Introduction
In prostate cancer, as in other malignancies, it is
important to assess the degree of malignancy or the prognosis of
patients in order to determine the appropriate treatment. Risk
classification, the grouping of patients on the basis of combining
several clinical factors, is being widely used in the clinical
setting. Several pre-treatment risk classification models for
prostate cancer have been proposed thus far, with the D’Amico
classification being the most widely-used one. According to this
classification model, in high-risk prostate cancer patients the
probability of recurrence subsequent to local treatment alone is
low (1). However, the present
study aimed to assess the outcome of radical prostatectomy (RP) in
high-risk patients with no pre-surgical treatment, with a view to
investigate the possibility of complete cure by RP alone in
Japanese high-risk prostate cancer patients.
Materials and methods
Patient characteristics and risk-group
classification
The patients underwent prostate biopsy and were
diagnosed with prostate cancer in the National Kyushu Cancer Center
(Fukuoka, Japan) and at additional associated institutions. Tissue
specimens, obtained from 436 patients between August 1998 and
December 2008 were reviewed in embedded whole-mount antegrade RP
specimens with adenocarcinoma. The patients underwent pelvic lymph
node dissection during the same time period. One hundred and
thirty-nine patients were excluded from this study, 69 patients due
to a past hormonal therapy, 6 patients due to an unclear biopsy or
prostatectomy specimen, and 64 patients due to an orchiectomy
during RP. At our institution, the one patient classified into the
high-risk group according to the D’Amico criteria underwent an
orchiectomy during the same time period until December 2004. The
patients were Japanese, (median age, 67 years; range, 47–77) and
the value of the prostate-specific antigen (PSA) ranged from 0.8 to
88.0 ng/ml (median, 7.4 ng/ml). A median follow-up period after
surgery was 60 months.
The patients were classified into three risk groups
according to the D’Amico criteria. The low- (PSA<10 and Gleason
score ≤6 and T1-T2a), intermediate- (PSA, 10.1–20.0 and/or Gleason
score, 7 and/or T2b) and high-risk (PSA>20 or Gleason score ≥8
or T2c) groups comprised 63 (21.2%), 122 (41.1%) and 112 (37.7%)
patients, respectively. Additional analyses were carried out using
the more restrictive definition according to which clinical stage
T2c is an intermediate- rather than a high-risk group
characteristic. Twenty-five patients likely to be classified into
the high-risk group by the standard definition and into the
intermediate-risk group by the more restrictive definition were
evaluated as a separate ‘intermediate/high’ group. One pathologist
evaluated the degree of malignancy of the biopsy and prostatectomy
specimens according to the 2005 International Society of Urological
Pathology (ISUP) Consensus Conference on Gleason grading system
(2) and pathological stage based
on the 2009 TNM classification (3).
Methods
Prostatectomy specimens were stained and fixed in
10% neutral-buffered formalin (NBF). The prostate was sectioned
into 3-mm sections in the plane perpendicular to the long axis of
the gland, from the prostate apex to the tip of the seminal
vesicles, followed by hematoxylin and eosin (H&E) staining and
determination of extra prostatic extension (EPE). EPE was defined
as a tumor extending from the prostate to the periprostatic soft
tissue. The presence of tumor cells at the stained margin of the
resection was evaluated as a positive resection margin (pRM).
Organ-confined disease (OCD) was defined as pT2 without lymph node
metastasis, and specimen-confined disease (SCD) was defined as
either pT2 or pT3 without pRM or lymph node metastasis. The
follow-up schedule following RP involved a PSA assay every 3 months
for the first 2 years, every 4 months for the next 3 years and
every 6 month thereafter. Disease recurrence or PSA failure was
determined as the time point when the serum PSA level was >0.2
ng/ml, or RP was performed if the PSA did not decrease below 0.2
ng/ml after surgery. A number of patients that underwent RP were
subsequently treated with radiation and/or hormone therapy prior to
the serum PSA level exceeding 0.2 ng/ml. Therefore, for these
patients the time point of the adjuvant therapy was defined as the
date of disease recurrence.
Statistical analysis
Statistical analyses were carried out using the
JMP® version 8 software (SAS Institute, Inc., Cary, NC,
USA). The PSA failure-free rate was determined using the
Kaplan-Meier method. The significance of the clinicopathological
parameters associated with PSA failure was assessed using the Cox
proportional hazards regression model. The log-rank test was used
to determine differences among each risk group. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics
according to risk group classificaction
The clinicopathological characteristics of the three
risk groups are shown in Table I.
