As an important metabolic organ in the human body,
the liver serves a crucial role in maintaining the homeostasis of
the internal environment and the normal function of the organism.
As a malignant tumor with high morbidity and mortality worldwide,
liver cancer has become one of the leading causes of cancer-related
deaths. According to the latest statistics, the incidence of liver
cancer in 2021 was 6.71/100,000 individuals, and the crude
mortality rate of liver cancer was 6.13/100,000 individuals
(1,2). Metabolic reprogramming of
hepatocellular carcinoma (HCC) cells is one of the central
mechanisms of tumor cell proliferation and development (3). In contrast to normal cells, which
rely primarily on oxidative phosphorylation (OXPHOS) for energy,
HCC cells tend to obtain energy through the glycolytic pathway even
under aerobic conditions, a phenomenon known as the 'Warburg
effect' (4). Although each
glucose molecule produces only two adenosine triphosphate (ATP)
molecules through aerobic glycolysis, while 36 ATP molecules can be
produced through OXPHOS, the faster rate of aerobic glycolysis
better meets the requirements for the rapid proliferation of tumor
cells (5). In addition, the
glycolytic product lactate can provide fuel for neighboring
oxygenated tumor cells, allowing the cooccurrence of glycolytic and
oxidative metabolism in the tumor microenvironment (TME) (6). Lactate dehydrogenase (LDH) is an
enzyme found in a wide range of tissues (such as heart muscle,
liver, kidney, skeletal muscle and lung) and is involved in the
final step of the glycolytic reaction, catalyzing the reversible
conversion between lactate and pyruvate (5). In tumor cells, LDH not only is
closely related to the rate of glycolysis and cofactor ratios but
also significantly affects the acidic environment of tumor cells
through the regulation of lactate production (7). LDH acts as a key regulator of tumor
development through the regulation of the biochemical environment
of the tumor, which has an important impact on processes such as
tumor cell invasion, immunosuppression, angiogenesis and metastasis
(7).
LDH serves an important role in the development,
diagnosis, treatment and prognosis of cardiovascular diseases,
infectious diseases, cancer and other diseases (8). LDH is not only an important marker
of myocardial injury but also serves an important role in the
development, progression and treatment of cardiovascular diseases
(9). Lactate dehydrogenase A
(LDHA) promotes cardiac remodeling by attenuating reactive oxygen
species (ROS) levels and promoting M2 macrophage polarization and
has the potential to be a target for myocardial infarction repair
(10,11). LDHA deficiency may lead to cardiac
hypertrophy and heart failure, and serum LDH levels are
significantly correlated with mortality in acute heart failure
(12,13). In addition, under high-glucose
conditions, LDHA may have some therapeutic potential, as it can
slow the progression of aortic coarctation (14). In hematologic diseases, elevation
of LDH levels is closely associated with a larger tumor load and
cell proliferation rate and can be used as an adjunctive diagnostic
and monitoring indicator for diseases such as leukemia and
pernicious anemia (15-18). Elevation of LDH levels in patients
with COVID-19 reflects cytokine-mediated tissue damage, multiorgan
dysfunction and hypercoagulability and significantly increases the
risk of severe illness and death, suggesting that elevated LDH
expression is closely related to poor prognosis (19-23). In scientific research, LDH is
often used in combination with other tumor markers as an important
marker of malignant tumors, and studies have shown that the degree
to which LDH levels are elevated is closely related to tumor load
and aggressiveness (8,24).
Although LDH serves an important role in disease
development, tumor metabolic reprogramming, regulation of the TME,
and has diagnostic and therapeutic potential, a systematic summary
and description of the specific role of LDH in liver cancer are
currently lacking. By reviewing the biological properties of LDH
and its role in the development of HCC, the present study aimed to
explore research progress related to the value of LDH in the
diagnosis, treatment and prognostic evaluation of HCC and to
provide novel targets and concepts for the diagnosis and treatment
of HCC.
LDH is an oxidoreductase consisting of four peptide
chains with a molecular weight of 134 kDa (25). LDH is a tetrameric enzyme
consisting of M (LDHA) and/or H [lactate dehydrogenase B (LDHB)]
subunits (7). LDHA and LDHB are
encoded by the LDHA gene located on chromosome 11 (11p15.1)
and the LDHB gene located on chromosome 12 (12p12.1),
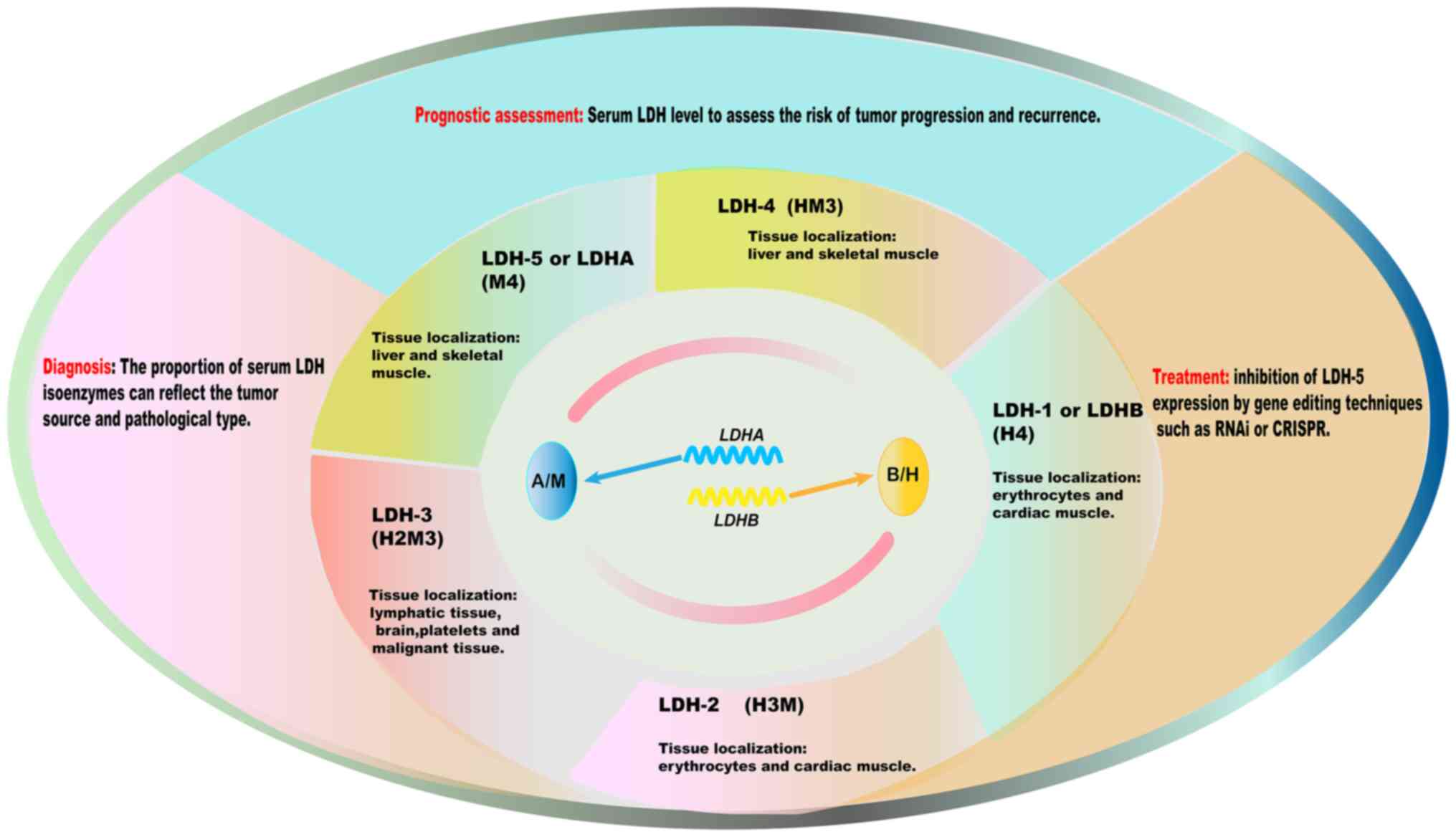
respectively (8). Five isoforms
of LDH, namely, LDH-1 (H4), LDH-2 (H3M), LDH-3 (H2M2), LDH-4 (HM3)
and LDH-5 (M4), can be produced by combining the A and B subunits
in different ways (7). LDH
isoforms are distributed in different tissues, with LDH-1 and LDH-2
being detected predominantly in the heart and erythrocytes, LDH-3
being detected predominantly in the brain, and LDH-5 and LDH-4
being detected predominantly in the liver and skeletal muscle
(26). There is also a
sperm-specific isozyme, LDH6, encoded by the LDHC gene
(located on chromosome 11) (5).
