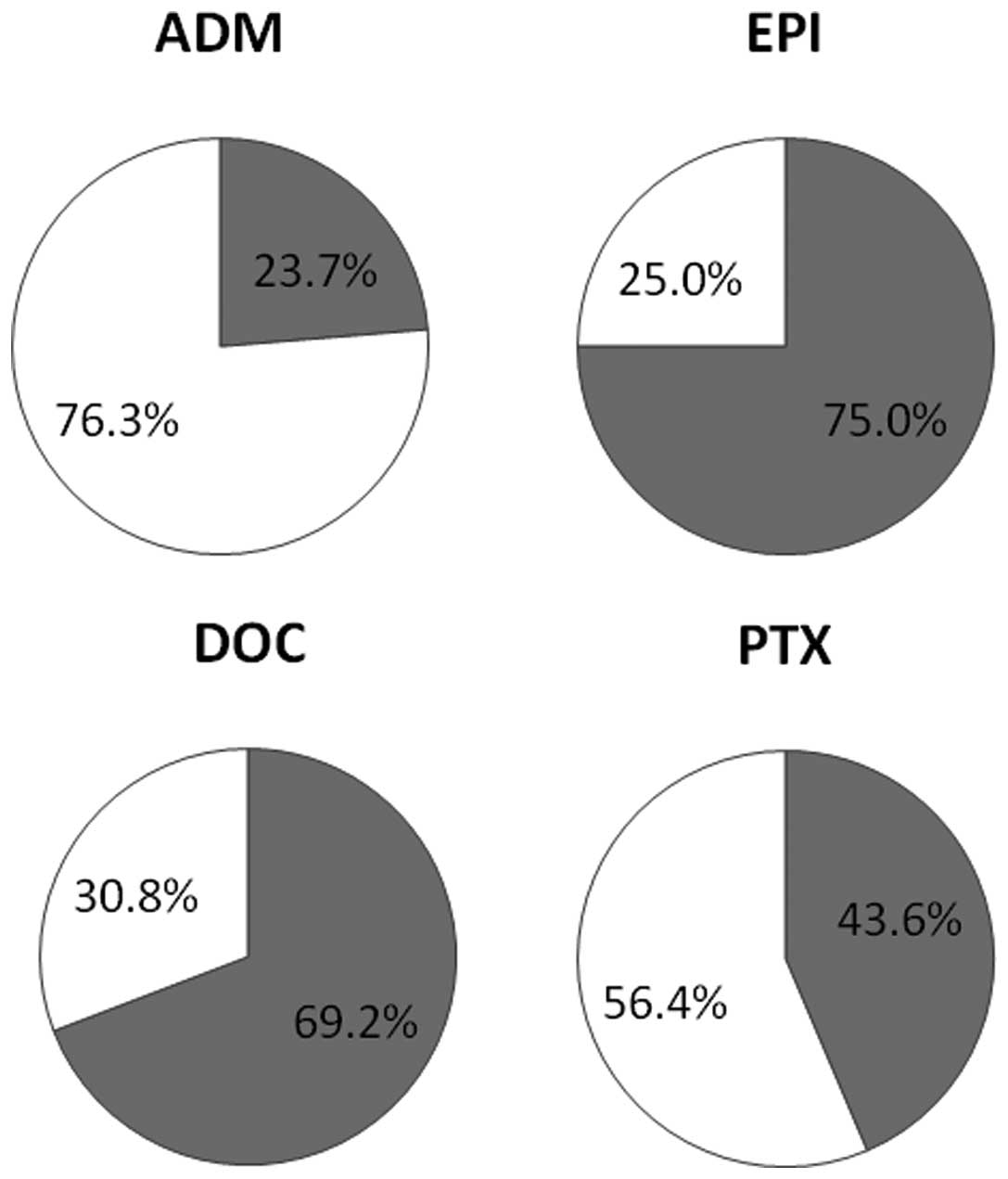
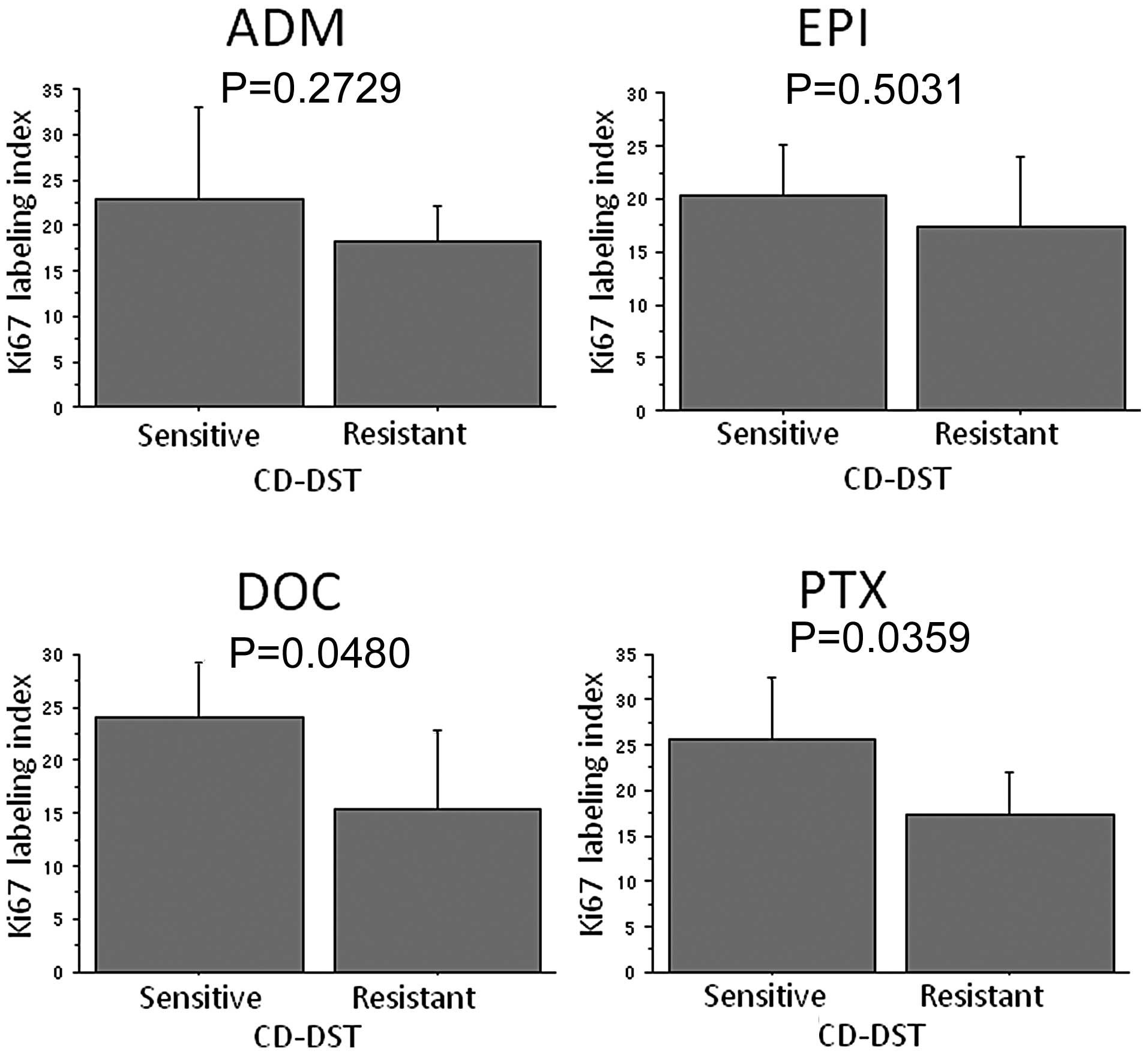
|
1.
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomized trials. Lancet. 365:1687–1717.
2005.
|
|
2.
|
Martin M, Pienkowski T, Mackey J, et al:
Breast Cancer International Research Group 001 Investigators:
Adjuvant docetaxel for node-positive breast cancer. N Engl J Med.
