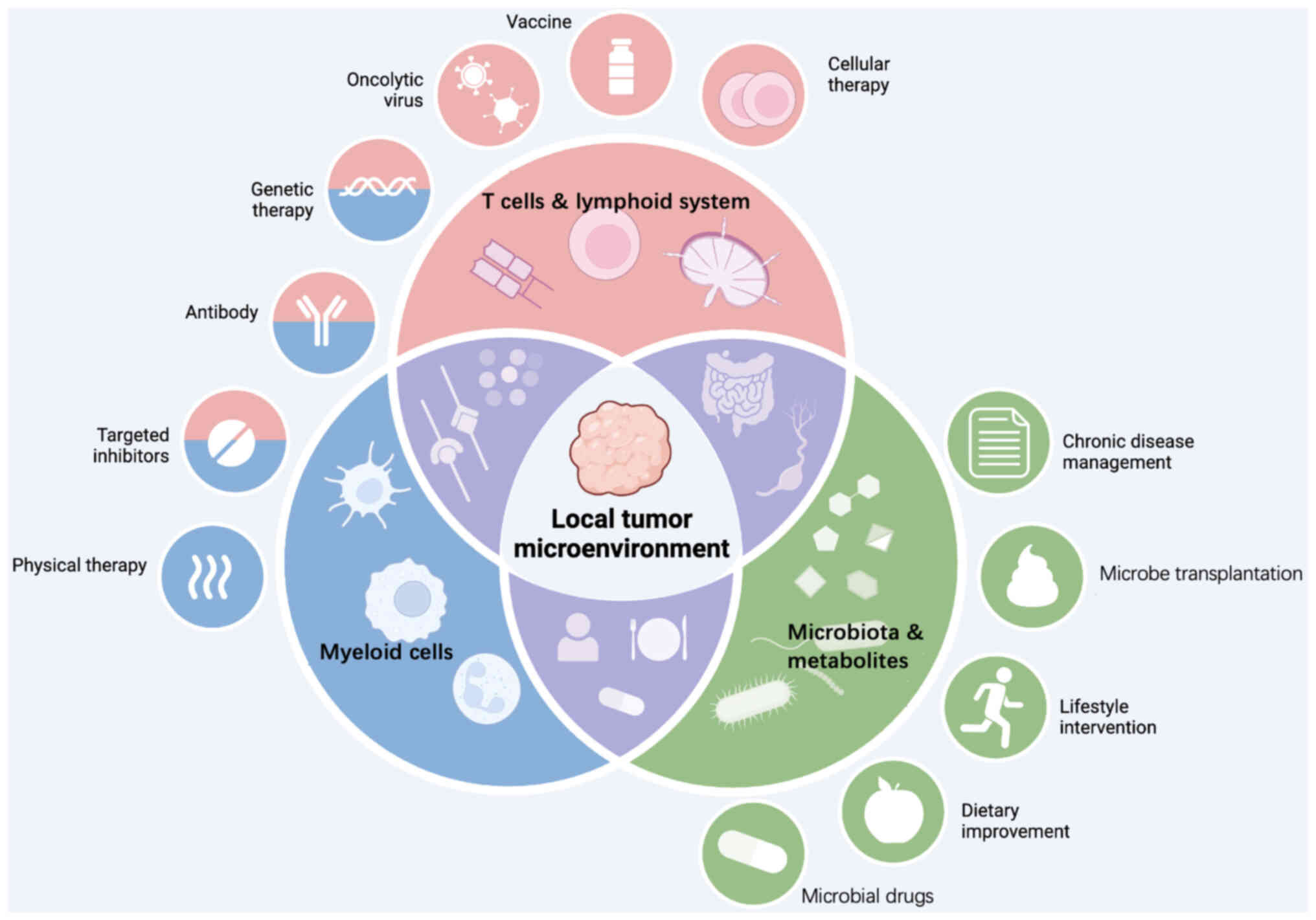
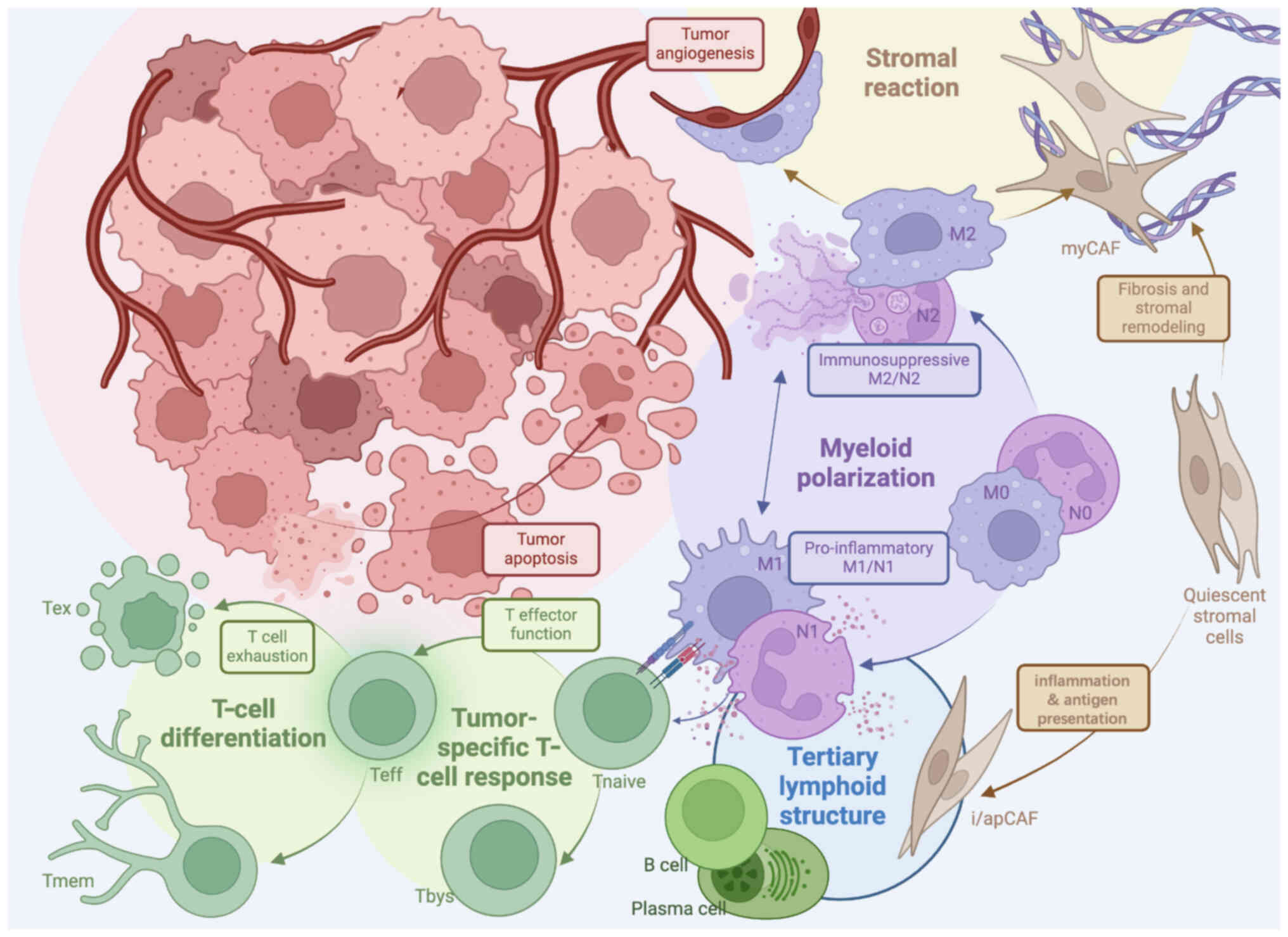
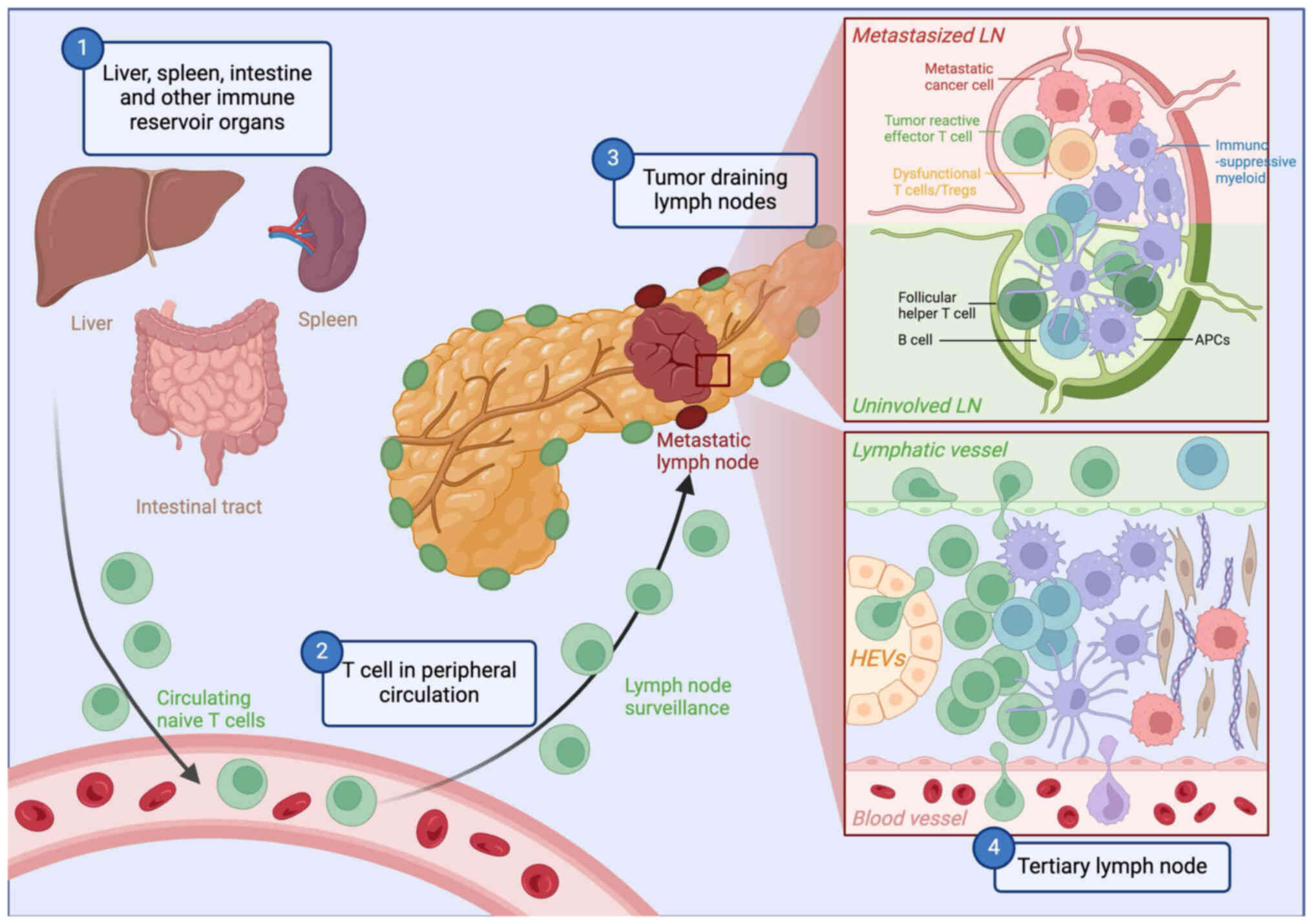
|
1
|
Anagnostou V, Niknafs N, Marrone K, Bruhm
DC, White JR, Naidoo J, Hummelink K, Monkhorst K, Lalezari F, Lanis
M, et al: Multimodal genomic features predict outcome of immune
checkpoint blockade in non-small-cell lung cancer. Nat Cancer.
