Programmed cell death (PCD) is defined as an
intrinsic component of physiological developmental programs or
tissue renewal, occurring independently of exogenous environmental
factors (1). Importantly,
accumulating evidence indicates that PCD has strong effects on a
number of lesions including chronic inflammation and cancer
(2-4). Ferroptosis, a term introduced by
Brent Stockwell in 2012, is a distinct form of iron-dependent PCD
characterized by the accumulation of lipid peroxides and subsequent
disruption of the cell membrane, ultimately leading to cell death
(5). Recent advances in molecular
biology have revealed the critical involvement of ferroptosis in
the pathophysiology of gastrointestinal diseases, establishing it
as a rapidly evolving research frontier in gastroenterology
(6).
According to the global cancer statistics in 2022
released by GLOBOCAN, gastric cancer (GC) ranks fifth in both
incidence and mortality worldwide, with >950,000 new cases and
>650,000 mortalities in the whole year (7). Helicobacter pylori (H.
pylori) infection, the most prevalent chronic bacterial
infection worldwide, is a major etiological factor for GC (8). Studies have demonstrated that H.
pylori mediates chronic inflammation by upregulating
proinflammatory cytokines, including interleukin (IL)-8, IL-1β and
tumor necrosis factor (TNF) (9-11).
H. pylori-associated chronic gastritis, also referred to as
type B gastritis, can progress to GC through a sequence of
histopathological changes, a process first described in 1975 and
termed as Correa's cascade (12).
Despite significant advancements in the prevention and treatment of
GC based on Correa's cascade, several critical challenges persist.
In the precancerous lesions of GC (PLGC), traditional therapies
such as H. pylori eradication and symptomatic treatment
often fail to effectively suppress chronic inflammation or halt the
progression of Correa's cascade (13,14). Moreover, the early detection of GC
is substantially hampered by the absence of specific clinical
manifestations in the initial stages, the suboptimal sensitivity of
current screening biomarkers, and the low popularity rate of
endoscopic screening (15,16).
Consequently, a substantial proportion of patients are diagnosed at
advanced stages of disease progression. In the management of
advanced GC, several therapeutic limitations remain unresolved,
including the development of chemoresistance, the paucity of novel
molecular targets for targeted therapies, and the low response rate
of immunotherapy, all of which represent pressing unmet needs in
contemporary oncology practice (17-20). Targeted regulation of
ferroptosis-related strategies, characterized by precision
targeting, antioxidant properties, anti-inflammatory effects and
potential anticancer activity, represents a promising approach to
overcoming these limitations.
This comprehensive review systematically
investigated the intricate interplay between H. pylori
infection and ferroptosis, while proposing novel promising
strategies for GC prevention and treatment through targeted
regulation of ferroptosis-related pathways across several different
stages of the Correa's cascade.
Oxygen serves as the terminal electron acceptor in
the majority of metabolic oxidation-reduction reactions for most
organisms, highlighting the necessity of oxidative stress.
Oxidative stress induces oxidative modifications of the cell's
bilayer membrane, particularly lipid oxidation, which impacts
various cellular physiological processes such as developmental
regulation, immune response, tumor suppression, metabolic balance
and aging. Ferroptosis, a form of PCD, is characterized by
extensive lipid peroxidation (21). Since its introduction in 2012,
ferroptosis research has centered around several fundamental
components: i) The systemic xc−-glutathione
(GSH)-glutathione peroxidase (GPX)4 ferroptosis suppression
pathway; ii) phospholipid hydroperoxides (PLOOHs); iii) iron
regulation; iv) GPX4-independent regulatory pathways; and v) other
important regulators such as tumor suppressor p53 and related
signaling pathways.
GSH is a crucial intracellular reductant, and also
functions as a cofactor for enzymes such as GPXs and
GSH-S-transferases. GSH biosynthesis relies on cysteine, which can
be imported from the environment via neutral amino acid
transporters, taken up in its oxidized form (cystine) through the
xc−-cystine/glutamate antiporter system
[comprising solute carrier family 7 member 11 and solute carrier
family 3 member 2 (SLC3A2 subunits)], or synthesized through the
trans-sulfuration pathway utilizing methionine and glucose
(21-23). The transporter protein in the
xc− system is a disulfide-linked heterodimer
consisting of a light chain (xCT) and a heavy chain (4F2hc)
(24). GPX4 plays a pivotal role
in the ferroptosis process, serving as the primary enzyme that
catalyzes the reduction and detoxification of PLOOHs in mammalian
cells (25).
As a type of lipid-derived reactive oxygen species
(ROS), PLOOHs mark the beginning of lipid peroxidation. This
process begins with the abstraction of a bisallylic hydrogen atom
from the polyunsaturated fatty acid (PUFA) acyl chain of
phospholipids within the lipid bilayer, generating a
carbon-centered phospholipid radical (PL•). This radical
subsequently reacts with molecular oxygen to form a phospholipid
peroxyl radical (PLOO•) (26),
which abstracts hydrogen from another PUFA, resulting in PLOOH
formation. In the absence of timely reduction of PLOOHs to their
corresponding alcohols (PLOH) by GPX4, the chain reaction products,
including lipid peroxide breakdown products [e.g., 4-hydroxynonenal
(4-HNE) and malondialdehyde] and oxidized/modified proteins,
disrupt membrane integrity and ultimately lead to organelle and/or
membrane breakdown (21).
The regulation of ferroptosis has emerged as a
critical focus in disease mechanism research. In the context of
ferroptosis modulation, two distinct mechanisms have been
identified: Erastin exerts its effect through indirect inhibition
of GPX4 by targeting system xc, thereby disrupting
cystine uptake, while RSL3 demonstrates direct GPX4 inhibition.
These compounds represent two fundamental classes of ferroptosis
inducers, as indicated in previous studies (27,28). Furthermore, PUFAs and
PUFA-containing lipids within biofilms are susceptible to direct
oxidation by certain lipoxygenases (LOXs), suggesting that LOXs may
also constitute a target for ferroptosis induction (29). Additionally, iron is also crucial
for the regulation of ferroptosis. Inhibition of GPX4 triggers the
Fenton reaction, leading to a rapid accumulation of PLOOHs, a
characteristic of iron toxicity (26). Moreover, it has been shown that
cytochrome P450 oxidoreductase can directly or indirectly trigger
lipid peroxidation by removing hydrogen from PUFAs or by reducing
ferric iron (Fe3+) to its ferrous form (Fe2+)
after its downstream electron acceptor is reduced (30).
Apart from the GSH-GPX4 inhibitory pathway, which is
recognized as the predominant ferroptosis regulatory system
(31), one such mechanism
involves ferroptosis suppressor protein 1 (FSP1, also known as
AIFM2), which inhibits lipid peroxidation and ferroptosis by
synthesizing panthenol and rejuvenating oxidized α-tocopherol
radicals (vitamin E) (32,33).
Another mechanism entails guanosine triphosphate cyclohydrolase 1
protecting against ferroptosis through its metabolites
tetrahydrobiopterin and dihydrobiopterin (34).
There is compelling evidence linking ferroptosis to
a spectrum of pathologies involving tissue damage, encompassing
cancer, neurodegeneration, inflammation and infection (35). Targeting ferroptosis mostly may
offer a therapeutic avenue for related disorders. However, a number
of cancer cells exhibit heightened vulnerability to ferroptosis,
suggesting its potential as an anticancer strategy. Ferroptosis has
been closely implicated in several cancer-associated signaling
pathways. A study on the interplay between energy stress and
ferroptosis has revealed that energy stress can inhibit ferroptosis
through AMP-activated protein kinase pathway (36). Furthermore, lactate produced by
cancer cells under energy stress may inhibit ferroapoptosis of
tumor cells and promote their metastatic spread (37). The phosphoinositide 3-kinase
(PI3K)-protein kinase B (AKT)-mammalian target of rapamycin (mTOR)
signaling axis has also been shown to shield cancer cells from
oxidative stress and ferroptosis through sterol regulatory
element-binding protein 1/stearoyl-CoA desaturase 1-mediated lipid
synthesis (38).