352:2302–2313. 2005.
|
|
3.
|
Martin M, Segui MA, Anton A, et al: Breast
Cancer International Research Group 001 Investigators: Adjuvant
docetaxel for node-positive breast cancer. N Engl J Med.
363:2200–2210. 2010.
|
|
4.
|
Sparano JA, Wang M, Martino S, et al:
Weekly paclitaxel in the adjuvant treatment of breast cancer. N
Engl J Med. 358:1663–1671. 2008.
|
|
5.
|
Conforti R, Boulet T, Tomasic G, et al:
Breast cancer molecular subclassification and estrogen receptor
expression to predict efficacy of adjuvant anthracyclines-based
chemotherapy: a biomarker study from two randomized trials. Ann
Oncol. 18:1477–1483. 2007.
|
|
6.
|
Berry DA, Cirrincione C, Henderson IC, et
al: Estrogen-receptor status and outcomes of modern chemotherapy
for patients with node-positive breast cancer. JAMA. 295:1658–1667.
2006.
|
|
7.
|
Regan MM, Viale G, Mastropasqua MG, et al:
Re-evaluating adjuvant breast cancer trials: assessing hormone
receptor status by immunohistochemical versus extraction assays. J
Natl Cancer Inst. 98:1571–1581. 2006.
|
|
8.
|
Bear HD, Anderson S, Brown A, et al: The
effect on tumor response adding sequential preoperative docetaxel
to preoperative doxorubicin and cyclophosphamide: preliminary
results from national surgical adjuvant breast and bowel project
protocol B-27. J Clin Oncol. 21:4165–4174. 2003.
|
|
9.
|
Stearns V, Singh B, Tsangaris T, et al: A
prospective randomized pilot study to evaluate predictors of
response in serial core biopsies to single agent neoadjuvant
doxorubicin or paclitaxel for patients with locally advanced breast
cancer. Clin Cancer Res. 9:124–133. 2003.
|
|
10.
|
Kuerer HM, Newman LA, Smith TL, et al:
Clinical course of breast cancer patients with complete pathologic
primary tumor and axillary lymph node response to doxorubicin-based
neoadjuvant chemotherapy. J Clin Oncol. 17:460–469. 1999.
|
|
11.
|
Paik S, Tang G, Shak S, et al: Gene
expression and benefit of chemotherapy in women with node negative,
estrogen receptor-positive breast cancer. J Clin Oncol.
24:3726–3734. 2006.
|
|
12.
|
Hayes DF, Thor AD, Dressler LG, et al;
Cancer and Leukemia Group B (CALGB) Investigators: HER2 and
response to paclitaxel in node-positive breast cancer. N Engl J
Med. 357:1496–1506. 2007.
|
|
13.
|
Hugh J, Hanson J, Cheang MCU, et al:
Breast cancer subtypes and response to docetaxel in node-positive
breast cancer: use of an immunohistochemical definition in the
BCIRG 001 trial. J Clin Oncol. 27:1168–1176. 2009.
|
|
14.
|
Penault-Llorca F, Andre F, Sagan C, et al:
Ki67 expression and docetaxel efficacy in patients with estrogen
receptor-positive breast cancer. J Clin Oncol. 27:2809–2815.
2009.
|
|
15.
|
Litman T, Druley TE, Stein WD, et al: From
MDR to MXR: new understanding of multidrug resistance systems,
their properties and clinical significance. Cell Mol Life Sci.
58:931–959. 2001.
|
|
16.
|
Yamamoto N, Tamura T, Murakami H, et al:
Randomized pharmacokinetic and pharmacodynamic study of docetaxel:
dosing based on body-surface area compared with individualized
dosing based on cytochrome P450 activity estimated using a urinary
metabolite of exogenous cortisol. J Clin Oncol. 23:1061–1069.
2005.
|
|
17.
|
Miyoshi Y, Taguchi T, Kim SJ, et al:
Prediction of response to docetaxel by immunohistochemical analysis
of CYP3A4 expression in human breast cancers. Breast Cancer.