1:99–111. 2020. View Article : Google Scholar
|
|
2
|
Wu B, Zhang B, Li B, Wu H and Jiang M:
Cold and hot tumors: From molecular mechanisms to targeted therapy.
Signal Transduct Target Ther. 9:2742024. View Article : Google Scholar :
|
|
3
|
Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y,
Lin D, Gao Q, Zhou H, Liao W and Yao H: Association of survival and
immune-related biomarkers with immunotherapy in patients with
non-small cell lung cancer: A meta-analysis and individual
patient-level analysis. JAMA Netw Open. 2:e1968792019. View Article : Google Scholar
|
|
4
|
Spitzer MH, Carmi Y, Reticker-Flynn NE,
Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR,
Chabon J, Bendall SC, et al: Systemic immunity is required for
effective cancer immunotherapy. Cell. 168:487–502.e15. 2017.
View Article : Google Scholar
|
|
5
|
Tselikas L, Dardenne A, de Baere T, Faron
M, Ammari S, Farhane S, Suzzoni S, Danlos FX, Raoult T, Susini S,
et al: Feasibility, safety and efficacy of human intra-tumoral
immuno-therapy. Gustave Roussy's initial experience with its first
100 patients. Eur J Cancer. 172:1–12. 2022. View Article : Google Scholar
|
|
6
|
Spranger S: Mechanisms of tumor escape in
the context of the T-cell-inflamed and the non-T-cell-inflamed
tumor microenvironment. Int Immunol. 28:383–391. 2016. View Article : Google Scholar :
|
|
7
|
Sadeghi Rad H, Monkman J, Warkiani ME,
Ladwa R, O'Byrne K, Rezaei N and Kulasinghe A: Understanding the
tumor microenvironment for effective immunotherapy. Med Res Rev.
41:1474–1498. 2021. View Article : Google Scholar :
|
|
8
|
Casalegno Garduño R, Spitschak A, Pannek T
and Pützer BM: CD8+ T cell subsets as biomarkers for predicting
checkpoint therapy outcomes in cancer immunotherapy. Biomedicines.
13:9302025. View Article : Google Scholar
|
|
9
|
Loi S, Adams S, Schmid P, Cortés J, Cescon
DW, Winer EP, Toppmeyer DL, Rugo HS, De Laurentiis M, Nanda R, et
al: LBA13-Relationship between tumor infiltrating lymphocyte (TIL)
levels and response to pembrolizumab (pembro) in metastatic
triple-negative breast cancer (mTNBC): Results from KEYNOTE-086.
Ann Oncol. 28(Suppl 5): v6082017. View Article : Google Scholar
|
|
10
|
Hegde PS and Chen DS: Top 10 challenges in
cancer immunotherapy. Immunity. 52:17–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to Anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018. View Article : Google Scholar
|
|
13
|
Cascio S, Chandler C, Zhang L, Sinno S,
Gao B, Onkar S, Bruno TC, Vignali DAA, Mahdi H, Osmanbeyoglu HU, et
al: Cancer-associated MSC drive tumor immune exclusion and
resistance to immunotherapy, which can be overcome by Hedgehog
inhibition. Sci Adv. 7:eabi57902021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grout JA, Sirven P, Leader AM, Maskey S,
Hector E, Puisieux I, Steffan F, Cheng E, Tung N, Maurin M, et al:
Spatial positioning and matrix programs of cancer-associated
fibroblasts promote T-cell exclusion in human lung tumors. Cancer
Discov. 12:2606–2625. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elyada E, Bolisetty M, Laise P, Flynn WF,
Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS,
et al: Cross-species single-cell analysis of pancreatic ductal
adenocarcinoma reveals antigen-presenting cancer-associated
fibroblasts. Cancer Discov. 9:1102–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Griffioen AW, Damen CA, Blijham GH and
Groenewegen G: Tumor angiogenesis is accompanied by a decreased
inflammatory response of tumor-associated endothelium. Blood.
88:667–673. 1996. View Article : Google Scholar
|
|
17
|
Nagl L, Horvath L, Pircher A and Wolf D:
Tumor endothelial cells (TECs) as potential immune directors of the
tumor microenvironment-new findings and future perspectives. Front
Cell Dev Biol. 8:7662020. View Article : Google Scholar
|
|
18
|
Sahu A, Kose K, Kraehenbuehl L, Byers C,
Holland A, Tembo T, Santella A, Alfonso A, Li M, Cordova M, et al:
In vivo tumor immune microenvironment phenotypes correlate with
inflammation and vasculature to predict immunotherapy response. Nat
Commun. 13:53122022. View Article : Google Scholar
|
|
19
|
Subramanian M, Kabir AU, Barisas D, Krchma
K and Choi K: Conserved angio-immune subtypes of the tumor
microenvironment predict response to immune checkpoint blockade
therapy. Cell Rep Med. 4:1008962023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steele MM, Jaiswal A, Delclaux I, Dryg ID,
Murugan D, Femel J, Son S, du Bois H, Hill C, Leachman SA, et al: T
cell egress via lymphatic vessels is tuned by antigen encounter and
limits tumor control. Nat Immunol. 24:664–675. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CX, Lao XM, Wang XY, Ren YZ, Lu YT,
Shi W, Wang YZ, Wu CY, Xu L, Chen MS, et al: Pericancerous
cross-presentation to cytotoxic T lymphocytes impairs
immunotherapeutic efficacy in hepatocellular carcinoma. Cancer
Cell. 42:2082–2097.e10. 2024. View Article : Google Scholar
|
|
22
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cristescu R, Aurora-Garg D, Albright A, Xu
L, Liu XQ, Loboda A, Lang L, Jin F, Rubin EH, Snyder A and
Lunceford J: Tumor mutational burden predicts the efficacy of
pembrolizumab monotherapy: A pan-tumor retrospective analysis of
participants with advanced solid tumors. J Immunother Cancer.
10:e0030912022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ricciuti B, Wang X, Alessi JV, Rizvi H,
Mahadevan NR, Li YY, Polio A, Lindsay J, Umeton R, Sinha R, et al:
Association of high tumor mutation burden in non-small cell lung
cancers with increased immune infiltration and improved clinical
outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA
Oncol. 8:1160–1168. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baharom F, Ramirez-Valdez RA, Khalilnezhad
A, Khalilnezhad S, Dillon M, Hermans D, Fussell S, Tobin KKS,
Dutertre CA, Lynn GM, et al: Systemic vaccination induces
CD8+ T cells and remodels the tumor microenvironment.
Cell. 185:4317–4332.e15. 2022. View Article : Google Scholar
|
|
26
|
Zheng M: Tumor mutation burden for
predicting immune checkpoint blockade response: The more, the
better. J Immunother Cancer. 10:e0030872022. View Article : Google Scholar
|
|
27
|
McGrail DJ, Pilié PG, Rashid NU, Voorwerk
L, Slagter M, Kok M, Jonasch E, Khasraw M, Heimberger AB, Lim B, et
al: High tumor mutation burden fails to predict immune checkpoint
blockade response across all cancer types. Ann Oncol. 32:661–672.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niknafs N, Balan A, Cherry C, Hummelink K,
Monkhorst K, Shao XM, Belcaid Z, Marrone KA, Murray J, Smith KN, et
al: Persistent mutation burden drives sustained anti-tumor immune
responses. Nat Med. 29:440–449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valpione S, Mundra PA, Galvani E, Campana
LG, Lorigan P, De Rosa F, Gupta A, Weightman J, Mills S, Dhomen N
and Marais R: The T cell receptor repertoire of tumor infiltrating
T cells is predictive and prognostic for cancer survival. Nat
Commun. 12:40982021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meier SL, Satpathy AT and Wells DK:
Bystander T cells in cancer immunology and therapy. Nat Cancer.
3:143–155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simoni Y, Becht E, Fehlings M, Loh CY, Koo
SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al:
Bystander CD8+ T cells are abundant and phenotypically
distinct in human tumour infiltrates. Nature. 557:575–579. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Zhao J, Yue S, Li Z, Duan X, Lin
Y, Yang Y, He J, Gao L, Pan Z, et al: An oncolytic virus delivering
tumor-irrelevant bystander T cell epitopes induces anti-tumor
immunity and potentiates cancer immunotherapy. Nat Cancer.
5:1063–1081. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lowery FJ, Krishna S, Yossef R, Parikh NB,
Chatani PD, Zacharakis N, Parkhurst MR, Levin N, Sindiri S, Sachs
A, et al: Molecular signatures of antitumor neoantigen-reactive T
cells from metastatic human cancers. Science. 375:877–884. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng Z, Rodriguez Ehrenfried A, Tan CL,
Steffens LK, Kehm H, Zens S, Lauenstein C, Paul A, Schwab M,
Förster JD, et al: Transcriptome-based identification of
tumor-reactive and bystander CD8+ T cell receptor
clonotypes in human pancreatic cancer. Sci Transl Med.
15:eadh95622023. View Article : Google Scholar
|
|
35
|
Kortekaas KE, Santegoets SJ, Sturm G,
Ehsan I, van Egmond SL, Finotello F, Rajanoski Z, Welters MJP, van
Poelgeest MIE and van der Burg SH: CD39 identifies the
CD4+ tumor-specific T-cell population in human cancer.
Cancer Immunol Res. 8:1311–1321. 2020. View Article : Google Scholar
|
|
36
|
Duhen T, Duhen R, Montler R, Moses J,
Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott
JE, et al: Co-expression of CD39 and CD103 identifies
tumor-reactive CD8 T cells in human solid tumors. Nat Commun.
9:27242018. View Article : Google Scholar
|
|
37
|
Qiao M, Zhou F, Liu X, Jiang T, Wang H,
Jia Y, Li X, Zhao C, Cheng L, Chen X, et al: Interleukin-10 induces
expression of CD39 on CD8+T cells to potentiate anti-PD1 efficacy
in EGFR-mutated non-small cell lung cancer. J Immunother Cancer.
10:e0054362022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Webb JR, Milne K and Nelson BH: PD-1 and
CD103 are widely coexpressed on prognostically favorable
intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol
Res. 3:926–935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Corgnac S, Malenica I, Mezquita L, Auclin
E, Voilin E, Kacher J, Halse H, Grynszpan L, Signolle N, Dayris T,
et al: CD103+CD8+ TRM cells accumulate in
tumors of anti-PD-1-responder lung cancer patients and are
tumor-reactive lymphocytes enriched with Tc17. Cell Rep Med.
