Introduction
Lung cancer remains the most common cause of
cancer-related mortality worldwide (1). Patients affected by lung cancer
exhibit a poor prognosis. Despite the significant advances in
surgical techniques, chemotherapy and radiotherapy, the relapse
rate is high and only a few patients achieve long-term survival,
with an overall 5-year survival rate of ∼15% (2). Evidence supports the cancer stem cell
(CSC) hypothesis, according to which CSCs may be responsible for
tumor initiation, metastasis, recurrence and resistance to
treatment (3,4). Due to the characteristics of CSCs,
the conventional therapies are unable to effectively eliminate
these cells. The residual CSCs continue to proliferate, leading to
the relapse of cancer. A variety of molecules have been
investigated as putative markers of CSCs in malignancies, including
lung cancer (5). Among the various
markers, CD133 is one of the most commonly used. It is widely
expressed in a number of malignancies, such as glioblastomas
(6), hepatocellular (7), ovarian (8), colon (9) and lung (10) carcinomas.
A growing number of CD133-positive cancer cells have
been identified in lung cancer (11,12).
Eramo et al (10) observed
that a rare population of CD133-positive cancer stem-like cells
were able to self-renew and generate an unlimited progeny of
non-tumorigenic cells, whereas the CD133-negative cancer cells
lacked this potential. However, the association between CD133
expression and the clinicopathological characteristics of lung
cancer remains unknown. Attempts to elucidate the association
between CD133-positive cancer cells and clinicopathological
characteristics in previous studies yielded controversial results
(13,14). The limited sample availability
resulted in discrepancies regarding the clinical significance
determined by different CSCs studies. Therefore, we performed a
systematic review of the literature with a meta-analysis to
determine the association between CSCs marker CD133 and the
clinicopathological characteristics of lung cancer and to
investigate the role of CSCs in the prognosis of lung cancer.
Materials and methods
Literature search
Studies were identified via an electronic search
through Medline, EMBASE and the China National Knowledge
Infrastructure (CNKI) databases, using the key words ‘lung cancer’
and ‘CD133’, completed by the personal bibliography of two of the
authors (Yaoxi Tan and Bo Chen). The bibliographies reported in all
the identified studies were used to complete the study search.
Review articles were scanned to identify additional eligible
studies. The search was completed on November 15, 2012. To be
eligible for inclusion in this systematic review, a study was
required to meet the following criteria: i) it only included
patients with primary lung cancer; ii) it investigated the
association between CD133 and clinicopathological characteristics;
iii) it was published as a full-text article in English or Chinese
and; iv) it reported the number of CD133-positive and -negative
patients. When duplicate studies were published, only the most
recent or most informative was included in the analysis, to avoid
overlap between cohorts.
Data extraction
The following information was extracted from each
study: i) year of publication and first author’s name; ii) sample
size, test method and cut-off level; iii) tumor data including
stage, grade, histological type and lymph node metastasis.
Information was carefully extracted from all the eligible studies
independently by two of the authors of the present study (Yaoxi Tan
and Bo Chen). Differences in the extraction of data were assessed
by a third investigator (Jianqing Wu).
Statistical analysis
To assess the association between CD133 and the
clinicopathological characteristics of lung cancer including stage,
grade, histological type and lymph node metastasis, odds ratios
(ORs) with 95% confidence intervals (CIs) were calculated. The
heterogeneity of combined ORs was initially evaluated by graphical
examination of the forest plots. Statistical assessment was then
performed using a χ2-based test of homogeneity and
evaluation of the inconsistency index (I2) statistic.
The I2 statistic was defined as the percentage of
variability due to heterogeneity rather than chance, with values
>50% representing the possibility of substantial heterogeneity
(15). P<0.05 was considered to
indicate a statistically significant difference. If no obvious
heterogeneity existed, the OR was calculated by the fixed-effects
model (the Mantel-Haenszel method) and χ2 tests.
Otherwise, the random-effects model (the DerSimonian-Laird method)
was used. In addition, evidence of publication bias was determined
using the Egger’s (16) and Begg’s
methods (17). All the
calculations were performed using the Stata statistical software
version 12.0 (StataCorp, College Station, TX, USA).
Results
Study characteristics
In total, 15 studies published between 2008 and 2012
were selected for this systematic review. The study sample size
ranged from 30 to 145 subjects. All studies investigated the
association between CD133 and the clinicopathological
characteristics of lung cancer. Cortes-Dericks et al
(18) used reverse
transcriptase-polymerase chain reaction (RT-PCR) to detect the
expression of CD133, whereas Herpel et al (19) used tissue microarray, Tirino et
al (20) used flow cytometry
and the remaining studies used immunohistochemistry (IHC) with
different cut-off levels. A total of 11 studies investigated
non-small-cell lung cancer (NSCLC) alone, one study investigated
adenocarcinoma and one squamous cell carcinoma. One study included
adenocarcinoma, squamous cell carcinoma and small-cell lung cancer
(SCLC). Li et al (21)
conducted the study on patients with neuroendocrine lung cancer.
The main characteristics of the 15 eligible publications are
presented in Table I.
 | Table I.Main characteristics of the 15
eligible studies. |
Table I.
