Introdution
Liver cancer is a malignant disease with a high
incidence and mortality, and is often diagnosed at an advanced
stage (1). Common therapies, such
as surgical resection, percutaneous or transarterial interventions
are of limited efficacy (2), as
chemotherapy, one of the main measures for a comprehensive
treatment, may induce adverse effects such as bone marrow
suppression, gastrointestinal reactions and lowered immunological
function.
Several Chinese herbs have been identified as
potential sources of antitumor drugs (3–5). The
effect-enhancing and toxicity-reducing activity of Chinese herbs in
radiotherapy and chemotherapy of tumors was previously confirmed
(6,7). However, no studies focusing on the
antitumor and toxicity-reducing effects of Hypericum
japonicum Thunb. (HJT) have been reported thus far. Therefore,
the present investigation was conducted to ascertain whether the
HJT extract enhanced the efficacy of 5-fluorouracil (5-FU)
treatment in murine liver tumor xenografts, and reduced toxicity of
chemotherapy in tumor-bearing mice.
Materials and methods
Reagents
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin G and streptomycin were obtained from Invitrogen Corp.
(Carlsbad, CA, USA). 5-FU was obtained from the Shanghai Xudong
Haipu Pharmaceutical Co., Ltd. (Shanghai, China). Murine H22
hepatoma cells were purchased from the Shanghai Institute of Cell
Biology at the Chinese Academy of Sciences (Shanghai, China).
Animals
Institute of Cancer Research (ICR) mice
(male/female, 50–50%; weight, 20±2 g) were purchased from the
Experimental Animal Center, Zhengzhou University (Zhengzhou,
China). The animals were kept in a standard laboratory, under a
12-h light/dark cycle, at room temperature (20±2°C), in a humid
(75±15%) and filtered laminar air flow-controlled room in the
animal facility of the Experimental Animal Center of the Zhengzhou
University. Mice were raised and cared for, given autoclaved water
and fed ad libitum with laboratory pellet chow. Experiments
were performed according to the regulations and guidelines for
animal experiments in China. This study was approved by the Ethics
Committee of Zhenghou University, Zhenghou, China.
Cell culture
H22 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS, 1×105 U/l penicillin and 100
mg/l streptomycin in a humidified atmosphere with 5% CO2
incubator at 37°C. The cells were subcultured until logarithmic
growth phase was reached.
Preparation of the HJT extract
HJT extract was obtained from Xi’an Kanger America
Biotechnology Co., Ltd. (Xi’an, China). Air-dried HJT samples were
ground and mixed with double-distilled water at 100°C for 10 min,
then cooled to room temperature. HJT was then stored at 4°C in dark
bottles and kept sterile until instilled intragastrically in the
mice.
Experimental design
ICR mice were randomized into A-F groups (n=10). The
animals were subcutaneously injected with H22 cells
(1×107 cells/mouse) into the armpit of the right hind
limb, with the exception of group A (normal group), which received
sodium suspension (0.9%). Group B served as the tumor control. In
groups C, D, E and F, 5-FU (20 mg/kg) was injected into the
abdominal cavity, once daily. Groups D (low-dose HJT group), E
(medium-dose HJT group) and F (high-dose HJT group) received
perfusion of HJT extract intragastrically at 3, 6 and 12 g/d per
kg, administered 24 h after the tumor inoculation, once daily for
10 days.
Determination of antitumor activity in
vivo
The general condition of mice such as movement, fur,
eating, drinking, body weight increase and toxic responses was
examined. Twenty four hours after the last dose, blood was
collected from the animals for the white blood cell (WBC) count by
retro-orbital puncture under slight anesthesia (diethyl ether).
Mice from each group were sacrificed under anesthesia using
CO2. The mice and the separated tumor were weighed.
Antitumor activity was evaluated by tumor weighing. The tumor
inhibition rate was calculated as: Tumor inhibitory rate (%) =
(1−average tumor weighing of administration group/average tumor
weighing of the tumor control group) × 100% (9). Simultaneously, the thymus and spleen
were excised and weighed. Indices were calculated as: Thymus index
= the thymus weight (mg)/body weight (g) and spleen index = the
spleen weight (mg)/body weight (g) (10).
