|
1
|
Price M, Neff C, Nagarajan N, Kruchko C,
Waite KA, Cioffi G, Cordeiro BB, Willmarth N, Penas-Prado M,
Gilbert MR, et al: CBTRUS statistical report: American brain tumor
association & NCI system tumors neuro-oncology branch
adolescent and young adult primary brain and other central nervous
diagnosed in the United States in 2016-2020. Neuro Oncol. 26(Suppl
3): iii1–iii53. 2024. View Article : Google Scholar
|
|
2
|
Weller M, Wen PY, Chang SM, Dirven L, Lim
M, Monje M and Reifenberger G: Glioma. Nat Rev Dis Primers.
10:332024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanders S, Herpai DM, Rodriguez A, Huang
Y, Chou J, Hsu FC, Seals D, Mott R, Miller LD and Debinski W: The
presence and potential role of ALDH1A2 in the glioblastoma
microenvironment. Cells. 10:24852021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schaff LR and Mellinghoff IK: Glioblastoma
and other primary brain malignancies in adults: A Review. JAMA.
329:574–587. 2023. View Article : Google Scholar : PubMed/NCBI
|
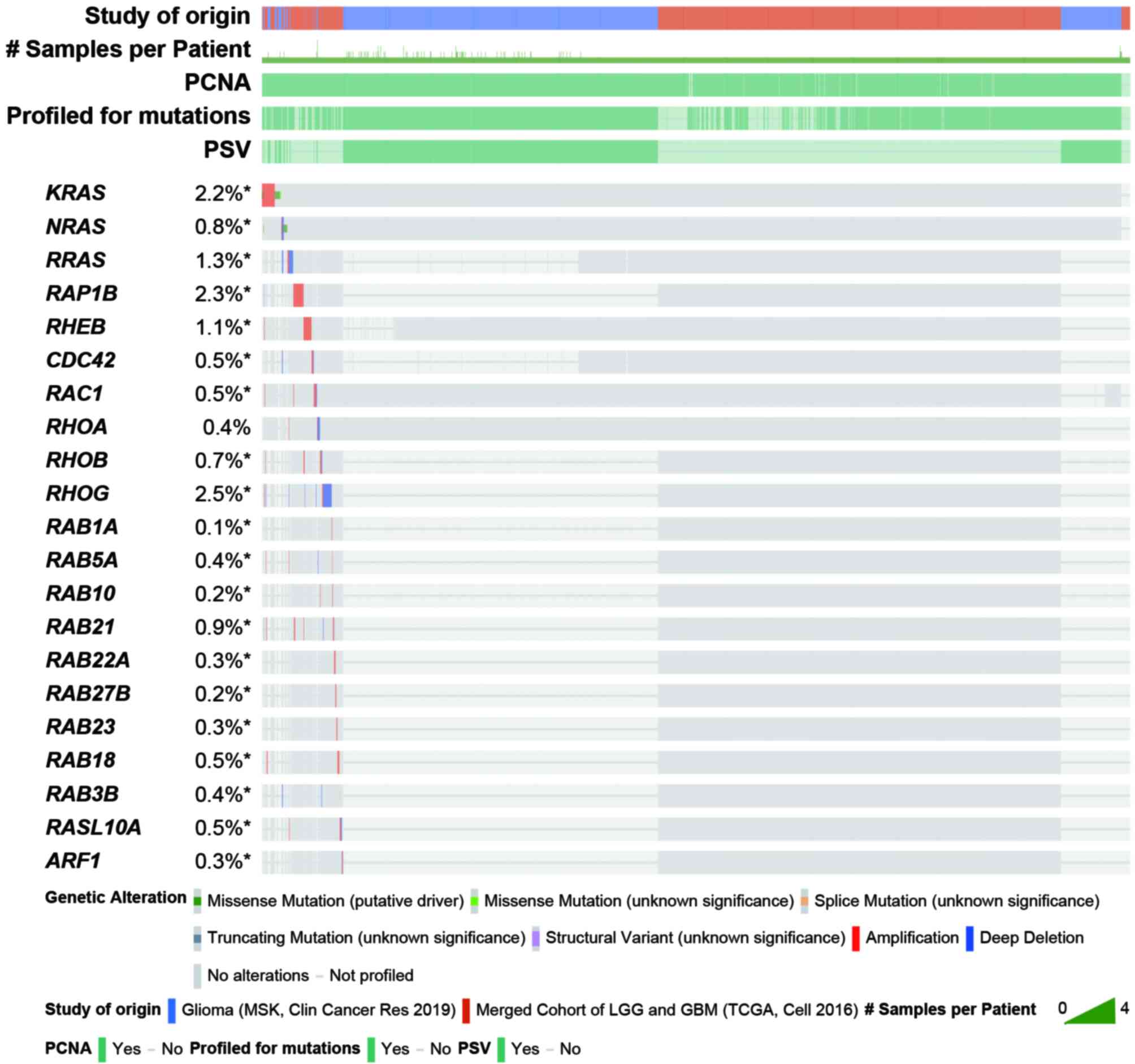
|
5
|
Yasinjan F, Xing Y, Geng H, Guo R, Yang L,
Liu Z and Wang H: Immunotherapy: A promising approach for glioma
treatment. Front Immunol. 14:12556112023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu S, Tang L, Li X, Fan F and Liu Z:
Immunotherapy for glioma: Current management and future
application. Cancer Lett. 476:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weller M, Wick W, Aldape K, Brada M,
Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R and
Reifenberger G: Glioma. Nat Rev Dis Primers. 1:150172015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang LM, Englander ZK, Miller ML and Bruce
JN: Malignant glioma. Adv Exp Med Biol. 1405:1–30. 2023. View Article : Google Scholar
|
|
9
|
Chen R, Smith-Cohn M, Cohen AL and Colman
H: Glioma subclassifications and their clinical significance.
Neurotherapeutics. 14:284–297. 2017. View Article : Google Scholar
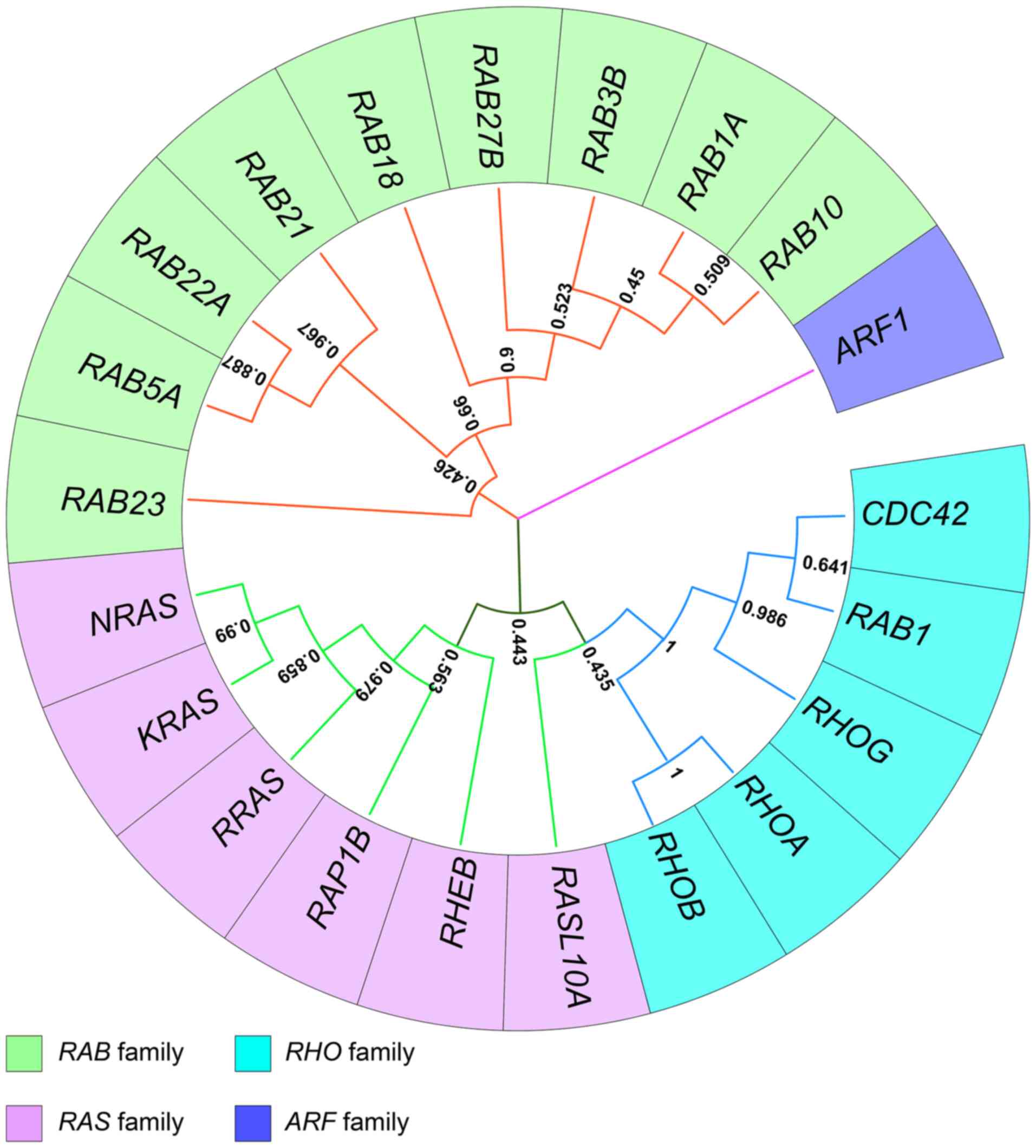
|
|
10
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar :
|
|
12
|
Luo Q, Liu Y, Yuan Z, Huang L and Diao B:
Expression of Rab3b in human glioma: Influence on cell
proliferation and apoptosis. Curr Pharm Des. 27:989–995. 2021.