Main characteristics of the 15
eligible studies.
| First author | Year | Histological
type | Method | Cut-off level | No. of patients | No. of case
groups | Refs. |
|---|
| Shien | 2012 | NSCLC | IHC | >1% staining | 30 | 9 | (23) |
| Bertolini | 2009 | NSCLC | IHC | Strong staining | 58 | 32 | (13) |
| Salnikov | 2010 | NSCLC | IHC | Any staining | 88 | 56 | (28) |
| Cortes-Dericks | 2012 | AD | RT-PCR | Median | 64 | 63 | (18) |
| Li | 2011 | NSCLC | IHC | >1% staining | 145 | 46 | (22) |
| Herpel | 2011 | NSCLC | Tissue
microarray | Diffuse expression or
distinct staining in at least 1 out of 4 tissue cores per
sample | 86 | 13 | (19) |
| Xu | 2011 | NSCLC | IHC | Any staining | 103 | 51 | (14) |
| Wei | 2008 | NSCLC | IHC | >10% staining | 77 | 40 | (26) |
| Li | 2011 | N/E LC | IHC | >10% staining | 90 | 44 | (21) |
| Yao | 2010 | LC | IHC | >10%
staining | 42 | 31 | (27) |
| Lin | 2009 | SQ | IHC | Any staining | 54 | 27 | (29) |
| Gu | 2010 | NSCLC | IHC | >10%
staining | 44 | 30 | (30) |
| Cheng | 2010 | NSCLC | IHC | >10%
staining | 65 | 45 | (31) |
| Sun | 2012 | NSCLC | IHC | Method described by
Xu and Yang (33) | 67 | 42 | (32) |
| Tirino | 2009 | NSCLC | Flow cytometry | NA | 89 | 64 | (20) |
Main results of the meta-analysis
Correlation of CD133 with tumor
stage
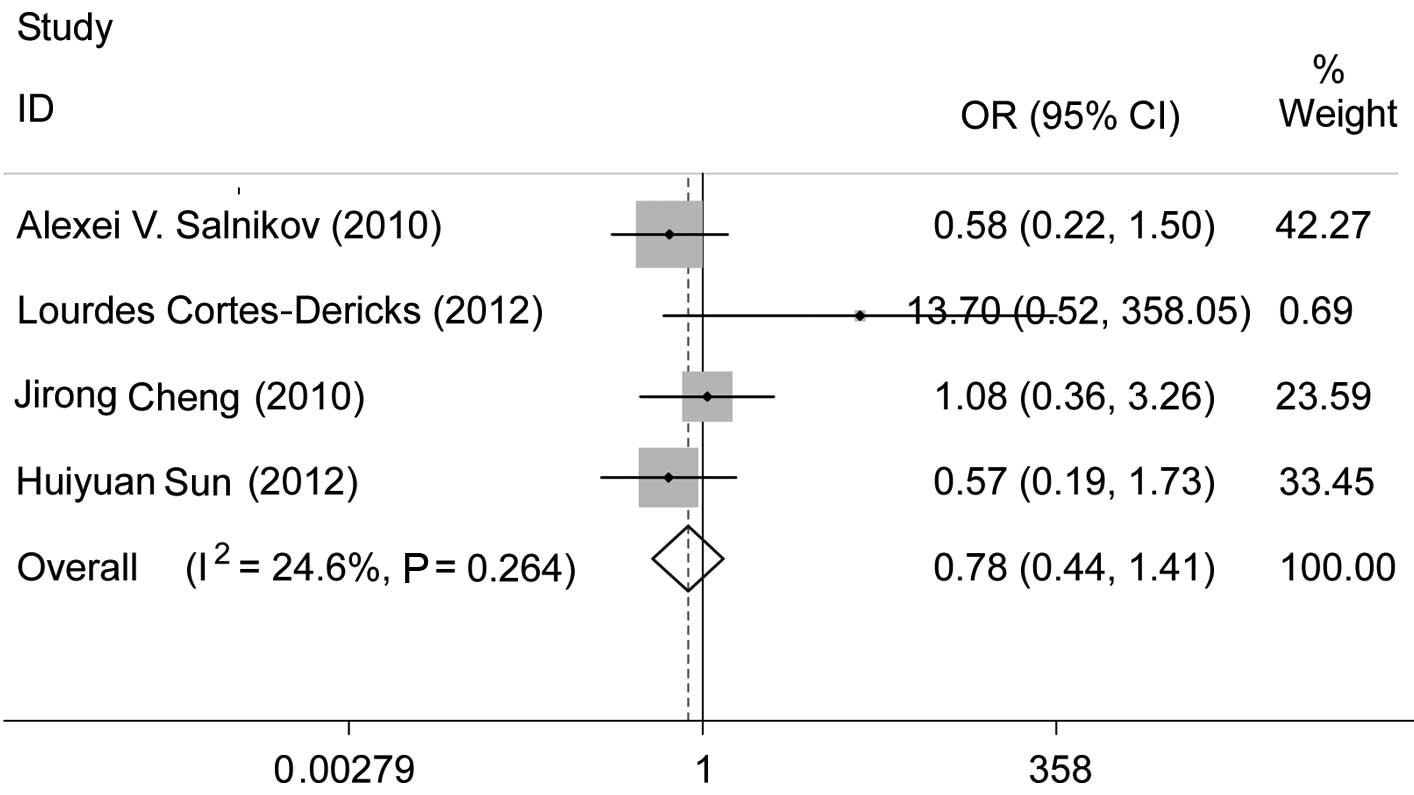
Among the selected studies, 8 analyzed the
association between CD133 and tumor stage. Cortes-Dericks et
al (18) and Li et al
(21) observed that positive CD133
expression exhibited a significant association with tumor stage. Of
the 8 studies, one study (22) was
limited to stage I and one study (19) assessed patients with stage I–II
disease. Two studies (13,21) were performed on stage I–IV lung
cancer of different histological types. Excluding the four studies
mentioned above, we performed a meta-analysis of the studies which
investigated stage I–III cancer. A comparison of stage I–II to
stage III revealed that there was no association between a positive
CD133 expression and tumor stage (pooled OR=0.78, 95% CI: 0.44–1.41
and P=0.411) (Fig. 1). There was
no evident publication bias (Egger’s test, P=0.089), a finding also
supported by the Begg’s funnel plots (figure not shown).
