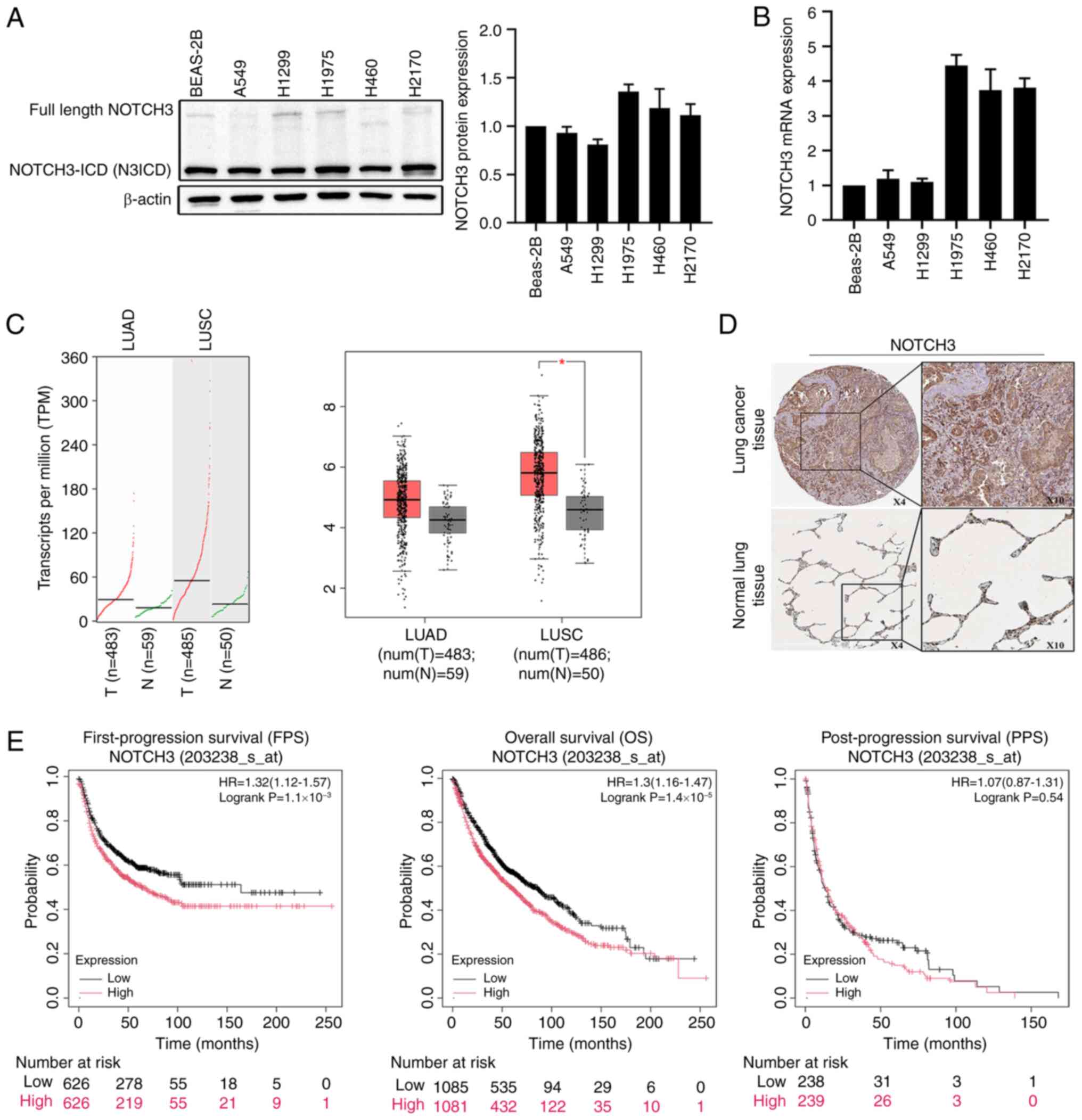
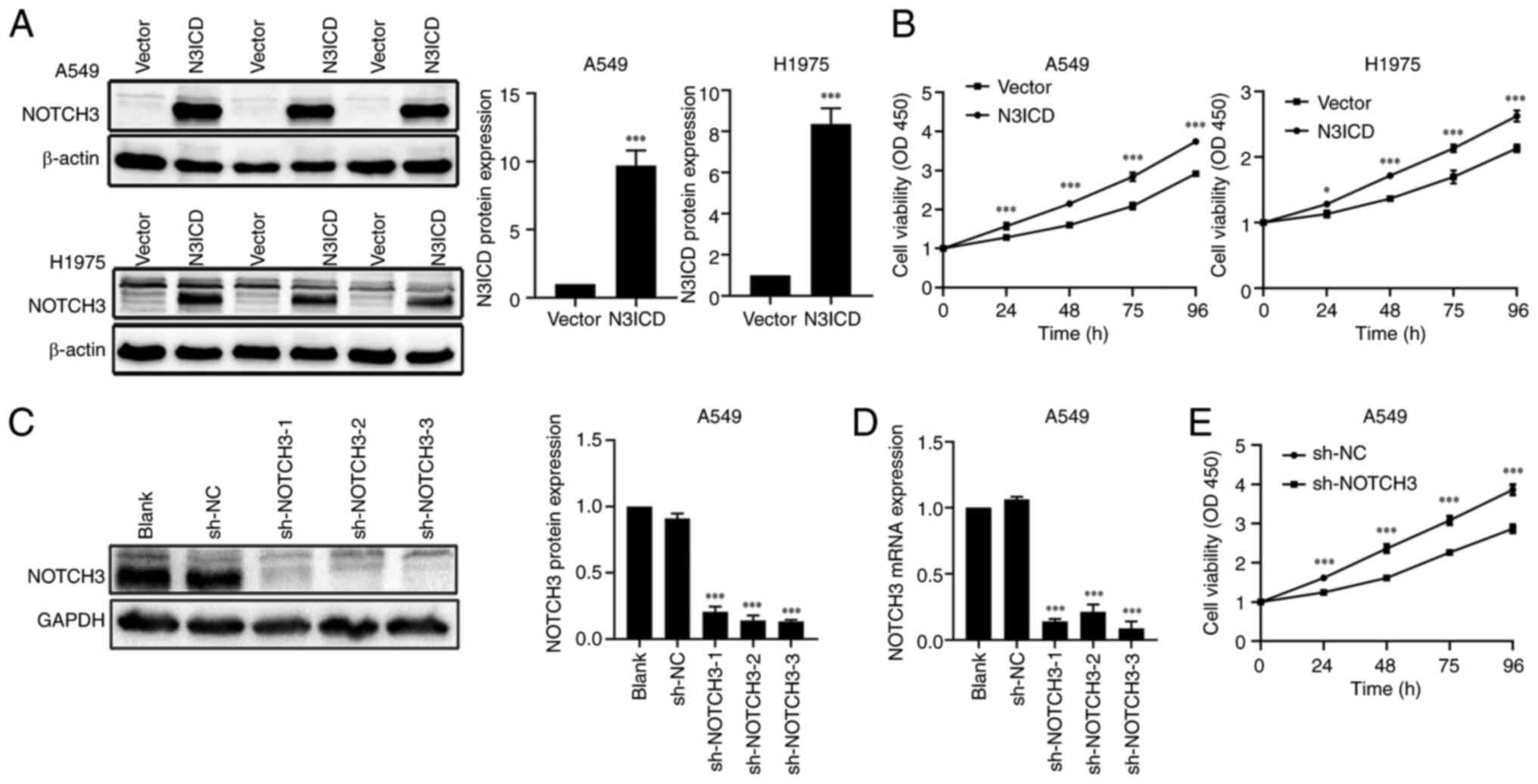
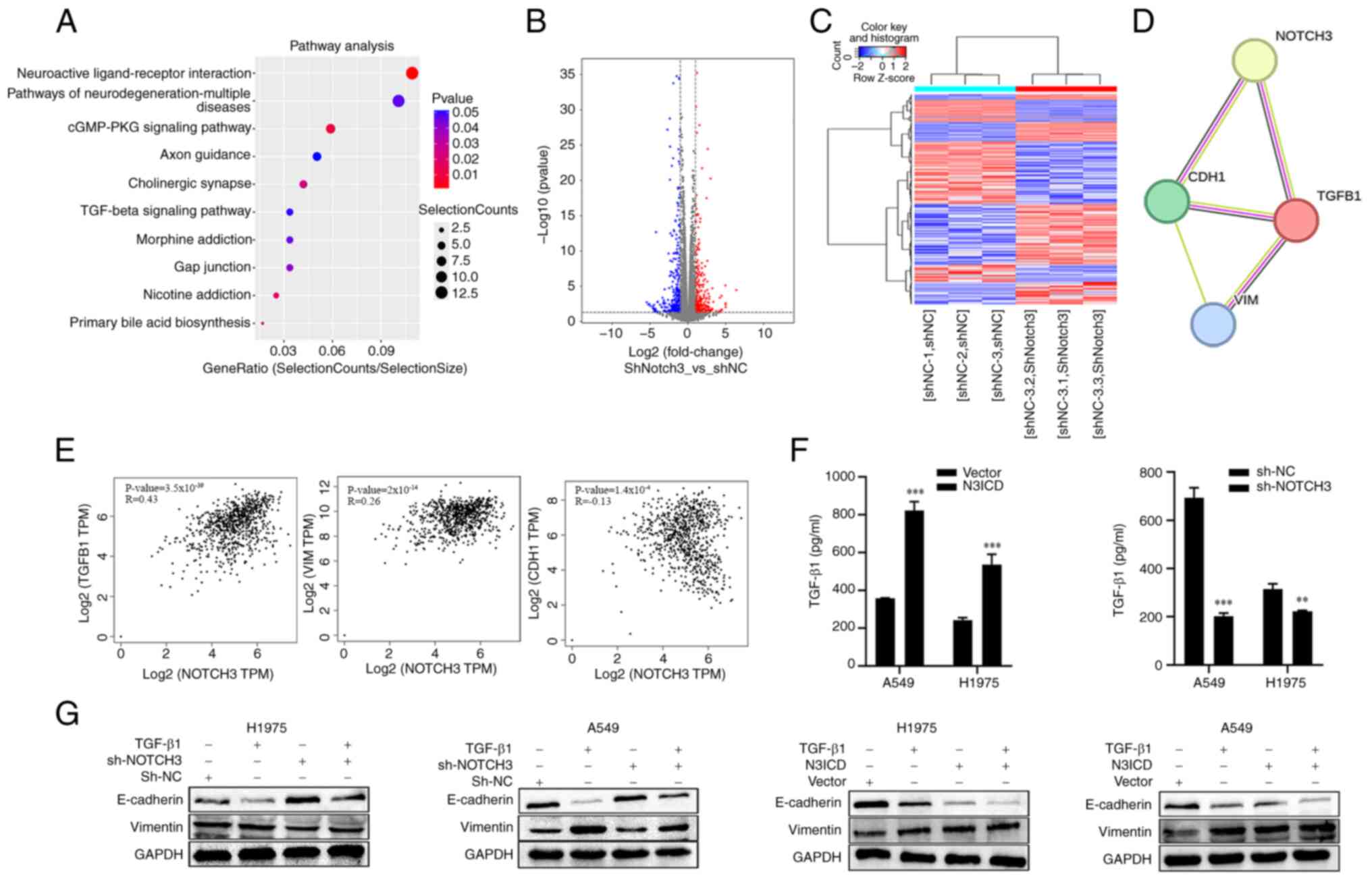
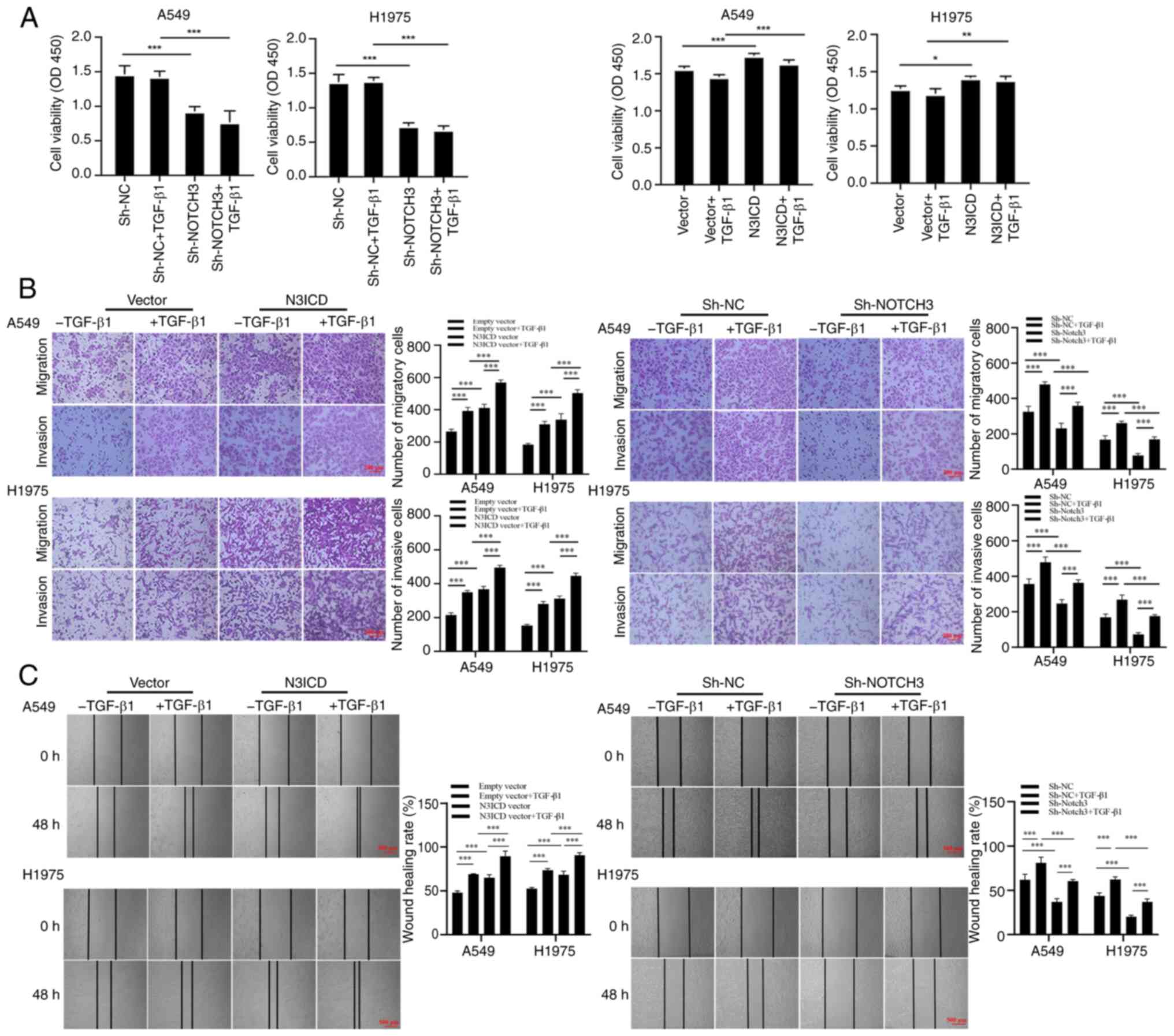
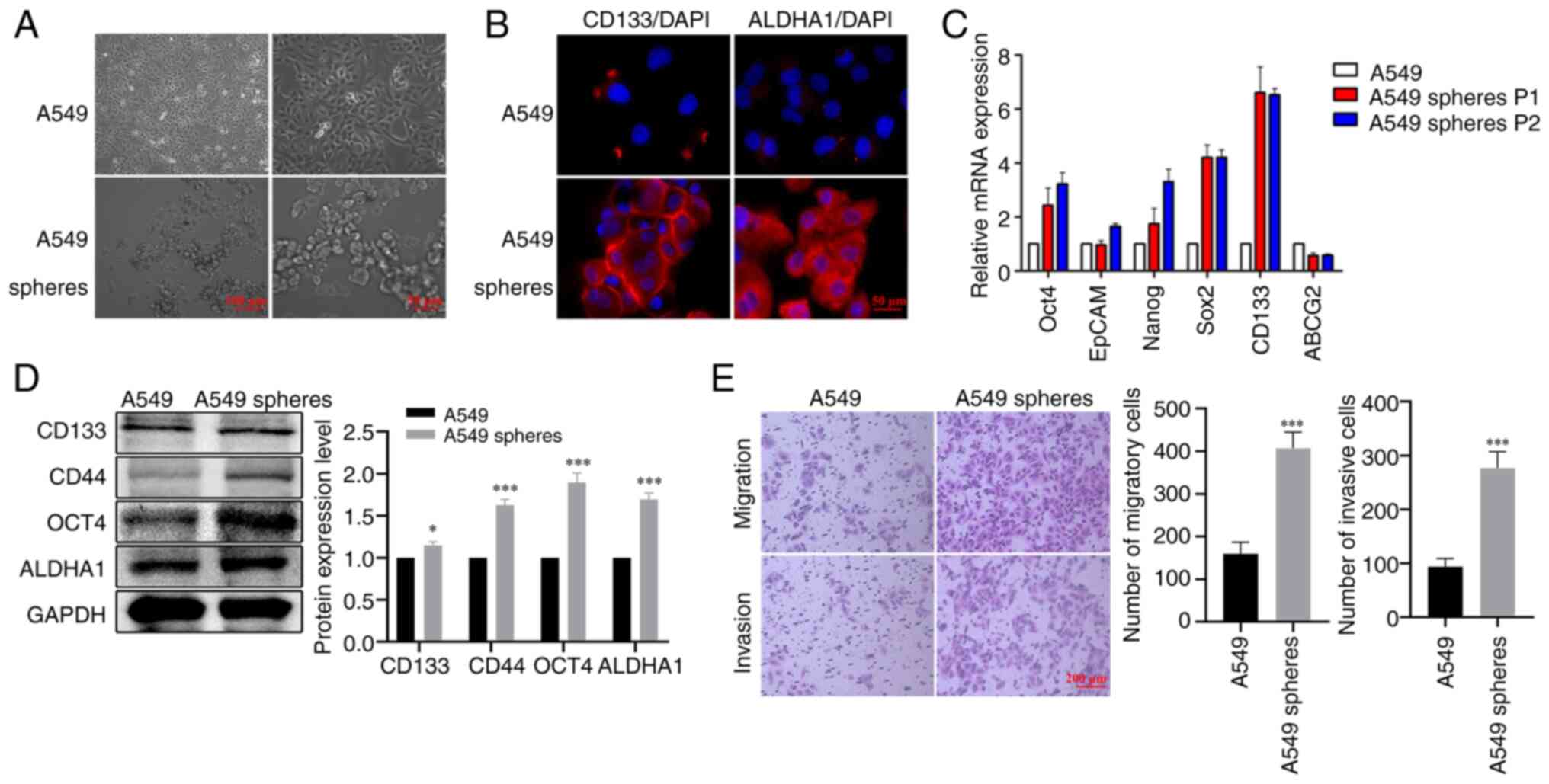
