Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most aggressive malignancies, characterized by late-stage
diagnosis, limited therapeutic options and poor prognosis. Despite
advancements in oncology, the 5-year survival rate remains dismal
at ~13% (1). While substantial
research has focused on genetic and environmental risk factors,
molecular changes and novel treatment options for PDAC, the
influence of biological sex on disease incidence, progression and
treatment response remains insufficiently understood.
Sex- and gender-specific factors are increasingly
recognized as important determinants of disease susceptibility,
progression, and treatment outcomes. Sex refers to biological
attributes, while gender encompasses socially constructed roles and
behaviors (2). Both biological
and sociocultural components of sex and gender can profoundly
influence healthcare delivery from prevention and screening to
diagnosis and therapy, highlighting the need for more
individualized sex- and gender-specific approaches (3,4).
Despite recent efforts, women remain underrepresented in clinical
studies, often due to inclusion biases related to fertility
concerns and hormonal variability (5). In oncology, these disparities remain
insufficiently addressed, hindering progress toward truly
personalized and effective treatment strategies (6). How sex-specific factors shape
disease biology and outcomes is particularly evident in colorectal
cancer. Although it affects both sexes, women over the age of 65
have higher mortality rates and a lower 5-year survival rate
compared with age-matched men (7). This survival disadvantage may, at
least in part, be related to the higher likelihood of women
developing right-sided colon cancer, which is often associated with
more aggressive tumor phenotypes, and to their greater
susceptibility to treatment-related toxicity (7,8).
These findings underscore the clinical relevance of sex-based
differences in cancer biology and outcomes and highlight
opportunities to develop strategies that optimize treatment based
on sex-specific factors.
By contrast, sex-specific differences in PDAC remain
poorly studied, resulting in limited evidence for sex-based
variations in pathophysiology and a lack of precision medicine
approaches tailored to both men and women. The present review
summarizes current evidence on the epidemiological, biological and
clinical aspects of sex differences in PDAC. By identifying key
disparities, the present review aims to highlight the need for
sex-specific strategies in prevention, diagnosis and treatment,
which could advance personalized and effective care, while offering
approaches to improve the integration of these variables into basic
and clinical research.
Epidemiology
PDAC displays a slightly higher incidence in men
than in women, with 269,709 vs. 241,283 respective new cases
reported in 2022, ranking it as the 15th and 16th most common
cancer in men and women, respectively (9). Globally, PDAC incidence rates are
rising, with a disproportionately steeper increase observed in
women. Between 1975 and 2016, incidence rates increased annually by
1.1% in women compared with 0.38% in men, highlighting a growing
burden of PDAC among women (10).
The highest incidence rates are reported in highly developed
regions, likely due to lifestyle and environmental factors, with
Western Europe currently exhibiting the highest lifetime risk of
PDAC (11).
Notably, the incidence and mortality of PDAC peak at
different ages between sexes: In men, the highest number of cases
and deaths occurs between ages 65-69 years, whereas in women, it
peaks between ages 75-79 years (12). Of particular concern is the rising
incidence of PDAC among women <40 years, who now show higher
rates compared with age-matched men (13,14). A study by Cavazzani et al
(13) reported the greatest
increase in PDAC incidence among women aged 18-26 years, with an
average annual percentage change (AAPC) of 9.37% [95% confidence
interval (CI), 7.36-11.41%; P<0.0001], compared with an AAPC of
4.43% in men of the same age group. Survival rates remain poor for
both sexes, with a 5-year survival of ~13% (1). While the age-standardized mortality
rate is higher in men overall, this varies by age group. The most
pronounced sex difference in relative survival is observed in
patients <44 years, with survival rates of 24.37% in women vs.
18.03% in men. However, this difference diminishes with age, and
survival rates become comparable in individuals >65 years (4.68%
in women vs. 4.44% in men) (15).
PDAC represents a growing global public health
challenge. The disproportionately steeper rise in incidence among
women, particularly at younger ages, highlights the urgent need for
sex-specific strategies in prevention, early detection, and
treatment.
Risk factors
Multiple risk factors for PDAC have been well
established, including tobacco smoking, alcohol consumption,
obesity, diabetes mellitus, chronic pancreatitis, and genetic
predisposition (14). However,
sex-based differences are often overlooked. This section provides
an overview of sex-specific variations in non-genetic PDAC risk
factors.
Cigarette smoking
Cigarette smoking is a well-documented risk factor
for PDAC, with a reported OR of 1.77 (95% CI, 1.38-2.26) for
current smokers compared with never smokers, and a dose- and
duration-dependent increase in risk (16). However, evidence regarding sex
differences in smoking-related PDAC risk remains conflicting. A
large meta-analysis found no significant difference in
smoking-related PDAC risk between men and women (16). By contrast, a multiethnic cohort
study involving 192,035 participants reported a notably higher risk
in women who began smoking before age 20 years, with a 71%
increased PDAC risk [hazard ratio (HR), 1.71; 95% CI, 1.14-2.57],
compared with a 49% increase in men (HR, 1.49; 95% CI, 1.03-2.15)
(17). These findings are
supported by data from the International Pancreatic Cancer
Case-Control Consortium, which reported a particularly elevated
risk among female smokers <65 years of age (18). Although smoking cessation
significantly reduces PDAC risk, the benefit appears to be less
pronounced in women. A pooled analysis from Japan found that female
former smokers remained at elevated risk, whereas the risk
normalized in men after quitting (19,20).
These findings suggest that smoking may pose a
particularly strong and persistent risk for younger women, even
when cessation occurs later in life. Thus, although smoking is a
well-established PDAC risk factor, sex- and age-related
differences, are not yet firmly supported and warrant further
high-quality prospective research. Key studies reporting
sex-specific differences in smoking-related PDAC risk are
summarized in Table I.
 | Table IComparative summary of studies on
smoking and pancreatic ductal adenocarcinoma risk by sex. |
Table I
Comparative summary of studies on
smoking and pancreatic ductal adenocarcinoma risk by sex.
| First author,
year | Number of
patients | Study design | Results | P-value | Evidence
levela | (Refs.) |
|---|
| Gram et al,
2023 | 1,936 | Prospective cohort
study, followed participants aged 45-75 years for a mean of 19.2
years | Current
smoker:
♀ HR, 1.49 (95% CI, 1.24 1.79)
♂ HR, 1.48 (95% CI, 1.22-1.79)
Early smoking:
♀ HR, 1.71 (95% CI, 1.14-2.57)
♂ HR, 1.49 (95% CI, 1.03-2.15) | N/A for interaction
by sex N/A for interaction by sex | B | (17) |
| Bosetti et
al, 2012 | 6,507 cases/12, 890
controls | Pooled case-control
(PanC4 Consortium) | Current smoker (15
to <25 cigarettes/day):
♀ OR, 2.60 (95% CI, 1.74-3.90)
♂ OR, 1.92 (95% CI, 1.55-2.38)
High consumption (≥25 cigarettes/day):
♀ OR, 3.84 (95% CI, 2.73-5.42)
♂ OR, 2.92 (95% CI, 2.01-4.23) | 0.195
0.286 | B | (18) |
| Koyanagi et
al, 2019 | 354,154 | Pooled analysis of
10 prospective population-based cohort studies in Japan | Current smoker
(compared with never smokers):
♀ HR, 1.81 (95% CI, 1.43-2.30)
♂ HR, 1.59 (95% CI, 1.32-1.91)
Former smoker (compared with never smokers):
♀ HR, 1.77 (95% CI, 1.19-2.62)
♂ HR, 1.10 (95% CI, 0.89-1.36)
Risk per 10 pack-years (compared with never smokers):
♀ HR, 1.06 (95% CI, 0.92-1.22)
♂ HR, 1.06 (95% CI, 1.03-1.10) | N/A for interaction
by sex
N/A for interaction by sex N/A for interaction by sex | A | (19) |
Body mass index (BMI) and obesity
Obesity is recognized as a modifiable risk factor
for PDAC, with growing evidence suggesting that women may be
particularly vulnerable to obesity-related risk (20). A study reported that women with a
BMI ≥40 had more than double the risk of PDAC [relative risk (RR),
2.76; 95% CI, 1.74-4.36] (21). A
pooled analysis from the PanScan Consortium further emphasized that
central adiposity, measured by the waist-to-hip ratio, is more
strongly associated with PDAC risk in women than in men (22). This finding suggests that fat
distribution may play a more critical role in PDAC risk among
women. An additional study indicated that components of metabolic
syndrome, defined as abdominal obesity, diabetes, hypertension and
dyslipidemia, increase PDAC risk more significantly in women
(23). Conversely, obesity during
early adulthood appears to be more closely associated with PDAC
mortality in men, highlighting the potential influence of both
timing and age of weight gain (24).
Table II provides
an overview of sex-specific effects of overweight and obesity on
PDAC risk. In summary, multiple large, prospective cohort and
pooled analyses support that obesity, particularly central or
severe obesity, confers a greater PDAC risk in women than in men,
although age and timing of weight gain may modify this
relationship. Further research should clarify the underlying
sex-specific metabolic and hormonal factors driving this
vulnerability.
 | Table IIComparative summary of studies on
obesity and PDAC risk by sex. |
Table II
Comparative summary of studies on
obesity and PDAC risk by sex.
