Introduction
Interstitial pneumonia (IP), although infrequently
encountered, is a serious adverse event that may occur during
anticancer chemotherapy. Two mechanisms have been proposed to
explain how anticancer drugs may injure the lung: anticancer drugs
damage the lung either directly through cytotoxic effects or
indirectly by activating the immune system of the patient. These
two mechanisms are involved in the initiation and progression of IP
(1). The onset of IP may differ
depending on the drug or pathogenic mechanism. Initial symptoms of
IP, including pyrexia, coughing and dyspnea, are usually
non-specific and unclear, and sometimes delay the appropriate
diagnosis (2). IP is significant
since a prolonged illness or severe respiratory sequelae could
interfere with subsequent anticancer treatments. Moreover, IP
itself could be fatal when not managed appropriately (2).
Neoadjuvant chemotherapy (NAC) is a promising
treatment for operable breast cancer cases since its feasibility
and efficacy have been previously demonstrated (3–6). It
is now the treatment of choice for patients with breast cancer as
it effectively improves the rate of breast conservation, as well as
the prognosis of the patient in a practical, clinical setting
(3,4). Fluorouracil, epirubicin and
cyclophosphamide (FEC) followed by weekly paclitaxel are the
standard NAC regimen in patients with operable advanced breast
cancer used in our institution. The pathological complete response
rate (pCR) of the patients treated with this regimen is as high as
39%. Additionally, this regimen has been administered effectively
in the out-patient setting and no life-threatening adverse events
have been observed (unpublished data).
The clinical courses of 5 patients who developed IP
during NAC at our institution are described in the present study.
The importance of an early diagnosis and treatment that promote
immediate recovery and prevent critical delays in the subsequent
anti-cancer therapy is discussed.
Patients and methods
Patients with IP during NAC
The adverse events of 95 women with locally advanced
breast cancer, who had been treated with FEC followed by weekly
paclitaxel between December, 2005 and February, 2010, were
retrospectively analyzed. Five patients discontinued NAC due to IP
(5.3%). The background characteristics of these 5 patients are
provided in Table I. These
patients were female, aged 45–63 years (average, 53). Clinically,
their initial diseases ranged from stage IIA to IIIC. One case also
had multiple sclerosis (Case 3) and another was a hepatitis B virus
carrier (Case 4). These diseases were not aggravated by the
therapy.
 | Table ICharacteristics of 5 patients with
interstitial pneumonia. |
Table I
Characteristics of 5 patients with
interstitial pneumonia.
| Case | Age (years) | Stage | ER | PgR | HER2 | Associated
disease | Smoking |
|---|
| 1 | 45 | IIIA | + | + | - | - | - |
| 2 | 50 | IIIB | - | - | - | - | - |
| 3 | 48 | IIB | - | - | - | Multiple
sclerosis | + |
| 4 | 63 | IIA | + | + | - | HBV carrier | - |
| 5 | 59 | IIIC | + | + | - | - | + |
NAC
The FEC 100 regimen involved intravenous
administration of 500 mg/m2 fluorouracil, 500
mg/m2 cyclophosphamide and 100 mg/m2
epirubicin on day 1. This regimen was administered every 21 days
for 4 cycles, followed by a once-weekly intravenous infusion of 80
mg/m2 paclitaxel for 12 weeks. Since all the cases had
HER-2-negative tumors, an anti-Her2 agent was not administered
during NAC. The protocol of this NAC regimen was approved by the
Chemotherapeutic Committee of the Osaka City University Hospital
(Osaka, Japan) and written informed consent was obtained from each
patient.
