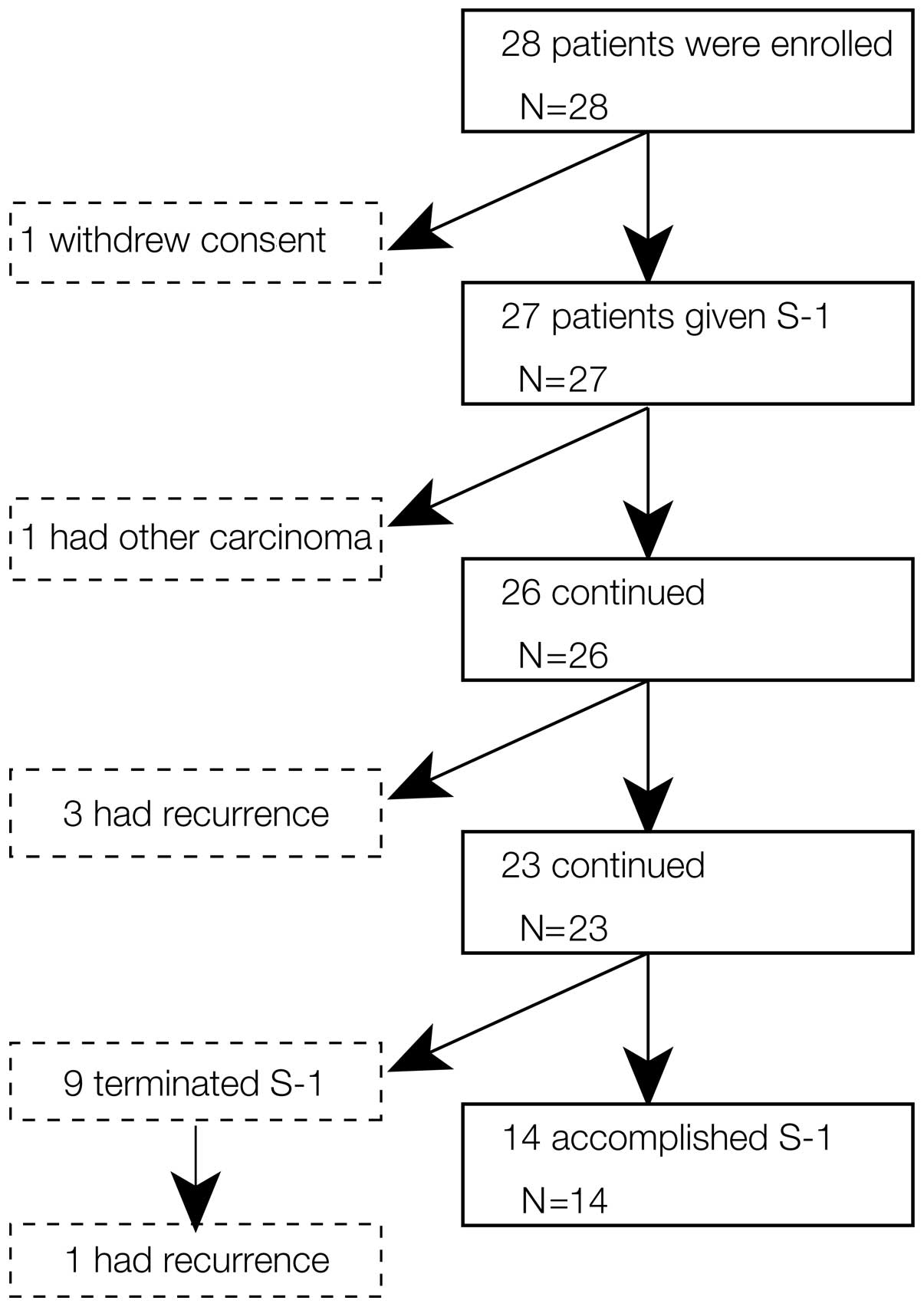
