Introduction
Thyroid tumors represent 1% of all neoplasms and
differentiated thyroid tumors (e.g., papillary and follicular
types) account for 80–85% of all thyroid tumors (1–4). The
majority of cases of differentiated thyroid tumors are associated
with favorable outcomes. Anaplastic thyroid carcinoma (ATC)
accounts for 5–15% of primary malignant thyroid tumors (5). The pronounced differences in the
biological behavior of the various histological types of thyroid
carcinoma are well known. In contrast to papillary and follicular
thyroid carcinomas, ATC is one of the most aggressive neoplasms in
humans. ATC is usually rapidly fatal, with a mean survival of 6
months after diagnosis (6,7). Since ATC is rare, there has not been
a sufficient number of available cases to investigate, in order to
obtain a better understanding of the natural history of this type
of tumor and the factors that may affect the response to treatment
and survival.
Numerous cancer immunotherapies that were developed
in experimental animals have been investigated in clinical trials.
Although some exhibited a moderate clinical efficacy, the majority
were not effective (8,9). Recent studies identified cells of
myeloid origin that are potent suppressors of tumor immunity and
represent a significant impediment to cancer immunotherapy
(8,9). Suppressive myeloid cells were
previously described in patients with cancer (10,11),
although their functional significance in the immune system has
only recently been evaluated. Accumulating evidence (12–15)
suggests that a population of cells with suppressive activity,
referred to as myeloid-derived suppressor cells (MDSCs), may
contribute to the negative regulation of immune responses during
cancer and other diseases. We previously characterized circulating
levels of MDSCs in patients with various types of malignant
diseases, including patients with thyroid cancer (16).
Interleukin (IL)-10 is a potent immunosuppressive
cytokine that is produced primarily by Th2 cells, macrophages and
activated B cells. This cytokine has a wide range of biological
activities and possesses immunosuppressive, anti-inflammatory and
immunomodulatory properties that promote the regulation of a
variety of immune cell differentiation and proliferation events
(17). IL-10 is also an
immunosuppressive effector cytokine produced by MDSCs (10,11).
Tumor development and growth occurs as a result of
interactions between the tumor and host immune/inflammatory cells,
with chronic inflammation being crucial in cancer development and
progression. The laboratory markers of systemic inflammatory
response and nutritional status, including C-reactive protein (CRP)
and neutrophil/lymphocyte ratio, have been investigated as
prognostic markers, with the evidence supporting their use being
optimally demonstrated in surgical patients (18,19).
The aim of the present study was to characterize
MDSC levels in normal volunteers and patients with thyroid cancer
and to investigate the association between MDSC levels and
immunosuppression, chronic inflammation and nutrition.
Materials and methods
Samples
Blood samples were collected from 49 patients with
thyroid cancer, including 38 patients with papillary, 6 with
anaplastic, 3 with medullary and 2 with follicular carcinoma.
Samples were also collected from 18 patients with benign thyroid
diseases [e.g., adenomatous goiter, follicular adenoma and
hyperthyroidism (Basedow’s disease)] and from 22 healthy volunteers
of similar age and gender distribution. Patients who received
treatment (e.g., surgery, chemotherapy or palliative care) and were
followed up at the Department of Organ Regulatory Surgery at
Fukushima Medical University between January, 2011 and January,
2013 were enrolled in this study. The patients were aged 18–90
years and had histologically confirmed thyroid cancer. Of the 49
patients, 8 had stage I, 1 had stage II, 6 had stage III and 34 had
stage IV disease. All patients were newly diagnosed and the blood
samples were collected prior to the initiation of any treatment.
Peripheral blood mononuclear cells (PBMCs) were separated on
Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). The isolated
PBMCs were washed twice with RPMI-1640 medium (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) and were maintained frozen at −80°C
in a freezing medium (BLC-1; Juji-Field Co., Ltd, Tokyo, Japan)
until use.