1:1001272020. View Article : Google Scholar
|
|
40
|
Wang Z, Ahmed S, Labib M, Wang H, Wu L,
Bavaghar-Zaeimi F, Shokri N, Blanco S, Karim S, Czarnecka-Kujawa K,
et al: Isolation of tumour-reactive lymphocytes from peripheral
blood via microfluidic immunomagnetic cell sorting. Nat Biomed Eng.
7:1188–1203. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gallerano D, Ciminati S, Grimaldi A,
Piconese S, Cammarata I, Focaccetti C, Pacella I, Accapezzato D,
Lancellotti F, Sacco L, et al: Genetically driven CD39 expression
shapes human tumor-infiltrating CD8+ T-cell functions.
Int J Cancer. 147:2597–2610. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Laumont CM, Wouters MCA, Smazynski J,
Gierc NS, Chavez EA, Chong LC, Thornton S, Milne K, Webb JR, Steidl
C and Nelson BH: Single-cell profiles and prognostic impact of
tumor-infiltrating lymphocytes coexpressing CD39, CD103, and PD-1
in ovarian cancer. Clin Cancer Res. 27:4089–4100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He J, Xiong X, Yang H, Li D, Liu X, Li S,
Liao S, Chen S, Wen X, Yu K, et al: Defined tumor antigen-specific
T cells potentiate personalized TCR-T cell therapy and prediction
of immunotherapy response. Cell Res. 32:530–542. 2022. View Article : Google Scholar
|
|
44
|
Liu B, Zhang Y, Wang D, Hu X and Zhang Z:
Single-cell meta-analyses reveal responses of tumor-reactive
CXCL13+ T cells to immune-checkpoint blockade. Nat
Cancer. 3:1123–1136. 2022. View Article : Google Scholar
|
|
45
|
Hanada KI, Zhao C, Gil-Hoyos R, Gartner
JJ, Chow-Parmer C, Lowery FJ, Krishna S, Prickett TD, Kivitz S,
Parkhurst MR, et al: A phenotypic signature that identifies
neoantigen-reactive T cells in fresh human lung cancers. Cancer
Cell. 40:479–493.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dai S, Zeng H, Liu Z, Jin K, Jiang W, Wang
Z, Lin Z, Xiong Y, Wang J, Chang Y, et al: Intratumoral
CXCL13+CD8+T cell infiltration determines
poor clinical outcomes and immunoevasive contexture in patients
with clear cell renal cell carcinoma. J Immunother Cancer.
9:e0018232021. View Article : Google Scholar
|
|
47
|
Aoki T, Chong LC, Takata K, Milne K,
Marshall A, Chavez EA, Miyata-Takata T, Ben-Neriah S, Unrau D,
Telenius A, et al: Single-cell profiling reveals the importance of
CXCL13/CXCR5 axis biology in lymphocyte-rich classic Hodgkin
lymphoma. Proc Natl Acad Sci USA. 118:e21058221182021. View Article : Google Scholar :
|
|
48
|
Eiva MA, Omran DK, Chacon JA and Powell DJ
Jr: Systematic analysis of CD39, CD103, CD137, and PD-1 as
biomarkers for naturally occurring tumor antigen-specific TILs. Eur
J Immunol. 52:96–108. 2022. View Article : Google Scholar :
|
|
49
|
Parkhurst M, Gros A, Pasetto A, Prickett
T, Crystal JS, Robbins P and Rosenberg SA: Isolation of T-cell
receptors specifically reactive with mutated tumor-associated
antigens from tumor-infiltrating lymphocytes based on CD137
expression. Clin Cancer Res. 23:2491–2505. 2017. View Article : Google Scholar
|
|
50
|
Yost KE, Satpathy AT, Wells DK, Qi Y, Wang
C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, et al:
Clonal replacement of tumor-specific T cells following PD-1
blockade. Nat Med. 25:1251–1259. 2019. View Article : Google Scholar
|
|
51
|
Tonnerre P, Wolski D, Subudhi S, Aljabban
J, Hoogeveen RC, Damasio M, Drescher HK, Bartsch LM, Tully DC, Sen
DR, et al: Differentiation of exhausted CD8+ T cells
after termination of chronic antigen stimulation stops short of
achieving functional T cell memory. Nat Immunol. 22:1030–1041.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Watowich MB, Gilbert MR and Larion M: T
cell exhaustion in malignant gliomas. Trends Cancer. 9:270–292.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Beltra JC, Manne S, Abdel-Hakeem MS,
Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ,
et al: Developmental relationships of four exhausted
CD8+ T cell subsets reveals underlying transcriptional
and epigenetic landscape control mechanisms. Immunity.
52:825–841.e8. 2020. View Article : Google Scholar
|
|
54
|
Sen DR, Kaminski J, Barnitz RA, Kurachi M,
Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et
al: The epigenetic landscape of T cell exhaustion. Science.
354:1165–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Z, Chen L, Chen H, Zhao J, Li K, Sun
J and Zhou M: Pan-cancer landscape of T-cell exhaustion
heterogeneity within the tumor microenvironment revealed a
progressive roadmap of hierarchical dysfunction associated with
prognosis and therapeutic efficacy. EBioMedicine. 83:1042072022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu Z, Yoshikawa T, Inoue S, Ito Y, Kasuya
H, Nakashima T, Zhang H, Kotaka S, Hosoda W, Suzuki S and Kagoya Y:
CD83 expression characterizes precursor exhausted T cell
population. Commun Biol. 6:2582023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Utzschneider DT, Charmoy M, Chennupati V,
Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F,
Hofmann M, Wieland D, et al: T cell factor 1-expressing memory-like
CD8(+) T cells sustain the immune response to chronic viral
infections. Immunity. 45:415–427. 2016. View Article : Google Scholar
|
|
58
|
Kim CG, Kim G, Kim KH, Park S, Shin S, Yeo
D, Shim HS, Yoon HI, Park SY, Ha SJ and Kim HR: Distinct exhaustion
features of T lymphocytes shape the tumor-immune microenvironment
with therapeutic implication in patients with non-small-cell lung
cancer. J Immunother Cancer. 9:e0027802021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wieland D, Kemming J, Schuch A, Emmerich
F, Knolle P, Neumann-Haefelin C, Held W, Zehn D, Hofmann M and
Thimme R: TCF1+ hepatitis C virus-specific
CD8+ T cells are maintained after cessation of chronic
antigen stimulation. Nat Commun. 8:150502017. View Article : Google Scholar
|
|
60
|
Jadhav RR, Im SJ, Hu B, Hashimoto M, Li P,
Lin JX, Leonard WJ, Greenleaf WJ, Ahmed R and Goronzy JJ:
Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as
resource cells during chronic viral infection and respond to PD-1
blockade. Proc Natl Acad Sci USA. 116:14113–14118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li X, Li Y, Dong L, Chang Y, Zhang X, Wang
C, Chen M, Bo X, Chen H, Han W and Nie J: Decitabine priming
increases anti-PD-1 antitumor efficacy by promoting CD8+ progenitor
exhausted T cell expansion in tumor models. J Clin Invest.
133:e1656732023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nagasaki J, Inozume T, Sax N, Ariyasu R,
Ishikawa M, Yamashita K, Kawazu M, Ueno T, Irie T, Tanji E, et al:
PD-1 blockade therapy promotes infiltration of tumor-attacking
exhausted T cell clonotypes. Cell Rep. 38:1103312022. View Article : Google Scholar
|
|
63
|
Codarri Deak L, Nicolini V, Hashimoto M,
Karagianni M, Schwalie PC, Lauener L, Varypataki EM, Richard M,
Bommer E, Sam J, et al: PD-1-cis IL-2R agonism yields better
effectors from stem-like CD8+ T cells. Nature.
610:161–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hashimoto M, Araki K, Cardenas MA, Li P,
Jadhav RR, Kissick HT, Hudson WH, McGuire DJ, Obeng RC, Wieland A,
et al: PD-1 combination therapy with IL-2 modifies CD8+
T cell exhaustion program. Nature. 610:173–181. 2022. View Article : Google Scholar :
|
|
65
|
Ren Z, Zhang A, Sun Z, Liang Y, Ye J, Qiao
J, Li B and Fu YX: Selective delivery of low-affinity IL-2 to PD-1+
T cells rejuvenates antitumor immunity with reduced toxicity. J
Clin Invest. 132:e1536042022. View Article : Google Scholar
|
|
66
|
Zehn D, Thimme R, Lugli E, de Almeida GP
and Oxenius A: 'Stem-like' precursors are the fount to sustain
persistent CD8+ T cell responses. Nat Immunol.
23:836–847. 2022. View Article : Google Scholar
|
|
67
|
Miller BC, Sen DR, Al Abosy R, Bi K,
Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al:
Subsets of exhausted CD8+ T cells differentially mediate
tumor control and respond to checkpoint blockade. Nat Immunol.
20:326–336. 2016. View Article : Google Scholar
|
|
68
|
Im SJ, Hashimoto M, Gerner MY, Lee J,
Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al:
Defining CD8+ T cells that provide the proliferative burst after
PD-1 therapy. Nature. 537:417–421. 2016. View Article : Google Scholar
|
|
69
|
Siddiqui I, Schaeuble K, Chennupati V,
Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ,
Scarpellino L, Gfeller D, Pradervand S, et al: Intratumoral
Tcf1+PD-1+CD8+ T cells with
stem-like properties promote tumor control in response to
vaccination and checkpoint blockade immunotherapy. Immunity.
50:195–211.e10. 2019. View Article : Google Scholar
|
|
70
|
Tabanelli V, Melle F, Motta G, Mazzara S,
Fabbri M, Agostinelli C, Calleri A, Del Corvo M, Fiori S, Lorenzini
D, et al: The identification of TCF1+ progenitor exhausted T cells
in THRLBCL may predict a better response to PD-1/PD-L1 blockade.
Blood Adv. 6:4634–4644. 2022. View Article : Google Scholar :
|
|
71
|
Zheng L, Qin S, Si W, Wang A, Xing B, Gao
R, Ren X, Wang L, Wu X, Zhang J, et al: Pan-cancer single-cell
landscape of tumor-infiltrating T cells. Science. 374:abe64742021.
View Article : Google Scholar
|
|
72
|
Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim
HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, et
al: TOX reinforces the phenotype and longevity of exhausted T cells
in chronic viral infection. Nature. 571:265–269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kim K, Park S, Park SY, Kim G, Park SM,
Cho JW, Kim DH, Park YM, Koh YW, Kim HR, et al: Single-cell
transcriptome analysis reveals TOX as a promoting factor for T cell
exhaustion and a predictor for anti-PD-1 responses in human cancer.