According to the D’Amico criteria, the low- (PSA<10 and Gleason
score ≤6 and T1-T2a), intermediate- (PSA, 10.1–20.0 and/or Gleason
score, 7 and/or T2b) and high-risk (PSA>20 or Gleason score ≥8
or T2c) groups comprised 63 (21.2%), 122 (41.1%) and 112 (37.7%)
patients, respectively. No differences were observed in the age of
the patients in the groups. According to the RP Gleason score, the
low-, intermediate- and high-risk groups had high-grade (Gleason
score ≥8) tumors in 14.3 (9/63), 15.6 (19/122) and 44.6% (50/112)
of patients, respectively. The tumor was organ-confined in 88.9
(56/63), 73.8 (90/122) and 65.2% (73/112) of patients,
respectively. Only one patient with lymph node metastasis in the
high-risk group was staged pT2. Lymph node involvement was observed
in one patient in the low-risk group and in two patients in the
high-risk group.
 | Table IClinicopathological characteristics
according to risk group classification. |
Table I
Clinicopathological characteristics
according to risk group classification.
| Characteristics | Risk groups
|
|---|
| Low | Intermediate | High |
|---|
| Total no. of
patients | 63 | 122 | 112 |
| Median age, years
(range) | 66 (47–77) | 67 (52–76) | 67 (48–77) |
| Clinical stage, n
(%) | | | |
| T1ab | 0 | 3 (2.4) | 3 (2.6) |
| T1c | 42 (66.7) | 64 (52.5) | 41 (36.6) |
| T2ab | 21 (33.3) | 55 (45.1) | 30 (26.8) |
| T2c | - | 0 | 33 (29.5) |
| T3 | - | - | 5 (4.5) |
| Preoperative PSA, n
(%) | | | |
| ≤4.0 | 8 (12.7) | 12 (9.9) | 6 (5.4) |
| 4.1–10.0 | 55 (87.3) | 78 (63.9) | 64 (57.1) |
| 10.1–20.0 | - | 32 (26.2) | 29 (25.9) |
| >20.1 | - | - | 13 (11.6) |
| Biopsy Gleason score,
n (%) | | | |
| 5 | 8 (12.7) | - | 2 (1.8) |
| 6 | 55 (87.3) | 12 (9.8) | 5 (4.5) |
| 7 | - | 110 (90.2) | 26 (23.2) |
| 8 | - | - | 27 (24.1) |
| 9 | - | - | 49 (43.7) |
| 10 | - | - | 3 (2.7) |
| Final Gleason score,
n (%) | | | |
| 5 | 2 (3.2) | 1 (0.8) | 2 (1.8) |
| 6 | 15 (23.8) | 11 (9.0) | 5 (4.5) |
| 7 | 37 (58.7) | 91 (74.6) | 55 (49.1) |
| 8 | 6 (9.5) | 6 (4.9) | 12 (10.7) |
| 9 | 3 (4.8) | 13 (10.7) | 38 (33.9) |
| Pathological stage, n
(%) | | | |
| pT2ab | 9 (14.3) | 18 (14.8) | 7 (6.3) |
| pT2c | 47 (74.6) | 72 (59.0) | 67 (59.8) |
| pT3a | 6 (9.5) | 31 (25.4) | 29 (25.9) |
| pT3b | 1 (1.6) | 1 (0.8) | 9 (8.0) |
| pN1 | 1 (1.6) | 0 | 2 (1.8) |
| RM1 | 6 (9.5) | 27 (22.1) | 22 (19.6) |
Concerning the high-risk group, the median PSA prior
to surgery was 8.6 ng/ml. Seventy-nine patients (70.5%) had a
biopsy Gleason score of ≥8. Forty-four patients (39.2%) had
non-palpable disease (cT1c). The Gleason scores for the RP
specimens were lower than those for the biopsy scores (downgraded)
in 46 patients (41.1%) and higher (upgraded) than those for the
biopsy scores in 20 patients (17.9%). Seven patients (15.2%) of the
downgraded groups had PSA failure, whereas 3 patients (15.0%) of
the upgraded and 10 (21.7%) of the same-graded groups had PSA
failure. Two patients from the same-graded groups with PSA failure
exhibited lymph node metastasis.
