Introduction
Outcomes are extremely poor in patients with
non-resectable gastric cancer, with a median survival period
ranging from 3 to 5 months, even with the best supportive care
(1,2). S-1 is an oral anticancer drug that
combines tegafur, a prodrug of fluorouracil, with
5-chloro-2,4-dihydropyrimidine (CDHP) and potassium oxonate at a
molar ratio of 1:0.4:1 (Taiho Pharmaceutical Co., Ltd., Tokyo,
Japan) (3). In a phase II study of
S-1, an ∼40% response rate was noted in patients with advanced
gastric cancer (4,5). Thus, S-1 chemotherapy has been widely
used as a basic treatment for patients with non-resectable gastric
cancer. Findings from the SPIRIT trial identified S-1 plus
cisplatin as a standard first-line treatment (6) and recommended its use in patients
with an expected survival period of at least 3 months. However, due
to the severe side effects, the S-1 plus cisplatin regimen [S-1:
40–60 mg/m2; in a 5-week cycle (3 weeks on and 2 weeks
off), in combination with 60 mg/m2 cisplatin on day 8]
was difficult to continue in patients with poor Eastern Cooperative
Oncology Group Performance Status (ECOG PS). Additionally, a number
of patients suffered from reduced quality of life (QOL) while
undergoing this medical treatment (7). However, Casaretto et al
(8) reported that chemotherapy
increased the 1-year survival rate, provided a longer symptom-free
period and improved the QOL of patients with non-resectable
advanced gastric cancer. Clinically, it is important to select
chemotherapeutic regimens that are most appropriate for the
patient’s condition.
The objective indicators determining suitable
chemotherapy regimens for patients with non-resectable gastric
cancer have been studied. The standard prognostic indicators in
oncology, such as tumor size, grade and stage, or molecular
biology, are less relevant in patients with advanced cancer. The
palliative prognostic (PaP) score was developed in the 1990s, as a
result of a series of prospective trials aimed to identify clinical
and biologic factors associated with the prognosis of advanced
cancer patients referred to hospice and to merge them into a
prognostic index (9). The survival
of patients with non-resectable or recurrent cancers can be
estimated using the PaP score even during chemotherapy (10).
In this study, the usefulness of the PaP score in
determining the first-line chemotherapy for patients with
non-resectable gastric cancer was examined retrospectively.
Materials and methods
Patients
Between 2003 and 2010, 558 patients with gastric
cancer were treated at the Tottori University Hospital, Yonago,
Japan. Forty-four patients (7.9%) were diagnosed as non-resectable.
Details of these 44 patients are shown in Table I. Patients were followed up at the
hospital until March 2012. During this period, gastrectomy was
performed on 3 patients (bleeding, 2 patients; perforation, 1
patient). All participants provided informed consent and the study
design was approved by the Ethics Review Board of Tottori
University.
 | Table IPatient data (n=44). |
Table I
Patient data (n=44).
| Variables | No. |
|---|
| Age (range, mean;
years) | 23–92, 66.5 |
| Gender
(male/female) | 24/20 |
| Ascites (yes/no) | 10/34 |
| ECOG PS (0/1/2) | 14/18/12 |
| Non-resectable
parameters | |
| Locally
advanced | 6 |
| Lymph node | 12 |
| Hematogenic
metastasis | 19 |
| Peritoneal
metastasis | 20 |
| Surgical
intervention | |
| No | 29 |
|
Probe-laparotomy | 1 |
| Bypass
operation | 11 |
| Gastrectomy | 3 |
Chemotherapy
First-line chemotherapy was received by 41 patients
(S-1, 7; S-1 plus cisplatin, 17; S-1 plus docetaxel, 13; other
chemotherapy, 4). Chemotherapy was terminated in the case of 3
patients with poor performance status (PS) and advanced age, who
then received best supportive care (BSC).
