|
1
|
Lu W and Kang Y: Epithelial-mesenchymal
plasticity in cancer progression and metastasis. Dev Cell.
49:361–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol. Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanchez-Tillo E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar
|
|
7
|
Goossens S, Vandamme N, Van Vlierberghe P
and Berx G: EMT transcription factors in cancer development
re-evaluated: Beyond EMT and MET. Biochim Biophys Acta Rev Cancer.
1868:584–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Y and Zhan H: Communication between
EMT and PD-L1 signaling: New insights into tumor immune evasion.
Cancer Lett. 468:72–81. 2020. View Article : Google Scholar
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tie Y, Tang F, Wei YQ and Wei XW:
Immunosuppressive cells in cancer: Mechanisms and potential
therapeutic targets. J Hematol Oncol. 15:612022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dongre A, Rashidian M, Reinhardt F,
Bagnato A, Keckesova Z, Ploegh HL and Weinberg RA:
Epithelial-to-mesenchymal transition contributes to
immunosuppression in breast carcinomas. Cancer Res. 77:3982–3989.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong P, Xiong Y, Yue J, Hanley SJB and
Watari H: Tumor-intrinsic PD-L1 signaling in cancer initiation,
development and treatment: Beyond immune evasion. Front Oncol.
8:3862018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
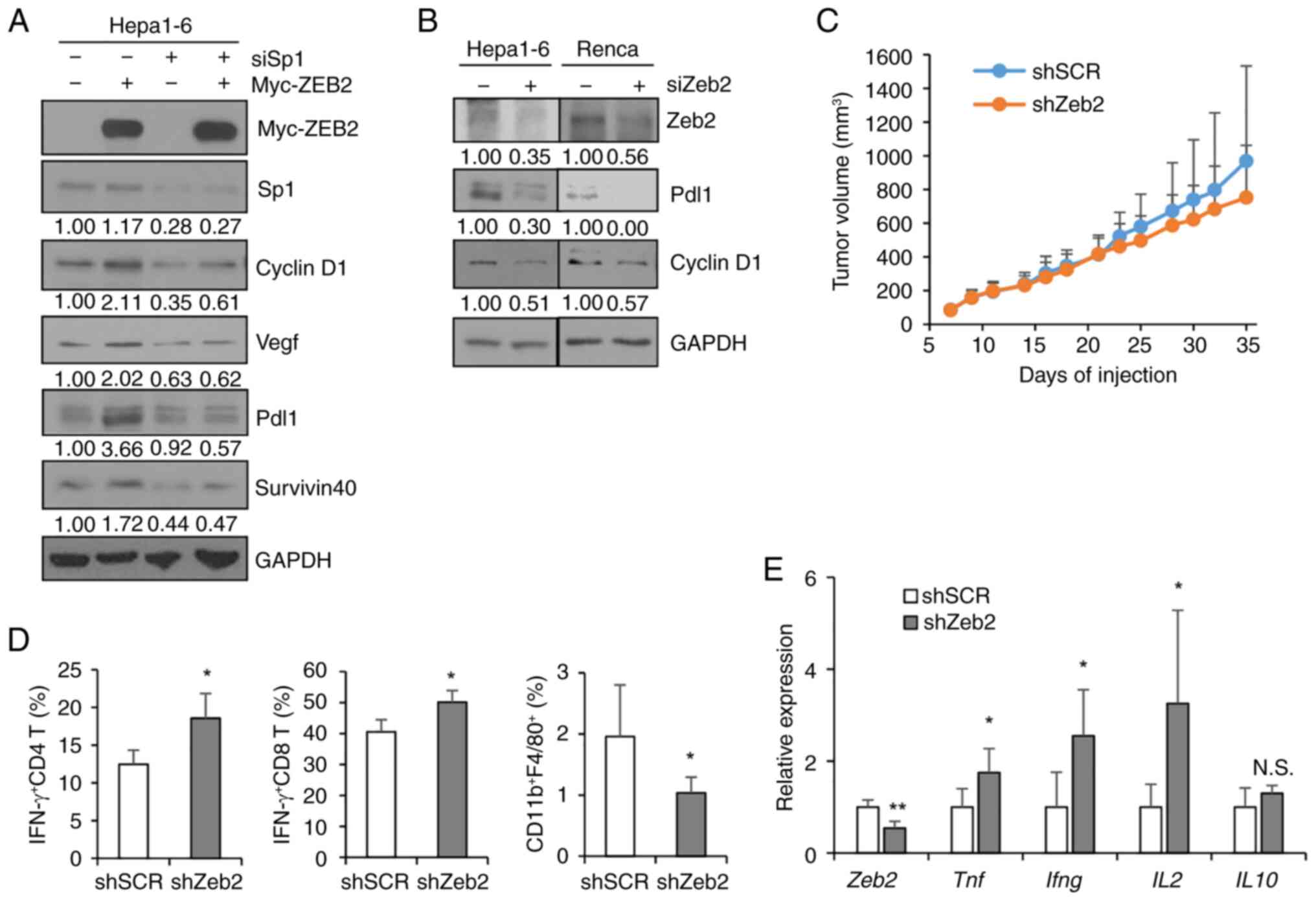
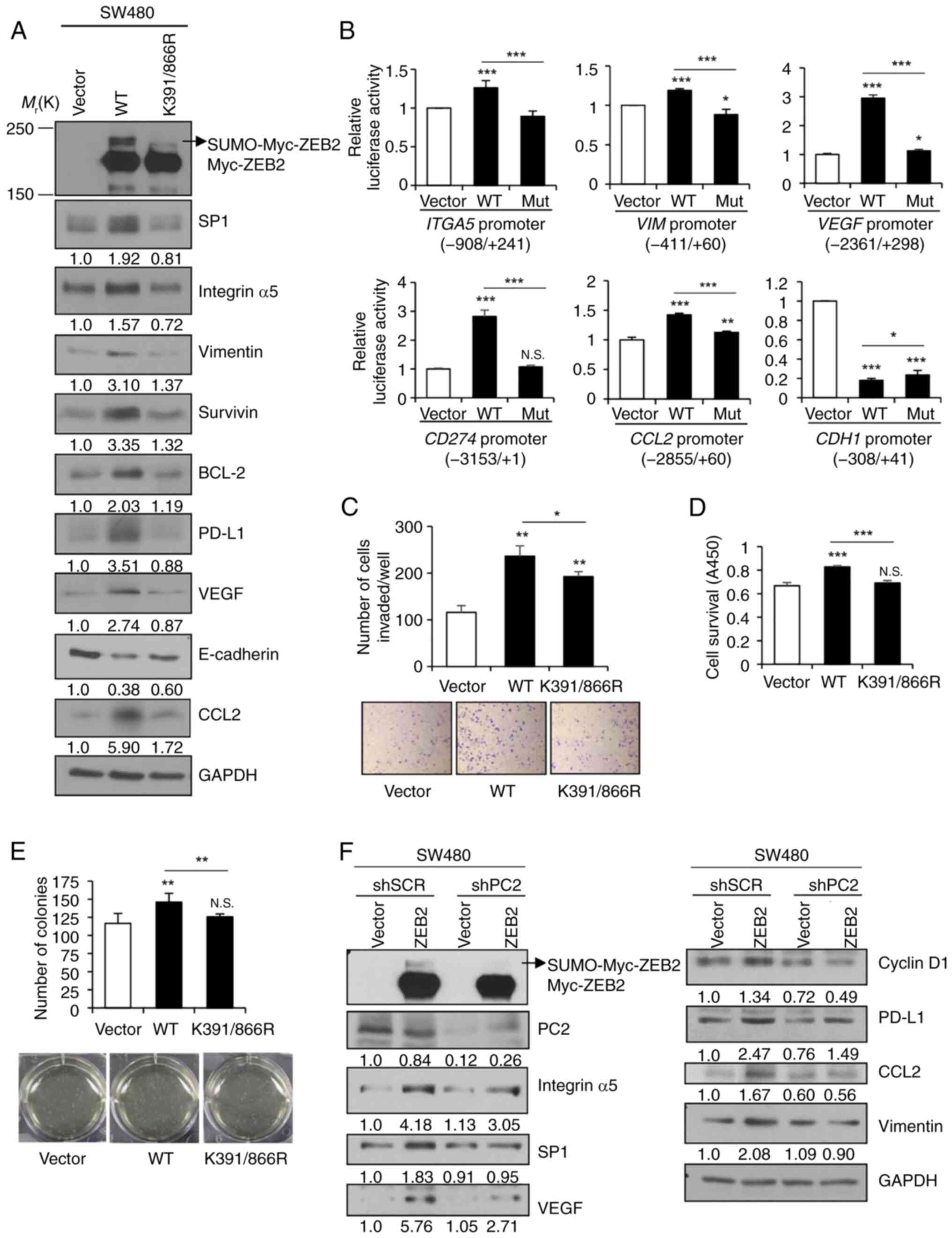
Nam EH, Lee Y, Park YK, Lee JW and Kim S:
ZEB2 upregulates integrin alpha5 expression through cooperation
with Sp1 to induce invasion during epithelial-mesenchymal
transition of human cancer cells. Carcinogenesis. 33:563–571. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nam EH, Lee Y, Zhao XF, Park YK, Lee JW
and Kim S: ZEB2-Sp1 cooperation induces invasion by upregulating
cadherin-11 and integrin alpha5 expression. Carcinogenesis.
35:302–314. 2014. View Article : Google Scholar
|
|
16
|
Ko D and Kim S: Cooperation between ZEB2
and Sp1 promotes cancer cell survival and angiogenesis during
metastasis through induction of survivin and VEGF. Oncotarget.
9:726–742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jang D, Kwon H, Choi M, Lee J and Pak Y:
Sumoylation of flotillin-1 promotes EMT in metastatic prostate
cancer by suppressing snail degradation. Oncogene. 38:3248–3260.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonello GB, Pham MH, Begum K, Sigala J,
Sataranatarajan K and Mummidi S: An evolutionarily conserved
TNF-alpha-responsive enhancer in the far upstream region of human
CCL2 locus influences its gene expression. J Immunol.
186:7025–7038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nam EH, Lee Y, Moon B, Lee JW and Kim S:
Twist1 and AP-1 cooperatively upregulate integrin alpha5 expression
to induce invasion and the epithelial-mesenchymal transition.
Carcinogenesis. 36:327–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pl12013. View Article : Google Scholar
|
|
21
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vasaikar S, Huang C, Wang X, Petyuk VA,
Savage SR, Wen B, Dou Y, Zhang Y, Shi Z, Arshad OA, et al:
Proteogenomic analysis of human colon cancer reveals new
therapeutic opportunities. Cell. 177:1035–1049.e1019. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon R, Lam A, Li MC, Ngan M, Menenzes S
and Zhao Y: Analysis of gene expression data using BRB-arraytools.