Adjuvant therapy subsequent to RP
Thirty-five patients had PSA failure subsequent to
RP. The PSA level in 4 patients did not decrease below 0.2 ng/ml
after surgery and, therefore, 4 of these patients received adjuvant
therapy. The PSA level of 21 patients was >0.2 ng/ml after
surgery, while 14 patients received adjuvant therapy. Ten patients
received adjuvant therapy prior to the serum PSA level reaching
>0.2 ng/ml.
PSA failure-free survival according to
risk group classification
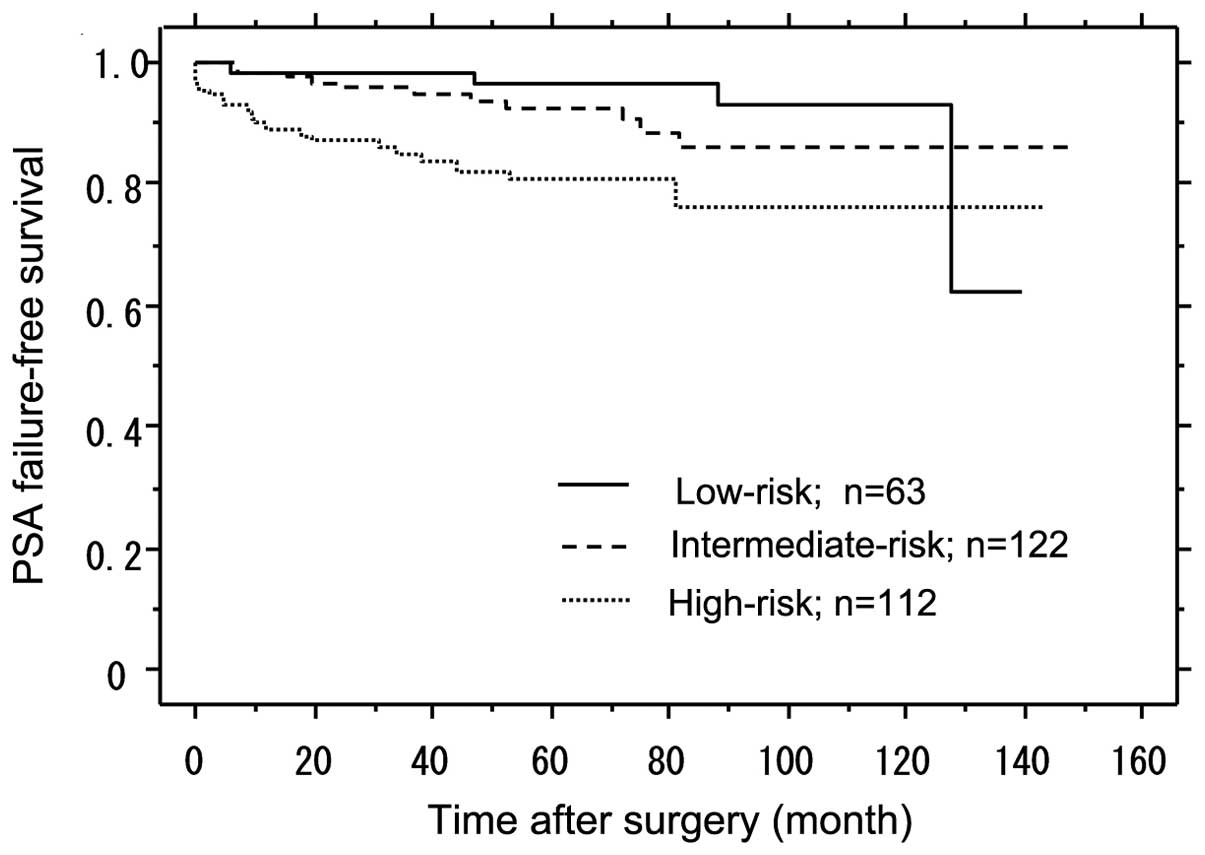
After the median follow-up period of 60 months, the
PSA failure-free rate in the low-, intermediate- and high-risk
groups was 96.5, 92.2 and 80.6%, respectively (Fig. 1). The difference between the high-
and intermediate-risk groups was statistically significant,
according to the log-rank test (P=0.017) (Fig. 1). The difference between the high-
and low-risk groups was statistically significant, according to the
log-rank test (P=0.009) (Fig
1).
