Introduction
Urothelial carcinoma (UC) is a neoplastic growth
that originates in the urinary tract lining and extends from the
renal pelvis to the urethra. It encompasses a range of histological
subtypes and anatomical locations, including upper tract UC (UTUC)
and primary urethral cancer (1).
UTUC accounts for 5-10% of UC cases, whereas primary urethral
cancer is even rarer (2). In the
United States, it is estimated that there were 87,840 new cases of
bladder cancer and 17,840 deaths in 2024, making it the fifth most
common cancer in men and the fourteenth in women. The incidence
rates of bladder cancer in the US are ~82.3 per 100,000 in men and
21.3 per 100,000 in women (3). In
Europe, bladder cancer ranks as the tenth most common cancer, with
~226,000 new cases and 69,000 deaths annually. The incidence rates
vary across European countries, with higher rates observed in
southern and eastern Europe (3).
In Asia, the incidence of UC is lower than that in Western
countries, but it still poses a significant health burden,
especially in rapidly industrializing regions. For instance, in
China, the incidence of bladder cancer is ~13.5 per 100,000, with
higher rates in urban areas (3).
However, bladder cancer remains a notable health issue in some
regions, with incidence rates influenced by factors such as
schistosomiasis infection. Globally, the aggressive nature and high
recurrence rate of UC make it a challenging disease to manage
globally.
Advanced UC, particularly in metastatic or
cisplatin-ineligible patients, is associated with poor prognosis
(4). The 5-year survival rate for
patients with advanced UC is ~10%, highlighting an urgent need for
more effective treatment strategies (5). Traditional treatment methods, such
as platinum-based chemotherapy, have limitations related to
achieving durable responses. For patients with advanced UC being
treated with platinum-based chemotherapy, the median OS is
typically less than 15 months (6). While the use of immune checkpoint
inhibitors (ICIs) has shown promise, their efficacy varies, and
numerous patients eventually develop resistance (7). A meta-analysis of 11 trials
including 1,630 previously treated patients with UC revealed that
the pooled objective response rate (ORR) for single targeted agents
was 10.7% (95% CI: 10.7-19.6%), the disease control rate was 33.2%
(95% CI: 25-41.4%), and the 1-year OS was 31% (95% CI: 23.6-39.4%)
(8). These findings underscore
the critical need for novel approaches to improve patient outcomes
in this patient population.
Over the past decade, the treatment landscape for UC
has evolved significantly with the advent of targeted therapies.
Erdafitinib, a fibroblast growth factor receptor (FGFR) inhibitor,
and enfortumab vedotin (EV), an antibody-drug conjugates (ADCs)
targeting Nectin-4, have demonstrated significant efficacy for the
treatment of UC. Erdafitinib achieved a 40% ORR in a phase II trial
for patients with FGFR2/3-altered UC (9). EV showed a robust ORR of 43% in a
phase I trial, even in patients whose cancer had previously
progressed on ICIs (10). These
advancements mark a significant shift from traditional chemotherapy
and underscore the importance of molecularly guided therapies in
improving the prognosis of patients with UC. Ongoing research
continues to explore the optimal sequencing of these therapies and
the development of next-generation agents to further improve
treatment outcomes.
Unlike prior reviews that have separately summarized
FGFR inhibitors or ADCs, the present article integrates real-world
evidence, biomarker analytics, and resistance biology into a single
comparative framework. Recent literature gaps include insufficient
head-to-head evaluation of FGFR inhibitors vs. ADCs sequences,
limited dissection of convergent resistance pathways (for example,
PI3K/AKT re-activation and TGF-β-mediated immune exclusion), and
absence of a unified roadmap guiding biomarker-driven combination
therapy. By synthesizing 2023-2025 trial data with spatial
transcriptomic and urinary circulating-tumor-DNA studies, the
present review offers a dynamic, systems-level perspective that
links genomic alteration, tumor microenvironment (TME) phenotype,
and clinical outcomes; such integration has not been previously
accomplished in UC-focused commentaries. Consequently, the
manuscript provides clinicians and translational researchers with
an evidence-based hierarchy for selecting FGFR or ADCs-based
regimens, forecasts emerging resistance mechanisms, and proposes
biomarker-adaptive trial designs to accelerate precision medicine
implementation.
Molecular biology of UC
In recent years, with advancements in genomics and
molecular biology technologies, the molecular landscape of UC has
been gradually unveiled. Various molecular alterations and
signaling pathways interact synergistically, driving tumor
development and progression (11). A deeper understanding of the
molecular biology of UC provides a strong foundation for the
development of targeted therapies. By identifying key molecular
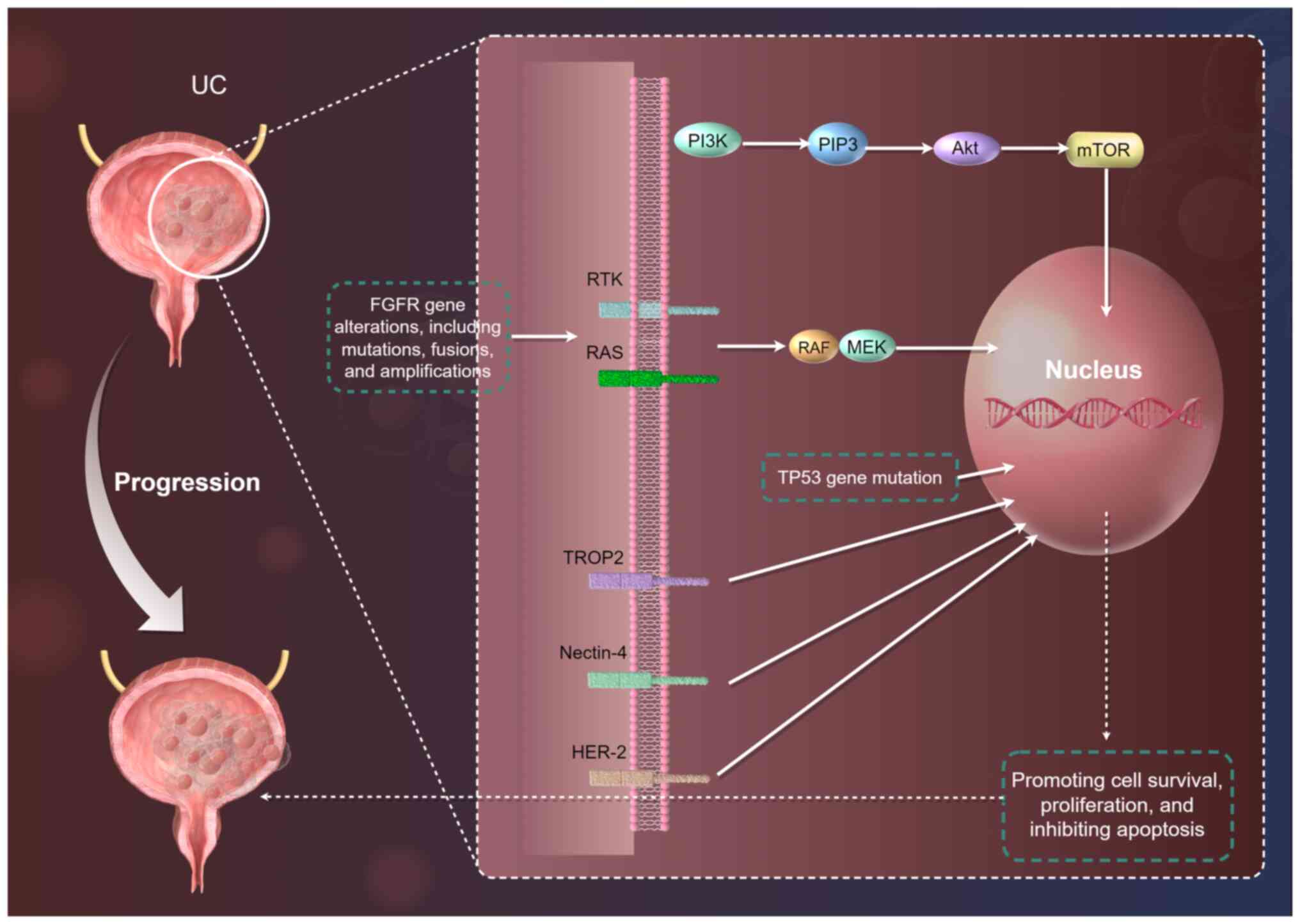
targets and pathways (Fig. 1),
researchers can develop more effective therapeutic strategies to
improve treatment outcomes and survival rates for patients with UC
(12).
Key molecular pathways
UC development and progression involve complex
molecular mechanisms. Among them, the Phosphatidylinositol 3-kinase
(PI3K)/Protein Kinase B(AKT)/Mammalian Target of Rapamycin (mTOR)
pathway plays a significant role (13). This pathway is frequently
activated due to mutations in genes such as PIK3CA, PTEN deletion,
or AKT1 mutation (14). PI3K
catalyzes the production of PIP3, which activates AKT. Activated
AKT, in turn, phosphorylates mTOR, promoting cell survival,
proliferation and inhibiting apoptosis (14). Studies have shown that PIK3CA
mutations are present in ~24-40% of UC cases (15). PTEN deletion occurs in ~17-30% of
cases (16). Activation of this
pathway contributes to UC progression and chemoresistance. For
example, in a particular study, PTEN-deficient mice were more prone
to developing UC and displayed more aggressive tumor
characteristics, such as increased proliferation rates and higher
metastatic potential (17).
The Receptor Tyrosine Kinase (RTK)/Rat Sarcoma
(RAS)/V-Raf Murine Sarcoma Viral Oncogene Homolog
(RAF)/Mitogen-Activated Protein Kinase Kinase (MEK)/Extracellular
Signal-Regulated Kinase (ERK) pathway is another critical signaling
pathway (18). Abnormal
activation of this pathway can be caused by mutations in FGFR, RAS,
or BRAF genes. FGFR gene alterations, including mutations, fusions
and amplifications, can lead to ligand-independent receptor
activation, subsequently activating downstream signaling pathways
(19). RAS mutations are found in
~15-30% of UC cases, and BRAF mutations occur in ~5-10% of cases
(20). Activation of this pathway
promotes cell proliferation, differentiation and angiogenesis. For
example, a study by Zhou et al (21) demonstrated that mutations in this
pathway are associated with tumor progression and poor prognosis in
patient with UC.
The Tumor Protein 53 (TP53) gene mutation pathway is
also crucial in UC. TP53 is a tumor suppressor gene, and its
encoded protein plays a pivotal role in cell cycle regulation, DNA
repair and apoptosis (22). TP53
mutations are detected in ~40-50% of UC cases. Mutations in this
gene lead to loss of its normal function, enabling cells with DNA
damage to continue proliferating, thereby promoting tumor
development and progression (22). Ecke et al (23) revealed that TP53 mutations are
associated with higher tumor grade and stage in UC and are linked
to poor patient prognosis.
FGFR alterations
FGFR gene alterations are prevalent in UC. FGFR
family includes FGFR1-4. Among them, FGFR3 alterations are the most
common in UC, occurring in ~10-30% of cases (24). FGFR3 gene mutations primarily
involve point mutations in the tyrosine kinase domain, such as
R248C, S249C and Y373C mutations, which lead to receptor
constitutive activation (25).
FGFR3 gene fusions, such as FGFR3-TACC3 fusions, can also result in
abnormal receptor activation. FGFR gene amplifications can increase
receptor expression levels, thereby enhancing downstream signaling
pathway activation (26). These
alterations contribute to tumor cell proliferation and progression.
For example, Liu et al (26) showed that FGFR3 mutations are
associated with tumor recurrence and progression in
non-muscle-invasive bladder cancer. FGFR3 alterations are more
common in non-muscle-invasive UC, with a detection rate of ~15-20%,
while in muscle-invasive UC, the detection rate is ~5-10%. FGFR2
alterations are relatively rare in UC but are also involved in
tumor progression.
Other molecular targets
In addition to the aforementioned molecular pathways
and FGFR alterations, other molecular targets also play important
roles in UC. TROP2 is a transmembrane glycoprotein that is highly
expressed in UC and other solid tumors. Its overexpression is
associated with tumor aggressiveness and poor prognosis (27). Sacituzumab govitecan, a
TROP2-targeted ADCs, has demonstrated significant efficacy in the
treatment of metastatic UC (28).
Nectin-4 is another molecule highly expressed in UC. EV, an ADCs
targeting Nectin-4, has shown remarkable therapeutic effects in
locally advanced or metastatic UC (29). HER-2 is overexpressed in a subset
of patients with UC. Trastuzumab deruxtecan, a HER-2-targeted ADCs,
has shown promising efficacy in HER-2-overexpressing UC (30). The expression levels of these
molecular targets are closely related to tumor biology and patient
prognosis.
