Introduction
Cervical carcinoma is the second most common cause
of carcinoma worldwide (1).
Radiotherapy (RT) is the major treatment modality for invasive
cervical carcinomas; however, the prognosis is poor in advanced
uterine cervical carcinoma (2,3).
In the 1990s, numerous attempts were made to improve
the prognosis for advanced uterine cervical carcinoma by
concurrently using radiotherapy and chemotherapy (CCRT). These
attempts led to the results of five randomized controlled trials
demonstrating that CCRT was effective against advanced uterine
cervical carcinoma, while decreasing the risk of mortality thereof
by 30–50%, announced by the National Cancer Institute (USA) in
February 1999 (4–8). However, data from these clinical
trials demonstrated several problems when this CCRT was employed in
Japanese patients. Some of the questions addressed were whether or
not the reported dose of cisplatin was appropriate for Japanese
women, whether the use of cisplatin was appropriate for patients
with advanced uterine cervical carcinoma given their possibly
reduced renal function, or to what extent platinum-containing drugs
are likely to be effective in such patients in the place of
cisplatin.
Nedaplatin (cis-diammine-glycoplatinum), a platinum
analog, was developed by the pharmaceutical company Shionogi &
Co., Ltd. (Tokushima, Japan), aiming to produce a treatment with
lower renal and gastrointestinal toxicity that is as effective as
cisplatin. Clinically, previous phase II studies conducted in Japan
suggested that nedaplatin had particularly strong clinical efficacy
on a squamous cell carcinoma of the head and neck, esophagus and
uterine cervix (9,10). In a phase II clinical trial,
nedaplatin demonstrated a 46% response rate in patients with
recurrent cervical carcinoma, which was slightly superior compared
to cisplatin (39%) (11).
Its lower incidence of nephrotoxicity in comparison
with cisplatin was demonstrated to be associated with a difference
in the kidney distribution of these drugs. When the two agents were
administered at the same dose, the accumulation of nedaplatin in
the kidney was ∼40% compared to cisplatin, leading to a lower
nephrotoxicity of nedaplatin (12,13).
Since nedaplatin exhibited minimal nephrotoxicity, it may be used
in patients with marginal renal function (14,15).
The radiosensitizing properties of nedaplatin have
been demonstrated in several preclinical studies (16,17).
However, the use of nedaplatin in the clinical setting of CCRT in
patients with cervical carcinoma is limited, CCRT with the
administration of nedaplatin at 80 mg/m2 on Days 1 and
29 has been reported to be safe and effective (18).
The present is a retrospective study conducted to
evaluate whether or not nedaplatin-based CCRT is safe and superior
to RT alone in Japanese patients with advanced stage of cervical
carcinoma.
Materials and methods
Patients
Patients with confirmed FIGO stage IIA to IVA
cervical carcinoma, who had been treated between 2006 and 2010 in
the Ehime University Hospital were eligible. No previous
chemotherapy or RT was allowed. Other eligibility criteria included
age (20–80 years) and performance status (score 0–2).
The patients had primary, previously untreated and
histologically confirmed carcinoma of the uterine cervix. The
pretreatment workup comprised a complete medical history and
physical examination, complete blood count, biochemistry panels,
chest X-ray and computed tomography (CT) of the abdomen and pelvis,
magnetic resonance imaging (MRI) and optional intravenous
pyelography and cystoscopy. Regarding lymph nodes measuring ≥10 mm
along their longest axis on CT or MRI were defined as metastatic
nodes. Patients with radiologic evidence of para-aortic disease
were excluded. MRI was used to evaluate the size and geometry of
the primary tumor. The maximal tumor diameter was measured on
T2-weighted images. The longest diameter was considered to be the
maximal tumor diameter.
Patients were staged clinically by both a
gynecological oncologist and a radiation oncologist, according to
the International Federation of Gynecology and Obstetrics staging
criteria, without general anesthesia. Patients, who received
extended-field RT were also excluded.