Genome Med. 12:222020. View Article : Google Scholar :
|
|
74
|
Abdel-Hakeem MS, Manne S, Beltra JC,
Stelekati E, Chen Z, Nzingha K, Ali MA, Johnson JL, Giles JR,
Mathew D, et al: Epigenetic scarring of exhausted T cells hinders
memory differentiation upon eliminating chronic antigenic
stimulation. Nat Immunol. 22:1008–1019. 2021. View Article : Google Scholar :
|
|
75
|
Pauken KE, Sammons MA, Odorizzi PM, Manne
S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al:
Epigenetic stability of exhausted T cells limits durability of
reinvigoration by PD-1 blockade. Science. 354:1160–1165. 2016.
View Article : Google Scholar
|
|
76
|
Gupta PK, Godec J, Wolski D, Adland E,
Yates K, Pauken KE, Cosgrove C, Ledderose C, Junger WG, Robson SC,
et al: CD39 expression identifies terminally exhausted CD8+ T
cells. PLoS Pathog. 11:e10051772015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tinoco R, Neubert EN, Stairiker CJ,
Henriquez ML and Bradley LM: PSGL-1 is a T cell intrinsic inhibitor
that regulates effector and memory differentiation and responses
during viral infection. Front Immunol. 12:6778242021. View Article : Google Scholar :
|
|
78
|
Tinoco R, Carrette F, Barraza ML, Otero
DC, Magaña J, Bosenberg MW, Swain SL and Bradley LM: PSGL-1 is an
immune checkpoint regulator that promotes T cell exhaustion.
Immunity. 44:1190–1203. 2016. View Article : Google Scholar
|
|
79
|
Vignali PDA, DePeaux K, Watson MJ, Ye C,
Ford BR, Lontos K, McGaa NK, Scharping NE, Menk AV, Robson SC, et
al: Hypoxia drives CD39-dependent suppressor function in exhausted
T cells to limit antitumor immunity. Nat Immunol. 24:267–279. 2023.
View Article : Google Scholar
|
|
80
|
Viramontes KM, Neubert EN, DeRogatis JM
and Tinoco R: PD-1 immune checkpoint blockade and PSGL-1 inhibition
synergize to reinvigorate exhausted T cells. Front Immunol.
13:8697682022. View Article : Google Scholar :
|
|
81
|
Moesta AK, Li XY and Smyth MJ: Targeting
CD39 in cancer. Nat Rev Immunol. 20:739–755. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tu E, McGlinchey K, Wang J, Martin P,
Ching SL, Floc'h N, Kurasawa J, Starrett JH, Lazdun Y, Wetzel L, et
al: Anti-PD-L1 and anti-CD73 combination therapy promotes T cell
response to EGFR-mutated NSCLC. JCI Insight. 7:e1428432022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ford BR and Poholek AC: Regulation and
immunotherapeutic targeting of the epigenome in exhausted CD8 T
cell responses. J Immunol. 210:869–879. 2023. View Article : Google Scholar
|
|
84
|
Franco F, Jaccard A, Romero P, Yu YR and
Ho PC: Metabolic and epigenetic regulation of T-cell exhaustion.
Nat Metab. 2:1001–1012. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gabriel SS, Tsui C, Chisanga D, Weber F,
Llano-León M, Gubser PM, Bartholin L, Souza-Fonseca-Guimaraes F,
Huntington ND, Shi W, et al: Transforming growth factor-β-regulated
mTOR activity preserves cellular metabolism to maintain long-term T
cell responses in chronic infection. Immunity. 54:1698–1714.e5.
2021. View Article : Google Scholar
|
|
86
|
Bengsch B, Johnson AL, Kurachi M, Odorizzi
PM, Pauken KE, Attanasio J, Stelekati E, McLane LM, Paley MA,
Delgoffe GM and Wherry EJ: Bioenergetic insufficiencies due to
metabolic alterations regulated by the inhibitory receptor PD-1 are
an early driver of CD8(+) T cell exhaustion. Immunity. 45:358–373.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guo Y, Xie YQ, Gao M, Zhao Y, Franco F,
Wenes M, Siddiqui I, Bevilacqua A, Wang H, Yang H, et al: Metabolic
reprogramming of terminally exhausted CD8+ T cells by
IL-10 enhances anti-tumor immunity. Nat Immunol. 22:746–756. 2021.
View Article : Google Scholar :
|
|
88
|
Tsui C, Kretschmer L, Rapelius S, Gabriel
SS, Chisanga D, Knöpper K, Utzschneider DT, Nüssing S, Liao Y,
Mason T, et al: MYB orchestrates T cell exhaustion and response to
checkpoint inhibition. Nature. 609:354–360. 2022. View Article : Google Scholar :
|
|
89
|
Stelekati E, Chen Z, Manne S, Kurachi M,
Ali MA, Lewy K, Cai Z, Nzingha K, McLane LM, Hope JL, et al:
Long-term persistence of exhausted CD8 T cells in chronic infection
is regulated by MicroRNA-155. Cell Rep. 23:2142–2156. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Utzschneider DT, Gabriel SS, Chisanga D,
Gloury R, Gubser PM, Vasanthakumar A, Shi W and Kallies A: Early
precursor T cells establish and propagate T cell exhaustion in
chronic infection. Nat Immun. 21:1256–1266. 2020. View Article : Google Scholar
|
|
91
|
Man K, Gabriel SS, Liao Y, Gloury R,
Preston S, Henstridge DC, Pellegrini M, Zehn D, Berberich-Siebelt
F, Febbraio MA, et al: Transcription factor IRF4 promotes
CD8+ T cell exhaustion and limits the development of
memory-like T cells during chronic infection. Immunity.
47:1129–1141.e5. 2017. View Article : Google Scholar
|
|
92
|
Seo H, González-Avalos E, Zhang W,
Ramchandani P, Yang C, Lio CJ, Rao A and Hogan PG: BATF and IRF4
cooperate to counter exhaustion in tumor-infiltrating CAR T cells.
Nat Immunol. 22:983–995. 2021. View Article : Google Scholar :
|
|
93
|
Russ BE, Tsyganov K, Quon S, Yu B, Li J,
Lee JKC, Olshansky M, He Z, Harrison PF, Barugahare A, et al:
Active maintenance of CD8+ T cell naïvety through
regulation of global genome architecture. bioRxiv: The preprint
server for biology. 2023.
|
|
94
|
Grusdat M, McIlwain DR, Xu HC, Pozdeev VI,
Knievel J, Crome SQ, Robert-Tissot C, Dress RJ, Pandyra AA, Speiser
DE, et al: IRF4 and BATF are critical for CD8+ T-cell
function following infection with LCMV. Cell Death Differ.
21:1050–1060. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jain N, Zhao Z, Feucht J, Koche R, Iyer A,
Dobrin A, Mansilla-Soto J, Yang J, Zhan Y, Lopez M, et al: TET2
guards against unchecked BATF3-induced CAR T cell expansion.
Nature. 615:315–322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jordan MS, Drury S, Giles JR, Manne S,
Huang H, Chen Z, Oldridge D, Wherry EJ and Baxter AE: TET2 controls
differentiation of terminally exhausted CD8 T cells. J Immunol.
206(1 Suppl): S14.072021. View Article : Google Scholar
|
|
97
|
Liu B, Hu X, Feng K, Gao R, Xue Z, Zhang
S, Zhang Y, Corse E, Hu Y, Han W and Zhang Z: Temporal single-cell
tracing reveals clonal revival and expansion of precursor exhausted
T cells during anti-PD-1 therapy in lung cancer. Nat Cancer.
3:108–121. 2022. View Article : Google Scholar
|
|
98
|
Luoma AM, Suo S, Wang Y, Gunasti L, Porter
CBM, Nabilsi N, Tadros J, Ferretti AP, Liao S, Gurer C, et al:
Tissue-resident memory and circulating T cells are early responders
to pre-surgical cancer immunotherapy. Cell. 185:2918–2935.e29.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li Z, Tuong ZK, Dean I, Willis C, Gaspal
F, Fiancette R, Idris S, Kennedy B, Ferdinand JR, Peñalver A, et
al: In vivo labeling reveals continuous trafficking of TCF-1+ T
cells between tumor and lymphoid tissue. J Exp Med.
219:e202107492022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kennedy BC, Dean I and Withers DR:
Migration of stem-like CD8 T cells between tissue microenvironments
underpins successful anti-tumour immune responses. Discov Immunol.
2:kyad0042023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fransen MF, Schoonderwoerd M, Knopf P,
Camps MG, Hawinkels LJ, Kneilling M, van Hall T and Ossendorp F:
Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint
therapy. JCI Insight. 3:e1245072018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liu Z, Yu Z, Chen D, Verma V, Yuan C, Wang
M, Wang F, Fan Q, Wang X, Li Y, et al: Pivotal roles of
tumor-draining lymph nodes in the abscopal effects from combined
immunotherapy and radiotherapy. Cancer Commun (Lond). 42:971–986.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tanaka R, Hiramitsu M, Shimizu S,
Kawashima S, Sato A and Iwase Y: Efficient drug delivery to lymph
nodes by intradermal administration and enhancement of anti-tumor
effects of immune checkpoint inhibitors. Cancer Treat Res Commun.
36:1007402023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
du Bois H, Heim TA and Lund AW:
Tumor-draining lymph nodes: At the crossroads of metastasis and
immunity. Sci Immunol. 6:eabg35512021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rahim MK, Okholm TLH, Jones KB, McCarthy
EE, Liu CC, Yee JL, Ki SJ, Marquez DM, Tenvooren I, Wai K, et al:
Dynamic CD8+ T cell responses to cancer immunotherapy in
human regional lymph nodes are disrupted in metastatic lymph nodes.
Cell. 186:1127–1143.e18. 2023. View Article : Google Scholar
|
|
106
|
Buchwald ZS, Nasti TH, Lee J, Eberhardt
CS, Wieland A, Im SJ, Lawson D, Curran W, Ahmed R and Khan MK:
Tumor-draining lymph node is important for a robust abscopal effect
stimulated by radiotherapy. J Immunother Cancer. 8:e0008672020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fear VS, Forbes CA, Neeve SA, Fisher SA,
Chee J, Waithman J, Ma SK, Lake R, Nowak AK, Creaney J, et al:
Tumour draining lymph node-generated CD8 T cells play a role in
controlling lung metastases after a primary tumour is removed but
not when adjuvant immunotherapy is used. Cancer Immunol Immunother.