Correlation between the characteristics
and PSA failure in the high-risk group
The correlation between the characteristics and PSA
failure in the high-risk group is shown in Table II. According to the Cox
proportional hazards analysis of the high-risk group, preoperative
variables, such as age, preoperative PSA, biopsy Gleason score and
clinical tumor stage were not significant predictors, and only
post-operative characteristics, such as the RP Gleason score,
organ-confined and specimen-confined status were significant
predictors based on the univariate analysis. In the multivariate
analysis, statistically significant differences were found in the
biopsy Gleason score and specimen-confined status in the patients
with and without PSA failure.
 | Table IICorrelation between characteristics
and PSA failure in the high-risk group. |
Table II
Correlation between characteristics
and PSA failure in the high-risk group.
| Characteristics | Hazard ratio | P-value | 95% Cl |
|---|
| Univariate
analysis | | | |
| Age <70 vs. ≥70
years | 0.903 | 0.824 | 0.368–2.217 |
| PSA | 1.012 | 0.362 | 0.986–1.039 |
| Biopsy Gleason
score ≤7 vs. ≥8 | 3.953 | 0.065 | 0.917–17.040 |
| cT1c vs. cT2 or
cT3 | 0.618 | 0.325 | 0.237–1.612 |
| RP Gleason score ≤7
vs. ≥8 | 2.917 | 0.028a | 1.119–7.600 |
| Organ-confined vs.
non-organ-confined | 3.379 | 0.007a | 1.378–8.286 |
| Specimen-confined
vs. non-specimen-confined | 4.718 | <0.001a | 1.955–11.389 |
| Multivariate
analysis | | | |
| Biopsy Gleason
score ≤7 vs. ≥8 | 4.332 | 0.017a | 1.247–27.289 |
| Specimen-confined
vs. non-specimen-confined | 5.024 | <0.001a | 2.047–12.337 |
PSA failure-free survival according to
SCD in the high-risk group
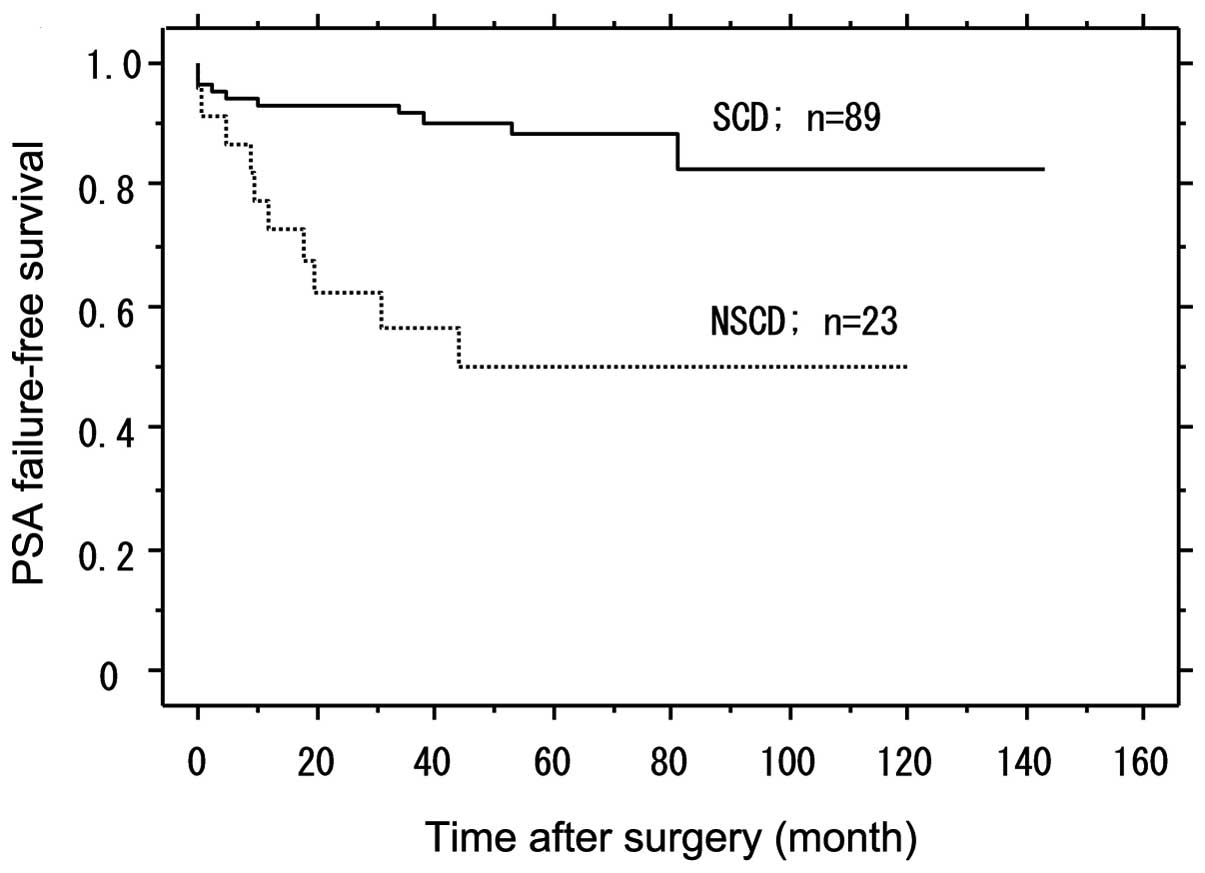
SCD was determined as either pT2 to pT3 without pRM
or lymph node metastasis, and it comprised 79.5% (89/112) of
patients in the high-risk group. The PSA failure-free survival with
SCD was significantly higher than in those with
non-specimen-confined disease (NSCD) (P<0.001). Patients (49.8%)
with NSCD had PSA failure, while in patients with SCD the PSA
