Immune checkpoint blockade (ICB) therapy has
demonstrated marked clinical efficacy, albeit limited to a subset
of patients with specific solid tumor types, such as melanoma,
non-small cell lung cancer (NSCLC), renal cell carcinoma and
urothelial carcinoma. Extensive research has been conducted to
elucidate the characteristics of responding patients and the
underlying mechanisms, aiming to identify suitable candidates for
precision therapy and potential therapeutic targets to overcome
resistance (1). A previous study
demonstrated that ICB therapy efficacy is associated with an
immunologically 'hot' phenotype of tumors (2). However, individual parameters,
including programmed death ligand 1 (PD-L1) expression, tumor
mutational burden (TMB) and T-cell infiltration (3), have proven insufficient as
predictive biomarkers for ICB response. Furthermore, given that the
immune response is an inherently systemic process, localized ICB
administration often fails to achieve complete tumor elimination
through immune-mediated mechanisms, such as cytotoxic T-cell
activation and infiltration, which are critical for targeting and
destroying tumor cells (4,5).
As with all immune responses, antitumor T cells
undergo a sequential process of priming, activation, circulation,
recruitment, infiltration, effector function, and ultimately,
resolution or memory formation. The intricate trafficking network
among the local tumor microenvironment (TME), surrounding lymphoid
tissues and peripheral circulation provides multiple opportunities
for tumors to abrogate effective antitumor immunity, necessitating
comprehensive strategies to address all immune escape mechanisms
for successful antitumor immune responses (6).
The recent advancement in multi-omics analysis has
provided deeper insights into the complexities of antitumor
immunity. Therefore, focusing on CD8+ T cells as the
principal effectors of antitumor immune responses, the present
review examines the comprehensive tumor ecosystem beyond the local
TME, with particular emphasis on the dynamic and systemic nature of
antitumor immunity, to provide direction for future basic and
translational research.
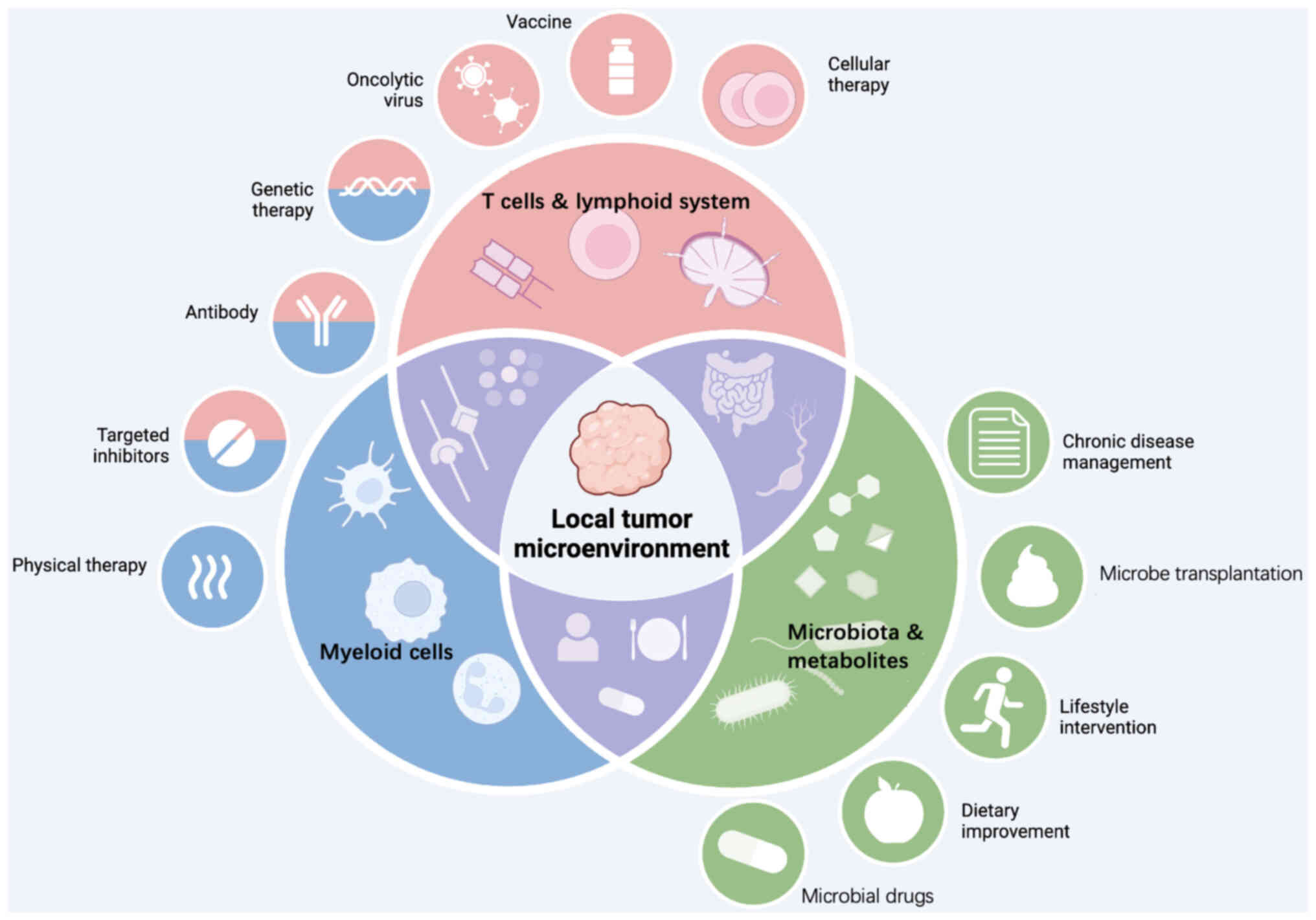
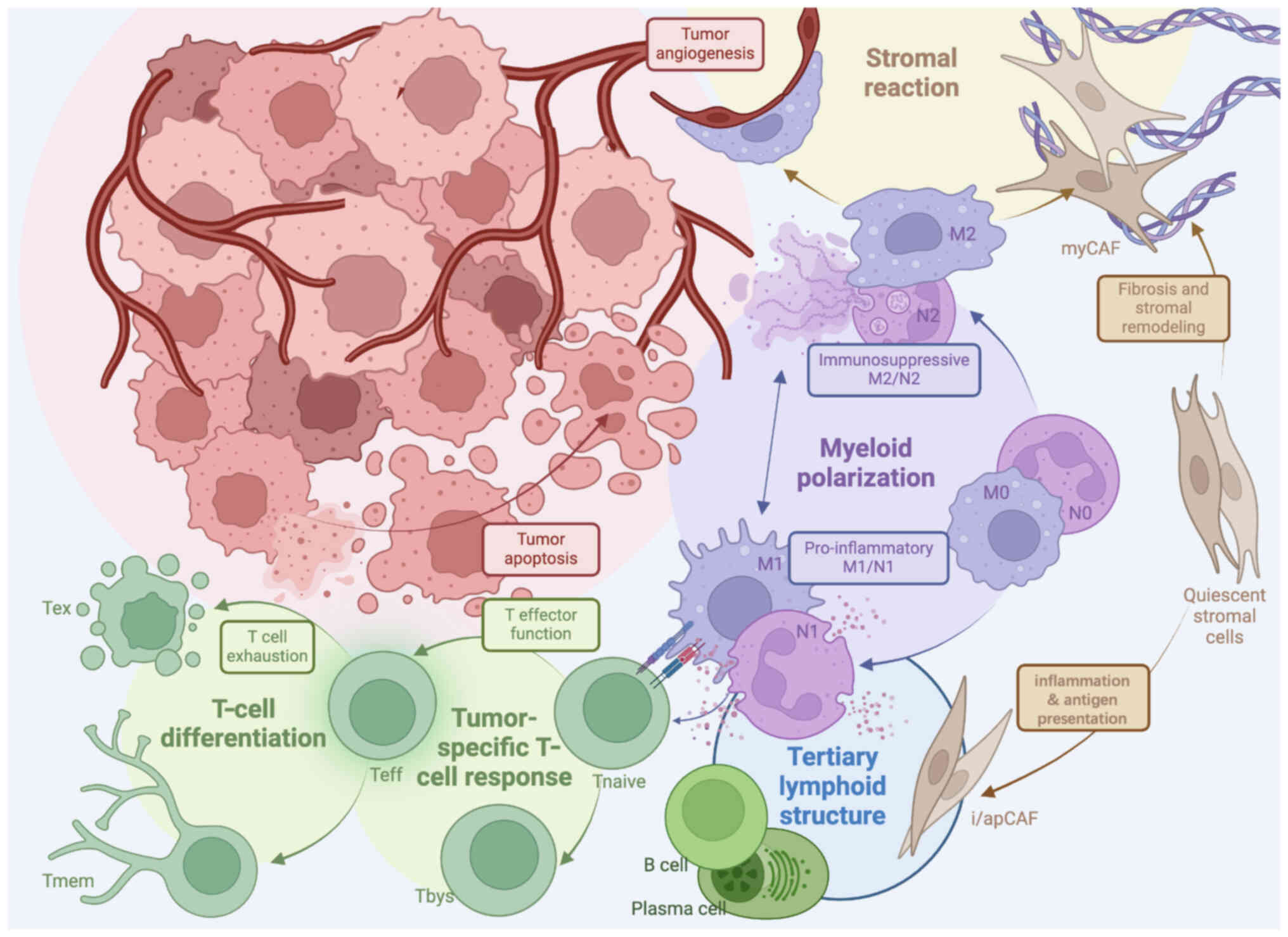
The TME is a complex ecosystem consisting of immune
and stromal cells, along with bioactive molecules that influence
tumor progression and immune responses (Fig. 1) (7). Key components include T cells, tumor
cells, cancer-associated fibroblasts (CAFs), myeloid-derived
suppressor cells and tumor endothelial cells (TECs). Additionally,
the extracellular matrix (ECM) and stromal molecules, such as
collagen, chemokines, cytokines and signaling molecules,
significantly impact tumor growth and T-cell function (Fig. 2). Among these, CD8+
tumor-infiltrating lymphocytes (TILs) are established prognostic
biomarkers and form the foundation of most immunotherapies
(8). However, their therapeutic
efficacy is limited by their inherent heterogeneity in composition,
phenotype and function, which complicates their correlation with
treatment outcomes.
Similarly, TECs impede T-cell infiltration through
abnormal vascular responses, including reduced adhesion molecule
expression. Abnormal responses of blood vessel TECs to inflammatory
cytokines and decreased expression of adhesion molecules impede
T-cell recruitment and infiltration (16,17). Studies have demonstrated that high
inflammation coupled with low vasculature correlates with optimal
ICB responses, as confirmed by two independent investigations
(18,19). Lymphatic TECs further exacerbate
immune exclusion by attracting C-X-C chemokine receptor type 4
(CXCR4)+ T cells away from tumors via C-X-C motif
chemokine ligand 12 (CXCL12) signaling (20). Collectively, these stromal cells
establish chronic inflammatory niches that hinder effective immune
responses. Recently, research (21) in hepatocellular carcinoma has
revealed that pericancerous macrophages cross-present antigens to
CD103+ CTLs via the endoplasmic reticulum-associated
degradation machinery-mediated cytosolic pathway, subsequently
activating the NLRP3 inflammasome in macrophages, thereby promoting
the progression of hepatoma and immunotherapy resistance.
T-cell specificity, driven by T-cell receptor (TCR)
recognition of major histocompatibility complex-I:antigen
complexes, underpins T-cell activation and antitumor immunity. TMB
and neoantigen availability strongly correlate with ICB efficacy in
immunologically 'hot' tumors such as melanoma and lung cancer
(22-24). Conversely, in 'cold' tumors with
low TMB, treatments such as chemotherapy, radiotherapy and tumor
vaccines can enhance antigenicity, converting cold tumors into hot
ones (25-28).
Despite these advances, most T cells in the TME are
bystander T cells (Tbys) that lack tumor antigen recognition but
still influence immune responses (29). Studies suggest that expressing
Tby-specific antigens in tumor cells could harness their antitumor
potential (30-32). By contrast, antigen-specific T
cells (Tas), which recognize tumor neoantigens, are the primary
effectors in immunotherapy (33).
These cells often exhibit exhaustion due to chronic stimulation
(34), characterized by markers
such as CD39 (31,35,36,37), CD103 (38-40) and programmed cell death protein 1
(PD-1) (41,42). Emerging markers such as CXCL13
(43-47) and CD137 (48,49) further refine Tas identification
and therapeutic targeting. Notably, the predominant expression of
exhaustion markers among Tas markers emphasizes the critical need
to address T-cell exhaustion in therapeutic strategies to maintain
clonal expansion and effector function (50).
Chronic antigen exposure in tumors induces T-cell
exhaustion, marked by impaired proliferation, reduced cytokine
production and elevated inhibitory receptor expression (51,52). T-cell exhaustion represents a
complex process orchestrated by dynamic alterations and
dysregulation in signaling pathways, transcription factors
[including T cell factor 1 (TCF1), T-box expressed in T cells and
thymocyte selection-associated high mobility group box protein
(TOX)], epigenetic programs and metabolic adaptations (53,54). Exhausted T cells (Tex) comprise a
heterogeneous population exhibiting a spectrum of phenotypic and
functional characteristics (55).
This spectrum is anchored by two distinct states: Progenitor Tex
(Tex-prog), which maintain stem-like and proliferative properties,
and terminally differentiated Tex (Tex-term).
Tex-int cells occupy an intermediate state between
functional effector T cells and Tex-term. These cells retain
proliferative capacity and respond robustly to ICB, serving as a
'stem-like' reservoir for sustained immune responses (68,69). The abundance of these cells
correlates with improved clinical outcomes, making them promising
targets for combination therapies such as vaccines and chimeric
antigen receptor (CAR)-T cells (53).