Of the LDH isoforms, LDH-5 (LDHA) has the strongest
ability to convert pyruvate to lactate because subunit M has a
strong ability to catalyze this reaction (27). By contrast, subunit H has the
ability to convert lactate back to pyruvate through the reverse
reaction (28). LDH is active
mainly in the cytoplasm and its main primary function is to
catalyze the reversible redox reaction between lactate and
pyruvate. LDH serves an important role in the balanced regulation
of the glycolytic and gluconeogenic pathways and participates in
cellular energy metabolism (29).
Under aerobic conditions, pyruvate enters mitochondria and the
tricarboxylic acid (TCA) cycle to produce large amounts of ATP
(30). During hypoxia or high
metabolic demand, LDH facilitates the reversible conversion of
pyruvate to lactate while oxidizing NADH to NAD+
(26). Catalysis of the
conversion of pyruvate to lactate by LDH helps to regulate the
intracellular NADH/NAD+ ratio, and a normal
intracellular NADH/NAD+ ratio is essential for various
cellular processes, including the oxidative stress response, DNA
repair and cell signaling (8).
Thus, LDH not only serves a key role in energy metabolism but is
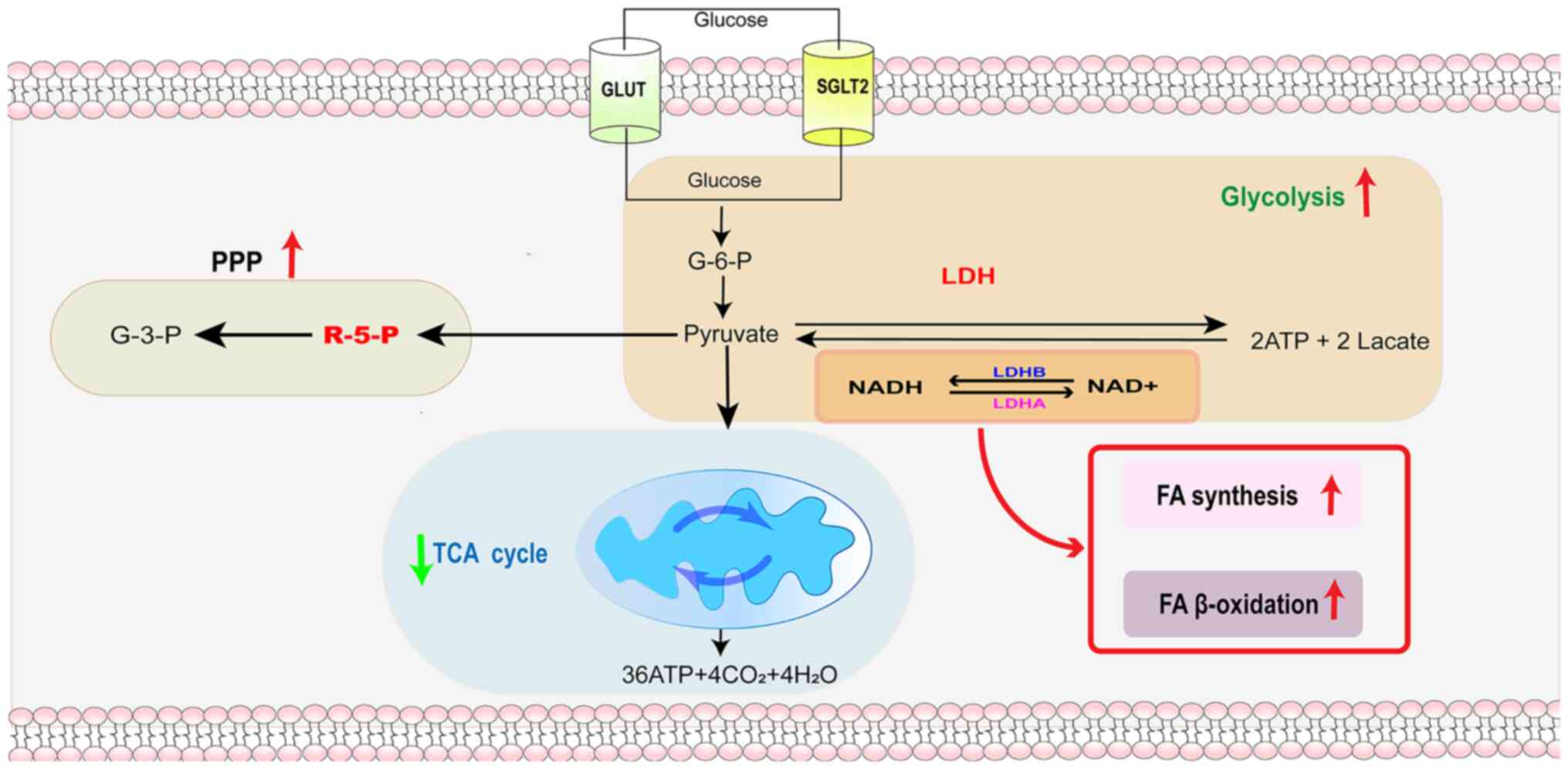
also involved in maintaining cellular redox homeostasis (27). LDH, an important catalytic enzyme
in glycolysis, can further support the metabolic demands of tumor
cells by converting lactate to pyruvate and promoting metabolic
synergy between glycolysis and OXPHOS (Fig. 1) (31).
LDH is a non-secreted enzyme and serum LDH activity
mainly reflects overall enzyme activity. According to standard
assays, the baseline activity of serum LDH is usually <247-248
U/l in adults (8,32). LDH activity in organs is closely
related to its metabolic activity and the physiological activity of
LDH in organs can reach as high as 9,000-25,000 U/g (26,33). There is a significant difference
in the expression levels of LDH in different organs and tissues;
LDH1 and LDH2 are more abundant in myocardium, while LDH5 and LDH4
are mainly in skeletal muscle and liver (26). LDH expression varies significantly
among different organs and is finely regulated by numerous
metabolic regulators, including the AMP-activated protein kinase
(AMPK) and mTOR signaling pathways (34). These key pathways directly
influence cellular metabolic adaptations by regulating LDH
expression and enzymatic activity. Under pathological conditions,
aberrant expression or activity of LDH is often closely associated
with the onset and progression of cardiovascular disease, liver
disease and cancer (8). In
addition, the metabolic activity of LDH is closely associated with
inflammatory responses and immunoregulatory networks, further
highlighting its complex role in pathophysiological processes
(35). LDH not only is an
important metabolic enzyme that serves a key pivotal role in
glycolysis and the TCA cycle but also performs multidimensional
functions in the regulation of cellular metabolism, tissue-specific
gene and protein expression and disease development. Elucidating
the biological properties and mechanism of action of LDH in
metabolic and pathological processes will provide an important
theoretical basis for understanding the pathogenesis of metabolic
diseases and tumors and LDH has potential value for the clinical
diagnosis and monitoring of diseases, in addition to serving as a
potential target for the development of novel therapeutic
strategies against these diseases.
As the metabolic center of the body, the liver not
only maintains glucose homeostasis by controlling multiple glucose
metabolic pathways (36) but also
participates in various lipid metabolism processes (37). HCC cells are metabolically
reprogrammed to meet the rapid proliferation needs of tumors
(34,38). HCC cells often exhibit enhanced
aerobic glycolysis, known as the 'Warburg effect' (34). Tumor cells usually produce lactic
acid through glycolysis in the presence of LDH (38). The study by Yang et al
(39) has shown that lactate can
promote the lactylation of adenylate kinase 2 at the K28 site,
which affects its enzyme activity, leading to an imbalance in
energy homeostasis in HCC cells and promoting tumor proliferation,
invasion and metastasis. Lactic acid produced by glycolysis can
also serve as a nutrient for cancer cells, which is closely related
to its ability to alleviate oxidative stress and facilitate lipid
synthesis, promoting tumor growth (34,40). Aerobic glycolysis in HCC cells
also provides important synthetic raw materials for macromolecular
synthesis, such as ribose 5-phosphate, a substrate of the
pentose-phosphate pathway, for nucleotide synthesis and the
glycolytic intermediates glycerol 3-phosphate and acetyl-coenzyme A
for lipid and protein synthesis (41,42). Abnormal lipid metabolism in HCC
cells can also drive the development of HCC (43). The increased ab initio
synthesis of lipids in HCC cells meets the energy and raw material
requirements for cell membrane synthesis and cell proliferation
(44). However, when the fatty
acid oxidation pathway is inhibited, the resulting lipid
accumulation enhances immunosuppression and promotes tumor growth
and metastasis; thus, it is often suggested that decreased fatty
acid metabolism is closely related to the aggressiveness of HCC
(45,46). In addition, the loss of
branched-chain amino acid catabolism has been shown to promote the
development of HCC (47).