FGFR pathway-directed therapies: Foundations
and clinical implementation
The increasing understanding of FGFR pathway
dysregulation in UC has driven the development of targeted
therapies. The molecular basis of FGFR abnormalities in UC are
addressed in the present section, the clinical data of FGFR
inhibitors such as erdafitinib and infigratinib are reviewed, and
resistance mechanisms are discussed, aiming to guide optimal use of
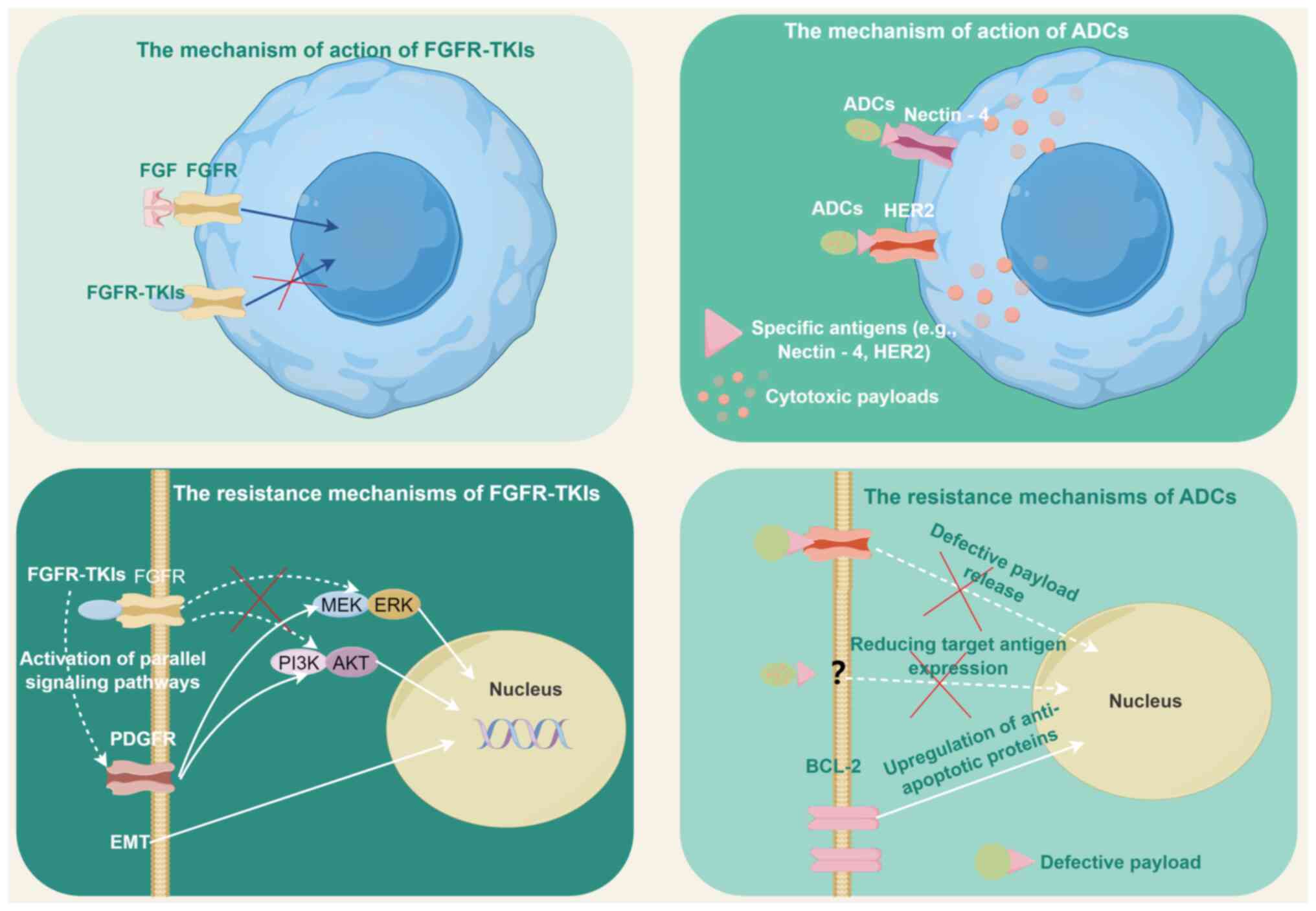
FGFR-targeted strategies in clinical practice (Fig. 2).
Molecular basis of FGFR dysregulation in
UC
Dysregulation of the FGFR signaling pathway through
amplifications, mutations and gene fusions has been implicated in
UC (31). FGFR signaling plays
crucial roles in tumor cell proliferation, angiogenesis, migration
and survival (32) (Table I). FGFR pathway dysregulation is a
hallmark of UC, primarily driven by genetic alterations in FGFR3
and, less frequently, FGFR2. These aberrations include activating
mutations, gene fusions and amplifications, which constitutively
activate downstream signaling cascades such as RAS/MAPK and
PI3K/AKT, promoting tumor proliferation and survival (33,34).
 | Table IRelated research on FGFR
dysregulation in urothelial carcinoma. |
Table I
Related research on FGFR
dysregulation in urothelial carcinoma.
| First author/s,
year | Aberration
types | Study types | Mechanism of
action | Relationship with
prognosis | (Refs.) |
|---|
| Ross et al,
2016; Necchi et al, 2019 | FGFR3
mutations/fusions | Genomic
profiling | Activating
mutations (for example, S249C, R248C) and fusions (for example,
*FGFR3-TACC3*) constitutively activate RAS/MAPK and PI3K/AKT
pathways. | Higher frequency in
advanced UC; associated with resistance to platinum chemotherapy
(ORR: 28% vs. 45% in wild-type). | (33,36) |
| Kim et al,
2018; Khalid et al, 2020 | FGFR3
mutations | Clinical cohort
analysis | Co-occurrence with
TP53 mutations enhances oncogenicity; promotes
ligand-independent kinase activation. | Correlates with
aggressive MIUC phenotypes; shorter OS in metastatic UC
(HR=1.42). | (34,40) |
| Gamallat et
al, 2023 | FGFR3
mutations | Subtype-specific
analysis | Enriched in large
nested variant UC (36%); drives subtype-specific signaling
dysregulation. | Subtype-specific
heterogeneity linked to differential therapeutic responses. | (35) |
| Necchi et
al, 2019 | FGFR3 mRNA
overexpression | Comparative DNA/RNA
analysis | Discordance between
DNA mutations and mRNA levels due to alternative splicing or
epigenetic regulation. | mRNA overexpression
correlates with DNA alterations in 65% of cases; prognostic value
context-dependent. | (36) |
| Song et al,
2023 | FGFR2
amplifications | Genomic and
microenvironmental | Activates
TGF-β-mediated immunosuppression; reduces CD8+ T-cell
infiltration. | Associated with
resistance to ICIs and aggressive phenotypes in advanced UC. | (41) |
| Okato et al,
2024 | FGFR3-mutant
TME |
Preclinical/translational study | M2 macrophage
polarization and immunosuppressive microenvironment; FGFR
inhibition enhances anti-PD-1 efficacy. | Improved survival
in murine models; synergy with immunotherapy in clinical
trials. | (42) |
| Mahmoud et
al, 2024 | FGFR3
overexpression | Immunohistochemical
analysis | Protein
overexpression detected in non-metastatic UC; lacks consistent
survival correlation. | Limited prognostic
significance in early-stage UC; potential biomarker for
FGFR-targeted therapies. | (43) |
| Shohdy et
al, 2019 | Co-alterations
(*CDKN2A/B*) | Mechanistic and
combination study | *CDKN2A/B*
deletions enhance dependency on CDK4/6; co-targeting FGFR and
CDK4/6 delays resistance. | Rationale for
combination therapies in FGFR3-altered UC. | (39) |
| Li et al,
2023 |
FGFR3-mediated resistance | Functional in
vitro study | P4HA2 stabilizes
HIF-1α, bypassing FGFR inhibition; metabolic adaptation drives
resistance. | Identifies novel
resistance mechanisms; informs strategies to overcome erdafitinib
resistance. | (38) |
Comprehensive genomic profiling of 295 advanced UC
cases revealed FGFR3 alterations in 15% of tumors, predominantly
point mutations (for example, S249C, R248C and Y375C) and fusions
(for example, FGFR3-TACC3) (33).
In muscle-invasive UC, FGFR3 mutations occur in 20% of cases, often
co-occurring with TP53 mutations, suggesting a synergistic
oncogenic role (34). Notably,
large nested variant UC exhibits a higher prevalence of FGFR3
mutations (36%), underscoring subtype-specific molecular
heterogeneity (35). At the mRNA
level, FGFR3 overexpression correlates with DNA-level mutations in
65% of advanced UC cases, though discordances exist due to
alternative splicing or epigenetic regulation (36).
FGFR3 mutations induce ligand-independent
dimerization and constitutive kinase activation. Huang et al
(37) demonstrated that FGFR3
silencing in UC cell lines suppressed RAS/MAPK pathway activity,
impairing invasion and proliferation. However, FGFR-driven tumors
often develop resistance via parallel pathways. For instance,
P4HA2-mediated HIF-1α stabilization was shown to bypass FGFR
inhibition in FGFR3-mutant UC, highlighting metabolic adaptation as
a resistance mechanism (38).
Additionally, co-alterations in CDKN2A/B (frequently deleted in
FGFR3-altered UC) may enhance dependency on CDK4/6, providing a
rationale for combination therapies (39).
While FGFR3 mutations were initially associated with
non-muscle-invasive UC and favorable prognosis, metastatic
FGFR3-altered UC correlates with poorer responses to platinum
chemotherapy (ORR: 28 vs. 45% in wild-type) (36). A meta-analysis of 1,574 patients
with UC confirmed that FGFR3 mutations independently predict
shorter OS in advanced stages (HR=1.42, 95% CI: 1.11-1.82)
(40). Conversely, FGFR2
amplifications, observed in 5-8% of UC, are linked to aggressive
phenotypes and resistance to ICIs due to TGF-β-mediated
immunosuppression (41,42).
Recent studies highlight the immunosuppressive
microenvironment in FGFR3-mutant UC, characterized by M2 macrophage
infiltration and reduced CD8+ T-cell activity.
Preclinical models demonstrated that FGFR inhibition synergizes
with anti-PD-1 therapy by reversing immunosuppression, offering a
compelling strategy for clinical translation (42). However, the prognostic value of
FGFR alterations remains context-dependent; for example, FGFR3
overexpression in non-metastatic UC lacks significant survival
correlation (43). Therefore,
FGFR dysregulation in UC is mechanistically diverse, influenced by
alteration type, coexisting genomic events, and TME factors.
Standardized detection methods and functional validation are
critical to optimize patient stratification for FGFR-targeted
therapies.
Application of FGFR-targeted strategies
in the treatment of UC
FGFR-targeted therapies have emerged as a
transformative approach in managing advanced UC, particularly for
patients with FGFR3 alterations. This section critically evaluates
the clinical efficacy, limitations and evolving strategies of FGFR
inhibitors, supported by pivotal trials and real-world evidence
(Table II).
 | Table IIApplication of FGFR-targeted
strategies in the treatment of urothelial carcinoma. |
Table II
Application of FGFR-targeted
strategies in the treatment of urothelial carcinoma.
| First author/s,
year | Intervention
strategies | Targets | Study types | Setting | Sample size
(N) | Therapeutic
effect | (Refs.) |
|---|
| Loriot et
al, 2019 | Erdafitinib
monotherapy | Pan-FGFR
(FGFR1-4) | Phase II BLC2001
trial | Post-platinum,
FGFR2/3-altered UC | N=99 | ORR: 40%; median
PFS: 5.5 months; median OS: 13.8 months in platinum-refractory
UC. | (44) |
| Loriot et
al, 2023 | Erdafitinib vs.
chemotherapy | Pan-FGFR
(FGFR1-4) | Phase III THOR
trial | FGFR3-altered,
post-ICI metastatic UC | N=266 | Improved median OS
(12.1 vs. 7.8 months; HR=0.64); ORR: 45.6% vs. 22.4%. | (47) |
| Siefker-Radtke
et al, 2024 | Erdafitinib vs.
pembrolizumab | Pan-FGFR
(FGFR1-4) | Phase III THOR
Cohort 2 | FGFR-altered,
PD-L1-high metastatic UC | N=352 | No OS benefit (10.9
vs. 11.1 months; HR=1.18) in PD-L1-high tumors. | (48) |
| Lyou et al,
2022 | Infigratinib
monotherapy | FGFR1-3 | Phase II trial | Post-platinum,
FGFR3-altered metastatic UC | N=67 | ORR: 25.4%; median
OS: 10.3 months in platinum-refractory UC. | (50) |
| Pal et al,
2022 | Infigratinib
adjuvant therapy | FGFR1-3 PROOF-302
trial | Phase III | FGFR3-mutant
muscle-invasive UC | N=630 | Preliminary data:
35% reduction in recurrence rates in FGFR3-mutant MIUC. | (51) |
| Sternberg et
al, 2023 | Rogaratinib
monotherapy | FGFR1-4 | Phase II/III FORT-1
trial | FGFR1/3 mRNA-high
metastatic UC | N=126 | ORR: 20.7% vs.
