Introduction
Liver cancer is a malignant tumor that poses a
serious threat to human health. According to statistics, there are
~700,000 new cases of liver cancer worldwide every year. Data
released by the International Agency for Research on Cancer of the
World Health Organization show that in 2022, there were an
estimated 900,000 new cases of liver cancer worldwide. The
incidence rate of liver cancer ranks sixth among malignant tumors
and has become the third leading cause of death in the world, with
830,000 deaths per year (1).
Among them, the number of new cases and deaths of liver cancer in
China is close to half of the total. Hepatocellular carcinoma (HCC)
is the fourth most common malignant tumor and the second leading
cause of cancer death in China. In 2022, there were 316,500 deaths
due to primary liver cancer, ranking second in both the number of
deaths and mortality rate. The etiology of liver cancer is diverse
and complex and it is currently thought to be related to hepatitis
virus infection, alcohol, nitrosamine substances, aflatoxin and
certain chemical carcinogens (2).
In addition to traditional liver tumor resection, further treatment
methods for liver cancer, including interventional therapy,
radiofrequency ablation, immunotherapy, targeted therapy,
hyperthermic perfusion therapy and liver transplantation, have
developed rapidly in recent years (3). Due to its difficult initial
diagnosis, rapid progress, significant risk factors after surgery
and poor prognosis (4), most
patients with liver cancer have missed the opportunity for radical
surgery at the time of clinical diagnosis and liver cancer still
has the possibility of further development, recurrence and
metastasis after palliative surgery. Immunotherapy and targeted
therapy are expensive and have not been popularized to date.
Primary prevention of liver cancer and the search
for new therapeutic drugs are the current research hotspots.
Although aspirin, sorafenib, regorafenib, lenvatinib and other
anti-liver cancer drugs are effective therapeutic drugs in clinical
practice, they still have shortcomings such as low efficacy,
serious side effects and drug resistance (5-7).
Although chemotherapy and immunotherapy are conventional options
for treatment, they have large toxic and side effects, while
natural products have lower systemic toxicity and fewer side
effects, which can provide better treatment options for patients
(8,9) and have unique advantages in the
treatment of liver cancer.
Natural products have played an important role in
medicine, particularly in the treatment of chronic and metabolic
diseases, and are the basis of most early drugs (10). Some of the world's best-known
drugs, including artemisinin, berberine, paclitaxel and elemene,
are compounds of natural origin (11). With the development of natural
medicinal chemistry technology, the determination of phytochemical
compositions and its application in drug development have become
possible. In the nearly 20 years, natural products still exist as
sources of new drugs and have a large share in new drug discovery
(12). Terpenoids are a class of
active natural products with a wide range of pharmacological
effects and represent a rich library of candidate compounds for
drug discovery (13). Terpenoids
can be divided into several subclasses according to their chemical
structures, including semi-terpenoids, monoterpenoids,
sesquiterpenoids, diterpenoids, ester terpenoids and triterpenoids.
Among them, the most distributed class of sesquiterpenoids is
mainly divided into acyclic, monocyclic, bicyclic, tricyclic and
tetracyclic sesquiterpenoids. They can also be divided into
five-membered ring, six-membered ring and seven-membered ring
sesquiterpenoids by the number of carbon atoms. Furthermore, it can
be divided into sesquiterpene alcohols, sesquiterpene ketones and
sesquiterpene lactones according to different oxygen-containing
groups (14). Various studies
have shown that sesquiterpenoid exhibits potential therapeutic
effects in anti-tumor, anti-inflammatory, antibacterial and
anti-cardiovascular diseases (15-22), and there are more new components
and physiological activities to be explored. There have been
numerous studies on the application of sesquiterpenoids in HCC, but
there is still a lack of systematic analysis of their therapeutic
applications. In the present review, the research progress of
natural products in the treatment of liver cancer in the past two
decades was summarized.
Methods
Prior to 2003, due to technical limitations, the
literature records were incomplete or difficult to obtain. A
20-year span is sufficient to present the development process of
this field from initial exploration to in-depth research,
facilitating the sorting of trends and key nodes; balancing
research resources and energy constraints, with a moderate duration
for detailed analysis of literature, to avoid an overly broad or
narrow scope. Adapting to the changes in liver cancer treatment
concepts, technologies and drugs, it is conducive to study the
effects of sesquiterpenes in combination with disease
characteristics. At the same time, it complies with the standards
and regulations of the medical research industry, making it easy to
compare and communicate with similar studies. Due to the above
reasons, the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://www.webofscience.com/), Science
Direct (https://www.sciencedirect.com) and
Springer databases (https://link.springer.com/) were searched for
information on sesquiterpenes for the treatment of HCC from 2003 to
2024. The search terms were 'sesquiterpenoid compound',
'sesquiterpene lactone', 'sesquiterpene', 'turmeric', 'curcuma
zedoary', 'Artemisia', 'atractylodes rhizome' and 'liver
cancer', 'hepatocellular carcinoma' and 'HCC'. The keyword
combinations 'liver cancer AND natural sesquiterpenes' and
'hepatocellular carcinoma and natural sesquiterpenes' were used to
find relevant articles. The inclusion criteria were as follows: i)
Studies with in vivo animal experiments showing the
anticancer efficacy of natural sesquiterpenes against HCC; ii)
clinical trials concerning the therapeutic benefit of HCC; and iii)
original research papers written in English. Review articles,
meta-analyses, cross-sectional studies, descriptive studies,
studies that did not provide sample data of cancer patients and
projects that used secondary data were excluded from this
review.
Previous studies were excluded from the review if
they were found to have major methodologic errors or lack
scientific merit. To aid in classification efforts, studies on
mixtures of different compounds or crude extracts were excluded
from this study, except for chemically ambiguous sesquiterpenoids.
Not all pharmacological effects of sesquiterpenoids have been
described in detail in the literature. For instance, the mechanism
by which artemisinin exerts its specific anti-HCC activity is still
elusive, which may limit its further development in the clinic.
Therefore, based on the results of the literature search, a
secondary literature search was performed for certain specific
medicinal plants obtained, and the search terms were the names of
representative compounds, including 'artemisinin', 'elemene' and
'parthenolide'. The gaps of the first search were supplemented and
the research system was markedly enriched. Those reviews also
includes compounds with undescribed mechanisms that nevertheless
have inhibitory effects on HCC cells, providing a more
comprehensive anti-liver cancer research direction for the future.
Finally, 46 sesquiterpenoids were obtained.
Results
Increasing evidence has shown that sesquiterpenoids
can effectively inhibit the progression of HCC and play a
therapeutic role in different stages of the disease. It can
effectively treat HCC by improving antioxidant capacity, enhancing
specific and non-specific immune function, inhibiting cell
proliferation and promoting cell apoptosis. The molecular structure
of sesquiterpenoids is presented in Fig. 1. Schematics illustrating pathways
included in the mechanisms of action of various sesquiterpenoids in
HCC are provided in Figs. 2 and
3. The basic information of
various natural sesquiterpenoids, including their source, molecular
formula and molecular mass, was presented in Table I. It summarizes the activity
features of the sesquiterpenoids, including IC50 and
dosage, mechanism of action and effects (Table II).
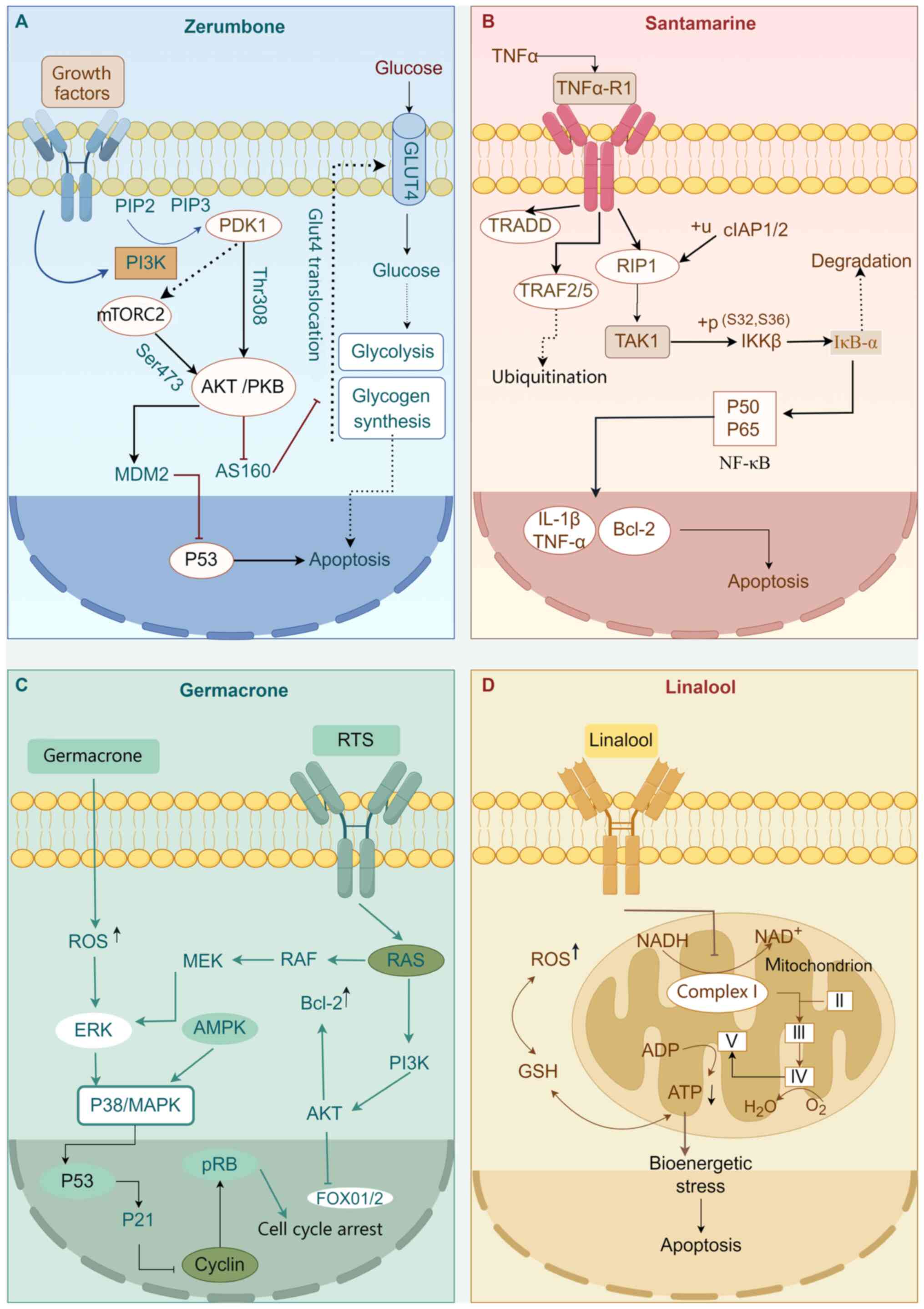 | Figure 2Mechanistic pathways of
sesquiterpenoids in hepatocellular carcinoma. (A) Gingerone, (B)
Santamarine, (C) Germacenone and (D) Linalool (figure was drawn
with Figdraw). PDK1, protein kinase domain containing 1; PI3K,
phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol(4,5)bisphosphate; PIP3,
phosphatidylinositol 3,4,5-triphosphate; mTORC2, mammalian target
of rapamycin complex 2; AKT(PKB), protein kinase B; MDM2, mdm2
proto-oncogene. TNF-α, tumor necrosis factor-α. TRADD, tumor
necrosis factor receptor 1-associated death domain protein;
TRAF2/5, tumor necrosis factor receptor-associated factor 2/5;
RIP1, receptor interacting serine/threonine-protein kinase 1; TAK1,
thymidine kinase 1; IKKβ, inhibitor of κB kinase β; IκB-α,
inhibitor of nuclear factor κB α; IL-1β, interleukin-1β; NF-κB,
nuclear factor-κB; Bcl-2, B-cell lymphoma-2; ROS, reactive oxygen
species; ERK, extracellular signal-regulated kinase; MAPK,
mitogen-activated protein kinases; AMPK,
adenosinemonophosphate-activated protein kinase; RAF, rapidly
accelerated fibrosarcoma; MEK, mitogen-activated protein kinase
kinase; GSH, glutathione; NADH, nicotinamide adenine dinucleotide;
ADP, adenosine diphosphate; ATP, adenosine triphosphate;
NAD+, nicotinamide adenine dinucleotide; RTS, radiation
therapy simulation. |
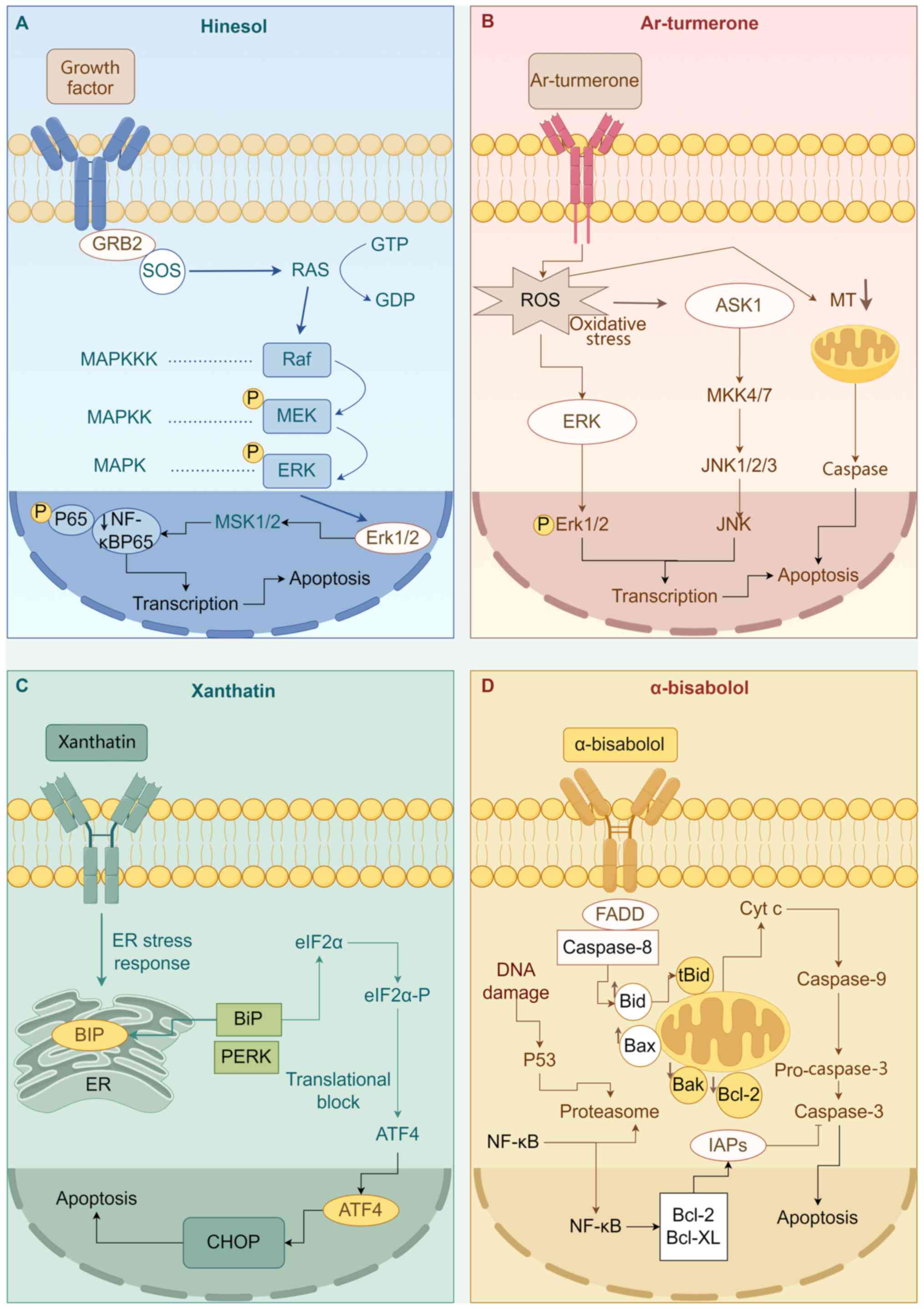 | Figure 3Mechanistic pathways of
sesquiterpenoids in hepatocellular carcinoma. (A) Hinesol, (B)
Ar-turmerone, (C) Xanthatin and (D) α-bisabolol (figure was drawn
with Figdraw). GRB2, growth factor receptor-bound protein 2; SOS,
suppressor of tumorigenicity 1; RAS, rat sarcoma; GTR, G-protein
coupled receptors; GDP, GDP-D-mannose pyrophosphorylase; RAF,
rapidly accelerated fibrosarcoma; MEK/MAPKK, MAPK kinase; ERK,
extracellular signal-regulated kinase; MAPKKK, MAPK kinase kinase;
MAPK, mitogen-activated protein kinases; NF-κB, nuclear factor-κB;
MSK1/2, mitogen- and stress-activated protein kinases; Erk1/2,
extracellular signal-regulated kinase; ASK1, apoptosis
signal-regulating kinase 1; MT, metallothionein; MKK4/7, MAPK
kinase 4/7; JNK, c-Jun N-terminal kinase; ER, endoplasmic
reticulum; BIP, binding immunoglobulin protein; CHOP, C/EBP
homologous protein; ATF4, activating transcription factor 4; PERK,
protein kinase R-like endoplasmic reticulum kinase; eIF2α,
eukaryotic translation initiation factor 2α; FADD, fas-associating
protein with a novel death domain; Bid, BH3-interacting domain
death agonist; Bax, BCL2-associated X; Bcl-2, B-cell lymphoma-2;
Cyt c, cytochrome C; Caspase-3, cysteinyl aspartate-specific
proteinase-3. |
 | Table IBasic information on various natural
sesquiterpenoids. |
Table I
Basic information on various natural
sesquiterpenoids.
