Introduction
Albumin is the most abundant plasma protein
synthesized primarily by liver cells. It is important in regulating
blood volume by maintaining the oncotic pressure and also serving
as a carrier for various molecules of low water solubility and
transport drugs. This highly soluble protein is present in human
plasma at a normal concentration between 35 and 50 g/l (1). The half-life of albumin is
approximately 19 days and accounts for at least 10% of liver
protein synthesis. This suggests that 10–15 g of albumin is
produced per day in healthy subjects (2). Serum albumin concentrations below the
normal range occur in a variety of disorders. Among these disorders
are those associated with malnutrition and malabsorption, where
protein is either not consumed in the diet or is lost through the
gastrointestinal tract (3).
Subsequently, a decrease in albumin concentration is frequently
observed in patients with liver disorders (4), and plasma albumin is also reduced in
cancer (5) and sepsis (6). Specifically, in nephrotic syndrome or
protein-losing gastroenteropathy, plasma albumin is highly reduced
to <20 g/l due to excessive albumin loss in urine or incomplete
albumin synthesis (7). Thus, lower
serum albumin (i.e., hypoalbuminaemia) is likely to be affected by
a higher binding affinity of certain drugs with albumin.
Sorafenib, 4-pyridine 2-carboxylic acid methylamide
4-methylbenzenesulfonate, is an orally administered multikinase
inhibitor that exhibits anti-angiogenic and anti-tumor activity
(8). This activity is caused by
targets in the kinase domains of vascular endothelial growth factor
(VEGF), platelet-derived growth factor (PDGF) and inhibition
signaling through the RAF kinases, including Raf-1 and Raf-B, or
RAF/mitogen-activated protein (MAP)/extracellular signal-regulated
kinase (ERK) kinase (MEK)/ERK (RAF/MEK/ERK) cascade (9,10).
Sorafenib as a single agent has demonstrated preclinical and
clinical activity against several types of tumorxx (11–15).
Sorafenib is known to bind to protein (>99.5%) and has a low
hepatic extraction ratio, suggesting that protein binding is
important in sorafenib pharmacokinetic variability (16). Thus, variations in sorafenib
binding may contribute to the variability in sorafenib exposure. A
recent study reported the characterization of in vitro
sorafenib binding properties to albumin and the effect of
albuminemia on sorafenib clearance and its disposition in cancer
patients (17). Similar reports
have shown that sorafenib is highly protein-bound in human plasma
with a higher affinity towards albumin and that limited free drugs
may play a role in its borderline clinical activity (18).
In this study, we examined the effect of both
extracellular (exogenous) and tissue (endogenous) albumin on
sorafenib-induced cytotoxicity using two major in vitro
culture cell lines, human hepatoma Huh-7 cells and
androgen-independent prostate cancer PC-3 cells. In addition, we
evaluated the cytotoxic effects of drug interaction with sorafenib
and warfarin, both of which exhibited a high affinity for albumin.
This is the first report to show that predominantly exogenous
albumin inhibits sorafenib-induced cytotoxicity resulting from its
extracellular binding properties. These data may be useful in the
clinical application of sorafenib.
Materials and methods
Materials and cell culture
Sorafenib was purchased from Toronto Research
Chemicals Incorporated (Ontario, Canada). Sorafenib was dissolved
in dimethyl sulfoxide (DMSO) and final concentrations were prepared
on the day of use from a stock solution. A concentration range of
0.1 to 60 μM was typically used in the different
experiments. Albumin, from human serum (catalog no. A1653, Sigma,
St. Louis, MO, USA) and all other reagents, unless otherwise
stated, were of the highest grade available and were purchased from
either Sigma or Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Cells were supplied by the Cell Resource Center for Biomedical
Research, Tohoku University (Sendai, Japan). The cells were
routinely cultured using standard methods as described in our
previous report (19) Research
protocols were approved by the Ethics Committees of Tohoku
Pharmaceutical University.
MTT assay
Cytotoxicity was assessed by the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
assay, a modification of our previously described method (20). Briefly, cells were seeded at
5×103 in 96-well plates and cultured overnight. The
cells were washed with serum-free medium. The medium was removed
and replaced with or without 4% albumin contents in serum-free
media. The cells were then incubated with sorafenib for 48 h,
followed by the addition of 10 μl MTT (5 mg/ml saline) to
each well. Samples were incubated for 90 min at 37°C, the
supernatant was aspirated, and the cells were lysed and solubilized
by the addition of 100 μl of 0.04 N HCl in isopropanol. The
absorbance of each well was determined at 590 nm using an Inter-med
model NJ-2300 Microplate Reader. The control cells were treated
with 0.5% DMSO (sorafenib vehicle). Cell viability was calculated
using the formula: absorbance in treated sample/absorbance in
control ×100 (%).
