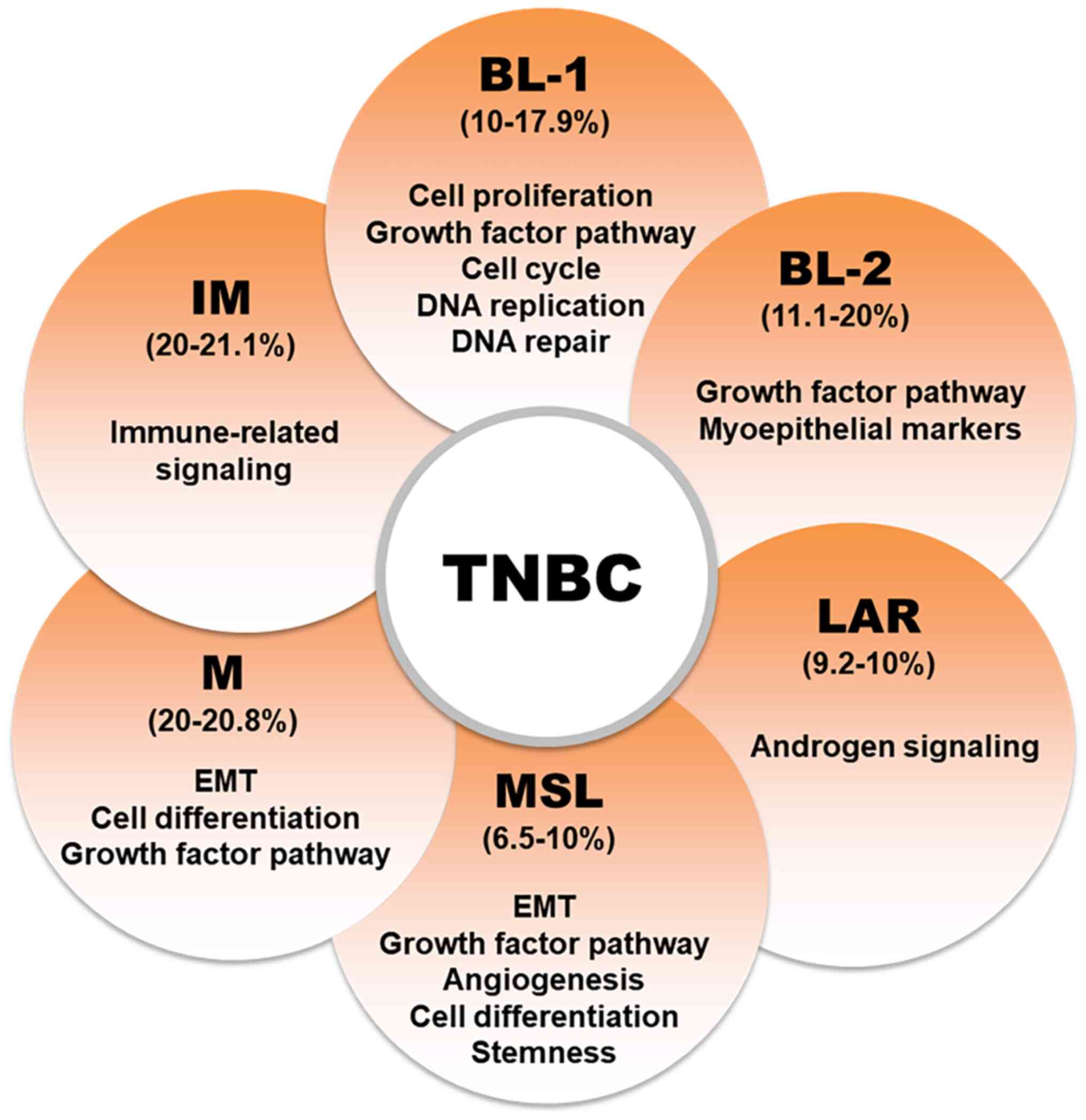
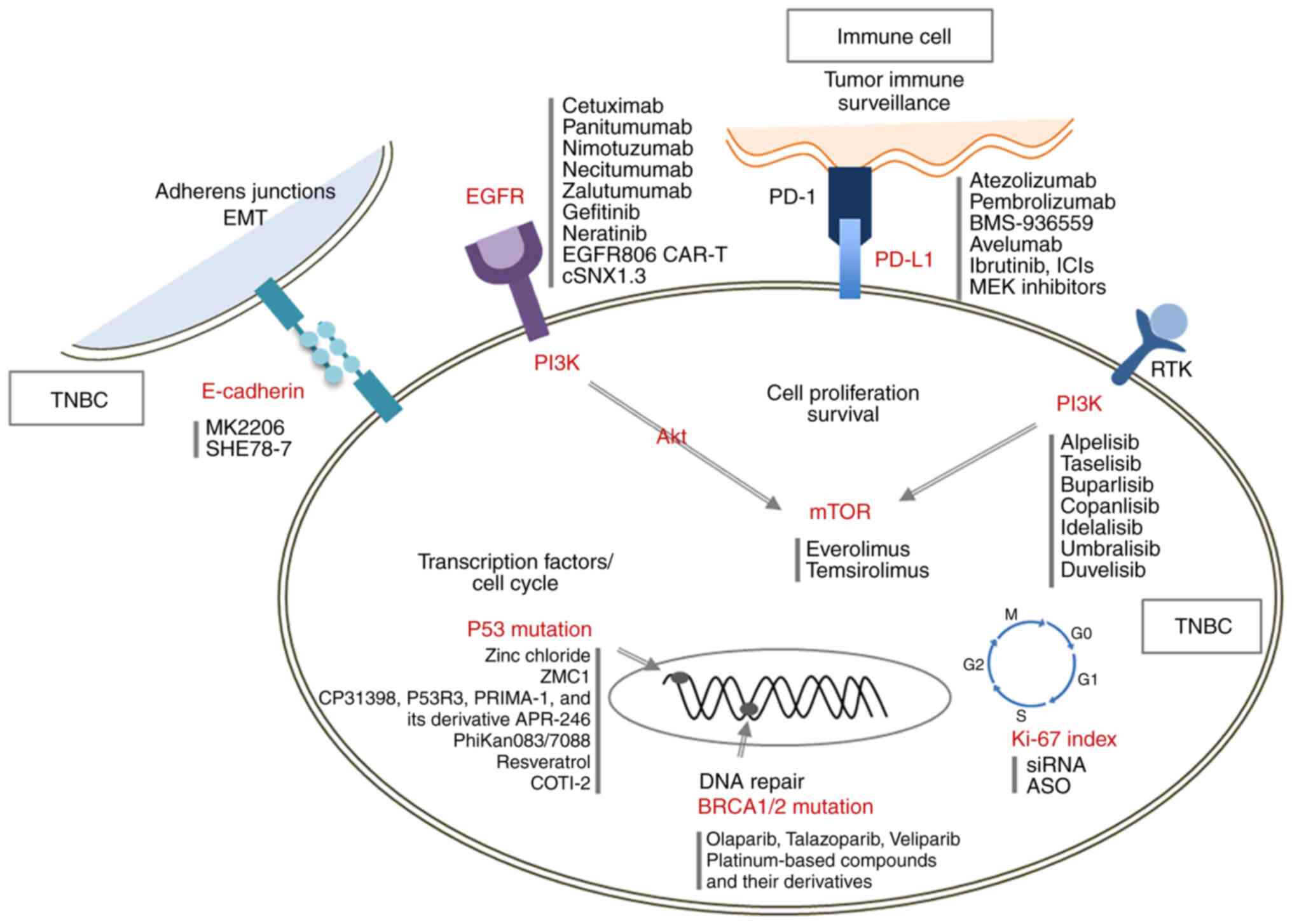
|
1
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ismail-Khan R and Bui MM: A review of
triple-negative breast cancer. Cancer Control. 17:173–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perou CM: Molecular stratification of
triple-negative breast cancers. Oncologist. 16(Suppl 1): S61–S70.
2011. View Article : Google Scholar
|
|
4
|
Bernardi R and Gianni L: Hallmarks of
triple negative breast cancer emerging at last? Cell Res.
24:904–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehanna J, Haddad FG, Eid R, Lambertini M
and Kourie HR: Triple-negative breast cancer: Current perspective
on the evolving therapeutic landscape. Int J Womens Health.
11:431–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prakash O, Hossain F, Danos D, Lassak A,
Scribner R and Miele L: Racial disparities in triple negative
breast cancer: A review of the role of biologic and non-biologic
factors. Front Public Health. 8:5769642020. View Article : Google Scholar
|
|
7
|
Asleh K, Riaz N and Nielsen TO:
Heterogeneity of triple negative breast cancer: Currentadvances in
subtyping and treatment implications. J Exp Clin Cancer Res.