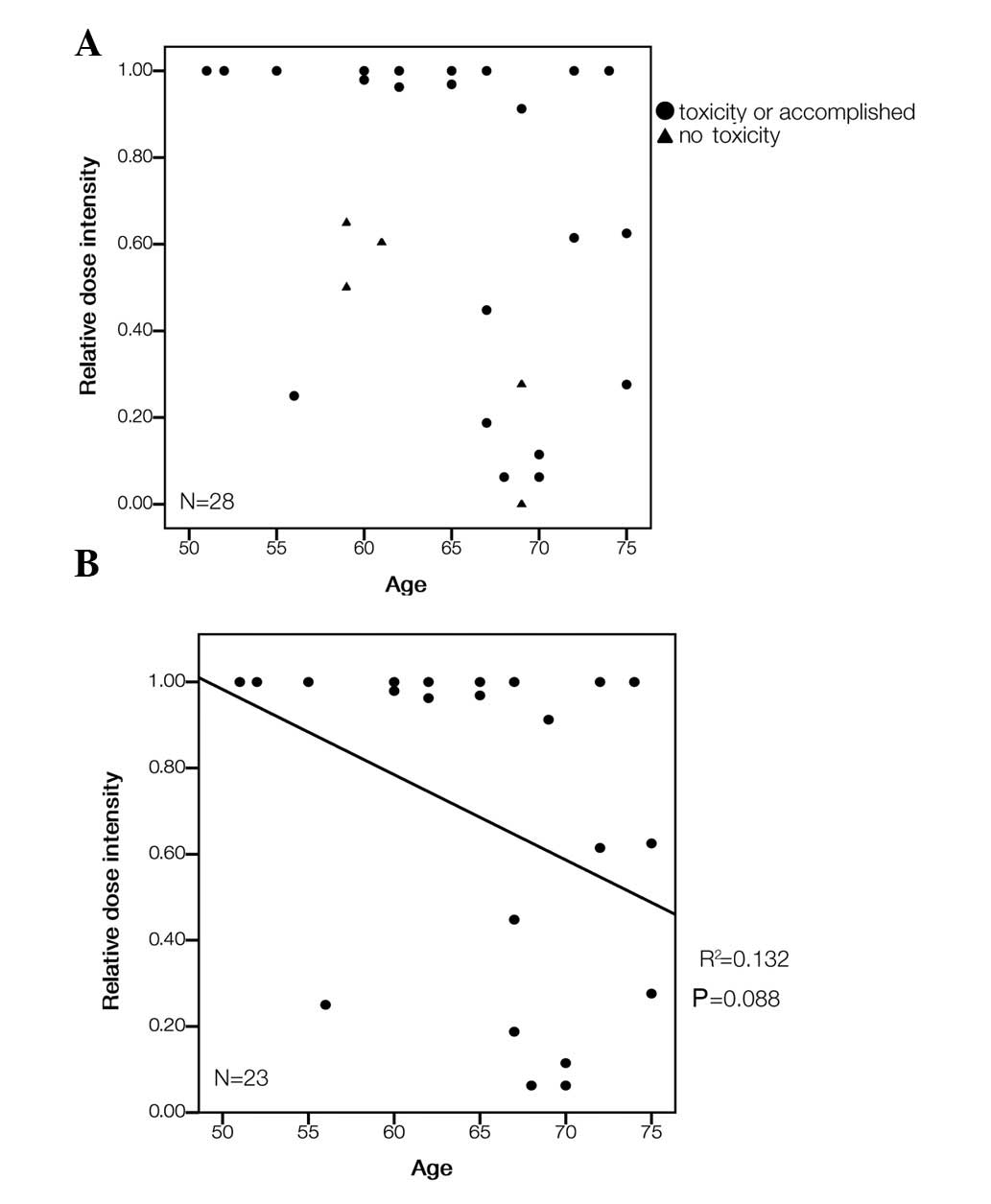