Metabolic reprogramming in HCC is regulated by oncogene activation
or inactivation, signaling pathway abnormalities and the TME
(48-50). The activation of oncogenes such as
c-Myc, KRAS and PI3K/AKT can promote metabolic reprogramming in HCC
cells by increasing the expression of genes related to glycolysis
and lipid metabolism (51-53).
Hypoxia, a low pH and a tumor environment containing inflammatory
cytokines can induce glycolytic reprogramming of HCC cells by
activating related signaling pathways, such as the
hypoxia-inducible factor (HIF) pathway (54). HIF-1α, which is stably expressed
under hypoxic conditions, upregulates the expression of various
glycolysis-related genes, such as LDH and promotes glycolysis and
lactic acid production (55).
High expression and activity of LDH are key factors
for maintaining continuous glycolysis in HCC cells (56). Owing to the rapid proliferation of
HCC cells, the local oxygen supply is relatively insufficient and
these cells rely on glycolysis for energy (57). LDH catalyzes the conversion of
pyruvate to lactate and ensures the smooth progression to
glycolysis through the regeneration of NAD+ (58,59). The study by Manerba et al
(60) found that the inhibition
of LDH activity or expression significantly reduces the rate of
glycolysis in HCC cells, leading to a decrease in intracellular ATP
levels, the inhibition of cell proliferation and the induction of
apoptosis. LDH deficiency also leads to an intracellular redox
imbalance, which affects the normal metabolism and function of
cells (61). The catalysis of
lactate production by LDH affects the proliferation and invasion of
HCC cells through the regulation of the glutathione system and the
production of ROS, which subsequently affects the proliferation,
invasion and metastasis of HCC cells (38,62,63). LDH also promotes tumor growth by
promoting lactate production and enhancing ferroptosis resistance
in HCC cells (40). LDH-catalyzed
lactate metabolism serves a key role in HCC development, not only
providing energy support to tumor cells but also promoting invasion
and metastasis through metabolic regulation.
Although LDH is often associated with the glucose
metabolism pathway, LDH interacts also closely with other metabolic
pathways through glucose metabolism (64). For example, lactate can enter
mitochondria through the lactate shuttle and be converted to
pyruvate through reverse catalysis by LDH, which participates in
the TCA and provides additional energy to cells (65). Lipid metabolism serves a key role
in the development and progression of HCC and increased lipid
synthesis promotes the progression of HCC (46,66). LDHA indirectly affects the
activity of lipid oxidases and reduces the precursors required for
lipid synthesis by regulating NAD+/NADH homeostasis
(67,68). LDHA also upregulates sterol
regulatory factor binding protein 1 through activation of the
HIF-1α/mTOR pathway, which in turn promotes the expression of lipid
synthases, such as fatty acid synthase and acetyl-CoA carboxylase
(69). Inhibition of LDHA may
result in the reduction of precursors required for lipid synthesis
by restoring TCA activity (42).
Thus, LDH maintains the homeostasis of glucose metabolism while
indirectly influencing the balance of other metabolic pathways,
thus resulting in the rapid proliferation and survival of HCC cells
(Fig. 2) (7).
The TME in HCC is a complex multicomponent
environment composed of immune cell populations (including T
lymphocytes, macrophages, neutrophils and dendritic cells) as well
as non-immune components [such as fibroblasts, vascular and
lymphatic endothelial cells and the extracellular matrix (ECM)]
(70). The TME not only exerts
tumor suppressive effects through immunosurveillance and
immunomodulation but also promotes tumor cell invasion and
metastasis as well as immune escape to achieve tumor progression
(71,72).
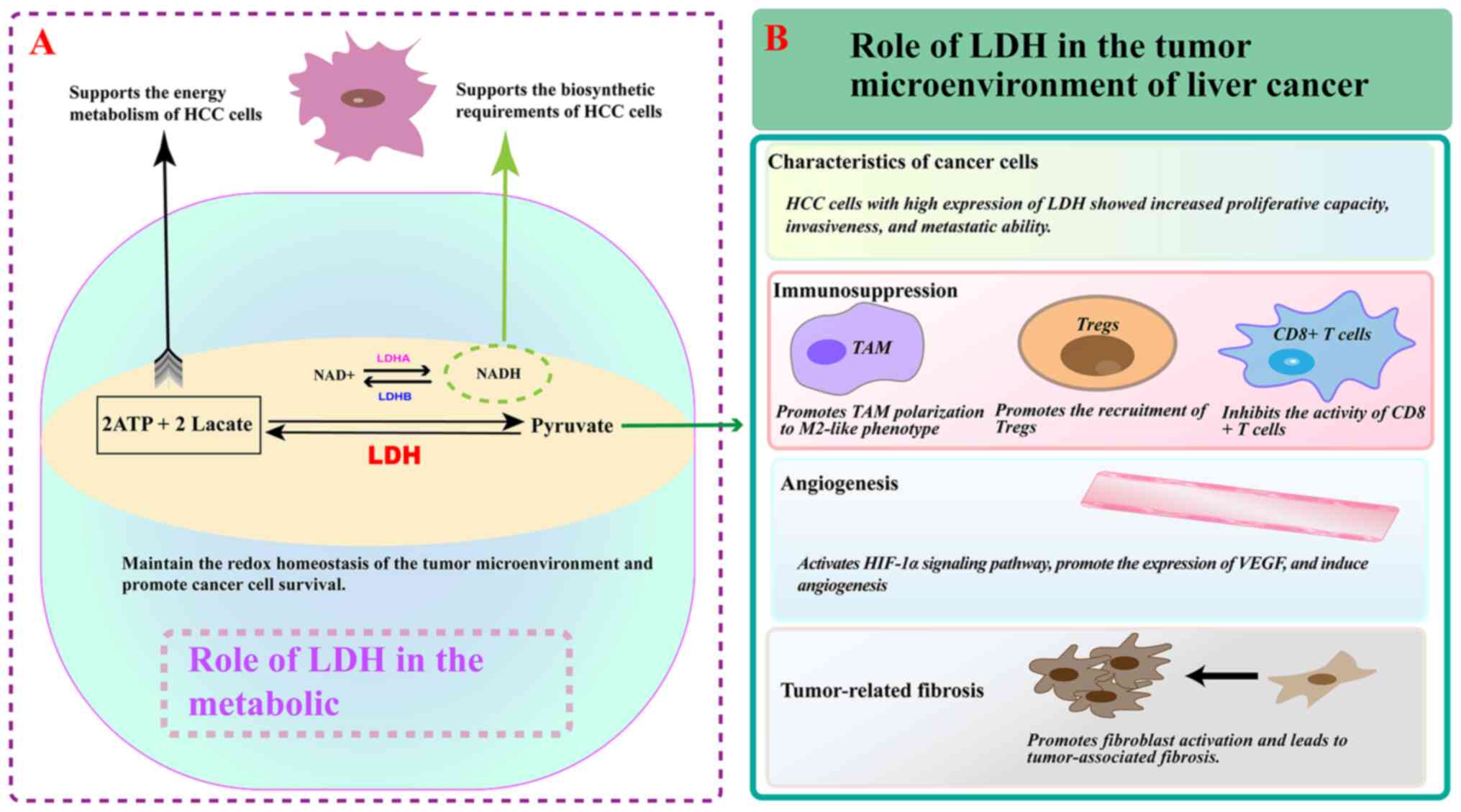
Synergistically, LDH and monocarboxylic acid
transporter protein coordinate lactate production and transport and
serve an important role in the regulation of the tumor immune
microenvironment (73-75). LDHA is expressed mainly in bone
and liver tissues, but numerous studies have shown that it is also
commonly expressed in malignant tumors (73-76,78). The stability of tumor-infiltrating
T regulatory cells (Treg cells) is affected by LDHA knockdown or
LDH inhibitors (77,78). In a previous study of immune cells
in tumors, LDHA levels were found to be significantly elevated in
activated lymphocytes, which can exert tumor suppressive effects by
upregulating LDHA and engaging in glycolysis (79). LDH can also be involved in
modulating the function of suppressor immune cells, such as T
lymphocytes, which help tumor cells evade immune surveillance and
promote the progression of HCC (80). The important role of LDH in the
TME was pointed out in a study by Verma et al (78), which reported that the inhibition
of LDH not only regulates the glycolytic balance between tumor
cells and Tregs, but also alters the functional state of antitumor
T cells (78). In HCC, LDH
regulates tumor-associated macrophages (TAMs) and tumor-associated
fibroblasts (TAFs) by modulating lactate production (81). Studies have shown that lactate can
contribute to the polarization of TAMs to an M2-like phenotype,
causing them to secrete cytokines and chemokines that promote tumor
cell growth, angiogenesis and metastasis and facilitate tumor
progression (82,83). In TAFs, lactate can stimulate the
production of ECM and growth factors to form a supportive stromal
environment for HCC cells (84).