Western blotting
The effects of signal transduction by sorafenib or
confirmation of the transfected assay were estimated by western
blotting (21). Briefly, the cells
were washed with PBS and lysed in CelLytic M (Sigma), according to
the manufacturer’s instructions. Samples of each protein (30
μg) were loaded onto a 10% SDS-polyacrylamide gel. After
electrophoresis, the protein was transferred to a polyvinylidene
difluoride (PVDF) membrane. The protein was blocked with blocking
solution (25 mM Tris-HCl, pH 7.4, 137 mM NaCl, 2.68 mM KCl and 5%
skim milk) for 4 h and reacted with antibody overnight at 4°C. The
membrane was then washed with blocking solution without skimmed
milk, and incubated with horseradish peroxidase-linked secondary
antibody for 1 h. After another wash, the protein levels were
analyzed by enhanced chemiluminescence with an ECL plus western
blotting detection system (Amersham, Arlington Heights, IL, USA).
The primary antibodies used were: VEGF (Calbiochem, Darmstadt,
Germany) and Raf-B (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). Albumin and any other antibodies were from Cell Signaling
(Beverly, MA, USA).
Transfection studies
siRNA-albumin (siALB) and siRNA-control as
non-targeting siRNA (Negative control: Neg) were transfected into
Huh-7 cells using HyperFect transfection reagent (Qiagen, Valencia,
CA, USA) according to the manufacturer's instructions. A
non-targeting siRNA was used as a control for the
non-sequence-specific effects of the transfected siRNAs. The siRNAs
(Qiagen) used were siALB from GeneSolution siRNA (catalog no.
1027416) and negative control from AllStars negative control siRNA
(catalog no. 1027281). Briefly, Huh-7 (1×105) cells
containing each siRNA (final concentration, 40 nM) and HyperFect