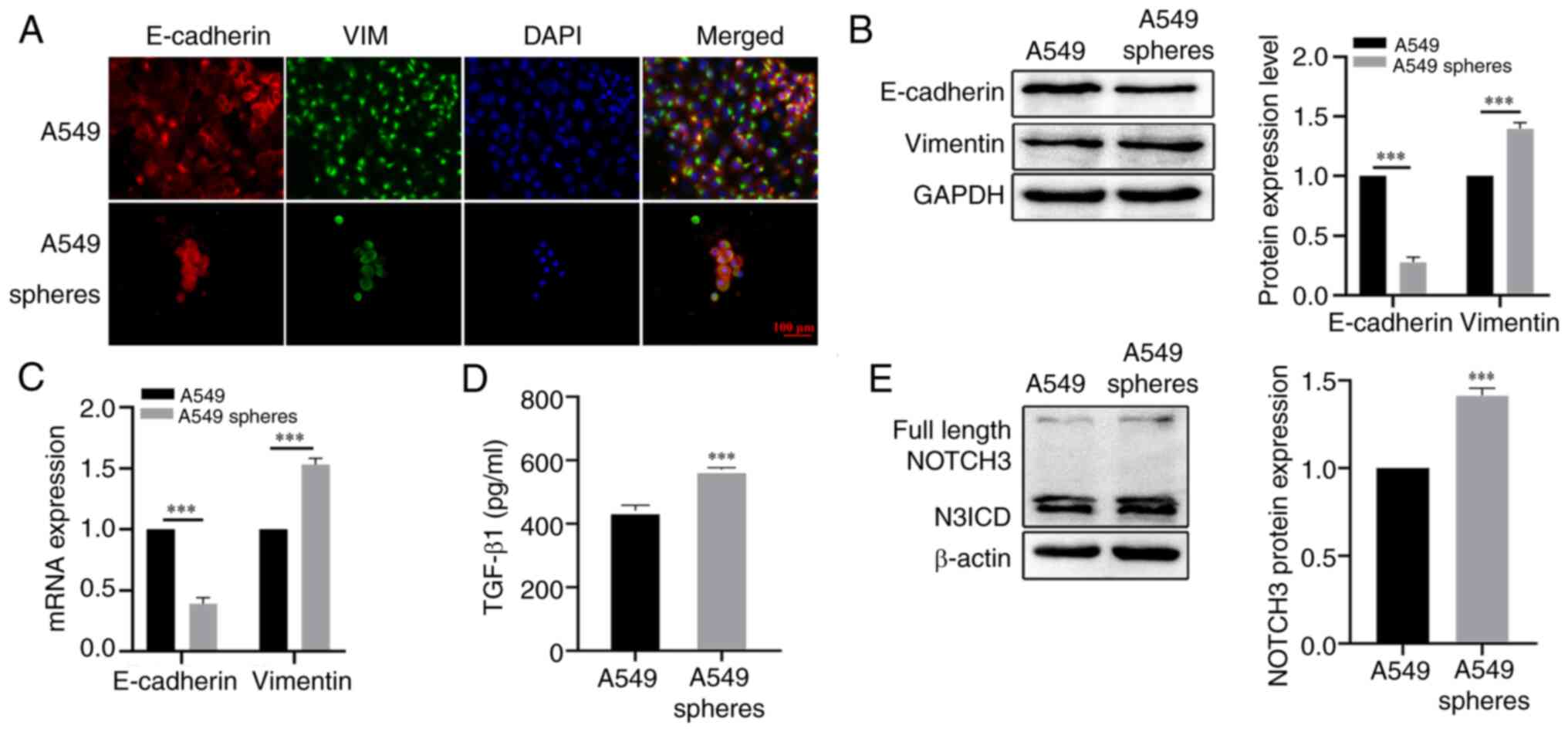
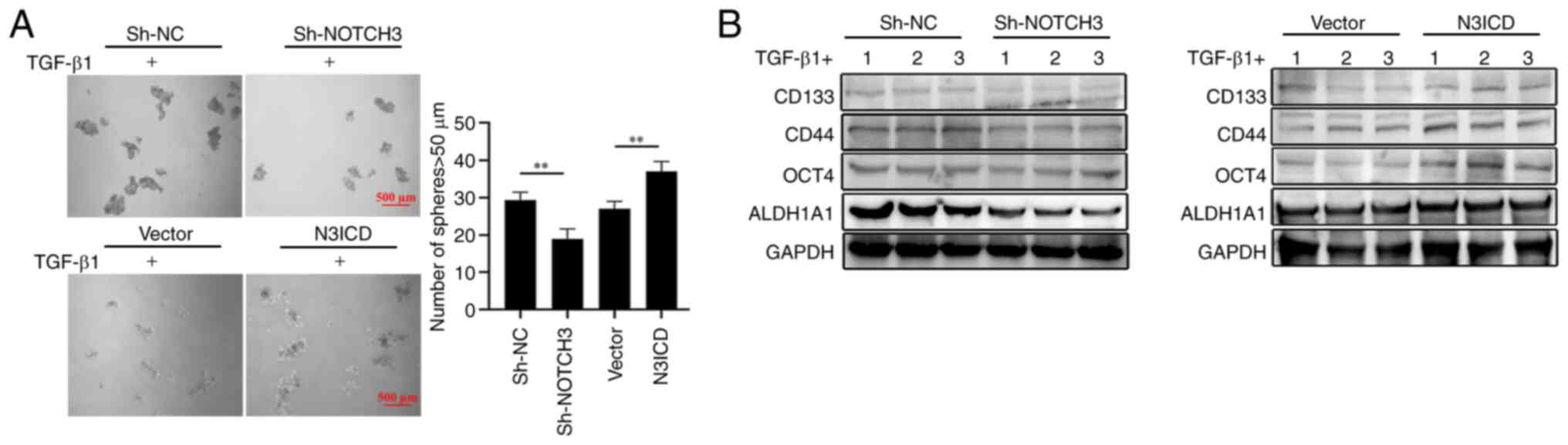
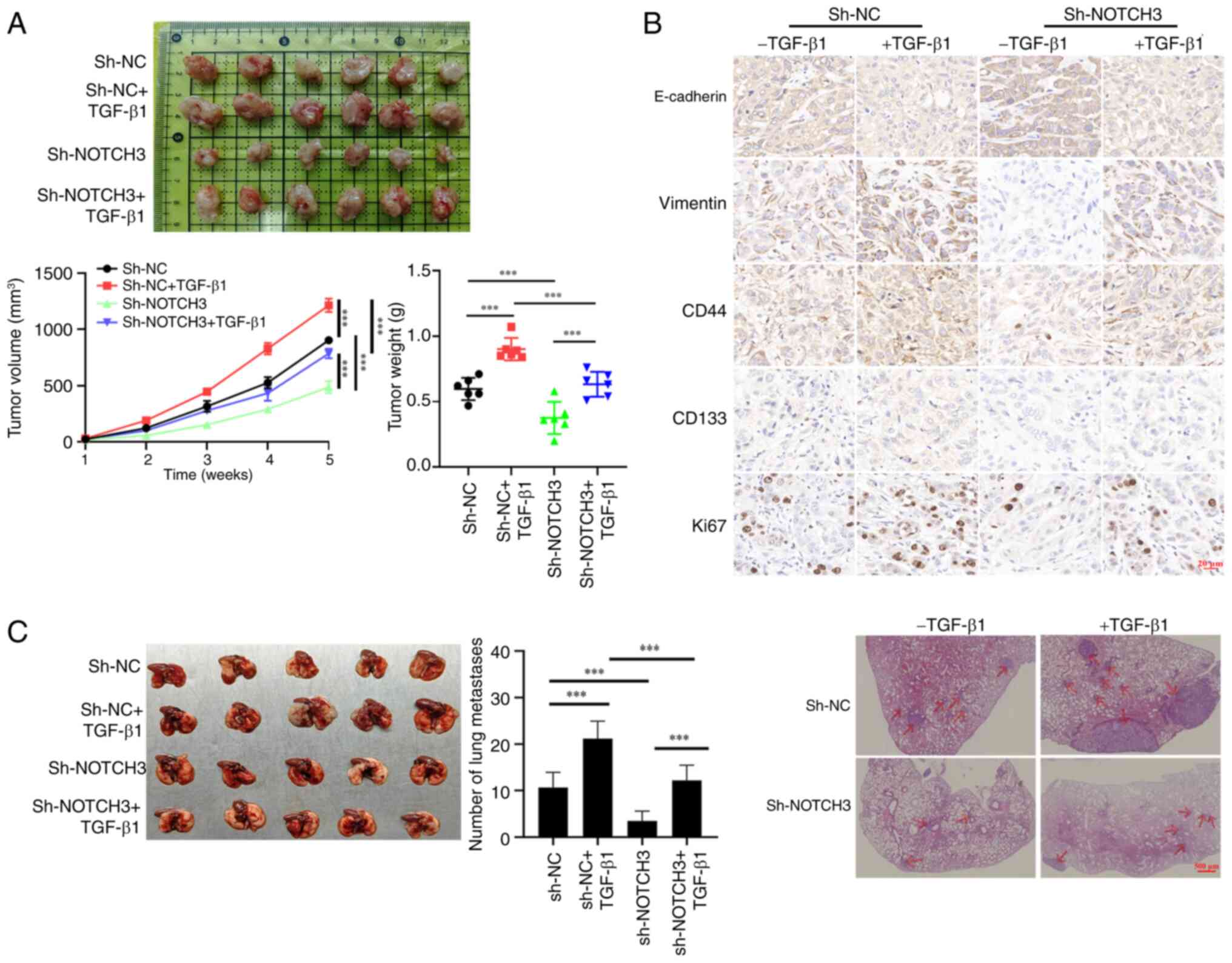
|
1
|
Altorki NK, Markowitz GJ, Gao D, Port JL,
Saxena A, Stiles B, McGraw T and Mittal V: The lung
microenvironment: An important regulator of tumour growth and
metastasis. Nat Rev Cancer. 19:9–31. 2019. View Article : Google Scholar :
|
|
2
|
Zaman A and Bivona TG: Emerging
application of genomics-guided therapeutics in personalized lung
cancer treatment. Ann Transl Med. 6:1602018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gregg JP, Li T and Yoneda KY: Molecular
testing strategies in non-small cell lung cancer: Optimizing the
diagnostic journey. Transl Lung Cancer Res. 8:286–301. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan AQ, Hasan A, Mir SS, Rashid K, Uddin
S and Steinhoff M: Exploiting transcription factors to target EMT
and cancer stem cells for tumor modulation and therapy. Semin
Cancer Biol. 100:1–16. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Babaei G, Aziz SG and Jaghi NZZ: EMT,
cancer stem cells and autophagy; The three main axes of metastasis.