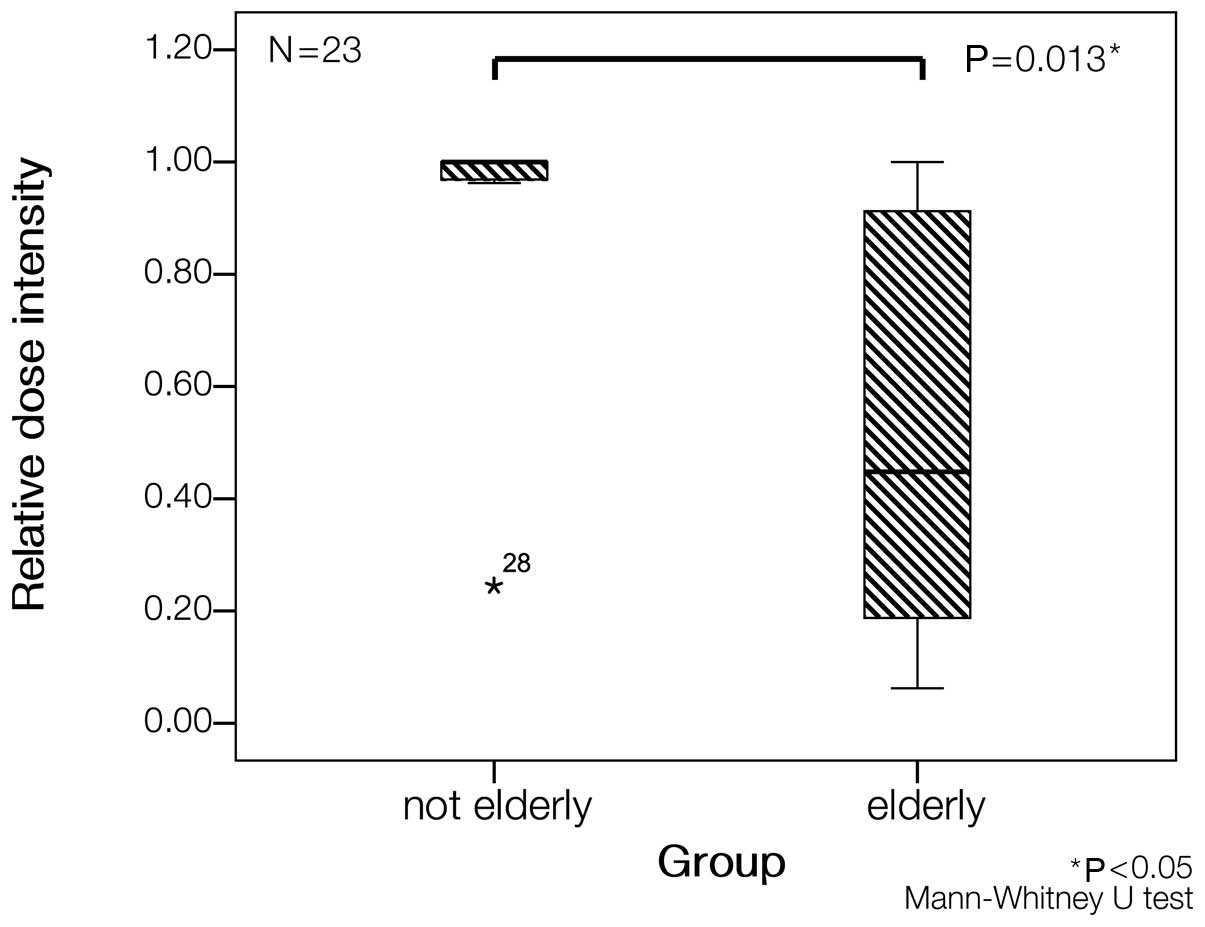