failure-free rate was 88.2% following a median follow-up of 60
months (Fig. 2).
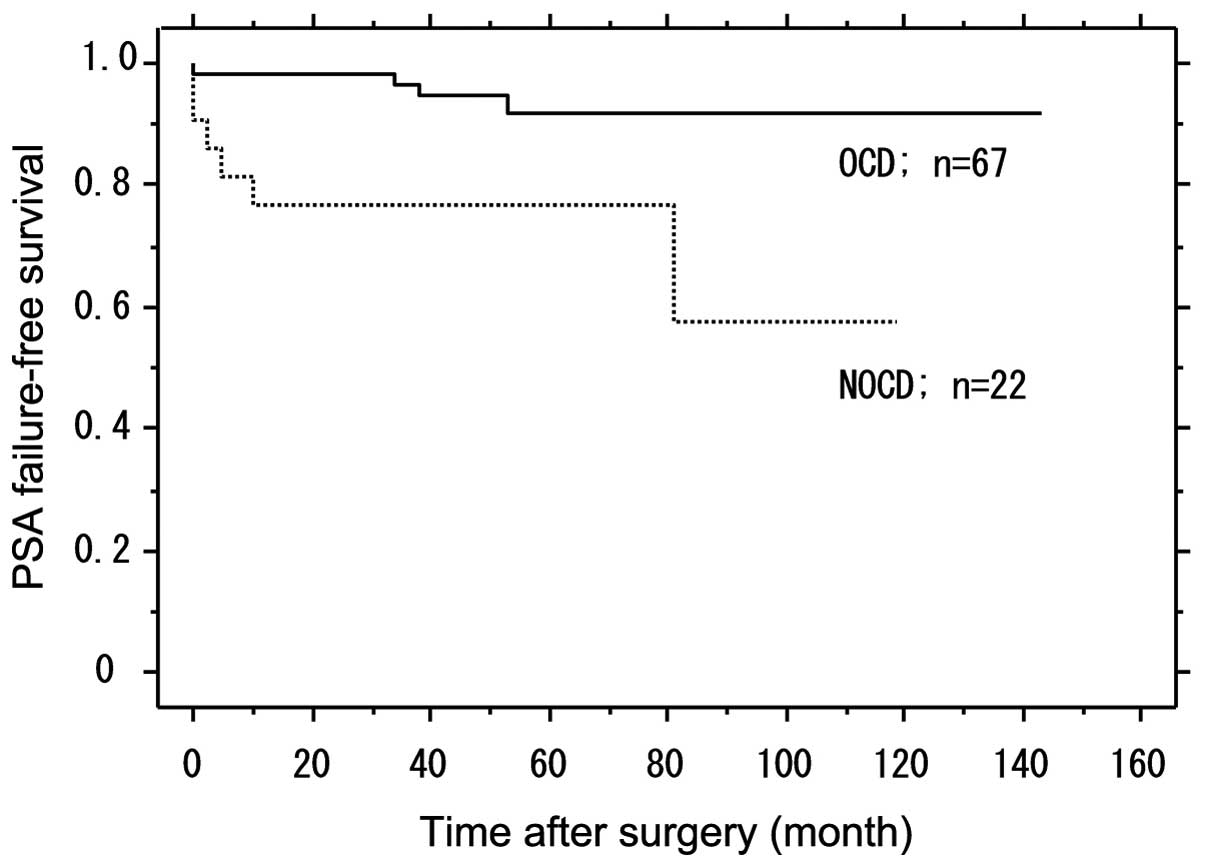
PSA failure-free survival according to
the OCD among SCD in the high-risk group
OCD was defined as pT2 without lymph node
metastasis. Among the SCD in the high-risk group, OCD was
identified in 75.3% (67/89) of the patients. The PSA failure-free
survival with OCD was significantly higher compared to patients
with non-OCD (P=0.004). Patients with non-OCD (23.4%) had PSA
failure, while in those with OCD the PSA failure-free rate was
92.1% following a median follow-up of 60 months (Fig. 3).
A more restrictive definition assigns
clinical stage T2c to intermediate- rather than high-risk
patients
Patients likely to be classified as high-risk
patients by the standard definition and intermediate-risk patients
by the more restrictive definition were evaluated as a separate
‘intermediate/high’ group. The intermediate/high-risk group
comprised 25 patients, belonging to the high-risk group by the
standard definition. By the restrictive definition, the low-,
intermediate-, high- and intermediate/high-risk groups comprised 63
(21.2%), 122 (41.1%), 87 (29.3%) and 25 (8.4%) patients,
respectively. In the intermediate/high-risk group, only one patient
exhibited PSA failure following surgery. After a median follow-up
period of 60 months, the PSA failure-free rates in the low-,
intermediate-, high- and intermediate/high-risk groups were 96.5,
92.2, 76.8 and 95.0%, respectively. The difference between the
high- and intermediate/high-risk groups was not statistically
significant by the log-rank test (P=0.064) (Fig. 4).
Discussion
High-risk prostate cancer classified according to
the D’Amico criteria is an important disease, accounting for 20–35%
of localized prostate cancer cases (4,5).
However, generally speaking, RP alone cannot achieve satisfactory
PSA control (1). Nevertheless,
individual disease state characteristics of the high-risk group are
not necessarily uniform and RP is also known to be likely to have