41:2652022. View Article : Google Scholar
|
|
8
|
Newton EE, Mueller LE, Treadwell SM,
Morris CA and Machado HL: Molecular targets of triple-negative
breast cancer: Where do we stand? Cancers (Basel). 14:4822022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HP, Jiang RY, Zhu JY, Sun KN, Huang
Y, Zhou HH, Zheng YB and Wang XJ: PI3K/AKT/mTOR signaling pathway:
An important driver and therapeutic target in triple-negative
breast cancer. Breast Cancer. 31:539–551. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Atchley DP, Albarracin CT, Lopez A, Valero
V, Amos CI, Gonzalez-Angulo AM, Hortobagyi GN and Arun BK: Clinical
and pathologic characteristics of patients with BRCA-positive and
BRCA-negative breast cancer. J Clin Oncol. 26:4282–4288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porta FM, Sajjadi E, Venetis K,
Frascarelli C, Cursano G, Guerini-Rocco E, Fusco N and Ivanova M:
Immune biomarkers in triple-negative breast cancer: Improving the
predictivity of current testing methods. J Pers Med. 13:11762023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ricciardi GR, Adamo B, Ieni A, Licata L,
Cardia R, Ferraro G, Franchina T, Tuccari G and Adamo V: Androgen
receptor (AR), E-cadherin, and Ki-67 as emerging targets and novel
prognostic markers in triple-negative breast cancer (TNBC)
patients. PLoS One. 10:e01283682015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerdes J, Li L, Schlueter C, Duchrow M,
Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E and Flad HD:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873.
1991.PubMed/NCBI
|
|
14
|
Schlüter C, Duchrow M, Wohlenberg C,
Becker MH, Key G, Flad HD and Gerdes J: The cell
proliferation-associated antigen of antibody Ki-67: A very large,
ubiquitous nuclear protein with numerous repeated elements,
representing a new kind of cell cycle-maintaining proteins. J Cell
Biol. 123:513–522. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Selz J, Stevens D, Jouanneau L, Labib A
and Le Scodan R: Prognostic value of molecular subtypes, ki67
expression and impact of postmastectomy radiation therapy in breast
cancer patients with negative lymph nodes after mastectomy. Int J
Radiat Oncol Bio Phys. 84:1123–1132. 2012. View Article : Google Scholar
|
|
16
|
Sobecki M, Mrouj K, Camasses A, Parisis N,
Nicolas E, Llères D, Gerbe F, Prieto S, Krasinska L, David A, et
al: The cell proliferation antigen Ki-67 organises heterochromatin.
Elife. 5:e137222016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishida S, Huang E, Zuzan H, Spang R, Leone
G, West M and Nevins JR: Role for E2F in control of both DNA
replication and mitotic functions as revealed from DNA microarray
analysis. Mol Cell Biol. 21:4684–4699. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sobecki M, Mrouj K, Colinge J, Gerbe F,
Jay P, Krasinska L, Dulic V and Fisher D: Cell-cycle regulation
accounts for variability in Ki-67 expression levels. Cancer Res.
77:2722–2734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim
JH, Lee SH, Han W, Kim DW, Kim TY, et al: Ki-67 can be used for
further classification of triple negative breast cancer into two
subtypes with different response and prognosis. Breast Cancer Res.
13:R222011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XQ, Pei DS, Qian GW, Yin XX, Cheng Q,
Li LT and Zheng JN: The effect of methylated oligonucleotide
targeting Ki-67 gene in human 786-0 renal carcinoma cells. Tumour
Biol. 32:863–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scholl SM, Pierga JY, Asselain B, Beuzeboc
P, Dorval T, Garcia-Giralt E, Jouve M, Palangié T, Remvikos Y,
Durand JC, et al: Breast tumour response to primary chemotherapy
predicts local and distant control as well as survival. Eur J
Cancer. 31A:1969–1975. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benini E, Rao S, Daidone MG, Pilotti S and
Silvestrini R: Immunoreactivity to MIB-1 in breast cancer:
Methodological assessment and comparison with other proliferation
indices. Cell Prolif. 30:107–115. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: Assessment of Ki67 in breast cancer: Recommendations from the
International Ki67 in Breast Cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cattoretti G, Becker MH, Key G, Duchrow M,
Schlüter C, Galle J and Gerdes J: Monoclonal antibodies against
recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect
proliferating cells in microwave-processed formalin-fixed paraffin
sections. J Pathol. 168:357–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muftah AA, Aleskandarany MA, Al-Kaabi MM,
Sonbul SN, Diez-Rodriguez M, Nolan CC, Caldas C, Ellis IO, Rakha EA
and Green AR: Ki67 expression in invasive breast cancer: The use of
tissue microarrays compared with whole tissue sections. Breast
Cancer Res Treat. 164:341–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Viale G, Hanlon Newell AE, Walker E,
Harlow G, Bai I, Russo L, Dell'Orto P and Maisonneuve P: Ki-67
(30-9) scoring and differentiation of luminal A- and luminal B-like
breast cancer subtypes. Breast Cancer Res Treat. 178:451–458. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W
and Zhou Q: Prognostic value of Ki-67 in patients with resected
triple-negative breast cancer: A meta-analysis. Front Oncol.
9:10682019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members:
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Penault-Llorca F and Radosevic-Robin N:
Ki67 assessment in breast cancer: An update. Pathology. 49:166–171.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kythreotou A, Siddique A, Mauri FA, Bower
M and Pinato DJ: PD-L1. J Clin Pathol. 71:189–194. 2018. View Article : Google Scholar
|
|
33
|
Thomas R, Al-Khadairi G and Decock J:
Immune checkpoint inhibitors in triple negative breast cancer
treatment: Promising future prospects. Front Oncol. 10:6005732021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oner G, Önder S, Karatay H, Ak N, Tükenmez
M, Müslümanoğlu M, İğci A, Dincçağ A, Özmen V, Aydiner A, et al:
Correction: Clinical impact of PD-L1 expression in triplenegative
breast cancer patients with residual tumor burden after neoadjuvant
chemotherapy. World J Surg Oncol. 21:542023. View Article : Google Scholar
|
|
36
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar
|
|
39
|
Gonzalez-Angulo AM, Ferrer-Lozano J,
Stemke-Hale K, Sahin A, Liu S, Barrera JA, Burgues O, Lluch AM,
Chen H, Hortobagyi GN, et al: PI3K pathway mutations and PTEN
levels in primary and metastatic breast cancer. Mol Cancer Ther.
10:1093–1101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Loi SM: Tumor-infiltrating lymphocytes,
breast cancer subtypes and therapeutic efficacy. OncoImmunology.
2:e247202013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dieci MV, Tsvetkova V, Orvieto E,
Piacentini F, Ficarra G, Griguolo G, Miglietta F, Giarratano T,
Omarini C, Bonaguro S, et al: Immune characterization of breast
cancer metastases: Prognostic implications. Breast Cancer Res.
20:622018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yeong J, Lim JCT, Lee B, Li H, Ong CCH,
Thike AA, Yeap WH, Yang Y, Lim AYH, Tay TKY, et al: Prognostic
value of CD8 + PD-1+ immune infiltrates and PDCD1 gene expression
in triple negative breast cancer. J Immunother Cancer. 7:342019.
View Article : Google Scholar
|
|
44
|
Lotfinejad P, Asghari Jafarabadi M, Abdoli
Shadbad M, Kazemi T, Pashazadeh F, Sandoghchian Shotorbani S,
Jadidi Niaragh F, Baghbanzadeh A, Vahed N, Silvestris N and
Baradaran B: Prognostic role and clinical significance of
tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1
(PD-L1) expression in triple-negative breast cancer (TNBC): A
systematic review and meta-analysis study. Diagnostics (Basel).
10:7042020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mittendorf EA, Zhang H, Barrios CH, Saji
S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al:
Neoadjuvant atezolizumab in combination with sequential
nab-paclitaxel and anthracycline-based chemotherapy versus placebo
and chemotherapy in patients with early-stage triple-negative
breast cancer (IMpassion031): A randomised, double-blind, phase 3
trial. Lancet. 396:1090–1100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baretta Z, Mocellin S, Goldin E, Olopade
OI and Huo D: Effect of BRCA germline mutations on breast cancer
prognosis: A systematic review and meta-analysis. Medicine
(Baltimore). 95:e49752016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hughes DJ, Ginolhac SM, Coupier I Corbex
M, Bressac-de-Paillerets B, Chompret A, Bignon YJ, Uhrhammer N,
Lasset C, Giraud S, et al: Common BRCA2 variants and modification
of breast and ovarian cancer risk in BRCA1 mutation carriers.