T-cell exhaustion results from persistent antigen
stimulation and is regulated by a complex interplay of signaling
pathways (71), transcription
factors and epigenetic programs. Factors such as mechanistic target
of rapamycin (mTOR), transforming growth factor-β (TGF-β) and PD-1
signaling influence transcriptional regulators, such as basic
leucine zipper transcription factor ATF-like (BATF), TOX and TCF1,
which maintain a delicate balance between T-cell activation and
exhaustion (83,84). Briefly, upstream molecular
pathways, such as mTOR and TGFβ (85), PD1 signaling (86) and IL-10 stimulation (87), orchestrate the activities of
transcription factors myeloblastosis (88), activator protein 1 (61,89), BATF (90-92), BTB and CNC Homology 2 (BACH2)
(90), special AT-rich
sequence-binding protein 1 (93),
interferon regulatory factor 4 and nuclear factor of activated T
cells cytoplasmic 1 (91,92,94). Multiple aspects of a T cell are
kept in a fine balance by these regulators; for example,
methylcytosine dioxygenase TET2 guards against BATF3-induced CAR-T
cell proliferation and ensuing genomic instability (95,96). With a more complete understanding
of this epigenetic programming, it is possible to fine tune T-cell
immunity and exhaustion while keeping proliferative responses and
general inflammation in check.
The systemic T-cell response to ICB is driven by two
key mechanisms: The expansion of pre-existing T-cell clones and the
infiltration of new clonotypes, a process termed clonal
rejuvenation (97). Pre-existing
clones provide a rapid antitumor response, while new clonotypes,
undetectable before treatment, emerge post-ICB therapy (98). This dynamic relies heavily on
continuous trafficking (99,100) between the lymphatic system and
blood circulation. Notably, blocking lymphocyte egress from lymph
nodes with the sphingosine-1-phosphate receptor inhibitor FTY720
abolishes ICB efficacy (101),
underscoring the critical role of the peripheral immune system in
T-cell activation and expansion.
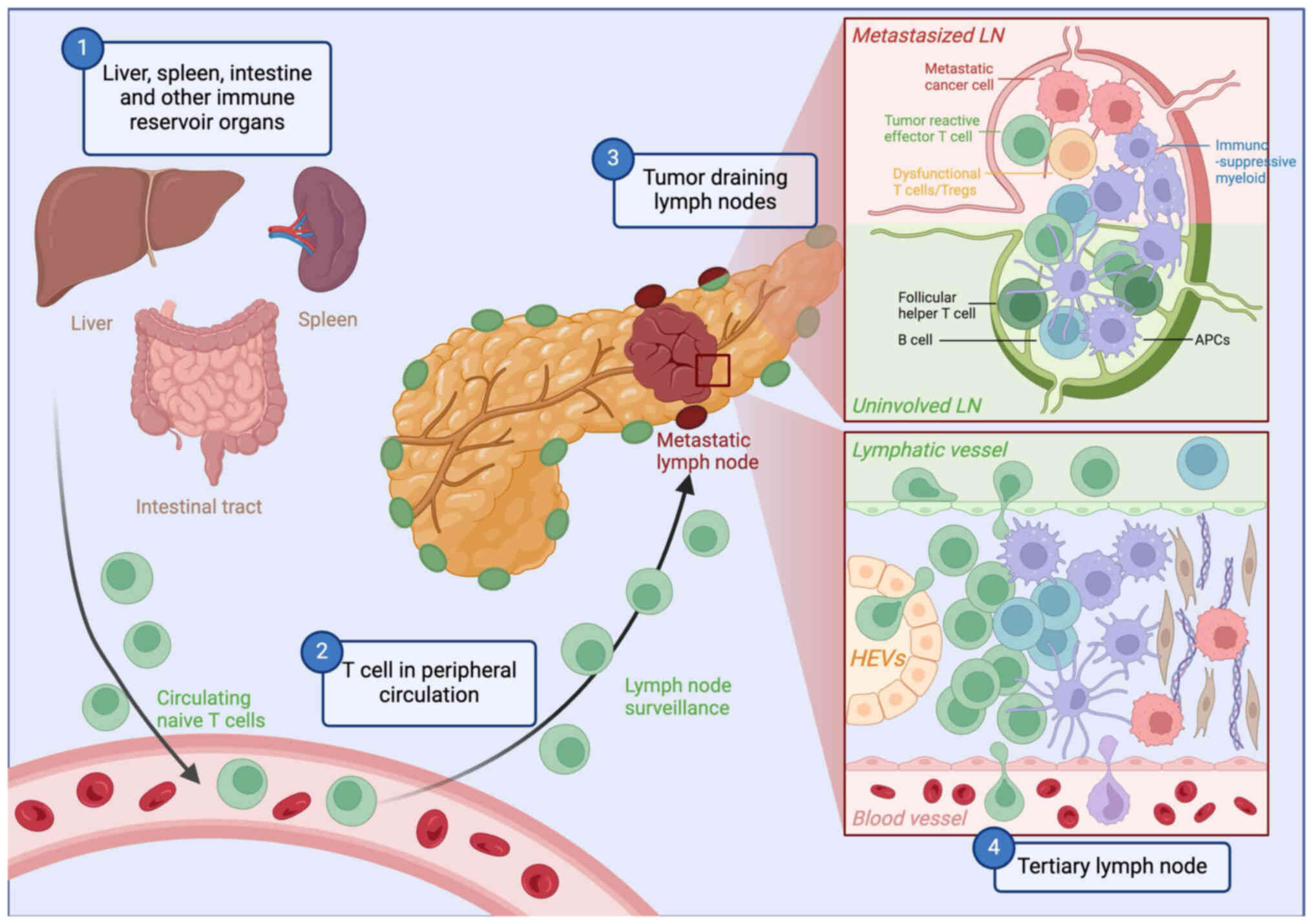
Peripheral lymphoid structures are central to
adaptive immunity, serving as hubs where antigens,
antigen-presenting cells (APCs) and lymphocytes interact within
specialized microenvironments (Fig.
3). These interactions occur in secondary lymphoid organs
(SLOs), such as tumor-draining lymph nodes (tdLNs), and in tertiary
lymphoid structures (TLSs), which form in response to chronic
inflammation (102). Both SLOs
and TLSs facilitate antigen-specific T-cell activation, playing
pivotal roles in antitumor immunity (103).
TLSs are organized immune cell aggregates that form
in chronic inflammatory conditions, including cancer (119-121). Structurally resembling SLOs,
TLSs support de novo and ongoing immune responses, promoting
T-cell activation and B-cell-mediated antibody production (122). Their presence is associated with
favorable outcomes across multiple cancer types, including breast
(123), colorectal (124) and pancreatic (125,126) cancer.
TLS formation is driven by chemokines such as
CXCL13, and their maturity, ranging from early lymphocyte
aggregation to fully developed germinal centers, impacts their
prognostic value (127-129). Mature TLSs are linked to better
outcomes, while immature TLSs may correlate with a poor prognosis
in certain cancer types, such as lung and breast cancer (130-132). Emerging therapeutic strategies
aim to enhance TLS formation (133-136) and maturation while mitigating
tumor-promoting inflammation.
The heterogeneity in TLS maturation, categorized
into early (lymphocyte aggregation), primary (follicular dendritic
cells without mature DCs) and secondary stages [active germinal
centers (GCs)] (137), is
evident across cancers and assessed via markers such as
CD138+ plasma cells (138) and CD23+ GC B cells
(139). For example, esophageal
cancer shows 26.9% TLS-negative, 30.2% immature and 42.9% mature
cases, with mature TLSs featuring proliferative/memory B cells,
plasma cells and CD4+ Th17 cells (140). Liver cancer exhibits 53%
TLS-negative cases, 26% lymphoid aggregates, 16% primary and 5%
secondary follicles, with the latter two linked to reduced
recurrence risk (141). In
squamous lung cancer, mature TLSs with GCs predict prognosis unless
diminished by corticosteroids during neoadjuvant chemotherapy
(128,142). Similarly, melanoma highlights
B-cell markers (activation-induced cytidine deaminase and CD21) for
mature TLSs and prognostic insights (143), while colorectal cancer
emphasizes the significance of follicular dendritic cells and
mature B cells (144).
The peripheral blood reflects systemic immune
status, with lymphocyte composition serving as a key biomarker for
antitumor immunity. Baseline lymphopenia, observed in various
cancer types (152), such as
metastatic breast cancer, correlates with disease burden and
treatment outcomes. While systemic chemotherapy often suppresses
peripheral immunity, localized treatments can preserve immune
function and synergize with ICB (153). Post-treatment lymphocyte counts
are predictive of ICB response, with higher levels correlating with
improved outcomes in melanoma, lung cancer and renal cell carcinoma
(RCC) (154). Relative
lymphocyte count demonstrates significant prognostic value in
melanoma (155,156), non-small cell lung cancer and
RCC (157).
The functional status of circulating T cells
significantly impacts cancer immunity (174). Memory T cells, particularly
central memory T cell (Tcm) and effector memory T cell (Tem)
subsets, are associated with favorable outcomes, while Tregs and
Tex-term often indicate a poor prognosis (175-177). Treatment-induced activation of
cytotoxic T cells and interferon-γ (IFNγ) production (174) are key markers of ICB response.
Elevated PD1+/CD8+ T cells and natural killer
(NK) cells (178) predict better
outcomes, whereas increased Tregs and naive T cells correlate with
a poor prognosis.
The composition of lymphocyte subsets reflects
distinct functional signatures defined by specific phenotypic
markers, with memory T-cell phenotypes generally linked to
favorable clinical outcomes. Key prognostic indicators include the
Tcm/effector T cell ratio (179), CD8+ Tem marked as C-C
chemokine receptor type 7
(CCR7)−/CD45RA−/CD27+/CD28+
(180,181), and CD4+ memory T
cells characterized by positivity for CD45RO/CCR7 and the absence
of CD62L (182). Elevated
baseline CD274 expression on T cells is also a significant
prognostic marker (183).
CD4+ T-cell subsets include central memory
(CD45RA−/CD62L+), effector memory
(CD45RA−/CD62L−) and non-senescent
(CD57−) cells (184).
Baseline PD1+ T cells and activated NK cells indicate
antitumor immunity (185), while
CD25+/FOXP3+/CD4+ Tregs are
negative prognostic factors (182,184), with PD1 expression on Tregs
linked to susceptibility to ICB-mediated activation and potential
tumor hyperprogression (186).
Tex-term (PD1+/T-cell immunoreceptor with Ig and ITIM
domains+/LAG3+ and
CD28−/CD57+/killer cell lectin-like receptor
subfamily G member 1+) (180,187,188) and PDL1+ NK cells
further highlight the complexity of immune dynamics in the TME
(157).
Treatment-induced immune changes, particularly early
T-cell activation and proliferation marked by Ki-67 expression in
PD1+/CD8+ Tex (189-193), cytotoxic activity, IFNγ
activation and effector memory phenotypes (194,195), are critical indicators of ICB
efficacy. A higher PD1+/CD8+ to
PD1+/CD4+ T-cell ratio (173,196) and increased NK cell populations
(197) correlate with favorable
outcomes, whereas Treg induction predicts a poor prognosis
(198). CD4+ Tcm
(CD27+/FAS−/CD45RA−/CCR7+)
play a vital role (199), and
post-treatment T cell immunoglobulin and mucin domain-containing
protein 3-positive Tex show tumor-specific prognostic value, being
unfavorable in NSCLC and RCC, but favorable in esophageal squamous
cell carcinoma (157,200). These findings suggest their
utility as biomarkers for immune activation rather than as direct
effectors. In hepatocellular carcinoma, an inflammatory signature
involving PDL1, CD8A, LAG3 and signal transducer and activator of
transcription 1 correlates with better outcomes, while
macrophage-associated gene patterns show no prognostic significance
(201). A composite biomarker
panel, including mitochondrial activation (peroxisome
proliferator-activated receptor γ coactivator 1 and reactive oxygen
species) and specific T-cell populations
(CD8+/PD-1hi and CD4+ T cells),
has demonstrated high predictive accuracy for PD-1 blockade therapy
outcomes (area under the curve, 0.96) (202).
irAEs are associated with enhanced therapeutic
responses and prolonged survival times (203,204) in ICB-treated patients. Specific
immune cell subsets, such as CXCR3+/CD8+ Tem
and CD11c+ APCs, are linked to both efficacy and
toxicity (205,206). Elevated Th17 (207) and Th1 (208) cells, coupled with reduced Treg
levels, predict irAEs, while CTLA4 (209) insufficiency exacerbates these
effects. Biomarkers such as post-treatment TCR clonality and
peripheral immune profiles hold promise for optimizing treatment
efficacy while minimizing irAEs (206,210).
Systemic inflammation plays a pivotal role in
regulating T-cell responses during immune defense against
malignancies. Pro-inflammatory mediators, such as IL-1β, TNF-α and
IL-6, create an inflammatory environment that facilitates
APC-mediated T-cell activation and expansion. While this process is
essential for initiating immune responses against infections and
cancer, chronic inflammation can lead to T-cell exhaustion
(211), marked by reduced
effector functions and increased expression of inhibitory receptors
such as PD-1 and CTLA-4.
Therapeutic strategies targeting systemic
inflammation aim to enhance T-cell functionality while preventing
exhaustion. Approaches include use of cytokine inhibitors (e.g.,
IL-6 or TNF-α antagonists) to mitigate excessive inflammation and
checkpoint inhibitors to restore T-cell activity (212). Emerging therapies such as
selective adjuvants that activate Toll-like receptors and metabolic
interventions targeting T cell-specific pathways offer promising
avenues to sustain T-cell function without inducing chronic
inflammation, particularly in cancer immunotherapy contexts
(213).