good cure rates. The present study aimed to retrospectively assess
the outcome of RP in high-risk patients with no pre-surgical
treatment, with a view to investigate the possibility of complete
cure by RP alone in Japanese high-risk prostate cancer patients
As shown in Table
I, the patients were classified into three groups according to
the D’Amico criteria: low-, intermediate-and high-risk groups,
accounting for 21.2% (63/297), 41.4% (122/297) and 37.7% (112/297),
respectively. In addition, while those cases in the high-risk group
with biopsy specimen Gleason scores of ≥8 accounted for 70.5%
(79/112) of this group, the proportion of cases with a Gleason
score from RP specimens of ≥8 in the low-, intermediate- and
high-risk groups was 14.3% (9/63), 15.6% (19/122) and 44.6%
(50/112), respectively. Recent studies have demonstrated that
>1/3 of the patients with a Gleason score of 8–10, according to
the biopsy findings are likely to have a Gleason score of ≤7 in the
RP specimen (6,7). In the high-risk group of the present
study, 48.1% (38/79) with a Gleason score of 8–10 on biopsy had a
Gleason score of ≤7 in the RP specimen. These findings suggest that
a number of the cases classified into the high-risk group according
to the D’Amico criteria in reference to the preoperative factor,
i.e., Gleason score on biopsy, are considered to have been
downgraded histopathologically with respect to their excised
specimens and are the cases in which RP alone may result in good
cure rates. However, the difference between patients with a Gleason
score of ≤7 in the RP specimen and patients with a Gleason score of
≥8 in the RP specimen was not statistically significant with regard
to the PSA failure-free survival, based on results of the log-rank
test (P=0.080), among the patients with a Gleason score of 8–10 on
biopsy of high-risk group. Consequently, the cases with an excised
specimen Gleason score downgraded with regard to the preoperative
factor were not considered to be cases in which complete cure may
be achieved via surgery alone.