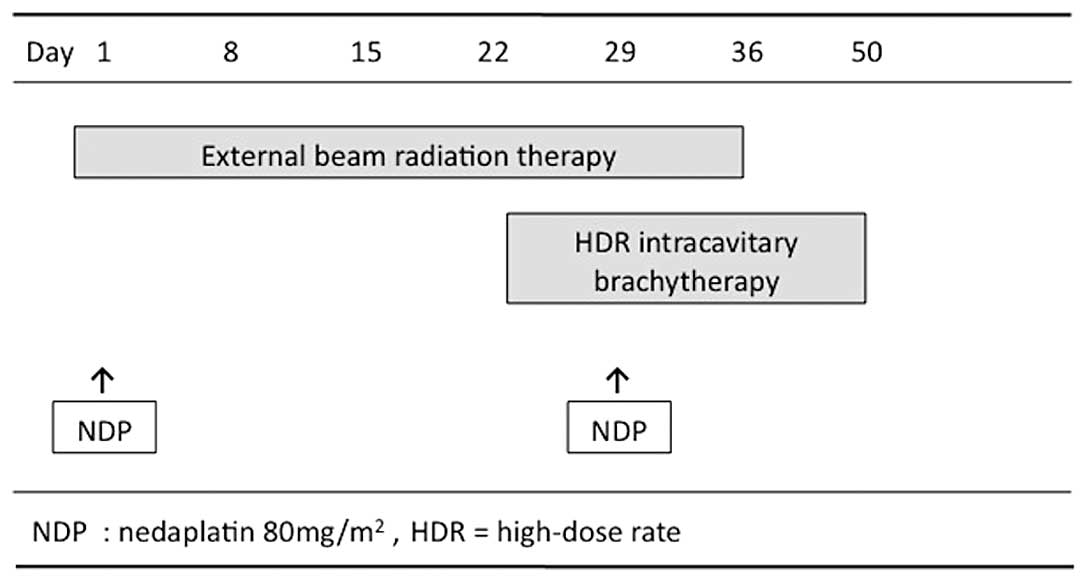
Radiotherapy
Patients were treated with conventional radiotherapy
consisting of external beam radiotherapy (EBRT) and high-dose rate
intracavitary brachytherapy (HDR-ICBT). The EBRT was performed
using a 10 megavolt (MV) X-ray from a linear accelerator. Using
anteroposterior parallel opposed portals, the external irradiation
was delivered to the whole pelvis at 2 Gy/fraction for 5
fractions/week, for a total of 25 fractions (50 Gy). The superior
margin of the external radiation field was placed on the upper
border of the fifth lumbar vertebra, while the inferior margin was
the inferior border of the obturator foramen, although this margin
extended inferiorly when there was vaginal invasion. Laterally, the
field extended 1.5–2 cm beyond the lateral margin of the bony
pelvic wall. A midline block (4 cm width at the midline) was
inserted into the central lower two-thirds length of the pelvic
field after 30 Gy had been delivered.
Subsequent to adequate tumor regression, the
HDR-ICBT was performed once a week during the course of the EBRT
with the centrally shielded field. Usually, the first HDR-ICBT was
applied after 30 Gy of EBRT. ICBT was administered to the patients
using a microselectron HDR. In patients with vaginal infiltration
or with a narrow vagina, a tandem with a vaginal cylinder was used.
The ICBT dose was delivered to point A, defined as 2 cm above the
cervical OS marker and 2 cm perpendicular to the uterine axis,
along the plane of the uterus. The planned total dose of HDR-ICBT
was 24 Gy in 4 fractions. No EBRT was performed on the same day as
HDR-ICBT (Fig. 1).