| Name of
compound | Molecular
formula | Relative molecular
mass | Source | (Refs.) |
|---|
| Acorusin E |
C15H22O5 | 282 | Acori
tatarinowii rhizoma | (150) |
| Ar-turmerone |
C15H20O | 236 | Turmeric | (104) |
| Artemeriopodin
G7 |
C29H36O6 | 516 | Artemisia
australis | (135) |
|
Artemiprincepsolides A |
C30H38O6 | 539 | Artemisia
princeps | (78) |
| Artemongolins
E |
C31H39O8 | 539 | Artemisia
mongolica | (155) |
| Artemisacrolide
B |
C19H24O5 | 377 | Artemisia
sacrorum | (157) |
| Artemisinin |
C15H22O5 | 284 | Artemisia
apiacea | (27) |
| Artemyriantholide
E |
C30H33O6Cl | 523 | Artemisia
lactiflora | (156) |
| Artesunate |
C19H28O8 | 380 | Artemisia
apiacea | (30) |
| Atractylon |
C15H20O | 236 | Rhizoma
atractylodis | (99) |
| Bigelovin |
C17H20O5 | 324 | Inula flower | (43) |
| Britanin |
C19H26O7 | 366 | Inula flower | (42) |
| Carpespene A |
C15H18O3 | 264 | Carpesium
faberi, Guizhou | (79) |
| Cis-Nerolidol |
C15H26O | 222 | Natural products
found in essential oils such as bitter neroli oil and Peruvian balm
oil | (90) |
| Costunolide |
C12H20O2 | 216 | Costustoot | (40) |
| Cryptomeridiol |
C15H28O2 | 260 | Mangnolia
officinalis | (142) |
| Curcumenol |
C15H24O2 | 260 | Curcuma
zedoary | (119) |
| Dehydrocostus
lactone |
C15H18O2 | 248 | Inula
racemosa | (39) |
|
Deoxyelephantopin |
C19H20O6 | 364 | Elephantopus
scaber | (59) |
|
Dihydroartemisinin |
C15H24O5 | 288 | Artemisia
apiacea | (28) |
|
Dimethylaminomicheliolide |
C17H28ClNO3 | 339 | Michelia
L. | (50) |
| Elemene |
C15H24 | 228 | Curcuma
zedoary | (146) |
| Furanodiene |
C15H20O | 232 | Curcuma
zedoary | (143) |
| Germacrone |
C15H22O | 236 | Curcuma
zedoary | (97) |
| Hemistepsin A |
C18H20O6 | 352 | Hemistepta
lyrata | (66) |
| Hinesol |
C15H26O | 244 | Rhizoma
atractylodis | (115) |
| Hirsutanol A |
C14H18O3 | 252 | Endophytic bacteria
of soft corals | (144) |
|
Isoalantolactone |
C15H20O2 | 252 |
Elecampane | (37) |
| Lavandiolide H |
C30H38O6 | 494 | Artemisia
atrovirens | (33) |
| Lavandiolide I |
C30H36O6 | 492 |
Artemisia | (158) |
| Linalool |
C10H18O | 172 | Coriandrum
sativum L. | (84) |
| Micheliolide |
C15H20O3 | 268 | Michelia
L. | (47) |
| Narjatamolide |
C19H24O4 | 316 | Rhizome of Pinus
officinalis | (44) |
| Parthenolide |
C15H20O3 | 268 | White daisy | (45) |
| Santamarine |
C15H20O3 | 268 | Aplotaxis
auriculata | (65) |
| Scabertopinolide
G |
C20H22O7 | 397 | Elephantopus
scaber | (63) |
| Senedensiscin
G |
C28H46O10 | 542 | Senedensiscin | (152) |
| Sulfoscorzonin
E |
C19H27O6NS | 397 | Scorzonera
divaricata | (153) |
| Thapsigargin |
C34H50O12 | 652 | Thapsia
garganica | (105) |
| Telekin |
C15H20O3 | 268 | Carpesium
divaricatum | (77) |
| Xanthatin |
C15H18O3 | 264 | Xanthium | (72) |
| Xanthorrhizol |
C15H22O | 236 | Turmeric | (88) |
| Zerumbone |
C15H22O | 236 | Zingiber
zerumbet | (129) |
|
(2S,7R,10S)-3-hydroxypseudotigerone
11-O-β-D-glucopyranoside |
C21H34O8 | 416 | Atractylis
lancea rhizome | (111) |
| β-elemene |
C15H24 | 228 | Curcuma
zedoary | (149) |
| α-bisabolol |
C15H26O | 222 | Chamomile | (94) |
 | Table IIMechanisms of action of
sesquiterpenoids against hepatocellular carcinoma. |
Table II
Mechanisms of action of
sesquiterpenoids against hepatocellular carcinoma.
| Sesquiterpene
compound |
IC50 | Effective dose | Pathways | Observed
effects | (Refs.) |
|---|
| Artemisinin | 250 μM
(HepG2), 290 μM (SMMC-7721) | - | via TIMP2 protein↑
and matrix metalloproteinase 2 protein↓ | Inhibited the
migration and invasion of HepG2 and SMMC-7721 cells | (35) |
|
Dihydroartemisinin | - | 20 and 40
μM | via inhibition of
cyclin B and cdc25c, G2/M phase arrest | Apoptosis of HepG2
and Hep3B cells was induced | (36) |
| Artesunate | 20 μM | - | via STAT3,
PI3K/AKT/mTOR pathway | The activity of
HepG2 cells was inhibited | (39) |
| Lavandiolide H | 3.8 μM
(HepG2), 4.6 μM (SMMC-7721), 4.5 μM (Huh7) | - | Bcl-2 protein↓ and
PARP-1↑ | Inhibited the
proliferation of HepG2, SMMC-7721 and Huh7 cells | (43) |
|
Isoalantolactone | 71.2 μM (12
h), 53.4 μM (24 h) | - | via
Ras/Raf/MEK | Inhibition of HepG2
cell proliferation | (45) |
| Dehydrocostus
lactone | 20.33
μM | - | via PI3K/AKT | Inhibition of HepG2
cell proliferation | (46) |
| Costunolide | 18.09±1.74
μM | - | Bax protein↑,
caspase-3↑, caspase-8↑, caspase-9↑ and Bcl-2 protein↓ | Promoted HepG2 cell
apoptosis | (48) |
| Britanin | 27.86±1.35
μM (HepG2), 28.92±1.09 μM (SMMC-7721), 15.69±1.58
μM (Huh7) | - | via AMPK was
regulated by activation of ROS | Induced apoptosis
and autophagy in HepG2, SMMC-7721 and Huh7 cells | (49) |
| Bigelovin | - | 5 and 10
μM | Caspase-3↑ and
PARP-1↑ | Induced apoptosis
of HepG2 and SMMC-7721 cells | (50) |
| Narjatamolide | 5.67±1.43
μM | - | via induced cell
cycle arrest at G2/M phase | Inhibition of
BEL-7402 cell proliferation | (51) |
| Parthenolide | - | - | ROS is generated,
resulting in blocking of the cell cycle | Apoptosis and
autophagy in HepG2 cells | (53) |
| Micheliolide | 31.46±5.33
μM (24 h), 13.4±1.39 μM (48 h), 8.13±1.29 μM
(72 h) | - | ROS inhibition
normalized MCL-induced ERS | inhibited the
development of hepatoma organoids | (56) |
| DMAMCL | 12.74±0.72
μM (HepG2), 13.82±0.54 μM (Hep3B), 12.91±0.83
μM (Huh7), 17.21±0.68 μM (SMMC-7221) | - | via PI3K/AKT | Apoptosis of HepG2,
SMMC-7721, Hep3B and Huh7 cell lines | (62) |
|
Deoxyelephantopin | 40 μM | - | via NF-κB | Inhibition of HepG2
cell proliferation and induction of apoptosis | (69) |
| Scabertopinolide
G | 7.0-10.3
μM | - | via increased ROS
production and decreased MMP | Autophagy was
induced in Hep3B and HepG2 cells | (70) |
| Santamarine | 70 μM | - | via NF-κB | Inhibition of HepG2
cell proliferation and induction of apoptosis | (72) |
| Hemistepsin A | 15.27±1.84
μM (Huh7), 26.5±6.07 μM (HepG2) | - | via AMP/AMPK,
STAT3 | Slowed down the
cell cycle progression of Huh7 and HepG2 cells and induced
apoptosis | (77) |
| Xanthatin | - | 20 and 40
μM | via PERK-eIF2α-ATF4
signaling pathway and target CHOP | Apoptosis of HepG2,
Bel-7402 and SMMC-7721 hepatoma cells | (82) |
| Telekin | 3.75-30
μM | - | via activation of
p38 and MAPKAPK-2 pathways | Inhibition of HepG2
cell viability and induction of apoptosis | (84) |
|
Artemiprincepsolides A | 9.9 μM | - | Bc1-2 protein↓ and
Bax protein↑ | Induced apoptosis
of HepG2 cells | (85) |
| Carpespene A | 5.17 μM | - | via triggering
excess ROS | Apoptosis of HepG2
cells | (90) |
| Linalool | - | 2 μM | via inhibition of
mitochondrial complex I and II activity | The viability of
HepG2 cells was reduced | (94) |
| Xanthorrhizol | 4.17
μg/ml | - | Promoted the
proteolytic cleavage of PARP and ICAD, Bcl-2 and Bcl-xl
protein↓ | Induced apoptosis
of HepG2 cells | (96) |
| Cis-Nerolidol | - | 150 μM | via mitochondrial
membrane potential by arresting the cell cycle in G1 phase | Cell death was
induced in HepG2/C3A cells | (100) |
| α-bisabolol | - | - | via NF-κB | Apoptosis of HepG2
cancer cells | (102) |
| Germacrone | - | 240 μM | via STAT3 and
JAK2 | Induced apoptosis
of HepG2 cells | (105) |
| Atractylon | 26.19 μM
(HepG2), 22.32 μM (SMCC7721), 34.14 μM (MHCC97H) | - | via mitochondrial
apoptosis pathway | Induced apoptosis
of HepG2, SMCC7721 and MHCC9H cells | (109) |
| Ar-turmerone | 64.8±7.1
μg/ml (HepG2), 102.5±11.5 μg/ml (Huh-7), 122.2±7.6
μg/ml (Hep3B) | - | via ERK/JNK | Apoptosis of HepG2,
Huh-7 and Hep3B cells | (111) |
| Thapsigargin | - | 1, 2, 4, 8
μM | via inhibition of
SERCA-ATPase in hepatoma cells and depletion of intracellular
Ca2+ pool | Apoptosis of
hepatoma cells | (115) |
|
(2S,7R,10S)-3-hydroxypseudotigerone-11-O-β-D-glucopyranoside | - | 10
μmol/l | - | Significant
protective effect on HepG2 cell injury induced by
N-acetyl-p-aminophenol | (121) |
| Hinesol | - | - | via MAPK and ERK;
NF-κB | Inhibition of
proliferation and induction of apoptosis in SMMC-7721 and LM3
cells | (124) |
| Curcumenol | - | - | via DJ-1, PTEN,
PI3K/AKT signal transduction pathway | Inhibition of the
proliferation of HepG2 hepatoma cells | (137) |
| Zerumbone | 6.20±0.7
μg/ml | 12.5
μg/ml | MMP-9, VEGF and
VEGF receptor proteins↓ | Induced apoptosis
of HepG2 cells | (138) |
| Artemeriopodin
G7 | 16.0 μM | - | CDC2 and p-CDC2
protein expression↓, it targets PDGFRA, affects AKT/STAT signaling
and induces G2/M cell cycle arrest | Inhibition of HepG2
cell migration and invasion, and induction of apoptosis | (147) |
| Cryptomeridiol | >50
μM | - | via IRE1α-ASK1-JNK,
resulting in loss of MMP | The viability of
HepG2, Hep3B and Huh-7 cells was reduced | (148) |
| Furanodiene | 70
μg/ml | 300 μM | via mitochondrial
caspase apoptosis and ERK/MAPK signals | Inhibition of HepG2
cell growth and induction apoptosis | (149) |
| Hirsutanol A | 14.54 (24 h), 6.71
(48 h), 3.59 (72 h) μmol/l | - | via activated
ROS | Autophagic cell
death was induced in Hep3B hepatoma cells | (150, 151) |
| Elemene | 63±2.1
μg/ml | - | GSTP1 gene
methylation was reversed and cell cycle was inhibited | Induced apoptosis
of QGY7703 cells | (155) |
| β-elemene | - | 100
μg/ml | Downregulation of
c-Met expression | Inhibited the
growth of H22 hepatoma cells | (156) |
| Acorusin E | 2.11-7.99
μM | - | - | Apoptosis of
SMMC-7721 cells | (157) |
| Senedensiscin
G | 9.5-11.5
μM | - | - | Showed inhibitory
activity on SMMC-7721 cells | (159) |
| Sulfoscorzonin
E | 4.21±0.56
μg/ml | - | - | Moderate cytotoxic
activity on HepG2 cells | (161) |
| Artemongolins
E | 88.6 μM
(HepG2), 59.1 μM (Huh7), 67.5 μM (SK-Hep-1) | - | - | The inhibitory
effect on HepG2, Huh7 and SK-Hep-1 cells was significant | (162) |
| Artemyriantholide
E | 14.2 μM
(HepG2), 9.0 μM (Huh7), 8.8 μM (SK-Hep-1) | - | MAP2K2 may be a
core gene | Good inhibitory
activity against HepG2, Huh7 and SK-Hep-1 cells | (163) |
| Artemisacrolide
B | 21.9 μM
(HepG2), 8.2 μM (Huh7), 16.9 μM (SK-Hep-1) | 200
μg/ml | - | Marked anti-liver
cancer activity against HepG2, Huh7 and SK-Hep-1 cells | (164) |
| Lavandiolide I | 12.1 μM
(HepG2) 18.4 μM (Huh7), 17.6 μM (SK-Hep-1) | - | - | Marked anti-liver
cancer activity against HepG2, Huh7 and SK-Hep-1 cells | (165) |
Sesquiterpene lactones
Sesquiterpene lactones are the most studied
sesquiterpene compounds at present. The chemical structure of
sesquiterpene lactones is based on a skeleton of 15 carbon atoms
and consists of three cyclic isoprene structures, one of which is a
five-membered (γ-) lactone group (cycloester) (23). This unique structure makes
sesquiterpene lactones have a variety of biological activities.