LDH-mediated production of lactate can also affect endothelial cell
function in the tumor vasculature, promoting angiogenesis and
increasing nutrient and oxygen supply to the tumor (85). Therefore, LDH can influence tumor
development by affecting the function of immune cells and
non-immune components in the immune microenvironment of HCC tumors
(Fig. 3).
Owing to its wide distribution in tissues and its
important role in metabolic reprogramming, LDH serves an important
role in the development of different diseases. Numerous studies
have suggested that LDH has an important role in the development of
liver diseases such as liver injury, liver fibrosis and HCC
(34,55,86). In a study of liver disease
development, LDHA was found to affect the interaction between LDHA
and HIF-1α in the classical Wnt signaling pathway, thereby
regulating glycolysis and liver fibrosis in hepatic stellate cells
(55). Salvianolic acid B can
inhibit M1 macrophage polarization by downregulating the expression
levels of LDHA, thus exerting a therapeutic effect on liver injury
(87). A recent study has shown
that the upregulation of LDHA expression in liver injury
contributes to pericentral regeneration of the liver (86). Protein arginine methyltransferase
3 promotes HCC growth by enhancing arginine methylation of LDHA
(88).
Since different organs contain different proportions
of LDH isoforms, the proportion of LDH isoforms in the blood can
help determine the source of released LDH (8,26).
By measuring the activity of different LDH isoforms, it is possible
to distinguish between different diseases, such as infections,
hemolytic anemia, liver disease, kidney disease, heart disease and
cancer (8,89). In studies of melanoma and
non-Hodgkin's lymphoma, elevated serum total LDH levels and the
levels of different subtypes of LDH were found to be useful not
only for diagnosing tumors but also for the prognostic assessment
of tumors (90,91). Serum LDH levels are often elevated
in patients with HCC (92).
Elevation of the serum LDH level is thought to be due to the
release of LDH from tumor cells into the bloodstream, which may be
due to cell necrosis or active secretion (27). Serum LDH levels have been shown to
be correlated with tumor size, stage and metastasis in HCC
(27,93). Measuring serum LDH activity can
aid the early screening and diagnosis of HCC (94,95). Changes in the proportions of
different LDH isoforms can also reflect the pathological type and
stage of HCC to a certain extent, which can improve the accuracy of
diagnosis (96,97). Combining serum LDH with other
traditional biomarkers, such as a-fetoprotein (AFP), can improve
diagnostic accuracy for HCC (98). In certain cases, patients with
normal AFP levels but elevated serum LDH levels may still be at
increased risk for HCC (99),
suggesting that LDH can be used as an adjunctive diagnostic
indicator, especially for AFP-negative HCC (100).
The expression patterns of LDH isoforms in tumor
tissues can provide valuable information for tumor diagnosis and
prognostic evaluation (101,102). LDH5 is usually upregulated in
HCC samples compared with that of normal liver tissue, and the
expression ratio of LDH5 to other LDH isoforms may be altered
(103,104). Increased levels of LDH5 in tumor
tissues are associated with more aggressive tumor behavior,
including increased rates of proliferation and greater invasion and
metastasis abilities (7,105). The expression levels of LDH
isozymes can be used to predict the response to therapy in patients
with HCC (101). Patients with
high LDH5-expressing tumors may have a poorer response to
conventional chemotherapy or targeted therapies, suggesting that
LDH isoform analysis can aid in selecting personalized treatment
regimens for patients with HCC (7). High serum LDH levels are associated
with poor prognosis in patients with HCC (106). Patients with HCC with elevated
serum LDH levels at multiple time points have shorter overall
survival and higher recurrence rates after treatment, including
surgical resection, liver transplantation and ablative therapy
(2). Previous studies have shown
that high preoperative serum LDH levels in patients with HCC tend
to portend a poorer prognosis, including shorter survival and
higher recurrence rates (2,106,107). The serum LDH level can be an
independent prognostic factor, even after adjusting for other
clinicopathological factors (108). LDH expression in tumor tissues
also has important prognostic significance (101). Patients with high levels of LDH
expression have a worse prognosis compared with that of patients
with low levels of LDH expression (7,56,101). Monitoring LDH expression in
tumor tissue may help clinicians stratify patients according to
outcomes and determine more appropriate treatment strategies
(109). However, the specificity
of serum LDH as a diagnostic biomarker is limited because elevated
LDH levels may be observed in other diseases, such as cirrhosis,
hepatitis and other malignancies (99,100,110). Therefore, serum LDH has some
applications in research as a tumor marker, especially in exploring
energy metabolism in and the microenvironment of tumor cells, but
it is not a commonly used tumor marker in clinical practice. Owing
to its lack of specificity and insufficient sensitivity, serum LDH
is usually not used as a preferred indicator for diagnosing or
monitoring tumors. In practice, physicians usually use LDH in
combination with other more specific tumor markers, imaging
techniques and pathology to comprehensively determine a patient's
condition (98-100).
Numerous small-molecule compounds are currently
being developed to treat HCC, with the aim of inhibiting the
activity of LDH and blocking abnormal glycolysis in cancer cells to
suppress the proliferation of HCC cells and induce their apoptosis
(111-114). Previous studies have suggested
that the regulation of angiogenesis through the VEGF/VEGF-receptor
signaling pathway, which is the target of tyrosine kinase
inhibitors such as sorafenib, serves a key role in the progression
of HCC (111,112). A previous study reported that
knockdown of LDHA significantly inhibits the proliferation,
migration and invasion of HCC cells and increases sensitivity to
sorafenib (113).
Polyadenylation-specific factor 6 causes changes in LDH to achieve
Warburg effect-mediated immune escape and angiogenesis; contributes
to cancer progression through c-Myc via the hexokinase, programmed
death-ligand 1 and VEGF pathways, and has the potential to
synergize with sorafenib to treat HCC (114). Given the key role of LDH in HCC
metabolism and progression, LDH inhibitors have emerged as
promising therapeutic agents for HCC (115,116). Several small-molecule inhibitors
of LDH, such as FX11 and GNE-140 (117,118), have been developed. These
inhibitors target the active site of LDH, block its enzymatic
activity and disrupt the glycolytic pathway in HCC cells (116).
In preclinical studies, the targeted inhibition of
LDH using drugs and other means has been shown to have significant
antitumor effects in HCC cell lines and animal models (119). Treatment with LDH inhibitors
results in decreased glycolytic flux, reduced ATP production and
induction of HCC cell apoptosis (7,120). In addition, combining LDH
inhibitors with other anticancer drugs, such as sorafenib, can, in
some cases, enhance antitumor efficacy and overcome drug resistance
(121). However, the translation
of LDH inhibitors from preclinical to clinical use is hampered by
issues such as poor solubility, off-target effects and limited
bioavailability (122). The
ability of gene therapy approaches such as RNA interference (RNAi)
and CRISPR/Cas9-mediated gene editing have also been explored
(123). RNAi-based strategies
can specifically reduce the expression of the LDH gene, which
results in reduced LDH protein levels and enzymatic activity
(124). However, there are
challenges related to RNAi and CRISPR/Cas9-based, such as achieving
effective delivery to tumor cells, potential immune responses and
off-target effects (125-127).
Overcoming these challenges is critical for the successful
development of gene therapy strategies targeting LDH for the
treatment of HCC. Combining LDH-targeted therapies with traditional
surgery, chemotherapy, radiotherapy and emerging immunotherapies to
achieve synergistic effects may further increase the efficacy of
HCC treatment and improve the quality of life and survival of
patients (Table I).
LDH serves a key multidimensional role in the
complex pathology of HCC and influences the developmental
trajectory of HCC in numerous aspects. From the perspective of
metabolic regulation, LDH actively maintains glycolysis to ensure
that tumor cells can efficiently produce energy in an aerobic
environment, providing a sufficient energy supply for the rapid
proliferation of cancer cells (128). Moreover, LDH also finely
regulates the redox balance, maintains the stability of the
intracellular environment, and creates favorable conditions for the
survival and proliferation of cancer cells (129). LDH also closely interacts with
other metabolic pathways, further strengthening the metabolic
adaptability and viability of tumor cells and promoting HCC
occurrence, development, and metastasis (64). In the TME, LDH regulates the
secretion of cytokines and chemokines to remodel the TME and
promote the invasion and metastasis of tumor cells (31).