12:11–15. 2005.
|
|
18.
|
Hasegawa S, Miyoshi Y, Egawa C, et al:
Mutational analysis of the class I beta-tubulin gene in human
breast cancer. Int J Cancer. 101:46–51. 2002.
|
|
19.
|
Burkhart CA, Kavallaris M and Band Horwitz
S: The role of beta-tubulin isotype in resistance to antimitotic
drugs. Biochim Biophys Acta. 1471:1–9. 2001.
|
|
20.
|
Coon JS, Marcus E, Gupta-Burt S, et al:
Amplification and overexpression of topoisomerase IIα predict
response to anthracycline-based therapy in locally advanced breast
cancer. Clin Cancer Res. 8:1061–1067. 2002.
|
|
21.
|
Anand S, Penrhyn-Lowe S and Venkitaraman
AR: AURORA-A amplification overrides the mitotic spindle assembly
checkpoint, inducing resistance to taxol. Cancer Cell. 3:51–62.
2003.
|
|
22.
|
Sjostrom J, Blomqvist C, Heikkila P, et
al: Predictive value of p53, mdm-2, p21, and mib-1 for chemotherapy
response in advanced breast cancer. Clin Cancer Res. 6:3103–3110.
2000.
|
|
23.
|
Sjostrom J, Blomqvist C, von Boguslawski
K, et al: The predictive value of bcl-2, bax, bcl-xL, bag-1, fas,
and fasL for chemotherapy response in advanced breast cancer. Clin
Cancer Res. 8:811–816. 2002.
|
|
24.
|
Yokomizo A, Ono M, Nanri H, et al:
Cellular levels of thioredoxin associated with drug sensitivity to
cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res.
55:4293–4296. 1995.
|
|
25.
|
Sahin AA, Ro J, Ro JY, et al: Ki67
immunostaining in node negative stage I/II breast carcinoma.
Significant correlation with prognosis. Cancer. 68:549–557.
1991.
|
|
26.
|
Domagala W, Markiewski M, Harezga B, et
al: Prognostic significance of tumor cell proliferation rate as
determined by the MIB-1 antibody in breast carcinoma: its
relationship with vimentin and p53 protein. Clin Cancer Res.
2:147–154. 1996.
|
|
27.
|
Trihia H, Murray S, Price K, et al: Ki67
expression in breast carcinoma. Its association with grading
systems, clinical parameters, and other prognostic factors - a
surrogate marker? Cancer. 97:1321–1331. 2003.
|
|
28.
|
Chang J, Ormerod M, Powles TJ, et al:
Apoptosis and proliferation as predictors of chemotherapy response
in patients with breast carcinoma. Cancer. 89:2145–2152. 2000.
|
|
29.
|
Archer CD, Parton M, Smith IE, et al:
Early changes in apoptosis and proliferation following primary
chemotherapy for breast cancer. Br J Cancer. 89:1035–1041.
2003.
|
|
30.
|
Goldhhirsch A, Ingle JN, Gelber RD, et al:
Thresholds for therapies: highlights of the St Gallen international
expert consensus on primary therapy early breast cancer. Ann Oncol.
20:1319–1329. 2009.
|
|
31.
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003.
|
|
32.
|
Yasuda H, Takada T, Wada K, et al: A new
in-vitro drug sensitivity test (collagen-gel droplet
embedded-culture drug sensitivity test) in carcinomas of pancreas
and biliary tract: possible clinical utility. J Hepatobiliary
Pancreat Surg. 5:261–268. 1998.
|
|
33.
|
Higashiyama M, Kodama K, Yokouchi H, et
al: Immunohistochemical p53 protein status in nonsmall cell lung
cancer is a promising indicator in determining in vitro
chemosensitivity to some anticancer drugs. J Surg Oncol. 68:19–24.
1998.
|
|
34.
|
Takamura Y, Kobayashi H, Taguchi T, et al:
Prediction of chemotherapeutic response by collagen gel droplet
embedded culture-drug sensitivity test in human breast cancers. Int
J Cancer. 98:450–455. 2002.
|
|
35.
|
Von Hoff DD: He’s not going to talk about
in vitro predictive assay again, is he? J Natl Cancer Inst.