Previous studies have shown that α-methylene-lactones of this kind
of compounds have anti-tumor activity, while α- and β-unsaturated
lactones have strong anti-inflammatory activity (24-26). Depending on the carboxy skeleton
and the type and position of the substituent, sesquiterpene
lactones can be divided into different subgroups, including the
germacranolides, guaianolides, pseudoguaianolides, eudesmanolides
and elemanolides. In recent years, it has been found that
sesquiterpene lactones with anti-tumor activity are mainly derived
from artemisinins, alantolides, descholinolides and
parthenolides.
Artemisinin and its derivatives
Sesquiterpenoids, particularly guaiacolactone,
artemisia, absinthium, sterolactone and barley fructosterol
lactone, are the main chemical components of Artemisia, and
some of them have shown a variety of significant biological
activities, including anti-tumor, anti-malaria, anti-inflammatory,
immunomodulatory, anti-ulcer, anti-parasite and anti-bacterial
(27).
The main component of the Artemisia and its
derivatives is artemisinin, dihydroartemisinin and artesunate are
derivatives of artemisinin, have strong anti-tumor activity. The
anticancer properties of artemisinin result from its unique
chemical structure, particularly its internal peroxide bridge
structure, which is critical for its biological activity. Its
mechanism of action mainly involves the generation of reactive
oxygen species (ROS) after interaction with intracellular iron
(28). Cancer cells, including
HCC, exhibit an abnormal iron metabolism, as indicated by elevated
transferrin receptor expression and iron accumulation. This
dependence makes them more vulnerable to oxidative damage caused by
ROS (29). Upon iron activation,
artemisinin produces cytotoxic free radicals that target cellular
macromolecules, leading to apoptosis and ferroptosis (30). Artemisinin has been shown in
studies of HCC to induce mitochondrial dysfunction, disrupting
cellular respiration and energy production. In addition, it can
regulate key signaling pathways, including the
phosphatidylinositol-3-hydroxykinase (PI3K)/protein kinase B
(AKT)/mammalian target of rapamycin (mTOR) axis, thereby inhibiting
cell proliferation and enhancing apoptosis (31). Another key mechanism involves the
regulation of angiogenesis. Artemisinin has been shown to inhibit
vascular endothelial growth factor (VEGF) signaling, which is
critical for tumor angiogenesis and metastasis (32). This is particularly important in
HCC, which relies heavily on neovascularization for growth and
spread. In addition, artemisinin enhances the effects of
conventional therapies, potentially reducing drug resistance by
making cancer cells more sensitive to chemotherapeutic agents and
radiotherapy (33). It is able to
reduce the production of inflammatory cytokines and reprogram
immune cells, such as the anti-tumor phenotype of macrophages, in a
certain direction. These immunomodulatory effects, coupled with its
direct cytotoxic effects, enhance its anticancer potential
(34). Artemisinin inhibited the
migration and invasion of HCC cell lines in a dose- and
time-dependent manner. The inhibitory effect of artemisinin on
invasion and metastasis of HCC cells is mediated by upregulation of
tissue inhibitors of metalloproteinase 2 (TIMP2) and downregulation
of matrix metalloproteinase 2 in vitro and in vivo
(35).
Dihydroartemisinin significantly inhibited the
growth of HCC cells in vitro and in vivo by inducing
G2/M cell cycle arrest and apoptosis in HCC cell lines. Induction
of p21 and inhibition of cyclin B and cell division cycle (CDC)25C
contributed to dihydroartemisinin-induced G2/M arrest.
Dihydroartemisinin-induced apoptosis was found to be associated
with mitochondrial membrane depolarization, cytochrome c release,
caspase activation and DNA fragmentation. In addition,
dihydroartemisinin was able to inhibit HCC growth in a xenograft
mouse model (36).
The mechanisms by which artesunate derivatives
inhibit tumor growth are not fully understood, among which the most
likely mechanism is that artesunate derivatives exhibit several
modes of action against HCC simultaneously in a variety of specific
ways. Whether artesunate drugs are used alone or synergistically in
combination, the underlying mechanisms are similar and may act in a
variety of specific ways, while exhibiting several modes of
anti-HCC action. Artesunate is a reduced artemisinin monoester
succinate that inhibits the activity of HepG2 and BWTG3 cells in a
dose- and time-dependent manner (37). It can induce apoptosis of HCC
cells by specifically inhibiting signal transducers and activators
of transcription (STAT3) (38).
Artesunate interfered with STAT3 dimerization in vitro,
inhibited constitutive and I-6-induced STAT3 and then regulated
STAT3-dependent procysteinyl aspartate-specific proteinase-3
(caspase-3), B-cell lymphoma/leukemia-2 (Bcl-2)-related X protein
(Bcl-xl) and survivin. Another study found that artesunate combined
with sorafenib induced apoptosis in HCC cells by inhibiting the
PI3K/AKT/mTOR pathway (39).
Lavandiolide H
Guaiacane-type sesquiterpene dimers, which contain a
large amount of sesquiterpene lactone, were isolated from
Artemisia and acted on three kinds of liver cancer cells
(40-42). The results showed that the
guaiacolide dimer sesquiterpene Lavandiolide H significantly
inhibited the proliferation of three different human hepatoma cell
lines. The sesquiterpene also induced G2/M-phase arrest and
apoptosis of HepG2 cells and downregulated the expression of the
Bcl-2 oncogene and poly(ADP-ribose) polymerase 1 (PARP-1), while
upregulating the expression of cleaved PARP-1 (43). The results of this study indicated
the potential of guaiactone dimer as a candidate natural small
molecule compound for the treatment of HCC.
Isoalantolactone
Isoalantolactone is derived from the dried roots of
elecampane, a plant of the genus Inula in Asteraceae, and
has wide-ranging therapeutic potential (44). As a promising candidate for cancer
research, as well as drug discovery and design, Isoalantolactone
exhibits potent anti-proliferative activity against HepG2 cells. By
promoting caspase-dependent apoptosis, inducing oxidative stress to
inhibit cell migration and cell invasion, and blocking Ras/Raf/MAPK
kinase signaling, it induces dose-dependent inhibition of
HepG2-cell proliferation (45).
Dehydrocostus lactone
Dehydrocostus lactone is a sesquiterpene lactone
with a high content in the essential oil of Inula racemosa
and the main bioactive component of Inula racemosa.
Dehydrocostus lactone inhibits HepG2 human hepatoblastoma cells by
downregulating the PI3K/AKT signaling pathway (46).
Costunolide
Costunolide is one of the main chemical components
and quality control components of Costustoot in traditional
Chinese medicine. Epidermal growth factor receptor (EGFR)
amplification and abnormal activity are closely related to the
occurrence and development of a variety of malignant tumors,
including liver cancer. Therefore, key molecules in the EGFR
signaling pathway are considered important oncogenic factors and
key therapeutic targets. Costunolide can increase the
ubiquitination of EGFR and reduce the distribution of EGFR
recycling to the cell membrane, thereby inhibiting EGF signaling
(47). In vitro studies
showed that costunolide could inhibit the proliferation and promote
apoptosis of HepG2 cells, and cause cell cycle arrest in G2/M phase
in a dose-dependent manner, and thus significantly induce apoptosis
of HepG2 cells. The mechanism is that costunolide may promote cell
apoptosis by upregulating the expression levels of Bcl-2-associated
X-protein (Bax), caspase-3, caspase-8 and caspase-9 and
downregulating the expression of Bcl-2 protein (48).
Britannin
Phytosterols, flavonoids, sesquiterpene lactones and
essential oils are the main chemical constituents of Inula
L. Britannin, a compound isolated from Inula L., which
can induce apoptosis and autophagy by activating ROS-regulated
adenosine monophosphate-activated protein kinase (AMPK) in liver
cancer cells. It provides a molecular basis for the development of
Britannin as an effective anti-liver cancer drug candidate
(49).
Bigelovin
Bigelovin, a sesquiterpene lactone isolated from
Inula, has been shown to have apoptosis-inducing,
anti-inflammatory and anti-angiogenic activities. Bigelovin was
found to have potential antitumor activity against human HCC in
vitro and in vivo. Bigelovin inhibited cell
proliferation and colony formation. Bigelovin induces apoptosis by
promoting the cleavage of caspase-3 and PARP-1. This process is
also accompanied by the activation of autophagy, which is
manifested as the increase of autophagosomes and the decrease of
light chain I protein type 3-II, Beclin-1 and ubiquitin-binding
protein p62 (50).
Narjatamolide
Narjatamolide, isolated from rhizoma nardostachyos,
exhibits an anti-proliferation effect on BEL-7402 cells in a
dose-dependent manner, and the results of cell cycle analysis show
that this compound can induce cell cycle arrest in G2/M phase
(51).
Parthenolide
Parthenolide is a gemmarane type sesquiterpene
lactone natural product extracted from Leucanthemella, which
was first isolated from the traditional herbal medicine white daisy
in 1965 (52). By inducing the
generation of ROS in HepG2 cells, blocking its cell cycle and
causing apoptosis and autophagy, it exerts an anti-tumor effect
(53).
Micheliolide
Micheliolide is a sesquiterpene lactone natural
product isolated from Michelia L. and Michelia x alba
(54), which is a guaiacane
sesquiterpene in structure. Compared with parthenolide, aconitolide
has at least three advantages: High stability, low toxicity and
continuous release of active drug (55). Micheliolide as a thioredoxin
reductase (TrxR) inhibitor with high potential to induce
immunogenic cell death (ICD). The magnitude of ICD-related effects
induced by micheliolide exposure in HCC cells was found to depend
on the generation of ROS-mediated endoplasmic reticulum (ER) stress
(ERS). Furthermore, ROS inhibition normalizes micheliolide-induced
ERS, whereas TrxR downregulation acts synergistically with
micheliolide-driven ERS, and micheliolide inhibits the development
of hepatoma organoids (56).
Dimethylaminomicheliolide
Dimethylaminomicheliolide is a pro-drug of
micheliolide, and in normal cells, the former has higher stability,
higher activity and lower toxicity than micheliolide. In addition
to their anti-tumor effects, micheliolide and
dimethylaminomicheliolide also have protective effects against
inflammation, hepatic steatosis, diabetic nephropathy and
rheumatoid arthritis (57-60).
Dimethylaminomicheliolide has minimal side effects in animals and
is a safe and promising drug for long-term in vivo treatment
(61). Dimethylaminomicheliolide
reduced the viability of HCC cells in a dose- and time-dependent
manner, and caused cell cycle arrest in G2/M phase and inhibited
cell invasion and epithelial-mesenchymal transition (EMT). It also
induces cell death through the intrinsic apoptotic pathway of HCC
cells, which can be blocked by the caspase inhibitor zVAD-fmk,
Bax/Bcl-2 antagonist/killer 1 (Bak) silencing or Bcl-2
overexpression. Dimethylaminomicheliolide inactivates the PI3K/AKT
pathway, leading to ROS production, thereby regulating
dimethylaminomicheliolide-induced apoptosis (62).
Deoxyelephantopin
Elephantopus scaber's chemical composition is
complex and its main components include sesquiterpene lactones,
triterpenes, flavonoids, steroids and anthrones. Modern
pharmacological studies have shown that it has the effects of liver
protection, as well as antibacterial, anti-tumor and
anti-inflammatory properties, and may be used in the treatment of
diabetes (63-65). Deoxyelephantopin is the main
component of Elephantopus scaber (66), which has a variety of biological
activities, including antibacterial, anti-diabetic,
anti-inflammatory, wound healing, liver protection and anti-cancer
activity (67,68). Deoxyelephantopin inhibits the
proliferation and induces apoptosis of HepG2 cells in a
dose-dependent manner, possibly related to the generation of ROS,
glutathione (GSH) depletion and reduced TrxR activity, disrupting
the mitochondrial membrane potential (MMP) and enhancing DNA
fragmentation. Further studies have shown that deoxyelephantopin
can reduce the phosphorylation of inhibitor of nuclear factor κB
(NF-κB)α (IκB-α) to inhibit the translocation of constitutive and
inducible NF-κB to the nucleus, and exert its anti-cancer effect
through oxidative stress. Deoxyelephantopin can be used as a
potential drug for effective treatment of liver cancer (69).
Scabertopinolide G
A total of seven new compounds, Scabertopinolide
A-G, were isolated from Elephantopus scaber, among which
Scabertopinolide G showed the strongest inhibitory effect on the
proliferation of three human tumor cell lines, HepG2, Hep3B and
MCF-7 (70). Scabertopinolide G
induces autophagy in Hep3B and HepG2 cells by increasing ROS
production and reducing the MMP. In addition, signaling pathways
including MAPK and AKT may play an important role in the induced
death of liver cancer cells (71).
Santamarine
Santamarine is one of the effective components of
Aplotaxis auriculata. The potential anticancer activity of
santamarine was achieved by inhibiting cell proliferation and
inducing cell apoptosis. Santamarine inhibited TNF-α-induced
translocation of NF-κB to the nucleus by reducing the
phosphorylation of IκB-α. In addition, santamarine inhibited STAT3
activation by reducing the phosphorylation of tyrosine 705.
Pretreatment with acetylcysteine reversed the effects of
santamarine-mediated cell death, NF-κB inhibition and STAT3
activity blockade, suggesting that oxidative stress is involved in
santamarine-mediated anticancer activity. The exact molecular
mechanism of santamarine-induced apoptosis needs to be further
studied to make it a lead drug for the treatment of liver cancer in
the future (72).
Hemistepsin A
Hemistepta lyrata is a plant of the
Asteraceae family, a wild Korean biennial herb that is
traditionally used to treat wounds, fever, bleeding and ulcers.
Hemistepsin A, one of the compounds extracted from Hemistepta
lyrata, is a potential candidate for the prevention of
hepatitis, steatosis and fibrosis (73-76). Hemistepsin A slows down the cell
cycle progression of HCC cells and induces cell senescence by
activating AMP-activated AMPK (77). Hemistepsin A induces apoptosis of
HCC cells by downregulating STAT3 and sensitizing them to
conventional chemotherapy drugs (78).
Xanthatin
Xanthatin is a bicyclic sesquiterpene lactone
isolated mainly from Xanthium (79). Studies have shown that xanthatin
has significant antitumor activity in a variety of cell lines of
colon, breast, lung, cervical and skin cancers (80,81). Xanthatin can trigger an ER stress
response in HCC cells and the pro-apoptotic effect is related to ER
stress. By increasing activating transcription factor 4 (ATF4) in
the nucleus, xanthatin promotes the PERK-eIF2α-ATF4 signaling
pathway and its downstream target of C/EBP homology
protein-mediated ER stress (82,83).
Telekin
Telekin is extracted from the traditional Chinese
herb Carpesium divaricatum. It was reported to inhibit HepG2
cell viability and induce apoptosis in a dose-dependent manner. In
addition, Telekin treatment induced cell cycle arrest at the G2/M
phase, with a significant increase in CDC25A and CDC2
phosphorylation and a decrease in cyclin B1 levels. Telekin induces
G2/M phase arrest in hepatoma cells by activating the p38 and
mitogen-activated protein kinase activated protein kinase 2
(MAPKAPK2) pathways, and has an inhibitory effect on hepatoma cells
(84).
Artemiprincepsolides A
Artemiprincepsolides A-F were isolated from
Artemisia princeps, and compounds 1-6 were evaluated for
their hepatotoxicity against three hepatoma cell lines. Among them,
Artemiprincepsolide A showed significant cytotoxicity against
HepG2, Huh7 and SK-Hep-1 cells, which was almost comparable to the
positive control sorafenib, inhibited the migration and invasion of
HepG2 cells in a dose-dependent manner by downregulating
phospho-CDC2 and upregulating cyclin B1 protein levels, and
significantly induced G2/M-phase arrest in HepG2 cells. Apoptosis
was induced by downregulating the expression of Bc1-2 and
upregulating the level of Bax (85).