Results
Representative case (Case 1)
A 45-year-old female was diagnosed with left breast
cancer (T2 N1 M0 stage IIB) and NAC regimen was administered. The
patient became aware of a cough and general fatigue 4 days
following the 10th once-weekly paclitaxel administration. Since an
abnormal shadow was not observed on chest X-ray, the 11th scheduled
once-weekly paclitaxel was administered. The cough and general
fatigue worsened and the patient began to exhibit pyrexia and
hypoxia, despite the administration of antibiotics. Chest X-ray
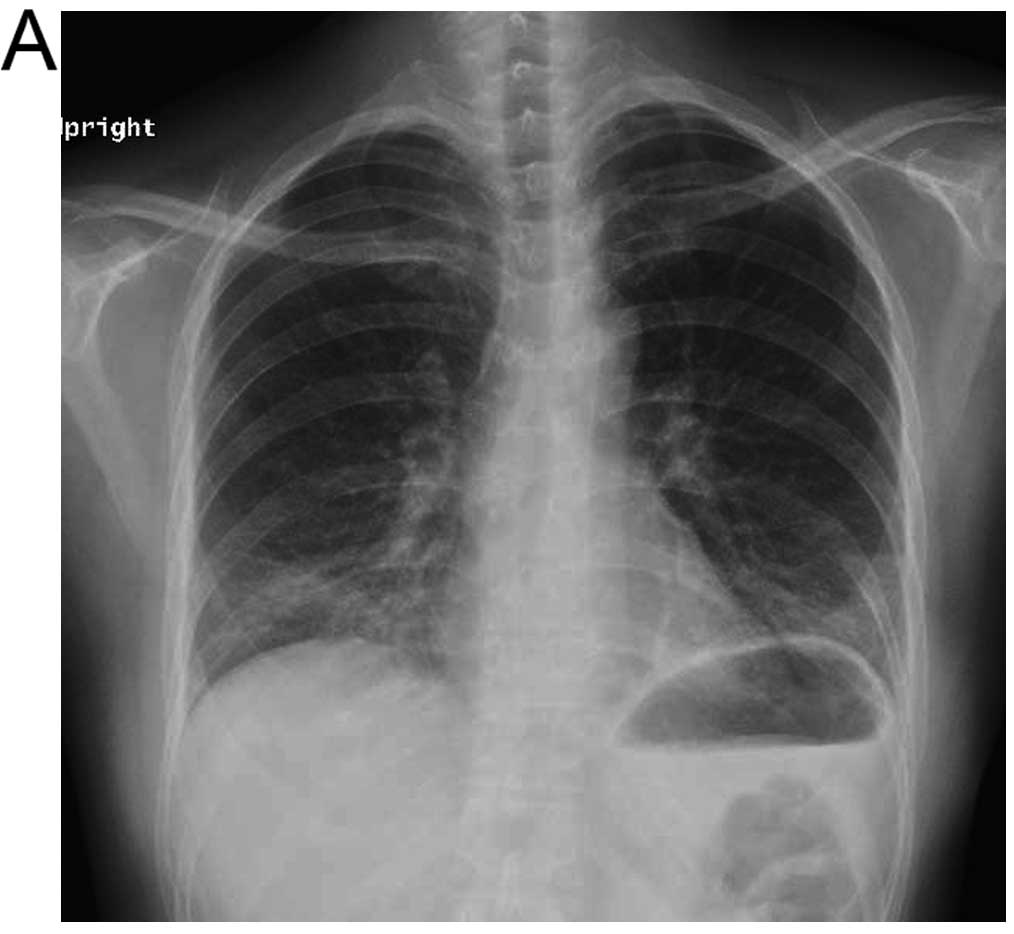
indicated ground glass opacity on the bilateral lower lung field
(Fig. 1A). A chest CT performed on
the same day showed diffuse ground glass opacity in the bilateral
lower lung field (Fig. 1B), and IP
was diagnosed. NAC (the 12th and last administration of once-weekly
paclitaxel) was suspended and steroid pulse therapy
(methylprednisolone 1 g for 3 days) was initiated upon admission.
The 12th paclitaxel dose was not administered. The symptoms
immediately improved and the abnormal X-ray and CT images were
eluminated (Fig. 1C and D). The
patient was discharged on the 5th hospital day. Breast-conserving
surgery with axillar lymph node dissection was performed 25 days
following the last dose of NAC, which was not behind the scheduled
timing. Local radiation therapy (50 Gy) was then added as scheduled
without complications. Pathological examination showed no residual
cancer cells. Goserelin and tamoxifen were administered as adjuvant
therapy for 37 months following the operation, and there were no
signs of recurrent disease. The patient was also free of any
respiratory symptoms.
Clinical course of the 5 patients with
IP
The clinical course of IP in the 5 patients is shown
in Table II. Most patients
developed cough, phlegm, general fatigue and pyrexia. Hypoxia
occurred in 3 cases. In all the cases, it was possible to initiate
treatment for IP within 2 weeks (8–14 days) from the appearance of
the initial symptoms. In one case with mild symptoms (Case 3), IP
was completely resolved by interrupting the NAC regimen. Steroid
pulse therapy was required in 2 cases, and additional treatment
with prednisolone for 3 weeks was required in the remaining 2
cases. Three patients had to be admitted to the hospital (3–11
days) due to hypoxia. In all the cases, the curative operation was
accomplished 3–6 weeks after the last administration of the
anticancer drug. Following recovery from IP, paclitaxel
administration was not resumed in any of the cases.
 | Table IIClinical characteristics and course of
5 patients with interstitial pneumonia (IP). |
Table II
Clinical characteristics and course of
5 patients with interstitial pneumonia (IP).