LDH has also shown value in the diagnosis and
treatment of liver cancer, as LDH serves a central role in the
disease (8). As a potential
diagnostic marker for HCC, LDH can act as a sensitive indicator in
the early stages of the disease, although it is not commonly used
in clinical practice because of its lack of specificity. The
combination of LDH with commonly used HCC markers can improve the
accuracy of HCC diagnosis and can assist in the early screening and
diagnosis of HCC (100). In
addition, the expression patterns of different isoforms of LDH are
important in the pathological typing and staging of HCC (8). Regarding the prognostic assessment
of HCC, serum LDH levels are correlated with survival and
recurrence rates and high LDH5 expression in tumor tissues is
correlated with aggressive tumor behavior as well as the
therapeutic response; thus, by using LDH, clinicians may accurately
assess disease progression and therapeutic effects and formulate
personalized therapeutic plans (7). Given the important role of LDH in
the metabolic reprogramming of HCC as well as in the TME, the use
of LDH as a therapeutic target is a key direction for the
development of novel anticancer drugs. Despite the potential of LDH
as a target for liver cancer therapy, numerous challenges to fully
utilizing the therapeutic potential of targeting LDH remain.
Currently, more specific and potent LDH inhibitors are needed;
specifically, the ability of inhibitors to target LDH in tumor
cells, their inhibitory effect and pharmacokinetic properties need
to be improved to ensure that the drugs can exert their effects
stably and efficiently in vivo and to reduce toxicity and
side effects on normal tissues. In addition, inefficiency in
delivering gene therapy components targeting LDH to cells as well
as the risk of off-target effects are key obstacles to clinical
application (122). Furthermore,
the mechanisms underlying the complex interactions between LDH and
other metabolic pathways and signaling pathways are not fully
understood and an in-depth analysis of these mechanisms will
provide strong support for the development of more precise and
effective therapeutic strategies for HCC. In the future,
LDH-related biomarkers have potential for use in clinical practice
for early diagnosis, precise prognostic prediction and the
selection of personalized therapeutic regimens for liver cancer.
With the continuous advances in precision medicine and tumor
metabolism research, targeting LDH may become an important approach
for the comprehensive treatment of HCC, bringing new treatment
opportunities for patients with liver cancer in the future.
Not applicable.
HJ wrote the original draft. QL wrote the original
draft and created the figures and tables. JL participated in the
literature search and analysis of the data to be included in the
review. SZ was assisted in the preparation of the figures and
table. BT acquired funding acquisition and helped with review
writing and editing. Data authentication is not applicable. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by grants from The National
Natural Science Foundation of China (grant no. 82460466), the
Science and Technology Plan Project of Guizhou Province [grant nos.
QIAN KE HE JI CHU-ZK (2024) YI BAN 323, QIAN KE HE JI CHU-ZK (2023)
ZHONG DIAN 058, and QIAN KE HE JI CHU-ZK (2022) YI BAN 646].
|
1
|
Satriano L, Lewinska M, Rodrigues PM,
Banales JM and Andersen JB: Metabolic rearrangements in primary
liver cancers: Cause and consequences. Nat Rev Gastroenterol
Hepatol. 16:748–766. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan EY, Danpanichkul P, Yong JN, Yu Z, Tan
DJH, Lim WH, Koh B, Lim RYZ, Tham EKJ, Mitra K, et al: Liver cancer
in 2021: Global burden of disease study. J Hepatol. 82:851–860.
2025. View Article : Google Scholar
|
|
3
|
Schmidt DR, Patel R, Kirsch DG, Lewis CA,
Vander Heiden MG and Locasale JW: Metabolomics in cancer research
and emerging applications in clinical oncology. CA Cancer J Clin.
71:333–358. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao M, Yao D, Wu L, Luo C, Wang Z, Zhang
J and Liu B: Targeting the Warburg effect: A revisited perspective
from molecular mechanisms to traditional and innovative therapeutic
strategies in cancer. Acta Pharm Sin B. 14:953–1008. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comandatore A, Franczak M, Smolenski RT,
Morelli L, Peters GJ and Giovannetti E: Lactate dehydrogenase and
its clinical significance in pancreatic and thoracic cancers. Semin
Cancer Biol. 86:93–100. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sonveaux P, Végran F, Schroeder T, Wergin
MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C,
Jordan BF, et al: Targeting lactate-fueled respiration selectively
kills hypoxic tumor cells in mice. J Clin Invest. 118:3930–3942.
2008.PubMed/NCBI
|
|
7
|
Sharma D, Singh M and Rani R: Role of LDH
in tumor glycolysis: Regulation of LDHA by small molecules for
cancer therapeutics. Semin Cancer Biol. 87:184–195. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Claps G, Faouzi S, Quidville V, Chehade F,
Shen S, Vagner S and Robert C: The multiple roles of LDH in cancer.
Nat Rev Clin Oncol. 19:749–762. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Kang K, Chen S, Su Q, Zhang W,
Zeng L, Lin X, Peng F, Lin J and Chai D: High serum lactate
dehydrogenase as a predictor of cardiac insufficiency at follow-up
in elderly patients with acute myocardial infarction. Arch Gerontol
Geriatr. 117:1052532024. View Article : Google Scholar
|
|
10
|
Feng H, Wu J, Chen P, Wang J, Deng Y, Zhu
G, Xian J, Huang L and Ouyang W: MicroRNA-375-3p inhibitor
suppresses angiotensin II-induced cardiomyocyte hypertrophy by
promoting lactate dehydrogenase B expression. J Cell Physiol.
234:14198–14209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai C, Li Q, May HI, Li C, Zhang G, Sharma
G, Sherry AD, Malloy CR, Khemtong C, Zhang Y, et al: Lactate
dehydrogenase A governs cardiac hypertrophic growth in response to
hemodynamic stress. Cell Rep. 32:1080872020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Wu G, Li M, Hesse M, Ma Y, Chen W,
Huang H, Liu Y, Xu W, Tang Y, et al: LDHA-mediated metabolic
reprogramming promoted cardiomyocyte proliferation by alleviating
ROS and inducing M2 macrophage polarization. Redox Biol.
56:1024462022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi S, Abe M, Arakaki T, Arasaki O
and Shimabukuro M: Prognostic value of lactate dehydrogenase for
mid-term mortality in acute decompensated heart failure: A
comparison to established biomarkers and brain natriuretic peptide.
Heart Lung Circ. 29:1318–1327. 2020. View Article : Google Scholar
|
|
14
|
Wu X, Ye J, Cai W, Yang X, Zou Q, Lin J,
Zheng H, Wang C, Chen L and Li Y: LDHA mediated degradation of
extracellular matrix is a potential target for the treatment of
aortic dissection. Pharmacol Res. 176:1060512022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ibraheem A, Nashwan AJ and Yassin MA:
Elderly patient with hematological and neurological manifestations
of undetermined origin: A diagnostic dilemma of pernicious anemia.
Cureus. 15:e430452023.PubMed/NCBI
|
|
16
|
Wahhab Ali KA, Ahmed AA and Mohammed ST:
Determination of serum myeloperoxidase (MPO) and lactate
dehydrogenase (LDH) as a tumour marker in chronic myeloid leukaemia
(CML). J Pak Med Assoc. 74(10 (Supple-8)): S283–S286. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oriaifo IA, Gerard JM and Thomas SM:
Diagnostic value of lactate dehydrogenase and uric acid as
screening tools for malignancies in children. Pediatr Emerg Care.
38:e1327–e1331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Labban H, Begum F, Paracha A, John V and
Islam M: Hemolytic anemia and pancytopenia secondary to vitamin B12
deficiency: Evaluation and clinical significance. Cureus.