Carpespene A
The main active components of Carpesium mainly
include sesquiterpene lactones and monoterpenoids (86,87), among which sesquiterpene lactones
show strong cytotoxic activity (88). Its antitumor activity is reflected
in its potent cytotoxic effect on a variety of tumor cell lines and
induction of apoptosis in vitro. Among them, the α- and
β-unsaturated lactone ring is the key active center (89). A total of 10 previously
undescribed sesquiterpenes, carpespenes A-J (nos. 1-10), and eight
known compounds (nos. 11-18), were isolated from the whole strain
of Carpesium faberi, Guizhou. Carpespene A is an
eudesmanolide-type sesquiterpene lactone containing an open
five-membered ring of C-2 and C-3. Mechanistically, Carpespene A
induced apoptosis in HepG2 cells by triggering excessive ROS
accumulation, and ROS-induced cytoprotective autophagy attenuated
the cytotoxicity of Carpespene A. Carpespene A also inhibited the
anti-phagocytosis and enhanced the cytotoxic effect of ROS on HepG2
cells (90).
Dimethylaminomicheliolide
Dimethylaminomicheliolide is a pro-drug of
micheliolide, in normal cells show than micheliolide higher
stability.
The active center of sesquiterpene alcohols has a
structure of 15 carbon atoms. The six most common sesquiterpene
alcohols are farnesol, nerolidol, petrolatol, patchoulic alcohol,
santalol and eucalyptol.
Linalool
Coriandrum sativum is used as an appetiser
for its flavoured leaves and seeds and is also considered a home
remedy in herbal and folk medicine. In addition to its ability to
reduce fertility, hyperglycemia, hyperlipidemia and oxidative
stress, cilanthus has antibacterial, anxiolytic and sedative
effects (91,92). Linalool is the main component of
coriandrum sativum and one of the chemicals commonly used in the
cosmetics and perfume industries. Safety evaluation studies have
shown that linalool is not irritating, phototoxic or allergenic,
but has low-grade acute toxicity (93). Linalool reduced HepG2 viability by
inhibiting mitochondrial complex I and II activity, increasing ROS
and reducing ATP and GSH levels (94).
Xanthorrhizol
Derived from Turmeric, Xanthorrhizol exhibits
anti-cancer activity against various types of cancer, liver, lung,
breast, cervical and colon cancer, by alleviating angiogenesis and
metastasis, activating apoptosis and inducing cell-cycle arrest
(95). Xanthorrhizol promoted the
proteolytic cleavage of PARP and inhibitor of caspase-activated
deoxyribonuclease (ICAD), and concomitantly, the expression of
anti-apoptotic Bcl-2 and Bcl-xl was also decreased in HepG2 cells,
The above results indicate that xanthorrhizol is an effective
anti-liver cancer drug, and its mechanism of action is to induce
apoptosis (96).
Cis-Nerolidol
Nerolidol, a compound found in numerous plant
species, has received considerable attention in various fields of
research due to its anti-inflammatory, anti-leishmaniasis and
anti-fungal activities (97,98). Nerolidol is a sesquiterpene and
exists in the form of two isomers, cis-nerolidol (C-NER) and
trans-nerolidol (99). Only C-NER
showed medium cell toxic activity (HepG2/C3A); C-NER did not show
genotoxic activity but altered MMP, reduced cell proliferation and
induced cell death by arresting the cell cycle at G1 phase
(100).
α-Bisabolol
Chamomile is a commonly used medicinal herb in
Europe and has historically been used in cosmetics and health foods
(101). Its main components are
blue balsamol and α-bisabolol. α-Bisabolol is a low-toxicity
sesquiterpene that can be used in the pharmaceutical, food and
hygiene industries, particularly for topical applications. Using
human hepatoma HepG2 cells as a model, higher concentrations of
cleaved caspase-3, -8 and -9 were found in α-bisabolol-treated
cells compared with untreated cells. In addition, α-bisabolol
reduced mitochondrial cytochrome c levels, increased the cytosolic
cytochrome c content and upregulated the downregulation of
pro-apoptotic proteins Bax and BH3-interacting domain death agonist
and the anti-apoptotic Bak and Bcl-2 proteins. After α-bisabolol
treatment, the expression of p53, NF-κB and Fas was increased,
suggesting that they play a role in mediating α-bisabolol-induced
apoptosis in cancer cells (102).
Sesquiterpene ketones
Ketones feature a carbonyl group (C=O) connected to
2 functional groups (R and R'). Sesquiterpene ketones generally
contain 15 carbon atoms, and structures with a number between 10
and 15 carbon atoms are also included in this group because of
their similar characteristics. Previous studies have shown that
sesquiterpenes containing α- and β-unsaturated ketones exhibit
significant anti-tumor activities (103).
Germacrones
Germacarones are found in plants from different
families, such as Zingeraceae, Geraniaceae and
Ericaceae. Currently, germacrone is mainly obtained from
Curcuma zedoary, and it can be used as a quality marker
component of Curcuma zedoary. Previous studies have shown
that germacarone has a significant inhibitory effect on HepG2 and
BeL-7402 cells and can induce G2/M-phase arrest of the cell cycle,
which is related to the significant reduction of cyclin B1 and
cyclin-dependent kinase 1 protein expression and the induction of
p21. Dose-related upregulation of Bax and downregulation of
Bcl-2/Bcl-xl resulted in an increase in the total number of
associated apoptotic cells, as well as upregulation of tumor
suppressor gene p53 and increased ROS (104). Germacarone can also reduce the
expression of STAT3 and Janus kinase 2 (JAK2), and induce apoptosis
of HepG2 cells through the JAK2/STAT3 signaling pathway (105).
Atractylon
Atractylon extracted from Atractylodes Lancea
(Thunb.) DC. and Atractylodes macrocephala significantly
inhibited proliferation and promoted apoptosis of hepatoblastoma
cell lines (106-108). In addition, the results showed
that atractylon decreased the MMP, increased ROS levels and
inhibited Bcl-2 expression. The activation of Bax and cleaved
caspase-3 indicated that atractylon induced apoptosis of HCC cells
through the mitochondrial apoptosis pathway. The results also
showed that atractylon inhibited the migration and invasion of HCC
cells by inhibiting the EMT process and downregulating the
expression of matrix metalloproteinases-2 and -9, and also
inhibited the growth of HCC cells. Furthermore, it has an
inhibitory effect on the EMT process in vivo (109). After atractylon treatment, the
proliferation ability of hepatoblastoma cells decreased, and the
apoptosis rate increased. The invasion and migration abilities of
HepG2 cells were significantly decreased. In addition, atractylon
was observed to regulate the expression of thymopoietin-antisense
RNA 1 and CCDC18 antisense RNA 1 and inhibit the invasion and
migration of liver cancer cells in vitro (110).
Ar-turmerone
Ar-turmerone is one of the active components of
Curcuma longa, and ar-turmerone induces apoptosis in HepG2
cells through ROS-mediated activation of ERK and c-Jun N-terminal
kinase (JNK) and triggering endogenous and exogenous caspase
activation, leading to apoptosis (111).
Other types of sesquiterpenes
Thapsigargin
Guaiane-type sesquiterpenoids are an important class
of natural products in nature, and hundreds of guaiane-type
sesquiterpenoids have been isolated and identified (112). Guaiane-type sesquiterpenoids
belong to the bicyclic sesquiterpenes. The basic mother nucleus is
formed by three isoprene units, and generally has a
4,10-dimethyl-7-isopropyl group substitution. Due to the different
position of side chains, there are also other types of
sesquiterpenes, including pseudoguaiane, patchoulane, carotane,
lactarane and daucane (113).
Thapsigargin, a guaiacan-type sesquicolide isolated from Thapsia
garganica, is an irreversible ER calcium ATPase inhibitor
(114), which inhibits
sarcoplasmic reticulum calcium pumps (SERCA-ATPase) in human HCC
cells. Depleting the intracellular Ca2+ pool induces
apoptosis. The X-ray structure of the thapsigargin-SERCA complex
provides a basis for understanding the structural conformation of
the complex, as well as the surrounding environment of the binding
site. It also provides detailed information for the design of
targeted prodrugs with thapsigargin as the active ingredient
(115). Thapsagargin (G202) has
completed phase II clinical trials for the treatment of
glioblastoma multiforme and HCC, and is expected to enter the
market in the near future (116,117).
(2S,7R,10S)-3-hydroxypseudotigerone-11-O-β-D-glucopyranoside
2S,7R,10S)-3-hydroxypseudotigerone-11-O-β-D-glucopyranoside is an
eucalanes type sesquiterpene. It's one of the main chemical
constituents of A. lancca (Thunb.) DC (118-120), which had obvious protective
effects on HepG2 cells induced by N-acetyl-p-aminophenol at a
concentration of 10 μM. The cell survival rate was 34.6%,
which is higher than that with bicyclol-positive drugs, indicating
that it exhibits a strong hepatoprotective effect (121).
Hinesol
Hinesol is a vanilloid type sesquiterpene, which has
been extracted from Atractylis lancea, Atractylodes
chinensis, Atractylodes japonica and Atractylodes
rhizome (106,122,123); it was observed to inhibit cell
proliferation and induce cell apoptosis by arresting the cell cycle
in G1 phase of SMMC-7721 and LM3 cells. The mechanism is related to
the inhibition of the phosphorylation of MAPK and ERK, and the
downregulation of NF-κB p65 and phosphorylated p65 in the nucleus
(124).
Curcumenol
Curcuma zedoary, traditionally due to its
wide range of plant components, has been reported to have numerous
biological activities and is used for many therapeutic effects
(125). Curcumenol, an oxidized
guaiacan-type sesquiterpene, has obvious anti-tumor, liver cancer
inhibition, liver protection and anti-inflammatory effects
(126). Aberrant expression of
microRNAs (miRs) in HCC can regulate the occurrence and development
of HCC (127-130). Previous studies have shown that
curcumenol can inhibit the expression of oncogene miR-2l to exert
anti-liver cancer effects (131-134). It can inhibit cell proliferation
and lead to a significant increase in the apoptotic ratio by
regulating the DJ-1 (Parkinsonism-associated deglycase gene
encoding a member of the peptidase C56 family of proteins),
phosphatase and tensin homolog and PI3K/AKT signal transduction
pathways. The drug effect of curcumenol depends on the action time
and dosage (135).
Zerumbone
As a representative of humulane sesquiterpenes,
zerumbone shows good anti-tumor activity, which is related to the
induction of cancer cell apoptosis and anti-proliferative effects
(136). It has been shown to
inhibit HCC cell proliferation by inducing apoptosis and thus G2/M
cell-cycle arrest through inhibition of the PI3K/AKT/mTOR and STAT3
signaling pathways (137), and
also by significantly reducing the expression of matrix
metalloproteinase-9, VEGF and VEGF receptor proteins in a
dose-dependent manner. Inhibition of the proliferation and
migration of the HepG2 cell line has been observed (138). Gingerone can significantly
increase the apoptosis of HepG2 cells in a certain period of time,
and this apoptosis is achieved by regulating the ratio of Bax/Bcl-2
(139-141).
Artemeriopodin G7
Artemisia is one of the largest genera in the
Asteraceae family, usually represented by 500 species of
small herbs and shrubs (142,143). It is widely distributed in the
Northern temperate zone of Asia, Europe and North America (144). Numerous artemisia species (e.g.,
Artemisia annua, Artemisia atrovirens, Capillary
artemisia, Artemisia mongolica and Artemisia
nilagirica) have been used as traditional medicines to treat a
variety of diseases (145), such
as malaria, dysmenorrhea, amenorrhea, hepatitis, inflammation,
bruising, jaundice, bleeding and cancer (27,146). Artemeriopodin G7, extracted from
Artemisia australis, can inhibit the migration and invasion
of HepG2 cells and induce apoptosis. G2/M cell-cycle arrest was
induced by downregulation of CDC2 and p-CDC2 levels. In addition,
artemeriopodin G7 targeted platelet-derived growth factor receptor
A and affected the AKT/STAT signaling pathway (147).
Cryptomeridiol
Cryptomeridiol, a naturally occurring sesquiterpene
derivative isolated from traditional Chinese medicine plant
Magnolia officinalis, was effective against HCC by
exacerbating the pre-activated unfolded protein response and
activating the silent nerve growth factor-induced gene B NGFI-B.
Mechanistically, Nur77 is induced to sense the inositol-requiring
enzyme 1 α/apoptosis signal-regulating kinase 1/JNK signal and
transduce to mitochondria, resulting in loss of MMPmitochondrial
membrane potential. Cryptomeridiol-induced heightened ER stress and
mitochondrial dysfunction resulted in increased cytotoxic products
of ROS. The in vivo anti-HCC activity of cryptomeridiol was
superior to that of sorafenib, which is currently used to treat
advanced HCC. Identification of Nur77 as a molecular target of
cryptomeridiol provides a basis for further development of improved
anti-HCC drugs (148).
Furanodiene
Furanodiene is a pure compound isolated from
Curcuma zedoary. Furanodiene inhibits HepG2 cell growth by
causing cell-cycle arrest at G2/M and inducing apoptosis. The
furanodiene-mediated mitochondrial caspase apoptosis pathway also
involves the activation of p38 and the inhibition of ERK and MAPK
signaling (149).
Hirsutanol A
Hirsutanol A is a novel sesquiterpenoid isolated
from the endophyte of Sarcophyton tortuosum, a soft coral in
the South China sea. The autophagic death of hepatoma carcinoma
cells is induced by the activation of ROS (150,151).
Elemene
Elemene is also extracted from Curcuma
zedoary as a non-cytotoxic antitumor agent with few side
effects, but may inhibit tumor cell proliferation, induce apoptosis
and differentiation, eliminate tumor cells, reverse multidrug
resistance and inhibit tumor metastasis, particularly in HCC
(152-154). Elemene induced apoptosis,
inhibited the cell cycle and reversed GSH S-transferase P1
methylation in QGY7703 cells (155).
β-elemene
β-elemene has been shown to have anti-cancer
effects on a variety of tumor diseases, including liver cancer, by
inhibiting tumor-cell growth or promoting apoptotic cell death.
c-Met is a tyrosine kinase receptor, which is widely found to be
overexpressed in tumor tissues and involved in cell proliferation,
migration, invasion and survival. Downregulation of c-Met
expression by β-elemene induced growth inhibition in mouse hepatoma
cells (156).
Sesquiterpenoids with an unspecified
mechanism of action but an anti-liver cancer effect
One new guaiane-type sesquiterpenoid-Acorusin E,
has been isolated from the rhizomes of Acorus tatarinowii.
In vitro cytotoxicity was evaluated in liver cancer cells
(SMMC-7721 and HepG2 cells) and showed moderate cytotoxicity with
IC50 values of 2.11-7.99 μmol (157).
Senecio is an important genus in Asteraceae,
with >200 species in China (158), most of which are widely used in
folk medicine due to their potent biological activity.
Senedensiscin G, which was isolated from Senedensiscin, showed a
broad spectrum of inhibitory activity against SMMC-7721 cells
(159).
The roots of Scorzonera divaricata have
antipyretic and detoxicant activities and are used in traditional
medicine to treat toxic ulcers and malignant gastric tumors
(160). Sulfoscorzonin E
isolated from the aboveground part of Scorzonera divaricata
exhibited moderate cytotoxic activity against hepatoma HepG2 cells
and possessed strong
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) and
2,2-diphenyl-1-picrylhydrazyl free radical scavenging abilities
(161).