70:3249–3258. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dammeijer F, van Gulijk M, Mulder EE,
Lukkes M, Klaase L, van den Bosch T, van Nimwegen M, Lau SP,
Latupeirissa K, Schetters S, et al: The PD-1/PD-L1-checkpoint
restrains T cell immunity in tumor-draining lymph nodes. Cancer
Cell. 38:685–700.e8. 2020. View Article : Google Scholar
|
|
109
|
Zhou Y, Slone N, Chrisikos TT, Kyrysyuk O,
Babcock RL, Medik YB, Li HS, Kleinerman ES and Watowich SS: Vaccine
efficacy against primary and metastatic cancer with in
vitro-generated CD103+ conventional dendritic cells. J
Immunother Cancer. 8:e0004742020. View Article : Google Scholar
|
|
110
|
Salmon H, Idoyaga J, Rahman A, Leboeuf M,
Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J,
Tung N, et al: Expansion and activation of CD103(+) dendritic cell
progenitors at the tumor site enhances tumor responses to
therapeutic PD-L1 and BRAF inhibition. Immunity. 44:924–938. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Huang Q, Wu X, Wang Z, Chen X, Wang L, Lu
Y, Xiong D, Liu Q, Tian Y, Lin H, et al: The primordial
differentiation of tumor-specific memory CD8+ T cells as
bona fide responders to PD-1/PD-L1 blockade in draining lymph
nodes. Cell. 185:4049–4066.e25. 2022. View Article : Google Scholar
|
|
112
|
Okamura K, Nagayama S, Tate T, Chan HT,
Kiyotani K and Nakamura Y: Lymphocytes in tumor-draining lymph
nodes co-cultured with autologous tumor cells for adoptive cell
therapy. J Transl Med. 20:2412022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Schenkel JM, Herbst RH, Canner D, Li A,
Hillman M, Shanahan SL, Gibbons G, Smith OC, Kim JY, Westcott P, et
al: Conventional type I dendritic cells maintain a reservoir of
proliferative tumor-antigen specific TCF-1+
CD8+ T cells in tumor-draining lymph nodes. Immunity.
54:2338–2353.e6. 2021. View Article : Google Scholar
|
|
114
|
Connolly KA, Kuchroo M, Venkat A, Khatun
A, Wang J, William I, Hornick NI, Fitzgerald BL, Damo M, Kasmani
MY, et al: A reservoir of stem-like CD8+ T cells in the
tumor-draining lymph node preserves the ongoing antitumor immune
response. Sci Immunol. 6:eabg78362021. View Article : Google Scholar
|
|
115
|
O'Melia MJ, Manspeaker MP and Thomas SN:
Tumor-draining lymph nodes are survival niches that support T cell
priming against lymphatic transported tumor antigen and effects of
immune checkpoint blockade in TNBC. Cancer Immunol Immunother.
70:2179–2195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Dominguez-Gutierrez PR, Kwenda EP, Donelan
W, Miranda M, Doty A, O'Malley P, Crispen PL and Kusmartsev S:
Detection of PD-L1-expressing myeloid cell clusters in the
hyaluronan-enriched stroma in tumor tissue and tumor-draining lymph
nodes. J Immunol. 208:2829–2836. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Núñez NG, Tosello Boari J, Ramos RN,
Richer W, Cagnard N, Anderfuhren CD, Niborski LL, Bigot J, Meseure
D, De La Rochere P, et al: Tumor invasion in draining lymph nodes
is associated with Treg accumulation in breast cancer patients. Nat
Commun. 11:32722020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yang H, Sun B, Ma W, Fan L, Xu K, Jia Y,
Xu J, Wang Z and Yao F: Multi-scale characterization of
tumor-draining lymph nodes in resectable lung cancer treated with
neoadjuvant immune checkpoint inhibitors. EBioMedicine.
84:1042652022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Schumacher TN and Thommen DS: Tertiary
lymphoid structures in cancer. Science. 375:eabf94192022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dieu-Nosjean MC, Giraldo NA, Kaplon H,
Germain C, Fridman WH and Sautès-Fridman C: Tertiary lymphoid
structures, drivers of the anti-tumor responses in human cancers.
Immunol Rev. 271:260–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Rodriguez AB, Peske JD, Woods AN, Leick
KM, Mauldin IS, Meneveau MO, Young SJ, Lindsay RS, Melssen MM,
Cyranowski S, et al: Immune mechanisms orchestrate tertiary
lymphoid structures in tumors via cancer-associated fibroblasts.
Cell Rep. 36:1094222021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ng KW, Boumelha J, Enfield KSS, Almagro J,
Cha H, Pich O, Karasaki T, Moore DA, Salgado R, Sivakumar M, et al:
Antibodies against endogenous retroviruses promote lung cancer
immunotherapy. Nature. 616:563–573. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang B, Liu J, Han Y, Deng Y, Li J and
Jiang Y: The presence of tertiary lymphoid structures provides new
insight into the clinicopathological features and prognosis of
patients with breast cancer. Front Immunol. 13:8681552022.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang Q, Shen X, An R, Bai J, Dong J, Cai
H, Zhu H, Zhong W, Chen W, Liu A and Du J: Peritumoral tertiary
lymphoid structure and tumor stroma percentage predict the
prognosis of patients with non-metastatic colorectal cancer. Front
Immunol. 13:9620562022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang WH, Wang WQ, Han X, Gao HL, Xu SS,
Li S, Li TJ, Xu HX, Li H, Ye LY, et al: Infiltrating pattern and
prognostic value of tertiary lymphoid structures in resected
non-functional pancreatic neuroendocrine tumors. J Immunother
Cancer. 8:e0011882020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Tanaka T, Masuda A, Inoue J, Hamada T,
Ikegawa T, Toyama H, Sofue K, Shiomi H, Sakai A, Kobayashi T, et
al: Integrated analysis of tertiary lymphoid structures in relation
to tumor-infiltrating lymphocytes and patient survival in
pancreatic ductal adenocarcinoma. J Gastroenterol. 58:277–291.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ukita M, Hamanishi J, Yoshitomi H, Yamanoi
K, Takamatsu S, Ueda A, Suzuki H, Hosoe Y, Furutake Y, Taki M, et
al: CXCL13-producing CD4+ T cells accumulate in the early phase of
tertiary lymphoid structures in ovarian cancer. JCI Insight.
7:e1572152022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Siliņa K, Soltermann A, Attar FM, Casanova
R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P,
Curioni-Fontecedro A, et al: Germinal centers determine the
prognostic relevance of tertiary lymphoid structures and are
impaired by corticosteroids in lung squamous cell carcinoma. Cancer
Res. 78:1308–1320. 2018. View Article : Google Scholar
|
|
129
|
Yang M, Lu J, Zhang G, Wang Y, He M, Xu Q,
Xu C and Liu H: CXCL13 shapes immunoactive tumor microenvironment
and enhances the efficacy of PD-1 checkpoint blockade in high-grade
serous ovarian cancer. J Immunother Cancer. 9:e0011362021.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Cabrita R, Lauss M, Sanna A, Donia M,
Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K,
Vallon-Christersson J, et al: Tertiary lymphoid structures improve
immunotherapy and survival in melanoma. Nature. 577:561–565. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Helmink BA, Reddy SM, Gao J, Zhang S,
Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et
al: B cells and tertiary lymphoid structures promote immunotherapy
response. Nature. 577:549–555. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Petitprez F, de Reyniès A, Keung EZ, Chen
TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A,
et al: B cells are associated with survival and immunotherapy
response in sarcoma. Nature. 577:556–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sawada J, Hiraoka N, Qi R, Jiang L,
Fournier-Goss AE, Yoshida M, Kawashima H and Komatsu M: Molecular
signature of tumor-associated high endothelial venules that can
predict breast cancer survival. Cancer Immunol Res. 10:468–481.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Martinet L, Garrido I, Filleron T, Le
Guellec S, Bellard E, Fournie JJ, Rochaix P and Girard JP: Human
solid tumors contain high endothelial venules: association with T-
and B-lymphocyte infiltration and favorable prognosis in breast
cancer. Cancer Res. 71:5678–5687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Asrir A, Tardiveau C, Coudert J, Laffont
R, Blanchard L, Bellard E, Veerman K, Bettini S, Lafouresse F, Vina
E, et al: Tumor-associated high endothelial venules mediate
lymphocyte entry into tumors and predict response to PD-1 plus
CTLA-4 combination immunotherapy. Cancer Cell. 40:318–334.e9. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li Z, Jiang Y, Li B, Han Z, Shen J, Xia Y
and Li R: Development and validation of a machine learning model
for detection and classification of tertiary lymphoid structures in
gastrointestinal cancers. JAMA Netw Open. 6:e22525532023.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Neyt K, Perros F, GeurtsvanKessel CH,
Hammad H and Lambrecht BN: Tertiary lymphoid organs in infection
and autoimmunity. Trends Immunol. 33:297–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hayashi Y, Makino T, Sato E, Ohshima K,
Nogi Y, Kanemura T, Honma K, Yamashita K, Saito T, Tanaka K, et al:
Density and maturity of peritumoral tertiary lymphoid structures in
oesophageal squamous cell carcinoma predicts patient survival and
response to immune checkpoint inhibitors. Br J Cancer.
128:2175–2185. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Deguchi S, Tanaka H, Suzuki S, Natsuki S,
Mori T, Miki Y, Yoshii M, Tamura T, Toyokawa T, Lee S, et al:
Clinical relevance of tertiary lymphoid structures in esophageal
squamous cell carcinoma. BMC Cancer. 22:6992022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ling Y, Zhong J, Weng Z, Lin G, Liu C, Pan
C, Yang H, Wei X, Xie X, Wei X, et al: The prognostic value and
molecular properties of tertiary lymphoid structures in oesophageal
squamous cell carcinoma. Clin Transl Med. 12:e10742022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Calderaro J, Petitprez F, Becht E, Laurent
A, Hirsch TZ, Rousseau B, Luciani A, Amaddeo G, Derman J, Charpy C,
et al: Intra-tumoral tertiary lymphoid structures are associated
with a low risk of early recurrence of hepatocellular carcinoma. J
Hepatol. 70:58–65. 2019. View Article : Google Scholar
|
|
142
|
Sun X, Liu W, Sun L, Mo H, Feng Y, Wu X,
Li C, Chen C, Li J, Xin Y, et al: Maturation and abundance of
tertiary lymphoid structures are associated with the efficacy of
neoadjuvant chemoimmunotherapy in resectable non-small cell lung
cancer. J Immunother Cancer. 10:e0055312022. View Article : Google Scholar :
|
|
143
|
Lynch KT, Young SJ, Meneveau MO, Wages NA,
Engelhard VH, Slingluff CL Jr and Mauldin IS: Heterogeneity in
tertiary lymphoid structure B-cells correlates with patient
survival in metastatic melanoma. J Immunother Cancer.
9:e0022732021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Posch F, Silina K, Leibl S, Mündlein A,
Moch H, Siebenhüner A, Samaras P, Riedl J, Stotz M, Szkandera J, et
al: Maturation of tertiary lymphoid structures and recurrence of
stage II and III colorectal cancer. Oncoimmunology. 7:e13788442017.
View Article : Google Scholar
|
|
145
|
Zhang Q and Wu S: Tertiary lymphoid
structures are critical for cancer prognosis and therapeutic
response. Front Immunol. 13:10637112023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Fridman WH, Meylan M, Petitprez F, Sun CM,
Italiano A and Sautès-Fridman C: B cells and tertiary lymphoid
structures as determinants of tumour immune contexture and clinical
outcome. Nat Rev Clin Oncol. 19:441–457. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Saito T, Nishikawa H, Wada H, Nagano Y,
Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, et
al: Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the
prognosis of colorectal cancers. Nat Med. 22:679–684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shalapour S, Font-Burgada J, Di Caro G,
Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M,
Strasner A, Hansel DE, et al: Immunosuppressive plasma cells impede
T-cell-dependent immunogenic chemotherapy. Nature. 521:94–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Finkin S, Yuan D, Stein I, Taniguchi K,
Weber A, Unger K, Browning JL, Goossens N, Nakagawa S, Gunasekaran
G, et al: Ectopic lymphoid structures function as microniches for
tumor progenitor cells in hepatocellular carcinoma. Nat Immunol.