19.3% (chemotherapy); median OS: 8.3 vs. 9.8 months (HR=1.02). | (52) |
| Necchi et
al, 2024 | Pemigatinib
monotherapy | FGFR1-3 | Phase II FIGHT-201
trial | FGFR-altered,
post-platinum metastatic UC | N=40 | ORR: 12%; no
survival benefit in UC. | (53) |
| Siefker-Radtke and
Loriot, 2022 | Erdafitinib +
pembrolizumab | Pan-FGFR +
PD-1 | Phase II trial | FGFR-altered,
ICI-naïve metastatic UC | N=58 | ORR: 47%; median
OS: 12.2 months; increased grade ≥3 AEs (e.g., rash,
diarrhea). | (55) |
| Necchi et
al, 2024 | Derazantinib +
atezolizumab | FGFR/CSF1R +
PD-L1 | Phase I/II
trial | FGFR-altered,
post-ICI metastatic UC | N=45 | ORR: 33%; CSF1R
inhibition counteracts macrophage-mediated resistance. | (56) |
Erdafitinib, a pan-FGFR inhibitor, is the first
FDA-approved FGFR inhibitors for metastatic UC harboring
susceptible FGFR2/3 alterations. The phase II BLC2001 trial (N=99)
demonstrated an ORR of 40%, median progression-free survival (PFS)
of 5.5 months, and median OS of 13.8 months in platinum-refractory
patients (44). However, the
single-arm design and absence of a control group limit the
interpretability of these outcomes, particularly in the context of
post-immunotherapy efficacy. Furthermore, the enrichment for
FGFR3-altered tumors, without stratification by mutation subtype,
may overestimate the true treatment effect, as FGFR3-TACC3 fusions
have been associated with more favorable responses than point
mutations (45).
Hyperphosphatemia, a class-effect toxicity due to FGFR1 inhibition,
occurred in 77% of patients but correlated with antitumor efficacy
(P=0.02) (46). Long-term
follow-up confirmed sustained responses, with a 24-month OS rate of
27% (9). The phase III THOR trial
validated erdafitinib's superiority over chemotherapy in
FGFR3-altered metastatic UC (Cohort 1: N=266), showing improved
median OS (12.1 vs. 7.8 months; HR=0.64) and ORR (45.6% vs. 22.4%)
(47). However, in Cohort 2
comparing erdafitinib to pembrolizumab in programmed death-Ligand
1(PD-L1)-high tumors, no OS benefit was observed (10.9 vs. 11.1
months; HR=1.18), underscoring the need for biomarker-driven
selection (48). Real-world
studies corroborated these findings, with Guercio et al
(49) reporting an ORR of 38% and
median OS of 11.2 months in 112 patients.
Infigratinib, an FGFR1-3 inhibitor, showed modest
activity in a phase II trial (N=67) with an ORR of 25.4% and median
OS of 10.3 months in platinum-refractory UC (50). Hyperphosphatemia again emerged as
a common adverse event (AE), observed in 73% of patients (46). The phase III PROOF-302 trial is
evaluating adjuvant infigratinib in FGFR3-mutant
muscle-invasive UC, with preliminary data suggesting a 35%
reduction in recurrence rates (51). Besides, Rogaratinib, an FGFR1-4
inhibitor, failed to outperform chemotherapy in the phase II/III
FORT-1 trial (N=126) despite selecting patients with high FGFR1/3
mRNA expression. ORR was comparable (20.7 vs. 19.3%), and median OS
was shorter (8.3 vs. 9.8 months; HR=1.02), highlighting challenges
in biomarker validation (52).
Furthermore, Pemigatinib, approved in cholangiocarcinoma, showed
limited efficacy in UC (ORR: 12%) in the FIGHT-201 trial (N=40),
with no survival benefit (53).
Its combination with pembrolizumab was explored in
cisplatin-ineligible patients but discontinued due to strategic
reasons (54).
Combining FGFR inhibitors with ICIs aims to overcome
immunosuppressive microenvironments. Erdafitinib plus pembrolizumab
achieved an ORR of 47% and median OS of 12.2 months in a phase II
trial (N=58), though grade ≥3 AEs (for example, rash and diarrhea)
increased significantly (47).
Preclinical models suggest FGFR inhibition reverses M2 macrophage
polarization and enhance CD8+ T-cell infiltration,
supporting this strategy (55).
Derazantinib, a dual FGFR/CSF1R inhibitor, combined with
atezolizumab (anti-PD-L1), showed promise in a phase I/II trial
(N=45) with an ORR of 33%. CSF1R targeting may counteract
tumor-associated macrophage-mediated resistance, offering a novel
therapeutic angle (56).
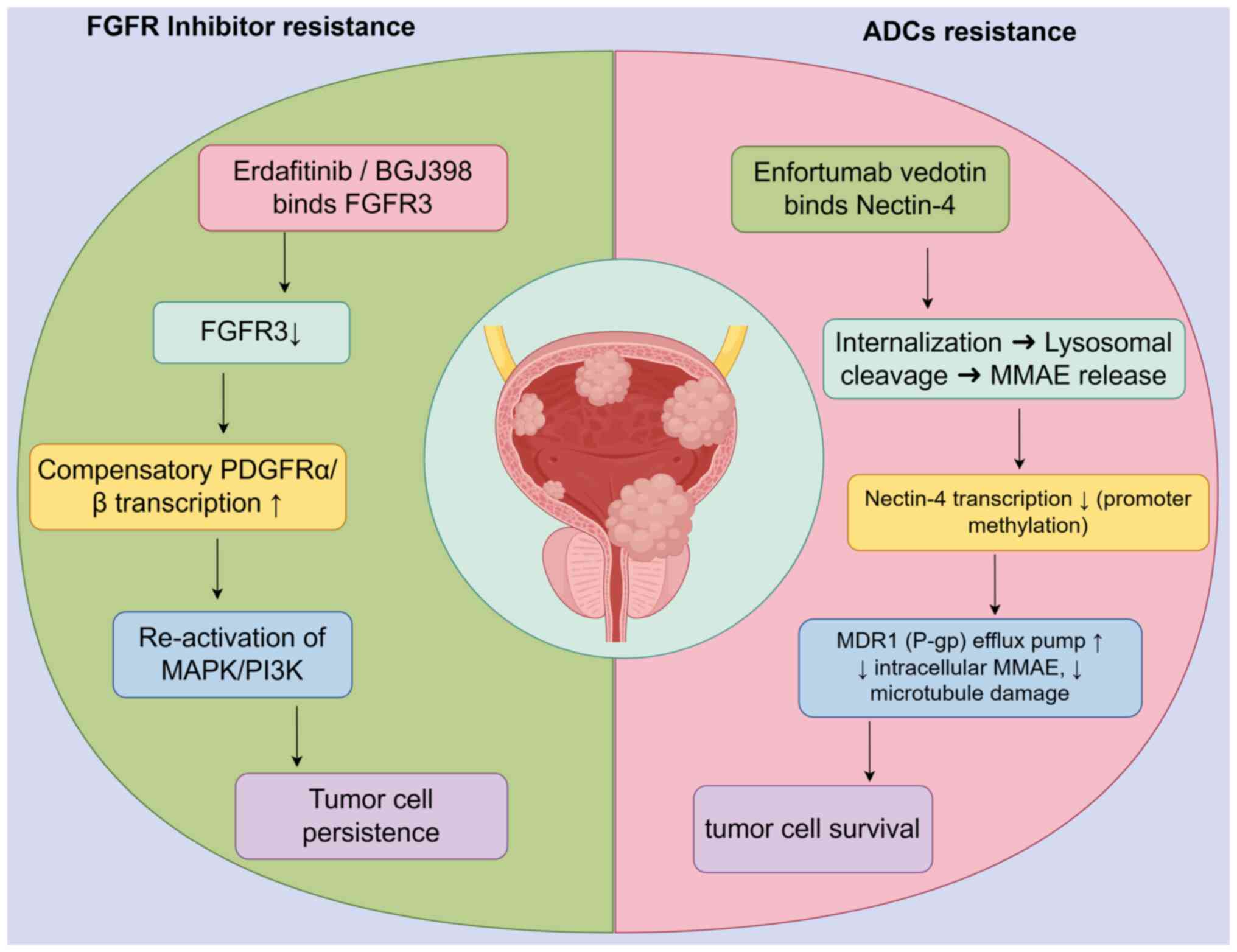
Resistance to FGFR-tyrosine kinase
inhibitors (TKIs)
Resistance to FGFR-TKIs remains a critical challenge
in UC, driven by diverse molecular mechanisms. Preclinical and
clinical studies have identified several pathways contributing to
acquired or intrinsic resistance, including compensatory signaling
activation, genomic evolution, and TME adaptations. Datta et
al (57) demonstrated that
Akt activation mediates resistance to BGJ398 (infigratinib) in
FGFR3-mutant UC models, highlighting compensatory PI3K/AKT/mTOR
pathway activation as a key escape mechanism. This finding aligns
with Pettitt et al (58),
who observed in vitro resistance to FGFR inhibitors via
multiple pathways, including MAPK reactivation and
epithelial-mesenchymal transition (EMT), underscoring the
heterogeneity of resistance mechanisms. Conversely, Kim et
al (59) reported that
FGFR1-overexpressing UC cells develop resistance to BGJ398 through
sustained MAPK/ERK signaling, which could be reversed by MEK
inhibitors, suggesting combinatorial strategies to delay
resistance.
Clinical studies further reveal the impact of FGFR
alterations on therapeutic outcomes. Rezazadeh Kalebasty et
al (60) observed that
patients with UC with FGFR mutations exhibited reduced clinical
benefit from anti-PD-(L)1 therapies post-FGFR inhibition,
suggesting cross-resistance linked to immunosuppressive TME
reprogramming. By contrast, Bellmunt et al (61) found that everolimus/pazopanib
combination therapy improved outcomes in genomically selected
patients with UC, including those with FGFR pathway alterations,
though efficacy was limited by mTOR pathway feedback activation.
Divergent resistance patterns are also influenced by tumor
histology. Brunelli et al (62) identified reduced TROP-2 and
NECTIN-4 expression in sarcomatoid UC variants, correlating with
poor ADCs responses and potential cross-resistance to FGFR-targeted
therapies. Additionally, Audisio et al (63) emphasized the role of clonal
evolution under FGFR inhibition, where subpopulations with
secondary FGFR2/3 mutations or parallel oncogenic drivers (for
example, EGFR) emerge, necessitating longitudinal genomic
profiling.
These studies collectively highlight the
multifactorial nature of FGFR-TKI resistance. While compensatory
signaling (for example, PI3K/AKT and MAPK) and genomic evolution
are predominant mechanisms, TME interactions and histological
subtypes further modulate therapeutic vulnerability. Future
research should prioritize combinatorial approaches (for example,
FGFR inhibitors + MEKi/mTORi) and biomarker-driven adaptive
therapies to circumvent resistance.
ADCs
ADCs have emerged as a transformative therapeutic
class in UC, leveraging tumor-specific antigens to deliver
cytotoxic payloads with precision. ADCs have redefined UC
treatment, with Nectin-4 and HER2 as validated targets (Fig. 2). Relevant studies have optimized
the selection of biomarkers, clarified the pathways of action, and
integrated ADCs into multimodal regimens (Table III). Real-world evidence and
innovative imaging modalities (for example, Nectin-4 PET) will
further personalize ADCs therapy.
 | Table IIIApplication of antibody-drug
conjugates strategies in the treatment of UC. |
Table III
Application of antibody-drug
conjugates strategies in the treatment of UC.
