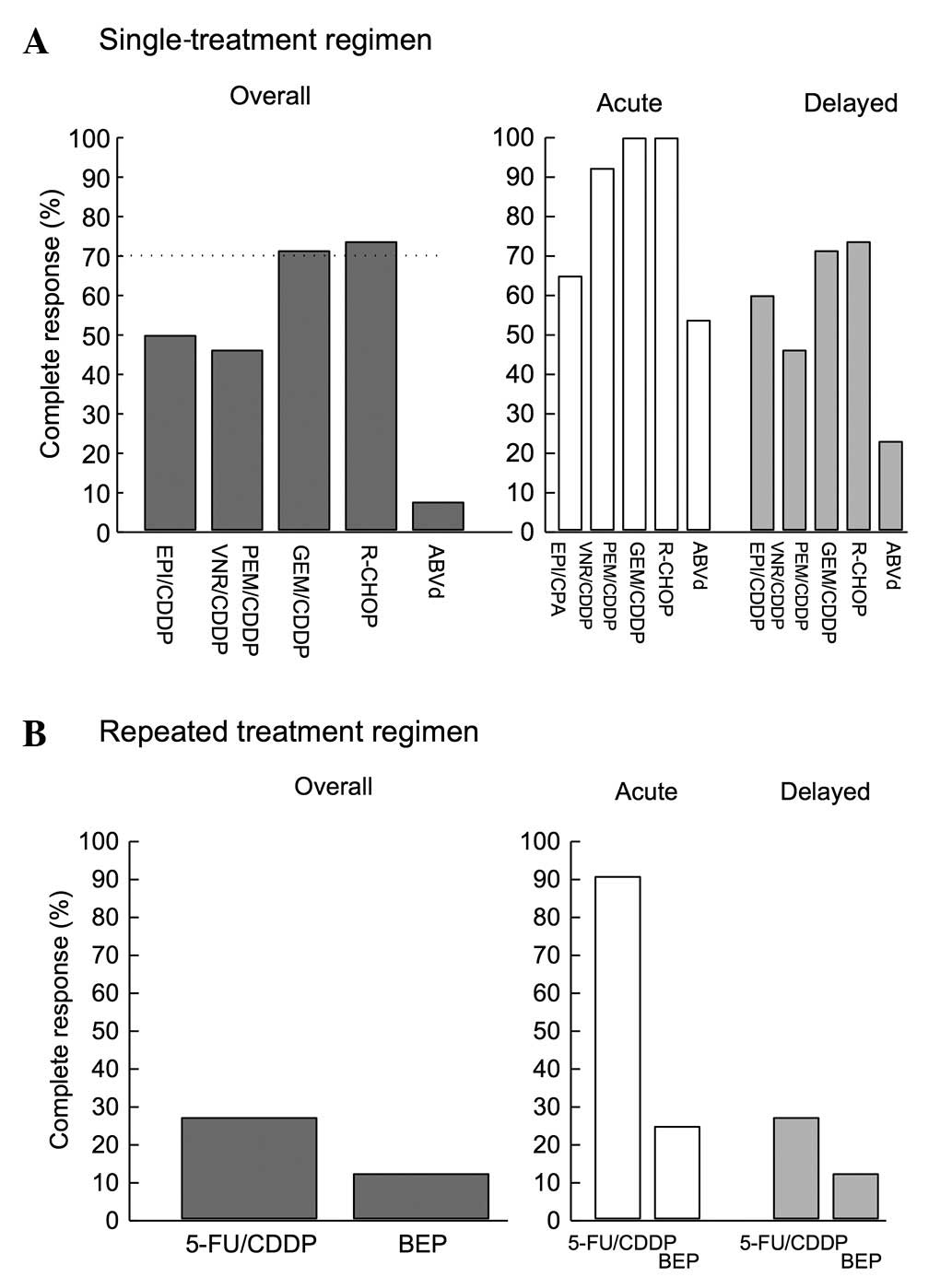