View Article : Google Scholar
|
|
13
|
Wang M, Jiang X, Yang Y, Chen H, Zhang C,
Xu H, Qi B, Yao C and Xia H: Rhoj is a novel target for progression
and invasion of glioblastoma by impairing cytoskeleton dynamics.
Neurotherapeutics. 17:2028–2040. 2020. View Article : Google Scholar
|
|
14
|
Xu J, Galvanetto N, Nie J, Yang Y and
Torre V: Rac1 promotes cell motility by controlling cell mechanics
in human glioblastoma. Cancers (Basel). 12:16672020. View Article : Google Scholar
|
|
15
|
Reiner DJ and Lundquist EA: Small GTPases.
WormBook. 2018:1–65. 2018. View Article : Google Scholar
|
|
16
|
Simanshu DK, Nissley DV and McCormick F:
RAS proteins and their regulators in human disease. Cell.
170:17–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ridley AJ: Life at the leading edge. Cell.
145:1012–1022. 2011. View Article : Google Scholar
|
|
18
|
Cherfils J and Zeghouf M: Regulation of
small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 93:269–309.
2013. View Article : Google Scholar
|
|
19
|
Chen K, Zhang Y, Qian L and Wang P:
Emerging strategies to target RAS signaling in human cancer
therapy. J Hematol Oncol. 14:1162021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuo T, Wong S, Buza N and Hui P: KRAS
mutation of extraovarian implants of serous borderline tumor:
Prognostic indicator for adverse clinical outcome. Mod Pathol.
31:350–357. 2018. View Article : Google Scholar
|
|
21
|
Zhao S, Li Z, Zhang M, Zhang L, Zheng H,
Ning J, Wang Y, Wang F, Zhang X, Gan H, et al: A brain somatic RHEB
doublet mutation causes focal cortical dysplasia type II. Exp Mol
Med. 51:1–11. 2019.
|
|
22
|
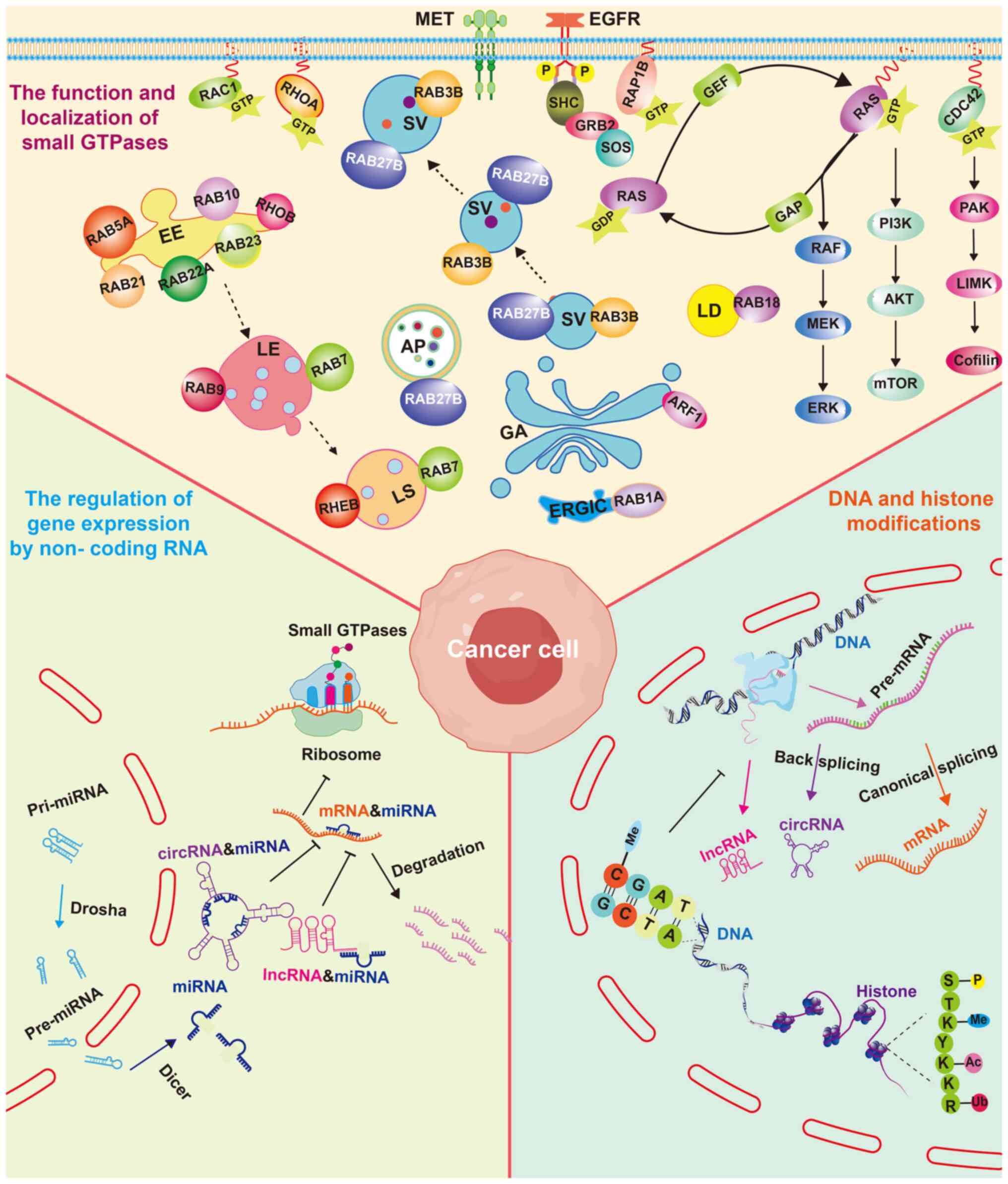
Gómez Del Pulgar T, Valdés-Mora F, Bandrés
E, Pérez-Palacios R, Espina C, Cejas P, García-Cabezas MA, Nistal
M, Casado E, González-Barón M, et al: Cdc42 is highly expressed in
colorectal adenocarcinoma and downregulates ID4 through an
epigenetic mechanism. Int J Oncol. 33:185–193. 2008.
|
|
23
|
Schnelzer A, Prechtel D, Knaus U, Dehne K,
Gerhard M, Graeff H, Harbeck N, Schmitt M and Lengyel E: Rac1 in
human breast cancer: Overexpression, mutation analysis, and
characterization of a new isoform, Rac1b. Oncogene. 19:3013–3020.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XR, Ji F, Ouyang J, Wu W, Qian LY and
Yang KY: Overexpression of RhoA is associated with poor prognosis
in hepatocellular carcinoma. Eur J Surg Oncol. 32:1130–1134. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazieres J, Antonia T, Daste G, Muro-Cacho
C, Berchery D, Tillement V, Pradines A, Sebti S and Favre G: Loss
of RhoB expression in human lung cancer progression. Clin Cancer
Res. 10:2742–2750. 2004. View Article : Google Scholar
|
|
26
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921. 2005.
View Article : Google Scholar
|
|
27
|
Li Y, Sun X, Ji D, Kong X, Liu D, Zhao Z,
Yan J and Chen S: Expression of Rab5a correlates with tumor
progression in pancreatic carcinoma. Virchows Arch. 470:527–536.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu X, Yin J, He R, Chao R and Zhu S:
Circ_KCNQ5 participates in the progression of childhood acute
myeloid leukemia by enhancing the expression of RAB10 via binding
to miR-622. Hematology. 27:431–440. 2022. View Article : Google Scholar
|
|
29
|
Ge J, Chen Q, Liu B, Wang L, Zhang S and
Ji B: Knockdown of Rab21 inhibits proliferation and induces
apoptosis in human glioma cells. Cell Mol Biol Lett. 22:302017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin Y, Zhang B, Wang W, Fei B, Quan C,
Zhang J, Song M, Bian Z, Wang Q, Ni S, et al: miR-204-5p inhibits
proliferation and invasion and enhances chemotherapeutic
sensitivity of colorectal cancer cells by downregulating RAB22A.