| First author/s,
year | Intervention
strategies | Targets | Study types | Setting | Sample size
(N) | Therapeutic
effect | (Refs.) |
|---|
| Li et al,
2024 | EV monotherapy | Nectin-4 | Phase II trial
(China) | Post-platinum
metastatic UC (Asian cohort) | N=60 | ORR 50%, median PFS
5.8 months in previously treated metastatic UC | (66) |
| Rizzo et al,
2025 | EV post-ICI
failure | Nectin-4 | Retrospective study
(ARON-2) | Post-ICI metastatic
UC | N=237 | Median OS 12.1
months vs. chemotherapy 8.3 months (HR=0.62) | (67) |
| Uchimoto et
al, 2025 | BMI impact on EV
efficacy | Nectin-4 | Cohort study
(Japan) | Post-ICI metastatic
UC (ULTRA-Japan) | N=166 | BMI ≥25: ORR 42.9%
vs. 23.1%, OS 14.2 vs. 9.1 months | (68) |
| Mishra et
al, 2024 | Nectin-4 PET-guided
dosing | Nectin-4 | Translational
imaging study | Metastatic UC with
heterogeneous Nectin-4 expression | N=12 | Tracer uptake
correlates with EV response (r=0.72, P=0.008) | (69) |
| Jindal et
al, 2023 | TP53/MDM2 biomarker
analysis | Nectin-4 | Biomarker
analysis | Post-ICI metastatic
UC | N=45 | TP53 wild-type: PFS
6.4 vs. 3.1 months (P=0.03) | (70) |
| Hovelroud et
al, 2024 | EV-associated
diabetic ketoacidosis | Nectin-4 | Case report | Post-ICI metastatic
UC | N=1 | Severe insulin
resistance in a nondiabetic patient | (71) |
| Desimpel et
al, 2024 | EV-associated lung
toxicity | Nectin-4 | Retrospective
analysis | Post-ICI metastatic
UC | N=1,892 | Interstitial lung
disease incidence 0.5% | (72) |
| Pipitone et
al, 2025 | EV extravasation
management | Nectin-4 | Case report &
literature review | Metastatic UC
receiving EV | N=1 (case) | Protocol for tissue
necrosis prevention | (73) |
| Zhu et al,
2025 | EV + pembrolizumab
cost-effectiveness | Nectin-4 | Cost-effectiveness
model | First-line
metastatic UC | Model-based | Incremental
cost-effectiveness ratio>$150,000/QALY; requires price
reduction | (74) |
| Sheng et al,
2024 | Disitamab vedotin
monotherapy | HER2 | Phase II trial | HER2-positive
metastatic UC | N=107 | HER2-positive
metastatic UC: ORR 51.2%, median OS 14.2 months | (77) |
| Chen et al,
2023 | Disitamab vedotin
monotherapy | HER2 | Phase II trial | HER2-positive
locally advanced or metastatic UC | N=76 | HER2-positive
metastatic UC: ORR 51.2%, median OS 14.2 months | (78) |
| Yan et al,
2025 | Disitamab vedotin
in HER2-low populations | HER2 | Phase II trial | HER2-negative
metastatic UC | N=82 | HER2-negative
metastatic UC: ORR 28.6%, median OS 11.8 months | (79) |
| Wang et al,
2025 | Disitamab vedotin
in HER2-null populations | HER2 | Real-world
study | HER2-low/null
metastatic UC | N=154 | ORR 18.2%, median
PFS 4.1 months | (80) |
| Yao et al,
2025 | Disitamab vedotin +
tislelizumab combination | HER2 + PD-1 | Phase II trial | HER2-expressing,
ICI-naïve metastatic UC | N=36 | ORR 58.3%, median
PFS 8.5 months | (82) |
| Ge et al,
2025 | RC48 + PD-1
inhibitors | HER2 + PD-1 | Real-world
study | HER2-expressing
metastatic UC | N=112 | ORR 54.5%, median
OS 16.1 months | (83) |
| Yang et al,
2025 | HER2 prognostic
value (shorter OS) | HER2 | Retrospective
cohort | Muscle-invasive
UC | N=412 | HER2+ associated
with HR=1.58 (P=0.02) in multivariable analysis | (84) |
| Chen et al,
2025 | HER2 in
bladder-preservation therapy | HER2 | Clinical
cohort | HER2+
muscle-invasive UC treated with bladder-sparing therapy | N=127 | No OS difference in
HER2+ MIBC patients | (85) |
| Chou et al,
2022 | TROP-2 in luminal
subtypes | TROP-2 | Molecular subtyping
analysis | Luminal papillary
UC cohort | N=120 | Enriched in luminal
papillary UC | (86) |
| Bahlinger et
al, 2024 | FGFR3/Nectin-4
co-alterations | FGFR3 | Translational
study | Advanced UC with
Nectin-4-high expression | N=200 | FGFR3 mutations in
25% of Nectin-4-high tumors | (87) |
Nectin-4-targeted ADCs
Nectin-4, a cell adhesion molecule overexpressed in
60-90% of UCs, has become a pivotal therapeutic target. EV, an ADCs
comprising a Nectin-4-directed antibody conjugated to monomethyl
auristatin E, received FDA approval based on the EV-301 trial
(64). The recent phase III
EV-302 trial (NCT04223856) established enfortumab vedotin plus
pembrolizumab as a new first-line standard for metastatic UC,
demonstrating superior OS and PFS compared with platinum-based
chemotherapy (median OS: 31.5 vs. 16.1 months; HR=0.47; median PFS:
12.5 vs. 6.3 months; HR=0.45) (65). Recent studies corroborate its
efficacy in diverse clinical settings (66-68). The phase II trial in Chinese
patients with previously treated metastatic UC demonstrated an ORR
of 50% and median PFS of 5.8 months, validating EV's activity in
Asian populations (66). Notably,
the aforementioned study lacked central imaging review and
biomarker stratification beyond Nectin-4 expression, raising
concerns about response assessment heterogeneity. Additionally, the
absence of post-progression treatment details introduces potential
immortal time bias, particularly in a single arm setting without
comparator arm. Real-world data from the ARON-2 retrospective study
(n=237) further revealed that EV achieved a median OS of 12.1
months post-ICI failure, outperforming chemotherapy (8.3 months;
HR=0.62, P<0.001) (67).
Notably, efficacy of EV was influenced by body mass index (BMI):
patients with BMI ≥25 had superior tumor response rates (ORR 42.9%
vs. 23.1%, P=0.04) and longer OS (14.2 vs. 9.1 months, P=0.02),
suggesting metabolic factors may modulate ADCs activity (68).
Biomarker-driven strategies are under exploration.
Mishra et al (69)
developed a Nectin-4 PET tracer to non-invasively quantify target
expression, revealing heterogeneous intratumoral distribution.
Higher tracer uptake correlated with EV response (r=0.72, P=0.008),
supporting personalized dosing. Additionally, TP53/MDM2 alterations
were associated with reduced EV benefit in a biomarker analysis
(n=45): TP53 wild-type tumors had longer PFS (6.4 vs. 3.1 months,
P=0.03) (70).
EV's toxicity profile remains consistent across
studies, with peripheral neuropathy (40-50%), rash (30%) and
hyperglycemia (5-10%) as key AEs (66,67). A pharmacovigilance study (n=1,892)
identified rare but severe AEs, including diabetic ketoacidosis
(0.3%) and interstitial lung disease (0.5%) (71,72). Extravasation management protocols
have been proposed following case reports of tissue necrosis
(73). In addition,
cost-effectiveness analyses weigh EV's benefits against its
economic burden. Zhu et al (74) modeled EV + pembrolizumab as
first-line therapy for metastatic UC, showing incremental
cost-effectiveness ratios exceeding $150,000/QALY, necessitating
price reductions for broader adoption.
HER2-directed ADCs
HER2 expression in UC is heterogeneous, with 5-15%
classified as HER2-positive (IHC 3+ or 2+/FISH+) and 30-40% as
HER2-low (IHC 1+ or 2+ with negative in situ hybridization),
consistent with the ASCO-CAP guidelines (75), which are commonly extrapolated to
UC in the absence of disease-specific criteria. Disitamab vedotin
(DV; RC48), an anti-HER2 ADCs, has shown promise across HER2
expression levels (76). In the
combined analysis of the phase II trials (n=107), DV monotherapy
achieved an ORR of 51.2% and median OS of 14.2 months in
HER2-positive metastatic UC, with grade ≥3 AEs (for example,
neutropenia: 20%) deemed manageable (77). Real-world studies reinforce these
findings: Chen et al (78)
reported an ORR of 48.6% and median PFS of 6.9 months in 76
HER2-positive patients treated with DV ± ICIs.
Intriguingly, DV exhibits activity even in
HER2-low/null cohorts. Yan et al (79) conducted a phase II trial (n=82) in
HER2-negative metastatic UC, demonstrating an ORR of 28.6% and
median OS of 11.8 months, suggesting off-target effects or HER2
detection limitations. Similarly, Wang et al (80) observed ORRs of 24.1% (HER2-low)
and 18.2% (HER2-null) in a real-world study (n=154), though
responses were less durable (median PFS: 4.1 vs. 5.3 months). These
findings challenge traditional HER2 thresholds and advocate for
refined scoring systems.
The combination of DV with ICIs has demonstrated
synergistic efficacy, representing a significant advancement. In
the phase Ib/II RC48-C014 trial (NCT04264936), DV plus the PD-1
inhibitor toripalimab achieved a confirmed ORR of 71.8% and a
median PFS of 9.2 months in patients with HER2-expressing (IHC
1+/2+/3+) locally advanced or metastatic UC (81). Notably, efficacy was consistent
across HER2-low (IHC 1+ or 2+/FISH-) and HER2-positive
(IHC 3+ or 2+/FISH+) subgroups,
challenging traditional HER2 positivity thresholds and suggesting
broader applicability.
DV-ICI combinations synergize efficacy. Yao et
al (82) reported an ORR of
58.3% and median PFS of 8.5 months in 36 ICI-naïve patients with
metastatic UC receiving DV + tislelizumab, with immune-related AEs
in 22%. Ge et al (83)
corroborated these results in a larger cohort (n=112; ORR: 54.5%,
median OS: 16.1 months), highlighting enhanced antitumor immunity.
While HER2 overexpression correlates with aggressive features (for
example, higher grade, nodal metastases), its prognostic value
remains contested. Yang et al (84) analyzed 412 UC specimens, finding
HER2 positivity (12.6%) associated with shorter OS (HR=1.58,
P=0.02) in multivariable analysis. Conversely, Chen et al
(85) reported no OS difference
in HER2-positive muscle-invasive UC treated with
bladder-preservation therapy, underscoring context-dependent
roles.
Emerging targets
TROP-2, expressed in 70% of UCs, is under
investigation with sacituzumab govitecan. Preclinical data reveal
TROP-2 overexpression in sarcomatoid UC (80%) vs. conventional UC
(40%), suggesting histology-specific targeting (62). Chou et al (86) identified TROP-2 enrichment in
luminal papillary subtypes, potentially guiding patient selection.
FGFR3-directed ADCs (for example, erdafitinib combinations) and
tissue factor-targeting agents (for example, tisotumab vedotin) are
in early trials. Bahlinger et al (87) observed FGFR3 mutations in 25% of
Nectin-4-high tumors, advocating dual-target approaches.
Translational insights: Biomarker-driven
patient selection
The advent of targeted therapies in UC has
underscored the critical role of biomarker-driven patient selection
to maximize therapeutic efficacy and minimize toxicity (88). This section evaluates the
translational insights from key clinical trials, focusing on FGFR
inhibitors and ADCs, and discusses the challenges and opportunities
in biomarker validation, heterogeneity, and clinical
implementation.
FGFR alterations: From discovery to
clinical validation
FGFR alterations, particularly FGFR3 mutations and
fusions, have emerged as pivotal oncogenic drivers in UC. These
genetic aberrations, identified in 20-40% of metastatic UC and
35-40% of high-risk non-muscle-invasive bladder cancer (NMIBC)
tumors, lead to constitutive activation of downstream signaling
pathways such as RAS-MAPK and PI3K-AKT, promoting tumor
proliferation, survival and angiogenesis (41). Preclinical studies highlighted
FGFR3's role in bladder carcinogenesis, spurring the development of
selective FGFR inhibitors. Erdafitinib, a first-in-class
pan-FGFR-TKI, demonstrated early promise in the phase 2 BLC2001
trial, achieving an ORR of 40% in FGFR-altered metastatic UC, which
laid the groundwork for subsequent phase 3 validation (47). The discovery of FGFR3's oncogenic
role and its high prevalence in UC established a strong rationale
for biomarker-driven therapeutic strategies, positioning FGFR
status as a critical predictive biomarker for patient
stratification.
The THOR trial (NCT03390504) marked a milestone in
the clinical validation of FGFR-targeted therapy. In this phase 3
study, erdafitinib significantly outperformed chemotherapy in
patients with FGFR3-altered metastatic UC, demonstrating a median
OS of 12.1 months vs. 7.8 months (HR=0.64) and a doubling of PFS
(5.6 vs. 2.7 months) (47).
Nevertheless, the trial's open-label design may introduce
assessment bias, particularly in subjective endpoints such as PFS.
Moreover, the exclusion of patients with prior immunotherapy limits
the generalizability of these findings to contemporary real-world
cohorts, where ICI exposure is now standard of care. These
transformative outcomes, coupled with durable responses and
manageable toxicity, led to erdafitinib's regulatory approvals in
China, USA and other regions, cementing FGFR3 alteration as a
validated biomarker for patient selection. However, challenges
persist, including tumor heterogeneity, the emergence of resistance
mutations (for example, FGFR3 gatekeeper mutations), and the need
for standardized biomarker testing protocols (89). Ongoing research focuses on
optimizing FGFR inhibitor sequencing, exploring combination
therapies with ICIs, and validating liquid biopsy-based FGFR
detection to address spatial and temporal heterogeneity in advanced
UC (42). These efforts aim to
refine precision medicine approaches and extend the benefits of
FGFR-targeted therapy to broader patient subsets.
HER2 as an emerging target: Expanding the
ADCs landscape
Human epidermal growth factor receptor 2 (HER2)
overexpression or amplification is observed in 10-20% of UC and has
emerged as a promising target for ADCs (90). Disitamab vedotin (DV), an
HER2-targeted ADCs, combined with the PD-1 inhibitor toripalimab,
demonstrated unprecedented efficacy in the RC48-C016 trial
(NCT04879329), achieving significant OS (11.5 vs. 9.5 months) and
PFS (4.2 vs. 2.9 months) benefits over chemotherapy in
HER2-expressing metastatic UC, including cisplatin-ineligible
patients (81). Notably, efficacy
was consistent across HER2-low and HER2-high subgroups, challenging
the traditional HER2 positivity thresholds and suggesting broader
applicability.
By contrast, EV, a NECTIN-4-directed ADCs, has shown
remarkable activity in unselected UC populations (ORR: 67.7%; CR:
29% in EV-302) (91). While EV
does not require biomarker preselection, retrospective analyses
suggest that NECTIN-4 expression levels correlate with response
durability, raising questions about the need for quantitative
biomarker thresholds (92). This
contrasts with HER2-targeted ADCs, where even low expression may
suffice for clinical benefit, as observed in DV trials. Such
differences underscore the need for target-specific biomarker
frameworks.
Navigating tumor heterogeneity and
resistance in biomarker-driven therapies
UC exhibits significant intratumoral heterogeneity,
with FGFR and HER2 status varying between primary and metastatic
lesions (93). For example, FGFR3
mutations are more common in primary NMIBC, while metastatic sites
often acquire additional genomic alterations (for example, TP53 and
RB1) (94). Longitudinal studies
reveal that FGFR alterations may be lost after BCG therapy or
chemotherapy, necessitating repeat biopsies for dynamic biomarker
assessment (95). Liquid biopsy
approaches [for example, circulating tumor DNA (ctDNA)] are under
investigation but require validation for real-time monitoring
(96).