| First author,
year | Number of
patients | Study design | Results | P-value | Evidence
levela | (Refs.) |
|---|
| Genkinger et
al, 2015 | 1,096,492 | Pooled analysis of
20 prospective cohort studies from the NCI BMI and Mortality Cohort
Consortium | Waist circumference
(per 10 cm):
♀ HR, 1.09 (95% CI, 1.03-1.15)
♂ HR, 1.08 (95% CI, 1.02-1.15)
Waist-to-hip ratio:
♀ HR, 1.10 (95% CI, 1.01-1.21)
♂ HR, 1.12 (95% CI, 1.02-1.23)
BMI in early adulthood (per 5 kg/m2):
♀ HR, 1.11 (95% CI, 1.03-1.21)
♂ HR, 1.25 (95% CI, 1.15-1.35) | 0.86
0.80
0.04 | A | (24) |
| Arslan et
al, 2010 | 2,170 PDAC
cases/2,209 matched controls | Pooled nested
case-control study (Pancreatic Cancer Cohort
Consortium-PanScan) | PDAC risk and body
composition BMI:
♀ OR, 1.34 (95% CI, 1.05-1.70)
♂ OR, 1.33 (95% CI, 1.04-1.69)
Waist-to-hip-ratio:
♀ OR, 1.87 (95% CI, 1.31-2.69)
♂ N/A, not significant | N/A for interaction
by sex
N/A for interaction by sex | A | (22) |
| Calle et al,
2003 | 900,053 | Prospective cohort
study (Cancer Prevention Study II), Follow-up 16 years | PDAC mortality by
BMI BMI, 25.0-29.9 kg/m2:
♀ RR, 1.11 (95% CI, 1.00-1.24)
♂ RR, 1.13 (95% CI, 1.03-1.25)
BMI, 30.0-34.9 kg/m2:
♀ RR, 1.28 (95% CI, 1.07-1.52)
♂ RR, 1.41 (95% CI, 1.19-1.66)
BMI, ≥40.0 kg/m2:
♀ RR, 2.76 (95% CI, 1.74-4.36)
♂ RR, 1.49 (95% CI, 0.99-2.22) | N/A for interaction
by sex
N/A for interaction by sex
N/A for interaction by sex | A | (21) |
| Johansen et
al, 2010 | 577,315/86 2 PDAC
cases | Pooled prospective
cohort study from the Metabolic Syndrome and Cancer Project
(Me-Can) | PDAC risk and
metabolic syndrome [sex-specific results (z-score-based
HRs)]
BMI:
♀ 0.92, (95% CI, 0.79-1.07)
♂ 0.90, (95% CI, 0.80-1.02)
Mid-blood pressure:
♀ 1.34, (95% CI, 1.08-1.66)
♂ 1.15, (95% CI, 0.97-1.35)
Glucose:
♀ 1.98, (95% CI, 1.41-2.76)
♂ 1.37, (95% CI, 1.01-1.85)
Cholesterol:
♀ 1.16, (95% CI, 0.96-1.41)
♂ 0.81, (95% CI, 0.69-0.95) | 0.45
0.06
0.02
0.08 | A | (23) |
| Jiao et al,
2010 | 952,494/2, 639 PDAC
cases | Pooled analysis of
7 prospective cohorts (US, Finland and China). Mean follow-up of
6.9 years | PDAC risk and
BMI
BMI (per 5 kg/m2 increment):
♀ RR, 1.12 (95% CI, 1.05-1.19)
♂ RR, 1.06 (95% CI, 0.99-1.13)
BMI, 30.0-34.9 kg/m2:
♀ RR, 1.34 (95% CI, 1.11-1.64)
♂ RR, 1.11 (95% CI, 0.95-1.30) | N/A for interaction
by sex
N/A for interaction by sex | A | (20) |
Diabetes mellitus
The association between diabetes mellitus and PDAC
appears to be largely independent of sex. While a study has
reported a higher prevalence of PDAC among male diabetic patients
(25), others found no
statistically significant difference in overall PDAC risk between
men and women (25-28). Although sex alone may not
significantly modify PDAC risk in diabetic patients, lifestyle
factors and comorbidity patterns, which often differ by sex, may
still influence overall risk and should be considered in prevention
strategies (26-28).
Alcohol
Heavy alcohol consumption (defined as ≥9 drinks per
day) is associated with an increased risk of PDAC, particularly in
men (29). Several large studies
and pooled analyses have shown that the association between heavy
alcohol intake and PDAC is more pronounced in men than in women
(29-31). However, this apparent sex
difference may partly reflect a statistical limitation: Female
heavy drinkers are often underrepresented in study cohorts,
limiting the ability to accurately assess risk in women (31). Nonetheless, a pooled analysis of
14 prospective cohort studies (n=2,187 PDAC cases) found a
statistically significant association between alcohol intake ≥30
g/day and PDAC in women (RR, 1.41; 95% CI, 1.07-1.85) (30). At even higher intake levels, the
risk was more substantial in men. Notably, the study could not
assess the impact of very high alcohol intake in women due to the
small number of female participants consuming ≥45 g/day (30). In regions with higher
socioeconomic status, a growing trend of alcohol consumption has
been observed among young women (32). If this trend continues, alcohol
may become an important contributor to an increased PDAC incidence
among women in the coming years.
Chronic pancreatitis (CP)
CP significantly increases the risk of PDAC, with a
reported 16- to 22-fold elevation, particularly within the first 2
years following a CP diagnosis (33,34). CP is more commonly diagnosed in
men, who also tend to develop the disease at a younger age compared
with women (33). This male
predominance is largely attributed to higher rates of alcohol
consumption and smoking (33,35). However, to date and to the best of
our knowledge, no significant sex-based differences have been
observed in the magnitude of PDAC risk among patients with CP
(33-35).
Hormonal factors
There is growing evidence suggesting that female sex
hormones may exert a protective effect against the development of
PDAC. However, studies examining hormone-related exposures have
yielded conflicting results (Table
III). A recent systematic review and meta-analysis of 21
observational studies involving 7,700 PDAC cases adds important
context: Ever-use of oral contraceptives (OCs) was associated with
a modest but statistically significant reduction in PDAC risk (RR,
0.85; 95% CI, 0.73-0.98) (36).
This inverse association was particularly evident in high-quality
studies and European cohorts, while no clear effect emerged in
studies from Asia or America (36). Notably, no dose-response
relationship was observed, as PDAC risk did not vary by duration of
OC use (36). Despite notable
heterogeneity, the meta-analysis supports a potential protective
role of exogenous estrogen.
 | Table IIIComparative summary of studies on
female hormonal determinants and PDAC risk. |
Table III
Comparative summary of studies on
female hormonal determinants and PDAC risk.
| First author/s,
year | Number of
patients | Study design | Results | P-value | Evidence
levela | (Refs.) |
|---|
| Ilic et al,
2021 | 21 studies (10
case-control + 11 cohort), 7,700 PDAC cases | Systematic review
and meta-analysis (Europe, America and Asia) | Ever use of OCs:
RR, 0.85 (95% CI, 0.73-0.98) No dose-response relationship for
duration: <1 year: RR, 1.08 (95% CI, 0.91-1.29) <5 years: RR,
1.07 (95% CI, 0.95-1.19) 5-10 years: RR, 1.08 (95% CI,
0.93-1.26) | 0.03
>0.05 for all | A | (36) |
| Andersson et
al, 2018 | 17,035/110 PDAC
cases | Prospective
population-based cohort study (Sweden) | Age at menarche
(continuous): HR, 1.17 (95% CI, 1.04-1.32) Ever use of any HRT: HR,
0.48 (95% CI, 0.23-1.00) Estrogen-only HRT: HR, 0.22 (95% CI,
0.05-0.90) Ever use of OC: HR, 0.68 (95% CI, 0.44-1.06) Parity,
breastfeeding and menopause timing: HR, ~1.00 | 0.008
0.049
0.035
0.091
>0.05 for all | B | (37) |
| Lee et al,
2013 | 118,164/323 PDAC
cases | Prospective cohort
study (USA) | Estrogen-only HRT,
current use: HR, 0.59 (95% CI, 0.42-0.84) Estrogen HRT ≥20 years:
HR, 0.55 (95% CI, 0.33-0.91) OC use ≥10 years: HR, 1.72 (95% CI,
1.19-2.49) High dose OC use ≥10 years: HR, 2.08 (95% CI, 1.05-4.12)
Age at menarche, parity, menopause and breastfeeding HR, ~1.00 | 0.003
0.036
0.014
0.024
>0.05 for all | A | (38) |
| Prizment et
al, 2006 | 37,459/228 PDAC
cases | Prospective
population-based cohort study (USA) | Age at menopause
≥55 vs. <45 years: HR, 0.35 (95% CI, 0.18-0.68) Age at menopause
(continuous, 5-year increase): HR, 0.81 (95% CI, 0.72-0.91) Ever
use of any HRT: HR, 1.07 (95% CI, 0.82-1.40) Ever use of OC: HR,
0.90 (95% CI, 0.62-1.30) Parity, menarche and age at first birth
HR, ~1.00 | 0.005
0.001
0.606
0.566
>0.05 for all | B | (39) |
| Duell and Holly,
2005 | 241 PDAC cases/818
controls | Population-based
case-control study (USA) | Age at menopause
≥55 vs. <45 years: OR, 1.9 (95% CI, 1.2-2.8) Estrogen-only HRT:
HR, 0.84 (95% CI, 0.62-1.1) Ever use of OC: HR, 0.95 (95% CI,
0.65-1.4) | 0.003
>0.05
>0.05 | B | (40) |
| Stevens et
al, 2009 | 995,1957/1,182 PDAC
cases | Prospective cohort
study (UK) | Parity (ever vs.
never): RR, 0.88 (95% CI, 0.79-0.98) Age at menarche, age at birth,
age at menopause, breastfeeding and number of children | 0.01
>0.05 for all | A | (41) |
In this context, findings from individual studies
illustrate the variability across hormone-related exposures.
Several studies investigating exogenous hormone therapy (HRT)
reported reduced PDAC risk: A Swedish cohort study of 17,035 women
linked high estrogen levels and HRT use to lower PDAC risk
(37), and another large cohort
likewise found a protective association with estrogen therapy
(38). However, these results
were not replicated in an American cohort of 37,459 women, in which
HRT showed no association with PDAC risk (39).
Studies assessing endogenous estrogen exposure show
similar inconsistency. In the American cohort, later menopause was
related to a lower PDAC risk and a significant inverse relationship
was observed between age at menopause and risk, suggesting a
protective effect of longer endogenous estrogen exposure (39). However, a separate case-control
study reported the opposite: An increased PDAC risk was associated
with later menopause (40). To
date and to the best of our knowledge, no consistent association
has been observed between PDAC risk and other reproductive factors
such as parity (number of children) or breastfeeding (37-39,41). In conclusion, current data suggest
that higher lifetime estrogen exposure may reduce PDAC risk, though
results are inconsistent and often lose significance after
adjustment, indicating potential residual confounding or
differences in population characteristics, hormone formulations or
follow-up duration. Overall, evidence is moderately strong but
lacks consistency and mechanistic clarity. While estrogen may have
a protective role, current data are insufficient to confirm
causality, warranting further research integrating hormonal and
genetic markers.
Pharmacokinetics and toxicity of
chemotherapy
Chemotherapeutic drugs are commonly prescribed at
standardized doses based on body weight or body surface area,
although therapeutic efficacy is more closely related to
circulating drug concentrations than to the administered dose
(6). As a result, interindividual
variations in drug metabolism can lead to suboptimal outcomes,
including reduced efficacy or increased toxicity (6). Pharmacokinetics and pharmacodynamics
differ between the sexes, yet this is often overlooked in clinical
dosing strategies. Factors such as higher body fat percentage in
women (42), greater renal
function in men (6), and
differences in muscle mass, total body water, cardiac output, and
liver perfusion (4) all influence
drug distribution and metabolism. A recent systematic review
identified significant sex-specific pharmacokinetic differences for
several drugs, including 5-fluorouracil (5-FU) and paclitaxel, both
of which are integral components of standard PDAC treatment
regimens (4,43). 5-FU, a key drug in the FOLFIRINOX
regimen (leucovorin, 5-FU, irinotecan, and oxaliplatin) (43), is cleared >20% more rapidly in
men than in women. Consequently, equivalent dosing may lead to
increased toxicity in women and a potential risk of underdosing in
men (44). Notably, this sex
difference in clearance remains significant even after adjusting
for age and dose (45). The
difference may be partially explained by sex-specific variation in
the activity of dihydropyrimidine dehydrogenase, the primary enzyme
responsible for metabolizing 5-FU (45). In addition, genetic variants of
methylenetetrahydrofolate reductase, an enzyme that influences 5-FU
cytotoxicity, have demonstrated sex-dependent effects, suggesting
that female patients may require lower 5-FU doses to avoid toxicity
(42). In line with these
findings, a study on colorectal cancer has shown that women
experience a significantly higher rate of side effects from
5-FU-based therapies, both in the adjuvant and metastatic settings
(4). In the treatment of
colorectal cancer, increased toxicity has been reported not only in
combination regimens but also with 5-FU monotherapy (4).