Clin Cancer Res. 20:6187–6199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JX, Huang XX, Cai MB, Tong ZT, Chen
JW, Qian D, Liao YJ, Deng HX, Liao DZ, Huang MY, et al:
Overexpression of the secretory small GTPase Rab27B in human breast
cancer correlates closely with lymph node metastasis and predicts
poor prognosis. J Transl Med. 10:2422012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
You X, Liu F, Zhang T, Li Y, Ye L and
Zhang X: Hepatitis B virus X protein upregulates oncogene Rab18 to
result in the dysregulation of lipogenesis and proliferation of
hepatoma cells. Carcinogenesis. 34:1644–1652. 2013. View Article : Google Scholar
|
|
33
|
de Bruijn I, Kundra R, Mastrogiacomo B,
Tran TN, Sikina L, Mazor T, Li X, Ochoa A, Zhao G, Lai B, et al:
Analysis and visualization of longitudinal genomic and clinical
data from the AACR Project GENIE biopharma collaborative in
cBioPortal. Cancer Res. 83:3861–3867. 2023. View Article : Google Scholar :
|
|
34
|
Wang XF, Shi ZM, Wang XR, Cao L, Wang YY,
Zhang JX, Yin Y, Luo H, Kang CS, Liu N, et al: MiR-181d acts as a
tumor suppressor in glioma by targeting K-ras and Bcl-2. J Cancer
Res Clin Oncol. 138:573–584. 2012. View Article : Google Scholar
|
|
35
|
Wang XR, Luo H, Li HL, Cao L, Wang XF, Yan
W, Wang YY, Zhang JX, Jiang T, Kang CS, et al: Overexpressed let-7a
inhibits glioma cell malignancy by directly targeting K-ras,
independently of PTEN. Neuro Oncol. 15:1491–1501. 2013. View Article : Google Scholar :
|
|
36
|
Shi Z, Chen Q, Li C, Wang L, Qian X, Jiang
C, Liu X, Wang X, Li H, Kang C, et al: MiR-124 governs glioma
growth and angiogenesis and enhances chemosensitivity by targeting
R-Ras and N-Ras. Neuro Oncol. 16:1341–1353. 2014. View Article : Google Scholar :
|
|
37
|
Zhang Y, Kim J, Mueller AC, Dey B, Yang Y,
Lee DH, Hachmann J, Finderle S, Park DM, Christensen J, et al:
Multiple receptor tyrosine kinases converge on microRNA-134 to
control KRAS, STAT5B, and glioblastoma. Cell Death Differ.
21:720–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Pang D, Wang C, Zhong S and Wang
S: MicroRNA-134 modulates glioma cell U251 proliferation and
invasion by targeting KRAS and suppressing the ERK pathway. Tumour
Biol. 37:11485–11493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
She X, Yu Z, Cui Y, Lei Q, Wang Z, Xu G,
Luo Z, Li G and Wu M: miR-181 subunits enhance the chemosensitivity
of temozolomide by Rap1B-mediated cytoskeleton remodeling in
glioblastoma cells. Med Oncol. 31:8922014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
She X, Yu Z, Cui Y, Lei Q, Wang Z, Xu G,
Xiang J, Wu M and Li G: miR-128 and miR-149 enhance the
chemosensitivity of temozolomide by Rap1B-mediated cytoskeletal
remodeling in glioblastoma. Oncol Rep. 32:957–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Z, Xu C, Ding B, Gao M, Wei X and Ji N:
Long non-coding RNA MALAT1 promotes proliferation and suppresses
apoptosis of glioma cells through derepressing Rap1B by sponging
miR-101. J Neurooncol. 134:19–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wan J, Guo AA and Chowdhury I: TRPM7
induces mechanistic target of Rap1b through the downregulation of
miR-28-5p in glioma proliferation and invasion. Front Oncol.
9:14132019. View Article : Google Scholar
|
|
43
|
Besse A, Sana J, Lakomy R, Kren L, Fadrus
P, Smrcka M, Hermanova M, Jancalek R, Reguli S, Lipina R, et al:
MiR-338-5p sensitizes glioblastoma cells to radiation through
regulation of genes involved in DNA damage response. Tumour Biol.
37:7719–7727. 2016. View Article : Google Scholar
|
|
44
|
Kalhori MR, Irani S, Soleimani M, Arefian
E and Kouhkan F: The effect of miR-579 on the PI3K/AKT pathway in
human glioblastoma PTEN mutant cell lines. J Cell Biochem.
120:16760–16774. 2019. View Article : Google Scholar
|
|
45
|
Kalhori MR, Arefian E, Fallah Atanaki F,
Kavousi K and Soleimani M: miR-548x and miR-4698 controlled cell
proliferation by affecting the PI3K/AKT signaling pathway in
Glioblastoma cell lines. Sci Rep. 10:15582020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schmidt N, Windmann S, Reifenberger G and
Riemenschneider MJ: DNA hypermethylation and histone modifications
downregulate the candidate tumor suppressor gene RRP22 on 22q12 in
human gliomas. Brain Pathol. 22:17–25. 2012. View Article : Google Scholar
|
|
47
|
Shi C, Ren L, Sun C, Yu L, Bian X, Zhou X,
Wen Y, Hua D, Zhao S, Luo W, et al: miR-29a/b/c function as
invasion suppressors for gliomas by targeting CDC42 and predict the
prognosis of patients. Br J Cancer. 117:1036–1047. 2017. View Article : Google Scholar
|
|
48
|
Sun G, Cao Y, Shi L, Sun L, Wang Y, Chen
C, Wan Z, Fu L and You Y: Overexpressed miRNA-137 inhibits human
glioma cells growth by targeting Rac1. Cancer Biother Radiopharm.
28:327–334. 2013.PubMed/NCBI
|
|
49
|
Qin W, Rong X, Dong J, Yu C and Yang J:
miR-142 inhibits the migration and invasion of glioma by targeting
Rac1. Oncol Rep. 38:1543–1550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang H, Wang Z, Liu X, Liu Q, Xu G, Li G
and Wu M: LRRC4 inhibits glioma cell growth and invasion through a
miR-185-dependent pathway. Curr Cancer Drug Targets. 12:1032–1042.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Q, Guo W, Zhang Y, Wu Y and Xiang J:
MiR-19a promotes cell proliferation and invasion by targeting RhoB
in human glioma cells. Neurosci Lett. 628:161–166. 2016. View Article : Google Scholar
|
|
52
|
Cai S, Shi CJ, Lu JX, Wang YP, Yuan T and
Wang XP: miR-124-3p inhibits the viability and motility of
glioblastoma multiforme by targeting RhoG. Int J Mol Med.
47:692021. View Article : Google Scholar
|
|
53
|
Quan Y, Song Q, Wang J, Zhao L, Lv J and
Gong S: MiR-1202 functions as a tumor suppressor in glioma cells by
targeting Rab1A. Tumour Biol. 39:10104283176975652017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu D, Yu J, Gao G, Lu G, Zhang Y and Ma P:
LncRNA DANCR functions as a competing endogenous RNA to regulate
RAB1A expression by sponging miR-634 in glioma. Biosci Rep.
38:BSR201716642018. View Article : Google Scholar :
|
|
55
|
Fu Z, Luo W, Wang J, Peng T, Sun G, Shi J,
Li Z and Zhang B: Malat1 activates autophagy and promotes cell
proliferation by sponging miR-101 and upregulating STMN1, RAB5A and
ATG4D expression in glioma. Biochem Biophys Res Commun.
492:480–486. 2017. View Article : Google Scholar
|
|
56
|
Gao W, Qiao M and Luo K: Long noncoding
RNA TP53TG1 contributes to radioresistance of glioma cells via
miR-524-5p/RAB5A axis. Cancer Biother Radiopharm. 36:600–612.
2021.
|
|
57
|
Zhang L, Chen H, Tian C and Zheng D:
Propofol represses cell growth and metastasis by modulating the
circular RNA Non-SMC condensin I complex subunit
G/MicroRNA-200a-3p/RAB5A axis in glioma. World Neurosurg.