Co-mutations (for example, TP53 with FGFR3) may
modulate response to targeted therapies. In the THOR trial,
patients with FGFR3-TACC3 fusions had superior outcomes compared
with those with FGFR3 mutations, suggesting fusion-specific
sensitivity (45). Similarly,
HER2 amplification often coexists with PI3K/AKT pathway activation,
which may confer resistance to DV unless combined with PI3K
inhibitors (97). These findings
highlight the importance of comprehensive genomic profiling to
identify co-targetable pathways.
Biomarker-driven selection has revolutionized UC
treatment, yet challenges in standardization, heterogeneity, and
resistance persist. FGFR and HER2 inhibitors exemplify the success
of precision medicine, while ADCs such as EV demonstrate the
potential of target-agnostic approaches. Future research must
prioritize biomarker validation, combinatorial strategies and
real-world evidence to bridge the gap between trial populations and
clinical practice. Recent evidence indicates that urine tumor DNA
(utDNA) assays achieve 91.4% sensitivity and 95.1% specificity for
detecting UC-associated FGFR3 or TERT mutations, outperforming
cytology and enabling longitudinal genotyping without repeated
cystoscopy (98). This high
diagnostic accuracy underscores the utility of utDNA in capturing
tumor-derived genetic material shed into the urine, thereby
providing a more comprehensive representation of intratumoral and
inter-lesional heterogeneity compared with single-site tissue
biopsies. In the prospective TAR-210 trial, real-time utDNA
screening for FGFR3 alterations increased trial-enrollment
efficiency by 36% and permitted early identification of emergent
FGFR3 gate-keeper mutations (99). By circumventing the spatial
limitations of tissue biopsies, utDNA offers a non-invasive means
to dynamically monitor clonal evolution and adapt therapeutic
strategies in response to molecular changes. Analogously, plasma
ctDNA panels that cover FGFR2/3, PIK3CA, TP53 and ERBB2 can be
performed every 4-6 weeks; rising variant-allele frequencies
precede radiological progression by a median of 4.2 months in
patients receiving erdafitinib or enfortumab vedotin, providing a
lead-time window for therapy adaptation (100). Thus, liquid biopsy platforms
already allow dynamic patient selection and early resistance
surveillance and are being integrated into adaptive trial designs
such as the ULTRA-switch study (100).
Resistance mechanisms and overcoming
therapeutic limitations
Resistance to FGFR inhibitors and ADCs in UC is
multifactorial, driven by convergent pathways such as kinase
switching, persistent downstream signaling and TME interactions
(101,102) (Fig. 3). However, emerging strategies,
including biomarker-guided combinations, next-generation ADCs and
epigenetic modulation, hold promise for overcoming these barriers.
Future studies must prioritize longitudinal biomarker validation
and innovative trial designs to translate preclinical insights into
clinical success.
Convergent resistance pathways between
FGFR-TKIs and ADCs
Resistance to FGFR inhibitors (for example,
erdafitinib) and ADCs (for example, EV) often involves compensatory
activation of parallel signaling pathways (103,104). For instance, FGFR inhibition in
metastatic UC induces upregulation of platelet-derived growth
factor receptor (PDGFR), enabling tumor cells to bypass FGFR
dependency via PDGF ligand stimulation (105). Preclinical studies in breast
cancer models demonstrated that FGFR inhibitor pemigatinib triggers
PDGFRα/β overexpression, reactivating MAPK/ERK signaling and
promoting minimal residual disease (MRD) survival (106). Similarly, resistance to
HER2-targeted ADCs (for example, disitamab vedotin) may involve MET
or HER2 amplification, as observed in non-small cell lung cancer
(NSCLC) EGFR-TKI resistance models (107). These findings highlight a shared
mechanism of 'kinase switching' across targeted therapies.
Both FGFR inhibitors and ADCs face resistance due to
sustained activation of downstream effectors. In FGFR3-altered UC,
MEK/ERK and PI3K/AKT pathways remain active despite FGFR
inhibition, driven by co-mutations (for example, TP53 and RB1) or
epigenetic adaptations (108,109). For ADCs, resistance may arise
from defective payload release or upregulation of anti-apoptotic
proteins (for example, BCL-2) (110). For example, EV-resistant UC cell
lines exhibit increased expression of multidrug resistance
transporters, reducing monomethyl auristatin E cytotoxicity
(104).
Tumor cells evade targeted therapies through EMT or
lineage plasticity (111). FGFR
inhibition in UC promotes EMT via STAT3 activation, enhancing
metastatic potential (103).
ADCs targeting NECTIN-4 or HER2 may similarly encounter resistance
due to loss of target expression during phenotypic shifts (112,113). Additionally, stromal
interactions in the TME play a pivotal role. Lung fibroblasts
secrete PDGF-AA to support MRD survival in FGFR inhibitor-treated
models, while cancer-associated fibroblasts shield tumor cells from
ADCs penetration by secreting extracellular matrix components
(114).
Novel strategies to circumvent
resistance
Dual inhibition of FGFR and compensatory pathways
(for example, PDGFR or PI3K) has shown promise (115,116). In preclinical UC models,
combining erdafitinib with the DNA methyltransferase 1 inhibitor
GSK3484862 delayed relapse by suppressing PDGFR upregulation and
epigenetic plasticity (90). For
ADCs, co-targeting HER2 and MET (for example, trastuzumab +
savolitinib) is under investigation in NSCLC, with potential
applicability to UC (90).
Bispecific ADCs (for example, targeting HER2 and
TROP2) or payload modifications (for example, TOP1 inhibitors) may
overcome resistance by broadening target engagement or enhancing
cytotoxicity (117). Erdafitinib
intravesical delivery systems (TAR-210) improve local efficacy
while reducing systemic toxicity, achieving 82% recurrence-free
survival in FGFR3-altered NMIBC (99). Similarly, FGFR3-specific degraders
(for example, PROTACs) are emerging to address kinase domain
mutations (118).
FGFR inhibitors may synergize with ICIs by
modulating the TME (119).
Erdafitinib increases T-cell infiltration and reduces
myeloid-derived suppressor cells in UC models, suggesting enhanced
immunogenicity (119). Clinical
trials evaluating erdafitinib + pembrolizumab (NCT05316155) are
underway, with preliminary data showing durable responses in
PD-L1-low populations. Furthermore, histone deacetylase (HDAC)
inhibitors reverse resistance-associated epigenetic silencing
(120). For example, low-dose
decitabine restored FGFR3 expression in erdafitinib-resistant UC
cells, re-sensitizing them to therapy. Metabolic reprogramming (for
example, valine restriction) combined with HDAC6 inhibitors has
shown efficacy in enhancing DNA damage in preclinical models,
offering a novel combinatorial approach (120).
In addition, longitudinal assessment of resistance
mutations via utDNA enables real-time adaptation of therapy
(100). The UI Seek assay, which
detects FGFR3/TERT mutations and methylation markers in urine,
demonstrated 91.37% sensitivity and 95.09% specificity for UC
diagnosis, outperforming traditional cytology (98). In the TAR-210 trial, utDNA-based
FGFR3 screening improved patient enrollment by 36%, highlighting
its utility in guiding adaptive therapies (121). Longitudinal ctDNA monitoring is
being integrated into adaptive trial designs. The ULTRA-ctDNA
sub-study (NCT05538680) will trigger crossover to alternative
targeted agents or combination regimens as soon as emergent FGFR3
secondary mutations or PI3K/AKT pathway alterations are detected,
aiming to prevent clinical relapse rather than merely documenting
it post hoc.
Clinical translation of resistance data is now
beginning to shape sequencing algorithms. In the multicenter
real-world APOLLO study (n=184), patients who progressed on
erdafitinib were systematically re-biopsied; 62% of cases acquired
PIK3CA or PTEN loss-of-function alterations that were absent at
baseline. Subsequent treatment with everolimus plus paclitaxel in
these molecularly selected patients yielded a 34% ORR and 8.1-month
median PFS, whereas non-selected historical controls achieved only
12% and 4.0 months, respectively (49). Similarly, longitudinal ctDNA
surveillance during EV therapy revealed that emergent NECTIN-4 loss
or TUBB3 mutations predicted resistance within 4-6 weeks; early
addition of taxane-based chemotherapy at the time of molecular
progression doubled median time-to-next-treatment compared with
waiting for radiological progression (7.3 vs. 3.5 months, HR 0.48,
P=0.02) (67).
Consequently, an adaptive 'biomarker-triggered'
sequence is being evaluated prospectively in the ULTRA-switch trial
(NCT05538680): Upon detection of FGFR3 gatekeeper or PI3K/AKT
pathway mutations, patients crossover from erdafitinib to a PI3Kβ
inhibitor plus paclitaxel, while NECTIN-4 loss triggers
EV-to-taxane switch. Early safety run-in data (n=42) show a 71%
clinical benefit rate without additive toxicity, supporting the
feasibility of real-time molecular triage.
Challenges and future directions
Resistance to targeted therapies poses a significant
challenge in the treatment of UC. With respect to FGFR inhibitors,
the mechanisms of resistance include secondary gene mutations and
compensatory signaling pathway activation (122). For example, studies have found
that FGFR gene mutations can lead to reduced inhibitor binding
affinity of the inhibitors to the target, thereby causing
resistance. Additionally, the activation of other signaling
pathways, such as the EGFR pathway, can compensate for the
inhibition of the FGFR pathway, promoting tumor cell survival and
proliferation (122,123). Similar resistance mechanisms
have been observed in the use of ADCs. Secondary gene mutations can
alter the structure of the target antigen, reducing the binding
ability of the ADCs (124).
Moreover, the upregulation of drug efflux pump expression can
decrease the intracellular concentration of the cytotoxic drugs,
leading to resistance (124).
To overcome these resistance mechanisms, several
strategies are being explored. Combination therapies represent a
promising approach. For instance, combining FGFR inhibitors with
immunotherapy or chemotherapy may enhance therapeutic efficacy
(125). Preclinical studies have
shown that the combination of FGFR inhibitors and anti-PD-1
antibodies can synergistically inhibit tumor growth by alleviating
immunosuppression in the TME (125,126). The development of
next-generation drugs is also crucial. Researchers are working on
designing FGFR inhibitors with higher selectivity and affinity to
overcome resistance caused by secondary mutations. Furthermore, the
use of ctDNA analysis may help in identifying resistance mechanisms
and guiding treatment adjustments in a timely manner (127).
Managing the AEs associated with targeted therapies
is essential for improving patient quality of life and treatment
adherence. FGFR inhibitors are commonly associated with ocular
toxicity and hyperphosphatemia. Ocular toxicity can manifest as
macular edema, serous retinopathy and blurred vision. Regular
ophthalmologic examinations are recommended for early detection and
management of these side effects early (128). Hyperphosphatemia can usually be
managed through dietary adjustments and the use of phosphate
binders. ADCs, on the other hand, can cause peripheral neuropathy
and myelosuppression. Peripheral neuropathy may require dose
adjustments or the use of neuroprotective agents. Myelosuppression
necessitates regular monitoring of blood cell counts and
appropriate supportive care. It is crucial to establish
standardized management protocols for these AEs to ensure the safe
and effective use of targeted therapies in clinical practice
(129).
Determining the optimal sequence of targeted
therapies, immunotherapy and chemotherapy in UC treatment
strategies remains an area of active research. Factors influencing
treatment sequence decisions include tumor molecular tumor
characteristics, patient performance status and prior treatment
history (130). For example,
patients with FGFR alterations may benefit from the use of FGFR
inhibitors as first-line therapy. However, the optimal timing for
introducing immunotherapy or chemotherapy in combination with
targeted therapies remains unclear. Further studies are needed to
investigate the interactions between different treatment modalities
and their impact on patient outcomes. Clinical trials evaluating
various treatment sequences are ongoing, and their results will
provide valuable insights into the best approaches for optimizing
treatment strategies for UC.
Combining targeted therapies with immunotherapy or
other treatments offers exciting prospects for UC therapy.
Preclinical and early clinical studies have demonstrated that such
combinations can increase antitumor activity. For instance,
combining FGFR inhibitors with ICIs may improve immune cell
infiltration and function in the TME (130). However, combination therapies
also present challenges, such as increased toxicity and trial
design complexity. Careful consideration of the potential side
effects and the development of effective toxicity management
strategies are necessary to ensure the safety of combination
therapies. Additionally, the design of clinical trials must account
for the interactions between different drugs and their
pharmacokinetic and pharmacodynamic characteristics. Despite these
challenges, the potential benefits of combination therapies make
them a promising direction for future research in UC treatment.
As our understanding of the molecular biology of UC
continues to deepen, new therapeutic targets and drugs are
emerging. In addition to the currently established targets such as
FGFR and TROP2, other molecules such as MET, AXL and Wnt/β-catenin
are gaining attention. MET amplification and mutations have been
identified in a subset of patients with UC and are associated with
poor prognosis. Inhibitors targeting MET are being developed and
have shown initial promise in preclinical studies. AXL
overexpression is linked to tumor progression and resistance to
therapy. Drugs targeting AXL may provide new treatment options for
patients with UC. The Wnt/β-catenin pathway plays a role in tumor
cell proliferation and survival. Modulating this pathway may offer
another avenue for therapeutic intervention. These novel targets
and drugs, along with advancements in genomics and molecular
biology technologies, are expected to further expand the
therapeutic landscape for UC and to improve treatment outcomes for
patients.