Chemotherapy
Chemotherapy was administered intravenously with
nedaplatin during the course of EBRT on Days 1 and 29, but was not
administered on the same day as ICBT. The dose of nedaplatin was 80
mg/m2 (range, 60–80). The drug was administered in a 2-h
infusion. Renal function and blood counts were assessed prior to
each cycle. Nedaplatin administration was suspended when the
granulocyte count was <1,500/μl or the platelet count was
<100,000/μl. During the weeks the patient did not receive
chemotherapy, radiation was continued, provided the white blood
cell count was >2,000/μl and the platelet count was
>50,000/μl.
Control patients
A non-randomized group of patients with stage IIA to
IVA cervical carcinoma treated with definitive RT alone between
2006 and 2010 served as the control group. RT for these patients
consisted of a combination of EBRT (50 Gy to the whole pelvis) and
HDR-ICBT (24 Gy to point A), which was the same as the treatment
for the CCRT-treated patients.
Toxicity
Clinical data regarding treatment-related
complications were also collected. Complications that occurred
within 90 days subsequent to the initiation of primary treatment
were considered to be acute complications. The severity of acute
complications was classified, according to the National Cancer
Institute Common Terminology Criteria for Adverse Events, version
2.0.
Follow-up
During the treatment, the patients were evaluated
weekly by pelvic examination and complete blood count. In patients
treated with chemoradiation, renal and liver function tests were
also performed on a weekly basis. Patients were monitored regularly
and examined for acute toxicity by both gynecological and radiation
oncologists. Subsequent to termination of the treatment, patient
follow-up occurred in an outpatient clinic on a monthly basis
during the first year, bimonthly during the second year, every 3
months during the third year, every 6 months during the fourth to
fifth years and annually thereafter. Follow-up procedures included
gynecological examination, cervical cytology and squamous cell
carcinoma antigen (SCCA) evaluation. Evaluation with CT was
repeated every 6 months during the first 2 years and once a year
thereafter. Pelvic failure, including central and parametrial
failure, was defined as persisting or recurring disease in the
pelvis. When pelvic examination or smears detected a potential
local recurrence, a biopsy was taken for confirmation whenever
possible. Distant failure was defined as a disease occurring
outside the pelvis, including the para-aortic lymph nodes. No
patient was lost to follow-up. The median duration of the follow-up
was 27.3 months (range, 10–67).
Statistical analysis
