinRed was enhanced; the fluorescence intensity decreased after
CALR was knocked down (Fig.
3G-I). This suggests that CALR enhanced remodeling of the
cellular cytoskeleton.
CALR reduces intracellular ROS
accumulation in ESCC cells
Levels of intracellular ROS accumulation were
measured using DCFH-DA fluorescent probe. In comparison with
controls, OE-CALR transfection decreased intracellular ROS
accumulation in KYSE150 or KYSE410 cells (Fig. 3J-L). Moreover, intracellular ROS
levels were increased in KYSE150 or KYSE410 cells with sh-CALR
transfections. Hence, CALR decreased intracellular ROS accumulation
in ESCC cells.
CALR increases CANX and PDIA3 expression
in ESCC cells
In comparison with controls, OE-CALR-treated KYSE150
or KYSE410 cells exhibited increased expression of CALR, CANX and
PDIA3 both in immunohistochemical and immunofluorescent staining
(Fig. 5A-N), whereas expression
levels were decreased in KYSE150 or KYSE410 cells with sh-CALR
transfection. Western blots demonstrated the enhanced expression of
CALR, CANX and PDIA3 in OE-CALR-treated KYSE150 or KYSE410 cells
but expression levels were decreased when CALR was knocked out
(Fig. 6A-K). Altogether, CALR
modulated CANX and PDIA3 expression in ESCC cells.
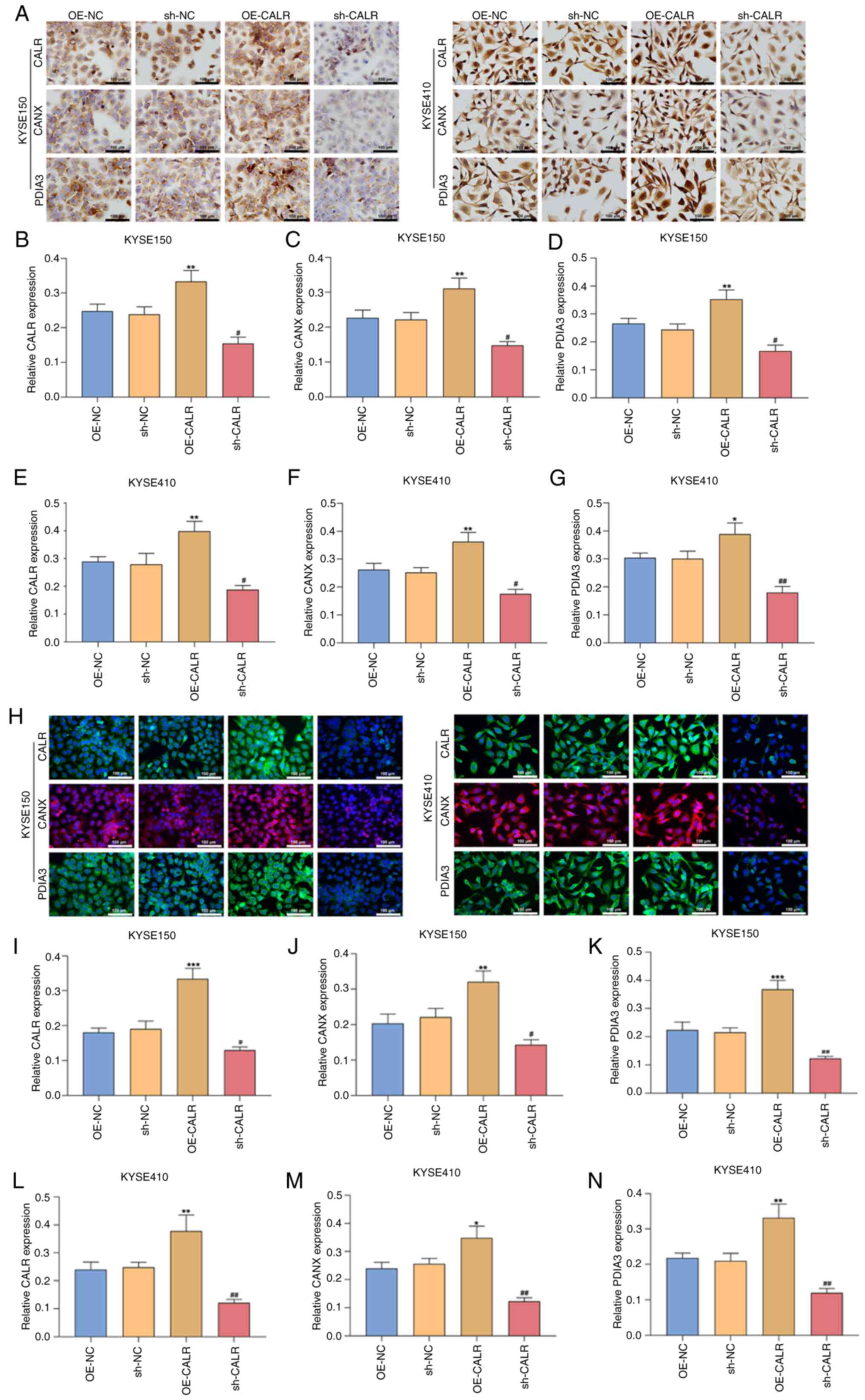 | Figure 5CALR increases CANX and PDIA3
expression in esophageal squamous cell carcinoma cells. (A)
Representative immunohistochemical staining. Relative expression of
(B) CALR, (C) CANX and (D) PDIA3 in KYSE150, relative expression of