Conclusion
Significant advancements in biomarker-driven
approaches and targeted therapies for UC have been made, offering
new treatment options and hope for patients. However, challenges
such as biomarker heterogeneity, resistance mechanisms, and the
need for optimized treatment strategies remain. Future research
should focus on improving biomarker validation, developing novel
combination therapies, and designing innovative clinical trials to
enhance the efficacy and precision of targeted treatments in
UC.
Availability of data and materials
Not applicable.
Authors' contributions
JD and YX made significant contributions to the
conception of the manuscript, wrote the first version of the
manuscript and prepared figures. TZ, HS and WL reviewed the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by Baiyin City Science and
Technology Plan Project (Preclinical study of c-MET targeted
therapy in bladder cancer; grant no. 2023-2-14Y).
References
|
1
|
Biasatti A, Bignante G, Ditonno F, Veccia
A, Bertolo R, Antonelli A, Lee R, Eun DD, Margulis V, Abdollah F,
et al: New insights into upper tract urothelial carcinoma: Lessons
learned from the ROBUUST collaborative study. Cancers (Basel).
17:16682025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nally E, Young M, Chauhan V, Wells C,
Szabados B, Powles T and Jackson-Spence F: Upper tract urothelial
carcinoma (UTUC): Prevalence, impact and management challenge.
Cancer Manag Res. 16:467–475. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jubber I, Ong S, Bukavina L, Black PC,
Compérat E, Kamat AM, Kiemeney L, Lawrentschuk N, Lerner SP, Meeks
JJ, et al: Epidemiology of Bladder Cancer in 2023: A Systematic
Review of Risk Factors. Eur Urol. 84:176–190. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moussa MJ, Campbell MT and Alhalabi O:
Revisiting treatment of metastatic urothelial cancer: Where do
cisplatin and platinum ineligibility criteria stand? Biomedicines.
12:5192024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rivera C, Jabbal IS, Yaghi M, Landau KS,
Muruve N, Saravia D, George TL, Nahleh ZA and Arteta-Bulos R:
Five-year survival comparison of different treatment modalities for
muscle invasive urothelial carcinoma, squamous cell carcinoma and
adenocarcinoma of the bladder: An analysis of the National cancer
database. J Clin Oncol. 40(6_suppl): S5752022. View Article : Google Scholar
|
|
6
|
Gupta S, Andreev-Drakhlin A, Fajardo O,
Fassò M, Garcia JA, Wee C and Schröder C: Platinum ineligibility
and survival outcomes in patients with advanced urothelial
carcinoma receiving first-line treatment. J Natl Cancer Inst.
116:547–554. 2024. View Article : Google Scholar
|
|
7
|
Mao L, Yang M, Fan X, Li W, Huang X, He W,
Lin T and Huang J: PD-1/L1 inhibitors can improve but not replace
chemotherapy for advanced urothelial carcinoma: A systematic review
and network meta-analysis. Cancer Innov. 2:191–202. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li XK and Wang WL: The role novel targeted
agents in the treatment of previously treated patients with
advanced urothelial carcinoma (UC): A meta-analysis. Eur Rev Med
Pharmacol Sci. 22:5165–5171. 2018.PubMed/NCBI
|
|
9
|
Siefker-Radtke AO, Necchi A, Park SH,
García-Donas J, Huddart RA, Burgess EF, Fleming MT, Rezazadeh
Kalebasty A, Mellado B, Varlamov S, et al: Efficacy and safety of
erdafitinib in patients with locally advanced or metastatic
urothelial carcinoma: Long-term follow-up of a phase 2 study.
Lancet Oncol. 23:248–258. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu EY, Petrylak DP, O'Donnell PH, Lee JL,
van der Heijden MS, Loriot Y, Stein MN, Necchi A, Kojima T,
Harrison MR, et al: Enfortumab vedotin after PD-1 or PD-L1
inhibitors in cisplatin-ineligible patients with advanced
urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2
trial. Lancet Oncol. 22:872–882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seront E and Machiels JP: Molecular
biology and targeted therapies for urothelial carcinoma. Cancer
Treat Rev. 41:341–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Glaser AP, Fantini D, Shilatifard A,
Schaeffer EM and Meeks JJ: The evolving genomic landscape of
urothelial carcinoma. Nat Rev Urol. 14:215–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ching CB and Hansel DE: Expanding
therapeutic targets in bladder cancer: The PI3K/Akt/mTOR pathway.
Lab Invest. 90:1406–1414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He F, Zhang F, Liao Y, Tang MS and Wu XR:
Structural or functional defects of PTEN in urothelial cells
lacking P53 drive basal/squamous-subtype muscle-invasive bladder
cancer. Cancer Lett. 550:2159242022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patel VG, McBride RB, Lorduy AC,
Castillo-Martin M, Cha EK, Berger MF, Wang L, Oh WK, Zhu J,
Cordon-Cardo C, et al: Prognostic significance of PIK3CA mutation
in patients with muscle-invasive urothelial carcinoma (UC). J Clin
Oncol. 34(15_suppl): e160022016. View Article : Google Scholar
|
|
16
|
Wang DS, Rieger-Christ K, Latini JM,
Moinzadeh A, Stoffel J, Pezza JA, Saini K, Libertino JA and
Summerhayes IC: Molecular analysis of PTEN and MXI1 in primary
bladder carcinoma. Int J Cancer. 88:620–625. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuruta H, Kishimoto H, Sasaki T, Horie Y,
Natsui M, Shibata Y, Hamada K, Yajima N, Kawahara K, Sasaki M, et
al: Hyperplasia and carcinomas in Pten-deficient mice and reduced
PTEN protein in human bladder cancer patients. Cancer Res.
66:8389–8396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imperial R, Toor OM, Hussain A,
Subramanian J and Masood A: Comprehensive pancancer genomic
analysis reveals (RTK)-RAS-RAF-MEK as a key dysregulated pathway in
cancer: Its clinical implications. Semin Cancer Biol. 54:14–28.
2019. View Article : Google Scholar
|
|
19
|
Degirmenci U, Wang M and Hu J: Targeting
aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells.
9:1982020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YS, Lee SC, Hwang IG, Lee SJ and Park
SH: Relationship between RAS, BRAF, PIK3CA and location of primary
tumor in urothelial carcinoma. Cancer Res. 79(13_Suppl): S12622019.
View Article : Google Scholar
|
|
21
|
Zhou H, Huang HY, Shapiro E, Lepor H,
Huang WC, Mohammadi M, Mohr I, Tang MS, Huang C and Wu XR:
Urothelial tumor initiation requires deregulation of multiple
signaling pathways: implications in target-based therapies.
Carcinogenesis. 33:770–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu G, Wang F, Li K, Li S, Zhao C, Fan C
and Wang J: Significance of TP53 mutation in bladder cancer disease
progression and drug selection. PeerJ. 7:e82612019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ecke TH, Sachs MD, Lenk SV, Loening SA and
Schlechte HH: TP53 gene mutations as an independent marker for
urinary bladder cancer progression. Int J Mol Med. 21:655–661.
2008.PubMed/NCBI
|
|
24
|
Khalid S, Basulaiman BM, Emack J, Booth
CM, Hernandez-Barajas D, Duran I, Smoragiewicz M, Amir E and
Vera-Badillo F: FGFR3 mutation as a prognostic indicator in
patients with urothelial carcinoma: A systematic review and
meta-analysis. J Clin Oncol. 37(7_suppl): S4112019. View Article : Google Scholar
|
|
25
|
Che X, Liu H, Qin J and Cao S: Landscape
of FGFR2/3 alterations in genitourinary cancer. J Clin Oncol.
41(16_suppl): e150472023. View Article : Google Scholar
|
|
26
|
Liu X, Zhang W, Geng D, He J, Zhao Y and
Yu L: Clinical significance of fibroblast growth factor receptor-3
mutations in bladder cancer: a systematic review and meta-analysis.
Genet Mol Res. 13:1109–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng P, Chen MB, Zhou LN, Tang M, Liu CY
and Lu PH: Impact of TROP2 expression on prognosis in solid tumors:
A Systematic Review and Meta-analysis. Sci Rep. 6:336582016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tagawa ST, Balar AV, Petrylak DP,
Kalebasty AR, Loriot Y, Fléchon A, Jain RK, Agarwal N, Bupathi M,
Barthelemy P, et al: TROPHY-U-01: A phase II open-label study of
sacituzumab govitecan in patients with metastatic urothelial
carcinoma progressing after platinum-based chemotherapy and
checkpoint inhibitors. J Clin Oncol. 39:2474–2485. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosenberg JE, Powles T, Sonpavde GP,
Loriot Y, Duran I, Lee JL, Matsubara N, Vulsteke C, Castellano D,
Mamtani R, et al: EV-301 long-term outcomes: 24-month findings from
the phase III trial of enfortumab vedotin versus chemotherapy in
patients with previously treated advanced urothelial carcinoma. Ann
Oncol. 34:1047–1054. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tagawa ST, Balar AV, Petrylak DP,
Rezazadeh A, Loriot Y, Flechon A, Jain RK, Agarwal N, Bupathi M,
Barthelemy P, et al: Updated outcomes in TROPHY-U-01 cohort 1, a
phase 2 study of sacituzumab govitecan (SG) in patients (pts) with
metastatic urothelial cancer (mUC) that progressed after platinum
(PT)-based chemotherapy and a checkpoint inhibitor (CPI). J Clin
Oncol. 41(6_suppl): S5262023. View Article : Google Scholar
|
|
31
|
Hall TG, Yu Y, Eathiraj S, Wang Y, Savage
RE, Lapierre JM, Schwartz B and Abbadessa G: Preclinical activity
of ARQ 087, a novel inhibitor targeting FGFR dysregulation. PLoS
One. 11:e01625942016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dienstmann R, Rodon J, Prat A,
Perez-Garcia J, Adamo B, Felip E, Cortes J, Iafrate AJ, Nuciforo P
and Tabernero J: Genomic aberrations in the FGFR pathway:
Opportunities for targeted therapies in solid tumors. Ann Oncol.
25:552–563. 2014. View Article : Google Scholar
|
|
33
|
Ross JS, Wang K, Khaira D, Ali SM, Fisher
HA, Mian B, Nazeer T, Elvin JA, Palma N, Yelensky R, et al:
Comprehensive genomic profiling of 295 cases of clinically advanced
urothelial carcinoma of the urinary bladder reveals a high
frequency of clinically relevant genomic alterations. Cancer.
122:702–711. 2016. View Article : Google Scholar
|
|
34
|
Kim YS, Kim K, Kwon GY, Lee SJ and Park
SH: Fibroblast growth factor receptor 3 (FGFR3) aberrations in
muscle-invasive urothelial carcinoma. BMC Urol. 18:682018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gamallat Y, Afsharpad M, El Hallani S,
Maher CA, Alimohamed N, Hyndman E and Bismar TA: Large, nested
variant of urothelial carcinoma is enriched with activating
mutations in fibroblast growth factor receptor-3 among other
targetable mutations. Cancers (Basel). 15:31672023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Necchi A, Lo Vullo S, Raggi D, Gloghini A,
Giannatempo P, Colecchia M and Mariani L: Prognostic effect of FGFR
mutations or gene fusions in patients with metastatic urothelial
carcinoma receiving first-line platinum-based chemotherapy: Results
from a large, single-institution cohort. Eur Urol Focus. 5:853–856.
2019. View Article : Google Scholar
|
|
37
|
Huang GK, Huang CC, Kang CH, Cheng YT,
Tsai PC, Kao YH and Chung YH: Genetic Interference of FGFR3 impedes
invasion of upper tract urothelial carcinoma cells by alleviating
RAS/MAPK signal activity. Int J Mol Sci. 24:17762023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Li Y, Liu B, Chen L, Lyu F, Zhang P,
He Q, Cheng L, Liu C, Song Y and Xing Y: P4HA2-mediated HIF-1α
stabilization promotes erdafitinib-resistance in FGFR3-alteration
bladder cancer. FASEB J. 37:e228402023. View Article : Google Scholar
|
|
39
|
Shohdy KS, Vlachostergios PJ, Abdel-Malek
RR and Faltas BM: Rationale for co-targeting CDK4/6 and FGFR
pathways in urothelial carcinoma. Expert Opin Ther Targets.
23:83–86. 2019. View Article : Google Scholar
|
|
40
|
Khalid S, Basulaiman BM, Emack J, Booth
CM, Duran I, Robinson AG, Berman D, Smoragiewicz M, Amir E and
Vera-Badillo FE: Fibroblast growth factor receptor 3 mutation as a
prognostic indicator in patients with urothelial carcinoma: A
systematic review and meta-analysis. Eur Urol Open Sci. 21:61–68.
2020. View Article : Google Scholar
|
|
41
|
Song Y, Peng Y, Qin C, Wang Y, Yang W, Du
Y and Xu T: Fibroblast growth factor receptor 3 mutation attenuates
response to immune checkpoint blockade in metastatic urothelial
carcinoma by driving immunosuppressive microenvironment. J
Immunother Cancer. 11:e0066432023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okato A, Utsumi T, Ranieri M, Zheng X,
Zhou M, Pereira LD, Chen T, Kita Y, Wu D, Hyun H, et al: FGFR
inhibition augments anti-PD-1 efficacy in murine FGFR3-mutant
bladder cancer by abrogating immunosuppression. J Clin Invest.