153:e46–e58. 2021. View Article : Google Scholar
|
|
58
|
Wu YJ, Yang QS, Chen H, Wang JT, Wang WB
and Zhou L: Long non-coding RNA CASC19 promotes glioma progression
by modulating the miR-454-3p/RAB5A axis and is associated with
unfavorable MRI features. Oncol Rep. 45:728–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shen G, Mao Y, Su Z, Du J, Yu Y and Xu F:
PSMB8-AS1 activated by ELK1 promotes cell proliferation in glioma
via regulating miR-574-5p/RAB10. Biomed Pharmacother.
122:1096582020. View Article : Google Scholar
|
|
60
|
Zhang X, Wang S, Lin G and Wang D:
Down-regulation of circ-PTN suppresses cell proliferation, invasion
and glycolysis in glioma by regulating miR-432-5p/RAB10 axis.
Neurosci Lett. 735:1351532020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Peng G, Su J, Xiao S and Liu Q: LINC00152
acts as a potential marker in gliomas and promotes tumor
proliferation and invasion through the LINC00152/miR-107/RAB10
axis. J Neurooncol. 154:285–299. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Song D, Ye L, Xu Z, Jin Y and Zhang L:
CircRNA hsa_ circ_0030018 regulates the development of glioma via
regulating the miR-1297/RAB21 axis. Neoplasma. 68:391–403. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xia Z, Liu F, Zhang J and Liu L: Decreased
expression of MiRNA-204-5p contributes to glioma progression and
promotes glioma cell growth, migration and invasion. PLoS One.
10:e01323992015. View Article : Google Scholar
|
|
64
|
Wang H, Wang Y, Bao Z, Zhang C, Liu Y, Cai
J and Jiang C: Hypomethylated Rab27b is a progression-associated
prognostic biomarker of glioma regulating MMP-9 to promote
invasion. Oncol Rep. 34:1503–1509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu Q, Tang H, Liu X, Liao Y, Li H, Zhao
Z, Yuan X and Jiang W: miR-200b as a prognostic factor targets
multiple members of RAB family in glioma. Med Oncol. 31:8592014.
View Article : Google Scholar
|
|
66
|
López-Ginés C, Navarro L, Muñoz-Hidalgo L,
Buso E, Morales JM, Gil-Benso R, Gregori-Romero M, Megías J, Roldán
P, Segura-Sabater R, et al: Association between epidermal growth
factor receptor amplification and ADP-ribosylation factor 1
methylation in human glioblastoma. Cell Oncol (Dordr). 40:389–399.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tamura K, Stecher G and Kumar S: MEGA11:
Molecular evolutionary genetics analysis version 11. Mol Biol Evol.
38:3022–3027. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen QW, Zhu XY, Li YY and Meng ZQ:
Epigenetic regulation and cancer (review). Oncol Rep. 31:523–532.
2014. View Article : Google Scholar
|
|
69
|
Gu M, Ren B, Fang Y, Ren J, Liu X, Wang X,
Zhou F, Xiao R, Luo X, You L and Zhao Y: Epigenetic regulation in
cancer. MedComm (2020). 5:e4952024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zou Y and Xu H: Involvement of long
noncoding RNAs in the pathogenesis of autoimmune diseases. J Transl
Autoimmun. 3:1000442020. View Article : Google Scholar
|
|
71
|
Zong L, Zhou L, Hou Y, Zhang L, Jiang W,
Zhang W, Wang L, Luo X, Wang S, Deng C, et al: Genetic and
epigenetic regulation on the transcription of GABRB2:
Genotype-dependent hydroxymethylation and methylation alterations
in schizophrenia. J Psychiatr Res. 88:9–17. 2017. View Article : Google Scholar
|
|
72
|
Bai B, Chen S, Zhang Q, Jiang Q and Li H:
Abnormal epigenetic regulation of the gene expression levels of
Wnt2b and Wnt7b: Implications for neural tube defects. Mol Med Rep.
13:99–106. 2016. View Article : Google Scholar
|
|
73
|
Li P, Han M, Zhao X, Ren G, Mei S and
Zhong C: Abnormal epigenetic regulations in the immunocytes of
sjögren's syndrome patients and therapeutic potentials. Cells.
11:17672022. View Article : Google Scholar
|
|
74
|
Agirre X, Martínez-Climent JÁ, Odero M and
Prosper F: Epigenetic regulation of miRNA genes in acute leukemia.
Leukemia. 26:395–403. 2012. View Article : Google Scholar
|
|
75
|
Zeng C, Tsoi LC and Gudjonsson JE:
Dysregulated epigenetic modifications in psoriasis. Exp Dermatol.
30:1156–1166. 2021. View Article : Google Scholar
|
|
76
|
Ramazi S, Dadzadi M, Sahafnejad Z and
Allahverdi A: Epigenetic regulation in lung cancer. MedComm (2020).
4:e4012023. View Article : Google Scholar
|
|
77
|
Kondo Y, Katsushima K, Ohka F, Natsume A
and Shinjo K: Epigenetic dysregulation in glioma. Cancer Sci.
105:363–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lu Y, Chan YT, Tan HY, Li S, Wang N and
Feng Y: Epigenetic regulation in human cancer: The potential role
of epi-drug in cancer therapy. Mol Cancer. 19:792020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Robertson KD: DNA methylation and
chromatin-unraveling the tangled web. Oncogene. 21:5361–5379. 2002.
View Article : Google Scholar
|
|
80
|
Moore LD, Le T and Fan G: DNA methylation
and its basic function. Neuropsychopharmacology. 38:23–38. 2013.
View Article : Google Scholar
|
|
81
|
Illingworth RS and Bird AP: CpG islands-'a
rough guide'. FEBS Lett. 583:1713–1720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Antequera F: Structure, function and
evolution of CpG island promoters. Cell Mol Life Sci. 60:1647–1658.
2003. View Article : Google Scholar
|
|
83
|
Lopez-Serra L, Ballestar E, Fraga MF,
Alaminos M, Setien F and Esteller M: A profile of methyl-CpG
binding domain protein occupancy of hypermethylated promoter CpG
islands of tumor suppressor genes in human cancer. Cancer Res.
66:8342–8346. 2006. View Article : Google Scholar
|
|
84
|
Hervouet E, Peixoto P, Delage-Mourroux R,
Boyer-Guittaut M and Cartron PF: Specific or not specific
recruitment of DNMTs for DNA methylation, an epigenetic dilemma.
Clin Epigenetics. 10:172018. View Article : Google Scholar :
|
|
85
|
Rasmussen KD and Helin K: Role of TET
enzymes in DNA methylation, development, and cancer. Genes Dev.
30:733–750. 2016. View Article : Google Scholar :
|
|
86
|
Hatakeyama A, Hartmann B, Travers A,
Nogues C and Buckle M: High-resolution biophysical analysis of the
dynamics of nucleosome formation. Sci Rep. 6:273372016. View Article : Google Scholar
|
|
87
|
Torres IO and Fujimori DG: Functional
coupling between writers, erasers and readers of histone and DNA
methylation. Curr Opin Struct Biol. 35:68–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang G, Weng R, Lan Y, Guo X, Liu Q, Liu
X, Lu C and Kang J: Synergetic effects of DNA methylation and
histone modification during mouse induced pluripotent stem cell
generation. Sci Rep. 7:395272017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ng MK and Cheung P: A brief histone in
time: Understanding the combinatorial functions of histone PTMs in
the nucleosome context. Biochem Cell Biol. 94:33–42. 2016.
View Article : Google Scholar
|
|
90
|
Ke XS, Qu Y, Rostad K, Li WC, Lin B,
Halvorsen OJ, Haukaas SA, Jonassen I, Petersen K, Goldfinger N, et
al: Genome-wide profiling of histone h3 lysine 4 and lysine 27
trimethylation reveals an epigenetic signature in prostate
carcinogenesis. PLoS One. 4:e46872009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tamura I, Maekawa R, Jozaki K, Ohkawa Y,
Takagi H, Doi-Tanaka Y, Shirafuta Y, Mihara Y, Taketani T, Sato S,
et al: Transcription factor C/EBPβ induces genome-wide H3K27ac and
upregulates gene expression during decidualization of human
endometrial stromal cells. Mol Cell Endocrinol. 520:1110852021.
View Article : Google Scholar
|
|
92
|
Sungalee S, Liu Y, Lambuta RA, Katanayeva
N, Donaldson Collier M, Tavernari D, Roulland S, Ciriello G and
Oricchio E: Histone acetylation dynamics modulates chromatin
conformation and allele-specific interactions at oncogenic loci.
Nat Genet. 53:650–662. 2021. View Article : Google Scholar
|
|
93
|
Reda A, Hategan LA, McLean TAB, Creighton
SD, Luo JQ, Chen SES, Hua S, Winston S, Reeves I, Padmanabhan A, et
al: Role of the histone variant H2A.Z.1 in memory, transcription,
and alternative splicing is mediated by lysine modification.