(E) CALR, (F) CANX and (G) PDIA3 in KYSE410 following OE-CALR or
sh-CALR transfection. (H) Representative immunofluorescent
staining. Scale bar, 100 µm. Relative expression of (I)
CALR, (J) CANX and (K) PDIA3 in KYSE150, relative expression of (L)
CALR, (M) CANX and (N) PDIA3 in KYSE410 with OE-CALR or sh-CALR
transfection. *P<0.05, **P<0.01,
***P<0.001 vs. OE-NC, #P<0.05,
##P<0.01 vs. sh-NC. CALR, calreticulin; CANX,
calnexin; PDIA3, protein disulfide isomerase A3; OE,
overexpression; sh, short hairpin; NC, negative control. |
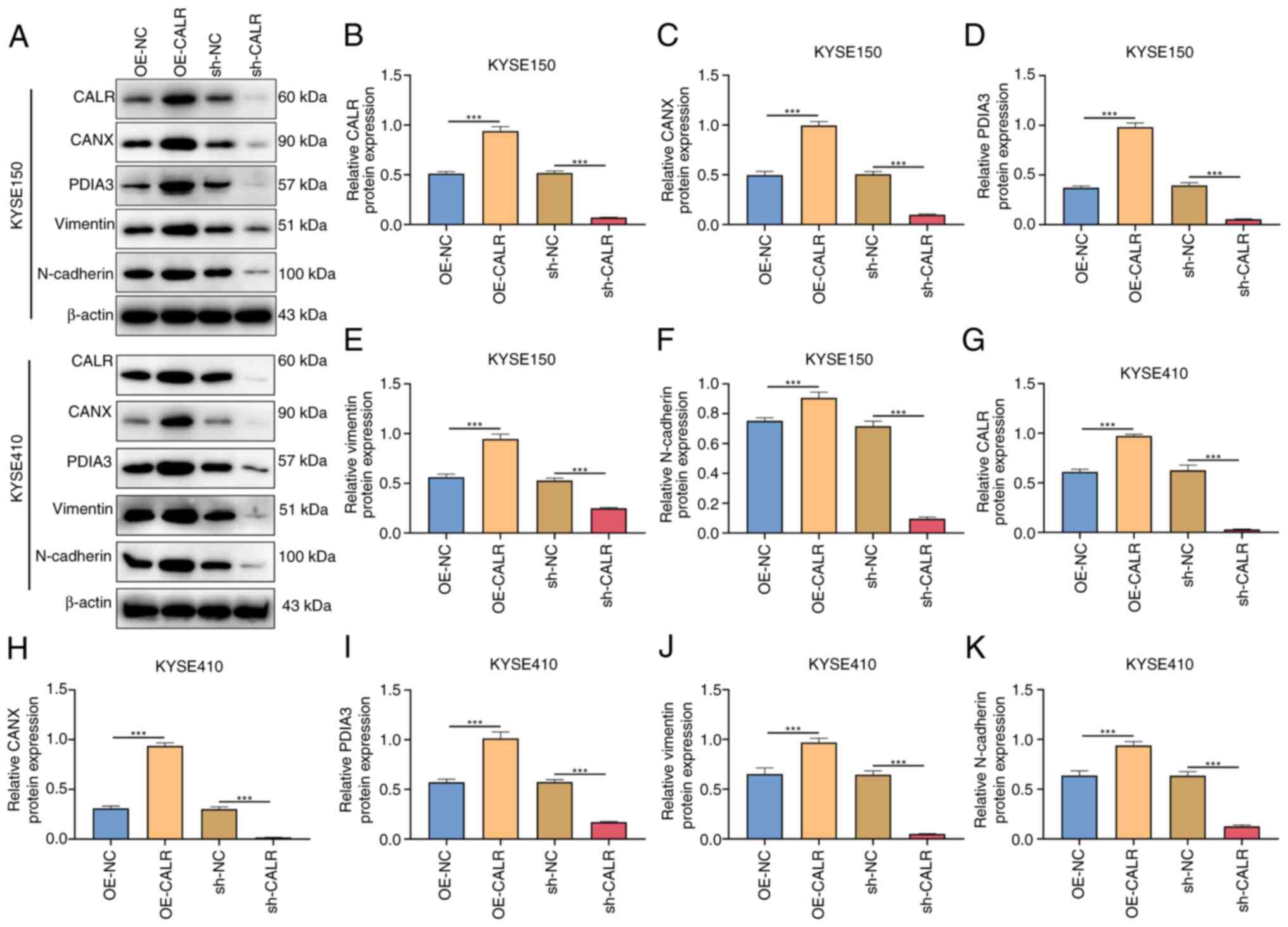 | Figure 6CALR induces epithelial-mesenchymal
transition of esophageal squamous cell carcinoma cells. (A)
Representative western blots. Relative protein expression of (B)
CALR, (C) CANX, (D) PDIA3, (E) vimentin and (F) N-cadherin in
KYSE150 with OE-CALR or sh-CALR transfection. Relative protein
expression of (G) CALR, (H) CANX, (I) PDIA3, (J) vimentin and (K)
N-cadherin in KYSE410 with OE-CALR or sh-CALR transfection.
***P<0.001. CALR, Calreticulin; CANX, calnexin;
PDIA3, protein disulfide isomerase A3; OE, overexpression; sh,
short hairpin; NC, negative control. |
CALR induces epithelial-mesenchymal
transition (EMT) of ESCC cells
Mesenchymal markers vimentin, and N-cadherin were
examined through western blots. In comparison with controls,
vimentin and N-cadherin expression levels were enhanced in KYSE150
or KYSE410 cells with OE-CALR transfection (Fig. 6A-K).