Artemongolins A-K (nos. 1-11), undescribed
sesquiterpenoid dimers isolated from Artemisia mongolica,
comprise a rare 5/7/5/5/10 hexocyclic system; their structures were
unambiguously elucidated through comprehensive spectroscopic
analyses, encompassing high-resolution electrospray ionization mass
spectrometry for precise molecular formula determination, infrared
spectroscopy to identify functional groups, one-dimensional nuclear
magnetic resonance (proton nuclear magnetic resonance, carbon-13
nuclear magnetic resonance, distortionless enhancement by
polarization transfer) and two-dimensional nuclear magnetic
resonance (correlation spectroscopy, heteronuclear single quantum
coherence, heteronuclear multiple-bond correlation, rotating-frame
Overhauser effect spectroscopy) experiments for detailed structural
connectivity and stereochemical assignment, and electron capture
detector calculations using the time-dependent density functional
theory to establish absolute configurations. Evaluation of the
anti-HCC activity of three human HCC cell lines showed that
Artemongolin E had the highest activity (162).
To date, 14 known compounds (nos. 12-25) and
Artemyriantholides A-K (nos. 1-11) have been isolated from
Artemisia lactiflora. All compounds were chemically
characterized as bluish sesquiterpene dimers. The anti-liver cancer
assay showed that the 11 compounds had obvious inhibitory effects
on HepG2 and Huh7 cells with IC50 values of 7.9-67.1
μM. Artemyriantholides E and H showed good inhibitory
activity on HCC. A computational prediction model suggested that
the target of Artemyriantholides is mitogen-activated protein
kinase kinase 2 (MAP2K2) (163).
At the concentration of 200 μg/ml, the
inhibitory effect of ethanol extract of artemisia sacrorum on HepG2
cells was 65.5%, and the inhibitory effect on Huh7 cells was 28.1%,
the inhibitory effect of ethyl acetate extract on HepG2 cells was
93.5%, and the inhibitory effect on Huh7 cells was 82.0%.
Artemisacrolide B was prominent in three HCC cell lines, with
IC50 of 21.9 μM (HepG2), 9.0 μM (Huh7) and
16.9 μM (SK-Hep-1), respectively (164).
Guaiacolide dimers are a unique class of natural
products with anticancer activity, but their low content in plants
limits in-depth pharmacological studies. Lavandiolide I, a
guaiacolide dimer isolated from Artemisia, showed potent
anti-HCC activity against the HepG2, Huh7 and SK-Hep-1 cell lines.
To explore more active oligomers, 33 derivatives of Lavandiolide I
were designed, synthesized and evaluated for their inhibitory
activity against human hepatoma cells. Of these derivatives, 10
were more active than Lavandolide I and Sorafenib against all three
cell lines. Among them, derivatives 25, 27 and 33 have 1.2-5.8
times higher anti-liver cancer activity than Lavandolide I, and
have lower toxicity and better safety against human liver cells
(THLE-2), with selection indices between 1.3 and 3.4, while
Lavandolide I is more toxic to THLE-2 cells (165).
Mechanism of action of compounds
The first mechanism is through the regulation of
the MAPK pathway, which includes stress-activated protein
kinase/JNK, ERK and MAPK14 (p38-MAPK) (166). In HCC tissues, activated ERK in
turn induces the expression of multiple genes, activates NF-κB and
phosphorylates cellular transcription factor-1, promotes cell
transition from G1 phase to S phase (167,168), and thus promotes the
proliferation of HCC cells. Activated ERK can also upregulate the
expression of genes including hypoxia-inducible factor-1α and
VEGF-α, and promote the glycolysis process and angiogenesis of HCC.
The use of ERK inhibitors can reduce the expression of ERK,
resulting in slow cell proliferation and cell apoptosis (169,170). For instance, the
furanodiene-mediated mitochondrial caspase apoptosis pathway also
involves the activation of p38 and the inhibition of ERK and MAPK
signaling.
The second regulatory pathway is inhibition of the
PI3K/AKT/mTOR pathway. PI3K can promote the production of
phosphatidylinositol-3,4,5-triphosphate, thereby promoting the
phosphorylation of AKT and further activating the serine/threonine
protein kinase mTOR, promoting protein synthesis and the growth and
invasion of liver cancer cells (170,172). For instance,
dimethylaminomicheliolide inactivates the PI3K/AKT pathway, leading
to ROS production, thereby regulating
dimethylaminomicheliolide-induced apoptosis.
As one of the important transcriptional regulators,
the third regulatory pathway NF-κB can exert biological effects by
regulating the transcriptional expression of a variety of genes.
NF-κB has a key role in inflammatory response and tumor
development, and plays an important regulatory role in the
progression of liver injury, liver fibrosis and HCC (173). In neutrophil-driven HCC, the
NF-κB gene can exert negative regulatory effects on the chemokine
network in neutrophils. However, NF-κB gene knockout can promote
the occurrence of liver cancer (174). For instance, deoxyelephantopin
can reduce the phosphorylation of IκB-α to inhibit the
translocation of constitutive and inducible NF-κB to the nucleus,
and exert its anti-cancer effect through oxidative stress.
The fourth regulatory pathway is the JAK2/STAT3
pathway. The activation of the JAK2/STAT3 pathway is a common
mechanism leading to the occurrence of liver cancer, and
upregulation of STAT3 is often found in HCC (113). The expression of STAT3, as a
driver, plays a key role in the occurrence, progression, metastasis
and immunosuppression of HCC, and is associated with poor prognosis
(175). For instance,
germacarone can also induce apoptosis of HepG2 cells by reducing
the expression of STAT3 and JAK2 signaling pathway components.
The last regulatory pathway is induction of
mitochondrial dysfunction, the key role of mitochondria in ROS
production and apoptotic signaling in tumor cells. Structural and
functional differences between mitochondria in normal cells and
cancer cells, result in cancer cells are more susceptible to
oxidative stress compared to normal cells (176). For instance, atractylon showed
that decreased the MMP, increased ROS levels and inhibited Bcl-2
expression. The activation of Bax and cleaved caspase-3 indicated
that atractylon induced apoptosis of HCC cells through the
mitochondrial apoptosis pathway.
The α- and β-unsaturated carbonyl and α-methylene
lactone groups and the conjugated aldehyde groups in sesquiterpene
lactones are considered to be reactive partial structures (25,177). Both its cytotoxic and
anti-inflammatory properties are partially mediated by
α,β-unsaturated carbonyl functions, such as cyclopentenone and
α-methylene γ-lactone (178).
For instance, when cancer cells were treated with parthenolide, the
ROS in tumor cells were increased due to a decrease in the cysteine
group of the antioxidant non-protein molecule GSH.
Applications of sesquiterpenes
Given the high cancer-related mortality and the
severe side effects of radiotherapy and chemotherapy treatments,
researchers and scientists have made great efforts to extract new
natural products from plants, microorganisms and other organisms to
evaluate their anticancer activity and explore their mechanisms of
action, as they are considered to have fewer toxic side effects
compared to traditional therapies such as chemotherapy. These
efforts have led to the development of anticancer drugs and the
introduction of natural products into clinical applications. In
1994, elemene oral emulsion and elemene injection were approved by
the China Food and Drug Administration. The drug has been on the
market and has become a national second-class anticancer drug with
Chinese intellectual property rights. It is used to treat various
diseases and bone metastasis. It features affinity for and
targeting of tumor cells, as well as sustained release, stability
and safety (179). Liposomal
elemene is a non-cytotoxic antineoplastic agent with a high content
of anticancer active ingredients (85% β-elemene) and is also called
a 'green therapy' for cancer treatment. In the clinic, elemene
liposome can be used alone or in combination with
chemoradiotherapy, or before and after surgery (180). After 20 years of clinical
research, elemene liposomes can inhibit a variety of cancer cells
through multiple targets and can improve immune function. In
particular, it has obvious advantages in improving the quality of
life of patients, prolonging survival time, resisting metastasis
and recurrence, and reversing multi-drug resistance. At present,
rimantagliene injection and oral emulsion have entered the
'National Medical Insurance Drug List' and are used in >3,000
hospitals in China, reaching >700,000 cancer patients, including
patients in Southeast Asia, Hong Kong, Japan, South Korea, Europe
and the United States (181).
However, it can cause serious adverse reactions, such as phlebitis
after intravenous injection. To date, various new delivery systems,
including solid lipid nanoparticles (NPs) (SLN), nanostructured
lipid carriers, long-circulating liposomes, active-targeting SLN,
drug-loaded liposomes and microemulsions, self-emulsified drug
delivery systems and active-targeting microemulsions, have been
developed. Already being actively developed, these systems have
contributed to numerous advances in elemene (182).
The development of drug resistance and dose-related
toxicity of natural products are the main disadvantages of
chemotherapeutic drugs in cancer treatment. Therefore, combination
therapy has become a feasible way to improve the efficacy and
reduce systemic toxicity of natural products. Although artemisinin
is the first choice for anti-malarial drugs, its derivatives have
shown remarkable efficacy in the treatment of laryngeal cancer,
uveal melanoma and pituitary macroadenoma, and are in phase I to II
clinical trials for the treatment of lupus nephritis and diseases
such as breast cancer, colon cancer and non-small cell lung cancer.
In the treatment of HCC, artesunate, a derivative of artemisinin,
can be used to sensitize sorafenib and play a synergistic
anti-tumor effect. Because artesunate is well tolerated and
affordable, the combination of artesunate and sorafenib can benefit
most patients with HCC. In addition, this combination therapy could
reduce the potential toxicity of sorafenib by reducing its
effective dose (183,184).
Numerous synthetic and herbal medicines have poor
oral bioavailability due to their low water solubility or inability
to cross biofilms, resulting in poor dissolution in biological
fluids and poor therapeutic efficacy. A growing number of reports
have highlighted the promise of phosphatidyl-based formulations as
effective drug delivery systems for natural bioactive ingredients.
Lipid NPs loaded with artemisinin provide a promising approach for
HCC treatment, harnessing the respective advantages of artemisinin
and lipid NP-based delivery systems (185). Encapsulation of artemisinin in
lipid NPs significantly reduced its systemic toxicity. By
facilitating targeted drug delivery specifically to the tumor site,
artemisinin's cytotoxic effects on healthy tissues can be
minimized. This targeted approach reduces the common side effects
of traditional chemotherapy, such as gastrointestinal discomfort,
liver toxicity and bone marrow suppression. In addition, the use of
biocompatible and biodegradable lipids in the formulation of NPs
further contributes to the reduction of toxicity. Another study
aimed to investigate the liver targeting and anti-HCC effects of
artesunate (ART)-loaded, glycyrrhetinic acid (GA)-modified
polyethylene glycol (PEG)-poly(lactic-acetic acid) (PLGA)
(ART/GA-PEG-PLGA) NPs. ART/GA-PEG-PLGA NPs have pro-apoptotic
effects on HepG2 cells, which are mainly achieved by inducing high
levels of ROS, reducing MMP and inducing cell cycle arrest.
Furthermore, ART/GA-PEG-PLGA NPs induced the endogenous apoptotic
pathway in HepG2 cells by upregulating the activity of cleaved
caspase-3/7 and the levels of cleaved PARP and phosphorylated
p38-MAPK. In addition, ART/GA-PEG-PLGA NPs accumulated more in the
liver and had a longer mean retention time, resulting in improved
bioavailability. Finally, ART/GA-PEG-PLGA NPs facilitated the
targeted distribution of ART in the liver, prolonged the retention
time of ART and enhanced its anti-tumor effect in vivo
(183).
Discussion
So far, a variety of sesquiterpenes have been found
in numerous traditional Chinese medicine and plants, such as the
Asteraceae, Gentianaceae, Zingiberaceae,
Umbelliferaceae, Olivaceae, Magnoliaceae and
Valeriaceae. Previous studies have shown that sesquiterpenes
exhibit potential therapeutic effects on anti-tumor,
anti-inflammatory, anti-bacterial and anti-cardiovascular diseases,
and more new components and physiological activities need to be
explored. In the present article, several types of sesquiterpenes
were reviewed, revealing that the biological activity of
sesquiterpene alcohols was not as good as that of sesquiterpene
ketones and sesquiterpene lactones. In the future, compounds with
the structure of sesquiterpene alcohols can be modified to
strengthen the structure-activity relationship.
A variety of natural substances can inhibit liver
cancer by causing growth arrest in cancer cells, inducing apoptosis
and inhibiting metastasis. Its regulatory mechanisms mainly include
the MAPK signaling pathway, PI3K/Akt/mTOR signaling pathway, NF-κB
signaling pathway, JAK2/STAT3 signaling pathway and mitochondrial
pathway. For instance, Hinesol has a certain inhibitory effect on
the MAPK and NF-κB signaling pathways, and NF-κB is one of the
downstream components of the ERK/MAPK signaling pathway. The
activation of MAPK can promote the dual phosphorylation and
degradation of IκB-α. The crosstalk between the MAPK signaling
pathway and the NF-κB pathway plays a crucial role in regulating
cell fate. For instance, JNK and p38-MAPK in the MAPK family can
activate NF-κB under certain conditions, thereby cooperating to
regulate cell survival and death; on the other hand, NF-κB can also
affect MAPK activity through a feedback mechanism. The complexity
of this interaction allows the two signaling pathways to have
different effects on apoptosis under different physiological and
pathological conditions. For instance, in the mechanistic pathway
of artesunate, AKT can indirectly affect STAT3 activity by
phosphorylating a variety of signaling molecules. AKT can
phosphorylate and inhibit glycogen synthase kinase 3 (GSK-3), and
inhibition of GSK-3 may lead to phosphorylation and activation of
STAT3 (187). mTORC1 can affect
STAT3 expression and activity by regulating protein synthesis.
mTORC1 activation can promote the synthesis of ribosomal proteins
and translation initiation factors, which may increase STAT3
expression and phosphorylation. The PI3K/AKT/mTOR signaling pathway
is regulated by multiple negative feedback signals that may also
affect STAT3 phosphorylation and activity. By inhibiting the
proliferation, invasion and differentiation of liver cancer cells
and delaying the expression of related factors, it interferes with
multiple signal transduction, so as to achieve the purpose of
treating liver cancer.
The fatal issues of sorafenib and cisplatin are
their drug resistance and severe toxicity, and natural products are
considered as a safe and effective alternative for cancer treatment
(188), so the chemopreventive
concept of plant-derived natural products is becoming increasingly
important. The use of natural product monomers from inception to
late combination and encapsulation in lipid nanoparticles minimizes
toxic effects by facilitating specific, targeted delivery to the
tumor site.
Conclusion
To date, numerous studies on sesquiterpenes from
the same source have been published, but there is a lack of certain
studies on the different effects of sesquiterpenes from different
sources. The different or identical efficacy of compounds from
various sources can be investigated in the future. Furthermore,
numerous studies on the activity of sesquiterpenes are only limited
to in vitro tests and animal studies, and further studies
are needed to evaluate the in vivo efficacy, mechanism of
action, structure-activity relationship and clinical application of
these compounds, so as to provide a reasonable and reliable
scientific basis for the development of a new generation of safe
and effective natural small molecular compounds and promote their
clinical application.
Availability of data and materials
Not applicable.
Authors' contributions
YL and JM conceived and designed the study. YL, JM
and XD carried out the analysis and wrote the manuscript. All
authors have read and agreed to the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This research was funded by the Chongqing Municipal Education
Commission Youth Project (grant no. KJQN202402816) and Chongqing
Natural Science Foundation General Project (grant no.
2023NSCQ-MSX1632).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hepatocellular carcinoma. Nat Rev Dis
Primers. 7:72021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar
|
|
4
|
Ramos S: Cancer chemoprevention and
chemotherapy: Dietary polyphenols and signalling pathways. Mol Nutr
Food Res. 52:507–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
García Rodríguez LA, Martín-Pérez M,
Hennekens CH, Rothwell PM and Lanas A: Bleeding risk with long-term
Low-dose aspirin: A systematic review of observational studies.
PLoS One. 11:e01600462016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laidlaw TM and Cahill KN: Current
knowledge and management of hypersensitivity to aspirin and NSAIDs.