| Case | Symptoms | Onset during
therapy | SpO2
(%) | WBC (/μl) | CRP (Mg/dl) | Span from onset to IP
therapy (days) | Therapy for IP | Admission period
(days) | Time from diagnosis
to operation (weeks) | Type of
operation | Effect of NAC |
|---|
| 1 | C, Fa, Fe | 11th | 93 | 5,500 | 1.84 | 10 | MPSL | 5 | 4 | Bp+Ax | pCR |
| 2 | C, Fa, Fe | 9th | 90 | 2,600 | 17.56 | 13 | MPSL→PSL | 11 | 5 | Bt+Ax | PR |
| 3 | C, Fa | 3rd | 99 | 2,200 | 0.43 | 14 | - | - | 3 | Bp+Ax | pCR |
| 4 | C, Fa, Fe | 8th | 89 | 5,000 | 9.77 | 13 | MPSL→PSL | 14 | 6 | Bp+Ax | PR |
| 5 | Fa, Fe | 6th | 94 | 3,800 | 5.28 | 8 | MPSL (0.5 g) | - | 5 | Bt+Ax | PR |
Comparison of the five IP cases with
patients without IP
The 5 IP cases were compared with other cases
administered NAC during the same period in our institution
(Table III). Age, stage of breast
cancer, response rate, and pCR rate were not significantly
different between the 2 groups. Although the patients with IP were
administered lower total doses of paclitaxel compared to those
without IP (since NAC was interrupted), the efficacy of the
treatment was not significantly reduced.
 | Table IIIEfficacy of NAC: comparison of cases
with and without interstitial pneumonia (IP). |
Table III
Efficacy of NAC: comparison of cases
with and without interstitial pneumonia (IP).
| With IP | Without IP |
|---|
| No. | 5 | 90 |
| Average age
(years) | 53.0 | 52.4 |
| Stage II/III | 2/3 | 66/24 |
| Response rate | 100% | 92.2% |
| pCR rate | 40% | 37.8% |
| Total dose of
paclitaxel | 592
mg/m2 | 960
mg/m2 |
Discussion
FEC followed by weekly paclitaxel is used as a
standard NAC regimen for patients with operable advanced breast
cancer in our institution. Although IP has been reported to occur
during FEC and paclitaxel therapy (2,7), IP
was diagnosed during the administration of paclitaxel in all the
cases reported in this study. However, it was not possible to
determine whether FEC or paclitaxel induced IP as it occurred at
any time following the initiation of administration. However, a
clinical study has shown that the incidence of IP during paclitaxel
treatment is <1% (8). By
contrast, 5 cases with IP are described in the present study
(5.3%). It is therefore possible that preceding paclitaxel therapy
with FEC may have had an additional effect regarding the occurrence
of IP, potentially by increasing injury to the lung.
Febrile neutropenia is one of the most common
adverse events that may occur during chemotherapy and patients
usually recover within a few days when antibiotics and
granulocyte-colony stimulating factor are provided. However, as the
present study has shown, in cases where abnormal inflammation
indices are prolonged, a chest CT should be performed to exclude
the possibility of IP. The criteria used for diagnosing
drug-induced IP include the following: prior administration of a
drug that could induce IP; the presence of clinical observations,
imaging and pathological findings that do not contradict the
possibility of drug-induced IP; improvement of IP symptoms
following withdrawal from administration or steroid therapy;
recurrence of symptoms when the drug is administered again
(9). In acute-type IP, the main
symptoms are pyrexia, dry cough, dyspnea, and sometimes respiratory
insufficiency. However, in chronic-type IP, the mild respiratory
symptoms gradually progress from several weeks to several months.
Additionally, the onset of pneumonia is modified by genetic
factors, age, total drug dose, additional cytotoxic drug therapy,
prior or concurrent radiotherapy treatment, pre-existing pulmonary
disease, history of smoking, oxygen therapy, bone marrow or stem
cell transplantation, and renal insufficiency (10). Therefore, it is sometimes difficult
to determine a final diagnosis of IP based on clinical
manifestations alone. As a result, the diagnosis of drug-induced IP
often depends on imaging findings as well as clinical symptoms.
Although clinical imaging findings vary depending on the pathology
pattern, ground glass opacity in chest X-rays or CT is the main
characteristic of IP (11).
Therefore, in cases with suspected respiratory disease during
chemotherapy, it may be necessary to perform imaging investigations
to diagnose IP.
Two mechanisms are believed to initiate drug-induced
IP following treatment with anticancer drugs: the drugs may injure
the lung directly via their cytotoxic effects or indirectly by
affecting the immune system (1).