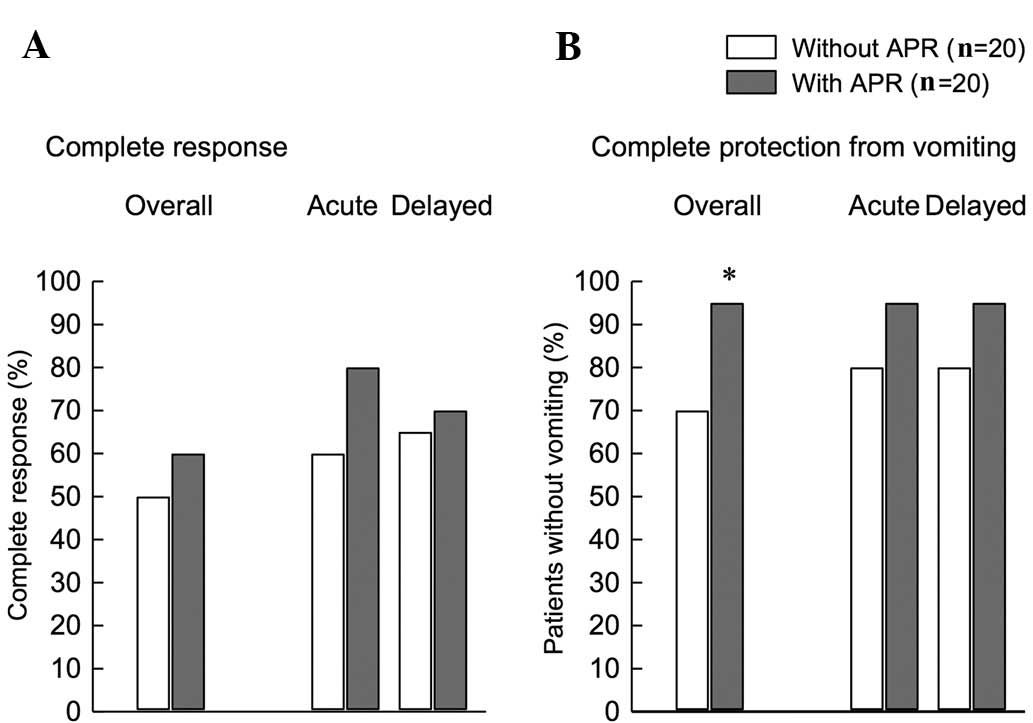
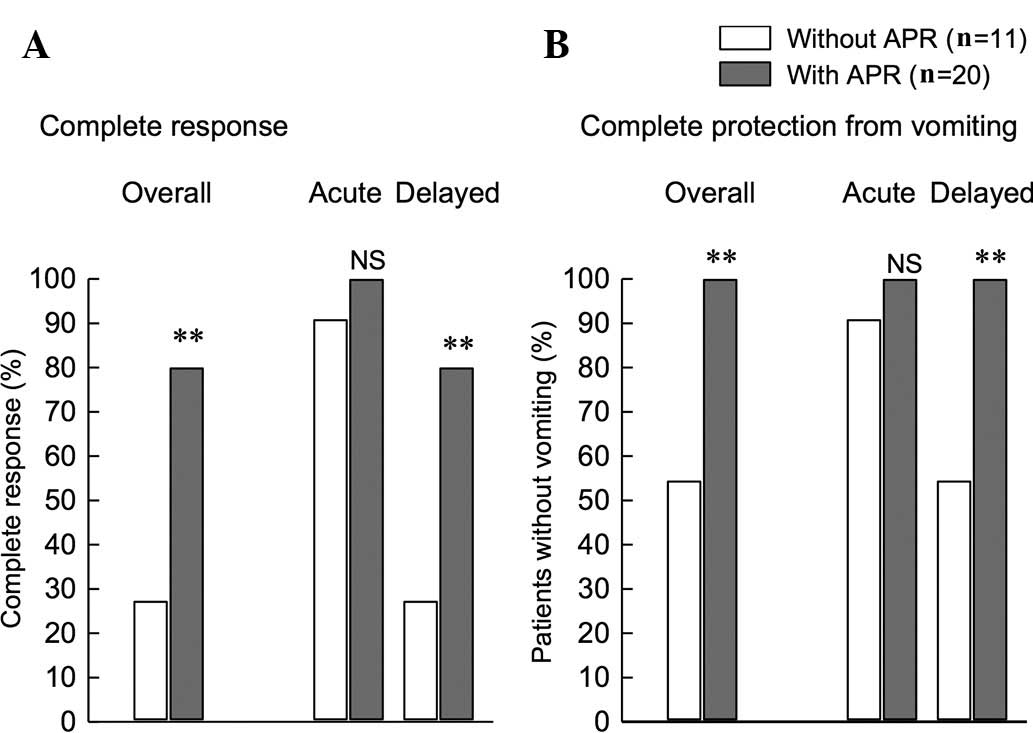