J Allergy Clin Immunol Pract. 5:537–545. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren W, Wang W and Guo Y: Analysis of
adverse reactions of aspirin in prophylaxis medication based on
FAERS database. Computational Mathematical Methods Med.
2022:78822772022. View Article : Google Scholar
|
|
8
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar :
|
|
9
|
Sayiner M, Golabi P and Younossi ZM:
Disease burden of hepatocellular carcinoma: A global perspective.
Dig Dis Sci. 64:910–917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chopra B and Dhingra AK: Natural products:
A lead for drug discovery and development. Phytother Res.
35:4660–4702. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng C, Zhuo S, Zhang B, Zhao X, Liu Y,
Liao C, Quan J, Li Z, Bode AM, Cao Y and Luo X: Treatment
implications of natural compounds targeting lipid metabolism in
nonalcoholic fatty liver disease, obesity and cancer. Int J Biol
Sci. 15:1654–1663. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the nearly four decades from 01/1981
to 09/2019. J Nat Prod. 83:770–803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang M, Lu JJ, Huang MQ, Bao JL, Chen XP
and Wang YT: Terpenoids: Natural products for cancer therapy.
Expert Opin Investig Drugs. 21:1801–1818. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Atanasov AG, Zotchev SB, Dirsch VM;
International Natural Product Sciences Taskforce; Supuran CT:
Natural products in drug discovery: Advances and opportunities. Nat
Rev Drug Discov. 20:200–216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langhasova L, Hanusova V, Rezek J,
Stohanslova B, Ambroz M, Kralova V, Vanek T, Lou JD, Yun ZL, Yang J
and Skalova L: Essential oil from Myrica rubra leaves inhibits
cancer cell proliferation and induces apoptosis in several human
intestinal lines. Industrial Crops Products. 59:20–26. 2014.
View Article : Google Scholar
|
|
16
|
Wang J, Li X, Bai Z, Chi BX, Wei Y and
Chen X: Curcumol induces cell cycle arrest in colon cancer cells
via reactive oxygen species and Akt/GSK3β/cyclin D1 pathway. J
Ethnopharmacol. 210:1–9. 2018. View Article : Google Scholar
|
|
17
|
Barcellos Marini M, Rodrigues de Freitas
W, Lacerda da Silva Machado F, Correa Ramos Leal I, Ribeiro Soares
A, Masahiko Kanashiro M and Frazão Muzitano M: Cytotoxic activity
of halogenated sesquiterpenes from Laurencia dendroidea. Phytother
Res. 32:1119–1125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choo SJ, Ryoo IJ, Kim KC, Na M, Jang JH,
Ahn JS and Yoo ID: Hypo-pigmenting effect of sesquiterpenes from
Inula britannica in B16 melanoma cells. Arch Pharm Res. 37:567–574.
2014. View Article : Google Scholar
|
|
19
|
Liu W, Wang X, Sun J, Yang Y, Li W and
Song J: Parthenolide suppresses pancreatic cell growth by
autophagy-mediated apoptosis. Onco Targets Ther. 10:453–461. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Wang C, Wu Z, Xue J, Shen B, Zuo
W, Wang Z and Wang SL: Artesunate suppresses the growth of
prostatic cancer cells through inhibiting androgen receptor. Biol
Pharm Bull. 40:479–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitsui T, Hayashi K, Kawai M, Kido M, Tani
H, Takaoka D, Matsuura N and Nozaki H: Culcitiolides E-J, six new
eremophilane-type sesquiterpene derivatives from Senecio
culcitioides. Chem Pharm Bull (Tokyo). 61:816–822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ko W, Park JS, Kim KW, Kim J, Kim YC and
Oh H: Nardosinone-type sesquiterpenes from the hexane fraction of
Nardostachys jatamansi attenuate NF-κB and MAPK signaling pathways
in lipopolysaccharide-stimulated BV2 microglial cells.
Inflammation. 41:1215–1228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheikh IA, El-Baba C, Youssef A, Saliba
NA, Ghantous A and Darwiche N: Lessons learned from the discovery
and development of the sesquiterpene lactones in cancer therapy and
prevention. Expert Opin Drug Discov. 17:1377–1405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matos MS, Anastácio JD and Nunes dos
Santos C: Sesquiterpene lactones: Promising natural compounds to
fight inflammation. Pharmaceutics. 13:9912021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Merfort I: Perspectives on sesquiterpene
lactones in inflammation and cancer. Curr Drug Targets.
12:1560–1573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hohmann MSN, Longhi-Balbinot DT, Guazelli
CFS, Navarro SA, Zarpelon AC, Casagrande R, Arakawa NS and Verri WA
Jr: Chapter 7-Sesquiterpene lactones: Structural diversity and
perspectives as anti-inflammatory molecules. Studies Natu Products
Chemistry. 49:243–264. 2016. View Article : Google Scholar
|
|
27
|
Ivanescu B, Miron A and Corciova A:
Sesquiterpene lactones from Artemisia Genus: Biological activities
and methods of analysis. J Anal Methods Chem. 2015:2476852015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ooko E, Saeed MEM, Kadioglu O, Sarvi S,
Colak M, Elmasaoudi K, Janah R, Greten HJ and Efferth T:
Artemisinin derivatives induce iron-dependent cell death
(ferroptosis) in tumor cells. Phytomedicine. 22:1045–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Efferth T: Molecular pharmacology and
pharmacogenomics of artemisinin and its derivatives in cancer
cells. Curr Drug Targets. 7:407–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia J, Qin Y, Zhang L, Guo C, Wang Y, Yue
X and Qian J: Artemisinin inhibits gallbladder cancer cell lines
through triggering cell cycle arrest and apoptosis. Mol Med Report.
13:4461–4468. 2016. View Article : Google Scholar
|
|
31
|
Zhang M, Wang L, Liu W, Wang T, De Sanctis
F, Zhu L, Zhang G, Cheng J, Cao Q, Zhou J, et al: Targeting
inhibition of accumulation and function of Myeloid-derived
suppressor cells by artemisinin via PI3K/AKT, mTOR, and MAPK
pathways enhances Anti-PD-L1 immunotherapy in melanoma and liver
tumors. J Immunol Res. 2022:22534362022.PubMed/NCBI
|
|
32
|
Tyagi N, Sharma GN, Shrivastava B, Saxena
P and Kumar N: Medicinal plants: Used in Anti-cancer treatment. Int
J Res Dev Pharmacy Life Sci. 6:2732–2739. 2017.
|
|
33
|
He GN, Bao NR, Wang S, Xi M, Zhang TH and
Chen FS: Ketamine induces ferroptosis of liver cancer cells by
targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther.
15:3965–3978. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
AbouAitah K and Lojkowski W: Delivery of
natural agents by means of mesoporous silica nanospheres as a
promising anticancer strategy. Pharmaceutics. 13:1432021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weifeng T, Feng S, Xiangji L, Changqing S,
Zhiquan Q, Huazhong Z, Peining Y, Yong Y, Mengchao W, Xiaoqing J
and Wan-Yee L: Artemisinin inhibits in vitro and in vivo invasion
and metastasis of human hepatocellular carcinoma cells.
Phytomedicine. 18:158–162. 2011. View Article : Google Scholar
|
|
36
|
Zhang CZ, Zhang H, Yun J, Chen GG and Lai
PB: Dihydroartemisinin exhibits antitumor activity toward
hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol.
83:1278–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vandewynckel YP, Laukens D, Geerts A,
Vanhove C, Descamps B, Colle I, Devisscher L, Bogaerts E, Paridaens
A, Verhelst X, et al: Therapeutic effects of artesunate in
hepatocellular carcinoma: repurposing an ancient antimalarial
agent. Eur J Gastroenterol Hepatol. 26:861–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ilamathi M, Santhosh S and
Sivaramakrishnan V: Artesunate as an anti-cancer agent targets
stat-3 and favorably suppresses hepatocellular carcinoma. Curr Top
Med Chem. 16:2453–2463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu J, Liu S, Xing Y, Min M, Runpeng Z, Jun
X and Dong H: Artesunate promotes sensitivity to sorafenib in
hepatocellular carcinoma. Biochem Biophysical Res Commun.
519:41–45. 2019. View Article : Google Scholar
|
|
40
|
Dong W, Ma WJ, Ma YB, Li FJ, Li TZ, Wang
YC, He XF, Geng CN, Zhang XM and Chen JJ: Guaiane-type
sesquiterpenoid dimers from Artemisia zhongdianensis and
antihepatoma carcinoma activity via the p38MAPK pathway. Chin J
Chemistry. 41:2453–2468. 2023. View Article : Google Scholar
|
|
41
|
Gao Z, Ma WJ, Li TZ, Ma YB, Hu J, Huang
XY, Geng CA, He XF, Zhang XM and Chen JJ: Artemidubolides A-T,
cytotoxic unreported guaiane-type sesquiterpenoid dimers against
three hepatoma cell lines from Artemisia dubia. Phytochemistry.
202:1132992022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Q, Zhang T, Ke CQ, Tang C, Yao S, Lin
L and Ye Y: Sesquiterpene lactone dimers from Artemisia
lavandulifolia inhibit interleukin-1β production in macrophages
through activating autophagy. Bioorg Chem. 105:1044512020.
View Article : Google Scholar
|
|
43
|
Su L, Zhang X, Ma Y, Geng C, Huang X, Hu
J, Li T, Tang S, Shen C, Gao Z, et al: New guaiane-type
sesquiterpenoid dimers from Artemisia atrovirens and their
antihepatoma activity. Acta Pharm Sin B. 11:1648–1666. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rasul A, Di J, Millimouno FM, Malhi M,
Tsuji I, Ali M, Li J and Li X: Reactive oxygen species mediate
isoalantolactone-induced apoptosis in human prostate cancer cells.
Molecules. 18:9382–9396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu ZC, Hui XG, Huo L, Sun DX, Peng W,
Zhang Y, Li XB, Ma T, Li WH, Liang J and Sun ZQ: Antiproliferative
effects of isoalantolactone in human liver cancer cells are
mediated through caspase-dependent apoptosis, ROS generation,
suppression of cell migration and invasion and targeting
Ras/Raf/MEK signalling pathway. Acta Biochim Pol. 69:299–304.
2022.PubMed/NCBI
|
|
46
|
Gu W, Zhao H, Yuan H and Zhao S:
Dehydrocostus lactone reduced malignancy of HepG2 human
hepatocellular carcinoma cells via Down-regulation of the PI3K/AKT
signaling pathway. Bull Exp Biol Med. 174:360–365. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Si H, Genna B, Zhuang X, Wang J, Burenbatu
B, Feng Q and Wang H: DaHuangWan targets EGF signaling to inhibit
the proliferation of hepatoma cells. PLoS One. 15:e02314662020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mao J, Yi M, Tao Y, Huang Y and Chen M:
Costunolide isolated from Vladimiria souliei inhibits the
proliferation and induces the apoptosis of HepG2 cells. Mol Med
Rep. 19:1372–1379. 2019.
|
|
49
|
Cui YQ, Liu YJ and Zhang F: The
suppressive effects of Britannin (Bri) on human liver cancer
through inducing apoptosis and autophagy via AMPK activation
regulated by ROS. Biochem Biophys Res Commun. 497:916–923. 2018.
View Article : Google Scholar
|
|
50
|
Wang B, Zhou TY, Nie CH, Wan DL and Zheng
SS: Bigelovin, a sesquiterpene lactone, suppresses tumor growth
through inducing apoptosis and autophagy via the inhibition of mTOR
pathway regulated by ROS generation in liver cancer. Biochem
Biophys Res Commun. 499:156–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chai T, Meng XH, Wang CB, Wang K, Ma LM,
Shi YP and Yang JL: Narjatamolide, an unusual homoguaiane
sesquiterpene lactone from Nardostachys jatamansi. J Org Chem.
86:11006–11010. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shimizu Y, Polavarapu R, Eskla KL,
Nicholson CK, Koczor CA, Wang R, Lewis W, Shiva S, Lefer DJ and
Calvert JW: Hydrogen sulfide regulates cardiac mitochondrial
biogenesis via the activation of AMPK. J Mol Cell Cardiol.
116:29–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu W, Ge K, Guo Y, Zhu W and Liu L:
ROS-dependent cell death Induced by parthenolide in human hepatoma
cell hepG2. Open Access Library J. 7:1–18. 2020.
|
|
54
|
Zhai JD, Li D, Long J, Zhang HL, Lin JP,
Qiu CJ, Zhang Q and Chen Y: Biomimetic semisynthesis of arglabin
from parthenolide. J Org Chem. 77:7103–7107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Q, Lu Y, Ding Y, Zhai J, Ji Q, Ma W,
Yang M, Fan H, Long J, Tong Z, et al: Guaianolide sesquiterpene
lactones, a source to discover agents that selectively inhibit
acute myelogenous leukemia stem and progenitor cells. J Med Chem.
55:8757–8769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu Z, Xu J, Sun S, Lin W, Li Y, Lu Q, Li
F, Yang Z, Lu Y, Liu W, et al: Mecheliolide elicits ROS-mediated
ERS driven immunogenic cell death in hepatocellular carcinoma.
Redox Biol. 54:1023512022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhong J, Gong W, Chen J, Qing Y, Wu S, Li
H, Huang C, Chen Y, Wang Y, Xu Z, et al: Micheliolide alleviates
hepatic steatosis in db/db mice by inhibiting inflammation and
promoting autophagy via PPAR-γ-mediated NF-кB and AMPK/mTOR
signaling. Int Immunopharmacol. 59:197–208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao Y, Chen SJ, Wang JC, Niu HX, Jia QQ,
Chen XW, Du XY, Lu L, Huang B, Zhang Q, et al: Sesquiterpene
lactones inhibit advanced oxidation protein product-induced MCP-1
expression in podocytes via an IKK/NF-κB-dependent mechanism. Oxid
Med Cell Longev. 2015:9340582015. View Article : Google Scholar
|
|
59
|
Xu H, Wang J, Wang C, Chang G, Lin Y,
Zhang H, Zhang H, Li Q and Pang T: Therapeutic effects of
micheliolide on a murine model of rheumatoid arthritis. Mol Med
Rep. 11:489–493. 2015. View Article : Google Scholar
|
|
60
|
Bian M, Fan R, Zhao S and Liu W: Targeting
the thioredoxin system as a strategy for cancer therapy:
Miniperspective. J Med Chem. 62:7309–7321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
An Y, Guo W, Li L, Xu C, Yang D, Wang S,
Lu Y, Zhang Q, Zhai J, Fan H, et al: Micheliolide derivative DMAMCL
inhibits glioma cell growth in vitro and in vivo. PLoS One.
10:e01162022015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yao S, Ye J, Yin M and Yu R: DMAMCL exerts
antitumor effects on hepatocellular carcinoma both in vitro and in
vivo. Cancer Lett. 483:87–97. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sulistyani N: Screening of anticancer,
hepatoprotective and nephroprotective effects of ethanol extract of
Elephantopus scaber L. Pak J Pharm Sci. 33:901–907. 2020.PubMed/NCBI
|
|
64
|
Fu L, Pei D, Yu M, Shang H, Si JG, Zhang
HW, Zhang T and Zou ZM: New phenolic acids from the whole herb of
Elephantopus scaber Linn. and their anti-inflammatory activity. Nat
Prod Res. 35:3667–3674. 2021. View Article : Google Scholar
|
|
65
|
Ariesta I and Sukma MG: Extract of
Elephantopus scaber as therapy for insulin resistance by decreasing
damage of heart and Liver in STZ-Na induced diabetic rats (Rattus
novergicus). Am Heart J. 229:1682020. View Article : Google Scholar
|
|
66
|
Divya GS, Mansoor KP, Rasheed SP and Kumar
A: PPAR gamma agonists: An effective strategy for cancer treatment.