Sex-related pharmacokinetic differences for
oxaliplatin are inconsistent. While a study has reported a 40%
lower clearance in women (46),
another found no significant sex-based differences (47). Data on irinotecan are similarly
mixed. Certain studies suggest that women may have lower clearance,
reduced volume of distribution and decreased bioavailability,
potentially influencing both efficacy and toxicity (48,49). However, another investigation
attributed this variability primarily to liver function, with
minimal differences attributable to sex (50). Although 5-FU, irinotecan, and
oxaliplatin are administered in combination in PDAC treatment, the
interactions and cumulative pharmacokinetic effects of these drugs
remain poorly understood. Although pharmacokinetic findings imply
differing toxicity risks, few studies have systematically evaluated
chemotherapy-related adverse events in PDAC by sex. Existing data
are often limited by small sample sizes or single-center designs.
Some report higher toxicity rates in women, such as constipation,
hand-foot syndrome and epigastric pain (51), and an increased incidence of
febrile neutropenia and agranulocytosis during FOLFIRINOX treatment
(52). Others, including a
prospective Korean study, found no significant overall differences
but noted more frequent dose reductions among female patients
(53), suggesting lower tolerance
or heightened susceptibility to toxicity.
Gemcitabine, either as a monotherapy or in
combination with nab-paclitaxel, is another well-established PDAC
treatment regimen for PDAC. To date and to the best of our
knowledge, no clinical evidence supports a sex-specific difference
in gemcitabine pharmacokinetics (54,55). While sex has been shown to
influence the clearance and toxicity of solvent-based paclitaxel
(56,57), this effect is not observed with
nab-paclitaxel, which displays no sex-related differences in
elimination (58), suggesting a
distinct pharmacological mechanism.
In summary, pharmacokinetic studies of 5-FU,
irinotecan and oxaliplatin suggest that standardized dosing does
not adequately account for sex-specific variability, with men at
risk of potential underdosing and women at higher risk of toxicity.
Major PDAC trials rarely stratify adverse events by sex,
underscoring the need for comprehensive, sex-disaggregated toxicity
analyses. Addressing this through novel therapy concepts is
essential to optimize therapeutic efficacy, minimize adverse events
and advance sex-sensitive treatment strategies in pancreatic
cancer.
Prognosis and therapy response
Few studies, albeit with large populations, have
explored sex differences in treatment allocation and prognostic
outcomes in PDAC. Notably, the Dutch Pancreatic Cancer Group
conducted two large retrospective studies analyzing sex-based
differences in tumor characteristics, treatment decisions, and
survival outcomes in both stage I-III and metastatic PDAC cohorts,
each including >6,000 patients (59,60). In both cohorts, female patients
were older, had worse performance status but fewer comorbidities
and were less likely to receive systemic chemotherapy compared with
male patients. The primary reason for this disparity was a higher
preference among women for best supportive care across all disease
stages. No sex-based difference was observed in the likelihood of
undergoing surgical resection in resectable PDAC, except among
women aged ≥80 years, who were less frequently offered surgery
(59,60). By contrast, two additional studies
reported a lower likelihood of curative treatment planning for
female patients. The first was a preliminary analysis of 100
patients from the Chemotherapy, Host Response and Molecular
dynamics in Periampullary cancer (CHAMP) study, which includes both
pancreatic and periampullary adenocarcinomas. The second was a
nationwide Swedish cohort study of 5,677 patients with
periampullary tumors, which similarly reported that fewer women
were planned for curative treatment (61,62). However, in the Swedish cohort,
this difference disappeared after adjustment for age and tumor
location, unlike in the CHAMP study. This discrepancy may be
explained by the inclusion of other periampullary tumors, such as
duodenal adenocarcinomas, which have different surgical and
prognostic profiles and may influence treatment decisions. The
largest study to date, conducted by the American College of
Surgeons and including 22,993 patients, identified male sex as a
risk factor for major morbidity following pancreaticoduodenectomy
(63). These findings highlight
that sex disparities in treatment outcomes are not unequivocal and
must be interpreted with caution. Most available studies are
retrospective and heterogeneous in design, with varying inclusion
criteria, treatment eras and adjustment for confounders, which
limits the strength of causal inference. Observed associations
between sex and prognosis in PDAC likely reflect the interplay of
multiple factors, including age, performance status, comorbidities,
socioeconomic status, access to specialized care and patient
preferences, rather than biological sex alone. Few studies have
stratified analyses or applied sensitivity models to disentangle
these sociocultural and clinical variables from intrinsic
biological differences. Nevertheless, several studies have
identified female sex as an independent prognostic factor for
improved overall survival (OS) in PDAC (59,60,64). Large cohort studies have reported
a statistically significant increase, or at least a trend toward,
increased OS in women, even after adjusting for confounders.
However, in the end, only two studies have directly examined sex
differences in response to FOLFIRINOX. A small cohort study by
Hohla et al (65), which
included 49 patients with unresectable PDAC, found a significantly
higher disease control rate in female patients treated with
FOLFIRINOX compared with males. Similarly, the PRODIGE 4/ACCORD 11
randomized trial observed a trend toward longer median OS in female
patients with metastatic disease receiving FOLFIRINOX, although
this did not reach statistical significance (64). These findings again raise
important questions about whether differences in chemotherapy
toxicity and metabolism contribute to improved survival outcomes in
women, underscoring the need for prospective studies with
sex-stratified analyses and standardized reporting to improve the
assessment of the biological vs. sociocultural determinants of
treatment response in PDAC (3).
Immune response and the tumor
microenvironment (TME)
Sex-based biological differences, particularly
within the immune system, are well recognized across multiple
diseases, including cancer (66-68). Generally, women exhibit stronger
innate and adaptive immune responses, which has been linked to a
higher prevalence of autoimmune disorders in women (66,67). The immune differences in men and
women are regulated by both gonadal hormones, primarily estrogen
and androgens and genetic mechanisms (66,69). Notably, stronger interferon
signaling in women may result from two key mechanisms: i)
Estrogen-mediated immune activation; and ii) X chromosome-linked
differences. Estrogen enhances interferon production through
Toll-like receptor 7 (TLR7) activation (70,71), and since TLR7 is encoded on the X
chromosome, its expression and downstream immune responses are
amplified in women (72). In
addition, T and B cells in women demonstrate enhanced signaling and
antibody responses to infections and vaccinations (67,73), which may contribute to more
effective tumor immune surveillance. Estrogen further shapes the
TME by modulating T cell activation and immune checkpoint
expression (67,68,74). Across several cancer entities, the
TMEs in women tend to display stronger immune activation than those
of men (68,75).
In PDAC, the TME is a key contributor to immune
evasion and poor prognosis; it is typically characterized by scarce
cytotoxic T cell infiltration, abundant regulatory T cells and a
dense, fibrotic stroma, which are features that also limit the
response to immunotherapy (76,77). As a result, checkpoint inhibitors
have shown limited efficacy in PDAC due to the inherently
immunosuppressive nature of the tumor (78). Nevertheless, emerging evidence
indicates that sex differences do influence immune pathways and the
TME in PDAC. For example, He et al (79) identified a typically
immunosuppressive subpopulation of formyl peptide receptor 2 (+) M2
macrophages that is specifically enriched in PDAC lesions from
female patients. These tumor associated macrophages (TAMs), induced
by estrogen, were associated with T cell exhaustion, reduced immune
cell infiltration and poorer survival in women, suggesting a
sex-specific immunosuppressive mechanism. In contrast to these
findings, a recent study reported that female patients with PDAC
exhibit higher levels of stromal biomarkers and increased tumor
stiffness, both of which were associated with longer survival
(80). The researchers further
showed that estrogen signaling shapes the TME by driving
cancer-associated fibroblasts toward a more tumor-suppressive
phenotype (80). However, these
estrogen-related effects were mediated not by systemic hormones but
by locally produced estrogens within the tumor tissue and therefore
do not fully explain the survival differences observed between
women and men (80). In another
study investigating patients with treatment-naive PDAC, women
exhibited elevated circulating levels of C-X-C motif chemokine
ligand (CXCL)9, IL-1β, IL-6, IL-10 and IL-13, supporting the notion
of enhanced systemic immune activation in women (81).
Sex differences may also impact immune responses to
chemotherapy. In a recent study by van Eijck et al (82), female sex was an independent
prognostic factor for 5-year OS in patients with localized PDAC
treated with neoadjuvant gemcitabine-based chemotherapy followed by
surgery (43% OS in females vs. 22% in males). Notably, after
neoadjuvant therapy, the TME in female patients showed fewer
immunosuppressive M2 macrophages (CD163+MRC1) and a
distinct transcriptomic profile with higher expression of
CXCL10 and CXCL11 and lower levels of CCL2 and
IL-34, suggesting an enhanced antitumor immune response
(82). Although data on sex
differences in immune responses in PDAC remain limited and
potentially confounded by complex interactions among hormones,
drugs and genetics, current evidence suggests that women may
exhibit a more immunosuppressive TME characterized by a higher
abundance of estrogen-influenced immunosuppressive macrophages,
contrary to the expected higher immune activation in women.
Notably, gemcitabine appears to induce greater macrophage
plasticity in women, indicating a potential pathway for immune
activation. Together, these observations highlight that sex and sex
hormones uniquely shape immune activity in the TME and modulate
responses to chemotherapy. Fig. 1
summarizes sex-related hormonal and genetic differences in PDAC,
highlighting the distinct roles of estrogens and androgens in
shaping tumor biology, immune responses and the TME in women and
men. Although immune-targeted therapies have shown limited success
in PDAC, emerging approaches such as neoantigen vaccination show
promise and may enhance immune-based treatment strategies (83). Accounting for sex-specific immune
regulation will therefore be critical as immunotherapies for PDAC
continue to advance.