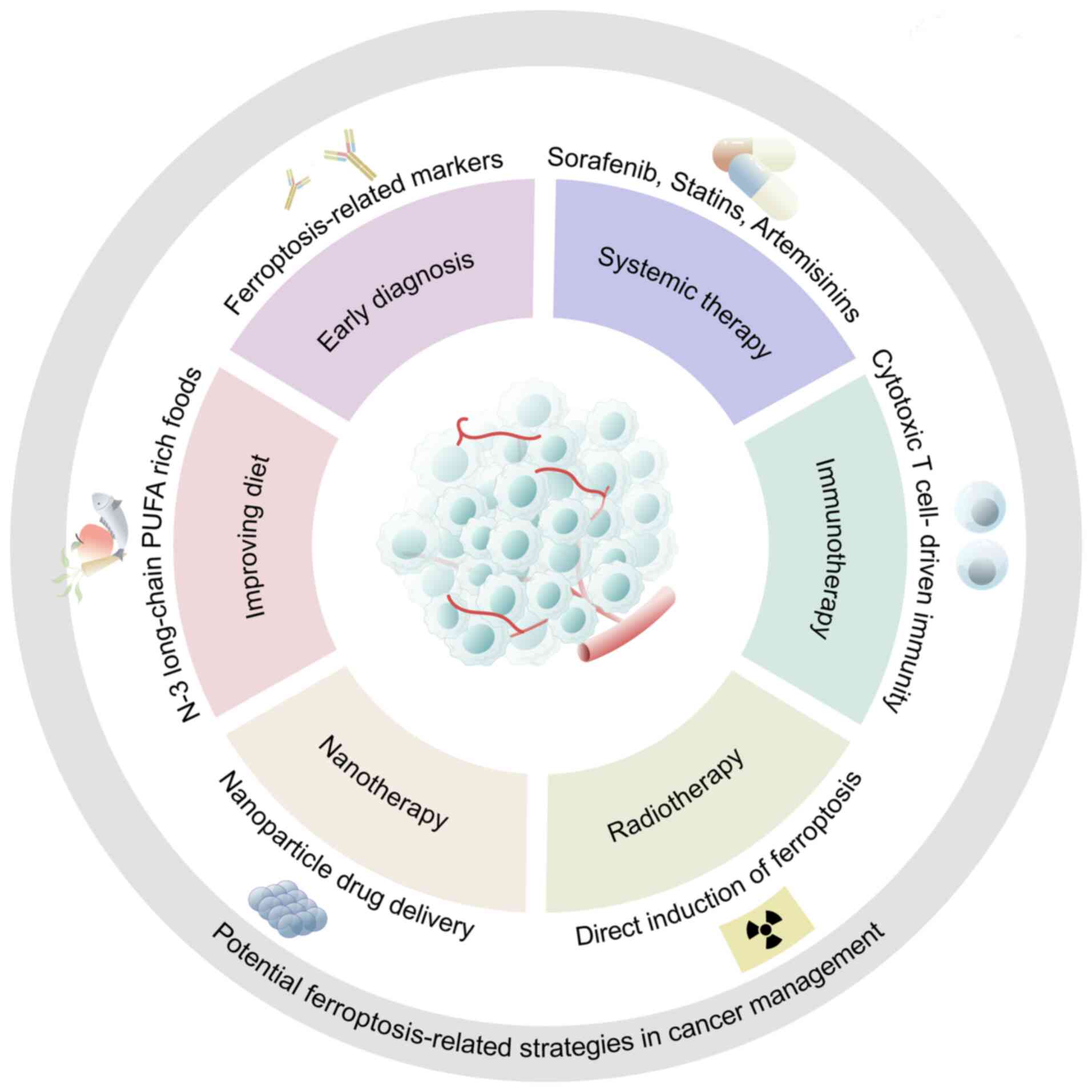
At present, cancer management research targeting
ferroptosis has achieved breakthroughs in a number of aspects
(Fig. 1). First, the
identification of ferroptosis-related biomarkers is one of the
promising strategies in cancer management (47,48). Secondly, in addition to being
transformed in chemoradiotherapy and immunotherapy, therapeutic
strategies to induce ferroptosis have also been applied in the
emerging field of nanotherapy (49). Additionally, some external factors
that can lead to the dysregulation of ferroptosis are also worthy
of attention. It has been documented that some infectious pathogens
such as H. pylori can cause dysregulation of ferroptosis
(50). Furthermore, N-3 PUFA
peroxidation has been shown to selectively induce ferroptosis in
cancer cells. Consequently, N-3 long-chain PUFA-rich foods may be a
dietary strategy for patients with cancer (51). In general, targeting ferroptosis
is a promising strategy for cancer treatment in the future.
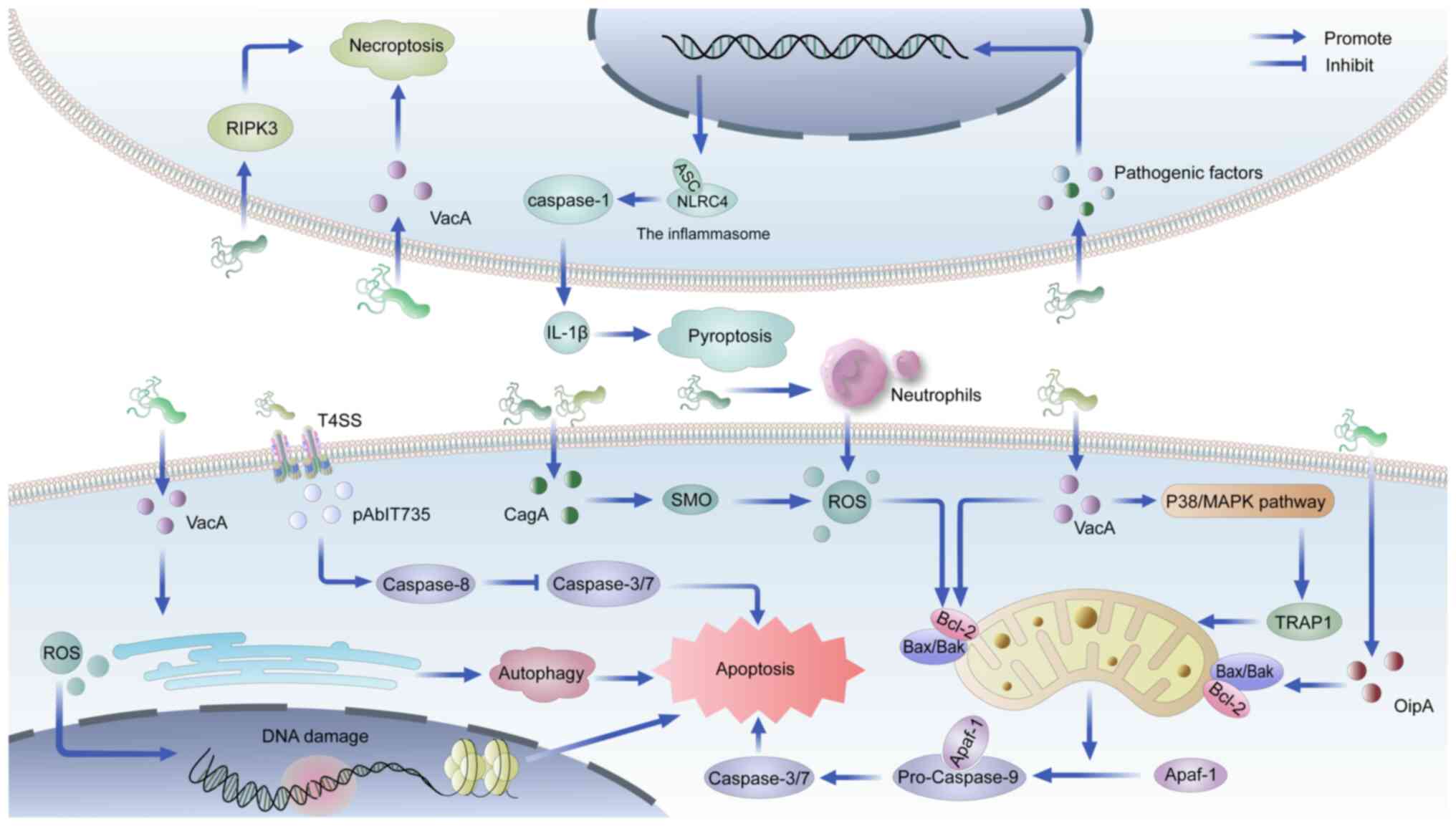
Autophagy and apoptosis represent the most prominent
forms of PCD in GC, with their dysregulation being closely linked
to specific virulence factors of H. pylori infection
(52,53). Vacuolar cell toxin (VacA), a key
virulence factor, induces autophagic cell death through endoplasmic
reticulum (ER) stress in gastric epithelial cells (54), while simultaneously promoting
apoptosis via the p38/MAPK pathway-mediated downregulation of TNF
receptor-associated protein 1 (55). Another critical virulence
determinant, cytotoxin-associated gene A (CagA), triggers
mitochondrial membrane depolarization by elevating hydrogen
peroxide (H2O2) levels through spermine
oxidase activation, subsequently initiating caspase-dependent
apoptosis (56). The oxidative
stress induced by H. pylori infection, characterized by
excessive production of ROS and reactive nitrogen species (RNS)
from neutrophils, leads to DNA damage (57,58), which may be mitigated through
apoptosis induction to prevent oncogenic mutations (59). The mitochondrial apoptotic pathway
is further regulated by H. pylori through modulation of the
B-cell lymphoma-2 (Bcl-2)-associated X/Bcl-2 ratio by outer
inflammatory protein A and VacA (60,61).
Accumulating evidence indicates that bacterial
infection can promote ferroptosis following tissue damage.