PaP score
The PaP score has four criteria: two symptoms
(anorexia and dyspnea), performance status measured by the
Karnofsky performance score, white blood cells (WBC) abnormalities
(high total WBC count and lymphopenia) and a physician’s survival
prediction measured in weeks (Table
II). Validated cut-off points based on the total PaP score were
established to classify the patients into three prognostic groups
for survival at 30 days: group A (>70% probability of a 1-month
survival period), 0 to 5.5 points; group B (30–70% probability of a
1-month survival period), 5.6 to 11 points; group C (<30%
probability of a 1-month survival), 11.1–17.5 points (10,11)
(Table II).
 | Table IIPaP score. |
Table II
PaP score.
| Item | Score |
|---|
| Symptoms
(presence/absence) | |
| Anorexia | 1.0/0.0 |
| Dyspnea | 1.5/0.0 |
| Karnofsky performance
status | |
| ≥50 | 0.0 |
| 30–40 | 0.0 |
| 10–20 | 2.5 |
| Clinical prediction
of survival (weeks) | |
| >12 | 0.0 |
| 11–12 | 2.0 |
| 9–10 | 2.5 |
| 7–8 | 2.5 |
| 5–6 | 4.5 |
| 3–4 | 6.0 |
| 1–2 | 8.5 |
| Total white blood
cells (/mm3) | |
| Normal
(4,800–8,500) | 0 |
| High
(8,501–11,000) | 0.5 |
| Very high
(>11,000) | 1.5 |
| Lymphocyte
percentage | |
| Normal
(20.0–40.0) | 0 |
| Low
(12.0–19.9) | 1.0 |
| Very low
(0–11.9) | 2.5 |
| PaP score groups | |
| A | 0–5.5 |
| B | 5.6–11.0 |
| C | 11.1–17.5 |
Statistical analysis
The terminology used in this study conforms to the
Japanese Classification of Gastric Carcinoma, 3rd English edition
(12). Statistical analysis was
carried out using χ2 tests. Overall survival was
calculated from the time of enrolment to death. Median survival
time (MST) was calculated using the Kaplan-Meier non-parametric
test, while comparison between the different patient cohorts was
performed using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Median survival time