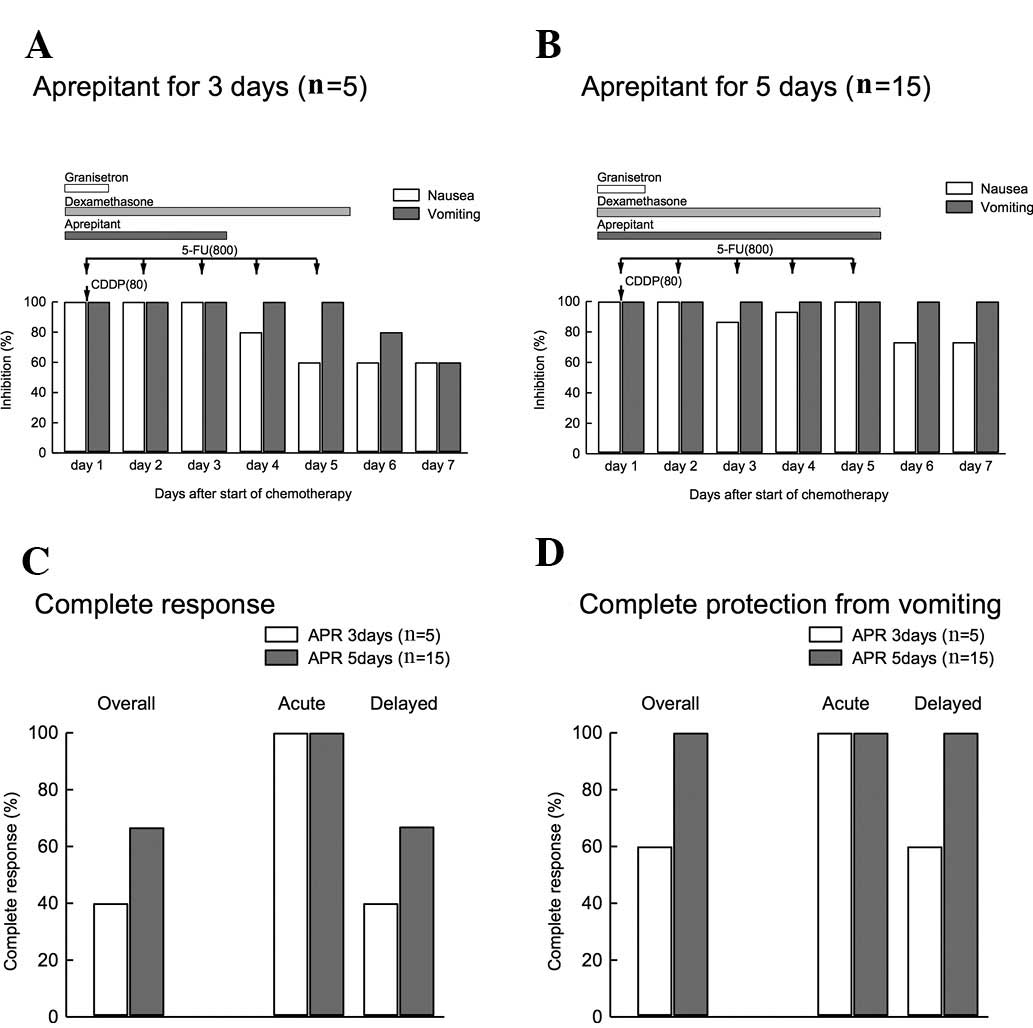
|
1.