reagent were incubated for 24 h, assessed by western blotting using
the expression of β-actin as the control and their cytotoxic
effects were evaluated by sorafenib.
The albumin was overexpressed using the vector
established in our previous study (19). PC-3 cells were transfected with
pcDNA 3.1 plasmid DNA alone (empty) or containing Albumin cDNA
(ALB) using Lipofectamine™ LTX reagent (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions. After being
cultured for 24 h without antibiotics, the transfected cells were
examined for western blotting using the expression of β-actin as
the control or using the cytotoxicity assay.
Statistical analysis
Statistical analysis of the results was performed
using a one-way analysis of variance (ANOVA) followed by the
Williams' type multiple comparison test or a Bonferroni test among
multiple groups. P<0.05 was considered statistically
significant.
Results
Comparison of the effect of cells
supplemented with or without albumin in serum-free media on
sorafenib-induced cytotoxicity
Albumin is contained in the serum of culture media.
To explore the essentially exogenous albumin by sorafenib-induced
cytotoxicity, we examined the human hepatoma Huh-7 or prostate
cancer PC-3 cells, supplemented with or without albumin in
serum-free media. To perform the assessment of exogenous albumin in
this assay, a concentration of 4% of normal physiologically serum
content was used. The concentration of sorafenib up to 1 μM
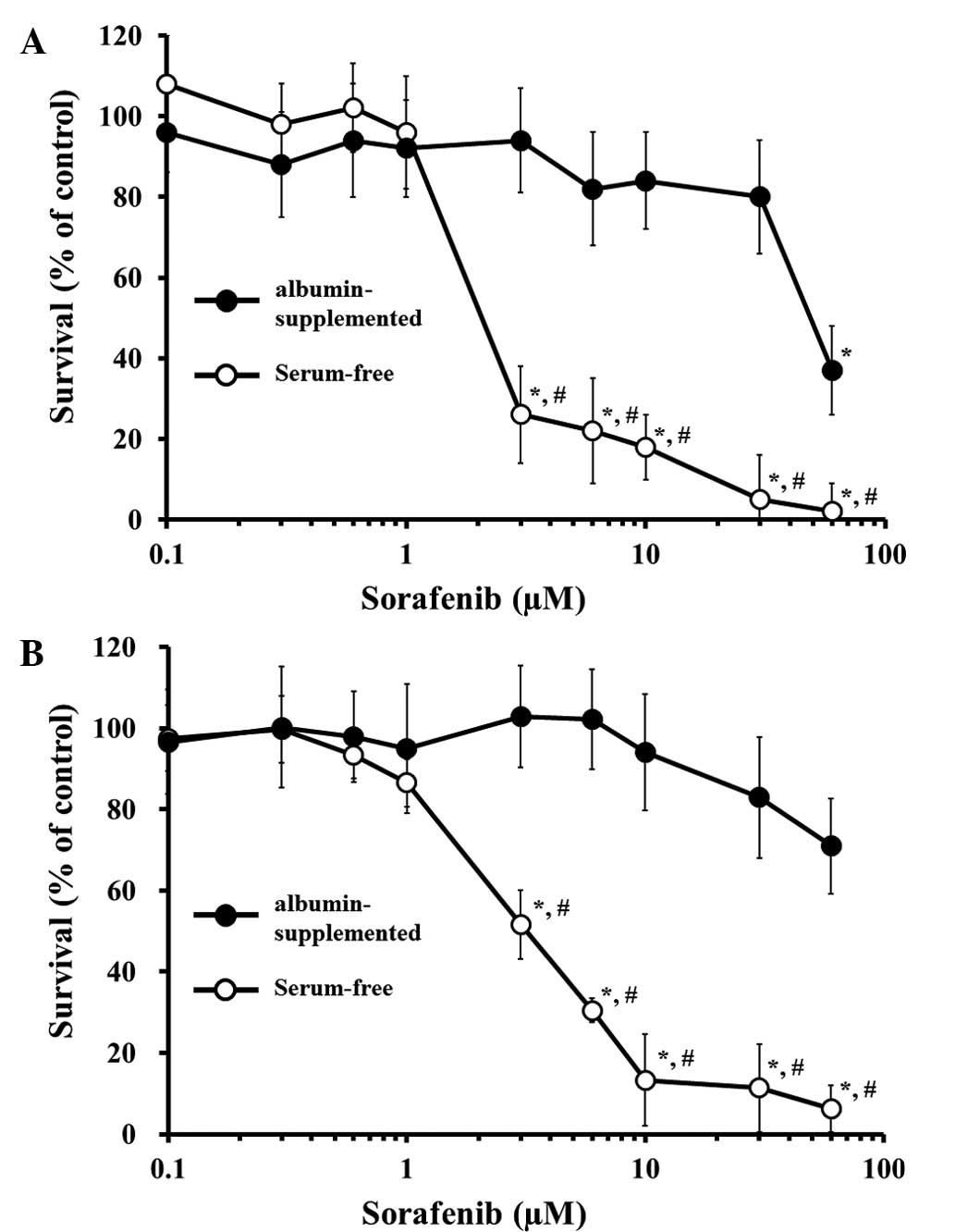
exhibited no cytotoxic effect on the two cell lines whether or not
they were albumin-supplemented or serum-free (Fig. 1A and B). The cell lines were
relatively robust to cell stress since when the serum was removed
from the culture media, no spontaneous apoptotic effects were