Mycobacterium tuberculosis (Mtb), for instance, secretes protein
tyrosine phosphatase A, which inhibits GPX4 expression, thereby
inducing ferroptosis and enhancing Mtb pathogenicity and
transmission (66). Similarly,
Pseudomonas aeruginosa utilizes host polyunsaturated
phosphatidylethanolamine to induce lipid peroxidation and
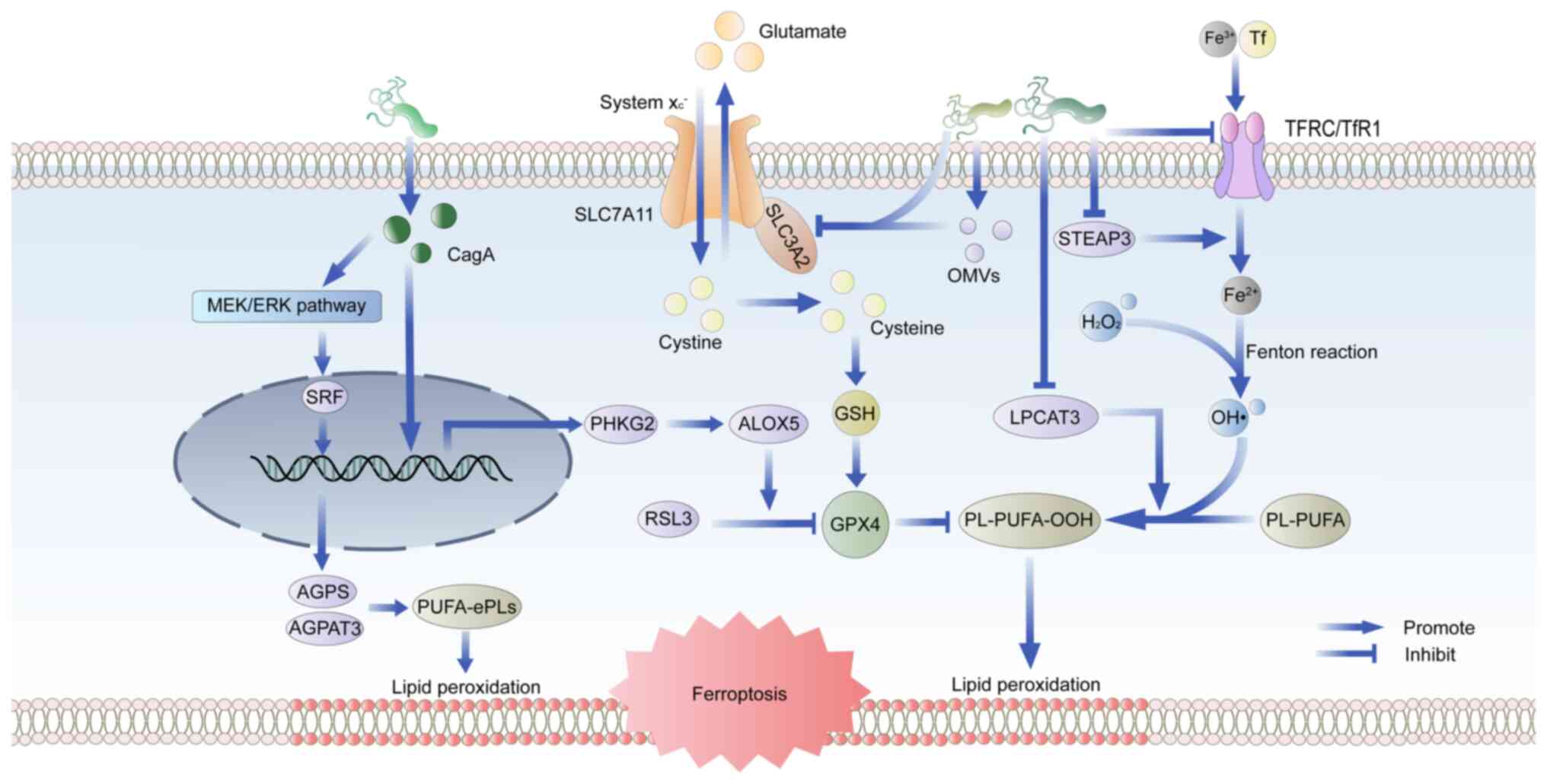
ferroptosis in bronchial epithelial cells (67). H. pylori infection elicits
a robust inflammatory response in the gastric mucosa, leading to
the generation of ROS and RNS, which in turn facilitates lipid
peroxidation (68). In H.
pylori infection, the release of virulence factors also affects
ferroptosis (Fig. 3). Inhibition
of GPX4 by RSL3 renders cells unable to eliminate accumulated lipid
hydroperoxides, ultimately leading to ferroptosis. A study has
corroborated that phosphorylase kinase G2 promotes RSL3-induced
ferroptosis in GC cells by enhancing arachidonate 5-lipoxygenase
expression in CagA-positive H. pylori infections, but the
mechanism of action of CagA in this process requires further
investigation (50). Another
study clarified that CagA could promote the synthesis of
polyunsaturated ether phospholipids through the MEK/ERK/serum
response factor pathway, leading to the susceptibility to
ferroptosis (69). An
investigation into the iron toxicity-associated gene tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein
epsilon (YWHAE) demonstrated significantly elevated YWHAE
expression levels in H. pylori-induced GC, which was
positively correlated with ferroptosis in GC (70).
Notably, ferroptosis interacts with other types of
PCD in the process of GC induced by H. pylori infection.
Ferroptosis-driven lipid peroxidation activates pro-apoptotic
signals, while apoptosis-related proteins (e.g., caspases) may
conversely enhance ferroptosis by degrading inhibitors such as GPX4
(42,73). Autophagy further promotes
ferroptosis sensitivity through ferritin degradation (releasing
free iron) or depletion of antioxidants such as GPX4 (74,75). Additionally, ferroptosis-induced
oxidative stress in the GC microenvironment activates the
nucleotide-binding oligomerization domain-like receptor protein 3
inflammasome, triggering IL-1β release and pyroptosis (76). These findings indicate that H.
pylori infection induces dysregulation of multiple PCD
pathways, which functionally interact during tumorigenesis.
Critically, H. pylori-driven PCD dysregulation-including
aberrant ferroptosis-significantly accelerates Correa's cascade of
GC, underscoring H. pylori eradication as a foundational
strategy for GC prevention (77).
Nevertheless, chronic inflammatory responses and pathological
progression frequently persist during intermediate to advanced
precancerous stages despite successful H. pylori eradication
therapy (78,79). This persistent pathological
progression highlights the potential therapeutic value of
strategies targeting various types of PCD, including ferroptosis,
as adjunctive interventions to complement conventional eradication
therapy in PLGC.
Studies on the regulation of ferroptosis not only
provide a new perspective on the pathogenesis of H. pylori
but also provide a direction for exploring new therapeutic targets
in Correa's cascade. The systematic review of ferroptosis in the
three key stages of chronic atrophic gastritis (CAG), intestinal
metaplasia (IM) and GC is conducive to bringing new breakthroughs
in the prevention and treatment of GC.
CAG is the initial stage in the 'inflammation-cancer
transformation model'. Knowing how to treat CAG timely and
accurately, and block or reverse the development of CAG to GC is
crucial for the prevention of GC. Conventional therapeutic
approaches for CAG primarily encompass H. pylori eradication
therapy, gastric mucosal protection and gastrointestinal function
enhancement. However, clinical evidence indicates that these
interventions demonstrate limited efficacy in reversing gastric
mucosal damage, particularly in patients presenting with extensive
or moderate-to-severe mucosal atrophy (80). Notably, accumulating evidence from
multiple studies has demonstrated that pharmacological inhibition
of ferroptosis can directly modulate the core pathological
processes of CAG through dual mechanisms: Attenuating gastric
mucosal injury and suppressing inflammatory responses (81,82).
A number of studies have focused on differentially
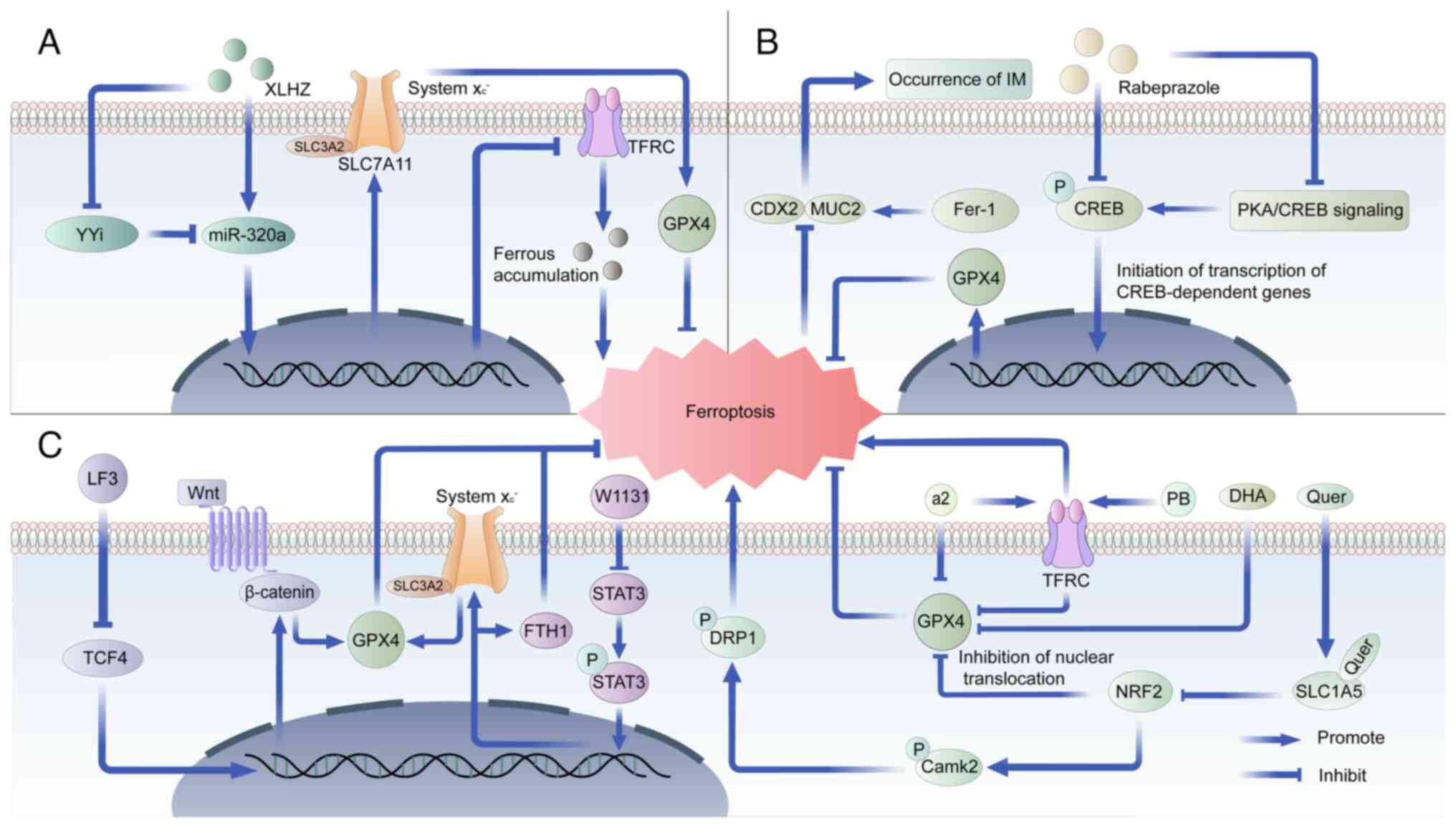
expressed genes associated with ferroptosis in CAG (83,84). Mechanistically, previous clinical
studies have revealed that patients with CAG frequently exhibit
abnormal iron metabolism or iron deficiency anemia, which is
associated with hepcidin, an antimicrobial polypeptide secreted by
gastric parietal cells (Fig. 4)
(85,86). Hepcidin inhibits iron efflux by
directly binding to ferroportin to cause conformational change and
trigger endocytosis and lysosomal degradation, which plays an
important role in regulating iron balance (87). Furthermore, CAG has been shown to
mediate hepcidin expression via the IL-6/signal transducer and
activator of transcription 3 (STAT3) signaling pathway, with
increased IL-6 expression being intimately linked to H.
pylori infection (84,88).