16:e572862024.PubMed/NCBI
|
|
19
|
Henry BM, Aggarwal G, Wong J, Benoit S,
Vikse J, Plebani M and Lippi G: Lactate dehydrogenase levels
predict coronavirus disease 2019 (COVID-19) severity and mortality:
A pooled analysis. Am J Emerg Med. 38:1722–1726. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Assiri A, Al-Tawfiq JA, Al-Rabeeah AA,
Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN,
Balkhy HH, Al-Hakeem RF, et al: Epidemiological, demographic, and
clinical characteristics of 47 cases of Middle East respiratory
syndrome coronavirus disease from Saudi Arabia: A descriptive
study. Lancet Infect Dis. 13:752–761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martinez-Outschoorn UE, Prisco M, Ertel A,
Tsirigos A, Lin Z, Pavlides S, Wang C, Flomenberg N, Knudsen ES,
Howell A, et al: Ketones and lactate increase cancer cell
'stemness', driving recurrence, metastasis and poor clinical
outcome in breast cancer: Achieving personalized medicine via
metabolo-genomics. Cell Cycle. 10:1271–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lippi G and Favaloro EJ: D-dimer is
associated with severity of coronavirus disease 2019: A pooled
analysis. Thromb Haemost. 120:876–878. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serrano-Lorenzo P, Coya ON, López-Jimenez
A, Blázquez A, Delmiro A, Lucia A, Arenas J and Martín MA; COVID-19
'12 Octubre' Hospital Clinical Biochemistry Study Group: Plasma
LDH: A specific biomarker for lung affectation in COVID-19? Pract
Lab Med. 25:e002262021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Qi M and Yang M: Current status
and future perspectives of lactate dehydrogenase detection and
medical implications: A review. Biosensors (Basel). 12:11452022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panteghini M: Lactate dehydrogenase: an
old enzyme reborn as a COVID-19 marker (and not only). Clin Chem
Lab Med. 58:1979–1981. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan AA, Allemailem KS, Alhumaydhi FA,
Gowder SJT and Rahmani AH: The biochemical and clinical
perspectives of lactate dehydrogenase: An enzyme of active
metabolism. Endocr Metab Immune Disord Drug Targets. 20:855–868.
2020. View Article : Google Scholar
|
|
27
|
Forkasiewicz A, Dorociak M, Stach K,
Szelachowski P, Tabola R and Augoff K: The usefulness of lactate
dehydrogenase measurements in current oncological practice. Cell
Mol Biol Lett. 25:352020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fondy TP and Kaplan NO: Structural and
functional properties of the H and M subunits of lactic
dehydrogenases. Ann N Y Acad Sci. 119:888–904. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hicks KG, Cluntun AA, Schubert HL, Hackett
SR, Berg JA, Leonard PG, Ajalla Aleixo MA, Zhou Y, Bott AJ,
Salvatore SR, et al: Protein-metabolite interactomics of
carbohydrate metabolism reveal regulation of lactate dehydrogenase.
Science. 379:996–1003. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eniafe J and Jiang S: The functional roles
of TCA cycle metabolites in cancer. Oncogene. 40:3351–3363. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tufail M, Jiang CH and Li N: Altered
metabolism in cancer: Insights into energy pathways and therapeutic
targets. Mol Cancer. 23:2032024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schumann G and Klauke R: New IFCC
reference procedures for the determination of catalytic activity
concentrations of five enzymes in serum: Preliminary upper
reference limits obtained in hospitalized subjects. Clin Chim Acta.
327:69–79. 2003. View Article : Google Scholar
|
|
33
|
Roman W: Quantitative estimation of
lactate dehydrogenase isoenzymes in serum. I. Review of methods and
distribution in human tissues. Enzymologia. 36:189–219.
1969.PubMed/NCBI
|
|
34
|
Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S,
Hao H and Xiong J: Metabolic dysregulation and emerging
therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin
B. 12:558–580. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta GS: The lactate and the lactate
dehydrogenase in inflammatory diseases and major risk factors in
COVID-19 patients. Inflammation. 45:2091–2123. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han HS, Kang G, Kim JS, Choi BH and Koo
SH: Regulation of glucose metabolism from a liver-centric
perspective. Exp Mol Med. 48:e2182016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Piccinin E, Villani G and Moschetta A:
Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: The
role of PGC1 coactivators. Nat Rev Gastroenterol Hepatol.
16:160–174. 2019. View Article : Google Scholar
|
|
38
|
Yang F, Hilakivi-Clarke L, Shaha A, Wang
Y, Wang X, Deng Y, Lai J and Kang N: Metabolic reprogramming and
its clinical implication for liver cancer. Hepatology.
78:1602–1624. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S,
Shen X, Wu Y, Zhang S, Wang X, et al: Lactylome analysis suggests
lactylation-dependent mechanisms of metabolic adaptation in
hepatocellular carcinoma. Nat Metab. 5:61–79. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z,
Cai K, Zhao Y and Luo Z: HCAR1/MCT1 regulates tumor ferroptosis
through the lactate-mediated AMPK-SCD1 activity and its therapeutic
implications. Cell Rep. 33:1084872020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ganapathy-Kanniappan S: Molecular
intricacies of aerobic glycolysis in cancer: Current insights into
the classic metabolic phenotype. Crit Rev Biochem Mol Biol.
53:667–682. 2018. View Article : Google Scholar
|
|
43
|
Borrelli A, Bonelli P, Tuccillo FM,
Goldfine ID, Evans JL, Buonaguro FM and Mancini A: Role of gut
microbiota and oxidative stress in the progression of non-alcoholic
fatty liver disease to hepatocarcinoma: Current and innovative
therapeutic approaches. Redox Biol. 15:467–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng Y, He J, Zuo B and He Y: Role of
lipid metabolism in hepatocellular carcinoma. Discov Oncol.
15:2062024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo D, Zhang X, Cui H, Yu D, Zhang H, Shi
X, Pang C, Li J, Guo W and Zhang S: ACADL functions as a tumor
suppressor in hepatocellular carcinoma metastasis by inhibiting
matrix metalloproteinase 14. Front Oncol. 12:8214842022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cai J, Chen T, Jiang Z, Yan J, Ye Z, Ruan
Y, Tao L, Shen Z, Liang X, Wang Y, et al: Bulk and single-cell
transcriptome profiling reveal extracellular matrix mechanical
regulation of lipid metabolism reprograming through YAP/TEAD4/ACADL
axis in hepatocellular carcinoma. Int J Biol Sci. 19:2114–2131.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ericksen RE, Lim SL, McDonnell E, Shuen
WH, Vadiveloo M, White PJ, Ding Z, Kwok R, Lee P, Radda GK, et al:
Loss of BCAA catabolism during carcinogenesis enhances mTORC1
activity and promotes tumor development and progression. Cell
Metab. 29:1151–1165.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tian LY, Smit DJ and Jücker M: The Role of
PI3K/AKT/mTOR signaling in hepatocellular carcinoma metabolism. Int
J Mol Sci. 24:26522023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bao MHR and Wong CCL: Hypoxia, metabolic
reprogramming, and drug resistance in liver cancer. Cells.
10:17152021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ng CKY, Dazert E, Boldanova T,
Coto-Llerena M, Nuciforo S, Ercan C, Suslov A, Meier MA, Bock T,
Schmidt A, et al: Integrative proteogenomic characterization of
hepatocellular carcinoma across etiologies and stages. Nat Commun.
13:24362022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
An J, Oh M, Kim SY, Oh YJ, Oh B, Oh JH,
Kim W, Jung JH, Kim HI, Kim JS, et al: PET-based radiogenomics
supports mTOR pathway targeting for hepatocellular carcinoma. Clin
Cancer Res. 28:1821–1831. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen J, Ding C, Chen Y, Hu W, Yu C, Peng
C, Feng X, Cheng Q, Wu W, Lu Y, et al: ACSL4 reprograms fatty acid
metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway.
Cancer Lett. 502:154–165. 2021. View Article : Google Scholar
|
|
53
|
Luo YD, Liu XY, Fang L, Yu HQ, Zhang YJ,
Chen M, Zhang LD and Xie CM: Mutant Kras and mTOR crosstalk drives
hepatocellular carcinoma development via PEG3/STAT3/BEX2 signaling.
Theranostics. 12:7903–7919. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lin D and Wu J: Hypoxia inducible factor
in hepatocellular carcinoma: A therapeutic target. World J
Gastroenterol. 21:12171–12178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang F, Chen L, Kong D, Zhang X, Xia S,
Liang B, Li Y, Zhou Y, Zhang Z, Shao J, et al: Canonical Wnt
signaling promotes HSC glycolysis and liver fibrosis through an
LDH-A/HIF-1α transcriptional complex. Hepatology. 79:606–623. 2024.
View Article : Google Scholar
|
|
56
|
Faloppi L, Bianconi M, Memeo R, Casadei
Gardini A, Giampieri R, Bittoni A, Andrikou K, Del Prete M, Cascinu
S and Scartozzi M: Lactate dehydrogenase in hepatocellular
carcinoma: something old, something new. Biomed Res Int.
2016:71962802016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai
W and Guo C: Emerging roles and the regulation of aerobic
glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res.
39:1262020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vaupel P, Schmidberger H and Mayer A: The
Warburg effect: Essential part of metabolic reprogramming and
central contributor to cancer progression. Int J Radiat Biol.