observed in the culture for 48 h (data not shown). In serum-free
conditions of >1 μM, sorafenib showed a significant
concentration-dependent cytotoxic effect in the two cells. By
contrast, albumin-supplemented conditions potently inhibited the
cytotoxic effect of sorafenib up to 10 or 30 μM in Huh-7 or
PC-3 cells, respectively. The calculated 50% cell growth inhibition
(IC50) of serum-free or albumin-supplemented conditions
in Huh-7 was 1.628 or 44.62 μM, and 3.319 or 153.7
μM, respectively, in PC-3. Thus, the presence of exogenous
albumin markedly blocked the sorafenib-induced cytotoxicity in the
two cells.
Concentration-dependent effects of
exogenous albumin on sorafenib-induced cytotoxicity
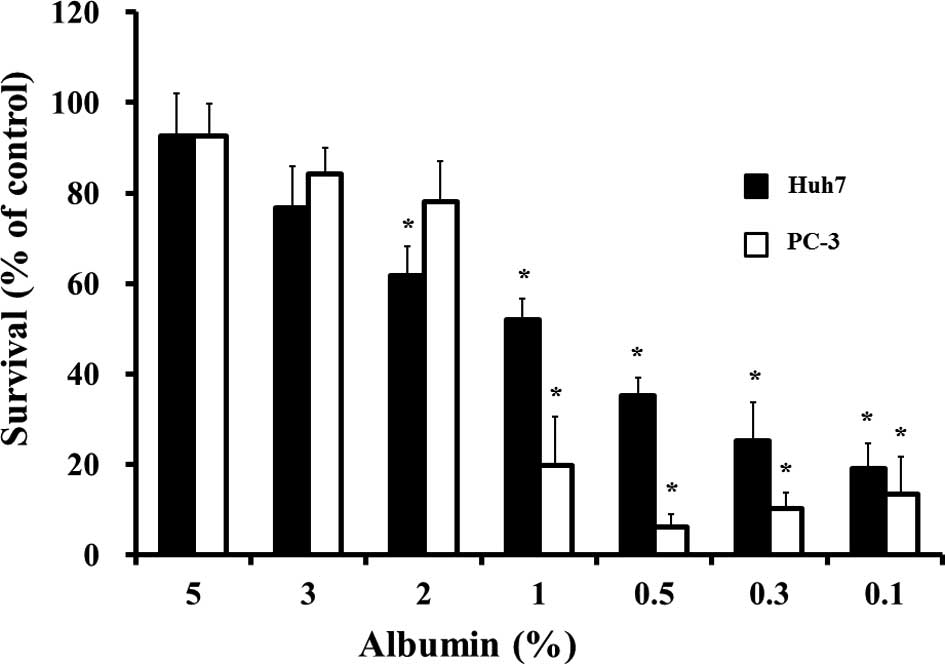
We examined the concentration-dependent effect of
exogenous albumin on 10 μM sorafenib-induced cytotoxicity in
Huh-7 or PC-3 cells. Exogenous lower albumin <2% in Huh-7, or 1%
in PC-3, respectively, exhibited an increased
concentration-dependent cytotoxic effect after 10 μM
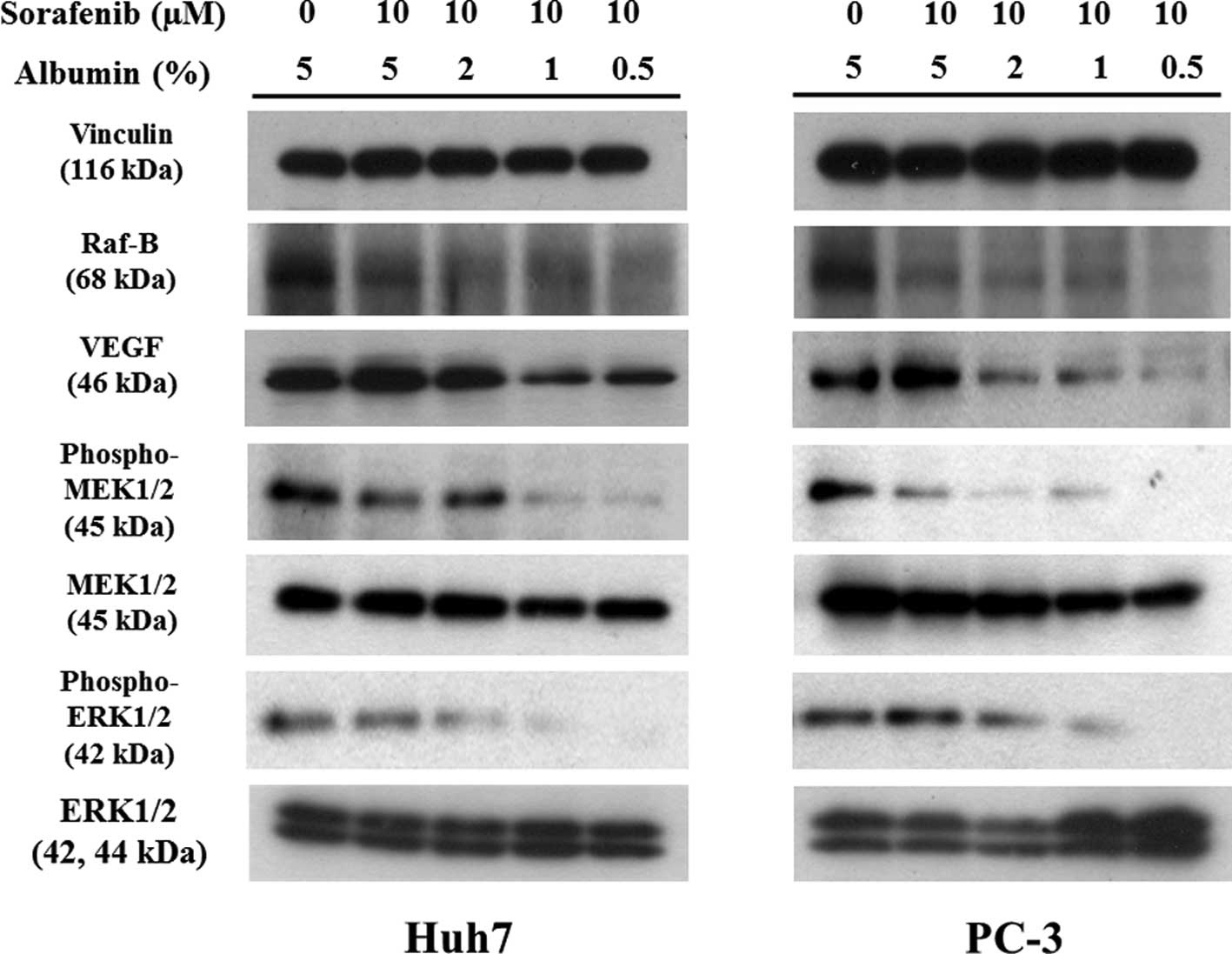
sorafenib was added in each of the cells (Fig. 2). Western blotting was used to
confirm the lower albumin concentration on the pharmacological
pathway of signal transduction by sorafenib in the two cells
(Fig. 3). No change was observed
in the expression of vinculin as the control or total MEK1/2 or
ERK1/2 proteins as each signal control. However, previous reported
changes of signal proteins, such as Raf-B, VEGF and the
phosphorylation of MEK1/2 or ERK1/2, following incubation with 10
μM sorafenib, correlated with the decrease in co-incubation
with albumin in a concentration-dependent manner. Accordingly,
these changes suggest that the reduction of cell survival and the
concentration of exogenous lower albumin may drive the effect of
sorafenib.