CALR promotes mitochondrial function of
ESCC cells
Mitochondrial membrane potential (indicated by JC-1
staining; Fig. 7A-C) and ratio of
NAD+/NADH content of KYSE150 and KYSE410 cells
overexpressing CALR was significantly increased, while transfection
with sh-CALR had the opposite effect (Fig. 7G and H). Compared with controls,
OE-CALR transfection resulted in increased mitochondrial perimeter
in KYSE150 or KYSE410 cell lines, demonstrating decreased
mitochondrial fission (Fig.
7D-F). Additionally, mitochondrial perimeter was decreased in
KYSE150 and KYSE410 cells with sh-CALR transfection. Altogether,
CALR may maintain mitochondrial function of ESCC cells.
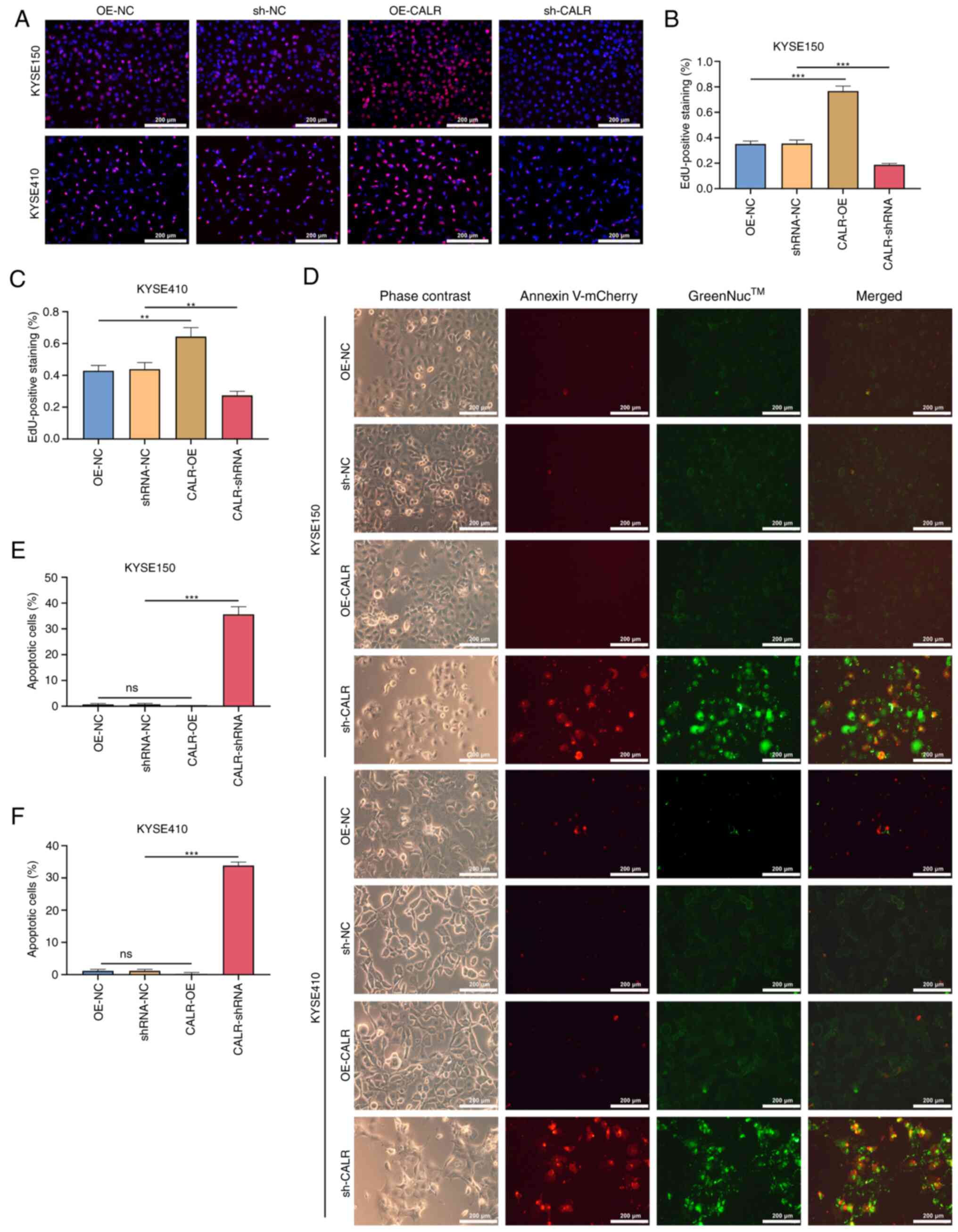
CALR promotes proliferation and inhibits
apoptosis of ESCC cells
The number of proliferating KYSE150 and KYSE410
cells overexpressing CALR increased significantly compared with the
control group, but decreased following sh-CALR transfection
(Fig. 8A-C). Similarly, in
CALR-overexpressing KYSE150 and KYSE410 cells, the number of
apoptotic cells was decreased, whereas the number of apoptotic
cells increased following sh-CALR transfection (Fig. 8D-F). These results indicated that
CALR promoted the proliferation and inhibited apoptosis of
esophageal carcinoma cells.