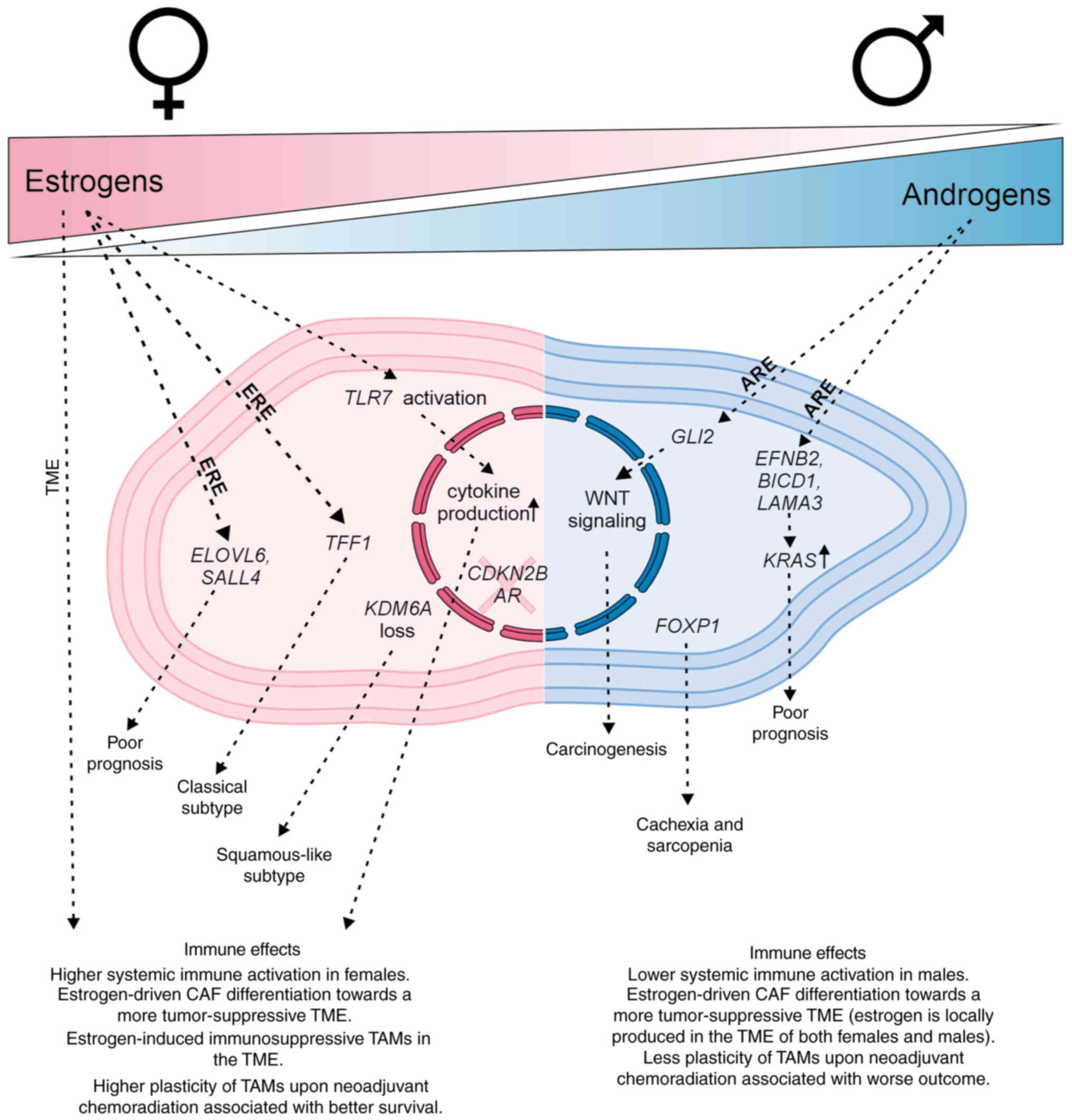 | Figure 1Overview of sex-related hormonal and
genetic differences for PDAC. Estrogens significantly shape immune
functions, including innate immune responsiveness through TLRs and
the regulation of cytokine production. In PDAC, estrogens influence
transcriptional subtypes and further modulate the TME by promoting
the differentiation of CAFs toward a more tumor-suppressive
phenotype. Conversely, estrogens can also affect TAMs, driving them
toward a more immunosuppressive state. Notably, TAMs in women show
greater plasticity following neoadjuvant chemoradiation with
gemcitabine, which is associated with improved survival. By
contrast, androgens are linked to carcinogenesis and cachexia.
Notably, the effects of estrogens are not restricted to women; men
can also produce estrogen locally within the tumor tissue,
similarly promoting CAF differentiation toward a more
tumor-suppressive TME. PDAC, pancreatic ductal adenocarcinoma; TME,
tissue microenvironment; ERE, estrogen response element; ARE,
androgen response element; AR, androgen receptor; TAM,
tumor-associated macrophage; CAF, cancer-associated fibroblasts;
TLR, Toll-like receptor; CDKN2B, cyclin-dependent kinase inhibitor
2B; TFF1, trefoil factor 1; KDM6A, lysine demethylase 6A; GLI2,
zinc finger protein 2; EFNB2, ephrin B2; BICD1, bicaudal D cargo
adaptor 1; LAMA3, laminin α3; ELOVL6,
elongation-of-very-long-chain-fatty acids 6; SALL4, Sal-like
protein 4. |
Tumor microbiota
Research on tumor microbiota has provided compelling
insights into its role in modulating the TME (84) and shaping systemic immune
responses, thereby influencing various diseases (85). In PDAC, the intratumoral
microbiota significantly impacts prognosis and therapeutic
efficacy, most notably by altering the TME (86), influencing tumor phenotype
(87), and degrading
chemotherapeutic agents such as gemcitabine (88,89). However, the influence of sex on
the tumor microbiota remains unclear. To date, few studies have
assessed bacterial species distribution by sex in patients with
PDAC and none have reported significant differences (86,90). This is further supported by
comparable rates of bacterial colonization in tumor tissue across
sexes (91), as well as a similar
prevalence of intratumoral gram-negative bacteria in male and
female patients (89). Although
it should be noted that none of the studies chose the investigation
of sex-specific differences as their primary endpoint.
Nonetheless, sex-specific differences in the oral
and gut microbiota have been reported in patients with PDAC
compared with healthy controls (92). These differences are also evident
in murine models, where a recent study identified increased
abundance of Ligilactobacillus and Acetatifactor in
female mice with PDAC (93).
While intriguing, the implications of these findings for immune
modulation or tumor progression remain unclear. Further
illustrating the role of microbiota in therapy, antibiotic
administration has been shown to enhance gemcitabine efficacy in
advanced PDAC (94), although
this effect was not associated with patient sex. While current
evidence does not show consistent sex-based differences in the
tumor microbiota of PDAC, emerging findings in gut and oral
microbiomes, as well as in preclinical models, underscore the need
for further investigation. Understanding how sex may modulate the
microbiome-immune-tumor axis could open new avenues for
personalized therapeutic strategies.
Biomarkers and genetics
Serum Biomarkers
The most commonly used biomarker for PDAC is
carbohydrate antigen 19-9 (CA19-9), although its specificity and
sensitivity remain limited (95).
Few studies have examined sex-specific differences and results are
inconsistent. A study reported more men (77.3%) with elevated
CA19-9 serum levels than women (66.7%) (96), although the clinical significance
of this difference remains unclear. However, several novel
biomarkers that are not used in clinical practice have shown
sex-based differences in expression patterns. For example, tissue
inhibitor of metalloproteinase 1 (TIMP1) has been shown to be
associated with liver metastases and poorer survival specifically
in male patients, a finding supported by mouse models showing
earlier metastasis with higher TIMP1 levels in males (97). In addition, thymidine kinase (TK)
levels have been reported to be higher in men: In one study, 21 men
vs. 9 women had TK levels above the median, while low TK levels
showed no sex difference (98).
Moreover, the serum marker adiponectin has been shown to be
associated with shorter survival in female patients only (99). These findings indicate that
certain biomarkers may have sex-specific prognostic value, although
none are currently used in clinical routine, to the best of our
knowledge. While some sex-related differences have been reported,
most data remain exploratory and lack consistent validation. The
continued absence of reliable biomarkers in PDAC remains a major
challenge for early detection and personalized therapy,
underscoring the need for large, well-designed studies that
systematically incorporate sex as a biological variable.
Genetic alterations
The four major driver mutations in PDAC affecting
KRAS, CDKN2A, TP53 and SMAD4 occur at similar
frequencies in both male and female patients (100,101). However, other tumor-related
genes show sex-specific mutation patterns; for example, alterations
in CDKN2B and the androgen receptor (AR) gene are
more frequently observed in female patients (100). Additionally, AR and estrogen
receptor binding sites in promoter regions contribute to distinct
sex-specific gene expression patterns in PDAC (102). A study found that among all
upregulated genes in male PDAC samples, 24 contained androgen
response elements (AREs), including EFNB2, BICD1 and
LAMA3, which strongly correlate with KRAS expression,
as well as GLI2, a key transcription factor involved in
carcinogenesis. These ARE-regulated genes in men are enriched in
tumor-related pathways, such as axon guidance and extracellular
matrix components, and are associated with poorer survival. By
contrast, among all genes upregulated in female PDAC samples
compared with normal tissue, only 3 genes, ELOVL6,
SALL4 and TFF1, are upregulated by estrogen response
elements. Overall, this suggests that AR-driven transcription plays
a more dominant and functionally relevant role in male PDAC biology
(102), while these sex-specific
molecular effects do not appear to be linked to differential
activity in the estrogen or pregnenolone pathways (103). Furthermore, no sex-specific
differences were described for single nucleotide polymorphisms
(SNPs) of DNA repair genes (104), although other SNPs had
sex-specific effects. For instance, the leptin receptor SNP
rs11585329 is associated with improved survival in men (99), while the APC I1307K variant
correlates with improved outcomes in women (74). While these findings expand our
understanding of sex-specific genomic features in PDAC, most
evidence is derived from retrospective or database-driven analyses
with limited functional validation. Linking these alterations to
biological function and therapeutic vulnerability will be crucial
to translate genomic sex differences into clinical relevance.
Transcriptional and epigenetic
regulation
Notably, the expression of certain transcription
factors contributes to sex-specific differences in tumor biology.
For example, the transcription factor Kaiso has been linked to
increased tumor invasiveness and poorer prognosis in male patients
(105). Additionally, the
transcription factors FoxP1 and activin have been associated with
the development of cachexia and sarcopenia, which appear to affect
male patients with PDAC more severely (106,107). However, findings on sex-specific
molecular tumor subtypes based on transcription profiles remain
inconsistent. Some studies suggest a higher prevalence of the more
aggressive quasi-mesenchymal or basal-like subtypes in men with
resected PDAC, potentially explaining poorer outcomes (108,109). By contrast, other studies report
no significant sex-based differences in gene expression signatures
used to predict treatment response to gemcitabine and FOLFIRINOX
(110,111). In the realm of epigenetics,
evidence indicates that loss of the histone demethylase, lysine
demethylase 6A (KDM6A), induces a squamous-like, metastatic PDAC
subtype specifically in female patients (112). In mouse models, male
Kdm6a-knockouts retained cancer protection through UTY, a
Y-chromosome-encoded homolog of Kdm6a, suggesting a sex-specific
regulation of chromatin remodeling (112). Hence, current evidence on
transcriptional and epigenetic regulation indicate a potential
impact of sex-differences, although data are scarce. Moreover,
variable study designs and limited validation contribute to
conflicting results. More specific approaches focusing on
sex-differences will be essential to clarify whether currently
described data represent true biological mechanisms or
context-dependent findings.