Drug interaction with warfarin and
sorafenib on cell survival reduction
Warfarin, a representative coumarin anticoagulant is
known for its interaction with numerous drugs. This drug has potent
binding activity with albumin. As stated above, the effect of
sorafenib pertains to exogenous albumin. Thus, we examined the
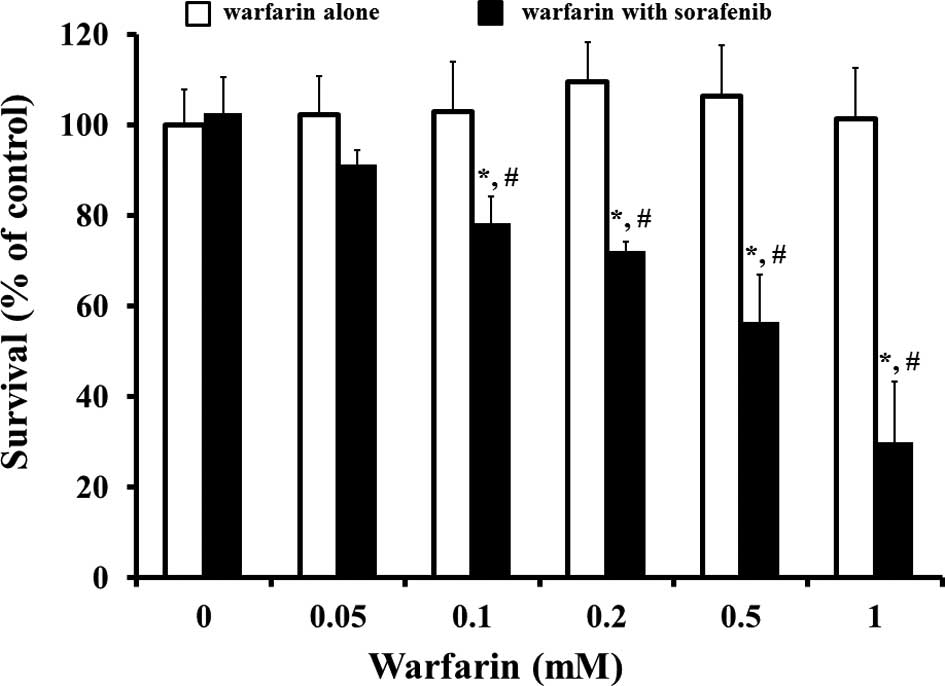
effect of warfarin on 3 μM sorafenb-induced cytotoxicity in
Huh-7 cells with albumin-supplemented conditions (Fig. 4). A single incubation with warfarin
up to 1 mM did not show any cytotoxic effect on Huh-7 during the 48
h culture. Co-incubation with warfarin of >0.05 mM and sorafenib
at 3 μM showed a significant warfarin
concentration-dependent increase in the reduction of cell survival.
Similarly, warfarin was found to potentiate the cytotoxic effect of
sorafenib in PC-3 cells (data not shown).
Effects of endogenous albumin on
sorafenib-induced cytotoxicity
In the physiological conditions, albumin exists in
extracellular (exogenous) form as serum and in intracellular form
on tissues (endogenous). Furthermore, we investigated the effect of
endogenous albumin expression on sorafenib-induced cytotoxicity
using a siRNA knock-down system in Huh-7 cells or the transfected
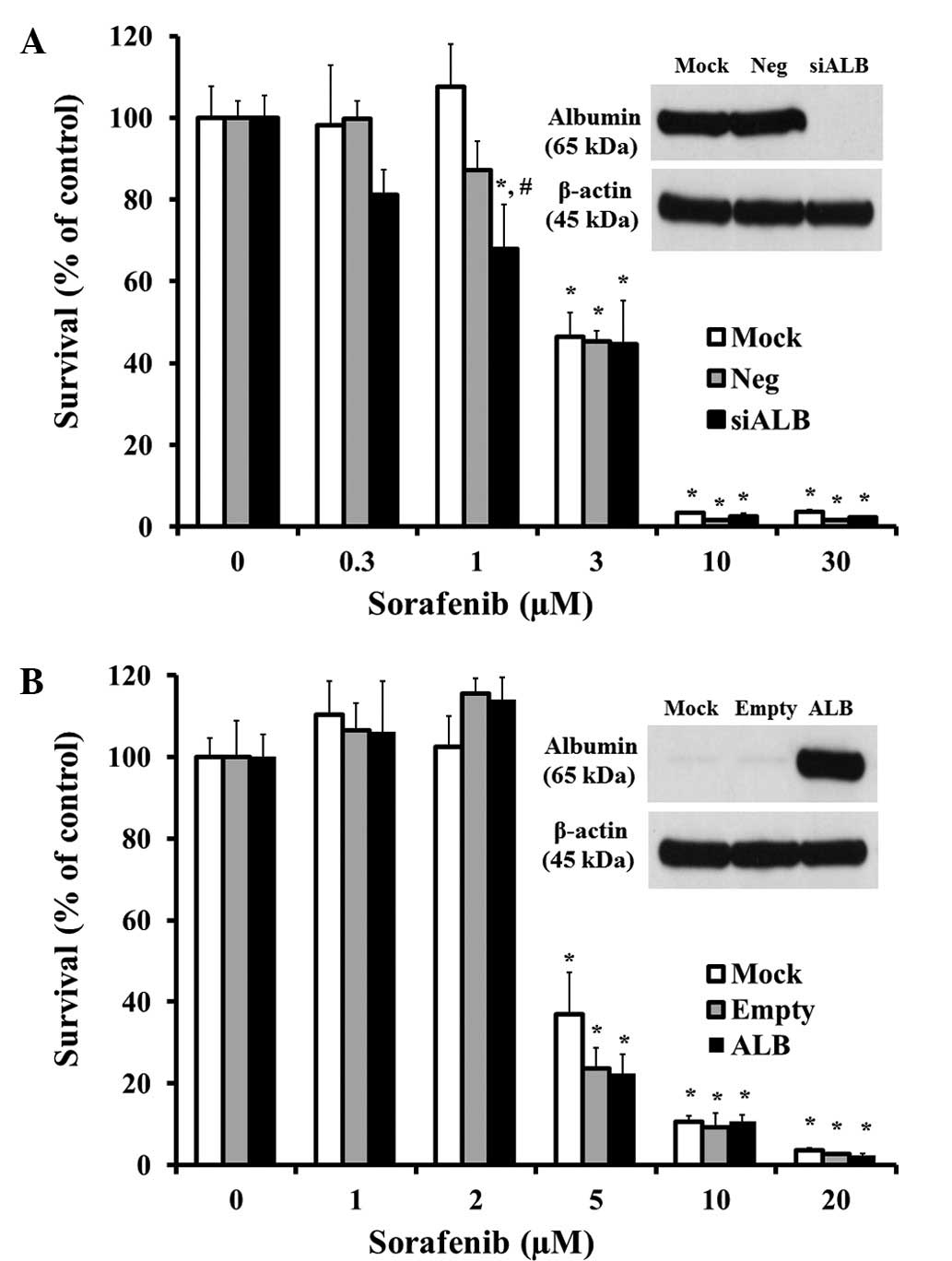
expression vector assay in PC-3 cells (Fig. 4A and B). In the Huh-7 hepatoma
cells, the constantly expressed albumin protein was derived from
liver tissues, and the albumin expression was almost completely
knocked down by transfection with siALB (Fig. 5A). Incubation with sorafenib at 0.3
to 30 μM has shown a concentration dependent cell survival
reduction in mock (not transfected with siRNA), siNeg (transfected
with non-specific siRNA as negative control) and siALB Huh-7 cells.
However, no significant changes occurred in the transfected cell
conditions, with the exception of incubation with 1 μM
sorafenib in the siALB cells. Prostate cancer as PC-3 did not
entirely express the albumin protein in the usual culture state,