Elevated hepcidin levels decrease the expression of divalent metal
transporter 1 and ferroportin 1 proteins, inhibiting duodenal iron
absorption and leading to disrupted iron metabolism and gastric
cell ferroptosis (84).
The current clinical drug mechanisms for the
treatment of CAG mainly include regulation of gastric acid
secretion, eradication of H. pylori, protection of gastric
mucosa and inhibition of inflammatory factors (89). However, due to the limitations and
adverse effects associated with the long-term use of conventional
medications, recent studies have explored the therapeutic efficacy
and underlying mechanisms of traditional Chinese medicine (TCM)
drugs and natural molecular compounds in CAG, particularly focusing
on their modulation of inflammation and ferroptosis (81,82). One notable study demonstrated that
Xianglianhuazhuo can regulate the Yin Yang 1/miR-320a/TFRC axis,
effectively inhibiting gastric epithelial cell proliferation,
promoting apoptosis, suppressing ferroptosis, ameliorating gastric
mucosa pathology and alleviating CAG symptoms (81). These beneficial effects are
postulated to be related to the anti-inflammatory, anticancer and
antioxidant properties of components like berberine (Fig. 4) (81). Similar studies have also reported
the potential value of Galangin targeting ferroptosis in the
treatment of CAG (82).
Therefore, the therapeutic strategy of inhibiting ferroptosis in
CAG through low-toxicity TCM and natural molecular compounds has
potential.
The most controversial issue regarding the stage of
IM is whether its progression can be reversed by therapeutic
strategies such as eradication of H. pylori (90,91). A previous study has revealed an
important role of apoptosis in the transformation of IM to GC
(92). However, to the best of
our knowledge, there are relatively few studies related to other
types of PCD in IM. Notably, several studies have identified
ferroptosis-related genes in IM as potential biomarkers for IM
diagnosis and novel therapeutic targets such as GOT1, ACSF2, SESN2,
HMOX1 and FTL (93-95). Furthermore, a recent study
reported that ranolrazole could attenuate IM by inhibiting GPX4
expression to enhance ferroptosis (Fig. 4) (96). However, in general, the regulatory
mechanism of ferroptosis in IM remains to be elucidated.
Although progress has been made in exploring
ferroptosis as a therapeutic target for PLGC, significant
limitations remain. First, systematic studies are lacking to
definitively establish whether dysregulated ferroptosis constitutes
a key mechanism driving lesion progression or regression. Due to
the well-documented relationship between ferroptosis and chronic
inflammation, research focusing on the inflammation-cancer
transformation axis may offer an insight in addressing this
fundamental question (97,98).
Secondly, suitable experimental models are still deficient for
elucidating the temporal dynamics and spatial heterogeneity of
ferroptosis regulation within PLGC, particularly in IM. Emerging
technologies such as single-cell sequencing and spatial
transcriptomics, alongside models such as spasmolytic polypeptide
expressing metaplasia, hold promise for providing novel insights
and guiding future experimental designs (99,100). Finally, the related research on
targeting ferroptosis in PLGC is still at the basic theoretical
stage and lacks evidence to achieve clinical translation. The
establishment of gastric organoids derived from CAG/IM patient
tissues may be a key model to highly mimic the in vivo
environment in the future (101).
At present, there are notable issues in the
first-line conventional treatment regimens and experimental novel
treatment regimens for GC. Current therapeutic strategies for GC
face significant challenges in both conventional first-line
treatments and emerging experimental regimens. Persistent issues
including acquired drug resistance, restricted patient eligibility
for targeted therapies and dose-limiting toxicities associated with
combination therapies necessitate urgent optimization (102,103). Furthermore, the clinical
application of innovative approaches such as CAR-T cell therapy is
constrained by suboptimal efficacy and the absence of well-defined
molecular targets, which substantially impedes the advancement of
novel treatment paradigms (104,105).
Emerging evidence has demonstrated that oxidative
stress plays a pivotal role in the initial phases of
inflammation-associated carcinogenesis. Mechanistic studies have
revealed that reduced iron uptake and diminished intracellular iron
reserves may significantly contribute to GC pathogenesis. These
findings provide a compelling rationale for developing targeted
therapeutic strategies against GC through selective induction of
ferroptosis in malignant cells (Table
I) (106-108). The systemic
xc−-GSH-GPX4 pathway plays a pivotal role in
ferroptosis inhibition, thereby promoting the development of GC.
Specifically, the transcription factor megakaryocytic leukemia
factor 1 binds to CArG box sites in the promoters of SLC3A2 and
SLC7A11, enhancing their transcription and subsequently increasing
GSH levels, which inhibits ferroptosis in GC cells (109). Glutamate-cysteine ligase, the
rate-limiting enzyme for GSH synthesis, is crucial for this process
(110). Furthermore, Aldo-keto
reductase 1 member B1 participates in lipid metabolism regulation
by removing the aldehyde group from GSH. It specifically modulates
GPX4 by decreasing ROS accumulation and lipid peroxidation,
lowering intracellular ferrous ion and malondialdehyde levels, and
increasing GSH expression, thereby inhibiting RSL3-induced
ferroptosis in GC.
Previous evidence has increasingly highlighted the
pivotal role of ferroptosis in the metastasis and invasion of GC.
Epithelial-mesenchymal transition (EMT) is well recognized as a
critical mechanism driving tumor metastasis. Specifically,
2,2'-dipyridinone hydrazide dithiocarbamate butyrate demonstrates
anticancer efficacy in gastric and esophageal cancer cells. It
inhibits transforming growth factor-β1 in GC cells by inducing
ferritinophagy and activating the p53 and prolyl hydroxylase domain
protein 2/hypoxia-inducible factor 1α (HIF-1α) pathways, ultimately
suppressing EMT (111,112). Additionally, its homolog,
2,2'-dipyridyl ketone hydrazine-thiocarbamate, also exhibits
inhibitory effects on EMT in GC cells through the induction of
ferritinophagy and activation of the p53/AKT/mTOR pathway (113). Furthermore, ferroptosis
triggered by ferritin autophagy, coupled with the generation of
excessive ROS, further mediates the suppression of EMT (112). Moreover, A previous study
revealed that the cystatin inhibitor, cystatin SN, regulates GPX4
protein stability by recruiting OTU domain-containing ubiquitin
aldehyde-binding protein 1 to inhibit ferroptosis, thereby
promoting GC metastasis (114).
Collectively, these findings suggest that targets associated with
ferroptosis may offer promising avenues for inhibiting tumor
metastasis and progression.
Epigenetic modulation of ferroptosis also
constitutes a pivotal mechanism in the development and progression
of GC. A recent study has demonstrated that mesenchymal GC cells
exhibit upregulated expression of very long chain fatty acid
elongation protein 5 and fatty acid desaturase 1, sensitizing them
to ferroptosis. Conversely, intestinal-type GC cells display
resistance to ferroptosis due to the silencing of these enzymes via
DNA methylation (115).
Additionally, non-coding RNAs are linked to ferroptosis regulation.
Furthermore, research on long non-coding RNA (lncRNA) PMAN has
revealed that HIF-1α inhibits ferroptosis in peritoneal metastasis
of GC by upregulating lncRNA-PMAN, which is highly expressed in
peritoneal metastases and is associated with poor prognosis
(116).
The induction of ferroptosis as a novel strategy for
the treatment of GC has made some achievements in recent years. On
the one hand, emerging studies indicate that novel molecular
compounds exert antitumor effects in GC through ferroptosis
induction, offering a promising therapeutic alternative for
patients with compromised tolerance to conventional
chemoradiotherapy-associated systemic toxicity (Fig. 4) (117-120). On the other hand, inducing
ferroptosis to improve the chemoresistance of GC has been shown to
be an indirect way to inhibit the development of GC. Related
studies have further explored and developed substances that can
regulate ferroptosis-related genes (121,122) (Fig. 4). Ferroptosis negative
regulation-related genes (GPX4, SLC7A11 and ferritin heavy chain 1)
and STAT3 have been reported to be upregulated in 5-FU-resistant
cells and xenografts (121).
W1131 can alleviate chemoresistance in GC by inducing ferroptosis
as a novel STAT3 inhibitor, which makes it combine with
chemotherapeutic drugs for the treatment of chemotherapy-resistant
GC (121). In addition to the
aforementioned strategies, there are some innovative studies that
provide novel perspectives for the treatment of GC. One study has
proposed that atanorin driven by nanomaterials superparamagnetic
iron oxide nanoparticles can be used to induce ferroptosis of GC
stem cells (122).
In summary, ferroptosis-targeting strategies hold
significant therapeutic promise for GC. However, several key
challenges require further elucidation. The current mechanistic
understanding remains insufficient. Critical unresolved questions
include the differential regulation of ferroptosis across molecular
GC subtypes and the influence of the tumor microenvironment on
ferroptosis sensitivity (123,124). Future investigations should
prioritize applying single-cell multi-omics analyses and GC
organoid/immune cell co-culture models to address these gaps
(124). In addition, the
clinical translation of ferroptosis induction faces substantial
limitations. Specifically, existing ferroptosis inducers lack
tumor-specific targeting, and the synergistic potential of
ferroptosis induction combined with immunotherapy or targeted
therapy lacks robust theoretical and experimental validation.
Consequently, future research efforts should focus on integrating
advanced drug delivery technologies (e.g., responsive nanocarriers)
and rigorously exploring novel combination therapeutic strategies
(125,126).