Effect of CALR on tumor growth and
expression of tumor-associated fibroblast marker protein in
mice
The tumor size of mice injected subcutaneously with
CALR knockdown ESCC cells was smaller than that of the control
group (Fig. 9A and B), indicating
that CALR knockdown inhibited tumor growth. The collagen and
reticular fibrosis were more obvious in the control group (Fig. 9C-F). Consistent with the results
of the cell experiments, the expression levels of CANX and PDIA3
were also decreased following the knockdown of CALR (Fig. 9G-N). Expression of
tumor-associated fibroblast activation markers including α-SMA,
FAP, FSP1, PDGFR, and TGF-β was also decreased in mice with CALR
knockdown (Fig. 9O-T).
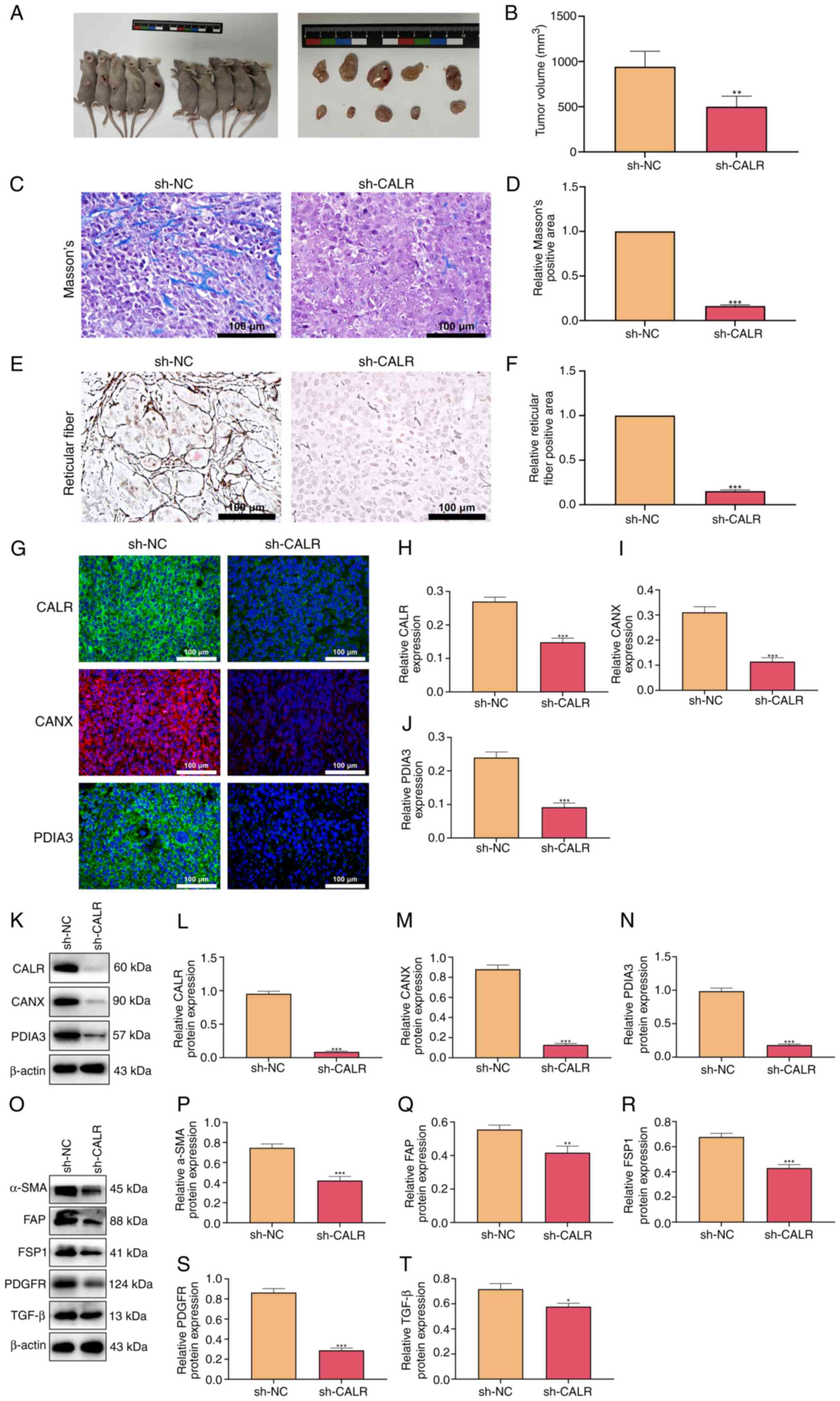 | Figure 9CALR regulates tumor growth and the
expression of tumor-associated fibroblast activation marker
proteins in mice. (A) Subcutaneous graft. (B) Size of the
subcutaneous graft. (C) Staining and (D) quantification of collagen
fibers by Masson's staining. (E) Staining and (F) quantification of
reticular fibers. (G) Expression of (H) CALR, (I) CANX and (J)
PDIA3 detected by immunofluorescent staining. Scale bar, 100
µm. (K) Expression of (L) CALR, (M) CANX and (N) PDIA3
detected by western blotting. (O) Representative western blots.