Cancer Epidemiol Biomarkers Prev. 14:265–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu K, Yang S and Zhao Y: Prognostic
significance of BRCA mutations in ovarian cancer: An updated
systematic review with meta-analysis. Oncotarget. 8:285–302. 2017.
View Article : Google Scholar :
|
|
49
|
Nanda R, Schumm LP, Cummings S, Fackenthal
JD, Sveen L, Ademuyiwa F, Cobleigh M, Esserman L, Lindor NM,
Neuhausen SL and Olopade OI: Genetic testing in an ethnically
diverse cohort of high-risk women: A comparative analysis of BRCA1
and BRCA2 mutations in American families of European and African
ancestry. JAMA. 294:1925–1933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wong-Brown MW, Meldrum CJ, Carpenter JE,
Clarke CL, Narod SA, Jakubowska A, Rudnicka H, Lubinski J and Scott
RJ: Prevalence of BRCA1 and BRCA2 germline mutations in patients
with triple-negative breast cancer. Breast Cancer Res Treat.
150:71–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee EH, Park SK, Park B, Kim SW, Lee MH,
Ahn SH, Son BH, Yoo KY and Kang D; KOHBRA Research Group; Korean
Breast Cancer Society: Effect of BRCA1/2 mutation on short-term and
long-term breast cancer survival: A systematic review and
meta-analysis. Breast Cancer Res Treat. 122:11–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Stoppa-Lyonnet D: The biological effects
and clinical implications of BRCA mutations: Where do we go from
here? Eur J Hum Genet. 24(Suppl 1): S3–S9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu L, Wang F, Xu R, Zhang S, Peng X, Feng
Y, Wang J and Lu C: Promoter methylation of BRCA1 in the prognosis
of breast cancer: A meta-analysis. Breast Cancer Res Treat.
142:619–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang C, Zhang J, Wang Y, Ouyang T, Li J,
Wang T, Fan Z, Fan T, Lin B and Xie Y: Prevalence of BRCA1
mutations and responses to neoadjuvant chemotherapy among BRCA1
carriers and non-carriers with triple-negative breast cancer. Ann
Oncol. 26:523–528. 2015. View Article : Google Scholar
|
|
56
|
Paluch-Shimon S, Friedman E, Berger R,
Papa M, Dadiani M, Friedman N, Shabtai M, Zippel D, Gutman M, Golan
T, et al: Neo-adjuvant doxorubicin and cyclophosphamide followed by
paclitaxel in triple-negative breast cancer among BRCA1 mutation
carriers and non-carriers. Breast Cancer Res Treat. 157:157–165.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fu X, Tan W, Song Q, Pei H and Li J: BRCA1
and breast cancer: Molecular mechanisms and therapeutic strategies.
Front Cell Dev Biol. 10:8134572022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Turk AA and Wisinski KB: PARP inhibitors
in breast cancer: Bringing synthetic lethality to the bedside.
Cancer. 124:2498–2506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Meyer P, Landgraf K, Högel B, Eiermann W
and Ataseven B: BRCA2 mutations and triple-negative breast cancer.
PLoS One. 7:e383612012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mendonsa AM, Na TY and Gumbiner BM:
E-cadherin in contact inhibition and cancer. Oncogene.
37:4769–4780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shen T, Zhang K, Siegal GP and Wei S:
Prognostic value of E-cadherin and β-catenin in triple-negative
breast cancer. Am J Clin Pathol. 146:603–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu JB, Feng CY, Deng M, Ge DF, Liu DC, Mi
JQ and Feng XS: E-cadherin expression phenotypes associated with
molecular subtypes in invasive non-lobular breast cancer: Evidence
from a retrospective study and meta-analysis. World J Surg Oncol.
15:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fang Y, Wang Y, Ma H, Guo Y, Xu R, Chen X,
Chen X, Lv Y, Li P and Gao Y: TFAP2A downregulation mediates
tumor-suppressive effect of miR-8072 in triple-negative breast
cancer via inhibiting SNAI1 transcription. Breast Cancer Res.
26:1032024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tang D, Xu S, Zhang Q and Zhao W: The
expression and clinical significance of the androgen receptor and
E-cadherin in triple-negative breast cancer. Med Oncol. 29:526–533.
2012. View Article : Google Scholar
|
|
65
|
Merikhian P, Eisavand MR and Farahmand L:
Triple-negative breast cancer: Understanding Wnt signaling in drug
resistance. Cancer Cell Int. 21:4192021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The E-cadherin and
N-cadherin switch in epithelial-to-mesenchymal transition:
Signaling, therapeutic implications, and challenges. Cells.
8:11182019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
De Leeuw WJ, Berx G, Vos CB, Peterse JL,
Van de Vijver MJ, Litvinov S, Van Roy F, Cornelisse CJ and
Cleton-Jansen AM: Simultaneous loss of E-cadherin and catenins in
invasive lobular breast cancer and lobular carcinoma in situ. J
Pathol. 183:404–411. 1997. View Article : Google Scholar
|
|
68
|
Corso G, Figueiredo J, De Angelis SP,
Corso F, Girardi A, Pereira J, Seruca R, Bonanni B, Carneiro P,
Pravettoni G, et al: E-cadherin deregulation in breast cancer. J
Cell Mol Med. 24:5930–5936. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Droufakou S, Deshmane V, Roylance R, Hanby
A, Tomlinson I and Hart IR: Multiple ways of silencing E-cadherin
gene expression in lobular carcinoma of the breast. Int J Cancer.
92:404–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brouxhon SM, Kyrkanides S, Teng X,
O'Banion MK, Clarke R, Byers S and Ma L: Soluble-E-cadherin
activates HER and IAP family members in HER2+ and TNBC human breast
cancers. Mol Carcinog. 53:893–906. 2014. View Article : Google Scholar
|
|
71
|
Kuhn PM, Russo GC, Crawford AJ,
Venkatraman A, Yang N, Starich BA, Schneiderman Z, Wu PH, Vo T,
Wirtz D and Kokkoli E: Local, sustained, and targeted co-delivery
of MEK inhibitor and doxorubicin inhibits tumor progression in
E-cadherin-positive breast cancer. Pharmaceutics. 16:9812024.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
De Schepper M, Vincent-Salomon A,
Christgen M, Van Baelen K, Richard F, Tsuda H, Kurozumi S, Brito
MJ, Cserni G, Schnitt S, et al: Results of a worldwide survey on
the currently used histopathological diagnostic criteria for
invasive lobular breast cancer. Mod Pathol. 35:1812–1820. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pai K, Baliga P and Shrestha BL:
E-cadherin expression: A diagnostic utility for differentiating
breast carcinomas with ductal and lobular morphologies. J Clin
Diagn Res. 7:840–844. 2013.PubMed/NCBI
|
|
74
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al: The genomic and transcriptomic architecture of 2,000 breast
tumours reveals novel subgroups. Nature. 486:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Padmanaban V, Krol I, Suhail Y, Szczerba
BM, Aceto N, Bader JS and Ewald AJ: E-cadherin is required for
metastasis in multiple models of breast cancer. Nature.
573:439–444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Russo GC, Crawford AJ, Clark D, Cui J,
Carney R, Karl MN, Su B, Starich B, Lih TS, Kamat P, et al:
E-cadherin interacts with EGFR resulting in hyper-activation of ERK
in multiple models of breast cancer. Oncogene. 43:1445–1462. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Song H, Wu T, Xie D, Li D, Hua K, Hu J and
Fang L: WBP2 downregulation inhibits proliferation by blocking YAP
transcription and the EGFR/PI3K/Akt signaling pathway in triple
negative breast cancer. Cell Physiol Biochem. 48:1968–1982. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim S, You D, Jeong Y, Yu J, Kim SW, Nam
SJ and Lee JE: Berberine down-regulates IL-8 expression through
inhibition of the EGFR/MEK/ERK pathway in triple-negative breast
cancer cells. Phytomedicine. 50:43–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
82
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Arrieta O, Cardona AF, Federico Bramuglia
G, Gallo A, Campos-Parra AD, Serrano S, Castro M, Avilés A, Amorin
E, Kirchuk R, et al: Genotyping non-small cell lung cancer (NSCLC)
in Latin America. J Thorac Oncol. 6:1955–1959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tilch E, Seidens T, Cocciardi S, Reid LE,
Byrne D, Simpson PT, Vargas AC, Cummings C, Fox SB, Lakhani SR and
Chenevix Trench G: Mutations in EGFR, BRAF and RAS are rare in
triple-negative and basal-like breast cancers from Caucasian women.