PD-1 blockade therapy has transformed cancer
treatment by reinvigorating T cell-mediated antitumor immunity.
PD-1, an immune checkpoint expressed on T cells, suppresses T-cell
activation to maintain self-tolerance, but is exploited by tumors
for immune evasion via the PD-1/PD-L1 axis (214). Monoclonal antibodies targeting
this pathway, such as nivolumab and pembrolizumab, have achieved
marked clinical success across various cancer types (215-217), such as non-small cell lung
cancer, head and neck squamous cell carcinoma, and melanoma.
Although these therapies provide durable responses and improved
survival, they can cause irAEs, necessitating careful management
(218,219). Combining PD-1 inhibitors with
other immunotherapies or conventional treatments has shown promise
in overcoming resistance and achieving synergistic effects
(220-223).
Advances in vaccine technologies have significantly
enhanced T cell-mediated antitumor immunity. Cancer vaccines
targeting tumor-associated antigens stimulate specific T-cell
responses by mimicking natural infections (224-227). The addition of adjuvants, such
as CpG oligodeoxynucleotides, further amplifies innate immune
responses, supporting T-cell activation and proliferation (228-231). Combining cancer vaccines with
checkpoint inhibitors has demonstrated synergistic effects,
improving T-cell responses and tumor clearance (225,232,233). Personalized cancer vaccines,
tailored to individual tumor antigenic profiles, represent a
promising breakthrough by generating broad-spectrum T-cell
responses while minimizing immune escape. These developments mark a
significant step forward in precision immunotherapy (234).
mRNA-based therapies have emerged as a powerful
tool in cancer immunotherapy, leveraging the ability of mRNA to
encode tumor-specific antigens and stimulate targeted immune
responses. Advances in delivery systems, such as lipid
nanoparticles, have improved mRNA stability and protection against
degradation (235-237). Structural modifications,
including nucleoside alterations, enhance translation efficiency
while minimizing immunogenicity (238-241). Co-delivery of immunomodulatory
molecules encoded by mRNA further amplifies T-cell responses and
counters immunosuppression (242). Clinical trials, particularly in
melanoma, have demonstrated the efficacy of personalized mRNA
vaccines in eliciting robust T-cell responses, underscoring their
potential in cancer treatment (243-245).
Lymphoid tissue-targeted nano delivery systems
offer an innovative approach to enhancing T-cell functionality in
cancer immunotherapy. Engineered nanoparticles, modified with
ligands targeting lymphoid tissue receptors (e.g., CCR7 and CXCR5),
enable precise delivery of immunomodulatory agents (246-249). Encapsulation of cytokines, such
as IL-2 and IL-12 (250-253), within these nanoparticles
enhances local T-cell responses while minimizing systemic side
effects. Recent advancements include delivering checkpoint
inhibitors directly to lymphoid tissues, addressing T-cell
exhaustion in the TME (254-257). By concentrating therapeutic
agents at desired sites, these systems improve treatment efficacy
and reduce adverse effects, representing a promising direction for
cancer immunotherapy.
OVs present a novel strategy to enhance T
cell-mediated antitumor responses by selectively infecting and
lysing cancer cells. This process triggers pro-inflammatory
responses that activate adaptive immunity while sparing healthy
tissue (258). OVs can also be
engineered to express immunomodulatory genes, such as IL-12 and
granulocyte-macrophage colony-stimulating factor, which enhance
T-cell recruitment and activation within the TME (259-262). Combining OVs with other
immunotherapies, particularly checkpoint inhibitors, has shown
synergistic effects in overcoming tumor-induced immunosuppression
(263). This dual mechanism,
namely direct tumor lysis and immune activation, positions OVs as a
promising component of combination immunotherapy strategies.
The present review underscores the intricate
interplay between local and systemic factors in T cell-mediated
antitumor immunity. Within the TME, T cells face challenges such as
physical exclusion, functional exhaustion and immunosuppression.
The distinction between Tex-prog and Tex-term has emerged as a key
factor influencing immunotherapy outcomes. Beyond the tumor site,
systemic immune responses mediated by lymphoid structures and
circulation are critical for sustaining effective antitumor
immunity.
Recent advancements in T-cell biology have paved
the way for novel therapeutic strategies, including mRNA-based
therapies, targeted nano delivery systems and engineered oncolytic
viruses. These innovations, coupled with improved biomarkers, such
as TCR repertoire analysis and circulating T-cell phenotyping,
enable more precise patient stratification and treatment
optimization.
Looking ahead, several challenges remain. Balancing
therapeutic efficacy with irAEs requires deeper insights into the
molecular mechanisms distinguishing beneficial from harmful immune
responses. Additionally, the role of systemic immunity,
particularly lymphoid structures and circulation, warrants further
exploration. Combination therapies targeting both T cells and the
TME hold significant potential but need systematic evaluation.
Finally, personalized immunotherapy, incorporating patient-specific
factors, such as tumor antigens and immune status, promises to
enhance treatment outcomes. Future clinical trials should consider
stratifying patients according to Tex-prog and Tex-term profiles to
optimize immunotherapy outcomes. Additionally, therapeutic
interventions that promote the formation and maturation of TLSs in
selected tumor types warrant systematic evaluation.
In conclusion, the rapid evolution of T cell-based
cancer immunotherapy offers exciting opportunities to overcome
current limitations. By advancing our understanding of immune
dynamics and leveraging emerging technologies, more effective and
personalized treatment strategies can be developed, ultimately
expanding the benefits of immunotherapy to a broader patient
population.
Not applicable.
XL, JH and CL wrote the daft. YZ and WW reviewed
and revised the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by grants from the National High Level
Hospital Clinical Research Funding (grant no. 2022-PUMCH-B-039 and
grant no. 2025-PUMCH-D-001), and the National College Student
Innovation and Entrepreneurship Training Program (grant nos.
202110023010, 2022zglc06080 and 2023zglc06022).
|
1
|
Anagnostou V, Niknafs N, Marrone K, Bruhm
DC, White JR, Naidoo J, Hummelink K, Monkhorst K, Lalezari F, Lanis
M, et al: Multimodal genomic features predict outcome of immune
checkpoint blockade in non-small-cell lung cancer. Nat Cancer.
1:99–111. 2020. View Article : Google Scholar
|
|
2
|
Wu B, Zhang B, Li B, Wu H and Jiang M:
Cold and hot tumors: From molecular mechanisms to targeted therapy.
Signal Transduct Target Ther. 9:2742024. View Article : Google Scholar :
|
|
3
|
Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y,
Lin D, Gao Q, Zhou H, Liao W and Yao H: Association of survival and
immune-related biomarkers with immunotherapy in patients with
non-small cell lung cancer: A meta-analysis and individual
patient-level analysis. JAMA Netw Open. 2:e1968792019. View Article : Google Scholar
|
|
4
|
Spitzer MH, Carmi Y, Reticker-Flynn NE,
Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR,
Chabon J, Bendall SC, et al: Systemic immunity is required for
effective cancer immunotherapy. Cell. 168:487–502.e15. 2017.
View Article : Google Scholar
|
|
5
|
Tselikas L, Dardenne A, de Baere T, Faron
M, Ammari S, Farhane S, Suzzoni S, Danlos FX, Raoult T, Susini S,
et al: Feasibility, safety and efficacy of human intra-tumoral
immuno-therapy. Gustave Roussy's initial experience with its first
100 patients. Eur J Cancer. 172:1–12. 2022. View Article : Google Scholar
|
|
6
|
Spranger S: Mechanisms of tumor escape in
the context of the T-cell-inflamed and the non-T-cell-inflamed
tumor microenvironment. Int Immunol. 28:383–391. 2016. View Article : Google Scholar :
|
|
7
|
Sadeghi Rad H, Monkman J, Warkiani ME,
Ladwa R, O'Byrne K, Rezaei N and Kulasinghe A: Understanding the
tumor microenvironment for effective immunotherapy. Med Res Rev.
41:1474–1498. 2021. View Article : Google Scholar :
|
|
8
|
Casalegno Garduño R, Spitschak A, Pannek T
and Pützer BM: CD8+ T cell subsets as biomarkers for predicting
checkpoint therapy outcomes in cancer immunotherapy. Biomedicines.
13:9302025. View Article : Google Scholar
|
|
9
|
Loi S, Adams S, Schmid P, Cortés J, Cescon
DW, Winer EP, Toppmeyer DL, Rugo HS, De Laurentiis M, Nanda R, et
al: LBA13-Relationship between tumor infiltrating lymphocyte (TIL)
levels and response to pembrolizumab (pembro) in metastatic
triple-negative breast cancer (mTNBC): Results from KEYNOTE-086.
Ann Oncol. 28(Suppl 5): v6082017. View Article : Google Scholar
|
|
10
|
Hegde PS and Chen DS: Top 10 challenges in
cancer immunotherapy. Immunity. 52:17–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to Anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018. View Article : Google Scholar
|
|
13
|
Cascio S, Chandler C, Zhang L, Sinno S,
Gao B, Onkar S, Bruno TC, Vignali DAA, Mahdi H, Osmanbeyoglu HU, et
al: Cancer-associated MSC drive tumor immune exclusion and
resistance to immunotherapy, which can be overcome by Hedgehog
inhibition. Sci Adv. 7:eabi57902021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grout JA, Sirven P, Leader AM, Maskey S,
Hector E, Puisieux I, Steffan F, Cheng E, Tung N, Maurin M, et al:
Spatial positioning and matrix programs of cancer-associated
fibroblasts promote T-cell exclusion in human lung tumors. Cancer
Discov. 12:2606–2625. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elyada E, Bolisetty M, Laise P, Flynn WF,
Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS,
et al: Cross-species single-cell analysis of pancreatic ductal
adenocarcinoma reveals antigen-presenting cancer-associated
fibroblasts. Cancer Discov. 9:1102–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Griffioen AW, Damen CA, Blijham GH and
Groenewegen G: Tumor angiogenesis is accompanied by a decreased
inflammatory response of tumor-associated endothelium. Blood.
88:667–673. 1996. View Article : Google Scholar
|
|
17
|
Nagl L, Horvath L, Pircher A and Wolf D:
Tumor endothelial cells (TECs) as potential immune directors of the
tumor microenvironment-new findings and future perspectives. Front
Cell Dev Biol. 8:7662020. View Article : Google Scholar
|
|
18
|
Sahu A, Kose K, Kraehenbuehl L, Byers C,
Holland A, Tembo T, Santella A, Alfonso A, Li M, Cordova M, et al:
In vivo tumor immune microenvironment phenotypes correlate with
inflammation and vasculature to predict immunotherapy response. Nat
Commun. 13:53122022. View Article : Google Scholar
|
|
19
|
Subramanian M, Kabir AU, Barisas D, Krchma
K and Choi K: Conserved angio-immune subtypes of the tumor
microenvironment predict response to immune checkpoint blockade
therapy. Cell Rep Med. 4:1008962023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steele MM, Jaiswal A, Delclaux I, Dryg ID,
Murugan D, Femel J, Son S, du Bois H, Hill C, Leachman SA, et al: T
cell egress via lymphatic vessels is tuned by antigen encounter and
limits tumor control. Nat Immunol. 24:664–675. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CX, Lao XM, Wang XY, Ren YZ, Lu YT,
Shi W, Wang YZ, Wu CY, Xu L, Chen MS, et al: Pericancerous
cross-presentation to cytotoxic T lymphocytes impairs
immunotherapeutic efficacy in hepatocellular carcinoma. Cancer
Cell. 42:2082–2097.e10. 2024. View Article : Google Scholar
|
|
22
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cristescu R, Aurora-Garg D, Albright A, Xu
L, Liu XQ, Loboda A, Lang L, Jin F, Rubin EH, Snyder A and
Lunceford J: Tumor mutational burden predicts the efficacy of
pembrolizumab monotherapy: A pan-tumor retrospective analysis of
participants with advanced solid tumors. J Immunother Cancer.
10:e0030912022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ricciuti B, Wang X, Alessi JV, Rizvi H,
Mahadevan NR, Li YY, Polio A, Lindsay J, Umeton R, Sinha R, et al:
Association of high tumor mutation burden in non-small cell lung
cancers with increased immune infiltration and improved clinical
outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA
Oncol. 8:1160–1168. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baharom F, Ramirez-Valdez RA, Khalilnezhad
A, Khalilnezhad S, Dillon M, Hermans D, Fussell S, Tobin KKS,
Dutertre CA, Lynn GM, et al: Systemic vaccination induces
CD8+ T cells and remodels the tumor microenvironment.
Cell. 185:4317–4332.e15. 2022. View Article : Google Scholar
|
|
26
|
Zheng M: Tumor mutation burden for
predicting immune checkpoint blockade response: The more, the
better. J Immunother Cancer. 10:e0030872022. View Article : Google Scholar
|
|
27
|
McGrail DJ, Pilié PG, Rashid NU, Voorwerk
L, Slagter M, Kok M, Jonasch E, Khasraw M, Heimberger AB, Lim B, et
al: High tumor mutation burden fails to predict immune checkpoint
blockade response across all cancer types. Ann Oncol. 32:661–672.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niknafs N, Balan A, Cherry C, Hummelink K,
Monkhorst K, Shao XM, Belcaid Z, Marrone KA, Murray J, Smith KN, et
al: Persistent mutation burden drives sustained anti-tumor immune
responses. Nat Med. 29:440–449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valpione S, Mundra PA, Galvani E, Campana
LG, Lorigan P, De Rosa F, Gupta A, Weightman J, Mills S, Dhomen N
and Marais R: The T cell receptor repertoire of tumor infiltrating
T cells is predictive and prognostic for cancer survival. Nat
Commun. 12:40982021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meier SL, Satpathy AT and Wells DK:
Bystander T cells in cancer immunology and therapy. Nat Cancer.