J Pharm Sci Innov. 2:1–3. 2013. View Article : Google Scholar
|
|
67
|
Bich Ngoc TT, Hoai Nga NT, My Trinh NT,
Thuoc TL and Phuong Thao DT: Elephantopus mollis Kunth extracts
induce antiproliferation and apoptosis in human lung cancer and
myeloid leukemia cells. J Ethnopharmacol. 263:1132222020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Beeran AA, Maliyakkal N, Rao CM and Udupa
N: The enriched fraction of Elephantopus scaber Triggers apoptosis
and inhibits Multi-drug resistance transporters in human epithelial
cancer cells. Pharmacogn Mag. 11:257–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mehmood T, Maryam A, Zhang H, Li Y, Khan M
and Ma T: Deoxyelephantopin induces apoptosis in HepG2 cells via
oxidative stress, NF-κB inhibition and mitochondrial dysfunction.
Biofactors. 43:63–72. 2017. View Article : Google Scholar
|
|
70
|
Bai M, Chen JJ, Xu W, Dong SH, Liu QB, Yao
GD, Lin B, Huang XX and Song SJ: Germacranolides from Elephantopus
scaber L. and their cytotoxic activities. Phytochemistry.
178:1124792020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bai M, Chen JJ, Xu W, Dong SH, Liu QB, Lin
B, Huang XX, Yao GD and Song SJ: Elephantopinolide AP,
germacrane-type sesquiterpene lactones from Elephantopus scaber
induce apoptosis, autophagy and G2/M phase arrest in hepatocellular
carcinoma cells. Eur J Med Chem. 198:1123622020. View Article : Google Scholar
|
|
72
|
Mehmood T, Maryam A, Tian X, Khan M and Ma
T: Santamarine inhibits NF-кB and STAT3 activation and induces
apoptosis in HepG2 liver cancer cells via oxidative stress. J
Cancer. 8:3707–3717. 2017. View Article : Google Scholar :
|
|
73
|
Kim JK, Cho IJ, Kim EO, Lee DG, Jung DH,
Ki SH, Ku SK and Kim SC: Hemistepsin A inhibits T0901317-induced
lipogenesis in the liver. BMB Rep. 54:1062021. View Article : Google Scholar :
|
|
74
|
Kim JK, Han NR, Park SM, Jegal KH, Jung
JY, Jung EH, Kim EO, Kim D, Jung DH, Lee JR, et al: Hemistepsin A
alleviates liver fibrosis by inducing apoptosis of activated
hepatic stellate cells via inhibition of nuclear factor-κB and Akt.
Food Chem Toxicol. 135:1110442020. View Article : Google Scholar
|
|
75
|
Kim JK, Cho IJ, Kim EO, Jung DH, Ku SK and
Kim SC: The effects of Hemistepta lyrata Bunge (Bunge) fractionated
extract on liver X receptor α-dependent lipogenic genes in
hepatocyte-derived cells. Herbal Formula Sci. 28:255–269. 2020.
|
|
76
|
Kim JK, Lee JE, Jung EH, Jung JY, Jung DH,
Ku SK, Cho IJ and Kim SC: Hemistepsin A ameliorates acute
inflammation in macrophages via inhibition of nuclear factor-κB and
activation of nuclear factor erythroid 2-related factor 2. Food
Chem Toxicol. 111:176–188. 2018. View Article : Google Scholar
|
|
77
|
Baek SY, Hwang UW, Suk HY and Kim YW:
Hemistepsin a inhibits cell proliferation and induces G0/G1-Phase
arrest, cellular senescence and apoptosis via the AMPK and p53/p21
signals in human hepatocellular carcinoma. Biomolecules.
10:7132020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cho IJ, Kim JK, Kim EO, Park SM, Kim SC,
Ki SH and Ku SK: Hemistepsin A induces apoptosis of hepatocellular
carcinoma cells by downregulating STAT3. Int J Mol Sci.
22:47432021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nibret E, Youns M, Krauth-Siegel RL and
Wink M: Biological activities of x1anthatin from Xanthium
strumarium leaves. Phytother Res. 25:1883–1890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kovács A, Vasas A, Forgo P, Réthy B, Zupkó
I and Hohmann J: Xanthanolides with antitumour activity from
Xanthium italicum. Z Naturforsch C J Biosci. 64:343–349. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ramírez-Erosa I, Huang Y, Hickie RA,
Sutherland RG and Barl B: Xanthatin and xanthinosin from the burs
of Xanthium strumarium L. as potential anticancer agents. Can J
Physiol Pharmacol. 85:1160–1172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shi T, Zhang L, Cheng Q, Yu JS, Liu J,
Shen YJ, Feng XJ and Shen YX: Xanthatin induces apoptosis by
activating endoplasmic reticulum stress in hepatoma cells. Eur J
Pharmacol. 843:1–11. 2019. View Article : Google Scholar
|
|
83
|
Kuck K, Jürgenliemk G, Lipowicz B and
Heilmann J: Sesquiterpenes from myrrh and their ICAM-1 inhibitory
activity in vitro. Molecules. 26:422020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li L, Zheng BB, Ma LS, Sun X, Chang JJ,
Xie WD and Li X: Telekin suppresses human hepatocellular carcinoma
cells in vitro by inducing G2/M phase arrest via the p38 MAPK
signaling pathway. Acta Pharmacol Sin. 35:1311–1322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Su LH, Ma WJ, Ma YB, Li TZ, Geng CA, Dong
W, He XF, Zhang XM and Chen JJ: Artemiprincepsolides A-F, Novel
Germacrane-guaiane and Eudesmane-guaiane Sesquiterpenoid Dimers
from Artemisia princeps and Their Antihepatoma Activity. Chin J
Chem. 41:2648–2656. 2023. View Article : Google Scholar
|
|
86
|
Wu H, Wu H, Wang W, Liu TT, Qi MG, Feng
JC, Li XY and Liu Y: Insecticidal activity of sesquiterpene
lactones and monoterpenoid from the fruits of Carpesium
abrotanoides. Industrial Crops Products. 92:77–83. 2016. View Article : Google Scholar
|
|
87
|
Wang F, Yang K, Ren FC and Liu JK:
Sesquiterpene lactones from Carpesium abrotanoides. Fitoterapia.
80:21–24. 2009. View Article : Google Scholar
|
|
88
|
Lee J, Min B, Lee S, Na M, Kwon B, Lee C,
Kim Y and Bae K: Cytotoxic sesquiterpene lactones from Carpesium
abrotanoides. Planta Med. 68:745–747. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yang C, Yuan C and Jia Z: Xanthanolides,
Germacranolides, and Other Constituents from Carpesium longifolium.
J Nat Prod. 66:1554–1557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yan Y, Chen J, Peng M, Zhang X, Feng E, Li
Q, Guo B, Ding X, Zhang Y and Tang L: Sesquiterpenes from Carpesium
faberi triggered ROS-induced apoptosis and protective autophagy in
hepatocellular carcinoma cells. Phytochemistry. 214:1138052023.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lo Cantore P, Iacobellis NS, De Marco A,
Capasso F and Senatore F: Antibacterial activity of Coriandrum
sativum L. and Foeniculum vulgare Miller var. vulgare (Miller)
essential oils. J Agric Food Chem. 52:7862–7866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Emamghoreishi M, Khasaki M and Aazam MF:
Coriandrum sativum: Evaluation of its anxiolytic effect in the
elevated plus-maze. J Ethnopharmacol. 96:365–370. 2005. View Article : Google Scholar
|
|
93
|
Bickers D, Calow P, Greim H, Hanifin JM,
Rogers AE, Saurat JH, Sipes IG, Smith RL and Tagami H: A
toxicologic and dermatologic assessment of linalool and related
esters when used as fragrance ingredients. Food Chem Toxicol.
41:919–942. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Usta J, Kreydiyyeh S, Knio K, Barnabe P,
Bou-Moughlabay Y and Dagher S: Linalool decreases HepG2 viability
by inhibiting mitochondrial complexes I and II, increasing reactive
oxygen species and decreasing ATP and GSH levels. Chem Biol
Interact. 180:39–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Simamora A, Timotius KH, Yerer MB,
Setiawan H and Mun'im A: Xanthorrhizol, a potential anticancer
agent, from Curcuma xanthorrhiza Roxb. Phytomedicine.
105:1543592022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tee TT, Cheah YH, Meenakshii N, Mohd
Sharom MY and Azimahtol Hawariah LP: Xanthorrhizol induced DNA
fragmentation in HepG2 cells involving Bcl-2 family proteins.
Biochem Biophys Res Commun. 420:834–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fonseca DV, Salgado PR, de Carvalho FL,
Salvadori MG, Penha AR, Leite FC, Borges CJ, Piuvezam MR, Pordeus
LC, Sousa DP and Almeida RN: Nerolidol exhibits antinociceptive and
anti-inflammatory activity: Involvement of the GABAergic system and
proinflammatory cytokines. Fundam Clin Pharmacol. 30:14–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Krist S, Banovac D, Tabanca N, Wedge DE,
Gochev VK, Wanner J, Schmidt E and Jirovetz L: Antimicrobial
activity of nerolidol and its derivatives against airborne microbes
and further biological activities. Nat Prod Commun. 10:143–148.
2015.PubMed/NCBI
|
|
99
|
Chan WK, Tan LT, Chan KG, Lee LH and Goh
BH: Nerolidol: A sesquiterpene alcohol with Multi-faceted
pharmacological and biological activities. Molecules. 21:5292016.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Biazi BI, Zanetti TA, Baranoski A,
Corveloni AC and Mantovani MS: Cis-Nerolidol induces endoplasmic
reticulum stress and cell death in human hepatocellular carcinoma
cells through extensive CYP2C19 and CYP1A2 oxidation. Basic Clin
Pharmacol Toxicol. 121:334–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sharifi-Rad M, Nazaruk J, Polito L,
Morais-Braga MFB, Rocha JE, Coutinho HDM, Salehi B, Tabanelli G,
Montanari C, Del Mar Contreras M, et al: Matricaria genus as a
source of antimicrobial agents: From farm to pharmacy and food
applications. Microbiol Res. 215:76–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Eddin LB, Jha NK, Goyal SN, Agrawal YO,
Subramanya SB, Bastaki SMA and Ojha S: Health benefits,
pharmacological effects, molecular mechanisms, and therapeutic
potential of α-bisabolol. Nutrients. 14:13702022. View Article : Google Scholar
|
|
103
|
Kreuger MRO, Grootjans S, Biavatti MW,
Vandenabeele P and D'Herde K: Sesquiterpene lactones as drugs with
multiple targets in cancer treatment: Focus on parthenolide.
Anticancer Drugs. 23:883–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu Y, Wang W, Fang B, Ma F, Zheng Q, Deng
P, Zhao S, Chen M, Yang G and He G: Anti-tumor effect of germacrone
on human hepatoma cell lines through inducing G2/M cell cycle
arrest and promoting apoptosis. Eur J Pharmacol. 698:95–102. 2013.
View Article : Google Scholar
|
|
105
|
Liu YY, Zheng Q, Fang B, Wang W, Ma FY,
Roshan S, Banafa A, Chen MJ, Chang JL, Deng XM, et al: Germacrone
induces apoptosis in human hepatoma HepG2 cells through inhibition
of the JAK2/STAT3 signalling pathway. Med Sci. 33:339–345.
2013.
|
|
106
|
Na-Bangchang K, Plengsuriyakam T and
Karbwang J: Research and development of atractylodes lancea (Thunb)
DC. As a promising candidate for cholangiocarcinoma
chemotherapeutics. Evid Based Complement Alternat Med.
2017:59292342017. View Article : Google Scholar
|
|
107
|
Cheng Y, Mai JY, Hou TL, Ping J and Chen
JJ: Antiviral activities of atractylon from Atractylodis Rhizoma.
Mol Med Rep. 14:3704–3710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Meng H, Li GY, Dai RH, Ma Y, Zhang K,
Zhang C, Li X and Wang J: Chemical constituents of Atractylodes
chinensis (DC.) koidz. Biochem Syst Ecol. 38:1220–1223. 2010.
View Article : Google Scholar
|
|
109
|
Cheng Y, Chen T, Yang X, Xue J and Chen J:
Atractylon induces apoptosis and suppresses metastasis in hepatic
cancer cells and inhibits growth in vivo. Cancer Manag Rese.
11:5883–5894. 2019. View Article : Google Scholar
|
|
110
|
Cheng Y, Ping J, Chen J, Fu Y, Zhao H and
Xue J: Molecular mechanism of atractylon in the invasion and
migration of hepatic cancer cells based on high-throughput
sequencing. Mol Med Rep. 25:1122022. View Article : Google Scholar :
|
|
111
|
Cheng SB, Wu LC, Hsieh YC, Wu CH, Chan YJ,
Chang LH, Chang CM, Hsu SL, Teng CL and Wu CC: Supercritical carbon
dioxide extraction of aromatic turmerone from Curcuma longa Linn.
Induces apoptosis through reactive oxygen species-triggered
intrinsic and extrinsic pathways in human hepatocellular carcinoma
HepG2 cells. J Agric Food Chem. 60:9620–9630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Abu-Izneid T, Rauf A, Shariati MA, Khalil
AA, Imran M, Rebezov M, Uddin MS, Mahomoodally MF and Rengasamy
KRR: Sesquiterpenes and their derivatives-natural anticancer
compounds: An update. Pharmacol Res. 161:1051652020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fraga BM: Natural sesquiterpenoids. Nat
Prod Rep. 30:1226–1264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wu L, Huang X, Kuang Y, Xing Z, Deng X and
Luo Z: Thapsigargin induces apoptosis in adrenocortical carcinoma
by activating endoplasmic reticulum stress and the JNK signaling
pathway: An in vitro and in vivo study. Drug Des Devel Ther.
13:2787–2798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Andersen TB, López CQ, Manczak T, Martinez
K and Simonsen HT: Thapsigargin-from Thapsia L. to mipsagargin.
Molecules. 20:6113–6127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Brennen WN, Rosen DM, Wang H, Isaacs JT
and Denmeade SR: Targeting carcinoma-associated fibroblasts within
the tumor stroma with a fibroblast activation protein-activated
prodrug. J Natl Cancer Inst. 104:1320–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Denmeade SR, Mhaka AM, Rosen DM, Brennen
WN, Dalrymple S, Dach I, Olesen C, Gurel B, Demarzo AM, Wilding G,
et al: Engineering a prostate-specific membrane antigen-activated
tumor endothelial cell prodrug for cancer therapy. Sci Transl Med.
4:140ra862012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Qu Z, Liu H, Zhang Z, Zheng P, Zhao S and
Hou W: Phytochemistry and pharmacology of sesquiterpenoids from
Atractylodes DC. Genus Rhizomes. Molecules. 29:13792024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tian XH, Hong LL, Jiao WH and Lin HW:
Natural sesquiterpene quinone/quinols: Chemistry, biological
activity, and synthesis. Nat Prod Reps. 40:718–749. 2023.
View Article : Google Scholar
|
|
120
|
Shulha O and Zidorn C: Sesquiterpene
lactones and their precursors as chemosystematic markers in the
tribe Cichorieae of the Asteraceae revisited: An update
(2008-2017). Phytochemistry. 163:149–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xu K, Feng ZM, Yang YN, Jiang JS and Zhang
PC: Eight new eudesmane-and eremophilane-type sesquiterpenoids from
Atractylodes lancea. Fitoterapia. 114:115–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ding HY, Wu YC and Linc HC: Phytochemical
and pharmacological studies on Chinese changzhu. J Chin Chem Soc.
47:561–566. 2000. View Article : Google Scholar
|
|
123
|
Nakai Y, Kido T, Hashimoto K, Kase Y,
Sakakibara I, Higuchi M and Sasaki H: Effect of the rhizomes of
Atractylodes lancea and its constituents on the delay of gastric
emptying. J Ethnopharmacol. 84:51–55. 2003. View Article : Google Scholar
|
|
124
|
Guo W, XU B, Meng Q, Zheng B, Li X, Liu M
and Du XD: Anti-tumor effect of Hinesol on liver cancer via
downregulating MEK/ERK and NF-κB pathway in SMMC-7721 and LM3cells.