This study was approved by the Ethics Committee of
Fukushima Medical University (1095) and written informed consent
was obtained from all the patients and healthy volunteers prior to
enrollment.
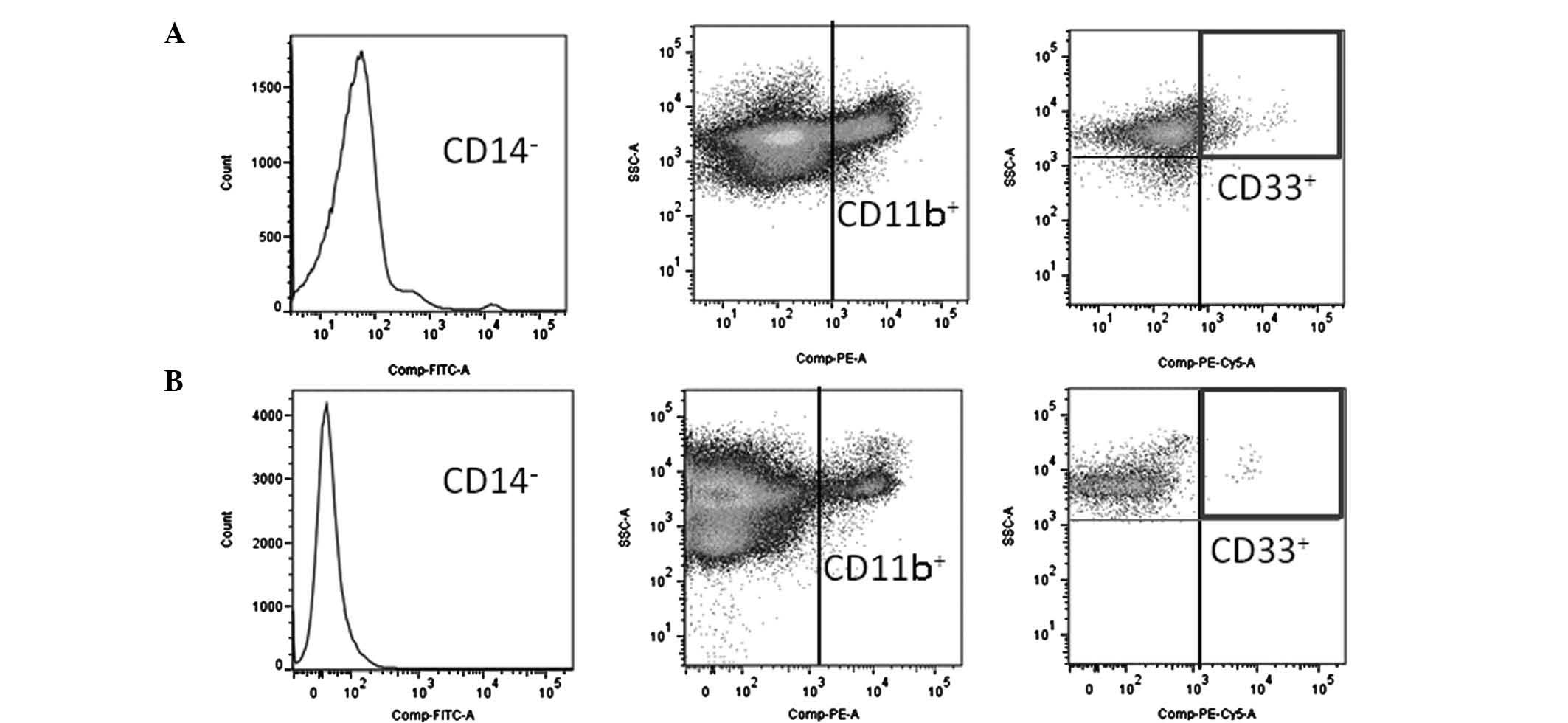
Flow cytometry
The cells were labeled with fluorescein
isothiocyanate (FITC), phycoerythrin (PE) and phycoerythrin-cyanin
5.1 (PC5). The antibodies used included those directed against
FITC-conjugated CD14 (Abcam, Cambridge, UK), PE-conjugated CD11b
(Beckman Coulter, Marseille, France) and PC5-conjugated CD33
(Beckman Coulter), diluted in phosphate-buffered saline (PBS) to 10
and 50 μg/ml. The cells were incubated with the antibodies
for 20 min at 4°C and were then washed with PBS. Data acquisition
and analysis were performed using a FACSAria II flow cytometer (BD
Biosciences, Mountain View, CA, USA), accompanied by FlowJo
software (TreeStar Inc., Ashland, OR, USA). The typical expression
patterns are shown in Fig. 1.