3:143–155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simoni Y, Becht E, Fehlings M, Loh CY, Koo
SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al:
Bystander CD8+ T cells are abundant and phenotypically
distinct in human tumour infiltrates. Nature. 557:575–579. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Zhao J, Yue S, Li Z, Duan X, Lin
Y, Yang Y, He J, Gao L, Pan Z, et al: An oncolytic virus delivering
tumor-irrelevant bystander T cell epitopes induces anti-tumor
immunity and potentiates cancer immunotherapy. Nat Cancer.
5:1063–1081. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lowery FJ, Krishna S, Yossef R, Parikh NB,
Chatani PD, Zacharakis N, Parkhurst MR, Levin N, Sindiri S, Sachs
A, et al: Molecular signatures of antitumor neoantigen-reactive T
cells from metastatic human cancers. Science. 375:877–884. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng Z, Rodriguez Ehrenfried A, Tan CL,
Steffens LK, Kehm H, Zens S, Lauenstein C, Paul A, Schwab M,
Förster JD, et al: Transcriptome-based identification of
tumor-reactive and bystander CD8+ T cell receptor
clonotypes in human pancreatic cancer. Sci Transl Med.
15:eadh95622023. View Article : Google Scholar
|
|
35
|
Kortekaas KE, Santegoets SJ, Sturm G,
Ehsan I, van Egmond SL, Finotello F, Rajanoski Z, Welters MJP, van
Poelgeest MIE and van der Burg SH: CD39 identifies the
CD4+ tumor-specific T-cell population in human cancer.
Cancer Immunol Res. 8:1311–1321. 2020. View Article : Google Scholar
|
|
36
|
Duhen T, Duhen R, Montler R, Moses J,
Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott
JE, et al: Co-expression of CD39 and CD103 identifies
tumor-reactive CD8 T cells in human solid tumors. Nat Commun.
9:27242018. View Article : Google Scholar
|
|
37
|
Qiao M, Zhou F, Liu X, Jiang T, Wang H,
Jia Y, Li X, Zhao C, Cheng L, Chen X, et al: Interleukin-10 induces
expression of CD39 on CD8+T cells to potentiate anti-PD1 efficacy
in EGFR-mutated non-small cell lung cancer. J Immunother Cancer.
10:e0054362022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Webb JR, Milne K and Nelson BH: PD-1 and
CD103 are widely coexpressed on prognostically favorable
intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol
Res. 3:926–935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Corgnac S, Malenica I, Mezquita L, Auclin
E, Voilin E, Kacher J, Halse H, Grynszpan L, Signolle N, Dayris T,
et al: CD103+CD8+ TRM cells accumulate in
tumors of anti-PD-1-responder lung cancer patients and are
tumor-reactive lymphocytes enriched with Tc17. Cell Rep Med.
1:1001272020. View Article : Google Scholar
|
|
40
|
Wang Z, Ahmed S, Labib M, Wang H, Wu L,
Bavaghar-Zaeimi F, Shokri N, Blanco S, Karim S, Czarnecka-Kujawa K,
et al: Isolation of tumour-reactive lymphocytes from peripheral
blood via microfluidic immunomagnetic cell sorting. Nat Biomed Eng.
7:1188–1203. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gallerano D, Ciminati S, Grimaldi A,
Piconese S, Cammarata I, Focaccetti C, Pacella I, Accapezzato D,
Lancellotti F, Sacco L, et al: Genetically driven CD39 expression
shapes human tumor-infiltrating CD8+ T-cell functions.
Int J Cancer. 147:2597–2610. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Laumont CM, Wouters MCA, Smazynski J,
Gierc NS, Chavez EA, Chong LC, Thornton S, Milne K, Webb JR, Steidl
C and Nelson BH: Single-cell profiles and prognostic impact of
tumor-infiltrating lymphocytes coexpressing CD39, CD103, and PD-1
in ovarian cancer. Clin Cancer Res. 27:4089–4100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He J, Xiong X, Yang H, Li D, Liu X, Li S,
Liao S, Chen S, Wen X, Yu K, et al: Defined tumor antigen-specific
T cells potentiate personalized TCR-T cell therapy and prediction
of immunotherapy response. Cell Res. 32:530–542. 2022. View Article : Google Scholar
|
|
44
|
Liu B, Zhang Y, Wang D, Hu X and Zhang Z:
Single-cell meta-analyses reveal responses of tumor-reactive
CXCL13+ T cells to immune-checkpoint blockade. Nat
Cancer. 3:1123–1136. 2022. View Article : Google Scholar
|
|
45
|
Hanada KI, Zhao C, Gil-Hoyos R, Gartner
JJ, Chow-Parmer C, Lowery FJ, Krishna S, Prickett TD, Kivitz S,
Parkhurst MR, et al: A phenotypic signature that identifies
neoantigen-reactive T cells in fresh human lung cancers. Cancer
Cell. 40:479–493.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dai S, Zeng H, Liu Z, Jin K, Jiang W, Wang
Z, Lin Z, Xiong Y, Wang J, Chang Y, et al: Intratumoral
CXCL13+CD8+T cell infiltration determines
poor clinical outcomes and immunoevasive contexture in patients
with clear cell renal cell carcinoma. J Immunother Cancer.
9:e0018232021. View Article : Google Scholar
|
|
47
|
Aoki T, Chong LC, Takata K, Milne K,
Marshall A, Chavez EA, Miyata-Takata T, Ben-Neriah S, Unrau D,
Telenius A, et al: Single-cell profiling reveals the importance of
CXCL13/CXCR5 axis biology in lymphocyte-rich classic Hodgkin
lymphoma. Proc Natl Acad Sci USA. 118:e21058221182021. View Article : Google Scholar :
|
|
48
|
Eiva MA, Omran DK, Chacon JA and Powell DJ
Jr: Systematic analysis of CD39, CD103, CD137, and PD-1 as
biomarkers for naturally occurring tumor antigen-specific TILs. Eur
J Immunol. 52:96–108. 2022. View Article : Google Scholar :
|
|
49
|
Parkhurst M, Gros A, Pasetto A, Prickett
T, Crystal JS, Robbins P and Rosenberg SA: Isolation of T-cell
receptors specifically reactive with mutated tumor-associated
antigens from tumor-infiltrating lymphocytes based on CD137
expression. Clin Cancer Res. 23:2491–2505. 2017. View Article : Google Scholar
|
|
50
|
Yost KE, Satpathy AT, Wells DK, Qi Y, Wang
C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, et al:
Clonal replacement of tumor-specific T cells following PD-1
blockade. Nat Med. 25:1251–1259. 2019. View Article : Google Scholar
|
|
51
|
Tonnerre P, Wolski D, Subudhi S, Aljabban
J, Hoogeveen RC, Damasio M, Drescher HK, Bartsch LM, Tully DC, Sen
DR, et al: Differentiation of exhausted CD8+ T cells
after termination of chronic antigen stimulation stops short of
achieving functional T cell memory. Nat Immunol. 22:1030–1041.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Watowich MB, Gilbert MR and Larion M: T
cell exhaustion in malignant gliomas. Trends Cancer. 9:270–292.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Beltra JC, Manne S, Abdel-Hakeem MS,
Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ,
et al: Developmental relationships of four exhausted
CD8+ T cell subsets reveals underlying transcriptional
and epigenetic landscape control mechanisms. Immunity.
52:825–841.e8. 2020. View Article : Google Scholar
|
|
54
|
Sen DR, Kaminski J, Barnitz RA, Kurachi M,
Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et
al: The epigenetic landscape of T cell exhaustion. Science.
354:1165–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Z, Chen L, Chen H, Zhao J, Li K, Sun
J and Zhou M: Pan-cancer landscape of T-cell exhaustion
heterogeneity within the tumor microenvironment revealed a
progressive roadmap of hierarchical dysfunction associated with
prognosis and therapeutic efficacy. EBioMedicine. 83:1042072022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu Z, Yoshikawa T, Inoue S, Ito Y, Kasuya
H, Nakashima T, Zhang H, Kotaka S, Hosoda W, Suzuki S and Kagoya Y:
CD83 expression characterizes precursor exhausted T cell
population. Commun Biol. 6:2582023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Utzschneider DT, Charmoy M, Chennupati V,
Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F,
Hofmann M, Wieland D, et al: T cell factor 1-expressing memory-like
CD8(+) T cells sustain the immune response to chronic viral
infections. Immunity. 45:415–427. 2016. View Article : Google Scholar
|
|
58
|
Kim CG, Kim G, Kim KH, Park S, Shin S, Yeo
D, Shim HS, Yoon HI, Park SY, Ha SJ and Kim HR: Distinct exhaustion
features of T lymphocytes shape the tumor-immune microenvironment
with therapeutic implication in patients with non-small-cell lung
cancer. J Immunother Cancer. 9:e0027802021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wieland D, Kemming J, Schuch A, Emmerich
F, Knolle P, Neumann-Haefelin C, Held W, Zehn D, Hofmann M and
Thimme R: TCF1+ hepatitis C virus-specific
CD8+ T cells are maintained after cessation of chronic
antigen stimulation. Nat Commun. 8:150502017. View Article : Google Scholar
|
|
60
|
Jadhav RR, Im SJ, Hu B, Hashimoto M, Li P,
Lin JX, Leonard WJ, Greenleaf WJ, Ahmed R and Goronzy JJ:
Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as
resource cells during chronic viral infection and respond to PD-1
blockade. Proc Natl Acad Sci USA. 116:14113–14118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li X, Li Y, Dong L, Chang Y, Zhang X, Wang
C, Chen M, Bo X, Chen H, Han W and Nie J: Decitabine priming
increases anti-PD-1 antitumor efficacy by promoting CD8+ progenitor
exhausted T cell expansion in tumor models. J Clin Invest.
133:e1656732023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nagasaki J, Inozume T, Sax N, Ariyasu R,
Ishikawa M, Yamashita K, Kawazu M, Ueno T, Irie T, Tanji E, et al:
PD-1 blockade therapy promotes infiltration of tumor-attacking
exhausted T cell clonotypes. Cell Rep. 38:1103312022. View Article : Google Scholar
|
|
63
|
Codarri Deak L, Nicolini V, Hashimoto M,
Karagianni M, Schwalie PC, Lauener L, Varypataki EM, Richard M,
Bommer E, Sam J, et al: PD-1-cis IL-2R agonism yields better
effectors from stem-like CD8+ T cells. Nature.
610:161–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hashimoto M, Araki K, Cardenas MA, Li P,
Jadhav RR, Kissick HT, Hudson WH, McGuire DJ, Obeng RC, Wieland A,
et al: PD-1 combination therapy with IL-2 modifies CD8+
T cell exhaustion program. Nature. 610:173–181. 2022. View Article : Google Scholar :
|
|
65
|
Ren Z, Zhang A, Sun Z, Liang Y, Ye J, Qiao
J, Li B and Fu YX: Selective delivery of low-affinity IL-2 to PD-1+
T cells rejuvenates antitumor immunity with reduced toxicity. J
Clin Invest. 132:e1536042022. View Article : Google Scholar
|
|
66
|
Zehn D, Thimme R, Lugli E, de Almeida GP
and Oxenius A: 'Stem-like' precursors are the fount to sustain
persistent CD8+ T cell responses. Nat Immunol.
23:836–847. 2022. View Article : Google Scholar
|
|
67
|
Miller BC, Sen DR, Al Abosy R, Bi K,
Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al:
Subsets of exhausted CD8+ T cells differentially mediate
tumor control and respond to checkpoint blockade. Nat Immunol.
20:326–336. 2016. View Article : Google Scholar
|
|
68
|
Im SJ, Hashimoto M, Gerner MY, Lee J,
Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al:
Defining CD8+ T cells that provide the proliferative burst after
PD-1 therapy. Nature. 537:417–421. 2016. View Article : Google Scholar
|
|
69
|
Siddiqui I, Schaeuble K, Chennupati V,
Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ,
Scarpellino L, Gfeller D, Pradervand S, et al: Intratumoral
Tcf1+PD-1+CD8+ T cells with
stem-like properties promote tumor control in response to
vaccination and checkpoint blockade immunotherapy. Immunity.
50:195–211.e10. 2019. View Article : Google Scholar
|
|
70
|
Tabanelli V, Melle F, Motta G, Mazzara S,
Fabbri M, Agostinelli C, Calleri A, Del Corvo M, Fiori S, Lorenzini
D, et al: The identification of TCF1+ progenitor exhausted T cells
in THRLBCL may predict a better response to PD-1/PD-L1 blockade.
Blood Adv. 6:4634–4644. 2022. View Article : Google Scholar :
|
|
71
|
Zheng L, Qin S, Si W, Wang A, Xing B, Gao
R, Ren X, Wang L, Wu X, Zhang J, et al: Pan-cancer single-cell
landscape of tumor-infiltrating T cells. Science. 374:abe64742021.
View Article : Google Scholar
|
|
72
|
Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim
HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, et
al: TOX reinforces the phenotype and longevity of exhausted T cells
in chronic viral infection. Nature. 571:265–269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kim K, Park S, Park SY, Kim G, Park SM,
Cho JW, Kim DH, Park YM, Koh YW, Kim HR, et al: Single-cell
transcriptome analysis reveals TOX as a promoting factor for T cell
exhaustion and a predictor for anti-PD-1 responses in human cancer.