Correlation of CD133 with tumor
differentiation
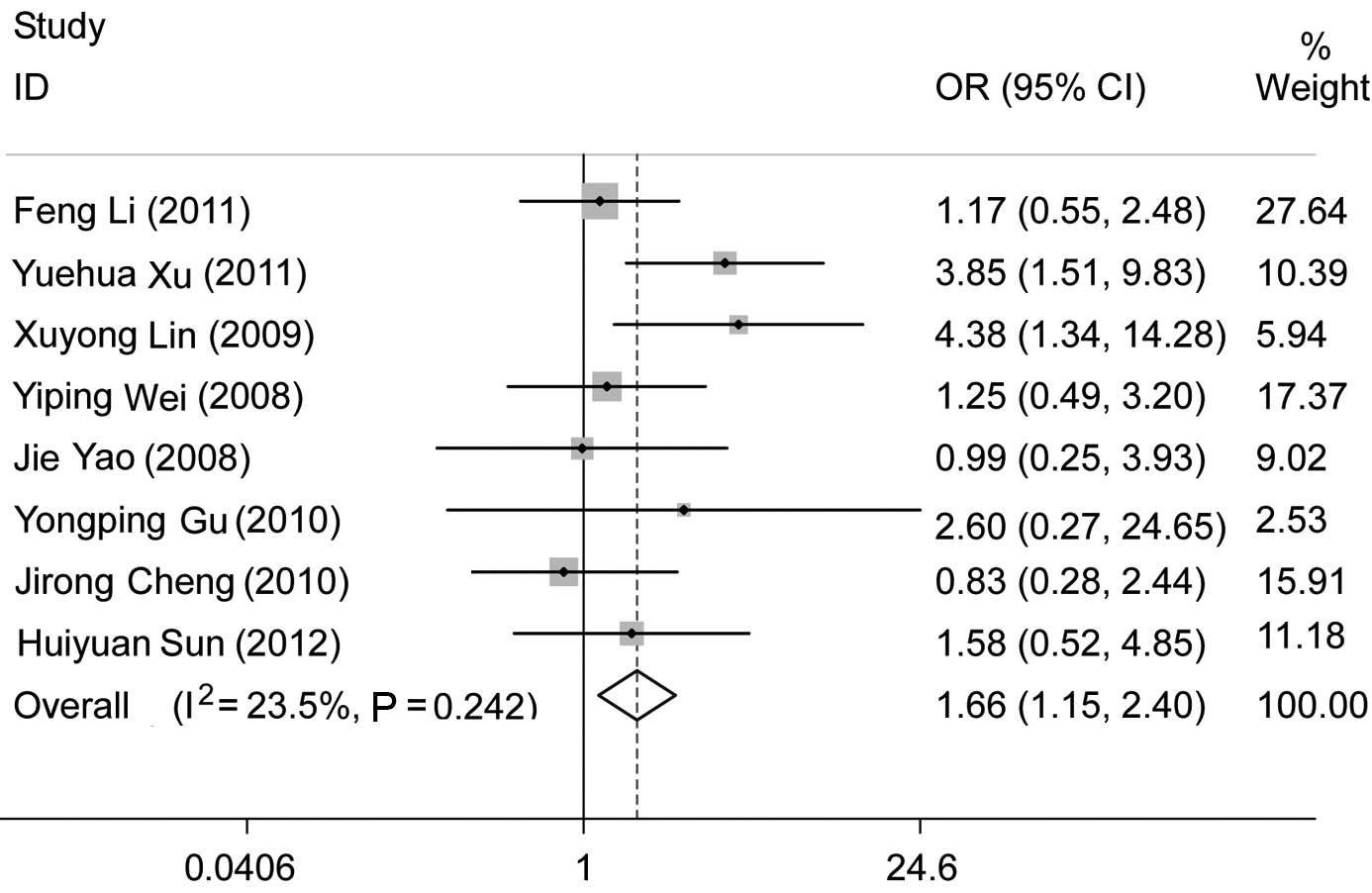
Eleven studies investigated the association between
CD133 and tumor differentiation. Three of those studies (13,14,18)
concluded that positive CD133 expression was associated with poorly
differentiated tumors. Significant heterogeneity (pooled OR=1.17,
95% CI: 0.68–2.00 and P=0.567; random effects, I2=55.3%
and P=0.013) existed when we analyzed all 11 studies. Excluding the
studies using RT-PCR (18) and
tissue microarray (19), we
performed a subgroup analysis among the studies using IHC. However,
significant heterogeneity (pooled OR=1.39, 95% CI: 0.81–2.37 and
P=0.229; random effects, I2=52.2% and P=0.033) was still
evident. We excluded one study (13) which was conducted on European
patients and analyzed the studies that investigated Asian patients
using IHC. The comparison of poor to high tumor differentiation
revealed that a positive CD133 expression was significantly
correlated with poor differentiation (pooled OR=1.66, 95% CI:
1.15–2.40 and P=0.006) without significant heterogeneity
(I2=23.5% and P=0.242) (Fig. 2). No evident publication bias
existed (Egger’s test, P=0.684), a finding also supported by the
Begg’s funnel plots (figure not shown).