Cytokine production by PBMCs
Approximately 106 PBMCs were incubated in
1 ml of RPMI-1640 medium containing 10% heat-inactivated fetal calf
serum (Gibco BRL, St. Louis, MO, USA) and 100 μg/ml
phytohemagglutinin (PHA; Sigma, Rockville, MD, USA) in 5%
CO2 at 37°C for 24 h. The aliquots of these supernatants
were then frozen and kept at −80°C until later use. The supernatant
samples were subsequently thawed and used for measurement of the
IL-10 concentration using enzyme-linked immunosorbent assay (ELISA)
test kits (R&D Systems, Minneapolis, MN, USA). Each sample was
only used once after thawing.
Lymphocyte proliferation assay
Lymphocyte proliferation assays were performed using
PBMCs suspended in RPMI-1640 medium (Wako Pure Chemical Industries)
containing 10% fetal calf serum (Sigma-Aldrich, St. Louis, MO,
USA). Following the addition of 10 μg/ml PHA into the PBMC
culture wells that were kept at 37°C in a 5% CO2
atmosphere, PHA mitogenesis was observed for 80 h.
3H-thymidine (Japan Radioisotope Association, Tokyo,
Japan) was added to the wells for the last 8 h of incubation. The
cells were harvested and 3H-thymidine incorporation was
determined using a liquid scintillation counter (Perkin-Elmer Inc.,
Waltham, MA, USA) and expressed as counts per minute (cpm). The
stimulation index (SI) was obtained by calculating total
cpm/control cpm. PBMCs that had not been subjected to PHA addition
were used as controls.
Markers of nutritional status and chronic
inflammation
To evaluate the nutritional status of the subjects,
the serum concentrations of albumin (determined by nephelometry)
were assessed. CRP was used as an indicator of inflammation.
Statistical analysis
The differences between the groups were determined
by Student’s t-tests. P<0.05 was considered to indicate a
statistically significant difference. Inadequate amounts of blood
were obtained from some patients; in these cases, certain
measurements were not possible.