16:1235–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Milutinovic S, Abe J, Godkin A, Stein JV
and Gallimore A: The dual role of high endothelial venules in
cancer progression versus immunity. Trends Cancer. 7:214–225. 2021.
View Article : Google Scholar
|
|
151
|
Sautès-Fridman C, Petitprez F, Calderaro J
and Fridman WH: Tertiary lymphoid structures in the era of cancer
immunotherapy. Nat Rev Cancer. 19:307–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ray-Coquard I, Cropet C, Van Glabbeke M,
Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P,
Labidi I, et al: Lymphopenia as a prognostic factor for overall
survival in advanced carcinomas, sarcomas, and lymphomas. Cancer
Res. 69:5383–5391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wu Z, Zhang J, Cai Y, Deng R, Yang L, Li J
and Deng Y: Reduction of circulating lymphocyte count is a
predictor of good tumor response after neoadjuvant treatment for
rectal cancer. Medicine (Baltimore). 97:e114352018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lee YJ, Park YS, Lee HW, Park TY, Lee JK
and Heo EY: Peripheral lymphocyte count as a surrogate marker of
immune checkpoint inhibitor therapy outcomes in patients with
non-small-cell lung cancer. Sci Rep. 12:6262022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Weide B, Martens A, Hassel JC, Berking C,
Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di
Giacomo AM, et al: Baseline biomarkers for outcome of melanoma
patients treated with pembrolizumab. Clin Cancer Res. 22:5487–5496.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Martens A, Wistuba-Hamprecht K, Geukes
Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B,
Capone M, et al: Baseline peripheral blood biomarkers associated
with clinical outcome of advanced melanoma patients treated with
ipilimumab. Clin Cancer Res. 22:2908–2918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Juliá EP, Mandó P, Rizzo MM, Cueto GR,
Tsou F, Luca R, Pupareli C, Bravo AI, Astorino W, Mordoh J, et al:
Peripheral changes in immune cell populations and soluble mediators
after anti-PD-1 therapy in non-small cell lung cancer and renal
cell carcinoma patients. Cancer Immunol Immunother. 68:1585–1596.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Cui JH, Lin KR, Yuan SH, Jin YB, Chen XP,
Su XK, Jiang J, Pan YM, Mao SL, Mao XF and Luo W: TCR repertoire as
a novel indicator for immune monitoring and prognosis assessment of
patients with cervical cancer. Front Immunol. 9:27292018.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gleason L, Porcu P and Nikbakht N: Reduced
overall T-cell receptor diversity as an indicator of aggressive
cutaneous T-cell lymphoma. Blood. 140(Suppl 1): 3539–3540. 2022.
View Article : Google Scholar
|
|
160
|
Manuel M, Tredan O, Bachelot T, Clapisson
G, Courtier A, Parmentier G, Rabeony T, Grives A, Perez S, Mouret
JF, et al: Lymphopenia combined with low TCR diversity (divpenia)
predicts poor overall survival in metastatic breast cancer
patients. Oncoimmunology. 1:432–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lin KR, Pang DM, Jin YB, Hu Q, Pan YM, Cui
JH, Chen XP, Lin YX, Mao XF, Duan HB and Luo W: Circulating
CD8+ T-cell repertoires reveal the biological
characteristics of tumors and clinical responses to chemotherapy in
breast cancer patients. Cancer Immunol Immunother. 67:1743–1752.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Cai G, Guan Z, Jin Y, Su Z, Chen X, Liu Q,
Wang C, Yin X, Zhang L, Ye G and Luo W: Circulating T-cell
repertoires correlate with the tumor response in patients with
breast cancer receiving neoadjuvant chemotherapy. JCO Precis Oncol.
6:e21001202022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Page DB, Yuan J, Redmond D, Wen YH, Durack
JC, Emerson R, Solomon S, Dong Z, Wong P, Comstock C, et al: Deep
sequencing of T-cell receptor DNA as a biomarker of clonally
expanded TILs in breast cancer after immunotherapy. Cancer Immunol
Res. 4:835–844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kuehm LM, Wolf K, Zahour J, DiPaolo RJ and
Teague RM: Checkpoint blockade immunotherapy enhances the frequency
and effector function of murine tumor-infiltrating T cells but does
not alter TCRβ diversity. Cancer Immunol Immunother. 68:1095–1106.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Rudqvist NP, Pilones KA, Lhuillier C,
Wennerberg E, Sidhom JW, Emerson RO, Robins HS, Schneck J, Formenti
SC and Demaria S: Radiotherapy and CTLA-4 blockade shape the TCR
repertoire of tumor-infiltrating T cells. Cancer Immunol Res.
6:139–150. 2018. View Article : Google Scholar :
|
|
166
|
Wu TD, Madireddi S, de Almeida PE,
Banchereau R, Chen YJ, Chitre AS, Chiang EY, Iftikhar H, O'Gorman
WE, Au-Yeung A, et al: Peripheral T cell expansion predicts tumour
infiltration and clinical response. Nature. 579:274–278. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Puig-Saus C, Sennino B, Peng S, Wang CL,
Pan Z, Yuen B, Purandare B, An D, Quach BB, Nguyen D, et al:
Neoantigen-targeted CD8+ T cell responses with PD-1
blockade therapy. Nature. 615:697–704. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Dong N, Moreno-Manuel A, Calabuig-Fariñas
S, Gallach S, Zhang F, Blasco A, Aparisi F, Meri-Abad M, Guijarro
R, Sirera R, et al: Characterization of circulating T cell receptor
repertoire provides information about clinical outcome after PD-1
blockade in advanced non-small cell lung cancer patients. Cancers
(Basel). 13:29502021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Han J, Duan J, Bai H, Wang Y, Wan R, Wang
X, Chen S, Tian Y, Wang D, Fei K, et al: TCR repertoire diversity
of peripheral PD-1+CD8+ T cells predicts
clinical outcomes after immunotherapy in patients with non-small
lung cancer. Cancer Immunol Res. 8:146–154. 2020. View Article : Google Scholar
|
|
170
|
Kato T, Kiyotani K, Tomiyama E, Koh Y,
Matsushita M, Hayashi Y, Nakano K, Ishizuya Y, Wang C, Hatano K, et
al: Peripheral T cell receptor repertoire features predict durable
responses to anti-PD-1 inhibitor monotherapy in advanced renal cell
carcinoma. Oncoimmunology. 10:18629482021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Snyder A, Nathanson T, Funt SA, Ahuja A,
Buros Novik J, Hellmann MD, Chang E, Aksoy BA, Al-Ahmadie H, Yusko
E, et al: Contribution of systemic and somatic factors to clinical
response and resistance to PD-L1 blockade in urothelial cancer: An
exploratory multi-omic analysis. PLoS Med. 14:e10023092017.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Arakawa A, Vollmer S, Tietze J, Galinski
A, Heppt MV, Bürdek M, Berking C and Prinz JC: Clonality of
CD4+ blood T cells predicts longer survival with CTLA4
or PD-1 checkpoint inhibition in advanced melanoma. Front Immunol.
10:13362019. View Article : Google Scholar
|
|
173
|
Zhu Q, Qiao G, Huang L, Xu C, Guo D, Wang
S, Zhao J, Song Y, Liu B, Chen Z, et al: Restored
CD8+PD-1+ T cells facilitate the response to
Anti-PD-1 for patients with pancreatic ductal adenocarcinoma. Front
Oncol. 12:8375602022. View Article : Google Scholar
|
|
174
|
Verronèse E, Delgado A,
Valladeau-Guilemond J, Garin G, Guillemaut S, Tredan O, Ray-Coquard
I, Bachelot T, N'Kodia A, Bardin-Dit-Courageot C, et al: Immune
cell dysfunctions in breast cancer patients detected through whole
blood multi-parametric flow cytometry assay. Oncoimmunology.
5:e11007912015. View Article : Google Scholar
|
|
175
|
Saleh R and Elkord E: FoxP3+ T
regulatory cells in cancer: Prognostic biomarkers and therapeutic
targets. Cancer Lett. 490:174–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune system.
Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Kotsakis A, Koinis F, Katsarou A,
Gioulbasani M, Aggouraki D, Kentepozidis N, Georgoulias V and
Vetsika EK: Prognostic value of circulating regulatory T cell
subsets in untreated non-small cell lung cancer patients. Sci Rep.
6:392472016. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Mamessier E, Sylvain A, Thibult ML,
Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P,
Romagné F, Thibault G, et al: Human breast cancer cells enhance
self tolerance by promoting evasion from NK cell antitumor
immunity. J Clin Invest. 121:3609–3622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Manjarrez-Orduño N, Menard LC, Kansal S,
Fischer P, Kakrecha B, Jiang C, Cunningham M, Greenawalt D, Patel
V, Yang M, et al: Circulating T cell subpopulations correlate with
immune responses at the tumor site and clinical response to PD1
inhibition in non-small cell lung cancer. Front Immunol.
9:16132018. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH,
Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al: Hyperprogressive
disease during PD-1/PD-L1 blockade in patients with non-small-cell
lung cancer. Ann Oncol. 30:1104–1113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wistuba-Hamprecht K, Martens A, Heubach F,
Romano E, Geukes Foppen M, Yuan J, Postow M, Wong P, Mallardo D,
Schilling B, et al: Peripheral CD8 effector-memory type 1 T-cells
correlate with outcome in ipilimumab-treated stage IV melanoma
patients. Eur J Cancer. 73:61–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kagamu H, Kitano S, Yamaguchi O, Yoshimura
K, Horimoto K, Kitazawa M, Fukui K, Shiono A, Mouri A, Nishihara F,
et al: CD4+ T-cell Immunity in the peripheral blood
correlates with response to Anti-PD-1 therapy. Cancer Immunol Res.
8:334–344. 2020. View Article : Google Scholar
|
|
183
|
Sade-Feldman M, Kanterman J, Klieger Y,
Ish-Shalom E, Olga M, Saragovi A, Shtainberg H, Lotem M and
Baniyash M: Clinical significance of circulating
CD33+CD11b+HLA-DR-myeloid cells in patients with stage IV melanoma
treated with ipilimumab. Clin Cancer Res. 22:5661–5672. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zuazo M, Arasanz H, Fernández-Hinojal G,
García-Granda MJ, Gato M, Bocanegra A, Martínez M, Hernández B,
Teijeira L, Morilla I, et al: Functional systemic CD4 immunity is
required for clinical responses to PD-L1/PD-1 blockade therapy.