Determination of the phagocytic function
of macrophage
After the last dose and 12-h fasting, chicken-red
cells (1 ml, 5%) were injected intraperitoneally into each group.
After 12 h, the mice were sacrificed and normal saline (2 ml) was
injected into the abdominal cavity. Peritoneal fluid (1 ml) was
then drawn for glass slides. Following incubation for 30 min,
peritoneal fluid was fixed with a mixture of acetone/methanol (1:1,
v/v) and stained with 4% Giemsa. After drying, peritoneal
macrophages were counted by microscopy. The effect of the HJT
extract on phagocytosis of enterocoelia macrophage was evaluated by
the chicken-red cell phagocytic rate and index, calculated using
the formulas: phagocytic index = chicken-red cells phagocyted/total
macrophages and phagocytic rate = (macrophages that phagocytized
red blood cells (RBC)/total macrophages) ×100% (11).
Determination of life prolongation rate
in the H22-bearing mice
The grouping and drug administration for this
experiment were the same as described above (n=10). H22 cells
(1×107 cells/mouse) were injected into the abdominal
cavity of each mouse. The survival time of the mice was recorded
for 35 days after terminating drug administration. The experiment
was considered invalid if the mortality rate in the control group
during the experiment was >20%, or the survival time of 20% mice
was >5 weeks. The life prolongation rate was determined as (the
mean living days of the mice in the treatment group/the mean living
days in the tumor control group–1) × 100%.
Statistical analysis
Values were expressed as the mean ± standard
deviation (SD). Statistical analysis was performed with one-way
analysis of variance (ANOVA) using the statistical software SPSS
17.0. P<0.05 was considered to indicate a statistically
significant difference.
Results
Antitumor and toxicity-reducing activity
of the HJT extract on hepatoma
H22-bearing mice in the tumor control group were not
lively, with dark fur, and exhibited loss of appetite as well as
slow weight gain. Additionally, mice in the 5-FU group died prior
to the last administration. By contrast, mice in the HJT extract
groups were vigorous, with shiny fur and a rapid body weight
increase, showing a good condition. The toxic effects in the 5-FU
group were severe, including anorexia, abdominal distention and
emaciation, while the mice in the HJT extract groups showed no or
little toxicity.
No statistically significant difference was detected
in the body weight between each group prior to treatment
(P>0.05) (Table I). Following
the experiment, the body weight of the tumor-bearing mice in the
groups increased to varying degrees. The body weight increase was
significantly higher in each HJT group compared with the tumor
control group (P<0.05), whereas the increase in the 5-FU group
was significantly lower compared with the other groups (P<0.05).
The tumor weight of three HJT groups and the 5-FU group was
significantly lower compared with the tumor control group
(P<0.05). Compared with the 5-FU group, the tumors of three HJT
groups showed a significant decrease in size, with the HJT dose
group increasing (P<0.05). The tumor inhibitory rates of the
5-FU group and HJT groups were 41.11, 54.44, 58.15 and 66.29%,
respectively.
 | Table IEffect of the HJT extract on body and
tumor weight of the H22-bearing mice [mean ± standard deviation
(SD), n=10]. |
Table I
Effect of the HJT extract on body and
tumor weight of the H22-bearing mice [mean ± standard deviation
(SD), n=10].