Breast Cancer Res Treat. 143:385–392. 2014. View Article : Google Scholar
|
|
85
|
Jacot W, Lopez-Crapez E, Thezenas S, Senal
R, Fina F, Bibeau F, Romieu G and Lamy PJ: Lack of EGFR-activating
mutations in European patients with triple-negative breast cancer
could emphasise geographic and ethnic variations in breast cancer
mutation profiles. Breast Cancer Res. 13:R1332011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al: Screening for epidermal growth factor receptor mutations in
lung cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pallis AG, Voutsina A, Kalikaki A,
Souglakos J, Briasoulis E, Murray S, Koutsopoulos A, Tripaki M,
Stathopoulos E, Mavroudis D and Georgoulias V: 'Classical' but not
'other' mutations of EGFR kinase domain are associated with
clinical outcome in gefitinib-treated patients with non-small cell
lung cancer. Br J Cancer. 97:1560–1566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yamane H, Ochi N, Yasugi M, Tabayashi T,
Yamagishi T, Monobe Y, Hisamoto A, Kiura K and Takigawa N:
Docetaxel for non-small-cell lung cancer harboring the activated
EGFR mutation with T790M at initial presentation. Onco Targets
Ther. 6:155–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lee HJ, Kim YT, Kang CH, Zhao B, Tan Y,
Schwartz LH, Persigehl T, Jeon YK and Chung DH: Epidermal growth
factor receptor mutation in lung adenocarcinomas: Relationship with
CT characteristics and histologic subtypes. Radiology. 268:254–264.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gupta GK, Collier AL, Lee D, Hoefer RA,
Zheleva V, Siewertsz van Reesema LL, Tang-Tan AM, Guye ML, Chang
DZ, Winston JS, et al: Perspectives on triple-negativebreast
cancer: Current treatment strategies, unmet needs, and potential
targets for future therapies. Cancers (Basel). 12:23922020.
View Article : Google Scholar
|
|
92
|
Gumuskaya B, Alper M, Hucumenoglu S,
Altundag K, Uner A and Guler G: EGFR expression and gene copy
number in triple-negative breast carcinoma. Cancer Genet Cytogenet.
203:222–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nakai K, Hung MC and Yamaguchi H: A
perspective on anti-EGFR therapies targeting triple-negative breast
cancer. Am J Cancer Res. 6:1609–1623. 2016.PubMed/NCBI
|
|
94
|
Choi J, Jung WH and Koo JS:
Clinicopathologic features of molecular subtypes of triple negative
breast cancer based on immunohistochemical markers. Histol
Histopathol. 27:1481–1493. 2012.PubMed/NCBI
|
|
95
|
Tan DSP, Marchió C, Jones RL, Savage K,
Smith IE, Dowsett M and Reis-Filho JS: Triple negative breast
cancer: Molecular profiling and prognostic impact in adjuvant
anthracycline-treated patients. Breast Cancer Res Treat. 111:27–44.
2008. View Article : Google Scholar
|
|
96
|
Martin V, Botta F, Zanellato E, Molinari
F, Crippa S, Mazzucchelli L and Frattini M: Molecular
characterization of EGFR and EGFR-downstream pathways in triple
negative breast carcinomas with basal like features. Histol
Histopathol. 27:785–792. 2012.PubMed/NCBI
|
|
97
|
Meseure D, Vacher S, Drak Alsibai K,
Trassard M, Susini A, Le Ray C, Lerebours F, Le Scodan R, Spyratos
F, Marc Guinebretiere J, et al: Profiling of EGFR mRNA and protein
expression in 471 breast cancers compared with 10 normal tissues: A
candidate biomarker to predict EGFR inhibitor effectiveness. Int J
Cancer. 131:1009–1010. 2012. View Article : Google Scholar
|
|
98
|
Medić-Milijić N, Jovanić I, Nedeljković M,
Marković I, Spurnić I, Milovanović Z, Ademović N, Tomić T and Tanić
N and Tanić N: Prognostic and clinical significance of PD-L1, EGFR
and androgen receptor (AR) expression in triple-negative breast
cancer (TNBC) patients. Life (Basel). 14:6822024.
|
|
99
|
Ueno NT and Zhang D: Targeting EGFR in
Triple negative breast cancer. J Cancer. 2:324–328. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Miricescu D, Totan A, Stanescu-Spinu II,
Badoiu SC, Stefani C and Greabu M: PI3K/AKT/mTOR signaling pathway
in breast cancer: From molecular landscape to clinical aspects. Int
J Mol Sci. 22:1732020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pascual J and Turner NC: Targeting the
PI3-kinase pathway in triple-negative breast cancer. Ann Oncol.
30:1051–1060. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fruman DA, Chiu H, Hopkins BD, Bagrodia S,
Cantley LC and Abraham RT: The PI3K pathway in human disease. Cell.
170:605–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yoshida T and Delafontaine P: Mechanisms
of IGF-1-mediated regulation of skeletal muscle hypertrophy and
atrophy. Cells. 9:19702020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shi X, Wang J, Lei Y, Cong C, Tan D and
Zhou X: Research progress on the PI3K/AKT signaling pathway in
gynecological cancer (Review). Mol. Med. Rep. 19:4529–4535.
2019.PubMed/NCBI
|
|
105
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Carpten JD, Faber AL, Horn C, Donoho GP,
Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage
S, et al: A transforming mutation in the pleckstrin homology domain
of AKT1 in cancer. Nature. 448:439–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chin YR, Yoshida T, Marusyk A, Beck AH,
Polyak K and Toker A: Targeting Akt3 signaling in triple-negative
breast cancer. Can Res. 74:964–973. 2014. View Article : Google Scholar
|
|
108
|
Li H, Prever L, Hirsch E and Gulluni F:
Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers
(Basel). 13:35172021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Costa RLB, Han HS and Gradishar WJ:
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast
cancer: A review. Breast Cancer Res Treat. 169:397–406. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Carey LA: Finding the positive in
triple-negative breast cancer. Nat Cancer. 2:476–478. 2021.
View Article : Google Scholar
|
|
111
|
Xia P and Xu XY: PI3K/Akt/mTOR signaling
pathway in cancer stem cells: From basic research to clinical
application. Am J Cancer Res. 5:1602–1609. 2015.PubMed/NCBI
|
|
112
|
Karami Fath M, Ebrahimi M, Nourbakhsh E,
Zia Hazara A, Mirzaei A, Shafieyari S, Salehi A, Hoseinzadeh M,
Payandeh Z and Barati G: PI3K/Akt/mTOR signaling pathway in cancer
stem cells. Pathol Res Pract. 237:1540102022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Reinhardt HC and Schumacher B: The p53
network: Cellular and systemic DNA damage responses in aging and
cancer. Trends Genet. 28:128–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sionov RV and Haupt Y: The cellular
response to p53: The decision between life and death. Oncogene.
18:6145–6157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kubbutat MH, Jones SN and Vousden KH:
Regulation of p53 stability by MDM2. Nature. 387:299–303. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lacroix M, Toillon RA and Leclercq G: p53
and breast cancer, an update. Endocr Relat Cancer. 13:293–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ozaki T and Nakagawara A: Role of p53 in
cell death and human cancers. Cancers (Basel). 3:994–1013. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Babikir HA, Afjei R, Paulmurugan R and
Massoud TF: Restoring guardianship of the genome: Anticancer drug
strategies to reverse oncogenic mutant p53 misfolding. Cancer Treat
Rev. 71:19–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Costa DCF, de Oliveira GAP, Cino EA,
Soares IN, Rangel LP and Silva JL: Aggregation and prion-like
properties of misfolded tumor suppressors: Is cancer a prion
disease? Cold Spring Harbor Perspect Biol. 8:a0236142016.
View Article : Google Scholar
|
|
120
|
Silva JL, De Moura Gallo CV, Costa DCF and
Rangel LP: Prion-like aggregation of mutant p53 in cancer. Trends
Biochem Sci. 39:260–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Eriksson SE, Ceder S, Bykov VJN and Wiman
KG: p53 as a hub in cellular redox regulation and therapeutic
target in cancer. J Mol Cell Biol. 11:330–341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
D'Orazi G and Givol D: p53 reactivation:
The link to zinc. Cell Cycle. 11:2581–2582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang G and Fersht AR: First-order
rate-determining aggregation mechanism of p53 and its implications.