The high diagnosis rate of advanced GC indicates
that the prevention and treatment of GC remain to be improved. The
prognostic markers related to ferroptosis screened by relevant
studies have important clinical significance in guiding the
treatment of GC (Table II). A
study from Japan investigated the relationship between GPX4, FSP1
and 4-HNE in tissues of patients with GC and their prognosis
(127). In this study, by
combining 163 pT3 or pT4 GC tissue samples and OS analysis, it was
found that patients with high GPX4 expression and low 4-HNE
accumulation had a poor prognosis (P=0.023), while patients with
low FSP1 expression and high 4-HNE accumulation had an improved
prognosis (P=0.033) (127). The
results also suggest that GPX4 and FSP1 may be potential
therapeutic targets for patients with GC with poor prognosis.
SLC2A3 is another ferroptosis marker. Univariate and multivariate
Cox regression analysis revealed that high expression of SLC2A3 was
associated with poor prognosis of patients with GC. Functional
enrichment analysis showed that SLC2A3 was related to
cytokine-cytokine receptor interaction, epithelial-mesenchymal
transition, T cell receptor signaling pathway, B cell receptor
signaling pathway, immune checkpoints and tumor microenvironment
regulation. SLC2A3 and related miRNAs are potential prognostic
biomarkers and therapeutics (128).
In addition, lncRNAs can regulate ferroptosis on the
epigenetic mechanism of GC, and the use of a variety of lncRNAs to
construct GC risk models has shown great advantages. A relative
study developed a novel ferroptosis-related prognostic model
incorporating 2 mRNAs and 15 lncRNAs to predict outcomes in
patients with GC. The model combined clinical features and key
factors, showed good predictive ability, and performed well in
external patient data validation, which is expected to improve the
clinical treatment effect of patients with GC (129). Another study identified 26
ferroptosis-related lncRNAs with independent prognostic value and
constructed a risk score model based on four high-risk lncRNAs
associated with poor prognosis of gastric adenocarcinoma (130).
Ferroptosis, a newly identified form of regulated
cell death, plays a key role in numerous physiological and
pathological processes. While significant progress has been made in
elucidating the molecular mechanisms of ferroptosis through basic
research, its precise role in diseases-particularly H.
pylori-associated GC-remains incompletely understood. In the
context of limited effective treatments for GC, systematic
investigations into ferroptosis dysregulation during H.
pylori pathogenesis and the identification of
ferroptosis-related therapeutic targets within Correa's cascade are
critical for developing novel and effective strategies. By
integrating multidisciplinary approaches, including systems
biology, nanotechnology and computational drug design, innovative
drug platforms can be developed to precisely modulate ferroptosis
pathways. These advancements could pave the way for novel
strategies to halt or even reverse the progression of Correa's
cascade. In conclusion, targeting ferroptosis represents a
promising strategy with significant potential for the timely
intervention of PLGC, as well as the early diagnosis and precision
treatment of GC.
The data generated in the present study may be
requested from the corresponding author.
CW wrote the manuscript. MW and CX revised the
manuscript. CY and MW contributed to the manuscript equally. All
authors have read and approved the final read manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
These authors declare that they have no competing
interests.
The authors would like to thank Dr. Huan Wang of
the First Affiliated Hospital of Nanchang University (Nanchang,
China) for her guidance in the development of the framework for the
article as well as her help in writing this paper.
This work was supported by the National Natural Science
Foundation of China (grant nos. 82100599 and 82560121); the Jiangxi
Provincial Department of Science and Technology (grant no.
20242BAB26122); the Science and Technology Plan of Jiangxi
Provincial Administration of Traditional Chinese Medicine (grant
no. 2023Z021); the Project of Jiangxi Provincial Academic and
Technical Leaders Training Program for Major Disciplines (grant no.
20243BCE51001); and the Ganpo Talent Program - Innovative High end
Talents (grant no. gpyc20240212).
|
1
|
Yuan J and Ofengeim D: A guide to cell
death pathways. Nat Rev Mol Cell Biol. 25:379–395. 2024. View Article : Google Scholar
|
|
2
|
Koren E and Fuchs Y: Modes of regulated
cell death in cancer. Cancer Discov. 11:245–265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolb JP, Oguin TH III, Oberst A and
Martinez J: Programmed Cell Death and Inflammation: Winter Is
Coming. Trends Immunol. 38:705–718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tong X, Tang R, Xiao M, Xu J, Wang W,
Zhang B, Liu J, Yu X and Shi S: Targeting cell death pathways for
cancer therapy: Recent developments in necroptosis, pyroptosis,
ferroptosis, and cuproptosis research. J Hematol Oncol. 15:1742022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Escuder-Rodríguez JJ, Liang D, Jiang X and
Sinicrope FA: Ferroptosis: Biology and Role in Gastrointestinal
Disease. Gastroenterology. 167:231–249. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
8
|
Moss SF, Shah SC, Tan MC and El-Serag HB:
Evolving Concepts in Helicobacter pylori Management.
Gastroenterology. 166:267–283. 2024. View Article : Google Scholar
|
|
9
|
Lei CQ, Wu X, Zhong X, Jiang L, Zhong B
and Shu HB: USP19 Inhibits TNF-α- and IL-1β-Triggered NF-κB
Activation by Deubiquitinating TAK1. J Immunol. 203:259–268. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartchewsky W Jr, Martini MR, Masiero M,
Squassoni AC, Alvarez MC, Ladeira MS, Salvatore D, Trevisan M,
Pedrazzoli J Jr and Ribeiro ML: Effect of Helicobacter pylori
infection on IL-8, IL-1beta and COX-2 expression in patients with
chronic gastritis and gastric cancer. Scand J Gastroenterol.
44:153–161. 2009. View Article : Google Scholar
|
|
11
|
El Filaly H, Desterke C, Outlioua A, Badre
W, Rabhi M, Karkouri M, Riyad M, Khalil A, Arnoult D and Akarid K:
CXCL-8 as a signature of severe Helicobacter pylori infection and a
stimulator of stomach region-dependent immune response. Clin
Immunol. 252:1096482023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correa P, Haenszel W, Cuello C, Tannenbaum
S and Archer M: A model for gastric cancer epidemiology. Lancet.
2:58–60. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tahara S, Tahara T, Horiguchi N, Kato T,
Shinkai Y, Yamashita H, Yamada H, Kawamura T, Terada T, Okubo M, et
al: DNA methylation accumulation in gastric mucosa adjacent to
cancer after Helicobacter pylori eradication. Int J Cancer.
144:80–88. 2019. View Article : Google Scholar
|
|
14
|
Qu X and Shi Y: Bile reflux and bile acids
in the progression of gastric intestinal metaplasia. Chin Med J
(Engl). 135:1664–1672. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuoka T and Yashiro M: Novel biomarkers
for early detection of gastric cancer. World J Gastroenterol.
29:2515–2533. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae S, Lee H, Her EY, Lee K, Kim JS, Ahn
J, Choi IJ, Jun JK, Choi KS and Suh M: Cost Utility analysis of
National cancer screening program for gastric cancer in Korea: A
markov model analysis. J Korean Med Sci. 40:e432025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugimoto N, Kawada J, Oka Y, Ueda S,
Murakami K, Nishikawa K, Kurokawa Y, Fujitani K, Kawakami H, Endo
S, et al: Salvage-line of capecitabine plus oxaliplatin therapy
(XELOX) for patients with inoperable/advanced gastric cancer
resistant/intolerant to cisplatin (OGSG1403). Anticancer Res.
45:307–313. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura Y, Kawazoe A, Lordick F,
Janjigian YY and Shitara K: Biomarker-targeted therapies for
advanced-stage gastric and gastro-oesophageal junction cancers: An
emerging paradigm. Nat Rev Clin Oncol. 18:473–487. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shitara K, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial.
Lancet. 392:123–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bannai S and Kitamura E: Transport
interaction of L-cystine and L-glutamate in human diploid
fibroblasts in culture. J Biol Chem. 255:2372–2376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato H, Tamba M, Kuriyama-Matsumura K,
Okuno S and Bannai S: Molecular cloning and expression of human
xCT, the light chain of amino acid transport system xc. Antioxid
Redox Signal. 2:665–671. 2000. View Article : Google Scholar
|
|
25
|
Ursini F, Maiorino M, Valente M, Ferri L
and Gregolin C: Purification from pig liver of a protein which
protects liposomes and biomembranes from peroxidative degradation
and exhibits glutathione peroxidase activity on phosphatidylcholine
hydroperoxides. Biochim Biophys Acta. 710:197–211. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conrad M and Pratt DA: The chemical basis
of ferroptosis. Nat Chem Biol. 15:1137–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang WS and Stockwell BR: Synthetic lethal
screening identifies compounds activating iron-dependent,
nonapoptotic cell death in oncogenic-RAS-harboring cancer cells.
Chem Biol. 15:234–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dolma S, Lessnick SL, Hahn WC and
Stockwell BR: Identification of genotype-selective antitumor agents
using synthetic lethal chemical screening in engineered human tumor
cells. Cancer Cell. 3:285–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Z, Liu X, Zhang W, Zhang K, Pan L, Zhu
M, Qin H, Zou C, Wang W, Zhang C, et al: Biomimetic macrophage
membrane-camouflaged nanoparticles induce ferroptosis by promoting
mitochondrial damage in glioblastoma. ACS Nano. 17:23746–23760.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zou Y, Li H, Graham ET, Deik AA, Eaton JK,
Wang W, Sandoval-Gomez G, Clish CB, Doench JG and Schreiber SL:
Cytochrome P450 oxidoreductase contributes to phospholipid
peroxidation in ferroptosis. Nat Chem Biol. 16:302–309. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Wan Y, Jiang Y, Zhang L and Cheng
W: GPX4: The hub of lipid oxidation, ferroptosis, disease and
treatment. Biochim Biophys Acta Rev Cancer. 1878:1888902023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar :
|
|
36
|
Lee H, Zandkarimi F, Zhang Y, Meena JK,
Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, et al:
Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat
Cell Biol. 22:225–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z,
Cai K, Zhao Y and Luo Z: HCAR1/MCT1 regulates tumor ferroptosis
through the lactate-mediated AMPK-SCD1 activity and its therapeutic
implications. Cell Rep. 33:1084872020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yi J, Zhu J, Wu J, Thompson CB and Jiang
X: Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu J, Minikes AM, Gao M, Bian H, Li Y,
Stockwell BR, Chen ZN and Jiang X: Intercellular interaction
dictates cancer cell ferroptosis via NF2-YAP signalling. Nature.