| Group | Dose (g/kg/day) | Body weight (g)
| Variation of body
weight (g) | Tumor weight (g) | Tumor inhibition rate
(%) |
|---|
| Pre-treatment | Post-treatment |
|---|
| Normal control | - | 20.05±2.07 | 24.35±3.24 |
4.30±3.47a,b | - | - |
| Tumor control | - | 20.11±1.94 | 21.07±2.02 | 0.96±2.13 | 2.70±1.13 | - |
| 5-FU | 0.02 | 19.98±2.03 | 20.55±1.86 | 0.57±1.89a | 1.59±0.59a | 41.11 |
| 0.02+3.00 | 20.01±2.25 | 21.49±1.94 |
1.48±1.65a,b |
1.23±0.54a,b | 54.44 |
| 5-FU + HJT | 0.02+6.00 | 20.07±2.06 | 22.15±1.75 |
2.08±2.03a,b |
1.13±0.42a,b | 58.15 |
| 0.02+12.00 | 19.96±1.95 | 23.24±2.05 |
3.28±2.19a,b |
0.91±0.63a,b | 66.29 |
Effect of the HJT extract on immune
organs and WBC count of H22-bearing mice
As shown in Fig. 1,
compared with the normal control group, the thymus and spleen
indices were significantly lower in the tumor control group and
further decreased in the 5-FU group (P<0.05). In the HJT extract
groups, atrophy of the immune organs induced by 5-FU was improved
(P<0.05). Compared with the tumor control group, the WBC count
showed a significant decrease in the 5-FU group (P<0.05), and
showed an increasing tendency in the HJT extract groups.
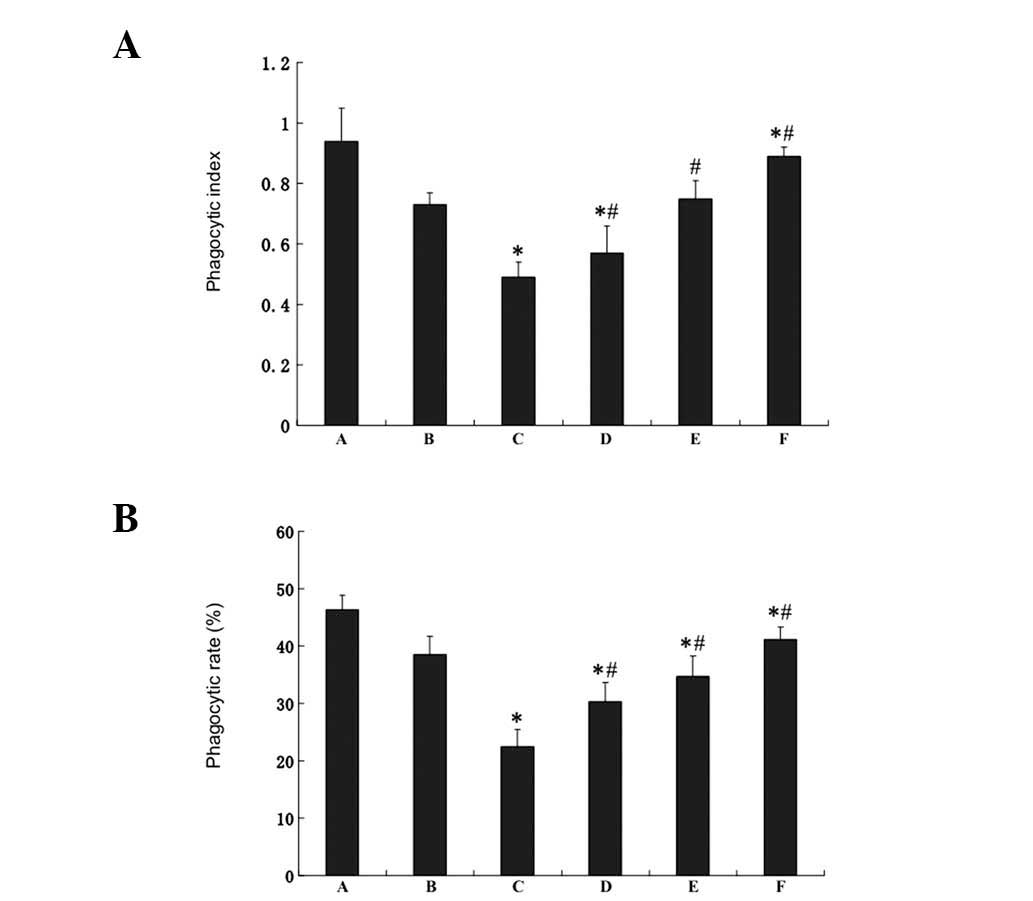
Effect of the HJT extract on the
phagocytic function of macrophages in H22-bearing mice
Compared with the tumor control group, 5-FU
decreased the phagocytic index and rate significantly (P<0.05)
(Fig. 2). However, the phagocytic
function of the macrophages of mice was increased in the HJT
groups. The phagocytic indices of the low-, medium- and high-dose
HJT groups were increased 16.32, 53.31 and 81.63%, respectively,
compared with the 5-FU group. The phagocytic rates of the low-,
medium- and high-dose HJT groups were increased by 35.07, 54.47 and
83.31%, respectively, compared with the 5-FU group.