Genome Med. 12:222020. View Article : Google Scholar :
|
|
74
|
Abdel-Hakeem MS, Manne S, Beltra JC,
Stelekati E, Chen Z, Nzingha K, Ali MA, Johnson JL, Giles JR,
Mathew D, et al: Epigenetic scarring of exhausted T cells hinders
memory differentiation upon eliminating chronic antigenic
stimulation. Nat Immunol. 22:1008–1019. 2021. View Article : Google Scholar :
|
|
75
|
Pauken KE, Sammons MA, Odorizzi PM, Manne
S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al:
Epigenetic stability of exhausted T cells limits durability of
reinvigoration by PD-1 blockade. Science. 354:1160–1165. 2016.
View Article : Google Scholar
|
|
76
|
Gupta PK, Godec J, Wolski D, Adland E,
Yates K, Pauken KE, Cosgrove C, Ledderose C, Junger WG, Robson SC,
et al: CD39 expression identifies terminally exhausted CD8+ T
cells. PLoS Pathog. 11:e10051772015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tinoco R, Neubert EN, Stairiker CJ,
Henriquez ML and Bradley LM: PSGL-1 is a T cell intrinsic inhibitor
that regulates effector and memory differentiation and responses
during viral infection. Front Immunol. 12:6778242021. View Article : Google Scholar :
|
|
78
|
Tinoco R, Carrette F, Barraza ML, Otero
DC, Magaña J, Bosenberg MW, Swain SL and Bradley LM: PSGL-1 is an
immune checkpoint regulator that promotes T cell exhaustion.
Immunity. 44:1190–1203. 2016. View Article : Google Scholar
|
|
79
|
Vignali PDA, DePeaux K, Watson MJ, Ye C,
Ford BR, Lontos K, McGaa NK, Scharping NE, Menk AV, Robson SC, et
al: Hypoxia drives CD39-dependent suppressor function in exhausted
T cells to limit antitumor immunity. Nat Immunol. 24:267–279. 2023.
View Article : Google Scholar
|
|
80
|
Viramontes KM, Neubert EN, DeRogatis JM
and Tinoco R: PD-1 immune checkpoint blockade and PSGL-1 inhibition
synergize to reinvigorate exhausted T cells. Front Immunol.
13:8697682022. View Article : Google Scholar :
|
|
81
|
Moesta AK, Li XY and Smyth MJ: Targeting
CD39 in cancer. Nat Rev Immunol. 20:739–755. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tu E, McGlinchey K, Wang J, Martin P,
Ching SL, Floc'h N, Kurasawa J, Starrett JH, Lazdun Y, Wetzel L, et
al: Anti-PD-L1 and anti-CD73 combination therapy promotes T cell
response to EGFR-mutated NSCLC. JCI Insight. 7:e1428432022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ford BR and Poholek AC: Regulation and
immunotherapeutic targeting of the epigenome in exhausted CD8 T
cell responses. J Immunol. 210:869–879. 2023. View Article : Google Scholar
|
|
84
|
Franco F, Jaccard A, Romero P, Yu YR and
Ho PC: Metabolic and epigenetic regulation of T-cell exhaustion.
Nat Metab. 2:1001–1012. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gabriel SS, Tsui C, Chisanga D, Weber F,
Llano-León M, Gubser PM, Bartholin L, Souza-Fonseca-Guimaraes F,
Huntington ND, Shi W, et al: Transforming growth factor-β-regulated
mTOR activity preserves cellular metabolism to maintain long-term T
cell responses in chronic infection. Immunity. 54:1698–1714.e5.
2021. View Article : Google Scholar
|
|
86
|
Bengsch B, Johnson AL, Kurachi M, Odorizzi
PM, Pauken KE, Attanasio J, Stelekati E, McLane LM, Paley MA,
Delgoffe GM and Wherry EJ: Bioenergetic insufficiencies due to
metabolic alterations regulated by the inhibitory receptor PD-1 are
an early driver of CD8(+) T cell exhaustion. Immunity. 45:358–373.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guo Y, Xie YQ, Gao M, Zhao Y, Franco F,
Wenes M, Siddiqui I, Bevilacqua A, Wang H, Yang H, et al: Metabolic
reprogramming of terminally exhausted CD8+ T cells by
IL-10 enhances anti-tumor immunity. Nat Immunol. 22:746–756. 2021.
View Article : Google Scholar :
|
|
88
|
Tsui C, Kretschmer L, Rapelius S, Gabriel
SS, Chisanga D, Knöpper K, Utzschneider DT, Nüssing S, Liao Y,
Mason T, et al: MYB orchestrates T cell exhaustion and response to
checkpoint inhibition. Nature. 609:354–360. 2022. View Article : Google Scholar :
|
|
89
|
Stelekati E, Chen Z, Manne S, Kurachi M,
Ali MA, Lewy K, Cai Z, Nzingha K, McLane LM, Hope JL, et al:
Long-term persistence of exhausted CD8 T cells in chronic infection
is regulated by MicroRNA-155. Cell Rep. 23:2142–2156. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Utzschneider DT, Gabriel SS, Chisanga D,
Gloury R, Gubser PM, Vasanthakumar A, Shi W and Kallies A: Early
precursor T cells establish and propagate T cell exhaustion in
chronic infection. Nat Immun. 21:1256–1266. 2020. View Article : Google Scholar
|
|
91
|
Man K, Gabriel SS, Liao Y, Gloury R,
Preston S, Henstridge DC, Pellegrini M, Zehn D, Berberich-Siebelt
F, Febbraio MA, et al: Transcription factor IRF4 promotes
CD8+ T cell exhaustion and limits the development of
memory-like T cells during chronic infection. Immunity.
47:1129–1141.e5. 2017. View Article : Google Scholar
|
|
92
|
Seo H, González-Avalos E, Zhang W,
Ramchandani P, Yang C, Lio CJ, Rao A and Hogan PG: BATF and IRF4
cooperate to counter exhaustion in tumor-infiltrating CAR T cells.
Nat Immunol. 22:983–995. 2021. View Article : Google Scholar :
|
|
93
|
Russ BE, Tsyganov K, Quon S, Yu B, Li J,
Lee JKC, Olshansky M, He Z, Harrison PF, Barugahare A, et al:
Active maintenance of CD8+ T cell naïvety through
regulation of global genome architecture. bioRxiv: The preprint
server for biology. 2023.
|
|
94
|
Grusdat M, McIlwain DR, Xu HC, Pozdeev VI,
Knievel J, Crome SQ, Robert-Tissot C, Dress RJ, Pandyra AA, Speiser
DE, et al: IRF4 and BATF are critical for CD8+ T-cell
function following infection with LCMV. Cell Death Differ.
21:1050–1060. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jain N, Zhao Z, Feucht J, Koche R, Iyer A,
Dobrin A, Mansilla-Soto J, Yang J, Zhan Y, Lopez M, et al: TET2
guards against unchecked BATF3-induced CAR T cell expansion.
Nature. 615:315–322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jordan MS, Drury S, Giles JR, Manne S,
Huang H, Chen Z, Oldridge D, Wherry EJ and Baxter AE: TET2 controls
differentiation of terminally exhausted CD8 T cells. J Immunol.
206(1 Suppl): S14.072021. View Article : Google Scholar
|
|
97
|
Liu B, Hu X, Feng K, Gao R, Xue Z, Zhang
S, Zhang Y, Corse E, Hu Y, Han W and Zhang Z: Temporal single-cell
tracing reveals clonal revival and expansion of precursor exhausted
T cells during anti-PD-1 therapy in lung cancer. Nat Cancer.
3:108–121. 2022. View Article : Google Scholar
|
|
98
|
Luoma AM, Suo S, Wang Y, Gunasti L, Porter
CBM, Nabilsi N, Tadros J, Ferretti AP, Liao S, Gurer C, et al:
Tissue-resident memory and circulating T cells are early responders
to pre-surgical cancer immunotherapy. Cell. 185:2918–2935.e29.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li Z, Tuong ZK, Dean I, Willis C, Gaspal
F, Fiancette R, Idris S, Kennedy B, Ferdinand JR, Peñalver A, et
al: In vivo labeling reveals continuous trafficking of TCF-1+ T
cells between tumor and lymphoid tissue. J Exp Med.
219:e202107492022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kennedy BC, Dean I and Withers DR:
Migration of stem-like CD8 T cells between tissue microenvironments
underpins successful anti-tumour immune responses. Discov Immunol.
2:kyad0042023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fransen MF, Schoonderwoerd M, Knopf P,
Camps MG, Hawinkels LJ, Kneilling M, van Hall T and Ossendorp F:
Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint
therapy. JCI Insight. 3:e1245072018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liu Z, Yu Z, Chen D, Verma V, Yuan C, Wang
M, Wang F, Fan Q, Wang X, Li Y, et al: Pivotal roles of
tumor-draining lymph nodes in the abscopal effects from combined
immunotherapy and radiotherapy. Cancer Commun (Lond). 42:971–986.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tanaka R, Hiramitsu M, Shimizu S,
Kawashima S, Sato A and Iwase Y: Efficient drug delivery to lymph
nodes by intradermal administration and enhancement of anti-tumor
effects of immune checkpoint inhibitors. Cancer Treat Res Commun.
36:1007402023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
du Bois H, Heim TA and Lund AW:
Tumor-draining lymph nodes: At the crossroads of metastasis and
immunity. Sci Immunol. 6:eabg35512021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rahim MK, Okholm TLH, Jones KB, McCarthy
EE, Liu CC, Yee JL, Ki SJ, Marquez DM, Tenvooren I, Wai K, et al:
Dynamic CD8+ T cell responses to cancer immunotherapy in
human regional lymph nodes are disrupted in metastatic lymph nodes.
Cell. 186:1127–1143.e18. 2023. View Article : Google Scholar
|
|
106
|
Buchwald ZS, Nasti TH, Lee J, Eberhardt
CS, Wieland A, Im SJ, Lawson D, Curran W, Ahmed R and Khan MK:
Tumor-draining lymph node is important for a robust abscopal effect
stimulated by radiotherapy. J Immunother Cancer. 8:e0008672020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fear VS, Forbes CA, Neeve SA, Fisher SA,
Chee J, Waithman J, Ma SK, Lake R, Nowak AK, Creaney J, et al:
Tumour draining lymph node-generated CD8 T cells play a role in
controlling lung metastases after a primary tumour is removed but
not when adjuvant immunotherapy is used. Cancer Immunol Immunother.
70:3249–3258. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dammeijer F, van Gulijk M, Mulder EE,
Lukkes M, Klaase L, van den Bosch T, van Nimwegen M, Lau SP,
Latupeirissa K, Schetters S, et al: The PD-1/PD-L1-checkpoint
restrains T cell immunity in tumor-draining lymph nodes. Cancer
Cell. 38:685–700.e8. 2020. View Article : Google Scholar
|
|
109
|
Zhou Y, Slone N, Chrisikos TT, Kyrysyuk O,
Babcock RL, Medik YB, Li HS, Kleinerman ES and Watowich SS: Vaccine
efficacy against primary and metastatic cancer with in
vitro-generated CD103+ conventional dendritic cells. J
Immunother Cancer. 8:e0004742020. View Article : Google Scholar
|
|
110
|
Salmon H, Idoyaga J, Rahman A, Leboeuf M,
Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J,
Tung N, et al: Expansion and activation of CD103(+) dendritic cell
progenitors at the tumor site enhances tumor responses to
therapeutic PD-L1 and BRAF inhibition. Immunity. 44:924–938. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Huang Q, Wu X, Wang Z, Chen X, Wang L, Lu
Y, Xiong D, Liu Q, Tian Y, Lin H, et al: The primordial
differentiation of tumor-specific memory CD8+ T cells as
bona fide responders to PD-1/PD-L1 blockade in draining lymph
nodes. Cell. 185:4049–4066.e25. 2022. View Article : Google Scholar
|
|
112
|
Okamura K, Nagayama S, Tate T, Chan HT,
Kiyotani K and Nakamura Y: Lymphocytes in tumor-draining lymph
nodes co-cultured with autologous tumor cells for adoptive cell
therapy. J Transl Med. 20:2412022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Schenkel JM, Herbst RH, Canner D, Li A,
Hillman M, Shanahan SL, Gibbons G, Smith OC, Kim JY, Westcott P, et
al: Conventional type I dendritic cells maintain a reservoir of
proliferative tumor-antigen specific TCF-1+
CD8+ T cells in tumor-draining lymph nodes. Immunity.
54:2338–2353.e6. 2021. View Article : Google Scholar
|
|
114
|
Connolly KA, Kuchroo M, Venkat A, Khatun
A, Wang J, William I, Hornick NI, Fitzgerald BL, Damo M, Kasmani
MY, et al: A reservoir of stem-like CD8+ T cells in the
tumor-draining lymph node preserves the ongoing antitumor immune
response. Sci Immunol. 6:eabg78362021. View Article : Google Scholar
|
|
115
|
O'Melia MJ, Manspeaker MP and Thomas SN:
Tumor-draining lymph nodes are survival niches that support T cell
priming against lymphatic transported tumor antigen and effects of
immune checkpoint blockade in TNBC. Cancer Immunol Immunother.
70:2179–2195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Dominguez-Gutierrez PR, Kwenda EP, Donelan
W, Miranda M, Doty A, O'Malley P, Crispen PL and Kusmartsev S:
Detection of PD-L1-expressing myeloid cell clusters in the
hyaluronan-enriched stroma in tumor tissue and tumor-draining lymph
nodes. J Immunol. 208:2829–2836. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Núñez NG, Tosello Boari J, Ramos RN,
Richer W, Cagnard N, Anderfuhren CD, Niborski LL, Bigot J, Meseure
D, De La Rochere P, et al: Tumor invasion in draining lymph nodes
is associated with Treg accumulation in breast cancer patients. Nat
Commun. 11:32722020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yang H, Sun B, Ma W, Fan L, Xu K, Jia Y,
Xu J, Wang Z and Yao F: Multi-scale characterization of
tumor-draining lymph nodes in resectable lung cancer treated with
neoadjuvant immune checkpoint inhibitors. EBioMedicine.