Expression levels of tumor-associated fibroblast activation marker
proteins (P) α-SMA, (Q) FAP, (R) FSP1, (S) PDGFR and (T) TGF-β were
detected by western blotting. **P<0.01,
***P<0.001. CALR, Calreticulin; CANX, calnexin;
PDIA3, protein disulfide isomerase A3; SMA, smooth muscle actin;
FAP, fibroblast Activation Protein; FSP1, fibroblast specific
protein 1; PDGFR, platelet derived growth factor receptors; sh,
short hairpin; NC, negative control. |
Discussion
Limited treatment options and inadequate
understanding of the molecular mechanisms of ESCC progression may
lead to poorer survival outcomes for patients with esophageal
cancer (25-27). ER stress is considered a novel
therapeutic target in cancer progression and associated with
overall survival of patients (28). Calcium homeostasis signaling is a
key factor in ER stress and CALR is responsible for the proper
folding of newly generated glycoproteins within the ER (29). However, it is not clear how CALR
regulates the biological behavior of esophageal cancer cells
through ER stress. The present results demonstrated CALR
upregulation in clinical ESCC compared with normal esophageal
tissues. CALR regulated ESCC cell proliferation and apoptosis,
migration capacity, Ca2+ accumulation, ER stress,
mitochondrial function, and ROS production and cytoskeletal
remodeling. CALR knockdown significantly decreased the expression
of CANX and PDIA3, inhibited the growth of xenografts and decreased
tumor-associated fibroblast levels. In conclusion, the present
results suggest that targeting CALR can reduce calcium ion levels
and energy metabolism in esophageal cancer cells to induce
apoptosis and inhibit ESCC progression.
CALR is involved in cellular processes, including
supporting Ca2+-dependent processes to maintain ER
function; activating CALR can initiate anti-tumor immunity
(30). Increased expression of
CALR indicates poor prognosis of nasopharyngeal carcinoma and
accelerates cell migration and invasion via activation of STAT3
(31). CALR regulates
intracellular free calcium-dependent chronic ER stress and induces
metastasis of pancreatic cancer cells; knockdown CALR reduces
subcutaneous tumor size and distant liver metastasis (32). Cell migration is the result of
induction of EMT (33). In the
present study, EMT markers in esophageal cancer cells were also
regulated by CALR and overexpression of CALR upregulated the
expression of vimentin and N-cadherin. CALR was found to regulate
the proliferation and apoptosis of esophageal cancer cells and
decrease the size of subcutaneous implant tumors, indicating the
role of CALR in division and proliferation of esophageal cancer
cells. Additionally, CALR heightens breast carcinogenesis as well
as progression via Wnt/β-catenin signaling activation (34). CALR, a reticular protein,
participates in a quality control system for newly synthesized
proteins and glycoproteins that relies on other chaperone proteins,
including CANX and PDIA3 (11,12). The present study demonstrated that
CALR can regulate the expression of CANX and PDIA3, which may
associated with Ca2+-dependent processes and ER stress,
thus promoting cancer. The CALR/CANX cycle prevents proteins from
misfolding (35). In bone
marrow-derived dendritic cells, Wntless deficiency leads to ER
stress response, increased macroautophagy/autophagy, reduced
calcium outflow from ER and disruption of the CALR/CANX cycle,
resulting in protein hypoglycation (12). The present study showed that CALR
knockdown could reduce CANX expression, hinder CALR/CANX cycling
and disrupt ER calcium homeostasis and mitochondrial function.
Intracellular Ca2+ serves a key role in
ER and mitochondrial homeostasis (36). Ca2+ homeostasis is a
key factor in tumorigenesis, development and metastasis.
Ca2+-dependent proteins of the ER can affect
mitochondrial function and ATP production, which is associated with
cell apoptosis (37). Targeting
abnormal Ca2+ signaling in ESCC cells represents a
developing therapeutic approach (38). In myeloproliferative tumors, CALR
mutation serves as an important marker for molecular pathological
diagnosis (39). CALR in mouse
and human acute myeloid leukemia cells is induced by activation of
AMPK when exposed to the cell surface and acts as a
damage-associated molecular pattern to stimulate an immune response
(40). In breast cancer, CALR
exposure and ATP release are involved in anti-tumor response
(41). The present study did not
assess tumor immune response but investigated the regulatory role
of CALR in esophageal cancer because CALR can affect ATP production
by regulating the levels of Ca2+ and mitochondrial
membrane potential in esophageal cancer cells, which is similar to
the aforementioned results, revealing that CALR can regulate
mitochondrial ATP production. ROS levels increased after CALR
knockdown, promoting apoptosis of esophageal cancer cells and
inducing cytoskeletal remodeling. These results highlighted the
regulatory role of CALR in ER and mitochondria. It was hypothesized
that CALR plays a bridging role between the ER and mitochondria,
which is not solely Ca2+-dependent. PDIA3 is a dynamic
feature of malignant progression in anaplastic thyroid carcinoma
(ATC); CALR exposure and ATP release are the key factors driving
malignant progression of ATC (42). Therefore, the role of CALR in
regulating mitochondria may be associated with regulation of PDIA3
expression. In future, studies should use cryo-electron microscopy
to determine the role of CALR in the connective structure of ER and
mitochondria.