572:402–406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim NG, Koh E, Chen X and Gumbiner BM:
E-cadherin mediates contact inhibition of proliferation through
Hippo signaling-pathway components. Proc Natl Acad Sci USA.
108:11930–11935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tarangelo A, Magtanong L, Bieging-Rolett
KT, Li Y, Ye J, Attardi LD and Dixon SJ: p53 suppresses metabolic
stress-induced ferroptosis in cancer cells. Cell Rep. 22:569–575.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J,
Zhong M, Yuan H, Zhang L, Billiar TR, et al: The tumor suppressor
p53 limits ferroptosis by blocking DPP4 activity. Cell Rep.
20:1692–1704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C,
Dai X, Li Z and Wu G: Ferroptosis: A novel anti-tumor action for
cisplatin. Cancer Res Treat. 50:445–460. 2018. View Article : Google Scholar :
|
|
46
|
Lachaier E, Louandre C, Godin C, Saidak Z,
Baert M, Diouf M, Chauffert B and Galmiche A: Sorafenib induces
ferroptosis in human cancer cell lines originating from different
solid tumors. Anticancer Res. 34:6417–6422. 2014.PubMed/NCBI
|
|
47
|
Zhang X, Hong B, Li H, Sun Z, Zhao J, Li
M, Wei D, Wang Y and Zhang N: Disulfidptosis and ferroptosis
related genes define the immune microenvironment and NUBPL serves
as a potential biomarker for predicting prognosis and immunotherapy
response in bladder cancer. Heliyon. 10:e376382024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kuang Y, Yang K, Meng L, Mao Y, Xu F and
Liu H: Identification and validation of ferroptosis-related
biomarkers and the related pathogenesis in precancerous lesions of
gastric cancer. Sci Rep. 13:160742023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu W, Liu D, Lu Y, Sun J, Zhu J, Xing Y,
Ma X, Wang Y, Ji M and Jia Y: PHKG2 regulates RSL3-induced
ferroptosis in Helicobacter pylori related gastric cancer. Arch
Biochem Biophys. 740:1095602023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dierge E, Debock E, Guilbaud C, Corbet C,
Mignolet E, Mignard L, Bastien E, Dessy C, Larondelle Y and Feron
O: Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the
acidic tumor environment leads to ferroptosis-mediated anticancer
effects. Cell Metab. 33:1701–1715.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Greenfield LK and Jones NL: Modulation of
autophagy by Helicobacter pylori and its role in gastric
carcinogenesis. Trends Microbiol. 21:602–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Genta RM: Helicobacter pylori,
inflammation, mucosal damage, and apoptosis: Pathogenesis and
definition of gastric atrophy. Gastroenterology. 113(6 Suppl):
S51–S55. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhu P, Xue J, Zhang ZJ, Jia YP, Tong YN,
Han D, Li Q, Xiang Y, Mao XH and Tang B: Helicobacter pylori VacA
induces autophagic cell death in gastric epithelial cells via the
endoplasmic reticulum stress pathway. Cell Death Dis. 8:32072017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Teng Y, Liu X, Han B, Ma Q, Liu Y, Kong H,
Lv Y, Mao F, Cheng P, Hao C, et al: Helicobacter
pylori-downregulated tumor necrosis factor receptor-associated
protein 1 mediates apoptosis of human gastric epithelial cells. J
Cell Physiol. 234:15698–15707. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chaturvedi R, Asim M, Romero-Gallo J,
Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo
MB, Yan F, et al: Spermine oxidase mediates the gastric cancer risk
associated with Helicobacter pylori CagA. Gastroenterology.
141:1696-1708.e1–e2. 2011. View Article : Google Scholar
|
|
57
|
Wu S, Chen Y, Chen Z, Wei F, Zhou Q, Li P
and Gu Q: Reactive oxygen species and gastric carcinogenesis: The
complex interaction between Helicobacter pylori and host.
Helicobacter. 28:e130242023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Salvatori S, Marafini I, Laudisi F,
Monteleone G and Stolfi C: Helicobacter pylori and gastric cancer:
Pathogenetic mechanisms. Int J Mol Sci. 24:28952023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Teymournejad O, Mobarez AM, Hassan ZM and
Talebi Bezmin Abadi A: Binding of the Helicobacter pylori OipA
causes apoptosis of host cells via modulation of Bax/Bcl-2 levels.
Sci Rep. 7:80362017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jain P, Luo ZQ and Blanke SR: Helicobacter
pylori vacuolating cytotoxin A (VacA) engages the mitochondrial
fission machinery to induce host cell death. Proc Natl Acad Sci
USA. 108:16032–16037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Posselt G, Wiesauer M, Chichirau BE,
Engler D, Krisch LM, Gadermaier G, Briza P, Schneider S, Boccellato
F, Meyer TF, et al: Helicobacter pylori-controlled c-Abl
localization promotes cell migration and limits apoptosis. Cell
Commun Signal. 17:102019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lin Y, Liu K, Lu F, Zhai C and Cheng F:
Programmed cell death in Helicobacter pylori infection and related
gastric cancer. Front Cell Infect Microbiol. 14:14168192024.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kumar S and Dhiman M: Inflammasome
activation and regulation during Helicobacter pylori pathogenesis.
Microb Pathog. 125:468–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cui G, Yuan A and Li Z: Occurrences and
phenotypes of RIPK3-positive gastric cells in Helicobacter pylori
infected gastritis and atrophic lesions. Dig Liver Dis.
54:1342–1349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qiang L, Zhang Y, Lei Z, Lu Z, Tan S, Ge
P, Chai Q, Zhao M, Zhang X, Li B, et al: A mycobacterial effector
promotes ferroptosis-dependent pathogenicity and dissemination. Nat
Commun. 14:14302023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dar HH, Tyurina YY, Mikulska-Ruminska K,
Shrivastava I, Ting HC, Tyurin VA, Krieger J, St Croix CM, Watkins
S, Bayir E, et al: Pseudomonas aeruginosa utilizes host
polyunsaturated phosphatidylethanolamines to trigger
theft-ferroptosis in bronchial epithelium. J Clin Invest.
128:4639–4653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Drake IM, Mapstone NP, Schorah CJ, White
KL, Chalmers DM, Dixon MF and Axon AT: Reactive oxygen species
activity and lipid peroxidation in Helicobacter pylori associated
gastritis: Relation to gastric mucosal ascorbic acid concentrations
and effect of H pylori eradication. Gut. 42:768–771. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Peng Y, Lei X, Yang Q, Zhang G, He S, Wang
M, Ling R, Zheng B, He J, Chen X, et al: Helicobacter pylori
CagA-mediated ether lipid biosynthesis promotes ferroptosis
susceptibility in gastric cancer. Exp Mol Med. 56:441–452. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu D, Peng J, Xie J and Xie Y:
Comprehensive analysis of the function of helicobacter-associated
ferroptosis gene YWHAE in gastric cancer through multi-omics
integration, molecular docking, and machine learning. Apoptosis.
29:439–456. 2024. View Article : Google Scholar
|
|
71
|
Melo J, Cavadas B, Pereira L, Figueiredo C
and Leite M: Transcriptomic remodeling of gastric cells by
Helicobacter pylori outer membrane vesicles. Helicobacter.
29:e130312024. View Article : Google Scholar
|
|
72
|
Shen C, Liu H, Chen Y, Liu M, Wang Q and
Liu J and Liu J: Helicobacter pylori induces GBA1 demethylation to
inhibit ferroptosis in gastric cancer. Mol Cell Biochem.
480:1845–1863. 2025. View Article : Google Scholar
|
|
73
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gryzik M, Asperti M, Denardo A, Arosio P
and Poli M: NCOA4-mediated ferritinophagy promotes ferroptosis
induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys
Acta Mol Cell Res. 1868:1189132021. View Article : Google Scholar
|
|
75
|
Chen X, Yu C, Kang R, Kroemer G and Tang
D: Cellular degradation systems in ferroptosis. Cell Death Differ.
28:1135–1148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu Y, Miao R, Xia J, Zhou Y, Yao J and
Shao S: Infection of Helicobacter pylori contributes to the
progression of gastric cancer through ferroptosis. Cell Death
Discov. 10:4852024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Piscione M, Mazzone M, Di Marcantonio MC,
Muraro R and Mincione G: Eradication of helicobacter pylori and
gastric cancer: A controversial relationship. Front Microbiol.
12:6308522021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
White JR, Winter JA and Robinson K:
Differential inflammatory response to Helicobacter pylori
infection: Etiology and clinical outcomes. J Inflamm Res.
8:137–147. 2015.PubMed/NCBI
|
|
80
|
Shah SC, Piazuelo MB, Kuipers EJ and Li D:
AGA clinical practice update on the diagnosis and management of
atrophic gastritis: Expert review. Gastroenterology.