Results
PBMCs obtained from 49 patients with thyroid cancer,
18 with non-cancerous thyroid diseases and 22 healthy volunteers
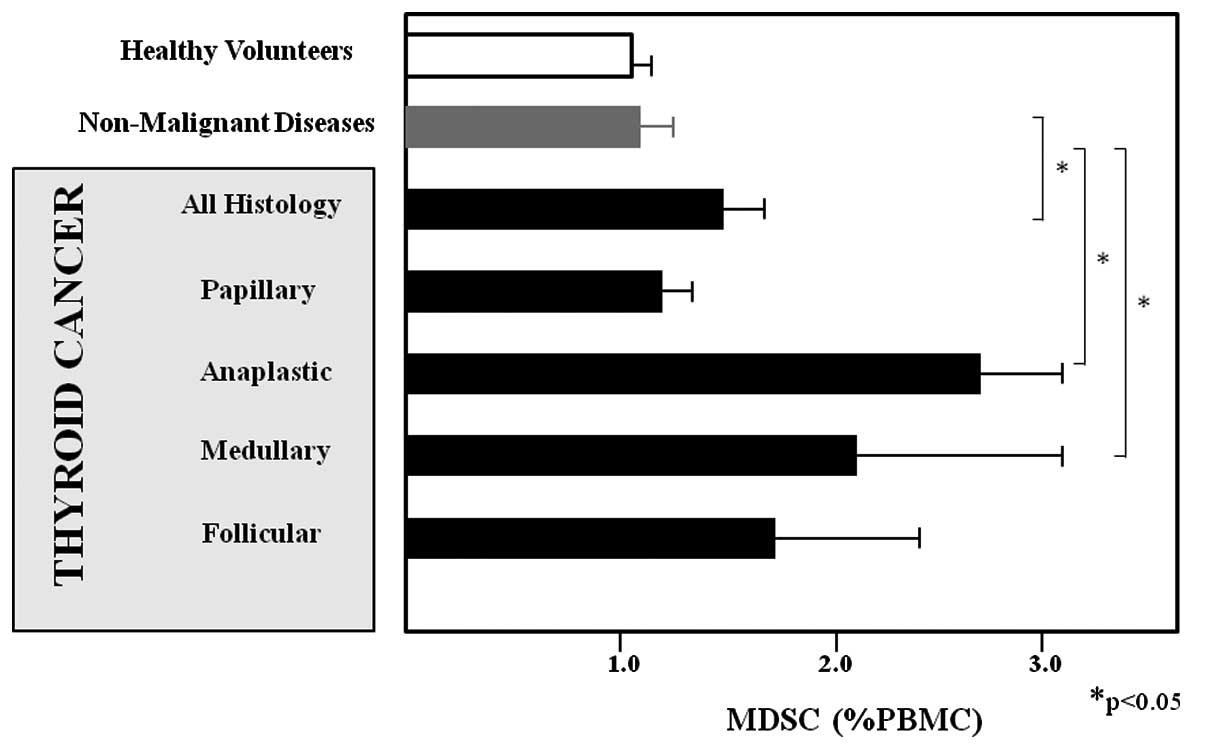
were investigated in this study. The concentrations of MDSCs were
significantly higher in patients with any type of thyroid cancer
(1.578±0.177, P<0.05), ATC (2.676±0.544, P<0.001), or
medullary thyroid carcinoma (2.063±1.028, P<0.05) when compared
to healthy volunteers (1.047±0.120 %PBMC). By contrast, there was
no significant difference in the MDSC levels when comparing
patients with papillary thyroid carcinoma (1.025±0.109),
non-cancerous thyroid diseases (1.188±0.161 %PBMC) and healthy
volunteers (Fig. 2). The levels of
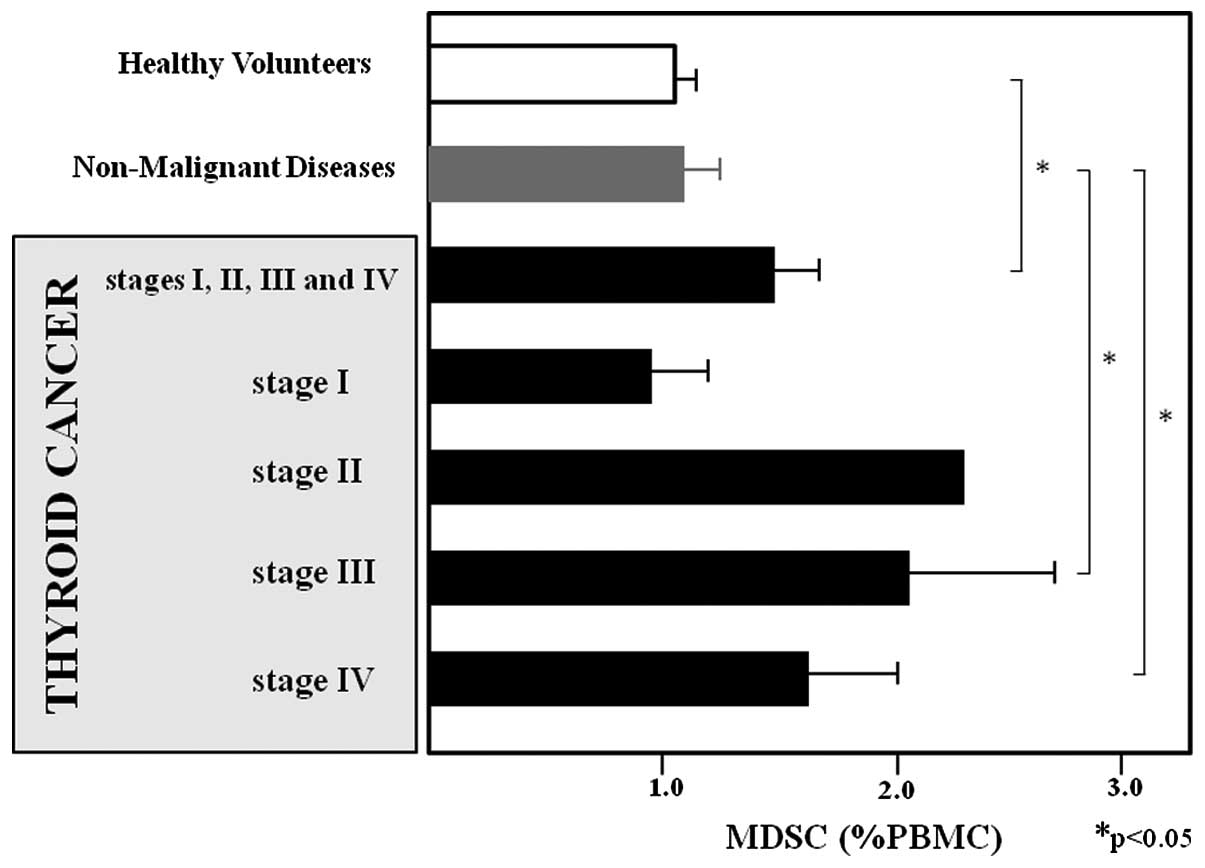
MDSCs according to the clinical stage of thyroid cancer are shown
in Fig. 3. The MDSC levels of
patients with stage I, II, III and IV thyroid cancer were
0.986±0.235, 2.36, 2.07±0.668 and 1.648±0.242 %PBMC, respectively,
and those of patients with stage III–IV disease were significantly
higher compared to those of healthy volunteers (P<0.05). Data
regarding SI, CRP and albumin levels of patients with non-cancerous
thyroid diseases and the histology of thyroid cancer are presented
in Table I. The SI and CRP levels
were significantly higher and the albumin levels were significantly