Histopathology and tissue biomarkers
Thus far and to the best of our knowledge, there is
no evidence that histopathological features, such as gland
formation, stromal density or entosis differ significantly between
male and female patients with PDAC (113-115). Similarly, the expression of
multiple diagnostic tissue biomarkers for PDAC, including CK7,
mucin-1 and SMAD4, does not show any sex-related variation in
histopathological examinations (Table IV). Due to retrospective
analyses, these results must be interpreted with caution. Up to
now, to the best of our knowledge, no clinically validated
sex-specific biomarkers for PDAC exist. Future studies
incorporating sex as a biological variable, particularly through
multi-omics approaches, are essential to uncover novel,
personalized biomarkers in PDAC.
 | Table IVCurrent evidence on biomarkers in
pancreatic ductal adenocarcinoma: Sex-specific differences. |
Table IV
Current evidence on biomarkers in
pancreatic ductal adenocarcinoma: Sex-specific differences.
| First author,
year | Marker | Number of
patients | Clinical
situation | Detection
method | Results | Evidence
levela | (Refs.) |
|---|
| Song et al,
2022 | CK7 | 55 | Resected | IHC | No significant sex
difference | B | (116) |
| Xiong et al,
2022 | CK8 | 176 | Resected | mRNA
expression | No significant sex
difference | B | (117) |
| Qian et al,
2022 | MUC1 | 420 | Resected | IHC | No significant sex
difference | B | (118) |
| Ermiah et
al, 2022 | CEA | 123 | Resected and
advanced |
Electrochemiluminescence immunoassay | No significant sex
difference | B | (119) |
| Ermiah et
al, 2022 | CA19-9 | 123 | Resected and
advanced |
Electrochemiluminescence immunoassay | No significant sex
difference | B | (119) |
| Qian et al,
2022 | MUC5AC | 407 | Resected | IHC | No significant sex
difference | B | (118) |
| Sumiyoshi et
al, 2024 | CA19-9/DUPAN2 | 521 | Resected | Serum level | No significant sex
difference | B | (120) |
| Liang et al,
2017 | CA 125/ MUC16 | 160 | Resected | mRNA
expression | No significant sex
difference | B | (121) |
| Liang et al,
2017 | CA125/ MUC16 | 110 | Resected | IHC | No significant sex
difference | B | (121) |
| Xiao et al,
2014 | CDX2 | 61 | Resected | IHC | No significant sex
difference | B | (122) |
| Amal et al,
2024 | SMAD4 | 60 | Resected and
advanced | IHC | No significant sex
difference | B | (123) |
| Amal et al,
2024 | S100P | 60 | Resected and
advanced | IHC | No significant sex
difference | B | (123) |
Discussion and future perspectives
PDAC exhibits clear sex-based differences across
multiple dimensions, including incidence, risk factors, tumor
biology, immune response and treatment outcomes. While men have
historically shown higher incidence and mortality rates, the burden
of disease is rising disproportionately in women, particularly in
younger women, underscoring the importance of sex-specific
research. Rising pancreatic cancer rates in young women likely
result from a combination of sex-based biological vulnerability and
gender-related behavioral patterns. Biologically, women appear more
susceptible to the carcinogenic effects of obesity, central fat
distribution and early-life smoking. Socially and behaviorally,
earlier smoking initiation, rising obesity rates and increasing
risky alcohol consumption intensify exposure to these factors.
These combined dynamics highlight the need for targeted, sex- and
gender-specific risk-prevention strategies.
Biological sex influences not only disease
susceptibility but also chemotherapy pharmacokinetics, toxicity
profiles, immune responses, and potentially tumor progression
pathways. Women often experience greater treatment-related
toxicity, while men may face subtherapeutic dosing, indicating that
standardized treatment regimens may inadequately account for
sex-based variability. In this context, existing therapeutic
drug-monitoring approaches for agents such as 5-FU and irinotecan
represent an important but underused opportunity; when applied
consistently, these tools could facilitate more precise dose
optimization for both sexes and help mitigate sex-related
differences in exposure and toxicity. In addition, gender and
sociocultural factors influence how cancer treatment is received
and tolerated, yet they remain largely unaddressed in research and
clinical practice. Despite accumulating evidence, most large
clinical trials and translational studies still fail to stratify or
analyze outcomes by sex, leaving a critical gap in our ability to
personalize therapy.
To advance toward precision medicine in PDAC, sex
must be systematically incorporated as a biological variable at
every stage of research and clinical development. For instance,
future PDAC trials should include sex as a predefined
stratification factor or covariate in randomization and outcome
analyses, ensuring adequate statistical power for sex-specific
endpoints. Adaptive trial designs could prospectively test
sex-based differences in efficacy or toxicity. Pharmacokinetic and
pharmacogenomic studies should explicitly evaluate sex differences
in drug metabolism, immune response, tolerance and
exposure-response associations to guide individualized dosing
strategies. Furthermore, translational studies should report
biomarker performance and prognostic value separately for men and
women, to identify sex-specific predictive signatures of response
or resistance. Finally, in preclinical models, the development of
sex-balanced patient-derived organoids and murine models should be
considered for dissecting sex-dependent mechanisms and therapeutic
vulnerabilities (Fig. 2).
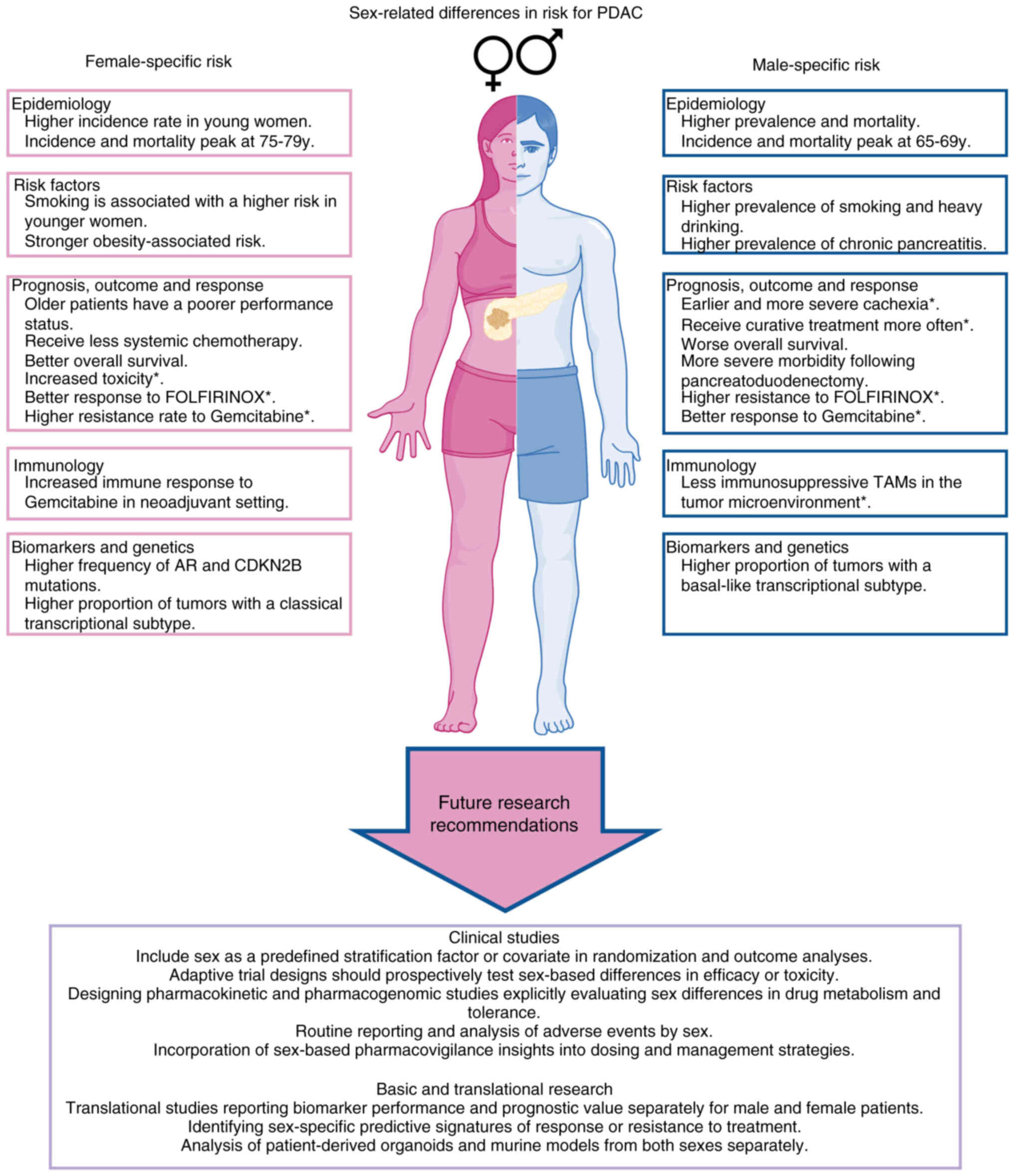 | Figure 2Structured overview of sex-related
differences in PDAC. The left panel summarizes aspects associated
with female patients, including epidemiological patterns, relevant
risk factors, prognostic considerations, immune-related features
and molecular characteristics. The right panel presents the
corresponding male-associated domains, following the same thematic
categories for direct comparison. The lower section synthesizes
overarching implications for future research, highlighting the need
to integrate sex as a biological variable in study design and
translational investigations. *trends have been shown,
but further studies are needed. PDAC, pancreatic ductal
adenocarcinoma; TAM, tumor-associated macrophage; AR, androgen
receptor; CDKN2B, cyclin-dependent kinase inhibitor 2B. |
It should be acknowledged that current evidence is
largely derived from retrospective analyses with heterogeneous
metho dologies and limited adjustment for confounders.
Nevertheless, the present review highlights that sex-stratified
data remain underreported in PDAC trials, and that preclinical
systems rarely account for hormonal or chromosomal sex effects.
Addressing these limitations will require coordinated efforts to
standardize sex reporting, design prospective studies with balanced
enrollment and integrate multi-omic datasets to unravel biological
from sociocultural determinants of outcome. In conclusion,
addressing sex disparities in PDAC is not merely a scientific
objective but a prerequisite for equitable precision oncology.
Incorporating sex-based insights into risk assessment, biomarker
discovery and therapeutic design will be essential to optimize
treatment efficacy and minimize toxicity, ultimately moving toward
more personalized and effective care for all patients with
PDAC.
Availability of data and materials
Not applicable.
Authors' contributions
All authors contributed to the conception, drafting
and critical revision of this review. SR, EOG, LAM, DKZ and MG
performed the primary literature search and SR and EOG drafted the
initial version of the manuscript. IR, JML and DÖ served as mentors
and provided guidance, supervision and critical feedback throughout
the preparation of the manuscript. SR and MG designed and prepared
the tables and figures. DKZ, MG and LAM revised and finalized
individual sections of the manuscript. All authors reviewed and
edited the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCIDs: Sophie Rauschenberg, 0009-0004-3408-1862;
Elisabeth Orgler-Gasche, 0000-0003-1904-2553; Didem Karakas Zeybek,
0000-0002-3781-6834; Ivonne Regel, 0000-0002-0206-4441; J.-Matthias
Löhr, 0000-0002-7647-198X; Daniel Öhlund, 0000-0002-5847-2778;
Michael Günther, 0009-0001-5091-5060; Lina Aguilera Munoz,
0000-0002-6317-8725.
Acknowledgments
This review was conducted as part of the
Pancreas2000 program, the postgraduate educational program of the
European Pancreas Club (EPC).
Funding
Not applicable.