Neuropsychopharmacology. 49:1285–1295. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nemeth K, Bayraktar R, Ferracin M and
Calin GA: Non-coding RNAs in disease: From mechanisms to
therapeutics. Nat Rev Genet. 25:211–232. 2024. View Article : Google Scholar
|
|
95
|
Sana J, Faltejskova P, Svoboda M and Slaby
O: Novel classes of non-coding RNAs and cancer. J Transl Med.
10:1032012. View Article : Google Scholar :
|
|
96
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar
|
|
97
|
Qin T, Li J and Zhang KQ: Structure,
regulation, and function of linear and circular long non-coding
RNAs. Front Genet. 11:1502020. View Article : Google Scholar :
|
|
98
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar :
|
|
99
|
Shang R, Lee S, Senavirathne G and Lai EC:
microRNAs in action: Biogenesis, function and regulation. Nat Rev
Genet. 24:816–833. 2023. View Article : Google Scholar
|
|
100
|
Uszczynska-Ratajczak B, Lagarde J,
Frankish A, Guigó R and Johnson R: Towards a complete map of the
human long non-coding RNA transcriptome. Nat Rev Genet. 19:535–548.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Khorkova O, Hsiao J and Wahlestedt C:
Basic biology and therapeutic implications of lncRNA. Adv Drug
Deliv Rev. 87:15–24. 2015. View Article : Google Scholar
|
|
102
|
Iwakiri J, Terai G and Hamada M:
Computational prediction of lncRNA-mRNA interactionsby integrating
tissue specificity in human transcriptome. Biol Direct. 12:152017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pisignano G, Michael DC, Visal TH, Pirlog
R, Ladomery M and Calin GA: Going circular: History, present, and
future of circRNAs in cancer. Oncogene. 42:2783–2800. 2023.
View Article : Google Scholar
|
|
104
|
Ma S, Kong S, Wang F and Ju S: CircRNAs:
Biogenesis, functions, and role in drug-resistant Tumours. Mol
Cancer. 19:1192020. View Article : Google Scholar :
|
|
105
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar
|
|
106
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yan H and Bu P: Non-coding RNA in cancer.
Essays Biochem. 65:625–639. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Elam C, Hesson L, Vos MD, Eckfeld K, Ellis
CA, Bell A, Krex D, Birrer MJ, Latif F and Clark GJ: RRP22 is a
farnesylated, nucleolar, Ras-related protein with tumor suppressor
potential. Cancer Res. 65:3117–3125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Riemenschneider MJ, Reifenberger J and
Reifenberger G: Frequent biallelic inactivation and transcriptional
silencing of the DIRAS3 gene at 1p31 in oligodendroglial tumors
with 1p loss. Int J Cancer. 122:2503–2510. 2008. View Article : Google Scholar
|
|
110
|
Tan Y, Zhang S, Xiao Q, Wang J, Zhao K,
Liu W, Huang K, Tian W, Niu H, Lei T and Shu K: Prognostic
significance of ARL9 and its methylation in low-grade glioma.
Genomics. 112:4808–4816. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rothhammer-Hampl T, Liesenberg F, Hansen
N, Hoja S, Delic S, Reifenberger G and Riemenschneider MJ: Frequent
epigenetic inactivation of DIRAS-1 and DIRAS-2 contributes to
chemo-resistance in gliomas. Cancers (Basel). 13:51132021.
View Article : Google Scholar
|
|
112
|
Bernal Astrain G, Nikolova M and Smith MJ:
Functional diversity in the RAS subfamily of small GTPases. Biochem
Soc Trans. 50:921–933. 2022. View Article : Google Scholar
|
|
113
|
Rásó E: Splice variants of
RAS-translational significance. Cancer Metastasis Rev.
39:1039–1049. 2020. View Article : Google Scholar
|
|
114
|
Prior IA, Hood FE and Hartley JL: The
frequency of ras mutations in cancer. Cancer Res. 80:2969–2974.
2020. View Article : Google Scholar
|
|
115
|
Knobbe CB, Reifenberger J and Reifenberger
G: Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and
BRAF in glioblastomas. Acta Neuropathol. 108:467–470. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:522017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sugiura R, Satoh R and Takasaki T: ERK: A
double-edged sword in cancer. ERK-dependent apoptosis as a
potential therapeutic strategy for cancer. Cells. 10:25092021.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar
|
|
119
|
An Z, Aksoy O, Zheng T, Fan QW and Weiss
WA: Epidermal growth factor receptor and EGFRvIII in glioblastoma:
Signaling pathways and targeted therapies. Oncogene. 37:1561–1575.
2018. View Article : Google Scholar
|
|
120
|
Yang J, Yan J and Liu B: Targeting
EGFRvIII for glioblastoma multiforme. Cancer Lett. 403:224–230.
2017. View Article : Google Scholar
|
|
121
|
Maruyama C, Tomisawa M, Wakana S, Yamazaki
H, Kijima H, Suemizu H, Ohnishi Y, Urano K, Hioki K, Usui T, et al:
Overexpression of human H-ras transgene is responsible for tumors
induced by chemical carcinogens in mice. Oncol Rep. 8:233–237.
2001.PubMed/NCBI
|
|
122
|
Tsunematsu S, Saito H, Kagawa T, Morizane
T, Hata J, Nakamura T, Ishii H, Tsuchiya M, Nomura T and Katsuki M:
Hepatic tumors induced by carbon tetrachloride in transgenic mice
carrying a human c-H-ras proto-oncogene without mutations. Int J
Cancer. 59:554–559. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Weber SM and Carroll SL: The role of R-Ras
proteins in normal and pathologic migration and morphologic change.
Am J Pathol. 191:1499–1510. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gutierrez-Erlandsson S, Herrero-Vidal P,
Fernandez-Alfara M, Hernandez-Garcia S, Gonzalo-Flores S,
Mudarra-Rubio A, Fresno M and Cubelos B: R-RAS2 overexpression in
tumors of the human central nervous system. Mol Cancer. 12:1272013.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Nakada M, Niska JA, Tran NL, McDonough WS
and Berens ME: EphB2/R-Ras signaling regulates glioma cell
adhesion, growth, and invasion. Am J Pathol. 167:565–576. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang YL, Wang RC, Cheng K, Ring BZ and Su
L: Roles of Rap1 signaling in tumor cell migration and invasion.
Cancer Biol Med. 14:90–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Altschuler D and Lapetina EG: Mutational
analysis of the cAMP-dependent protein kinase-mediated
phosphorylation site of Rap1b. J Biol Chem. 268:7527–7531. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wittchen ES, Aghajanian A and Burridge K:
Isoform-specific differences between Rap1A and Rap1B GTPases in the
formation of endothelial cell junctions. Small GTPases. 2:65–76.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lin KT, Yeh YM, Chuang CM, Yang SY, Chang
JW, Sun SP, Wang YS, Chao KC and Wang LH: Glucocorticoids mediate
induction of microRNA-708 to suppress ovarian cancer metastasis
through targeting Rap1B. Nat Commun. 6:59172015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yang Y, Li M, Yan Y, Zhang J, Sun K, Qu
JK, Wang JS and Duan XY: Expression of RAP1B is associated with
poor prognosis and promotes an aggressive phenotype in gastric
cancer. Oncol Rep. 34:2385–2394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Heard JJ, Fong V, Bathaie SZ and Tamanoi
F: Recent progress in the study of the Rheb family GTPases. Cell
Signal. 26:1950–1957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Armijo ME, Campos T, Fuentes-Villalobos F,
Palma ME, Pincheira R and Castro AF: Rheb signaling and
tumorigenesis: mTORC1 and new horizons. Int J Cancer.
138:1815–1823. 2016. View Article : Google Scholar
|
|
133
|
Basso AD, Mirza A, Liu G, Long BJ, Bishop
WR and Kirschmeier P: The farnesyl transferase inhibitor (FTI)
SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR
signaling. Role in FTI enhancement of taxane and tamoxifen
anti-tumor activity. J Biol Chem. 280:31101–31108. 2005. View Article : Google Scholar
|
|
134
|
Mecca C, Giambanco I, Donato R and Arcuri
C: Targeting mTOR in Glioblastoma: Rationale and
Preclinical/Clinical Evidence. Dis Markers. 2018:92304792018.
View Article : Google Scholar
|
|
135
|
Colardo M, Segatto M and Di Bartolomeo S:
Targeting RTK-PI3K-mTOR axis in gliomas: An update. Int J Mol Sci.