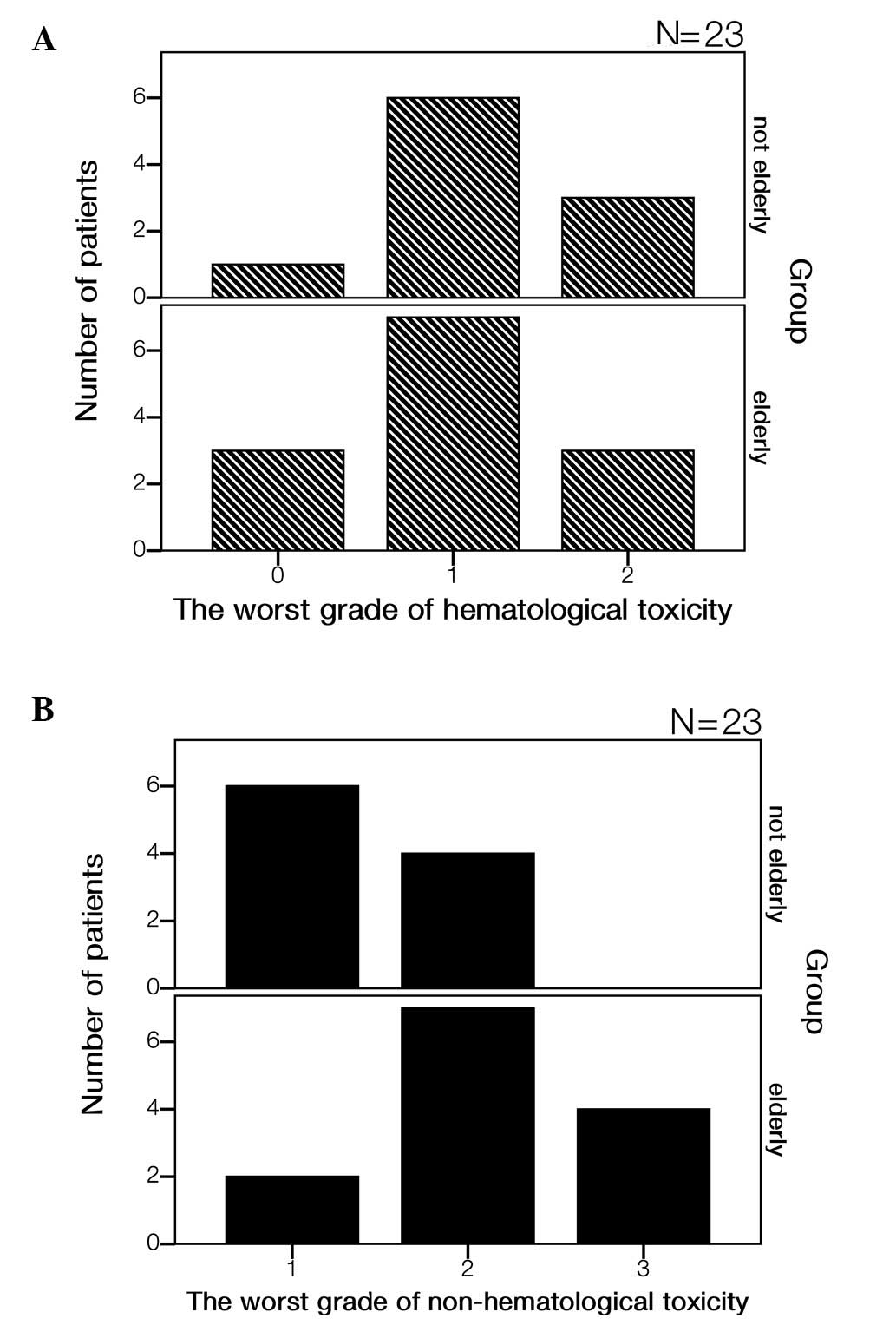
|
1.