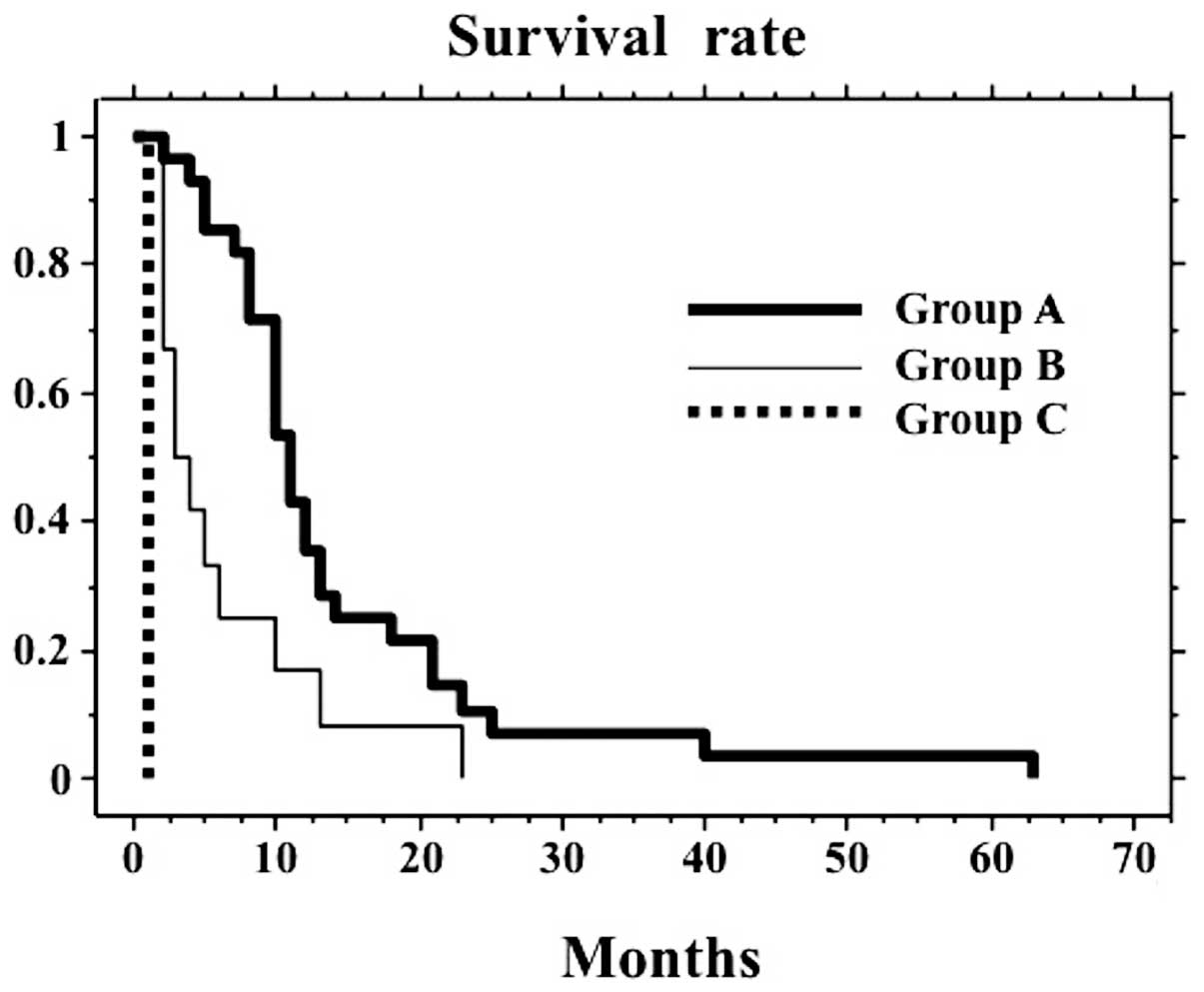
The MST of the 44 patients was 10 months. Patients
were divided into 3 subgroups, according to their PaP score. The
MST of 28 patients in group A (11 months) was much better compared
to the 12 patients in group B (3 months) or the 4 patients in group
C (1 month, P<0.0001, Fig. 1).
In the 40 patients in groups A and B, the correlation between
prognosis and factors considered to affect the prognosis was
analyzed (Table III). The presence
or absence of ascites or bypass surgery did not affect patient
survival.
 | Table IIIPrognosis of patients in PaP score
groups A and B. |
Table III
Prognosis of patients in PaP score
groups A and B.
| N | MST (months) | P-value |
|---|
| PaP score group | | | |
| A | 28 | 11 | 0.0045 |
| B | 12 | 3 | |
| Ascites | | | |
| Absent | 31 | 10 | 0.7548 |
| Present | 9 | 10 | |
| Surgical
intervention | | | |
| No | 25 | 10 | 0.7238 |
| Yes | 15 | 11 | |
Correlation between the PaP score and the
first-line chemotherapy regimens
The correlation between the PaP score and the
first-line chemotherapy regimens are shown in Table IV. The S-1 plus cisplatin regimen
was commonly used as first-line chemotherapy in PaP group A.
However, due to renal dysfunction, cisplatin was not used in a
number of patients in group B, thus S-1 plus docetaxel or S-1 alone
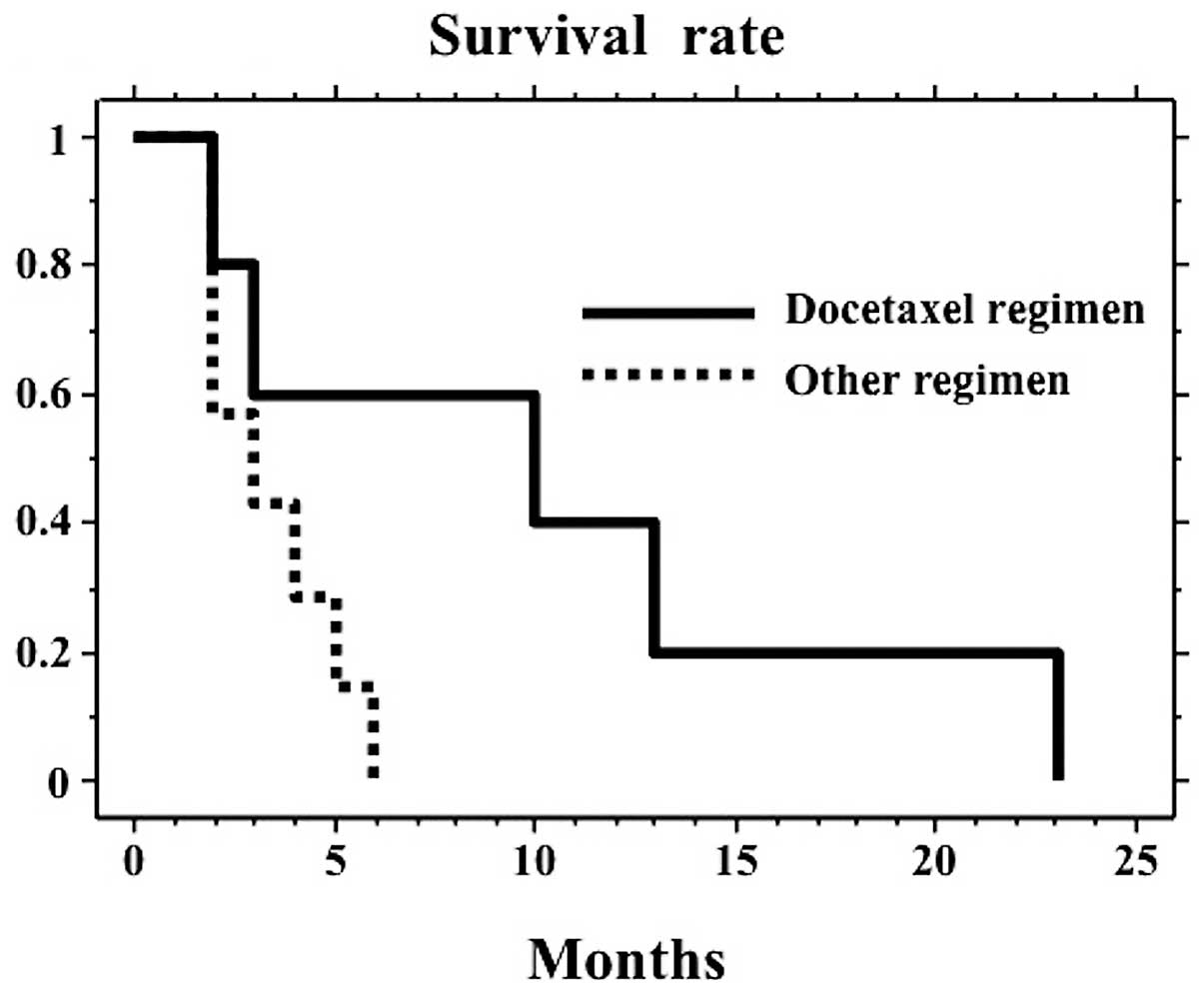
was selected in this group instead. In the 28 patients in group A,
the MST using the cisplatin regimen (10 months, n=16) did not
differ from the other regimens (11 months, n=12, P=0.221). Although
the difference was not significant (P=0.062), in the 12 patients in
group B, the docetaxel regimen prolonged the survival from 3 (other
regimens, MST, 3 months, n=7) to 10 months (docetaxel regimen, MST,
10 months, n=5, Fig. 2).
 | Table IVCorrelation between PaP score and
first-line chemotherapy. |
Table IV