Correlation of CD133 with histological
type
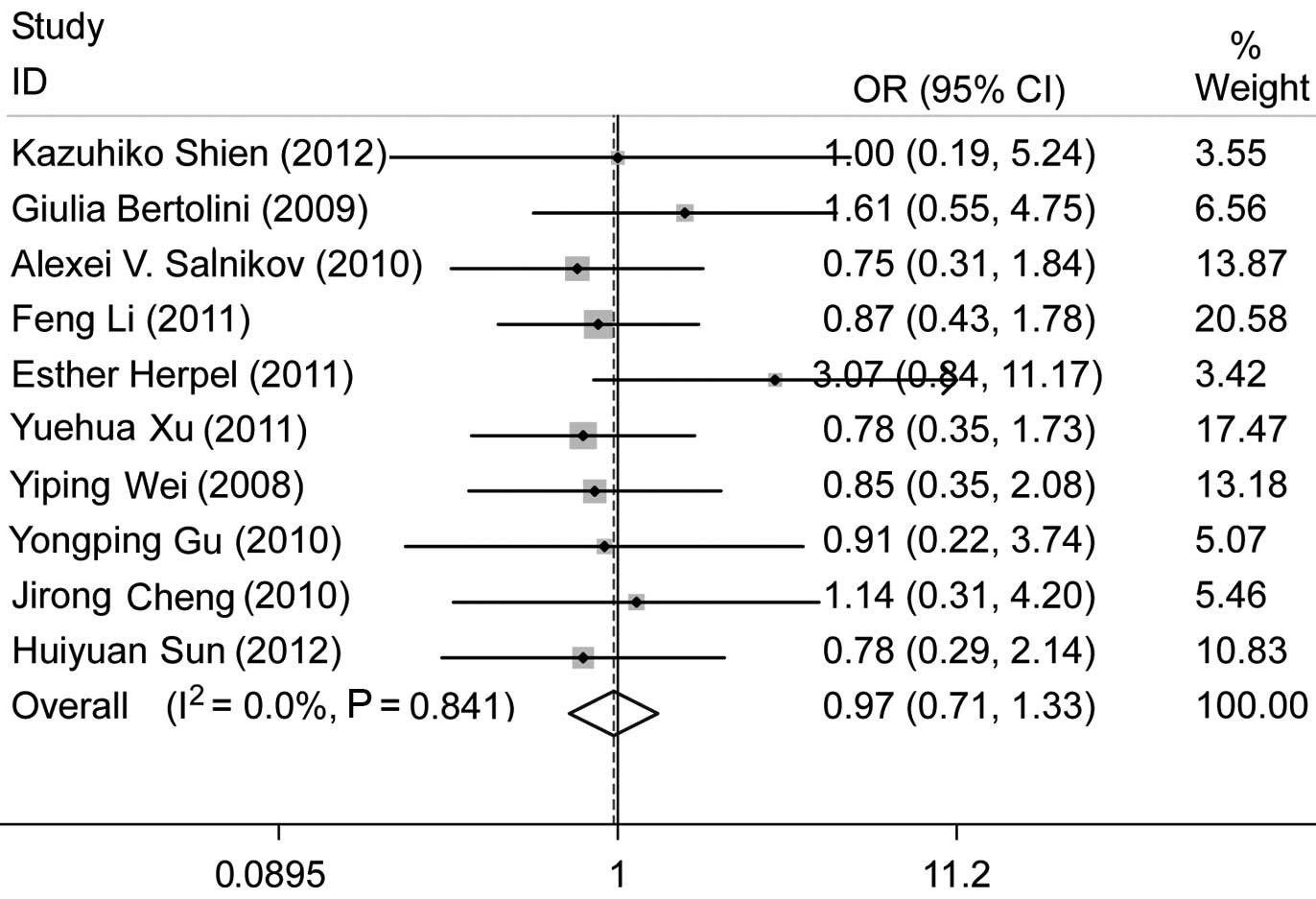
A total of 12 studies were found to be eligible for
the analysis of the association between a positive CD133 expression
and histological type. Of these, Bertolini et al (13) observed a correlation between a
positive CD133 expression and adenocarcinoma. We excluded two more
studies, one of which (18)
investigated adenocarcinoma alone, while the other (21) investigated neuroendocrine lung
cancer. We analyzed the remaining 9 studies which were conducted on
patients with NSCLC. There was no significant association between
positive CD133 expression and histological type (adenocarcinoma vs.
non-adenocarcinoma), with pooled OR=0.97, 95% CI: 0.71–1.33 and
P=0.86 (Fig. 3). No evident
publication bias existed (Egger’s test, P=0.143), a finding also
supported by the Begg’s funnel plots (figure not shown).