The differences between the groups with respect to
stage, histology, the site of recurrence and treatment-related
toxicity were assessed using Fisher’s exact test. The maximum tumor
diameter, pretreatment hemoglobin and SCCA levels were compared
using Welch’s t-test. The overall survival (OS) and
progression-free survival (PFS) curves were calculated according to
the Kaplan-Meier method. P<0.05 was considered to indicate a
statistically significant difference. Analyses were performed using
the SPSS15.0 software.
Results
Patient characteristics
Between January, 2006 and December, 2010, 17
patients with FIGO stage IIA to IVA cervical carcinoma treated with
nedaplatin-based CCRT (n=8) or RT alone (n=9) were identified. The
clinicopathological characteristics of these patients are shown in
Table I. The characteristics of
the patients in the CCRT group were similar to those in the RT
group. No significant differences were found among the patient
characteristics, with the exception of age.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | CCRT-group (%) | RT-group (%) | P-value |
|---|
| No. of patients | 8 | 9 | |
| Age (years) | | | |
| Median | 63.5 | 74.1 | 0.0356 |
| Range | 51–78 | 58–79 | |
| Clinical stage | | | |
| IIA | 0 (0) | 3 (33.3) | 0.1781 |
| IIB | 2 (25.0) | 0 (0) | |
| IIIA | 1 (12.5) | 2 (22.2) | |
| IIIB | 4 (50.0) | 4 (44.4) | |
| IVA | 1 (12.5) | 0 (0) | |
| Histology | | | |
| Squamous cell | 6 (75.0) | 9 (10) | 0.2794 |
| Adenocarcinoma | 1 (12.5) | 0 | |
| Adenosquamous | 1 (12.5) | 0 | |
| Tumor diameter
(mm) | | | |
| Median | 47.5 | 60.0 | 0.0700 |
| Range | 44–87 | 54–86 | |
| Pretreatment
hemoglobin level (g/dl) | | | |
| Median | 11.5 | 11.1 | 0.6583 |
| Range | 9.3–14.8 | 8.0–12.8 | |
| Pretreatment SCCA
level (ng/ml) | | | |
| Median | 7.7 | 34.2 | 0.1034 |
| Range | 1.1–51.6 | 0.8–250 | |
Treatment outcome
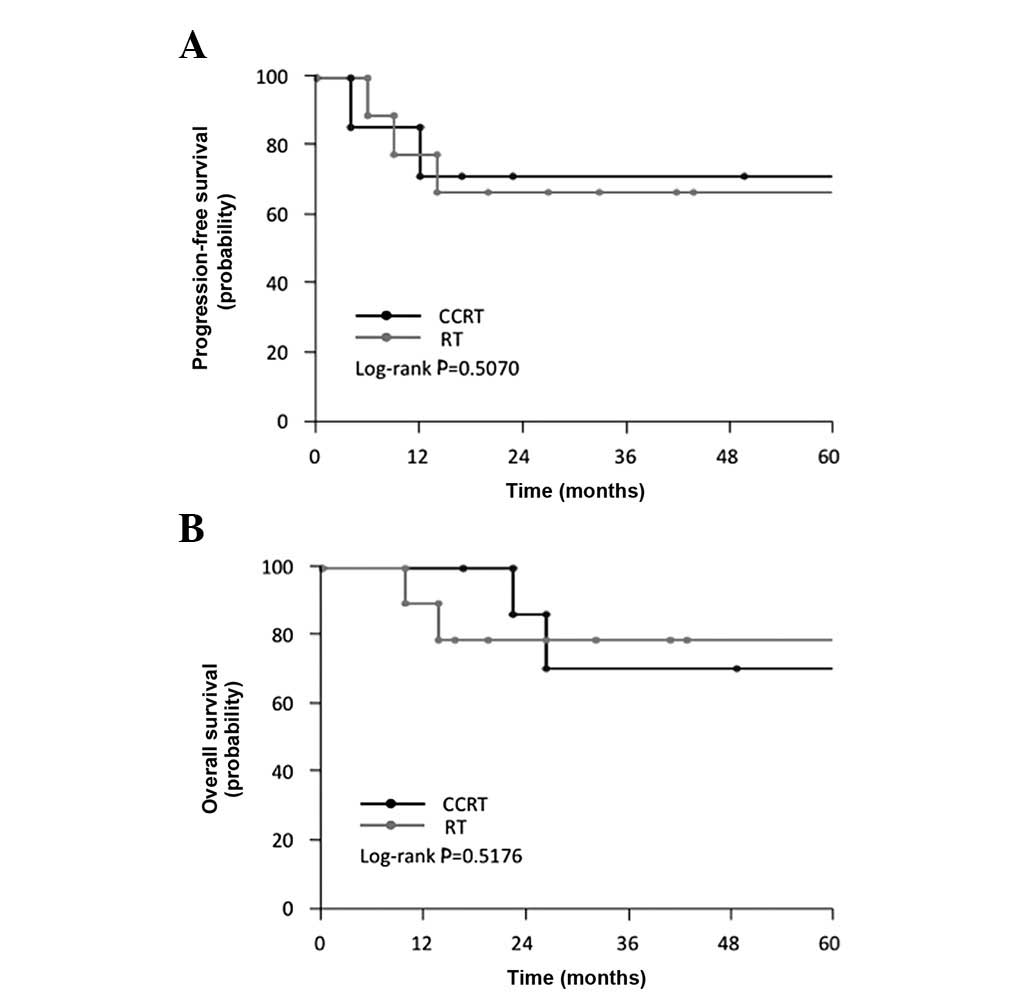
As shown in Table
II, among the CCRT-treated patients, the median and mean PFS
were 30.5 and 33.6 months, respectively, while the median and mean
OS were 38.5 and 41.5 months, respectively. In CCRT-treated
patients, the 5-year OS rate was 68.6%. However, in the RT group,
the median and mean PFS were 27.3 and 28.5 months, respectively,
while the median and mean OS were 27.3 and 29.9 months,
respectively (Table II). In
RT-treated patients the 5-year OS rate was 77.8%. When compared to
the RT group, the CCRT group showed no statistically significant
differences in terms of PFS (log-rank; P= 0.5070) and OS (log-rank;
P= 0.5176) (Fig. 2A and B). These
findings indicate that the addition of concurrent nedaplatin to
pelvic EBRT plus HDR-ICBT did not significantly improve the
prognosis in this patient population.