Cancer Inform. 3:11–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Wang J, Chen Z, Luo J, Guo W, Sun
L and Lin L: Targeting M2-like tumor-associated macrophages is a
potential therapeutic approach to overcome antitumor drug
resistance. NPJ Precis Oncol. 8:312024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
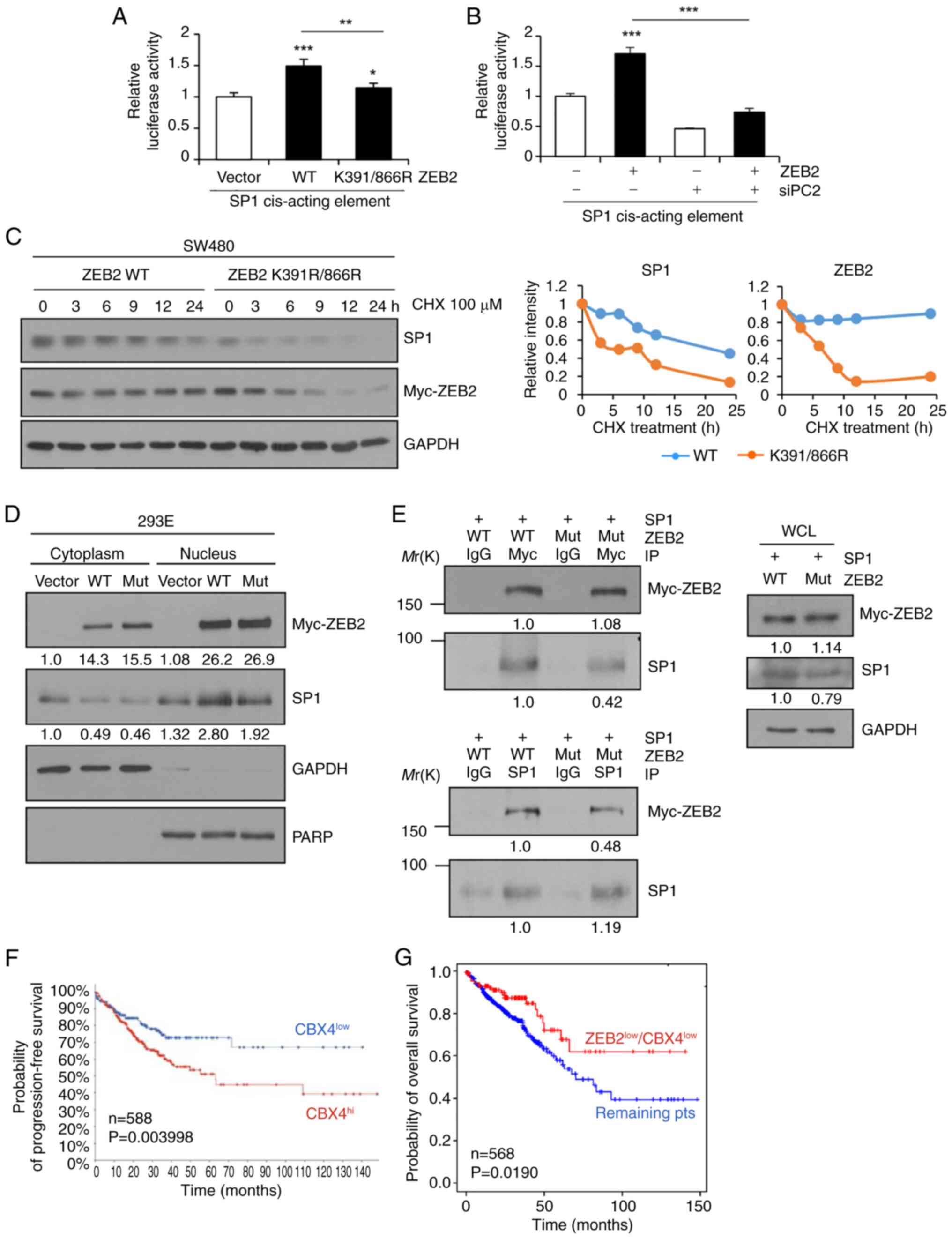
Long J, Zuo D and Park M: Pc2-mediated
sumoylation of Smad-interacting protein 1 attenuates
transcriptional repression of E-cadherin. J Biol Chem.
280:35477–35489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Low-Marchelli JM, Ardi VC, Vizcarra EA,
van Rooijen N, Quigley JP and Yang J: Twist1 induces CCL2 and
recruits macrophages to promote angiogenesis. Cancer Res.