whereas the introduction of the albumin expression vector
apparently overexpressed albumin in this study (Fig. 5B). Similarly, these cells exhibited
concentration-dependent cytotoxic effects by sorafenib, but there
were no differences regarding whether or not they were transfected
with the albumin vector. These data indicated that tissue albumin
rarely affects sorafenib-induced cytotoxicity.
Discussion
Sorafenib is known to bind to albumin resulting in a
strong effect on sorafenib clearance in albumine mia and therefore
on its disposition in adult cancer patients with advanced solid
tumors (17,18). In several pathological states,
endogenous ligands may accumulate to relatively high concentrations
able to displace drugs highly bound to albumin, resulting in a
significant increase in the unbound fraction of drugs (22). Patients with advanced solid tumors
frequently exhibit denutrition and they have severe renal or
hepatic impairment. Under those circumstances, hypoalbuminemia,
hypertriglyceridemia or hyperbilirubinemia may occur. Therefore, an
increase in sorafenib unbound fraction resulting in an enhanced
clearance should be expected. This study demonstrates that
exogenous albumin inhibits sorafenib-induced cytotoxicity in human
cancer cell lines. This result suggests that in the case of serious
side effects, depending on the higher plasma concentration of
sorafenib in cancer patients following sorafenib treatments,
exogenous albumin may be used as an antidote drug for overdose of
sorafenib. In serum-free media, albumin is often used instead of
fetal bovine serum in the cell culture media, improving the
performance of a wide range of cell types, including stem and
primary cells (23). Thus, albumin
is added to the cell culture media as a supplement to increase the
growth and productivity of cells (24). Our in vitro study may not
completely reflect cell growth, but the albumin-supplemented groups
did not show any change, compared with the usual culture conditions
in the experimental period.