 | Table IITreatment and survival. |
Table II
Treatment and survival.
| Variables | CCRT-group (n=8) | RT-group (n=9) | P-value |
|---|
| Dose of nedaplatin
administered (mg/m2) | | | |
| Median | 75.0 | - | |
| Range | 60–80 | - | |
| Duration of RT
(days) | | | |
| Median | 50.9 | 49.0 | 0.7677 |
| Range | 44–61 | 41–61 | |
| Patients with
recurrence (%) | | | |
| Local | 2 | 0 | 0.1428 |
| Distant | 1 | 4 | |
| Initial response | | | |
| CR | 7 | 9 | 0.4706 |
| PR | 1 | 0 | |
| PFS (months) | | | |
| Median | 30.5 | 27.3 | 0.6182 |
| Range | 4–63 | 6–61 | |
| Mean | 33.6 | 28.5 | |
| OS (months) | | | |
| Median | 38.5 | 27.3 | 0.2312 |
| Range | 17–67 | 10–63 | |
| Mean | 41.5 | 29.9 | |
As shown in Table
III, treatment failure was observed in 3 patients (37.5%) in
the CCRT group and in 4 patients (44.4%) in the RT group. Of the 3
patients with treatment failures in the CCRT group, the first site
of relapse was pelvic in 2 patients, while 1 patient developed
distant relapse. The pelvic failures involved uterine recurrence.
In the RT group, the 4 patients with treatment failure showed
distant relapse. The distant site of relapse involved para-aortic
lymph node, bone, lung and liver. The rate of distant relapse was
higher in the RT compared to the CCRT group. However, the
difference was not statistically significant (P=0.1428). No patient
showed synchronous pelvic and distant relapse sites. The failure
rate was not different in the two groups (P=0.5806).
 | Table IIIPatterns of failure. |
Table III
Patterns of failure.
| Site of relapse, time
until recurrence (month)
| |
|---|
| CCRT-group (n=8) | RT-group (n=9) | P-value |
|---|
| No. patients with
recurrence | 3 (37.5%) | 4 (44.4%) | 0.5806 |
| Type | | | |
| Pelvic | | | |
| 1 uterus, 4 | 0 | 0.1428 |
| 1 uterus, PLN,
12 | | |
| Distant | | | |
| 1 PAN, 38 | 1 PAN, bone, 5 | |
| | 1 liver, 6 | |
| | 1 lung, 9 | |
| | 1 PAN, 14 | |
Adverse effects
Generally, nedaplatin-based CCRT was well-tolerated.
Among the patients in the CCRT group, the most frequently observed
acute toxicity was hematologic. As shown in Table IV, in the CCRT group, grade 3 acute
toxicity was observed in 3 patients (37.5%). Of these patients, 1
had grade 3 neutropenia and required colony-simulating growth
factor for 2 days. There were no grade 3–4 non-hematologic
toxicities in either group.
 | Table IVAcute toxicity. |
Table IV
Acute toxicity.