However, the two mechanisms may act together to induce IP (1). In the present study, symptoms
improved immediately following the termination of cytotoxic drug
treatment and the initiation of steroid therapy. Thus, both the
drug itself and alterations in the immune system may be involved in
the initiation of IP.
The standard drug therapy for IP is 0.5–1.0 mg/kg of
prednisolone/day (12). Steroid
pulse therapy (1,000 mg of methyl-prednisolone/day for 3 days) may
be necessary for cases with severe symptoms. The 4 cases treated
with steroid pulse therapy in this study showed significant
improvement in their IP symptoms, suggesting that steroid pulse
therapy is suitable for inducing an immediate response in cases of
drug-induced IP during NAC.
Since patients administered chemotherapy should be
considered as compromised hosts, Pneumocystis pneumonia and
cytomegalovirus infection should be excluded. Therefore, in the
present study, high spectrum antibiotics, co-trimoxazole, and
antiviral medication were provided during the steroid therapy until
it was clear that these infectious origins were not present, as
indicated by negative results of the tests measuring β-D-glucan and
C7-HRP.
Regarding the management of IP that arises during
chemo-therapy, the most important objectives are to immediately
diagnose drug-induced IP and to suspend chemotherapy in order to
prevent disease aggravation. Therefore, physicians should always be
aware of the fact that IP may occur at any time during
chemotherapy, in particular for patients who are administered NAC.
This is because IP may interfere with scheduled future anticancer
treatment plans, particularly when it is prolonged or aggravated by
delays in appropriate management. Furthermore, it should be
emphasized that early steroid pulse therapy allows for a rapid and
complete cure of IP that arises during chemotherapy.
References
|
1.
|
Delaunois LM: Mechanisms in pulmonary
toxicology. Clin Chest Med. 25:1–14. 2004. View Article : Google Scholar
|
|
2.
|
Nagata S, Ueda N, Yoshida Y, et al: Severe
interstitial pneumonitis associated with the administration of
taxanes. J Infect Chemother. 16:340–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wolmark N, Wang J, Mamounas E, et al:
Preoperative chemotherapy in patients with operable breast cancer:
nine-year results from National Surgical Adjuvant Breast and Bowel
Project B-18. J Natl Cancer Inst Monogr. 30:96–102. 2001.
|
|
4.
|
van der Hage JA, van de Velde CJ, Julien
JP, et al: Preoperative chemotherapy in primary operable breast
cancer: results from the European Organization for Research and
Treatment of Cancer trial 10902. J Clin Oncol. 19:4224–4237.
2001.
|
|
5.
|
Bear HD, Anderson S, Brown A, et al: The
effect on tumor response of adding sequential preoperative
docetaxel to preoperative doxorubicin and cyclophosphamide:
preliminary results from National Surgical Adjuvant Breast and
Bowel Project Protocol B-27. J Clin Oncol. 21:4165–4174. 2003.
View Article : Google Scholar
|
|
6.
|
Bear HD, Anderson S, Smith RE, et al:
Sequential preoperative or postoperative docetaxel added to
preoperative doxorubicin plus cyclophosphamide for operable breast
cancer: National Surgical Adjuvant Breast and Bowel Project
Protocol B-27. J Clin Oncol. 24:2019–2027. 2006. View Article : Google Scholar
|
|
7.
|
Segura A, Yuste A, Cercos A, et al:
Pulmonary fibrosis induced by cyclophosphamide. Ann Pharmacother.
35:894–897. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Khan A, McNally D, Tutschka PJ and
Bilgrami S: Paclitaxel-induced acute bilateral pneumonitis. Ann
Pharmacother. 31:1471–1474. 1997.PubMed/NCBI
|
|
9.
|
Ellis SJ, Cleverley JR and Müller NL:
Drug-induced lung disease: high-resolution CT findings. AJR Am J
Roentgenol. 175:1019–1024. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Camus PH, Foucher P, Bonniaud PH and Ask
K: Drug-induced infiltrative lung disease. Eur Respir J Suppl.
32:S93–S100. 2001.
|
|
11.
|
Elliot TL, Lynch DA, Newell JD Jr, et al:
High-resolution computed tomography features of nonspecific
interstitial pneumonia and usual interstitial pneumonia. J Comput
Assist Tomogr. 29:339–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wang GS, Yang KY and Perng RP:
Life-threatening hypersensitivity pneumonitis induced by docetaxel
(taxotere). Br J Cancer. 85:1247–1250. 2001. View Article : Google Scholar : PubMed/NCBI
|