22:48992021. View Article : Google Scholar
|
|
136
|
Liu M, Bi F, Zhou X and Zheng Y: Rho
GTPase regulation by miRNAs and covalent modifications. Trends Cell
Biol. 22:365–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hodge RG and Ridley AJ: Regulating Rho
GTPases and their regulators. Nat Rev Mol Cell Biol. 17:496–510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
O'Connor K and Chen M: Dynamic functions
of RhoA in tumor cell migration and invasion. Small GTPases.
4:141–147. 2013. View Article : Google Scholar
|
|
139
|
Yin M, Lu Q, Liu X, Wang T, Liu Y and Chen
L: Silencing Drp1 inhibits glioma cells proliferation and invasion
by RHOA/ROCK1 pathway. Biochem Biophys Res Commun. 478:663–668.
2016. View Article : Google Scholar
|
|
140
|
Xiong W, Yin A, Mao X, Zhang W, Huang H
and Zhang X: Resveratrol suppresses human glioblastoma cell
migration and invasion via activation of RhoA/ROCK signaling
pathway. Oncol Lett. 11:484–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Talamillo A, Grande L, Ruiz-Ontañon P,
Velasquez C, Mollinedo P, Torices S, Sanchez-Gomez P, Aznar A,
Esparis-Ogando A, Lopez-Lopez C, et al: ODZ1 allows glioblastoma to
sustain invasiveness through a Myc-dependent transcriptional
upregulation of RhoA. Oncogene. 36:1733–1744. 2017. View Article : Google Scholar
|
|
142
|
Ridley AJ: Rho GTPases and actin dynamics
in membrane protrusions and vesicle trafficking. Trends Cell Biol.
16:522–529. 2006. View Article : Google Scholar
|
|
143
|
Huang M and Prendergast GC: RhoB in cancer
suppression. Histol Histopathol. 21:213–218. 2006.
|
|
144
|
Zaoui K and Duhamel S: RhoB as a tumor
suppressor: It's all about localization. Eur J Cell Biol.
102:1513132023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wei LJ, Li JA, Bai DM and Song Y:
miR-223-RhoB signaling pathway regulates the proliferation and
apoptosis of colon adenocarcinoma. Chem Biol Interact. 289:9–14.
2018. View Article : Google Scholar
|
|
146
|
Prendergast GC: Actin' up: RhoB in cancer
and apoptosis. Nat Rev Cancer. 1:162–168. 2001. View Article : Google Scholar
|
|
147
|
Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng
Y and Bi F: miR-21 targets the tumor suppressor RhoB and regulates
proliferation, invasion and apoptosis in colorectal cancer cells.
FEBS Lett. 585:2998–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Cimbora-Zovko T, Fritz G, Mikac N and
Osmak M: Downregulation of RhoB GTPase confers resistance to
cisplatin in human laryngeal carcinoma cells. Cancer Lett.
295:182–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kazanietz MG and Caloca MJ: The Rac GTPase
in cancer: From old concepts to new paradigms. Cancer Res.
77:5445–5451. 2017. View Article : Google Scholar :
|
|
150
|
Steffen A, Ladwein M, Dimchev GA, Hein A,
Schwenkmezger L, Arens S, Ladwein KI, Margit Holleboom J, Schur F,
Victor Small J, et al: Rac function is crucial for cell migration
but is not required for spreading and focal adhesion formation. J
Cell Sci. 126:4572–4588. 2013.PubMed/NCBI
|
|
151
|
Bosco EE, Mulloy JC and Zheng Y: Rac1
GTPase: A 'Rac' of all trades. Cell Mol Life Sci. 66:370–374. 2009.
View Article : Google Scholar
|
|
152
|
Leng R, Liao G, Wang H, Kuang J and Tang
L: Rac1 expression in epithelial ovarian cancer: effect on cell EMT
and clinical outcome. Med Oncol. 32:3292015. View Article : Google Scholar
|
|
153
|
Dokmanovic M, Hirsch DS, Shen Y and Wu WJ:
Rac1 contributes to trastuzumab resistance of breast cancer cells:
Rac1 as a potential therapeutic target for the treatment of
trastuzumab-resistant breast cancer. Mol Cancer Ther. 8:1557–1569.
2009. View Article : Google Scholar
|
|
154
|
Heid I, Lubeseder-Martellato C, Sipos B,
Mazur PK, Lesina M, Schmid RM and Siveke JT: Early requirement of
Rac1 in a mouse model of pancreatic cancer. Gastroenterology.
141:719–730. 730.e1–7. 2011. View Article : Google Scholar
|
|
155
|
Kwiatkowska A, Didier S, Fortin S, Chuang
Y, White T, Berens ME, Rushing E, Eschbacher J, Tran NL, Chan A and
Symons M: The small GTPase RhoG mediates glioblastoma cell
invasion. Mol Cancer. 11:652012. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Cerione RA: Cdc42: New roads to travel.
Trends Cell Biol. 14:127–132. 2004. View Article : Google Scholar
|
|
157
|
Etienne-Manneville S: Cdc42-the centre of
polarity. J Cell Sci. 117:1291–1300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
Cdc42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar
|
|
159
|
Chen QY, Jiao DM, Yao QH, Yan J, Song J,
Chen FY, Lu GH and Zhou JY: Expression analysis of Cdc42 in lung
cancer and modulation of its expression by curcumin in lung cancer
cell lines. Int J Oncol. 40:1561–1568. 2012.
|
|
160
|
Zucman-Rossi J, Legoix P and Thomas G:
Identification of new members of the Gas2 and Ras families in the
22q12 chromosome region. Genomics. 38:247–254. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhen Y and Stenmark H: Cellular functions
of Rab GTPases at a glance. J Cell Sci. 128:3171–3176. 2015.
|
|
162
|
Stenmark H: Rab GTPases as coordinators of
vesicle traffic. Nat Rev Mol Cell Biol. 10:513–525. 2009.
View Article : Google Scholar
|
|
163
|
Homma Y, Hiragi S and Fukuda M: Rab family
of small GTPases: An updated view on their regulation and
functions. FEBS J. 288:36–55. 2021. View Article : Google Scholar :
|
|
164
|
Liu Y, Wang X, Zhang Z, Xiao B, An B and
Zhang J: The overexpression of Rab9 promotes tumor progression
regulated by XBP1 in breast cancer. Onco Targets Ther.
12:1815–1824. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Li Z, Li Y, Jia Y, Ding B and Yu J: Rab1A
knockdown represses proliferation and promotes apoptosis in gastric
cancer cells by inhibition of mTOR/p70S6K pathway. Arch Biochem
Biophys. 685:1083522020. View Article : Google Scholar
|
|
166
|
Zhao Z, Liu XF, Wu HC, Zou SB, Wang JY, Ni
PH, Chen XH and Fan QS: Rab5a overexpression promoting ovarian
cancer cell proliferation may be associated with APPL1-related
epidermal growth factor signaling pathway. Cancer Sci.
101:1454–1462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhang D, Lu C and Ai H: Rab5a is
overexpressed in oral cancer and promotes invasion through ERK/MMP
signaling. Mol Med Rep. 16:4569–4576. 2017. View Article : Google Scholar
|
|
168
|
Ge J and Ge C: Rab14 overexpression
regulates gemcitabine sensitivity through regulation of Bcl-2 and
mitochondrial function in pancreatic cancer. Virchows Arch.
474:59–69. 2019. View Article : Google Scholar
|
|
169
|
Yang XZ, Li XX, Zhang YJ,
Rodriguez-Rodriguez L, Xiang MQ, Wang HY and Zheng XF: Rab1 in cell
signaling, cancer and other diseases. Oncogene. 35:5699–5704. 2016.
View Article : Google Scholar
|
|
170
|
Zenner HL, Yoshimura S, Barr FA and Crump
CM: Analysis of Rab GTPase-activating proteins indicates that
Rab1a/b and Rab43 are important for herpes simplex virus 1
secondary envelopment. J Virol. 85:8012–8021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Nuoffer C, Davidson HW, Matteson J,
Meinkoth J and Balch WE: A GDP-bound of rab1 inhibits protein
export from the endoplasmic reticulum and transport between Golgi
compartments. J Cell Biol. 125:225–237. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Zhang Y, Wang L, Lv Y, Jiang C, Wu G, Dull
RO, Minshall RD, Malik AB and Hu G: The GTPase Rab1 is required for
NLRP3 inflammasome activation and inflammatory lung injury. J
Immunol. 202:194–206. 2019. View Article : Google Scholar
|
|
173
|
Yang XZ, Cui SZ, Zeng LS, Cheng TT, Li XX,
Chi J, Wang R, Zheng XF and Wang HY: Overexpression of Rab1B and
MMP9 predicts poor survival and good response to chemotherapy in
patients with colorectal cancer. Aging (Albany NY). 9:914–931.