|
Bloechl-Daum B, Deuson RR, Mavros P,
Hansen M and Herrstedt J: Delayed nausea and vomiting continue to
reduce patients’ quality of life after highly and moderately
emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol.
24:4472–4478. 2006.
|
|
2.
|
Lachaine J, Yelle L, Kaizer L, Dufour A,
Hopkins S and Deuson R: Chemotherapy-induced emesis: quality of
life and economic impact in the context of current practice in
Canada. Support Cancer Ther. 2:181–187. 2005.
|
|
3.
|
Sun CC, Bodurka DC, Weaver CB, et al:
Rankings and symptom assessments of side effects from chemotherapy:
insights from experienced patients with ovarian cancer. Support
Care Cancer. 13:219–227. 2005.
|
|
4.
|
Roscoe JA, Morrow GR, Aapro MS,
Molassiotis A and Olver I: Anticipatory nausea and vomiting.
Support Care Cancer. 19:1533–1538. 2011.
|
|
5.
|
Roila F, Herrstedt J, Aapro M, et al:
Guideline update for MASCC and ESMO in the prevention of
chemotherapy- and radiotherapy-induced nausea and vomiting: results
of the Perugia consensus conference. Ann Oncol. 21:S232–S243.
2010.
|
|
6.
|
Basch E, Prestrud AA, Hesketh PJ, et al:
Antiemetics: American society of clinical oncology clinical
practice guideline update. J Clin Oncol. 29:4189–4198. 2011.
|
|
7.
|
National Comprehensive Cancer Network
(NCCN): Clinical Practice Guidelines in Oncology. Antiemesis.
Version I, 2011. (Available at: http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf).
Accessed April 12, 2012.
|
|
8.
|
de Wit R, Herrstedt J, Rapoport B, et al:
Addition of the oral NK1 antagonist aprepitant to
standard antiemetics provides protection against nausea and
vomiting during multiple cycles of cisplatin-based chemotherapy. J
Clin Oncol. 21:4105–4111. 2003.
|
|
9.
|
Grunberg S, Chua D, Maru A, et al:
Single-dose fosaprepitant for the prevention of
chemotherapy-induced nausea and vomiting associated with cisplatin
therapy: randomized, double-blind study protocol - EASE. J Clin
Oncol. 29:1495–1501. 2011.
|
|
10.
|
Hesketh PJ, Grunberg SM, Gralla RJ, et al:
The oral neurokinin-1 antagonist aprepitant for the prevention of
chemotherapy-induced nausea and vomiting: a multinational,
randomized, double-blind, placebo-controlled trial in patients
receiving high-dose cisplatin - the Aprepitant Protocol 052 Study
Group. J Clin Oncol. 21:4112–4119. 2003.
|
|
11.
|
Rapoport BL, Jordan K, Boice JA, et al:
Aprepitant for the prevention of chemotherapy-induced nausea and
vomiting associated with a broad range of moderately emetogenic
chemotherapies and tumor types: a randomized, double-blind study.