Disrupting mitochondrial homeostasis is an
anti-neoplastic target in ESCC (43). For example, isocitrate
dehydrogenase 2 results in radio-therapeutic resistance in ESCC
through heightening mitochondrial function (44). Deferoxamine alleviates ESCC growth
through ERK1/2-dependent mitochondrial dysfunction (45). CALR downregulations lead to
mitochondrial Ca2+ overload and permeability transition
pore-mediated cell death in tumor cells (46). Compared with studies of non-small
cell lung cancer (NSCLC), the present results are different
(19,47-48): In this study, CALR was
overexpressed in esophageal cancer cells and promoted the invasion
of tumor-associated fibroblasts. However, the level of CALR
expression is low in some NSCLCS. Chen et al (19) showed that CALR inhibited non-small
cell lung cancer progression by facilitating dendritic cell
infiltration in NSCLC tissues, Stoll et al (47) has detected in a small subset of
NSCLC that the loss of CALR expression, those may be due to
different tumor tissue sources. Studies have shown that the impact
of total or membrane exposure CALR levels on prognosis vary
depending on the type of cancer (10,48). As one of the immunogenic cell
death hallmarks, previous findings also have shown a significant
association between CALR and immune cell infiltration in esophageal
cancer: Patients with esophageal cancer with high expression of
CALR exhibit longer disease-free survival (49). Transportation of CALR to the
plasma membrane surface induces immunogenic cell death. However,
the present study only focused on the regulation induced by
knocking down the expression of total CALR in cells, which
demonstrates that different CALR expression have different effects
on cancer cells in different types of tissues and cell, especially
in the mechanism of the immune system against cancer. In addition
to downregulating the expression of CANX and PDIA3, CALR knockdown
also decreased tumor-associated fibroblast levels in mice,
accompanied by a decrease expression in fibroblast markers. Cell
and mouse experiments showed that CALR mediated cell proliferation
and apoptosis and inhibited tumor-related fibroblast
infiltration.
In summary, the present study revealed the
carcinogenic role of CALR in ESCC. CALR promoted the progression of
ESCC by regulating ER stress and mitochondrial function, mediating
ATP production, cytoskeletal remodeling, cell proliferation and
apoptosis through CANX and PDIA3. Knockdown CALR significantly
inhibited tumor-associated fibroblast infiltration and is a
potential drug target for ESCC.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YM and WL designed the experiments. XW, YH, LZ, FZ
and SY performed experiments. YL, YW and XM wrote and revised the
manuscript and analyzed the data. HY, HL and FH analyzed the data.
YM and SY confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the World Medical Association Declaration of Helsinki and
Guidelines for the Care and Use of Laboratory Animals. Approval of
the experiments involving patient samples and animals was granted
by the Ethics Committee of the General Hospital of Ningxia Medical
University (approval no. 2020-880).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Natural Science Foundation of
Ningxia (grant nos. 2023AAC03529 and 2024AAC03702) and Ningxia Hui
Autonomous Region Key Research and Development Project (grant no.
2020BEG03001).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Z, Gu S, Lu T, Wu K, Li L, Dong C and
Zhou Y: IFI6 depletion inhibits esophageal squamous cell carcinoma
progression through reactive oxygen species accumulation via
mitochondrial dysfunction and endoplasmic reticulum stress. J Exp
Clin Cancer Res. 39:1442020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang J, Xu J, Chen Y, Zhuang W, Zhang Y,
Chen Z, Chen J, Zhang H, Niu Z, Fan Q, et al: Camrelizumab versus
investigator's choice of chemotherapy as second-line therapy for
advanced or metastatic oesophageal squamous cell carcinoma
(ESCORT): A multicentre, randomised, open-label, phase 3 study.
Lancet Oncol. 21:832–842. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Tang H, Fang Y, Tan L, Yin J, Shen
Y, Zeng Z, Zhu J, Hou Y, Du M, et al: Morbidity and mortality of
patients who underwent minimally invasive esophagectomy after
neoadjuvant chemoradiotherapy vs. neoadjuvant chemotherapy for
locally advanced esophageal squamous cell carcinoma: A randomized
clinical trial. JAMA Surg. 156:444–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab
combination therapy in advanced esophageal squamous-cell carcinoma.
N Engl J Med. 386:449–462. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng
J, Yang S, Fan Y, Shi J, Zhang X, et al: Toripalimab plus
chemotherapy in treatment-naïve, advanced esophageal squamous cell
carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell.
40:277–288.e3. 2022. View Article : Google Scholar
|
|
7
|
Yamamoto S and Kato K: JUPITER-06
establishes immune checkpoint inhibitors as essential first-line
drugs for the treatment of advanced esophageal squamous cell
carcinoma. Cancer Cell. 40:238–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma F, Li Y, Xiang C, Wang B, Lv J, Wei J,
Qin Z, Pu Y, Li K, Teng H, et al: Proteomic characterization of
esophageal squamous cell carcinoma response to immunotherapy
reveals potential therapeutic strategy and predictive biomarkers. J
Hematol Oncol. 17:112024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miao Z, Li J, Zeng S, Lv Y, Jia S, Ding D,
Li W and Liu Q: Endoplasmic reticulum-targeting AIE
photosensitizers to boost immunogenic cell death for immunotherapy
of bladder carcinoma. ACS Appl Mater Interfaces. 16:245–260. 2024.
View Article : Google Scholar
|
|
10
|
Fucikova J, Spisek R, Kroemer G and
Galluzzi L: Calreticulin and cancer. Cell Res. 31:5–16. 2021.