|
Pisters KM and Le Chevalier T: Adjuvant
chemotherapy in completely resected non-small-cell lung cancer. J
Clin Oncol. 23:3270–3278. 2005.
|
|
2.
|
Pignon JP, Tribodet H, Scagliotti GV, et
al: Lung adjuvant cisplatin evaluation: a pooled analysis by the
LACE Collaborative Group. J Clin Oncol. 26:3552–3559. 2008.
|
|
3.
|
Arriagada R, Auperin A, Burdett S, et al:
Adjuvant chemotherapy, with or without postoperative radiotherapy,
in operable non-small-cell lung cancer: two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010.
|
|
4.
|
Pisters KM, Evans WK, Azzoli CG, et al:
Cancer Care Ontario and American Society of Clinical Oncology
adjuvant chemotherapy and adjuvant radiation therapy for stages
I-IIIA resectable non small-cell lung cancer guideline. J Clin
Oncol. 25:5506–5518. 2007.
|
|
5.
|
Crino L, Weder W, van Meerbeeck J and
Felip E: Early stage and locally advanced (non-metastatic)
non-small-cell lung cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 21:v103–v115.
2010.
|
|
6.
|
Douillard JY, Tribodet H, Aubert D, et al:
Adjuvant cisplatin and vinorelbine for completely resected
non-small cell lung cancer: subgroup analysis of the Lung Adjuvant
Cisplatin Evaluation. J Thorac Oncol. 5:220–228. 2010.
|
|
7.
|
Kato H, Ichinose Y, Ohta M, et al: A
randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
|
|
8.
|
Hamada C, Tsuboi M, Ohta M, et al: Effect
of postoperative adjuvant chemotherapy with tegafur-uracil on
survival in patients with stage IA non-small cell lung cancer: an
exploratory analysis from a meta-analysis of six randomized
controlled trials. J Thorac Oncol. 4:1511–1516. 2009.
|
|
9.
|
Fruh M, Rolland E, Pignon JP, et al:
Pooled analysis of the effect of age on adjuvant cisplatin-based
chemotherapy for completely resected non-small-cell lung cancer. J
Clin Oncol. 26:3573–3581. 2008.
|
|
10.
|
Shirasaka T, Nakano K, Takechi T, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma ortho-topically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.
|
|
11.
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
|
|
12.
|
Iwasa S, Yamada Y, Fukagawa T, et al:
Management of adjuvant S-1 therapy after curative resection of
gastric cancer: dose reduction and treatment schedule modification.
Gastric Cancer. 14:28–34. 2011.
|
|
13.
|
Kim WY, Nakata B and Hirakawa K:
Alternative pharmacokinetics of S-1 components, 5-fluorouracil,
dihydrofluorouracil and alpha-fluoro-beta-alanine after oral
administration of S-1 following total gastrectomy. Cancer Sci.
98:1604–1608. 2007.
|
|
14.
|
Kochi M, Fujii M, Kanamori N, et al:
Effect of gastrectomy on the pharmacokinetics of S-1, an oral
fluoropyrimidine, in resectable gastric cancer patients. Cancer
Chemother Pharmacol. 60:693–701. 2007.
|
|
15.
|
Kawahara M, Furuse K, Segawa Y, et al:
Phase II study of S-1, a novel oral fluorouracil, in advanced
non-small-cell lung cancer. Br J Cancer. 85:939–943. 2001.
|
|
16.
|
Yano T, Yamazaki K, Maruyama R, et al:
Feasibility study of postoperative adjuvant chemotherapy with S-1
(tegaful, gimeracil, oteracil potassium) for non-small cell lung
cancer-LOGIK 0601 study. Lung Cancer. 67:184–187. 2010.
|
|
17.
|
Sobin L and Witthekind C: TNM
Classification of Malignant Tumours. 6th edition. Wiley-Liss; New
York: pp. 99–103. 2002
|
|
18.