EMBO Mol Med. 11:e102932019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Mazzaschi G, Facchinetti F, Missale G,
Canetti D, Madeddu D, Zecca A, Veneziani M, Gelsomino F, Goldoni M,
Buti S, et al: The circulating pool of functionally competent NK
and CD8+ cells predicts the outcome of anti-PD1 treatment in
advanced NSCLC. Lung Cancer. 127:153–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Kamada T, Togashi Y, Tay C, Ha D, Sasaki
A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, et al:
PD-1+ regulatory T cells amplified by PD-1 blockade
promote hyperprogression of cancer. Proc Natl Acad Sci USA.
116:9999–10008. 2019. View Article : Google Scholar
|
|
187
|
Jacquelot N, Roberti MP, Enot DP,
Rusakiewicz S, Ternès N, Jegou S, Woods DM, Sodré AL, Hansen M,
Meirow Y, et al: Predictors of responses to immune checkpoint
blockade in advanced melanoma. Nat Commun. 8:5922017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ferrara R, Naigeon M, Auclin E, Duchemann
B, Cassard L, Jouniaux JM, Boselli L, Grivel J, Desnoyer A,
Mezquita L, et al: Circulating T-cell immunosenescence in patients
with advanced non-small cell lung cancer treated with single-agent
PD-1/PD-L1 inhibitors or platinum-based chemotherapy. Clin Cancer
Res. 27:492–503. 2021. View Article : Google Scholar
|
|
189
|
Griffiths JI, Wallet P, Pflieger LT,
Stenehjem D, Liu X, Cosgrove PA, Leggett NA, McQuerry JA, Shrestha
G, Rossetti M, et al: Circulating immune cell phenotype dynamics
reflect the strength of tumor-immune cell interactions in patients
during immunotherapy. Proc Natl Acad Sci USA. 117:16072–16082.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Kwon M, An M, Klempner SJ, Lee H, Kim KM,
Sa JK, Cho HJ, Hong JY, Lee T, Min YW, et al: Determinants of
response and intrinsic resistance to PD-1 blockade in
microsatellite instability-high gastric cancer. Cancer Discov.
11:2168–2185. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Kamphorst AO, Pillai RN, Yang S, Nasti TH,
Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al:
Proliferation of PD-1+ CD8 T cells in peripheral blood after
PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci
USA. 114:4993–4998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee
SH, Ahn JS, Cheon J, Min YJ, Park SH, et al: The first-week
proliferative response of peripheral blood
PD-1+CD8+ T cells predicts the response to
anti-PD-1 therapy in solid tumors. Clin Cancer Res. 25:2144–2154.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Krieg C, Nowicka M, Guglietta S, Schindler
S, Hartmann FJ, Weber LM, Dummer R, Robinson MD, Levesque MP and
Becher B: High-dimensional single-cell analysis predicts response
to anti-PD-1 immunotherapy. Nat Med. 24:144–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
de Coaña YP, Wolodarski M, Poschke I,
Yoshimoto Y, Yang Y, Nyström M, Edbäck U, Brage SE, Lundqvist A,
Masucci GV, et al: Ipilimumab treatment decreases monocytic MDSCs
and increases CD8 effector memory T cells in long-term survivors
with advanced melanoma. Oncotarget. 8:21539–21553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Duchemann B, Naigeon M, Auclin E, Ferrara
R, Cassard L, Jouniaux JM, Boselli L, Grivel J, Desnoyer A, Danlos
FX, et al: CD8+PD-1+ to
CD4+PD-1+ ratio (PERLS) is associated with
prognosis of patients with advanced NSCLC treated with PD-(L)1
blockers. J Immunother Cancer. 10:e0040122022. View Article : Google Scholar
|
|
197
|
Youn JI, Park SM, Park S, Kim G, Lee HJ,
Son J, Hong MH, Ghaderpour A, Baik B, Islam J, et al: Peripheral
natural killer cells and myeloid-derived suppressor cells correlate
with anti-PD-1 responses in non-small cell lung cancer. Sci Rep.
10:90502020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Dodagatta-Marri E, Meyer DS, Reeves MQ,
Paniagua R, To MD, Binnewies M, Broz ML, Mori H, Wu D, Adoumie M,
et al: α-PD-1 therapy elevates Treg/Th balance and increases tumor
cell pSmad3 that are both targeted by α-TGFβ antibody to promote
durable rejection and immunity in squamous cell carcinomas. J
Immunother Cancer. 7:622019. View Article : Google Scholar
|
|
199
|
Takeuchi Y, Tanemura A, Tada Y, Katayama
I, Kumanogoh A and Nishikawa H: Clinical response to PD-1 blockade
correlates with a sub-fraction of peripheral central memory CD4+ T
cells in patients with malignant melanoma. Int Immunol. 30:13–22.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Kato R, Yamasaki M, Urakawa S, Nishida K,
Makino T, Morimoto-Okazawa A, Kawashima A, Iwahori K, Suzuki S,
Ueda R, et al: Increased Tim-3+ T cells in PBMCs during
nivolumab therapy correlate with responses and prognosis of
advanced esophageal squamous cell carcinoma patients. Cancer
Immunol Immunother. 67:1673–1683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Sangro B, Melero I, Wadhawan S, Finn RS,
Abou-Alfa GK, Cheng AL, Yau T, Furuse J, Park JW, Boyd Z, et al:
Association of inflammatory biomarkers with clinical outcomes in
nivolumab-treated patients with advanced hepatocellular carcinoma.
J Hepatol. 73:1460–1469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kim HR, Park SM, Seo SU, Jung I, Yoon HI,
Gabrilovich DI, Cho BC, Seong SY, Ha SJ and Youn JI: The ratio of
peripheral regulatory T cells to Lox-1+
polymorphonuclear myeloid-derived suppressor cells predicts the
early response to Anti-PD-1 therapy in patients with non-small cell
lung cancer. Am J Respir Crit Care Med. 199:243–246. 2019.
View Article : Google Scholar :
|
|
203
|
Jia XH, Geng LY, Jiang PP, Xu H, Nan KJ,
Yao Y, Jiang LL, Sun H, Qin TJ and Guo H: The biomarkers related to
immune related adverse events caused by immune checkpoint
inhibitors. J Exp Clin Cancer Res. 39:2842020. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Schweizer C, Schubert P, Rutzner S,
Eckstein M, Haderlein M, Lettmaier S, Semrau S, Gostian AO, Frey B,
Gaipl US, et al: Prospective evaluation of the prognostic value of
immune-related adverse events in patients with non-melanoma solid
tumour treated with PD-1/PD-L1 inhibitors alone and in combination
with radiotherapy. Eur J Cancer. 140:55–62. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Chuah S, Lee J, Song Y, Kim HD, Wasser M,
Kaya NA, Bang K, Lee YJ, Jeon SH, Suthen S, et al: Uncoupling
immune trajectories of response and adverse events from anti-PD-1
immunotherapy in hepatocellular carcinoma. J Hepatol. 77:683–694.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Zhu H, Galdos FX, Lee D, Waliany S, Huang
YV, Ryan J, Dang K, Neal JW, Wakelee HA, Reddy SA, et al:
Identification of pathogenic immune cell subsets associated with
checkpoint inhibitor-induced myocarditis. Circulation. 146:316–335.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Franken A, Van Mol P, Vanmassenhove S,
Donders E, Schepers R, Van Brussel T, Dooms C, Yserbyt J, De Crem
N, Testelmans D, et al: Single-cell transcriptomics identifies
pathogenic T-helper 17.1 cells and pro-inflammatory monocytes in
immune checkpoint inhibitor-related pneumonitis. J Immunother
Cancer. 10:e0053232022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Kim KH, Hur JY, Cho J, Ku BM, Koh J, Koh
JY, Sun JM, Lee SH, Ahn JS, Park K, et al: Immune-related adverse
events are clustered into distinct subtypes by T-cell profiling
before and early after anti-PD-1 treatment. Oncoimmunology.
9:17220232020. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Wei SC, Meijers WC, Axelrod ML, Anang
NAAS, Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L,
Courand PY, et al: A genetic mouse model recapitulates immune
checkpoint inhibitor-associated myocarditis and supports a
mechanism-based therapeutic intervention. Cancer Discov.
11:614–625. 2021. View Article : Google Scholar :
|
|
210
|
Fairfax BP, Taylor CA, Watson RA, Nassiri
I, Danielli S, Fang H, Mahé EA, Cooper R, Woodcock V, Traill Z, et
al: Peripheral CD8+ T cell characteristics associated
with durable responses to immune checkpoint blockade in patients
with metastatic melanoma. Nat Med. 26:193–199. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Chia S, Bedard PL, Hilton J, Amir E,
Gelmon K, Goodwin R, Villa D, Cabanero M, Tu D, Tsao M and Seymour
L: A phase Ib trial of durvalumab in combination with trastuzumab
in HER2-positive metastatic breast cancer (CCTG IND.229).
Oncologist. 24:1439–1445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Soler MF, Abaurrea A, Azcoaga P, Araujo AM
and Caffarel MM: New perspectives in cancer immunotherapy:
Targeting IL-6 cytokine family. J Immunother Cancer.
11:e0075302023. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Wattenberg MM and Beatty GL: Overcoming
immunotherapeutic resistance by targeting the cancer inflammation
cycle. Semin Cancer Biol. 65:38–50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Tobias J, Steinberger P, Drinić M and
Wiedermann U: Emerging targets for anticancer vaccination: PD-1.
ESMO Open. 6:1002782021. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y,
Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of
immune-checkpoint inhibitors in human cancers. Front Immunol.
13:9644422022. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Yang Y, Yu Y and Lu S: Effectiveness of
PD-1/PD-L1 inhibitors in the treatment of lung cancer: Brightness
and challenge. Sci China Life Sci. 63:1499–1514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Reck M, Remon J and Hellmann MD:
First-line immunotherapy for non-small-cell lung cancer. J Clin
Oncol. 40:586–597. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal
AN, Gadgeel SM, Girshman J, Kris MG, Riely GJ, Yu HA and Hellmann
MD: Severe immune-related adverse events are common with sequential
PD-(L)1 blockade and osimertinib. Ann Oncol. 30:839–844. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Jhawar SR, Wang SJ, Thandoni A, Bommareddy
PK, Newman JH, Marzo AL, Kuzel TM, Gupta V, Reiser J, Daniels P, et
al: Combination oncolytic virus, radiation therapy, and immune
checkpoint inhibitor treatment in anti-PD-1-refractory cancer. J
Immunother Cancer. 11:e0067802023. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Gebrael G, Sahu KK, Agarwal N and Maughan
BL: Update on combined immunotherapy for the treatment of advanced
renal cell carcinoma. Hum Vaccin Immunother. 19:21935282023.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Cafri G, Gartner JJ, Zaks T, Hopson K,
Levin N, Paria BC, Parkhurst MR, Yossef R, Lowery FJ, Jafferji MS,
et al: mRNA vaccine-induced neoantigen-specific T cell immunity in
patients with gastrointestinal cancer. J Clin Invest.