Correlation of CD133 with lymph node
metastasis
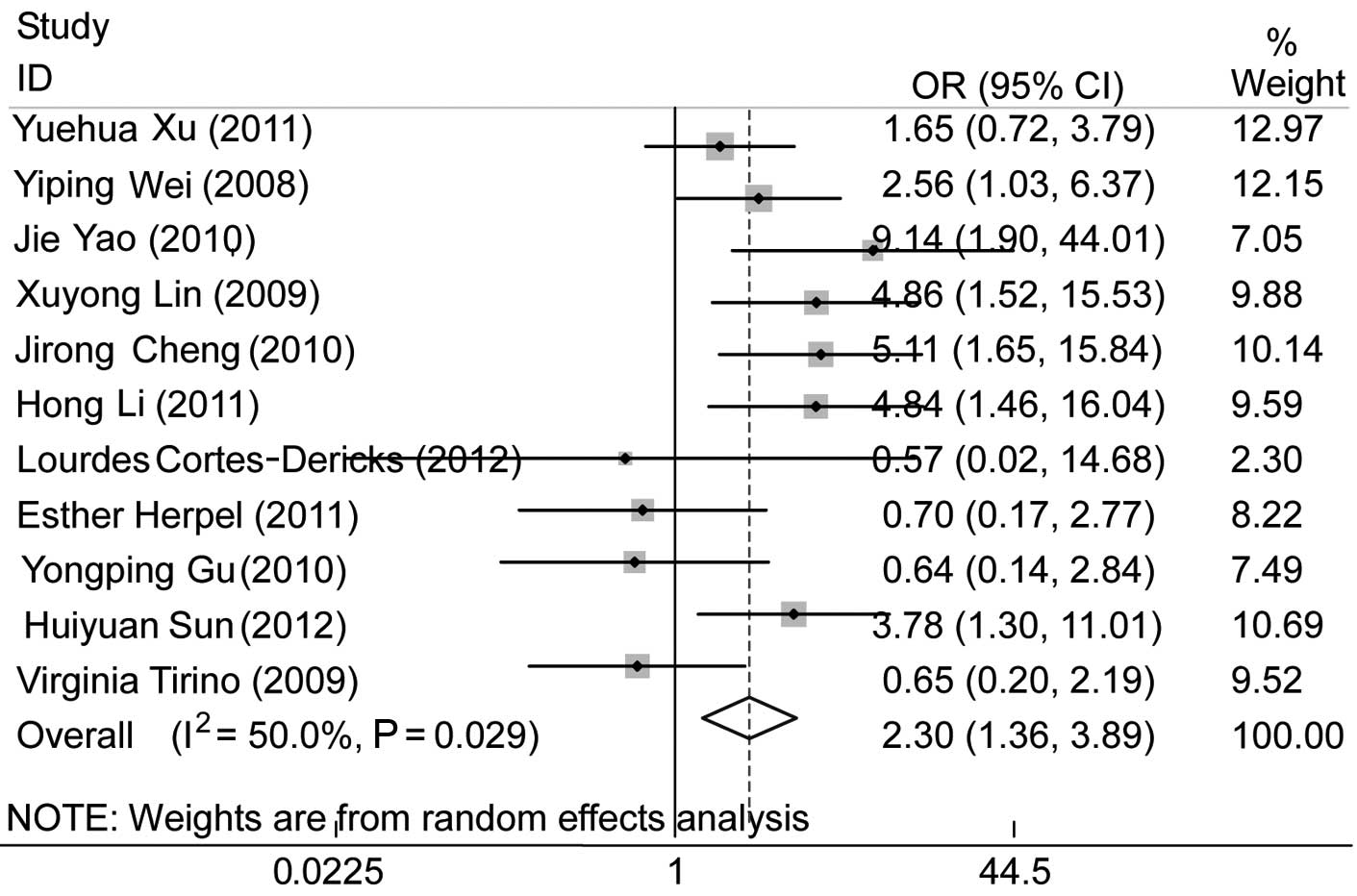
Eleven publications investigated the association
between CD133 expression and lymph node metastasis. One study
(23), which was limited to
patients with N2 or N3 NSCLC undergoing induction chemoradiotherapy
(CRT), was excluded. We observed that CD133 was associated with
nodal status (pooled OR=2.30, 95% CI: 1.36–3.89 and P=0.002), with
a positive CD133 expression in tumors with lymph node metastasis
(N+ compared to N0) (Fig. 4). No
evident publication bias existed (Egger’s test, P=0.639), a finding
also supported by the Begg’s funnel plots (figure not shown).