The cytotoxic effect of sorafenib has shown a
statistically significant increase below the concentration of 2%
albumin compared with the 5% albumin-supplemented conditions
(Fig. 2). In clinical cases,
however, a 2% albumin concentration in plasma is regarded as a
serious pathological event. Despite the enhanced effect of a lower
concentration of albumin in our experimental study, the use of
sorafenib may be used in cancer patients with albuminemia. A
decrease in the expression in Raf-B and the phosphorylation of MEK
by sorafenib showed no change for either the 5 or 2% albumin
concentration in the two cells (Fig.
3). These results suggest that sorafenib inhibits kinase
activation without exhibiting significant cytotoxic effects. In
contrast to standard antineoplastics, sorafenib was also shown to
be suitable for long-term administration due to its good safety
profile (25). Currently,
sorafenib is approved for the treatment of patients with advanced
renal cell carcinoma and those with hepatocellular carcinoma. In
this study, we used two distinct human cancer cells, hepatoma and
prostate cancer. Patients with metastatic androgen-independent
prostate cancer have a poor prognosis with few therapeutic options,
all of which are palliative. The effect of sorafenib on PC-3, a
hormone refractory, metastatic prostate carcinoma cell line was
also examined. In their study, Ullén et al indicated that
sorafenib induced apoptosis and autophagy in two hormone refractory
prostate cancer cells in vitro, including PC-3 (26). Our results partly supported
previous data which showed that sorafenib can be widely applied in
other tumor types. As shown in Fig.
4, co-incubation with warfarin enhanced the sorafenib-induced
cytotoxicity; however, the experimental concentration of warfarin
used in this study was much higher than that of the clinical plasma
concentration at almost 567.6±123.3 ng/ml (1.72±0.37 μM)
(27). Although no serious side
effects of the interaction between sorafenib and warfarin have been
known to occur in clinical patients, the combination should be used
with care. Of note, combined use of sorafenib with warfarin is
written as ‘attention’ on the information provided in the
NEXAVAR® (sorafenib) package insert.
Drug distribution is a function of both plasma
protein and tissue protein bindings. One of the physiological
actions of albumin involves transporting drugs. If tissue albumin
attracts or blocks sorafenib in its high affinity towards albumin,
it may serve as a good target or delivery carrier for cancer
chemotherapy. Thus, we hypothesized that change in tissue albumin
may affect sorafenib-induced cytotoxicity. In contrast to our
expectation, sorafenib-induced cytotoxicity has changed little in
tissue albumin, regardless of whether or not albumin was
overexpressed or knocked-down in experimental cells (Fig. 5A and B). Currently, no reports are
available examining the cancer chemotherapeutic drug effects of the
change of tissue albumin. Thus, the cytotoxic effect of sorafenib
is not likely to be correlated with the expression of tissue
albumin as existing cancer chemotherapeutic drugs.
In conclusion, sorafenib-induced cytotoxicity is
inhibited by extracellular albumin but not tissue albumin in human
cancer cell lines. These mechanisms depend on the high-bound
properties of sorafenib and exogenous albumin. Our results suggest
that in case of serious side effects in cancer patients receiving
sorafenib treatments, exogenous albumin may be used as an antidote
drug for overdose of sorafenib. However, even then care should be
taken when adminstering a combined use of drugs with high-bound
affinity towards albumin, such as warfarin, in cancer patients with
albuminemia. Therefore, our findings may be useful in the cancer
therapeutic strategy by sorafenib.
References
|
1.
|
Peters T: All About Albumin: Biochemistry,
Genetics, and Medical Applications. Academic Press; San Diego:
1996
|
|
2.
|
Ballmer PE, McNurlan MA, Milne E, Heys SD,
Buchan V, Calder AG and Garlick PJ: Measurement of albumin
synthesis in humans: a new approach employing stable isotopes. Am J
Physiol. 259:E797–E803. 1990.
|
|
3.
|
Waldmann TA: Protein-losing enteropathy
and kinetic studies of plasma protein metabolism. Semin Nucl Med.
2:251–263. 1972.
|
|
4.
|
Ballmer PE, Walshe D, McNurlan MA, Watson
H, Brunt PW and Garlick PJ: Albumin synthesis rates in cirrhosis:
correlation with Child-Turcotte classification. Hepatology.
18:292–297. 1993.
|
|
5.
|
Pasanisi F, Orban A, Scalfi L, Alfonsi L,
Santarpia L, Zurlo E, Celona A, Potenza A and Contaldo F:
Predictors of survival in terminal-cancer patients with
irreversible bowel obstruction receiving home parenteral nutrition.