Proc Natl Acad Sci USA. 109:13590–13595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ghosh S, Salot S, Sengupta S, Navalkar A,
Ghosh D, Jacob R, Das S, Kumar R, Jha NN, Sahay S, et al: p53
amyloid formation leading to its loss of function: Implications in
cancer pathogenesis. Cell Death Differ. 24:1784–1798. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li JP, Zhang XM, Zhang Z, Zheng LH, Jindal
S and Liu YJ: Association of p53 expression with poor prognosis in
patients with triple-negative breast invasive ductal carcinoma.
Medicine (Baltimore). 98:e154492019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar
|
|
127
|
Neilsen PM, Noll JE, Suetani RJ, Schulz
RB, Al-Ejeh F, Evdokiou A, Lane DP and Callen DF: Mutant p53 uses
p63 as a molecular chaperone to alter gene expression and induce a
pro-invasive secretome. Oncotarget. 2:1203–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lim LY, Vidnovic N, Ellisen LW and Leong
CO: Mutant p53 mediates survival of breast cancer cells. Br J
Cancer. 101:1606–1612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Adorno M, Cordenonsi M, Montagner M,
Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo
V, et al: A mutant-p53/Smad complex opposes p63 to empower
TGFbeta-induced metastasis. Cell. 137:87–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Muller PAJ, Trinidad AG, Timpson P, Morton
JP, Zanivan S, van den Berghe PVE, Nixon C, Karim SA, Caswell PT,
Noll JE, et al: Mutant p53 enhances MET trafficking and signalling
to drive cell scattering and invasion. Oncogene. 32:1252–1265.
2013. View Article : Google Scholar :
|
|
131
|
Huang G, Zhong X, Yao L, Ma Q, Liao H, Xu
L, Zou J, Sun R, Wang D and Guo X: MicroRNA-449a inhibits cell
proliferation and migration by regulating mutant p53 in MDA-MB-468
cells. Exp Ther Med. 22:10202021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Marvalim C, Datta A and Lee SC: Role of
p53 in breast cancer progression: An insight into p53 targeted
therapy. Theranostics. 13:1421–1442. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shah SP, Roth A, Goya R, Oloumi A, Ha G,
Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, et al: The clonal
and mutational evolution spectrum of primary triple-negative breast
cancers. Nature. 486:395–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bae SY, Nam SJ, Jung Y, Lee SB, Park BW,
Lim W, Jung SH, Yang HW and Jung SP: Differences in prognosis and
efficacy of chemotherapy by p53 expression in triple-negative
breast cancer. Breast Cancer Res Treat. 172:437–444. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Jasar D, Smichkoska S, Kubelka K,
Filipovski V and Petrushevska G: Expression of p53 protein product
in triple negative breast cancers and relation with clinical and
histopathological parameters. Pril (Makedon Akad Nauk Umet Odd Med
Nauki). 36:69–79. 2015.PubMed/NCBI
|
|
136
|
Karamitopoulou E, Perentes E, Tolnay M and
Probst A: Prognostic significance of MIB-1, p53, and bcl-2
immunoreactivity in meningiomas. Hum Pathol. 29:140–145. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Geyer FC, Rodrigues DN, Weigelt B and
Reis-Filho JS: Molecular classification of estrogen
receptor-positive/luminal breast cancers. Adv Anat Pathol.
19:39–53. 2012. View Article : Google Scholar
|
|
138
|
Zizi-Sermpetzoglou A, Moustou E,
Petrakopoulou N, Arkoumani E, Tepelenis N and Savvaidou V: Atypical
polypoid adenomyoma of the uterus. A case report and a review of
the literature. Eur J Gynaecol Oncol. 33:118–121. 2012.PubMed/NCBI
|
|
139
|
Ibrahim T, Farolfi A, Scarpi E, Mercatali
L, Medri L, Ricci M, Nanni O, Serra L and Amadori D: Hormonal
receptor, human epidermal growth factor receptor-2, and Ki67
discordance between primary breast cancer and paired metastases:
Clinical impact. Oncology. 84:150–157. 2013. View Article : Google Scholar
|
|
140
|
Niikura N, Masuda S, Kumaki N, Xiaoyan T,
Terada M, Terao M, Iwamoto T, Oshitanai R, Morioka T, Tuda B, et
al: Prognostic significance of the Ki67 scoring categories in
breast cancer subgroups. Clin Breast Cancer. 14:323–329.e3. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Polley MYC, Leung SCY, McShane LM, Gao D,
Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F,
Bartlett JMS, et al: An international Ki67 reproducibility study. J
Natl Cancer Inst. 105:1897–1906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Polley MYC, Leung SCY, Gao D, Mastropasqua
MG, Zabaglo LA, Bartlett JMS, McShane LM, Enos RA, Badve SS, Bane
AL, et al: An international study to increase concordance in Ki67
scoring. Mod Pathol. 28:778–786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Baum M, Buzdar A, Cuzick J, Forbes J,
Houghton J, Howell A and Sahmoud T; ATAC (Arimidex Tamoxifen Alone
or in Combination) Trialists' Group: Anastrozole alone or in
combination with tamoxifen versus tamoxifen alone for adjuvant
treatment of postmenopausal women with early-stage breast cancer:
Results of the ATAC (Arimidex, Tamoxifen Alone or in Combination)
trial efficacy and safety update analyses. Cancer. 98:1802–1810.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wyatt CA, Geoghegan JC and Brinckerhoff
CE: Short hairpin RNA-mediated inhibition of matrix
metalloproteinase-1 in MDA-231 cells: Effects on matrix destruction
and tumor growth. Cancer Res. 65:11101–11108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zuckerman JE and Davis ME: Clinical
experiences with systemically administered siRNA-based therapeutics
in cancer. Nat Rev Drug Discov. 14:843–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zheng JN, Ma TX, Cao JY, Sun XQ, Chen JC,
Li W, Wen RM, Sun YF and Pei DS: Knockdown of Ki-67 by small
interfering RNA leads to inhibition of proliferation and induction
of apoptosis in human renal carcinoma cells. Life Sci. 78:724–729.
2006. View Article : Google Scholar
|
|
147
|
Zheng JN, Sun YF, Pei DS, Liu JJ, Ma TX,
Han RF, Li W, Zheng DB, Chen JC and Sun XQ: Treatment with
vector-expressed small hairpin RNAs against Ki67 RNA-induced cell
growth inhibition and apoptosis in human renal carcinoma cells.
Acta Biochim Biophys Sin (Shanghai). 38:254–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Burnett JC and Rossi JJ: RNA-based
therapeutics: Current progress and future prospects. Chem Biol.
19:60–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
de Carvalho Vicentini FTM,
Borgheti-Cardoso LN, Depieri LV, de Macedo Mano D, Abelha TF and
Petrilli R: Delivery systems and local administration routes for
therapeutic siRNA. Pharm Res. 30:915–931. 2013. View Article : Google Scholar
|
|
150
|
Conde J, Edelman ER and Artzi N:
Target-responsive DNA/RNA nanomaterials for microRNA sensing and
inhibition: The jack-of-all-trades in cancer nanotheranostics? Adv
Drug Deliv Rev. 81:169–183. 2015. View Article : Google Scholar
|
|
151
|
Bischoff JR, Kirn DH, Williams A, Heise C,
Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A and
McCormick F: An adenovirus mutant that replicates selectively in
p53-eficient human tumor cells. Science. 274:373–376. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yu DC, Chen Y, Dilley J, Li Y, Embry M,
Zhang H, Nguyen N, Amin P, Oh J and Henderson DR: Antitumor synergy
of CV787, a prostate cancer-specific adenovirus, and paclitaxel and
docetaxel. Cancer Res. 61:517–525. 2001.PubMed/NCBI
|
|
153
|
Rajecki M, Kanerva A, Stenman UH, Tenhunen
M, Kangasniemi L, Särkioja M, Ala-Opas MY, Alfthan H, Sankila A,
Rintala E, et al: Treatment of prostate cancer with Ad5/3Delta24hCG
allows non-invasive detection of the magnitude and persistence of
virus replication in vivo. Mol Cancer Ther. 6:742–751. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen RF, Li YY, Li LT, Cheng Q, Jiang G
and Zheng JN: Novel oncolytic adenovirus sensitizes renal cell
carcinoma cells to radiotherapy via mitochondrial apoptotic cell
death. Mol Med Rep. 11:2141–2146. 2015. View Article : Google Scholar
|
|
155
|
Toth K and Wold WSM: Increasing the
efficacy of oncolytic adenovirus vectors. Viruses. 2:1844–1866.