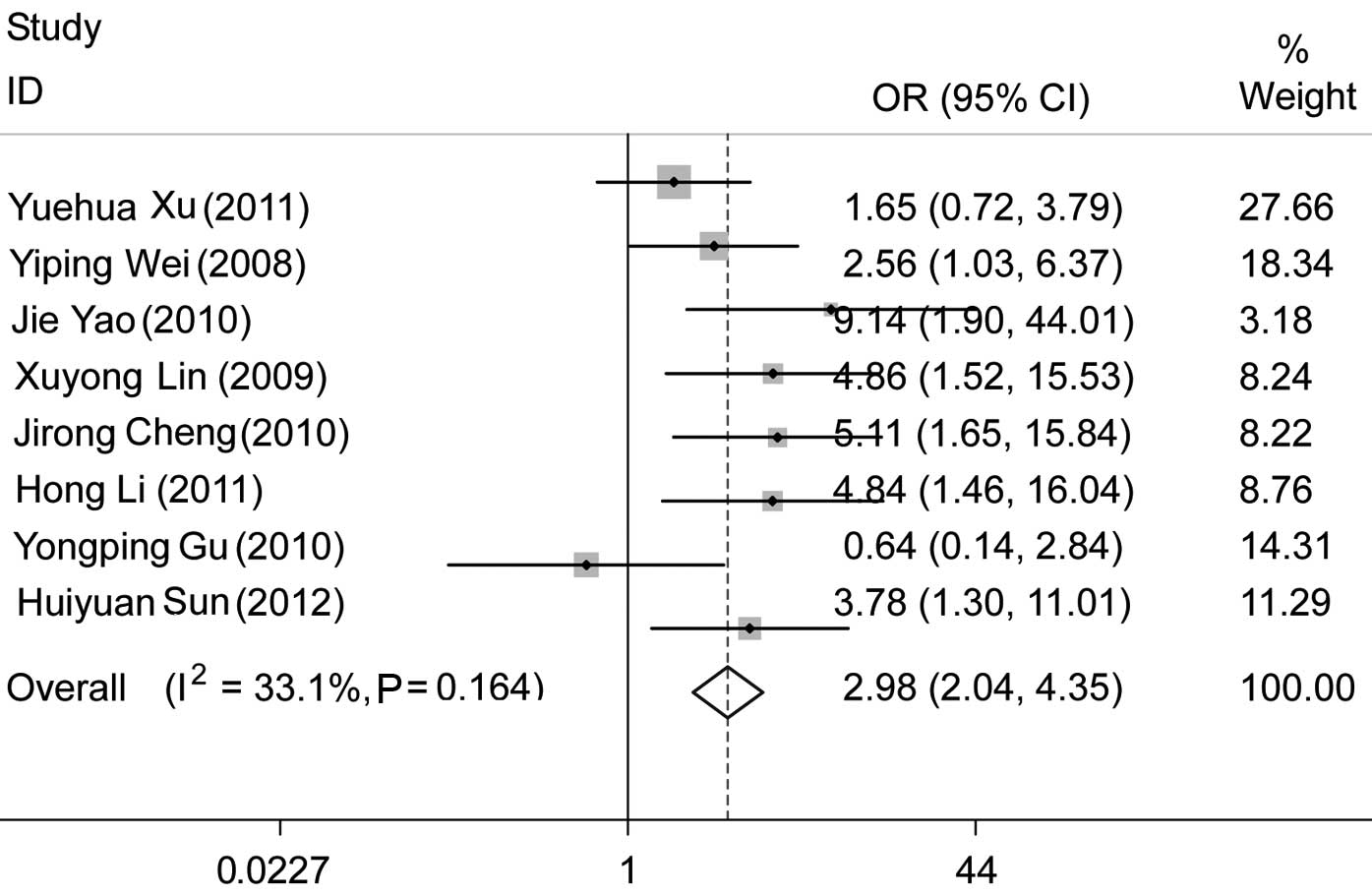
However, there was significant heterogeneity. We performed a
subgroup analysis among the studies that used IHC and observed that
positive CD133 expression was associated with nodal status (pooled
OR=2.98, 95% CI, 2.04–4.35 and P<0.001), without significant
heterogeneity (Fig. 5).
Discussion
Accumulating evidence indicates that specific
subpopulations of cancer cells with stem cell characteristics
within the majority of tumors may be crucial in the pathogenesis of
malignant tumors, including lung cancer. Several methods were used
to identify and enrich CSCs from lung cancer, e.g., side
populations and stem cell markers of normal tissue (24). CD133 is one of the most extensively
used markers in lung cancer, although its function in lung cancer
has not been fully elucidated. It remains controversial whether
CD133 is associated with clinicopathological characteristics and
prognosis of lung cancer. The aim of this current meta-analysis was
to investigate the correlation between CD133 expression and the
clinicopathological characteristics in patients with lung cancer,
since therapeutic decisions are directly associated with
clinicopathological characteristics, such as tumor type and
differentiation. Our results indicated that a positive CD133
expression was significantly associated with differentiation and
lymph node metastasis, although it was not associated with tumor
stage or histological type.