Chin J Pharmacol Toxicol. 32:282. 2018.
|
|
125
|
Lobo R, Prabhu KS and Shirwaikar A:
Curcuma zedoaria Rosc. (White turmeric): A review of its chemical,
pharmacological and ethnomedicinal properties. J Pharm Pharmacol.
61:13–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mao Z, Zhong L, Zhuang X, Liu H and Peng
Y: Curcumenol targeting YWHAG inhibits the pentose phosphate
pathway and enhances antitumor effects of cisplatin. Evid Based
Complement Alternat Med. 2022:39889162022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wen Y, Han J, Chen J, Dong J, Xia Y, Liu
J, Jiang Y, Dai J, Lu J, Jin G, et al: Plasma mi RNA s as early
biomarkers for detecting hepatocellular carcinoma. Int J Cancer.
137:1679–1690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yerukala Sathipati S and Ho SY: Novel
miRNA signature for predicting the stage of hepatocellular
carcinoma. Scie Rep. 10:144522020. View Article : Google Scholar
|
|
129
|
Sartorius K, Sartorius B, Winkler C,
Chuturgoon A and Makarova J: The biological and diagnostic role of
miRNA's in hepatocellular carcinoma. Front Biosci (Landmark Ed).
23:1701–1720. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
130
|
Qadir MI and Rizvi SZ: miRNA in
hepatocellular carcinoma: Pathogenesis and therapeutic approaches.
Crit Rev Eukaryot Gene Expr. 27:355–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Elhefnawi M, Salah Z and Soliman B: The
promise of miRNA replacement therapy for hepatocellular carcinoma.
Curr Gene Ther. 19:290–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang X, Gao J, Zhou B, Xie J, Zhou G and
Chen Y: Identification of prognostic markers for hepatocellular
carcinoma based on miRNA expression profiles. Life Sci.
232:1165962019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li D, Zhang J and Li J: Role of miRNA
sponges in hepatocellular carcinoma. Clin Chim Acta. 500:10–19.
2020. View Article : Google Scholar
|
|
134
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang R, Zhong L, Sun K, Liu J, Wang Q,
Mao D, Fang G and Long F: A study on curcumol influencing
proliferation and apoptosis of hepatocellular carcinoma cells
through DJ-1/PTEN/PI3K/AKT Pathway. Biomed Res Int.
2022:99127762022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kirana C, McIntosh GH, Record IR and Jones
GP: Antitumor activity of extract of Zingiber aromaticum and its
bioactive sesquiterpenoid zerumbone. Nutr Cancer. 45:218–225. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wani NA, Zhang B, Teng K, Barajas JM,
Motiwala T, Hu P, Yu L, Brüschweiler R, Ghoshal K and Jacob ST:
Reprogramming of glucose metabolism by zerumbone suppresses
hepatocarcinogenesis. Mol Cancer Res. 16:256–268. 2018. View Article : Google Scholar
|
|
138
|
Samad NA, Abdul AB, Rahman HS, Rasedee A,
Tengku Ibrahim TA and Keon YS: Zerumbone suppresses angiogenesis in
HepG2 cells through inhibition of matrix metalloproteinase-9,
vascular endothelial growth factor, and vascular endothelial growth
factor receptor expressions. Pharmacogn Mag. 13(Suppl 4):
S731–S736. 2017.
|
|
139
|
Abdul ABH, Al-Zubairi AS, Tallan ND, Wahab
SIA, Zain ZNM, Ruslay S and Syam MM: Anticancer activity of natural
compound (zerumbone) extracted from Zingiber zerumbet in human HeLa
cervical cancer cells. Int J Pharmacol. 4:1602008. View Article : Google Scholar
|
|
140
|
Sakinah SA, Handayani ST and Hawariah LP:
Zerumbone induced apoptosis in liver cancer cells via modulation of
Bax/Bcl-2 ratio. Cancer Cell Int. 7:42007. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Taha MME, Abdul AB, Abdullah R, Ibrahim
TA, Abdelwahab SI and Mohan S: Potential chemoprevention of
diethylnitrosamine-initiated and 2-acetylaminofluorene-promoted
hepatocarcinogenesis by zerumbone from the rhizomes of the
subtropical ginger (Zingiber zerumbet). Chem Biol Interact.
186:295–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Bora KS and Sharma A: Phytochemical and
pharmacological potential of Medicago sativa: A review. Pharm Biol.
49:211–220. 2011. View Article : Google Scholar
|
|
143
|
Rustaiyan A and Masoudi S: Chemical
constituents and biological activities of Iranian Artemisia
species. Phytochemistry Lett. 4:440–447. 2011. View Article : Google Scholar
|
|
144
|
He ZZ, Yan JF, Song ZJ, Ye F, Liao X, Peng
SL and Ding LS: Chemical constituents from the aerial parts of
Artemisia minor. J Nat Prod. 72:1198–1201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Martínez MJA, Del Olmo LMB, Ticona LA and
Benito PB: The Artemisia L. genus: A review of bioactive
sesquiterpene lactones. Stud Natu Products Chem. 37:43–65. 2012.
View Article : Google Scholar
|
|
146
|
Suresh J, Mahesh NM, Ahuja J and Santilna
KS: Review on Artemisia nilagirica (Clarke) pamp. J Biol Active
Products Nat. 1:97–104. 2011.
|
|
147
|
He X, Ma W, Hu J, Li T, Geng C, Ma Y, Wang
M, Yang K, Zhang X and Chen JJ: Diverse structures and antihepatoma
effect of sesquiterpenoid dimers from Artemisia eriopoda by
AKT/STAT signaling pathway. Signal Transduct Target Ther. 8:642023.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li X, Chen Q, Liu J, Lai S, Zhang M, Zhen
T, Hu H, Gao X, Wong AST and Zeng JZ: Orphan nuclear receptor Nur77
mediates the lethal endoplasmic reticulum stress and therapeutic
efficacy of cryptomeridiol in hepatocellular carcinoma. Cells.
11:38702022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yu X, Yang FQ, Li SP, Gao JL, Hu G, Lao
SC, Conceição EL, Fung KP, Wangl YT and Lee SM: Furanodiene induces
G2/M cell cycle arrest and apoptosis through MAPK signaling and
mitochondria-caspase pathway in human hepatocellular carcinoma
cells. Cancer Biol Ther. 6:1044–1050. 2014.
|
|
150
|
Yang F, Chen WD, Deng R, Li DD, Wu KW,
Feng GK, Li HJ and Zhu XF: Hirsutanol A induces apoptosis and
autophagy via reactive oxygen species accumulation in breast cancer
MCF-7 cells. J Pharmacol Sci. 119:214–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yang F, Chen WD, Deng R, Zhang H, Tang J,
Wu KW, Li DD, Feng GK, Lan WJ, Li HJ and Zhu XF: Hirsutanol A, a
novel sesquiterpene compound from fungus Chondrostereum sp.,
induces apoptosis and inhibits tumor growth through
mitochondrial-independent ROS production: Hirsutanol A inhibits
tumor growth through ROS production. J Transl Med. 11:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang J, Mao Y, Hou L and Cui X: The
effect of beta-elemene on alpha-tubulin polymerization in human
hepatoma HepG2 cells. Chin J Cancer Res. 25:770–776. 2013.
|
|
153
|
Peng X, Zhao Y, Liang X, Wu L, Cui S, Guo
A and Wang W: Assessing the quality of RCTs on the effect of
beta-elemene, one ingredient of a Chinese herb, against malignant
tumors. Contemp Clin Trials. 27:70–82. 2006. View Article : Google Scholar
|
|
154
|
Sun Y, Liu G, Zhang Y, Zhu H, Ren Y and
Shen YM: Synthesis and in vitro anti-proliferative activity of
β-elemene monosubstituted derivatives in HeLa cells mediated
through arrest of cell cycle at the G1 phase. Bioorg Med Chem.
17:1118–1124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Wu B, Jiang Y, Zhu F, Sun D and Huang H:
Demethylation effects of elemene on the GSTP1 gene in HCC cell line
QGY7703. Oncol Lett. 11:2545–2551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Qin Y, Guo Y, Wei W, Wang B, Jin H, Sun J,
Qi J, Ren S and Zuo Y: Anti-tumor effect of β-elemene in murine
hepatocellular carcinoma cell line H22 depends on the level of
c-Met downregulation. Biomedicine Preventive Nutr. 2:91–98. 2012.
View Article : Google Scholar
|
|
157
|
Ni G, Shi GR, Zhang D, Fu NJ, Yang HZ,
Chen XG and Yu DQ: Cytotoxic Lignans and Sesquiterpenoids from the
Rhizomes of Acorus tatarinowii. Planta Medica. 82:632–638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Chen JJ, Wei HB, Xu YZ, Hu SC and Gao K:
Senedensiscins A-F: Six new eudesmane sesquiterpenoid glucosides
from Senecio densiserratus. Tetrahedron. 69:10598–10603. 2013.
View Article : Google Scholar
|
|
159
|
Yang ML, Chen JJ, Wei HB and Gao K:
Cytotoxic sesquiterpenoids from Senecio densiserratus.
Phytochemistry Lett. 16:236–240. 2016. View Article : Google Scholar
|
|
160
|
Tsevegsuren N, Edrada RA, Lin W, Ebel R,
Torre C, Ortlepp S, Wray V and Proksch P: Biologically active
natural products from Mongolian medicinal plants Scorzonera
divaricata and Scorzonera pseudodivaricata. J Nat Prod. 70:962–967.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wu Q, He XF, Jiang CX, Zhang W, Shi ZN, Li
HF and Zhu Y: Two novel bioactive sulfated guaiane sesquiterpenoid
salt alkaloids from the aerial parts of Scorzonera divaricata.
Fitoterapia. 124:113–119. 2018. View Article : Google Scholar
|
|
162
|
Shang C, Ma YB, Wang Y, He XF, Li TZ and
Chen JJ: Artemongolins A-K, undescribed germacrane-guaiane
sesquiterpenoid dimers from Artemisia mongolica and their
antihepatoma activities. Arch Pharm Res. 46:782–794. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang MF, Li TZ, Ma YB, Ma WJ, Wang YC, Li
FJ and Chen JJ: Artemyriantholides A-K, guaiane-type
sesquiterpenoid dimers from Artemisia myriantha var. pleiocephala
and their antihepatoma activity. Phytochemistry. 222:1141002024.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
He XF, Ma YB, Li TZ and Chen JJ: Highly
oxygenated guaiane-type sesquiterpene lactones from Artemisia
sacrorum and their antihepatoma activity. Phytochemistry.
217:1139302024. View Article : Google Scholar
|
|
165
|
Wang X, Li TZ, Ma YB, Ma WJ, Xue D and
Chen JJ: Synthesis and antihepatoma activity of guaianolide dimers
derived from lavandiolide I. Bioorg Med Chem Lett. 104:1297082024.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1-to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Yang F, Li J, Zhu J, Wang D, Chen S and
Bai X: Hydroxysafflor yellow A inhibits angiogenesis of
hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling
pathway in H22 tumor-bearing mice. Eur J Pharmacol. 754:105–114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Cusimano A, Foderà D, D'Alessandro N,
Lampiasi N, Azzolina A, Montalto G and Cervello M: Potentiation of
the antitumor effects of both selective cyclooxygenase-1 and
cyclooxygenase-2 inhibitors in human hepatic cancer cells by
inhibition of the MEK/ERK pathway. Cancer Biol Ther. 6:1457–1464.
2007. View Article : Google Scholar
|
|
170
|
Wang XD, Meng FC and Mao JX: Progress of
natural sesquiterpenoids in the treatment of hepatocellular
carcinoma. Front Oncol. 14:14452222024. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Li Y, Wang T, Sun Y, Huang T and Li C, Fu
Y, Li Y and Li C: p53-mediated PI3K/AKT/mTOR pathway played a role
in PtoxDpt-induced EMT inhibition in liver cancer cell
lines. Oxid Med Cell Longev. 2019:25314932019.
|
|
172
|
Kahraman DC, Kahraman T and Cetin-Atalay
R: Targeting PI3K/Akt/mTOR pathway identifies differential
expression and functional role of IL8 in liver cancer stem cell
enrichment. Mol Cancer Ther. 18:2146–2157. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Luedde T and Schwabe RF: NF-κB in the
liver-linking injury, fibrosis and hepatocellular carcinoma. Nat
Rev Gastroenterol Hepatol. 8:108–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wilson CL, Jurk D, Fullard N, Banks P,
Page A, Luli S, Elsharkawy AM, Gieling RG, Chakraborty JB, Fox C,
et al: NFκB1 is a suppressor of neutrophil-driven hepatocellular
carcinoma. Nat Commun. 6:68182015. View Article : Google Scholar
|
|
175
|
Lee C and Cheung ST: STAT3: An emerging
therapeutic target for hepatocellular carcinoma. Cancers.
11:16462019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Boland ML, Chourasis AH and Macleod KF:
Mitochondrial dysfunction in cancer. Front Oncol. 3:2922013.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lee KH, Huang ES, Piantadosi C, Pagano JS
and Geissman TA: Cytotoxicity of sesquiterpene lactones. Cancer
Res. 31:1649–1654. 1971.PubMed/NCBI
|
|
178
|
Lopez-Anton N, Hermann C, Murillo R,
Merfort I, Wanner G, Vollmar AM and Dirsch VM: Sesquiterpene
lactones induce distinct forms of cell death that modulate human
Monocyte-derived macrophage responses. Apoptosis. 12:141–153. 2007.
View Article : Google Scholar
|
|
179
|
Bai Z, Yao C, Zhu J, Xie Y, Ye XY, Bai R
and Xie T: Anti-tumor drug discovery based on natural product
β-elemene: Anti-tumor mechanisms and structural modification.
Molecules. 26:14992021. View Article : Google Scholar
|
|
180
|
Xie T, Li CL and Wang SL: Basic research
progress of natural anticancer target drugs of elemene liposomes
series. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34:507–512. 2014.In
Chinese. PubMed/NCBI
|
|
181
|
Zhai B, Zeng Y, Zeng Z, Zhang N, Li C,
Zeng Y, You Y, Wang S, Chen X, Sui X and Xie T: Drug delivery
systems for elemene, its main active ingredient β-elemene, and its
derivatives in cancer therapy. Int J Nanomedicine. 13:6279–6296.
2018. View Article : Google Scholar :
|
|
182
|
Xie T, Cl L, Wang SL, Zeng ZW, Wang F and
Zhao R: Advances in the research of Elemene Liposome series
targeted anticancer natural drugs. Chin J Integr Trad West Med.
34:507–512. 2014.
|
|
183
|
Li H, Xu K, Pian G and Sun S: Artesunate
and sorafenib: Combinatorial inhibition of liver cancer cell
growth. Oncol Lett. 18:4735–4743. 2019.PubMed/NCBI
|
|
184
|
Yao X, Zhao CR, Yin H, Wang K and Gao JJ:
Synergistic antitumor activity of sorafenib and artesunate in
hepatocellular carinoma cells. Acta Pharmacol Sin. 41:1609–1620.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Alven S and Aderibigbe BA: Nanoparticles
formulations of artemisinin and derivatives as potential
therapeutics for the treatment of cancer, leishmaniasis and
malaria. Pharmaceutics. 12:7482020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Pan XW, Huang JS, Liu SR, Shao YD, Xi JJ,
He RY, Shi TT, Zhuang RX and Bao JF: Evaluation of the liver
targeting and anti-liver cancer activity of artesunate-loaded and
glycyrrhetinic acid-coated nanoparticles. Exp Ther Med. 26:5162023.
View Article : Google Scholar
|
|
187
|
Hermida MA, Kumar JD and Leslie NR: GSK3
and its interactions with the PI3K/AKT/mTOR signalling network. Adv
Biol Regul. 65:5–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: Theoretical basis
and therapeutic aspects. Signal Transduct Target Ther. 5:872020.
View Article : Google Scholar : PubMed/NCBI
|