161:1325–1332.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Guo Y, Jia X, Du P, Wang J, Du Y, Li B,
Xue Y, Jiang J, Cai Y and Yang Q: Mechanistic insights into the
ameliorative effects of Xianglianhuazhuo formula on chronic
atrophic gastritis through ferroptosis mediated by
YY1/miR-320a/TFRC signal pathway. J Ethnopharmacol. 323:1176082024.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yang T, Lu M, Jiang W, Jin D, Sun M, Mao H
and Han H: Galangin alleviates gastric mucosal injury in rats with
chronic atrophic gastritis by reducing ferroptosis. Histol
Histopathol. January 24–2025.Epub ahead of print.
|
|
83
|
Pan W, Liu C, Ren T, Chen X, Liang C, Wang
J and Yang J: Exploration of lncRNA/circRNA-miRNA-mRNA network in
patients with chronic atrophic gastritis in Tibetan plateau areas
based on DNBSEQ-G99 RNA sequencing. Sci Rep. 14:92122024.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhao Y, Zhao J, Ma H, Han Y, Xu W, Wang J,
Cai Y, Jia X, Jia Q and Yang Q: High hepcidin levels promote
abnormal iron metabolism and ferroptosis in chronic atrophic
gastritis. Biomedicines. 11:23382023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lanser L, Fuchs D, Kurz K and Weiss G:
Physiology and inflammation driven pathophysiology of iron
homeostasis-mechanistic insights into anemia of inflammation and
its treatment. Nutrients. 13:37322021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Schwarz P, Kübler JA, Strnad P, Müller K,
Barth TF, Gerloff A, Feick P, Peyssonnaux C, Vaulont S, Adler G and
Kulaksiz H: Hepcidin is localised in gastric parietal cells,
regulates acid secretion and is induced by Helicobacter pylori
infection. Gut. 61:193–201. 2012. View Article : Google Scholar
|
|
87
|
Ganz T and Nemeth E: Hepcidin and
disorders of iron metabolism. Annu Rev Med. 62:347–360. 2011.
View Article : Google Scholar
|
|
88
|
Santos MP, Pereira JN, Delabio RW, Smith
MAC, Payão SLM, Carneiro LC, Barbosa MS and Rasmussen LT: Increased
expression of interleukin-6 gene in gastritis and gastric cancer.
Braz J Med Biol Res. 54:e106872021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jia J, Zhao H, Li F, Zheng Q, Wang G, Li D
and Liu Y: Research on drug treatment and the novel signaling
pathway of chronic atrophic gastritis. Biomed Pharmacother.
176:1169122024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhu F, Zhang X, Li P and Zhu Y: Effect of
Helicobacter pylori eradication on gastric precancerous lesions: A
systematic review and meta-analysis. Helicobacter. 28:e130132023.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liang Y, Yang Y, Nong R, Huang H, Chen X,
Deng Y, Huang Z, Huang J, Cheng C, Ji M, et al: Do atrophic
gastritis and intestinal metaplasia reverse after Helicobacter
pylori eradication? Helicobacter. 29:e130422024. View Article : Google Scholar
|
|
92
|
Bir F, Calli-Demirkan N, Tufan AC, Akbulut
M and Satiroglu-Tufan NL: Apoptotic cell death and its relationship
to gastric carcinogenesis. World J Gastroenterol. 13:3183–3188.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li T, Yang Q, Liu Y, Jin Y, Song B, Sun Q,
Wei S, Wu J and Li X: Machine learning identify ferroptosis-related
genes as potential diagnostic biomarkers for gastric intestinal
metaplasia. Technol Cancer Res Treat. 23:153303382412720362024.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Song B, Li T, Zhang Y, Yang Q, Pei B, Liu
Y, Wang J, Dong G, Sun Q, Fan S and Li X: Identification and
verification of ferroptosis-related genes in gastric intestinal
metaplasia. Front Genet. 14:11524142023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hamedi Asl D, Naserpour Farivar T, Rahmani
B, Hajmanoochehri F, Emami Razavi AN, Jahanbin B, Soleimani Dodaran
M and Peymani A: The role of transferrin receptor in the
Helicobacter pylori pathogenesis; L-ferritin as a novel marker for
intestinal metaplasia. Microb Pathog. 126:157–164. 2019. View Article : Google Scholar
|
|
96
|
Xie J, Liang X, Xie F, Huang C, Lin Z, Xie
S, Yang F, Zheng F, Geng L, Xu W, et al: Rabeprazole suppressed
gastric intestinal metaplasia through activation of GPX4-mediated
ferroptosis. Front Pharmacol. 15:14090012024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ebrahimi N, Adelian S, Shakerian S,
Afshinpour M, Chaleshtori SR, Rostami N, Rezaei-Tazangi F,
Beiranvand S, Hamblin MR and Aref AR: Crosstalk between ferroptosis
and the epithelial-mesenchymal transition: Implications for
inflammation and cancer therapy. Cytokine Growth Factor Rev.
64:33–45. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang M, Zhong J, Song Z, Xu Q, Chen Y and
Zhang Z: Regulatory mechanisms and potential therapeutic targets in
precancerous lesions of gastric cancer: A comprehensive review.
Biomed Pharmacother. 177:1170682024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gu L, Chen H, Geng R, Sun M, Shi Q, Chen
Y, Chang J, Wei J, Ma W, Xiao J, et al: Single-cell and Spatial
transcriptomics reveals ferroptosis as the most enriched programmed
cell death process in hemorrhage stroke-induced
oligodendrocyte-mediated white matter injury. Int J Biol Sci.
20:3842–3862. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Miao ZF, Sun JX, Adkins-Threats M, Pang
MJ, Zhao JH, Wang X, Tang KW, Wang ZN and Mills JC: DDIT4 licenses
only healthy cells to proliferate during injury-induced metaplasia.
Gastroenterology. 160:260–271.e10. 2021. View Article : Google Scholar
|
|
101
|
Pang MJ, Burclaff JR, Jin R,
Adkins-Threats M, Osaki LH, Han Y, Mills JC, Miao ZF and Wang ZN:
Gastric organoids: Progress and remaining challenges. Cell Mol
Gastroenterol Hepatol. 13:19–33. 2022. View Article : Google Scholar
|
|
102
|
Hu X, Ma Z, Xu B, Li S, Yao Z, Liang B,
Wang J, Liao W, Lin L, Wang C, et al: Glutamine metabolic
microenvironment drives M2 macrophage polarization to mediate
trastuzumab resistance in HER2-positive gastric cancer. Cancer
Commun (Lond). 43:909–937. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang F, Li A, Liu H and Zhang H: Gastric
cancer combination therapy: Synthesis of a hyaluronic acid and
cisplatin containing lipid prodrug coloaded with sorafenib in a
nanoparticulate system to exhibit enhanced anticancer efficacy and
reduced toxicity. Drug Des Devel Ther. 12:3321–3333. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Qi C, Gong J, Li J, Liu D, Qin Y, Ge S,
Zhang M, Peng Z, Zhou J, Cao Y, et al: Claudin18.2-specific CAR T
cells in gastrointestinal cancers: Phase 1 trial interim results.
Nat Med. 28:1189–1198. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yasuda T and Wang YA: Gastric cancer
immunosuppressive microenvironment heterogeneity: Implications for
therapy development. Trends Cancer. 10:627–642. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fonseca-Nunes A, Agudo A, Aranda N, Arija
V, Cross AJ, Molina E, Sanchez MJ, Bueno-de-Mesquita HB, Siersema
P, Weiderpass E, et al: Body iron status and gastric cancer risk in
the EURGAST study. Int J Cancer. 137:2904–2914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Noto JM, Piazuelo MB, Shah SC,
Romero-Gallo J, Hart JL, Di C, Carmichael JD, Delgado AG, Halvorson
AE, Greevy RA, et al: Iron deficiency linked to altered bile acid
metabolism promotes Helicobacter pylori-induced inflammation-driven
gastric carcinogenesis. J Clin Invest. 132:e1478222022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Dai ZT, Wu YL, Li XR and Liao XH: MKL-1
suppresses ferroptosis by activating system Xc- and increasing
glutathione synthesis. Int J Biol Sci. 19:4457–4475. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar :
|
|
111
|
Guan D and Li C, Li Y, Li Y, Wang G, Gao F
and Li C: The DpdtbA induced EMT inhibition in gastric cancer cell
lines was through ferritinophagy-mediated activation of p53 and
PHD2/hif-1α pathway. J Inorg Biochem. 218:1114132021. View Article : Google Scholar
|
|
112
|
Guan D, Zhou W, Wei H, Wang T, Zheng K,
Yang C, Feng R, Xu R, Fu Y, Li C, et al: Ferritinophagy-mediated
ferroptosis and activation of Keap1/Nrf2/HO-1 pathway were
conducive to EMT inhibition of gastric cancer cells in action of
2,2'-Di-pyridineketone hydrazone dithiocarbamate butyric acid
ester. Oxid Med Cell Longev. 2022:39206642022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Xu Z, Feng J, Li Y, Guan D, Chen H, Zhai
X, Zhang L and Li C and Li C: The vicious cycle between
ferritinophagy and ROS production triggered EMT inhibition of
gastric cancer cells was through p53/AKT/mTor pathway. Chem Biol
Interact. 328:1091962020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li D, Wang Y, Dong C, Chen T, Dong A, Ren
J, Li W, Shu G, Yang J, Shen W, et al: CST1 inhibits ferroptosis
and promotes gastric cancer metastasis by regulating GPX4 protein
stability via OTUB1. Oncogene. 42:83–98. 2023. View Article : Google Scholar :
|
|
115
|
Lee JY, Nam M, Son HY, Hyun K, Jang SY,
Kim JW, Kim MW, Jung Y, Jang E, Yoon SJ, et al: Polyunsaturated
fatty acid biosynthesis pathway determines ferroptosis sensitivity
in gastric cancer. Proc Natl Acad Sci USA. 117:32433–32442. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lin Z, Song J, Gao Y, Huang S, Dou R,
Zhong P, Huang G, Han L, Zheng J, Zhang X, et al: Hypoxia-induced
HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the
cytoplasmic translocation of ELAVL1 in peritoneal dissemination
from gastric cancer. Redox Biol. 52:1023122022. View Article : Google Scholar
|
|
117
|
Liu Y, Song Z, Liu Y, Ma X, Wang W, Ke Y,
Xu Y, Yu D and Liu H: Identification of ferroptosis as a novel
mechanism for antitumor activity of natural product derivative a2
in gastric cancer. Acta Pharm Sin B. 11:1513–1525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hu C, Zu D, Xu J, Xu H, Yuan L, Chen J,
Wei Q, Zhang Y, Han J, Lu T, et al: Polyphyllin B suppresses
gastric tumor growth by modulating iron metabolism and inducing
ferroptosis. Int J Biol Sci. 19:1063–1079. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ding L, Dang S, Sun M, Zhou D, Sun Y, Li
E, Peng S, Li J and Li G: Quercetin induces ferroptosis in gastric
cancer cells by targeting SLC1A5 and regulating the p-Camk2/p-DRP1
and NRF2/GPX4 Axes. Free Radic Biol Med. 213:150–163. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang H, Lu C, Zhou H, Zhao X, Huang C,
Cheng Z, Liu G and You X: Synergistic effects of dihydroartemisinin
and cisplatin on inducing ferroptosis in gastric cancer through
GPX4 inhibition. Gastric Cancer. 28:187–210. 2025. View Article : Google Scholar
|
|
121
|
Ouyang S, Li H, Lou L, Huang Q, Zhang Z,
Mo J, Li M, Lu J, Zhu K, Chu Y, et al: Inhibition of
STAT3-ferroptosis negative regulatory axis suppresses tumor growth
and alleviates chemoresistance in gastric cancer. Redox Biol.