Correlation between PaP score and
first-line chemotherapy.
| First-line
chemotherapy | PaP score group
|
|---|
| A | B | C |
|---|
| S-1 plus
cisplatin | 16 | 1 | 0 |
| S-1 plus
docetaxel | 8 | 5 | 0 |
| S-1 plus CPT-11 | 2 | 2 | 0 |
| S-1 only | 2 | 4 | 1 |
| BSC | 0 | 0 | 3 |
Discussion
Large-scale randomized phase III clinical trials may
reveal effective chemotherapeutic regimens for patients with
advanced cancers, with the exception of those of advanced age or
with poor PS. However, in clinical situations, it is difficult to
decide the most suitable chemotherapeutic regimen for patients with
short life expectancy or poor PS, such as patients with
non-resectable gastric cancer. Identifying the patients that may
benefit from palliative chemotherapy is quite difficult and its
usefulness when controlling symptoms and maintaining QOL has not
yet been proven (13,14).
The PaP score contains five parameters (symptom, PS,
inflammation, immunity and physician’s survival prediction)
associated with cancer patient survival. Findings of previous
studies have indicated that the PaP score may accurately estimate
pre-terminal patient survival (15–17).
Using the PaP score in 44 patients with non-resectable advanced
gastric cancer, the correlation between PaP score groups and
chemotherapeutic regimens was investigated. The findings showed
that in the PaP score group A, the S-1 plus cisplatin regimen was
commonly used and differences in chemotherapeutic regimens did not
affect the survival of the patients in this group. In comparison,
the survival of patients in PaP group B was extremely poor,
although the S-1 plus docetaxel regimen prolonged the survival of
these patients from 3 to 10 months. Although this study is
retrospective and the number of objective cases is small, the
docetaxel regimen may have a survival advantage in patients with a
poor prognosis.