2010. View Article : Google Scholar
|
|
156
|
Liu J, Fang L, Cheng Q, Li L, Su C, Zhang
B, Pei D, Yang J, Li W and Zheng J: Effects of G250 promoter
controlled conditionally replicative adenovirus expressing
Ki67-siRNA on renal cancer cell. Cancer Sci. 103:1880–1888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Gaudet D, Alexander VJ, Baker BF, Brisson
D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham
MJ, et al: Antisense inhibition of apolipoprotein C-III in patients
with hypertriglyceridemia. N Engl J Med. 373:438–447. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kennedy BWC: Mongersen, an oral SMAD7
antisense oligonucleotide, and Crohn's disease. N Engl J Med.
372:24612015. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wheeler TM, Leger AJ, Pandey SK, MacLeod
AR, Nakamori M, Cheng SH, Wentworth BM, Bennett CF and Thornton CA:
Targeting nuclear RNA for in vivo correction of myotonic dystrophy.
Nature. 488:111–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Natale R, Blackhall F, Kowalski D, Ramlau
R, Bepler G, Grossi F, Lerchenmüller C, Pinder-Schenck M, Mezger J,
Danson S, et al: Evaluation of antitumor activity using change in
tumor size of the survivin antisense oligonucleotide LY2181308 in
combination with docetaxel for second-line treatment of patients
with non-small-cell lung cancer: a randomized open-label phase II
study. J Thorac Oncol. 9:1704–1708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Sen M, Thomas SM, Kim S, Yeh JI, Ferris
RL, Johnson JT, Duvvuri U, Lee J, Sahu N, Joyce S, et al:
First-in-human trial of a STAT3 decoy oligonucleotide in head and
neck tumors: Implications for cancer therapy. Cancer Discov.
2:694–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kausch I, Lingnau A, Endl E, Sellmann K,
Deinert I, Ratliff TL, Jocham D, Sczakiel G, Gerdes J and Böhle A:
Antisense treatment against Ki-67 mRNA inhibits proliferation and
tumor growth in vitro and in vivo. Int J Cancer. 105:710–716. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Kausch I, Jiang H, Ewerdwalbesloh N, Doehn
C, Krüger S, Sczakiel G and Jocham D: Inhibition of Ki-67 in a
renal cell carcinoma severe combined immunodeficiency disease mouse
model is associated with induction of apoptosis and tumour growth
inhibition. BJU Int. 95:416–420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lingnau A, Steiner U, Kurzidim H, Jocham D
and Kausch I: Phase I dose-escalation study of intravesical
instillation of antisense oligonucleotide FFC15-01 against Ki-67 in
patients with non-muscle invasive bladder cancer. Debates Bladder
Cancer. 2:12010.
|
|
165
|
Ratilainen T, Holmén A, Tuite E, Nielsen
PE and Nordén B: Thermodynamics of sequence-specific binding of PNA
to DNA. Biochemistry. 39:7781–7791. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Thomas SM, Sahu B, Rapireddy S, Bahal R,
Wheeler SE, Procopio EM, Kim J, Joyce SC, Contrucci S, Wang Y, et
al: Antitumor effects of EGFR antisense guanidine-based peptide
nucleic acids in cancer models. ACS Chem Biol. 8:345–352. 2013.
View Article : Google Scholar :
|
|
167
|
Thompson ED, Taube JM, Asch-Kendrick RJ,
Ogurtsova A, Xu H, Sharma R, Meeker A, Argani P, Emens LA and
Cimino-Mathews A: PD-L1 expression and the immune microenvironment
in primary invasive lobular carcinomas of the breast. Mod Pathol.
30:1551–1560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Sabatier R, Finetti P, Mamessier E,
Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D and
Bertucci F: Prognostic and predictive value of PDL1 expression in
breast cancer. Oncotarget. 6:5449–5464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Baracco EE, Pietrocola F, Buqué A, Bloy N,
Senovilla L, Zitvogel L, Vacchelli E and Kroemer G: Inhibition of
formyl peptide receptor 1 reduces the efficacy of anticancer
chemotherapy against carcinogen-induced breast cancer.
Oncoimmunology. 5:e11392752016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Rey-Cárdenas M, Guerrero-Ramos F, Gómez de
Liaño Lista A, Carretero-González A, Bote H, Herrera-Juárez M,
Carril-Ajuria L, Martín-Soberón M, Sepulveda JM, Billalabeitia EG,
et al: Recent advances in neoadjuvant immunotherapy for urothelial
bladder cancer: What to expect in the near future. Cancer Treat
Rev. 93:1021422021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Reck M, Remon J and Hellmann MD:
First-line immunotherapy for non-small-cell lung cancer. J Clin
Oncol. 40:586–597. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Huang AC and Zappasodi R: A decade of
checkpoint blockade immunotherapy in melanoma: Understanding the
molecular basis for immune sensitivity and resistance. Nat Immunol.
23:660–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Sendur MAN: Adjuvant immunotherapy for
renal cell carcinoma. Lancet Oncol. 23:1110–1111. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Romero D: Benefit in patients with
PD-L1-positive TNBC. Nat Rev Clin Oncol. 16:62019.
|
|
175
|
Loi S, Drubay D, Adams S, Pruneri G,
Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria
S, et al: Tumor-infiltrating lymphocytes and prognosis: A pooled
individual patient analysis of early-stage triple-negative breast
cancers. J Clin Oncol. 37:559–569. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Yarchoan M, Johnson BR, Lutz ER, Laheru DA
and Jaffee EM: Targeting neoantigens to augment antitumour
immunity. Nat Rev Cancer. 17:209–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Dudley JC, Lin MT, Le DT and Eshleman JR:
Microsatellite instability as a biomarker for PD-1 blockade. Clin
Cancer Res. 22:813–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017:PO.17.000732017.PubMed/NCBI
|
|
179
|
Lipson EJ, Forde PM, Hammers HJ, Emens LA,
Taube JM and Topalian SL: Antagonists of PD-1 and PD-L1 in cancer
treatment. Semin Oncol. 42:587–600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Pusztai L, Karn T, Safonov A, Abu-Khalaf
MM and Bianchini G: New strategies in breast cancer: Immunotherapy.
Clin Cancer Res. 22:2105–2110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Loi S, Dushyanthen S, Beavis PA, Salgado
R, Denkert C, Savas P, Combs S, Rimm DL, Giltnane JM, Estrada MV,
et al: RAS/MAPK activation is associated with reduced
tumor-infiltrating lymphocytes in triple-negative breast cancer:
Therapeutic cooperation between MEK and PD-1/PD-L1 immune
checkpoint inhibitors. Clin Cancer Res. 22:1499–1509. 2016.
View Article : Google Scholar :
|
|
183
|
Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng
PP, Chang BY and Levy R: Therapeutic antitumor immunity by
checkpoint blockade is enhanced by ibrutinib, an inhibitor of both
BTK and ITK. Proc Natl Acad Sci USA. 112:E966–E972. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Hodgson D, Lai Z, Dearden S, Barrett JC,
Harrington EA, Timms K, Lanchbury J, Wu W, Allen A, Senkus E, et
al: Analysis of mutation status and homologous recombination
deficiency in tumors of patients with germline BRCA1 or BRCA2
mutations and metastatic breast cancer: OlympiAD. Ann Oncol.
32:1582–1589. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
U.S. Food and Drug Administration (FDA):
TALZENNA® (talazoparib) prescribing information. FDA; Silver
Spring, MD: 2025, https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217439s000lbl.pdf
(May 2025). Accessed on May 9 2025.
|
|
186
|
Litton JK, Scoggins ME, Hess KR, Adrada
BE, Murthy RK, Damodaran S, DeSnyder SM, Brewster AM, Barcenas CH,
Valero V, et al: Neoadjuvant talazoparib for patients with operable
breast cancer with a germline BRCA pathogenic variant. J Clin
Oncol. 38:388–394. 2020. View Article : Google Scholar :
|
|
187
|
Pop L, Suciu ID, Ionescu P and Ionescu OD:
The dual blockade in the neoadjuvant setting of HER-2 positive
early-stage breast cancer. J Med Life. 12:329–331. 2019. View Article : Google Scholar
|
|
188
|
Isakoff SJ, Overmoyer B, Tung NM, Gelman
RS, Giranda VL, Bernhard KM, Habin KR, Ellisen LW, Winer EP and
Goss PE: A phase II trial of the PARP inhibitor veliparib (ABT888)
and temozolomide for metastatic breast cancer. J Clin Oncol. 28(15
Suppl): S10192010. View Article : Google Scholar
|
|
189
|
Rodler ET, Gralow J, Kurland BF, Griffin
M, Yeh R, Thompson JA, Porter P, Swisher EM, Gadi VK, Korde LA, et
al: Phase I: Veliparib with cisplatin (CP) and vinorelbine (VNR) in
advanced triple-negative breast cancer (TNBC) and/or BRCA
mutation-associated breast cancer. J Clin Oncol. 32(15 Suppl):
S25692014. View Article : Google Scholar
|
|
190
|
Samol J, Ranson M, Scott E, Macpherson E,
Carmichael J, Thomas A and Cassidy J: Safety and tolerability of
the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib
(AZD2281) in combination with topotecan for the treatment of
patients with advanced solid tumors: A phase I study. Invest New
Drugs. 30:1493–1500. 2012. View Article : Google Scholar
|
|
191
|
Dent RA, Lindeman GJ, Clemons M, Wildiers
H, Chan A, McCarthy NJ, Singer CF, Lowe ES, Watkins CL and
Carmichael J: Phase I trial of the oral PARP inhibitor olaparib in
combination with paclitaxel for first- or second-line treatment of
patients with metastatic triple-negative breast cancer. Breast
Cancer Res. 15:R882013. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Marullo R, Werner E, Degtyareva N, Moore
B, Altavilla G, Ramalingam SS and Doetsch PW: Cisplatin induces a
mitochondrial-ROS response that contributes to cytotoxicity
depending on mitochondrial redox status and bioenergetic functions.