134:e1692412024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahmoud SF, Holah NS, Alhanafy AM and
Serag El-Edien MM: Do fibroblast growth factor receptor (FGFR) 2
and 3 proteins play a role in prognosis of invasive urothelial
bladder carcinoma? Iran J Pathol. 19:81–88. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Loriot Y, Necchi A, Park SH, Garcia-Donas
J, Huddart R, Burgess E, Fleming M, Rezazadeh A, Mellado B,
Varlamov S, et al: Erdafitinib in locally advanced or metastatic
urothelial carcinoma. N Engl J Med. 381:338–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Parker Kerrigan BC, Ledbetter D, Kronowitz
M, Phillips L, Gumin J, Hossain A, Yang J, Mendt M, Singh S,
Cogdell D, et al: RNAi technology targeting the FGFR3-TACC3 fusion
break-point: An opportunity for precision medicine. Neurooncol Adv.
2:vdaa1322020.
|
|
46
|
Lyou Y, Grivas P, Rosenberg JE,
Hoffman-Censits J, Quinn DI, P Petrylak D, Galsky M, Vaishampayan
U, De Giorgi U, Gupta S, et al: Hyperphosphatemia secondary to the
selective fibroblast growth factor receptor 1-3 inhibitor
infigratinib (BGJ398) is associated with antitumor efficacy in
fibroblast growth factor receptor 3-altered advanced/metastatic
urothelial carcinoma. Eur Urol. 78:916–924. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Loriot Y, Matsubara N, Park SH, Huddart
RA, Burgess EF, Houede N, Banek S, Guadalupi V, Ku JH, Valderrama
BP, et al: Erdafitinib or chemotherapy in advanced or metastatic
urothelial carcinoma. N Engl J Med. 389:1961–1971. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Siefker-Radtke AO, Matsubara N, Park SH,
Huddart RA, Burgess EF, Özgüroğlu M, Valderrama BP, Laguerre B,
Basso U, Triantos S, et al: Erdafitinib versus pembrolizumab in
pretreated patients with advanced or metastatic urothelial cancer
with select FGFR alterations: cohort 2 of the randomized phase III
THOR trial. Ann Oncol. 35:107–117. 2024. View Article : Google Scholar
|
|
49
|
Guercio BJ, Sarfaty M, Teo MY, Ratna N,
Duzgol C, Funt SA, Lee CH, Aggen DH, Regazzi AM, Chen Z, et al:
Clinical and genomic landscape of FGFR3-altered urothelial
carcinoma and treatment outcomes with erdafitinib: A real-world
experience. Clin Cancer Res. 29:4586–4595. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lyou Y, Rosenberg JE, Hoffman-Censits J,
Quinn DI, Petrylak D, Galsky M, Vaishampayan U, De Giorgi U, Gupta
S, Burris H, et al: Infigratinib in early-line and salvage therapy
for FGFR3-Altered metastatic urothelial carcinoma. Clin Genitourin
Cancer. 20:35–42. 2022. View Article : Google Scholar :
|
|
51
|
Pal SK, Somford DM, Grivas P, Sridhar SS,
Gupta S, Bellmunt J, Sonpavde G, Fleming MT, Lerner SP, Loriot Y,
et al: Targeting FGFR3 alterations with adjuvant infigratinib in
invasive urothelial carcinoma: The phase III PROOF 302 trial.
Future Oncol. 18:2599–2614. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sternberg CN, Petrylak DP, Bellmunt J,
Nishiyama H, Necchi A, Gurney H, Lee JL, van der Heijden MS,
Rosenbaum E, Penel N, et al: FORT-1: Phase II/III study of
rogaratinib versus chemotherapy in patients with locally advanced
or metastatic urothelial carcinoma selected based on FGFR1/3 mRNA
expression. J Clin Oncol. 41:629–639. 2023. View Article : Google Scholar :
|
|
53
|
Necchi A, Pouessel D, Leibowitz R, Gupta
S, Fléchon A, García-Donas J, Bilen MA, Debruyne PR, Milowsky MI,
Friedlander T, et al: Pemigatinib for metastatic or surgically
unresectable urothelial carcinoma with FGF/FGFR genomic
alterations: Final results from FIGHT-201. Ann Oncol. 35:200–210.
2024. View Article : Google Scholar
|
|
54
|
Gong J, Mita AC, Wei Z, Cheng HH, Mitchell
EP, Wright JJ, Ivy SP, Wang V, Gray RC, McShane LM, et al: Phase II
study of erdafitinib in patients with tumors with FGFR
amplifications: results from the NCI-MATCH ECOG-ACRIN trial
(EAY131) subprotocol K1. JCO Precis Oncol. 8:e23004062024.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Siefker-Radtke AO and Loriot Y:
Erdafitinib for locally advanced or metastatic urothelial
carcinoma. Am J Health Syst Pharm. 79:824–825. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Necchi A, Ramlau R, Falcón González A,
Chaudhry A, Todenhöfer T, Tahbaz R, Fontana E, Giannatempo P,
Deville JL, Pouessel D, et al: Derazantinib alone and with
atezolizumab in metastatic urothelial carcinoma with activating
FGFR aberrations. JNCI Cancer Spectr. 8:pkae0302024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Datta J, Damodaran S, Parks H, Ocrainiciuc
C, Miya J, Yu L, Gardner EP, Samorodnitsky E, Wing MR, Bhatt D, et
al: Akt activation mediates acquired resistance to fibroblast
growth factor receptor inhibitor BGJ398. Mol Cancer Ther.
16:614–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pettitt GA, Hurst CD, Khan Z, McPherson
HR, Dunning MC, Alder O, Platt FM, Black EV, Burns JE and Knowles
MA: Development of resistance to FGFR inhibition in urothelial
carcinoma via multiple pathways in vitro. J Pathol. 259:220–232.
2023. View Article : Google Scholar :
|
|
59
|
Kim SH, Ryu H, Ock CY, Suh KJ, Lee JY, Kim
JW, Lee JO, Kim JW, Kim YJ, Lee KW, et al: BGJ398, A Pan-FGFR
inhibitor, overcomes paclitaxel resistance in urothelial carcinoma
with FGFR1 overexpression. Int J Mol Sci. 19:31642018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rezazadeh Kalebasty A, Benjamin DJ, Loriot
Y, Papantoniou D, Siefker-Radtke AO, Necchi A, Naini V, Carcione
JC, Santiago-Walker A, Triantos S and Burgess EF: Outcomes of
patients with advanced urothelial carcinoma after anti-programmed
Death-(ligand) 1 therapy by fibroblast growth factor receptor gene
alteration status: An observational study. Eur Urol Open Sci.
47:48–57. 2022. View Article : Google Scholar
|
|
61
|
Bellmunt J, Lalani AA, Jacobus S,
Wankowicz SA, Polacek L, Takeda DY, Harshman LC, Wagle N, Moreno I,
Lundgren K, et al: Everolimus and pazopanib (E/P) benefit
genomically selected patients with metastatic urothelial carcinoma.
Br J Cancer. 119:707–712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Brunelli M, Gobbo S, Malpeli G,
Sirgiovanni G, Caserta C, Munari E, Francesconi S, Caliò A,
Martignoni G, Cimadamore A, et al: TROP-2, NECTIN-4 and predictive
biomarkers in sarcomatoid and rhabdoid bladder urothelial
carcinoma. Pathologica. 116:55–61. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Audisio M, Buttigliero C, Turco F,
Delcuratolo MD, Pisano C, Parlagreco E, Di Stefano RF, Di Prima L,
Crespi V, Farinea G, et al: Metastatic urothelial carcinoma: Have
we take the road to the personalized medicine? Cells. 11:16142022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Heath EI and Rosenberg JE: The biology and
rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev
Urol. 18:93–103. 2021. View Article : Google Scholar
|
|
65
|
Gupta S, Loriot Y, Van der Heijden MS,
Bedke J, Valderrama BP, Kikuchi E, Fléchon A, Petrylak D, De Santis
M, Galsky MD, et al: Enfortumab vedotin plus pembrolizumab versus
chemotherapy in patients with previously untreated locally advanced
or metastatic urothelial cancer (EV-302): Patient-reported outcomes
from an open-label, randomised, controlled, phase 3 study. Lancet
Oncol. 26:795–805. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li S, Shi Y, Dong H, Guo H, Xie Y, Sun Z,
Zhang X, Kim E, Zhang J, Li Y, et al: Phase 2 trial of enfortumab
vedotin in patients with previously treated locally advanced or
metastatic urothelial carcinoma in China. Cancer Med.
13:e703682025. View Article : Google Scholar
|
|
67
|
Rizzo M, Morelli F, Ürün Y, Buti S, Park
SH, Bourlon MT, Grande E, Massari F, Landmesser J, Poprach A, et
al: Real-life impact of enfortumab vedotin or chemotherapy in the
sequential treatment of advanced urothelial carcinoma: The ARON-2
retrospective experience. Cancer Med. 14:e704792025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Uchimoto T, Iwatsuki K, Komura K,
Fukuokaya W, Adachi T, Hirasawa Y, Hashimoto T, Yoshizawa A, Saruta
M, Hashimoto M, et al: Association of body mass index and tumor
response in metastatic urothelial carcinoma treated with enfortumab
vedotin: data from the ULTRA-Japan consortium. Int J Clin Oncol.
30:761–769. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mishra A, Sharma AK, Gupta K, Banka DR,
Johnson BA, Hoffman-Censits J, Huang P, McConkey DJ and Nimmagadda
S: Nectin-4 Pet for optimizing enfortumab vedotin dose-response in
urothelial carcinoma. bioRxiv [Preprint]. 2024.12.25.630315.
2024.
|
|
70
|
Jindal T, Zhu X, Bose R, Kumar V,
Maldonado E, Deshmukh P, Shipp C, Feng S, Johnson MS, Angelidakis
A, et al: Somatic alterations of TP53 and MDM2 associated with
response to enfortumab vedotin in patients with advanced urothelial
cancer. Front Oncol. 13:11610892023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hovelroud R, Goh Xiu Ming S, McLeod DSA,
Donovan PJ, Ng G and Mungomery M: A case of enfortumab
vedotin-associated diabetic ketoacidosis with severe insulin
resistance in a nondiabetic woman. JCEM Case Rep. 2:luae2122024.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Desimpel G, Zammit F, Lejeune S, Grisay G
and Seront E: Lung toxicity occurring during enfortumab vedotin
treatment: From a priming case report to a retrospective analysis.
Pharmaceuticals (Basel). 17:15472024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pipitone S, Vitale MG, Baldessari C,
Sabbatini R, Dominici M, Porretta Serapiglia C, Ricchi L and Rivasi
M: Extravasation of enfortumab vedotin: A case report and
literature review on antibody-drug conjugates. Eur J Hosp
Pharmejhpharm. 2024-0043232025.Epub ahead of print. View Article : Google Scholar
|
|
74
|
Zhu Y, Liu K, Zhu H, Li S and Yuan D:
Enfortumab vedotin plus pembrolizumab for previously untreated
locally advanced or metastatic urothelial carcinoma: A
cost-effectiveness analysis. Ther Adv Med Oncol.
17:175883592412955442025. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo
H, Shi B, Liu J, He Z, Yu G, et al: Open-label, multicenter, phase
II Study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in
patients with locally advanced or metastatic urothelial carcinoma.
Clin Cancer Res. 27:43–51. 2021. View Article : Google Scholar
|
|
77
|
Sheng X, Wang L, He Z, Shi Y, Luo H, Han
W, Yao X, Shi B, Liu J, Hu C, et al: Efficacy and safety of
disitamab vedotin in patients with human epidermal growth factor
receptor 2-positive locally advanced or metastatic urothelial
carcinoma: A combined analysis of two phase II clinical trials. J
Clin Oncol. 42:1391–1402. 2024. View Article : Google Scholar
|
|
78
|
Chen M, Yao K, Cao M, Liu H, Xue C, Qin T,
Meng L, Zheng Z, Qin Z, Zhou F, et al: HER2-targeting antibody-drug
conjugate RC48 alone or in combination with immunotherapy for
locally advanced or metastatic urothelial carcinoma: A multicenter,
real-world study. Cancer Immunol Immunother. 72:2309–2318. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yan X, Li J, Xu H, Liu Y, Zhou L, Li S, Wu
X, Tang B, Chi Z, Cui C, et al: Efficacy and safety of DV in
HER2-negative and HER2-low locally advanced or metastatic
urothelial carcinoma: Results of a phase 2 study. Med.
6:1006372025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang D, Cao M, Zhang Y, Bi L, Chen M, Ni
M, Zheng Q, Yao K, Liu Z, Yang X, et al: RC48-ADC monotherapy or in
combination with immunotherapy for locally advanced or metastatic
urothelial carcinoma with HER2 low and null expression: A
multicenter, real-world, retrospective study. BMC Cancer.