2017. View Article : Google Scholar
|
|
174
|
Wang X, Liu F, Qin X, Huang T, Huang B,
Zhang Y and Jiang B: Expression of Rab1A is upregulated in human
lung cancer and associated with tumor size and T stage. Aging
(Albany NY). 8:2790–2798. 2016. View Article : Google Scholar
|
|
175
|
Xu M, Shao X, Kuai X, Zhang L, Zhou C and
Cheng Z: Expression analysis and implication of Rab1A in
gastrointestinal relevant tumor. Sci Rep. 9:133842019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Ren B, Wang L, Nan Y, Liu T, Zhao L, Ma H,
Li J, Zhang Y and Ren X: RAB1A regulates glioma cellular
proliferation and invasion via the mTOR signaling pathway and
epithelial-mesenchymal transition. Future Oncol. 17:3203–3216.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lanzetti L, Palamidessi A, Areces L, Scita
G and Di Fiore PP: Rab5 is a signalling GTPase involved in actin
remodelling by receptor tyrosine kinases. Nature. 429:309–314.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Chiariello M, Bruni CB and Bucci C: The
small GTPases Rab5a, Rab5b and Rab5c are differentially
phosphorylated in vitro. FEBS Lett. 453:20–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Tan JY, Jia LQ, Shi WH, He Q, Zhu L and Yu
B: Rab5a-mediated autophagy regulates the phenotype and behavior of
vascular smooth muscle cells. Mol Med Rep. 14:4445–4453. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Li Y, Zhao Y, Hu J, Xiao J, Qu L, Wang Z,
Ma D and Chen Y: A novel ER-localized transmembrane protein, EMC6,
interacts with RAB5A and regulates cell autophagy. Autophagy.
9:150–163. 2013. View Article : Google Scholar
|
|
181
|
Yu MH, Luo Y, Qin SL and Zhong M:
Increased expression of Rab5A predicts metastasis and poor
prognosis in colorectal cancer patients. Int J Clin Exp Pathol.
8:6974–6980. 2015.
|
|
182
|
Engebraaten O, Yau C, Berg K, Borgen E,
Garred Ø, Berstad MEB, Fremstedal ASV, DeMichele A, Veer LV',
Esserman L and Weyergang A: RAB5A expression is a predictive
biomarker for trastuzumab emtansine in breast cancer. Nat Commun.
12:64272021. View Article : Google Scholar
|
|
183
|
English AR and Voeltz GK: Rab10 GTPase
regulates ER dynamics and morphology. Nat Cell Biol. 15:169–178.
2013. View Article : Google Scholar
|
|
184
|
Chen YT, Holcomb C and Moore HP:
Expression and localization of two low molecular weight GTP-binding
proteins, Rab8 and Rab10, by epitope tag. Proc Natl Acad Sci USA.
90:6508–6512. 1993. View Article : Google Scholar
|
|
185
|
Leaf DS and Blum LD: Analysis of rab10
localization in sea urchin embryonic cells by three-dimensional
reconstruction. Exp Cell Res. 243:39–49. 1998. View Article : Google Scholar
|
|
186
|
Cardoso CM, Jordao L and Vieira OV: Rab10
regulates phagosome maturation and its overexpression rescues
Mycobacterium-containing phagosomes maturation. Traffic.
11:221–235. 2010. View Article : Google Scholar
|
|
187
|
Babbey CM, Bacallao RL and Dunn KW: Rab10
associates with primary cilia and the exocyst complex in renal
epithelial cells. Am J Physiol Renal Physiol. 299:F495–F506. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Lerner DW, McCoy D, Isabella AJ, Mahowald
AP, Gerlach GF, Chaudhry TA and Horne-Badovinac S: A
Rab10-dependent mechanism for polarized basement membrane secretion
during organ morphogenesis. Dev Cell. 24:159–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Isabella AJ and Horne-Badovinac S:
Rab10-mediated secretion synergizes with tissue movement to build a
polarized basement membrane architecture for organ morphogenesis.
Dev Cell. 38:47–60. 2016. View Article : Google Scholar
|
|
190
|
Etoh K and Fukuda M: Rab10 regulates
tubular endosome formation through KIF13A and KIF13B motors. J Cell
Sci. 132:jcs2269772019. View Article : Google Scholar
|
|
191
|
Zhuo J, Han J, Zhao Y, Hao R, Shen C, Li
H, Dai L, Sheng A, Yao H, Yang X and Liu W: RAB10 promotes breast
cancer proliferation migration and invasion predicting a poor
prognosis for breast cancer. Sci Rep. 13:152522023. View Article : Google Scholar :
|
|
192
|
Wang W, Jia WD, Hu B and Pan YY: RAB10
overexpression promotes tumor growth and indicates poor prognosis
of hepatocellular carcinoma. Oncotarget. 8:26434–26447. 2017.
View Article : Google Scholar :
|
|
193
|
Scita G and Di Fiore PP: The endocytic
matrix. Nature. 463:464–473. 2010. View Article : Google Scholar
|
|
194
|
Simpson JC and Jones AT: Early endocytic
Rabs: Functional prediction to functional characterization. Biochem
Soc Symp. 72:99–108. 2005. View Article : Google Scholar
|
|
195
|
Simpson JC, Griffiths G, Wessling-Resnick
M, Fransen JA, Bennett H and Jones AT: A role for the small GTPase
Rab21 in the early endocytic pathway. J Cell Sci. 117(Pt 26):
6297–6311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Evans TM, Ferguson C, Wainwright BJ,
Parton RG and Wicking C: Rab23, a negative regulator of hedgehog
signaling, localizes to the plasma membrane and the endocytic
pathway. Traffic. 4:869–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Pellinen T, Arjonen A, Vuoriluoto K,
Kallio K, Fransen JA and Ivaska J: Small GTPase Rab21 regulates
cell adhesion and controls endosomal traffic of beta1-integrins. J
Cell Biol. 173:767–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zhou Y, Wu B, Li JH, Nan G, Jiang JL and
Chen ZN: Rab22a enhances CD147 recycling and is required for lung
cancer cell migration and invasion. Exp Cell Res. 357:9–16. 2017.
View Article : Google Scholar
|
|
199
|
Takeda M, Koseki J, Takahashi H, Miyoshi
N, Nishida N, Nishimura J, Hata T, Matsuda C, Mizushima T, Yamamoto
H, et al: Disruption of Endolysosomal RAB5/7 efficiently eliminates
colorectal cancer stem cells. Cancer Res. 79:1426–1437. 2019.
View Article : Google Scholar
|
|
200
|
Chen Y, Ng F and Tang BL: Rab23 activities
and human cancer-emerging connections and mechanisms. Tumour Biol.
37:12959–12967. 2016. View Article : Google Scholar
|
|
201
|
Lledo PM, Johannes L, Vernier P, Zorec R,
Darchen F, Vincent JD, Henry JP and Mason WT: Rab3 proteins: Key
players in the control of exocytosis. Trends Neurosci. 17:426–432.
1994. View Article : Google Scholar
|
|
202
|
Martin S and Parton RG: Characterization
of Rab18, a lipid droplet-associated small GTPase. Methods Enzymol.
438:109–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Gerondopoulos A, Bastos RN, Yoshimura S,
Anderson R, Carpanini S, Aligianis I, Handley MT and Barr FA: Rab18
and a Rab18 GEF complex are required for normal ER structure. J
Cell Biol. 205:707–720. 2014. View Article : Google Scholar :
|
|
204
|
Dejgaard SY, Murshid A, Erman A, Kizilay
O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig TB, Pepperkok R,
Simpson JC and Presley JF: Rab18 and Rab43 have key roles in
ER-Golgi trafficking. J Cell Sci. 121(Pt 16): 2768–2781. 2008.
View Article : Google Scholar
|
|
205
|
Fukuda M: Rab27 effectors, pleiotropic
regulators in secretory pathways. Traffic. 14:949–963. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Ostrowski M, Carmo NB, Krumeich S, Fanget
I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et
al: Rab27a and Rab27b control different steps of the exosome
secretion pathway. Nat Cell Biol. 12:19–30. 2010. View Article : Google Scholar
|
|
207
|
Li Z, Fang R, Fang J, He S and Liu T:
Functional implications of Rab27 GTPases in cancer. Cell Commun
Signal. 16:442018. View Article : Google Scholar
|
|
208
|
Donaldson JG and Jackson CL: ARF family G
proteins and their regulators: Roles in membrane transport,
development and disease. Nat Rev Mol Cell Biol. 12:362–375. 2011.