84:1042652022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Schumacher TN and Thommen DS: Tertiary
lymphoid structures in cancer. Science. 375:eabf94192022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dieu-Nosjean MC, Giraldo NA, Kaplon H,
Germain C, Fridman WH and Sautès-Fridman C: Tertiary lymphoid
structures, drivers of the anti-tumor responses in human cancers.
Immunol Rev. 271:260–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Rodriguez AB, Peske JD, Woods AN, Leick
KM, Mauldin IS, Meneveau MO, Young SJ, Lindsay RS, Melssen MM,
Cyranowski S, et al: Immune mechanisms orchestrate tertiary
lymphoid structures in tumors via cancer-associated fibroblasts.
Cell Rep. 36:1094222021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ng KW, Boumelha J, Enfield KSS, Almagro J,
Cha H, Pich O, Karasaki T, Moore DA, Salgado R, Sivakumar M, et al:
Antibodies against endogenous retroviruses promote lung cancer
immunotherapy. Nature. 616:563–573. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang B, Liu J, Han Y, Deng Y, Li J and
Jiang Y: The presence of tertiary lymphoid structures provides new
insight into the clinicopathological features and prognosis of
patients with breast cancer. Front Immunol. 13:8681552022.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang Q, Shen X, An R, Bai J, Dong J, Cai
H, Zhu H, Zhong W, Chen W, Liu A and Du J: Peritumoral tertiary
lymphoid structure and tumor stroma percentage predict the
prognosis of patients with non-metastatic colorectal cancer. Front
Immunol. 13:9620562022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang WH, Wang WQ, Han X, Gao HL, Xu SS,
Li S, Li TJ, Xu HX, Li H, Ye LY, et al: Infiltrating pattern and
prognostic value of tertiary lymphoid structures in resected
non-functional pancreatic neuroendocrine tumors. J Immunother
Cancer. 8:e0011882020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Tanaka T, Masuda A, Inoue J, Hamada T,
Ikegawa T, Toyama H, Sofue K, Shiomi H, Sakai A, Kobayashi T, et
al: Integrated analysis of tertiary lymphoid structures in relation
to tumor-infiltrating lymphocytes and patient survival in
pancreatic ductal adenocarcinoma. J Gastroenterol. 58:277–291.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ukita M, Hamanishi J, Yoshitomi H, Yamanoi
K, Takamatsu S, Ueda A, Suzuki H, Hosoe Y, Furutake Y, Taki M, et
al: CXCL13-producing CD4+ T cells accumulate in the early phase of
tertiary lymphoid structures in ovarian cancer. JCI Insight.
7:e1572152022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Siliņa K, Soltermann A, Attar FM, Casanova
R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P,
Curioni-Fontecedro A, et al: Germinal centers determine the
prognostic relevance of tertiary lymphoid structures and are
impaired by corticosteroids in lung squamous cell carcinoma. Cancer
Res. 78:1308–1320. 2018. View Article : Google Scholar
|
|
129
|
Yang M, Lu J, Zhang G, Wang Y, He M, Xu Q,
Xu C and Liu H: CXCL13 shapes immunoactive tumor microenvironment
and enhances the efficacy of PD-1 checkpoint blockade in high-grade
serous ovarian cancer. J Immunother Cancer. 9:e0011362021.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Cabrita R, Lauss M, Sanna A, Donia M,
Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K,
Vallon-Christersson J, et al: Tertiary lymphoid structures improve
immunotherapy and survival in melanoma. Nature. 577:561–565. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Helmink BA, Reddy SM, Gao J, Zhang S,
Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et
al: B cells and tertiary lymphoid structures promote immunotherapy
response. Nature. 577:549–555. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Petitprez F, de Reyniès A, Keung EZ, Chen
TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A,
et al: B cells are associated with survival and immunotherapy
response in sarcoma. Nature. 577:556–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sawada J, Hiraoka N, Qi R, Jiang L,
Fournier-Goss AE, Yoshida M, Kawashima H and Komatsu M: Molecular
signature of tumor-associated high endothelial venules that can
predict breast cancer survival. Cancer Immunol Res. 10:468–481.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Martinet L, Garrido I, Filleron T, Le
Guellec S, Bellard E, Fournie JJ, Rochaix P and Girard JP: Human
solid tumors contain high endothelial venules: association with T-
and B-lymphocyte infiltration and favorable prognosis in breast
cancer. Cancer Res. 71:5678–5687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Asrir A, Tardiveau C, Coudert J, Laffont
R, Blanchard L, Bellard E, Veerman K, Bettini S, Lafouresse F, Vina
E, et al: Tumor-associated high endothelial venules mediate
lymphocyte entry into tumors and predict response to PD-1 plus
CTLA-4 combination immunotherapy. Cancer Cell. 40:318–334.e9. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li Z, Jiang Y, Li B, Han Z, Shen J, Xia Y
and Li R: Development and validation of a machine learning model
for detection and classification of tertiary lymphoid structures in
gastrointestinal cancers. JAMA Netw Open. 6:e22525532023.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Neyt K, Perros F, GeurtsvanKessel CH,
Hammad H and Lambrecht BN: Tertiary lymphoid organs in infection
and autoimmunity. Trends Immunol. 33:297–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hayashi Y, Makino T, Sato E, Ohshima K,
Nogi Y, Kanemura T, Honma K, Yamashita K, Saito T, Tanaka K, et al:
Density and maturity of peritumoral tertiary lymphoid structures in
oesophageal squamous cell carcinoma predicts patient survival and
response to immune checkpoint inhibitors. Br J Cancer.
128:2175–2185. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Deguchi S, Tanaka H, Suzuki S, Natsuki S,
Mori T, Miki Y, Yoshii M, Tamura T, Toyokawa T, Lee S, et al:
Clinical relevance of tertiary lymphoid structures in esophageal
squamous cell carcinoma. BMC Cancer. 22:6992022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ling Y, Zhong J, Weng Z, Lin G, Liu C, Pan
C, Yang H, Wei X, Xie X, Wei X, et al: The prognostic value and
molecular properties of tertiary lymphoid structures in oesophageal
squamous cell carcinoma. Clin Transl Med. 12:e10742022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Calderaro J, Petitprez F, Becht E, Laurent
A, Hirsch TZ, Rousseau B, Luciani A, Amaddeo G, Derman J, Charpy C,
et al: Intra-tumoral tertiary lymphoid structures are associated
with a low risk of early recurrence of hepatocellular carcinoma. J
Hepatol. 70:58–65. 2019. View Article : Google Scholar
|
|
142
|
Sun X, Liu W, Sun L, Mo H, Feng Y, Wu X,
Li C, Chen C, Li J, Xin Y, et al: Maturation and abundance of
tertiary lymphoid structures are associated with the efficacy of
neoadjuvant chemoimmunotherapy in resectable non-small cell lung
cancer. J Immunother Cancer. 10:e0055312022. View Article : Google Scholar :
|
|
143
|
Lynch KT, Young SJ, Meneveau MO, Wages NA,
Engelhard VH, Slingluff CL Jr and Mauldin IS: Heterogeneity in
tertiary lymphoid structure B-cells correlates with patient
survival in metastatic melanoma. J Immunother Cancer.
9:e0022732021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Posch F, Silina K, Leibl S, Mündlein A,
Moch H, Siebenhüner A, Samaras P, Riedl J, Stotz M, Szkandera J, et
al: Maturation of tertiary lymphoid structures and recurrence of
stage II and III colorectal cancer. Oncoimmunology. 7:e13788442017.
View Article : Google Scholar
|
|
145
|
Zhang Q and Wu S: Tertiary lymphoid
structures are critical for cancer prognosis and therapeutic
response. Front Immunol. 13:10637112023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Fridman WH, Meylan M, Petitprez F, Sun CM,
Italiano A and Sautès-Fridman C: B cells and tertiary lymphoid
structures as determinants of tumour immune contexture and clinical
outcome. Nat Rev Clin Oncol. 19:441–457. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Saito T, Nishikawa H, Wada H, Nagano Y,
Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, et
al: Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the
prognosis of colorectal cancers. Nat Med. 22:679–684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shalapour S, Font-Burgada J, Di Caro G,
Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M,
Strasner A, Hansel DE, et al: Immunosuppressive plasma cells impede
T-cell-dependent immunogenic chemotherapy. Nature. 521:94–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Finkin S, Yuan D, Stein I, Taniguchi K,
Weber A, Unger K, Browning JL, Goossens N, Nakagawa S, Gunasekaran
G, et al: Ectopic lymphoid structures function as microniches for
tumor progenitor cells in hepatocellular carcinoma. Nat Immunol.
16:1235–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Milutinovic S, Abe J, Godkin A, Stein JV
and Gallimore A: The dual role of high endothelial venules in
cancer progression versus immunity. Trends Cancer. 7:214–225. 2021.
View Article : Google Scholar
|
|
151
|
Sautès-Fridman C, Petitprez F, Calderaro J
and Fridman WH: Tertiary lymphoid structures in the era of cancer
immunotherapy. Nat Rev Cancer. 19:307–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ray-Coquard I, Cropet C, Van Glabbeke M,
Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P,
Labidi I, et al: Lymphopenia as a prognostic factor for overall
survival in advanced carcinomas, sarcomas, and lymphomas. Cancer
Res. 69:5383–5391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wu Z, Zhang J, Cai Y, Deng R, Yang L, Li J
and Deng Y: Reduction of circulating lymphocyte count is a
predictor of good tumor response after neoadjuvant treatment for
rectal cancer. Medicine (Baltimore). 97:e114352018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lee YJ, Park YS, Lee HW, Park TY, Lee JK
and Heo EY: Peripheral lymphocyte count as a surrogate marker of
immune checkpoint inhibitor therapy outcomes in patients with
non-small-cell lung cancer. Sci Rep. 12:6262022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Weide B, Martens A, Hassel JC, Berking C,
Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di
Giacomo AM, et al: Baseline biomarkers for outcome of melanoma
patients treated with pembrolizumab. Clin Cancer Res. 22:5487–5496.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Martens A, Wistuba-Hamprecht K, Geukes
Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B,
Capone M, et al: Baseline peripheral blood biomarkers associated
with clinical outcome of advanced melanoma patients treated with
ipilimumab. Clin Cancer Res. 22:2908–2918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Juliá EP, Mandó P, Rizzo MM, Cueto GR,
Tsou F, Luca R, Pupareli C, Bravo AI, Astorino W, Mordoh J, et al:
Peripheral changes in immune cell populations and soluble mediators
after anti-PD-1 therapy in non-small cell lung cancer and renal
cell carcinoma patients. Cancer Immunol Immunother. 68:1585–1596.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Cui JH, Lin KR, Yuan SH, Jin YB, Chen XP,
Su XK, Jiang J, Pan YM, Mao SL, Mao XF and Luo W: TCR repertoire as
a novel indicator for immune monitoring and prognosis assessment of
patients with cervical cancer. Front Immunol. 9:27292018.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gleason L, Porcu P and Nikbakht N: Reduced
overall T-cell receptor diversity as an indicator of aggressive
cutaneous T-cell lymphoma. Blood. 140(Suppl 1): 3539–3540. 2022.
View Article : Google Scholar
|
|
160
|
Manuel M, Tredan O, Bachelot T, Clapisson
G, Courtier A, Parmentier G, Rabeony T, Grives A, Perez S, Mouret
JF, et al: Lymphopenia combined with low TCR diversity (divpenia)
predicts poor overall survival in metastatic breast cancer
patients. Oncoimmunology. 1:432–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lin KR, Pang DM, Jin YB, Hu Q, Pan YM, Cui
JH, Chen XP, Lin YX, Mao XF, Duan HB and Luo W: Circulating
CD8+ T-cell repertoires reveal the biological
characteristics of tumors and clinical responses to chemotherapy in
breast cancer patients. Cancer Immunol Immunother. 67:1743–1752.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Cai G, Guan Z, Jin Y, Su Z, Chen X, Liu Q,
Wang C, Yin X, Zhang L, Ye G and Luo W: Circulating T-cell
repertoires correlate with the tumor response in patients with
breast cancer receiving neoadjuvant chemotherapy. JCO Precis Oncol.
6:e21001202022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Page DB, Yuan J, Redmond D, Wen YH, Durack
JC, Emerson R, Solomon S, Dong Z, Wong P, Comstock C, et al: Deep
sequencing of T-cell receptor DNA as a biomarker of clonally
expanded TILs in breast cancer after immunotherapy. Cancer Immunol
Res. 4:835–844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kuehm LM, Wolf K, Zahour J, DiPaolo RJ and
Teague RM: Checkpoint blockade immunotherapy enhances the frequency
and effector function of murine tumor-infiltrating T cells but does
not alter TCRβ diversity. Cancer Immunol Immunother. 68:1095–1106.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Rudqvist NP, Pilones KA, Lhuillier C,
Wennerberg E, Sidhom JW, Emerson RO, Robins HS, Schneck J, Formenti
SC and Demaria S: Radiotherapy and CTLA-4 blockade shape the TCR
repertoire of tumor-infiltrating T cells. Cancer Immunol Res.