Support Care Cancer. 18:423–431. 2010.
|
|
12.
|
Hesketh PJ, Grunberg SM, Herrstedt J, et
al: Combined data from two phase III trials of the NK1
antagonist aprepitant plus a 5HT3 antagonist and a
corticosteroid for prevention of chemotherapy-induced nausea and
vomiting: effect of gender on treatment response. Support Care
Cancer. 14:354–360. 2006.
|
|
13.
|
von der Maase H, Hansen SW, Roberts JT, et
al: Gemcitabine and cisplatin versus methotrexate, vinblastine,
doxorubicin, and cisplatin in advanced or metastatic bladder
cancer: results of a large, randomized, multinational, multicenter,
phase III study. J Clin Oncol. 18:3068–3077. 2000.
|
|
14.
|
Tobinai K, Ogura M, Itoh K, et al:
Randomized phase II study of concurrent and sequential combinations
of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine
and prednisolone) chemotherapy in untreated indolent B-cell
non-Hodgkin lymphoma: 7-year follow-up results. Cancer Sci.
101:2579–2585. 2010.
|
|
15.
|
Piccart MJ, Di Leo A, Beauduin M, et al:
Phase III trial comparing two dose levels of epirubicin combined
with cyclophosphamide with cyclophosphamide, methotrexate, and
fluorouracil in node-positive breast cancer. J Clin Oncol.
19:3103–3110. 2001.
|
|
16.
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008.
|
|
17.
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323.
2007.
|
|
18.
|
Ogura M, Itoh K, Kinoshita T, et al: Phase
II study of ABVd therapy for newly diagnosed clinical stage II–IV
Hodgkin lymphoma: Japan Clinical Oncology Group study (JCOG 9305).
Int J Hematol. 92:713–724. 2010.
|
|
19.
|
Lefebvre JL, Chevalier D, Luboinski B,
Kirkpatrick A, Collette L and Sahmoud T: Larynx preservation in
pyriform sinus cancer: preliminary results of a European
Organization for Research and Treatment of Cancer phase III trial.
EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst.
88:890–899. 1996.
|
|
20.
|
de Wit R, Roberts JT, Wilkinson PM, et al:
Equivalence of three or four cycles of bleomycin, etoposide, and
cisplatin chemotherapy and of a 3- or 5-day schedule in
good-prognosis germ cell cancer: a randomized study of the European
Organization for Research and Treatment of Cancer Genitourinary
Tract Cancer Cooperative Group and the Medical Research Council. J
Clin Oncol. 19:1629–1640. 2001.
|
|
21.
|
Vitolo U, Chiappella A, Ferreri AJ, et al:
First-line treatment for primary testicular diffuse large B-cell
lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral
testis irradiation: final results of an international phase II
trial. J Clin Oncol. 29:2766–2772. 2011.
|
|
22.
|
Dogliotti L, Carteni G, Siena S, et al:
Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as
first-line chemotherapy in advanced transitional cell carcinoma of
the urothelium: results of a randomized phase 2 trial. Eur Urol.
52:134–141. 2007.
|
|
23.
|
Warr DG, Hesketh PJ, Gralla RJ, et al:
Efficacy and tolerability of aprepitant for the prevention of
chemotherapy-induced nausea and vomiting in patients with breast
cancer after moderately emetogenic chemotherapy. J Clin Oncol.
23:2822–2830. 2005.
|
|
24.
|
Poli-Bigelli S, Rodrigues-Pereira J,
Carides AD, et al: Addition of the neurokinin 1 receptor antagonist
aprepitant to standard antiemetic therapy improves control of
chemotherapy-induced nausea and vomiting. Results from a
randomized, double-blind, placebo-controlled trial in Latin
America. Cancer. 97:3090–3098. 2003.
|
|
25.
|
Chawla SP, Grunberg SM, Gralla RJ, et al:
Establishing the dose of the oral NK1 antagonist
aprepitant for the prevention of chemo-therapy-induced nausea and
vomiting. Cancer. 97:2290–2300. 2003.
|
|
26.
|
Jordan K, Kinitz I, Voigt W, Behlendorf T,
Wolf HH and Schmoll HJ: Safety and efficacy of a triple antiemetic
combination with the NK-1 antagonist aprepitant in highly and
moderately emetogenic multiple-day chemotherapy. Eur J Cancer.
45:1184–1187. 2009.
|