| Grade 0 n, (%) | Grade 1 n, (%) | Grade 2 n, (%) | Grade 3 n, (%) | Grade 4 n, (%) |
|---|
| CCRT-group | | | | | |
| Hematologic | | | | | |
| Leukopenia | 1 (12.5) | 3 (37.5) | 1 (12.5) | 3 (37.5) | 0 (0.0) |
|
Neutropenia | 1 (12.5) | 2 (25.0) | 4 (50.0) | 1 (12.5) | 0 (0.0) |
| Anemia | 1 (12.5) | 6 (75.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) |
|
Thrombocytopenia | 8 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Non-hematologic | | | | | |
|
Nausea/vomiting | 7 (87.5) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 5 (62.5) | 2 (25.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) |
| Radiation
dermatitis | 7 (87.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| RT-group | | | | | |
| Hematologic | | | | | |
| Leukopenia | 4 (44.5) | 3 (33.3) | 2 (22.2) | 0 (0.0) | 0 (0.0) |
|
Neutropenia | 5 (55.6) | 2 (22.2) | 2 (22.2) | 0 (0.0) | 0 (0.0) |
| Anemia | 3 (33.3) | 2 (22.2) | 4 (44.5) | 0 (0.0) | 0 (0.0) |
|
Thrombocytopenia | 9 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Non-hematologic | | | | | |
|
Nausea/vomiting | 9 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 5 (55.6) | 3 (33.3) | 1 (11.1) | 0 (0.0) | 0 (0.0) |
| Radiation
dermatitis | 9 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
In the RT group, although grade 1–2 acute
toxicities, such as hematologic or gastrointestinal toxicity were
commonly observed, no grade 3–4 non-hematologic toxicities were
detected. The incidence of grade 3–4 acute toxicity was higher in
the CCRT compared to the RT group. No other severe side effect was
observed in either the CCRT or the RT group.
Discussion
CCRT, usually involving weekly cisplatin (CDDP) (40
mg/m2 for 6 weeks) in combination with RT, has been
established as the standard treatment for locally advanced cervical
carcinoma (4–8). However, CDDP has severe renal
toxicity, thus heavy hydration is then required to undergo
treatment-using CDDP. Nevertheless, nedaplatin is a derivative of
cisplatin with lower renal toxicity, although similar to
cisplatin.
Nedaplatin, an antineoplastic drug containing a
platinum complex, was developed in order to provide superior
antitumor effects to cisplatin and lower renal and gastrointestinal
toxicity. Based on the results of a phase I clinical trial of
nedaplatin, the drug was suggested to be administered as an
intravenous infusion of 100 mg/m2 at 4-week intervals
(19). A phase II clinical trial
using this dose regimen demonstrated a response rate of 46.3%
(19/41 patients) in patients with uterine cervical carcinoma
(11), which was superior to that
obtained with cisplatin (35.9%; 14/39 patients). Regarding adverse
drug reactions, although the nephrotoxicity of nedaplatin was
confirmed to be milder compared to cisplatin, certain patients
developed grade 3 or 4 myelosuppression (33.6 and 31.1% for
thrombocytopenia and leukopenia, respectively). Nedaplatin has been
used clinically in Japan as an alternative to cisplatin for
patients with cervical carcinoma. Previous studies examining
nedaplatin-based CCRT have demonstrated that nedaplatin is an
effective and well-tolerated regimen for invasive cervical
carcinoma (20–22). However, the use of this agent in
the clinical setting of CCRT in patients with cervical carcinoma is
limited.
In their collaborative dose escalation study, Hatae
et al suggested that CCRT with the administration of
nedaplatin at 80 mg/m2 on Days 1 and 29 for cervical
carcinoma was safe and effective (18). In this study, the main side effects
for the nedaplatin dose of 80 mg/m2 were digestive
disorders including nausea and anorexia and bone marrow
suppression, such as leukopenia, neutropenia and thrombopenia. The
efficacy was partial response (PR) or better in the patients
(18). In the present study, a
retrospective analysis was carried out to evaluate whether or not
CCRT with the administration of nedaplatin at 80 mg/m2
on Days 1 and 29 is safe and superior to RT alone in Japanese
patients with advanced stage of cervical carcinoma.