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003.
|
|
19.
|
Aoyama T, Yoshikawa T, Watanabe T, et al:
Safety and feasibility of S-1 adjuvant chemotherapy for gastric
cancer in elderly patients. Gastric Cancer. 15:76–82. 2011.
|
|
20.
|
Tsushima T, Hironaka S, Boku N, et al:
Safety and efficacy of S-1 monotherapy in elderly patients with
advanced gastric cancer. Gastric Cancer. 13:245–250. 2010.
|
|
21.
|
Hirata K, Horikoshi N, Aiba K, et al:
Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor
drug. Clin Cancer Res. 5:2000–2005. 1999.
|
|
22.
|
Fujita K, Yamamoto W, Endo S, et al:
CYP2A6 and the plasma level of 5-chloro-2, 4-dihydroxypyridine are
determinants of the pharmacokinetic variability of tegafur and
5-fluorouracil, respectively, in Japanese patients with cancer
given S-1. Cancer Sci. 99:1049–1054. 2008.
|
|
23.
|
Ikeda M, Furukawa H, Imamura H, et al:
Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor
agent in animal model and in patients with impaired renal function.
Cancer Chemother Pharmacol. 50:25–32. 2002.
|
|
24.
|
Fujita K, Nakayama H, Ichikawa W, et al:
Pharmacokinetics of 5-fluorouracil in elderly Japanese patients
with cancer treated with S-1 (a combination of tegafur and
dihydropyrimidine dehydrogenase inhibitor
5-chloro-2,4-dihydroxypyridine). Drug Metab Dispos. 37:1375–1377.
2009.
|
|
25.
|
Kimura Y, Kikkawa N, Iijima S, et al: A
new regimen for S-1 therapy aiming at adverse reaction mitigation
and prolonged medication by introducing a 1-week drug-free interval
after each 2-week dosing session: efficacy and feasibility in
clinical practice. Gastric Cancer. 6:34–39. 2003.
|
|
26.
|
Tsukuda M, Kida A, Fujii M, et al:
Randomized scheduling feasibility study of S-1 for adjuvant
chemotherapy in advanced head and neck cancer. Br J Cancer.
93:884–889. 2005.
|
|
27.
|
Sakuma K, Hosoya Y, Arai W, et al:
Alternate-day treatment with S-1 in patients with gastric cancer: a
retrospective study of strategies for reducing toxicity. Int J Clin
Oncol. 15:166–171. 2010.
|
|
28.
|
Arai W, Hosoya Y, Hyodo M, et al:
Alternate-day oral therapy with TS-1 for advanced gastric cancer.
Int J Clin Oncol. 9:143–148. 2004.
|
|
29.
|
Shin SJ, Jeong JH, Park YS, et al: Phase
II trial of S-1 mono-therapy in elderly or frail patients with
metastatic colorectal cancer. Invest New Drugs. 29:1073–1080.
2010.
|
|
30.
|
Zhu AX, Clark JW, Ryan DP, et al: Phase I
and pharmacokinetic study of S-1 administered for 14 days in a
21-day cycle in patients with advanced upper gastrointestinal
cancer. Cancer Chemother Pharmacol. 59:285–293. 2007.
|
|
31.
|
Hanagiri T, Baba T, So T, et al: Time
trends of surgical outcome in patients with non-small cell lung
cancer. J Thorac Oncol. 5:825–829. 2010.
|
|
32.
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
|
|
33.
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323.
2007.
|
|
34.
|
Okamoto I, Yoshioka H, Morita S, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naive patients with advanced
non-small-cell lung cancer: results of a west Japan oncology group
study. J Clin Oncol. 28:5240–5246. 2010.
|
|
35.
|
Kubota K, Sakai H, Yamamoto N, et al: A
multi-institution phase I/II trial of triweekly regimen with S-1
plus cisplatin in patients with advanced non-small cell lung
cancer. J Thorac Oncol. 5:702–706. 2010.
|