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2025. American Cancer Society; Atlanta: 2025
|
|
2
|
Kaufman MR, Eschliman EL and Karver TS:
Differentiating sex and gender in health research to achieve gender
equity. Bull World Health Organ. 101:666–671. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mauvais-Jarvis F, Bairey Merz N, Barnes
PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN,
Govindan R, Klein SL, et al: Sex and gender: Modifiers of health,
disease, and medicine. Lancet. 396:565–582. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delahousse J, Wagner AD, Borchmann S,
Adjei AA, Haanen J, Burgers F, Letsch A, Quaas A, Oertelt-Prigione
S, Özdemir BC, et al: Sex differences in the pharmacokinetics of
anticancer drugs: A systematic review. ESMO Open. 9:1040022024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sosinsky AZ, Rich-Edwards JW, Wiley A,
Wright K, Spagnolo PA and Joffe H: Enrollment of female
participants in United States drug and device phase 1-3 clinical
trials between 2016 and 2019. Contemp Clin Trials. 115:1067182022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner AD, Oertelt-Prigione S, Adjei A,
Buclin T, Cristina V, Csajka C, Coukos G, Dafni U, Dotto GP,
Ducreux M, et al: Gender medicine and oncology: Report and
consensus of an ESMO workshop. Ann Oncol. 30:1914–1924. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SE, Paik HY, Yoon H, Lee JE, Kim N and
Sung MK: Sex- and gender-specific disparities in colorectal cancer
risk. World J Gastroenterol. 21:5167–5175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdel-Rahman O: Impact of sex on
chemotherapy toxicity and efficacy among patients with metastatic
colorectal cancer: Pooled analysis of 5 randomized trials. Clin
Colorectal Cancer. 18:110–115 e2. 2019.PubMed/NCBI
|
|
9
|
Ferlay JEM, Lam F, Laversanne M, Colombet
M, Mery L, Piñeros M, Znaor A, Soerjomataram I and Bray F: Global
Cancer Observatory: Cancer Today. International Agency for Research
on Cancer; Lyon: Available from: https://gco.iarc.who.int/today2024.
|
|
10
|
Khalaf N, El-Serag HB, Abrams HR and
Thrift AP: Burden of pancreatic cancer: From epidemiology to
practice. Clin Gastroenterol Hepatol. 19:876–884. 2021. View Article : Google Scholar
|
|
11
|
Wang S, Zheng R, Li J, Zeng H, Li L, Chen
R, Sun K, Han B, Bray F, Wei W and He J: Global, regional, and
national lifetime risks of developing and dying from
gastrointestinal cancers in 185 countries: A population-based
systematic analysis of GLOBOCAN. Lancet Gastroenterol Hepatol.
9:229–237. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
GBD 2017 Pancreatic Cancer Collaborators:
The global, regional, and national burden of pancreatic cancer and
its attributable risk factors in 195 countries and territories,
1990-2017: A systematic analysis for the Global Burden of disease
study 2017. Lancet Gastroenterol Hepatol. 4:934–947. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cavazzani A, Angelini C, Gregori D and
Cardone L: Cancer incidence (2000-2020) among individuals under 35:
An emerging sex disparity in oncology. BMC Med. 22:3632024.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Lok V, Ngai CH, Zhang L, Yuan J,
Lao XQ, Ng K, Chong C, Zheng ZJ and Wong MCS: Worldwide Burden of,
risk factors for, and trends in pancreatic cancer.
Gastroenterology. 160:744–754. 2021. View Article : Google Scholar
|
|
15
|
ECIS-European Cancer Information System.
2025, Available from: https://ecis.jrc.ec.europa.eu/explorer.php.
|
|
16
|
Lynch SM, Vrieling A, Lubin JH, Kraft P,
Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross
M, et al: Cigarette smoking and pancreatic cancer: A pooled
analysis from the pancreatic cancer cohort consortium. Am J
Epidemiol. 170:403–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gram IT, Park SY, Wilkens LR, Le Marchand
L and Setiawan VW: Smoking and pancreatic cancer: A sex-specific
analysis in the Multiethnic Cohort study. Cancer Causes Control.
34:89–100. 2023.
|
|
18
|
Bosetti C, Lucenteforte E, Silverman DT,
Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH, et
al: Cigarette smoking and pancreatic cancer: An analysis from the
International pancreatic cancer case-control consortium (Panc4).
Ann Oncol. 23:1880–1888. 2012. View Article : Google Scholar :
|
|
19
|
Koyanagi YN, Ito H, Matsuo K, Sugawara Y,
Hidaka A, Sawada N, Wada K, Nagata C, Tamakoshi A, Lin Y, et al:
Smoking and pancreatic cancer incidence: A pooled analysis of 10
population-based cohort studies in Japan. Cancer Epidemiol
Biomarkers Prev. 28:1370–1378. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiao L, Berrington de Gonzalez A, Hartge
P, Pfeiffer RM, Park Y, Freedman DM, Gail MH, Alavanja MC, Albanes
D, Beane Freeman LE, et al: Body mass index, effect modifiers, and
risk of pancreatic cancer: A pooled study of seven prospective
cohorts. Cancer Causes Control. 21:1305–1314. 2010.PubMed/NCBI
|
|
21
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arslan AA, Helzlsouer KJ, Kooperberg C,
Shu XO, Steplowski E, Bueno-de-Mesquita HB, Fuchs CS, Gross MD,
Jacobs EJ, Lacroix AZ, et al: Anthropometric measures, body mass
index, and pancreatic cancer: A pooled analysis from the pancreatic
cancer cohort consortium (PanScan). Arch Intern Med. 170:791–802.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johansen D, Stocks T, Jonsson H, Lindkvist
B, Bjorge T, Concin H, Almquist M, Häggström C, Engeland A, Ulmer
H, et al: Metabolic factors and the risk of pancreatic cancer: A
prospective analysis of almost 580,000 men and women in the
Metabolic Syndrome and cancer project. Cancer Epidemiol Biomarkers
Prev. 19:2307–2317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Genkinger JM, Kitahara CM, Bernstein L,
Berrington de Gonzalez A, Brotzman M, Elena JW, Giles GG, Hartge P,
Singh PN, Stolzenberg-Solomon RZ, et al: Central adiposity, obesity
during early adulthood, and pancreatic cancer mortality in a pooled
analysis of cohort studies. Ann Oncol. 26:2257–2266. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao Y, Tao M, Jia X, Xu H, Chen K, Tang H
and Li D: Effect of diabetes mellitus on survival in patients with
pancreatic cancer: A systematic review and meta-analysis. Sci Rep.
5:171022015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mellenthin C, Balaban VD, Dugic A and
Cullati S: Risk factors for pancreatic cancer in patients with
new-onset diabetes: A systematic review and meta-analysis. Cancers
(Basel). 14:46842022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song S, Wang B, Zhang X, Hao L, Hu X, Li Z
and Sun S: Long-term diabetes mellitus is associated with an
increased risk of pancreatic cancer: A meta-analysis. PLoS One.
10:e01343212015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huxley R, Ansary-Moghaddam A, Berrington
de Gonzalez A, Barzi F and Woodward M: Type-II diabetes and
pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer.
92:2076–2083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lucenteforte E, La Vecchia C, Silverman D,
Petersen GM, Bracci PM, Ji BT, Bosetti C, Li D, Gallinger S, Miller
AB, et al: Alcohol consumption and pancreatic cancer: A pooled
analysis in the International pancreatic cancer case-control
consortium (PanC4). Ann Oncol. 23:374–382. 2012. View Article : Google Scholar
|
|
30
|
Genkinger JM, Spiegelman D, Anderson KE,
Bergkvist L, Bernstein L, van den Brandt PA, English DR,
Freudenheim JL, Fuchs CS, Giles GG, et al: Alcohol intake and
pancreatic cancer risk: A pooled analysis of fourteen cohort
studies. Cancer Epidemiol Biomarkers Prev. 18:765–776. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiao L, Silverman DT, Schairer C, Thiebaut
AC, Hollenbeck AR, Leitzmann MF, Schatzkin A and
Stolzenberg-Solomon RZ: Alcohol use and risk of pancreatic cancer:
The NIH-AARP Diet and Health Study. Am J Epidemiol. 169:1043–1051.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arab JP, Dunn W, Im G and Singal AK:
Changing landscape of alcohol-associated liver disease in younger
individuals, women, and ethnic minorities. Liver Int. 44:1537–1547.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Santos R, Coleman HG, Cairnduff V and
Kunzmann AT: Clinical prediction models for pancreatic cancer in
general and at-risk populations: A systematic review. Am J
Gastroenterol. 118:26–40. 2023. View Article : Google Scholar
|
|
34
|
Gandhi S, de la Fuente J, Murad MH and
Majumder S: Chronic pancreatitis is a risk factor for pancreatic
cancer, and incidence increases with duration of disease: A
systematic review and meta-analysis. Clin Transl Gastroenterol.
13:e004632022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kirkegard J, Mortensen FV and
Cronin-Fenton D: Chronic pancreatitis and pancreatic cancer risk: A
systematic review and meta-analysis. Am J Gastroenterol.
112:1366–1372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ilic M, Milicic B and Ilic I: Association
between oral contraceptive use and pancreatic cancer risk: A
systematic review and meta-analysis. World J Gastroenterol.
27:2643–2656. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Andersson G, Borgquist S and Jirstrom K:
Hormonal factors and pancreatic cancer risk in women: The Malmo
diet and cancer study. Int J Cancer. 143:52–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee E, Horn-Ross PL, Rull RP, Neuhausen
SL, Anton-Culver H, Ursin G, Henderson KD and Bernstein L:
Reproductive factors, exogenous hormones, and pancreatic cancer
risk in the CTS. Am J Epidemiol. 178:1403–1413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prizment AE, Anderson KE, Hong CP and
Folsom AR: Pancreatic cancer incidence in relation to female
reproductive factors: Iowa women's health study. JOP. 8:16–27.
2007.PubMed/NCBI
|
|
40
|
Duell EJ and Holly EA: Reproductive and
menstrual risk factors for pancreatic cancer: A population-based
study of San Francisco Bay Area women. Am J Epidemiol. 161:741–747.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stevens RJ, Roddam AW, Green J, Pirie K,
Bull D, Reeves GK and Beral V; Million Women Study Collaborators:
Reproductive history and pancreatic cancer incidence and mortality
in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers
Prev. 18:1457–1460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ioannou C, Ragia G, Balgkouranidou I,
Xenidis N, Amarantidis K, Koukaki T, Biziota E, Kakolyris S and
Manolopoulos VG: MTHFR c.665C>T guided fluoropyrimidine therapy
in cancer: Gender-dependent effect on dose requirements. Drug Metab
Pers Ther. 37:323–327. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Conroy T, Pfeiffer P, Vilgrain V, Lamarca
A, Seufferlein T, O'Reilly EM, Hackert T, Golan T, Prager G,
Haustermans K, et al: Pancreatic cancer: ESMO Clinical Practice
Guideline for diagnosis, treatment and follow-up. Ann Oncol.
34:987–1002. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mueller F, Buchel B, Koberle D, Schurch S,
Pfister B, Krahenbuhl S, Froehlich TK, Largiader CR and Joerger M:
Gender-specific elimination of continuous-infusional 5-fluorouracil
in patients with gastrointestinal malignancies: Results from a
prospective population pharmacokinetic study. Cancer Chemother
Pharmacol. 71:361–370. 2013. View Article : Google Scholar
|
|
45
|
Milano G, Etienne MC, Cassuto-Viguier E,
Thyss A, Santini J, Frenay M, Renee N, Schneider M and Demard F:
Influence of sex and age on fluorouracil clearance. J Clin Oncol.