Several studies (14,18,21,23,25–27)
reported that CD133 expression was correlated with poor prognosis
in lung cancer, whereas other authors (13,19,22,28)
observed no such association. The patient populations varied among
these studies. For example, Herpel et al (19) conducted the study on previously
untreated stage I–II NSCLC patients, while Shien et al
(23) investigated patients with
locally advanced N2 or N3 NSCLC, who underwent induction CRT
followed by surgery. The methods used to detect CD133 expression
were also different, including IHC, RT-PCR and tissue microarray.
Even among the studies using IHC, the cut-off level and the
antibodies used varied widely. In addition, certain studies
reported disease-free survival, whereas others reported overall
survival with different follow-up times. Therefore, due to these
considerable differences, we were not able to directly perform a
systematic review to assess the correlation between CD133
expression and the prognosis of lung cancer.
The studies mentioned previously provided important
information regarding the indirect prognostic value of CD133 and
the therapy targeting CSCs in lung cancer. Eramo et al
(10) observed that CD133-positive
stem-like cancer cells were capable of self-renewal. The injection
of immunocompromised mice with CD133-positive lung cancer cells
readily generated tumor xenografts phenotypically identical to the
original tumor, leading to the conclusion that CD133 was a reliable
marker of lung cancer. We inferred that there was a larger
percentage of CSCs in the majority of lung cancer tissue, with a
higher number of CD133-positive lung cancer cells. One of the
properties of CSCs was the ability to undergo asymmetrical
division, leading to pluripotential differentiation and metastasis
(28). This finding was consistent
with the results of our meta-analysis, suggesting that a positive
CD133 expression was clearly associated with poor tumor
differentiation and lymph node metastasis. Poor differentiation and
metastasis were significantly associated with poor survival of
cancer. In brief, positive CD133 expression was most likely
correlated with poor prognosis of lung cancer. It should also be
noted that, since CD133 positivity was correlated with the
expression of resistance-related proteins (28), the proportion of CSCs in patients
who had received radiotherapy and/or chemotherapy was higher
compared to that in patients who received surgery alone. As a
result, it was not clear whether CD133 expression was of higher
prognostic value in patients who received radio-therapy and/or
chemotherapy. Further studies are required to elucidate the direct
association between CD133 expression and the prognosis of lung
cancer.
A meta-analysis is a quantitative approach in which
individual study findings on the same topic are statistically
integrated and analyzed. With more samples, the results of a
meta-analysis are more reliable compared to those of a single
study. However, the present meta-analysis had certain limitations.
When analyzing whether CD133 expression was associated with tumor
differentiation or lymph node metastasis, there was significant
heterogeneity. The methods used to detect CD133 expression varied
widely among the studies and heterogeneity was eliminated following
exclusion of the studies that did not use IHC. Although IHC was the
most commonly applied method, the cut-off level was defined
differently among the studies. In addition, the IHC results were
based on the primary antibody used and different antibodies were
used by the eligible studies. The dilution of the antibody also
varied, leading to differences in the sensitivity of the method,
depending on the antibody concentration. Another factor was the
effect of ethnicity. The majority of the studies included were
conducted on Asian patients. There is the possibility that
different ethnic groups exhibit differences in CD133 expression,
leading to heterogeneity and bias.
Although there was no evident publication bias in
this meta-analysis, we were not able to completely exclude biases.
For example, the study was restricted to studies published in
English and Chinese, which may lead to bias.
In conclusion, our meta-analysis suggests that CD133
expression is significantly associated with poor differentiation
and lymph node metastasis in lung cancer. CD133 expression is most
likely correlated with poor prognosis of lung cancer. However,
further studies are required, with larger patient samples, unified
methods and cut-off levels to detect CD133 expression, classified
by tumor stage, therapeutic schedule, follow-up time and survival
events, to confirm the findings of the present meta-analysis.
Acknowledgements
This study was a project funded by the
Priority Academic Program Development of Jiangsu Higher Education
Institutions and also supported by the National Natural Science
Foundation of China (no. 81272602).
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
2.
|
Alberg AJ, Ford JG and Samet JM; American
College of Chest Physicians: Epidemiology of lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132(Suppl 3): S29–S55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Dontu G, Liu S and Wicha MS: Stem cells in
mammary development and carcinogenesis: implications for prevention
and treatment. Stem Cell Rev. 1:207–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Donnenberg VS and Donnenberg AD: Multiple
drug resistance in cancer revisited: the cancer stem cell
hypothesis. J Clin Pharmacol. 45:872–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Toyooka S, Mitsudomi T, Soh J, et al:
Molecular oncology of lung cancer. Gen Thorac Cardiovasc Surg.
59:527–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
7.
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006.
|
|
8.
|
Ferrandina G, Bonanno G, Pierelli L, et
al: Expression of CD133-1 and CD133-2 in ovarian cancer. Int J
Gynecol Cancer. 18:506–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kojima M, Ishii G, Atsumi N, Fujii S,
Saito N and Ochiai A: Immunohistochemical detection of CD133
expression in colorectal cancer: a clinicopathological study.