Biomed Pharmacother. 133:1109092021. View Article : Google Scholar
|
|
9
|
Zhang C, Ding XP, Zhao QN, Yang XJ, An SM,
Wang H, Xu L, Zhu L and Chen HZ: Role of α7-nicotinic acetylcholine
receptor in nicotine-induced invasion and epithelial-to-mesenchymal
transition in human non-small cell lung cancer cells. Oncotarget.
7:59199–59208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han J, Won M, Kim JH, Jung E, Min K,
Jangili P and Kim JS: Cancer stem cell-targeted bio-imaging and
chemotherapeutic perspective. Chem Soc Rev. 49:7856–7878. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lytle NK, Barber AG and Reya T: Stem cell
fate in cancer growth, progression and therapy resistance. Nat Rev
Cancer. 18:669–680. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kovall RA, Gebelein B, Sprinzak D and
Kopan R: The canonical notch signaling pathway: Structural and
biochemical insights into shape, sugar, and force. Dev Cell.
41:228–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luca VC, Kim BC, Ge C, Kakuda S, Wu D,
Roein-Peikar M, Haltiwanger RS, Zhu C, Ha T and Garcia KC:
Notch-Jagged complex structure implicates a catch bond in tuning
ligand sensitivity. Science. 355:1320–1324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun J, Dong M, Xiang X, Zhang S and Wen D:
Notch signaling and targeted therapy in non-small cell lung cancer.
Cancer Lett. 585:2166472024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Y, de la Cruz CC, Sayles LC,
Alleyne-Chin C, Vaka D, Knaak TD, Bigos M, Xu Y, Hoang CD, Shrager
JB, et al: A rare population of CD24(+)ITGB4(+)Notch(hi) cells
drives tumor propagation in NSCLC and requires Notch3 for
self-renewal. Cancer Cell. 24:59–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: Gamma-secretase inhibitor
prevents Notch3 activation and reduces proliferation in human lung
cancers. Cancer Res. 67:8051–8057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Li M, Si J, Xiong Y, Lu F, Zhang J,
Zhang L, Zhang P and Yang Y: Blockade of Notch3 inhibits the
stem-like property and is associated with ALDH1A1 and CD44 via
autophagy in non-small lung cancer. Int J Oncol. 48:2349–2358.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML,
Chang BW and Zhang YB: Notch3 overexpression associates with poor
prognosis in human non-small-cell lung cancer. Med Oncol.
30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S,
Chen Y and Wu K: Meta-analysis reveals the correlation of Notch
signaling with non-small cell lung cancer progression and
prognosis. Sci Rep. 5:103382015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hassan WA, Yoshida R, Kudoh S, Motooka Y
and Ito T: Evaluation of role of Notch3 signaling pathway in human
lung cancer cells. J Cancer Res Clin Oncol. 142:981–993. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Martin M: Cutadapt removes adapter
sequences from high-throughput sequencing reads. EMBnet J.
17:10–12. 2011. View Article : Google Scholar
|
|
25
|
Kim D, Langmead B and Salzberg SL: HISAT:
A fast spliced aligner with low memory requirements. Nat Methods.
12:357–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar
|
|
27
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar
|
|
28
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
30
|
Leiter A, Veluswamy RR and Wisnivesky JP:
The global burden of lung cancer: Current status and future trends.
Nat Rev Clin Oncol. 20:624–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zong D, Ouyang R, Li J, Chen Y and Chen P:
Notch signaling in lung diseases: Focus on Notch1 and Notch3. Ther
Adv Respir Dis. 10:468–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wael H, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 signaling controls cell
proliferation, apoptosis and differentiation in lung carcinoma.