82:96–101. 1990.
|
|
36.
|
Kern DH, Drogemuller CR, Kennedy MC, et
al: Development of miniaturized, improved nucleic acid precursor
incorporation assay for chemosensitivity testing of human solid
tumors. Cancer Res. 45:5436–5441. 1985.
|
|
37.
|
Fruehauf JP and Bosanquet AG: In vitro
determination of drug response: a discussion of clinical
applications. Principles Prac Oncol Updates. 7:121993.
|
|
38.
|
Carmichael J, De Graff WG, Gazdar AF, et
al: Evaluation of a tetrazolium-based semiautomated colorimetric
assay: assessment of chemosensitivity testing. Cancer Res.
47:936–942. 1987.
|
|
39.
|
Wilbur DW, Camacho ES, Hilliard DA, et al:
Chemotherapy of non-small cell lung carcinoma guided by an in vitro
drug resistance assay measuring total tumour cell kill. Br J
Cancer. 65:27–32. 1992.
|
|
40.
|
Mechetner E, Kyshtoobayeva A, Zonis S, et
al: Levels of multidrug resistance (MDR1) P-glycoprotein expression
by human breast cancer correlate with in vitro resistance to taxol
and doxorubicin. Clin Cancer Res. 4:389–398. 1998.
|
|
41.
|
Furukawa T, Kubota T and Hoffman RM:
Clinical application of the histoculture drug response assay. Clin
Cancer Res. 1:305–311. 1995.
|
|
42.
|
Yamamoto Y, Watanabe Y, Ishida N, et al:
Collagen gel droplet-embedded culture drug sensitivity test in
human breast cancer. Gan To Kagaku Ryoho. 35:793–796. 2008.(In
Japanese).
|
|
43.
|
Paik S, Bryant J, Park C, et al: erbB-2
and response to doxorubicin in patients with axillary lymph
node-positive, hormone receptor-negative breast cancer. J Natl
Cancer Inst. 90:1361–1370. 1998.
|
|
44.
|
Henderson IC, Berry DA, Demetri GD, et al:
Improved outcomes from adding sequential paclitaxel but not from
escalating doxorubicin dose in an adjuvant chemotherapy regimen for
patients with node-positive primary breast cancer. J Clin Oncol.
21:976–983. 2003.
|
|
45.
|
Tham YL, Gomez LF, Mohsin S, et al:
Clinical response to neoadjuvant docetaxel predicts improved
outcome in patients with large locally advanced breast cancers.
Breast Cancer Res Treat. 94:279–284. 2005.
|
|
46.
|
Learn PA, Yeh IT, McNutt M, et al:
HER-2/neu expression as a predictor of response to neoadjuvant
docetaxel in patients with operable breast carcinoma. Cancer.
103:2252–2260. 2005.
|
|
47.
|
de Azambuja E, Cardoso F, de Castro G Jr,
et al: Ki67 as prognostic marker in early breast cancer: a
meta-analysis of published studies involving 12,155 patients. Br J
Cancer. 96:1504–1513. 2007.
|
|
48.
|
Cheang MC, Chia SK, Voduc D, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009.
|
|
49.
|
Stuart-Harris R, Caldas C, Pinder SE and
Pharoah P: Proliferation markers and survival in early breast
cancer: a systematic review and meta-analysis of 85 studies in
32,825 patients. Breast. 17:323–334. 2008.
|
|
50.
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005.
|
|
51.
|
Paik S, Shak S, Tang G, et al: A multigene
assay to predict recurrence of tamoxifen-treated, node-negative
breast cancer. N Engl J Med. 351:2817–2826. 2004.
|
|
52.
|
van de Vijver MJ, He YD, van’t Veer LJ, et
al: A gene-expression signature as a predictor of survival in
breast cancer. N Engl J Med. 347:1999–2009. 2002.
|
|
53.
|
Sparano JA and Paik S: Development of the
21-gene assay and its application in clinical practice and clinical
trials. J Clin Oncol. 26:721–728. 2008.
|
|
54.
|
Cardoso F, van’t Veer L, Rutgers E, et al:
Clinical application of the 70-gene profile: the MINDACT trial. J
Clin Oncol. 26:729–735. 2008.
|