Our retrospective study demonstrated no significant
improvement in the initial response, PFS and OS in patients treated
with nedaplatin-based CCRT, compared to RT alone. Mainly due to the
small number of patients enrolled, as well as the retrospective
nature of this study, other potential biases, such as the age of
the patients may have influenced the results.
Nedaplatin-based CCRT was well-tolerated in our
patient population. Grade 3–4 leukopenia was observed in 3/8
(37.5%) of patients treated with nedaplatin-based CCRT, which was
higher compared to the RT-group. This incidence of grade 3–4
leukopenia was lower compared to CCRT with weekly nedaplatin in
advanced uterine cervical carcinoma (44.5–50.0%) (21,22).
One patient treated with nedaplatin-based CCRT required granulocyte
colony-stimulating factor (G-CSF) injections for 2 days. No grade 3
or greater non-hematologic toxicity was observed in these treatment
groups. Nevertheless, no significant differences were observed in
the length of radiotherapy in these treatment groups, suggesting
that nedaplatin-based CCRT using the present regimen was clinically
well-tolerated.
As shown in Table
III, the difference in the failure patterns was not
statistically significant (P= 0.1428), while nedaplatin-based CCRT
resulted in fewer distant relapses in this patient population.
However, only one patient had distant recurrence in
nedaplatin-based CCRT, the 4 patients with relapse had distant
recurrence in the RT group. This finding suggests that
nedaplatin-based CCRT may control distant relapses.
Nevertheless, the statistical power of this study is
limited due to several reasons, such as the relatively small cohort
of patients, the heterogeneity of the patient population, as well
as its retrospective nature. Consequently, additional studies
conducted on a mature population are strongly recommended.
References
|
1.
|
Goonatillake S, Khong R and Hoskin P:
Chemoradiation in gynaecological cancer. Clin Oncol (R Coll
Radiol). 21:566–572. 2009.
|
|
2.
|
Pearcey R, Brundage M, Drouin P, et al:
Phase III trial comparing radical radiotherapy with and without
cisplatin chemotherapy in patients with advanced squamous cell
cancer of the cervix. J Clin Oncol. 20:966–972. 2002.
|
|
3.
|
Tzioras S, Pavlidis N, Paraskevaidis E and
Ioannidis JP: Effects of different chemotherapy regimens on
survival for advanced cervical cancer: systematic review and
meta-analysis. Cancer Treat Rev. 33:24–38. 2007.
|
|
4.
|
Keys HM, Bundy BN, Stehman FB, et al:
Cisplatin, radiation, and adjuvant hysterectomy compared with
radiation and adjuvant hysterectomy for bulky stage IB cervical
carcinoma. N Engl J Med. 340:1154–1161. 1999.
|
|
5.
|
Morris M, Eifel PJ, Lu J, et al: Pelvic
radiation with concurrent chemotherapy compared with pelvic and
para-aortic radiation for high-risk cervical cancer. N Engl J Med.
340:1137–1143. 1999.
|
|
6.
|
Rose PG, Bundy BN, Watkins EB, et al:
Concurrent cisplatin-based radiotherapy and chemotherapy for
locally advanced cervical cancer. N Engl J Med. 340:1144–1153.
1999.
|
|
7.
|
Whitney CW, Sause W, Bundy BN, et al:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stage IIB–IVA
carcinoma of the cervix with negative para-aortic lymph nodes: a
gynecologic oncology group and southwest oncology group study. J
Clin Oncol. 17:1339–1348. 1999.
|
|
8.
|
Peters WA III, Liu PY, Barrett RJ II, et
al: Concurrent chemotherapy and pelvic radiation therapy compared
with pelvic radiation therapy alone as adjuvant therapy after
radical surgery in high-risk early-stage cancer of the cervix. J
Clin Oncol. 18:1606–1613. 2000.
|
|
9.
|
Fukuda M, Shinkai T, Eguchi K, et al:
Phase II study of (glycolate-O,O′) diammineplatinum(II), a novel
platinum complex, in the treatment of non-small cell lung cancer.