View Article : Google Scholar
|
|
209
|
D'Souza-Schorey C and Chavrier P: ARF
proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell
Biol. 7:347–358. 2006. View Article : Google Scholar
|
|
210
|
Donaldson JG and Honda A: Localization and
function of Arf family GTPases. Biochem Soc Trans. 33(Pt 4):
639–642. 2005. View Article : Google Scholar
|
|
211
|
Gillingham AK and Munro S: The small G
proteins of the Arf family and their regulators. Annu Rev Cell Dev
Biol. 23:579–611. 2007. View Article : Google Scholar
|
|
212
|
Schlienger S, Campbell S and Claing A:
ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast
cancer cell invasion. Mol Biol Cell. 25:17–29. 2014. View Article : Google Scholar
|
|
213
|
Boulay PL, Schlienger S, Lewis-Saravalli
S, Vitale N, Ferbeyre G and Claing A: ARF1 controls proliferation
of breast cancer cells by regulating the retinoblastoma protein.
Oncogene. 30:3846–3861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Ma H, Fang W, Li Q, Wang Y and Hou SX:
Arf1 ablation in colorectal cancer cells activates a super signal
complex in DC to enhance anti-tumor immunity. Adv Sci (Weinh).
10:e23050892023. View Article : Google Scholar
|
|
215
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Rong L, Li N and Zhang Z: Emerging
therapies for glioblastoma: Current state and future directions. J
Exp Clin Cancer Res. 41:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Weller M, van den Bent M, Preusser M, Le
Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven
L, et al: EANO guidelines on the diagnosis and treatment of diffuse
gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186. 2021.
View Article : Google Scholar
|
|
218
|
Li T, Li J, Chen Z, Zhang S, Li S, Wageh
S, Al-Hartomy OA, Al-Sehemi AG, Xie Z, Kankala RK and Zhang H:
Glioma diagnosis and therapy: Current challenges and
nanomaterial-based solutions. J Control Release. 352:338–370. 2022.
View Article : Google Scholar
|
|
219
|
Liu F, Wang Y, Gu H and Wang X:
Technologies and applications of single-cell DNA methylation
sequencing. Theranostics. 13:2439–2454. 2023. View Article : Google Scholar :
|
|
220
|
Alshammari E, Zhang Y, Sobota J and Yang
Z: Aberrant DNA methylation of tumor suppressor genes and oncogenes
as cancer biomarkers. Genomic and Epigenomic Biomarkers of
Toxicology and Disease: Clinical and Therapeutic Actions. Sahu SC:
Wiley; Oxford: pp. 251–271. 2022, View Article : Google Scholar
|
|
221
|
Da Costa EM, McInnes G, Beaudry A and
Raynal NJ: DNA Methylation-targeted drugs. Cancer J. 23:270–276.
2017. View Article : Google Scholar
|
|
222
|
Diesch J, Zwick A, Garz AK, Palau A,
Buschbeck M and Götze KS: A clinical-molecular update on
azanucleoside-based therapy for the treatment of hematologic
cancers. Clin Epigenetics. 8:712016. View Article : Google Scholar :
|
|
223
|
Federici L, Capelle L, Annereau M, Bielle
F, Willekens C, Dehais C, Laigle-Donadey F, Hoang-Xuan K, Delattre
JY, Idbaih A, et al: 5-Azacitidine in patients with IDH1/2-mutant
recurrent glioma. Neuro Oncol. 22:1226–1228. 2020. View Article : Google Scholar
|
|
224
|
He W, Lin S, Guo Y, Wu Y, Zhang LL, Deng
Q, Du ZM, Wei M, Zhu W, Chen WJ, et al: Targeted demethylation at
ZNF154 promotor upregulates ZNF154 expression and inhibits the
proliferation and migration of Esophageal squamous carcinoma cells.
Oncogene. 41:4537–4546. 2022. View Article : Google Scholar
|
|
225
|
Zaib S, Rana N and Khan I: Histone
modifications and their role in epigenetics of cancer. Curr Med
Chem. 29:2399–2411. 2022. View Article : Google Scholar
|
|
226
|
Chen R, Zhang M, Zhou Y, Guo W, Yi M,
Zhang Z, Ding Y and Wang Y: The application of histone deacetylases
inhibitors in glioblastoma. J Exp Clin Cancer Res. 39:1382020.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Wang F, Jin Y, Wang M, Luo HY, Fang WJ,
Wang YN, Chen YX, Huang RJ, Guan WL, Li JB, et al: Combined
anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal
cancer: A randomized phase 2 trial. Nat Med. 30:1035–1043. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Gao Y, He H, Li X, Zhang L, Xu W, Feng R,
Li W, Xiao Y, Liu X, Chen Y, et al: Sintilimab (anti-PD-1 antibody)
plus chidamide (histone deacetylase inhibitor) in relapsed or
refractory extranodal natural killer T-cell lymphoma (SCENT): A
phase Ib/II study. Signal Transduct Target Ther. 9:1212024.
View Article : Google Scholar :
|
|
229
|
Monje M, Cooney T, Glod J, Huang J, Peer
CJ, Faury D, Baxter P, Kramer K, Lenzen A, Robison NJ, et al: Phase
I trial of panobinostat in children with diffuse intrinsic pontine
glioma: A report from the Pediatric brain tumor consortium
(PBTC-047). Neuro Oncol. 25:2262–2272. 2023. View Article : Google Scholar :
|
|
230
|
Mueller S, Kline C, Stoller S, Lundy S,
Christopher L, Reddy AT, Banerjee A, Cooney TM, Raber S, Hoffman C,
et al: PNOC015: Repeated convection-enhanced delivery of MTX110
(aqueous panobinostat) in children with newly diagnosed diffuse
intrinsic pontine glioma. Neuro Oncol. 25:2074–2086. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Xu K, Ramesh K, Huang V, Gurbani SS,
Cordova JS, Schreibmann E, Weinberg BD, Sengupta S, Voloschin AD,
Holdhoff M, et al: Final report on clinical outcomes and tumor
recurrence patterns of a pilot study assessing efficacy of
belinostat (PXD-101) with chemoradiation for newly diagnosed
glioblastoma. Tomography. 8:688–700. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Raedler LA: Farydak (Panobinostat): First
HDAC inhibitor approved for patients with relapsed multiple
myeloma. Am Health Drug Benefits. 9(Spec Feature): 84–87.
2016.PubMed/NCBI
|
|
233
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38:532019. View Article : Google Scholar :
|
|
234
|
Li Z and Rana TM: Therapeutic targeting of
microRNAs: current status and future challenges. Nat Rev Drug
Discov. 13:622–638. 2014. View Article : Google Scholar
|
|
235
|
Petrescu GED, Sabo AA, Torsin LI, Calin GA
and Dragomir MP: MicroRNA based theranostics for brain cancer:
Basic principles. J Exp Clin Cancer Res. 38:2312019. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Mathupala SP, Mittal S, Guthikonda M and
Sloan AE: MicroRNA and brain tumors: A cause and a cure? DNA Cell
Biol. 26:301–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Morokoff A, Jones J, Nguyen H, Ma C,
Lasocki A, Gaillard F, Bennett I, Luwor R, Stylli S, Paradiso L, et
al: Serum microRNA is a biomarker for post-operative monitoring in
glioma. J Neurooncol. 149:391–400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Yue X, Lan F, Hu M, Pan Q, Wang Q and Wang
J: Downregulation of serum microRNA-205 as a potential diagnostic
and prognostic biomarker for human glioma. J Neurosurg.
124:122–128. 2016. View Article : Google Scholar
|
|
239
|
Sun Y, Jing Y and Zhang Y: Serum
lncRNA-ANRIL and SOX9 expression levels in glioma patients and
their relationship with poor prognosis. World J Surg Oncol.
19:2872021. View Article : Google Scholar :
|
|
240
|
Tan SK, Pastori C, Penas C, Komotar RJ,
Ivan ME, Wahlestedt C and Ayad NG: Serum long noncoding RNA HOTAIR
as a novel diagnostic and prognostic biomarker in glioblastoma
multiforme. Mol Cancer. 17:742018. View Article : Google Scholar :
|
|
241
|
Stella M, Falzone L, Caponnetto A, Gattuso
G, Barbagallo C, Battaglia R, Mirabella F, Broggi G, Altieri R,
Certo F, et al: Serum extracellular vesicle-derived circHIPK3 and
circS-MARCA5 are two novel diagnostic biomarkers for glioblastoma
multiforme. Pharmaceuticals (Basel). 14:6182021. View Article : Google Scholar
|
|
242
|
Li P, Xu Z, Liu T, Liu Q, Zhou H, Meng S,
Feng Z, Tang Y, Liu C, Feng J, et al: Circular RNA sequencing
reveals serum exosome circular RNA panel for high-grade astrocytoma
diagnosis. Clin Chem. 68:332–343. 2022. View Article : Google Scholar
|