Effects of the HJT extract on the life
prolongation rate in H22-bearing mice
As shown in Table
II, the survival time of the ascites tumor mice in the HJT
groups was significantly higher compared with those of the tumor
control (P<0.05) and 5-FU groups (P<0.05). Survival time of
the 5-FU group was slightly higher compared with that of the tumor
control group, with no significant difference being detected
between the two groups. The life prolongation rates in the low-,
medium- and high-dose HJT groups were 25.97, 43.74 and 62.27%,
respectively, which were significantly higher compared with those
of the 5-FU group.
 | Table IIEffects of the HJT extract on the life
prolongation rate in the H22-bearing mice [mean ± standard
deviation (SD), n=10]. |
Table II
Effects of the HJT extract on the life
prolongation rate in the H22-bearing mice [mean ± standard
deviation (SD), n=10].
| | | Life prolongation
rate (%)
|
|---|
| Group | Dose (g/kg/day) | Survival time
(day) | vs. tumor control
group | vs. 5-FU group |
|---|
| Tumor control | - | 15.98±4.85 | - | - |
| 5-FU | 0.02 | 16.23±4.03 | 1.56 | - |
| 0.02+3.00 |
20.13±3.89a,b | 25.97 | 24.03 |
| 5-FU + HJT | 0.02+6.00 |
22.97±4.31a,b | 43.74 | 41.53 |
| 0.02+12.00 |
25.93+5.07a,b | 62.27 | 59.77 |
Discussion
Previous studies have noted the bioactivies of the
Hypericum genus including the antitumor, antioxidant,
antimicrobial and natural killer (NK) cell-activating activity
(8–12). HJT, an annual herb from the genus
Hypericum L., known in traditional Chinese medicine as
Tian-ji-huang, grows mainly throughout southern China. HJT has been
used in a Chinese herbal compound formula for many years for the
treatment of bacterial diseases; infectious, acute and chronic
hepatitis; gastrointestinal disorder; internal hemorrhage and
tumors (13,14). Data in the present study
demonstrated that the HJT extract significantly inhibited the
growth of H22-transplanted tumors and had a synergistic
tumor-inhibiting effect with the 5-FU group.
Reduction of the chemotherapy-induced side effects
in cancer treatment has gained increasing attention. The results of
this study demonstrated that the HJT extract exhibited an improved
efficacy of supporting treatment in chemotherapy. The increase in
body weight markedly reduced toxic reactions and the higher life
prolongation rate demonstrated that the HJT extract had
toxicity-reducing activity.
The immune function is important in the course of
radiotherapy and chemotherapy for patients with malignant tumors.
Findings of the present study have shown that the thymus and spleen
indices, WBC count and the phagocytic function were elevated in the
HJT groups, indicating that the HJT extract efficaciously
antagonized the immunosuppression caused by 5-FU and may be used as
an immune response modifier in chemotherapy. It is possible to
estimate that HJT likely improves the immune function of patients
suffering from a malignant disease.
The antitumor and toxicity-reducing effects of HJT
extract are potentially associated with components such as
flavonoids, xanthones, glucoside and embelin in HJT, which also
contribute to the immune activity on the tumor cells (15–18).
However, in the present study, the whole herb extract was used,
while the concrete mechanism of action remains to be
elucidated.
Acknowledgements
This study was supported by the Talent
Recruitment Research Grant of the Zhengzhou University, Zhengzhou,
China.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Dai LC, Wang X, Yao X, Lu YL, Ping JL and
He JF: Enhanced therapeutic effects of combined chemotherapeutic
drugs and midkine antisense oligonucleotides for hepatocellular
carcinoma. World J Gastroenterol. 13:1989–1994. 2007. View Article : Google Scholar
|
|
3
|
Yu J, Liu H, Lei J, Tan W, Hu X and Zou G:
Antitumor activity of chloroform fraction of Scutellaria
barbata and its active constituents. Phytother Res. 21:817–822.