130:5976–5988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Ott PA, Hu-Lieskovan S, Chmielowski B,
Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD,
Lin JJ, et al: A phase Ib trial of personalized neoantigen therapy
plus anti-PD-1 in patients with advanced melanoma, non-small cell
lung cancer, or bladder cancer. Cell. 183:347–362.e24. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Keskin DB, Anandappa AJ, Sun J, Tirosh I,
Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E,
et al: Neoantigen vaccine generates intratumoral T cell responses
in phase Ib glioblastoma trial. Nature. 565:234–239. 2019.
View Article : Google Scholar :
|
|
227
|
Platten M, Bunse L, Wick A, Bunse T, Le
Cornet L, Harting I, Sahm F, Sanghvi K, Tan CL, Poschke I, et al: A
vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature.
592:463–468. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Kochenderfer JN, Chien CD, Simpson JL and
Gress RE: Maximizing CD8+ T cell responses elicited by peptide
vaccines containing CpG oligodeoxynucleotides. Clin Immunol.
124:119–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Haining WN, Davies J, Kanzler H, Drury L,
Brenn T, Evans J, Angelosanto J, Rivoli S, Russell K, George S, et
al: CpG oligodeoxynucleotides alter lymphocyte and dendritic cell
trafficking in humans. Clin Cancer Res. 14:5626–5634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Song YC, Cheng HY, Leng CH, Chiang SK, Lin
CW, Chong P, Huang MH and Liu SJ: A novel emulsion-type adjuvant
containing CpG oligodeoxynucleotides enhances CD8+ T-cell-mediated
anti-tumor immunity. J Control Release. 173:158–165. 2014.
View Article : Google Scholar
|
|
231
|
Baumgaertner P, Costa Nunes C, Cachot A,
Maby-El Hajjami H, Cagnon L, Braun M, Derré L, Rivals JP, Rimoldi
D, Gnjatic S, et al: Vaccination of stage III/IV melanoma patients
with long NY-ESO-1 peptide and CpG-B elicits robust CD8+
and CD4+ T-cell responses with multiple specificities
including a novel DR7-restricted epitope. Oncoimmunology.
5:e12162902016. View Article : Google Scholar
|
|
232
|
Zhu P, Li SY, Ding J, Fei Z, Sun SN, Zheng
ZH, Wei D, Jiang J, Miao JL, Li SZ, et al: Combination
immunotherapy of glioblastoma with dendritic cell cancer vaccines,
anti-PD-1 and poly I:C. J Pharm Anal. 13:616–624. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Soltani M, Savvateeva LV,
Ganjalikhani-Hakemi M and Zamyatnin AA: Clinical combinatorial
treatments based on cancer vaccines: Combination with checkpoint
inhibitors and beyond. Curr Drug Targets. 23:1072–1084. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
van der Burg SH, Arens R, Ossendorp F, van
Hall T and Melief CJ: Vaccines for established cancer: Overcoming
the challenges posed by immune evasion. Nat Rev Cancer. 16:219–233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Reichmuth AM, Oberli MA, Jaklenec A,
Langer R and Blankschtein D: mRNA vaccine delivery using lipid
nanoparticles. Ther Deliv. 7:319–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Kon E, Ad-El N, Hazan-Halevy I,
Stotsky-Oterin L and Peer D: Targeting cancer with mRNA-lipid
nanoparticles: Key considerations and future prospects. Nat Rev
Clin Oncol. 20:739–754. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Tenchov R, Bird R, Curtze AE and Zhou Q:
Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a
landscape of research diversity and advancement. ACS Nano.
15:16982–17015. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Karikó K, Muramatsu H, Welsh FA, Ludwig J,
Kato H, Akira S and Weissman D: Incorporation of pseudouridine into
mRNA yields superior nonimmunogenic vector with increased
translational capacity and biological stability. Mol Ther.
16:1833–1840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Ramos da Silva J, Bitencourt Rodrigues K,
Formoso Pelegrin G, Silva Sales N, Muramatsu H, de Oliveira Silva
M, Porchia BFMM, Moreno ACR, Aps LRMM, Venceslau-Carvalho AA, et
al: Single immunizations of self-amplifying or non-replicating
mRNA-LNP vaccines control HPV-associated tumors in mice. Sci Transl
Med. 15:eabn34642023. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Sittplangkoon C, Alameh MG, Weissman D,
Lin PJC, Tam YK, Prompetchara E and Palaga T: mRNA vaccine with
unmodified uridine induces robust type I interferon-dependent
anti-tumor immunity in a melanoma model. Front Immunol.
13:9830002022. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Meulewaeter S, Aernout I, Deprez J,
Engelen Y, De Velder M, Franceschini L, Breckpot K, Van Calenbergh
S, Asselman C, Boucher K, et al: Alpha-galactosylceramide improves
the potency of mRNA LNP vaccines against cancer and intracellular
bacteria. J Control Release. 370:379–391. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Yang J, Arya S, Lung P, Lin Q, Huang J and
Li Q: Hybrid nanovaccine for the co-delivery of the mRNA antigen
and adjuvant. Nanoscale. 11:21782–21789. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Carvalho T: Personalized anti-cancer
vaccine combining mRNA and immunotherapy tested in melanoma trial.
Nat Med. 29:2379–2380. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Podaza E, Carri I, Aris M, von Euw E,
Bravo AI, Blanco P, Ortiz Wilczyñski JM, Koile D, Yankilevich P,
Nielsen M, et al: Evaluation of T-cell responses against shared
melanoma associated antigens and predicted neoantigens in cutaneous
melanoma patients treated with the CSF-470 allogeneic cell vaccine
plus BCG and GM-CSF. Front Immunol. 11:11472020. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Sebastian M, Schröder A, Scheel B, Hong
HS, Muth A, von Boehmer L, Zippelius A, Mayer F, Reck M,
Atanackovic D, et al: A phase I/IIa study of the mRNA-based cancer
immunotherapy CV9201 in patients with stage IIIB/IV non-small cell
lung cancer. Cancer Immunol Immunother. 68:799–812. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Zilio S, Bicciato S, Weed D and Serafini
P: CCR1 and CCR5 mediate cancer-induced myelopoiesis and
differentiation of myeloid cells in the tumor. J Immunother Cancer.
10:e0031312022. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Badr G, Al-Sadoon MK, Rabah DM and Sayed
D: Snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles
induce apoptosis and growth arrest in human prostate cancer cells.
Apoptosis. 18:300–314. 2013. View Article : Google Scholar
|
|
248
|
Badr G, Al-Sadoon MK and Rabah DM:
Therapeutic efficacy and molecular mechanisms of snake
(Walterinnesia aegyptia) venom-loaded silica nanoparticles in the
treatment of breast cancer- and prostate cancer-bearing
experimental mouse models. Free Radic Biol Med. 65:175–189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
249
|
An S, Tiruthani K, Wang Y, Xu L, Hu M, Li
J, Song W, Jiang H, Sun J, Liu R and Huang L: Locally trapping the
C-C chemokine receptor type 7 by gene delivery nanoparticle
inhibits lymphatic metastasis prior to tumor resection. Small.
15:e18051822019. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Liu JQ, Zhang C, Zhang X, Yan J, Zeng C,
Talebian F, Lynch K, Zhao W, Hou X, Du S, et al: Intratumoral
delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for
cancer immunotherapy. J Control Release. 345:306–313. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Li Y, Su Z, Zhao W, Zhang X, Momin N,
Zhang C, Wittrup KD, Dong Y, Irvine DJ and Weiss R: Multifunctional
oncolytic nanoparticles deliver self-replicating IL-12 RNA to
eliminate established tumors and prime systemic immunity. Nat
Cancer. 1:882–893. 2020. View Article : Google Scholar
|
|
252
|
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins
JJ, Wagner ES, Gabaldon TA and Zaharoff DA: Localized
interleukin-12 for cancer immunotherapy. Front Immunol.
11:5755972020. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Xu S, Xu Y, Solek NC, Chen J, Gong F,
Varley AJ, Golubovic A, Pan A, Dong S, Zheng G and Li B:
Tumor-tailored ionizable lipid nanoparticles facilitate IL-12
circular RNA delivery for enhanced lung cancer immunotherapy. Adv
Mater. 36:e24003072024. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Fu S, Li G, Zang W, Zhou X, Shi K and Zhai
Y: Pure drug nano-assemblies: A facile carrier-free nanoplatform
for efficient cancer therapy. Acta Pharm Sin B. 12:92–106. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Reda M, Ngamcherdtrakul W, Nelson MA,
Siriwon N, Wang R, Zaidan HY, Bejan DS, Reda S, Hoang NH, Crumrine
NA, et al: Development of a nanoparticle-based immunotherapy
targeting PD-L1 and PLK1 for lung cancer treatment. Nat Commun.
13:42612022. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Han X, Wei Q, Lv Y, Weng L, Huang H, Wei
Q, Li M, Mao Y, Hua D, Cai X, et al: Ginseng-derived nanoparticles
potentiate immune checkpoint antibody efficacy by reprogramming the
cold tumor microenvironment. Mol Ther. 30:327–340. 2022. View Article : Google Scholar :
|
|
257
|
Liu L, Wang Y, Miao L, Liu Q, Musetti S,
Li J and Huang L: Combination immunotherapy of MUC1 mRNA
nano-vaccine and CTLA-4 blockade effectively inhibits growth of
triple negative breast cancer. Mol Ther. 26:45–55. 2018. View Article : Google Scholar :
|
|
258
|
Ma R, Li Z, Chiocca EA, Caligiuri MA and
Yu J: The emerging field of oncolytic virus-based cancer
immunotherapy. Trends Cancer. 9:122–139. 2023. View Article : Google Scholar :
|
|
259
|
Hu H, Zhang S, Cai L, Duan H, Li Y, Yang
J, Wang Y and Liu B, Dong S, Fang Z and Liu B: A novel cocktail
therapy based on quintuplet combination of oncolytic herpes simplex
virus-2 vectors armed with interleukin-12, interleukin-15, GM-CSF,
PD1v, and IL-7 × CCL19 results in enhanced antitumor efficacy.
Virol J. 19:742022. View Article : Google Scholar
|
|
260
|
Kim KJ, Moon D, Kong SJ, Lee YS, Yoo Y,
Kim S, Kim C, Chon HJ, Kim JH and Choi KJ: Antitumor effects of
IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1.
Gene Ther. 28:186–198. 2021. View Article : Google Scholar
|
|
261
|
Oh E, Oh JE, Hong J, Chung Y, Lee Y, Park
KD, Kim S and Yun CO: Optimized biodegradable polymeric
reservoir-mediated local and sustained co-delivery of dendritic
cells and oncolytic adenovirus co-expressing IL-12 and GM-CSF for
cancer immunotherapy. J Control Release. 259:115–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Zhang SN, Choi IK, Huang JH, Yoo JY, Choi
KJ and Yun CO: Optimizing DC vaccination by combination with
oncolytic adenovirus coexpressing IL-12 and GM-CSF. Mol Ther.
19:1558–1568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Malhotra J and Kim ES: Oncolytic viruses
and cancer immunotherapy. Curr Oncol Rep. 25:19–28. 2023.
View Article : Google Scholar
|