52:1023172022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ni Z, Nie X, Zhang H, Wang L, Geng Z, Du
X, Qian H, Liu W and Liu T: Atranorin driven by nano materials
SPION lead to ferroptosis of gastric cancer stem cells by weakening
the mRNA 5-hydroxymethylcytidine modification of the Xc-/GPX4 axis
and its expression. Int J Med Sci. 19:1680–1694. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cui JX, Xu XH, He T, Liu JJ, Xie TY, Tian
W and Liu JY: L-kynurenine induces NK cell loss in gastric cancer
microenvironment via promoting ferroptosis. J Exp Clin Cancer Res.
42:522023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kumar V, Ramnarayanan K, Sundar R,
Padmanabhan N, Srivastava S, Koiwa M, Yasuda T, Koh V, Huang KK,
Tay ST, et al: Single-cell atlas of lineage states, tumor
microenvironment, and subtype-specific expression programs in
gastric cancer. Cancer Discov. 12:670–691. 2022. View Article : Google Scholar
|
|
125
|
Zhang Q, Kuang G, Li W, Wang J, Ren H and
Zhao Y: Stimuli-responsive gene delivery nanocarriers for cancer
therapy. Nanomicro Lett. 15:442023.PubMed/NCBI
|
|
126
|
Cheng X, Dai E, Wu J, Flores NM, Chu Y,
Wang R, Dang M, Xu Z, Han G, Liu Y, et al: Atlas of metastatic
gastric cancer links ferroptosis to disease progression and
immunotherapy response. Gastroenterology. 167:1345–1357. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tamura K, Tomita Y, Kanazawa T, Shinohara
H, Sakano M, Ishibashi S, Ikeda M, Kinoshita M, Minami J, Yamamoto
K, et al: Lipid peroxidation regulators GPX4 and FSP1 as prognostic
markers and therapeutic targets in advanced gastric cancer. Int J
Mol Sci. 25:92032024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lin L, Que R, Wang J, Zhu Y, Liu X and Xu
R: Prognostic value of the ferroptosis-related gene SLC2A3 in
gastric cancer and related immune mechanisms. Front Genet.
13:9193132022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu Y, Liu Y, Ye S, Feng H and Ma L: A new
ferroptosis-related signature model including messenger RNAs and
long non-coding RNAs predicts the prognosis of gastric cancer
patients. J Transl Int Med. 11:145–155. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Cai Y, Wu S, Jia Y, Pan X and Li C:
Potential key markers for predicting the prognosis of gastric
adenocarcinoma based on the expression of ferroptosis-related
lncRNA. J Immunol Res. 2022:12492902022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ryu MH, Lee KH, Shen L, Yeh KH, Yoo C,
Hong YS, Park YI, Yang SH, Shin DB, Zang DY, et al: Randomized
phase II study of capecitabine plus cisplatin with or without
sorafenib in patients with metastatic gastric cancer (STARGATE).
Cancer Med. 12:7784–7794. 2023. View Article : Google Scholar :
|
|
132
|
Xu X and Li Y, Wu Y, Wang M, Lu Y, Fang Z,
Wang H and Li Y: Increased ATF2 expression predicts poor prognosis
and inhibits sorafenib-induced ferroptosis in gastric cancer. Redox
Biol. 59:1025642023. View Article : Google Scholar
|
|
133
|
Zhuang J, Liu X, Yang Y, Zhang Y and Guan
G: Sulfasalazine, a potent suppressor of gastric cancer
proliferation and metastasis by inhibition of xCT: Conventional
drug in new use. J Cell Mol Med. 25:5372–5380. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Shitara K, Doi T, Nagano O, Fukutani M,
Hasegawa H, Nomura S, Sato A, Kuwata T, Asai K, Einaga Y, et al:
Phase 1 study of sulfasalazine and cisplatin for patients with
CD44v-positive gastric cancer refractory to cisplatin (EPOC1407).
Gastric Cancer. 20:1004–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang Y, Zheng L, Shang W, Yang Z, Li T,
Liu F, Shao W, Lv L, Chai L, Qu L, et al: Wnt/beta-catenin
signaling confers ferroptosis resistance by targeting GPX4 in
gastric cancer. Cell Death Differ. 29:2190–2202. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wu C, Wang S, Huang T, Xi X, Xu L, Wang J,
Hou Y, Xia Y, Xu L, Wang L and Huang X: NPR1 promotes cisplatin
resistance by inhibiting PARL-mediated mitophagy-dependent
ferroptosis in gastric cancer. Cell Biol Toxicol. 40:932024.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Fu D, Wang C, Yu L and Yu R: Induction of
ferroptosis by ATF3 elevation alleviates cisplatin resistance in
gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol
Biol Lett. 26:262021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhou Q, Liu T, Qian W, Ji J, Cai Q, Jin Y,
Jiang J and Zhang J: HNF4A-BAP31-VDAC1 axis synchronously regulates
cell proliferation and ferroptosis in gastric cancer. Cell Death
Dis. 14:3562023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Qu X, Liu B, Wang L, Liu L, Zhao W, Liu C,
Ding J, Zhao S, Xu B, Yu H, et al: Loss of cancer-associated
fibroblast-derived exosomal DACT3-AS1 promotes malignant
transformation and ferroptosis-mediated oxaliplatin resistance in
gastric cancer. Drug Resist Updat. 68:1009362023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yao L, Hou J, Wu X, Lu Y, Jin Z, Yu Z, Yu
B, Li J, Yang Z, Li C, et al: Cancer-associated fibroblasts impair
the cytotoxic function of NK cells in gastric cancer by inducing
ferroptosis via iron regulation. Redox Biol. 67:1029232023.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sun J, Li J, Pantopoulos K, Liu Y, He Y,
Kang W and Ye X: The clustering status of detached gastric cancer
cells inhibits anoikis-induced ferroptosis to promote metastatic
colonization. Cancer Cell Int. 24:772024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yao X, He Z, Qin C, Deng X, Bai L, Li G
and Shi J: SLC2A3 promotes macrophage infiltration by glycolysis
reprogramming in gastric cancer. Cancer Cell Int. 20:5032020.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li Y, Xu X, Wang X, Zhang C, Hu A and Li
Y: MGST1 expression is associated with poor prognosis, enhancing
the Wnt/β-catenin pathway via regulating AKT and inhibiting
ferroptosis in gastric cancer. ACS Omega. 8:23683–23694. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wang C, Shi M, Ji J, Cai Q, Zhao Q, Jiang
J, Liu J, Zhang H, Zhu Z and Zhang J: Stearoyl-CoA desaturase 1
(SCD1) facilitates the growth and anti-ferroptosis of gastric
cancer cells and predicts poor prognosis of gastric cancer. Aging
(Albany NY). 12:15374–15391. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sun X, Yang S, Feng X, Zheng Y, Zhou J,
Wang H, Zhang Y, Sun H and He C: The modification of ferroptosis
and abnormal lipometabolism through overexpression and knockdown of
potential prognostic biomarker perilipin2 in gastric carcinoma.
Gastric Cancer. 23:241–259. 2020. View Article : Google Scholar
|
|
146
|
Zang J, Cui M, Xiao L, Zhang J and Jing R:
Overexpression of ferroptosis-related genes FSP1 and CISD1 is
related to prognosis and tumor immune infiltration in gastric
cancer. Clin Transl Oncol. 25:2532–2544. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Li X, Qian J, Xu J, Bai H, Yang J and Chen
L: NRF2 inhibits RSL3 induced ferroptosis in gastric cancer through
regulation of AKR1B1. Exp Cell Res. 442:1142102024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Tu RH, Wu SZ, Huang ZN, Zhong Q, Ye YH,
Zheng CH, Xie JW, Wang JB, Lin JX, Chen QY, et al: Neurotransmitter
receptor HTR2B regulates lipid metabolism to inhibit ferroptosis in
gastric cancer. Cancer Res. 83:3868–3885. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Qu H, Liang Y, Guo Q, Lu L, Yang Y, Xu W,
Zhang Y and Qin Y: Identifying CTH and MAP1LC3B as ferroptosis
biomarkers for prognostic indication in gastric cancer decoding.
Sci Rep. 14:43522024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wang S, Zhang S, Li X, Leng C, Li X, Lv J,
Zhao S, Qiu W and Guo J: Development of oxidative stress- and
ferroptosis-related prognostic signature in gastric cancer and
identification of CDH19 as a novel biomarker. Hum Genomics.
18:1212024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Jiang Y, Li L, Li W, Liu K, Wu Y and Wang
Z: NFS1 inhibits ferroptosis in gastric cancer by regulating the
STAT3 pathway. J Bioenerg Biomembr. 56:573–587. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Jiang Y, Li W, Zhang J, Liu K, Wu Y and
Wang Z: NFS1 as a candidate prognostic biomarker for gastric cancer
correlated with immune infiltrates. Int J Gen Med. 17:3855–3868.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu W, Zhang F, Yang K and Yan Y:
Comprehensive analysis regarding the prognostic significance of
downregulated ferroptosis-related gene AKR1C2 in gastric cancer and
its underlying roles in immune response. PLoS One. 18:e02809892023.
View Article : Google Scholar : PubMed/NCBI
|