View Article : Google Scholar :
|
|
11
|
Fabarius A, Samra V, Drews O, Mörz H,
Bierbaum M, Darwich A, Weiss C, Brendel S, Kleiner H, Seifarth W,
et al: Evidence for recombinant GRP78, CALR, PDIA3 and GPI as
mediators of genetic instability in human CD34+ cells. Cancers
(Basel). 14:28832022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang LT, Lin MH, Liu KY, Chiou SS, Wang
SN, Chai CY, Tseng LW, Chiou HC, Wang HC, Yokoyama KK, et al:
WLS/wntless is essential in controlling dendritic cell homeostasis
via a WNT signaling-independent mechanism. Autophagy. 17:4202–4217.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
da Silva DC, Valentão P, Andrade PB and
Pereira DM: Endoplasmic reticulum stress signaling in cancer and
neurodegenerative disorders: Tools and strategies to understand its
complexity. Pharmacol Res. 155:1047022020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antoniotti V, Bellone S, Correia FP, Peri
C, Tini S, Ricotti R, Mancioppi V, Gagliardi M, Spadaccini D,
Caputo M, et al: Calreticulin and PDIA3, two markers of endoplasmic
reticulum stress, are associated with metabolic alterations and
insulin resistance in pediatric obesity: A pilot study. Front
Endocrinol (Lausanne). 13:10039192022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan AA, Allemailem KS, Almatroudi A,
Almatroodi SA, Mahzari A, Alsahli MA and Rahmani AH: Endoplasmic
reticulum stress provocation by different nanoparticles: An
innovative approach to manage the cancer and other common diseases.
Molecules. 25:53362020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Qi F, Su H, Zhang C, Zhang Q, Chen
Y, Chen P, Su L, Chen Y, Yang Y, et al: GRP75-faciliated
mitochondria-associated ER membrane (MAM) integrity controls
cisplatin-resistance in ovarian cancer patients. Int J Biol Sci.
18:2914–2931. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Zhao X, Qin Z, Li J, Sun B and Liu
L: Regulation of calcium homeostasis in endoplasmic
reticulum-mitochondria crosstalk: Implications for skeletal muscle
atrophy. Cell Commun Signal. 23:172025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SY, Oh JY, Kang TH, Shin HS, Cheng MA,
Farmer E, Wu TC and Hung CF: Endoplasmic reticulum stress enhances
the antigen-specific T cell immune responses and therapeutic
antitumor effects generated by therapeutic HPV vaccines. J Biomed
Sci. 26:412019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen R, Huang M, Yang X, Chen XH, Shi MY,
Li ZF, Chen ZN and Wang K: CALR-TLR4 complex inhibits non-small
cell lung cancer progression by regulating the migration and
maturation of dendritic cells. Front Oncol. 11:7430502021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fucikova J, Kepp O, Kasikova L, Petroni G,
Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G and Galluzzi L:
Detection of immunogenic cell death and its relevance for cancer
therapy. Cell Death Dis. 11:10132020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu X, Chen L, Li Y, Hu Z and He F:
Ferroptosis, necroptosis, and pyroptosis in the tumor
microenvironment: Perspectives for immunotherapy of SCLC. Semin
Cancer Biol. 86:273–285. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Song N, Liu Y, Liu Y, Li J, Ding J
and Tong Z: Efficient induction of anti-tumor immune response in
esophageal squamous cell carcinoma via dendritic cells expressing
MAGE-A3 and CALR antigens. Cell Immunol. 295:77–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Song X, Cheng G, Zhang J, Dong L,
Bai J, Luo D, Xiong Y, Li S, Liu F, et al: The regulatory mechanism
and biological significance of mitochondrial calcium uniporter in
the migration, invasion, angiogenesis and growth of gastric cancer.
Onco Targets Ther. 13:11781–11794. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47:W556–W560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pelosof L, Saung MT, Donoghue M, Casak S,
Mushti S, Cheng J, Jiang X, Liu J, Zhao H, Khazraee M, et al:
Benefit-Risk summary of nivolumab for the treatment of patients
with unresectable advanced, recurrent, or metastatic esophageal
squamous cell carcinoma after prior fluoropyrimidine- and
platinum-based chemotherapy. Oncologist. 26:318–324. 2021.
View Article : Google Scholar :
|
|
26
|
Sugimura K, Miyata H, Tanaka K, Makino T,
Takeno A, Shiraishi O, Motoori M, Yamasaki M, Kimura Y, Hirao M, et
al: Multicenter randomized phase 2 trial comparing
chemoradiotherapy and docetaxel plus 5-fluorouracil and cisplatin
chemotherapy as initial induction therapy for subsequent conversion
surgery in patients with clinical T4b esophageal cancer: Short-term
results. Ann Surg. 274:e465–e472. 2021. View Article : Google Scholar
|
|
27
|
Yamamoto S, Kawakami H, Kii T, Hara H,
Kawabata R, Kawada J, Takeno A, Matsuyama J, Ueda S, Okita Y, et
al: Randomized phase II study of docetaxel versus paclitaxel in
patients with esophageal squamous cell carcinoma refractory to
fluoropyrimidine- and platinum-based chemotherapy: OGSG1201. Eur J
Cancer. 154:307–315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Xu N, Xu J, Zhang C, Li X, Xu H,
Zhu W, Li J, Liang D and Zhou W: A risk signature based on
endoplasmic reticulum stress-associated genes predicts prognosis
and immunity in pancreatic cancer. Front Mol Biosci.
10:12980772023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ciftciler R and Balasar O: A rare CALR
variant mutation and efficient peginterferon alfa-2a response in a
patient with essential thrombocythemia. Cancer Genet.