PSA failure-free survival rates were examined for
each group and yielded the following results: 93.7% (59/63) for the
low-risk group; 91.0% (111/122) for the intermediate-risk group and
82.1% (92/112) for the high-risk group (Fig. 1). In their study, Kawamorita et
al (8) investigated these
rates only in Japanese patients and concluded that the PSA
failure-free rates in the low-, intermediate- and high-risk groups
were 87.8, 87.3 and 64.5%, respectively. Compared to these
findings, although the PSA failure-free survival rates at this
institution following RP alone for the high-risk group were good,
compared to the low- and intermediate-risk groups, the PSA
failure-free survival rates for the high-risk group were low
(P=0.017, 0.009). The reason for this difference was that the
results included cases in which control via surgery alone is
difficult. Nevertheless, urologists are aware of the fact that in
several high-risk cases, the treatment outcomes of surgery alone
are good. By contrast, the correlation between the characteristics
and PSA failure were examined in the high-risk group (Table II). According to results of the
multivariate analysis, only the biopsy Gleason score was found to
be a significant predictor in patients with and without PSA failure
(P=0.017), among the pre-operative variables. Results of the
univariate and multivariate analyses did not reveal statistically
significant differences in preoperative variables, such as
preoperative PSA and clinical tumor stage that were risk profiles
in the D’Amico risk classification (P=0.362, P=0.325).
Post-operative variables, such as the RP Gleason score and the
organ-confined status, were found to be significant predictors
based on the univariate analysis (P=0.028, P=0.007), while a
post-operative variable, such as specimen-confined status was alone
a significant predictor, in the univariate and multivariate
analyses (P<0.001).
Fig. 2 shows PSA
failure-free survival according to SCD, indicating that the PSA
failure-free rate was 88.8% (79/89) in patients with NSCD, while
PSA failure-free survival with SCD was significantly higher
compared to patients with NSCD (P<0.001). Mian et al
(9) reported that among 188
patients with high-grade cancer, the subgroup with SCD had an 84%
PSA failure-free rate and emphasized that it is important to resect
high-risk prostate cancer completely by RP. In this study, among
the patients with SCD, those with OCD had a higher PSA failure-free
rate compared to the patients with non-organ-confined disease
(NOCD). In this study, of the 112 patients in the high-risk group,
the subgroup with SCD had an 88.2% PSA failure-free rate, following
a median follow-up of 60 months (Fig.
2). Of the patients with SCD, those with OCD had a higher PSA
failure-free rate compared to patients with NOCD (P=0.004)
(Fig. 3). Therefore, the selection
of patients who are expected to have pathologically OCD is
especially important for the surgical treatment of high-risk
disease.
Byar et al (10) reported that when pathologically
examined, tumors apparently unilateral on rectal examination are
bilateral in ∼70% of patients, whereas adenocarcinoma of the
prostate is multifocal in >85% of patients. Additionally, we
often experience cases in which, although a prostate biopsy detects
cancer in a unilateral lobe of the prostate, testing of the excised
specimens demonstrates a prostate cancer in the bilateral lobes. In
the present study, 97 patients were diagnosed with prostate cancer
in the bilateral prostate lobe in the prostatectomy specimen out of
the 106 patients considered to have unilateral cancer based on the
findings of rectal examinations. Prostate biopsies were performed
in 1,580 cases during the same period as this study. RP was
performed in 154/287 cases in which cancer was detected only in a
unilateral lobe of the prostate, and the surgical findings
demonstrated prostate cancer in the bilateral lobes in 128 cases
(83.1%). Thus, these findings do not confirm whether or not cT2c
should be used to identify high-risk patients.