6:139–150. 2018. View Article : Google Scholar :
|
|
166
|
Wu TD, Madireddi S, de Almeida PE,
Banchereau R, Chen YJ, Chitre AS, Chiang EY, Iftikhar H, O'Gorman
WE, Au-Yeung A, et al: Peripheral T cell expansion predicts tumour
infiltration and clinical response. Nature. 579:274–278. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Puig-Saus C, Sennino B, Peng S, Wang CL,
Pan Z, Yuen B, Purandare B, An D, Quach BB, Nguyen D, et al:
Neoantigen-targeted CD8+ T cell responses with PD-1
blockade therapy. Nature. 615:697–704. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Dong N, Moreno-Manuel A, Calabuig-Fariñas
S, Gallach S, Zhang F, Blasco A, Aparisi F, Meri-Abad M, Guijarro
R, Sirera R, et al: Characterization of circulating T cell receptor
repertoire provides information about clinical outcome after PD-1
blockade in advanced non-small cell lung cancer patients. Cancers
(Basel). 13:29502021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Han J, Duan J, Bai H, Wang Y, Wan R, Wang
X, Chen S, Tian Y, Wang D, Fei K, et al: TCR repertoire diversity
of peripheral PD-1+CD8+ T cells predicts
clinical outcomes after immunotherapy in patients with non-small
lung cancer. Cancer Immunol Res. 8:146–154. 2020. View Article : Google Scholar
|
|
170
|
Kato T, Kiyotani K, Tomiyama E, Koh Y,
Matsushita M, Hayashi Y, Nakano K, Ishizuya Y, Wang C, Hatano K, et
al: Peripheral T cell receptor repertoire features predict durable
responses to anti-PD-1 inhibitor monotherapy in advanced renal cell
carcinoma. Oncoimmunology. 10:18629482021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Snyder A, Nathanson T, Funt SA, Ahuja A,
Buros Novik J, Hellmann MD, Chang E, Aksoy BA, Al-Ahmadie H, Yusko
E, et al: Contribution of systemic and somatic factors to clinical
response and resistance to PD-L1 blockade in urothelial cancer: An
exploratory multi-omic analysis. PLoS Med. 14:e10023092017.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Arakawa A, Vollmer S, Tietze J, Galinski
A, Heppt MV, Bürdek M, Berking C and Prinz JC: Clonality of
CD4+ blood T cells predicts longer survival with CTLA4
or PD-1 checkpoint inhibition in advanced melanoma. Front Immunol.
10:13362019. View Article : Google Scholar
|
|
173
|
Zhu Q, Qiao G, Huang L, Xu C, Guo D, Wang
S, Zhao J, Song Y, Liu B, Chen Z, et al: Restored
CD8+PD-1+ T cells facilitate the response to
Anti-PD-1 for patients with pancreatic ductal adenocarcinoma. Front
Oncol. 12:8375602022. View Article : Google Scholar
|
|
174
|
Verronèse E, Delgado A,
Valladeau-Guilemond J, Garin G, Guillemaut S, Tredan O, Ray-Coquard
I, Bachelot T, N'Kodia A, Bardin-Dit-Courageot C, et al: Immune
cell dysfunctions in breast cancer patients detected through whole
blood multi-parametric flow cytometry assay. Oncoimmunology.
5:e11007912015. View Article : Google Scholar
|
|
175
|
Saleh R and Elkord E: FoxP3+ T
regulatory cells in cancer: Prognostic biomarkers and therapeutic
targets. Cancer Lett. 490:174–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune system.
Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Kotsakis A, Koinis F, Katsarou A,
Gioulbasani M, Aggouraki D, Kentepozidis N, Georgoulias V and
Vetsika EK: Prognostic value of circulating regulatory T cell
subsets in untreated non-small cell lung cancer patients. Sci Rep.
6:392472016. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Mamessier E, Sylvain A, Thibult ML,
Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P,
Romagné F, Thibault G, et al: Human breast cancer cells enhance
self tolerance by promoting evasion from NK cell antitumor
immunity. J Clin Invest. 121:3609–3622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Manjarrez-Orduño N, Menard LC, Kansal S,
Fischer P, Kakrecha B, Jiang C, Cunningham M, Greenawalt D, Patel
V, Yang M, et al: Circulating T cell subpopulations correlate with
immune responses at the tumor site and clinical response to PD1
inhibition in non-small cell lung cancer. Front Immunol.
9:16132018. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH,
Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al: Hyperprogressive
disease during PD-1/PD-L1 blockade in patients with non-small-cell
lung cancer. Ann Oncol. 30:1104–1113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wistuba-Hamprecht K, Martens A, Heubach F,
Romano E, Geukes Foppen M, Yuan J, Postow M, Wong P, Mallardo D,
Schilling B, et al: Peripheral CD8 effector-memory type 1 T-cells
correlate with outcome in ipilimumab-treated stage IV melanoma
patients. Eur J Cancer. 73:61–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kagamu H, Kitano S, Yamaguchi O, Yoshimura
K, Horimoto K, Kitazawa M, Fukui K, Shiono A, Mouri A, Nishihara F,
et al: CD4+ T-cell Immunity in the peripheral blood
correlates with response to Anti-PD-1 therapy. Cancer Immunol Res.
8:334–344. 2020. View Article : Google Scholar
|
|
183
|
Sade-Feldman M, Kanterman J, Klieger Y,
Ish-Shalom E, Olga M, Saragovi A, Shtainberg H, Lotem M and
Baniyash M: Clinical significance of circulating
CD33+CD11b+HLA-DR-myeloid cells in patients with stage IV melanoma
treated with ipilimumab. Clin Cancer Res. 22:5661–5672. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zuazo M, Arasanz H, Fernández-Hinojal G,
García-Granda MJ, Gato M, Bocanegra A, Martínez M, Hernández B,
Teijeira L, Morilla I, et al: Functional systemic CD4 immunity is
required for clinical responses to PD-L1/PD-1 blockade therapy.
EMBO Mol Med. 11:e102932019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Mazzaschi G, Facchinetti F, Missale G,
Canetti D, Madeddu D, Zecca A, Veneziani M, Gelsomino F, Goldoni M,
Buti S, et al: The circulating pool of functionally competent NK
and CD8+ cells predicts the outcome of anti-PD1 treatment in
advanced NSCLC. Lung Cancer. 127:153–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Kamada T, Togashi Y, Tay C, Ha D, Sasaki
A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, et al:
PD-1+ regulatory T cells amplified by PD-1 blockade
promote hyperprogression of cancer. Proc Natl Acad Sci USA.
116:9999–10008. 2019. View Article : Google Scholar
|
|
187
|
Jacquelot N, Roberti MP, Enot DP,
Rusakiewicz S, Ternès N, Jegou S, Woods DM, Sodré AL, Hansen M,
Meirow Y, et al: Predictors of responses to immune checkpoint
blockade in advanced melanoma. Nat Commun. 8:5922017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ferrara R, Naigeon M, Auclin E, Duchemann
B, Cassard L, Jouniaux JM, Boselli L, Grivel J, Desnoyer A,
Mezquita L, et al: Circulating T-cell immunosenescence in patients
with advanced non-small cell lung cancer treated with single-agent
PD-1/PD-L1 inhibitors or platinum-based chemotherapy. Clin Cancer
Res. 27:492–503. 2021. View Article : Google Scholar
|
|
189
|
Griffiths JI, Wallet P, Pflieger LT,
Stenehjem D, Liu X, Cosgrove PA, Leggett NA, McQuerry JA, Shrestha
G, Rossetti M, et al: Circulating immune cell phenotype dynamics
reflect the strength of tumor-immune cell interactions in patients
during immunotherapy. Proc Natl Acad Sci USA. 117:16072–16082.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Kwon M, An M, Klempner SJ, Lee H, Kim KM,
Sa JK, Cho HJ, Hong JY, Lee T, Min YW, et al: Determinants of
response and intrinsic resistance to PD-1 blockade in
microsatellite instability-high gastric cancer. Cancer Discov.
11:2168–2185. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Kamphorst AO, Pillai RN, Yang S, Nasti TH,
Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al:
Proliferation of PD-1+ CD8 T cells in peripheral blood after
PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci
USA. 114:4993–4998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee
SH, Ahn JS, Cheon J, Min YJ, Park SH, et al: The first-week
proliferative response of peripheral blood
PD-1+CD8+ T cells predicts the response to
anti-PD-1 therapy in solid tumors. Clin Cancer Res. 25:2144–2154.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Krieg C, Nowicka M, Guglietta S, Schindler
S, Hartmann FJ, Weber LM, Dummer R, Robinson MD, Levesque MP and
Becher B: High-dimensional single-cell analysis predicts response
to anti-PD-1 immunotherapy. Nat Med. 24:144–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
de Coaña YP, Wolodarski M, Poschke I,
Yoshimoto Y, Yang Y, Nyström M, Edbäck U, Brage SE, Lundqvist A,
Masucci GV, et al: Ipilimumab treatment decreases monocytic MDSCs
and increases CD8 effector memory T cells in long-term survivors
with advanced melanoma. Oncotarget. 8:21539–21553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Duchemann B, Naigeon M, Auclin E, Ferrara
R, Cassard L, Jouniaux JM, Boselli L, Grivel J, Desnoyer A, Danlos
FX, et al: CD8+PD-1+ to
CD4+PD-1+ ratio (PERLS) is associated with
prognosis of patients with advanced NSCLC treated with PD-(L)1
blockers. J Immunother Cancer. 10:e0040122022. View Article : Google Scholar
|
|
197
|
Youn JI, Park SM, Park S, Kim G, Lee HJ,
Son J, Hong MH, Ghaderpour A, Baik B, Islam J, et al: Peripheral
natural killer cells and myeloid-derived suppressor cells correlate
with anti-PD-1 responses in non-small cell lung cancer. Sci Rep.
10:90502020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Dodagatta-Marri E, Meyer DS, Reeves MQ,
Paniagua R, To MD, Binnewies M, Broz ML, Mori H, Wu D, Adoumie M,
et al: α-PD-1 therapy elevates Treg/Th balance and increases tumor
cell pSmad3 that are both targeted by α-TGFβ antibody to promote
durable rejection and immunity in squamous cell carcinomas. J
Immunother Cancer. 7:622019. View Article : Google Scholar
|
|
199
|
Takeuchi Y, Tanemura A, Tada Y, Katayama
I, Kumanogoh A and Nishikawa H: Clinical response to PD-1 blockade
correlates with a sub-fraction of peripheral central memory CD4+ T
cells in patients with malignant melanoma. Int Immunol. 30:13–22.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Kato R, Yamasaki M, Urakawa S, Nishida K,
Makino T, Morimoto-Okazawa A, Kawashima A, Iwahori K, Suzuki S,
Ueda R, et al: Increased Tim-3+ T cells in PBMCs during
nivolumab therapy correlate with responses and prognosis of
advanced esophageal squamous cell carcinoma patients. Cancer
Immunol Immunother. 67:1673–1683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Sangro B, Melero I, Wadhawan S, Finn RS,
Abou-Alfa GK, Cheng AL, Yau T, Furuse J, Park JW, Boyd Z, et al:
Association of inflammatory biomarkers with clinical outcomes in
nivolumab-treated patients with advanced hepatocellular carcinoma.
J Hepatol. 73:1460–1469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kim HR, Park SM, Seo SU, Jung I, Yoon HI,
Gabrilovich DI, Cho BC, Seong SY, Ha SJ and Youn JI: The ratio of
peripheral regulatory T cells to Lox-1+
polymorphonuclear myeloid-derived suppressor cells predicts the
early response to Anti-PD-1 therapy in patients with non-small cell
lung cancer. Am J Respir Crit Care Med. 199:243–246. 2019.
View Article : Google Scholar :
|
|
203
|
Jia XH, Geng LY, Jiang PP, Xu H, Nan KJ,
Yao Y, Jiang LL, Sun H, Qin TJ and Guo H: The biomarkers related to
immune related adverse events caused by immune checkpoint
inhibitors. J Exp Clin Cancer Res. 39:2842020. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Schweizer C, Schubert P, Rutzner S,
Eckstein M, Haderlein M, Lettmaier S, Semrau S, Gostian AO, Frey B,
Gaipl US, et al: Prospective evaluation of the prognostic value of
immune-related adverse events in patients with non-melanoma solid
tumour treated with PD-1/PD-L1 inhibitors alone and in combination
with radiotherapy. Eur J Cancer. 140:55–62. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Chuah S, Lee J, Song Y, Kim HD, Wasser M,
Kaya NA, Bang K, Lee YJ, Jeon SH, Suthen S, et al: Uncoupling
immune trajectories of response and adverse events from anti-PD-1
immunotherapy in hepatocellular carcinoma. J Hepatol. 77:683–694.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Zhu H, Galdos FX, Lee D, Waliany S, Huang
YV, Ryan J, Dang K, Neal JW, Wakelee HA, Reddy SA, et al:
Identification of pathogenic immune cell subsets associated with
checkpoint inhibitor-induced myocarditis. Circulation. 146:316–335.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Franken A, Van Mol P, Vanmassenhove S,
Donders E, Schepers R, Van Brussel T, Dooms C, Yserbyt J, De Crem
N, Testelmans D, et al: Single-cell transcriptomics identifies
pathogenic T-helper 17.1 cells and pro-inflammatory monocytes in
immune checkpoint inhibitor-related pneumonitis. J Immunother
Cancer. 10:e0053232022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Kim KH, Hur JY, Cho J, Ku BM, Koh J, Koh
JY, Sun JM, Lee SH, Ahn JS, Park K, et al: Immune-related adverse
events are clustered into distinct subtypes by T-cell profiling
before and early after anti-PD-1 treatment. Oncoimmunology.
9:17220232020. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Wei SC, Meijers WC, Axelrod ML, Anang
NAAS, Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L,
Courand PY, et al: A genetic mouse model recapitulates immune
checkpoint inhibitor-associated myocarditis and supports a
mechanism-based therapeutic intervention. Cancer Discov.