lower in patients with ATC compared to those in patients with
non-cancerous thyroid diseases (P<0.05). However, there was no
significant difference in these parameters when comparing patients
with non-cancerous thyroid diseases to patients with papillary,
follicular and medullary thyroid carcinomas.
 | Table I.Stimulation index (SI) and serum
concentrations of C-reactive protein (CRP) and albumin in patients
with thyroid cancer according to histological type. |
Table I.
Stimulation index (SI) and serum
concentrations of C-reactive protein (CRP) and albumin in patients
with thyroid cancer according to histological type.
| Histological
type | SI | CRP (mg/dl) | Albumin (mg/dl) |
|---|
| Non-malignant disease
(18) | 796.6+184.2 | 0.08+0.03 | 3.98+0.12 |
| Papillary carcinoma
(38) | 513.1+78.1 | 0.64+0.33 | 3.72+0.10 |
| Anaplastic carcinoma
(6) | 281.5+212.8a | 7.51+2.78a | 2.96+0.56a |
| Medullary carcinoma
(3) | 450.5+116.8 | 0.10+0.01 | 4.13+0.13 |
| Follicular carcinoma
(2) | 353.3+102.3 | 0.07+0.02 | 4.02+0.01 |
Data regarding SI, CRP and albumin levels in
patients with non-cancerous thyroid diseases according to the
clinical stage of thyroid cancer are presented in Table II. The SI was significantly lower
in patients with stage III–IV thyroid cancer compared to that in
patients with non-cancerous thyroid diseases (P<0.05).