25:8122025. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou L, Yang KW, Zhang S, Yan XQ, Li SM,
Xu HY, Li J, Liu YQ, Tang BX, Chi ZH, et al: Disitamab vedotin plus
toripalimab in patients with locally advanced or metastatic
urothelial carcinoma (RC48-C014): A phase Ib/II dose-escalation and
dose-expansion study. Ann Oncol. 36:331–339. 2025. View Article : Google Scholar
|
|
82
|
Yao JW, Zhong JL, Zhou Q and Guo J:
Efficacy and safety of disitamab vedotin in combination with immune
checkpoint inhibitors in patients with locally advanced or
metastatic urothelial carcinoma. World J Urol. 43:1542025.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ge H, Liu C, Shen C, Hu D, Zhao X and Wang
Y, Ge H, Qin R, Ma X and Wang Y: The effectiveness and safety of
RC48 alone or in combination with PD-1 inhibitors for locally
advanced or metastatic urothelial carcinoma: A multicenter,
real-world study. J Transl Med. 23:2432025. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yang M, Yao Y, Wang K, Qi L, Yang B,
Khudadad M, Guo Y, Wang Y, Liu Y, Li L, et al: Clinicopathological
characteristics and prognostic significance of HER2 status
evaluation in patients with urothelial carcinoma: A retrospective
single-center experience in China. Virchows Arch. Feb 26–2025.Epub
ahead of print. View Article : Google Scholar
|
|
85
|
Chen Y, Luo F, Zhang T and Li J: Impact of
HER2 expression on the prognosis of muscle-invasive bladder cancer
patients treated with bladder-preservation comprehensive therapy.
Biol Proced Online. 27:22025. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chou J, Trepka K, Sjöström M, Egusa EA,
Chu CE, Zhu J, Chan E, Gibb EA, Badura ML, Contreras-Sanz A, et al:
TROP2 expression across molecular subtypes of urothelial carcinoma
and enfortumab vedotin-resistant cells. Eur Urol Oncol. 5:714–718.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bahlinger V, Branz A, Strissel PL, Strick
R, Lange F, Geppert CI, Klümper N, Hölzel M, Wach S, Taubert H, et
al: Associations of TACSTD2/TROP2 and NECTIN-4/NECTIN-4 with
molecular subtypes, PD-L1 expression, and FGFR3 mutational status
in two advanced urothelial bladder cancer cohorts. Histopathology.
84:863–876. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Katims AB, Reisz PA, Nogueira L, Truong H,
Lenis AT, Pietzak EJ, Kim K and Coleman JA: Targeted therapies in
advanced and metastatic urothelial carcinoma. Cancers (Basel).
14:54312022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shvartsbart A, Roach JJ, Witten MR,
Koblish H, Harris JJ, Covington M, Hess R, Lin L, Frascella M,
Truong L, et al: Discovery of potent and selective inhibitors of
wild-type and gatekeeper mutant fibroblast growth factor receptor
(FGFR) 2/3. J Med Chem. 65:15433–15442. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shih CH, Lin YH, Luo HL and Sung WW:
Antibody-drug conjugates targeting HER2 for the treatment of
urothelial carcinoma: Potential therapies for HER2-positive
urothelial carcinoma. Front Pharmacol. 15:13262962024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Khosravanian MJ, Mirzaei Y, Mer AH,
Keyhani-Khankahdani M, Abdinia FS, Misamogooe F, Amirkhani Z,
Bagheri N, Meyfour A, Jahandideh S, et al: Nectin-4-directed
antibody-drug conjugates (ADCs): Spotlight on preclinical and
clinical evidence. Life Sci. 352:1229102024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chu CE, Sjöström M, Egusa EA, Gibb EA,
Badura ML, Zhu J, Koshkin VS, Stohr BA, Meng MV, Pruthi RS, et al:
Heterogeneity in NECTIN4 expression across molecular subtypes of
urothelial cancer mediates sensitivity to enfortumab vedotin. Clin
Cancer Res. 27:5123–5130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Guancial EA, Werner L, Bellmunt J, Bamias
A, Choueiri TK, Ross R, Schutz FA, Park RS, O'Brien RJ, Hirsch MS,
et al: FGFR3 expression in primary and metastatic urothelial
carcinoma of the bladder. Cancer Med. 3:835–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ross JS, Gay LM, Ferry EK, Jacob J,
Shapiro O, Hoffman-Censits JH, Millis SZ, Elvin JA, Chung J,
Vergilio JA, et al: FGFR3 Driven Metastatic Urothelial Carcinoma of
the Urinary Bladder (mUCB): A Comprehensive Genomic Profiling
Study. J Clin Oncol. 36(15_suppl): 4531. 2018. View Article : Google Scholar
|
|
95
|
Redig AJ, Costa DB, Taibi M, Boucher D,
Johnson BE, Jänne PA and Jackman DM: Prospective study of repeated
biopsy feasibility and acquired resistance at disease progression
in patients with advanced EGFR mutant lung cancer treated with
erlotinib in a phase 2 trial. JAMA Oncol. 2:1240–1242. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kang M and Ku JH: Emerging role of liquid
biopsy of cell-free tumor DNA for bladder cancer surveillance.
Transl Androl Urol. 6:590–592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bambury RM and Rosenberg JE: Advanced
urothelial carcinoma: Overcoming treatment resistance through novel
treatment approaches. Front Pharmacol. 4:32013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang P, Shi Y, Zhang J, Shou J, Zhang M,
Zou D, Liang Y, Li J, Tan Y, Zhang M, et al: UCseek: Ultrasensitive
early detection and recurrence monitoring of urothelial carcinoma
by shallow-depth genome-wide bisulfite sequencing of urinary
sediment DNA. EBioMedicine. 89:1044372023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pichler R, van Creij NCH, Subiela JD,
Cimadamore A, Caño-Velasco J, Tully KH, Mori K, Contieri R, Afferi
L, Mari A, et al: Biological and therapeutic implications of FGFR
alterations in urothelial cancer: A systematic review from
non-muscle-invasive to metastatic disease. Actas Urol Esp (Engl
Ed). 49:5017192025.In English, Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang R, Zang J, Jin D, Xie F, Shahatiaili
A, Wu G, Zhang L, Wang L, Zhang Y, Zhao Z, et al: Urinary tumor DNA
MRD analysis to identify responders to neoadjuvant immunotherapy in
muscle-invasive bladder cancer. Clin Cancer Res. 29:4040–4046.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Benjamin DJ and Mita AC: FGFR-altered
urothelial carcinoma: Resistance mechanisms and therapeutic
strategies. Target Oncol. 20:1–11. 2025. View Article : Google Scholar
|
|
102
|
Yajima S and Masuda H: Immune checkpoint
inhibitors and antibody-drug conjugates in urothelial carcinoma:
Current landscape and future directions. Cancers (Basel).
17:15942025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Katoh M, Loriot Y, Brandi G, Tavolari S,
Wainberg ZA and Katoh M: FGFR-targeted therapeutics: Clinical
activity, mechanisms of resistance and new directions. Nat Rev Clin
Oncol. 21:312–329. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Khoury R, Saleh K, Khalife N, Saleh M,
Chahine C, Ibrahim R and Lecesne A: Mechanisms of resistance to
antibody-drug conjugates. Int J Mol Sci. 24:96742023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Szklener K, Chmiel P, Michalski A and
Mańdziuk S: New directions and challenges in targeted therapies of
advanced bladder cancer: The role of FGFR inhibitors. Cancers
(Basel). 14:14162022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Khajah MA, Mathew PM and Luqmani YA:
Inhibitors of PI3K/ERK1/2/p38 MAPK Show preferential activity
against endocrine-resistant breast cancer cells. Oncol Res.
25:1283–1295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Feldt SL and Bestvina CM: The role of MET
in resistance to EGFR inhibition in NSCLC: A review of mechanisms
and treatment implications. Cancers (Basel). 15:29982023.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Manzano RG, Catalan-Latorre A and
Brugarolas A: RB1 and TP53 co-mutations correlate strongly with
genomic biomarkers of response to immunity checkpoint inhibitors in
urothelial bladder cancer. BMC Cancer. 21:4322021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ungaro A, Tucci M, Audisio A, Di Prima L,
Pisano C, Turco F, Delcuratolo MD, Di Maio M, Scagliotti GV and
Buttigliero C: Antibody-Drug conjugates in urothelial carcinoma: A
new therapeutic opportunity moves from bench to bedside. Cells.
11:8032022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wise AR, Maloney S, Hering A, Zabala S,
Richmond GE, VanKlompenberg MK, Nair MT and Prosperi JR: Bcl-2
Up-Regulation Mediates Taxane Resistance Downstream of APC Loss.
Int J Mol Sci. 25:67452024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Das C, Adhikari S, Bhattacharya A,
Chakraborty S, Mondal P, Yadav SS, Adhikary S, Hunt CR, Yadav KK,
Pandita S, et al: Epigenetic-metabolic interplay in the DNA damage
response and therapeutic resistance of breast cancer. Cancer Res.
83:657–666. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Klümper N, Ralser DJ, Ellinger J, Roghmann
F, Albrecht J, Below E, Alajati A, Sikic D, Breyer J, Bolenz C, et
al: Membranous NECTIN-4 expression frequently decreases during
metastatic spread of urothelial carcinoma and is associated with
enfortumab vedotin resistance. Clin Cancer Res. 29:1496–1505. 2023.
View Article : Google Scholar :
|
|
113
|
Qu M, Zhou L, Yan X, Li S, Wu X, Xu H, Li
J, Guo J, Zhang X, Li H and Sheng X: Advances in HER2-Targeted
treatment for advanced/metastatic urothelial carcinoma. Bladder
(San Franc). 10:e212000122023.PubMed/NCBI
|
|
114
|
Watts TL, Cui R, Szaniszlo P, Resto VA,
Powell DW and Pinchuk IV: PDGF-AA mediates mesenchymal stromal cell
chemotaxis to the head and neck squamous cell carcinoma tumor
microenvironment. J Transl Med. 14:3372016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen CH, Changou CA, Hsieh TH, Lee YC, Chu
CY, Hsu KC, Wang HC, Lin YC, Lo YN, Liu YR, et al: Dual inhibition
of PIK3C3 and FGFR as a new therapeutic approach to treat bladder
cancer. Clin Cancer Res. 24:1176–1189. 2018. View Article : Google Scholar
|
|
116
|
Hosaka K, Yang Y, Seki T, Du Q, Jing X, He
X, Wu J, Zhang Y, Morikawa H, Nakamura M, et al: Therapeutic
paradigm of dual targeting VEGF and PDGF for effectively treating
FGF-2 off-target tumors. Nat Commun. 11:37042020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Schlam I, Moges R, Morganti S, Tolaney SM
and Tarantino P: Next-generation antibody-drug conjugates for
breast cancer: Moving beyond HER2 and TROP2. Crit Rev Oncol
Hematol,. 190:1040902023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Shibata N, Cho N, Koyama H and Naito M:
Development of a degrader against oncogenic fusion protein
FGFR3-TACC3. Bioorg Med Chem Lett. 60:1285842022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pinkerneil M, Hoffmann MJ, Schulz WA and
Niegisch G: HDACs and HDAC inhibitors in urothelial
carcinoma-perspectives for an antineoplastic treatment. Curr Med
Chem. 24:4151–4165. 2017. View Article : Google Scholar
|
|
121
|
Jaromin M, Konecki T and Kutwin P:
Revolutionizing treatment: Breakthrough approaches for
BCG-unresponsive non-muscle-invasive bladder cancer. Cancers
(Basel). 16:13662024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yue S, Li Y, Chen X, Wang J, Li M, Chen Y
and Wu D: FGFR-TKI resistance in cancer: Current status and
perspectives. J Hematol Oncol. 14:232021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Facchinetti F, Hollebecque A, Braye F,
Vasseur D, Pradat Y, Bahleda R, Pobel C, Bigot L, Déas O, Florez
Arango JD, et al: Resistance to selective FGFR inhibitors in
FGFR-Driven urothelial cancer. Cancer Discov. 13:1998–2011. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ruder S, Martinez J, Palmer J, Arham AB
and Tagawa ST: Antibody-drug conjugates in urothelial carcinoma:
Current status and future. Curr Opin Urol. 35:292–300. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ruan R, Li L, Li X, Huang C, Zhang Z,
Zhong H, Zeng S, Shi Q, Xia Y, Zeng Q, et al: Unleashing the
potential of combining FGFR inhibitor and immune checkpoint
blockade for FGF/FGFR signaling in tumor microenvironment. Mol
Cancer. 22:602023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li R, Linscott J, Catto JWF, Daneshmand S,
Faltas BM, Kamat AM, Meeks JJ, Necchi A, Pradere B, Ross JS, et al:
FGFR inhibition in urothelial carcinoma. Eur Urol. 87:110–122.
2025. View Article : Google Scholar
|
|
127
|
Jogo T, Nakamura Y, Shitara K, Bando H,
Yasui H, Esaki T, Terazawa T, Satoh T, Shinozaki E, Nishina T, et
al: Circulating tumor DNA analysis detects FGFR2 Amplification and
concurrent genomic alterations associated with FGFR inhibitor
efficacy in advanced gastric cancer. Clin Cancer Res. 27:5619–5627.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kommalapati A, Tella SH, Borad M, Javle M
and Mahipal A: FGFR inhibitors in oncology: Insight on the
management of toxicities in clinical practice. Cancers (Basel).
13:29682021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Rugo HS: Management of toxicities from
antibody-drug conjugates. Clin Adv Hematol Oncol. 23:96–99.
2025.PubMed/NCBI
|
|
130
|
Zhang M, Liu C, Tu J, Tang M, Ashrafizadeh
M, Nabavi N, Sethi G, Zhao P and Liu P: Advances in cancer
immunotherapy: Historical perspectives, current developments, and
future directions. Mol Cancer. 24:1362025. View Article : Google Scholar : PubMed/NCBI
|