95:912–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Y, Li W, Bian Y, Li Y and Cong L:
Multifaceted roles of aerobic glycolysis and oxidative
phosphorylation in hepatocellular carcinoma. PeerJ. 11:e147972023.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Manerba M, Di Ianni L, Govoni M, Roberti
M, Recanatini M and Di Stefano G: LDH inhibition impacts on heat
shock response and induces senescence of hepatocellular carcinoma
cells. Eur J Pharm Sci. 105:91–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sun Z, Liu L, Liang H and Zhang L:
Nicotinamide mononucleotide induces autophagy and ferroptosis via
AMPK/mTOR pathway in hepatocellular carcinoma. Mol Carcinog.
63:577–588. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Certo M, Tsai CH, Pucino V, Ho PC and
Mauro C: Lactate modulation of immune responses in inflammatory
versus tumour microenvironments. Nat Rev Immunol. 21:151–161. 2021.
View Article : Google Scholar
|
|
63
|
Jeong DW, Cho IT, Kim TS, Bae GW, Kim IH
and Kim IY: Effects of lactate dehydrogenase suppression and
glycerol-3-phosphate dehydrogenase overexpression on cellular
metabolism. Mol Cell Biochem. 284:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin J, Rao D, Zhang M and Gao Q: Metabolic
reprogramming in the tumor microenvironment of liver cancer. J
Hematol Oncol. 17:62024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang RN and Fan JG: Lipid
metabolism-related long noncoding RNAs: A potential prognostic
biomarker for hepatocellular carcinoma. World J Gastroenterol.
30:3799–3802. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Koukourakis MI, Kakouratos C, Kalamida D,
Bampali Z, Mavropoulou S, Sivridis E and Giatromanolaki A:
Hypoxia-inducible proteins HIF1α and lactate dehydrogenase LDH5,
key markers of anaerobic metabolism, relate with stem cell markers
and poor post-radiotherapy outcome in bladder cancer. Int J Radiat
Biol. 92:353–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
He W, Li Q and Li X: Acetyl-CoA regulates
lipid metabolism and histone acetylation modification in cancer.
Biochim Biophys Acta Rev Cancer. 1878:1888372023. View Article : Google Scholar
|
|
69
|
Zhao X, Jiang P, Deng X, Li Z, Tian F, Guo
F, Li X and Wang S: Inhibition of mTORC1 signaling sensitizes
hepatocellular carcinoma cells to glycolytic stress. Am J Cancer
Res. 6:2289–2298. 2016.PubMed/NCBI
|
|
70
|
Sas Z, Cendrowicz E, Weinhäuser I and
Rygiel TP: Tumor microenvironment of hepatocellular carcinoma:
Challenges and opportunities for new treatment options. Int J Mol
Sci. 23:37782022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Neophytou CM, Panagi M, Stylianopoulos T
and Papageorgis P: The role of tumor microenvironment in cancer
metastasis: Molecular mechanisms and therapeutic opportunities.
Cancers (Basel). 13:20532021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Peng X, He Z, Yuan D, Liu Z and Rong P:
Lactic acid: The culprit behind the immunosuppressive
microenvironment in hepatocellular carcinoma. Biochim Biophys Acta
Rev Cancer. 1879:1891642024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Luo Y, Li L, Chen X, Gou H, Yan K and Xu
Y: Effects of lactate in immunosuppression and inflammation:
Progress and prospects. Int Rev Immunol. 41:19–29. 2022. View Article : Google Scholar
|
|
75
|
Zhang Y, Zhai Z, Duan J, Wang X, Zhong J,
Wu L, Li A, Cao M, Wu Y, Shi H, et al: Lactate: The mediator of
metabolism and immunosuppression. Front Endocrinol (Lausanne).
13:9014952022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Maeda M, Ko M, Mane MM, Cohen IJ, Shindo
M, Vemuri K, Serganova I and Blasberg R: Genetic and drug
inhibition of LDH-A: Effects on murine gliomas. Cancers (Basel).
14:23062022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sarkar T, Dhar S and Sa G:
Tumor-infiltrating T-regulatory cells adapt to altered metabolism
to promote tumor-immune escape. Curr Res Immunol. 2:132–141. 2021.
View Article : Google Scholar
|
|
78
|
Verma S, Budhu S, Serganova I, Dong L,
Mangarin LM, Khan JF, Bah MA, Assouvie A, Marouf Y, Schulze I, et
al: Pharmacologic LDH inhibition redirects intratumoral glucose
uptake and improves antitumor immunity in solid tumor models. J
Clin Invest. 134:e1776062024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
O'Neill LA, Kishton RJ and Rathmell J: A
guide to immunometabolism for immunologists. Nat Rev Immunol.
16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Niu D, Luo T, Wang H, Xia Y and Xie Z:
Lactic acid in tumor invasion. Clin Chim Acta. 522:61–69. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Han S, Bao X, Zou Y, Wang L, Li Y, Yang L,
Liao A, Zhang X, Jiang X, Liang D, et al: d-lactate modulates M2
tumor-associated macrophages and remodels immunosuppressive tumor
microenvironment for hepatocellular carcinoma. Sci Adv.
9:eadg26972023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang Y, Han Q, Zhao H and Zhang J:
Promotion of epithelial-mesenchymal transformation by
hepatocellular carcinoma-educated macrophages through
Wnt2b/β-catenin/c-Myc signaling and reprogramming glycolysis. J Exp
Clin Cancer Res. 40:132021. View Article : Google Scholar
|
|
83
|
Li D, Zhang T, Guo Y, Bi C, Liu M and Wang
G: Biological impact and therapeutic implication of
tumor-associated macrophages in hepatocellular carcinoma. Cell
Death Dis. 15:4982024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jin M, Cao W, Chen B, Xiong M and Cao G:
Tumor-derived lactate creates a favorable niche for tumor via
supplying energy source for tumor and modulating the tumor
microenvironment. Front Cell Dev Biol. 10:8088592022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ye J, Gao X, Huang X, Huang S, Zeng D, Luo
W, Zeng C, Lu C, Lu L, Huang H, et al: Integrating single-cell and
spatial transcriptomics to uncover and elucidate GP73-mediated
pro-angiogenic regulatory networks in hepatocellular carcinoma.
Research (Wash D C). 7:03872024.PubMed/NCBI
|
|
86
|
Wang S, Wang X, Shan Y, Tan Z, Su Y, Cao
Y, Wang S, Dong J, Gu J and Wang Y: Region-specific cellular and
molecular basis of liver regeneration after acute pericentral
injury. Cell Stem Cell. 31:341–358.e7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hu S, Yang Z, Li L, Yan Q, Hu Y, Zhou F,
Tan Y and Pei G: Salvianolic acid B alleviates liver injury by
regulating lactate-mediated histone lactylation in macrophages.
Molecules. 29:2362024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lei Y, Han P, Chen Y, Wang H, Wang S, Wang
M, Liu J, Yan W, Tian D and Liu M: Protein arginine
methyltransferase 3 promotes glycolysis and hepatocellular
carcinoma growth by enhancing arginine methylation of lactate
dehydrogenase A. Clin Transl Med. 12:e6862022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Agarwala SS, Keilholz U, Gilles E,
Bedikian AY, Wu J, Kay R, Stein CA, Itri LM, Suciu S and Eggermont
AM: LDH correlation with survival in advanced melanoma from two
large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur J
Cancer. 45:1807–1814. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bouafia F, Drai J, Bienvenu J, Thieblemont
C, Espinouse D, Salles G and Coiffier B: Profiles and prognostic
values of serum LDH isoenzymes in patients with haematopoietic
malignancies. Bull Cancer. 91:E229–E240. 2004.PubMed/NCBI
|
|
91
|
Ho J, de Moura MB, Lin Y, Vincent G,
Thorne S, Duncan LM, Hui-Min L, Kirkwood JM, Becker D, Van Houten B
and Moschos SJ: Importance of glycolysis and oxidative
phosphorylation in advanced melanoma. Mol Cancer. 11:762012.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rafaqat S, Sattar A, Khalid A and Rafaqat
S: Role of liver parameters in diabetes mellitus-a narrative
review. Endocr Regul. 57:200–220. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dercle L, Ammari S, Roblin E, Bigorgne A,
Champiat S, Taihi L, Plaian A, Hans S, Lakiss S, Tselikas L, et al:
High serum LDH and liver metastases are the dominant predictors of
primary cancer resistance to anti-PD(L)1 immunotherapy. Eur J
Cancer. 177:80–93. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhu W and Huang Y: Research progress of
serum biomarkers for early screening of hepatocellular carcinoma.