Furthermore, the CRP and albumin levels were higher in patients
with stage IV thyroid cancer compared to those in patients with
non-cancerous thyroid diseases (P<0.05).
 | Table II.Stimulation index (SI) and serum
concentrations of C-reactive protein (CRP) and albumin in patients
with thyroid cancer according to tumor stage. |
Table II.
Stimulation index (SI) and serum
concentrations of C-reactive protein (CRP) and albumin in patients
with thyroid cancer according to tumor stage.
| Tumor stage | SI | CRP (mg/dl) | Albumin (mg/dl) |
|---|
| Non-malignant disease
(18) | 796.6+184.2 | 0.08+0.03 | 3.98+0.12 |
| Stage I (8) | 708.3+184.2 | 0.10+0.05 | 3.93+0.11 |
| Stage II (1) | 700.8 | 0.05 | 4.10 |
| Stage III (6) | 422.2+132.2a | 0.08+0.03 | 3.92+0.18 |
| Stage IV (34) | 420.9+55.6a | 1.91+0.64b | 3.56+0.12a |
Subsequently, patients with thyroid carcinoma were
categorized into one of two groups according to a %PBMC of MDSC
cut-off level of 1.578, which was the average %PBMC of MDSC in
patients with any type of thyroid carcinoma. As shown in Table III, the size of the thyroid nodule
tended to be larger (P<0.10) in patients with higher MDSC
levels. Furthermore, in patients with higher MDSC levels, the
production of CRP and IL-10 was significantly higher (P<0.05)
and the levels of albumin were significantly lower (P<0.05)
compared to those in patients with lower MDSC levels.
 | Table III.Comparison of patients according to
level of myeloid-derived suppressor cells (MDSCs). |
Table III.
Comparison of patients according to
level of myeloid-derived suppressor cells (MDSCs).
| Variables | High MDSC levels
(n=35) | Low MDSC levels
(n=19) | P-value |
|---|
| Age (years) | 63.15+2.52 | 59.57+3.19 | 0.451 |
| Tumor diameter
(mm) | 25.16+8.1 | 13.81+1.96 | 0.07 |
| MDSC (%PBMC) | 2.850+0.353 | 0.885+0.051 | 1.43E-09 |
| CRP (mg/dl) | 3.351+1.395 | 0.709+0.359 | 0.017 |
| Albumin (mg/dl) | 3.318+0.185 | 3.806+0.120 | 0.028 |
| IL-10 production
(μg/ml) | 3664.6+767.0 | 1503.8+508.4 | 0.021 |
Discussion
The aim of this study was to characterize MDSC
levels in patients with thyroid cancer, patients with benign
thyroid diseases and healthy volunteers. Patients with ATC
exhibited significantly elevated levels of circulating MDSCs, SI
and CRP and significantly decreased levels of serum albumin
compared to patients with non-cancerous thyroid diseases.
Furthermore, MDSC levels were higher and SI was lower in patients
with stage III and IV thyroid cancer compared to healthy
volunteers. The CRP and albumin levels were lower in patients with
stage IV thyroid cancer compared to those in patients with
non-cancerous thyroid diseases. Additionally, the levels of CRP
were significantly higher, the albumin concentration was lower and
the production of IL-10 was elevated in patients with higher
compared to those with lower MDSC levels. These data suggest that
MDSC levels are increased in patients with ATC and advanced thyroid
cancer. In addition, these patients exhibited impaired
cell-mediated immune responses, chronic inflammation and
nutritional impairment.