274-275:51–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye J, Qi L, Du Z, Yu L, Chen K, Li R, Feng
R and Zhai W: Calreticulin: A potential diagnostic and therapeutic
biomarker in gallbladder cancer. Aging (Albany NY). 13:5607–5620.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han Y, Liao Q, Wang H, Rao S, Yi P, Tang
L, Tian Y, Oyang L, Wang H, Shi Y and Zhou Y: High expression of
calreticulin indicates poor prognosis and modulates cell migration
and invasion via activating Stat3 in nasopharyngeal carcinoma. J
Cancer. 10:5460–5468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sheng W, Wang G, Tang J, Shi X, Cao R, Sun
J, Lin YH, Jia C, Chen C, Zhou J and Dong M: Calreticulin promotes
EMT in pancreatic cancer via mediating Ca(2+) dependent acute and
chronic endoplasmic reticulum stress. J Exp Clin Cancer Res.
39:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Chen J, Zuo Q, Wu C, Yu T, Zheng
P, Huang H, Deng J, Fang L, Liu H, et al: Calreticulin enhances
gastric cancer metastasis by dimethylating H3K9 in the E-cadherin
promoter region mediating by G9a. Oncogenesis. 11:292022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Xie P, Hao N, Zhang M, Liu Y, Liu
P, Semenza GL, He J and Zhang H: HIF-1-regulated expression of
calreticulin promotes breast tumorigenesis and progression through
Wnt/β-catenin pathway activation. Proc Natl Acad Sci USA.
118:e21091441182021. View Article : Google Scholar
|
|
35
|
Lam STT and Lim CJ: Cancer biology of the
endoplasmic reticulum lectin chaperones calreticulin, calnexin and
PDIA3/ERp57. Prog Mol Subcell Biol. 59:181–196. 2021. View Article : Google Scholar
|
|
36
|
Liu Y, Liu Z and Wang K: The
Ca(2+)-activated chloride channel ANO1/TMEM16A: An emerging
therapeutic target for epithelium-originated diseases? Acta Pharm
Sin B. 11:1412–1433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song LL, Qu YQ, Tang YP, Chen X, Lo HH, Qu
LQ, Yun YX, Wong VKW, Zhang RL, Wang HM, et al: Hyperoside
alleviates toxicity of β-amyloid via endoplasmic
reticulum-mitochondrial calcium signal transduction cascade in
APP/PS1 double transgenic Alzheimer's disease mice. Redox Biol.
61:1026372023. View Article : Google Scholar
|
|
38
|
Chang Y, Funk M, Roy S, Stephenson E, Choi
S, Kojouharov HV, Chen B and Pan Z: Developing a mathematical model
of intracellular calcium dynamics for evaluating combined
anticancer effects of afatinib and RP4010 in esophageal cancer. Int
J Mol Sci. 23:17632022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Andrews C, Conneally E and Langabeer SE:
Molecular diagnostic criteria of myeloproliferative neoplasms.
Expert Rev Mol Diagn. 23:1077–1090. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mondesir J, Ghisi M, Poillet L, Bossong
RA, Kepp O, Kroemer G, Sarry JE, Tamburini J and Lane AA: AMPK
activation induces immunogenic cell death in AML. Blood Adv.
7:7585–7596. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Calvillo-Rodríguez KM, Mendoza-Reveles R,
Gómez-Morales L, Uscanga-Palomeque AC, Karoyan P, Martínez-Torres
AC and Rodríguez-Padilla C: PKHB1, a thrombospondin-1 peptide
mimic, induces anti-tumor effect through immunogenic cell death
induction in breast cancer cells. Oncoimmunology. 11:20543052022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu T, Zhu C, Song F, Zhang W, Yuan M, Pan
Z and Huang P: Immunological characteristics of immunogenic cell
death genes and malignant progression driving roles of TLR4 in
anaplastic thyroid carcinoma. BMC Cancer. 23:11312023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamashita K, Miyata H, Makino T, Masuike
Y, Furukawa H, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y,
Yamasaki M, et al: High expression of the mitophagy-related protein
pink1 is associated with a poor response to chemotherapy and a poor
prognosis for patients treated with neoadjuvant chemotherapy for
esophageal squamous cell carcinoma. Ann Surg Oncol. 24:4025–4032.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Zhuo S, Xu W, Chen X, Huang D, Sun
X and Cheng Y: Isocitrate dehydrogenase 2 contributes to radiation
resistance of oesophageal squamous cell carcinoma via regulating
mitochondrial function and ROS/pAKT signalling. Br J Cancer.
123:126–136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lan L, Wei W, Zheng Y, Niu L, Chen X,
Huang D, Gao Y, Mo S, Lu J, Guo M, et al: Deferoxamine suppresses
esophageal squamous cell carcinoma cell growth via ERK1/2 mediated
mitochondrial dysfunction. Cancer Lett. 432:132–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han A, Li C, Zahed T, Wong M, Smith I,
Hoedel K, Green D and Boiko AD: Calreticulin is a critical cell
survival factor in malignant neoplasms. PLoS Biol. 17:e30004022019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stoll G, Iribarren K, Michels J, Leary A,
Zitvogel L, Cremer I and Kroemer G: Calreticulin expression:
Interaction with the immune infiltrate and impact on survival in
patients with ovarian and non-small cell lung cancer.
Oncoimmunology. 5:e11776922016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Méndez-Ferrer S, Bonnet D, Steensma DP,
Hasserjian RP, Ghobrial IM, Gribben JG, Andreeff M and Krause DS:
Bone marrow niches in haematological malignancies. Nat Rev Cancer.
20:285–298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo H, Sun Y, Wang L, Liu H, Zhao R, Song
M and Ge H: Targeting endoplasmic reticulum associated degradation
pathway combined with radiotherapy enhances the immunogenicity of
esophageal cancer cells. Cancer Biol Ther. 24:21667632023.
View Article : Google Scholar : PubMed/NCBI
|