Cancer Chemother Pharmacol. 26:393–396. 1990.
|
|
10.
|
Inuyama Y, Miyake H, Horiuchi M, Hayasaki
K, Komiyama S and Ota K: [A late phase II clinical study of
cis-diammine glycolato platinum, 254-S, for head and neck cancers].
Gan To Kagaku Ryoho. 19:871–877. 1992.
|
|
11.
|
Kato T, Nishimura H, Yakushiji M, et al:
[Phase II study of 254-S (cis-diammine glycolato platinum) for
gynecological cancer]. Gan To Kagaku Ryoho. 19:695–701. 1992.
|
|
12.
|
Uehara T, Watanabe H, Itoh F, et al:
Nephrotoxicity of a novel antineoplastic platinum complex,
nedaplatin: a comparative study with cisplatin in rats. Arch
Toxicol. 79:451–460. 2005.
|
|
13.
|
Kawai Y, Taniuchi S, Okahara S, Nakamura M
and Gemba M: Relationship between cisplatin or nedaplatin-induced
nephrotoxicity and renal accumulation. Biol Pharm Bull.
28:1385–1388. 2005.
|
|
14.
|
Kameyama Y, Okazaki N, Nakagawa M, Koshida
H, Nakamura M and Gemba M: Nephrotoxicity of a new platinum
compound, 254-S, evaluated with rat kidney cortical slices. Toxicol
Lett. 52:15–24. 1990.
|
|
15.
|
Sasaki Y, Amano T, Morita M, et al: Phase
I study and pharmacological analysis of
cis-diammine(glycolato)platinum (254-S; NSC 375101D) administered
by 5-day continuous intravenous infusion. Cancer Res. 51:1472–1477.
1991.
|
|
16.
|
Nakamura Y, Hasegawa M, Hayakawa K, et al:
Induction of p53-dependent apoptosis in vivo by nedaplatin
and ionizing radiation. Oncol Rep. 7:261–265. 2000.
|
|
17.
|
Tanaka T, Yukawa K and Umesaki N:
Radiation reduces carboplatin sensitivity and enhances nedaplatin
sensitivity in cervical squamous cell carcinoma in vitro. Eur J
Gynaecol Oncol. 28:352–355. 2007.
|
|
18.
|
Hatae M, Takahashi T, Kodama S, et al: [A
dose escalation study of concurrent chemoradiation therapy with
nedaplatin for cervical cancer]. Gan To Kagaku Ryoho. 32:473–478.
2005.
|
|
19.
|
Ota K, Wakui A, Majima H, et al: [Phase I
study of a new platinum complex 254-S, cis-diammine
(glycolato)-platinum (II)]. Gan To Kagaku Ryoho. 19:855–861.
1992.
|
|
20.
|
Mabuchi S, Ugaki H, Isohashi F, et al:
Concurrent weekly nedaplatin, external beam radiotherapy and
high-dose-rate brachytherapy in patients with FIGO stage IIIb
cervical cancer: a comparison with a cohort treated by radiotherapy
alone. Gynecol Obstet Invest. 69:224–232. 2010.
|
|
21.
|
Yokoyama Y, Takano T, Nakahara K, et al: A
phase II multicenter trial of concurrent chemoradiotherapy with
weekly nedaplatin in advanced uterine cervical carcinoma: Tohoku
Gynecologic Cancer Unit Study. Oncol Rep. 19:1551–1556. 2008.
|
|
22.
|
Niibe Y, Tsunoda S, Jobo T, et al: Phase
II study of radiation therapy combined with weekly nedaplatin in
locally advanced uterine cervical carcinoma (LAUCC): kitasato
gynecologic radiation oncology group (KGROG 0501)-initial analysis.
Eur J Gynaecol Oncol. 29:222–224. 2008.
|