The ATC tissue produces large amounts of cytokines
(e.g., IL-8 and IL-6) and growth factors (e.g., granulocyte
colony-stimulating factor) (20–22).
The exact mechanism underlying the increased production of immature
myeloid cells in cancer patients has not been elucidated. However,
the soluble factors produced by the ATC cells may affect the normal
pathway of cell differentiation, resulting in the accumulation of
MDSCs and increased clinical aggressiveness of ATC. The serum CRP
level is a sensitive marker of inflammation and it is found to be
increased in response to tissue damage or infection. The CRP level
is also considered to be a prognostic factor in cancer patients
(23,24). This acute phase reactant is
produced primarily in the liver and its expression is upregulated
by pro-inflammatory cytokines, such as IL-6 and IL-8 (25,26).
The host immune system responds to tumor growth via elevated levels
of inflammatory cytokines, which may further increase CRP levels.
Hypoalbuminemia, typically observed in patients with cancer
cachexia, is frequently associated with chronic inflammation.
Furthermore, nutritional impairment associated with inflammation
and immune suppression is observed in patients with ATC, which
appears to be independent of the clinical aggressiveness of
ATC.
The present study demonstrated that MDSC levels were
higher in patients with systemic inflammation and hypoalbuminemia.
In addition, immune suppression was closely associated with the
Th2-dominant condition. Previous studies suggested that the MDSC
levels may decrease following administration of chemotherapeutic or
certain anti-inflammatory agents (15,27).
Therefore, the regulation of MDSC levels using these strategies may
facilitate anti-ATC treatment, including immunotherapy. However,
further studies are required to verify this hypothesis.
Acknowledgements
The authors would like to thank Dr
Takeshi Machida and Professor Hideharu Sekine (Department of
Immunology, Fukushima Medical University) for their assistance in
the flow cytometric experiments and Mrs. Hideko Taguchi for the
management of research funding and facilities.
References
|
1.
|
Franceschi S, Boyle P, Maisonneuve P, La
Vecchia C, Burt AD, Kerr DJ and MacFarlane GJ: The epidemiology of
thyroid carcinoma. Crit Rev Oncog. 4:25–52. 1993.
|
|
2.
|
Reeve TS and Delbridge L: Thyroid cancers
of follicular cell origin. Prog Surg. 19:78–88. 1988.
|
|
3.
|
De Groot LJ, Kaplan EL, McCormick M and
Straus FH: Natural history, treatment, and course of papillary
thyroid carcinoma. J Clin Endocrinol Metab. 71:414–424.
1990.PubMed/NCBI
|
|
4.
|
Samaan NA, Schultz PN, Hickey RC, Goepfert
H, Haynie TP, Johnston DA and Ordonez NG: The results of various
modalities of treatment of well differentiated thyroid carcinomas:
a retrospective review of 1,599 patients. J Clin Endocrinol Metab.
75:714–720. 1992.PubMed/NCBI
|
|
5.
|
Franssila KO: Prognosis of thyroid
carcinoma. Cancer. 36:1138–1146. 1975. View Article : Google Scholar
|
|
6.
|
Ain KB: Anaplastic thyroid carcinoma:
behavior, biology, and therapeutic approaches. Thyroid. 8:715–726.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Giuffrida D and Gharib H: Anaplastic
thyroid carcinoma: current diagnosis and treatment. Ann Oncol.
11:1083–1089. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pardoll D and Allison J: Cancer
immunotherapy: breaking the barriers to harvest the crop. Nat Med.
10:887–902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gabrilovich DI and Nagaj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ostrand-Rosenberg S and Sinha P:
Myeloid-derived suppressor cells: linking inflammation and cancer.