Lung Cancer. 85:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Zhang S, Lu Y, Zhang Y, Wang E and
Cui Z: The roles of Notch3 on the cell proliferation and apoptosis
induced by CHIR99021 in NSCLC cell lines: A functional link between
Wnt and Notch signaling pathways. PLoS One. 8:e846592013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Z, Xiao J, Liu M, Cui J, Lian B, Sun Y
and Li C: Notch3 regulates ferroptosis via ROS-induced lipid
peroxidation in NSCLC cells. FEBS Open Bio. 12:1197–1205. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bakir B, Chiarella AM, Pitarresi JR and
Rustgi AK: EMT, MET, plasticity, and tumor metastasis. Trends Cell
Biol. 30:764–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang YE and Stuelten CH: Alternative
splicing in EMT and TGF-β signaling during cancer progression.
Semin Cancer Biol. 101:1–11. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong C, Wu K, Gu S, Wang W, Xie S and Zhou
Y: PTBP3 mediates TGF-β-induced EMT and metastasis of lung
adenocarcinoma. Cell Cycle. 21:1406–1421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su Q, Wang JJ, Ren JY, Wu Q, Chen K, Tu
KH, Zhang Y, Leong SW, Sarwar A, Han X, et al: Parkin deficiency
promotes liver cancer metastasis by TMEFF1 transcription activation
via TGF-β/Smad2/3 pathway. Acta Pharmacol Sin. 45:1520–1529. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oh MK, Joo H and Kim IS: Prohaptoglobin
inhibits the transforming growth factor-β-induced
epithelial-to-mesenchymal transition in vitro by increasing Smad1/5
activation and suppressing the Smad2/3 signaling pathway in SK-Hep1
liver cancer cells. PLoS One. 17:e02664092022. View Article : Google Scholar
|
|
40
|
Su J, Morgani SM, David CJ, Wang Q, Er EE,
Huang YH, Basnet H, Zou Y, Shu W, Soni RK, et al: TGF-β
orchestrates fibrogenic and developmental EMTs via the RAS effector
RREB1. Nature. 577:566–571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gough NR, Xiang X and Mishra L: TGF-β
signaling in liver, pancreas, and gastrointestinal diseases and
cancer. Gastroenterology. 161:434–452.e15. 2021. View Article : Google Scholar
|
|
42
|
Liu L, Chen X, Wang Y, Qu Z, Lu Q, Zhao J,
Yan X, Zhang H and Zhou Y: Notch3 is important for TGF-β-induced
epithelial-mesenchymal transition in non-small cell lung cancer
bone metastasis by regulating ZEB-1. Cancer Gene Ther. 21:364–372.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Danielpour D, Corum S, Leahy P and
Bangalore A: Jagged-1 is induced by mTOR inhibitors in renal cancer
cells through an Akt/ALK5/Smad4-dependent mechanism. Curr Res
Pharmacol Drug Discov. 3:1001172022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen WJ, Zhong HT, Wu HT, Hou YY, Wu Z,
Fang ZX and Liu J: NOTCH3 inhibits transcription factor ZEB1
expression and metastasis of breast cancer cells via
transcriptionally upregulating miR-223. J Cancer. 15:192–203. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ohashi S, Natsuizaka M, Naganuma S, Kagawa
S, Kimura S, Itoh H, Kalman RA, Nakagawa M, Darling DS, Basu D, et
al: A NOTCH3-mediated squamous cell differentiation program limits
expansion of EMT-competent cells that express the ZEB transcription
factors. Cancer Res. 71:6836–6847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lei ZN, Teng QX, Koya J, Liu Y, Chen Z,
Zeng L, Chen ZS, Fang S, Wang J, Liu Y and Pan Y: The correlation
between cancer stem cells and epithelial-mesenchymal transition:
Molecular mechanisms and significance in cancer theragnosis. Front
Immunol. 15:14172012024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiu M, Wang Y, Li B, Wang X, Xiao F, Chen
S, Zhang L, Zhou B and Hua F: The role of Notch3 signaling in
cancer stemness and chemoresistance: Molecular mechanisms and
targeting strategies. Front Mol Biosci. 8:6941412021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chang Z, Gao Y, Chen P, Gao W, Zhao W, Wu
D, Liang W, Chen Z, Chen L and Xi H: THBS2 promotes gastric cancer
progression and stemness via the Notch signaling pathway. Am J
Cancer Res. 14:3433–3450. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Z, Miao L, Li K, Ma Q, Pan L, Shen C,
Ge Q, Du Y, Yin L, Yang H, et al: A hypothalamic-amygdala circuit
underlying sexually dimorphic aggression. Neuron. 112:3176–3191.e7.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Day CP, Merlino G and Van Dyke T:
Preclinical mouse cancer models: A maze of opportunities and
challenges. Cell. 163:39–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Prendergast BJ, Onishi KG and Zucker I:
Female mice liberated for inclusion in neuroscience and biomedical
research. Neurosci Biobehav Rev. 40:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|