73:662–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lyons JG, Patel V, Roue NC, Fok SY, Soon
LL, Halliday GM and Gutkind JS: Snail up-regulates proinflammatory
mediators and inhibits differentiation in oral keratinocytes.
Cancer Res. 68:4525–4530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katsura A, Tamura Y, Hokari S, Harada M,
Morikawa M, Sakurai T, Takahashi K, Mizutani A, Nishida J, Yokoyama
Y, et al: ZEB1-regulated inflammatory phenotype in breast cancer
cells. Mol Oncol. 11:1241–1262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gschwandtner M, Derler R and Midwood KS:
More than just attractive: How CCL2 influences myeloid cell
behavior beyond chemotaxis. Front Immunol. 10:27592019. View Article : Google Scholar
|
|
31
|
Kudo-Saito C, Shirako H, Ohike M,
Tsukamoto N and Kawakami Y: CCL2 is critical for immunosuppression
to promote cancer metastasis. Clin Exp Metastasis. 30:393–405.
2013. View Article : Google Scholar
|
|
32
|
Gao L, Wang FQ, Li HM, Yang JG, Ren JG, He
KF, Liu B, Zhang W and Zhao YF: CCL2/EGF positive feedback loop
between cancer cells and macrophages promotes cell migration and
invasion in head and neck squamous cell carcinoma. Oncotarget.
7:87037–87051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol.
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang F, Wang H, Wang X, Jiang G, Liu H,
Zhang G, Wang H, Fang R, Bu X, Cai S and Du J: TGF-β induces
M2-like macrophage polarization via SNAIL-mediated suppression of a
pro-inflammatory phenotype. Oncotarget. 7:52294–52306. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HR, Seo CW, Han SJ, Lee JH and Kim J:
Zinc finger E-box binding homeobox 2 as a prognostic biomarker in
various cancers and its correlation with infiltrating immune cells
in ovarian cancer. Curr Issues Mol Biol. 44:1203–1214. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie H, Wu Z, Li Z, Huang Y, Zou J and Zhou
H: Significance of ZEB2 in the immune microenvironment of colon
cancer. Front Genet. 13:9953332022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Wang H, Yao H, Li C, Fang JY and
Xu J: Regulation of PD-L1: emerging routes for targeting tumor
immune evasion. Front Pharmacol. 9:5362018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar :
|
|
39
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yi M, Niu M, Xu L, Luo S and Wu K:
Regulation of PD-L1 expression in the tumor microenvironment. J
Hematol Oncol. 14:102021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen L, Gibbons DL, Goswami S, Cortez MA,
Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al: Metastasis
is regulated via microRNA-200/ZEB1 axis control of tumour cell
PD-L1 expression and intratumoral immunosuppression. Nat Commun.
5:52412014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scott CL and Omilusik KD: ZEBs: Novel
players in immune cell development and function. Trends Immunol.
40:431–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bogachek MV, De Andrade JP and Weigel RJ:
Regulation of epithelial-mesenchymal transition through SUMOylation
of transcription factors. Cancer Res. 75:11–15. 2015. View Article : Google Scholar :
|
|
44
|
Seeler JS and Dejean A: SUMO and the
robustness of cancer. Nat Rev Cancer. 17:184–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Y, Xia Z, Wang X, Zhao X, Sheng Z, Ye
Y, He G, Zhou L, Zhu H, Xu N and Liang S: Small-molecule inhibitors
targeting protein sumoylation as novel anticancer compounds. Mol
Pharmacol. 94:885–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brackett CM and Blagg BSJ: Current status
of sumoylation inhibitors. Curr Med Chem. 28:3892–3912. 2021.
View Article : Google Scholar :
|
|
47
|
Shibue T and Weinberg RA: EMT, CSCs and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|