10:1171–1175. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bastian G, Barrail A and Urien S:
Population pharmacokinetics of oxaliplatin in patients with
metastatic cancer. Anticancer Drugs. 14:817–824. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nikanjam M, Stewart CF, Takimoto CH,
Synold TW, Beaty O, Fouladi M and Capparelli EV: Population
pharmacokinetic analysis of oxaliplatin in adults and children
identifies important covariates for dosing. Cancer Chemother
Pharmacol. 75:495–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Klein CE, Gupta E, Reid JM, Atherton PJ,
Sloan JA, Pitot HC, Ratain MJ and Kastrissios H: Population
pharmacokinetic model for irinotecan and two of its metabolites,
SN-38 and SN-38 glucuronide. Clin Pharmacol Ther. 72:638–647. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Berg AK, Buckner JC, Galanis E, Jaeckle
KA, Ames MM and Reid JM: Quantification of the impact of
enzyme-inducing antiepileptic drugs on irinotecan pharmacokinetics
and SN-38 exposure. J Clin Pharmacol. 55:1303–1312. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chabot GG, Abigerges D, Catimel G, Culine
S, de Forni M, Extra JM, Mahjoubi M, Hérait P, Armand JP, Bugat R,
et al: Population pharmacokinetics and pharmacodynamics of
irinotecan (CPT-11) and active metabolite SN-38 during phase I
trials. Ann Oncol. 6:141–151. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
De Francia S, Mancardi D, Berchialla P,
Armando T, Storto S, Allegra S, Soave G, Racca S, Chiara F,
Carnovale J, et al: Gender-specific side effects of chemotherapy in
pancreatic cancer patients. Can J Physiol Pharmacol. 100:371–377.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim J, Ji E, Jung K, Jung IH, Park J, Lee
JC, Kim JW, Hwang JH and Kim J: Gender differences in patients with
metastatic pancreatic cancer who received FOLFIRINOX. J Pers Med.
11:832021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Keum J, Lee HS, Kang H, Jo JH, Chung MJ,
Park JY, Park SW, Song SY and Bang S: Single-center risk factor
analysis for FOLFIRINOX associated febrile neutropenia in patients
with pancreatic cancer. Cancer Chemother Pharmacol. 85:651–659.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sugiyama E, Kaniwa N, Kim SR, Hasegawa R,
Saito Y, Ueno H, Okusaka T, Ikeda M, Morizane C, Kondo S, et al:
Population pharmacokinetics of gemcitabine and its metabolite in
Japanese cancer patients: Impact of genetic polymorphisms. Clin
Pharmacokinet. 49:549–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang X, Galettis P, Links M, Mitchell PL
and McLachlan AJ: Population pharmacokinetics of gemcitabine and
its metabolite in patients with cancer: Effect of oxaliplatin and
infusion rate. Br J Clin Pharmacol. 65:326–333. 2008. View Article : Google Scholar
|
|
56
|
Lim HS, Bae KS, Jung JA, Noh YH, Hwang AK,
Jo YW, Hong YS, Kim K, Lee JL, Park SJ, et al: Predicting the
efficacy of an oral paclitaxel formulation (DHP107) through
modeling and simulation. Clin Ther. 37:402–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Joerger M, Kraff S, Huitema AD, Feiss G,
Moritz B, Schellens JH, Beijnen JH and Jaehde U: Evaluation of a
pharmacology-driven dosing algorithm of 3-weekly paclitaxel using
therapeutic drug monitoring: A pharmacokinetic-pharmacodynamic
simulation study. Clin Pharmacokinet. 51:607–617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen N, Li Y, Ye Y, Palmisano M, Chopra R
and Zhou S: Pharmacokinetics and pharmacodynamics of nab-paclitaxel
in patients with solid tumors: Disposition kinetics and
pharmacology distinct from solvent-based paclitaxel. J Clin
Pharmacol. 54:1097–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pijnappel EN, Schuurman M, Wagner AD, de
Vos-Geelen J, van der Geest LGM, de Groot JB, Koerkamp BG, de Hingh
IHJT, Homs MYV, Creemers GJ, et al: Sex, gender and age differences
in treatment allocation and survival of patients with metastatic
pancreatic cancer: A nationwide study. Front Oncol. 12:8397792022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gehrels AM, Wagner AD, Besselink MG,
Verhoeven RHA, van Eijck CHJ, van Laarhoven HWM, Wilmink JW and van
der Geest LG; Dutch Pancreatic Cancer Group: Gender differences in
tumor characteristics, treatment allocation and survival in stage
I-III pancreatic cancer: A nationwide study. Eur J Cancer.
206:1141172024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Olsson Hau S, Williamsson C, Andersson B,
Eberhard J and Jirstrom K: Sex and gender differences in treatment
intention, quality of life and performance status in the first 100
patients with periampullary cancer enrolled in the CHAMP study. BMC
Cancer. 23:3342023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Williamsson C, Rystedt J and Andersson B:
An analysis of gender differences in treatment and outcome of
periampullary tumours in Sweden-A national cohort study. HPB
(Oxford). 23:847–853. 2021. View Article : Google Scholar
|
|
63
|
Pastrana Del Valle J, Mahvi DA,
Fairweather M, Wang J, Clancy TE, Ashley SW, Urman RD, Whang EE and
Gold JS: Associations of gender, race, and ethnicity with
disparities in short-term adverse outcomes after pancreatic
resection for cancer. J Surg Oncol. 125:646–657. 2022. View Article : Google Scholar
|
|
64
|
Lambert A, Jarlier M, Gourgou Bourgade S
and Conroy T: Response to FOLFIRINOX by gender in patients with
metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11
randomized trial. PLoS One. 12:e01832882017. View Article : Google Scholar
|
|
65
|
Hohla F, Hopfinger G, Romeder F,
Rinnerthaler G, Bezan A, Stattner S, Hauser-Kronberger C, Ulmer H
and Greil R: Female gender may predict response to FOLFIRINOX in
patients with unresectable pancreatic cancer: A single institution
retrospective review. Int J Oncol. 44:319–326. 2014. View Article : Google Scholar
|
|
66
|
Dunn SE, Perry WA and Klein SL: Mechanisms
and consequences of sex differences in immune responses. Nat Rev
Nephrol. 20:37–55. 2024. View Article : Google Scholar
|
|
67
|
Klein SL and Flanagan KL: Sex differences
in immune responses. Nat Rev Immunol. 16:626–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ye Y, Jing Y, Li L, Mills GB, Diao L, Liu
H and Han L: Sex-associated molecular differences for cancer
immunotherapy. Nat Commun. 11:17792020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Weinstein Y, Ran S and Segal S:
Sex-associated differences in the regulation of immune responses
controlled by the MHC of the mouse. J Immunol. 132:656–661. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Griesbeck M, Ziegler S, Laffont S, Smith
N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart
M, et al: Sex differences in plasmacytoid dendritic cell levels of
IRF5 drive higher IFN-α production in women. J Immunol.
195:5327–5336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Webb K, Peckham H, Radziszewska A, Menon
M, Oliveri P, Simpson F, Deakin CT, Lee S, Ciurtin C, Butler G, et
al: Sex and pubertal differences in the type 1 interferon pathway
associate with both X chromosome number and serum sex hormone
concentration. Front Immunol. 9:31672019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hagen SH, Henseling F, Hennesen J, Savel
H, Delahaye S, Richert L, Ziegler SM and Altfeld M: Heterogeneous
escape from x chromosome inactivation results in sex differences in
type I IFN responses at the single human pDC level. Cell Rep.
33:1084852020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Umlauf BJ, Haralambieva IH, Ovsyannikova
IG, Kennedy RB, Pankratz VS, Jacobson RM and Poland GA:
Associations between demographic variables and multiple
measles-specific innate and cell-mediated immune responses after
measles vaccination. Viral Immunol. 25:29–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Abramenko N, Vellieux F, Vesela K, Kejik
Z, Hajduch J, Masarik M, Babula P, Hoskovec D, Pacák K, Martásek P,
et al: Investigation of the potential effects of estrogen receptor
modulators on immune checkpoint molecules. Sci Rep. 14:30432024.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Han J, Yang Y, Li X, Wu J, Sheng Y, Qiu J,
Wang Q, Li J, He Y, Cheng L and Zhang Y: Pan-cancer analysis
reveals sex-specific signatures in the tumor microenvironment. Mol
Oncol. 16:2153–2173. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ho WJ, Jaffee EM and Zheng L: The tumour
microenvironment in pancreatic cancer-clinical challenges and
opportunities. Nat Rev Clin Oncol. 17:527–540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Clark CE, Hingorani SR, Mick R, Combs C,
Tuveson DA and Vonderheide RH: Dynamics of the immune reaction to
pancreatic cancer from inception to invasion. Cancer Res.
67:9518–9527. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
He F, Tay AHM, Calandigary A, Malki E,
Suzuki S, Liu T, Wang Q, Fernández-Moro C, Kaisso M, Olofsson-Sahl
P, et al: FPR2 shapes an immune-excluded pancreatic tumor
microenvironment and drives T-cell exhaustion in a sex-dependent
manner. Cancer Res. 83:1628–1645. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Manoukian P, van Schie DM, van Schelt AS,
Wassenaar NPM, Dings MPG, Lansbergen MF, Waasdorp C, Leite de
Oliveira R, Wickenhagen WV, Heijboer AC, et al: Estrogen production
in pancreatic cancer shapes a tumor suppressive stromal
microenvironment. Cancer Res. Nov 19–2025.Epub ahead of print.
PubMed/NCBI
|
|
81
|
Ahmed A, Kohler S, Klotz R, Giese N,
Hackert T, Springfeld C, Jäger D and Halama N: Sex differences in
the systemic and local immune response of pancreatic cancer
patients. Cancers (Basel). 15:18152023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
van Eijck CWF, Mustafa DAM, Vadgama D, de
Miranda NFCC, Groot Koerkamp B, van Tienhoven G, van der Burg SH,
Malats N and van Eijck CHJ; Dutch Pancreatic Cancer Group (DPCG):
Enhanced antitumour immunity following neoadjuvant
chemoradiotherapy mediates a favourable prognosis in women with
resected pancreatic cancer. Gut. 73:311–324. 2024. View Article : Google Scholar
|
|
83
|
Sethna Z, Guasp P, Reiche C, Milighetti M,
Ceglia N, Patterson E, Lihm J, Payne G, Lyudovyk O, Rojas LA, et
al: RNA neoantigen vaccines prime long-lived CD8(+) T cells in
pancreatic cancer. Nature. 639:1042–1051. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li Y, Chang RB, Stone ML, Delman D,
Markowitz K, Xue Y, Coho H, Herrera VM, Li JH, Zhang L, et al:
Multimodal immune phenotyping reveals microbial-T cell interactions
that shape pancreatic cancer. Cell Rep Med. 5:1013972024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Andrews MC, Duong CPM, Gopalakrishnan V,
Iebba V, Chen WS, Derosa L, Khan MAW, Cogdill AP, White MG, Wong
MC, et al: Gut microbiota signatures are associated with toxicity
to combined CTLA-4 and PD-1 blockade. Nat Med. 27:1432–1441. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Riquelme E, Zhang Y, Zhang L, Montiel M,
Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et
al: Tumor microbiome diversity and composition influence pancreatic
cancer outcomes. Cell. 178:795–806 e12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guo W, Zhang Y, Guo S, Mei Z, Liao H, Dong
H, Wu K, Ye H, Zhang Y, Zhu Y, et al: Tumor microbiome contributes
to an aggressive phenotype in the basal-like subtype of pancreatic
cancer. Commun Biol. 4:10192021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nejman D, Livyatan I, Fuks G, Gavert N,
Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E,
et al: The human tumor microbiome is composed of tumor
type-specific intracellular bacteria. Science. 368:973–980. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Guenther M, Gil L, Surendran SA, Palm MA,
Heinemann V, von Bergwelt-Baildon M, Mayerle J, Engel J, Werner J,
Boeck S and Ormanns S: Bacterial lipopolysaccharide as a negative
predictor of adjuvant gemcitabine efficacy in pancreatic cancer.