2007. View
Article : Google Scholar
|
|
4
|
Vickers A: Botanical medicines for the
treatment of cancer: rationale, overview of current data, and
methodological considerations for phase I and II trials. Cancer
Invest. 20:1069–1079. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu F, Wang JG, Wang SY, Li Y, Wu YP and
Xi SM: Antitumor effect and mechanism of Gecko on human esophageal
carcinoma cell lines in vitro and xenografted sarcoma 180 in
Kunming mice. World J Gastroenterol. 14:3990–3996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL and
Pan CE: Effect of resveratrol and in combination with 5-FU on
murine liver cancer. World J Gastroenterol. 10:3048–3052.
2004.PubMed/NCBI
|
|
7
|
Cao Y, Xia QH, Meng H and Zhong AP:
Antitumor and synergistic effect of Chinese medicine ‘bushen huayu
jiedu recipe’ and chemotherapy on transplanted animal
hepatocarcinoma. World J Gastroenterol. 11:5218–5220. 2005.
|
|
8
|
Dongre SH, Badami S, Natesan S and H RC:
Antitumor activity of the methanol extract of Hypericum
hookerianum stem against Ehrlich ascites carcinoma in Swiss
albino mice. J Pharmacol Sci. 103:354–359. 2007.PubMed/NCBI
|
|
9
|
Radulović N, Stankov-Jovanović V,
Stojanović G, Šmelcerović A, Spiteller M and Asakawa Y: Screening
of in vitro antimicrobial and antioxidant activity of nine
Hypericum species from the Balkans. Food Chem. 103:15–21.
2007.
|
|
10
|
Jayasuriya H, Clark AM and McChesney JD:
New antimicrobial filicinic acid derivatives from Hypericum
drummondii. J Nat Prod. 54:1314–1320. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jayasuriya H, McChesney JD, Swanson SM and
Pezzuto JM: Antimicrobial and cytotoxic activity of rottlerin-type
compounds from Hypericum drummondii. J Nat Prod. 52:325–331.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Helgason CM, Frank JL, Johnson DR, Frank
MG and Hendricks SE: The effects of St. John’s Wort
(Hypericum perforatum) on NK cell activity in vitro.
Immunopharmacology. 46:247–251. 2000.
|
|
13
|
Ishiguro K, Yamaki M, Kashihara M and
Takagi S: Sarothralen A and B, new antibiotic compounds from
Hypericum japonicum. Planta Med. 52:288–290. 1986.
View Article : Google Scholar
|
|
14
|
Wu QL, Wang SP, Du LJ, Yang JS and Xiao
PG: Xanthones from Hypericum japonicum and H. henryi.
Phytochemistry. 49:1395–1402. 1998.PubMed/NCBI
|
|
15
|
Wang XW, Mao Y, Wang NL and Yao XS: A new
phloroglucinol diglycoside derivative from Hypericum japonicum
Thunb. Molecules. 13:2796–2803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai Y, Desano J, Qu Y, Tang W, Meng Y,
Lawrence TS and Xu L: Natural IAP inhibitor Embelin enhances
therapeutic efficacy of ionizing radiation in prostate cancer. Am J
Cancer Res. 1:128–143. 2011.PubMed/NCBI
|
|
17
|
Ding M, Zhao J, Bowman L, Lu Y and Shi X:
Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE
pathway by quercitrin. Int J Oncol. 36:59–67. 2010.PubMed/NCBI
|
|
18
|
Lin JP, Yang JS, Lu CC, Chiang JH, Wu CL,
Lin JJ, Lin HL, Yang MD, Liu KC, Chiu TH and Chung JG: Rutin
inhibits the proliferation of murine leukemia WEHI-3 cells in vivo
and promotes immune response in vivo. Leuk Res. 33:823–828. 2009.
View Article : Google Scholar : PubMed/NCBI
|