PLoS One. 8:e811622013. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Song X, Kong F, Zong ZF, Ren M, Meng Q, Li
Y and Sun Z: miR-124 and miR-142 enhance cisplatin sensitivity of
non-small cell lung cancer cells through repressing autophagy via
directly targeting SIRT1. RSC Adv. 9:5234–5243. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Pabla N and Dong Z: Curtailing side
effects in chemotherapy: A tale of PKCδ in cisplatin treatment.
Oncotarget. 3:107–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
McCabe N, Turner NC, Lord CJ, Kluzek K,
Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka
MZ, et al: Deficiency in the repair of DNA damage by homologous
recombination and sensitivity to poly(ADP-ribose) polymerase
inhibition. Cancer Res. 66:8109–8115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Jia X, Wang K, Xu L, Li N, Zhao Z and Li
M: A systematic review and meta-analysis of BRCA1/2 mutation for
predicting the effect of platinum-based chemotherapy in
triple-negative breast cancer. Breast. 66:31–39. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Teo K, Gómez-Cuadrado L, Tenhagen M, Byron
A, Rätze M, van Amersfoort M, Renes J, Strengman E, Mandoli A,
Singh AA, et al: E-cadherin loss induces targetable autocrine
activation of growth factor signalling in lobular breast cancer.
Sci Rep. 8:154542018. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Bajrami I, Marlow R, van de Ven M, Brough
R, Pemberton HN, Frankum J, Song F, Rafiq R, Konde A, Krastev DB,
et al: E-cadherin/ROS1 inhibitor synthetic lethality in breast
cancer. Cancer Discov. 8:498–515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Mateus AR, Simões-Correia J, Figueiredo J,
Heindl S, Alves CC, Suriano G, Luber B and Seruca R: E-cadherin
mutations and cell motility: A genotype-phenotype correlation. Exp
Cell Res. 315:1393–1402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Watabe M, Nagafuchi A, Tsukita S and
Takeichi M: Induction of polarized cell-cell association and
retardation of growth by activation of the E-cadherin-catenin
adhesion system in a dispersed carcinoma line. J Cell Biol.
127:247–256. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Green SK, Francia G, Isidoro C and Kerbel
RS: Antiadhesive antibodies targeting E-cadherin sensitize
multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer
Ther. 3:149–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Masuda H, Zhang D, Bartholomeusz C,
Doihara H, Hortobagyi GN and Ueno NT: Role of epidermal growth
factor receptor in breast cancer. Breast Cancer Res Treat.
136:331–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ,
Kussie P and Ferguson KM: Structural basis for inhibition of the
epidermal growth factor receptor by cetuximab. Cancer Cell.
7:301–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y,
Li W, Xu N, Lin LZ, Wu Q, et al: Efficacy and tolerability of
first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin
(FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type
metastatic colorectal cancer: The open-label, randomized, phase III
TAILOR trial. J Clin Oncol. 36:3031–3039. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Hirsch FR, Redman MW, Moon J, Agustoni F,
Herbst RS, Semrad TJ, Varella-Garcia M, Rivard CJ, Kelly K, Gandara
DR and Mack PC: EGFR high copy number together with high EGFR
protein expression predicts improved outcome for cetuximab-based
therapy in squamous cell lung cancer: Analysis from SWOG S0819, a
phase III trial of chemotherapy with or without cetuximab in
advanced NSCLC. Clin Lung Cancer. 23:60–71. 2022. View Article : Google Scholar :
|
|
207
|
Cai WQ, Zeng LS, Wang LF, Wang YY, Cheng
JT, Zhang Y, Han ZW, Zhou Y, Huang SL, Wang XW, et al: The latest
battles between EGFR monoclonal antibodies and resistant tumor
cells. Front Oncol. 10:12492020. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Xu MJ, Johnson DE and Grandis JR:
EGFR-targeted therapies in the post-genomic era. Cancer Metastasis
Rev. 36:463–473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Mazorra Z, Lavastida A, Concha-Benavente
F, Valdés A, Srivastava RM, García-Bates TM, Hechavarría E,
González Z, González A, Lugiollo M, et al: Nimotuzumab induces NK
cell activation, cytotoxicity, dendritic cell maturation and
expansion of EGFR-specific T cells in head and neck cancer
patients. Front Pharmacol. 8:3822017. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Benmebarek MR, Karches CH, Cadilha BL,
Lesch S, Endres S and Kobold S: Killing mechanisms of chimeric
antigen receptor (CAR) T cells. Int J Mol Sci. 20:12832019.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Byrd TT, Fousek K, Pignata A, Szot C,
Samaha H, Seaman S, Dobrolecki L, Salsman VS, Oo HZ, Bielamowicz K,
et al: TEM8/ANTXR1-specific CAR T cells as a targeted therapy for
triple-negative breast cancer. Cancer Res. 78:489–500. 2018.
View Article : Google Scholar :
|
|
212
|
Xia L, Zheng Z, Liu JY, Chen YJ, Ding J,
Hu GS, Hu YH, Liu S, Luo WX, Xia NS and Liu W: Targeting
triple-negative breast cancer with combination therapy of EGFR CAR
T cells and CDK7 inhibition. Cancer Immunol Res. 9:707–722. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Hübner J, Raschke M, Rütschle I, Gräßle S,
Hasenberg T, Schirrmann K, Lorenz A, Schnurre S, Lauster R,
Maschmeyer I, et al: Simultaneous evaluation of anti-EGFR-induced
tumour and adverse skin effects in a microfluidic human 3D
co-culture model. Sci Rep. 8:150102018. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Jungbluth AA, Stockert E, Huang HJ,
Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM,
Gullick WJ, et al: A monoclonal antibody recognizing human cancers
with amplification/overexpression of the human epidermal growth
factor receptor. Proc Natl Acad Sci USA. 100:639–644. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Scott AM, Lee FT, Tebbutt N, Herbertson R,
Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, et al:
A phase I clinical trial with monoclonal antibody ch806 targeting
transitional state and mutant epidermal growth factor receptors.
Proc Natl Acad Sci USA. 104:4071–4076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Vitanza N, Gust J, Wilson A, Huang W,
Perez F, Wright J, Leary S, Cole B, Albert C, Pinto N, et al:
IMMU-03. Updates on brainchild-01, -02, and -03: Phase 1
locoregional car T cell trials targeting HER2, EGFR, and B7-H3 for
children with recurrent CNS tumors and DIPG. Neuro Oncol. 22(Suppl
3): iii3602020. View Article : Google Scholar :
|
|
217
|
Huerta JJ, Diaz-Trelles R, Naves FJ,
Llamosas MM, Del Valle ME and Vega JA: Epidermal growth factor
receptor in adult human dorsal root ganglia. Anat Embryol (Berl).
194:253–257. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Atwell B, Chen CY, Christofferson M,
Montfort WR and Schroeder J: Sorting nexin-dependent therapeutic
targeting of oncogenic epidermal growth factor receptor. Cancer
Gene Ther. 30:267–276. 2023. View Article : Google Scholar :
|
|
219
|
André F, Ciruelos E, Rubovszky G, Campone
M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al:
Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced
breast cancer. N Engl J Med. 380:1929–1940. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Cataldo ML, De Placido P, Esposito D,
Formisano L, Arpino G, Giuliano M, Bianco R, De Angelis C and
Veneziani BM: The effect of the alpha-specific PI3K inhibitor
alpelisib combined with anti-HER2 therapy in HER2+/PIK3CA mutant
breast cancer. Front Oncol. 13:11082422023. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Juric D, Krop I, Ramanathan RK, Wilson TR,
Ware JA, Sanabria Bohorquez SM, Savage HM, Sampath D, Salphati L,
Lin RS, et al: Phase I dose-escalation study of taselisib, an oral
PI3K inhibitor, in patients with advanced solid tumors. Cancer
Discov. 7:704–715. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Schmid P, Zaiss M, Harper-Wynne C,
Ferreira M, Dubey S, Chan S, Makris A, Nemsadze G, Brunt AM,
Kuemmel S, et al: Abstract GS2-07: MANTA-a randomized phase II
study of fulvestrant in combination with the dual mTOR inhibitor
AZD2014 or everolimus or fulvestrant alone in estrogen
receptor-positive advanced or metastatic breast cancer. Cancer Res.