JNCI Cancer Spectr. 6:pkac0392022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Stein-Thoeringer CK, Renz BW, De Castilhos
J, von Ehrlich-Treuenstatt V, Wirth U, Tschaidse T, Hofmann FO,
Koch DT, Beirith I, Ormanns S, et al: Microbiome dysbiosis with
enterococcus presence in the upper gastrointestinal tract is a risk
factor for mortality in patients undergoing surgery for pancreatic
cancer. Ann Surg. 281:615–623. 2025. View Article : Google Scholar
|
|
91
|
Nalluri H, Jensen E and Staley C: Role of
biliary stent and neoadjuvant chemotherapy in the pancreatic tumor
microbiome. BMC Microbiol. 21:2802021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Khan ZA, Ghorbani M, Heffinger L,
Damdimopoulos A, Moro CF, Björnstedt M, Löhr JM, Heuchel R, Chen MS
and Sarhan D: Genderized gut and oral microbiome shifts: Uncovering
sex-specific dysbiosis in pancreatic cancer. bioRxiv. Oct
3–2024.Epub ahead of print. View Article : Google Scholar
|
|
93
|
Kaune T, Griesmann H, Theuerkorn K,
Hammerle M, Laumen H, Krug S, Plumeier I, Kahl S, Junca H, Gustavo
Dos Anjos Borges L, et al: Gender-specific changes of the gut
microbiome correlate with tumor development in murine models of
pancreatic cancer. iScience. 26:1068412023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fulop DJ, Zylberberg HM, Wu YL, Aronson A,
Labiner AJ, Wisnivesky J, Cohen DJ, Sigel KM and Lucas AL:
Association of Antibiotic receipt with survival among patients with
metastatic pancreatic ductal adenocarcinoma receiving chemotherapy.
JAMA Netw Open. 6:e2342542023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
O'Neill RS and Stoita A: Biomarkers in the
diagnosis of pancreatic cancer: Are we closer to finding the golden
ticket? World J Gastroenterol. 27:4045–4087. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Balaban DV, Marin FS, Manucu G, Zoican A,
Ciochina M, Mina V, Patoni C, Vladut C, Bucurica S, Costache RS, et
al: Clinical characteristics and outcomes in carbohydrate antigen
19-9 negative pancreatic cancer. World J Clin Oncol. 13:630–640.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hermann CD, Schoeps B, Eckfeld C,
Munkhbaatar E, Kniep L, Prokopchuk O, Wirges N, Steiger K, Häußler
D, Knolle P, et al: TIMP1 expression underlies sex disparity in
liver metastasis and survival in pancreatic cancer. J Exp Med.
218:e202109112021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Asplund E, Bergqvist M, Krook M and Lohr
JM: Plasma thymidine kinase activity as a prognostic biomarker in
pancreatic ductal adenocarcinoma: A single-center prospective
study. Scand J Gastroenterol. 58:1044–1048. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Babic A, Wang QL, Lee AA, Yuan C, Rifai N,
Luo J, Tabung FK, Shadyab AH, Wactawski-Wende J, Saquib N, et al:
Sex-specific associations between adiponectin and leptin signaling
and pancreatic cancer survival. Cancer Epidemiol Biomarkers Prev.
32:1458–1469. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang X, Mao T, Zhang B, Xu H, Cui J, Jiao
F, Chen D, Wang Y, Hu J, Xia Q, et al: Characterization of the
genomic landscape in large-scale Chinese patients with pancreatic
cancer. EBioMedicine. 77:1038972022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Boeck S, Jung A, Laubender RP, Neumann J,
Egg R, Goritschan C, Vehling-Kaiser U, Winkelmann C, Fischer von
Weikersthal L, Clemens MR, et al: EGFR pathway biomarkers in
erlotinib-treated patients with advanced pancreatic cancer:
Translational results from the randomised, crossover phase 3 trial
AIO-PK0104. Br J Cancer. 108:469–476. 2013. View Article : Google Scholar :
|
|
102
|
Ramezankhani R, Ghavidel AA, Rashidi S,
Rojhannezhad M, Abolkheir HR, Mirhosseini M, Taleahmad S and
Vosough M: Gender-related differentially expressed genes in
pancreatic cancer: Possible culprits or accomplices? Front Genet.
13:9669412022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Peduzzi G, Archibugi L, Katzke V,
Gentiluomo M, Capurso G, Milanetto AC, Gazouli M, Goetz M, Brenner
H, Vermeulen RCH, et al: Common variability in oestrogen-related
genes and pancreatic ductal adenocarcinoma risk in women. Sci Rep.
12:181002022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li D, Suzuki H, Liu B, Morris J, Liu J,
Okazaki T, Li Y, Chang P and Abbruzzese JL: DNA repair gene
polymorphisms and risk of pancreatic cancer. Clin Cancer Res.
15:740–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jones J, Mukherjee A, Karanam B, Davis M,
Jaynes J, Reams RR, Dean-Colomb W and Yates C: African Americans
with pancreatic ductal adenocarcinoma exhibit gender differences in
Kaiso expression. Cancer Lett. 380:513–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Schonk MM, Ducharme JB, Neyroud D, Nosacka
RL, Tucker HO, Judge SM and Judge AR: Myofiber-specific FoxP1
knockout protects against pancreatic cancer-induced muscle wasting
in male but not female mice. bioRxiv. Sep 21–2024.Epub ahead of
print. PubMed/NCBI
|
|
107
|
Zhong X, Narasimhan A, Silverman LM, Young
AR, Shahda S, Liu S, Wan J, Liu Y, Koniaris LG and Zimmers TA: Sex
specificity of pancreatic cancer cachexia phenotypes, mechanisms,
and treatment in mice and humans: Role of Activin. J Cachexia
Sarcopenia Muscle. 13:2146–2161. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Muckenhuber A, Berger AK, Schlitter AM,
Steiger K, Konukiewitz B, Trumpp A, Eils R, Werner J, Friess H,
Esposito I, et al: Pancreatic ductal adenocarcinoma subtyping using
the biomarkers hepatocyte nuclear Factor-1A and cytokeratin-81
correlates with outcome and treatment response. Clin Cancer Res.
24:351–359. 2018. View Article : Google Scholar
|
|
109
|
Guenther M, Surendran SA, Steinke LM, Liou
I, Palm MA, Heinemann V, Haas M, Boeck S and Ormanns S: The
prognostic, predictive and clinicopathological implications of
KRT81/HNF1A- and GATA6-Based transcriptional subtyping in
pancreatic cancer. Biomolecules. 15:4262025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nicolle R, Bachet JB, Harle A, Iovanna J,
Hammel P, Rebours V, Turpin A, Ben Abdelghani M, Wei A, Mitry E, et
al: Prediction of adjuvant gemcitabine sensitivity in resectable
pancreatic adenocarcinoma using the GemPred RNA signature: An
ancillary study of the PRODIGE-24/CCTG PA6 clinical trial. J Clin
Oncol. 42:1067–1076. 2024. View Article : Google Scholar :
|
|
111
|
Fraunhoffer N, Hammel P, Conroy T, Nicolle
R, Bachet JB, Harle A, Rebours V, Turpin A, Ben Abdelghani M, Mitry
E, et al: Development and validation of AI-assisted transcriptomic
signatures to personalize adjuvant chemotherapy in patients with
pancreatic ductal adenocarcinoma. Ann Oncol. 35:780–791. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Andricovich J, Perkail S, Kai Y, Casasanta
N, Peng W and Tzatsos A: Loss of KDM6A activates super-enhancers to
induce gender-specific squamous-like pancreatic cancer and confers
sensitivity to BET inhibitors. Cancer Cell. 33:512–526 e8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kouchi Y, Takano S, Harada-Kagitani S,
Shinomiya Y, Yogi N, Sakamoto T, Mishima T, Fugo K, Kambe M, Nagai
Y, et al: Complex glandular pattern is an aggressive morphology
that predicts poor prognosis of pancreatic ductal adenocarcinoma.
Ann Diagn Pathol. 64:1521102023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Grochowski P, Grosser B, Sommer F, Probst
A, Waidhauser J, Schenkirsch G, Reitsam NG and Märkl B: The concept
of stroma areactive invasion front areas (SARIFA) as a new
prognostic biomarker for lipid-driven cancers holds true in
pancreatic ductal adenocarcinoma. BMC Cancer. 24:7682024.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hayashi A, Yavas A, McIntyre CA, Ho YJ,
Erakky A, Wong W, Varghese AM, Melchor JP, Overholtzer M, O'Reilly
EM, et al: Genetic and clinical correlates of entosis in pancreatic
ductal adenocarcinoma. Mod Pathol. 33:1822–1831. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Song J, Ruze R, Chen Y, Xu R, Yin X, Wang
C, Xu Q and Zhao Y: Construction of a novel model based on
cell-in-cell-related genes and validation of KRT7 as a biomarker
for predicting survival and immune microenvironment in pancreatic
cancer. BMC Cancer. 22:8942022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Xiong F, Guo T, Wang X, Wu G, Liu W, Wang
Q, Wang B and Chen Y: Keratin 8 is an inflammation-induced and
prognosis-related marker for pancreatic adenocarcinoma. Dis
Markers. 2022:81595372022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Qian Y, Gong Y, Liu Y, Chen Y, Wang R, Dai
Z, Zou X, Tasiheng Y, Luo G, Lin X, et al: Atypical mucin
expression predicts worse overall survival in resectable pancreatic
ductal adenocarcinoma. J Immunol Res. 2022:73535722022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ermiah E, Eddfair M, Abdulrahman O,
Elfagieh M, Jebriel A, Al-Sharif M, Assidi M and Buhmeida A:
Prognostic value of serum CEA and CA19-9 levels in pancreatic
ductal adenocarcinoma. Mol Clin Oncol. 17:1262022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sumiyoshi T, Uemura K, Shintakuya R, Okada
K, Baba K, Harada T, Serikawa M, Ishii Y, Nakamura S, Arihiro K, et
al: Clinical utility of the combined use of CA19-9 and DUPAN-2 in
pancreatic adenocarcinoma. Ann Surg Oncol. 31:4665–4672. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu
W, Liu J, Xiang J, Liang D, Hu Q, et al: Oncogenic KRAS targets
MUC16/CA125 in pancreatic ductal adenocarcinoma. Mol Cancer Res.
15:201–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Xiao W, Hong H, Awadallah A, Zhou L and
Xin W: Utilization of CDX2 expression in diagnosing pancreatic
ductal adenocarcinoma and predicting prognosis. PLoS One.
9:e868532014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Amal AM, Ahmed MSM, Hareedy AAM, Khattab
HMH and Mahmoud EK: The utility of SMAD4 and S100P as diagnostic
immunohistochemical markers for pancreatic ductal adenocarcinomas
in egyptian patients. J Microsc Ultrastruct. Jun 25–2024.Epub ahead
of print. View Article : Google Scholar
|
