Zhonghua Gan Zang Bing Za Zhi. 29:308–312. 2021.In Chinese.
PubMed/NCBI
|
|
95
|
Liao XM, Zhao SR, Dai WC and Fan R:
Research advances of metabolomics in early diagnosis of
hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi.
30:803–808. 2022.In Chinese. PubMed/NCBI
|
|
96
|
Sevinc A, Sari R and Fadillioglu E: The
utility of lactate dehydrogenase isoenzyme pattern in the
diagnostic evaluation of malignant and nonmalignant ascites. J Natl
Med Assoc. 97:79–84. 2005.PubMed/NCBI
|
|
97
|
Nagasue N: Changes in lactic dehydrogenase
isoenzymes after hepatic artery ligation in patients with hepatic
carcinoma. Gastroenterol Jpn. 10:150–156. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yan Q, Sun YS, An R, Liu F, Fang Q, Wang
Z, Xu T, Chen L and Du J: Application and progress of the detection
technologies in hepatocellular carcinoma. Genes Dis. 10:1857–1869.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Faloppi L, Scartozzi M, Bianconi M,
Svegliati Baroni G, Toniutto P, Giampieri R, Del Prete M, De
Minicis S, Bitetto D, Loretelli C, et al: The role of LDH serum
levels in predicting global outcome in HCC patients treated with
sorafenib: Implications for clinical management. BMC Cancer.
14:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang T and Zhang KH: New blood biomarkers
for the diagnosis of AFP-negative hepatocellular carcinoma. Front
Oncol. 10:13162020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kong W, Zuo X, Liang H, Hu J, Zhang H,
Wang X and Chen W: Prognostic value of lactate dehydrogenase in
patients with hepatocellular carcinoma: A meta-analysis. Biomed Res
Int. 2018:17231842018. View Article : Google Scholar
|
|
102
|
Augoff K and Grabowski K: Significance of
lactate dehydrogenase measurements in diagnosis of malignancies.
Pol Merkur Lekarski. 17:644–647. 2004.In Polish.
|
|
103
|
Lee SC, Kao MC, Yin SJ and Lin CY: Serum
lactate dehydrogenase isoenzymes in patients with hepatocellular
carcinoma. Taiwan Yi Xue Hui Za Zhi. 81:218–223. 1982.PubMed/NCBI
|
|
104
|
Urbańska K and Orzechowski A:
Unappreciated role of LDHA and LDHB to control apoptosis and
autophagy in tumor cells. Int J Mol Sci. 20:20852019. View Article : Google Scholar
|
|
105
|
Yuan C, Li Z, Wang Y, Qi B, Zhang W, Ye J,
Wu H, Jiang H, Song LN, Yang J and Cheng J: Overexpression of
metabolic markers PKM2 and LDH5 correlates with aggressive
clinicopathological features and adverse patient prognosis in
tongue cancer. Histopathology. 65:595–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Su K, Huang W, Li X, Xu K, Gu T, Liu Y,
Song J, Qian K, Xu Y, Zeng H, et al: Evaluation of lactate
dehydrogenase and alkaline phosphatase as predictive biomarkers in
the prognosis of hepatocellular carcinoma and development of a new
nomogram. J Hepatocell Carcinoma. 10:69–79. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY and
Cheng NS: Prognostic value of alkaline phosphatase, gamma-glutamyl
transpeptidase and lactate dehydrogenase in hepatocellular
carcinoma patients treated with liver resection. Int J Surg.
36:143–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li J, Wu MF, Lu HW, Chen Q, Lin ZQ and
Wang LJ: Pretreatment serum lactate dehydrogenase is an independent
prognostic factor for patients receiving neoadjuvant chemotherapy
for locally advanced cervical cancer. Cancer Med. 5:1863–1872.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hu Z, Yuan Y, Hu Z, Liu Q, Fu Y, Hou J,
Sun X, Li S, Duan W and Chen M: Development and validation of
prognostic nomograms for hepatocellular carcinoma after hepatectomy
based on inflammatory markers. J Hepatocell Carcinoma. 9:1403–1413.
2022. View Article : Google Scholar
|
|
110
|
Krishnamurthy K, Medina AM and Howard L:
The utility of elevated serum lactate dehydrogenase in current
clinical practice. Lab Med. 52:e17–e22. 2021. View Article : Google Scholar
|
|
111
|
Han L, Lin X, Yan Q, Gu C, Li M, Pan L,
Meng Y, Zhao X, Liu S and Li A: PBLD inhibits angiogenesis via
impeding VEGF/VEGFR2-mediated microenvironmental cross-talk between
HCC cells and endothelial cells. Oncogene. 41:1851–1865. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Man S, Yao J, Lv P, Liu Y, Yang L and Ma
L: Curcumin-enhanced antitumor effects of sorafenib via regulating
the metabolism and tumor microenvironment. Food Funct.
11:6422–6432. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sim DY, Lee HJ, Ahn CH, Park J, Park SY,
Kil BJ, Shim BS, Kim B and Kim SH: Negative regulation of CPSF6
suppresses the warburg effect and angiogenesis leading to tumor
progression via c-Myc signaling network: Potential therapeutic
target for liver cancer therapy. Int J Biol Sci. 20:3442–3460.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fiume L, Manerba M, Vettraino M and Di
Stefano G: Inhibition of lactate dehydrogenase activity as an
approach to cancer therapy. Future Med Chem. 6:429–445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase A in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Schwab M, Thunborg K, Azimzadeh O, von
Toerne C, Werner C, Shevtsov M, Di Genio T, Zdralevic M, Pouyssegur
J, Renner K, et al: Targeting cancer metabolism breaks
radioresistance by impairing the stress response. Cancers (Basel).
13:37622021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Alobaidi B, Hashimi SM, Alqosaibi AI,
AlQurashi N and Alhazmi S: Targeting the monocarboxylate
transporter MCT2 and lactate dehydrogenase A LDHA in cancer cells
with FX-11 and AR-C155858 inhibitors. Eur Rev Med Pharmacol Sci.
27:6605–6617. 2023.PubMed/NCBI
|
|
119
|
Li X, Lu P, Li B, Yang R, Chu Y, Zhang Z,
Wan H, Niu C, Wang C and Luo K: Sensitization of hepatocellular
carcinoma cells to irradiation by miR-34a through targeting lactate
dehydrogenase-A. Mol Med Rep. 13:3661–3667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Paul SK, Dutta Chowdhury K, Dey SR, Paul A
and Haldar R: Exploring the possibility of drug repurposing for
cancer therapy targeting human lactate dehydrogenase A: A
computational approach. J Biomol Struct Dyn. 41:9967–9976. 2023.
View Article : Google Scholar
|
|
121
|
Brower V: Sorafenib plus cisplatin for
hepatocellular carcinoma. Lancet Oncol. 17:e4242016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Han JH, Lee EJ, Park W, Ha KT and Chung
HS: Natural compounds as lactate dehydrogenase inhibitors:
Potential therapeutics for lactate dehydrogenase inhibitors-related
diseases. Front Pharmacol. 14:12750002023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zheng R, Fang X, Chen X, Huang Y, Xu G, He
L, Li Y, Niu X, Yang L, Wang L, et al: Knockdown of lactate
dehydrogenase by adeno-associated virus-delivered CRISPR/Cas9
system alleviates primary hyperoxaluria type 1. Clin Transl Med.
10:e2612020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ariceta G, Barrios K, Brown BD, Hoppe B,
Rosskamp R and Langman CB: Hepatic lactate dehydrogenase A: An RNA
interference target for the treatment of all known types of primary
hyperoxaluria. Kidney Int Rep. 6:1088–1098. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Tang Q and Khvorova A: RNAi-based drug
design: Considerations and future directions. Nat Rev Drug Discov.
23:341–364. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Alshaer W, Zureigat H, Al Karaki A,
Al-Kadash A, Gharaibeh L, Hatmal MM, Aljabali AAA and Awidi A:
siRNA: Mechanism of action, challenges, and therapeutic approaches.
Eur J Pharmacol. 905:1741782021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Cheng X, Fan S, Wen C and Du X:
CRISPR/Cas9 for cancer treatment: Technology, clinical applications
and challenges. Brief Funct Genomics. 19:209–214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Van Wilpe S, Koornstra R, Den Brok M, De
Groot JW, Blank C, De Vries J, Gerritsen W and Mehra N: Lactate
dehydrogenase: A marker of diminished antitumor immunity.
Oncoimmunology. 9:17319422020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lin Y, Wang Y and Li PF: Mutual regulation
of lactate dehydrogenase and redox robustness. Front Physiol.
13:10384212022. View Article : Google Scholar : PubMed/NCBI
|