Cancer Sci. 99:1578–1583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Janikova M, Skarda J, Dziechciarkova M, et
al: Identification of CD133+/nestin+ putative
cancer stem cells in non-small cell lung cancer. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 154:321–326. 2010.
|
|
12.
|
Cui F, Wang J, Chen D and Chen YJ: CD133
is a temporary marker of cancer stem cells in small cell lung
cancer, but not in non-small cell lung cancer. Oncol Rep.
25:701–708. 2011.PubMed/NCBI
|
|
13.
|
Bertolini G, Roz L, Perego P, et al:
Highly tumorigenic lung cancer CD133+ cells display
stem-like features and are spared by cisplatin treatment. Proc Natl
Acad Sci USA. 106:16281–16286. 2009.PubMed/NCBI
|
|
14.
|
Xu YH, Wang JM, Zhang GB and Hu HC:
Expression and clinical significance of CD133 and B7-H4 in
non-small cell lung cancer. Jiangsu Med J. 4:412–415. 2011.
|
|
15.
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cortes-Dericks L, Galetta D, Spaggiari L,
Schmid RA and Karoubi G: High expression of octamer-binding
transcription factor 4A, prominin-1 and aldehyde dehydrogenase
strongly indicates involvement in the initiation of lung
adenocarcinoma resulting in shorter disease-free intervals. Eur J
Cardiothorac Surg. 41:e173–e181. 2012. View Article : Google Scholar
|
|
19.
|
Herpel E, Jensen K, Muley T, et al: The
cancer stem cell antigens CD133, BCRP1/ABCG2 and CD117/c-KIT are
not associated with prognosis in resected early-stage non-small
cell lung cancer. Anticancer Res. 31:4491–4500. 2011.PubMed/NCBI
|
|
20.
|
Tirino V, Camerlingo R, Franco R, et al:
The role of CD133 in the identification and characterisation of
tumour-initiating cells in non-small-cell lung cancer. Eur J
Cardiothorac Surg. 36:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Li H, Wang Y, Yu L and Zhao P:
Clinicopathological significance of expression of CD133 protein in
neuroendocrine lung carcinoma tissues. Chin J Cancer Prev Treat.
18:29–31. 2011.
|
|
22.
|
Li F, Zeng H and Ying K: The combination
of stem cell markers CD133 and ABCG2 predicts relapse in stage I
non-small cell lung carcinomas. Med Oncol. 28:1458–1462. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shien K, Toyooka S, Ichimura K, et al:
Prognostic impact of cancer stem cell-related markers in non-small
cell lung cancer patients treated with induction chemoradiotherapy.
Lung Cancer. 77:162–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rivera C, Rivera S, Loriot Y, Vozenin MC
and Deutsch E: Lung cancer stem cell: new insights on experimental
models and preclinical data. J Oncol. 2011:5491812011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Woo T, Okudela K, Mitsui H, et al:
Prognostic value of CD133 expression in stage I lung
adenocarcinomas. Int J Clin Exp Pathol. 4:32–42. 2010.PubMed/NCBI
|
|
26.
|
Wei YP, Wang M, Hua P, et al: Expression
of tumor stem cell marker CD133 in non-small cell lung carcinoma
and its clinical significance. J Sun Yat-Sen Univ (Med Sci).
29:312–316. 2008.
|
|
27.
|
Yao J, Wang ZG, Tong XW, Li ZJ and Yu ZG:
Expression of tumor stem cell marker CD133 and CD44 in original
tumor tissues and metastatic lymph nodes of lung cancer. Med J Natl
Defend Force Southwest China. 20:1300–1303. 2010.
|
|
28.
|
Salnikov AV, Gladkich J, Moldenhauer G,
Volm M, Mattern J and Herr I: CD133 is indicative for a resistance
phenotype but does not represent a prognostic marker for survival
of non-small cell lung cancer patients. Int J Cancer. 126:950–958.
2010.PubMed/NCBI
|
|
29.
|
Lin X, Liu S, Liu N, Yang X, Xu H and Wang
E: Expression and significance of stem cell markers CK19, Notch3,
CD133, P75NTR, STRO-1 and ABCG2 in pulmonary squamous carcinomas.
Zhongguo Fe. 12:316–321. 2009.(In Chinese).
|
|
30.
|
Gu YP, Sun MM, Gu LQ, Zhang H and Xie F:
Expression and significance of cancer stem cell marker CD133, ABCG2
and p75NTR in non-small cell lung carcinoma. Suzhou Univ J Med Sci.
30:513–516. 2010.
|
|
31.
|
Cheng JR, Wang SQ, Zu MRT and Zou J:
Expressions of CD133 and CD105 in lung cancer tissue and their
clinical significance. Tumor. 30:334–337. 2010.
|
|
32.
|
Sun HY, Yang M, Zheng MJ, Ren ZJ and Liu
H: Expression of CD133 and ALDH1 in non-small cell lung cancer and
their clinical significance. J Clin Exp Pathol. 28:813–815.
2012.
|
|
33.
|
Xu LZ and Yang WT: The criteria of
immunohistochemical reaction results. China Oncol. 6:229–231.
1996.
|