Nutrition. 17:581–584. 2001.
|
|
6.
|
Ruot B, Breuillé D, Rambourdin F, Bayle G,
Capitan P and Obled C: Synthesis rate of plasma albumin is a good
indicator of liver albumin synthesis in sepsis. Am J Physiol
Endocrinol Metab. 279:E244–E251. 2000.
|
|
7.
|
Ballmer PE: Causes and mechanisms of
hypoalbuminaemia. Clin Nutr. 20:271–273. 2001.
|
|
8.
|
Wilhelm S, Carter C, Lynch M, Lowinger T,
Dumas J, Smith RA, Schwartz B, Simantov R and Kelley S: Discovery
and development of sorafenib: a multikinase inhibitor for treating
cancer. Nat Rev Drug Discov. 5:835–844. 2006.
|
|
9.
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109.
2004.
|
|
10.
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006.
|
|
11.
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib for treatment of renal cell carcinoma: final efficacy and
safety results of the phase III treatment approaches in renal
cancer global evaluation trial. J Clin Oncol. 27:3312–3318.
2009.
|
|
12.
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008.
|
|
13.
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008.
|
|
14.
|
Kloos RT, Ringel MD, Knopp MV, et al:
Phase II trial of sorafenib in metastatic thyroid cancer. J Clin
Oncol. 27:1675–1684. 2009.
|
|
15.
|
Dahut WL, Scripture C, Posadas E, et al: A
phase II clinical trial of sorafenib in androgen-independent
prostate cancer. Clin Cancer Res. 14:209–214. 2008.
|
|
16.
|
Jain L, Woo S, Gardner ER, Dahut WL, Kohn
EC, Kummar S, Mould DR, Giaccone G, Yarchoan R, Venitz J and Figg
WD: Population pharmacokinetic analysis of sorafenib in patients
with solid tumours. Br J Clin Pharmacol. 72:294–305. 2011.
|
|
17.
|
Tod M, Mir O, Bancelin N, et al:
Functional and clinical evidence of the influence of sorafenib
binding to albumin on sorafenib disposition in adult cancer
patients. Pharm Res. 28:3199–3207. 2011.
|
|
18.
|
Villarroel MC, Pratz KW, Xu L, Wright JJ,
Smith BD and Rudek MA: Plasma protein binding of sorafenib, a multi
kinase inhibitor: in vitro and in cancer patients. Invest New
Drugs. Nov 17–2011, (E-pub ahead of print).
|
|
19.
|
Kanno S, Kurauchi K, Tomizawa A, Yomogida
S and Ishikawa M: Albumin modulates docosahexaenoic acid-induced
cytotoxicity in human hepatocellular carcinoma cell lines. Toxicol
Lett. 200:154–161. 2011.
|
|
20.
|
Kanno S, Higurashi A, Watanabe Y, Shouji
A, Asou K and Ishikawa M: Susceptibility to cytosine arabinoside
(Ara-C)-induced cytotoxicity in human leukemia cell lines. Toxicol
Lett. 152:149–158. 2004.
|
|
21.
|
Kanno SI, Maeda N, Tomizawa A, Yomogida S,
Katoh T and Ishikawa M: Involvement of p21waf1/cip1
expression in the cytotoxicity of the potent histone deacetylase
inhibitor spiruchostatin B towards susceptible NALM-6 human B cell
leukemia cells. Int J Oncol. 40:1391–1396. 2012.
|
|
22.
|
Tesseromatis C and Alevizou A: The role of
the protein-binding on the mode of drug action as well the
interactions with other drugs. Eur J Drug Metab Pharmacokinet.
33:225–230. 2008.
|
|
23.
|
Mather JP: Making informed choices:
medium, serum, and serum-free medium. How to choose the appropriate
medium and culture system for the model you wish to create. Methods
Cell Biol. 57:19–30. 1998.
|
|
24.
|
Fanali G, di Masi A, Trezza V, Marino M,
Fasano M and Ascenzi P: Human serum albumin: from bench to bedside.
Mol Aspects Med. 33:209–290. 2012.
|
|
25.
|
Blanchet B, Billemont B, Barete S,
Garrigue H, Cabanes L, Coriat R, Francès C, Knebelmann B and
Goldwasser F: Toxicity of sorafenib: clinical and molecular
aspects. Expert Opin Drug Saf. 9:275–287. 2010.
|
|
26.
|
Ullén A, Farnebo M, Thyrell L, Mahmoudi S,
Kharaziha P, Lennartsson L, Grandér D, Panaretakis T and Nilsson S:
Sorafenib induces apoptosis and autophagy in prostate cancer cells
in vitro. Int J Oncol. 37:15–20. 2010.
|
|
27.
|
Sun S, Wang M, Su L, Li J, Li H and Gu D:
Study on warfarin plasma concentration and its correlation with
international normalized ratio. J Pharm Biomed Anal. 42:218–222.
2006.
|