J Immunol. 182:4499–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zea AH, Rodriguez PC, Atkins MB, Hernandez
C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A,
O’Neill A and Ochoa AC: Arginase-producing myeloid suppressor cells
in renal cell carcinoma patients: a mechanism of tumor evasion.
Cancer Res. 65:3044–3048. 2005.PubMed/NCBI
|
|
13.
|
Ochoa AC, Zea AH, Hernandez C and
Rodriguez PC: Arginase, prostaglandins, and myeloid-derived
suppressor cells in renal cell carcinoma. Clin Cancer Res.
13:721s–726s. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garett-Mayre E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden, and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar
|
|
15.
|
Gabitass RF, Annels NE, Crawshaw J, Pandha
HS and Middleton GE: Use of gemcitabine-(GEM) and
fluoropyrimidine-based chemotherapy to reduce myeloid-derived
suppressor cells (MDSCs) in pancreatic (PC) and esophago-gastric
cancer (EGC) (abs. 2588). In: Proceedings of the 2011 Annual
meeting of Am Soc Clin Oncol; 3–7 June 2011; Chicago
|
|
16.
|
Ohki S, Shibata M, Gonda K, Machida T,
Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H and
Takenoshita S: Circulating myeloid-derived suppressor cells are
increased and correlate to immune suppression, inflammation and
hypoalbuminemia in patients with cancer. Oncol Rep. 28:453–458.
2012.PubMed/NCBI
|
|
17.
|
Moore KW, de Waal Malefyt R, Coffmann RL
and O’Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Blackwill F and Mantovani A: Cancer and
inflammation: implications for pharmacology and therapeutics. Clin
Pharmacol Ther. 87:401–406. 2010. View Article : Google Scholar
|
|
19.
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Enomoto T, Sugawa H, Inoue T, Miyamoto M,
Kosugi S, Takahashi T, Kitamura N, Yamamoto I, Konishi J, Mori T
and Imura H: Establishment of a human undifferentiated human
thyroid carcinoma cell line producing several growth factors and
cytokines. Cancer. 65:1971–1979. 1990. View Article : Google Scholar
|
|
21.
|
Iwasa K, Noguchi M, Mori K, Ohta N,
Miyazaki I, Nonomura A, Mizukami Y, Nakamura S and Michigishi T:
Anaplastic thyroid carcinoma producing the granulocyte colony
stimulating factor (G-CSF): report of a case. Surg Today.
25:158–160. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Murabe H, Akamizu T, Kubota A and Kusaka
S: Anaplastic thyroid carcinoma with prominent cardiac metastasis
accompanied by a marked leukocytosis with a neutrophilia and high
GM-CSF level in serum. Intern Med. 31:1107–1111. 1992. View Article : Google Scholar
|
|
23.
|
Allin KH, Bojesen SE and Nordestgaard BG:
Baseline C-reactive protein is associated with incident cancer and
survival in patients with cancer. J Clin Oncol. 27:2217–2224. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chen HH, Chen IH, Liao CT, Wei FC, Lee LY
and Huang SF: Preoperative circulating C-reactive protein levels
predict pathological aggressiveness in oral squamous cell
carcinoma: a retrospective clinical study. Clin Otolaryngol.
36:147–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Il’yasova D, Colbert LH, Harris TB, Newman
AB, Bauer DC, Satterifield S and Kritchevsky SB: Circulating levels
of inflammatory markers and cancer risk in the health aging and
body comparison cohort. Cancer Epidemiol Biomarkers Prev.
14:2413–2418. 2005.PubMed/NCBI
|
|
26.
|
Erlinger TP, Platz EA, Rifai N and
Helzlsouer KJ: C-reactive protein and the risk of incident
colorectal cancer. JAMA. 291:585–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kao J, Ko EC, Eisenstein S, et al:
Targeting immune suppressing myeloid-derived suppressor cells in
oncology. Cri Rev Oncol Hematol. 77:12–19. 2011. View Article : Google Scholar : PubMed/NCBI
|