78(4 Suppl): GS2–07. 2018. View Article : Google Scholar
|
|
223
|
Dent S, Cortés J, Im YH, Diéras V, Harbeck
N, Krop IE, Wilson TR, Cui N, Schimmoller F, Hsu JY, et al: Phase
III randomized study of taselisib or placebo with Fulvestrant in
estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced
breast cancer: The SANDPIPER trial. Ann Oncol. 32:197–207. 2021.
View Article : Google Scholar
|
|
224
|
Baselga J, Im SA, Iwata H, Cortés J, De
Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, et
al: Buparlisib plus fulvestrant versus placebo plus fulvestrant in
postmenopausal, hormone receptor-positive, HER2-negative, advanced
breast cancer (BELLE-2): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:904–916. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Mallick S, Duttaroy AK and Dutta S: The
PIK3CA gene and its pivotal role in tumor tropism of
triple-negative breast cancer. Transl Oncol. 50:1021402024.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Xu S, Li S, Guo Z, Luo J, Ellis MJ and Ma
CX: Combined targeting of mTOR and AKT is an effective strategy for
basal-like breast cancer in patient-derived xenograft models. Mol
Cancer Ther. 12:1665–1675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Mishra R, Patel H, Alanazi S, Kilroy MK
and Garrett JT: PI3K inhibitors in cancer: Clinical implications
and adverse effects. Int J Mol Sci. 22:34642021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Kornblum N, Zhao F, Manola J, Klein P,
Ramaswamy B, Brufsky A, Stella PJ, Burnette B, Telli M, Makower DF,
et al: Randomized phase II trial of fulvestrant plus everolimus or
placebo in postmenopausal women with hormone receptor-positive,
human epidermal growth factor receptor 2-negative metastatic breast
cancer resistant to aromatase inhibitor therapy: Results of
PrE0102. J Clin Oncol. 36:1556–1563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Carlino F, Diana A, Terminiello M,
Ventriglia A, Piccolo A, Bruno V, Lobianco L, Caterino M,
Ciardiello F, Danielee B, et al: 302P Clinical implication of
tissue re-biopsy in metastatic breast cancer (MBC) patients: A
single centre retrospective analysis. Ann Oncol. 32:S495–S496.
2021. View Article : Google Scholar
|
|
231
|
Basho RK, Gilcrease M, Murthy RK, Helgason
T, Karp DD, Meric-Bernstam F, Hess KR, Herbrich SM, Valero V,
Albarracin C, et al: Targeting the PI3K/AKT/mTOR pathway for the
treatment of mesenchymal triple-negative breast cancer: Evidence
from a phase 1 trial of mTOR inhibition in combination with
liposomal doxorubicin and bevacizumab. JAMA Oncol. 3:509–515. 2017.
View Article : Google Scholar
|
|
232
|
Kastenhuber ER and Lowe SW: Putting p53 in
context. Cell. 170:1062–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Joerger AC and Fersht AR: Structural
biology of the tumor suppressor p53 and cancer-associated mutants.
Adv Cancer Res. 97:1–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Loh SN: Follow the mutations: Toward
class-specific, small-molecule reactivation of p53. Biomolecules.
10:3032020. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Puca R, Nardinocchi L, Porru M, Simon AJ,
Rechavi G, Leonetti C, Givol D and D'Orazi G: Restoring p53 active
conformation by zinc increases the response of Mutant p53 tumor
cells to anticancer drugs. Cell Cycle. 10:1679–1689. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Kwan K, Castro-Sandoval O, Gaiddon C and
Storr T: Inhibition of p53 protein aggregation as a cancer
treatment strategy. Curr Opin Chem Biol. 72:1022302023. View Article : Google Scholar
|
|
237
|
P SS, Naresh P, A J, Wadhwani A, M SK and
Jubie S: Dual modulators of p53 and cyclin D in ER alpha signaling
by albumin nanovectors bearing zinc chaperones for ER-positive
breast cancer therapy. Mini Rev Med Chem. 21:792–802. 2012.
View Article : Google Scholar
|
|
238
|
Yu X, Na B, Zaman S, Withers T, Gilleran
J, Blayney AJ, Bencivenga AF, Blanden AR, Liu Y, Boothman DA, et
al: Abstract 3432: Zinc metallochaperones for mutant p53
reactivation in cancer therapeutics. Cancer Res. 80(16 Suppl):
S34322020. View Article : Google Scholar
|
|
239
|
Parrales A, Ranjan A, Iyer SV, Padhye S,
Weir SJ, Roy A and Iwakuma T: DNAJA1 controls the fate of misfolded
mutant p53 through the mevalonate pathway. Nat Cell Biol.
18:1233–1243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Alalem M, Bhosale M, Ranjan A, Yamamoto S,
Kaida A, Nishikawa S, Parrales A, Farooki S, Anant S, Padhye S and
Iwakuma T: Mutant p53 depletion by novel inhibitors for
HSP40/J-domain proteins derived from the natural compound
plumbagin. Cancers (Basel). 14:41872022. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Kamada R, Toguchi Y, Nomura T, Imagawa T
and Sakaguchi K: Tetramer formation of tumor suppressor protein
p53: Structure, function, and applications. Biopolymers.
106:598–612. 2016. View Article : Google Scholar
|
|
242
|
Synnott NC, Murray A, McGowan PM, Kiely M,
Kiely PA, O'Donovan N, O'Connor DP, Gallagher WM, Crown J and Duffy
MJ: Mutant p53: A novel target for the treatment of patients with
triple-negative breast cancer? Int J Cancer. 140:234–246. 2017.
View Article : Google Scholar
|
|
243
|
Rangel LP, Ferretti GDS, Costa CL, Andrade
SMMV, Carvalho RS, Costa DCF and Silva JL: p53 reactivation with
induction of massive apoptosis-1 (PRIMA-1) inhibits amyloid
aggregation of mutant p53 in cancer cells. J Biol Chem.
294:3670–3682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Duffy MJ, Synnott NC and Crown J: Mutant
p53 in breast cancer: Potential as a therapeutic target and
biomarker. Breast Cancer Res Treat. 170:213–219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Reza MN, Ferdous N, Emon MTH, Islam MS,
Mohiuddin AKM and Hossain MU: Pathogenic genetic variants from
highly connected cancer susceptibility genes confer the loss of
structural stability. Sci Rep. 11:192642021. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Tonsing-Carter E, Bailey BJ, Saadatzadeh
MR, Ding J, Wang H, Sinn AL, Peterman KM, Spragins TK, Silver JM,
Sprouse AA, et al: Potentiation of carboplatin-mediated DNA damage
by the Mdm2 modulator nutlin-3a in a humanized orthotopic
breast-to-lung metastatic model. Mol Cancer Therapeut.
14:2850–2863. 2015. View Article : Google Scholar
|
|
247
|
da Costa DCF, Campos NPC, Santos RA,
Guedes-da-Silva FH, Martins-Dinis MMDC, Zanphorlin L, Ramos C,
Rangel LP and Silva JL: Resveratrol prevents p53 aggregation in
vitro and in breast cancer cells. Oncotarget. 9:29112–29122. 2018.
View Article : Google Scholar
|
|
248
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar :
|
|
249
|
Hassin O and Oren M: Drugging p53 in
cancer: One protein, many targets. Nat Rev Drug Discov. 22:127–144.
2023. View Article : Google Scholar
|
|
250
|
Ubby I, Krueger C, Rosato R, Qian W, Chang
J and Sabapathy K: Cancer therapeutic targeting using
mutant-p53-specific siRNAs. Oncogene. 38:3415–3427. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Braicu C, Pileczki V, Irimie A and
Berindan-Neagoe I: p53siRNA therapy reduces cell proliferation,
migration and induces apoptosis in triple negative breast cancer
cells. Mol Cell Biochem. 381:61–68. 2013. View Article : Google Scholar : PubMed/NCBI
|