Docetaxel is reported to have a low rate of grade
3/4 leucopenia and neutropenia (19.4 and 10.6%) and rare, severe
non-hematologic toxicities (18).
Docetaxel chemotherapy with or without S-1 has been a suitable
treatment for patients with advanced gastric cancer, advanced age
or poor PS (19).
In conclusion, in the treatment of advanced
non-resectable gastric cancer, the PaP score should be used to
select patients and chemotherapeutic regimens. The S-1 plus
docetaxel regimen is expected to improve outcomes in patients with
a poor PS.
References
|
1
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glimelius B, Hoffman K, Haglund U, Nyren O
and Sjoden PO: Initial or delayed chemotherapy with best supportive
care in advanced gastric cancer. Ann Oncol. 5:189–190.
1994.PubMed/NCBI
|
|
3
|
Shirasaka T, Shimamato Y, Ohshima H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koizumi W, Kurihara M, Nakano S and
Hasegawa K: Phase II study of S-1, a novel oral derivative of
5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative
Gastric Cancer Study Group. Oncology. 58:191–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H and
Takeuchi M: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): a phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koo DH, Ryu MH, Ryoo BY, Lee SS, Moon JH,
Chang HM, Lee JL, Kim TW and Kang YK: Three-week combination
chemotherapy with S-1 and cisplatin as first-line treatment in
patients with advanced gastric cancer: a retrospective study with
159 patients. Gastric Cancer. 15:305–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casaretto L, Sousa PLR and Mari JJ:
Chemotherapy versus support cancer treatment in advanced gastric
cancer: a meta-analysis. Braz J Med Biol Res. 39:431–440. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pirovano M, Maltoni M, Nanni O, Marinari
M, Indelli M, Zaninetta G, Petrella V, Bami S, Zecca E, Scarpi E,
Labianca R, Amadori D and Luporini G: A new palliative prognostic
score: A first step for the staging of terminally ill cancer
patients. J Pain Symptom Manage. 17:231–239. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tassinari D, Montanari L, Maltoni M,
Ballardini M, Piancastelli A, Musi M, Porzio G, Minotti V, Caraceni
A, Poggi B, Stella A, Aielli F and Scarpi E: The palliative
prognostic score and survival in patients with advanced solid
tumors receiving chemotherapy. Support Care Cancer. 16:359–370.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maltoni M, Nanni O, Pirovano M, Scarpi E,
Indelli M, Martini C, Monti M, Amoldi E, Piva L, Ravaioli A,
Cruciani G, Labianca R and Amadori D: Successful validation of the
palliative prognostic score in terminally ill cancer patients. J
Pain Symptom Manage. 17:240–247. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma. 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geels P, Eisenhauer E, Bezjak A, Zee B and
Day A: Palliative effect of chemotherapy: objective tumor response
is associated with symptom improvement in patients with metastatic
breast cancer. J Clin Oncol. 18:2395–2405. 2000.PubMed/NCBI
|
|
14
|
Browner I and Carducci MA: Palliative
chemotherapy: historical perspective, applications and
controversies. Semin Oncol. 32:145–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glare PA, Eychmueller S and McMahon P:
Diagnostic accuracy of the palliative prognostic score in
hospitalized patients with advanced cancer. J Clin Oncol.
22:4823–4828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stone CA, Tiernan E and Dooley BA:
Prospective validation of the palliative prognostic index in
patients with cancer. J Pain Symptom Manage. 35:617–622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stiel S, Bertram L, Neuhaus S, Nauck F,
Ostgathe C, Elsner F and Radbruch L: Evaluation and comparison of
two prognostic scores and the physicians’ estimate of survival in
terminally ill patients. Support Care Cancer. 18:43–49. 2010.
|
|
18
|
Massacesi C, Marcucci F, Rocchi MB,
Mazzanti P, Pilone A and Bonsignori M: Factors predicting
docetaxel-related toxicity: experience at a single institution. J
Chemother. 16:86–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii M: Chemotherapy for advanced gastric
cancer: ongoing phase III study of S-1 alone versus S-1 and
docetaxel combination (JACCRO GC03 study). Int J Clin Oncol.
13:201–205. 2008. View Article : Google Scholar : PubMed/NCBI
|