11:614–625. 2021. View Article : Google Scholar :
|
|
210
|
Fairfax BP, Taylor CA, Watson RA, Nassiri
I, Danielli S, Fang H, Mahé EA, Cooper R, Woodcock V, Traill Z, et
al: Peripheral CD8+ T cell characteristics associated
with durable responses to immune checkpoint blockade in patients
with metastatic melanoma. Nat Med. 26:193–199. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Chia S, Bedard PL, Hilton J, Amir E,
Gelmon K, Goodwin R, Villa D, Cabanero M, Tu D, Tsao M and Seymour
L: A phase Ib trial of durvalumab in combination with trastuzumab
in HER2-positive metastatic breast cancer (CCTG IND.229).
Oncologist. 24:1439–1445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Soler MF, Abaurrea A, Azcoaga P, Araujo AM
and Caffarel MM: New perspectives in cancer immunotherapy:
Targeting IL-6 cytokine family. J Immunother Cancer.
11:e0075302023. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Wattenberg MM and Beatty GL: Overcoming
immunotherapeutic resistance by targeting the cancer inflammation
cycle. Semin Cancer Biol. 65:38–50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Tobias J, Steinberger P, Drinić M and
Wiedermann U: Emerging targets for anticancer vaccination: PD-1.
ESMO Open. 6:1002782021. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y,
Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of
immune-checkpoint inhibitors in human cancers. Front Immunol.
13:9644422022. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Yang Y, Yu Y and Lu S: Effectiveness of
PD-1/PD-L1 inhibitors in the treatment of lung cancer: Brightness
and challenge. Sci China Life Sci. 63:1499–1514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Reck M, Remon J and Hellmann MD:
First-line immunotherapy for non-small-cell lung cancer. J Clin
Oncol. 40:586–597. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal
AN, Gadgeel SM, Girshman J, Kris MG, Riely GJ, Yu HA and Hellmann
MD: Severe immune-related adverse events are common with sequential
PD-(L)1 blockade and osimertinib. Ann Oncol. 30:839–844. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Jhawar SR, Wang SJ, Thandoni A, Bommareddy
PK, Newman JH, Marzo AL, Kuzel TM, Gupta V, Reiser J, Daniels P, et
al: Combination oncolytic virus, radiation therapy, and immune
checkpoint inhibitor treatment in anti-PD-1-refractory cancer. J
Immunother Cancer. 11:e0067802023. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Gebrael G, Sahu KK, Agarwal N and Maughan
BL: Update on combined immunotherapy for the treatment of advanced
renal cell carcinoma. Hum Vaccin Immunother. 19:21935282023.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Cafri G, Gartner JJ, Zaks T, Hopson K,
Levin N, Paria BC, Parkhurst MR, Yossef R, Lowery FJ, Jafferji MS,
et al: mRNA vaccine-induced neoantigen-specific T cell immunity in
patients with gastrointestinal cancer. J Clin Invest.
130:5976–5988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Ott PA, Hu-Lieskovan S, Chmielowski B,
Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD,
Lin JJ, et al: A phase Ib trial of personalized neoantigen therapy
plus anti-PD-1 in patients with advanced melanoma, non-small cell
lung cancer, or bladder cancer. Cell. 183:347–362.e24. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Keskin DB, Anandappa AJ, Sun J, Tirosh I,
Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E,
et al: Neoantigen vaccine generates intratumoral T cell responses
in phase Ib glioblastoma trial. Nature. 565:234–239. 2019.
View Article : Google Scholar :
|
|
227
|
Platten M, Bunse L, Wick A, Bunse T, Le
Cornet L, Harting I, Sahm F, Sanghvi K, Tan CL, Poschke I, et al: A
vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature.
592:463–468. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Kochenderfer JN, Chien CD, Simpson JL and
Gress RE: Maximizing CD8+ T cell responses elicited by peptide
vaccines containing CpG oligodeoxynucleotides. Clin Immunol.
124:119–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Haining WN, Davies J, Kanzler H, Drury L,
Brenn T, Evans J, Angelosanto J, Rivoli S, Russell K, George S, et
al: CpG oligodeoxynucleotides alter lymphocyte and dendritic cell
trafficking in humans. Clin Cancer Res. 14:5626–5634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Song YC, Cheng HY, Leng CH, Chiang SK, Lin
CW, Chong P, Huang MH and Liu SJ: A novel emulsion-type adjuvant
containing CpG oligodeoxynucleotides enhances CD8+ T-cell-mediated
anti-tumor immunity. J Control Release. 173:158–165. 2014.
View Article : Google Scholar
|
|
231
|
Baumgaertner P, Costa Nunes C, Cachot A,
Maby-El Hajjami H, Cagnon L, Braun M, Derré L, Rivals JP, Rimoldi
D, Gnjatic S, et al: Vaccination of stage III/IV melanoma patients
with long NY-ESO-1 peptide and CpG-B elicits robust CD8+
and CD4+ T-cell responses with multiple specificities
including a novel DR7-restricted epitope. Oncoimmunology.
5:e12162902016. View Article : Google Scholar
|
|
232
|
Zhu P, Li SY, Ding J, Fei Z, Sun SN, Zheng
ZH, Wei D, Jiang J, Miao JL, Li SZ, et al: Combination
immunotherapy of glioblastoma with dendritic cell cancer vaccines,
anti-PD-1 and poly I:C. J Pharm Anal. 13:616–624. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Soltani M, Savvateeva LV,
Ganjalikhani-Hakemi M and Zamyatnin AA: Clinical combinatorial
treatments based on cancer vaccines: Combination with checkpoint
inhibitors and beyond. Curr Drug Targets. 23:1072–1084. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
van der Burg SH, Arens R, Ossendorp F, van
Hall T and Melief CJ: Vaccines for established cancer: Overcoming
the challenges posed by immune evasion. Nat Rev Cancer. 16:219–233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Reichmuth AM, Oberli MA, Jaklenec A,
Langer R and Blankschtein D: mRNA vaccine delivery using lipid
nanoparticles. Ther Deliv. 7:319–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Kon E, Ad-El N, Hazan-Halevy I,
Stotsky-Oterin L and Peer D: Targeting cancer with mRNA-lipid
nanoparticles: Key considerations and future prospects. Nat Rev
Clin Oncol. 20:739–754. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Tenchov R, Bird R, Curtze AE and Zhou Q:
Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a
landscape of research diversity and advancement. ACS Nano.
15:16982–17015. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Karikó K, Muramatsu H, Welsh FA, Ludwig J,
Kato H, Akira S and Weissman D: Incorporation of pseudouridine into
mRNA yields superior nonimmunogenic vector with increased
translational capacity and biological stability. Mol Ther.
16:1833–1840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Ramos da Silva J, Bitencourt Rodrigues K,
Formoso Pelegrin G, Silva Sales N, Muramatsu H, de Oliveira Silva
M, Porchia BFMM, Moreno ACR, Aps LRMM, Venceslau-Carvalho AA, et
al: Single immunizations of self-amplifying or non-replicating
mRNA-LNP vaccines control HPV-associated tumors in mice. Sci Transl
Med. 15:eabn34642023. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Sittplangkoon C, Alameh MG, Weissman D,
Lin PJC, Tam YK, Prompetchara E and Palaga T: mRNA vaccine with
unmodified uridine induces robust type I interferon-dependent
anti-tumor immunity in a melanoma model. Front Immunol.
13:9830002022. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Meulewaeter S, Aernout I, Deprez J,
Engelen Y, De Velder M, Franceschini L, Breckpot K, Van Calenbergh
S, Asselman C, Boucher K, et al: Alpha-galactosylceramide improves
the potency of mRNA LNP vaccines against cancer and intracellular
bacteria. J Control Release. 370:379–391. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Yang J, Arya S, Lung P, Lin Q, Huang J and
Li Q: Hybrid nanovaccine for the co-delivery of the mRNA antigen
and adjuvant. Nanoscale. 11:21782–21789. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Carvalho T: Personalized anti-cancer
vaccine combining mRNA and immunotherapy tested in melanoma trial.
Nat Med. 29:2379–2380. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Podaza E, Carri I, Aris M, von Euw E,
Bravo AI, Blanco P, Ortiz Wilczyñski JM, Koile D, Yankilevich P,
Nielsen M, et al: Evaluation of T-cell responses against shared
melanoma associated antigens and predicted neoantigens in cutaneous
melanoma patients treated with the CSF-470 allogeneic cell vaccine
plus BCG and GM-CSF. Front Immunol. 11:11472020. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Sebastian M, Schröder A, Scheel B, Hong
HS, Muth A, von Boehmer L, Zippelius A, Mayer F, Reck M,
Atanackovic D, et al: A phase I/IIa study of the mRNA-based cancer
immunotherapy CV9201 in patients with stage IIIB/IV non-small cell
lung cancer. Cancer Immunol Immunother. 68:799–812. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Zilio S, Bicciato S, Weed D and Serafini
P: CCR1 and CCR5 mediate cancer-induced myelopoiesis and
differentiation of myeloid cells in the tumor. J Immunother Cancer.
10:e0031312022. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Badr G, Al-Sadoon MK, Rabah DM and Sayed
D: Snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles
induce apoptosis and growth arrest in human prostate cancer cells.
Apoptosis. 18:300–314. 2013. View Article : Google Scholar
|
|
248
|
Badr G, Al-Sadoon MK and Rabah DM:
Therapeutic efficacy and molecular mechanisms of snake
(Walterinnesia aegyptia) venom-loaded silica nanoparticles in the
treatment of breast cancer- and prostate cancer-bearing
experimental mouse models. Free Radic Biol Med. 65:175–189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
249
|
An S, Tiruthani K, Wang Y, Xu L, Hu M, Li
J, Song W, Jiang H, Sun J, Liu R and Huang L: Locally trapping the
C-C chemokine receptor type 7 by gene delivery nanoparticle
inhibits lymphatic metastasis prior to tumor resection. Small.
15:e18051822019. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Liu JQ, Zhang C, Zhang X, Yan J, Zeng C,
Talebian F, Lynch K, Zhao W, Hou X, Du S, et al: Intratumoral
delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for
cancer immunotherapy. J Control Release. 345:306–313. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Li Y, Su Z, Zhao W, Zhang X, Momin N,
Zhang C, Wittrup KD, Dong Y, Irvine DJ and Weiss R: Multifunctional
oncolytic nanoparticles deliver self-replicating IL-12 RNA to
eliminate established tumors and prime systemic immunity. Nat
Cancer. 1:882–893. 2020. View Article : Google Scholar
|
|
252
|
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins
JJ, Wagner ES, Gabaldon TA and Zaharoff DA: Localized
interleukin-12 for cancer immunotherapy. Front Immunol.
11:5755972020. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Xu S, Xu Y, Solek NC, Chen J, Gong F,
Varley AJ, Golubovic A, Pan A, Dong S, Zheng G and Li B:
Tumor-tailored ionizable lipid nanoparticles facilitate IL-12
circular RNA delivery for enhanced lung cancer immunotherapy. Adv
Mater. 36:e24003072024. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Fu S, Li G, Zang W, Zhou X, Shi K and Zhai
Y: Pure drug nano-assemblies: A facile carrier-free nanoplatform
for efficient cancer therapy. Acta Pharm Sin B. 12:92–106. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Reda M, Ngamcherdtrakul W, Nelson MA,
Siriwon N, Wang R, Zaidan HY, Bejan DS, Reda S, Hoang NH, Crumrine
NA, et al: Development of a nanoparticle-based immunotherapy
targeting PD-L1 and PLK1 for lung cancer treatment. Nat Commun.
13:42612022. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Han X, Wei Q, Lv Y, Weng L, Huang H, Wei
Q, Li M, Mao Y, Hua D, Cai X, et al: Ginseng-derived nanoparticles
potentiate immune checkpoint antibody efficacy by reprogramming the
cold tumor microenvironment. Mol Ther. 30:327–340. 2022. View Article : Google Scholar :
|
|
257
|
Liu L, Wang Y, Miao L, Liu Q, Musetti S,
Li J and Huang L: Combination immunotherapy of MUC1 mRNA
nano-vaccine and CTLA-4 blockade effectively inhibits growth of
triple negative breast cancer. Mol Ther. 26:45–55. 2018. View Article : Google Scholar :
|
|
258
|
Ma R, Li Z, Chiocca EA, Caligiuri MA and
Yu J: The emerging field of oncolytic virus-based cancer
immunotherapy. Trends Cancer. 9:122–139. 2023. View Article : Google Scholar :
|
|
259
|
Hu H, Zhang S, Cai L, Duan H, Li Y, Yang
J, Wang Y and Liu B, Dong S, Fang Z and Liu B: A novel cocktail
therapy based on quintuplet combination of oncolytic herpes simplex
virus-2 vectors armed with interleukin-12, interleukin-15, GM-CSF,
PD1v, and IL-7 × CCL19 results in enhanced antitumor efficacy.
Virol J. 19:742022. View Article : Google Scholar
|
|
260
|
Kim KJ, Moon D, Kong SJ, Lee YS, Yoo Y,
Kim S, Kim C, Chon HJ, Kim JH and Choi KJ: Antitumor effects of
IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1.
Gene Ther. 28:186–198. 2021. View Article : Google Scholar
|
|
261
|
Oh E, Oh JE, Hong J, Chung Y, Lee Y, Park
KD, Kim S and Yun CO: Optimized biodegradable polymeric
reservoir-mediated local and sustained co-delivery of dendritic
cells and oncolytic adenovirus co-expressing IL-12 and GM-CSF for
cancer immunotherapy. J Control Release. 259:115–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Zhang SN, Choi IK, Huang JH, Yoo JY, Choi
KJ and Yun CO: Optimizing DC vaccination by combination with
oncolytic adenovirus coexpressing IL-12 and GM-CSF. Mol Ther.
19:1558–1568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Malhotra J and Kim ES: Oncolytic viruses
and cancer immunotherapy. Curr Oncol Rep. 25:19–28. 2023.
View Article : Google Scholar
|