Of those cases classified into the high-risk group
according to the D’Amico criteria, we re-classified the high-risk
ones selected due exclusively to their cT2c stage, in order to
re-examine the PSA failure-free survival (Fig. 4). Using the more restrictive,
definition-assigned clinical stage T2c patients as
intermediate/high-risk within the high-risk group, 25 patients
(8.4%) were assigned to the intermediate/high-risk group and 87
patients (29.3 %) to the high-risk group. After a median follow-up
period of 60 months, the PSA failure-free rates in the low-,
intermediate-, high- and intermediate/high-risk groups were 96.5,
92.2, 76.8 and 95.0%, respectively. However, the difference between
the high- and intermediate/high-risk groups was not statistically
significant, based on the log-rank test (P=0.064). This is the
reason for the small number of patients in the
intermediate/high-risk group compared to the high-risk group. These
findings demonstrate, that the outcomes of the cases classified
into the standard high-risk group due exclusively to their cT2c
stage, are good even with RP alone. It is, therefore, believed that
by excluding the cases classified into the standard high-risk group
due exclusively to their cT2c stage, the cases in which a complete
cure is difficult to achieve via RP alone should be considered to
belong to the high-risk group. In their study, Cooperberg et
al (11) reported that the
clinical stage T2c alone should not classify a patient into the
high-risk group. Patients likely to be classified into the
high-risk group based only on the presence of T2c stage disease had
a markedly lower risk of recurrence compared to patients evaluated
as high-risk patients using the more restrictive definition, as
well as to patients classified into the intermediate-risk group.
These descriptions are consistent with the observations in the
present study. Although RP is not recommended for the high-risk
patients, the patients classified into the high-risk group on the
basis of their T2c stage only are likely to benefit more from
treatment by RP alone.
We retrospectively assessed the outcome of RP alone
in Japanese patients with high-risk prostate cancer. The cases
classified into the high-risk group based on cT2c stage only are
believed to be patients likely to achieve complete cure via surgery
alone. Consequently, such cases should not be evaluated as
high-risk cases, based on their cT2c stage only, according to the
risk classification system.
References
|
1
|
D’Amico AV, Whittington R, Malkowicz SB,
et al: Biochemical outcome after radical prostatectomy, external
beam radiation therapy, or interstitial radiation therapy for
clinically localized prostate cancer. JAMA. 280:969–974. 1998.
|
|
2
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Eqevad LL; ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) Consensus Conference on Gleason
Grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sobin LH, Gospodarowicz MK and Wittekind
Ch: TNM Classification of Malignant Tumors. 7th edition.
Wiley-Blackwell; Oxford: 2009
|
|
4
|
Grossfeld GD, Latini DM, Lubeck DP,
Broering JM, Li YP, Mehta SS and Carroll PR: Predicting disease
recurrence in intermediate and high-risk patients undergoing
radical prostatectomy using percent positive biopsies: results from
CaPSURE. Urology. 59:560–565. 2002. View Article : Google Scholar
|
|
5
|
D’Amico AV, Whittington R, Malkowicz SB,
et al: Predicting prostate specific antigen outcome preoperatively
in the prostate specific antigen era. J Urol. 166:2185–2188.
2001.PubMed/NCBI
|
|
6
|
Manoharan M, Bird VG, Kim SS, Civantos F
and Soloway MS: Outcome after radical prostatectomy with a
pretreatment prostate biopsy Gleason score of >/=8. BJU Int.
92:539–544. 2003.
|
|
7
|
Boorjian SA, Kames RJ, Crispen PL, Ragel
LJ, Berqstralh EJ, Sebo TJ and Blute ML: The impact of discordance
between biopsy and pathological Gleason scores on survival after
radical prostatectomy. J Urol. 181:95–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawamorita N, Saito S, Ishidoya S, Ito A,
Saito H, Kato M and Arai Y: Radical prostatectomy for high-risk
prostate cancer: biochemical outcome. Int J Urol. 16:733–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mian BM, Troncoso P, Okihara K, Bhadkamkar
V, Johnston D, Reves AO and Babaian RJ: Outcome of patients with
Gleason score 8 or higher prostate cancer following radical
prostatectomy alone. J Urol. 167:1675–1680. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Byar DP and Mostofi FK: Carcinoma of the
prostate: prognostic evaluation of certain pathologic features in
208 radical prostatectomies. Examined by the step-section
technique. Cancer. 30:5–13. 1972. View Article : Google Scholar
|
|
11
|
Cooperberg MR, Cowan J, Broering JM and
Carroll PR: High-risk prostate cancer in the United States,
1990–2007. World J Urol. 26:211–218. 2008.
|